Abstract
Dbl is the prototype of a large family of GDP-GTP exchange factors for small GTPases of the Rho family. In vitro, Dbl is known to activate Rho and Cdc42 and to induce a transformed phenotype. Dbl is specifically expressed in brain and gonads, but its in vivo functions are largely unknown. To assess its role in neurogenesis and gametogenesis, targeted deletion of the murine Dbl gene was accomplished in embryonic stem cells. Dbl-null mice are viable and did not show either decreased reproductive performances or obvious neurological defects. Histological analysis of mutant testis showed normal morphology and unaltered proliferation and survival of spermatogonia. Dbl-null brains indicated a correct disposition of the major neural structures. Analysis of cortical stratification indicated that Dbl is not crucial for neuronal migration. However, in distinct populations of Dbl-null cortical pyramidal neurons, the length of dendrites was significantly reduced, suggesting a role for Dbl in dendrite elongation.
The Dbl gene encodes a prototype protein for a large family of GDP-GTP exchange factors (GEFs) for Rho-like small GTPases (40, 42). GTPases function as molecular switches, being inactive when bound to GDP and active when bound to GTP. Inactivation is brought about by the intrinsic GTP hydrolytic capability, which is further increased by GTPase-activating proteins. In contrast, activation, which occurs in response to a variety of stimuli, including growth factors and cytokines, adhesion, and cell cycle progression, is mediated by GEFs, molecules which promote the dissociation of bound GDP and subsequent GTP association. The exchange of GTP for GDP induces a conformational change of the GTPase, which allows its effector domain to interact with downstream targets and eventually induces a wide range of cellular responses (35).
The Dbl protein, together with more than 40 distinct homologues (35), interacts with Rho-like GTPases by a specific subdomain, termed the Dbl homology (DH) domain, which comprises approximately 180 amino acids and is located in the center of the molecule (in the human Dbl it spans amino acids 498 to 674). This sequence has no significant homology to exchange factors for GTPases of other families, like Ras and ARF, indicating a specificity of the DH domain for Rho family members (3). Deletion studies showed that this domain is not only important for interaction with the GTPases but also essential for GEF activity (17, 45). Moreover, recent evidence suggests that the Dbl DH domain is necessary for the formation of homo-oligomers, which in turn are essential for the full activation of Rho GTPases (44). Another shared structural motif present in Dbl and all of its homologues is the pleckstrin homology (PH) domain. This motif is invariably located at the C-terminal end of the DH domain (in the human Dbl it spans amino acids 703 to 812), it consists of about 100 amino acids, and, together with the DH domain, it forms the minimal module responsible for GEF biological activity (40). By protein-protein or protein-lipid interactions, the PH domains are thought to target proteins to the plasma membrane (2). Accordingly to this view, the Dbl PH domain is necessary for the targeting of the protein to the Triton X-100-insoluble cellular fraction (43). Moreover, recent studies indicate that the Dbl PH domain binds to phosphoinositides in vivo and in vitro and that this interaction down-regulates GEF activity (31). Unlike other Rho exchange factors, Dbl contains a putative coiled-coil N-terminal sequence that shares moderate homology with the vimentin intermediate filament (30, 42). Recent findings suggest that this part of the protein is involved in an intramolecular interaction with the PH domain, keeping Dbl in an autoinhibited, inactive state (1).
Dbl has been found to activate Cdc42 and RhoA in cultured cells and in in vitro biochemical assays (16, 17, 28, 31). RhoA and Cdc42 are known to regulate actin cytoskeleton organization (15) and various physiological processes including cell movement, cell proliferation, cytokinesis, and apoptosis (35). Thus, the cellular functions of Dbl are considered to be intimately linked to the ability of activating these GTPases (40, 42). Consistent with the wide spectrum of actions of Rho-like proteins on growth regulation, Dbl and most Dbl-like proteins, including Vav, Ect2, Tim, Ost, Dbs, Lbc, Lfc, Lsc, Net, Bcr, Cdc24, RasGRF, and Sos, possess oncogenic potential. Indeed, Dbl was first identified as a transforming agent by transfection of fibroblasts with DNA derived from a human diffuse-B-cell lymphoma (10, 30). Deregulated expression of proto-Dbl per se is sufficient to cause a highly transformed phenotype in NIH 3T3 fibroblasts; however, maximal oncogenic activation results from the expression of onco-Dbl, a truncated form of Dbl that, by lacking the first 497 N-terminal residues (10, 30), contains a constitutively active DH-PH domain (42). The precise mechanism by which Dbl-dependent activation of RhoA and Cdc42 causes transformation is not clear. However, Dbl-mediated generation of transformed foci of NIH 3T3 cells is probably induced not by cytoskeletal remodeling but rather by altered gene expression. Consistently with this hypothesis, Dbl has been found to activate JNK and to stimulate transcription from NF-κB responsive elements and cyclin D1 promoter (5, 39, 41).
In naturally occurring tumors, Dbl is expressed in a restricted pattern of histiotypes, including tumors of neuroectodermal and neuroendocrine origin and Ewing's sarcoma (7, 36). Despite these findings, an attempt to reproduce Dbl-induced neoplastic transformation in transgenic mice failed to generate tumors (11) except in the absence of p53 (4). In contrast to the extensive studies on Dbl function in cellular transformation, little is known about its physiological roles. Analysis of the expression pattern in human and mouse tissues revealed that Dbl is specifically expressed in the nervous system and in gonads of both sexes, in maturing germ cells (12, 30). In order to gain deeper insight into the roles of Dbl in neurogenesis and gametogenesis, we generated by gene targeting technology a mutant mouse strain lacking Dbl expression. Here we report that Dbl-null mice are viable and fertile and do not present any major phenotype in gonads. Moreover, we show that, although Dbl is not crucial for brain development and neuronal migration, it plays a role in controlling dendritic growth of distinct subpopulations of cortical neurons.
MATERIALS AND METHODS
Generation of Dbl-null mice.
