Abstract
The sperm mitochondria-associated cysteine-rich protein (SMCP) is a cysteine- and proline-rich structural protein that is closely associated with the keratinous capsules of sperm mitochondria in the mitochondrial sheath surrounding the outer dense fibers and axoneme. To investigate the function of SMCP, we generated mice with a targeted disruption of the gene Smcp by homologous recombination. Homozygous mutant males on a mixed genetic background (C57BL/6J × 129/Sv) are fully fertile, while they are infertile on the 129/Sv background, although spermatogenesis and mating are normal. Homozygous Smcp−/− female mice are fertile on both genetic backgrounds. Electron microscopical examination demonstrated normal structures of sperm head, mitochondria, and tail. In vivo experiments with sperm of Smcp−/− 129/Sv mice revealed that the migration of spermatozoa from the uterus into the oviduct is reduced. This result is supported by the observation that sperm motility as determined by the computer-assisted semen analysis system (CASA) is significantly affected as compared to wild-type spermatozoa. In vitro fertilization assays showed that Smcp-deficient spermatozoa are able to bind to the oocyte but that the number of fertilized eggs is reduced by more than threefold relative to the wild-type control. However, removal of the zona pellucida resulted in an unaffected sperm-egg fusion which was monitored by the presence of pronuclei and generation of blastocyts. These results indicate that the infertility of the male Smcp−/− mice on the 129/Sv background is due to reduced motility of the spermatozoa and decreased capability of the spermatozoa to penetrate oocytes.
Mitochondria are confined to a structure known as the mitochondrial sheath in the midpiece of mammalian spermatozoa. The mitochondria in the sheath are elongated, crescent shaped, and aligned end-to-end in helices wrapped around the outer dense fibers which in turn surround the flagellar axoneme (21). Pallini et al. (22) purified sonication- and sodium dodecyl sulfate (SDS)-resistant structures from sperm mitochondria, known as capsules, that retain the size and shape of the outer membranes of sperm mitochondria. The integrity of capsules is maintained by disulfide bridges, and purified capsules from bull sperm contain three proteins, including a peculiar hydrophilic protein containing a large amount of proline (26%) and cysteine (18%), the sperm mitochondria-associated cysteine-rich protein (SMCP). For many years, SMCP was thought to be a 20-kDa selenoprotein and the predominant capsular protein (7), but recent work demonstrates that those attributes belong to phospholipid hydroxide glutathione peroxidase, which functions as a cytosolic enzyme in somatic cells and an enzymatically inactive structural protein in the mitochondrial capsule (33). Electron microscope immunocytochemistry localizes mouse SMCP to the outer mitochondrial membranes and intermitochondrial spaces of the mitochondrial sheath (8). Since SMCP is neither a selenoprotein nor the major capsule protein, earlier names (mitochondrial capsule protein and mitochondrial capsule selenoprotein) seem inappropriate.
Smcp cDNAs have been isolated from mouse, human, rat, and hamster (2, 4, 15, 19). In mice, expression of the Smcp mRNA is restricted to haploid spermatogenic cells and the mRNA is present throughout most of the haploid phase (26). The Smcp mRNA is stored as a translationally inactive free mRNP in early haploid cells and is translated actively on polysomes in late haploid cells (8, 14). The Smcp gene is present as a single copy in the mouse genome and encodes a 143-amino-acid protein. It is composed of two exons and lacks the typical TATA and CAAT box motifs.
Several functions have been proposed for SMCP. The observation that SMCP becomes associated with mitochondria after the mitochondrial sheath has formed indicates that it is not necessary for the formation of the sheath (8). However, SMCP could still function to stabilize the mitochondrial capsules and sheath and/or attach the sheath to the outer dense fibers (8). Cummins et al. (10) have suggested that SMCP may be involved in the maternal inheritance of mitochondrial DNA by targeting proteolytic degradation of paternal mitochondria after fertilization. SMCP is also a major autoantigen in the Lewis rat and a candidate for the target of an immunocontraceptive (11).
To investigate the physiological role of the SMCP, we disrupted the Smcp gene in mice. Smcp-deficient mice are viable and females are fertile; however, male fertility depends strongly on the genetic background and is associated with defects in sperm motility.
MATERIALS AND METHODS
Generation of Smcp mutant mice.
A λ phage clone carrying the mouse Smcp gene was isolated from a 129/Sv genomic mouse library (Stratagene, La Jolla, Calif.) by using the rat Smcp cDNA (2). For the determination of the restriction map of the Smcp locus and localization of the exonic sequences, the isolated phage clone was examined by Southern blot analysis. The Smcp-targeting vector was constructed by using the plasmid pPNT (32), which contained a neomycin resistance gene driven by a PGK promoter (pgk-neo) and a herpes simplex virus thymidine kinase gene (tk) cassette. A 4.5-kb SpeI/SalI fragment containing the 5′-flanking region of the Smcp gene was isolated and ligated with XhoI-digested pPNT vector after filling the end with Klenow enzyme (clone Smcp/1). Finally, the 3-kb XbaI/SalI fragment (SalI site from polylinker of phage clone) containing the 3′-flanking region of the Smcp gene was isolated and inserted into the XbaI-digested clone Smcp/1 by blunt end ligation. The resulting targeting construct was linearized with NotI (Fig. 1A) and transfected into RI embryonic stem cells (35), and colonies resistant to G418 (400 μg/ml) and ganciclovir (GANC) (2 μM) were selected.
