Abstract
The family of cytoplasmic Janus (Jak) tyrosine kinases plays an essential role in cytokine signal transduction, regulating cell survival and gene expression. Ligand-induced receptor dimerization results in phosphorylation of Jak2 on activation loop tyrosine Y1007 and stimulation of its catalytic activity, which, in turn, results in activation of several downstream signaling cascades. Recently, the catalytic activity of Jak2 has been found to be subject to negative regulation through various mechanisms including association with SOCS proteins. Here we show that the ubiquitin-dependent proteolysis pathway is involved in the regulation of the turnover of activated Jak2. In unstimulated cells Jak2 was monoubiquitinated, and interleukin-3 or gamma interferon stimulation induced polyubiquitination of Jak2. The polyubiquitinated Jak2 was rapidly degraded through proteasomes. By using different Jak2 mutants we show that tyrosine-phosphorylated Jak2 is preferentially polyubiquitinated and degraded. Furthermore, phosphorylation of Y1007 on Jak2 was required for proteasomal degradation and for SOCS-1-mediated downregulation of Jak2. The proteasome inhibitor treatment stabilized the Jak2-SOCS-1 protein complex and inhibited the proteolysis of Jak2. In summary, these results indicate that the ubiquitin-proteasome pathway negatively regulates tyrosine-phosphorylated Jak2 in cytokine receptor signaling, which provides an additional mechanism to control activation of Jak2 and maintain cellular homeostasis.
Most cytokines that regulate the growth and differentiation of immune and hematopoietic cells function through transmembrane receptors belonging to the cytokine receptor superfamily (19, 44). The binding of cytokines to their cognate receptors leads to dimerization or oligomerization of the receptor chains and activation of the receptor-associated Janus (Jak) family of tyrosine kinases. Jak kinases mediate essential and nonredundant functions in cytokine signaling, and individual Jaks are selectively activated by various cytokine receptors. For example, Jak2 is required for erythropoietin, interleukin-3 (IL-3), and gamma interferon (IFN-γ) signal transduction (39, 45). Jaks associate with the membrane-proximal regions of cytokine receptors, and ligand-induced aggregation of the receptor chains allows auto- and transphosphorylation of Jaks on critical tyrosine residues within the activation loop of the kinase domain. Activation of Jaks results in phosphorylation of a number of signaling proteins, such as the signal transducers and activators of transcription (STATs), phosphatidylinositol 3-kinase, and Shc, and leads to activation of intracellular signaling pathways and expression of target genes.
Regulation of Jak activity is a critical point in the modulation of cytokine responses, and recently several mechanisms for regulating Jak activation have been described. Intramolecular interactions control the activity of the tyrosine kinase domain in Jak kinases (41, 53). The SH2 domain-containing tyrosine phosphatases SHP-1 and SHP-2 have been shown to have both stimulatory and inhibitory effects on cytokine receptor signaling (23). An important mechanism for negative regulation of cytokine signaling is mediated through members of the recently identified SOCS (suppressor of cytokine signaling) family of proteins (2, 5). The SOCS family consists of eight members that have highly specialized functions in regulation of cytokine signaling. One of the family members, SOCS-1, also termed JAB (Jak binding protein) or SSI-1 (STAT-induced STAT inhibitor 1), was identified through its ability to inhibit IL-6 signal transduction and bind to Jak2 (11, 34, 46). SOCS-1 has been shown to have a crucial function in regulation of Jak2 activation, IFN-γ responses, and T-cell differentiation (1, 3, 30).
The SOCS proteins contain a central SH2 domain, which interacts either with the autophosphorylation site tyrosines in Jaks or with the phosphorylated tyrosine residues in cytokine receptors (36, 40). The hallmark of the SOCS family is a C-terminal homology domain referred to as the SOCS box, which has been found in a large number of proteins: WD-40 repeat-containing proteins, SPRY domain-containing proteins, ankyrin repeat-containing proteins, and small GTPases (18). The SOCS boxes of SOCS-1 and SOCS-3 were found to mediate interaction with the elongin B/C complex, and the SOCS box contains a conserved elongin B/C binding motif (BC box) in the N terminus (57). The elongin B/C complex was initially identified as a component of the multiprotein von Hippel-Lindau tumor suppressor E3 ligase complex, which also contains RING finger protein Rbx1 as a bridging factor and Cullin-2 (10, 22). The interaction between the SOCS box and elongins B and C implicates the ubiquitin-proteasome pathway in regulation of SOCS function and protein turnover. SOCS-3 is rapidly degraded through the proteasome pathway, suggesting that the interaction with elongins B and C directs the multiprotein complex to proteasomes for degradation (57). However, for SOCS-1, the SOCS box and interaction with elongins B and C is reported to inhibit degradation of SOCS-1 (21, 35). The possible role of the SOCS-elongin B/C complex in regulation of Jak kinases in cytokine signaling is currently not known.
The ubiquitin-proteasome pathway mediates specific degradation of regulatory proteins and plays an important role in controlling a variety of cellular functions such as DNA repair, cell cycle control, antigen presentation, intracellular translocation of proteins and apoptosis (6). Conjugation of ubiquitin to the substrate proceeds in three distinctive steps: first, ubiquitin is activated by the ubiquitin-activating enzyme (E1) in an ATP-dependent reaction; second, activated ubiquitin is transferred onto a ubiquitin carrier protein (E2); third, the ubiquitin moieties are transferred onto a ubiquitin ligase (E3), which catalyzes the covalent modification of lysine residues on the substrate with the ATP-activated ubiquitin. Polyubiquitinated proteins are then recognized by the 19S complex of the 26S proteasomes and degraded into short peptides with ubiquitin recycled via the action of isopeptidases (48).
Several lines of evidence have also implicated the ubiquitin-proteasome pathway in regulation of cytokine receptor signaling. Various components of the ubiquitin pathway are regulated by cytokine-induced signals. For example, IFNs have been shown to induce the expression of the UbcH family of ubiquitin-conjugating enzymes, and different isozymes of deubiquitinating enzymes are induced by cytokines in a Jak-dependent manner (8, 37). Proteasome inhibitors have been shown to prolong activation of the Jak/STAT pathway in response to IL-2, IL-3, growth hormone (GH), ciliary neurotrophic factor, IFN, and erythropoietin stimulations (4, 14, 24, 29, 49, 50, 56). The underlying molecular mechanisms by which this occurs are still, however, poorly understood. The tyrosine-phosphorylated forms of STAT4, STAT5, and STAT6, but not other STATs, have been shown to be stabilized by proteasome inhibitors, but ubiquitination of these proteins was not detected (51). Cell type-dependent differences, for example, in expression of critical regulatory proteins, may also account for the specificity of the ubiquitin-proteasome pathway. For example, STAT1 becomes ubiquitinated after IFN-γ stimulation in HeLa cells (24), and tyrosine-phosphorylated Stat1 has been found to be stabilized by proteasome inhibitors in fibroblasts without any indication of ubiquitination (16). In addition, certain cytokine receptors such as EpoR, GHR, IL-2Rβ, and IL-9R have been shown to become ubiquitinated, and proteasomes control the turnover of these receptors (50).
Here we present evidence that the ubiquitin-proteasome pathway directly regulates the activated form of Jak2. We show that Jak2 is ubiquitinated in vivo and in vitro and that IL-3 and IFN-γ stimulation increases the accumulation of polyubiquitinated forms of Jak2 that are stabilized by proteasome inhibitor treatment. Tyrosine phosphorylation was found to be a requirement for efficient ubiquitination and proteasomal degradation of Jak2. These findings led us to investigate the role of SOCS proteins in this regulation, and proteasome inhibitors were found to stabilize the interaction between Jak2 and SOCS-1. Expression of SOCS-1 enhanced proteasome-dependent degradation of the activated form of Jak2. Importantly, ubiquitination and protein levels of Jak2YF, a Jak2 mutant with a Y1007F mutation that fails to interact with SOCS-1, were not affected by coexpression of SOCS-1. Thus, the Jak2 protein is a target for ubiquitin-proteasome-mediated regulation of cytokine signaling, in which tyrosine phosphorylation of Jak2 and the interaction with SOCS-1 function as regulatory mechanisms.
MATERIALS AND METHODS
Antibodies and reagents.
The following antibodies were used: antiphosphotyrosine clone 4G10 (Upstate Biotechnology, Lake Placid, N.Y.), a polyclonal anti-Jak2 antibody, kind gift from J. N. Ihle (45), an anti-influenza virus hemagglutinin (HA) epitope antibody (clone 16B12; Berkeley-Antibody, Richmond, Calif.), antiubiquitin mouse monoclonal antibody mAb-Ubi-1 (Sigma-Aldrich RBI), and anti-SOCS-1 and anti-SOCS-3 monoclonal antibodies (57). Recombinant murine IL-3 was purchased from PeproTech (London, England), and recombinant human IFN-γ was purchased from Immugenex. Proteasome inhibitors MG132 (carbobenzoxyl-l-leucyl-l-leucyl-l-leucinal; Z-LLL-CHO), clasto-lactacystin-β-lactone (β-lactacystin), and ubiquitin aldehyde were purchased from Calbiochem-Novabiochem (Darmstadt, Germany); MG132 and β-lactacystin were dissolved in dimethyl sulfoxide. Biotinylated antimouse and antirabbit antibodies were purchased from Dako A/S, and streptavidin-biotinylated horseradish peroxidase was purchased from Amersham Pharmacia Biotech. Rabbit reticulocyte lysates were purchased from Promega (Madison, Wis.), and ubiquitin, hexokinase, d-glucose, and ATP for the in vitro ubiquitination reaction were purchased from Sigma. Pervanadate was prepared as follows: 100 μl of 100 mM Na3VO4 was mixed with 88 μl of Tris-buffered saline (TBS) and 12 μl of 30% H2O2. The mixture was used within 5 min of preparation.
