Abstract
Telomeres of Drosophila melanogaster contain arrays of the retrotransposon-like elements HeT-A and TART. Their transposition to broken chromosome ends has been implicated in chromosome healing and telomere elongation. We have developed a genetic system which enables the determination of the frequency of telomere elongation events and their mechanism. The frequency differs among lines with different genotypes, suggesting that several genes are in control. Here we show that the Su(var)2-5 gene encoding heterochromatin protein 1 (HP1) is involved in regulation of telomere length. Different Su(var)2-5 mutations in the heterozygous state increase the frequency of HeT-A and TART attachment to the broken chromosome end by more than a hundred times. The attachment occurs through either HeT-A/TART transposition or recombination with other telomeres. Terminal DNA elongation by gene conversion is greatly enhanced by Su(var)2-5 mutations only if the template for DNA synthesis is on the same chromosome but not on the homologous chromosome. The Drosophila lines bearing the Su(var)2-5 mutations maintain extremely long telomeres consisting of HeT-A and TART for many generations. Thus, HP1 plays an important role in the control of telomere elongation in D. melanogaster.
Telomeres are specialized DNA-protein complexes at the termini of linear chromosomes that ensure the stability of eukaryotic genomes (47, 64, 65). Specialized mechanisms have evolved to add DNA to the ends of eukaryotic chromosomes, balancing the loss from terminal DNA underreplication (10, 47). In most eukaryotes, a special reverse transcriptase, telomerase, adds telomeric DNA repeats to the chromosome ends, using an internal RNA template (10, 28, 29, 47). In contrast, telomeres of Drosophila melanogaster consist of multiple copies of HeT-A and TART elements having features of non-long-terminal-repeat retrotransposons (6, 40, 47, 48), in particular, an oligo(A) tract at the 3′ end. HeT-A and TART in telomeres are arranged head to tail (6, 36, 47, 61).
Terminal deletions in Drosophila have been obtained (5, 7, 27, 35, 41, 57). Again in contrast to mammalian chromosomes, broken Drosophila chromosomes behave as capped ones: they are stably transmitted through many generations (7). HeT-A and TART were found to be transposed to the ends of broken chromosomes (3, 4, 8, 18, 54, 57). It was shown that Drosophila telomeres may be elongated by the transposition of mobile elements to receding chromosome ends (6, 9, 27, 40, 47, 48), as well as by gene conversion using the homologous telomeric sequences as template and by recombination between the telomeric sequences (32, 42). The existence of different mechanisms for telomere elongation prompts one to think that telomere length may be regulated by an as-yet-uncharacterized protein complex bound thereto.
Heterochromatin protein 1 (HP1) has been reported recently to mediate normal telomere behavior in Drosophila (23). HP1 is a conserved nonhistone chromosomal protein that is best known for its preferential binding to pericentric heterochromatin (31, 51). The gene encoding HP1, Su(var)2-5, is a dosage-dependent regulator of heterochromatin silencing in Drosophila (20, 21). HP1 is found in all Drosophila telomeres, including the ends of stable terminal deletions (23). The lack of HP1 results in multiple telomere-telomere fusions producing a remarkable spectrum of abnormal chromosome configurations. Thus, HP1 is an important functional component of both heterochromatin and telomere.
In this study, we assess the role of HP1 in the regulation of telomere length. Truncated chromosomes with breaks within the yellow gene have been used to assess the frequency and study the mechanism of telomere shortening and elongation (2, 3, 5, 7, 8, 32, 42). The yellow gene is required for larval and adult cuticle pigmentation (60). The enhancers controlling yellow expression in the wings and body cuticle are located in the upstream region of the yellow gene, whereas the enhancer controlling yellow expression in bristles resides in the intron (5, 25, 39). Therefore, flies with terminal DNA breakpoints in the upstream region that remove the wing and body enhancers display a y2-like phenotype: wild-type pigmentation in bristles and lack of pigmentation in the body cuticle, wing blade, and aristae (5). Here we found that additions of HeT-A or TART to the upstream yellow sequences activate yellow expression only in aristae. Terminal deficiencies with breaks at the yellow promoter or within the yellow transcription unit result in a y1-like phenotype, i.e., complete repression of yellow function (5, 7, 32). Additions of HeT-A restore yellow expression in the bristles and aristae (7, 32), owing to the presence of the promoter at the HeT-A 3′ end (13).
Using these observations, we have developed a genetic screen system to monitor the frequency of HeT-A/TART attachment to the yellow receding end. Previously we found that transposition of HeT-A depended on the line genotype and ranged from less than 2 × 10−5 to 2 × 10−3 (32). Here, we show that mutations in the Su(var)2-5 gene in the heterozygous state raise the frequency of HeT-A/TART addition to the ends of terminally truncated chromosomes by more than a hundred times. The results of Southern blot analysis and sequencing suggest that attachment takes place either by transposition to the chromosome end or by recombination between the 3′ terminus of the telomeric HeT-A or TART element and the receding end of the yellow regulatory region.
Previously, we observed that the ends of yellow terminal deficiencies could also be elongated by gene conversion if the yellow gene on the homologous chromosome served as template (42). Su(var)2-5 mutations do not significantly increase the frequency of gene conversion with the homologous chromosome as the template source. In contrast, these mutations dramatically potentiate terminal DNA elongation by gene conversion if the template for DNA synthesis is on the same chromosome.
The existence of extremely large arrays of HeT-A and TART in fly lines bearing Su(var)2-5 mutations for many generations suggests that HP1 is required for regulation of telomere length in D. melanogaster.
MATERIALS AND METHODS
Genetic crosses.
All Drosophila stocks were maintained at 25°C on a standard yeast extract medium. Genetic symbols of the yellow alleles and their origin were described elsewhere (32, 42). Most of genetic markers used were described by Lindsley and Zimm (37). The yac chromosome has a deletion of the yellow and achaete genes but not of any vital genes and thus provides an opportunity to examine the behavior of the yellow gene on the homologue in the absence of other yellow sequences. The In(I)wm4h; Su(var)2-501/SM1 line, In(I)wm4h; Su(var)2-504/In(2L)tIn(2R)Cy Cy1Roi1pR1cn1 line, and In(I)wm4h; Su(var)2-505/In(2L)Cy1 In(2R)Cy line were obtained from the Umeå stock center. The Df(1)w y1w67c23; Su(var)2-502/CyO y+ line, Df(1)w y1w67c23; Su(var)2-504/CyO y+ line, Df(1)w y1w67c23; Su(var)2-505/CyO y+ line, and yw; Dp(2, 2)p90/CyO y+ line were obtained from J. Eissenberg and described in Lu et al. (38). The ytataw line kindly provided by C.-T. Wu is described in Morris et al. (43). The Gaiano line was obtained from M. Gatti.
For determination of the yellow phenotype, the extent of pigmentation in different tissues of adult flies was estimated visually for 3- to 5-day-old females developing at 25°C.
Molecular methods.
For Southern blot hybridization, DNA from adult flies was isolated using the protocol described by Ashburner (1). Treatment of DNA with restriction endonucleases, blotting, fixation, and hybridization with radioactive probes prepared by random primer extension were performed as described in the protocols for the Hybond-N+ nylon membrane (Amersham, Arlington Heights, Ill.) and in the laboratory manual (52). Phages with cloned regions of the yellow locus were obtained from J. Modolell. The clones of HeT-A and TART were obtained from M. L. Pardue and K. L. Traverse. The probes were made from gel-isolated fragments after appropriate restriction digestion of plasmid subclones.
The junctions between newly transposed mobile elements and the DNA terminus were cloned by DNA amplification with two oligonucleotide primers. The primers used in DNA amplification were from the yellow gene and HeT-A. The numbers of nucleotide map positions are given in brackets in accordance with the yellow (26) and HeT-A (8) sequences. The primers in yellow are as follows: y1 (CCTGGAACATTGCAC [3053 to 3039]), y2 (AAGACGGCGTCACCAAGGTGATC [3101 to 3078]), and y3 (ACTTCCACTTACCATCACGCCAG [3293 to 3271]). The primers in HeT-A are as follows: h1 (TGTTGCAAGTGGCGCGCATCC [456 to 434]) and h2 (GGTGCTTCCGTACTTCTGGCGG [359 to 338]). The primer in TART is t1 (CGAAACGCAACAACAAAATGG [1124 to 1144] [TARTC]).
The products of amplification were fractionated by electrophoresis in 1.5% agarose gels in Tris-acetate-EDTA buffer. The successfully amplified products were cloned in a Bluescript plasmid (Stratagene, La Jolla, Calif.) and sequenced using the Amersham sequencing kit (Amersham).
RNA extraction and Northern hybridization were done as described by Danilevskaya et al. (15). The HeT-A probe, kindly provided by M. L. Pardue, was from element 23Zn-1 (GenBank accession no. U06920); the open reading frame spanned nucleotides 1746 to 4421 (46).
RESULTS
The frequency of HeT-A and TART attachment to a broken chromosome end is much higher on a Su(var)2-5 mutant background.
First, we tested whether a decrease of functional HP1 concentration induced by the Su(var)2-5 mutations could change the frequency of HeT-A/TART attachment to the broken chromosome end. HP1 is a likely candidate for the control of telomere elongation, as it binds to telomeres, and some mutations in the Su(var)2-5 gene encoding HP1 affect the normal behavior of telomeres (23). We have tested several available Su(var)2-5 mutations for their influence on the frequency of the HeT-A/TART attachment to the broken chromosome end by using a modification of the previously described system (32, 42).
In three Su(var)2-5 alleles, HP1 fails to interact with telomeres and terminally deficient chromosomes. These mutations are independently obtained recessive lethals (63). Su(var)2-505 is formally equivalent to a null mutation (20, 22). Su(var)2-504 encodes a truncated HP1 protein that lacks part of the domain required for its nuclear localization (22). Su(var)2-501 is a point mutation at the first nucleotide of the last intron leading to formation of two aberrant transcripts (21). In contrast to these, Su(var)2-502 is a point mutation changing just one amino acid in a conserved region of the protein (50) known as the chromo domain (CHD) (49). In the Su(var)2-502 allele, HP1 is present in telomeres and no chromosome abnormalities are observed (23).
