Abstract
The androgen receptor (AR) is a nuclear hormone receptor superfamily member that conveys both trans repression and ligand-dependent trans-activation function. Activation of the AR by dihydrotestosterone (DHT) regulates diverse physiological functions including secondary sexual differentiation in the male and the induction of apoptosis by the JNK kinase, MEKK1. The AR is posttranslationally modified on lysine residues by acetylation and sumoylation. The histone acetylases p300 and P/CAF directly acetylate the AR in vitro at a conserved KLKK motif. To determine the functional properties governed by AR acetylation, point mutations of the KLKK motif that abrogated acetylation were engineered and examined in vitro and in vivo. The AR acetylation site point mutants showed wild-type trans repression of NF-κB, AP-1, and Sp1 activity; wild-type sumoylation in vitro; wild-type ligand binding; and ligand-induced conformational changes. However, acetylation-deficient AR mutants were selectively defective in DHT-induced trans activation of androgen-responsive reporter genes and coactivation by SRC1, Ubc9, TIP60, and p300. The AR acetylation site mutant showed 10-fold increased binding of the N-CoR corepressor compared with the AR wild type in the presence of ligand. Furthermore, histone deacetylase 1 (HDAC1) bound the AR both in vivo and in cultured cells and HDAC1 binding to the AR was disengaged in a DHT-dependent manner. MEKK1 induced AR-dependent apoptosis in prostate cancer cells. The AR acetylation mutant was defective in MEKK1-induced apoptosis, suggesting that the conserved AR acetylation site contributes to a pathway governing prostate cancer cellular survival. As AR lysine residue mutations that abrogate acetylation correlate with enhanced binding of the N-CoR repressor in cultured cells, the conserved AR motif may directly or indirectly regulate ligand-dependent corepressor disengagement and, thereby, ligand-dependent trans activation.
Steroid receptors, including the androgen receptor (AR), are members of the nuclear receptor (NR) superfamily which generally function as ligand-dependent transcriptional regulators (9, 31, 84). The AR is expressed in a variety of cell types and plays an important role in development, male sexual differentiation, and prostate cellular proliferation. The functional domains of the AR (termed A to F) are conserved with other members of the classical receptor subclass. The C-terminal region of the AR, including the hinge region and ligand-binding domain (LBD), is responsible for ligand binding and dimerization. The well-conserved DNA binding domain consists of 68 amino acids with two zinc finger structures. The N-terminal region contributes to transcriptional activation through its activation function 1 (AF-1) (5). In contrast to several other hormone-regulated NRs, the AR lacks an intrinsic AF-2 function in the LBD. The LBD, which consists of 12 α helices projecting away from the hormone-binding pocket in the absence of ligand, undergoes substantial conformational changes in the presence of ligand. The folding of the most carboxyl-terminal helix 12 over the ligand-binding pocket in turn creates new structural surfaces that bind coactivators required for efficient transactivation.
Several AR coactivators have been identified, including the p160 proteins, the p300/CREB-binding protein (CBP) family, Ubc9, ARA70, ARA55, and TIP60 (1, 5, 15, 68, 92). The efficient recruitment of coactivators to the AR involves an association between both the AR amino terminus and the LBD (5, 34). The coactivator proteins regulate gene expression through several distinct mechanisms. CBP and the related functional homologue p300 (CBP/p300) convey a bridging function between the DNA-bound transcription factor and the basal apparatus and provide a scaffold to assemble high-molecular-weight enhanceosomes (reviewed in reference 26). In addition, the cointegrator proteins p300/CBP share the capacity to acetylate histones, which correlates, under certain circumstances, with their transcriptional coactivator function (54, 87). Acetylation facilitates binding of transcription factors to specific target DNA sequences by destabilizing nucleosomes bound to the promoter region of a target gene (45, 61). Furthermore, cointegrators may directly acetylate nonhistone proteins, including transcription factor, to regulate their activity (reviewed in references 81 and 87). Acetylation of the tumor suppressor p53 (51), the transcription factor erythroid Kruppel-like factor (95), and the erythroid cell differentiation factor GATA-1 (33) enhanced their transactivation functions. More recently nuclear hormone receptors (NHR) and NR coactivators were shown to serve as targets of acetylation. Direct acetylation of the nuclear receptor coactivator ACTR (18) or estrogen receptor α (86) contributed to ligand-dependent transcriptional attenuation. The AR is acetylated in vitro and in cultured cells at a conserved KLKK motif (23). Mutation of the AR KLKK motif abrogated acetylation of the AR in cultured cells and reduced ligand-dependent activity (23). However, the role of the AR acetylation site in regulating the other diverse functions of the AR is not known.
The AR conveys both trans activation and trans-repression function. The mechanism by which the AR represses expression of other transcription factors is a relatively poorly understood process. The AR inhibits activity of both c-Jun and c-Fos through mechanisms that may involve direct protein-protein interaction or competition for limiting coactivators (62, 76). Several genes downregulated by androgens contain NF-κB binding sites in their promoters, and components of tumor necrosis factor alpha (TNF-α)-dependent antiproliferation required the AR, suggesting an important function for AR-NF-κB cross talk in prostate cancer cells. The AR binds to RelA and attenuates RelA-mediated trans activation at an NF-κB site, consistent with a role for direct physical binding of NF-κB proteins in AR trans repression (62).
The activity of several NHR, such as the thyroid hormone receptor and the retinoic acid receptor, is actively repressed in the absence of ligand by binding to the corepressor protein N-CoR or SMRT. N-CoR and SMRT are largely modular proteins that interact with NHR through their C termini. The N-terminal repression domain interacts with histone deacetylase (HDAC) complexes. Upon recognition of their substrates, acetylated lysine residues of histone, HDACs remove acetyl groups from lysine residues, resulting in a more compact chromatin structure, decreasing the accessibility of the chromatin for transcription factors. In the case of the Rev-erbβ NHR superfamily member, N-CoR contacts both the NHR and the basal transcription apparatus. As some components of the basal apparatus are acetylated, it has been proposed that deacetylation may also decrease transcriptional activity. The mechanisms responsible for repressing the activity of the AR are not well understood. The cell cycle regulatory protein cyclin D1 and an intracellular transducer of transforming growth factor β signaling, Smad3, are reported to inhibit the activity of the ligand-treated AR (29, 44, 73). Smad3 repression of other NRs involves, at least in part, hormone-sensitive interactions with the corepressors N-CoR, SMRT, and Alien, which bind to the SIN3 proteins and recruit HDAC activity (17, 43). The finding that coactivator and corepressor surfaces of NHR overlap substantially (32, 66) is compatible with a dynamic model in which enzymatic modifications of the NR coordinate sequential disengagement of corepressors followed by coactivator binding (56). Whether such processes regulate AR activity remains to be determined.
In addition to responding to ligands, the AR is modified by kinase signaling pathways. The mitogen-activated protein kinase (MAPK) kinase kinase 1 (MEKK1) leads to activation of MKK4 and JNK (46). Constitutively active alleles of MEKK1 can induce cellular apoptosis (25, 89). Activity of the AR is enhanced by constitutively active MEKK1 expression that induces cellular apoptosis in an AR-dependent manner (2). The AR is also posttranslationally modified by sumoylation. The modification of proteins by SUMO-1 (sumoylation) is a reversible, conserved, enzymatic event targeted to a lysine within the motif ψKXE (where ψ represents a large hydrophobic amino acid and X represents any amino acid) within the protein target. Conjugation of the small ubiquitin-like modifier SUMO-1/SMT3C/Sentrin-1 to cellular substrates involves an E1-like enzyme known as SAE1/SAE2. SUMO-1 is transferred from the E1 to a cysteine within the SUMO-specific residue E2-conjugating enzyme (Ubc9). Ubc9 then catalyzes an isopeptide bond between SUMO-1 and the ɛ-amino group of lysine in the target protein. A proportion of the AR is sumoylated in cultured cells (70), and like sumoylation of c-Jun (57), sumoylation of the AR attenuates AR transactivation function (70). The specificity for the target protein of sumoylation is thought to reside with Ubc9 itself. Ubc9 binds the AR within the hinge region (68) that includes the site of direct acetylation, raising the possibility that AR acetylation may in turn affect modification by SUMO-1 and, thereby, transactivation.
The role of AR acetylation in AR trans repression and trans activation for specific target genes and the molecular mechanisms by which the AR acetylation site regulates transcriptional coregulator complex recruitment are unknown. Furthermore, the functional consequence of the AR acetylation site on cellular phenotype is unknown. As similar types of questions either remain to be addressed or are controversial with other transcription factors that are directly acetylated, such as p53 (72), it is likely that analysis of the AR acetylation site may provide insight into the function of other acetylated transcription factors. In this study, we investigated the functional significance of the conserved lysine residue motif within the AR hinge region that was previously shown to serve as a substrate for acetylation by the histone acetyltransferase (HAT) p300 and P/CAF (23). The AR mutations at the acetylation site maintained trans-repression function, bound ligands with wild-type affinity, were sumoylated, and conveyed 80 to 90% of the basal activity of the AR wild type (ARwt). The dihydrotestosterone (DHT)-induced activity of the androgen-responsive reporter genes, prostate-specific antigen (PSA), mouse mammary tumor virus (MMTV), and an androgen-responsive element (ARE) reporter was abrogated. Coactivation by SRC1, p300, Ubc9, and TIP60 was abolished. Binding of the AR mutant to the corepressor N-CoR in the presence of ligand was increased, suggesting a critical role for these lysine residues in ligand-dependent recruitment of N-CoR. Prostate cancer cell lines stably expressing either ARwt or AR acetylation mutants demonstrated a resistance of the AR acetylation site mutant lines to several apoptosis-inducing agents. Mutation of the AR acetylation site abrogated MEKK1-induced apoptosis. These studies indicate that the conserved AR lysine residues, which are acetylated in vitro and in cultured cells, play a role in coordinating a subset of AR functions, including corepressor disengagement, ligand-induced transactivation, and cellular apoptosis.
MATERIALS AND METHODS
Reporter genes and expression vectors.
The expression vector pCMVHA-p300 (4) and the AR-regulated reporter genes PSA-LUC, a 600-bp fragment of the PSA promoter with an additional 2.4-kb enhancer sequence cloned upstream of luciferase (PSA P/E-luc), MMTV-LUC, and ARE-LUC were used (23, 93). The NF-κB-responsive reporter 3xRel-LUC (3), the AP-1-responsive promoter 3xAP-1-LUC, and the (UAS)5-E1B-TATA-LUC reporter were as described previously (21). The wild-type human AR was subcloned from pARO into pcDNA3, and the AR acetylation site mutants ARK630R, ARK630A, ARK(632/633)A, and ARK(630/632/633)R were derived by PCR with sequence-specific primers and cloned into pcDNA3. The integrity of all constructs was confirmed by sequence analysis. The expression vectors for Gal4-Sp1 (65), pCMVSRC1a (38), TIP60 (35), ARA70 (92), ARA55, Ubc9 (68), Smad3, Smad3ΔC (40), pCEP4-MEKK1wt (67), pCMV-FlagN-CoR (6), pCMV-HDAC1 (13), and pCMV-EGFP were previously described.
Cell culture, DNA transfection, and luciferase assays.
