Abstract
Transcription factor gene AP-2γ belongs to a family of four closely related genes. AP-2γ had been implicated in multiple functions during proliferation and differentiation based on its expression pattern in trophoblast, neural crest, and ectoderm cells in murine embryos. In order to address the question of the role of AP-2γ during mammalian development, we generated mice harboring a disrupted AP-2γ allele. AP-2γ heterozygous mice are viable and display reduced body sizes at birth but are fertile. Mice deficient for AP-2γ, however, are growth retarded and die at days 7 to 9 of embryonic development. Immunohistochemical analysis revealed that the trophectodermal cells that are found to express AP-2γ fail to proliferate, leading to failure of labyrinth layer formation. As a consequence, the developing embryo suffers from malnutrition and dies. Analysis of embryo cultures suggests that AP-2γ is also implicated in the regulation of the adenosine deaminase (ADA) gene, a gene involved in purine metabolism found expressed at the maternal-fetal interface. Therefore, AP-2γ seems to be required in early embryonic development because it regulates the genetic programs controlling proliferation and differentiation of extraembryonic trophectodermal cells.
The AP-2 transcription factor gene family consists of four different genes referred to as AP-2α, AP-2β, AP-2γ, and the recently discovered AP-2δ gene (4, 15, 17, 19, 22, 37, 40). All members of the AP-2 family share a characteristic protein structure containing a unique C-terminal helix-span-helix motif that mediates protein dimerization; together with a basic domain they are involved in DNA binding with varying affinity to GC-rich elements. An N-terminal proline- and glutamine-rich region mediates transcriptional activation (33, 36).
Despite the expression of all AP-2 genes in the extraembryonic trophoblast cells, it remained unclear if there was a redundant function in placental gene regulation. Gene knockout experiments with AP-2α and AP-2β indicate that the AP-2 proteins carry out individual functions during mouse development. While AP-2α is predominantly essential for craniofacial development and ventral body wall closure (29, 39), lack of AP-2β leads to polycystic kidney disease (20). Both AP-2α- and AP-2β-deficient mice display unaltered implantation and placentation. Is AP-2γ a key regulator of placental development? Prior to implantation, AP-2γ is expressed in the trophoblast cells starting at day 3.5 of murine development. After implantation, the expression of AP-2γ continues in the trophoblast cells and its derivatives, the primary giant cells and the diploid cells of the polar trophectoderm. With ongoing proliferation of the trophoblast cells, AP-2γ is expressed in the ectoplacental cone and the extraembryonic ectoderm. At the time of chorio-allantoic fusion, AP-2γ expression is increased in all derivatives of the trophoblast lineage (27, 30). Thus, AP-2γ, together with the T-box gene Eomes (26), is the only transcription factor gene found to be expressed in all trophoblast lineages throughout placental development. This suggests that AP-2γ might play a role in regulating trophoblast gene expression programs. In fact, AP-2γ has been shown to regulate the genes for adenosine deaminase (ADA) (30, 31), human placental lactogen (24), and human chorionic gonadotropin-β (11). Nevertheless, the function of AP-2γ during murine development remained to be elucidated.
In the study presented here, we addressed the question of the role of AP-2γ using gene knockout technologies. We demonstrate that AP-2γ is essential for early embryogenesis, due to the fact that the mutants display a significant retardation of growth by embryonic day 7.5 (E7.5) and are resorbed by E9.5. The loss of AP-2γ leads to a reduction in cell proliferation in the extraembryonic ectoderm and the ectoplacental cone; in addition, loss of AP-2γ leads to a reduced number of giant cells. As a consequence the labyrinth layer, derived from chorion and allantois, fails to form. The embryo suffers from deprivation of nutrients and, as a result, retards in growth and dies.
In addition, in vitro experiments show that the expression of adenosine deaminase (ADA) is highly reduced in trophectoderm outgrowths of AP-2γ mutant animals compared to wild-type trophectoderm cells. ADA is a purine metabolic enzyme that converts cytotoxic deoxyadenosine to deoxyinosine (1). Thus, we hypothesize that the reduced ADA expression leads to the accumulation of the toxin, and to cell death. This could be a further mechanism leading to the death and resorption of the null mutant. The work outlined here helps in understanding the molecular processes controlled by transcription factor gene AP-2γ.
MATERIALS AND METHODS
Construction of the targeting vector.
A genomic clone of murine AP-2γ gene (kindly provided by Pascal Dollé, IGMBC, Strasbourg, France) was used to design the targeting vector by standard recombinant techniques. A 6-kb genomic AP-2γ fragment spanning exons 2 to 7 was subcloned into pBluescript II KS (+/−). loxP sites flanked exon 5, which had been shown to be critical for AP-2 function. The 5′-loxP site contained an additional Asp718 site at the 5′ end and was inserted in intron 4 at the PstI site. A floxed selection cassette containing the pgk-neo and the HSV-tk genes (kind gift of Peter Mombaerts [18]) mediating positive and negative selections, respectively, was inserted in intron 5 at the XbaI site.
Targeting of ES cells and generation of AP-2γ-deficient mice.