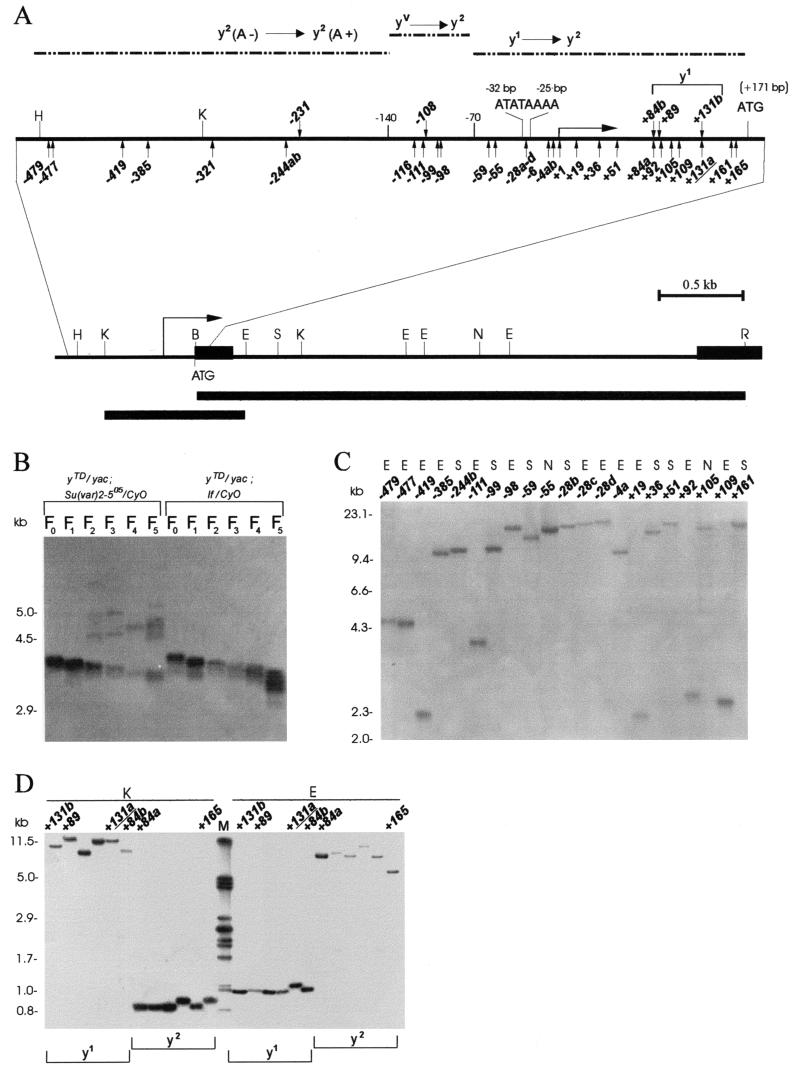
To study the frequency of HeT-A and TART attachment to broken chromosome ends, we used the terminally truncated chromosomes, with breaks in the yellow gene designated yellow terminal deficiencies (yTD). Breaks that place the end of the chromosome at the yellow promoter or within the yellow transcription unit between positions −140 and +171 (ATG codon) relative to the yellow transcription start site result in a variegated-bristle (yv-like) or y1-like phenotype (Fig. 1A). Addition of a promoter-containing HeT-A to the end of the deficient chromosome (between bp −140 and +171) generates y2-like alleles, i.e., y1 → y2 or yv → y2 (32). Breaks between bp −1200 and −140 result in the y2-like phenotype with yellow-colored aristae, y2(A−) (Fig. 1A). Addition of either a HeT-A or a TART sequence restores aristal pigmentation. This observation allowed us to monitor the attachment of both HeT-A and TART to the yellow terminal sequences.
FIG. 1.
Molecular structure of the broken chromosome end in the yellow gene. (A) A schematic presentation of terminal yellow deficiencies associated with different y phenotypes. The promoter (ATATAAAA) and start of translation (ATG) locations are indicated relative to the transcription start site of the yellow gene. The coding yellow region is shown as a black box. The location of the start of the yellow transcription region is shown by a horizontal arrow on the uppermost solid horizontal line. The dotted horizontal lines show the regions of the yellow sequence in which the termini of the yTD line that correspond to the same classes of y phenotype have been mapped. The KpnI-EcoRV and BamHI-EcoRI genomic fragments used as a probe for Southern blot analysis are indicated by the bottommost thick horizontal line and the line just above, respectively. The points of HeT-A attachment are shown by small vertical arrows below the uppermost solid horizontal line. The points of TART attachment are shown by small vertical arrows above the same line. Restriction enzyme abbreviations: B, BamHI; K, KpnI; H, HindIII; E, EcoRV; N, NruI; S, SpeI; R, EcoRI. (B) Effect of the Su(var)2-505 mutation on the rate of terminal DNA shortening. Southern blot analysis of DNAs prepared from 10 to 14 yTD/yac females of the Su(var)2-505 and control lines taken in six subsequent generations as described in the Fig. 2A legend. DNAs were digested with EcoRI. The filter was hybridized with the BamHI-EcoRI probe. The additional bands correspond to new DNA attachments to the receding yellow sequences at the end of truncated chromosome. (C) HeT-A and TART transpositions to the broken chromosome end in the yellow gene. DNAs were digested with EcoRV (E), NruI (N), and SpeI (S). The filter was hybridized with the KpnI-EcoRV probe. (D) HeT-A and TART transpositions obtained in the progeny of one yTD/yac; Su(var)2-505/CyO female displaying a y1 phenotype. In the next generation, all yTD/yac; If/CyO daughters with either a y1- or a y2-like phenotype were individually crossed for DNA isolation. DNAs were digested with KpnI (K) and EcoRV (E). The filter was hybridized with the KpnI-EcoRV probe. The junctions between yellow and new DNA attachments were cloned and sequenced in the cases designated with numbers (see Fig. 3).
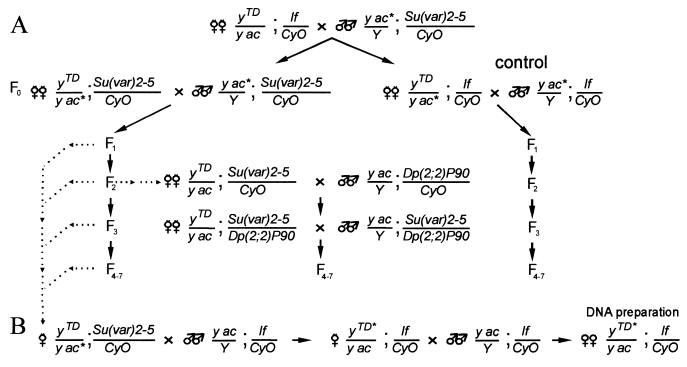
Five yTD/yac; If/CyO lines carrying deficiencies terminating in the region from bp −700 to −300 were selected with the aid of Southern blot hybridization from among the flies displaying a y2(A−) phenotype. The y2TD/yac; If/CyO females were crossed to yac; Su(var)2-5/CyO males as shown in Fig. 2A. In the offspring, y2TD/yac; Su(var)2-5/CyO females were crossed to yac; Su(var)2-5/CyO males for five successive generations to determine the appearance of flies with the y2(A+) phenotype. As a control, y2TD/yac; If/CyO females were crossed to yac; If/CyO males. Since similar frequencies of terminal DNA addition resulting in the y2(A+) phenotype have been observed for different yTD lines, these results are combined in Table 1.
FIG. 2.
The scheme used to study the role of Su(var) mutations in terminal DNA elongation. (A) The main scheme of crosses. The mutations of Su(var)2-5 tested were Su(var)2-501, Su(var)2-502, Su(var)2-504, or Su(var)2-505. Depending on the purpose of the experiment, the yTD chromosome contains different y alleles at the end of the terminally truncated chromosome. In some experiments, yac chromosome (yac*) was substituted for either the yw or the ytataw chromosome. (B) The scheme for isolation of individual stable lines bearing terminally truncated chromosomes with new DNA attachments. yTD* indicates a y allele with a new phenotype (i.e., a new DNA attachment).
TABLE 1.
Frequencies of HeT-A/TART attachment in the presence of Su(var)2-5 mutationsa
| Su(var) mutation | No. of flies displaying a new y phenotype/total no. in generation:
|
% Q (F2-F7)b | ||||||
|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | ||
| If/CyO | 1/1,100 | 2/1,320 | 0/1,560 | 1/1,820 | 0/1,920 | 2/2,440 | 1/1,469 | 0.06 |
| Su(var)2-501 | 2/740 | 226/1,253 | 332/1,817 | 616/2,211 | 364/1,271 | 145/967 | 143/802 | 22 |
| Su(var)2-502 | 6/800 | 140/983 | 303/1,122 | 171/791 | 108/620 | 43/273 | 62/353 | 20 |
| Su(var)2-504 | 0/525 | 181/733 | 130/515 | 96/584 | 18/146 | 21 | ||
| Su(var)2-505 | 0/460 | 98/820 | 128/578 | 70/459 | 61/311 | 67/720 | 14 | |
| Su(var)2-5/Dp(2;2)P90 | 80/806 | 9/1,457 | 2/1,365 | 4/1,417 | 0.35c | |||
The scheme of crosses is shown in Fig. 2. All tested lines have genotypes yTD/yac; Su(var)2-5/CyO and yTD/yac; If/CyO.
Q, average frequency of visible events signifying a new y phenotype(s) versus total number of flies scored (as a percentage).
In the case of the Su(var)2-5/Dp(2;2)P90 combination, Q was calculated for generations F5 to F7 only.