Cell culture.
Cos-7 cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL) supplemented with 10% fetal calf serum (FCS), 100 U of penicillin/ml, and 100 μg of streptomycin/ml. IL-3-dependent myeloid progenitor 32D cells were maintained in RPMI medium (Gibco-BRL) supplemented with 4% WEHI supernatant, 10% FCS, and antibiotics. Starvation was performed by washing the cells with phosphate-buffered saline (PBS) prior to incubation in medium containing 0.5% FCS for 12 to 14 h.
Plasmid construction and transfections.
The plasmids expressing Jak2WT-HA and Jak2KN-HA have been described previously (41). Jak2KN-HA contains a K882E substitution in the lysine involved in the phosphotransfer reaction. The Y1007F Jak2 mutant is identical with Jak2WT-HA except for the Y1007F substitution created by direct PCR mutagenesis using the following primers: 5′TGC CGC AGG ACA AAG AAT TCT ACA AAG TAA AGG AGC CA and 3′TGG CTC CTT TAC TTT GTA GAA TTC TTT GTC CTG CTG CGG CA. HA-tagged ubiquitin and His6-ubiquitin were kind gifts from D. Bohman (EMBL Heidelberg, Germany). SOCS-1, SOCS-1ΔSB, and SOCS-3 expression plasmids have been previously described (18, 58). Cos-7 cells were transfected by electroporation using a Bio-Rad Pulse apparatus.
Pulse-chase experiments.
Transfected Cos-7 cells were pretreated with proteasome inhibitor MG132 for 1 h before lysis, and the proteasome inhibitors were maintained throughout the experiments. Cells were then transferred to methionine-free and cysteine-free DMEM (Gibco-BRL) for 30 min, pulsed with 0.1 mCi of [35S]methionine and [35S]cysteine (ProMix; Amersham) for 15 min, and chased with DMEM followed by stimulations (see Fig. 7).
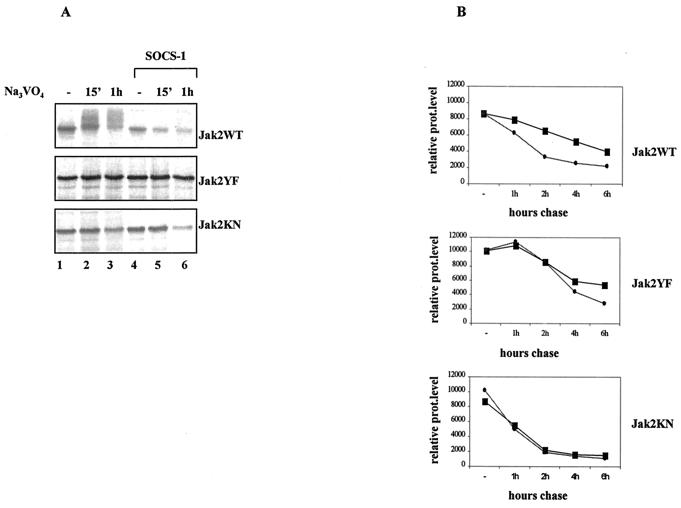
FIG. 7.
Phosphorylation-dependent proteasomal degradation of Jak2WT is enhanced by SOCS-1 coexpression. (A) SOCS-1 coexpression enhances the degradation of Jak2WT but not Jak2YF or Jak2KN in vivo. Cos-7 cells were transfected with Jak2 plasmid (3 μg) either alone or with the SOCS-1 plasmid (5 μg) and pretreated with proteasome inhibitor MG132 (20 μM) for 1 h before 35S metabolic labeling. The inhibitor was maintained throughout the pulse-labeling experiment. Cells were then treated with pervanadate (25 μM) and lysed in NP-40 lysis buffer supplemented with 25 μM MG132. Jak2 protein levels were analyzed by immunoprecipitation using an anti-HA antibody followed by autoradiographic detection. (B) Half-lives of different Jak2 mutants in the absence (square) or presence (circle) of SOCS-1 coexpression. Cos-7 cells were transfected with different Jak2 plasmids alone or together with the SOCS-1 plasmid and pulse-labeled with 35S for 15 min and chased for the indicated periods. Jak2 protein (prot) levels were analyzed by anti-HA immunoprecipitation followed by autoradiographic exposure. Images were scanned and quantified by using the Quan-Image5 program, and the relative protein amounts were adjusted so that time zero represents 100.
Immunoprecipitation and Western blotting.
The cells were harvested in ice-cold PBS and lysed in NP-40 lysis buffer (50 mM Tris-HCl [pH 7.4], 10% glycerol, 50 mM NaCl, 0.5% sodium deoxycholate, 1% NP-40, 20 mM NaF, 0.2 mM Na3VO4) supplemented with protease inhibitors. After 30 min of incubation on ice, lysates were cleared by centrifugation for 20 min at 4°C. For detection of ubiquitination in endogenous Jak2, isopeptidase activity was inhibited by addition of 20 μg of ubiquitin aldehyde/ml to the lysis buffer or, alternatively, the cells were lysed by being boiled in PBS-2% sodium dodecyl sulfate (SDS) buffer as described previously (47). The protein amount was determined by a Bio-Rad Dc protein assay kit (Bio-Rad Laboratories). Immunoprecipitations were carried out as previously described (25). For antiubiquitin detection, immunoprecipitations were performed in the presence of 25 μM MG132 and immunoblotting was carried out as described previously (38). The stripping was performed by incubating the filters in 62.5 mM Tris-HCl-100 mM 2-mercaptoethanol-2% SDS for 45 min at 56°C followed by extensive washings and blocking with 5% nonfat dry milk in TBS.
Immunodetection.
After SDS-polyacrylamide gel electrophoresis the proteins were transferred to a nitrocellulose membrane (Protran; Schleicher & Schuell GmbH) and blocked with 5% nonfat dried milk in TBS-0.1% Tween 20. The membranes were incubated with specific antibodies diluted in TBS-0.05% Tween 20. Immunodetection was performed using the enhanced chemiluminescence method (Amersham Pharmacia Biotech).
In vitro ubiquitination of Jak2.
Jak2 was immunoprecipitated from transfected Cos-7 cells by using an anti-Jak2 antibody and purified with protein A-Sepharose beads. After extensive washes, the beads were resuspended in 30 μl of buffer containing 40 mM Tris-HCl (pH 7.5), 5 mM MgCl2, 2 mM dithiothreitol (DTT), 2 mM ATP, 5 μg of ubiquitin/ml, 12.5 μM MG132, and 20 μg of ubiquitin aldehyde/ml. To deplete endogenous ATP, hexokinase (1 mg/ml) and 2-deoxyglucose (20 mM) were also included. Five microliters of crude rabbit reticulocyte lysates was added to each reaction mixture, and the mixture was incubated for 1.5 h at 30°C. The beads were then extensively washed and resuspended in SDS loading buffer. Cell lysates from Cos-7 cells transfected with SOCS-1, SOCS-1ΔSB, or a control were prepared by washing the cells twice with ice-cold PBS and lysing them in 250 μl of lysis buffer (20 mM HEPES [pH 7.2], 10 mM KCl, 1.5 mM MgCl2, 1 mM DTT, 25 μM MG132, and protease and phosphatase inhibitors). Lysates were sonicated for two cycles of 30 s followed by centrifugation for 30 min. For the ubiquitination reaction, immunoprecipitated Jak2 (as described above) bound to protein A-Sepharose was resuspended in 50 μl of reaction buffer (20 mM HEPES [pH 7.2], 10 mM MgCl2, 1 mM DTT, 1 mM ATP, 1 mg of hexokinase/ml, 20 mM d-glucose, 25 μM MG132, 5 μg of ubiquitin/ml) containing 100 μg of cell lysates and then the suspension was incubated for 90 min at 30°C, after which the bound Jak2 was washed and resuspended in SDS loading buffer.
RESULTS
Jak2 is ubiquitinated in vivo, and its ubiquitination is regulated by cytokines.
Proteasome inhibitors have been shown to prolong the Jak/STAT signaling pathway, but the underlying molecular mechanisms remain poorly understood. We wanted to investigate the role of the proteasome pathway in regulation of Jak activation and initially examined whether Jak2 is subject to ubiquitination in response to IL-3 stimulation. For these experiments, IL-3-dependent myeloid 32D cells were starved overnight and stimulated with IL-3 in the presence or absence of proteasome inhibitors. Two different proteasome inhibitors were used, MG132, which is a reversible 26S proteasome inhibitor, and β-lactacystin, which is an irreversible proteasome inhibitor that blocks the isopeptidase activities as well. 32D cells were lysed with a nondenaturing buffer containing proteasome inhibitors as well as ubiquitin aldehyde to inhibit isopeptidase activities. Jak2 was immunoprecipitated and subjected to antiubiquitin and anti-Jak2 immunoblotting. IL-3 stimulation resulted in rapid tyrosine phosphorylation of Jak2, and MG132 treatment stabilized the tyrosine phosphorylation of Jak2 as previously reported (4) (data not shown). A protein whose molecular weight corresponded to that of Jak2 was detected by the antiubiquitin antibody in both unstimulated and IL-3-stimulated 32D cells (Fig. 1A). Stripping and reblotting the filter with an anti-Jak2 antibody indicated that the protein was Jak2. Based on the molecular weight this band represented a monoubiquitinated form of Jak2. IL-3 stimulation induced a smear above the Jak2 band, which is characteristic of polyubiquitination. This smear could also be detected upon longer exposures using antiphosphotyrosine antibodies (data not shown). The presence of ubiquitin aldehyde in the lysis buffer was critical for detection of the polyubiquitinated forms of Jak2. Pretreatment of 32D cells with MG132 did not affect the level of monoubiquitinated Jak2 but increased the accumulation of polyubiquitinated Jak2. Control immunoprecipitations with antibodies against STAT1, STAT6, and PU.1 indicated that the antiubiquitin antibody detected a faint nonspecific band slightly above Jak2 (data not shown). Preclearing the lysates with irrelevant antibodies did not affect the intensity of the Jak2 band detected by antiubiquitin blotting.
