Abstract
The Tax oncoprotein of human T-cell leukemia virus type 1 (HTLV-1) induces leukemia in transgenic mice and permanent T-cell growth in vitro. In transformed lymphocytes, it acts as an essential growth factor. Tax stimulates the cell cycle in the G1 phase by activating the cyclin-dependent kinase (CDK) CDK4 and CDK6 holoenzyme complexes. Here we show that Tax directly interacts with CDK4. This binding to CDK4 was specific, since Tax did not bind to either CDK2 or CDK1. The interaction with CDK4/cyclin D complexes was observed in vitro, in transfected fibroblasts, in HTLV-1-infected T cells, and in adult T-cell leukemia-derived cultures. Binding studies with several point and deletion mutants indicated that the N terminus of Tax mediates the interaction with CDK4. The Tax/CDK complex represented an active holoenzyme which capably phosphorylates the Rb protein in vitro and is resistant to repression by the inhibitor p21CIP. Binding-deficient Tax mutants failed to activate CDK4, indicating that direct association with Tax is required for enhanced kinase activity. Tax also increased the association of CDK4 with its positive cyclin regulatory subunit. Thus, protein-protein contact between Tax and the components of the cyclin D/CDK complexes provides a further mechanistic explanation for the mitogenic and immortalizing effects of this HTLV-1 oncoprotein.
The deregulation of the enzymatic machinery that controls the G1- to S-phase transition is causatively linked with viral transformation and tumorigenesis. Many viral oncoproteins from herpesviruses (vCyc and kCyc), adenoviruses (E1A), and papovaviruses (simian virus 40 [SV40] large T antigen and human papillomavirus E7) affect G1-specific cyclin-dependent kinases (CDKs) and/or their cognate substrate, retinoblastoma (Rb) protein (pRb) (22, 25, 50). CDK4 and its close relative CDK6 bind to cyclin D isotypes and, together with CDK2 complexes, integrate mitogenic and growth-inhibitory signals. These cyclin/CDK complexes are the first to be activated. CDK4/CDK6 activities allow the cell cycle to pass the restriction point within the mid-G1 phase, thus committing it to enter the S phase. By pRb hyperphosphorylation they mediate the release of E2F transcription factors that stimulate the expression of S-phase-specific genes (45, 46).
Human T-cell leukemia virus type 1 (HTLV-1) causes an aggressive and fatal disease of CD4+ T lymphocytes termed adult T-cell leukemia (ATL) and a neurodegenerative disease called HTLV-1-associated myelopathy or tropical spastic paraparesis. The leukemogenic properties of the virus are accompanied by its capacity to stimulate the growth of normal human lymphocytes in nonleukemogenic patients as well as in vitro (7, 12, 16, 19, 20). Observations made with HTLV-1-transformed cells indicate an abnormal regulation of the cell cycle. Compared to HTLV-1-negative CD4+ T cells, HTLV-1-transformed cells express decreased amounts of cyclin D3 and increased levels of the cyclin kinase inhibitor p21CIP (2, 8); interleukin 2 (IL-2)-independent HTLV-1-transformed cells display constitutive cyclinE/CDK2 activity accompanied by the depletion of the cyclin kinase inhibitor p27KIP from these kinase complexes (9).
Several lines of evidence indicate that the HTLV-1 regulatory protein p40tax is responsible for the leukocyte-transforming and oncogenic features of the virus (1, 15, 17). The growth of primary human lymphocytes conditionally immortalized by Tax depends on tax expression, demonstrating that this protein is necessary and sufficient for transformed cell growth. Moreover, the proliferation of these cells is reversibly arrested in the G1 phase when tax transcription is suppressed, thus verifying the role of Tax in the G1- to S-phase transition of immortalized T lymphocytes (42). Finally, singular expression of Tax can induce various tumors (including leukemia) in transgenic mice (17).
The mechanism by which Tax influences the growth and G1- to S-phase transition of transformed primary human T cells is not fully understood. Different Tax functions may cooperate to influence cellular growth. In addition to its function as a modulator of cellular transcription, Tax may play a role in the stimulation of host cell proliferation, since this protein affects the expression of several genes relevant to growth. It activates genes encoding proto-oncogenes, the α chain of the IL-2 receptor, cytokines (52), cyclin D2 (21, 41), and the CDK inhibitor p21CIP (8, 11). The Tax protein also represses the expression of DNA polymerase β, an enzyme important for DNA repair (23), p18INK-4C (49), and Bax (5).
Tax directly interferes with the functions of cell cycle regulatory proteins (24). It inhibits the transactivating function of the tumor suppressor p53 (33, 36), and it binds to p16INK-4A (28, 48) as well as to cyclin D1/cyclin D3 (34). In the presence of Tax, the CDKs CDK4 and CDK6 are activated (35, 42), suggesting that this viral protein is involved in CDK4/CDK6 stimulation. Since CDK4 activity is required to respond to IL-2 (30), it could be crucial for the IL-2 responsiveness of Tax-transformed T cells. Therefore, this CDK4 stimulation may essentially contribute to mitogenic and immortalizing Tax effects. CDK activation may be explained in part by the direct binding of Tax to p16INK-4A (28, 48), an inhibitor of the CDKs CDK4 and CDK6. However, this cannot be the sole mechanism by which Tax activates CDKs, since this protein also enhances kinase activity in cells null for p16INK-4A expression (27, 34).
Many viral proteins can directly activate cellular kinases. We thus investigated whether Tax might activate CDKs through a direct physical interaction. Here we show that Tax, through its N-terminal sequences, specifically binds CDK4 in vitro and in HTLV-1-infected cells. Furthermore, we found that the association of Tax with the CDK4 holoenzyme resulted in enhanced kinase activity. Because binding-deficient Tax mutants failed to enhance CDK4 activity, we concluded that the stimulation of kinase function requires direct physical contact.
MATERIALS AND METHODS
Cells, plasmids, and antibodies.
HuT-102, C91PL, and MT-2 are human T-cell lines infected with HTLV-1. Jurkat is a T-cell line derived from CD4+ acute lymphoblastic T-cell leukemia. All cells were cultivated in RPMI 1640 supplemented with 10% fetal calf serum (FCS), glutamine, and the antibiotics streptomycin and penicillin. Taxi-1 and Tesi are both derived from primary lymphocytes transduced with Tax-expressing recombinant rhadinovirus vectors (3, 13, 14, 42). They were maintained in RPMI 1640 supplemented with 20% FCS, glutamine, antibiotics, and interleukin 2 (IL-2) (20 to 40 U/ml). The HTLV-1-positive ATL-derived cell lines JuanaW and StEd were cultured as previously described (40). 293T, an adenovirus-transformed human embryonic kidney cell line carrying the SV40 large T antigen, was cultured in Dulbecco's modified Eagle's medium supplemented with 10% FCS, glutamine, and antibiotics.
Human cyclin D3 cDNA was donated by A. Arnold, Boston, Mass. (4). A fragment containing the entire coding sequence of human cyclin D3 was isolated by PCR and cloned into the BamHI site of the pcDNA3 vector (Invitrogen, Groningen, The Netherlands). The cDNAs for CDK4, cyclin D2, and the expression construct for p21CIP were obtained from J. O. Funk, Erlangen, Germany, and P. Jansen-Dürr, Heidelberg, Germany, and inserted into pcDNA3. The CDK6 expression vector was donated by J. U. Jung, Southborough, Mass., and the CDK2 and CDK1 expression vectors were donated by H. Stöppler, National Institutes of Health. For the production of glutathione S-transferase (GST) fused to pRb, we used an expression vector that contains the Rb coding region from codons 379 to 928 and that was supplied by J. U. Jung (24). The Tax mutants were previously described (44, 47). GST-Tax mutants were constructed with pGEX vectors. The purification of proteins from Escherichia coli, including GST column chromatography, was performed according to the column manufacturer's instructions (Pharmacia, Uppsala, Sweden).
Rabbit antibodies to Tax were prepared against full-length recombinant Tax protein. Rabbit antibodies to CDK4, CDK6, CDK2, cyclin D2, and p21CIP were obtained from Santa Cruz Biotechnology Inc., Santa Cruz, Calif.; the mouse antibody to cyclin D3 was obtained from Transduction Laboratories, San Diego, Calif.; and mouse antibodies to Tax were derived from the hybridoma cell lines 168B17-46-34 and 168B17-46-50 (provided by B. Langton through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases).
Protein binding assays.