Cell culture, DNA transfection, and luciferase assays were performed as previously described (16, 21). The prostate cancer cell line DU145 and the HEK293 cell line were cultured in Dulbecco's minimal essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% penicillin, and 1% streptomycin. In studies of cellular apoptosis with MEKK1, transfections were performed exactly as previously described (2). Cells were plated at a density of 5 × 105 cells in a 60-mm-diameter dish on the day prior to transfection. For DU145 cells, Lipofectamine Plus (Gibco BRL) was used. The DNA-Lipofectamine mix was added to the cells in optimem. Cells were incubated in media containing 10% charcoal-stripped fetal bovine serum prior to experimentation with DHT (23). At least two different plasmid preparations of each construct were used. In cotransfection experiments, a dose response was determined in each experiment with 300 and 600 ng of expression vector and the promoter reporter plasmids (2.4 μg). Luciferase activity was normalized for transfection with β-galactosidase reporters as internal controls. Luciferase assays were performed at room temperature with an Autolumat LB 953 (EG&G Berthold) (88). The fold effect was determined for 300 to 600 ng of expression vector with comparison made to the effect of the empty expression vector cassette, and statistical analyses were performed by using the Mann-Whitney U test.
In vitro SUMO conjugation assays.
SAE2/SAE1, Ubc9, and SUMO-1 were expressed in Escherichia coli B834 and purified as described previously (82). In vitro transcription-translation of proteins was performed by using 1 μg of plasmid DNA and a wheat germ coupled transcription-translation system according to the instructions provided by the manufacturer (Promega, Madison, Wis.). [35S]methionine (Amersham) was used in the reactions to generate radiolabeled proteins. SUMO conjugation assays were performed in 10-μl volumes containing 1 μl of [35S]methionine-labeled substrate (AR or promyelocytic leukemia [PML] protein), 10 μg of SUMO-1, 120 ng of SAE1/SAE2, and 650 ng of Ubc9 in 50 mM Tris (pH 7.5), 5 mM MgCl2, 2 mM ATP, and 10 mM creatine phosphate (containing 3.5 U of creatine kinase/ml and 0.6 U of inorganic pyrophosphatase/ml). The reaction mixtures were incubated at 37°C for 120 min. After termination with sodium dodecyl sulfate (SDS) sample buffer containing β-mercaptoethanol, reaction products were fractionated by electrophoresis in 8% polyacrylamide gels containing SDS, stained, destained, and dried before analysis by phosphorimaging.
Protease sensitivity and ligand-binding assays.
Protease sensitivity assays were performed as previously described (10), with minor modifications. In vitro [35S]methionine-labeled ARwt and ARK630A proteins were prepared by coupled transcription-translation with a Promega TNT coupled reticulocyte lysate kit (Promega) with 1.0 μg of plasmid DNA in a total volume of 50 μl. The translated products were subsequently separated into 22.5-μl aliquots and treated with or without DHT (10 nM) for 20 min at 25°C. The mixtures were then separated into 4.5-μl aliquots and incubated with increasing amounts of trypsin as indicated for 10 min at 25°C. The digestion was terminated by the addition of 20 μl of denaturing gel loading buffer and boiling for 5 min. The products of the digestion were separated by SDS-12% polyacrylamide gel electrophoresis (PAGE) and visualized by autoradiography.
Ligand-binding assays were performed as described previously (79) with equal amounts of in vitro-translated recombinant wild-type AR and mutant ARs diluted to a total volume of 2 μl in ligand-binding buffer (50 mM Tris, 1 mM EDTA, 10% glycerol, 1 mM dithiothreitol [pH 7.5]). Fifty microliters of this dilution was mixed with 50 μl of different concentrations of [1,2,4,5,6,7-3H]DHT (127 Ci/mmol) with or without a 200-fold excess of unlabeled DHT and incubated at 4°C for 2 h. Unbound steroids were removed with HAP (79), and counts per minute were determined by liquid scintillation counting.
Western blots, IP, and electrophoretic mobility shift assays (EMSAs).
Western and immunoprecipitation (IP)-Western blotting were performed as previously described (73). The antibodies used in Western blot analysis were rabbit anti-AR antibody (N-20), the polyclonal p300 antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.) (4), anti-Flag antibody (M2; Sigma) (73), and anti-HDAC1 antibody (Upstate Biotechnology, Lake Placid, N.Y.). The guanine nucleotide dissociation inhibitor antibody (a generous gift from Perry Bickel, Washington University, St. Louis, Mo.) was used as an internal control for protein abundance (47). For the detection of protein, the membrane was incubated with anti-AR (N-20) (1:1,000), anti-p300 (N-20) (1:1,000), anti-HDAC1, and anti-M2 Flag antibody for Flag N-CoR at room temperature for 2 h or at 4°C overnight. The blots were then washed three times with 0.5% Tween 20-phosphate-buffered saline and incubated with the appropriate horseradish peroxidase-conjugated secondary antibody. The proteins were visualized by the enhanced chemiluminescence system (Amersham Pharmacia Biotech). The abundance of immunoreactive protein was quantified by phosphorimaging with an Image Quant, version 1.11, computing densitometer (Molecular Dynamics, Sunnyvale, Calif.).
IP-Western blotting was performed as previously described (73) with the lysates from HEK293 cells transfected with pcDNA3AR, pcDNA3ARK630A, pcDNA3ARK(632/633)A, pCMVHA-p300, pCMV-FlagN-CoR, or an empty expression vector cassette as a control. The cells were treated for 24 h with 100 nM DHT or vehicle. Cells were rinsed with phosphate-buffered saline, harvested by scraping, pelleted, and lysed in buffer (50 mM HEPES [pH 7.2], 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.1% Tween 20, 0.1 mM phenylmethylsulfonyl fluoride, 2.5 μg of leupeptin/ml, 0.1 mM sodium orthovanadate [Sigma]). The extracts were cleared by centrifugation and further precleared by rocking at 4°C with washed protein A-agarose beads (Roche Molecular Biochemicals, Indianapolis, Ind.). The precleared extracts were immunoprecipitated with 0.5 μg of AR antibody or equivalent amounts of the appropriate control immunoglobulin G and 50 μl of protein A-agarose for 8 to 12 h at 4°C. The beads were washed five times with lysis buffer and boiled in SDS sample buffer, and the released proteins were resolved by SDS-PAGE. The gels were transferred to nitrocellulose, and Western blotting was performed.
EMSAs were performed as previously described by using the ARE sequence (74). Oligonucleotides encoding the high-affinity androgen response element (74) (5′-ATG CAT TGG GTA CAT CTT GTT CAC ATA GAC A-3′) and its complementary strand were used for EMSAs as recently described (8). In vitro translation products of ARwt, ARK630A, and ARK630R were generated by using the TNT T7/SP6 Coupled Reticulocyte Lysate system (Promega) and 1 μg of plasmid DNA for ARwt, ARK630A, and ARK630R. The level of the ARs synthesized was assessed by Western blot analysis and shown to be similar. The binding reaction was performed with 3 μl of in vitro-translated AR, 100 fmol of [γ-32P]ATP-labeled probe, and 1.0 μg of poly[d(I-C)] in 20 mM Tris (pH 7.9), 0.5 mM EDTA, 2.5 mM MgCl2, 1.5 mM dithiothreitol, 100 mg of Pefabloc SC/ml, 10% glycerin, 100 nM DHT, and 200 μM ZnCl2. The reaction mixture was incubated on ice for 30 min. The complexes were separated on 5% polyacrylamide gels in 0.5× Tris-borate-EDTA. The gels were vacuum dried at 80°C for 2 h, and the protein-DNA complexes were visualized by autoradiography.
Apoptosis assays.
The thiazolyl blue (MTT) assay, which is a quantitative colorimetric assay for mammalian cell survival and proliferation, was performed as previously described (93). Briefly, 2 × 103 cells of the DU145 stable cell lines for ARwt, ARK630A, and ARK(632/633)A were grown in 96-well plates in 50 μl of DMEM with 10% charcoal-stripped FBS. After 24 h, the cells were treated with either vehicle or 10 nM DHT for approximately 20 to 30 min to engage the endogenous AR (71) and then treated with either cycloheximide (10 μg/ml), TNF-related apoptosis-inducing ligand (TRAIL) (10 ng/ml) (R&D Systems, Minneapolis, Minn.), TNF-α (10 ng/ml) (Promega), or tetradecanoyl phorbol acetate (TPA) (100 ng/ml). After 6 h, 20 μl of MTT (5 mg/ml; Sigma) was added to each plate for 3 h at 37°C. After incubation, 200 μl of 0.04 N HCl in isopropanol was added to each well. After several rounds of pipetting and 5 min of incubation at room temperature, the absorbency was read at a test wavelength of 490 nm. Comparisons were made within each cell line to the vehicle-treated standard (control), as it could not be assumed that cellular division rates were identical between the cell lines (78).
In separate experiments, the stable cell lines were treated as described above and then analyzed directly for morphological features of apoptosis after direct fixation of all cells with 10% paraformaldehyde. This procedure ensured fixation of both adherent and nonadherent cells that had undergone apoptosis. At least three high-power fields chosen at random were assessed for each treatment to ensure the counting of at least 300 cells. The percent apoptotic cells was scored for blebbing and chromatin condensation. Data are shown as the relative change in apoptosis by the inducing agent in the presence of 10 nM DHT.
In studies with MEKK1, apoptosis was detected by morphological analysis of green fluorescent protein (GFP)-transfected cells (2). Equal amounts of GFP were transfected into the wells with either the ARwt or the AR mutants; thus, GFP itself was not an independent variable between the groups compared. At least 200 cells were counted by using a fluorescent microscope, and cells were scored for blebbing and chromatin condensation by an investigator blinded to the experimental condition. At 24 h posttransfection, cells were treated with either 100 nM DHT or vehicle, and at 48 h, the cells were rinsed with phosphate-buffered saline, fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.5% Triton X-100, and stained for DNA with Hoechst 3325 dye (5 μg/ml).
RESULTS
DHT-induced AR activity involves lysine residues acetylated in vitro.
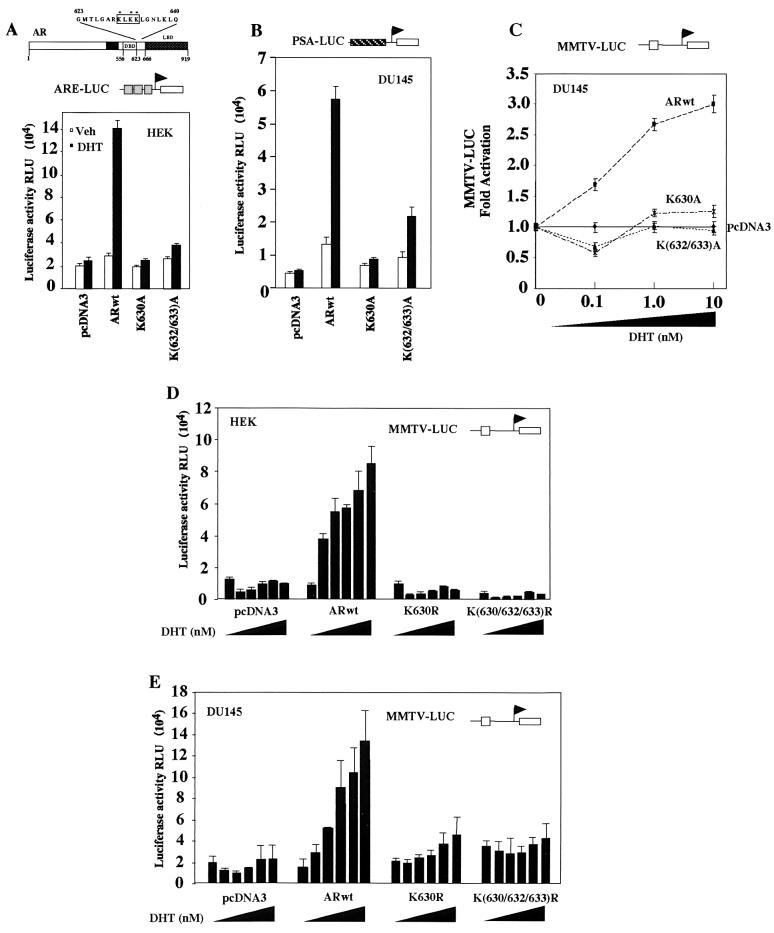
In previous studies, the AR was shown to be acetylated in cultured cells with anti-acetyl lysine antibodies (23). Either p300 or P/CAF was capable of acetylating the AR in vitro, and Edman degradation analysis of the acetylated AR products demonstrated that lysines 630, 632, and 633 were preferentially acetylated, constituting an acetylation motif that is conserved between species (Fig. 1A). Only monoacetylated lysine-containing peptides were detected in the samples by matrix-assisted laser desorption ionization-time of flight mass spectrometry, indicating that the product analyzed by Edman degradation was a heterogeneous population of peptides, each acetylated at a single site. We assessed the role of the in vitro AR acetylation sites in ligand sensitivity with point mutants of the AR acetylation sites and reporter genes encoding a specific ARE (ARE-LUC), an endogenous androgen-responsive gene (PSA-LUC), or the androgen-responsive MMTV-LUC.