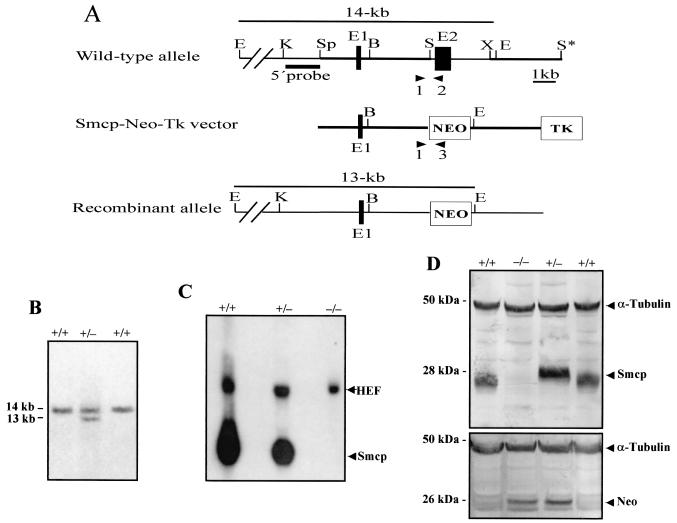
FIG. 1.
Targeted disruption of Smcp gene. (A) The structures of the wild type, targeting vector (Smcp-Neo-Tk), and mutant allele are shown together with the relevant restriction sites. A 2.8-kb SalI/XbaI fragment containing exon 2 of the Smcp gene was replaced by a pgk-neo selection cassette (NEO). TK, Thymidine kinase cassette; B, BamHI; E, EcoRI; K, KpnI; S, SalI; Sp, SpeI; X, XbaI. Primers 1, 2, and 3, which were used to amplify the wild-type and mutated alleles by PCR, are indicated. (B) Southern blot analysis of ES clones. Genomic DNA extracted from ES clones was digested with EcoRI and probed with a 5′ external probe shown in panel A. Homologous recombination events yielded a 13-kb hybridizing band detected in heterozygous (+/−) cell lines, while wild-type (+/+) cell lines showed a 14-kb band. (C) Northern blotting of testicular RNA from the three genotypes. Hybridization with the mouse Smcp cDNA probe revealed a 0.8-kb mRNA prominent in Smcp+/+, reduced in Smcp+/−, and absent in Smcp−/− mice. Rehybridization was performed with human elongation factor 2 cDNA (HEF2). (D) Western blots with testicular proteins from wild-type (+/+), heterozygous (+/−), and knockout (−/−) mice were incubated with anti-SMCP, anti-neomycin, and anti-α-tubulin antiserum. The immunoreactive 28-kDa SMCP protein was detectable in wild-type and heterozygous mice but not in Smcp−/− mice. The immunoreactive ∼26-kDa neomycin phosphotransferase II protein (Neo) was detectable only in Smcp−/− and Smcp+/− mice. The α-tubulin antiserum revealed equal protein loading.
Genomic DNA extracted from embryonic stem (ES) cell clones using standard methods (16) was digested with EcoRI, electrophoresed, and blotted onto Hybond N membranes (Amersham, Braunschweig, Germany). Filters were hybridized with a 32P-labeled 1.5-kb KpnI/SpeI fragment (Fig. 1A) at 65°C overnight and washed twice at 65°C to final stringency at 0.2× SSC-0.1% SDS (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). To confirm a correct homologous recombination event of the targeted Smcp gene and the absence of additional random integration of the targeting construct, a neomycin fragment was used to reprobe Southern blots. One ES clone carrying the disrupted Smcp allele was injected into C57BL/6J blastocyts (35), and two male chimeric mice were generated. These males were mated to C57BL/6J and 129/Sv females, and the resulting F1 offspring were genotyped by PCR analyses. Heterozygous Smcp animals were crossed to obtain homozygous mice.
Genomic DNA was extracted from mouse tails by using standard protocols (12). PCR was carried out for 35 cycles using the following conditions: 30 s at 94°C, 30 s at 57°C, and 45 s at 72°C. The following primers were used to discriminate wild-type and mutant alleles: 1 (Smcp sense), 5′-GAGCCCTTCTCCAGAGTTTGG-3′; 2 (Smcp antisense), 5′-GTCTTAGTTTTTACCCTGGAG-3′; 3 (Pgk antisense), 5′-TTCCATTGCTCAGGGTGCTG-3′. The amplification products were analyzed on 2% agarose gels. A 480-bp fragment of the mutant allele was amplified with primers 2 and 3 whereas primers 1 and 2 (Fig. 1A) amplified a 550-bp wild-type product with template DNA from both heterozygous and wild-type animals.
Northern blot analysis.
Total RNA was extracted from mouse testes using the RNA Now kit (ITC Biotechnologies, Heidelberg, Germany) according to the manufacturer's recommendation. Twenty micrograms of RNA were size fractionated by electrophoresis on a 1% agarose gel containing formaldehyde and transferred to a nylon membrane. The membrane was hybridized with a 32P-labeled Smcp cDNA fragment (2) and reprobed with elongation factor-2 cDNA (24) to ensure equal loading.
Western blot analysis.
Testes were homogenized in 10 volumes of SEM buffer (0.32 M sucrose, 1 mM EDTA, 0.1% [vol/vol] mercaptoethanol) and adjusted to a final protein concentration of 10 μg/μl. Twenty micrograms of each protein homogenate was loaded onto a precast 4 to 12% NuPAGE Bis-Tris gel (Invitrogen, Groningen, The Netherlands). After electrophoresis, the proteins were blotted to polyvinylidene difluoride membranes (Machery & Nagel, Düren, Germany) as previously described (17). Smcp was probed with Smcp antisera (8). Neomycin phosphotransferase II and α-tubulin were detected using commercially available antibodies (5Prime→3Prime, Boulder, Colo.; Sigma-Aldrich Chemie, Deisenhofen, Germany). For detection of bound antibodies, filters were incubated with a 1:10,000 dilution of alkaline phosphatase-conjugated goat anti-rabbit or goat anti-mouse immunoglobulin G (Sigma-Aldrich Chemie), and proteins were visualized with 0.35 mg of nitroblue tetrazolium/ml and 0.18 mg of 5-bromo-4-chloro-indolylphosphate substrate (BCIP)/ml.