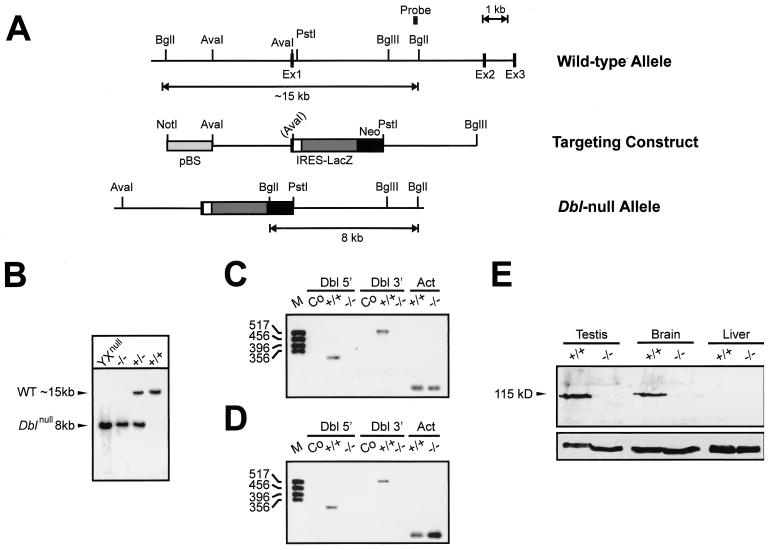
To generate Dbl-null mice, a genomic clone containing the first three Dbl exons was isolated from a phage library of 129Sv genomic DNA using as a probe the first 850 bp of the human Dbl cDNA (BamHI-HindIII fragment). The Dbl gene was disrupted by inserting into an AvaI site, downstream of the ATG start codon, the internal ribosome entry site (IRES)-LacZ cassette followed by the neomycin resistance gene (kindly provided by R. Fässler, Martinsried, Germany) (19). The targeting construct (see Fig. 1A) was electroporated into R1 embryonic stem (ES) cells in two distinct transfection experiments. Genomic DNA obtained from G418-resistant clones was digested with BglI and screened by Southern blot analysis using a BstEII-BglI external probe. This probe detects a 15-kb band and an 8-kb band in the wild-type and the homologous recombinant allele, respectively. Single-copy insertion of the targeting construct was detected with an internal probe corresponding to the neomycin resistance gene. Two correctly targeted independent clones were injected into C57B6 blastocysts and germ line-transmitting chimeras were bred to C57B6 and 129Sv mice to generate an outbred and an inbred colony.
FIG. 1.
Targeted disruption of the mouse Dbl gene. (A) Structure of the wild-type allele, targeting construct, and recombinant locus. Ex, exon. The probe used for detection of homologous recombinants is represented by a black bar. The AvaI site in brackets was lost during cloning. (B) Southern blot analysis of BglI-digested genomic DNA from Dbl-null ES cells (XYnull) and from the progeny obtained by crossing heterozygous and hemizygous mice. (C and D) RT-PCR analysis of Dbl expression in the testis (C) and brain (D) of wild-type (+/+) and Dbl-null (−/−) mice. Oligonucleotides were designed to amplify Dbl exon 1 (Dbl 5′; 315 bp containing the AvaI site used to insert the resistance cassette) and a murine Dbl cDNA sequence downstream of exon 3 (Dbl 3′; 517 bp spanning the DH and part of the PH domain). A fragment of the actin cDNA (Act) was amplified for normalization. Co, control amplification with mRNA alone. (E) Western blot analysis of testis, brain, and liver extracts from wild-type (+/+) and Dbl-null mice (−/−), using anti-Dbl antibody (top); the blot was reprobed with an anti-focal adhesion kinase antibody to confirm loading of equal amounts of proteins (bottom).
RNA analysis.
For Dbl expression analysis, total RNA was extracted using RNeasy columns (Qiagen, Hilden, Germany) and subsequently retrotranscribed with Moloney murine leukemia virus reverse transcriptase (RT; Life Technologies, Gaithersburg, Md.). Amplification by PCR was obtained with the following primers: 5′-AATACCACCCAAGAAGCAGC-3′ and 5′-GGGGTTAACTGCTTGTCATC-3′ for Dbl 5′ (amplifying nucleotides 38 to 352 of the murine Dbl cDNA), 5′-TGATCTTATGCCACCTCTCC-3′ and 5′-TTTGTAGCACCTTTCCGGTG-3′ for Dbl 3′ (amplifying nucleotides 1709 to 2251 of the murine Dbl cDNA), and 5′-TCCTGCGTCTGGACCTGG-3′ and 5′-CCATCTCTTGCTCGAAGT-3′ for actin. Expression of lacZ was evaluated with two primers located in the 3′ portion of the gene: 5′-CAACTGGTAATGGTAGCGA-3′ and 5′-AACCAGCCATCGCCATCT-3′. Analysis of marker gene expression during the first stages of spermatogenesis was performed as described above with primers amplifying genes for Mvh (5′-CCAAAAGTGACATATATACCC-3′ and 5′-TTGGTTGATCAGTTCTCGAG-3′), Sycp3 (5′-GGTGGAAGAAAGCATTCTGG-3′ and 5′-CAGCTCCAAATTTTTCCAGC-3′), and calmegin (5′-ATATGCGTTTCCAGGGTGTTGGAC-3′ and 5′-GTATGCACCTCCACAATCAATACC-3′) as previously reported (32).
Western blotting.
Tissues were homogenized in Laemmli's buffer containing 10 μg of aprotinin, 10 μg of leupeptin, and 10 μg of pepstatin per ml. Then 100 μg of protein was subjected to electrophoresis on a 6% gel, transferred to a nitrocellulose membrane, and blocked with 5% milk in Tris-buffered saline with 0.05% Tween 20. Membranes were incubated with anti-Dbl rabbit polyclonal antibody raised against a peptide mapping within amino acids 800 to 850, a region downstream of the DH-PH domains of Dbl (SC-89; Santa Cruz, Santa Cruz, Calif.) diluted 1:500. Primary antibody was then detected with horseradish peroxidase-conjugated antibody, and the signal was visualized with an ECL system (Amersham Pharmacia Biotech, Piscataway, N.J.).