To produce an S tag-Tax-His tag fusion protein, Tax cDNA was cloned via PCR into the pET29b vector (Novagen, Darmstadt, Germany). An E. coli BL21 culture containing pET29b Tax was grown to an A600 of 0.5, and protein expression was induced for 3 h with 0.5 mM isopropyl-1-thio-β-d-galactopyranoside. Cells were harvested by centrifugation and resuspended in 10 ml of lysis puffer (6 M guanidinium hydrochloride, 10 mM Tris [pH 8.0]) overnight at 4°C. The insoluble fraction was removed by centrifugation. The supernatant was loaded into a nickel-nitrilotriacetic acid-agarose-column (Qiagen, Hilden, Germany), which subsequently was washed twice with buffer containing 8 M urea and 10 mM Tris (pH 6.0). The bound protein was removed from the agarose with elution buffer (8 M urea, 10 mM Tris [pH 8.0]), which contained increasing amounts of imidazole (50 mM to 1 M), and was collected in fractions. The fractions containing the highest Tax concentrations were incubated with S protein-agarose (Novagen) for 2 h at 4°C. Following binding, elution buffer was exchanged with radioimmunoprecipitation (RIPA) buffer (10 mM Tris [pH 7.4], 150 mM NaCl, 2 mM EDTA, 1% Nonidet P-40, 0.5% desoxycholate, 0.1% sodium dodecyl sulfate [SDS]).
35S-methionine-labeled proteins were generated by in vitro translation with rabbit reticulocyte lysates (TnT transcription and translation kit; Promega). For affinity chromatography, comparable amounts of the in vitro translated proteins were added to 700 μl of RIPA buffer supplemented with 3% bovine serum albumin and 5 μl of S protein-agarose-bound Tax protein. The reaction mixtures were incubated at 4°C for 1 h. Bound proteins were precipitated by centrifugation, washed four times with RIPA buffer at 4°C, and recovered by boiling the beads in 25 μl of 2× loading buffer (20 mM Tris [pH 6.8], 2% SDS, 10% β-mercaptoethanol, 20% glycerol, 0.2% bromophenol blue). Proteins were sized on an SDS-15% polyacrylamide gel, quantitated by using a phosphorimager, and visualized by autoradiography.
Transfection, coimmunoprecipitation, and immune complex kinase assay.
Human 293T cells were transfected with plasmids by using Lipofectamine PLUS reagents (Life Technologies, Bethesda, Md.). For coimmunoprecipitation, the cells were lysed in buffer containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 0.5% Nonidet P-40, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and 10 μg of aprotinin ml−1; frozen; thawed; and clarified by centrifugation (10,000 × g for 15 min at 4°C). The protein supernatant (0.5 to 1 mg) was immunoprecipitated with 1 μg of the corresponding antibody by incubation for 1 h at 4°C, and the immune complexes were collected by using either protein A-Sepharose CL-4B (Pharmacia) beads for monoclonal antibodies or Pansorbin (Calbiochem, San Diego, Calif.) for polyclonal antibodies (1 h at 4°C). Subsequently, the beads with the precipitated proteins were washed four times with lysis buffer. For the detection of protein complexes, the immunoprecipitates were separated on SDS-12% polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore, Bedford, Mass.) by using 2.5 mM Tris-19.2 mM glycine buffer. To block nonspecific binding, the membranes were incubated with 5% nonfat dry milk in phosphate-buffered saline containing 0.2% Tween 20 before antibodies were added. After being washed with phosphate-buffered saline containing 0.2% Tween 20, the membranes were incubated with a 1:2,500 dilution of anti-mouse or anti-rabbit immunoglobulin G-horseradish peroxidase conjugate (Amersham, Freiburg, Germany). Bound antibodies were visualized with an enhanced chemiluminescence detection system (Amersham). For the immune complex kinase assay, the cells were lysed in buffer consisting of 50 mM HEPES (pH 7.5), 150 mM NaCl, 2.5 mM EGTA, 0.1% Tween 20, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin ml−1, 1 mM sodium orthovanadate, and 5 mM NaF. The lysates were treated as described above. In order to precipitate the kinase complexes, either the anti-CDK4 antibody or one of the monoclonal anti-Tax antibodies was used together with protein A-Sepharose. The kinase reaction was performed as previously described (42).
Generation of Tax deletion mutants.
All Tax deletion mutants were generated via PCR (37). In order to introduce the internal deletion, five different primers were used, two outside 28-mer oligonucleotides spanning the 5′ and 3′ ends of the Tax open reading frame (Taxs and Taxas) and three chimeric oligonucleotides designed to carry the 5′ and 3′ sequences flanking the deleted regions. After three rounds of PCR with Pwo polymerase (Roche, Mannheim, Germany), deletion clones TD99, TD150, and TD254 were created. To engineer the N-terminal TD1 and C-terminal TD319 deletion clones, one round of PCR was performed by using an internal 5′ primer or 3′ primer in combination with the corresponding outside primer. The resulting PCR products were digested with BamHI and KpnI and ligated via these sites into the pcDNA3.1.Amyc expression vector (Invitrogen). The resulting clones were verified by nucleotide sequencing.
Oligonucleotides.
The oligonucleotide sequences were as follows: Taxs, 5′-ATTTAAGGATCCACCATGGCCCACTTC-3′ (outer primer); Taxas, 5′-ATTTAGGGTACCGACTTCTGTTTC-3′ (outer primer); TD1s, 5′-ATTTAAGGATCCATGGCCCGCCTACATC-3′ ; TD99s, 5′-CCATCGGTAAATGTCCAGGCCCCTGTGGTAAGGG-3′ ; TD150s, 5′-CAATCACTCATACAACCCTGTACACCCTCTGGGG-3′ ; TD254s, 5′-GGACATTTACCGATGGCCCCTCATTTTTACTCTC-3′ ; and TD319as, 5′-ATTTCGGGTACCAGAAATGGGGATGTTG-3′ .
RESULTS
Tax stimulates CDK4 activity in fibroblasts.
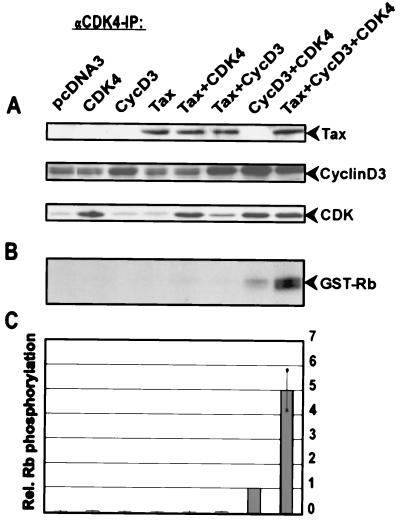
In order to understand the mechanism of Tax-mediated CDK4 activation, human 293T fibroblasts were transfected with various combinations of Tax, cyclin D3, and CDK4 expression constructs, and the effects on CDK4 activity were determined. In vitro kinase assays were conducted by immunoprecipitating CDK4 and using recombinant pRb as a substrate (Fig. 1). The activity of the endogenous kinase was very low (pcDNA3) and, in this setting, could not be detectably stimulated by Tax alone. Coexpression of either CDK4 or cyclin D3 with Tax also did not increase Rb kinase activity. Since transfected Tax also failed to significantly stimulate the activity of CDK4 immunoprecipitates, it seems likely that neither the interaction of Tax with p16INK-4A nor the stimulation by Tax of cyclin D expression plays a significant role in CDK4 activation under these circumstances. On the other hand, cells triply transfected with Tax, cyclin D3, and CDK4 revealed kinase activity which was fivefold enhanced compared to that in cells transfected with cyclin D3 and CDK4. This augmented kinase activity was not due to increased cyclin D3 or CDK4 expression, since the amounts of both proteins were unchanged in cells in the absence or presence of Tax (Fig. 1A). Therefore, the results obtained with Tax/cyclin D3/CDK4 suggest a Tax-mediated process which cannot simply be explained by previously reported Tax activities, such as direct binding to CDK inhibitors (CKIs) or Tax-enhanced expression of cyclins.
FIG. 1.
Tax stimulates the activity of CDK4. 293T cells were cotransfected with 500 ng of CDK4, cyclin D3, and Tax expression vectors in different combinations. All transfections were equalized for the amount of total DNA by addition of the empty vector (pcDNA3). CDK4 complexes were precipitated by using specific polyclonal antibodies. Kinase activity was assessed in vitro by using recombinant pRb as the substrate. The radioactivity incorporated into the substrate was quantified by phosphorimaging. (A) Immunoblots of the lysates used for immunoprecipitation (IP). (B) Representative autoradiograph of the phosphorylated Rb substrate. (C) Relative (Rel.) CDK4 activities in three independent experiments (mean and standard deviation).