Fertility test.
To investigate the fertility of the Smcp-deficient males on a mixed background (C57BL/6J × 129/Sv) and on a 129/Sv genetic background, respectively, five sexually mature male Smcp−/− mice from each genetic background were mated, each with two females, for 3 months. Females were checked for the presence of vaginal plugs and/or pregnancy. Pregnant females were removed to holding cages to give birth. The number and size of litters sired by each group of males were determined in a 3-month mating period.
Furthermore, 8-week-old CD1 female mice were superovulated by intraperitoneal injections of 5 IU of pregnant mare serum gonadotrophin (PMSG) (Intergonan; Intervet, Tönisvorst, Germany) followed by 5 IU of human chorionic gonadotrophin (HCG) (Predalon; Organon, Oberschleiβheim, Germany) 46 to 48 h later, and they were mated with Smcp+/+ or Smcp−/− males of the 129/Sv genetic background. Oocytes from females with a copulatory plug were isolated. The oviducts were dissected out and flushed with M2 medium (Sigma, St. Louis, Mo.). The oocytes were treated with M2 medium containing hyaluronidase (300 μg/ml) to remove the cumulus cells, washed in M2, maintained in M16 (Sigma-Aldrich Chemie), and examined for the presence of male and female pronuclei. The eggs were then cultured in M16 covered with mineral oil to check for embryonic development.
In vitro fertilization assays.
Sexually mature Smcp+/+ and Smcp−/− male mice of 129/Sv genetic background were used for these experiments. Female CD1 mice were superovulated as described before, and oocytes were collected 10 to 12 h after hCG administration. The cumulus cells were removed by hyaluronidase treatment, and the oocytes were washed in fertilization medium (MediCult, Jyllinge, Denmark) and then maintained in this medium. To remove the zona pellucida, oocytes were treated with acidic Tyrode's and washed three times with phosphate-buffered saline (PBS) as previously described (12). Spermatozoa were isolated from the cauda epididymis and vas deferens of each male group, capacitated in Tyrode's medium at 37°C for 1.5 h, and then added to the intact and the zona-free oocytes in 100-μl drops of fertilization medium and incubated for 6 h at 37°C in 5% CO2 covered with mineral oil. Using a large bore micropipette, eggs were washed in M16, photographed, and cultured further in M16 as described above.
Sperm analysis.
From five Smcp−/− and Smcp+/+ male mice of the 129/Sv genetic background, the epididymides were collected and dissected in Tyrode's medium. Sperm number in corpus and cauda epididymis was determined using the Neubauer cell chamber. To investigate the acrosome reaction, spermatozoa were capacitated for 1.5 h in Tyrode's medium and then incubated for 5 min at 37°C in 5% CO2 with Tyrode's medium plus 20 μM calcium ionophor A23187 (Sigma-Aldrich Chemie). For the determination of the percentage of sperm that had undergone an acrosome reaction, sperm were fixed and stained with Coomassie brilliant blue R250 as described by Thaler and Cardullo (29). At least 200 spermatozoa from each male were examined for the presence or absence of the characteristic dark blue acrosomal crescent. To investigate the sperm migration in the female reproductive tract, five Smcp+/+ and Scmp−/− males each were mated with two mature CD1 females. Six hours after mating, uteri and oviducts from females with vaginal plug were flushed with M2 medium and the sperm number was determined.
Sperm motility analyses.
Epididymes of wild-type and mutant homozygous Smcp mice were dissected in in vitro fertilization (IVF) medium (Medi-Cult, Jyllinge, Denmark). Spermatozoa were allowed to swim out of the epididymes and were incubated for 1.5, 3.5, or 5.5 h at 37°C. A drop of the sperm suspension was transferred to the incubation chamber, which was set at a temperature of 37°C. Sperm movement was quantified using the CEROS computer-assisted semen analysis system (version 10; Hamilton Thorne Research, Beverly, Mass.). Then, 6,000 to 11,000 spermatozoa from each of four Smcp+/+ and four Smcp−/− mice were analyzed using the following parameters: negative phase-contrast optics; recording, 60 frames/s; minimum contrast, 60; minimum cell size, 6 pixels; straightness (STR) threshold, ≥50%; cutoff of the average path velocity (VAP) and straight line velocity (VSL) were 25 and 30 μm/s, respectively; minimum progressive average path velocity (VAP), 75 μm/s; slow cells motile, no (this limit avoids counting sperm moved by others or Brownian motion and low-velocity nonprogressive cells); and minimum static contrast, 15 pixels.
For statistical analysis, frequencies of the six sperm motility parameters VAP, VSL, VCL, ALH, BCF, and STR were examined by probability plots categorized by mouse type (wild-type/mutant Smcp) and by time of observation (1.5, 3.5 and 5.5 h after preparation). VAP, VSL, VCL, and BCF were log normally distributed, but ALH and STR were not. For statistical testing, sperm motility measurements of each parameter were pooled for mouse type and for time of observation. Considering the log-normal distribution, Student's tests for independent observations were applied in order to define differences in VAP, VSL, VCL, and BCF means (normalized by natural logarithms) comparing wild-type and mutant Smcp mice. For the same purpose, the nonparametric ALH and STR distributions were tested by Friedman's analysis of variance. Statistical analyses were performed by Statistica (StatSoft, Inc., Tulsa, Okla.).