Histology and immunohistochemistry. For staining with hematoxylin-eosin and bromodeoxyuridine (BrdU) and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) immunohistochemistry, tissues were fixed in 4% buffered paraformaldehyde (PAF), paraffin embedded, and sectioned at a thickness of 5 μm. For routine histology, sections were stained with hematoxylin-eosin according to standard protocols. For BrdU labeling, BrdU (Sigma-Aldrich, St. Louis, Mo.) was injected intraperitoneally (100 μg/g of body mass). Injected animals were killed 1 h later, and the fixed testes were paraffin embedded, sectioned, and stained by using BrdU labeling kit II (Boehringer Mannheim, Mannheim, Germany) following the manufacturer's instructions. Apoptotic cells were detected by TUNEL using an in situ cell death detection kit (Roche Diagnostics, Mannheim, Germany) according to the instructions of the manufacturer. For overall nervous system histology, sagittal sections of PAF-perfused brains were Nissl stained with 2% cresyl violet. Cryostat coronal and tangential sections (50 μm thick) were reacted for both cytochrome oxidase and NADPH-diaphorase (NADPH-d) (38) histochemistry, in order to study the barrel field in somatosensory cortex and the laminar distribution of nitric oxide-synthesizing interneurons. Briefly, sections were incubated first for 1 h in a solution of 1 mg of NADPH (Sigma, St. Louis, Mo.) per ml and 0.2 mg of nitroblue tetrazolium (Sigma) per ml in phosphate buffer containing 0.5 to 1% Triton X-100 and then for 12 to 24 h in a medium containing cytochrome c (Sigma) and diamino-benzidine. For in situ detection of LacZ activity, mutant tissues were fixed, cryosectioned (10 μm), and stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) as described previously (19).
Analysis of neuronal processes.
In a series of embryonic and neonatal animals, the early development of retinal projections to the primary visual centers and of pyramidal neurons in the cerebral cortex was studied. For this purpose, we inserted crystals of the lipophilic carbocyanine dye 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; Molecular Probes) (37) in PAF-fixed brains, as follows: (i) in the optic nerve, to label retinotectal and retinogeniculate projections; (ii) in the superior colliculus, to label corticocollicular pyramidal neurons; and (iii) in the corpus callosum, to label callosally projecting corticocortical neurons. After several weeks at 37°C (depending on the length of the pathway to be traced by DiI), 150-μm-thick coronal sections of brains were cut with a vibratome (Leica, Heidelberg, Germany) and analyzed with a Nikon Eclipse 600 fluorescence microscope. Selected pyramidal neurons were drawn using a computer-interfaced microscope with a motorized stage and Neurolucida software (13). The dendritic arbors and the cell bodies of pyramidal neurons were quantitatively studied by using Neuroexplorer software.
RESULTS
Generation of Dbl-null mice.
To generate an inactive Dbl allele by gene targeting, a murine genomic clone containing the Dbl locus was identified by using human Dbl cDNA (30) as a probe. This strategy led to the identification of a clone spanning 15 kbp and containing the first coding exon (exon 1) and the next two exons (Fig. 1A). Sequence analysis revealed that the fragment of the murine Dbl locus analyzed has the same genomic structure as its human counterpart and that the length of introns and exons is conserved (29). To inactivate Dbl expression, exon 1 (Fig. 1A) was interrupted downstream of the starting codon by the insertion of a neomycin resistance cassette. Of 310 G418-resistant ES cell clones, 11 showed by Southern blot hybridization the restriction pattern expected after homologous recombination (Fig. 1B). Interestingly, recombinant ES cells showed a single band corresponding to the mutant allele (Fig. 1B). This observation supports the localization of murine Dbl in the X chromosome and is consistent with the X chromosome mapping of the human Dbl gene (12, 29). In fact, R1 ES cells derive from a male embryo (26), and therefore, mutant clones carry only the Dbl-null allele.
In vitro culture of Dbl-null ES cells did not show any abnormality in cell shape or growth, in agreement with the absence of Dbl expression in wild-type ES cells (as assessed by RT-PCR; data not shown). Two Dbl-null clones were injected into recipient blastocysts, and chimeras with extensive ES cell contribution were produced. This result indicated that the presence of cells hemizygous for the Dbl-null allele was compatible with normal development of chimeras. Mating of male chimeras with wild-type mice revealed no impairment in germ line transmission, initially suggesting that the mutation did not affect spermatogenesis in the context of a chimeric gonad. Inbred 129Sv and outbred 129Sv/C57 mutant strains were established and showed a normal X-linked distribution of the Dbl-null allele: of 364 mice obtained by crossing hemizygous males with heterozygous females, 90 (24.2%) were wild-type males, 93 (25.5%) were mutant males, 88 (24.2%) were heterozygous females, and 95 (26.1%) were homozygous females. The insertion of the IRES-LacZ cassette into exon 1 was used to track Dbl expression in situ in tissues known to express Dbl, such as brain and gonads. However, possibly because of the combination of the low activity of the Dbl promoter (12) and the partial inefficiency of the IRES sequence (19), expression of β-galactosidase could not be detected by X-Gal staining but only by RT-PCR analysis (data not shown).
To test whether the Dbl-null allele abolished Dbl expression, RT-PCR experiments were carried out with oligonucleotides amplifying exon 1 and a 3′ region corresponding to the murine Dbl cDNA sequence (12). As expected for the specific expression of Dbl in tissues of neuroectodermal origin and in gonads (12, 30), the Dbl transcript was found in total mRNA extracted from wild-type testis (Fig. 1C) and brain (Fig. 1D). Conversely, the amplification bands were absent in all tissues homozygous for the Dbl-null allele (Fig. 1C and D). The upstream and downstream primers amplifying the 3′ region of Dbl are located in the DH and PH domains, respectively, and the result of the amplification thus demonstrates that the sequence containing these two domains is no longer transcribed in the mutant organs. The lack of expression in mutant mice of the protein encoded by the Dbl gene was further proven by Western blot analysis with an antibody raised against an epitope mapping at the C-terminal tail of Dbl. Wild-type controls showed expression of Dbl in adult testis and brain but not in liver extracts. In contrast, in all samples derived from Dbl-null homozygous mice, Dbl was undetectable (Fig. 1E).
Dbl is dispensable for gonad development and function.