FIG. 1.
The AR acetylation site is required for DHT-mediated activation of androgen-responsive reporter genes. (A) Schematic representation of the human AR indicating the DNA binding domain (DBD), the LBD, and the conserved KXKK motif (indicated by asterisks). The androgen- responsive reporter gene ARE-LUC (A), PSA-LUC (B), or MMTV-LUC (C) was transfected into DU145 cells with either the ARwt or AR acetylation site mutant expression plasmids. The cells were treated for 24 h with either vehicle, DHT (10−7 M), or DHT at concentrations ranging from 0.1 to 10 nM (C) as indicated. Luciferase activity was determined, and the data are shown as means ± standard deviations for at least six separate transfections. The MMTV-LUC reporter was transfected into DU145 (D) or HEK293 (E) cells either alone or with the expression plasmid encoding ARwt or the AR acetylation site mutant (arginine substitution) expression plasmids. The cells were treated with either vehicle or DHT (0.01, 0.1, 1, 10, 100 nM) as indicated for 24 h, and luciferase activity was determined. RLU, relative luciferase units.
In the presence of DHT, ARE-LUC activity was induced six- to sevenfold by the ARwt compared with the empty pcDNA3 vector but was induced less than 50% by either of the AR acetylation site mutants (Fig. 1A). We next assessed the role for the AR lysine residues in regulating basal and ligand-treated AR function with the androgen-responsive PSA gene promoter linked to the luciferase reporter gene in the prostate cancer cell line DU145 (Fig. 1B). The ARwt enhanced basal AR activity 2.5-fold. The addition of DHT (10−6 M) induced AR activity 4.3-fold. The expression plasmid encoding the AR acetylation site mutant (ARK630A) increased basal AR activity 1.5-fold compared with the empty expression vector cassette; however, there was no significant increase in DHT-induced activity of the PSA-LUC reporter. Expression of the ARK(632/633)A construction enhanced basal AR activity 1.7-fold; however, DHT-induced activity was reduced 50 to 60% compared with the wild type (Fig. 1B). To assess whether ligand sensitivity was affected at subphysiological concentrations, dose-response curves were examined. The androgen-responsive MMTV-LUC reporter was induced threefold by ARwt compared with the empty pcDNA3 vector with 1 to 10 nM DHT in DU145 cells; however, the AR acetylation mutants were not induced (Fig. 1C).
Similar studies were performed with point mutants in which the AR lysine residues were replaced with arginine (Fig. 1D and E). In HEK cells, the MMTV-LUC reporter was induced by ARwt four- to fivefold; however, the arginine substitution mutants were not induced by DHT. Similar observations were made in DU145 prostate cancer cells (Fig. 1E). In previous studies of DU145 cells, it has been shown that the ligand-treated AR does not induce either the pA3LUC vector or several other luciferase reporter genes (RSV-LUC, cyclin E-LUC, and c-Fos-LUC) (73), suggesting that the induction of the PSA-LUC and MMTV-LUC reporters is promoter specific. The expression plasmids encoding point mutations of the AR acetylation site were shown to be expressed in numbers equal to those of the wild type in cultured cells (see Fig. 4G, left panel). Together these studies suggest that the AR acetylation sites reduce both basal and ligand-induced activity of androgen-responsive reporter genes.
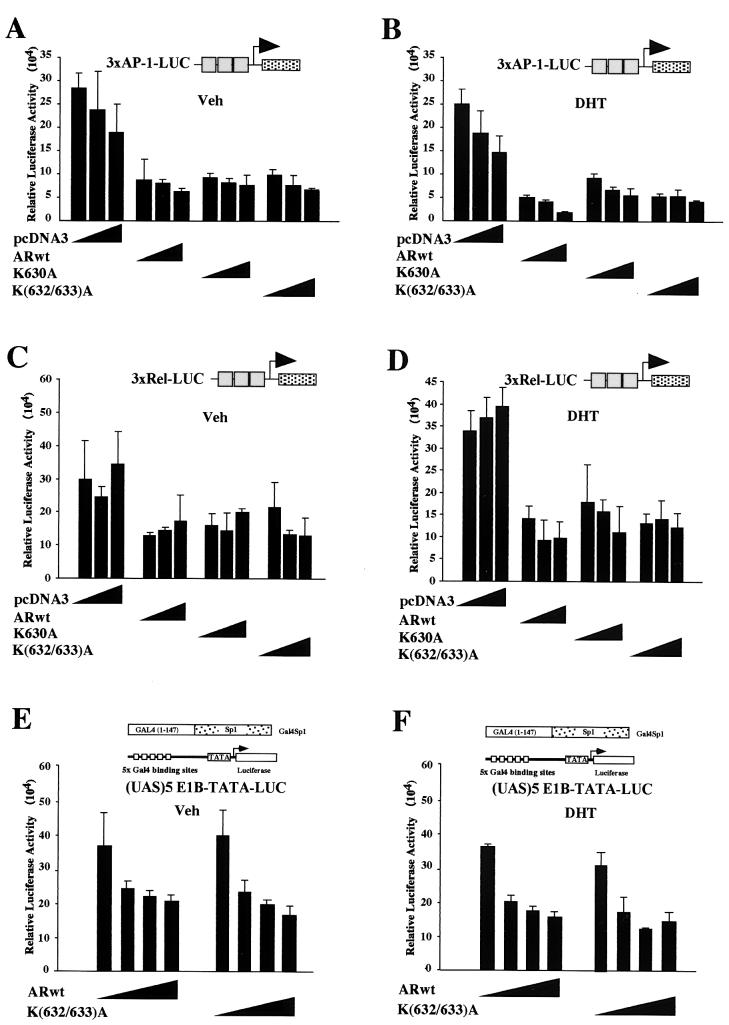
FIG. 4.
The AR acetylation site regulates coactivator-mediated induction of ligand-treated AR. The MMTV-LUC reporter was cotransfected with expression vectors for either the wild-type or mutant AR and the candidate AR coactivators TIP60 (A), SRC1 (B), ARA55 (C), ARA70 (D), Ubc9 (E), and p300 (F) or equal amounts of the empty expression vector cassettes (pCMV). The cells were treated with either DHT (10−7 M) or vehicle for 24 h, and luciferase activity was assessed. The data are shown as the means ± standard deviations for at least six separate transfections. DHT treatment (10−7 M) was for 24 h. (G) The ARwt and AR acetylation site mutant were transfected into HEK293 cells, and Western blotting was performed for AR, p300, and the loading control guanine nucleotide dissociation inhibitor (GDI). In the right panel, equal amounts of the cellular extracts were immunoprecipitated with the AR antibody, and Western blotting was performed with either the AR or the p300 antibody. +, present; −, absent.
The AR acetylation site does not regulate AR trans-repression function.
The AR inhibits AP-1 activity in different cell types, which likely involves several different mechanisms (39, 76, 77). The ability of the AR to inhibit AP-1 activity was assessed by using the AP-1 site from the collagenase promoter. The ARwt and the AR acetylation mutants inhibited AP-1 activity approximately 60 to 75% (Fig. 2A). In the presence of DHT, ARwt repressed AP-1 activity by 80% and the AR mutants repressed AP-1 activity by 70% (Fig. 2B). Several genes downregulated by androgen contain NF-κB binding sites, and the AR attenuates NF-κB reporter activity in a dose-dependent manner (62). We assessed NF-κB activity with the well-characterized 3xRel-LUC reporter gene (3). The ARwt and the AR mutants inhibited 3xRel-LUC reporter activity 50% in the absence of ligand and 60 to 70% in the presence of DHT (Fig. 2C and D). The AR forms a physical interaction with Sp1 and shares a common coactivator, SNURF (52, 69). The ability of the ARwt to regulate Sp1 function was examined in DU145 cells with a heterologous reporter gene system. The Sp1 coding region was linked to the Gal4 DNA binding domain, and activity was assessed with a heterologous reporter consisting of multimerized Gal4 DNA binding sites linked to the luciferase reporter gene (UAS)5-E1B-TATA-LUC (Fig. 2E). The ARwt repressed Sp1 activity 30 to 50%, and similar repression was observed with the AR acetylation mutant (Fig. 2E and F). Together these studies suggest that the ability of the AR to trans repress the activity of several distinct signaling pathways is not affected by the mutation of the AR acetylation site.
FIG. 2.
The trans-repression function of the AR acetylation site mutants is preserved. The expression vectors for either the wild-type or mutant AR or equal amounts of the empty expression vector cassettes (pCMV) were cotransfected into HEK293 cells with the luciferase reporter genes for AP-1 (3xAP-1-LUC) (A and B), NF-κB activity (3xRel-LUC) (C and D), or the (UAS)5-E1B-TATA-LUC heterologous reporter (E and F), and the chimeric transcription factor for Sp-1 was fused to the Gal4 DNA binding domain. The cells were treated with either DHT (10−7 M) or vehicle (Veh) for 24 h, and luciferase activity was assessed. The data are shown as means ± standard deviations. The DHT treatment (10−7 M) was for 24 h.
In vitro sumoylation and DNA binding of the AR are not dependent upon the AR acetylation site.