Electron microscopy.
Testes and epididymides were fixed with 5% glutaraldehyde in 0.2 M phosphate buffer, postfixed with 2% osmium tetroxide, and embedded in epoxy (Epon) resin. Selected areas were thin sectioned and examined by electron microscopy.
RESULTS
Generation of Smcp-deficient mice.
The mouse Smcp gene contains a single intron, and the second exon contains the complete 143-amino-acid coding region (13). To disrupt the Scmp gene, a replacement-targeting construct was achieved by replacing exon 2 of the Smcp gene with the neomycin phosphotransferase II gene (Pgk-neo). The herpex simplex virus thymidine kinase (tk) gene at the 3′ end of the construct enabled us to use positive and negative selection (Fig. 1A) (18). R1 ES cells were transfected with the targeting construct and selected for homologous recombination events. Drug-resistant clones were selected, and DNA was isolated and screened by Southern blot analysis using a 1.5-kb KpnI/SpeI fragment. A probe upstream of the targeting construct detected a 14-kb EcoRI fragment in the wild-type allele and 13-kb recombinant fragment in the disrupted allele (Fig. 1A and B). One ES cell clone injected into C57BL/6J blastocyts gave rise to two chimeric mice that transmitted the mutant Smcp allele to their offspring. Chimeric mice were intercrossed to C57BL/6J and 129/Sv females in order to establish the Smcp-disrupted allele on a C57BL/6J × 129/Sv hybrid background and on a 129/Sv background, respectively. In both genetic backgrounds male and female heterozygotes developed normally and were fertile. The heterozygous animals were crossed, and PCR analyses revealed that approximately 25% (47 of 197 and 49 of 202) of the offspring were homozygous for the mutant Smcp−/−.
The targeted disruption of the Smcp gene resulted in the absence of expression of the Smcp RNA and protein. Northern blot analysis of RNA derived from the testes of the different genotypes revealed the absence of the 0.8-kb Smcp transcript in the homozygous mutant mice (Fig. 1C). Western blot analysis with polyclonal anti-Smcp antisera identified the 28-kDa Smcp protein in testicular extracts of wild-type and heterozygous mice but not in extracts of Smcp−/− homozygous mice (Fig. 1D). Probing the Western blot with neomycin phosphotransferase II antibodies revealed that the Neo protein derived from the targeting construct was expressed in the testes of Smcp+/− and Smcp−/− mice and that it was not detectable in wild-type mice. Anti-α-tubulin antibodies established equal protein loading in the Western blots (Fig. 1D).
Infertility of Smcp homozygous mutant males on 129/Sv background.
To investigate the consequences of disruption of the Smcp gene for male fertility, we mated the Smcp−/− males on mixed C57BL6J × 129/Sv and 129/Sv genetic backgrounds, each with two females for 3 months. All matings with Smcp−/− males on the hybrid background were productive, and the average litter size was not significantly different from that of breeding of wild-type litter males with wild-type females (Table 1). In contrast, all Smcp−/− males on the background 129/Sv were infertile despite normal sexual behavior towards female mice and production of copulation plugs. The 129/Sv background was utilized in all of the remaining experiments described below.
TABLE 1.
Fertility of Smcp+/+, Smcp+/−, and Smcp−/− mice on genetic backgrounds C57BL/6J × 129/Sv and 129/Sv
| Genotype of male | No. of mice borna | Avg litter size |
|---|---|---|
| C57BL/6J × 129/Sv | ||
| +/+ | 178 | 7.1 |
| +/− | 177 | 7.0 |
| −/− | 153 | 6.1 |
| 129/Sv | ||
| +/+ | 138 | 5.3 |
| +/− | 126 | 5.0 |
| −/− | 0 | 0 |
Five males and 10 females mated.
To further evaluate Smcp−/− male fertility on the 129/Sv background, we performed in vivo and in vitro fertilization assays. Wild-type females were mated with wild-type and Smcp−/− males, and oocytes were collected 12 h later and scored for fertilization. Eighty-four percent of the oocytes (165 of 196) harvested from females inseminated by wild-type males had male pronuclei, whereas all oocytes examined from females inseminated by Smcp−/− 129/Sv males lacked male pronuclei (0 of 188).
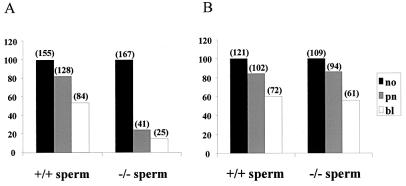
To study whether the infertility of the Smcp-deficient mice on 129/Sv is due to the failure of sperm to penetrate the zona pellucida or due to the failure of sperm to fuse with the egg plasma membrane, we performed in vitro experiments. As summarized in Fig. 2A, only 24.5% of oocytes with intact zona pellucida were fertilized by Smcp-deficient sperm and 14% of all oocytes developed into normal blastocyts, whereas 82.6% of eggs were fertilized with sperm of the wild-type counterparts and 54% of them developed into blastocysts. In contrast, insemination of zona-free oocytes by sperm from Scmp−/− and wild-type mice did not result in significant differences in the fertilization rate (Fig. 2B). Our findings indicate that the lack of Smcp significantly affects the ability of sperm to penetrate the zona pellucida of the oocytes but does not prevent the fusion of sperm and egg plasma membranes.
FIG. 2.
Zona-intact (A) and zona-free (B) oocytes were incubated with sperm of Smcp+/+ and Smcp−/− mice, and in vitro fertilization was assessed by examination for male pronuclei and early development. The number of oocytes (no) incubated with sperm is given as 100%. The number of oocytes with two pronuclei (pn) and the number of oocytes that developed to the blastocyst stage (bl) appear above the bars, and the bars are the percentages of oocytes incubated that had two pronuclei or that developed to the blastocyst stage.