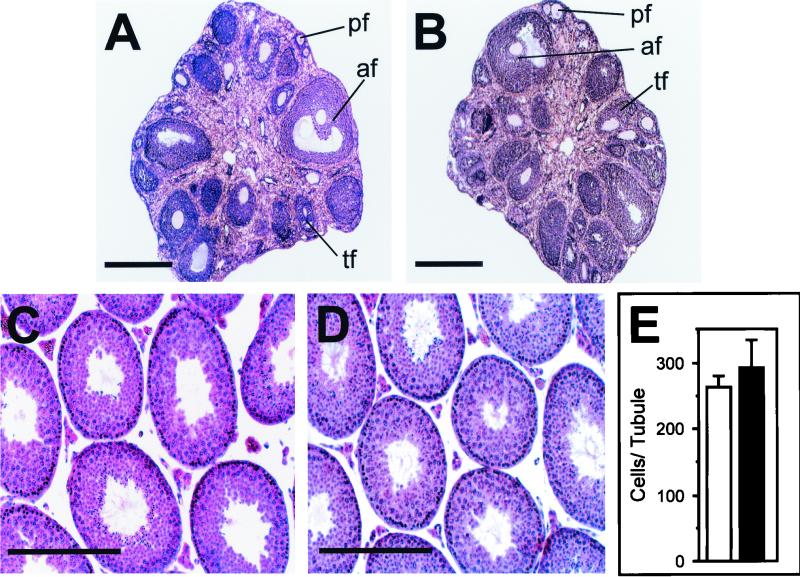
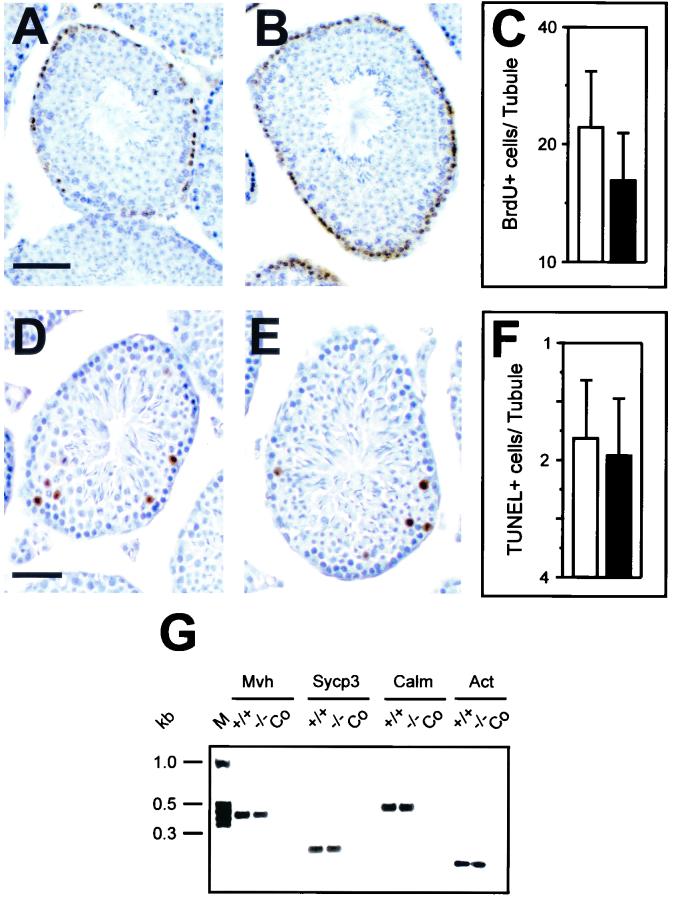
Mice deficient for Dbl were intercrossed to establish their ability to produce offspring. Despite the presence of Dbl in gonads of both sexes, no impairment of fertility was detected. Analysis of breeding performance revealed that Dbl-null females produced litters comparable in frequency and size to those of their wild-type counterparts. Histological analysis of Dbl-null homozygous ovaries at 8 weeks of age did not show any overt abnormality, and all stages of follicular maturation were detected equally in the two genotypes (Fig. 2A and B). Like females, Dbl-null males were able to generate progeny to a normal extent (data not shown). Consistently, histological analysis of adult seminiferous tubules confirmed that spermatogenesis can proceed correctly in the absence of Dbl (Fig. 2C and D). Moreover, measurements of tubular morphology and cellularity did not reveal significant differences in diameter and germ cell number (Fig. 2E). Since Dbl is involved in the control of cell proliferation and survival (40-42), it is possible that, in its absence, subtle differences in the production of male germ cells may occur without altering fertility. To test this hypothesis, proliferation and survival of spermatogonia were analyzed by BrdU and TUNEL labeling assays. Measurements of proliferating cells in mature tubules were not different in mice of the two genotypes (Fig. 3A to C). Similarly, the number of apoptotic cells was not significantly altered in Dbl-null versus wild-type testes (Fig. 3D to F). To investigate whether the lack of Dbl could influence testis development, histology of wild-type and mutant masculine gonads was analyzed at 10 and 20 days after birth, but no major differences were detected (data not shown). To further test whether Dbl-null spermatogonia correctly entered the initial meiotic division, the expression of specific marker genes for early spermatogenic cells (32) was followed by RT-PCR analysis of 15-day-old testes (Fig. 3G). The proper expression of Mvh (present from the primordial germ cell stage throughout the meiotic division), Sycp3 (expressed from leptotene), and calmegin (present from pachytene) indicates that Dbl-null spermatocytes develop normally to and beyond the pachytene stage.
FIG. 2.
Histological analysis of ovary and testis. (A and B) Hematoxylin-eosin-stained sections of wild-type (A) and Dbl-null (B) adult ovaries. Various follicular maturation stages are equally present in both genotypes. pf, primary follicle; tf, atresic follicle; af, antral follicle. Bar, 1 mm. (C and D) Hematoxylin-eosin stained sections of wild-type (C) and Dbl-null (D) adult testes. Bar, 300 μm. (E) Number of cells per seminiferous tubule in sections of wild-type (white bar) and Dbl-null (black bar) testis. Nuclei were counted in 10 random tubule sections from the testes of five 8-week-old mice of both genotypes. Values are means ± standard deviations.
FIG. 3.
Analysis of spermatogenesis. (A and B) BrdU labeling of proliferating spermatogonial cells in wild-type (A) and Dbl-null (B) testes. Bar, 100 μm. (C) Frequency of BrdU-labeled cells is equivalent in seminiferous tubules of adult wild-type mice (white bar) and homozygous mutants (black bar). BrdU-labeled cells were counted in 10 random tubule sections from the testes of five 8-week-old mice of both genotypes. Values are means ± standard deviations. (D and E) TUNEL staining in wild-type (D) and Dbl-null (E) adult testes. Bar, 100 μm. (F) The number of apoptotic cells in seminiferous tubules is not altered by the lack of Dbl. Means ± standard deviations were calculated as for panel C. (G) RT-PCR analysis of the sperm-specific marker genes for Mvh, Sycp3, and calmegin (Calm). A fragment of actin cDNA (Act) was amplified for normalization.
Dbl is dispensable for neuronal migration but is required for correct extension of basal dendrites.