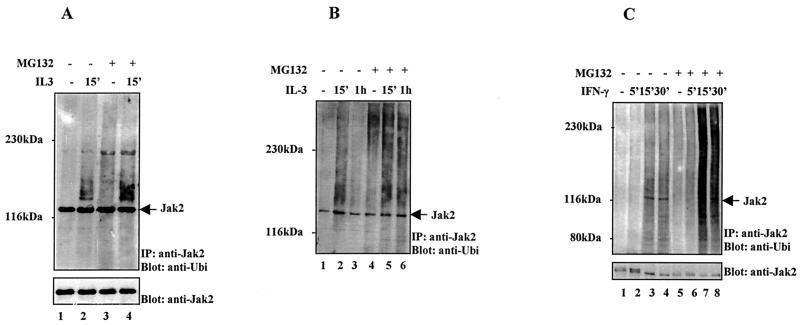
FIG. 1.
Ubiquitination of Jak2 in response to cytokine stimulation. (A) 32D cells were starved overnight and pretreated with proteasome inhibitor MG132 (20 μM) or vehicle for 1 h before IL-3 (100 ng/ml) stimulation. The cells were lysed in NP-40 lysis buffer supplied with 20 μg of MG132/ml and 20 μg of ubiquitin aldehyde/ml to inhibit isopeptidase activities. Jak2 was immunoprecipitated (IP) and subjected to immunoblotting using an antiubiquitin antibody. After being stripped the filter was reblotted with an anti-Jak2 antibody. (B) 32D cells were treated as described in for panel A. Cell extracts were prepared under denaturing lysis conditions, and the immunoprecipitated Jak2 was analyzed by immunoblotting using an antiubiquitin antibody. (C) Cos-7 cells were starved overnight and pretreated with proteasome inhibitor MG132 (20 μM) or vehicle for 1 h before IFN-γ (100 ng/ml) stimulation. Cell extracts were prepared under denaturing lysis conditions, and the immunoprecipitated Jak2 was analyzed by immunoblotting using an antiubiquitin antibody. After being stripped the filter was reblotted with an anti-Jak2 antibody.
Ubiquitination of Jak2 was next investigated by using a denaturing lysis protocol to inhibit the proteasome as well as the isopeptidase activities. In 32D cells IL-3 stimulation induced rapid polyubiquitination of Jak2, which decreased by 1 h (Fig. 1B). The proteasome inhibitor treatment stabilized the polyubiquitinated form of Jak2, thus suggesting that the polyubiquitinated forms of Jak2 are normally rapidly removed through the proteasome pathway. Similar results were obtained with another IL-3-dependent myeloid cell line, FDCP-1 (data not shown). We also investigated whether other cytokine signals would regulate ubiquitination of Jak2. IFN-γ stimulation induced ubiquitination of the endogenous Jak2 protein, and MG132 treatment enhanced and stabilized the polyubiquitination of Jak2 (Fig. 1C). Taken together, these data indicate that the ubiquitin-proteasome pathway is involved in regulation of Jak2 in IL-3 and IFN-γ signal transduction.
Ubiquitination of Jak2 in intact cells and in a cell-free system.
Modulation of cytokine receptors by the proteasome pathway is a potential mechanism for the effects of proteasome inhibitors and for regulation of Jak2 activity. To exclude this, ubiquitination of Jak2 in Cos-7 cells, where Jaks can be activated by overexpression in a receptor-independent mechanism, was next investigated. Cos-7 cells were transiently transfected with plasmids encoding HA epitope-tagged wild-type Jak2 (Jak2WT-HA) and ubiquitin. The Jak2 protein was immunoprecipitated with an anti-Jak2 antibody and subjected to SDS-polyacrylamide gel electrophoresis and immunoblotting with antiubiquitin and anti-HA antibodies. As shown in Fig. 2A, endogenous Jak2 from untransfected cells was weakly ubiquitinated (lane 1) and overexpression of Jak2WT-HA enhanced the ubiquitination of Jak2. Coexpression of the ubiquitin expression plasmid with that for Jak2WT-HA did not have any significant effect on the level of Jak2 ubiquitination, suggesting that the availability of ubiquitin is not a rate-limiting step in this reaction. After being stripped, the filter was probed with the anti-HA antibody, which indicated that the band detected by antiubiquitin was the Jak2 protein. β-Lactacystin treatment resulted in accumulation of multiubiquitinated forms of Jak2, detected as the characteristic high-molecular-weight smear. Preclearing the lysates with an irrelevant monoclonal antibody did not affect the ubiquitin signal of Jak2 (data not shown)
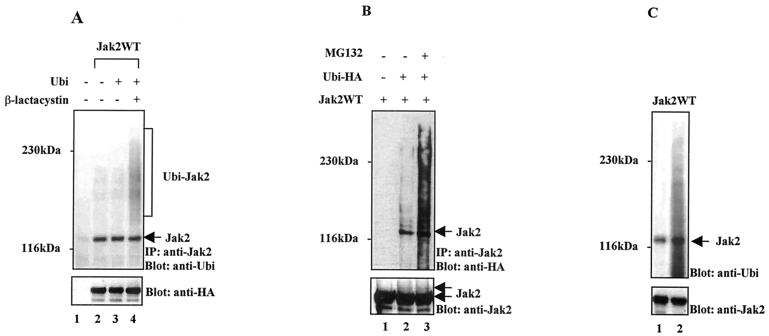
FIG. 2.
In vivo and in vitro ubiquitination of Jak2. (A) Ubiquitination of Jak2 in Cos-7 cells. Cos-7 cells were transiently transfected with plasmids encoding Jak2WT-HA and ubiquitin (Ubi). The sample from lane 4 was pretreated with proteasome inhibitor β-lactacystin (10 μM) for 12 h before harvesting. Cell extracts were immunoprecipitated (IP) with an anti-Jak2 antibody and analyzed by immunoblotting with an antiubiquitin antibody. After being stripped the filter was blotted with an anti-HA antibody. (B) Detection of Jak2 ubiquitination using an anti-HA antibody. Cos-7 cells were transfected with Jak2WT (lanes 1 to 3) and Ubi-HA (lanes 2 and 3) expression plasmids, and Jak2 was immunoprecipitated with anti-Jak2 antibodies and analyzed by immunoblotting with an anti-HA antibody. Lane 3 represents the sample pretreated with MG132 (10 μM) for 3 h before harvesting. The filter was stripped and reblotted with an anti-Jak2 antibody. (C) In vitro ubiquitination of Jak2. Immunopurified Jak2WT-HA from transfected Cos-7 cells was subjected to an in vitro ubiquitination reaction. After the reaction, Western blotting was performed using an antiubiquitin antibody. Lane 1, input for the ubiquitination reaction; lane 2, immunopurified Jak2 after a 1.5-h ubiquitination reaction.
To confirm the ubiquitination of Jak2, Cos-7 cells were transiently transfected with Jak2WT (without the HA tag) and HA epitope-tagged ubiquitin (Ubi-HA) expression plasmids (Fig. 2B). Jak2 was immunoprecipitated with the anti-Jak2 antibody, and ubiquitination was detected by anti-HA blotting. The anti-HA antibody detected Jak2 only when Ubi-HA was cotransfected, and MG132 treatment increased the ubiquitination. The anti-HA and anti-Jak2 blots show typical laddering caused by sequentially attached ubiquitin molecules. Thus, these results confirmed that Jak2WT was monoubiquitinated in Cos-7 cells, and proteasome inhibitor treatment enhanced polyubiquitination of Jak2.
We also set up an in vitro assay to analyze if Jak2 is subject to ubiquitination in a cell-free system. Such a system was previously successfully used in demonstrating the ubiquitination of the epidermal growth factor receptor (EGFR) in vitro (28). As starting material for the reaction we used immunopurified Jak2WT from transfected Cos-7 cells, crude rabbit reticulocyte lysate, ATP, and ubiquitin. The reaction was allowed to proceed for 1.5 h. Immunoblotting with the antiubiquitin antibody demonstrated that the input Jak2 protein was already monoubiquitinated and that the in vitro ubiquitination reaction enhanced the monoubiquitinated and polyubiquitinated forms of Jak2 (Fig. 2C).
Polyubiquitination and degradation of Jak2 are regulated through tyrosine phosphorylation.