Tax interacts specifically and directly with CDK4 and CDK6 in fibroblasts and lymphocytes.
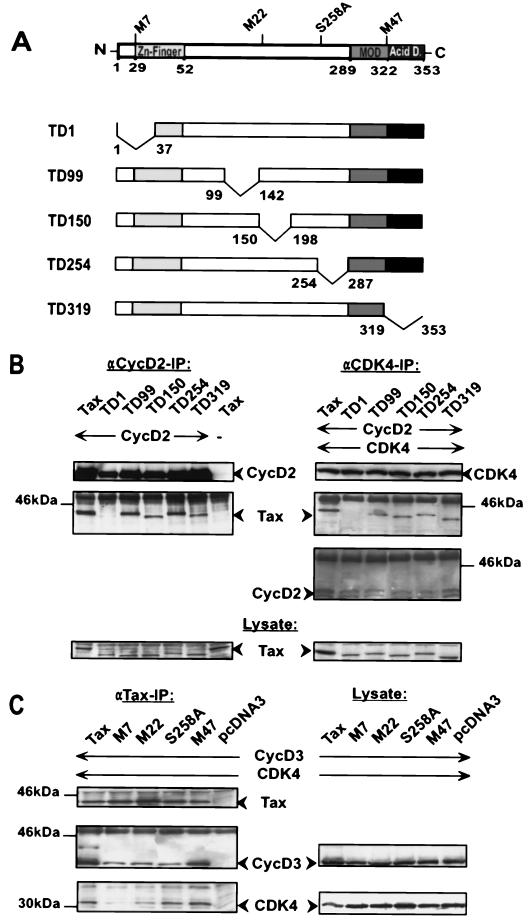
Viral regulatory proteins frequently activate cellular kinases by direct binding. We thus considered the possibility that Tax could bind CDKs directly. Consequently, human fibroblasts (293T) were transfected in various combinations with expression vectors for Tax and for various CDKs, either CDK4, CDK6, CDK2, or CDK1 (Fig. 2). Tax was immunoprecipitated, and potential binding partners were examined by Western blotting (Fig. 2A). Although all CDKs were expressed in comparable amounts in the lysate controls, CDK4 and CDK6, but not CDK2 and CDK1, were found to coprecipitate with Tax. Complementary experiments in which a CDK was precipitated first and potentially bound Tax protein was then assayed confirmed the specific association of Tax with CDK4 and CDK6 and the lack of binding to CDK2 and CDK1 (Fig. 2B).
FIG.2.
CDK4 and CDK6, but not CDK1 and CDK2, are specifically bound by Tax. For coimmunoprecipitation experiments, Tax was coexpressed with various CDKs in 293T cells. (A, left panel) The cell extracts were precipitated with a monoclonal anti-Tax antibody and analyzed in separate immunoblots with antibodies directed individually against Tax, CDK4, CDK6, CDK2, or hemagglutinin-tagged CDK1. IP, immunoprecipitation. (B, left panel) CDK-associated Tax protein was also detected in the reverse experiment with specific anti-CDK antibodies for immunoprecipitation. (A and B, right panels) The presence of CDKs and Tax in the lysates was determined by immunoblot analysis. (C) GST pull-down assays probing a Jurkat cell lysate (lane 1) with either GST alone (lane 2) or GST-Tax (lane 3). After equilibration with Jurkat cell lysate, each column was extensively washed, and bound proteins were eluted. Eluates were analyzed by Western blotting with anti-CDK4 or anti-CDK2 antibodies.
The association of Tax with CDK4 was further tested in pull-down assays. Here, lysates prepared from Jurkat T cells (Fig. 2C) were chromatographed over columns individually saturated with either GST alone (Fig. 2C, lane 2) or GST-Tax (Fig. 2C, lane 3). After equilibration with lysate, the columns were extensively washed with buffer and then eluted for bound proteins. We assayed the eluates with an antiserum specific to either CDK4 (Fig. 2C, top) or CDK2 (Fig. 2C, bottom). In Western blotting analysis, CDK4, but not CDK2, was found to bind GST-Tax but not GST alone. These pull-down results are in good agreement with the above coimmunoprecipitation findings (Fig. 2A and B).
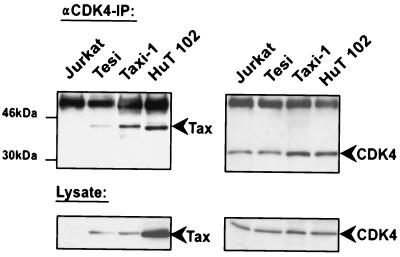
Next, the Tax/CDK interaction in HTLV-1-transformed cells (HuT-102) and Tax-transformed T cells (Taxi-1 and Tesi) was investigated. As a control, we used Tax-negative Jurkat cells. HuT-102, Tesi, and Taxi-1 cells express physiological amounts of Tax and CDKs. Coimmunoprecipitation results obtained with these cells reaffirmed the specific CDK4/Tax interaction documented above in cotransfection experiments (Fig. 3). In these T-lymphocytic backgrounds, CDK6 rather than CDK1 was also observed to bind Tax (data not shown).
FIG. 3.
Tax binds CDK4 in Tax-transformed HTLV-1-infected human T cells. For coimmunoprecipitations, Tax-immortalized cells (Tesi and Taxi-1), the HTLV-1-infected cell line HuT-102, and a negative control (Jurkat cells) were used. Immunoprecipitation (IP) was performed with anti-CDK4 antibodies and 1 mg of protein from the whole-cell lysate. The upper panels show the precipitated Tax and CDK4 proteins detected by Western blot analysis. The lower panels show the endogenous expression levels of these proteins in the different cell lines used for immunoprecipitation.
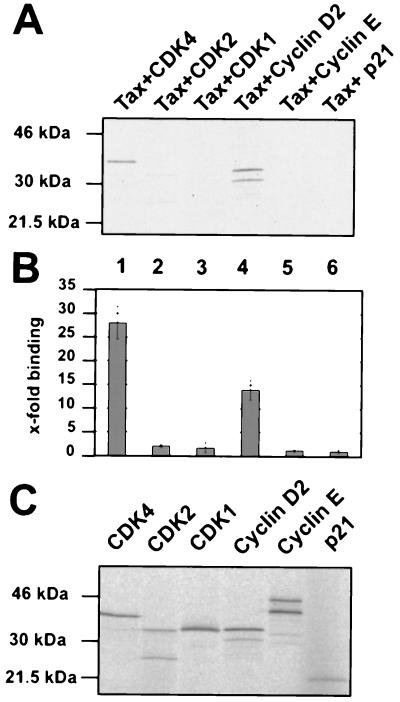
To analyze whether the interaction between the proteins is direct, we performed binding assays in vitro (Fig. 4A and B). The tax cDNA was attached to sequences encoding an S tag, and fusion proteins were purified from recombinant E. coli. Radioactively labeled binding partners (CDK4 and cyclin D2) and controls (CDK1, CDK2, cyclin E, and p21CIP1) were obtained as in vitro translation products and incubated with Tax fixed to S protein-agarose (Fig. 4A). The amounts of in vitro translated proteins were checked prior to the binding reactions and found to be comparable (Fig. 4C). In the binding assays, only CDK4 and cyclin D2 were capable of interacting with Tax. Quantification of protein binding in three independent experiments revealed that CDK4 was bound with a 14-fold higher efficiency than CDK1 or CDK2; the binding of cyclin D2 was 11-fold higher than that of cyclin E (Fig. 4B). In confirmation of previous observations (28), no detectable direct interaction of Tax with p21CIP1 could be observed. Thus, the p21CIP protein coprecipitating with Tax from HTLV-1-infected cells is indirectly linked to Tax, probably by binding to cyclin-CDK. In contrast, the in vitro binding assays clearly indicated that the interactions of Tax with both CDK4 and cyclin D are direct. Hence, as the results obtained with independent methods (i.e., coimmunoprecipitations, pull-down assays, and in vitro binding assays) and different cells (i.e., fibroblastic and lymphocytic cells) all supported a specific and direct CDK4/Tax interaction, we concluded that this association could be physiologically important.
FIG. 4.
