Abstract
Atm-deficient mice die of malignant thymic lymphomas characterized by translocations within the Tcrα/δ locus, suggesting that tumorigenesis is secondary to aberrant responses to double-stranded DNA (dsDNA) breaks that occur during RAG-dependent V(D)J recombination. We recently demonstrated that development of thymic lymphoma in Atm−/− mice was not prevented by loss of RAG-2. Thymic lymphomas that developed in Rag2−/− Atm−/− mice contained multiple chromosomal abnormalities, but none of these involved the Tcrα/δ locus. These findings indicated that tumorigenesis in Atm−/− mice is mediated by chromosomal translocations secondary to aberrant responses to dsDNA breaks and that V(D)J recombination is an important, but not essential, event in susceptibility. In contrast to these findings, it was recently reported that Rag1−/− Atm−/− mice do not develop thymic lymphomas, a finding that was interpreted as demonstrating a requirement for RAG-dependent recombination in the susceptibility to tumors in Atm-deficient mice. To test the possibility that RAG-1 and RAG-2 differ in their roles in tumorigenesis, we studied Rag1−/− Atm−/− mice in parallel to our previous Rag2−/− Atm−/− study. We found that thymic lymphomas occur at high frequency in Rag1−/− Atm−/− mice and resemble those that occur in Rag2−/− Atm−/− mice. These results indicate that both RAG-1 and RAG-2 are necessary for tumorigenesis involving translocation in the Tcrα/δ locus but that Atm deficiency leads to tumors through a broader RAG-independent predisposition to translocation, related to a generalized defect in dsDNA break repair.
Ataxia telangiectasia (AT) is an autosomal recessive disease caused by mutation of the ataxia telangiectasia mutated (ATM) gene (16). Absence of the ATM protein, which plays an important role in cellular responses to the presence of double-stranded DNA (dsDNA) breaks and induction of cell cycle checkpoints (6, 18), results in a pleiotrophic phenotype characterized by progressive cerebellar ataxia, ocular telangiectasias, immunodeficiencies, increased sensitivity to ionizing radiation, and increased incidence of lymphoid cancers (2, 17, 20). While the exact pathophysiological mechanism relating the genetic defect to the clinical manifestations of the disease has not been elucidated, it is plausible that several components of the AT phenotype, including the predisposition to development of lymphoid malignancies, result from aberrant recognition and repair of dsDNA breaks such as those that normally occur during V(D)J recombination in lymphoid cells (15).
Support for this hypothesis comes from studies of the murine model of AT. Karyotypic analysis of the thymic lymphomas that characteristically develop in Atm-deficient mice (1, 3, 22) has identified consistent translocations within the Tcrα/δ locus, suggesting the involvement of V(D)J recombination in the process of lymphomagenesis (11). Because V(D)J recombination is dependent upon the recombinase-activating genes (Rag1 and Rag2) (13, 19), Liao and Van Dyke generated Rag1−/− Atm−/− mice to assess the requirement for V(D)J recombination in tumorigenesis (8). Consistent with the hypothesis that V(D)J recombination is involved in tumorigenesis in Atm-deficient mice, Liao and Van Dyke reported that no thymic lymphomas developed in Rag1−/− Atm−/− mice, whereas all Rag1+/− Atm−/− mice died of thymic lymphoma. In contrast, an analogous study conducted by our group observed that Rag2−/− Atm−/− mice did develop thymic lymphomas and did so through a mechanism that did not involve Tcr α/δ translocation (14). Given that absence of either RAG-1 or RAG-2 eliminates V(D)J recombination (13, 19), the disparity in the results of these two tumor incidence studies was surprising. A difference in lymphoma susceptibility between Rag1−/− Atm−/− mice and Rag2−/− Atm−/− mice could signify previously unidentified differences in RAG-1 and RAG-2 function. Alternatively the difference in results of previously reported studies of Rag1−/− Atm−/− and Rag2−/− Atm−/− mice could be attributable to experimental variables such as differences in genetic background and/or environmental differences in mouse colonies used in these studies. To more directly compare the roles of RAG-1 and RAG-2 in the generation of thymic lymphomas, we have studied Rag1−/− Atm−/− mice under the conditions previously used in characterizing susceptibility to lymphomas in Rag2−/− Atm−/− mice.
MATERIALS AND METHODS
Mice.