To address the question whether the fertility of Smcp−/− mice might be influenced by an acrosomal defect, we examined the response of spermatozoa from Smcp−/− and wild-type mice to the calcium ionophore A23187. There was no significant difference in the assay of acrosome reaction between Smcp−/− and wild-type spermatozoa (Table 2).
TABLE 2.
Sperm analysis of Smcp+/+ and Smcp−/− mice (129/Sv background)a
| Genotype | No. of sperm in:
|
Acrosome reaction (%) | |||
|---|---|---|---|---|---|
| Corpus epididymis (107) | Cauda epididymis (107) | Uterus (106) | Oviduct | ||
| +/+ | 1.0 ± 0.2 | 1.8 ± 0.3 | 0.8 ± 0.2 | 301 ± 15 | 81 ± 7 |
| −/− | 0.8 ± 0.4 | 1.6 ± 0.6 | 0.9 ± 0.3 | 78 ± 7b | 77 ± 8 |
Data from the sperm analysis represent the mean ± standard error of the mean of the individual measurements (five in each case).
The value for this parameter of Smcp−/− mice is significantly different from that of Smcp+/+ mice (P < 0.05).
Sperm analysis of Smcp mutant males on 129/Sv background.
For a closer investigation into the causes of male infertility, sperm numbers were determined in the corpus and cauda epididymis as well as in uteri and oviducts of females inseminated by Smcp−/− and wild-type males. As shown in Table 2, a significant reduction (P < 0.05) in sperm number was found only in oviducts of females mated with Smcp−/− males. This result let us to suggest that the migration of Smcp-deficient spermatozoa through the female genital tract is disturbed.
We therefore measured sperm motility after 1.5-, 3.5-, and 5.5-h incubations in vitro. No significant differences between the motility of spermatozoa of wild-type and heterozygous mice were observed (data not shown). After 1.5 h, the proportion of motile spermatozoa of Smcp−/− mice was slightly reduced and the proportion that exhibited progressive movement in Smcp−/− mice was reduced compared with those of wild-type mice, 24.7% versus 36% (Table 3). However, after 3.5 and 5.5 h, the proportion of motile and progressively motile spermatozoa in Smcp−/− and wild-type mice had decreased similarly (Table 3).
TABLE 3.
Analysis of sperm motility of Smcp+/+ and Smcp−/− mice
| Genotype of mice | Incubation time (h) | Total no. of measured spermatozoa | No. of motile spermatozoa (%) | No. of spermatozoa with progressive movement (%) |
|---|---|---|---|---|
| +/+ | 1.5 | 10,077 | 5,531 (54.9) | 3,623 (36.0) |
| 3.5 | 11,189 | 5,352 (47.8) | 3,273 (29.3) | |
| 5.5 | 8,329 | 3,119 (37.4) | 1,688 (20.3) | |
| −/− | 1.5 | 8,474 | 4,006 (47.3) | 2,096 (24.7) |
| 3.5 | 8,950 | 3,865 (43.2) | 1,946 (21.7) | |
| 5.5 | 6,409 | 2,338 (36.5) | 1,210 (18.9) |
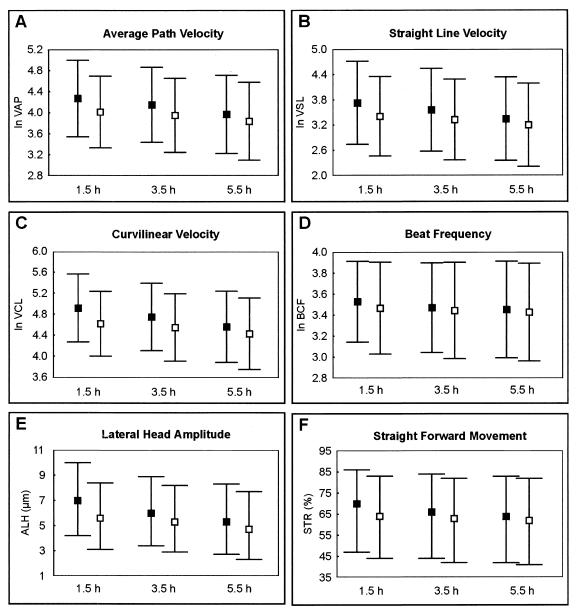
The following parameters were evaluated in more detail: curvilinear velocity (VCL), average path velocity (VAP), straight line velocity (VSL), beat cross frequency (BCF), straightness (STR), linearity (LIN), and amplitude of the lateral head displacement (ALH) (Fig. 3). For the linearity no significant alterations were found (P < 0.16). For all other parameters, a significant reduction for Smcp−/− spermatozoa in comparison to the wild-type sperm was observed (P < 0.001). A decrease of 20 to 30% was found for the velocity parameters VAP, VSL, and VCL. The lower velocity of the Smcp−/− spermatozoa could be due to a reduction of the BCF and the ALH. The ALH was approximately 18% diminished in the Smcp-deficient sperm in comparison to wild-type spermatozoa. After 5.5 h of incubation time, the differences between the sperm motility of Smcp−/− and wild-type mice was found to be reduced (for example, 13 to 15% for sperm velocities).
FIG. 3.