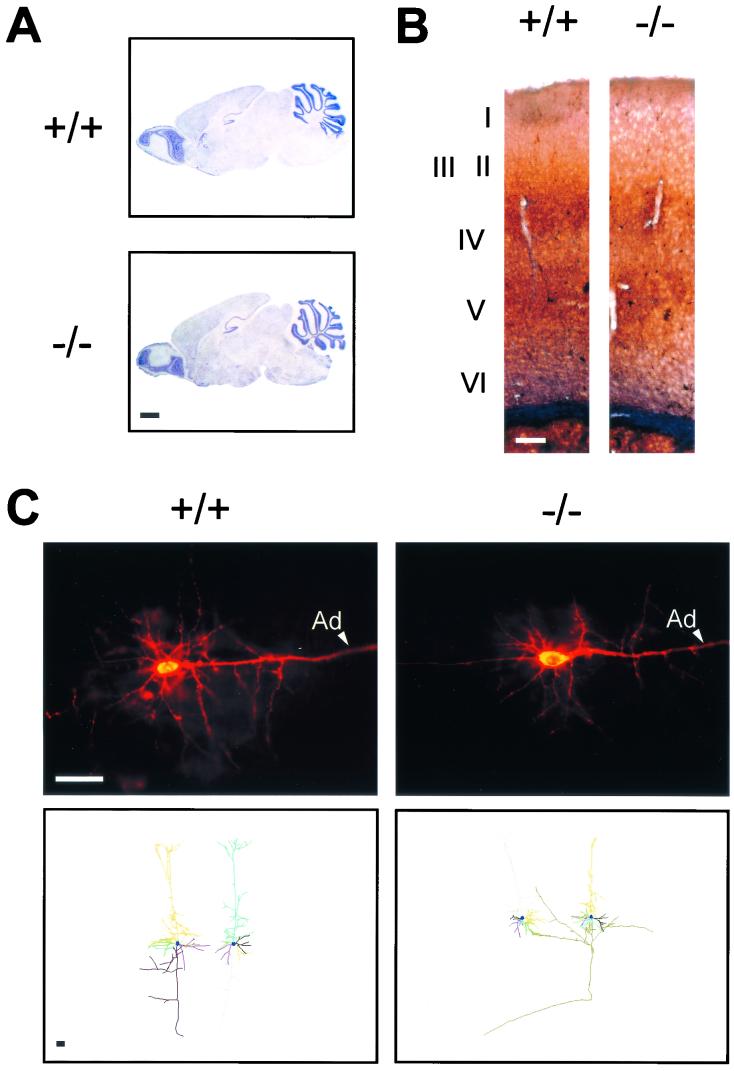
Despite the expression of Dbl in the nervous system (7, 30), mutant mice did not show any major alteration of behavior, particularly with respect to posture, coordination of movements, sensorial discrimination, nourishing activity, and socialization. To investigate whether the lack of Dbl caused subtle alterations in the morphology of the nervous system, adult brains were sectioned and Nissl stained. As shown in Fig. 4A, sections of wild-type and Dbl-null brains were indistinguishable. To further understand whether Dbl, like its Rho GTPase downstream effectors (23), played a role in controlling migration of nervous cells, the distribution of neuronal populations was analyzed in the cortex. When both Nissl-stained (Fig. 4A) and cytochrome oxidase-stained (data not shown) sections were examined, cortical lamination and cortical columns in Dbl-null mice appeared to be identical to those of wild-type controls. Similarly, the barrel field in the somatosensory cortex was equally well organized in mice of both genotypes (data not shown). Differential staining procedures were further used to examine the positions of specific populations of cortical neurons. NO-synthesizing inhibitory GABAergic interneurons were evidenced by the NADPH-d reaction (Fig. 4B). Conversely, the localization of pyramidal neurons was analyzed by retrograde transport of DiI inserted in the superior colliculus (Fig. 4C) or in the corpus callosum. Both methods revealed in Dbl-null brains a normal distribution of cells in the cortical layers, suggesting that Dbl is not essential for the migration of interneurons and pyramidal neurons. Since Rho GTPases play an important role in the development of axons and dendrites (23), DiI-labeled neuronal processes were analyzed by fluorescence microscopy using computer-assisted morphometric techniques. As determined by anterograde labeling of retinocollicular and retinogeniculate projections at embryonic day 18, the distribution of axons was similar in control and Dbl-null brains (data not shown). Conversely, to study definitive dendrite arborization, measurements were focused on dendrites, at 7 days after birth, of two well-characterized distinct cortical pyramidal neurons: the corticocollicular and the callosally projecting cells. Statistical analysis revealed that the basal dendrites in both corticocollicular and callosally projecting Dbl-null neurons were significantly shorter than those in matching wild-type cells (Table 1). Similarly, apical dendrites showed a strong tendency to be shorter in mutant brains (Table 1). Although the number of nodes per dendrite did not appear to be different in the two genotypes, these results suggest a specific role for Dbl in the fine control of dendrite elongation in cortical cells.
FIG. 4.
Brain histology. (A) Nissl staining of sagittal sections of wild-type (+/+) and Dbl-deficient (−/−) mice. Bar, 1 mm. (B) Analysis of cortical neuron stratification by NADPH-d staining. Distribution and density of labeled cells are equal in the two genotypes. Cortical layers are indicated by roman numbers. Bar, 200 μm. (C) (Upper panels) Fluorescence microscopy of vibratome sections of P7 wild-type (left) and Dbl-null (right) brains showing DiI-labeled corticocollicular neurons. Ad, apical dendrite. (Lower panels) Drawings of individual callosally projecting neurons from P7 wild-type (left) and Dbl-null (right) brains using Neurolucida software. Single dendrites are shown in different colors. Bars, 100 μm.
TABLE 1.
Morphometric analysis of wild-type and Dbl-null cortical dendrites
| Dendrite type | Mouse genotype | Mean ± SD (n) [Pa]
|
|
|---|---|---|---|
| Dendrite length (μm) | No. of nodes/dendrite | ||
| Callosally projecting | |||
| Basalb | Wild type | 401.23 ± 216.01 (50) | 3.31 ± 2.13 |
| Dbl null | 264.29 ± 198.61 (36) [0.003] | 2.92 ± 2.64 | |
| Apical | Wild type | 3,755 ± 1,208 (8) | 31 ± 11 |
| Dbl null | 2,178 ± 579 (15) [0.007] | 22 ± 6 | |
| Corticocollicular | |||
| Basalb | Wild type | 443.95 ± 248.66 (43) | 3.36 ± 2.13 |
| Dbl null | 320.53 ± 215.24 (69) [0.008] | 2.93 ± 2.47 | |
| Apical | Wild type | 2,054 ± 856 (6) | 17 ± 7 |
| Dbl null | 1,396 ± 633 (7) [0.15] | 13 ± 6 | |
P values were calculated by Student's t test and show significance compared with the wild-type value.