Linearized vector DNA was introduced by electroporation into E14-KPA murine embryonic stem (ES) cells (kindly provided by Klaus Peter Knobeloch, FMP, Berlin, Germany) and cultured following standard procedures (28). After selection with G418 (active substance, 250 μg/ml), two correctly targeted clones of 176 clones were identified by Southern blotting and subsequently subjected to cre-mediated loop-out reaction. Following selection with ganciclovir, 104 of 111 clones had lost the complete insert and thus carried loss-of-function alleles. To generate chimeric mice, C57BL/6J blastocysts injected with AP-2γ+/− ES clones were transferred into pseudopregnant foster mice. Offspring of germ line-transmitting chimeric mice were screened for the presence of the disrupted AP-2γ gene. Mice heterozygous for the disrupted AP-2γ allele were mated to obtain animals homozygous for the mutation.
PCR assays.
Genotyping of mice and blastocysts was performed as follows. Tail biopsy or inner cell mass cells were lysed at 55°C in 200 or 20 μl, respectively, of lysis buffer (50 mM KCl, 10 mM Tris HCl [pH 8.3], 2.5 M MgCl2, 0.1 mg of gelatin per ml, 0.45% NP-40, 0.45% Tween 20, 1 mg of proteinase K per ml) for 2 h. Boiling for 10 min inactivated the proteinase K, and 10 μl of each sample was used for PCR analysis. Three primers were used to detect wild-type and null-mutant alleles: P1 (5′-AACAGGTTATCATTTGGTTGGGATT-3′), P2 (5′-CAATTTTGTCCAACTTCTCCCTCAA-3′), and P3 (5′-AATAGTCAGCCACCGCTTTACT AGG-3′), which amplified 300-bp (wild-type) and 700-bp (null-allele) fragments. The following PCR cycle profile was used: 1 cycle of 94°C for 10 min, followed by 37 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s, and finally 1 cycle of 72°C for 7 min. PCR products were electrophoresed on a 2% standard agarose gel and documented using Eagle-Eye gel documentation system (Stratagene, Heidelberg, Germany).
Whole-mount in situ hybridization.
Digoxigenin-labeled probes were generated with recombinant clones containing full-length cDNAs of Brachyury (T) with T7 and SP6 RNA polymerase. E8 embryos were hybridized according to standard protocols as previously described (14) (http://stratus.lifesci.ucla.edu./hhmi/derobertis/). Embryos were photographed by using a Prog.Res. digital camera (Jenoptik, Jena, Germany) fitted to a Zeiss-Axiovert microscope (Zeiss, Jena, Germany). Pictures were assembled by using Adobe Photoshop and Illustrator software.
Histological sections.
Deciduae at E7.5 to E8.5 resulting from heterozygous intercrosses were collected and fixed in 4% (wt/vol) paraformaldehyde in phosphate-buffered saline (PBS) overnight at 4°C, processed, and embedded in paraffin following standard procedures. Sections of 7-μm thickness were prepared, processed, and used for hematoxylin and eosin and PCNA staining.
PCNA staining.
After removal of paraffin the sections were rinsed twice with distilled H2O, incubated for 10 min in 2 N HCl, and washed again twice with distilled H2O. After incubation in methanol-0.3% H2O2 for 20 min and washing in distilled H2O, the sections were blocked for 10 min in blocking buffer (0.05 M Tris HCl, 0.15 M NaCl [pH 7.6], 0.5% Tween 20) containing 10% horse serum and 0.5% mouse serum. After being washed twice in PBS, the sections were incubated with PCNA-specific antibody diluted 1:100 in blocking buffer for 1 to 2 h and rinsed twice with PBS. Anti-PCNA antibody binding was detected with horseradish peroxidase-conjugated secondary antibody diluted 1:50 in blocking buffer according to the manufacturer's instructions (Vector ABC-Elite and AEC kits). The sections were counterstained with hematoxylin for 30 s, embedded in Immunomount (Shandon-Lipshah, Runcorn, United Kingdom), and photographed.
Immunohistochemical staining of sections.
After removal of paraffin the sections were rinsed twice with PBS and incubated in methanol-1% H2O2 for 30 min. After being washed three times in PBS the sections were boiled five times in a microwave (5 min each) in 10 mM sodium citrate (pH 6) and thereafter blocked in 10% horse serum plus 2% milk powder in PBS for 30 min at room temperature. After being rinsed with PBS the sections were incubated with an antibody specific to AP-2 (1:200; Geneka, Munich, Germany) at 4°C overnight. After two washes in PBS the secondary antibody (1:400 in PBS plus 2% milk powder) was applied for 1 h. The subsequent steps of the procedure were the same as for the PCNA assay described above.
Immunohistochemical staining of blastocyst cultures.
The blastocyst cultures were fixed in acetone for 20 min at −20°C and washed twice in PBS. After incubation in methanol-0.3% H2O2 for 5 min and three washes in PBS, the cultures were blocked in PBS-3% (wt/vol) milk powder for 30 min at room temperature and incubated with an antibody specific to ADA (1:100; Santa Cruz Biotechnology) in PBS-3% milk powder at 4°C overnight. The cultures were washed in PBS and incubated with the secondary antibody (1:400 in PBS-3% milk powder) for 1 h at room temperature. The subsequent steps of the procedure were the same as for the PCNA assay described above.
In vitro culture of blastocysts.