All four Su(var)2-5 mutations tested have a strongly dominant effect on the frequency of HeT-A/TART attachment to yellow (Table 1). In the first generation, the frequency of HeT-A attachment is comparable in the yTD/yac; Su(var)2-5/CyO and control yTD/yac; If/CyO lines. However, in the subsequent generations, the frequency of HeT-A/TART attachment was more than 100 times higher on the Su(var)2-5 mutant background. The lack of attachment in the first generation may be explained by a significant maternal contribution of HP1 (38). Much the same frequencies of HeT-A/TART addition have been obtained with all Su(var)2-5 mutations, including Su(var)2-505, a null allele, and Su(var)2-502, which does not interfere with HP1 binding to telomeres or with telomere behavior (23).
The Su(var)2-5 mutations differ in their origins, which diminishes the possible effect of a hypothetical linked but unrelated mutation. To further eliminate the possible influence of the genotype, we introduced into the yTD/yac; Su(var)2-502/CyO and yTD/yac; Su(var)2-505/CyO lines the Dp(2; 2)P90 duplication covering the Su(var)2-5 gene (Fig. 2A). Dp(2; 2)P90, in combination with the In(I)wm4h chromosome, enhances the effect of position on variegation, indicating that there is a functional Su(var)2-5 gene in this duplication. In the presence of Dp(2; 2)P90, the frequency of HeT-A/TART attachment was strongly diminished (Table 1), confirming the role of mutations in the Su(var)2-5 gene in the activation of HeT-A/TART attachment.
In terminal chromosomal deficiencies, 70 to 75 bp are lost per generation (2, 3, 5). To study the rate of DNA loss in the presence of the Su(var)2-5 mutations, we isolated the DNAs from yTD/yac; Su(var)2-5/CyO flies and control yTD/yac; If/CyO flies over six consecutive generations. In every generation, the size of terminal fragments was independently measured using Southern blot analysis (Fig. 1B). In both Su(var)2-5 mutant and control lines, the chromosomes lost DNA sequences from the broken end at the same rate of 70 to 80 bp per generation. Thus, Su(var)2-5 mutations do not significantly influence the stability of the terminal chromosomal deficiencies.
Possible mechanisms of HeT-A and TART attachment to the terminal yellow sequences.
Previously, we have shown that HeT-A attachment to a receding chromosome end containing a HeT-A sequence can occur via transposition, conversion, or recombination with HeT-A 3′ termini of other telomeres (32). To determine the molecular nature of DNA attachment in the presence of the Su(var)2-5 mutations, the Su(var)2-5 mutations were crossed out from the individual yTD/yac; Su(var)2-5/CyO females as shown in Fig. 2B. As a result, new HeT-A and TART additions were selected in stable yTD/yac; If/CyO progeny.
To reveal new DNA attachments that could not be selected by phenotype, we also examined all progeny obtained from 10 females. In the progeny of one female, all daughters acquired new DNA attachments (Fig. 1D). A number of the derivatives obtained in the progeny of one female had identical restriction maps of the new DNA attachments, suggesting that the mutation happened at an early premeiotic stage in the germ line. However, in most cases the new DNA attachments had a different restriction map (Fig. 1C), suggesting that they arose independently at later stages in the germ line. Frequently, yTD females displaying mosaic pigmentation of bristles appeared. In the progeny of such females, daughters displayed either a y2-like (new DNA attachments) or an original y1-like (no DNA attachments) phenotype (data not shown). This suggests that the DNA attachments were in somatic cells.
Many HeT-A elements have sites for KpnI and EcoRI restriction endonucleases in the 3′ region and for SpeI and EcoRV in the central region and no sites for NruI (3, 7, 8). On the other hand, all these endonucleases have sites in the yellow transcription unit in the vicinity of the terminal deficiency (Fig. 1A). Therefore, these enzymes were used for DNA hydrolysis in the analysis of HeT-A attachments (Fig. 1C). The KpnI-EcoRV fragment subcloned from the yellow gene was used as a probe (Fig. 1A). The TART elements have a more complex and variable restriction map (15, 54).
To prove the nature of the attached elements, the junctions between terminal yellow sequences and new DNA attachments were cloned by PCR and sequenced (Fig. 3). The PCR primers were located in the yellow gene and in the conserved regions from the 3′ ends of HeT-A or TART.
FIG. 3.
Diagram of HeT-A and TART attachment to the receding yellow sequences. All HeT-A and TART additions are indicated as described in the legends to Fig. 1, 4, and 5. The numbers in parentheses show the approximate sizes of the attachments. The base pair locations at the junctions between HeT-A or TART and yellow are shown. The lowercase letters indicate substitutions in the conserved sequences at the 3′ end of HeT-A or TART. Adenine bases at the junctions may originate either from the yellow sequences or from the 3′ oligo(A) tail of HeT-A or TART.
Most of the DNA attachments had KpnI and EcoRI sites at the 3′ end, as is typical of HeT-A. The sizes of new DNA additions differed greatly, from 1.4 to more than 20 kb (Fig. 1C and 3). In the case of large DNA additions, we could precisely estimate only the minimal size of attached DNA because of the possible existence of additional restriction sites within the attached DNA sequence.
Analysis of junctions between terminal yellow sequences and HeT-A sequences shows that HeT-A elements are attached to random yellow terminal sequences (Fig. 3). Only one of the yellow sequences, i.e., AAAA in the promoter region, was independently targeted four times (−28a, −28b, −28c, and −28d) (Fig. 3). In all cases, a string of adenine residues was present between the yellow and HeT-A sequences. Sequences of the HeT-A 3′ termini (5′ CCAGCAAAGTT 3′) were conserved in half of the HeT-A attachments (19 of 38). In other HeT-A attachments, the last T was omitted (15 cases) or last two to three nucleotides were missing (4 cases). In some of these cases, short additional sequences appeared at the junction between yellow and HeT-A (Fig. 3). These sequences are similar to the sequences located at the 3′ end (−981) or in the 5′ untranslated sequences (−470) of HeT-A. In two cases (−55 and −59), DNA attachments had two tandem copies of the HeT-A 3′ end with a string of adenine residues.
The small size of the attached DNA and the presence of oligo(A) and of a conserved HeT-A terminus suggest transposition of HeT-A to the receding yellow. All these criteria were met in 7 of 11 short DNA additions. Conversely, the large size of DNA additions, presence of several A bases at the end of the target yellow sequence, and existence of base substitutions in the normally conserved 3′ terminal GTT triplet argue in favor of recombination between the receding yellow and the 3′ terminus of a telomeric HeT-A element. In four cases, all these features are combined, and in three others, the presence of oligo(A) in the yellow gene is combined with the large size of attachment. These attachments may have appeared as a result of recombination. Still, in most cases Southern blot analysis and sequencing data do not allow one to discriminate between the two possible mechanisms of HeT-A attachment, transposition and recombination. It seems most probable that Su(var)2-5 mutations induce both mechanisms.
The attachment of TART appears to be rarer (5 events) than that of HeT-A (38 events). The TART attachments had more variable sequences at the 3′ end and a very long string of adenine residues at the junction with yellow sequences than HeT-A attachments (Fig. 3). In one case (+131a), we found a chimeric attachment that contains 126 bp of the 3′ end of HeT-A followed by TART.
The results obtained indicate that all tested Su(var)2-5 mutations in the heterozygous state enhance the number of HeT-A and TART attachments to the receding yellow sequences by at least 100 times.
Su(var)2-5 mutations do not activate terminal gene conversion from a template located on a homologous chromosome.
Previously we found that the broken ends of Drosophila chromosomes may be extended by gene conversion by using the sequences of a homologous chromosome as template (42). As the frequency of terminal DNA elongation is strongly dependent on the genetic background of a given line, we have examined the terminal gene conversion on the Su(var)2-5 mutant background.
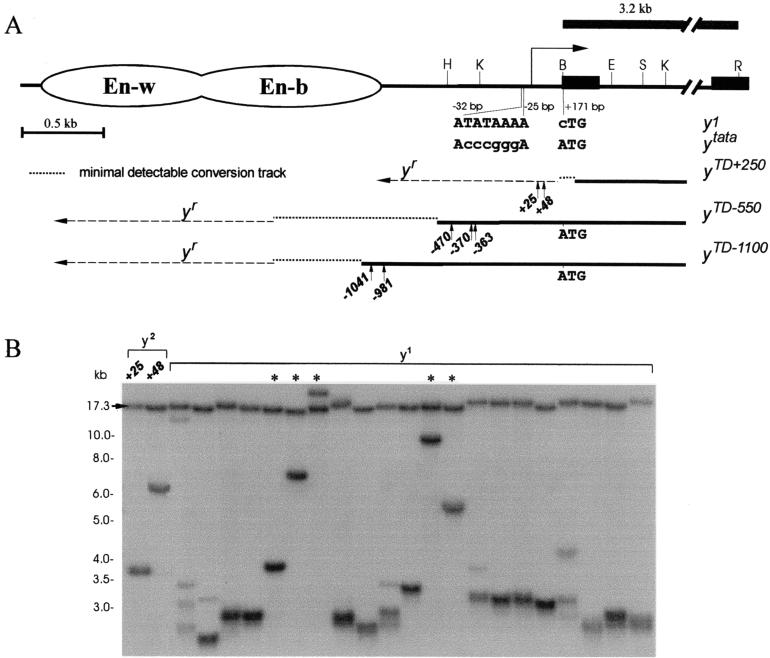
In the first series of experiments, we checked how Su(var)2-5 mutations can activate extensive DNA elongation by gene conversion. Two terminal deficiencies (yTD/yac) were selected (Fig. 4A), terminating at about bp −550 (yTD−550) and −1100 (yTD−1100). The template for gene conversion was the y1 allele (yw chromosome) generated by a single base pair change (ATG → CTG) in the yellow first codon (43). This y1 allele has the intact regulatory region but a nonproductive coding region and therefore yields a null phenotype, namely, lack of pigmentation in all parts of the cuticle. The attachment of HeT-A or TART to the end of the deficient chromosome in the yTD line leads to a y2(A+) phenotype. The addition of at least the body enhancer (bp −1600) to the ends of the deficient chromosomes via gene conversion partially restores yellow expression in the body (yellow revertant, or yr). Further addition of yellow sequences gradually increases the extent of pigmentation of the body cuticle and wing blades (42). Thus, it is possible to monitor (Fig. 4A) conversion tracks longer than 1,050 (yTD−550) or 500 bp (yTD−1100).