Basal dendrites were measured in 15 cells derived from three brains per genotype at postnatal day 7.
DISCUSSION
Dbl, the prototype GDP/GTP exchange factor for small GTPases of the Rho family, was found to play crucial roles in vitro in cell proliferation (40, 42) and cytoskeletal remodeling (6), but its in vivo function remained largely unknown. In an attempt to elucidate this role, mice lacking Dbl expression were generated by gene targeting. Previous reports indicated that deletion of other GEFs of the Dbl family that modulate Rho could cause severe developmental defects. For example, the inactivation of the Drosophila melanogaster protein DrhoGEF2 determines defective gastrulation and the formation of embryos lacking mesoderm (14). Given the high degree of structural and functional conservation of the Dbl family genes, the finding that Dbl-null mice can normally develop was thus unexpected. Nonetheless, the restricted expression pattern of Dbl in adult brain and gonads suggested that it could play crucial roles in specialized tasks such as gametogenesis and neurogenesis.
In agreement with the hypothesis of Dbl playing a role in ovary function, experimental evidence indicates that control of Rho GTPases are important for oogenesis. An example is the targeted inactivation of the Rho upstream modulator RhoGDIα, which, in mice, causes severe infertility of females (34). In contrast, in Dbl-deficient females, the normal morphology of ovaries, the presence of unaltered numbers of follicles at all maturation stages, and the equal litter sizes in wild-type and mutant mice strongly indicate that the lack of Dbl does not influence ovary function.
The expression of Dbl in testes, however, pointed to the possible presence of subtle phenotypes that could negatively influence spermatogenesis or spermatozoan functions. In addition, experimental evidence suggests that Dbl-dependent activation of Rho might play a role in this process. Dbl-dependent activation of Rho could influence the activity of Rho effectors like ROCK and citron kinase, which are known to play crucial roles during cytokinesis (9, 21, 25) of various cell types, possibly including spermatogonia. In agreement with this hypothesis, citron kinase-deficient mice show testicular atrophy (F. Di Cunto, personal communication). In addition, the role of Rho in sperm production is further supported by the finding that mice lacking RhoGDIα have abnormal seminiferous tubules and a reduced number of germinal cells and are sterile (34). Finally, as showed by inhibition of Rho by ADP-ribosylation using the C3 exoenzyme, Rho GTPases have a prominent role in motility of bovine sperm (18). Despite all these indications, Dbl-deficient males did not show major alterations of testicular morphology and were fertile. Moreover, the findings that Dbl-deficient testes do not display abnormal cell proliferation and survival and that entering the meiotic cycle is not affected further prove that Dbl is dispensable for spermatogenesis.
The second major district of Dbl expression is the central nervous system, where Dbl appears to be widely distributed in developing neurons (7). Despite this fact, no major alterations of brain morphology were detected in Dbl-null mice. This finding was unexpected, especially because control over the activity of Rho GTPases is considered crucial for neuronal proliferation and migration (23). For example, in mice lacking the Dbl homologue Trio, the brain develops to a normal size but specific abnormalities of neural organization are seen in the olfactory bulb and in the dentate gyrus (27). In contrast, our data show that involvement of Dbl in the control of neuronal migration is very unlikely. Although it is still possible that subtle morphological alterations of Dbl-deficient mice might have escaped detection, histological examination of mutant cortex using different labeling techniques (Fig. 4) did not reveal any defective neuronal organization, clearly excluding a role for Dbl in cortical neuron migration. The other possible function of Dbl could be to control Rho-like GTPases in axon pathfinding and dendrite arborization. Following a commonly accepted model, Rac and Cdc42 activation promotes growth cone advance, whereas Rho activation inhibits growth or promotes retraction (8, 20, 23). Interestingly, a statistically significant (30%) reduction of total basal dendritic length was evidenced in cortical neurons of 7-day-old Dbl-deficient mice. This observation strongly suggests that Dbl might be necessary for the correct elongation of basal dendrites. This conclusion is indeed supported by the finding that two distinct neuronal populations (corticocollicular and callosally projecting neurons) have very similar phenotypes. In addition, analysis of apical dendrite extension, although it did not reach statistical significance in corticocollicular cells (possibly due to an insufficient number of samples), indicates that the lack of Dbl causes a tendency to size reduction. Interestingly, observation of retinotectal and retinogeniculate projections at embryonic day 18 indicated that the lack of Dbl does not affect axonal elongation and arborization, suggesting that Dbl might be particularly important only in dendrite development. Consistent with this hypothesis, several lines of evidence indicate that specific members of the Rho GTPase family might have distinct roles in regulating outgrowth and retraction of neurites. For example, in Drosophila, whereas the Rac homologue Drac appears to control axon but not dendrite elongation, the Cdc42 homologue Dcdc42 governs the extension of both types of projections (23).
From in vitro experiments using cultured cells and biochemical assays, Dbl is known to activate mainly Rho and Cdc42. Given the opposing activities of these two GTPases, which promote growth retraction and elongation, respectively, the finding that Dbl-deficient dendrites are shorter suggests that, in vivo, Dbl might have a stronger effect on Cdc42 than on RhoA. In fact, in vivo analysis of RhoA function indicates that, in Drosophila, this specific GTPase is not required for the growth of axonal projections but is necessary to restrain the extension of dendrites (22). On the other hand, Cdc42 appears to play an opposite role: mutations of the Drosophila homologue Dcdc42 cause defective dendrite and axon elongation (24). In agreement, in vitro experiments show that transfection of a dominant negative Cdc42 in vertebrate pyramidal neurons affects dendrite formation (33).
Technical factors limited morphometric analysis of cortex to 7-day-old brains: in fact, during the first postnatal week developing dendrites appeared to be too short for a significant quantification, and later, DiI tracing is less effective in filling all dendritic arbors. Although the dendritic tree is fairly complete in mice at 1 week of age, we cannot determine whether the phenotype of cortical neurons is only transient, occurring just after a period of rapid dendritic growth, or is stable and maintained at the adult stage. Although adult mutant mice do not display important neurological phenotypes, it is possible that, because of the effect seen in 1-week-old brains, adult Dbl-deficient mice could display aberrant scores in behavioral model systems.