Natural matings between heterozygous mice were used to obtain embryos of all genotypes. Embryos were staged according to the detection of vaginal plugs resulting from the crosses (noon of day 1 of plugging was E0.5). Blastocysts at E3.5 were flushed from the uterine horns and cultured individually in 50 μl of ES cell medium without leukemia inhibitory factor on gelatin-coated chamber slides (Nunc, Wiesbaden, Germany) for up to 1 week. Photographs of the cultures were taken with a Zeiss Axiocam fitted to an inverted microscope (Zeiss Axiovert), and genotype was determined by PCR of picked inner cell mass.
RESULTS
Targeted disruption of the AP-2γ allele.
A 6-kb genomic fragment spanning exons 2 to 7 was used to introduce a floxed neo/tk cassette 3′ of exon 5 as well as a solitary loxP site 5′ of exon 5 (Fig. 1A, upper panel). After electroporation into ES cells and selection with G418, 2 correctly targeted clones of 173 analyzed were identified by Southern blot analysis (Fig. 1A, middle panel, and Fig. 1B). cre-mediated excision removed the region between the loxP sites, generating the AP-2γ-null allele (Fig. 1A, lower panel). A PCR assay was used to verify the cre-mediated loop-out reaction (Fig. 1C). To generate mice harboring the AP-2γ-null allele, cells were injected into C57BL/6 blastocysts, resulting in several chimeric animals. Offspring of germ line-transmitting animals were tested for the presence of the null allele. Mice heterozygous for the mutation appeared slightly growth retarded after birth but ultimately reached normal sizes and were fertile (not shown).
FIG. 1.
(A) Targeting strategy of the AP-2γ genomic locus. Schematics of the targeting vector (top), wild-type locus (upper middle), the mutated locus (lower middle), and the null allele (bottom) after cre-mediated loop-out reaction are shown. Shaded boxes indicate exons. Arrows indicate the positions of the primers (P1, P2, and P3) used for detection of the cre-mediated excision. (B) Southern blot analysis of genomic DNA isolated from ES cell clones digested with XhoI (left) and XhoI/Asp718 (right) hybridized to a 5′ external probe (indicated in panel A) is shown. The 4.5-kb (wt) and 8.5-kb (mut) bands and 3.7-kb (wt) and 2.5-kb (mut) bands specific to the wild-type and the targeted alleles, respectively, confirmed homologous recombination and integration of the 5′ loxP sequence. (C) The generation of the AP-2γ null allele by cre-mediated loop-out reaction was checked by PCR amplification of ES cell DNA using primers detecting wild-type (wt) as well as null allele (null). Abbreviations: A, Asp 718; P, PstI; X, XhoI; wt, wild type; mut, mutant; tk, HSV-tk (herpes simplex virus thymidine kinase); neo, neomycin resistance gene.
Lack of AP-2γ results in lethality during early gestation.
To obtain mice homozygous for the AP-2γ mutation, heterozygous animals were mated. However, we did not recover any live- born AP-2γ-deficient animal, indicating that complete loss of AP-2γ is not compatible with proper embryonic development. To determine the nature of the phenotype as well as the time point of the lethality we dissected pregnant animals at various stages of embryonic development. We found that the AP-2γ-deficient animals were present at expected Mendelian ratios up to 3.5 days postcoitum (of 27 blastocysts isolated, 7 [26%] were wild type, 14 [51%] were heterozygous, and 6 [23%] were deficient for the AP-2γ allele), indicating that the mutation does not interfere with preimplantation development. After implantation, however, the AP-2γ-deficient embryos were found to be growth retarded by day 6 of development, died, and were resorbed by day 9 of embryonic development.
AP-2γ mutant embryos are reduced in size and fail to gastrulate.
In order to examine the gross morphology of the mutant embryos and to compare them to their wild-type littermates, we dissected embryos at E8.0. Figure 2A shows a mutant embryo next to a control littermate. The mutant embryo is clearly retarded, and volumetric calculation indicated that mutants are up to 50-fold smaller than their littermates. At E6.5, gastrulation is initiated, resulting in the formation of the third germ layer, the mesoderm. In order to determine to what extent gastrulation is affected, we performed a whole-mount in situ analysis using Brachyury (T) as a probe (35). As seen in Fig. 2A, mesodermal cells are clearly visible in the wild-type embryo (Fig. 2A, right embryo), while almost no signal is detected on the AP-2γ mutant animals (Fig. 2A, left embryo). These results indicate that the mutant embryo is retarded in overall growth and patterning processes. AP-2γ, however, is not expressed in the embryo proper at this stage of development but can be detected in the cells of the ectoplacental cone and the trophectoderm-derived giant cells. Therefore, we decided to analyze histological sections of wild-type and mutant conceptuses and found that not only the embryo proper but also the ectoplacental cone was growth retarded. (Fig. 2B and C). It is very likely that the growth retardation of the embryo results from a defect in the extraembryonic tissues.
FIG. 2.