Computer-assisted analysis of sperm motility. The results of analyses of wild-type (▪) and Smcp−/− (□) spermatozoa are shown. Sperm velocities (micrometers/second), forward movement (percent), lateral amplitude of the sperm head (micrometers), and beat frequency (hertz) were measured after 1.5, 3.5, and 5.5 h. For velocities (VAP, VSL, and VCL) and the beat frequency (BCF), the means and appropriate standard deviations are illustrated. For amplitude (ALH) and straightness (STR), the medians and quartiles are shown. The Smcp-deficient spermatozoa exhibit highly significantly reduced velocities, beat frequency, amplitude and forward movement in comparison to wild-type sperm (P < 0.001). (A) VAP; (B) VSL; (C) VCL; (D) BCF; (E) ALH; (F) STR.
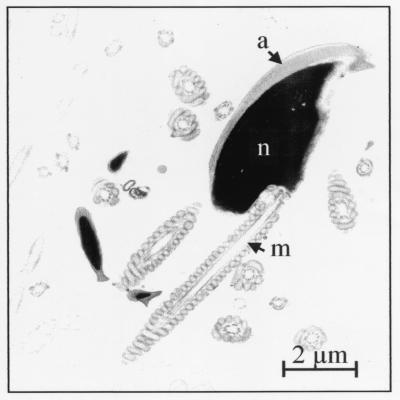
To determine whether the Smcp-deficient spermatozoa have structural abnormalities, light and electron microscopical analyses were performed. Neither in spermatogenesis nor in spermatozoa were structural defects observed. Because SMCP is a mitochondrial protein, these organelles were analyzed in more detail, and they were found to be normal (Fig. 4).
FIG. 4.
Transmission electron micrograph of Smcp-deficient sperm. Ultrastructure of spermatozoa including mitochondria appears to be normal. a, acrosome; m, mitochondrial sheath; n, nucleus.
DISCUSSION
SMCP is a conserved, cysteine-rich protein that is closely associated with the keratinous mitochondrial capsules of bull and mouse spermatozoa (8, 22). To determine the function of this unusual protein, we generated a null mutation of the mouse Smcp locus and examined the effect of Smcp deletion on the morphology of spermatozoa and male fertility.
Breeding of the Smcp−/− mice revealed that the effects of the null mutation on fertility depend on the genetic background. The infertility of Smcp−/− males on the 129/Sv background and the normal fertility of Smcp−/− males on the hybrid C57Bl/6 × 129/Sv background indicate that the mutation of the Smcp locus interacts with as-yet-unknown modifying genes. Interestingly, highly variable penetrance on male infertility on different genetic backgrounds has also been reported for mice carrying targeted null mutations of other genes affecting spermatogenesis, including the Pou homeodomain, transition proteins 1 and 2 genes, and desert hedgehog genes (3, 6, 23, 36).
The reduced number of spermatozoa recovered from the oviducts of females inseminated by Smcp−/− 129/Sv males pointed toward a defect in sperm motility. This hypothesis was confirmed by significant decreases in sperm velocities measured in vitro. Furthermore, we found that the ability of the sperm of Smcp−/− mice to fertilize zona pellucida-intact oocytes was strongly reduced, even though the fusion of mutant sperm with the egg plasma membrane was unaffected after removal of the zona pellucida, judging from the generation of male pronuclei and development to the blastocyst stage. Taken together, our data suggest that the infertility of the male Smcp−/− 129/Sv mice is caused by reduced sperm motility in the female reproductive tract and an unknown role of SMCP in penetrating the zona pellucida. Our data also show that the failure to penetrate the zona pellucida is not caused by an impaired acrosome reaction. Conceivably, the impaired motility of the SMCP-deficient spermatozoa affects hypermotility, which is required for zona pellucida penetration (5, 27, 28).
The loss of SMCP function leads to a higher proportion of immotile spermatozoa, and the motile spermatozoa exhibited lower velocities and reduced beat frequency and amplitude. Loss or reduction in sperm motility is known as asthenozoospermia and is one of the primary causes of untreatable infertility or subfertility in men (1, 30). In about 20 to 30% of patients with asthenozoospermia, the impaired sperm movement is not correlated with abnormalities in sperm structure (9, 34) and is therefore similar to the phenotype of the Smcp-deficient mice. The human SMCP gene has also been identified (4), and it is reasonable to speculate that mutations in SMCP and/or proteins that interact with SMCP could cause asthenozoospermia in humans too. Recently, we have shown that mice lacking a functional dynein heavy chain also exhibit severe asthenozoospermia (20). Little is known about inherited defects in sperm motility, and few mouse models exist for the disruption of a gene resulting in abnormal sperm movement (25, 31). The Smcp- and dynein-deficient mice provide experimental models to study the mechanisms of asthenozoospermia.
SMCP was initially thought to be the predominant structural protein in the sperm mitochondrial capsules (7, 22). However, rat sperm mitochondrial capsules purified by sonication and treatment with SDS and trypsin contain a single protein (7), phospholipid hydroperoxide glutathione peroxidase (33). The absence of SMCP suggests that SMCP is not the predominant capsular protein, but it does not demonstrate that SMCP is not part of the capsule, because these capsules were purified with trypsin, an enzyme that is expected to rapidly degrade SMCP because it contains ∼10% lysine (2). The finding that SMCP copurifies with bull sperm mitochondrial capsules prepared without trypsin and electron microscope immunocytochemistry localization of SMCP to the sperm mitochondrial membranes and intermitochondrial spaces (8, 22) imply that SMCP is closely associated with, but not necessarily an integral part, of the capsule. Herr et al. (11) confirmed the localization of SMCP to the mitochondrial sheath of rat spermatozoa by light microscope immunocytochemistry. Our results show that the mitochondrial sheath develops normally in Smcp−/− sperm, demonstrating that SMCP is not necessary for the formation of the sheath. Further studies are needed to determine whether the impaired motility of the SMCP-deficient spermatozoa is correlated with subtle ultrastructural changes. It is also possible that SMCP deficiency impairs motility by reducing the production or transfer of respiratory energy to the flagellum.