Our results indicated that cytokine stimulation induced polyubiquitination of Jak2. Next, we wanted to examine directly the role of tyrosine phosphorylation in ubiquitination and degradation of Jak2. For this purpose Cos-7 cells were transiently transfected with Jak2WT-HA or kinase-negative Jak2 (Jak2KN-HA) expression plasmids (see Materials and Methods). Overexpression results in tyrosine phosphorylation and activation of Jak2WT. For these experiments the expression level of Jak2 was titrated to a level which allowed the regulation of Jak2 tyrosine phosphorylation by tyrosine phosphatase inhibitor pervanadate. The cells were treated with different concentrations of pervanadate for 1 h to induce tyrosine phosphorylation of Jak2. Jak2 was immunoprecipitated with the anti-HA antibody and analyzed by Western blotting. As illustrated in Fig. 3, pervanadate treatment induced, in a dose-dependent manner, ubiquitination of Jak2, which correlated with enhanced tyrosine phosphorylation of Jak2. Treatment of the cells with 50 μM pervanadate caused phosphorylation and polyubiquitination of Jak2 and disappearance of the monoubiquitinated form of Jak2. Interestingly, anti-HA blotting showed that 50 μM pervanadate treatment resulted in a dramatic decrease in Jak2 protein levels. Inhibition of proteasomes by β-lactacystin enhanced both polyubiquitination and tyrosine phosphorylation of Jak2 and stabilized the phosphorylated Jak2 protein (Fig. 3). Thus, these results suggest that phosphorylation of Jak2 serves as a signal for the ubiquitination reaction and for the degradation of Jak2 through the proteasome pathway.
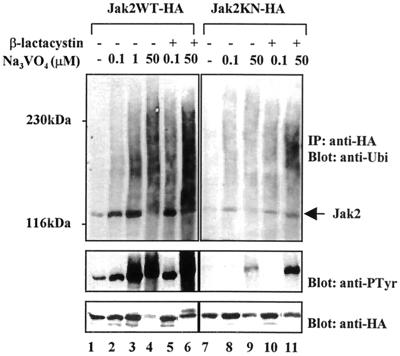
FIG. 3.
Tyrosine phosphorylation of Jak2 correlates with its ubiquitination and degradation. Jak2WT-HA (left) and Jak2KN-HA (right) were transiently expressed in Cos-7 cells. After 48 h the cells were starved overnight and pretreated with proteasome inhibitor β-lactacystin (20 μM) or vehicle for 1 h before treatment with different concentrations of pervanadate for 1 h. Total-cell lysates were immunoprecipitated (IP) with an anti-HA antibody and analyzed by immunoblotting using antiubiquitin or antiphosphotyrosine (anti-PTyr) antibodies. The antiubiquitin and antiphosphotyrosine blots from Jak2WT-HA and Jak2KN-HA panels were exposed for the same time. The filters were then stripped and blotted with an anti-HA antibody (bottom).
Jak2KN was found to be monoubiquitinated at low levels in Cos-7 cells (Fig. 3). Jak2KN is not significantly tyrosine phosphorylated when overexpressed in Cos-7 cells, but a high concentration of pervanadate resulted in low levels of tyrosine phosphorylation of Jak2KN, probably due to transphosphorylation. A modest increase in mono- and polyubiquitination of Jak2KN was detected after pervanadate treatment, and β-lactacystin pretreatment enhanced ubiquitination as well as tyrosine phosphorylation of Jak2KN. Pervanadate treatment (50 μM) decreased Jak2KN protein levels slightly. These results further substantiate the correlation between tyrosine phosphorylation and ubiquitination of Jak2 and the involvement of proteasomes in regulation of the tyrosine-phosphorylated form of Jak2.
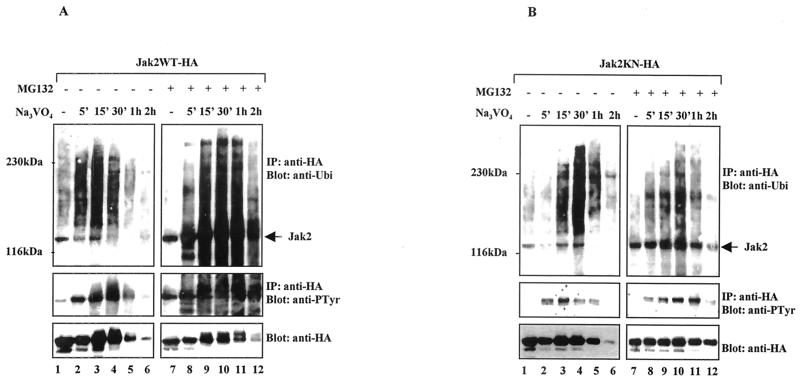
To analyze in more detail the role of tyrosine phosphorylation in regulation of Jak2 via the ubiquitin-proteasome pathway, we performed kinetic studies using pervanadate (50 μM) and proteasome inhibitor MG132 (25 μM). Cos-7 cells were transiently transfected with Jak2WT-HA (Fig. 4A) or Jak2KN-HA (Fig. 4B). Jak2 was immunoprecipitated using the anti-HA antibody and analyzed by Western blotting. As shown in Fig. 4A, pervanadate treatment rapidly increased the polyubiquitination of Jak2, with a peak at 15 min, after which the level of ubiquitination decreased and became undetectable after 2 h. Tyrosine phosphorylation of Jak2 also showed a time dependence, with pervanadate treatment stimulating the phosphorylation of Jak2, followed by a decline in the level of phosphorylation. Anti-HA blotting indicated that the decreased levels of phosphorylation and ubiquitination correlated with degradation of Jak2. Preincubation with MG132 enhanced ubiquitination and phosphorylation of Jak2. Furthermore, MG132 treatment stabilized the Jak2 protein levels, particularly the lower-mobility forms of Jak2, which are likely to represent phosphorylated forms of Jak2 proteins.
FIG. 4.
Kinetics of Jak2 ubiquitination and degradation in vivo. Jak2WT-HA (A) and Jak2KN-HA (B) were transiently expressed in Cos-7 cells. After 48 h the cells were starved overnight and stimulated with pervanadate (50 μM) as indicated. Right blots (A and B), lysates from cells pretreated with proteasome inhibitor MG132 (25 μM) for 1 h before pervanadate treatment. Jak2 was assessed by immunoblotting using an antiubiquitin or antiphosphotyrosine (anti-PTyr) antibody followed by stripping and anti-HA blotting. The antiubiquitin and antiphosphotyrosine blots for Jak2WT-HA and Jak2KN-HA were exposed for the same time. IP, immunoprecipitation.
The effects of pervanadate treatment on ubiquitination, tyrosine phosphorylation, and degradation of Jak2KN and of Jak2WT showed clear differences in magnitude and slight differences in time course (Fig. 4B). Tyrosine phosphorylation of Jak2KN occurred at low levels, and polyubiquitination appeared slightly later, showing a peak at 30 min. The anti-HA Western blot indicated that, compared to those of Jak2WT, the Jak2KN protein levels were less affected by pervanadate treatment, a finding which correlates with the lower level of tyrosine phosphorylation and ubiquitination. MG132 stabilized the protein levels and enhanced both tyrosine phosphorylation and ubiquitination of Jak2KN.
SOCS-1 regulates the degradation of Jak2 through the proteasome pathway.
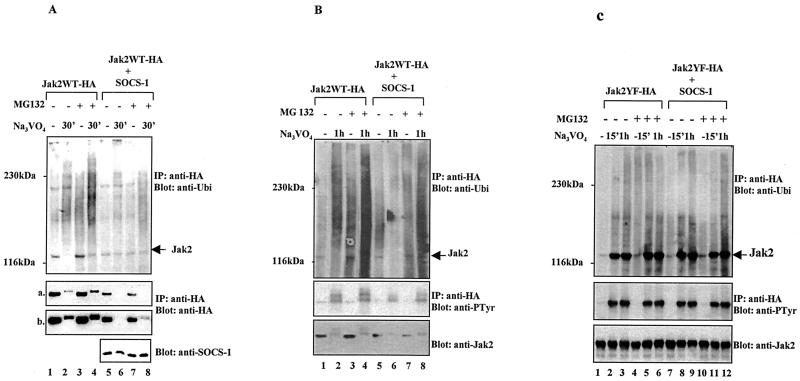
SOCS-1 is reported to bind to the phosphorylated activation loop tyrosine in Jak2 and to inhibit the catalytic activity of Jak2 (52). The association of SOCS proteins with elongins B and C has, however, raised the possibility that the SOCS-mediated regulation of cytokine signaling may also involve the proteasome pathway (21). Our results indicated that tyrosine phosphorylation regulates the degradation of Jak2 via the ubiquitin-proteasome pathway, and therefore we wanted to investigate whether SOCS proteins could be involved in ubiquitin-mediated degradation of Jak2. To test this hypothesis, Cos-7 cells were transiently transfected with plasmids expressing Jak2WT-HA alone or together with SOCS-1, and the cells were treated with pervanadate (25 μM) in the presence or absence of proteasome inhibitor MG132 (20 μM). Coexpression of SOCS-1 resulted in reduced levels of ubiquitinated Jak2 compared to expression of Jak2 alone (Fig. 5A). Anti-HA immunoblotting indicated that coexpression of Jak2 with SOCS-1 caused a reduction in Jak2 protein levels (Fig. a, lanes 1 and 5), which were further decreased by pervanadate treatment. Pretreatment of the cells with MG132 partially stabilized Jak2 protein levels in the presence of SOCS-1, as shown in longer exposures of the anti-HA blot (Fig. b). These results suggest that SOCS-1 enhanced proteasomal degradation of Jak2. An anti-SOCS-1 Western blot showed that pervanadate had only a marginal effect on SOCS-1 protein levels.
FIG. 5.