AP-2γ-deficient embryos are severely retarded in development. (A) Whole-mount in situ staining for the primitive streak marker Brachyury (T) of a wild-type conceptus (right) and an AP-2γ-deficient conceptus (left) at E8.0 of murine development. The blue staining indicates cells expressing Brachyury (arrows). (B to E) Histological sections of wild-type (wt) and AP-2γ-deficient (−/−) embryos in utero at E7.5 of murine development. Sagittal section through the decidua (dc) harboring a wild-type (B) and an AP-2γ-deficient embryo (C). Note the reduced size of the ectoplacental cone in panel C compared to that in panel B (epc; arrow). PCNA staining of wt conceptus (D) and AP-2γ-deficient (−/−) conceptus (E) at E7.5 of development. Note the difference in proliferation (red signal) in the ectoplacental cone and in the extraembryonic ectoderm for the wild-type conceptus and for the mutant. Arrowheads mark erythrocytes corresponding to forming blood lacunas indicative of the beginning of resorption of the mutant conceptus. Abbreviations: hf, head fold; wt, wild type; dc, decidua; epc, ectoplacental cone. Scale bars, 500 μm (A to C) and 100 μm (D and E).
Reduced proliferation of trophectodermal cells in AP-2γ mutant conceptuses.
Most of the extraembryonic tissues are derived from trophectodermal cells. Trophectodermal cells are among the first specialized cells which arise from the fertilized egg. In mice, there are three principal subtypes of trophoblast cells known: (i) the cells of the extraembryonic ectoderm that are the self-renewing stem cells, (ii) the intermediate population of the ectoplacental cone, and (iii) the giant cells surrounding the conceptus that are in direct contact with the maternal decidua (9). Since AP-2γ is found expressed in all cells of the trophectodermal lineage at the stage spanning E6.5 to E9.5 (27, 30), we decided to analyze these cells in more detail. Recently, it was shown that one possible function of AP-2 genes might be the regulation of cellular proliferation by suppression of genes implicated in differentiation and apoptotic processes (23). We used paraffin sections and PCNA immunohistochemistry to detect cell proliferation. Figure 2D shows that the majority of cells of the ectoplacental cone in the control animal are positive for PCNA staining. The AP-2γ-deficient conceptus, on the other hand, displays highly reduced PNCA staining (Fig. 2E) in cells of the ectoplacental cone, indicating that lack of AP-2γ results in reduced proliferation of extraembryonic tissue.
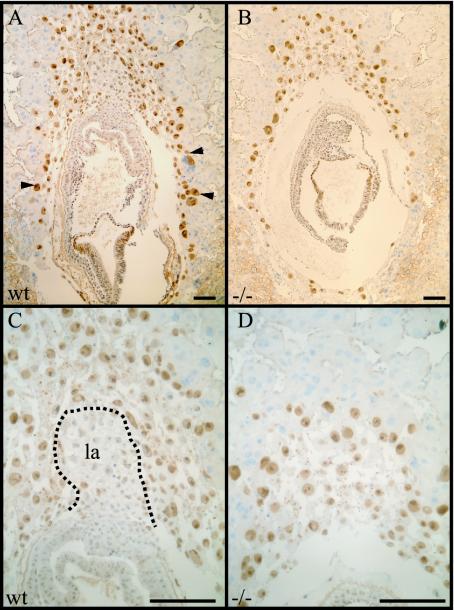
AP-2γ-deficient embryos display a reduced number of giant cells and fail to develop the labyrinth layer.
Beginning from day 7.5 of development, the labyrinth layer forms as a consequence of a concerted interaction of chorion and allantois (9). Since extraembryonic ectodermal cells expressing AP-2γ contribute to the chorion, we investigated the sizes and structures of the extraembryonic tissues by using a pan-AP-2 antibody at day 8.0 of development (30). The pan-AP-2 antibody detects all AP-2 isoforms and can therefore be used on wild-type and AP-2γ-deficient mice to stain all derivatives of the trophectodermal lineage. As seen in Fig. 3, there were fewer trophectodermal giant cells seen in AP-2γ mutant animals (Fig. 3B) than in the wild-type littermate (Fig. 3A). Furthermore, the mutant embryo failed to form a labyrinth layer (Fig. 3D), which was clearly detectable at this stage in the wild-type animal (Fig. 3C).
FIG. 3.
AP-2γ-deficient embryos show decreased numbers of giant cells and fail to develop a labyrinth layer. Immunohistochemical staining of sagittal sections through the decidua of a wild-type (wt) (A) and an AP-2γ-deficient embryo (−/−) (B) at E8.0 of murine development using a pan-AP-2 antibody is shown. Note the lack of secondary giant cells in the null mutant compared to the wild type (arrowheads in panel A). The AP-2γ-deficient embryo (D) fails to form the functional labyrinth anlage (la), which is clearly visible in the control embryo (C). Scale bar, 100 μm.
Taken together, these results indicate that loss of AP-2γ leads to a deficiency of proliferation in extraembryonic tissues. Lack of AP-2γ cannot be compensated by the other known members of the AP-2 gene family, indicating a unique role for AP-2γ in controlling the development of extraembryonic tissues.
Cultured trophoblast cells lose epithelial morphology.