A likely explanation for absent or subtle phenotypes in Dbl-deficient mice is redundancy or compensation. Dbl is a member of a growing family of homologues that not only have structural similarities but also exhibit similar functions and expression patterns (40, 42). In fact, other than Dbl, the Rho activators Tiam, Abr, and Bcr are present in neurons and germ cells (40, 42). In addition, a large number of Rho GEFS, like Dbs, Ost, Lbc, Lfc, Lsc, Trio, Ephexin, and Kalirin, are concomitantly expressed in the brain. It is therefore possible that multiple Rho GEFs are coexpressed in the same cells and that this redundancy might mask the lack of a single member. The establishment of further mutant strains lacking the expression of other Rho GEFs and their intercrossing will help to address this question.
Acknowledgments
We thank F. Di Cunto for his valuable advice and for sharing unpublished information. We are grateful to G. Tarone for critically reading the manuscript. We thank Immacolata Carfora, Davide Barberio, and Sara Imarisio for technical assistance.
This work was supported by a grant from CNR PF Biotecnologie, MURST ex 60% and 40%, Italian Association for Cancer Research (AIRC).
REFERENCES
- 1.Bi, F., B. Debreceni, K. Zhu, B. Salani, A. Eva, and Y. Zheng. 2001. Autoinhibition mechanism of proto-Dbl. Mol. Cell. Biol. 21:1463-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bottomley, M. J., K. Salim, and G. Panayotou. 1998. Phospholipid-binding protein domains. Biochim. Biophys. Acta 1436:165-183. [DOI] [PubMed] [Google Scholar]
- 3.Cherfils, J., and P. Chardin. 1999. GEFs: structural basis for their activation of small GTP-binding proteins. Trends Biochem. Sci. 24:306-311. [DOI] [PubMed] [Google Scholar]
- 4.Colucci-D'Amato, G. L., G. Santelli, A. D'Alessio, G. Chiappetta, A. Mineo, G. Manzo, G. Vecchio, and V. de Franciscis. 1995. Dbl expression driven by the neuron specific enolase promoter induces tumor formation in transgenic mice with a p53(+/−) genetic background. Biochem. Biophys. Res. Commun. 216:762-770. [DOI] [PubMed] [Google Scholar]
- 5.Coso, O. A., M. Chiariello, J. C. Yu, H. Teramoto, P. Crespo, N. Xu, T. Miki, and J. S. Gutkind. 1995. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell 81:1137-1146. [DOI] [PubMed] [Google Scholar]
- 6.Defilippi, P., C. Olivo, M. Venturino, L. Dolce, L. Silengo, and G. Tarone. 1999. Actin cytoskeleton organization in response to integrin-mediated adhesion. Microsc. Res. Tech. 47:67-78. [DOI] [PubMed] [Google Scholar]
- 7.de Franciscis, V., R. Rosati, G. L. Colucci-D'Amato, A. Eva, and G. Vecchio. 1991. Preferential expression of the dbl protooncogene in some tumors of neuroectodermal origin. Cancer Res. 51:4234-4237. [PubMed] [Google Scholar]
- 8.Dickson, B. J. 2001. Rho GTPases in growth cone guidance. Curr. Opin. Neurobiol. 11:103-110. [DOI] [PubMed] [Google Scholar]
- 9.Di Cunto, F., S. Imarisio, E. Hirsch, V. Broccoli, A. Bulfone, A. Migheli, C. Atzori, E. Turco, R. Triolo, G. P. Dotto, L. Silengo, and F. Altruda. 2000. Defective neurogenesis in citron kinase knockout mice by altered cytokinesis and massive apoptosis. Neuron 28:115-127. [DOI] [PubMed] [Google Scholar]
- 10.Eva, A., and S. A. Aaronson. 1985. Isolation of a new human oncogene from a diffuse B-cell lymphoma. Nature 316:273-275. [DOI] [PubMed] [Google Scholar]
- 11.Eva, A., G. Graziani, M. Zannini, L. M. Merin, J. S. Khillan, and P. A. Overbeek. 1991. Dominant dysplasia of the lens in transgenic mice expressing the dbl oncogene. New Biol. 3:158-168. [PubMed] [Google Scholar]
- 12.Galland, F., V. Pirisi, O. de Lapeyriere, and D. Birnbaum. 1991. Restriction and complexity of Mcf2 proto-oncogene expression. Oncogene 6:833-839. [PubMed] [Google Scholar]
- 13.Glaser, J. R., and E. M. Glaser. 1990. Neuron imaging with Neurolucida--a PC-based system for image combining microscopy. Comput. Med. Imaging Graph. 14:307-317. [DOI] [PubMed] [Google Scholar]
- 14.Hacker, U., and N. Perrimon. 1998. DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila. Genes Dev. 12:274-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509-514. [DOI] [PubMed] [Google Scholar]
- 16.Hart, M. J., A. Eva, T. Evans, S. A. Aaronson, and R. A. Cerione. 1991. Catalysis of guanine nucleotide exchange on the CDC42Hs protein by the dbl oncogene product. Nature 354:311-314. [DOI] [PubMed] [Google Scholar]
- 17.Hart, M. J., A. Eva, D. Zangrilli, S. A. Aaronson, T. Evans, R. A. Cerione, and Y. Zheng. 1994. Cellular transformation and guanine nucleotide exchange activity are catalyzed by a common domain on the dbl oncogene product. J. Biol. Chem. 269:62-65. [PubMed] [Google Scholar]
- 18.Hinsch, K. D., B. Habermann, I. Just, E. Hinsch, S. Pfisterer, W. B. Schill, and K. Aktories. 1993. ADP-ribosylation of Rho proteins inhibits sperm motility. FEBS Lett. 334:32-36. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch, E., V. L. Katanaev, C. Garlanda, O. Azzolino, L. Pirola, L. Silengo, S. Sozzani, A. Mantovani, F. Altruda, and M. P. Wymann. 2000. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science 287:1049-1053. [DOI] [PubMed] [Google Scholar]
- 20.Kaibuchi, K., S. Kuroda, and M. Amano. 1999. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu. Rev. Biochem. 68:459-486. [DOI] [PubMed] [Google Scholar]
- 21.Kosako, H., H. Goto, M. Yanagida, K. Matsuzawa, M. Fujita, Y. Tomono, T. Okigaki, H. Odai, K. Kaibuchi, and M. Inagaki. 1999. Specific accumulation of Rho-associated kinase at the cleavage furrow during cytokinesis: cleavage furrow-specific phosphorylation of intermediate filaments. Oncogene 18:2783-2788. [DOI] [PubMed] [Google Scholar]