The creation of Atm-deficient mice (allele designation Atmins5790neo) has been described previously (1). Progeny of heterozygote matings were genotyped by PCR (9). Rag1-deficient mice (C57BL/6J-sv/129; Jackson Labs) were identified phenotypically by flow cytometric analysis of peripheral blood lymphocytes as described previously (14). To generate mice deficient for both Rag1 and Atm, double heterozygotes were crossed and progeny were typed as described above.
Tumor incidence and histopathological analyses.
As described previously (14), mice were examined daily for evidence of morbidity and mortality. Mice were sacrificed when death was judged to be imminent and prepared for necropsy. Organs from mice that were found dead were similarly preserved. Tissues were fixed, sectioned, stained, and examined by light microscopy (L. G. Luna, Histopathological methods and color atlas of special stains and tissue, American Histolabs, Inc., Publications Division, Gaithersburg, Md.).
Statistical analysis.
Kaplan-Meier survival curves were generated as previously described (7, 14). Statistical significance was determined by the Mantel-Haenszel method (12). Individual P values are provided after adjustment for multiple comparisons according to the method of Hochberg (4). All P values are two-tailed.
Thymic lymphoma cell culture.
Thymic lymphomas were harvested, and single-cell suspensions were cultured in complete medium supplemented with 100 IU of human recombinant interleukin 2 per ml and 6 ng of recombinant murine interleukin 7 per ml as described previously (14). Tail DNA was isolated from all Rag1−/− Atm−/− mice developing thymic lymphomas and genotyped by PCR for both Rag1 and Rag2 to verify genotypes. Rag2 PCR typing was carried out as previously described (5), and Rag1 typing was carried out under identical conditions, with substitution of Rag1-specific primers as follows: RAG-1 forward, CAA CAT CTG CCT TCA CTT C; RAG-1 reverse, TAC CCT GAG CTT CAG TTC TG; neo, TAT CAG GAC ATA GCG TTG GCG.
SKY and fluorescent in situ hybridization (FISH).
Metaphase chromosomes were prepared from tumor cell lines at early-passage numbers (0 to 2) as described previously (14). Spectral karyotyping (SKY) of the tumor cell lines was performed following published procedures (10, 14, 21). Six to ten metaphases were analyzed for each tumor. BAC clones (Genome Systems, St. Louis, Mo.) for the TcrCa and TcrVd were labeled with digoxigenin-dUTP and biotin-dUTP (Roche Biomolecular, Indianapolis, Ind.) and detected with fluorescein- and rhodamine-conjugated avidin and antibody as described previously (11). Images were acquired with a Leica DMRXA microscope (Leica, Wetzlar, Germany) and Q-FISH software (Leica Imaging Systems, Cambridge, United Kingdom).
RESULTS AND DISCUSSION
Incidence of malignant thymic lymphomas in mice deficient in both Atm and Rag1.
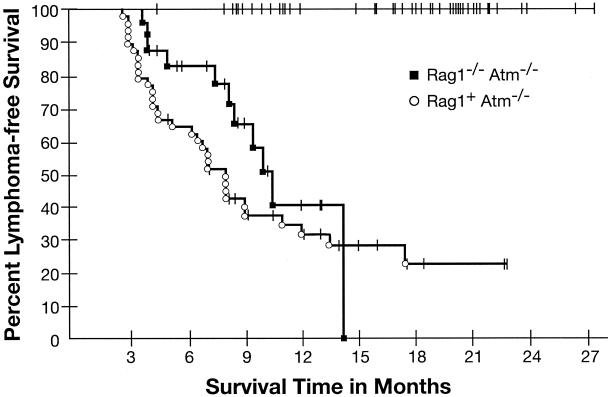
As in our previous study, Rag1−/− Atm−/− mice, like Rag2−/− Atm−/− mice, were found to develop malignant thymic lymphomas (Fig. 1). Of 24 Rag1−/− Atm−/− mice monitored, 11 (46%) were diagnosed with malignant thymic lymphoma at necropsy compared with 0 of 33 Rag1+ Atm+/+ mice (P < 0.001 by Mantel-Haenszel test comparing overall probability as a function of time). Median age at death for Rag1−/− Atm−/− mice was 8.3 months (range of times to death, 3.75 to 14.25 months). As expected, Rag1+ Atm−/− littermate controls also developed malignant thymic lymphomas, with 33 of 48 Rag1+ Atm−/− mice (69%) having this diagnosis at necropsy (Fig 1) and a median age at death of 7.0 months (range, 2.75 to 17.5 months).