FIG. 4.
The model system to study terminal DNA elongation by gene conversion on the Su(var)2-5 mutant background in the presence of a template on the homologous chromosome. (A) Schematic presentation of the terminal yellow deficiencies associated with different y alleles. The molecular structures of the y1 and ytata mutations are shown. The wing (En-w) and body (En-b) enhancers are indicated by ovoids. The approximate regions of the ends of terminally truncated chromosomes in the yTD alleles are shown by three thin horizontal black lines. The dotted horizontal lines show the regions of yellow sequence in which the termini of yTD line with original phenotype have been mapped. The dashed horizontal lines show the regions of yellow sequence in which the termini of yTD line acquiring a new y phenotype have been mapped. The 3.2-kb BamHI-EcoRI genomic fragment used as a probe for Southern blot analysis is indicated by the uppermost thick line. The HeT-A attachment points are shown by small vertical arrows below each of the three thin horizontal black lines. The sequences at the junctions between yellow and HeT-A are shown in Fig. 3. Other designations are as defined in the Fig. 1 legend. (B) Southern blot analysis of DNAs prepared from F2 progeny of individual yTD+250/ytataw; Su(var)2-5/CyO flies. DNAs were digested with EcoRI. The filter was hybridized with the 3.2-kb BamHI-EcoRI probe. The 17.3-kb band corresponds to the DNA fragment between two EcoRI sites in the ytata allele. Asterisks indicate yTD lines displaying a y1-like phenotype that acquired new DNA attachments. The presence of additional bands indicates the heterogeneity of the progeny, suggesting that in some sisters, terminally truncated chromosomes acquired new DNA sequences (HeT-A or TART).
The yTD/yw; Su(var)2-504/CyO and yTD/yw; Su(var)2-505/CyO lines were constructed as described in the Fig. 2A legend. New y phenotypes were monitored for three subsequent generations (Table 2). Gene conversion generated yr derivatives, while HeT-A or TART attachment to the ends of the deficient chromosomes generated y2(A+) derivatives. In the first generation (F1), the Su(var)2-5 mutant and control lines had similar frequencies of gene conversion and DNA attachment. However, in the next two generations, the frequencies of HeT-A and TART attachment increased 100-fold, while the frequencies of DNA elongation by terminal gene conversion remained unchanged.
TABLE 2.
Frequencies of HeT-A additions and terminal gene conversions in the yTD/y1 linesa
| Generation | Su(var)2-5 mutation | Total no. of flies scored | Gene conversionb
|
DNA attachmentb
|
||
|---|---|---|---|---|---|---|
| No. | % Q | No. | % Q | |||
| F1 | Su(var)2-5+ | 4,522 | 12 | 0.27 | 2 | 0.04 |
| Su(var)2-502 | 3,067 | 14 | 0.45 | 1 | 0.03 | |
| Su(var)2-504 | 1,290 | 3 | 0.23 | 0 | <0.07 | |
| Su(var)2-505 | 2,175 | 10 | 0.45 | 1 | 0.05 | |
| F2 | Su(var)2-5+ | 3,821 | 15 | 0.40 | 1 | 0.03 |
| Su(var)2-502 | 1,246 | 3 | 0.24 | 162 | 13 | |
| Su(var)2-504 | 1,548 | 1 | 0.06 | 375 | 24 | |
| Su(var)2-505 | 1,266 | 2 | 0.16 | 243 | 20 | |
| F3 | Su(var)2-5+ | 2,484 | 18 | 0.72 | 0 | <0.04 |
| Su(var)2-502 | 648 | 2 | 0.31 | 102 | 15 | |
| Su(var)2-504 | 984 | 1 | 0.10 | 162 | 16 | |
| Su(var)2-505 | 1,690 | 2 | 0.12 | 281 | 17 | |
The scheme of crosses is shown in Fig. 2.
Abbreviations: No., total number of flies displaying a new y phenotype that is generated either by DNA elongation via gene conversion or by attachment of the telomere-specific mobile elements; Q, average frequency of visible events signifying a new y phenotype versus total number of flies scored (in percent).
To directly show that our genetic system distinguished HeT-A/TART attachment to the receding yellow sequences from addition of yellow sequences by gene conversion, the Su(var)2-5 mutations were crossed out from the individual yTD/yac; Su(var)2-5/CyO females as shown in Fig. 2B. DNAs of the selected derivatives displaying new y phenotypes were studied by Southern blot analysis (data not shown). All tested y2-like alleles yielding pigmented aristae had new DNA attachments at the ends, while yr-like alleles displaying darker pigmentation had the yellow enhancers at the end of the deficient chromosomes. The addition of HeT-As to the broken ends was proved by PCR cloning and sequencing of the junctions between HeT-A and yellow in five y2(A+)-like alleles (Fig. 3 and 4A).
Although the results obtained argue that the Su(var)2-5 mutations only enhance the HeT-A/TART attachment, it is still possible that small conversion tracks do exist. To examine this possibility, we developed a special genetic system to monitor conversion tracks in the range of 100 to 200 bp (Fig. 4A). Using Southern blot analysis, we selected the yTD+250 line carrying deficiencies terminating in the coding region of the yellow gene. The yTD+250 chromosome was balanced over the ytata chromosome, in which the yellow TATA promoter (TATAAA) was substituted for CCCGGG (43). As a result, ytata flies displayed yellow bristles. It has been shown that the HeT-A promoter can activate yellow transcription and the chimeric HeT-A-yellow mRNA produces a functional protein if the ATG start codon is not removed (32). Thus, the attachment of HeT-A restores yellow pigmentation in bristles if the ATG codon in the yellow gene has been introduced at the end of the deficient chromosome by gene conversion.
Six independent yTD+250/ytataw; Su(var)2-5/CyO lines were constructed with either Su(var)2-504 or Su(var)2-505 (Fig. 2A). According to Southern blot analysis, all constructed lines in F1 carried deficiencies terminating in the region around position +250 downstream of the yellow transcription start site (data not shown). Thus, these lines had lost the ATG start codon of the yellow gene (+171). The yTD lines were propagated for three generations. For each of the six lines, we examined from 600 to 1,500 flies (altogether, about 5,000 flies). As a result, only two y2-like females were found (Fig. 4B). The junctions between HeT-A and the yellow gene were amplified and sequenced (Fig. 3 and 4A). In both cases HeT-A was attached to the yellow sequences upstream of the ATG start codon, suggesting elongation of the yellow sequences prior to the HeT-A attachment.
To directly examine the frequency of DNA attachment and gene conversion, the F2 progeny of individual yTD+250/ytataw; Su(var)2-5/CyO flies were taken for Southern blot analysis (Fig. 4B). In 5 of 21 tested lines, i.e., in ca. 24% of cases, terminal deficiencies had new DNA attachments. In several other lines, the presence of weak bands corresponding to DNA fragments of larger size suggests that new DNA attachments were formed in the progeny of selected females.
Thus, Su(var)2-5 mutations greatly increase the frequency of HeT-A and TART attachment to the ends of truncated chromosomes rather than that of gene conversion if the template for DNA synthesis is on a homologous chromosome.
Su(var)2-5 mutations greatly raise the frequency of gene conversion if the template for DNA synthesis is on the same chromosome.
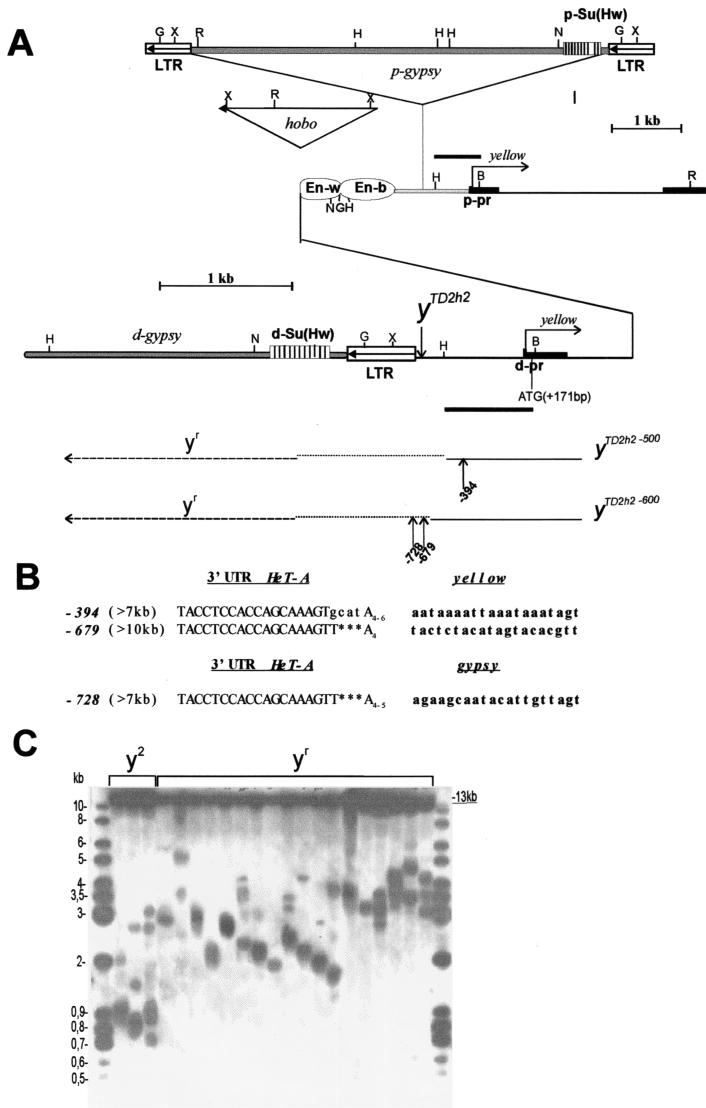
Thereafter, we studied the effect of Su(var)2-5 mutations on the ratio of DNA attachment to gene conversion in the presence of a template for gene conversion on the same terminally truncated chromosome. For this, derivatives of the yTD2h2 line were used. This line contains a terminally truncated X chromosome which has a duplication of the yellow sequences extending from bp +875 to the chromosome end (Fig. 5A). In addition to the yellow duplication, a gypsy retrotransposon is inserted between the yellow enhancers and promoter at position −700. The yTD2h2 flies have a y2-like phenotype because the gypsy insulator blocks the interaction between the wing and body enhancers and the promoter (24, 26).