- 22.Lee, T., C. Winter, S. S. Marticke, A. Lee, and L. Luo. 2000. Essential roles of Drosophila RhoA in the regulation of neuroblast proliferation and dendritic but not axonal morphogenesis. Neuron 25:307-316. [DOI] [PubMed] [Google Scholar]
- 23.Luo, L. 2000. Rho GTPases in neuronal morphogenesis. Nat. Rev. Neurosci. 1:173-180. [DOI] [PubMed] [Google Scholar]
- 24.Luo, L., Y. J. Liao, L. Y. Jan, and Y. N. Jan. 1994. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 8:1787-1802. [DOI] [PubMed] [Google Scholar]
- 25.Madaule, P., M. Eda, N. Watanabe, K. Fujisawa, T. Matsuoka, H. Bito, T. Ishizaki, and S. Narumiya. 1998. Role of citron kinase as a target of the small GTPase Rho in cytokinesis. Nature 394:491-494. [DOI] [PubMed] [Google Scholar]
- 26.Nagy, A., J. Rossant, R. Nagy, W. Abramow-Newerly, and J. C. Roder. 1993. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl. Acad. Sci. USA 90:8424-8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Brien, S. P., K. Seipel, Q. G. Medley, R. Bronson, R. Segal, and M. Streuli. 2000. Skeletal muscle deformity and neuronal disorder in Trio exchange factor-deficient mouse embryos. Proc. Natl. Acad. Sci. USA 97:12074-12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olivo, C., C. Vanni, P. Mancini, L. Silengo, M. R. Torrisi, G. Tarone, P. Defilippi, and A. Eva. 2000. Distinct involvement of cdc42 and RhoA GTPases in actin organization and cell shape in untransformed and Dbl oncogene transformed NIH3T3 cells. Oncogene 19:1428-1436. [DOI] [PubMed] [Google Scholar]
- 29.Palmieri, G., V. de Franciscis, A. Casamassimi, G. Romano, A. Torino, P. Pingitore, D. Califano, G. Santelli, A. Eva, G. Vecchio, M. D'Urso, and A. Ciccodicola. 2000. Human dbl proto-oncogene in 85 kb of xq26, and determination of the transcription initiation site. Gene 253:107-115. [DOI] [PubMed] [Google Scholar]
- 30.Ron, D., S. R. Tronick, S. A. Aaronson, and A. Eva. 1988. Molecular cloning and characterization of the human dbl proto-oncogene: evidence that its overexpression is sufficient to transform NIH/3T3 cells. EMBO J. 7:2465-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russo, C., Y. Gao, P. Mancini, C. Vanni, M. Porotto, M. Falasca, M. R. Torrisi, Y. Zheng, and A. Eva. 2001. Modulation of oncogenic DBL activity by phosphoinositol phosphate binding to pleckstrin homology domain. J. Biol. Chem. 276:19524-19531. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka, S. S., Y. Toyooka, R. Akasu, Y. Katoh-Fukui, Y. Nakahara, R. Suzuki, M. Yokoyama, and T. Noce. 2000. The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev. 14:841-853. [PMC free article] [PubMed] [Google Scholar]
- 33.Threadgill, R., K. Bobb, and A. Ghosh. 1997. Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron 19:625-634. [DOI] [PubMed] [Google Scholar]
- 34.Togawa, A., J. Miyoshi, H. Ishizaki, M. Tanaka, A. Takakura, H. Nishioka, H. Yoshida, T. Doi, A. Mizoguchi, N. Matsuura, Y. Niho, Y. Nishimune, S. Nishikawa, and Y. Takai. 1999. Progressive impairment of kidneys and reproductive organs in mice lacking Rho GDIα. Oncogene 18:5373-5380. [DOI] [PubMed] [Google Scholar]
- 35.Van Aelst, L., and C. D'Souza-Schorey. 1997. Rho GTPases and signaling networks. Genes Dev. 11:2295-2322. [DOI] [PubMed] [Google Scholar]
- 36.Vecchio, G., A. O. Cavazzana, T. J. Triche, D. Ron, C. P. Reynolds, and A. Eva. 1989. Expression of the dbl proto-oncogene in Ewing's sarcomas. Oncogene 4:897-900. [PubMed] [Google Scholar]
- 37.Vercelli, A., M. Repici, D. Garbossa, and A. Grimaldi. 2000. Recent techniques for tracing pathways in the central nervous system of developing and adult mammals. Brain Res. Bull. 51:11-28. [DOI] [PubMed] [Google Scholar]
- 38.Vincent, S. R., and H. Kimura. 1992. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience 46:755-784. [DOI] [PubMed] [Google Scholar]
- 39.Westwick, J. K., R. J. Lee, Q. T. Lambert, M. Symons, R. G. Pestell, C. J. Der, and I. P. Whitehead. 1998. Transforming potential of Dbl family proteins correlates with transcription from the cyclin D1 promoter but not with activation of Jun NH2-terminal kinase, p38/Mpk2, serum response factor, or c-Jun. J. Biol. Chem. 273:16739-16747. [DOI] [PubMed] [Google Scholar]
- 40.Whitehead, I. P., S. Campbell, K. L. Rossman, and C. J. Der. 1997. Dbl family proteins. Biochim. Biophys. Acta 1332:F1-F23. [DOI] [PubMed] [Google Scholar]
- 41.Whitehead, I. P., Q. T. Lambert, J. A. Glaven, K. Abe, K. L. Rossman, G. M. Mahon, J. M. Trzaskos, R. Kay, S. L. Campbell, and C. J. Der. 1999. Dependence of Dbl and Dbs transformation on MEK and NF-κB activation. Mol. Cell. Biol. 19:7759-7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng, Y. 2001. Dbl family guanine nucleotide exchange factors. Trends Biochem. Sci. 26:724-732. [DOI] [PubMed] [Google Scholar]
- 43.Zheng, Y., D. Zangrilli, R. A. Cerione, and A. Eva. 1996. The pleckstrin homology domain mediates transformation by oncogenic dbl through specific intracellular targeting. J. Biol. Chem. 271:19017-19020. [DOI] [PubMed] [Google Scholar]
- 44.Zhu, K., B. Debreceni, F. Bi, and Y. Zheng. 2001. Oligomerization of DH domain is essential for Dbl-induced transformation. Mol. Cell. Biol. 21:425-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu, K., B. Debreceni, R. Li, and Y. Zheng. 2000. Identification of Rho GTPase-dependent sites in the Dbl homology domain of oncogenic Dbl that are required for transformation. J. Biol. Chem. 275:25993-26001. [DOI] [PubMed] [Google Scholar]