The mammalian sperm tail contains several specialized structures, the mitochondrial capsule and sheath, the outer dense fibers, and the fibrous sheath. Our results suggest that a subtle modification in the mid-piece can have profound effects on sperm motility, a process that is under intense evolutionary pressure because it affects reproductive success directly. Targeted gene deletion provides a powerful approach to dissect the assembly and function of the specialized structures of the sperm tail.
Acknowledgments
K. Nayernia and I. M. Adham contributed equally to this work.
We are grateful to M. Schindler and H. Riedesel for assistance with the generation of knock-out mice. We thank C. Müller for help with particular experiments and A. Winkler for secretarial help.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (through SFB 271) to W.E.
REFERENCES
- 1.Acacio, B., T. Gottfried, R. Israel, and R. Z. Sokol. 2000. Evaluation of a large cohort of men presenting for a screening semen analysis. Fertil. Steril. 73:595-597. [DOI] [PubMed] [Google Scholar]
- 2.Adham, I. M., D. Tessmann, K. A. Soliman, D. Murphy, H. Kremling, C. Szpirer, and W. Engel. 1996. Cloning, expression, and chromosomal localization of the rat mitochondrial capsule selenoprotein gene (MCS): the reading frame does not contain potential UGA selenocysteine codons. DNA Cell. Biol. 15:159-166. [DOI] [PubMed] [Google Scholar]
- 3.Adham, I. M., K. Nayernia, E. Burkhardt-Göttges, O. Topaloglu, C. Dixkens, A. F. Holstein, and W. Engel. 2001. Teratozoospermia in mice lacking the transition protein 2 (Tnp2). Mol. Hum. Reprod. 7:513-520. [DOI] [PubMed] [Google Scholar]
- 4.Aho, H., M. Schwemmer, D. Tessmann, D. Murphy, G. Mattei, and W. Engel. 1996. Isolation, expression, and chromosomal localization of the human mitochondrial capsule selenoprotein gene (MCSP). Genomics 32:184-190. [DOI] [PubMed] [Google Scholar]
- 5.Bedford, J. M. 1998. Mammalian fertilization misread? Sperm penetration of the eutherian zona pellucida is unlikely to be a lytic event. Biol. Reprod. 59:1275-1287. [DOI] [PubMed] [Google Scholar]
- 6.Bitgood, M. J., L. Shen, and A. P. McMahon. 1996. Stertoli cell signalling by Desert hedgehog regulates the male germline. Curr. Biol. 6:298-304. [DOI] [PubMed] [Google Scholar]
- 7.Calvin, H. I., G. W. Cooper, and E. Wallace. 1981. Evidence that selenium in rat sperm is associated with a cysteine-rich structural protein of the mitochondrial capsules. Gamete Res. 4:139-149. [Google Scholar]
- 8.Cataldo, L., K. Baig, R. Oko, M. A. Mastrangelo, and K. C. Kleene. 1996. Developmental expression, intracellular localization, and selenium content of the cysteine-rich protein associated with the mitochondrial capsules of mouse sperm. Mol. Reprod. Dev. 45:320-331. [DOI] [PubMed] [Google Scholar]
- 9.Courtade, M., C. Lagorce, L. Bujan, C. Caratero, and R. Mieusset. 1998. Clinical characteristics and light and transmission electron microscopie sperm defects of infertile men with persistent unexplained asthenozoospermia. Fertil. Steril. 70:297-304. [DOI] [PubMed] [Google Scholar]
- 10.Cummins, J. M., T. Wakayama, and R. Yanagimachi. 1997. Fate of microinjected sperm components in the mouse oocyte and embryo. Zygote 5:301-308. [DOI] [PubMed] [Google Scholar]
- 11.Herr, J. C., D. Thomas, L. A. Bush, S. Coonrod, V. Khole, S. S. Howards, and C. J. Flickinger. 1999. Sperm mitochondria-associated cysteine-rich protein (SMCP) is an autoantigen in Lewis rats. Biol. Reprod. 61:428-435. [DOI] [PubMed] [Google Scholar]
- 12.Hogan, B., F. Costantini, and E. Lacy. 1986. Manipulating the mouse embryo. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 13.Karimpour, I., M. Custer, D. Shih, J. Smith, and K. C. Kleene. 1992. Nucleotide sequence of the gene encoding the mitochondrial capsule selenoprotein in mouse sperm. Identification of three in-phase UGA codons. DNA Cell Biol. 11:693-699. [DOI] [PubMed] [Google Scholar]
- 14.Kleene, K. C. 1989. Poly (A) shortening accompanies the activation translation of five mRNAs during spermiogenesis in the mouse. Development 106:367-373. [DOI] [PubMed] [Google Scholar]
- 15.Kleene, K. C., J. Smith, A. Borzorgzadeh, L. Hahn, M. Harris, I. Karimpour, and J. Gerstel. 1990. Sequence and developmental expression of the mRNA encoding the selenoprotein of the sperm mitochondrial capsule in the mouse. Dev. Biol. 137:395-402. [DOI] [PubMed] [Google Scholar]
- 16.Laird, P. W., A. Zijdererreld, K. Linders, M. A. Radnicki, R. Jaenisch, and A. Berns. 1991. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 19:4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemaire, L., A. Senftleben, and U. A. Heinlein. 1992. Characterization by enriched polyclonal antibodies of developmentally regulated and cell type specific mouse testis antigens. Life Sci. 51:439-448. [DOI] [PubMed] [Google Scholar]
- 18.Mansour, S. L., K. R. Thomas, and M. R. Capecchi. 1988. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutation to non-selectable gene. Nature 336:348-352. [DOI] [PubMed] [Google Scholar]
- 19.Nam, S. Y., S. Maeda, M. Fujisawa, M. Kurohmaru, and Y. Hayashi. 1998. Cloning and expression of the mitochondrial capsule selenoprotein gene in the golden hamster. J. Vet. Med. Sci. 60:1113-1118. [DOI] [PubMed] [Google Scholar]
- 20.Neesen, J., R. Kirschner, M. Ochs, A. N. Schmiedl, B. Habermann, C. Müller, F. A. Holstein, T. Nuesslein, I. Adham, and W. Engel. 2001. Disruption of an inner arm dynein heavy chain gene results in asthenozoospermia and reduced ciliary beat frequency. Hum. Mol. Genet. 10:1117-1128. [DOI] [PubMed] [Google Scholar]
- 21.Otani, H., O. Tanaka, K. K. Kasai, and T. Yoshioka. 1998. Development of mitochondrial sheath in the middle piece of mouse spermatid tail: regular dispositions and synchronized changes. Anat. Res. 222:26-33. [DOI] [PubMed] [Google Scholar]
- 22.Pallini, V., B. Baccetti, and A. G. Burrini. 1979. A peculiar cysteine-rich polypeptide related to some unusual properties of mammalian sperm mitochondria, p. 141-151. In D. W. Fawcett and J. M. Bedford (ed.), The spermatozoon. Urban and Schwartzenberg Inc., Baltimore, Md.