FIG. 5.
Model system to study terminal DNA elongation by gene conversion on a Su(var)2-5 mutant background in the presence of a template on the same chromosome. (A) Schematic presentation of the yTD2h2 allele and its derivatives associated with different y phenotypes. The approximate end of the truncated chromosome in the yTD2h2 allele is shown by a downward-pointing vertical arrow. The coordinates (kilobases) in the yellow gene and gypsy element are defined from the transcription start site of the distal yellow promoter. The gypsy element is inserted −700 bp upstream of the transcription start site. The Su(Hw) binding sites are indicated by the stripes in the striped boxes. The wing (En-w) and body (En-b) enhancers are indicated by ovoids. The triangle and arrowhead indicate the hobo element and its direction, respectively. Abbreviations: d-pr, distal yellow promoter; p-pr, proximal yellow promoter; d-Su(Hw), distal gypsy insulator; p-Su(Hw), proximal gypsy insulator; d-gypsy, distal gypsy retrotransposon; p-gypsy, proximal gypsy retrotransposon. The approximate ends of the truncated chromosomes in two yTD2h2 derivatives are shown by the bottommost two thin black lines. The dotted horizontal lines show the regions of the yellow sequence in which the termini of the yTD line with the y2-like phenotype have been mapped. The dashed horizontal lines show the regions of the yellow sequence where the termini of yTD line acquiring a yr (yellow revertant)-like phenotype have been mapped. The HindIII-BamHI genomic fragment used as a probe for Southern blot analysis is indicated by thick line segments. The points of HeT-A attachment are shown by small arrows with numbers. The sequences at the junctions between yellow and HeT-A are shown in Fig. 3. Restriction enzyme abbreviations: B, BamHI; K, KpnI; H, HindIII; N, NcoI; G, BglII; X, XhoI. Other designations are defined in the Fig. 1 legend. (B) Diagram of HeT-A attachment to the receding yellow or gypsy sequences. All HeT-A additions are indicated as in Fig. 5A. Other designations are defined in the Fig. 3 legend. (C) Southern blot analysis of DNAs prepared from F2 progeny of individual yTD2h2/yac; Su(var)2-5/CyO02 flies. DNAs were digested with BamHI. The filter was hybridized with the HindIII-BamHI probe. The 13-kb band corresponds to the DNA fragment that hybridized with the proximal HindIII-BamHI probe. The presence of additional bands indicates the heterogeneity of the progeny, suggesting that in some sisters, terminally truncated chromosomes acquired new DNA sequences.
It has been shown that a second gypsy insulator placed upstream of the yellow enhancers neutralizes the enhancer-blocking activity of the first one (24). As a result, addition of the second gypsy insulator to the end of the deficient chromosome restores yellow expression in the body and wings (yr). In yTD2h2 flies, the gypsy sequences may be duplicated to the end of the deficient chromosome by gene conversion using the homologous yellow and gypsy sequences located on the same chromosome as the template. Thus, the frequency of intrachromosomal gene conversion can be monitored by scoring flies according to the presence of darker pigmentation of the wing blades and body cuticle (yr phenotype).
Two yTD2h2/yac lines were selected by Southern blot analysis. The ends of deficient chromosomes were located at bp −500 and −600 relative to the yellow transcription start site (Fig. 5A). Thus, to activate yellow expression in the body and wings, the minimal span of the terminal DNA elongation by gene conversion should be 700 to 800 bp. To study the role of HP1, we used the Su(var)2-502 and Su(var)2-505 mutations. The yTD2h2/yac; Su(var)2-505/CyO [or yTD2h2/yac; Su(var)2-502] lines were constructed as described in the Fig. 2A legend. yTD2h2/yac; Su(var)2-5/CyO females were individually crossed to yac/Y; Su(var)2-5/CyO males over four subsequent generations. yTD2h2/yac; If/CyO flies obtained in the same crosses were used as an internal control.
As in the experiments described above, we did not find any detectable difference between the mutant and control lines in F1 (Table 3). However, in the subsequent generations, flies with darker wing and body pigmentation (yr phenotype) appeared among y2-like females with a frequency of up to 23%. To show that the yr derivatives were generated by gene conversion, the progeny of individual yr females was taken for DNA preparation. Southern blot analysis showed a tight correlation between the y phenotype and the size of terminal DNA elongation in the yr derivatives (Fig. 5C). The point mutation in the HP1 CHD [Su(var)2-502] and complete inactivation of HP1 [Su(var)2-505] enhanced terminal gene conversion to approximately the same level. Thus, HP1 (and its CHD, in particular) is responsible for repression of terminal DNA elongation by gene conversion in yTD2h2 flies.
TABLE 3.
Extents and frequencies of elongation of terminal sequences in yTD2h2 derivativesa
| Generation | Su(var)2-5 mutation | Gene conversionb
|
DNA attachmentb
|
||||
|---|---|---|---|---|---|---|---|
| Totalc | Events | % Q | Totald | Events | % Q | ||
| F1 | Su(var)2-502 | 1,400 | 2 | 0.14 | 54 | 1 | 2 |
| Su(var)2-505 | 1,650 | 3 | 0.18 | 69 | 0 | <2 | |
| Su(var)2-5+ | 1,200 | 2 | 0.16 | 41 | 0 | <3 | |
| F2 | Su(var)2-502 | 1,250 | 186 | 15 | 46 | 3 | 6.5 |
| Su(var)2-505 | 1,900 | 445 | 23 | 72 | 2 | 2.8 | |
| Su(var)2-5+ | 2,100 | 7 | 0.33 | 86 | 0 | <2 | |
| F3 | Su(var)2-502 | 900 | 153 | 7.9 | 36 | 2 | 5.6 |
| Su(var)2-505 | 1,400 | 302 | 21.5 | 44 | 2 | 4.6 | |
| Su(var)2-5+ | 1,600 | 4 | 0.25 | 55 | 1 | 2 | |
| F4 | Su(var)2-502 | 750 | 59 | 20.1 | 34 | 1 | 3 |
| Su(var)2-505 | 1,200 | 168 | 18.2 | 48 | 2 | 2.1 | |
| Su(var)2-5+ | 1,450 | 4 | 0.28 | 44 | 0 | <3 | |
The scheme of crosses is shown in Fig. 2.
Events, numbers of females displaying a new y phenotype that is generated either by DNA elongation via gene conversion or by attachment of telomere-specific mobile elements; Q, average frequency of visible events signifying a new y phenotype versus number of flies scored (in percent).
Total number of scored females.
Total number of females examined for attachment of HeT-A and TART.
To determine the frequency of HeT-A and TART transposition, we monitored new DNA attachments between the primers in yellow and HeT-A for all females in whose progeny we found no yr flies by using Southern blot analysis and PCR amplification (Table 3). The average frequency of DNA attachment was found to be 4.1%, that is, lower than in other experiments. In three cases, the junction between the attached HeT-A and yellow sequence was cloned and sequenced, confirming HeT-A attachment to the end of the terminally deficient chromosomes (Fig. 5B). The results obtained suggest that the presence of two tandem copies of homologous yellow sequences at the end of a terminally deficient chromosome significantly reduces the frequency of new DNA attachments induced by the Su(var)2-5 mutations.
Su(var) mutations activate HeT-A transcription.
As HP1 is known to be a transcriptional repressor (19, 21), the Su(var)2-5 mutations may derepress transcription of HeT-As and thereby increase the frequency of HeT-A transposition via reverse transcription.
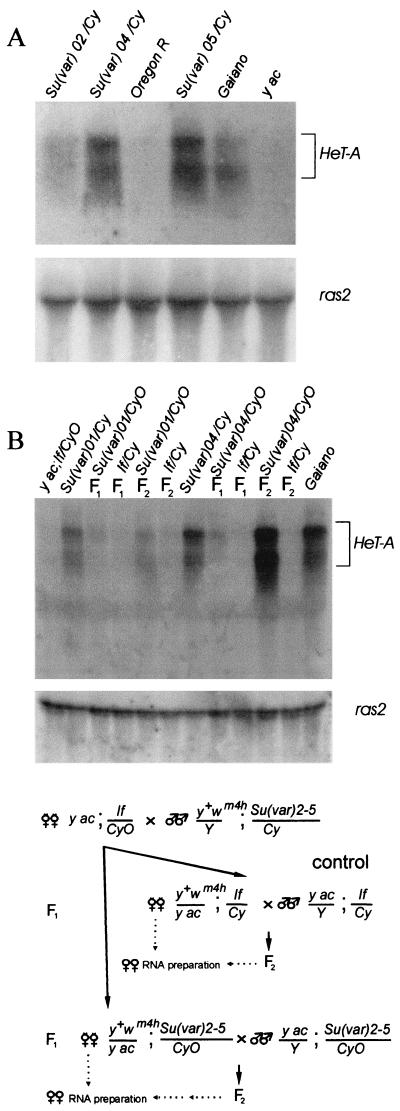
To examine this possibility, we compared HeT-A transcription for a wild-type line and on the Su(var)2-5 mutant background. It was shown that when RNA from flies is probed with sequences from any part of HeT-A, the major species of RNA detected is a sense-strand transcript of ∼6 kb (14, 15). This is the size expected for a full-length transcript of HeT-A. As a probe, we used a DNA fragment containing the HeT-A open reading frame (bp 1746 to 4421). In the Su(var)2-501, Su(var)2-502, Su(var)2-504, and Su(var)2-505 mutants, as in the Gaiano line, HeT-A transcripts were 10 times as abundant as in the control lines, Oregon and yac (Fig. 6).