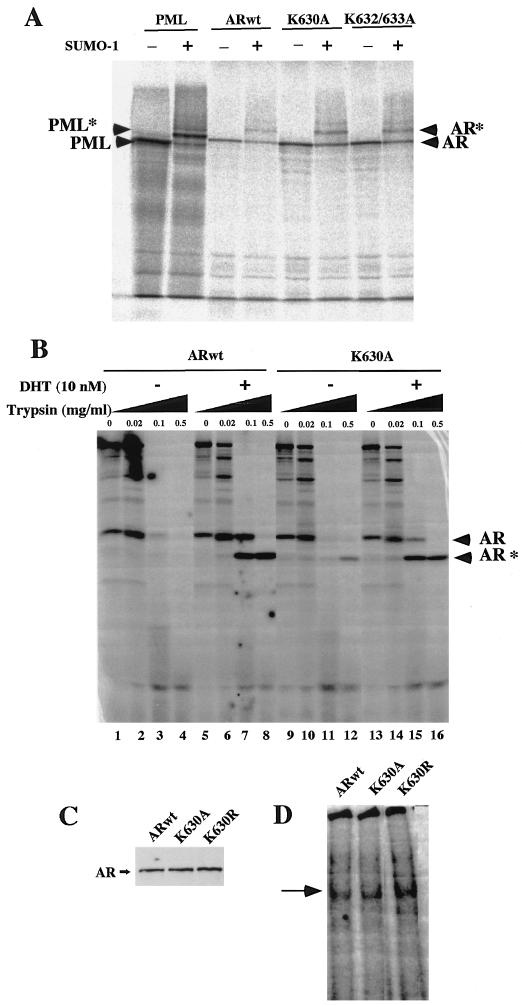
In order to investigate the mechanisms responsible for the defect in ligand-dependent transactivation of the AR acetylation site mutants, we examined the possibility that the acetylation site may regulate AR sumoylation, AR gross structure in the presence of ligand, and DNA binding. Acceptors of SUMO-1 modification are not targeted for degradation, unlike the majority of ubiquitinated proteins; however, the transcriptional activity of specific proteins appears to be affected. The AR binds Ubc9 in a region that includes the AR acetylation motif (68), and Ubc9 has the capacity to serve as an E3 for sumoylation. The ligand-dependent transactivation of the AR is regulated by sumoylation (70); therefore, we investigated the possibility that the AR acetylation site may play a role in the defective ligand-dependent transactivation of the AR acetylation mutants. Modification of the PML protein by sumoylation targets it to distinct nuclear bodies (20), and 35S-labeled in vitro-translated PML serves as an ideal substrate for in vitro sumoylation assays with Ubc9 (82). As expected, PML was modified by SUMO-1 that occurred at several sites (Fig. 3A). The ARwt was also sumoylated, and there was no significant difference in the pattern or proportion of sumoylation of the AR acetylation mutants (Fig. 3A).
FIG. 3.
AR in vitro sumoylation, protease cleavage pattern, and in vitro DNA binding are not affected by the AR acetylation site. (A) The in vitro SUMO-1 conjugation assays were conducted as described previously (82) with in vitro-translated 35S-labeled PML, ARwt, or AR acetylation mutants as indicated. The unconjugated proteins are indicated by arrows, and the SUMO-1-conjugated forms are indicated by asterisks. (B) Protease sensitivity assays were conducted with ARwt or AR mutants by using increasing concentrations of trypsin and either vehicle or DHT as indicated. The full-length in vitro-translated 35S-labeled AR and the major trypsin digestion products are shown. (C) Equal amounts of in vitro-translated 35S-labeled AR (shown by autoradiography) were used in an EMSA with a 32P-labeled high-affinity ARE (74). The DNA-protein complex of delayed migration is indicated by an arrow. +, present; −, absent.
It is known that the addition of DHT induces a conformational change in the AR and that ligand-induced conformational changes of NHR can be assessed by limited proteolytic digestion (10). To determine whether the AR lysine substitution mutations altered conformation of the full-length AR protein in the presence of ligand, limited proteolytic digestion was performed comparing the ARwt and the ARK630A. Trypsin addition induced a dose-dependent cleavage pattern, with a specific pattern induced in the presence of ligand (Fig. 3B, lanes 3 and 4 versus lanes 7 and 8). The proteolytic pattern of the ARK630A mutant in the presence or absence of ligand was similar to that of the ARwt (Fig. 3B, lanes 7 and 8 versus lanes 15 and 16). The induction of trypsin cleavage upon ligand addition was consistent with the similar in vitro ligand-binding properties of the ARwt and the AR mutant within the physiological range of the ligand (for ARwt, kDa = 0.84 nM; for ARK630A, kDa = 1.3 nM). In addition, the DNA binding properties of the AR mutants were assayed. As several different cellular proteins have been proposed to regulate the DNA binding properties of the AR, we performed EMSAs with an ARE previously identified as a selective AR-binding site (74) and compared binding by using equal amounts of in vitro-translated full-length AR protein (Fig. 3C). A specific DNA-protein complex formed in the presence of the ARwt (Fig. 3D) that was competed by a specific cognate competitor (data not shown) and which was of mobility and intensity similar to those of the mutant ARs.
Mutation of the conserved AR lysine residue abrogates coactivator-dependent induction.
Several distinct coactivators have been described which can enhance AR activity in either the presence or the absence of ligand. To investigate the possibility that reduced ligand-dependent transactivation of the AR acetylation mutants was due to a selective defect in activation of the AR by a previously described coactivator, transient expression studies were conducted in DU145 cells with the androgen-responsive MMTV-LUC reporter gene. TIP60 encodes an AR-interactive protein with a domain homologous to the HAT domain of p300 and conveys DNA-dependent ATPase activity that plays a role in DNA repair (35). The activity of the ARwt was augmented 4-fold by TIP60 in the absence of ligand and 12-fold in the presence of ligand (Fig. 4A). The basal activity of the AR mutant ARK630A was induced fourfold by TIP60; however, in the presence of ligand there was no significant additional induction (Fig. 4A). SRC1 binds the non-ligand-treated AR through AF-1 (12), recruits p300 (91), and is known to augment ligand-treated AR function in fibroblasts (12). Coexpression of SRC1 (38) increased the activity of the ligand-treated ARwt sixfold, whereas the ARK630A was not induced (Fig. 4B). The AR-binding proteins, ARA55 and ARA70, induced AR activity in some but not all studies (12, 92). In the present studies, neither ARA55 nor ARA70 induced either the ARwt or the AR mutants (Fig. 4C and D). The ARK(632/633)A mutant was also defective in ligand-induced activation by each of the coactivators (data not shown). We examined Ubc9, a homologue of the class E2 ubiquitin-conjugating enzymes, which is thought to function as an AR transactivator independently of its ubiquitin conjugation function (68). The AR 629 to 633 region was necessary for Ubc9-induced AR activity. Ubc9 augmented ligand-dependent activation of the ARwt three- to fourfold, whereas activity of ARK630A was not enhanced by coexpression of Ubc9 (Fig. 4E). Consistent with CBP induction of the AR in CV1 cells (1), coexpression of p300 increased DHT-induced activity three- to fourfold (Fig. 4F). In contrast, the ARK630A mutant was not significantly induced by p300 in the presence of ligand (Fig. 4F). Together, these studies suggest that the AR acetylation mutants are defective in transactivation by several distinct AR coactivators.
Several models have been proposed to explain the mechanisms by which p300 augments NR activity. p300 may augment ligand-treated AR activity either by bridging AF-1 and AF-2 (34) or by interacting with the AR N terminus and the LBD (22). Alternatively, p300 may, upon recruitment through SRC coactivators (83, 85), facilitate histone acetylation and basal machinery interactions to promote gene expression. To determine whether failed activation of the ARK630A by p300 was due to reduced binding in cultured cells, IP-Western blotting was performed. The ARwt and the AR mutants were transfected into AR-deficient HEK293 cells with the p300 expression vector. The ARwt and the mutants showed similar levels of expression by Western blotting, and p300 levels were also similar (Fig. 4G, left panel). IP showed equal amounts of the AR in the immunoprecipitate (Fig. 4G, right panel). The amount of p300 associated with AR was reduced in the ARK630A and ARK(632/633)A mutants compared with ARwt (Fig. 4G, lane 4 versus lanes 5 and 6).
TSA induction and DHT-induced AR activity involve lysine residues acetylated in vitro.
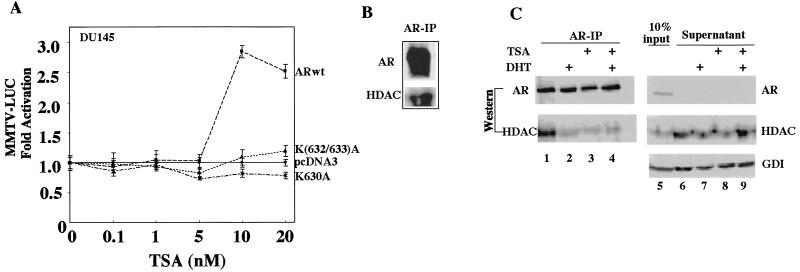
In previous studies, the androgen-responsive synthetic reporter gene MMTV-LUC was shown to be induced in the presence of ARwt by the specific HDAC inhibitor trichostatin A (TSA) (23, 53). We therefore assessed the role of the in vitro AR acetylation sites on TSA sensitivity with point mutants of the AR acetylation sites and the androgen-responsive reporter gene MMTV-LUC in the human prostate cancer cell line DU145. Increasing concentrations of TSA induced the ARwt 2.5 to 3-fold at 10 to 20 nM compared with the empty vector (pcDNA3) (Fig. 5A). In contrast, neither of the AR acetylation site mutants, ARK630A and ARK(632/633)A, was induced by TSA. TSA failed to augment ligand-treated AR activity (23), suggesting that the mechanisms governing ligand-treated activity and TSA sensitivity involve a common pathway. As TSA is a specific inhibitor of HDACs, we investigated the possibility that HDAC1 may physically associate with the AR in vivo. Cellular extracts from the murine liver, which expresses the AR (73), were immunoprecipitated with an AR-specific antibody (73). Western blotting of the AR with an HDAC1-specific antibody demonstrated the presence of HDAC1 in the AR IP (Fig. 5B). The addition of DHT (10−7 M) reduced the abundance of HDAC1 in the AR IP (Fig. 5C, lane 1 versus lane 2). TSA treatment also reduced the amount of HDAC1 coprecipitated with the AR (Fig. 5C, lane 1 versus lane 3). The addition of TSA to DHT did not significantly alter the amount of HDAC1 bound to the AR (Fig. 5C, lane 3 versus lane 4). The treatment with DHT and TSA did not affect the abundance of HDAC1 in the cells (Fig. 5C, lane 6 versus lanes 7 and 8). Thus, HDAC1 coprecipitates with the AR in vivo and in cultured cells and the association between HDAC1 and the AR is regulated by DHT or TSA.
FIG. 5.
TSA induction of the AR involves the AR acetylation site. (A) The expression plasmids encoding the wild-type and mutant AR were transfected into DU145 cells with the MMTV-LUC reporter. The cells were treated with either vehicle or TSA at the dose indicated for 24 h, and luciferase activity was determined. The data are means ± standard errors of the means for at least six separate transfections. (B) IP was performed with murine hepatic extracts with an AR-specific antibody, with sequential Western blotting for either AR or HDAC1. (C) Hepatocellular extracts were treated with either vehicle, TSA (30 nM), or DHT (100 nM) and subjected to IP with an AR-specific antibody. The AR IP was electrophoresed on an SDS-PAGE gel, and Western blotting was performed for AR or HDAC1 (lanes 1 to 4). In the right panel (lanes 5 to 9), the supernatant was analyzed by Western blotting. The absence of AR in the IP supernatant indicates that the IP is saturating. Similar levels of HDAC1 are seen in the supernatant treated with vehicle, TSA, or DHT. +, present; GDI, guanine nucleotide dissociation inhibitor.
Increased N-CoR binding of the AR acetylation mutant.