AP-2γ is detected as early as the blastocyst stage in the cells of the trophectoderm. We sought to establish an in vitro system in order to further analyze the cellular and molecular consequences of the AP-2γ deficiency. For this purpose, heterozygous mice were mated, the resulting embryos were flushed from the uterine horns at day 3.5 of gestation, and blastocyst microdrop cultures were initiated. The cultures were checked daily, and photographs were taken. A total of 27 blastocyst cultures were initiated, monitored, and genotyped. We found that the trophoblast cells of the AP-2γ-deficient animals were not distinguishable from the control cultures. However, after 48 h, when the trophoblast cells had attached onto the tissue culture dish, their morphology changed and the epithelial morphology was lost after 5 days in culture (Fig. 4A and B). Since it is known that AP-2 genes are involved in the regulation of e-cadherin (10), a molecule involved in cell adhesion, we decided to check the AP-2γ-deficient cultures with an antibody specific to e-cadherin. However, we could not see any difference in staining intensity (data not shown). Thus, cultured trophoblast cells derived from AP-2γ-deficient blastocysts display a change in morphology; this difference is not based on altered levels of e-cadherin in these cells.
FIG. 4.
AP-2γ-deficient blastocyst outgrowths show changed morphology. Photographs of outgrowths of wild-type (wt) (A) and AP-2γ-deficient (−/−) (B) blastocysts after 5 days in culture are shown. Trophectodermal cells (te) and inner cell mass (icm) of AP-2γ-deficient blastocysts display altered morphology when cultured in vitro. (C and D) Reduced expression of ADA in AP-2γ-deficient blastocyst outgrowths. Trophectodermal cells of a wt culture (C) and an AP-2γ-deficient culture (−/−) (D) were incubated with an ADA-specific antibody. Cells expressing ADA display brown cytoplasmic staining. Nuclei were counterstained with hematoxylin (blue). The trophectodermal cells of the control were positive for ADA expression (C), whereas in the AP-2γ-deficient cells the staining was much reduced (D). Abbreviations: icm, inner cell mass; te, trophectodermal cells.
AP-2γ-deficient trophoblast cells display reduced expression of ADA.
ADA is an essential enzyme of the purine metabolism, which is found enriched at the maternal-fetal interface throughout postimplantation development. Its cellular function is to convert adenosine, a toxic product, into inosine. ADA is expressed in maternal decidual cells, in embryo-derived trophoblast cells, and in giant cells lining the implantation chamber of the embryo (1). Since blocking experiments of ADA resulted in severe growth retardation and death of the embryos by E10.5 (6, 38) and ADA has been reported to be regulated by AP-2 transcription factors, we tested whether the level of embryonic ADA is affected by loss of AP-2γ. Because of the close proximity of giant trophoblast cells and the secondary deciduum, it would have been difficult to assess the relative pattern and level of zygotically derived ADA expression in the gestational site. Therefore, we decided to determine embryonic ADA levels in embryo culture using an antibody to ADA. As seen in Fig. 4C and D, AP-2γ-deficient trophoblast cells had significantly reduced levels of ADA. This finding suggests that one molecular consequence of the AP-2γ deficiency is the lack of zygotically derived ADA expression. It was previously reported that the trophoblast cells are more resistant to lack of ADA than are the cells of the embryo proper (1-3). In fact, upon closer morphological examination, all inner cell mass outgrowths derived from AP-2γ-deficient blastocyst cultures displayed an altered morphology indicative of differentiation and apoptotic processes (Fig. 4A and B).
DISCUSSION
In the study presented here, we have addressed the functional role of transcription factor AP-2γ by using homologous recombination in murine ES cells to generate and analyze mice lacking this transcription factor. We provide the first genetic evidence that AP-2γ plays an essential role in early postimplantation development. The phenotypic alterations seen are in agreement with the expression pattern of AP-2γ, namely in the trophoblast giant cells, the ectoplacental cone, and the extraembryonic ectoderm.
Animals lacking AP-2γ display severe growth retardation, die, and are resorbed up to day 9.5 of murine embryonic development. Whole-mount in situ analysis reveals that the embryos do not gastrulate and therefore fail to form mesoderm. This failure is most likely a secondary consequence of the growth retardation, since there is no expression of AP-2γ in the embryo proper at this point of murine development.
Several studies have shown that AP-2γ is expressed in trophectodermal cells as early as the blastocyst stage and is maintained in all derivatives of trophoblast cells, i.e., the primary and secondary giant cells as well as the cells of the ectoplacental cone (27, 30). Our studies show that the preimplantation and early postimplantation development of AP-2γ-deficient animals is not affected, indicating that other molecules might compensate for the function. While all derivatives of the trophoblast cells in AP-2γ-deficient animals are formed, both the embryo and the extraembryonic tissues are severely growth retarded. This growth retardation is based on a reduced proliferation of the cells of the ectoplacental cone and a reduced number of giant cells. A role for AP-2 genes in regulating proliferation has been published by us and others (7, 23). AP-2 has been shown to activate the proliferative markers c-erbB2 and c-erbB3 (7) and to repress genes which induce differentiation and apoptosis such as Stra 13, Mtd (or Bok-1), and KLF-4 (23).
While these experiments demonstrate that AP-2γ is essential for the development of extraembryonic tissues, the function of this gene in the embryo proper remains to be elucidated. Studies show that AP-2γ is expressed in migrating neural crest cells and in cells of the basal layer of the skin (5). Since we have been constructing ES cells harboring a conditional allele, experiments that address the role of AP-2γ in the embryo proper or even specific tissues will be possible (34).