FIG. 6.
Analysis of HeT-A transcripts in the Su(var)2-5 mutant strains. (A) Northern blot hybridization with total RNA isolated from progeny from the In(I)wm4h; Su(var)2-504/In(2L)tIn(2R)Cy[Su(var)04/Cy], Df(1)w, and y1w67c23; Su(var)2-502/CyO y+[Su(var)02/Cy]; Oregon R lines and the In(I)wm4h; Su(var)2-505/In(2L)Cy1 In(2R)Cy[Su(var)05/Cy]; Gaiano and yac lines. The double-strand probe from the open reading frame of HeT-A detects a prominent RNA of ∼6 kb. The probe from the ras2 gene gives rise to a 1.6-kb transcript that is used as a marker for the amount of RNA. (B) Northern blot hybridization with total RNA isolated from flies obtained in the crosses is depicted in the lower part of the figure. Gaiano and If/CyO lines were used as controls with high and low HeT-A transcription, respectively. The In(I)wm4h; Su(var)2-501/SM1 [Su(var)01/Cy] and In(I)wm4h; Su(var)2-504/In(2L)tIn(2R)Cy Cy1Roi1pR1cn1[Su(var)04/Cy] lines were used in the crosses.
To exclude the possible contribution of increased copy numbers of HeT-A elements to the high HeT-A transcription rate, we also examined HeT-A transcription in Su(var)2-5/CyO and If/CyO females obtained in F1 and F2 after crosses of yac; If/CyO females to Su(var)2-5 males (Fig. 6B). In F1 females, approximately the same level of content in HeT-A transcripts was found in Su(var)2-5/CyO and If/CyO (Fig. 6B). This correlates with a low rate of HeT-A attachment to the deficient chromosomes and may be explained by a significant maternal contribution of HP1. However, in F2 the Su(var)2-5/CyO females displayed high HeT-A expression compared with that of the control If/CyO females. Southern blot analysis showed that samples from Su(var)2-5/CyO and If/CyO females had approximately the same levels of HeT-A content (data not shown).
Thus, Su(var)2-5 mutations activate HeT-A transcription, and HP1 appears to repress HeT-A transcription in telomeres. The activation of the HeT-A transcription may partly contribute to the high rate of the transposition-mediated HeT-A additions on the Su(var)2-5 mutant background.
Drosophila lines bearing Su(var)2-5 mutations often have very high HeT-A and TART content.
The results obtained demonstrate that the Su(var)2-5 mutations greatly raise the frequency of DNA attachment to a terminally deficient chromosome. To study the possible effect of this phenomenon on the length of Drosophila telomeres, we measured the content of HeT-A and TART in different lines bearing the Su(var)2-5 mutations.
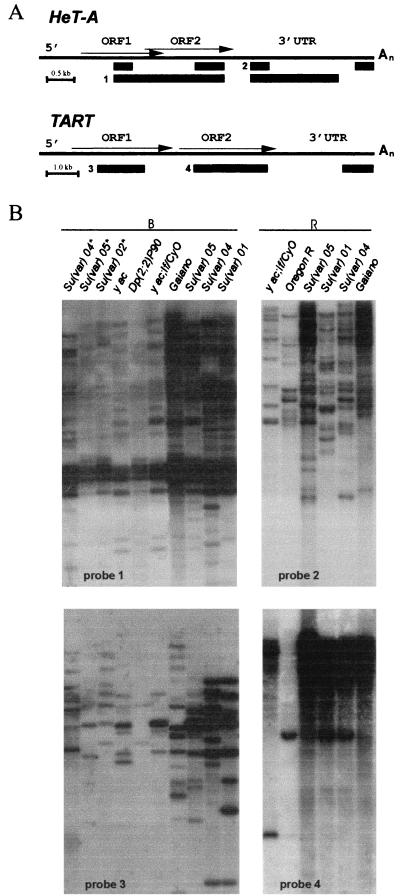
As controls, we used Oregon, yac, and Gaiano lines. It was shown previously that the Gaiano line obtained from natural Drosophila populations has very long telomeres (40). As hybridization probes, we used the fragments subcloned from different parts of HeT-A and TART (Fig. 7A).
FIG. 7.
The content of HeT-A and TART elements in the Su(var)2-5 mutants. (A) Diagrams of HeT-A and TART. The bars under each diagram indicate the sequences used as probes for Southern blot analysis. (B) Southern blot analysis of HeT-A and TART copy numbers in the Su(var)2-5 lines. Asterisks indicate new lines obtained from J. Eissenberg (38) as follows: Su(var)02*, Df(1)w y1w67c23; Su(var)2-502/CyO y+; Su(var)04*, Df(1)w y1w67c23; Su(var)2-504/CyO y+; Su(var)05*, and Df(1)w y1w67c23; Su(var)2-505/CyO y+. The controls were Gaiano (very long telomeres), yac; If/CyO, and Oregon R (normal telomeres). Results from progeny from the Su(var)01, If/CyO In(I)wm4h; Su(var)2-501/SM; Su(var)04, Df(1)w y1w67c23; Su(var)2-504/CyO y+; Su(var)05, In(I)wm4h; Su(var)2-505/In(2L)Cy1 In(2R)Cy, Dp(2, 2)P90 yw, and Dp (2, 2)P90/CyOy+ lines are shown. DNAs were digested with BamHI (B) or EcoRI (R). The filters were probed with different fragments indicated in panel A.
We found that DNAs isolated from the Su(var)2-501, Su(var)2-504, and Su(var)2-505 lines hybridized with HeT-A and TART probes with approximately the same intensity as with DNA isolated from Gaiano line (Fig. 7B), i.e., much more strongly than with DNA from Oregon or yac strains. This means that these Su(var)2-5 lines, like Gaiano, have very long telomeric arrays of HeT-A and TART elements.
However, in the y; Su(var)2-502/CyO y+, y; Su(var)2-504/CyO y+, and y; Su(var)2-505/CyO y+ lines obtained from Joel Eissenberg, the lengths of HeT-A/TART stretches were much smaller. The latter three lines were constructed a year and a half before our study (38), while other Su(var)2-5 lines have been maintained for many years. One might suggest that the increase in telomere length on the Su(var)2-5 mutant background is a slow process that takes several years.
DISCUSSION
HP1 is required for control of telomere elongation in Drosophila melanogaster.
HP1 is the first identified capping protein of Drosophila telomeres (23). No other component of the Drosophila telomeric capping complex has yet been identified, in contrast to those of mammal and yeast species (40). The bulk of HP1 is localized on the strongly heterochromatic fourth chromosome and in the pericentric heterochromatin (33, 34). In addition, it has also been clearly located at telomeres (23), where it may be a component of the telomeric end-capping complex. Mutations in the gene encoding HP1, Su(var)2-5, are suppressors of centromeric position-effect variegation (21). These mutations do not affect telomeric silencing (12, 23, 59), suggesting that HP1 is not a component of the telomere-associated sequence-binding protein complex, which is mainly responsible for telomeric silencing. However, a complex consisting of HP1, the origin recognition complex (ORC), and HP1/ORC-associated protein appears to bind to the telomere-associated sequence (53). HP1 mutations show phenotypes consistent with the role of HP1 in telomere capping; that is, they lead to frequent telomere-telomere attachment during mitosis and meiosis (23). Thus, HP1 may play an important role in protection of the telomere ends.
The role of HP1 in regulation of terminal DNA elongation was studied using terminal deficiencies located within the yellow gene. Broken chromosomes in Drosophila behave as capped chromosomes; they are transmitted through many generations (2, 3, 7), and HP1 is present at the ends of terminal deficiencies (23). Thus, the telomere-binding proteins can bind the ends of chromosomes in a sequence-independent manner, and the yellow sequences located at the end of the deficient chromosome have the properties of the real telomere.
Here we found that the Su(var)2-5 mutations resulted in more than a 100-fold increase in the frequency of terminal DNA elongation that was realized either via mobile element attachment or via terminal gene conversion if the homologous sequence was located near the same chromosome terminus. The strong dominant effect of the Su(var)2-5 mutations suggests that the regulation of telomere length is sensitive to HP1 concentration. The role of HP1 in regulation of telomere length is supported by the finding that the HeT-A and TART arrays are extremely long in Su(var)2-5 lines maintained for many generations.
HP1 contains a single amino-terminal CHD motif and a single carboxy-terminal chromo shadow domain (CSD) motif separated by a linker of variable length (hinge region) (19). The CSD specifically binds nucleosomal H4 N-terminal peptides and is important for self-association of HP1 (66). This result may explain the non-sequence-specific binding of HP1 to telomeres and also the fact that CHD inactivation in the Su(var)2-502 mutant does not influence HP1-telomere binding (23). Also, a variety of factors have been reported to interact directly with HP1 through CSD (19). Some of these proteins may play a role in recruiting HP1 to telomeres.
The Su(var)2-502 mutation does not induce chromosomal abnormalities as other Su(var)2-5 mutations inactivating HP1 do (23). However, in our assays, Su(var)2-502 had the same dominant effect on the terminal DNA elongation as Su(var)2-505, the null mutation in the Su(var)2-5 gene. As CHD is not necessary for HP1 binding to telomeres and their stability, these functions can be separated from the suppression of telomere elongation, for which CHD is essential. So far, no direct interactions of HP1 that occur solely through CHD have been described (19). CHD appears to be important for interaction with ORC (45). In mammalian cells, HP1 binds to H3 through its CHD (44). CHD may also be required for modulation of CSD interaction with other proteins that may have a role in formation of the protein complex on telomeres, like the lamin B receptor and Ku-70 (19, 56). These interactions may be critical for the control of telomere elongation. A recent study showed that the Drosophila HP1 recognizes a “histone code” involving Lys9 (methyl-K9) in histone H3 (30). It was found that the methyl-K9 binding of HP1 occurs via its CHD. Thus, CHD may be also involved in the effective binding of HP1 to telomeres.