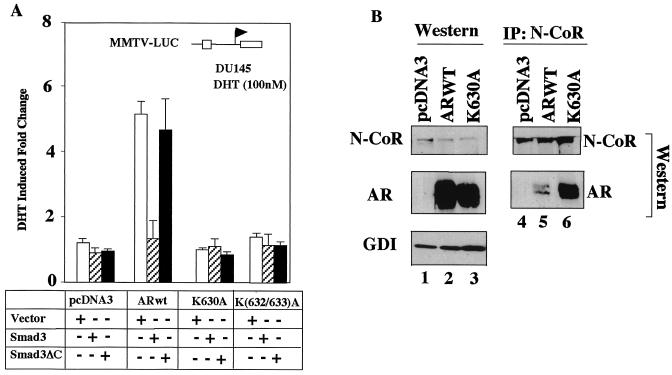
HDAC1 is found in a large transcriptional regulatory complex that includes the corepressors mSIN3 and N-CoR (6, 30, 59). In recent studies, N-CoR was shown to bind Smad3 (reviewed in reference 43) and Smad3 was shown to inhibit ligand-induced AR activity in CV1 cells (29). We therefore investigated the possibility that Smad3 may repress ligand-induced AR activity in prostate cancer cells. Cotransfection of the AR with Smad3 showed that the ligand-treated AR activity was repressed 70% by Smad3 (Fig. 6A). The carboxyl terminus of Smad3 was required for protein-protein interaction with the AR (29). The C-terminal deletion mutant of Smad3 (Smad3ΔC) failed to repress the activity of the ligand-treated AR. Smad3 had no effect on the activity of either ARK630A or ARK(632/633)A (Fig. 6A). Smad3 repression is thought to be mediated through N-CoR/HDAC1. As Smad3 repressed ARwt to the level of activity seen for ARK630A or ARK(632/633)A, we considered the possibility that the reduced activity of the ligand-treated AR acetylation mutants may be a function of their enhanced binding to corepressors of the Smad3/N-CoR complex. As Smad3 repression is thought to involve N-CoR binding (29, 43), we examined the interaction between N-CoR and the AR. IP-Western blotting was performed with HEK293 cells transfected with equal amounts of Flag-tagged N-CoR and AR. Western blotting showed that similar levels of N-CoR were expressed in transfected cells and that the expression levels of the ARwt and AR mutant were similar (Fig. 6B, lanes 2 and 3). IP with the anti-Flag antibody showed similar amounts of N-CoR by IP (Fig. 6B, lanes 4 to 6). The amount of N-CoR bound to ARK630A was 10-fold higher than that with the ARwt in the presence of DHT (Fig. 6B, right panel, lane 5 versus lane 6).
FIG. 6.
Enhanced N-CoR binding of the AR acetylation site mutant in cultured cells. (A) Smad3 binds components of the HDAC regulatory complex (HDAC1, N-CoR). Smad3 or a Smad3 mutant with the carboxyl terminus deleted (Smad3ΔC) was transfected with the MMTV-LUC reporter into DU145 cells with either ARwt or the AR acetylation site mutant, and the cells were treated with DHT for 24 h. (B) The binding of N-CoR to the AR was determined by IP-Western blotting. HEK293 cells transfected with expression vectors for FlagN-CoR and either ARwt or the AR acetylation site mutant were assessed by Western blotting in the presence of DHT for expression levels. Similar levels of AR are shown by Western blotting. Equal amounts of cell extracts were subjected to IP with anti-Flag antibody (M2) to precipitate N-CoR and Western blotting performed for Flag (N-CoR) and the AR. AR immunoreactivity is detected in the N-CoR IP. +, present; −, absent; GDI, guanine nucleotide dissociation inhibitor.
The AR acetylation site regulates AR function induced by JNK in prostate cancer cells.
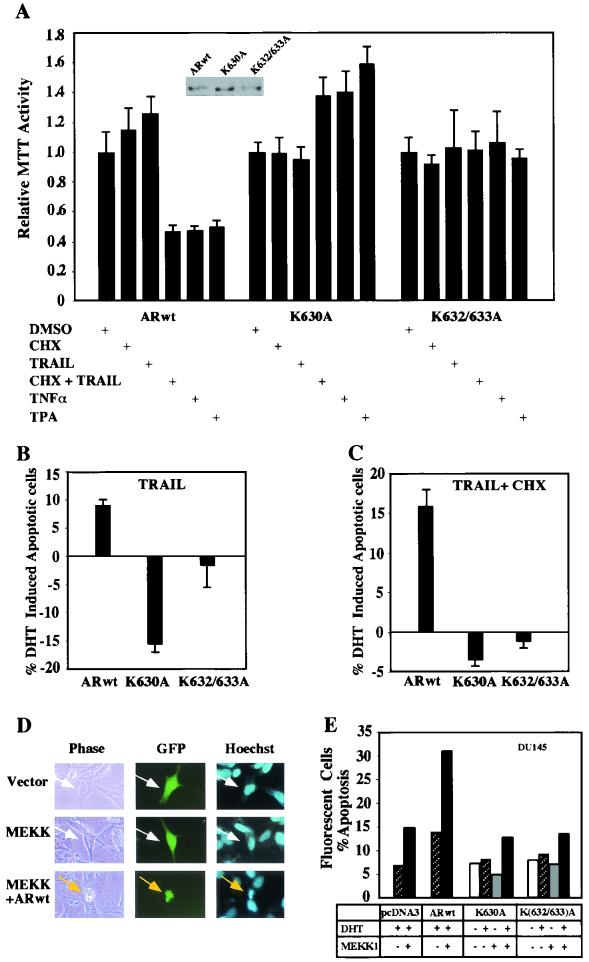
It is known that the AR can induce cell cycle arrest or apoptosis when introduced into AR-negative cells and that overexpression of the AR in transgenic animals is associated with increased cellular apoptosis (11, 80). TRAIL and TNF-α in the presence of cycloheximide induce prostate cancer cellular apoptosis (7, 58, 60, 94). To further investigate the functional properties of the AR acetylation site, stable prostate cancer cell lines (DU145) expressing either ARwt, AR acetylation site mutant, or control vector pcDNA3 were made and examined for responses to cellular apoptosis-inducing agents. Analyses were performed with at least three separate experimental analyses of each line, and the findings were representative of at least three separate stable lines. Initial experiments were conducted with the MTT assay. The cells were treated with either vehicle or DHT (100 nM) for approximately 30 min to engage the AR with ligand (71), and then the cells were treated with apoptosis-inducing agents. Treatment of the ARwt DU145 cells with TRAIL (50 ng/ml) in the presence of cycloheximide (2 μM) inhibited MTT activity 55% compared with vehicle, and either TNF-α or TPA inhibited activity by 40 to 50% (Fig. 7A). In contrast, the AR acetylation mutant stable lines showed no change in activity with any of these treatments (Fig. 7A) (n = 6, P < 0.01).
FIG. 7.
Induction of apoptosis and the AR acetylation site. (A) Induction of apoptosis by TRAIL is reduced in stable cell lines expressing the AR acetylation site mutants. DU145 cells stably expressing either ARwt, ARK630A, or ARK(632/633)A were grown in 96-well plates in 50 μl of DMEM with 10% charcoal-stripped FBS. After 24 h, the cells were treated with vehicle or 100 nM DHT and then treated with either cycloheximide (CHX; 10 μg/ml), TRAIL (10 ng/ml), TNF-α (10 ng/ml), or TPA (100 ng/ml) as indicated. The MTT assay was performed after 6 h. The data are shown as means ± standard deviations for six separate experiments. Vehicle-treated cells are established as 100%. (B and C) DU145 stable lines were treated with vehicle or 100 nM DHT for 30 min, and then apoptosis-inducing agents were added as indicated, and apoptotic cells were scored (see Materials and Methods). Data are shown as the relative change in apoptosis by the inducing agent in the presence of 100 nM DHT. A representative experiment is shown. (D) Induction of apoptosis by Jun kinase kinase (MEKK1) through the AR is abrogated by the mutation of the AR acetylation site. DU145 cells were transfected with expression vectors for the AR, MEKK1, and pCMV-GFP or equal amounts of empty vector control. The cells were treated for 24 h with DHT and scored for apoptosis 48 h posttransfection as previously described (2). The morphology of the transfected DU145 cells is shown, in phase contrast. White arrows indicate GFP-positive cells, and the yellow arrows indicate GFP-positive cells with chromatin condensation. (E) The graph represents independent experiments in which 200 green fluorescent cells were counted and scored for cytoplasmic blebbing and chromatin condensation. The results are representative of three separate experiments. +, present; −, absent; DMSO, dimethyl sulfoxide.
Direct measurement of morphological features of apoptosis was also performed, as both cellular proliferation and apoptosis may affect activity in the MTT assay. Cells may become detached upon apoptosis and may become underrepresented in analysis; therefore, cells were fixed in a manner to include both adherent and nonadherent cells and scored for apoptosis by morphological features in separate experiments (48, 78). The cells were pretreated with vehicle or 100 nM DHT for 30 min and then treated with apoptosis-inducing agent for 6 h (Fig. 7B and C). At least 300 cells were scored in three independent high-power fields as described in Materials and Methods. The relative apoptosis rate of the ARwt-expressing stable DU145 cells was increased by either TRAIL or TRAIL with cycloheximide in the presence of ligand compared with the vehicle control. In contrast, the stable DU145 cell line expressing the AR acetylation mutants did not show the same induction of apoptosis in the presence of DHT (Fig. 7B and C).
Transient expression studies were also conducted to examine the role of the AR acetylation site in AR mutant proteins. To assess the role of the AR acetylation site in AR-mediated apoptosis, a previously described experimental approach was used (2). AR activity is induced by the MAPK kinase kinase, MEKK1, which induces phosphorylation of MKK4 (SEK1) and JNK as well as IκB kinase (37, 46). Activation of MEKK1 induces apoptosis of prostate cancer cells in an AR-dependent manner through the JNK pathway (2). To examine the functional significance of the AR acetylation site in MEKK1-induced prostate cellular apoptosis, studies were performed in DU145 cells exactly as previously described (2). Cells were cotransfected with either wild-type or mutant ARs and either the MEKK1 expression vector or control empty vector. A GFP expression vector was used to monitor the transfected cells. The cells were treated for 24 h with either DHT (10−7 M) or control vehicle and scored for apoptosis with morphological markers of blebbing or nuclear condensation (Fig. 7D). The expression of either the ARwt or MEKK1 alone did not affect basal apoptosis compared with equal amounts of empty vector cassette (Fig. 7E). In contrast, the coexpression of MEKK1 and the ARwt in the presence of ligand induced apoptosis to approximately 30% as previously described (2). Coexpression of MEKK1 with either ARK630A or ARK(632/633)A resulted in an apoptosis rate similar to that of vector controls (Fig. 7E). These studies demonstrate an important function of the AR acetylation site in regulating MEKK1-dependent apoptosis in cultured human prostate cancer cells.
DISCUSSION
The recent findings that nonhistone proteins serve as direct targets of histone acetylases have led to further mechanistic analysis of the substrate residues in transcription factor function (81, 86). The AR conveys both trans-repression and trans- activation function. In the present studies, the AR acetylation site selectively regulated the AR trans activation but not the trans-repression functions. The sumoylation of the AR was unaffected by the mutation of the AR acetylation site in vitro. Ligand-induced conformational changes, assessed by limited protease digestion, showed a similar pattern induced by the ligand in the ARwt and the AR acetylation mutant. Intriguingly, the AR acetylation mutants showed increased N-CoR binding and reduced p300 binding compared with the wild-type AR. As p300 and N-CoR regulate ligand-dependent gene expression of other NHR, these studies suggest that the AR lysine residues may play an important role in a subset of AR functions by regulating recruitment of hormone-dependent coregulators.