SOCS-1 coexpression enhances proteasomal degradation of Jak2WT but not Jak2YF. Cos-7 cells were transiently transfected with plasmids encoding Jak2WT-HA (A and B) or Jak2YF-HA (C) either alone or together with plasmids encoding SOCS-1. After 48 h the cells were starved overnight and pretreated with proteasome inhibitor MG132 (20 μM) for 1 h before pervanadate treatment (25 μM). Jak2 was analyzed by immunoprecipitation (IP) with an anti-HA antibody followed by immunoblotting with the indicated antibodies. (A, a and b) Different exposures of the same blot. SOCS-1 protein levels were determined by Western blotting 20 μg of total-cell lysates using an anti-SOCS-1 antibody.
To analyze the Jak2-SOCS-1 interaction in more detail, similar experiments were performed with autophosphorylation site mutant Jak2YF mutant, which has been shown to fail to interact or to interact only weakly with SOCS-1 (52). Interestingly, pervanadate treatment induced monoubiquitination and tyrosine phosphorylation of Jak2YF but only very low levels of polyubiquitnation (Fig. 5C). In striking contrast to the results obtained with Jak2WT (Fig. 5A and B), the protein levels of Jak2YF were not affected by pervanadate treatment. Furthermore, SOCS-1 expression did not have any effect on ubiquitination, tyrosine phosphorylation, or protein levels of Jak2YF (Fig. 5C).
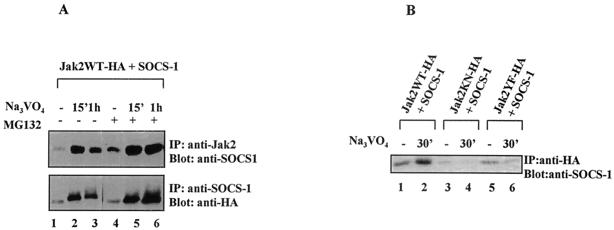
The results in Fig. 5 suggest that autophosphorylation site Y1007 and the interaction with SOCS-1 regulate the ubiquitin-mediated degradation of Jak2. To validate this hypothesis, we analyzed the interaction between Jak2 and SOCS-1 directly. Cos-7 cells were cotransfected with Jak2WT-HA and SOCS-1, and the association between Jak2 and SOCS-1 was analyzed. As shown in Fig. 6A, pervanadate enhanced the association between SOCS-1 and Jak2 in anti-Jak2 immunoprecipitations but the amount of coimmunoprecipitated SOCS-1 decreased after 1 h of treatment. MG132 pretreatment stabilized the Jak2-SOCS-1 complex. Similar results were obtained when the experiments were performed in the reverse direction by immunoblotting SOCS-1 immunoprecipitates with anti-Jak2 for Jak2. These results, together with the finding that SOCS-1 protein levels were not affected by pervanadate treatment (Fig. 5A), suggest that MG132 inhibited the degradation of the ubiquitinated Jak2 in the Jak2-SOCS-1 complex. The interaction between SOCS-1 and Jak2 mutants was also analyzed, and, as shown in Fig. 6B, SOCS-1 showed only very weak association with Jak2KN-HA and Jak2YF-HA, suggesting that the association of SOCS-1 with Jak2 requires phosphorylation of Jak2 on tyrosine 1007.
FIG. 6.
Proteasome inhibitor MG132 stabilizes the interaction between Jak2WT and SOCS-1. (A) Coimmunoprecipitation of Jak2 and SOCS-1. Cos-7 cells were transfected with Jak2WT-HA and SOCS-1 as indicated and treated with MG132 and pervanadate as described for Fig. 5. Total-cell lysates were immunoprecipitated (IP) with anti-HA or anti-SOCS-1 antibodies and analyzed by immunoblotting using anti-SOCS-1 or anti-HA antibodies. (B) Coimmunoprecipitation of SOCS-1 with different Jak2 mutants. Cos-7 cells were transfected with Jak2WT-HA (lanes 1 and 2), Jak2KN-HA (lanes 3 and 4), and Jak2YF-HA (lanes 5 and 6) together with SOCS-1. Cells were pretreated with MG132 (25 μM) for 1 h before the pervanadate treatment (25 μM). After anti-HA immunoprecipitation, the filter was immunoblotted with an anti-SOCS-1 antibody.
The results obtained from Western blotting were confirmed by metabolic-labeling experiments. Cos-7 cells were transfected with Jak2WT-HA or Jak2KN-HA and Jak2YF-HA alone or together with SOCS-1 and then pulse labeled with 35S in the presence of MG132. Pervanadate treatment caused a mobility shift in Jak2WT but not in Jak2 mutants (Fig. 7A). SOCS-1 coexpression decreased the tyrosine-phosphorylated slower-migrating forms of Jak2WT, while the protein levels of Jak2KN-HA and Jak2YF-HA were not affected by SOCS-1 coexpression or pervanadate treatment. A slight decrease in Jak2KN protein level was noticed after 1 h of pervanadate treatment (Fig. 7A, bottom, lane 6).
Pulse-chase experiments were conducted to investigate the effect of SOCS-1 on the half-lives of different Jak2 proteins. Cos-7 cells were transfected with different Jak2 plasmids alone or together with SOCS-1 and pulse-labeled with 35S and then chased for different time periods (Fig. 7B). Jak2 was immunoprecipitated with the anti-HA antibody. After autoradiography the amount of the immunoprecipitated Jak2 protein was quantified. Jak2KN was found to have a shorter half-life than Jak2WT or Jak2YF for currently unknown reasons. However, in accordance with results shown above, the half-lives of Jak2KN-HA and Jak2YF-HA mutants were not affected by SOCS-1 while the degradation of Jak2WT was accelerated in the presence of SOCS-1.
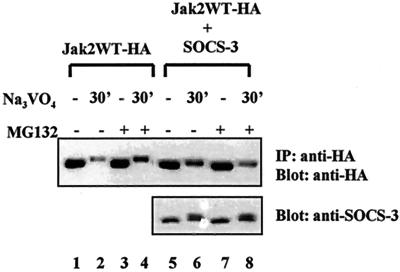
We wanted to validate the specificity of SOCS-1 in the ubiquitin-mediated degradation of Jak2 by analyzing the effect of related protein SOCS-3 on Jak2 protein turnover in Cos-7 cells. Cotransfection of SOCS-3 had no effect on Jak2 protein levels (Fig. 8). Pervanadate treatment caused tyrosine phosphorylation of SOCS-3, as shown by detection of the low-mobility forms of the protein (7), and a slight reduction of SOCS-3 protein, which was stabilized by MG132 treatment.
FIG. 8.
SOCS-3 does not affect Jak2 protein levels in Cos-7 cells. Cos-7 cells were transiently transfected with Jak2WT (4 μg) or with SOCS-3 (4 μg) plasmids. After 48 h the cells were starved overnight and treated with MG132 and pervanadate as described for Fig. 5. Jak2 was analyzed by immunoprecipitation (IP) and immunoblotting using an anti-HA antibody. SOCS-3 protein levels were determined by Western blotting 20 μg of total-cell lysates using an anti-SOCS-3 antibody.
The SOCS box is required for ubiquitination of Jak2.
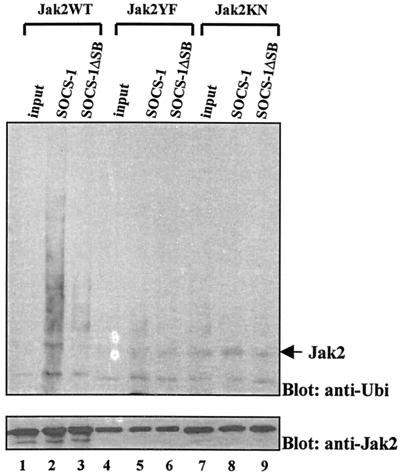
The SOCS box of SOCS-1 interacts with elongins B and C and may target the bound molecules through proteasomal degradation (57). To determine the role of the SOCS box in the ubiquitination of Jak2, we analyzed the effects of SOCS-1 and SOCS-1ΔSB lacking the SOCS box (58) in an in vitro assay (55). Jak2WT-HA, Jak2YF-HA, and Jak2KN-HA were immunopurified from Cos-7 cells and incubated in the presence of SOCS-1, SOCS-1ΔSB, or control lysates. The ubiquitination reaction was allowed to proceed for 1 h, and the ubiquitination of Jak2 was analyzed by Western blotting. The results (Fig. 9) show that SOCS-1 induced ubiquitination of Jak2WT, whereas SOCS-1ΔSB had no significant effect. SOCS-1 did not affect the ubiquitination of Jak2YF and Jak2KN mutants. These results suggest that SOCS-1 induces polyubiquitination of Jak2WT, which requires the presence of the SOCS box of SOCS-1 and phosphorylation of Y1007 on Jak2.
FIG. 9.
SOCS-1 enhances the ubiquitination of Jak2WT in vitro. Immunopurified Jak2WT, Jak2YF, and Jak2KN were subjected to an in vitro ubiquitination reaction in the presence SOCS-1, SOCS-1ΔSB, or control lysates. The ubiquitination of Jak2 was analyzed after 1 h of in vitro reaction by antiubiquitin Western blotting. The filter was stripped and reblotted with an anti-Jak2 antibody.
DISCUSSION
Appropriate biological responses through cytokine receptors require stringent control of the activation signals. The importance of negative regulation is well exemplified by the results obtained from deregulated and aberrantly activated Jak tyrosine kinases that cause autonomous cell growth and cancer (26, 33). Here we show that the ubiquitin-proteasome pathway directly regulates tyrosine-phosphorylated Jak2 in cytokine receptor signaling.