Su(var)2-5 mutations activate different types of terminal DNA elongation, depending on the presence of a terminal sequence duplication on the same chromosome.
DNA addition to terminal yellow sequences derepressed by Su(var)2-5 mutations may occur either via attachment of mobile element HeT-A or TART by transposition or recombination or via elongation of the yellow sequences by gene conversion using the homologous sequences on the same chromosome as a template. If a template is absent or located on a homologous chromosome, the Su(var)2-5 mutations markedly increase the frequency of the attachment of both HeT-A and TART to the end of the deficient chromosome but do not affect the frequency of terminal DNA elongation by gene conversion.
We also found that Su(var)2-5 mutations strongly enhanced HeT-A transcription. As most of the HeT-A elements are located at telomeres (47, 48), HP1 appears to be responsible for repression of the HeT-A transcription in telomeres. The elevated frequency of HeT-A transposition induced by Su(var)2-5 mutations may be partly explained by a high content of HeT-A RNA, which mediates HeT-A transposition via reverse transcription (40, 47). However, the activation of HeT-A and TART transcription cannot influence terminal DNA elongation by recombinational mechanisms, which is also strongly elevated on the Su(var)2-5 mutant background. Thus, HP1 appears to be directly involved in regulation of telomere length.
Two telomere-binding proteins, TRF1 and TRF2, are engaged in telomere length control in human cells (55, 58). TRF1 and TRF2 were shown to be components of a negative feedback mechanism that restricts telomere elongation and ensures stable telomere length. TRF2 can remodel telomeric DNA into t loops in vitro, and TRF1 has several biochemical activities likely to promote t-loop formation (29). The long stretches of the double-stranded telomere DNA are looped around and the single-stranded terminus is tucked back inside the double-stranded DNA molecule, thus protecting the chromosome terminus from DNA damage response and regulating the access to telomerase (28). It was proposed that long telomeres recruit large amounts of the TRF1 and TRF2 proteins that facilitate remodeling of the telomeres into t loops (55). In the t-loop state, telomerase would no longer be able to elongate the telomere, leading to loss of sequences with cell divisions (28, 29).
Smogorzewska et al. (55) also suggested that the same mechanism is operative in telomere length control in the yeast Saccharomyces cerevisiae. TRF1 and TRF2 lack significant homology with the proteins Rap1p, Rif1p, and Rif2p that regulate telomere elongation in S. cerevisiae (11). Rif1p and Rif2p are in vivo telomere-binding proteins (12) that are brought to the telomere by virtue of their ability to interact with the carboxyl terminus of Rap1p (62). Deletion of either Rif1p or Rif2p results in telomere lengthening, and deletion of both has a synergistic effect (62). Thus, S. cerevisiae cells appear to assess the telomere length by monitoring the amount of telomere-bound Rap1p and Rif proteins. There are several pieces of indirect evidence for the presence of t loops in S. cerevisiae, which are remodeled by Rap1p and Rif proteins in the same way that TRF1 and TRF2 make t loops in human cells (16, 17). It is proposed that the S. cerevisiae telomere folds back onto the subtelomeric regions to form an approximately 3-kb loop (16).
It seems possible that HP1 also regulates Drosophila telomere length by participating in the formation of t loops. In the t-loop state, transpositions of HeT-A and TART are repressed. If homologous sequences are absent from the end of a yellow terminal deficiency, the HP1 concentration appears to be critical for loop formation. As a result, the Su(var)2-5 mutations in heterozygous state can strongly activate HeT-A and TART attachment. Tandem repeats of yellow sequences at the end of the truncated chromosome may facilitate loop formation, which partially diminishes the role of the HP1 concentration in suppression of HeT-A/TART attachment. Thus, Su(var) mutations mainly enhance terminal gene conversion if a homologous sequence is located on the same chromosome. At the same time, the frequency of mobile element attachment is diminished.
Further studies of the role of HP1 and other telomere-binding proteins in the control of Drosophila telomere elongation are required for understanding the detailed mechanisms of the process. However, it seems possible that, despite the different organization and structures of telomeric chromatin in humans, yeast species, and Drosophila, they share similar basic mechanisms for the control of telomere length.
Acknowledgments
We are sincerely grateful to Y. B. Schwartz for helpful suggestions and discussions during this work. We thank J. Eissenberg for critical comments on the manuscript. We also thank J. Eissenberg, M. L. Pardue, K. L. Traverse, C.-T. Wu, J. Modolell, M. Gatti, and the Umeå Stock Center for Drosophila stocks and plasmids.
This work was supported by the Russian state program Frontiers in Genetics, the Russian Foundation for Basic Research, and by an International Research Scholar award from the Howard Hughes Medical Institute to P.G.
REFERENCES
- 1.Ashburner, M. 1989. Drosophila: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 2.Biessmann, H., S. B. Carter, and J. M. Mason. 1990. Chromosome ends in Drosophila without telomeric DNA sequences. Proc. Natl. Acad. Sci. USA 87:1758-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biessmann, H., L. E. Champion, K. O'Hair, K. Ikenaga, B. Kasravi, and J. M. Mason. 1992. Frequent transpositions of Drosophila melanogaster HeT-A transposable elements to receding chromosome ends. EMBO J. 11:4459-4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biessmann, H., B. Kasravi, T. Bui, G. Fujiwara, L. E. Champion, and J. M. Mason. 1994. Comparison of two active HeT-A retroposons of Drosophila melanogaster. Chromosoma 103:90-98. [DOI] [PubMed] [Google Scholar]
- 5.Biessmann, H., and J. M. Mason. 1988. Progressive loss of DNA sequences from terminal chromosome deficiencies in Drosophila melanogaster. EMBO J. 7:1081-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biessmann, H., and J. M. Mason. 1997. Telomere maintenance without telomerase. Chromosoma 106:63-69. [DOI] [PubMed] [Google Scholar]
- 7.Biessmann, H., J. M. Mason, K. Ferry, M. d'Hulst, K. Valgeirsdottir, K. L. Traverse, and M. L. Pardue. 1990. Addition of telomere-associated HeT DNA sequences “heals” broken chromosome ends in Drosophila. Cell 61:663-673. [DOI] [PubMed] [Google Scholar]
- 8.Biessmann, H., K. Valgeirsdottir, A. Lofsky, C. Chin, B. Ginther, R. W. Levis, and M. L. Pardue. 1992. HeT-A, a transposable element specifically involved in healing broken chromosome ends in Drosophila melanogaster. Mol. Cell. Biol. 12:3910-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biessmann, H., M. F. Walter, and J. M. Mason. 1997. Drosophila telomere elongation. Ciba Found. Symp. 211:53-67. [DOI] [PubMed] [Google Scholar]
- 10.Blasco, M. A., S. M. Gasser, and J. Lingner. 1999. Telomeres and telomerase. Genes Dev. 13:2353-2359. [DOI] [PubMed] [Google Scholar]
- 11.Bourns, B. D., M. K. Alexander, A. M. Smith, and V. A. Zakian. 1998. Sir proteins, Rif proteins, and Cdc13p bind Saccharomyces telomeres in vivo. Mol. Cell. Biol. 18:5600-5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crydermann, D. E., E. J. Morris, H. Biessmann, S. C. R. Elgin, and L. L. Wallrath. 1999. Silencing at Drosophila telomeres: nuclear organization and chromatin structure play critical roles. EMBO J. 18:3724-3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danilevskaya, O. N., I. R. Arkhipova, K. L. Traverse, and M. L. Pardue. 1997. Promoting in tandem: the promoter for telomere transposon HeT-A and implications for the evolution of retroviral LTRs. Cell 88:647-655. [DOI] [PubMed] [Google Scholar]
- 14.Danilevskaya, O. N., F. Slot, K. L. Traverse, N. C. Hogan, and M. L. Pardue. 1994. The Drosophila telomere transposon HeT-A produces a transcript with tightly bound protein. Proc. Natl. Acad. Sci. USA 91:6679-6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danilevskaya, O. N., K. L. Traverse, N. C. Hogan, P. G. DeBaryshe, and M. L. Pardue. 1999. The two Drosophila telomeric transposable elements have very different patterns of transcription. Mol. Cell. Biol. 19:873-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Bruin, D., S. M. Kantrow, R. A. Liberatore, and V. A. Zakian. 2000. Telomere folding is required for the stable maintenance of telomere position effects in yeast. Mol. Cell. Biol. 20:7991-8000.11027269 [Google Scholar]
- 17.de Bruin, D., Z. Zaman, R. A. Liberatore, and M. Ptashne. 2001. Telomere looping permits gene activation by a downstream UAS in yeast. Nature 409:109-113. [DOI] [PubMed] [Google Scholar]
- 18.Drake, J. W. 1993. Rates of spontaneous mutation among RNA viruses. Proc. Natl. Acad. Sci. USA 90:4171-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eissenberg, J. C., and S. C. R. Elgin. 2000. The HP1 protein family: getting a grip on chromatin. Curr. Opin. Genet. Dev. 10:204-210. [DOI] [PubMed] [Google Scholar]
- 20.Eissenberg, J. C., and T. Hartnett. 1993. A heat shock-activated cDNA rescues the recessive lethality of mutations in the heterochromatin-associated protein HP1 of Drosophila melanogaster. Mol. Gen. Genet. 240:333-338. [DOI] [PubMed] [Google Scholar]