The in vitro experiments performed here indicate that AP-2γ-deficient trophoblasts display markedly reduced levels of ADA. ADA-deficient mice suffer from severely disturbed purine metabolism and die perinatally (16, 32). Treatment of mice with the potent ADA inhibitor 2′-deoxycoformycin results in a phenocopy of the AP-2γ mutant (2, 3, 12, 13), which supports the idea of AP-2γ regulating ADA (6).
We have shown that loss of AP-2γ leads to a reduction of the proliferative capacity of extraembryonic tissues. Furthermore, in vitro cultures show that AP-2γ-deficient blastocyst cultures display lack of ADA, an enzyme essential for detoxification of adenosine and deoxyadenosine. The data we presented here lead us to a model which pinpoints AP-2γ as a key player in trophoblast development. Lack of AP-2γ leads to reduction of proliferation and lack of expression of ADA in the extraembryonic cells. As a consequence the embryo proper suffers from malnutrition and intoxication leading to growth retardation and subsequently death and resorption by E9.5. However, the set of genes being regulated by AP-2γ has yet to be determined.
With this study three of four AP-2 genes in mice were deleted by gene knockout strategies. While animals were mostly unaffected in the heterozygous state, the respective null mutants displayed either cranio-abdominoschisis (29, 39), polycystic kidneys (20), or failure of the trophoblast cells, i.e., lethal phenotypes. In light of the fact that all AP-2 genes are expressed in overlapping patterns (21, 22) and display a high degree of homology on the DNA and protein levels, it is surprising that the single gene deletions result in such diverse and extensive phenotypes. This shows that there are specific functions for individual AP-2 genes which cannot be carried out by the other AP-2 genes.
Defects in placentation have also been reported for mice with knockouts of other genes such as Eomes (26), Hand 1 (25), and Mash2 (8). While some defects are quite similar to the AP-2γ knockout phenotype, there are also clear differences. The T-box gene Eomes is found expressed throughout the differentiation of the trophoblast lineages. Loss-of-function studies revealed that Eomes is required for the differentiation of trophectoderm and the formation of trophoblast stem cells (26). In contrast to AP-2γ-deficient trophectodermal cells, Eomes-deficient blastocyst cultures fail to form trophectodermal outgrowths in vitro, suggesting that this gene acts earlier in extraembryonic development. It remains to be seen if Eomes acts upstream of AP-2γ in the cascade of transcription factors.
Mice deficient in the basic helix-loop-helix transcription factor Hand1 are found to be growth retarded by day 7.5 of development due to a block in trophoblast giant cell differentiation and a smaller ectoplacental cone (25). Thus, Hand1 seems to be required for a specific subset of trophectodermal derivatives. Mash2 knockout mice die from placental failure at day 10 of development; the spongiotrophoblast cells and their precursors are absent, and chorionic ectoderm is reduced (8). Mash2 is expressed in the ectoplacental cone, the chorion, and their derivatives but is absent in primary and secondary giant cells and the allantois. Thus, the expression pattern of Mash2 is only partially overlapping with AP-2γ, making it unlikely that this gene is a potential target molecule.
The experiments described above help us to understand the molecular processes underlying placental development in mice. In an evolutionary context, the rodent and human placentations are quite similar; therefore, results from murine models that elucidate the molecular basis of placental development should facilitate the design of strategies to reduce fetal loss caused by placental dysfunction such as preeclampsia and intrauterine growth restriction in humans.
Acknowledgments
We thank Peter Mombaerts for the pLTNL selection cassette, Pascal Dolle for the AP-2γ genomic clone, Rolf Kemler for the e-cadherin antibody, Martin Blum for the Brachyury probe, and Ivan Horak and Klaus Peter Knobeloch for the KPA-ES cells. We thank Andrea Jacob, Norma Howells, Judith Dahmen, Inge Heim, and Yvonne Petersen for excellent technical assistance and maintenance of the animal colony. Very special thanks to Richard “Ritchie” Jäger for critically reading the manuscript.
This work was supported by a grant of the Deutsche Forschungsgemeinschaft to H.S. (DFG no. 503-3).