The role of direct transcription factor acetylation in hormone signaling to endogenous androgen-regulated target genes was largely unknown. In the present studies, point mutation of the AR lysine residues, which was previously shown to abrogate acetylation of the full-length AR protein (23), substantially reduced DHT-dependent activation of several androgen-responsive reporters, including the PSA promoter, the MMTV promoter, and a simple synthetic androgen response element. PSA, an endogenous androgen-regulated target gene, is a prostate-specific kallikrein, the expression of which is used to monitor patients with prostate cancer (24). The PSA promoter has been well characterized as an androgen-responsive gene. In the present studies, mutation of the AR acetylation site reduced the transcriptional activity of androgen-responsive reporter gene coactivation by several AR regulators (Ubc9, SRC1, TIP60). Ubc9 trans activation of the AR occurs independently of its ubiquitin-ligase function. The failure of Ubc9 to induce ARK630A activity is consistent with previous findings that the AR hinge region, which includes the lysine motif, is required for Ubc9 binding and activation (68). The interaction of SRC1 with the AR involves the glutamine-rich region of SRC1 and the AF-2 region of the AR. Coactivation of AR by SRC1 requires AD1 of SRC1, the same region responsible for recruitment of p300/CBP, and does not require the LXXLL motif that interacts with AF-2 (12). Thus, failed SRC1 coactivation of the acetylation site mutants is consistent with defective p300 coactivation. TIP60 binds the AR through a region which includes the acetylation site (15), conveys intrinsic HAT activity (15, 90), and regulates DNA repair and apoptosis (35). As TIP60 augmented the activity of the ligand-treated ARwt but not the AR mutant and MEKK1 augmented AR apoptosis in the presence of ligand, it will be of interest to further evaluate the role of TIP60 in AR-mediated apoptosis.
What common features shared by this subset of coactivators (SRC1, Ubc9, TIP60, and p300) might explain their reduced ligand-dependent coactivation of the AR acetylation site mutants? The bromodomains of several coactivators serve as recognition motifs providing recruitment to acetylated lysine residues (36), and it is feasible that one or more of the bromodomain-containing coactivators (p300, P/CAF) serves as a docking module through the AR-acetylated residues for sequential recruitment of other coactivators. P/CAF, which failed to activate the acetylation site mutants (23), is an important regulator of AR function in prostate cancer cells. P/CAF binds the AR in vivo (73), binds p300, and contains a bromodomain that is required for AR binding. p300 is viewed as a limiting coactivator for many NR, including the AR; therefore, although the reduction in p300 binding to the AR mutants was modest, reduced recruitment of coactivators may contribute to the defective trans activation of the AR acetylation mutants.
Transcriptional repression by N-CoR involves a multiprotein complex that includes HDAC complexes, chromatin remodeling proteins, and a transducin β-like protein that interacts with histones (41, 59). In the present studies, the nuclear corepressor N-CoR showed proportionally more binding to the AR lysine point mutants in cultured DU145 cells. The enhanced N-CoR binding to the AR lysine point mutants may provide an important mechanistic link to the reduced activity of the AR acetylation-defective mutants. The finding that coactivator and corepressor surfaces of NR overlap substantially (32, 66) is compatible with a dynamic model in which enzymatic modifications of the NR coordinate the sequential disengagement of corepressors followed by coactivator binding (56). Enhanced corepressor binding of the AR acetylation-defective mutants suggests a role for acetylation in the disengagement of corepressors. Acetylation of upstream binding factor also correlates with reduced transcriptional repression by the pRB-recruited HDAC (64). Together these studies provide evidence for a model in which acetylation disengages corepressors to sequentially recruit coactivators (45).
Several observations in the present studies suggest that the defective transactivation of the AR acetylation mutants may relate to altered coactivator-corepressor binding and does not result from reduced expression levels or ligand binding. Firstly, expression levels of the ARwt and the ARK630A or ARK(632/633)A mutant proteins were similar in cultured cells, suggesting that reduced transactivation was not due to reduced expression. The AR acetylation site does not fall within the ligand-binding pocket deduced from the crystal structure (55), the AR acetylation site mutants demonstrated wild-type ligand-induced protease sensitivity, and in vitro ligand-binding assays showed normal ligand-binding affinity at the physiological concentrations of ligand. Thus, the reduced ligand-induced transactivation of the AR acetylation mutants does not appear to be due to reduced expression or altered ligand binding. The recruitment of HDAC1/N-CoR-containing complexes to the promoters of target genes induces a repressive chromatin state through the functions of HDAC1 (19, 50). It has been proposed that ligand-dependent activation of NHR involves both recruitment of coactivators and disengagement of corepressors (27). The present studies of the AR are consistent with a model in which the selective defects in coactivation of the AR acetylation mutants may be due to increased binding of N-CoR regulated through the acetylation site.
The cellular phenotype regulated by direct acetylation of specific transcription factor events remains to be determined. Several studies have implicated direct transcription factor acetylation in regulating reporter gene expression (81, 87). Thus, acetylation regulates the transcriptional activity of p53 (28, 75), GATA-1 (14), erythroid Kruppel-like factor (95), Xenopus NF-Y (49), and the human immunodeficiency virus transactivator protein (Tat) (42) in reporter assays. Only recently has the functional cellular phenotype governed by transcription factor acetylation been examined (63). Acetylation site mutations of p53 were defective in repression of Ras-induced transformation, suggesting an important functional role for p53 acetylation in vivo (63). In previous studies, the AR KXKK motif was both necessary and sufficient for acetylation by either p300 or P/CAF (23). This motif resembles the C-terminal p53 acetylation motif and is highly conserved between NHR, suggesting an important biological function (86). Activation of the MEKK1 pathway enhances AR activity and induces cellular apoptosis in prostate cancer cells (2). In the present studies, AR acetylation was a critical determinant of the apoptotic response induced by MEKK1 in prostate cancer cells. Furthermore, stable cell lines expressing the AR acetylation mutants showed no enhancement of apoptosis by several agents in the presence of the ligand DHT. AR-dependent apoptosis has been observed in cultured cells and in transgenic mice in which the AR was targeted to the prostate by the probasin promoter (80). Several agents can induce apoptosis through AR-dependent and AR-independent pathways. Ligand-dependent events correlate with AR-mediated nuclear events, as the AR is thought to be required for nuclear DHT function. Therefore, in the present studies, comparison was made between the vehicle and the ligand to examine those apoptotic events most likely regulated by the AR in the stable cell lines. It remains possible, although unlikely, that DHT may govern non-AR-dependent apoptotic events that were selectively altered by the AR acetylation site, for example, through altered expression of a paracrine growth factor. The mechanisms by which the AR acetylation site regulates cellular apoptosis remain to be further explored. The evasion of cellular apoptosis contributes to aberrant growth control during tumorigenesis in multiple settings. The identification of acetylation as a key posttranslational modification required for activation of the AR by multiple distinct coactivators suggests that these residues may form an ideal target for AR inactivation and tumor therapies.
Acknowledgments
We thank R. Evans, E. Kalkhoven, J. Kyriakis, B. O'Malley, Y. Nakatani, N. Schreiber-Agus, and E. R. Stanley for plasmids and helpful discussions.
This work was supported by grants from the NIH (R01CA86072 to R.G.P. and R01CA72038-01 to S.A.W.F.) and The Susan Komen Breast Cancer Foundation (to R.G.P.). R.T.H. and E.J. were supported by the Medical Research Council. Y.-G.Y. is supported by grant CA26504 to E. R. Stanley. Work conducted at the Albert Einstein College of Medicine was supported by Cancer Center Core National Institutes of Health grant 5-P30-CA13330-26.
REFERENCES
- 1.Aarnisalo, P., J. J. Palvimo, and O. A. Janne. 1998. CREB-binding protein in androgen receptor-mediated signaling. Proc. Natl. Acad. Sci. USA 95:2122-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abreu-Martin, M. T., A. Chari, A. A. Palladino, N. A. Craft, and C. L. Sawyers. 1999. Mitogen-activated protein kinase kinase kinase 1 activates androgen receptor-dependent transcription and apoptosis in prostate cancer. Mol. Cell. Biol. 19:5143-5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akama, K. T., C. Albanese, R. G. Pestell, and L. J. Van Eldik. 1998. Amyloid β-peptide stimulates nitric oxide production in astrocytes through an NFκB-dependent mechanism. Proc. Natl. Acad. Sci. USA 95:5795-5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albanese, C., M. D'Amico, A. T. Reutens, M. Fu, G. Watanabe, R. J. Lee, R. N. Kitsis, B. Henglein, M. Avantaggiati, K. Somasundaram, B. Thimmapaya, and R. G. Pestell. 1999. Activation of the cyclin D1 gene by the E1A-associated protein p300 through AP-1 inhibits cellular apoptosis. J. Biol. Chem. 274:34186-34195. [DOI] [PubMed] [Google Scholar]
- 5.Alen, P., F. Claessens, G. Verhoeven, W. Rombauts, and B. Peeters. 1999. The androgen receptor amino-terminal domain plays a key role in p160 coactivator-stimulated gene transcription. Mol. Cell. Biol. 19:6085-6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alland, L., R. Muhle, H. J. Hou, J. Potes, L. Chin, N. Schreiber-Agus, and R. A. DePinho. 1997. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature 387:49-55. [DOI] [PubMed] [Google Scholar]