Tax directly interacts with CDK4 and cyclin D2. For these affinity chromatography experiments, equal amounts of recombinant Tax protein bound to S protein-agarose were incubated with the following in vitro translated 35S-labeled proteins: CDK4 (lane 1), CDK2 (lane 2), CDK1 (lane 3), cyclin D2 (lane 4), cyclin E (lane 5), and p21CIP1 (lane 6). (A) Tax-bound proteins were sized on an SDS-polyacrylamide gel and visualized by autoradiography. (B) Quantitative evaluations of three independent experiments. Error bars show standard deviations. (C) Autoradiogram showing 5% of the in vitro translated proteins used for one of the binding experiments. The quantitative evaluation of these proteins served to even out slight differences in the amounts of labeled proteins used in the in vitro interaction assays.
Association of Tax with active cyclin D/CDK complexes.
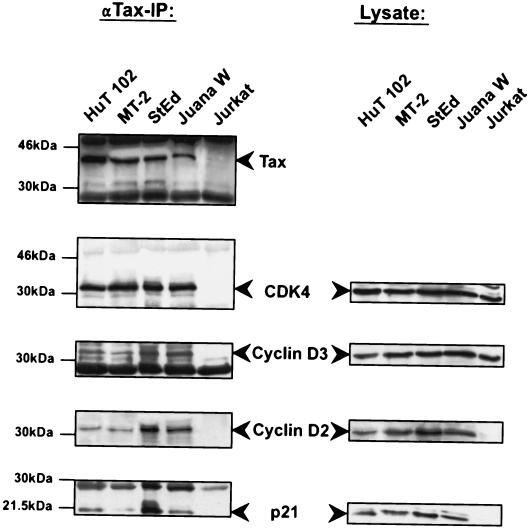
The findings above are compatible with Tax requiring both the cyclin and the CDK components to promote kinase function. We next examined whether Tax interacted with the holoenzyme cyclin D/CDK4 complex. Immunoprecipitations were performed with Tax-, cyclin D3-, cyclin D2-, or CDK4-transfected 293T cells (data not shown) as well as Tax-expressing HuT-102, MT-2, StEd, and JuanaW cells (Fig. 5). StEd and JuanaW are lymphocyte cultures that were recently established from ATL patients (40); the HTLV-1-negative T-cell line Jurkat served as a negative control. Tax protein was precipitated with specific antiserum, and associated proteins were then analyzed by immunoblotting. We found Tax-associated proteins to include cyclin D3, as reported previously (34), as well as cyclin D2 and CDK4. Of note, Tax simultaneously coprecipitated CDK4, cyclin D, and p21CIP1, suggesting that this oncoprotein associates with a holoenzyme complex inside cells. Moreover, immunohistochemical analysis demonstrated that Tax colocalizes with cyclin D2, CDK4, and p21 in nuclear speckles in Tax-expressing T cells (Tesi). From these experiments, we deduce that in transfected fibroblasts and HTLV-1-infected ATL-derived cultures, Tax most likely binds to CDK4 holoenzyme complexes by direct contact with CDK4 and cyclin D.
FIG. 5.
Tax binds to the holoenzyme cyclin D/CDK4 complex in HTLV-1-infected T cells derived from ATL patients. Precipitations with the Tax protein were performed with long-term-cultured IL-2-independent HTLV-1-infected cell lines (HuT-102 and MT-2), IL-2-dependent cultures derived from ATL patients (StEd and JuanaW) (40), and the T-cell line Jurkat as a negative control. For the immunoprecipitation (IP) experiments, a monoclonal anti-Tax antibody was used to bind Tax from 1 mg of protein from the whole-cell lysate. Precipitated Tax and associated cyclin D2, cyclin D3, CDK4, and the inhibitor p21CIP1 were detected by Western blot analysis. The panels on the right show the endogenous expression levels of the Tax-bound proteins in the cell lines used for coimmunoprecipitation experiments.
To determine whether the Tax/cyclin D/CDK complex represents an active kinase, anti-Tax antibodies were used to immunoprecipitate protein complexes from various Tax-transformed cells (Taxi-1) and HTLV-1-infected cells (HuT-102, C91PL, and MT-2). The Tax-containing complexes were assayed for in vitro kinase activity on GST-Rb (Fig. 6). The degree of Rb phosphorylation for Tax-expressing cells was 8- to 16-fold higher (Fig. 6, lanes 2 to 5) than that for control Jurkat cells (Fig. 6, lane 1). The increase in kinase activity correlated well with the degree of Tax expression in the various cells. In parallel, similar experiments were performed with the Tesi cell line (Fig. 6, lanes 6 and 7), whose expression of Tax is suppressed in the presence of tetracycline (42). Tetracycline-positive and tetracycline-negative Tesi cell samples were then compared for the amounts of Tax-associated active Rb kinase. We found that treatment with tetracycline (which suppressed Tax expression) reproducibly reduced Rb phosphorylation by at least fourfold. The minor increase in background activity present in tetracycline-positive Tesi cells (Fig. 6, lane 7) is due to some leakage of the tetracycline repressor system that controls Tax expression. These results corroborate the findings for cells which constitutively express Tax (Fig. 6, lanes 2 to 5). In all, the findings indicate strongly that in cells, Tax associates with an active Rb kinase and upregulates its enzymatic activity.
FIG. 6.
Tax-associated proteins can phosphorylate the CDK4 substrate, Rb. Tax and associated proteins were immunoprecipitated from cell extracts with a monoclonal anti-Tax antibody and tested for kinase activity with GST-Rb as a substrate. The upper panels show the levels of Tax present in the investigated cell lines, the middle panels show an immune complex kinase assay (autoradiography), and the lower panels show the quantified results. The lower panels depict the mean and standard deviation of three independent experiments. IP, immunoprecipitation; Rel., relative. Lanes: 1, Jurkat; 2, Taxi-1; 3, HuT-102; 4, C91PL; 5, MT-2; 6 and 7, Tesi cells containing a tetracycline-repressible Tax gene. Tesi cells were cultured in either the absence (lane 6) or the presence (lane 7) of tetracycline (tet) (1 μg/ml) for 1 week, lysed, and subjected to an immune complex kinase assay.
The N terminus of Tax interacts with CDK4/cyclin D complexes.
To better understand how Tax associates with CDK4, five Tax mutants containing deletions linearly spanning the whole reading frame of the protein were generated (Fig. 7A). These mutants were individually coexpressed in 293T cells with cyclin D2 and CDK4 (Fig. 7B). Transfected cells were separately precipitated with anti-CDK4 antibodies, which revealed that except for the N-terminal deletion mutant TD1, all other Tax mutants associated with a cyclin/CDK4 complex. The corresponding lysate control verified that all Tax deletion mutants were comparably synthesized, suggesting that the failure of mutant TD1 to form a complex is not trivially explained by failed or unstable expression. Similar results were observed in interaction experiments with cyclin D2 and Tax mutants alone. The results accrued with the deletion mutants were confirmed in similar coimmunoprecipitation experiments with well-defined point mutants (Fig. 7C). In this regard, mutant M7, bearing a mutation in the postulated N-terminal zinc finger domain (mutated at amino acids 29 and 30), exhibited significantly reduced binding to CDK4 and relatively reduced association with cyclin D3 compared to either wild-type Tax or mutant M47 (mutated at amino acids 319 and 320).
FIG. 7.
N-terminal Tax sequences are essential for the interaction with CDK4/cyclin D complexes. (A) Schematic representation of the point and deletion mutants used in the following experiments; structural features of the Tax protein are indicated. MOD, modulator domain; Acid D., acidic domain. (B) Coimmunoprecipitation experiment with the Tax deletion mutants. For the experiments, 293T cells were transfected with 500 ng of wild type Tax or 800 ng of five different Tax deletion mutants and 500 ng of a cyclin D2 expression construct. The blots on the upper left represent precipitation with polyclonal anti-cyclin D2 antibodies. The same antibodies were used for cyclin D2 Western blot analysis. In order to detect Tax and the mutant proteins, a rabbit anti-Tax serum was used. The blots on the upper right show a triple transfection with Tax, cyclin D2, and CDK4 (500 ng) constructs. The precipitation and the subsequent Western blot analysis were done with polyclonal anti-CDK4 antibodies. The lower panels (lysate control) depict the expression of wild-type Tax and the deletion mutants in the lysates prepared for the coimmunoprecipitation experiments. IP, immunoprecipitation. (C) Coimmunoprecipitation experiment with the Tax point mutants. Cells were cotransfected with 500 ng of expression constructs for Tax point mutants, cyclin D3, and CDK4. Tax was immunoprecipitated from whole-cell lysates with a monoclonal anti-Tax antibody. Coprecipitated proteins were detected with a monoclonal anti-cyclin D3 antibody and a polyclonal anti-CDK4 antibodies. Precipitated proteins are shown on the left. Corresponding lysate controls are shown on the right.