FIG. 1.
Kaplan-Meier analysis of tumor incidence. Thymic lymphoma-free survival is plotted versus time in months for Rag1−/− Atm−/− mice and littermate controls. A drop in the curve represents the sacrifice or death of an animal diagnosed at necropsy with malignant thymic lymphoma. Tick marks represent the sacrifice or death of an animal that was not subsequently diagnosed with malignant thymic lymphoma. As no Rag1−/− Atm+/+ or Rag1+ Atm+/+ animals were diagnosed with malignant thymic lymphomas, the curves for these two groups are at 100% event-free survival.
In addition to malignant thymic lymphomas, Atm-deficient mice also showed an increased incidence of nonthymic tumors compared with control mice. Four of 24 Rag1−/− Atm−/− mice (17%) and 6 of 48 Rag1+ Atm−/− mice (13%) developed various nonthymic tumors (carcinomas and sarcomas) compared to 0 of 33 Rag1+ Atm+/+ mice (P = 0.0002 and P < 0.0001, respectively, based on actuarial probability of developing nonthymic tumors and after adjustment for both comparisons). The occurrence of tumors other than thymic lymphomas was similar to our previously reported observation with Rag2−/− Atm−/− mice.
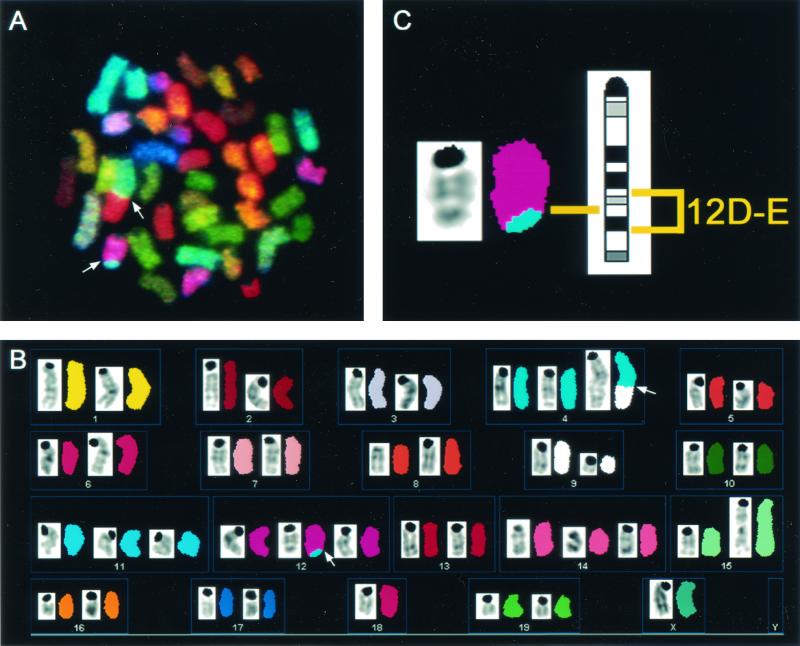
To investigate potential mechanisms of tumorigenesis in Rag1-deficient mice, molecular cytogenetic analysis using SKY was employed to identify chromosomal aberrations in early-passage cells cultured from three Rag1−/− Atm−/− thymic lymphomas. Thymic lymphoma cells from Rag1−/− Atm−/− mice had multiple translocations in each tumor (Table 1). A representative metaphase from tumor no. 20154 is shown in Fig. 2. This tumor had a translocation [T(12;4)] in addition to other chromosomal aberrations. Remarkably, each of three Rag1−/− Atm−/− tumors analyzed had a chromosomal translocation involving chromosome 12, bands D and E. This same breakpoint region has been identified in both Atm−/− and Rag2−/− Atm−/− thymic lymphomas (11, 14). Hybridization using a FISH probe containing the Igh-6 gene (at 12F1) confirmed that the region distal to the breakpoint was lost (data not shown). One tumor (no. 20343) revealed a complex translocation involving chromosome 14. In order to exclude the involvement of the Tcr locus in this rearrangement, we used FISH with BAC clones for the constant and variable regions of the TCR α/δ locus, respectively. All metaphase spreads and interphase nuclei analyzed (n = 19) showed colocalization of the differentially labeled probes. We can therefore exclude the possibility that intrachromosomal rearrangements, such as inversions, disrupt the integrity of the Tcr locus.