- 7.Avdi, N. J., J. A. Nick, B. B. Whitlock, M. A. Billstrom, P. M. Henson, G. L. Johnson, and G. S. Worthen. 2001. Tumor necrosis factor-alpha activation of the c-Jun N-terminal kinase pathway in human neutrophils. Integrin involvement in a pathway leading from cytoplasmic tyrosine kinases apoptosis. J. Biol. Chem. 276:2189-2199. [DOI] [PubMed] [Google Scholar]
- 8.Barbulescu, K., C. Geserick, I. Schuttke, W. D. Schleuning, and B. Haendler. 2001. New androgen response elements in the murine pem promoter mediate selective transactivation. Mol. Endocrinol. 15:1803-1816. [DOI] [PubMed] [Google Scholar]
- 9.Beato, M., and A. Sanchez-Pacheco. 1996. Interaction of steroid hormone receptors with the transcription initiation complex. Endocr. Rev. 17:587-609. [DOI] [PubMed] [Google Scholar]
- 10.Berger, J., P. Bailey, C. Biswas, C. A. Cullinan, T. W. Doebber, N. S. Hayes, R. Saperstein, R. G. Smith, and M. D. Leibowitz. 1996. Thiazolidinediones produce a conformational change in peroxisomal proliferator-activated receptor-gamma: binding and activation correlate with antidiabetic actions in db/db mice. Endocrinology 137:4189-4195. [DOI] [PubMed] [Google Scholar]
- 11.Berthon, P., A. S. Waller, J. M. Villette, L. Loridon, O. Cussenot, and N. J. Maitland. 1997. Androgens are not a direct requirement for the proliferation of human prostatic epithelium in vitro. Int. J. Cancer 73:910-916. [DOI] [PubMed] [Google Scholar]
- 12.Bevan, C. L., S. Hoare, F. Claessens, D. M. Heery, and M. G. Parker. 1999. The AF1 and AF2 domains of the androgen receptor interact with distinct regions of SRC1. Mol. Cell. Biol. 19:8383-8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouzahzah, B., M. Fu, A. Iavarone, V. M. Factor, S. S. Thorgeirsson, and R. G. Pestell. 2000. Transforming growth factor β1 recruits histone deacetylase 1 to a p130 repressor complex in transgenic mice in vivo. Cancer Res. 60:4531-4537. [PubMed] [Google Scholar]
- 14.Boyes, J., P. Byfield, Y. Nakatani, and V. Ogryzko. 1998. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature 396:594-598. [DOI] [PubMed] [Google Scholar]
- 15.Brady, M. E., D. M. Ozanne, L. Gaughan, I. Waite, S. Cook, D. E. Neal, and C. N. Robson. 1999. Tip60 is a nuclear hormone receptor coactivator. J. Biol. Chem. 274:17599-17604. [DOI] [PubMed] [Google Scholar]
- 16.Bromberg, J. F., M. H. Wrzeszczynska, G. Devgan, Y. Zhao, R. G. Pestell, C. Albanese, and J. E. Darnell. 1999. Stat3 as an oncogene. Cell 98:295-303. [DOI] [PubMed] [Google Scholar]
- 17.Burke, L. J., and A. Baniahmad. 2000. Co-repressors 2000. FASEB J. 14:1876-1888. [DOI] [PubMed] [Google Scholar]
- 18.Chen, H., R. J. Lin, W. Xie, D. Wilpitz, and R. M. Evans. 1999. Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase. Cell 98:675-686. [DOI] [PubMed] [Google Scholar]
- 19.David, G., L. Alland, S. H. Hong, C. W. Wong, R. A. DePinho, and A. Dejean. 1998. Histone deacetylase associated with mSin3A mediates repression by the acute promyelocytic leukemia-associated PLZF protein. Oncogene 16:2549-2556. [DOI] [PubMed] [Google Scholar]
- 20.Duprez, E., A. J. Saurin, J. M. Desterro, V. Lallemand-Breitenbach, K. Howe, M. N. Boddy, E. Solomon, H. de The, R. T. Hay, and P. S. Freemont. 1999. SUMO-1 modification of the acute promyelocytic leukaemia protein PML: implications for nuclear localisation. J. Cell Sci. 112:381-393. [DOI] [PubMed] [Google Scholar]
- 21.Fan, S., J.-A. Wang., R. Yuan, Y. Ma, Q. Meng, M. R. Erdos, R. G. Pestell, F. Yuan, K. J. Auborn, I. D. Goldberg, and E. M. Rosen. 1999. BRCA1 inhibition of estrogen receptor signaling in transfected cells. Science 284:1354-1356. [DOI] [PubMed] [Google Scholar]
- 22.Fronsdal, K., N. Engedal, T. Slagsvold, and F. Saatcioglu. 1998. CREB binding protein is a coactivator for the androgen receptor and mediates cross-talk with AP-1. J. Biol. Chem. 273:31853-31859. [DOI] [PubMed] [Google Scholar]
- 23.Fu, M., C. Wang, A. T. Reutens, R. Angelletti, L. Siconolfi-Baez, V. Ogryzko, M. L. Avantaggiati, and R. G. Pestell. 2000. p300 and P/CAF acetylate the androgen receptor at sites governing hormone-dependent transactivation. J. Biol. Chem. 275:20853-20860. [DOI] [PubMed] [Google Scholar]
- 24.Garnick, M., and W. Fair. 1996. Prostate cancer: emerging concepts. Part II. Ann. Intern. Med. 125:205-212. [DOI] [PubMed] [Google Scholar]
- 25.Gibson, S., C. Widmann, and G. L. Johnson. 1999. Differential involvement of MEK kinase 1 (MEKK1) in the induction of apoptosis in response to microtubule-targeted drugs versus DNA damaging agents. J. Biol. Chem. 274:10916-10922. [DOI] [PubMed] [Google Scholar]
- 26.Giordano, A., and M. L. Avantaggiati. 1999. p300 and CBP: partners for life and death. J. Cell. Physiol. 181:218-230. [DOI] [PubMed] [Google Scholar]
- 27.Glass, C. K., and M. G. Rosenfeld. 2000. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 14:121-141. [PubMed] [Google Scholar]
- 28.Gu, W., and R. G. Roeder. 1997. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90:595-606. [DOI] [PubMed] [Google Scholar]
- 29.Hayes, S., M. Zarnegar, M. Sharma, F. Yang, D. M. Peehl, P. ten Dijke, and Z. Sun. 2001. SMAD3 represses androgen receptor-mediated transcription. Cancer Res. 61:2112-2118. [PubMed] [Google Scholar]
- 30.Heinzel, T., R. M. Lavinsky, T. M. Mullen, M. Soderstrom, C. D. Laherty, J. Torchia, W. M. Yang, G. Brard, S. D. Ngo, J. R. Davie, E. Seto, R. N. Eisenman, D. W. Rose, C. K. Glass, and M. G. Rosenfeld. 1997. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature 387:43-48. [DOI] [PubMed] [Google Scholar]
- 31.Horwitz, K. B., T. A. Jackson, D. L. Bain, J. K. Richer, G. S. Takimoto, and L. Tung. 1996. Nuclear receptor coactivators and corepressors. Mol. Endocrinol. 10:1167-1177. [DOI] [PubMed] [Google Scholar]
- 32.Hu, X., Y. Li, and M. Lazar. 2001. Determinants of CoRNR-dependent repression complex assembly on nuclear hormone receptors. Mol. Cell. Biol. 21:1747-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hung, H. L., J. Lau, A. Y. Kim, M. J. Weiss, and G. A. Blobel. 1999. CREB-binding protein acetylates hematopoietic transcription factor GATA-1 at functionally important sites. Mol. Cell. Biol. 19:3496-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikonen, T., J. J. Palvimo, and O. A. Janne. 1997. Interaction between the amino- and carboxyl-terminal regions of the rat androgen receptor modulates transcriptional activity and is influenced by nuclear receptor coactivators. J. Biol. Chem. 272:29821-29828. [DOI] [PubMed] [Google Scholar]
- 35.Ikura, T., V. V. Ogryzko, M. Grigoriev, R. Groisman, J. Wang, M. Horikoshi, R. Scully, J. Qin, and Y. Nakatani. 2000. Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell 102:463-473. [DOI] [PubMed] [Google Scholar]
- 36.Jackson, R. H., A. G. Ladurner, D. S. King, and R. Tjian. 2000. Structure and function of a human TAFII250 double bromodomain module. Science 288:1422-1425. [DOI] [PubMed] [Google Scholar]
- 37.Joyce, D., C. Albanese, J. Steer, M. Fu, B. Bouzahzah, and R. G. Pestell. 2001. NF-kB and cell-cycle regulation: the cyclin connection. Cytokine Growth Factor Rev. 12:73-90. [DOI] [PubMed] [Google Scholar]
- 38.Kalkhoven, E., J. E. Valentine, D. M. Heery, and M. G. Parker. 1998. Isoforms of steroid receptor co-activator 1 differ in their ability to potentiate transcription by the oestrogen receptor. EMBO J. 17:232-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kallio, P. J., H. Poukka, A. Moilanen, O. A. Janne, and J. J. Palvimo. 1995. Androgen receptor-mediated transcriptional regulation in the absence of direct interaction with a specific DNA element. Mol. Endocrinol. 9:1017-1028. [DOI] [PubMed] [Google Scholar]
- 40.Kang, H.-Y., H.-K. Lin, Y.-C. Hu, K.-E. Huang, and C. Chang. 2001. From transforming growth factor-b signaling to androgen action: identification of Smad3 as an androgen receptor coregulator in prostate cancer cells. Proc. Natl. Acad. Sci. USA 98:3018-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kao, H. Y., M. Downes, P. Ordentlich, and R. M. Evans. 2000. Isolation of a novel histone deacetylase reveals that class I and class II deacetylases promote SMRT-mediated repression. Genes Dev. 14:55-66. [PMC free article] [PubMed] [Google Scholar]
- 42.Kiernan, R., C. Vanhulle, L. Schiltz, E. Adam, H. Xiao, F. Maudoux, C. Calomne, A. Burny, Y. Nakatani, K.-T. Jeang, M. Benkirane, and C. Van Lint. 1999. HIV Tat transcriptional activity is regulated by acetylation. EMBO J. 18:6106-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knoepfler, P. S., and R. N. Eisenman. 1999. Sin meets NuRD and other tails of repression. Cell 99:447-450. [DOI] [PubMed] [Google Scholar]
- 44.Knudson, K. E., W. K. Cavenee, and K. C. Arden. 1999. D-type cyclins complex with the androgen receptor and inhibit its transcriptional transactivation ability. Cancer Res. 59:2297-2301. [PubMed] [Google Scholar]
- 45.Kouzarides, T. 2000. Acetylation: a regulatory modification to rival phosphorylation. EMBO J. 19:1176-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kyriakis, J. M., and J. Avruch. 1996. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J. Biol. Chem. 271:24313-24316. [DOI] [PubMed] [Google Scholar]
- 47.Lee, R. J., C. Albanese, M. Fu, M. D'Amico, B. Lin, G. Watanabe, G. K. Haines III, P. M. Siegel, M.-C. Hung, Y. Yarden, J. M. Horowitz, W. J. Muller, and R. G. Pestell. 2000. Cyclin D1 is required for transformation by activated Neu and is induced through an E2F-dependent signaling pathway. Mol. Cell. Biol. 20:672-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levkau, B., H. Koyama, E. W. Raines, B. E. Clurman, B. Herren, K. Orth, J. M. Roberts, and R. Ross. 1998. Cleavage of p21Cip1/Waf1 and p27Kip1 mediates apoptosis in endothelial cells through activation of cdk2: role of a caspase cascade. Mol. Cell 1:553-563. [DOI] [PubMed] [Google Scholar]
- 49.Li, Q., M. Herrler, N. Landsberger, N. Kaludov, V. V. Ogryzko, Y. Nakatani, and A. P. Wolffe. 1998. Xenopus NF-Y pre-sets chromatin to potentiate p300 and acetylation-responsive transcription from the Xenopus hsp70 promoter in vivo. EMBO J. 17:6300-6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin, R. J., L. Nagy, S. Inoue, W. Shao, W. H. Miller, Jr., and R. M. Evans. 1998. Role of the histone deacetylase complex in acute promyelocytic leukaemia. Nature 391:811-814. [DOI] [PubMed] [Google Scholar]
- 51.Liu, L., D. M. Scolnick, R. C. Trievel, H. B. Zhang, R. Marmorstein, T. D. Halazonetis, and S. L. Berger. 1999. p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol. Cell. Biol. 19:1202-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu, S., G. Jenster, and D. E. Epner. 2000. Androgen induction of cyclin-dependent kinase inhibitor p21 gene: role of androgen receptor and transcription factor Sp1 complex. Mol. Endocrinol. 14:753-760. [DOI] [PubMed] [Google Scholar]