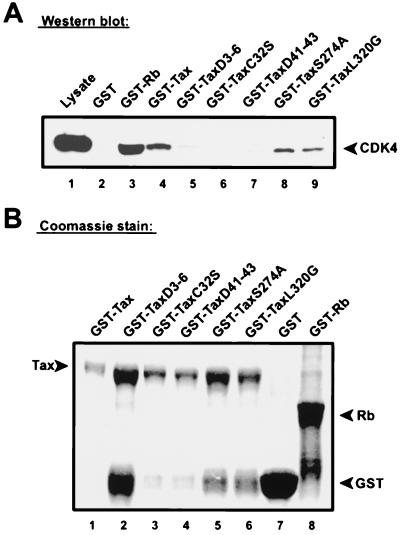
As further verification, pull-down assays were applied for the definition of the CDK4-binding Tax domain (Fig. 8). GST-Tax (Fig. 8A, lane 4) and five different GST-Tax mutants (Fig. 8A, lanes 5 to 9) were used separately to probe Jurkat T-cell lysates containing physiological amounts of cell cycle regulatory proteins, including CDK4 and cyclin. In parallel, GST alone (Fig. 8A, lane 2) and GST-Rb (Fig. 8A, lane 3) served as negative and positive controls. After equilibration with lysates, each column was extensively washed and bound proteins were eluted. Column-bound CDK4 was assayed by Western blotting of eluates with anti-CDK4 antibodies. Interestingly, CDK4 failed to bind to three Tax variants with mutations in the N terminus (TaxΔ3-6, TaxC23S, and TaxΔ41-43) but bound well to the two Tax mutants with changes in the C terminus (TaxS274A and TaxL320G). The minor differences in the amounts of proteins detected with Coomassie blue-stained protein electrophoresis gels (Fig. 8B) cannot explain the phenotypic differences between wild-type Tax and N-terminal and C-terminal mutants. For instance, although wild-type Tax was used at the lowest abundance, it resulted in a high yield of CDK4 binding. In contrast, mutant TaxΔ3-6, which was expressed at a relatively high level, did not bind any CDK4. No differences in amounts of proteins were detectable between the binding-deficient N-terminal mutant C23S and the binding-competent C-terminal mutant L320G. This result does fully agree with a requirement for an intact Tax N terminus for binding to CDK4, even in the presence of competing T-cell proteins at physiological concentrations.
FIG. 8.
N-terminal Tax mutants do not bind CDK4 from Jurkat cell lysates. (A) CDK4 binds to Tax mutated in the C terminus but not to counterpart proteins mutated in the N terminus. Jurkat cell lysates were equilibrated with columns saturated with GST alone (negative control, lane 2), GST-Rb (positive control, lane 3), GST-Tax (lane 4), or several GST-Tax mutants (lanes 5 to 9). Proteins retained on columns were eluted and analyzed by immunoblotting with anti-CDK4 antibodies. (B) Visualization of Tax mutant and control proteins used to construct the GST columns. GST alone, GST-Rb, GST-Tax, and GST-Tax mutant proteins were purified and electrophoresed on a denaturing SDS gel, followed by Coomassie blue staining.
Stimulation of CDK4 correlates with direct binding by Tax.
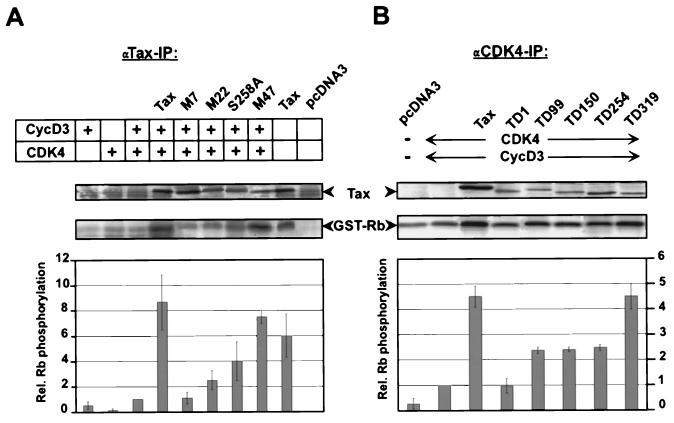
To investigate whether Tax binding was required for the stimulation of CDK4/cyclin D kinase, we tested the ability of binding-defective Tax mutants to activate CDK4 (Fig. 9). In vitro kinase assays were performed after transfection of 293T cells individually with wild-type, point mutant, and deletion mutant Tax expression vectors followed by precipitation of Tax-associated CDK4 complexes with a monoclonal anti-Tax antibody (Fig. 9A). High GST-Rb kinase activities were found associated with wild-type Tax and C-terminal Tax mutants (MycS258A and MycM47), while low kinase activities were recovered with Tax proteins mutated in the N terminus (MycM7 and MycM22). Interestingly, the anti-Tax antibody could precipitate CDK activity even in the absence of transfected expression plasmids for cyclins and/or CDKs. This result suggests that the interactions are efficient in 293T cells and that Tax binds well to the relatively low levels of endogenous CDK4/cyclin complexes. Overall, the amount of phosphorylated GST-Rb correlated well with the degree of Tax protein binding to CDK4 observed in coimmunoprecipitations (Fig. 7C). Similar levels of expression were confirmed for all Tax mutants by Western blotting analysis of the cell lysates used for the kinase assays.
FIG. 9.
N-terminal mutations affect the capacity of Tax to enhance kinase activity. 293T cells were cotransfected with expression constructs for cyclin D3, CDK4, and different Tax mutants as described in the legend to Fig. 7. The CDK/Tax complexes were precipitated and subjected to kinase assays with GST-Rb as a substrate. (A) Association of CDK activity with Tax point mutants. Tax and associated proteins were precipitated with monoclonal anti-Tax antibodies. The upper panels show a representative autoradiograph of phosphorylated pRb. The results of three individual experiments were quantified; the lower panel shows the mean and standard deviation. (B) Stimulation of CDK4 kinase activity by Tax deletion mutants. Tax/CDK complexes were precipitated with an anti-CDK4 antibody. The upper panels show Rb phosphorylation in a representative autoradiograph. The lower panel shows the relative (Rel.) kinase activities quantified by phosphorimaging. IP, immunoprecipitation. Error bars show standard deviations. The upper panels of panels A and B also show Western blot analysis with a rabbit anti-Tax serum verifying that equal amounts of Tax wild type and point mutants were expressed.
In a complementary set of experiments, we transfected cells with Tax or Tax deletion mutants and then immunoprecipitated proteins by using an excess of anti-CDK4 serum. These experiments monitored the overall level of CDK4 activity within transfected cells. Here, the induction of kinase activity (Fig. 9B) again correlated with the ability of Tax or Tax mutants to bind the cyclin D/CDK4 complex (Fig. 7B). In particular, the binding-deficient N-terminal deletion mutant TD1 did not stimulate kinase activity compared to the negative control (cyclin D3 and CDK4) (Fig. 9B). In contrast, Tax mutants with internal deletions (TD99, TD150, and TD254), which maintain, albeit at reduced affinity, binding to the CDK4/cyclin D complex, commensurately stimulated kinase activity. The C-terminal deletion mutant TD319, which strongly binds the cyclin D/CDK4 complex, activated this kinase complex nearly as efficiently as wild-type Tax. In summary, these results correlate direct protein-protein contact between Tax and the cyclin D/CDK4 complex with the stimulatory effect of the former on kinase activity.
Tax stimulates the association of CDK4 and cyclin D and counteracts the inhibitory activity of p21CIP1.