The irreversible nature of the proteasomal degradation of proteins demands strict control for these processes. The specificity is mainly determined through a large family of E3 ubiquitin ligases, which recognize substrate proteins in highly ordered protein complexes (6). Protein phosphorylation has been shown to function as a regulatory mechanism for ubiquitination and proteasomal degradation of various signaling proteins. Several cell surface receptors as well as cellular and transcriptional regulators such as cyclins, cyclin-dependent kinase inhibitors, Src kinases, IκB-α, p53, β-catenin, c-Jun, and RNA polymerase have been shown to be regulated via phosphorylation-dependent proteasomal degradation (27). Tyrosine phosphorylation was also a critical regulator of ubiquitination and degradation of Jak2. Tyrosine phosphorylation stimulated polyubiquitination of Jak2, which was stabilized by proteasome inhibitor treatment, thus indicating that polyubiquitinated Jak2 is rapidly degraded through the proteasome pathway. We did not observe any significant downregulation in Jak2 protein levels in 32D cells after IL-3 stimulation. Based on our experience, only a small fraction of cellular Jak2 becomes activated and tyrosine phoshorylated after IL-3 stimulation, and the degradation of this fraction is likely to be difficult to detect on the total-protein level. The relatively short half-life of unstimulated Jak2 is also consistent with the concept that the turnover of Jak2 functions as a regulatory mechanism in cytokine signaling (43).
A likely mechanism for the tyrosine phosphorylation-dependent regulation of ubiquitination is specific recruitment of the ubiquitination enzyme complex to the protein. The catalytic activation of Jak2 involves auto- or transphosphorylation of the activation loop Y1007, which is likely to cause a conformational change that allows substrates and ATP to access the catalytic pocket (12). Here we show that proteasomal degradation of Jak2 required phosphorylation of Jak2 on Y1007. The phosphorylated Y1007 on Jak2 serves also as a docking site for the extended SH2 domain of SOCS-1, but an additional 12 N-terminal amino acids, constituting what is termed the kinase-inhibitory region, confer high-affinity binding to the kinase domain and inhibitory interaction with the catalytic pocket of Jak2 (52). The interaction of the SOCS box with elongins B and C suggested that the regulation via SOCS proteins may involve the ubiquitin-proteasome pathway (57). Recent results from mice with a deletion of the SOCS box of SOCS-1 demonstrate a clear physiological regulatory function for the SOCS box (58). These mice have a phenotype similar to but less severe than that of SOCS-1−/− mice and show increased IFN-γ responsiveness and develop a lethal inflammatory disease. We showed that SOCS-1 enhanced ubiquitination of Jak2 in a SOCS box-dependent manner and that this effect was regulated by Y1007 on Jak2. Thus SOCS-1 is likely to recruit, along with elongins, the E3 ligase to the phosphorylated Jak2. The exact composition of the SOCS-1-elongin B/C ubiquitination complex is currently unknown but it is likely to resemble that of the von Hippel-Lindau complex and to consist of RING finger protein Rbx1 as a bridging factor, Cullin-2, and an E2-conjugating enzyme. In cotransfection experiments SOCS-1 also induced Jak2 degradation which was dependent on Y1007. SOCS-1 has also been shown to induce degradation of the VAV protein through the ubiquitin-proteasome pathway (9). We did not observe any significant reduction in total Jak2 protein levels in IFN-γ-treated thymocytes from SOCS-1−/− mice (data not shown). However, there was a decrease in antiubiquitin-reactive high-molecular-weight protein complexes, which are typically observed with polyubiquitinated proteins. These results are consistent with the concept that ubiquitination of Jak2 is reduced in SOCS-1−/− thymocytes, but more-detailed studies are required to resolve this issue. Interestingly, SOCS-3, which associates weakly with Jak2 (42), did not have any effect on Jak2 protein levels. These findings support the concept that different SOCS proteins have highly specialized functions in regulation of cytokine receptor signaling.
Proteasome inhibitors stabilized the Jak2-SOCS-1 complex and inhibited proteolysis of Jak2. It is of interest that SOCS-1 protein levels were not markedly affected by pervanadate treatment. We could not detect significant levels of ubiquitination of SOCS-1 (data not shown); thus, while SOCS-1 may act as part of an E3 ubiquitin ligase complex, it is not a substrate of the E3 ligase. These findings together with results from mice with a SOCS box deletion are consistent with a sequential-regulation model for SOCS-1, which is initiated by an inhibitory interaction with activated Jak2, followed by recruitment of the E3 ubiquitin ligase complex and resulting in ubiquitination and subsequent targeting of Jak2 to proteasomes for degradation. In line with this hypothesis, cytokine stimulation has been shown to enhance the interaction between SOCS-1 and elongins B and C, possibly due to conformational changes induced by SOCS-1-Jak2 association (57).
Jak2 was found to be monoubiquitinated to various degrees in different cells. This finding was somewhat surprising, but monoubiquitination of Jak2 was observed by using three different lysis conditions as well as an HA-tagged ubiquitin expression vector in Cos-7 cells. Monoubiquitination of Jak2 did not appear to be strictly tyrosine phosphorylation dependent, as both Jak2KN in Cos-7 cells and endogenous Jak2 in unstimulated cells were monoubiquitinated. Induction of tyrosine phosphorylation, however, stimulated both monoubiquitination and polyubiquitination of Jak2. Attachment of a single ubiquitin molecule on a target substrate (i.e., monoubiquitination) either modulates the activity or localization of the protein or targets the protein to the proteasomes (6, 17). Intriguingly, monoubiquitination of proteins may increase the efficiency of the polyubiquitination reaction (31). For Jak2, monoubiquitination may “sensitize” the activated kinase to the subsequent SOCS-1-mediated polyubiquitination, ensuring that activated Jak2 is degraded in a timely fashion. The regulatory mechanisms for the monoubiquitination of Jak2 are currently unknown, and it remains possible that the monoubiquitination reaction involves an E3 ubiquitin ligase complex different from the one which catalyzes the polyubiquitination of the tyrosine-phosphorylated Jak2.
Proteasome inhibitors stabilized the tyrosine-phosphorylated form of Jak2, which suggests that the physiological function of the ubiquitin-proteasome pathway is to remove the active tyrosine kinase and prevent aberrant cytokine signaling. Recently SOCS-1 was shown to mediate the growth inhibition of TEL-Jak2-transformed cells and to accelerate the proteasomal degradation of the oncogenic, hyperactive TEL-Jak2 and gyrase B-Jak2 chimeras, neither of which is activated in response to cytokines (13, 20). These results are in accordance with our results showing that IL-3 and IFN-γ stimulations and tyrosine phosphorylation of Jak2 on Y1007 regulate ubiquitination and degradation of the cellular Jak2. Regulation of hyperactivated and oncogenic forms of Src kinases through ubiquitin-mediated degradation has also been shown; thus proteasomal degradation may be a common mechanism to control the activity of tyrosine kinases (15, 55). In agreement with this hypothesis, mutations that inhibit the ubiquitin ligase activity of RING finger protein c-Cbl are oncogenic and cause failure to ubiquitinate and desensitize EGFR (28). The regulatory mechanisms involved in these processes have to possess high degrees of specificity, and E3 ligase E6AP appears to be responsible for ubiquitination of Src kinases, and c-Cbl mediates the ubiquitination of EGFR (15, 38), while the SOCS-1-recruited ubiquitination complex represents one mechanism involved in regulation of Jak2.
The SOCS-1-mediated ubiquitination and degradation of Jak2 show some analogy to the c-Cbl-mediated desensitization of EGFR (28). The phosphorylated Y1045 on EGFR serves as a docking site for RING finger protein c-Cbl, which regulates the ubiquitination of the receptor. Phosphorylation of c-Cbl on Y371 flanking its RING finger domain is critical for the ubiquitination process and subsequent degradation of the activated EGFR. The Jak2-SOCS-1 interaction does not involve direct tyrosine phosphorylation of SOCS-1 (data not shown), but whether the function of SOCS-1 is regulated by other posttranslational modifications or protein interactions remains to be investigated. In this regard it is interesting that cytokine stimulation has been shown to promote the interaction between SOCSs and elongins B and C (57).
It is becoming evident that the ubiquitin-proteasome pathway plays an integral part in regulation of cytokine receptor signaling. Several cytokine receptors were shown to be ubiquitinated upon ligand stimulation, and their turnover and intracellular sorting are regulated through proteasomes (49, 54). SOCS family protein Cis has been implicated in proteasome-mediated regulation of EpoR (49). Here we show that ubiquitination plays a critical role in selecting the activated Jak2 for degradation. Interaction between SOCS-1 and tyrosine-phosphorylated Jak2 stimulates proteasomal degradation of Jak2, which may regulate, e.g., IFN-γ and granulocyte-macrophage colony-stimulating factor signaling (32). Various SOCS proteins display highly specific functions in regulation of individual cytokine signaling pathways, and it is likely that other SOCSs or distinct adapter proteins and ubiquitination enzyme complexes regulate other cytokine signaling pathways. Finally, proteasome inhibitors stabilize transcriptionally active tyrosine-phosphorylated STAT5 via an ubiquitin-independent mechanism, which represents a distinct mechanism of proteasomal regulation (51). In summary, regulation of cytokine receptor signaling cascades that activate Jak2 and STAT transcription factors involves several highly specific ubiquitin-proteasome-dependent regulatory mechanisms, and the specificity of this regulation is determined by interactions with ancillary proteins such as SOCSs.