REFERENCES
- 1.Blackburn, M. R., and R. E. Kellems. 1996. Regulation and function of adenosine deaminase in mice. Prog. Nucleic Acid Res. Mol. Biol. 55:195-226. [DOI] [PubMed] [Google Scholar]
- 2.Blackburn, M. R., T. B. Knudsen, and R. E. Kellems. 1997. Genetically engineered mice demonstrate that adenosine deaminase is essential for early postimplantation development. Development 124:3089-3097. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn, M. R., M. Wakamiya, C. T. Caskey, and R. E. Kellems. 1995. Tissue-specific rescue suggests that placental adenosine deaminase is important for fetal development in mice. J. Biol. Chem. 270:23891-23894. [DOI] [PubMed] [Google Scholar]
- 4.Bosher, J. M., N. F. Totty, J. J. Hsuan, T. Williams, and H. C. Hurst. 1996. A family of AP-2 proteins regulates c-erbB-2 expression in mammary carcinoma. Oncogene 13:1701-1707. [PubMed] [Google Scholar]
- 5.Chazaud, C., M. Oulad-Abdelghani, P. Bouillet, D. Decimo, P. Chambon, and P. Dolle. 1996. AP-2.2, a novel gene related to AP-2, is expressed in the forebrain, limbs and face during mouse embryogenesis. Mech. Dev. 54:83-94. [DOI] [PubMed] [Google Scholar]
- 6.Gao, X., M. R. Blackburn, and T. B. Knudsen. 1994. Activation of apoptosis in early mouse embryos by 2′-deoxyadenosine exposure. Teratology 49:1-12. [DOI] [PubMed] [Google Scholar]
- 7.Grooteclaes, M., D. Vernimmen, S. Plaza, F. Pasleau, D. Hodzic, and R. Winkler-Gol. 1999. A new cis element is involved in the HER2 gene overexpression in human breast cancer cells. Cancer Res. 59:2527-2531. [PubMed] [Google Scholar]
- 8.Guillemot, F., A. Nagy, A. Auerbach, J. Rossant, and A. L. Joyner. 1994. Essential role of Mash-2 in extraembryonic development. Nature 371:333-336. [DOI] [PubMed] [Google Scholar]
- 9.Hemberger, M., and J. C. Cross. 2001. Genes governing placental development. Trends Endocrinol. Metab. 12:162-168. [DOI] [PubMed] [Google Scholar]
- 10.Hennig, G., O. Lowrick, W. Birchmeier, and J. Behrens. 1996. Mechanisms identified in the transcriptional control of epithelial gene expression. J. Biol. Chem. 271:595-602. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, W., and J. L. Jameson. 1999. AP-2 (activating protein 2) and Sp1 (selective promoter factor 1) regulatory elements play distinct roles in the control of basal activity and cyclic adenosine 3′,5′-monophosphate responsiveness of the human chorionic gonadotropin-beta promoter. Mol. Endocrinol. 13:1963-1975. [DOI] [PubMed] [Google Scholar]
- 12.Knudsen, T. B., M. K. Gray, J. K. Church, M. R. Blackburn, M. J. Airhart, R. E. Kellems, and R. G. Skalko. 1989. Early postimplantation embryolethality in mice following in utero inhibition of adenosine deaminase with 2′-deoxycoformycin. Teratology 40:615-626. [DOI] [PubMed] [Google Scholar]
- 13.Lecka-Czernik, B., C. K. Lumpkin, Jr., and S. Goldstein. 1995. An overexpressed gene transcript in senescent and quiescent human fibroblasts encoding a novel protein in the epidermal growth factor-like repeat family stimulates DNA synthesis. Mol. Cell. Biol. 15:120-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maldonado-Saldivia, J., B. Funke, R. K. Pandita, T. Schuler, B. E. Morrow, and H. Schorle. 2000. Expression of Cdcrel-1 (Pnutl1), a gene frequently deleted in velo-cardio-facial syndrome/DiGeorge syndrome. Mech. Dev. 96:121-124. [DOI] [PubMed] [Google Scholar]
- 15.McPherson, L. A., V. R. Baichwal, and R. J. Weigel. 1997. Identification of ERF-1 as a member of the AP2 transcription factor family. Proc. Natl. Acad. Sci. USA 94:4342-4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Migchielsen, A. A., M. L. Breuer, M. A. van Roon, H. te Riele, C. Zurcher, F. Ossendorp, S. Toutain, M. S. Hershfield, A. Berns, and D. Valerio. 1995. Adenosine-deaminase-deficient mice die perinatally and exhibit liver-cell degeneration, atelectasis and small intestinal cell death. Nat. Genet. 10:279-287. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell, P. J., C. Wang, and R. Tjian. 1987. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell 50:847-861. [DOI] [PubMed] [Google Scholar]
- 18.Mombaerts, P., F. Wang, C. Dulac, S. K. Chao, A. Nemes, M. Mendelsohn, J. Edmondson, and R. Axel. 1996. Visualizing an olfactory sensory map. Cell 87:675-686. [DOI] [PubMed] [Google Scholar]
- 19.Moser, M., A. Imhof, A. Pscherer, R. Bauer, W. Amselgruber, F. Sinowatz, F. Hofstadter, R. Schule, and R. Buettner. 1995. Cloning and characterization of a second AP-2 transcription factor: AP-2 beta. Development 121:2779-2788. [DOI] [PubMed] [Google Scholar]
- 20.Moser, M., A. Pscherer, C. Roth, J. Becker, G. Mucher, K. Zerres, C. Dixkens, J. Weis, L. Guay-Woodford, R. Buettner, and R. Fassler. 1997. Enhanced apoptotic cell death of renal epithelial cells in mice lacking transcription factor AP-2beta. Genes Dev. 11:1938-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moser, M., J. Ruschoff, and R. Buettner. 1997. Comparative analysis of AP-2 alpha and AP-2 beta gene expression during murine embryogenesis. Dev. Dyn. 208:115-124. [DOI] [PubMed] [Google Scholar]