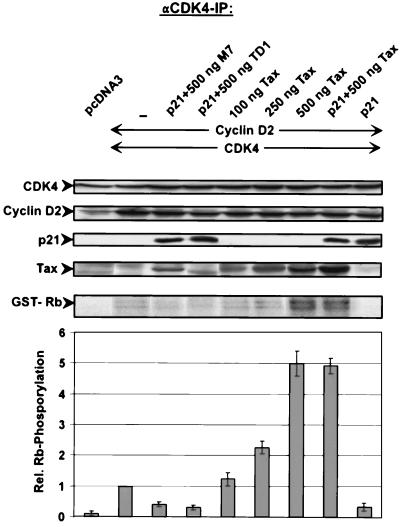
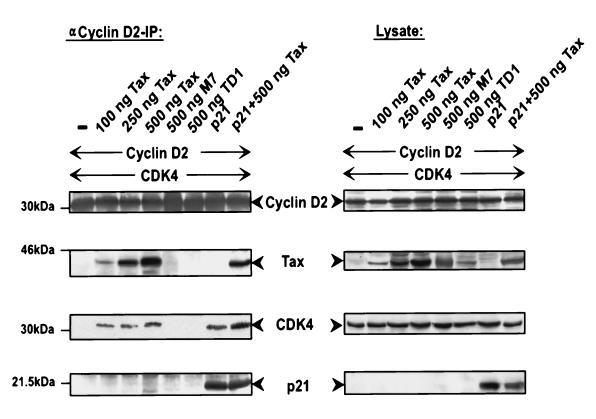
In HTLV-1-infected cells, the p21CIP1 repressor of CDK is markedly upregulated (2, 8). To address the question of whether Tax can stimulate CDK4 even in the presence of the inhibitor, we determined Tax-mediated stimulation in the presence of large amounts of p21 in transfected 293T cells (Fig. 10). CDK4, cyclin D2, p21CIP1, Tax or, as negative controls, the binding-deficient Tax mutants M7 and TD1 were coexpressed in various combinations. CDK4 activity was determined. The results showed that even in the presence of p21CIP1, Tax was able to stimulate Rb kinase activity (Fig. 10, compare eighth and ninth lanes). The level of CDK4 activity was the same as the activity measured without the inhibitor (Fig. 10, compare seventh and eighth lanes). The CDK4-repressing activity of p21CIP1 could be demonstrated (Fig. 10, compare second and ninth lanes). The ability to counteract the p21 repressive activity was dependent on the capacity of Tax to bind CDK4, since the binding-deficient mutants could not stimulate CDK4 activity (Fig. 10, third and fourth lanes). In summary, this experiment showed that p21 bound to Tax-associated CDK/cyclin complexes is inefficient in repressing kinase activity. This finding provides an explanation of how Tax can stimulate CDK activation in the presence of large amounts of p21CIP1. This finding also explains the Rb kinase activity of CDK4/cyclin D2/Tax/p21CIP1 complexes isolated from HTLV-1-infected cells.
FIG. 10.
Tax counteracts the inhibition of CDK activity by p21CIP1 through binding to the CDK complex. For immune complex kinase assays, various amounts of Tax and binding-deficient Tax mutants were coexpressed with cyclin D2, CDK4, and p21CIP1 in 293T cells. CDK4 complexes were precipitated, and kinase activity was assessed in vitro with pRb as a substrate. The upper panels show the levels of expression of the relevant proteins in the lysates used for immunoprecipitation (IP), the GST-Rb panel shows pRb substrate phosphorylation (autoradiograph), and the lower panel shows the relative (Rel.) CDK4 activities in three different experiments (mean and standard deviation).
The capacity of Tax to directly bind both CDK4 and cyclin D resembles that of p21CIP1. Since the latter protein has been described to stimulate the association of CDK and cyclin, we considered the possibility that Tax could do the same. To investigate this hypothesis, we performed coimmunoprecipitation experiments with cyclin D2 antibodies (Fig. 11). The experiments showed that the presence of Tax drastically increased the amounts of CDK4 molecules coprecipitated with cyclin D2. The effect was comparable to that of p21CIP1 and was dependent on the dose of Tax and its capacity to bind CDK. Binding-deficient mutants (M7 and TD1) were not able to mediate this effect. Since the association of CDK and cyclin is crucial for kinase activity, the observed Tax-mediated enhancement of CDK/cyclin complex formation may explain at least in part the stimulation of CDK activity.
FIG. 11.
Tax promotes the assembly of CDK4/cyclin D complexes in fibroblasts. 293T cells were cotransfected with 500 ng of expression constructs for cyclin D2, CDK4, and p21CIP1 and various amounts of Tax expression plasmids. (Left panels) Cyclin D2-associated complexes were precipitated with a specific polyclonal antiserum from a cell lysate containing 700 μg of protein. The compositions of the complexes were analyzed by immunoblotting. IP, immunoprecipitation. (Right panels) Western blots of the corresponding lysate controls.
DISCUSSION
The Tax oncoprotein, an essential growth factor for HTLV-1-infected lymphocytes, can stimulate the G1- to S-phase progression through the activation of CDK4 (42). Here, we have expanded upon that observation by showing that the N terminus of Tax specifically contacts an active cyclin D/CDK4 complex. Using binding-defective mutants, we showed that direct binding of Tax correlates with the ability of this oncoprotein to activate cyclin D/CDK4. Furthermore, our experiments demonstrated the resistance of Tax-bound CDK4 complexes to the inhibitor p21CIP1 and suggested that Tax stimulates, by directly binding cyclin and CDK, the assembly of active complexes, thus providing a mechanistic explanation of Tax-mediated CDK activation.
Tax binds CDK4 and CDK6 (18) but neither CDK2 nor CDK1, suggesting preferential affinity and specificity for the former set of cyclin-dependent kinases. The CDK4 and CDK6 kinases are closely related (∼70%) (29) and have similar functions in the G1 phase of the cell cycle. Therefore, one might deduce that Tax binds to a region conserved between CDK4 and CDK6 (although we have yet to directly investigate this point). Our binding experiments with mutated Tax proteins did reveal that association with CDK4 is mediated by the N terminus of Tax. The N-terminal domain contains a putative Zn finger motif (43), which has been suggested to play a role in protein-protein interactions.
We also found Tax associated with cyclin D3 and cyclin D2. These findings reaffirm earlier observations that Tax can bind cyclins D3 and D1 (34). The domain required for cyclin binding also was located in the N terminus of Tax. This finding implies that the binding sites within Tax for CDK4, CDK6, and D cyclins are adjacent. The possibility that binding to one of them mediates an indirect interaction with the other can be ruled out, since Tax directly contacts in vitro each of the proteins in the absence of the other. In addition, the following observations corroborate the idea that the binding of Tax to CDK4 is not mediated by a bridging function of endogenous cyclin D: (i) abundant coexpression of transfected Tax and CDK4 resulted in a high degree of binding, despite a low level of endogenous cyclin D in the cells, and (ii) the resulting immunoprecipitated CDK/Tax complexes were inactive, indicating the probable absence of the cyclin component. On the other hand, in transient overexpression settings and in HTLV-1-infected cells, Tax could be precipitated simultaneously with both the kinase and the cyclin. One interpretation of these findings is that Tax can indeed sufficiently bind CDK4 and CDK6 kinases; however, such binding in the absence of associated cyclins results in an ineffective interaction. In contrast, the binding of Tax to a CDK4/cyclin complex results in activation of the latter. The in vitro binding assays suggested that within a complex of Tax, CDK4 or CDK6, and cyclin D, Tax could provide independent contact sites for both CDK4 or CDK6 and cyclin D. Furthermore, because the Tax-bound cyclin D/CDK complex potently activates Rb phosphorylation, one can conclude that Tax binding does not interfere with the binding of CDK to cyclin. Moreover, as coimmunopreciptations have shown, Tax seems to stimulate the association of CDK with cyclin. Hence, we favor the idea that two closely proximal binding sites exist within the N terminus of Tax for cyclin D and CDK4 or CDK6. Such adjacent binding sites are not without precedent. For instance, separate binding sites for cyclin and CDK sequences lie in close proximity in p21CIP1 and p27KIP, wherein a relatively short N-terminal peptide of about 70 amino acids is sufficient to form a stable interaction with both components of the CDK complex (31, 32). Like Tax, p21CIP1 has been reported to stimulate the association of CDK and cyclin (26).
We do not favor the idea that a ternary complex of Tax, CDK4 or CDK6, and cyclin D might be mediated through surreptitious Tax binding to p16INK-4A (28, 48) for the following reasons. First, since both p16INK-4A and cyclin bind to the same site within CDK4 (10), p16INK-4A binding to CDK is known to interfere with CDK binding to cyclin. Second, we have observed that a CDK mutant that is not repressible by p16INK-4A can be stimulated by Tax (data not shown). Finally, the Tax region relevant for binding CDK4/cyclin D tightly correlates with the same region needed for CDK activation. This observation is most readily explained by Tax activation of the kinase through direct contact with the holoenzyme complex. The requirement of the CDK4 interaction for Tax-mediated transformation is suggested by the observation that the binding-deficient Tax mutant M7 is incapable of transforming T cells (39). The capacity of mutant M47 to transform correlates well with its potential to bind and activate CDK4 (38).