Acknowledgments
We thank Paula Kosonen for the excellent technical assistance and D. Bohman for kindly providing reagents. The SOCS-1−/− cells were generously provided by J. Ihle and E. Parganas.
This work was supported by grants from the Medical Research Council of Academy of Finland, Medical Research Fund of Tampere University Hospital, the Finnish Foundation for Cancer Research, and the Sigrid Jusélius Foundation and a grant from the National Institutes of Health (CA22556).
REFERENCES
- 1.Alexander, W. S., R. Starr, J. E. Fenner, C. L. Scott, E. Handman, N. S. Sprigg, J. E. Corbin, A. L. Cornish, R. Darwiche, C. M. Owczarek, T. W. Kay, N. A. Nicola, P. J. Hertzog, D. Metcalf, and D. J. Hilton. 1999. SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell 98:597-608. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, W. S., R. Starr, D. Metcalf, S. E. Nicholson, A. Farley, A. G. Elefanty, M. Brysha, B. T. Kile, R. Richardson, M. Baca, J. G. Zhang, T. A. Willson, E. M. Viney, N. S. Sprigg, S. Rakar, J. Corbin, S. Mifsud, L. DiRago, D. Cary, N. A. Nicola, and D. J. Hilton. 1999. Suppressors of cytokine signaling (SOCS): negative regulators of signal transduction. J. Leukoc. Biol. 66:588-592. [DOI] [PubMed] [Google Scholar]
- 3.Brysha, M., J. G. Zhang, P. Bertolino, J. E. Corbin, W. S. Alexander, N. A. Nicola, D. J. Hilton, and R. Starr. 2001. Suppressor of cytokine signaling-1 attenuates the duration of interferon gamma signal transduction in vitro and in vivo. J. Biol. Chem. 276:22086-22089. [DOI] [PubMed] [Google Scholar]
- 4.Callus, B. A., and B. Mathey-Prevot. 1998. Interleukin-3-induced activation of the JAK/STAT pathway is prolonged by proteasome inhibitors. Blood 91:3182-3192. [PubMed] [Google Scholar]
- 5.Chen, X. P., J. A. Losman, and P. Rothman. 2000. SOCS proteins, regulators of intracellular signaling. Immunity 13:287-290. [DOI] [PubMed] [Google Scholar]
- 6.Ciechanover, A. 1998. The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J. 17:7151-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohney, S. J., D. Sanden, N. A. Cacalano, A. Yoshimura, A. Mui, T. S. Migone, and J. A. Johnston. 1999. SOCS-3 is tyrosine phosphorylated in response to interleukin-2 and suppresses STAT5 phosphorylation and lymphocyte proliferation. Mol. Cell. Biol. 19:4980-4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Andrea, A., and D. Pellman. 1998. Deubiquitinating enzymes: a new class of biological regulators. Crit. Rev. Biochem. Mol. Biol. 33:337-352. [DOI] [PubMed] [Google Scholar]
- 9.De Sepulveda, P., S. Ilangumaran, and R. Rottapel. 2000. Suppressor of cytokine signaling-1 inhibits VAV function through protein degradation. J. Biol. Chem. 275:14005-14008. [DOI] [PubMed] [Google Scholar]
- 10.Duan, D. R., A. Pause, W. H. Burgess, T. Aso, D. Y. Chen, K. P. Garrett, R. C. Conaway, J. W. Conaway, W. M. Linehan, and R. D. Klausner. 1995. Inhibition of transcription elongation by the VHL tumor suppressor protein. Science 269:1402-1406. [DOI] [PubMed] [Google Scholar]
- 11.Endo, T. A., M. Masuhara, M. Yokouchi, R. Suzuki, H. Sakamoto, K. Mitsui, A. Matsumoto, S. Tanimura, M. Ohtsubo, H. Misawa, T. Miyazaki, N. Leonor, T. Taniguchi, T. Fujita, Y. Kanakura, S. Komiya, and A. Yoshimura. 1997. A new protein containing an SH2 domain that inhibits JAK kinases. Nature 387:921-924. [DOI] [PubMed] [Google Scholar]
- 12.Feng, J., B. A. Witthuhn, T. Matsuda, F. Kohlhuber, I. M. Kerr, and J. N. Ihle. 1997. Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol. Cell. Biol. 17:2497-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frantsve, J., J. Schwaller, D. W. Sternberg, J. Kutok, and D. G. Gilliland. 2001. Socs-1 Inhibits TEL-JAK2-mediated transformation of hematopoietic cells through inhibition of JAK2 kinase activity and induction of proteasome-mediated degradation. Mol. Cell. Biol. 21:3547-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebert, C. A., S. H. Park, and D. J. Waxman. 1999. Termination of growth hormone pulse-induced STAT5b signaling. Mol. Endocrinol. 13:38-56. [DOI] [PubMed] [Google Scholar]
- 15.Harris, K. F., I. Shoji, E. M. Cooper, S. Kumar, H. Oda, and P. M. Howley. 1999. Ubiquitin-mediated degradation of active Src tyrosine kinase. Proc. Natl. Acad. Sci. USA 96:13738-13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haspel, R. L., M. Salditt-Georgieff, and J. E. Darnell, Jr. 1996. The rapid inactivation of nuclear tyrosine phosphorylated Stat1 depends upon a protein tyrosine phosphatase. EMBO J. 15:6262-6268. [PMC free article] [PubMed] [Google Scholar]
- 17.Hicke, L. 2001. Protein regulation by monoubiquitin. Nat. Rev. Mol. Cell. Biol. 2:195-201. [DOI] [PubMed] [Google Scholar]
- 18.Hilton, D. J., R. T. Richardson, W. S. Alexander, E. M. Viney, T. A. Willson, N. S. Sprigg, R. Starr, S. E. Nicholson, D. Metcalf, and N. A. Nicola. 1998. Twenty proteins containing a C-terminal SOCS box form five structural classes. Proc. Natl. Acad. Sci. USA 95:114-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ihle, J. N., B. A. Witthuhn, F. W. Quelle, K. Yamamoto, and O. Silvennoinen. 1995. Signaling through the hematopoietic cytokine receptors. Annu. Rev. Immunol. 13:369-398. [DOI] [PubMed] [Google Scholar]
- 20.Kamizono, S., T. Hanada, H. Yasukawa, S. Minoguchi, R. Kato, M. Minoguchi, K. Hattori, S. Hatakeyama, M. Yada, S. Morita, T. Kitamura, H. Kato, K. Nakayama, and A. Yoshimura. 2001. The SOCS box of SOCS-1 accelerates ubiquitin-dependent proteolysis of TEL-JAK2. J. Biol. Chem. 276:12530-12538. [DOI] [PubMed] [Google Scholar]
- 21.Kamura, T., S. Sato, D. Haque, L. Liu, W. G. Kaelin, Jr., R. C. Conaway, and J. W. Conaway. 1998. The Elongin BC complex interacts with the conserved SOCS-box motif present in members of the SOCS, ras, WD-40 repeat, and ankyrin repeat families. Genes Dev. 12:3872-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kibel, A., O. Iliopoulos, J. A. DeCaprio, and W. G. Kaelin, Jr. 1995. Binding of the von Hippel-Lindau tumor suppressor protein to Elongin B and C. Science 269:1444-1446. [DOI] [PubMed] [Google Scholar]
- 23.Kim, H., and H. Baumann. 1999. Dual signaling role of the protein tyrosine phosphatase SHP-2 in regulating expression of acute-phase plasma proteins by interleukin-6 cytokine receptors in hepatic cells. Mol. Cell. Biol. 19:5326-5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim, T. K., and T. Maniatis. 1996. Regulation of interferon-gamma-activated STAT1 by the ubiquitin-proteasome pathway. Science 273:1717-1719. [DOI] [PubMed] [Google Scholar]
- 25.Kovanen, P. E., I. Junttila, K. Takaluoma, P. Saharinen, L. Valmu, W. Li, and O. Silvennoinen. 2000. Regulation of Jak2 tyrosine kinase by protein kinase C during macrophage differentiation of IL-3-dependent myeloid progenitor cells. Blood. 95:1626-1632. [PubMed] [Google Scholar]
- 26.Lacronique, V., A. Boureux, V. D. Valle, H. Poirel, C. T. Quang, M. Mauchauffe, C. Berthou, M. Lessard, R. Berger, J. Ghysdael, and O. A. Bernard. 1997. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science 278:1309-1312. [DOI] [PubMed] [Google Scholar]
- 27.Laney, J. D., and M. Hochstrasser. 1999. Substrate targeting in the ubiquitin system. Cell 97:427-430. [DOI] [PubMed] [Google Scholar]
- 28.Levkowitz, G., H. Waterman, S. A. Ettenberg, M. Katz, A. Y. Tsygankov, I. Alroy, S. Lavi, K. Iwai, Y. Reiss, A. Ciechanover, S. Lipkowitz, and Y. Yarden. 1999. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol. Cell 4:1029-1040. [DOI] [PubMed] [Google Scholar]
- 29.Malek, R. L., and S. W. Halvorsen. 1999. Ciliary neurotrophic factor and phorbol ester each decrease selected STAT3 pools in neuroblastoma cells by proteasome-dependent mechanisms. Cytokine 11:192-199. [DOI] [PubMed] [Google Scholar]