- 21.Eissenberg, J. C., T. C. James, D. M. Foster-Hartnett, T. Hartnett, V. Ngan, and S. C. Elgin. 1990. Mutation in a heterochromatin-specific chromosomal protein is associated with suppression of position-effect variegation in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 87:9923-9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eissenberg, J. C., G. D. Morris, G. D. Reuter, and T. Hartnett. 1992. The heterochromatin-associated protein HP1 is an essential protein in Drosophila melanogaster with dosage-dependent effects on position-effect variegation. Genetics 131:345-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fanti, L., G. Giovinazzo, M. Berloco, and S. Pimpinelli. 1998. The heterochromatin protein 1 prevents telomere fusions in Drosophila. Mol. Cell 2:527-538. [DOI] [PubMed] [Google Scholar]
- 24.Gause, M., H. Hovhannisyan, T. Kahn, S. Kuhfittig, V. Mogila, and P. Georgiev. 1998. hobo induced rearrangements in the yellow locus influence the insulation effect of the gypsy su(Hw)-binding region in Drosophila melanogaster. Genetics 149:1393-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geyer, P. K., and V. G. Corces. 1987. Separate regulatory elements are responsible for the complex pattern of tissue-specific and developmental transcription of the yellow locus in Drosophila melanogaster. Genes Dev. 1:996-1004. [DOI] [PubMed] [Google Scholar]
- 26.Geyer, P. K., C. Spana, and V. G. Corces. 1986. On the molecular mechanism of gypsy-induced mutations at the yellow locus of Drosophila melanogaster. EMBO J. 5:2657-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golubovsky, M. D., A. Y. Konev, M. F. Walter, H. Biessmann, and J. M. Mason. 2001. Terminal retrotransposons activate a subtelomeric white transgene at the 2L telomere in Drosophila. Genetics 158:1111-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greider, C. W. 1999. Telomeres do D-loop-T-loop. Cell 97:419-422. [DOI] [PubMed] [Google Scholar]
- 29.Griffith, J. D., L. Comeau, S. Rosenfield, R. M. Stansel, A. Bianchi, H. Moss, and T. de Lange. 1999. Mammalian telomeres end in a large duplex loop. Cell 97:503-514. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs, S. A., S. D. Taverna, Y. Zhang, S. D. Briggs, J. Li, J. C. Eissenberg, C. D. Allis, and S. Khorasanizadeh. 2001. Specificity of the HP1 chromo domain for the methylated N-terminus of histone H3. EMBO J. 20:5232-5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.James, T. C., and S. C. R. Elgin. 1986. Identification of nonhistone chromosomal protein associated with heterochromatin in Drosophila and its gene. Mol. Cell. Biol. 6:3862-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kahn, T., M. Savitsky, and P. Georgiev. 2000. Attachment of HeT-A sequences to chromosome termini in Drosophila melanogaster may occur by different mechanisms. Mol. Cell. Biol. 20:7634-7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kellum, R., and B. Alberts. 1995. Heterochromatin protein 1 is required for correct chromosome segregation in Drosophila embryos. J. Cell Sci. 108:1419-1431. [DOI] [PubMed] [Google Scholar]
- 34.Kellum, R., J. W. Raff, and B. Alberts. 1995. Heterochromatin protein 1 distribution during development and during cell cycle in Drosophila embryos. J. Cell Sci. 108:1407-1418. [DOI] [PubMed] [Google Scholar]
- 35.Levis, R. W. 1989. Viable deletions of a telomere from a Drosophila chromosome. Cell 58:791-801. [DOI] [PubMed] [Google Scholar]
- 36.Levis, R. W., R. Ganesan, K. Houtchens, L. A. Tolar, and F.-M. Sheen. 1993. Transposons in place of telomere repeats at a Drosophila telomere. Cell 75:1083-1093. [DOI] [PubMed] [Google Scholar]
- 37.Lindsley, D. L., and G. G. Zimm. 1992. The genome of Drosophila melanogaster. Academic Press, New York, N.Y.
- 38.Lu, B. Y., P. C. R. Emtage, B. J. Duyf, A. J. Hilliker, and J. C. Eissenberg. 2000. Heterochromatin protein 1 is required for the normal expression of two heterochromatin genes in Drosophila. Genetics 155:699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin, M., Y. B. Meng, and W. Chia. 1989. Regulatory elements involved in the tissue-specific expression of the yellow gene of Drosophila. Mol. Gen. Genet. 218:118-126. [DOI] [PubMed] [Google Scholar]
- 40.Mason, J. M., A. Haoudi, A. Y. Konev, E. Kurenova, M. F. Walter, and H. Biessmann. 2000. Control of telomere elongation and telomeric silencing in Drosophila melanogaster. Genetica 109:61-70. [DOI] [PubMed] [Google Scholar]
- 41.Mason, J. M., E. Strobel, and M. M. Green. 1984. mu-2: mutator gene in Drosophila that potentiates the induction of terminal deficiencies. Proc. Natl. Acad. Sci. USA 81:6090-6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mikhailovsky, S., T. Belenkaya, and P. Georgiev. 1999. Broken chromosome ends can be elongated by conversion in Drosophila melanogaster. Chromosoma 108:114-120. [DOI] [PubMed] [Google Scholar]
- 43.Morris, J. R., P. K. Geyer, and C.-T. Wu. 1998. Core promoter elements can regulate transcription on a separate chromosome in trans. Genes Dev. 13:253-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neilsen, A. L., M. Oulad-Abdelghani, J. A. Ortiz, E. Remboutsika, P. Chambon, and R. Losson. 2001. Heterochromatin formation in mammalian cells: interaction between histones and HP1 proteins. Mol. Cell 7:729-739. [DOI] [PubMed] [Google Scholar]
- 45.Pak, D. T. S., M. Pflumm, I. Chesnokov, D. W. Huang, R. Kellum, J. Marr, P. Romanowski, and M. R. Botchan. 1997. Association of the origin recognition complex with heterochromatin and HP1 in higher eukaryotes. Cell 91:311-323. [DOI] [PubMed] [Google Scholar]
- 46.Pardue, M. L., O. N. Danilevskaya, K. Lowenhaupt, J. Wong, and K. Erby. 1996. The gag coding region of the Drosophila telomeric retrotransposon, HeT-A, has an internal frame shift and a length polymorphic region. J. Mol. Evol. 43:572-583. [DOI] [PubMed] [Google Scholar]
- 47.Pardue, M. L., and P. G. DeBaryshe. 1999. Telomeres and telomerase: more than the end of the line. Chromosoma 108:73-82. [DOI] [PubMed] [Google Scholar]
- 48.Pardue, M.-L., and P. G. Debaryshe. 2000. Drosophila telomere transposons: genetically active elements in heterochromatin. Genetica 109:45-52. [DOI] [PubMed] [Google Scholar]
- 49.Paro, R., and D. S. Hogness. 1991. The polycomb protein shares a homologous domain with a heterochromatin-associated protein of Drosophila. Proc. Natl. Acad. Sci. USA 88:263-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Platero, J. S., T. Hartnett, and J. C. Eissenberg. 1995. Functional analysis of the chromo domain of HP1. EMBO J. 14:3977-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Powers, J. A., and J. C. Eissenberg. 1993. Overlapping domains of the heterochromatin-associated protein HP1 mediate nuclear localization and heterochromatin binding. J. Cell Biol. 120:291-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 53.Shareef, M. M., C. King, M. Damaj, R. K. Badagu, D. W. Huang, and R. Kellum. 2001. Drosophila heterochromatin protein 1 (HP1)/origin recognition complex (ORC) protein associated with HP1 and ORC and functions in heterochromatin-induced silencing. Mol. Biol. Cell 12:1671-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheen, F. M., and R. W. Levis. 1994. Transposition of the LINE-like retrotransposon TART to Drosophila chromosome termini. Proc. Natl. Acad. Sci. USA 91:12510-12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smogorzewska, A., B. van Steensel, A. Bianchi, S. Oelmann, M. R. Schaefer, G. Schnapp, and T. de Lange. 2000. Control of human telomere length by TRF1 and TRF2. Mol. Cell. Biol. 20:1659-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song, K., Y. Jung, D. Jung, and I. Lee. 2001. Human Ku70 interacts with heterochromatin protein 1alpha. J. Biol. Chem. 276:8321-8327. [DOI] [PubMed] [Google Scholar]
- 57.Traverse, K. L., and M. L. Pardue. 1988. A spontaneously opened ring chromosome of Drosophila melanogaster has acquired HeT-A DNA sequences at both new telomeres. Proc. Natl. Acad. Sci. USA 85:8116-8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Steensel, B., and T. de Lange. 1997. Control of telomere length by the human telomeric protein TRF1. Nature 385:740-743. [DOI] [PubMed] [Google Scholar]
- 59.Wallrath, L. L., and S. C. R. Elgin. 1995. Position effect variegation in Drosophila is associated with altered chromatin structure. Genes Dev. 9:1263-1277. [DOI] [PubMed] [Google Scholar]
- 60.Walter, M. F., B. C. Black, G. Afshar, A.-Y. Kermabon, T. R. F. Wright, and H. Biessmann. 1991. Temporal and spatial expression of the yellow gene in correlation with cuticle formation and DOPA decarboxylase activity in Drosophila development. Dev. Biol. 147:32-45. [DOI] [PubMed] [Google Scholar]
- 61.Walter, M. F., C. Jang, B. Kasravi, J. Donath, B. M. Mechler, J. M. Mason, and H. Biessmann. 1995. DNA organization and polymorphism of a wild-type Drosophila telomere region. Chromosoma 104:229-241. [DOI] [PubMed] [Google Scholar]
- 62.Wotton, D., and D. Shore. 1997. A novel Rap1p-interacting factor, Rif2p, cooperates with Rif1p to regulate telomere length in Saccharomyces cerevisiae. Genes Dev. 11:748-760. [DOI] [PubMed] [Google Scholar]
- 63.Wustmann, G., J. Sydona, H. Taubert, and G. Reuter. 1989. The genetics of position-effect variegation modifying loci in Drosophila melanogaster. Mol. Gen. Genet. 217:520-527. [DOI] [PubMed] [Google Scholar]
- 64.Zakian, V. A. 1995. Telomeres: beginning to understand the end. Science 270:1601-1607. [DOI] [PubMed] [Google Scholar]
- 65.Zakian, V. A. 1996. Telomere functions: lessons from yeast. Trends Cell Biol. 6:29-33. [DOI] [PubMed] [Google Scholar]
- 66.Zhao, T., T. Heyduk, C. D. Allis, and J. C. Eissenberg. 2000. Heterochromatin protein 1 binds to nucleosomes and DNA in vitro. J. Biol. Chem. 275:28322-28338. [DOI] [PubMed] [Google Scholar]