- 22.Oulad-Abdelghani, M., P. Bouillet, C. Chazaud, P. Dolle, and P. Chambon. 1996. AP-2.2: a novel AP-2-related transcription factor induced by retinoic acid during differentiation of P19 embryonal carcinoma cells. Exp. Cell Res. 225:338-347. [DOI] [PubMed] [Google Scholar]
- 23.Pfisterer, P., J. Ehlermann, M. Hegen, and H. Schorle. 2002. A subtractive gene expression screen suggests a role of transcription factor AP-2alpha in control of proliferation and differentiation. J. Biol. Chem. 277:6637-6644. [DOI] [PubMed] [Google Scholar]
- 24.Richardson, B. D., R. A. Langland, C. J. Bachurski, R. G. Richards, C. A. Kessler, Y. H. Cheng, and S. Handwerger. 2000. Activator protein-2 regulates human placental lactogen gene expression. Mol. Cell. Endocrinol. 160:183-192. [DOI] [PubMed] [Google Scholar]
- 25.Riley, P., L. Anson-Cartwright, and J. C. Cross. 1998. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat. Genet. 18:271-275. [DOI] [PubMed] [Google Scholar]
- 26.Russ, A. P., S. Wattler, W. H. Colledge, S. A. Aparicio, M. B. Carlton, J. J. Pearce, S. C. Barton, M. A. Surani, K. Ryan, M. C. Nehls, V. Wilson, and M. J. Evans. 2000. Eomesodermin is required for mouse trophoblast development and mesoderm formation. Nature 404:95-99. [DOI] [PubMed] [Google Scholar]
- 27.Sapin, V., P. Bouillet, M. Oulad-Abdelghani, B. Dastugue, P. Chambon, and P. Dolle. 2000. Differential expression of retinoic acid-inducible (Stra) genes during mouse placentation. Mech. Dev. 92:295-299. [DOI] [PubMed] [Google Scholar]
- 28.Schorle, H., T. Holtschke, T. Hunig, A. Schimpl, and I. Horak. 1991. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature 352:621-624. [DOI] [PubMed] [Google Scholar]
- 29.Schorle, H., P. Meier, M. Buchert, R. Jaenisch, and P. J. Mitchell. 1996. Transcription factor AP-2 essential for cranial closure and craniofacial development. Nature 381:235-238. [DOI] [PubMed] [Google Scholar]
- 30.Shi, D., and R. E. Kellems. 1998. Transcription factor AP-2gamma regulates murine adenosine deaminase gene expression during placental development. J. Biol. Chem. 273:27331-27338. [DOI] [PubMed] [Google Scholar]
- 31.Shi, D., J. H. Winston, M. R. Blackburn, S. K. Datta, G. Hanten, and R. E. Kellems. 1997. Diverse genetic regulatory motifs required for murine adenosine deaminase gene expression in the placenta. J. Biol. Chem. 272:2334-2341. [PubMed] [Google Scholar]
- 32.Wakamiya, M., M. R. Blackburn, R. Jurecic, M. J. McArthur, R. S. Geske, J. Cartwright, Jr., K. Mitani, S. Vaishnav, J. W. Belmont, R. E. Kellems, et al. 1995. Disruption of the adenosine deaminase gene causes hepatocellular impairment and perinatal lethality in mice. Proc. Natl. Acad. Sci. USA 92:3673-3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wankhade, S., Y. Yu, J. Weinberg, M. A. Tainsky, and P. Kannan. 2000. Characterization of the activation domains of AP-2 family transcription factors. J. Biol. Chem. 275:29701-29708. [DOI] [PubMed] [Google Scholar]
- 34.Werling, U., and H. Schorle. 2002. Conditional inactivation of transcription factor AP-2γ by using the Cre/loxP recombination system. Genesis 32:127-129. [DOI] [PubMed] [Google Scholar]
- 35.Wilkinson, D. G., S. Bhatt, and B. G. Herrmann. 1990. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature 343:657-659. [DOI] [PubMed] [Google Scholar]
- 36.Williams, T., A. Admon, B. Luscher, and R. Tjian. 1988. Cloning and expression of AP-2, a cell-type-specific transcription factor that activates inducible enhancer elements. Genes Dev. 2:1557-1569. [DOI] [PubMed] [Google Scholar]
- 37.Williamson, J. A., J. M. Bosher, A. Skinner, D. Sheer, T. Williams, and H. C. Hurst. 1996. Chromosomal mapping of the human and mouse homologues of two new members of the AP-2 family of transcription factors. Genomics 35:262-264. [DOI] [PubMed] [Google Scholar]
- 38.Wubah, J. A., M. M. Ibrahim, X. Gao, D. Nguyen, M. M. Pisano, and T. B. Knudsen. 1996. Teratogen-induced eye defects mediated by p53-dependent apoptosis. Curr. Biol. 6:60-69. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, J., S. Hagopian-Donaldson, G. Serbedzija, J. Elsemore, D. Plehn-Dujowich, A. P. McMahon, R. A. Flavell, and T. Williams. 1996. Neural tube, skeletal and body wall defects in mice lacking transcription factor AP-2. Nature 381:238-241. [DOI] [PubMed] [Google Scholar]
- 40.Zhao, F., M. Satoda, J. D. Licht, Y. Hayashizaki, and B. D. Gelb. 2001. Cloning and characterization of a novel mouse AP-2 transcription factor, AP-2delta, with unique DNA binding and transactivation properties. J. Biol. Chem. 276:40755-40760. [DOI] [PubMed] [Google Scholar]