- 23.Pearse, R. V., D. W. Drolet, K. A. Kalla, R. Hooshmand, J. R. Bermingham, and M. G. Rosenfeld. 1997. Reduced fertility in mice deficient for the POU protein sperm-1. Proc. Natl. Acad. Sci. USA 94:7555-7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rapp, G., J. Klaudiny, G. Hagendorff, M. R. Luck, and K. H. Scheit. 1989. Complete sequence of the coding region of human elongation factor 2 (EF-2) by enzymatic amplification of cDNA from human ovarian granulosa cells. Biol. Chem. Hoppe-Seyler 370:1071-1075. [DOI] [PubMed] [Google Scholar]
- 25.Ren, D., B. Navarro, G. Perez, A. C. Jackson, S. Hsu, Q. Shi, J. L. Tilly, and D. E. Clapham. 2001. A sperm ion channel required for sperm motility and male fertility. Nature 413:603-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shih, D. M., and K. C. Kleene. 1992. A study by in situ hybridization of stage of appearance and disappearance of the transition protein 2 and mitochondrial capsule selenoprotein mRNA during spermatogenesis in the mouse. Mol. Reprod. Dev. 33:222-227. [DOI] [PubMed] [Google Scholar]
- 27.Stauss, C. R., T. J. Votta, and S. S. Suarez. 1995. Sperm motility hyperactivation facilitates penetration of the hamster zona pellucida. Biol. Reprod. 53:1280-1285. [DOI] [PubMed] [Google Scholar]
- 28.Suarrez, S. S. 1996. Hyperactivated motility in sperm. J. Androl. 17:331-335. [PubMed] [Google Scholar]
- 29.Thaler, C. D., and R. A. Cardullo. 1995. Biochemical characterization of a glycosyl-phosphatidylinositol-linked hyaluronidase on mouse sperm. Biochemistry 34:7788-7795. [DOI] [PubMed] [Google Scholar]
- 30.Thonneau, P., S. Marchand, A. Tallec, M. L. Ferial, B. Ducot, J. Lansac, P. Lopes, J. M. Tabaste, and A. Spira. 1991. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989). Hum. Reprod. 6:811-816. [DOI] [PubMed] [Google Scholar]
- 31.Tian, M., H. E. Broxmeyer, Y. Fan, Z. Lai, S. Zang, S. Aronica, S. Cooper, R. M. Bigsby, R. Steinmetz, S. J. Engle, A. Mestek, J. D. Pollock, M. N. Lehmann, H. T. Jansen, M. Ying, P. J. Stambrook, J. A. Tischfeld, and L. Yu. 1997. Altered hematopoiesis, behavior, and sexual function in mu opioid receptor-deficient mice. J. Exp. Med. 185:1517-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tybulewicz, V. L., C. E. Grawford, P. K. Jackson, R. T. Bronson, and R. C. Mulligan. 1991. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl protooncogene. Cell 65:1153-1163. [DOI] [PubMed] [Google Scholar]
- 33.Ursini, F., S. Heim, M. Kiess, M. Maiorini, A. Roveri, J. Wissing, and L. Flohe. 1999. Dual function of the selenoprotein PHGPx during sperm maturation. Science 285:1393-1396. [DOI] [PubMed] [Google Scholar]
- 34.Wilton, L. J., P. D. Temple-Smith, and D. M. de Kretser. 1992. Quantitative ultrastructural analysis of sperm tails reveals flagellar defects aassociated with persistent asthenozoospermia. Hum. Reprod. 7:510-516. [DOI] [PubMed] [Google Scholar]
- 35.Wurst, W., and A. L. Joyner. 1993. Production of embryonic stem cell clones, p. 33-61. In A. L. Joyner (ed.), Gene targeting: a practical approach. IRL Press, Oxford, England.
- 36.Yu, Y. E., Y. Zhang, E. Unni, C. R. Shirley, J. M. Deng, L. D. Russel, M. M. Weil, R. R. Behringer, and M. L. Meistrich. 2000. Abnormal spermatogenesis and reduced fertility in transition nuclear protein 1-deficient mice. Proc. Natl. Acad. Sci. USA 79:4683-4688. [DOI] [PMC free article] [PubMed] [Google Scholar]