- 30.Marine, J. C., D. J. Topham, C. McKay, D. Wang, E. Parganas, D. Stravopodis, A. Yoshimura, and J. N. Ihle. 1999. SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell 98:609-616. [DOI] [PubMed] [Google Scholar]
- 31.Mastrandrea, L. D., J. You, E. G. Niles, and C. M. Pickart. 1999. E2/E3-mediated assembly of lysine 29-linked polyubiquitin chains. J. Biol. Chem. 274:27299-27306. [DOI] [PubMed] [Google Scholar]
- 32.Metcalf, D., W. S. Alexander, A. G. Elefanty, N. A. Nicola, D. J. Hilton, R. Starr, S. Mifsud, and L. Di Rago. 1999. Aberrant hematopoiesis in mice with inactivation of the gene encoding SOCS-1. Leukemia 13:926-934. [DOI] [PubMed] [Google Scholar]
- 33.Meydan, N., T. Grunberger, H. Dadi, M. Shahar, E. Arpaia, Z. Lapidot, J. S. Leeder, M. Freedman, A. Cohen, A. Gazit, A. Levitzki, and C. M. Roifman. 1996. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature 379:645-648. [DOI] [PubMed] [Google Scholar]
- 34.Naka, T., M. Narazaki, M. Hirata, T. Matsumoto, S. Minamoto, A. Aono, N. Nishimoto, T. Kajita, T. Taga, K. Yoshizaki, S. Akira, and T. Kishimoto. 1997. Structure and function of a new STAT-induced STAT inhibitor. Nature 387:924-929. [DOI] [PubMed] [Google Scholar]
- 35.Narazaki, M., M. Fujimoto, T. Matsumoto, Y. Morita, H. Saito, T. Kajita, K. Yoshizaki, T. Naka, and T. Kishimoto. 1998. Three distinct domains of SSI-1/SOCS-1/JAB protein are required for its suppression of interleukin 6 signaling. Proc. Natl. Acad. Sci. USA 95:13130-13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicholson, S. E., D. De Souza, L. J. Fabri, J. Corbin, T. A. Willson, J. G. Zhang, A. Silva, M. Asimakis, A. Farley, A. D. Nash, D. Metcalf, D. J. Hilton, N. A. Nicola, and M. Baca. 2000. Suppressor of cytokine signaling-3 preferentially binds to the SHP-2-binding site on the shared cytokine receptor subunit gp130. Proc. Natl. Acad. Sci. USA 97:6493-6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nyman, T. A., S. Matikainen, T. Sareneva, I. Julkunen, and N. Kalkkinen. 2000. Proteome analysis reveals ubiquitin-conjugating enzymes to be a new family of interferon-alpha-regulated genes. Eur. J. Biochem. 267:4011-4019. [DOI] [PubMed] [Google Scholar]
- 38.Oda, H., S. Kumar, and P. M. Howley. 1999. Regulation of the Src family tyrosine kinase Blk through E6AP-mediated ubiquitination. Proc. Natl. Acad. Sci. USA 96:9557-9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parganas, E., D. Wang, D. Stravopodis, D. J. Topham, J. C. Marine, S. Teglund, E. F. Vanin, S. Bodner, O. R. Colamonici, J. M. van Deursen, G. Grosveld, and J. N. Ihle. 1998. Jak2 is essential for signaling through a variety of cytokine receptors. Cell 93:385-395. [DOI] [PubMed] [Google Scholar]
- 40.Ram, P. A., and D. J. Waxman. 1999. SOCS/CIS protein inhibition of growth hormone-stimulated STAT5 signaling by multiple mechanisms. J. Biol. Chem. 274:35553-35561. [DOI] [PubMed] [Google Scholar]
- 41.Saharinen, P., K. Takaluoma, and O. Silvennoinen. 2000. Regulation of the Jak2 tyrosine kinase by its pseudokinase domain. Mol. Cell. Biol. 20:3387-3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sasaki, A., H. Yasukawa, A. Suzuki, S. Kamizono, T. Syoda, I. Kinjyo, M. Sasaki, J. A. Johnston, and A. Yoshimura. 1999. Cytokine-inducible SH2 protein-3 (CIS3/SOCS3) inhibits Janus tyrosine kinase by binding through the N-terminal kinase inhibitory region as well as SH2 domain. Genes Cells 4:339-351. [DOI] [PubMed] [Google Scholar]
- 43.Siewert, E., W. Muller-Esterl, R. Starr, P. C. Heinrich, and F. Schaper. 1999. Different protein turnover of interleukin-6-type cytokine signalling components. Eur. J. Biochem. 265:251-257. [DOI] [PubMed] [Google Scholar]
- 44.Silvennoinen, O., P. Saharinen, K. Paukku, K. Takaluoma, and P. Kovanen. 1997. Cytokine receptor signal transduction through Jak tyrosine kinases and Stat transcription factors. APMIS 105:497-509. [DOI] [PubMed] [Google Scholar]
- 45.Silvennoinen, O., B. A. Witthuhn, F. W. Quelle, J. L. Cleveland, T. Yi, and J. N. Ihle. 1993. Structure of the murine Jak2 protein-tyrosine kinase and its role in interleukin 3 signal transduction. Proc. Natl. Acad. Sci. USA 90:8429-8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Starr, R., T. A. Willson, E. M. Viney, L. J. Murray, J. R. Rayner, B. J. Jenkins, T. J. Gonda, W. S. Alexander, D. Metcalf, N. A. Nicola, and D. J. Hilton. 1997. A family of cytokine-inducible inhibitors of signalling. Nature 387:917-921. [DOI] [PubMed] [Google Scholar]
- 47.Strous, G. J., P. van Kerkhof, R. Govers, A. Ciechanover, and A. L. Schwartz. 1996. The ubiquitin conjugation system is required for ligand-induced endocytosis and degradation of the growth hormone receptor. EMBO J. 15:3806-3812. [PMC free article] [PubMed] [Google Scholar]
- 48.Tanaka, K., and T. Chiba. 1998. The proteasome: a protein-destroying machine. Genes Cells 3:499-510. [DOI] [PubMed] [Google Scholar]
- 49.Verdier, F., S. Chretien, O. Muller, P. Varlet, A. Yoshimura, S. Gisselbrecht, C. Lacombe, and P. Mayeux. 1998. Proteasomes regulate erythropoietin receptor and signal transducer and activator of transcription 5 (STAT5) activation. Possible involvement of the ubiquitinated Cis protein. J. Biol. Chem. 273:28185-28190. [DOI] [PubMed] [Google Scholar]
- 50.Verdier, F., P. Walrafen, N. Hubert, S. Chretien, S. Gisselbrecht, C. Lacombe, and P. Mayeux. 2000. Proteasomes regulate the duration of erythropoietin receptor activation by controlling down-regulation of cell surface receptors. J. Biol. Chem. 275:18375-18381. [DOI] [PubMed] [Google Scholar]
- 51.Wang, D., R. Moriggl, D. Stravopodis, N. Carpino, J. C. Marine, S. Teglund, J. Feng, and J. N. Ihle. 2000. A small amphipathic alpha-helical region is required for transcriptional activities and proteasome-dependent turnover of the tyrosine-phosphorylated Stat5. EMBO J. 19:392-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yasukawa, H., H. Misawa, H. Sakamoto, M. Masuhara, A. Sasaki, T. Wakioka, S. Ohtsuka, T. Imaizumi, T. Matsuda, J. N. Ihle, and A. Yoshimura. 1999. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J. 18:1309-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeh, T. C., E. Dondi, G. Uze, and S. Pellegrini. 2000. A dual role for the kinase-like domain of the tyrosine kinase Tyk2 in interferon-alpha signaling. Proc. Natl. Acad. Sci. USA 97:8991-8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yen, C. H., Y. C. Yang, S. K. Ruscetti, R. A. Kirken, R. M. Dai, and C. C. Li. 2000. Involvement of the ubiquitin-proteasome pathway in the degradation of nontyrosine kinase-type cytokine receptors of IL-9, IL-2, and erythropoietin. J. Immunol. 165:6372-6380. [DOI] [PubMed] [Google Scholar]
- 55.Yokouchi, M., T. Kondo, A. Sanjay, A. Houghton, A. Yoshimura, S. Komiya, H. Zhang, and R. Baron. 2001. Src-catalyzed phosphorylation of c-Cbl leads to the interdependent ubiquitination of both proteins. J. Biol. Chem. 276:35185-35193. [DOI] [PubMed] [Google Scholar]
- 56.Yu, C. L., and S. J. Burakoff. 1997. Involvement of proteasomes in regulating Jak-STAT pathways upon interleukin-2 stimulation. J. Biol. Chem. 272:14017-14020. [DOI] [PubMed] [Google Scholar]
- 57.Zhang, J. G., A. Farley, S. E. Nicholson, T. A. Willson, L. M. Zugaro, R. J. Simpson, R. L. Moritz, D. Cary, R. Richardson, G. Hausmann, B. J. Kile, S. B. Kent, W. S. Alexander, D. Metcalf, D. J. Hilton, N. A. Nicola, and M. Baca. 1999. The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation. Proc. Natl. Acad. Sci. USA 96:2071-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang, J. G., D. Metcalf, S. Rakar, M. Asimakis, C. J. Greenhalgh, T. A. Willson, R. Starr, S. E. Nicholson, W. Carter, W. S. Alexander, D. J. Hilton, and N. A. Nicola. 2001. The SOCS box of suppressor of cytokine signaling-1 is important for inhibition of cytokine action in vivo. Proc. Natl. Acad. Sci. USA 98:13261-13265. [DOI] [PMC free article] [PubMed] [Google Scholar]