While it has been reported that Tax stimulates the cyclin D2 promoter (2, 21, 41) when attached to a reporter gene, the intracellular impact of this observation on cyclin D2 protein expression is difficult to detect in human lymphocytes conditionally immortalized by Tax (42). It is possible that the upregulation may vary from cell type to cell type and that it has an additional stimulatory effect in some cell lines by increasing the amounts of CDK4/cyclin D2 complexes which can be activated by Tax. In Tax-transformed human lymphocytes, the levels of none of the other major regulatory proteins involved in G1 control (CDK4, CDK6, cyclin D3, and p16INK-4A) are strongly affected by the presence or absence of Tax (42). Thus, in view of our current results and suggestions raised elsewhere (34), we propose that protein-protein posttranslational effects rather than transcriptional modulatory roles of Tax in gene expression play the predominant physiological role in CDK activation. A mechanism explaining how an association of Tax with the CDK4 complex could affect kinase function is the possible stimulation of CDK and cyclin assembly. In addition, Tax acting as a shuttle protein (6) could bind to cytoplasmic CDK/cyclin complexes and mediate their nuclear transport. The presence of Tax in the complexes overcomes the inhibitory effect of large amounts of the p21CIP protein.
In addition to HTLV-1, other viruses also developed different mechanisms to activate cyclin/CDK, for instance, the E1A protein of adenovirus, the large T antigen of SV40, and the E7 protein of human papillomavirus type 16 (22). Notably, E7 and E1A interact with cyclin E/CDK2 complexes. Additionally, some transforming herpesviruses even encode viral homologues of cellular D cyclins which form stable complexes with CDK4 and CDK6. In contrast to their cellular counterparts, these viral cyclins are completely resistant to inhibition by CKIs (25, 50). Interestingly, the capacity of DNA tumor viruses to stimulate the G1- to S-phase progression is frequently complemented by a p53-inactivating function (22). Similarly, HTLV-1 Tax was shown here to promote the activation of CDK/cyclins important for the G1-to-S phase transition and has been shown elsewhere to inactivate p53 function as well (36, 51). Together, these independent effects serve to subvert G1 arrest and the induction of apoptosis as a response to genotoxic damage. This strategy appears to be shared by many diverse viruses and potentially contributes to the oncogenic nature of these viruses.
Acknowledgments
This work was supported by DFG (SFB 466, TP-C3) and the Wilhelm-Sander Foundation.
We thank Brigitte Biesinger, Sabine Lang, and Tobias Ruckes for helpful discussions. The technical assistance of Domenica Saul is appreciated.
REFERENCES
- 1.Akagi, T., and K. Shimotono. 1993. Proliferative response of Tax 1-transduced primary human T cells to anti-CD3 antibody stimulation by an interleukin-2-independent pathway. J. Virol. 67:1211-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akagi, T., H. Ono, and K. Shimotohno. 1996. Expression of cell-cycle regulatory genes in HTLV-I infected T-cell lines: possible involvement of Tax1 in the altered expression of cyclin D2, p18Ink4 and p21Waf1/Cip1/Sid1. Oncogene 12:1645-1652. [PubMed] [Google Scholar]
- 3.Alt, M., B. Fleckenstein, and R. Grassmann. 1991. A pair of selectable herpesvirus vectors for simultaneous gene expression in human lymphoid cells. Gene 102:265-269. [DOI] [PubMed] [Google Scholar]
- 4.Arnold, A., H. G. Kim, R. D. Gaz, R. L. Eddy, Y. Fukushima, M. G. Byers, T. B. Shows, and H. M. Kronenberg. 1989. Molecular cloning and chromosomal mapping of DNA rearranged with the parathyroid hormone gene in a parathyroid adenoma. J. Clin. Investig. 83:2034-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braunweiler, A., J. E. Garrus, J. C. Reed, and J. K. Nyborg. 1997. Repression of bax gene expression by the HTLV-1 Tax protein: implications for suppression of apoptosis in virally infected cells. Virology 231:135-140. [DOI] [PubMed] [Google Scholar]
- 6.Burton, M., C. D. Upadhyaya, B. Maier, T. J. Hope, and O. J. Semmes. 2000. Human T-cell leukemia virus type 1 Tax shuttles between functionally discrete subcellular targets. J. Virol. 74:2351-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavrois, M., I. Leclercq, O. Gout, A. Gessain, S. Wain-Hobson, and E. Wattel. 1998. Persistent oligoclonal expansion of human T-cell leukemia virus type 1-infected circulating cells in patients with tropical spastic paraparesis/HTLV-1 associated myelopathy. Oncogene 17:77-82. [DOI] [PubMed] [Google Scholar]
- 8.Cereseto, A., F. Diella, J. C. Mulloy, A. Cara, P. Michieli, R. Grassmann, G. Franchini, and, M. E. Klotman.1996. p53 functional impairment and high p21waf1/cip1 expression in human T-cell lymphotropic/leukemia virus type I-transformed T-cells. Blood 88:1551-1560. [PubMed] [Google Scholar]
- 9.Cereseto, A., R. Washington Parks, E. Rivadeneira, and G. Fanchini. 1999. Limiting amounts of p27Kip1 correlates with constitutive activation of cyclin E-CDK2 complex in HTLV-I-transformed T-cells. Oncogene 18:2441-2450. [DOI] [PubMed] [Google Scholar]
- 10.Coleman, K. G., B. S. Waulet, D. Morrissey, J. Mulehorn, S. A. Sedman, P. Brinkley, S. Price, and K. R. Webster. 1997. Identification of CDK4 sequences involved in cyclin D1 and p16 binding. J. Biol. Chem. 272:18869-18874. [DOI] [PubMed] [Google Scholar]
- 11.Fuente, C., F. Santiago, S. Y. Chong, L. Deng, T. Mayhood, P. Fu, D. Stein, T. Denny, F. Coffman, N. Azimi, R. Mahieux, and F. Kashanchi. 2000. Overexpression of p21waf1 in human T-cell lymphotropic virus type 1-infected cells and its association with cyclin A/cdk2. J. Virol. 74:7270-7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabet, A. S., F. Mortreux, A. Talarmin, Y. Plumelle, I. Leclercq, A. Leroy, A. Gessain, E. Clity, M. Joubert, and E. Wattel. 2000. High circulating proviral load with oligoclonal expansion of HTLV-1 bearing T cells in HTLV-1 carriers with strongyloidiasis. Oncogene 19:4954-4960. [DOI] [PubMed] [Google Scholar]
- 13.Grassmann, R., C. Dengler, I. Mueller Fleckenstein, B. Fleckenstein, K. McGuire, M. C. Dokhelar, J. G. Sodroski, and A. W. Haseltine. 1989. Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type I X-region genes transduced by herpesvirus saimiri vector. Proc. Natl. Acad. Sci. USA 86:3351-3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grassmann, R., and B. Fleckenstein. 1989. Selectable recombinant herpesvirus saimiri is capable of persisting in a human T-cell line. J. Virol. 63:1818-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grassmann, R., S. Berchtold, I. Radant, M. Alt, B. Fleckenstein, J. G. Sodroski, W. A. Haseltine, and U. Ramstedt. 1992. Role of human T-cell leukemia virus type 1 X region proteins in immortalization of primary human lymphocytes in culture. J. Virol. 66:4570-4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grassmann, R., B. Fleckenstein, and R. C. Desrosiers. 1994. Viral transformation of human T-lymphocytes. Adv. Cancer Res. 63:211-243. [DOI] [PubMed] [Google Scholar]
- 17.Grossman, W., J. T. Kimata, F. H. Wong, M. Zutter, and L. Ratner. 1995. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA 92:1057-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haller, K., T. Ruckes, I. Schmitt, D. Saul, E. Derow, and R. Grassmann. 2000. Tax-dependent stimulation of G1 phase-specific cyclin-dependent kinases and increased expression of signal transduction genes characterize HTLV type 1-transformed T cells. AIDS Res. Hum. Retrovir. 16:1683-1688. [DOI] [PubMed] [Google Scholar]