TABLE 1.
Chromosomal aberrations in Rag1−/− Atm−/− thymic lymphomas
| Rag1−/− Atm−/− tumor no. | SKY-detected structural aberrations | SKY-detected gains and losses |
|---|---|---|
| 8567 | Del(9) | +5, +10, +15 |
| T(12D-E;9) | −7 | |
| T(17;15) | ||
| 20343 | T(9;15;14) | +18 |
| T(12D-E;15) | ||
| T(Dp(14);15;9) | ||
| 20154 | T(4;9) | +3, +4 |
| Del(9) | −6 | |
| T(12D-E;4) | ||
| Dp(15B-D) |
FIG. 2.
Spectral karyotyping analysis of a metaphase cell from Rag1−/− Atm−/− thymic lymphoma no. 20154 displaying chromosome aberrations. The entire metaphase is shown in display colors in panel A, with arrows indicating translocated chromosomes. The spectrum-based classification karyotype is shown in panel B, with each pseudocolored chromosome positioned next to its inverted 4′,6′-diamidino-2-phenylindole-banded image. The T(12;4) is enlarged in panel C next to the G-band ideogram for chromosome 12, to highlight the band region involved in the translocation. The full karyotype of this metaphase is 42, X, T(4D;9A), Del(9B-F), T(12D-E;4D), Dp(15B-D), +4, +11, +12, +14, −18, −Y. Note that this representative metaphase for tumor no. 20154 does not contain every clonal aberration that is described in Table 1.
The results of this study further support our previous finding that V(D)J recombination is not required for tumorigenesis in Atm-deficient mice (14). Rag1−/− Atm−/− mice, like Rag2−/− Atm−/− mice, develop malignant thymic lymphomas. The basis for the difference between the findings reported here and those of Liao and Van Dyke (8), who failed to observe thymic lymphomas in Rag1−/− Atm−/− mice, is not clear but may reflect variables including genetic differences in the strains employed and/or environmental conditions under which animals were housed. Tumors from Rag1−/− Atm−/− thymic lymphomas resemble those from Rag2−/− Atm−/− thymic lymphomas in several aspects, including cell surface phenotype (variable levels of CD4 and CD8 expression and absence of TCR α/β expression; data not shown), the presence of multiple translocations in individual tumor cells, and the absence of Tcr α/δ locus translocation—a translocation which is consistently seen in Atm−/− thymic lymphomas (11, 14). It thus appears that the mechanism of thymic lymphoma development in Rag1−/− Atm−/− mice, as in Rag2−/− Atm−/− mice, is related to abnormal repair of dsDNA breaks that occur secondary to processes other than V(D)J recombination. The observance of multiple chromosomal aberrations as well as the lack of translocations within the Tcr α/δ locus in Rag1−/− Atm−/− thymic lymphomas is consistent with this hypothesis. Of note is the frequent translocation site at chromosome 12D-E seen in thymic lymphomas from Atm−/− mice, Rag2−/− Atm−/− mice, and Rag1−/− Atm−/− mice. Further studies to elucidate the genes involved in this region may help to define the mechanism of tumorigenesis in Atm-deficient mice.
Acknowledgments
L.K.P., Z.W., and M.V. contributed equally to this work.
We thank Genevieve Sanchez, Amy Werling, Wendy Davis, and staff members at Bioqual for providing excellent animal care and Corinne Epperly for expert technical assistance. Sincere appreciation is extended to Andre Nussenzweig for critical reading of the manuscript.
REFERENCES
- 1.Barlow, C., S. Hirotsune, R. Payulor, et al. 1996. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86:159-171. [DOI] [PubMed] [Google Scholar]
- 2.Boder, E. 1985. Ataxia-telangiectasia: an overview, p. 1-63. In R. A. Gatti and M. Swift (ed.), Ataxia-telangiectasia: genetics, neuropathology, and immunology of a degenerative disease of childhood. Liss, New York, N.Y.