- 53.Luo, R. X., A. A. Postigo, and D. C. Dean. 1998. Rb interacts with histone deacetylase to repress transcription. Cell 92:463-473. [DOI] [PubMed] [Google Scholar]
- 54.Martinez-Balbas, M. A., A. J. Bannister, K. Martin, P. Haus-Seuffert, M. Meisterernst, and T. Kouzarides. 1998. The acetyltransferase activity of CBP stimulates transcription. EMBO J. 17:2886-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matias, P. M., P. Donner, R. Coelho, M. Thomaz, C. Peixoto, S. Macedo, N. Otto, S. Joschko, P. Scholz, A. Wegg, S. Basler, M. Schafers, U. Egner, and M. A. Carrondo. 2000. Structural evidence for ligand specificity in the binding domain of the human androgen receptor. J. Biol. Chem. 275:26164-26171. [DOI] [PubMed] [Google Scholar]
- 56.McKenna, J., R. B. Lanz, and B. W. O'Malley. 1999. Nuclear receptor co-regulators: cellular and molecular biology. Endocr. Rev. 20:321-344. [DOI] [PubMed] [Google Scholar]
- 57.Muller, S., M. Berger, F. Lehembre, J. S. Seeler, Y. Haupt, and A. Dejean. 2000. c-Jun and p53 activity is modulated by SUMO-1 modification. J. Biol. Chem. 275:13321-13329. [DOI] [PubMed] [Google Scholar]
- 58.Munshi, A., G. Pappas, T. Honda, T. J. McDonnell, A. Younes, Y. Li, and R. E. Meyn. 2001. TRAIL (APO-2L) induces apoptosis in human prostate cancer cells that is inhibitable by Bcl-2. Oncogene 20:3757-3765. [DOI] [PubMed] [Google Scholar]
- 59.Nagy, L., H. Y. Kao, D. Chakravarti, R. J. Lin, C. A. Hassig, D. E. Ayer, S. L. Schreiber, and R. M. Evans. 1997. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A and histone deacetylase. Cell 89:373-380. [DOI] [PubMed] [Google Scholar]
- 60.Nimmanapalli, R., C. L. Perkins, M. Orlando, E. O'Bryan, D. Nguyen, and K. N. Bhalla. 2001. Pretreatment with paclitaxel enhances apo-2 ligand/tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis of prostate cancer cells by inducing death receptors 4 and 5 protein levels. Cancer Res. 61:759-763. [PubMed] [Google Scholar]
- 61.Ogryzko, V. V., R. L. Schiltz, V. Russanova, B. H. Howard, and Y. Nakatani. 1996. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87:953-959. [DOI] [PubMed] [Google Scholar]
- 62.Palvimo, J. J., P. Reinikainen, T. Ikonen, P. J. Kallio, A. Moilanen, and O. A. Jänne. 1996. Mutual transcriptional interference between RelA and androgen receptor. J. Biol. Chem. 271:24151-24156. [DOI] [PubMed] [Google Scholar]
- 63.Pearson, M., R. Carbone, C. Sebastiani, M. Cioce, M. Fagioli, S. Saito, Y. Higashimoto, E. Appella, S. Minucci, P. P. Pandolfi, and P. G. Pelicci. 2000. PML regulates p53 acetylation and premature senescence induced by oncogenic Ras. Nature 406:207-210. [DOI] [PubMed] [Google Scholar]
- 64.Pelletier, G., V. Y. Stefanovsky, M. Faubladier, I. Hirschler-Laszkiewicz, J. Savard, L. I. Rothblum, J. Cote, and T. Moss. 2000. Competitive recruitment of CBP and Rb-HDAC regulates UBF acetylation and ribosomal transcription. Mol. Cell 6:1059-1066. [DOI] [PubMed] [Google Scholar]
- 65.Pena, P., A. T. Reutens, M. D'Amico, C. Albanese, G. Watanabe, A. Donner, I.-W. Shu, T. Williams, and R. G. Pestell. 1999. Activator protein-2 mediates transcriptional activation of the CYP11A1 gene by interaction with Sp1 rather than binding to DNA. Mol. Endocrinol. 13:1402-1416. [DOI] [PubMed] [Google Scholar]
- 66.Perissi, V., L. M. Staszewski, E. M. McInerney, R. Kurokawa, A. Krones, D. W. Rose, M. H. Lambert, M. V. Milburn, C. K. Glass, and M. G. Rosenfeld. 1999. Molecular determinants of nuclear receptor-corepressor interaction. Genes Dev. 13:3198-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pombo, C. M., J. H. Kehrl, I. Sanchez, P. Katz, J. Avruch, L. I. Zon, J. R. Woodgett, T. Force, and J. M. Kyriakis. 1995. Activation of the SAPK pathway by the human STE20 homologue germinal centre kinase. Nature 377:750-754. [DOI] [PubMed] [Google Scholar]
- 68.Poukka, H., P. Aarnisalo, U. Karvonen, J. J. Palvimo, and O. A. Janne. 1999. Ubc9 interacts with the androgen receptor and activates receptor-dependent transcription. J. Biol. Chem. 274:19441-19446. [DOI] [PubMed] [Google Scholar]
- 69.Poukka, H., P. Aarnisalo, H. Santti, O. A. Janne, and J. J. Palvimo. 2000. Coregulator small nuclear RING finger protein (SNURF) enhances Sp1- and steroid receptor-mediated transcription by different mechanisms. J. Biol. Chem. 275:571-579. [DOI] [PubMed] [Google Scholar]
- 70.Poukka, H., U. Karvonen, O. A. Janne, and J. J. Palvimo. 2000. Covalent modification of the androgen receptor by small ubiquitin-like modifier 1 (SUMO-1). Proc. Natl. Acad. Sci. USA 97:14145-14150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Poukka, H., U. Karvonen, N. Yoshikawa, H. Tanaka, J. J. Palvimo, and O. A. Jänne. 2000. The RING finger protein SNURF modulates nuclear trafficking of the androgen receptor. J. Cell Sci. 113:2991-3001. [DOI] [PubMed] [Google Scholar]
- 72.Prives, C., and J. L. Manley. 2001. Why is p53 acetylated? Cell 107:815-818. [DOI] [PubMed] [Google Scholar]
- 73.Reutens, A. T., M. Fu, G. Watanabe, C. Albanese, M. J. McPhaul, S. P. Balk, O. A. Janne, J. J. Palvimo, and R. G. Pestell. 2001. Cyclin D1 governs androgen receptor function by ligand-dependent regulation of P/CAF. Mol. Endocrinol. 15:797-811. [DOI] [PubMed] [Google Scholar]
- 74.Roche, P. J., S. A. Hoare, and M. G. Parker. 1992. A consensus DNA-binding site for the androgen receptor. Mol. Endocrinol. 6:2229-2235. [DOI] [PubMed] [Google Scholar]
- 75.Sakaguchi, K., J. E. Herrera, S. Saito, T. Miki, M. Bustin, A. Vassilev, C. W. Anderson, and E. Appella. 1998. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 12:2831-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sato, N., M. D. Sadar, N. Bruchovsky, F. Saatcioglu, P. S. Rennie, S. Sato, P. H. Lange, and M. E. Gleave. 1997. Androgenic induction of prostate-specific antigen gene is repressed by protein-protein interaction between the androgen receptor and AP-1/c-Jun in the human prostate cancer cell line LNCaP. J. Biol. Chem. 272:17485-17494. [DOI] [PubMed] [Google Scholar]
- 77.Shemshedini, L., R. Knauthe, P. Sassone-Corsi, A. Pornon, and H. Gronemeyer. 1992. Cell-specific inhibitory and stimulatory effects of Fos and Jun on transcription activation by nuclear receptors. EMBO J. 11:167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soengas, M. S., R. M. Alarcon, H. Yoshida, A. J. Giaccia, R. Hakem, T. W. Mak, and S. W. Lowe. 1999. Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science 284:156-159. [DOI] [PubMed] [Google Scholar]
- 79.Sperry, T. S., and P. Thomas. 1999. Characterization of two nuclear androgen receptors in Atlantic croaker: comparison of their biochemical properties and binding specificities. Endocrinology 140:1602-1611. [DOI] [PubMed] [Google Scholar]
- 80.Stanbrough, M., I. Leav, P. W. L. Kwan, G. J. Bubley, and S. P. Balk. 2001. Prostatic intraepithelial neoplasia in mice expressing an androgen receptor transgene in prostate epithelium. Proc. Natl. Acad. Sci. USA 98:10823-10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sterner, D. E., and S. L. Berger. 2000. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 64:435-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tatham, M. H., E. Jaffray, O. A. Vaughan, J. M. Desterro, C. H. Botting, J. H. Naismith, and R. T. Hay. 2001. Polymeric chains of SUMO-2 and SUMO-3 are conjugated to protein substrates by SAE1/SAE2 and Ubc9. J. Biol. Chem. 276:35368-35374. [DOI] [PubMed] [Google Scholar]
- 83.Torchia, J., D. W. Rose, J. Inostroza, Y. Kamei, S. Westin, C. K. Glass, and M. G. Rosenfeld. 1997. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature 387:677-684. [DOI] [PubMed] [Google Scholar]
- 84.Tsai, M. J., and B. W. O'Malley. 1994. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 63:451-486. [DOI] [PubMed] [Google Scholar]
- 85.Voegel, J. J., M. J. S. Heine, M. Tini, V. Vivat, P. Chambon, and H. Gronemeyer. 1998. The co-activator TIF2 contains three nuclear receptor binding motifs and mediates transactivation through CBP binding-dependent and -independent pathways. EMBO J. 17:507-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang, C., M. Fu, R. Angeletti, L. Siconolfi-Baez, A. Reutens, M. P. Lisanti, B. Katzenellenbogen, S. Kato, T. Hopp, S. A. W. Fuqua, P. J. Kushner, and R. G. Pestell. 2001. Direct acetylation of the estrogen receptor α hinge region by p300 regulates transactivation and hormone sensitivity. J. Biol. Chem. 276:18375-18383. [DOI] [PubMed] [Google Scholar]
- 87.Wang, C., M. Fu, S. Mani, S. Wadler, A. M. Senderowicz, and R. G. Pestell. 2001. Histone acetylation and the cell-cycle in cancer. Front. Biosci. 6:D610-D629. [DOI] [PubMed]
- 88.Watanabe, G., C. Albanese, R. J. Lee, A. Reutens, G. Vairo, B. Henglein, and R. G. Pestell. 1998. Inhibition of cyclin D1 kinase activity is associated with E2F-mediated inhibition of cyclin D1 promoter activity through E2F and Sp1. Mol. Cell. Biol. 18:3212-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Widmann, C., P. Gerwins, N. L. Johnson, M. B. Jarpe, and G. L. Johnson. 1998. MEK kinase 1, a substrate for DEVD-directed caspases, is involved in genotoxin-induced apoptosis. Mol. Cell. Biol. 18:2416-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yamamoto, T., and M. Horikoshi. 1997. Novel substrate specificity of the histone acetyltransferase activity of HIV-1-Tat interactive protein Tip60. J. Biol. Chem. 272:30595-30598. [DOI] [PubMed] [Google Scholar]
- 91.Yao, T. P., G. Ku, N. Zhou, R. Scully, and D. M. Livingston. 1996. The nuclear hormone receptor coactivator SRC-1 is a specific target of p300. Proc. Natl. Acad. Sci. USA 93:10626-10631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yeh, S., and C. Chang. 1996. Cloning and characterization of a specific coactivator, ARA70, for the androgen receptor in human prostate cells. Proc. Natl. Acad. Sci. USA 93:5517-5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yeh, S., Y.-C. Hu, M. Rahman, H.-K. Lin, C.-L. Hsu, H.-J. Ting, H.-Y. Kang, and C. Chang. 2000. Increase of androgen-induced cell death and androgen receptor transactivation by BRCA1 in prostate cancer cells. Proc. Natl. Acad. Sci. USA 97:11256-11261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu, R., S. Mandlekar, S. Ruben, J. Ni, and A. N. Kong. 2000. Tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in androgen-independent prostate cancer cells. Cancer Res. 60:2384-2389. [PubMed] [Google Scholar]
- 95.Zhang, W., and J. J. Bieker. 1998. Acetylation and modulation of erythroid Kruppel-like factor (EKLF) activity by interaction with histone acetyltransferases. Proc. Natl. Acad. Sci. USA 95:9855-9860. [DOI] [PMC free article] [PubMed] [Google Scholar]