- 19.Hanon, E., S. Hall, G. P. Taylor, M. Saito, R. Davis, Y. Tanaka, K. Usuku, M. Osame, J. N. Weber, and C. R. Bangham. 2000. Abundant tax protein expression in CD4+T cells infected with human T-cell lymphotropic virus type I (HTLV-I) is prevented by cytotoxic T lymphocytes. Blood 95:1386-1392. [PubMed] [Google Scholar]
- 20.Hanon, E., R. E. Asquith, G. P. Taylor, Y. Tanaka, J. N. Weber, and C. R. Bangham. 2000. High frequency of viral protein expression in human T cell lymphotropic virus type 1-infected peripheral blood mononuclear cells. AIDS Res. Hum. Retrovir. 16:1711-1715. [DOI] [PubMed] [Google Scholar]
- 21.Huang, J., K. Ohtani, R. Iwanaga, Y. Matsumura, and M. Nakamura. 2001. Direct trans-activation of the human cyclin D2 gene by the oncogene product Tax of human T-cell leukemia virus type I. Oncogene 20:1094-1102. [DOI] [PubMed] [Google Scholar]
- 22.Jansen-Dürr, P. 1996. How viral oncogenes make the cell cycle. Trends Genet. 12:270-275. [DOI] [PubMed] [Google Scholar]
- 23.Jeang, K. T., S. G. Widen, O. J. Semmes, and S. H. Wilson. 1990. HTLV-I transactivator protein, tax, is a trans-repressor of the human beta-polymerase gene. Science 274:1082-1084. [DOI] [PubMed] [Google Scholar]
- 24.Jin, D. Y., F. Spencer, and K. T. Jeang. 1998. Human T cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell 93:81-91. [DOI] [PubMed] [Google Scholar]
- 25.Jung, J. U., M. Stäger, and R. C. Desrosiers. 1994. Virus-encoded cyclin. Mol. Cell. Biol. 14:7235-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaBaer, J., M. D. Garrett, A. Stevenson, J. M. Slingerland, C. Sandhu, H. S. Chou, A. Fattaey, and E. Harlow. 1997. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11:847-862. [DOI] [PubMed] [Google Scholar]
- 27.Lemasson, I., S. Thebault, C. Sardet, C. Devaux, and J.-M. Mesnard. 1998. Activation of E2F-mediated transcription by human T-cell leukemia virus type I Tax protein in a p16 INK4A-negative T-cell line. J. Biol. Chem. 273:23598-23608. [DOI] [PubMed] [Google Scholar]
- 28.Low, K. G., L. F. Dorner, D. B. Fernando, J. Grossman, K. T. Jeang, and M. J. Comb. 1997. Human T-cell leukemia virus type 1 Tax releases cell cycle arrest induced by p16INK4A. J. Virol. 71:1956-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyerson, M., G. H. Enders, C.-L. Wu, L.-K. Su, C. Gorka, C. Nelson, E. Harlow, and L.-H. Tsai. 1992. A family of human cdc2-related protein kinases. EMBO J. 11:2909-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Modiano, J. F., J. Mayor, C. Ball, M. K. Fuentes, and D. S. Linthicum. 2000. CDK4 expression and activity are required for cytokine responsiveness in T cells. J. Immunol. 165:6693-6702. [DOI] [PubMed] [Google Scholar]
- 31.Morgan, D. O. 1995. Principles of CDK regulation. Nature 374:131-134. [DOI] [PubMed] [Google Scholar]
- 32.Morgan, D. O. 1997. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu. Rev. Cell Dev. Biol. 13:261-291. [DOI] [PubMed] [Google Scholar]
- 33.Mulloy, J. C., T. Kislyakova, A. Cereseto, L. Casareto, A. LoMonico, J. Fullen, M. V. Lorenzi, A. Cara, C. Nicot, C. Giam, and G. Franchini. 1998. Human T-cell lymphotropic/leukemia virus type 1 Tax abrogates p53-induced cell cycle arrest and apoptosis through its CREB/ATF functional domain. J. Virol. 72:8852-8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neuveut, C., K. G. Low, F. Maldarelli, I. Schmitt, F. Majone, R. Grassmann, and K.-T. Jeang. 1998. Human T-cell leukemia virus type 1 Tax and cell cycle progression: role of cyclin D-cdk and p110Rb. Mol. Cell. Biol. 18:3620-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohtani, K., Ritsuko, I., Masaaki, A., Yongping, H., Yuuki, M., and M. Nakamura. 2000. Cell type-specific E2F activation and cell cycle progression induced by the oncogene product Tax of human T-cell leukemia virus type I. J. Biol. Chem. 275:11154-11163. [DOI] [PubMed] [Google Scholar]
- 36.Pise-Masison, C. A., R. Mahieux, H. Jiang, M. Ashcroft, M. Radonovich, J. Duvall, C. Guillerm, and, J. N. Brady. 2000. Inactivation of p53 by human T-cell lymphotropic virus type 1 Tax requires activation of the NF-κB pathway and is dependent on p53 phosphorylation. Mol. Cell. Biol. 20:3377-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pont-Kingdon, G. Creation of chimeric junctions, deletions, and insertions by PCR. Methods Mol. Biol. 67:167-172. [DOI] [PubMed]
- 38.Robek, M. D., and L. Ratner. 2000. Immortalization of T lymphocytes by human T-cell leukemia virus type 1 is independent of the Tax-CBP/p300 interaction. J. Virol. 74:11988-11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosin, O., C. Koch, I. Schmitt, O. J. Semmes, K. T. Jeang, and R. Grassmann. 1998. A human T-cell leukemia virus Tax variant incapable of activating NF-kappaB retains its immortalizing potential for primary T-lymphocytes. J. Biol. Chem. 273:6698-6703. [DOI] [PubMed] [Google Scholar]
- 40.Ruckes, T., D. Saul, J. Van Snick, O. Hermine, and R. Grassmann. 2001. Autocrine antiapoptotic stimulation of cultured adult T-cell leukemia cells by overexpression of the chemokine I-309. Blood 98:1150-1159. [DOI] [PubMed] [Google Scholar]
- 41.Santiago, F., E. Clark, S. Chong, C. Molina, F. Mozafari, R. Mahieux, M. Fujii, N. Azimi, and F. Kashanchi. 1999. Transcriptional up-regulation of the cyclin D2 gene and acquisition of new cyclin-dependent kinase partners in human T-cell leukemia virus type 1-infected cells. J. Virol. 73:9917-9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitt, I., O. Rosin, P. Rohwer, M. Gossen, and R. Grassmann. 1998. Stimulation of cyclin-dependent kinase activity and G1- to S-phase transition in human lymphocytes by the human T-cell leukemia/lymphotropic virus type 1 Tax protein. J. Virol. 72:633-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Semmes, O. J., and K. T. Jeang. 1992. HTLV-I Tax is a zinc-binding protein: role of zinc in Tax structure and function. Virology 188:754-764. [DOI] [PubMed] [Google Scholar]
- 44.Semmes, O. J., and K.-T. Jeang. 1992. Mutational analysis of human T-cell leukemia virus type I Tax: regions necessary for function determined with 47 mutant proteins. J. Virol. 66:7183-7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sherr, C. J. 1994. G1 phase progression: cycling on cue. Cell 79:551-555. [DOI] [PubMed] [Google Scholar]
- 46.Sherr, C. J. 1995. D-type cyclins. Trends Biochem. Sci. 20:187-190. [DOI] [PubMed] [Google Scholar]
- 47.Smith, M. R., and W. C. Greene. 1990. Identification of HTLV-I tax transactivator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 4:1875-1885. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki, T., S. Kitao, H. Matsushime, and M. Yoshida. 1996. HTLV-1 Tax protein interacts with cyclin-dependent kinase inhibitor p16INK4A and counteracts its inhibitory activity towards CDK4. EMBO J. 15:1607-1614. [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki, T., T. Narita, M. Uchida-Toita, and M. Yoshida. 1999. Down-regulation of the INK4 family of cyclin-dependent kinase inhibitors by tax protein of HTLV-1 through two distinct mechanisms. Virology 259:384-391. [DOI] [PubMed] [Google Scholar]
- 50.Swanton, C., D. J. Mann, B. Fleckenstein, F. Neipel, G. Peters, and N. Jones. 1997. Herpes viral cyclin/Cdk6 complexes evade inhibition by CDK inhibitor proteins. Nature 390:184-187. [DOI] [PubMed] [Google Scholar]
- 51.Van, P. L., K.-W. Yim, D.-Y. Jin, G. Dapolito, A. Kurimasa, and K.-T. Jeang. 2001. Genetic evidence of a role for ATM in functional interaction between human T-cell leukemia virus type 1 Tax and p53. J. Virol. 75:396-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshida, M., T. Suzuki, J. Fujisawa, and H. Hirai. 1995. HTLV-1 oncoprotein tax and cellular transcription factors. Curr. Top. Microbiol. Immunol. 193:79-89. [DOI] [PubMed] [Google Scholar]