- 3.Elson, A., Y. Wang, C. J. Daugherty, et al. 1996. Pleiotropic defects in ataxia-telangiectasia protein-deficient mice. Proc. Natl. Acad. Sci. USA 93:13084-13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hochberg, Y. 1988. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75:800-802. [Google Scholar]
- 5.Horton, R. M., P. I. Karachunski, and B. M. Conti-Fine. 1995. PCR screening of transgenic RAG-2 knockout immunodeficient mice. BioTechniques 19:690-691. [PubMed] [Google Scholar]
- 6.Jeggo, P. A., A. M. Carr, and A. R. Lehmann. 1998. Splitting the ATM: distinct repair and checkpoint defects in ataxia-telangiectasia. Trends Genet. 14:312-316. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan, E., and P. Meier. 1958. Non-parametric estimation from incomplete observations. J. Am. Stat. Assoc. 53:457-481. [Google Scholar]
- 8.Liao, M.-J., and T. Van Dyke. 1999. Critical role for Atm in suppressing V(D)J recombination-driven thymic lymphoma. Genes Dev. 13:1246-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao, M.-J., C. Yin, C. Barlow, A. Wynshaw-Boris, and T. Van Dyke. 1999. Atm is dispensable for p53 apoptosis and tumor suppression triggered by cell cycle dysfunction. Mol. Cell. Biol. 19:3095-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liyanage, M., A. Coleman, S. Du Manoir, et al. 1996. Multicolor spectral karyotyping of mouse chromosomes. Nat. Genet. 14:312-315. [DOI] [PubMed] [Google Scholar]
- 11.Liyanage, M., Z. Weaver, C. Barlow, A. Coleman, D. G. Pankratz, S. Anderson, A. Wynshaw-Boris, and T. Ried. 2000. Abnormal rearrangement within the α/δ T-cell receptor locus in lymphomas from Atm-deficient mice. Blood 96:1940-1946. [PubMed] [Google Scholar]
- 12.Mantel, N. 1966. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chem. Rep. 50:163-170. [PubMed] [Google Scholar]
- 13.Mombaerts, P., J. Iacomini, R. S. Johnson, K Herrup, S. Tonegawa, and V. E. Papaionnaou. 1992. RAG-1 deficient mice have no mature B and T lymphocytes. Cell 68:869-877. [DOI] [PubMed] [Google Scholar]
- 14.Petiniot, L. K., Z. Weaver, C. Barlow, et al. 2000. Recombinase-activating gene (RAG) 2-mediated V(D)J recombination is not essential for tumorigenesis in Atm-deficient mice. Proc. Natl. Acad. Sci. USA 97:6664-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rotman, G., and Y. Shiloh. 1998. ATM: from gene to function. Hum. Mol. Genet. 7:1555-1563. [DOI] [PubMed] [Google Scholar]
- 16.Savitsky, K., A. Bar-Shira, S. Gilad, et al. 1995. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 268:1749-1753. [DOI] [PubMed] [Google Scholar]
- 17.Sedwick, R. P., and E. Boder. 1972. Ataxia-telangiectasia, p. 267-339. In P. J. Vinken and G. W. Bruyn (ed.), Handbook of clinical neurology, vol. 14. Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- 18.Shiloh, Y. 2001. ATM and ATR: networking cellular responses to DNA damage. Curr. Opin. Genet. Dev. 11:71-77. [DOI] [PubMed] [Google Scholar]
- 19.Shinkai, Y., G. Rathbun, E. Lam, et al. 1992. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68:855-867. [DOI] [PubMed] [Google Scholar]
- 20.Waldmann, T. A. 1982. Immunological abnormalities in ataxia-telangiectasia, p. 37-52. In B. A. Bridges, and D. G. Harnden (ed.), Ataxia-telangiectasia: a cellular and molecular link between cancer, neuropathology and immune deficiency. John Wiley, New York, N.Y.
- 21.Weaver, Z. A., S. J. McCormack, M. Liyanage, et al. 1999. A recurring pattern of chromosomal aberrations in mammary gland tumors of MMTV-cmyc transgenic mice. Genes Chromosomes Cancer 25:251-260. [PubMed] [Google Scholar]
- 22.Xu, Y., T. Ashley, E. E. Brainerd, R. T. Bronson, M. S. Meyn, and D. Baltimore. 1996. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 10:2411-2422. [DOI] [PubMed] [Google Scholar]