Abstract
The Raf-1 kinase activates the ERK (extracellular-signal-regulated kinase) pathway. The cyclic AMP (cAMP)-dependent protein kinase (PKA) can inhibit Raf-1 by direct phosphorylation. We have mapped all cAMP-induced phosphorylation sites in Raf-1, showing that serines 43, 259, and 621 are phosphorylated by PKA in vitro and induced by cAMP in vivo. Serine 43 phosphorylation decreased the binding to Ras in serum-starved but not in mitogen-stimulated cells. However, the kinase activity of a RafS43A mutant was fully inhibited by PKA. Mutation of serine 259 increased the basal Raf-1 activity and rendered it largely resistant to inhibition by PKA. cAMP increased Raf-1 serine 259 phosphorylation in a PKA-dependent manner with kinetics that correlated with ERK deactivation. PKA also decreased Raf-1 serine 338 phosphorylation of Raf-1, previously shown to be required for Raf-1 activation. Serine 338 phosphorylation of a RafS259A mutant was unaffected by PKA. Using RafS259 mutants we also demonstrate that Raf-1 is the sole target for PKA inhibition of ERK and ERK-induced gene expression, and that Raf-1 inhibition is mediated mainly through serine 259 phosphorylation.
The Raf-1 kinase is at the interface of a signaling pathway that connects cell surface receptors to nuclear transcription factors. Raf-1 is the entry point to the ERK/MAPK (extracellular-signal-regulated kinase/mitogen-activated protein kinase) pathway. It phosphorylates and activates MEK (MAPK/ERK kinase), which in turn phosphorylates and activates ERK. Raf-1 activation is initiated by binding of Raf-1 to GTP-loaded Ras, which results in the translocation of Raf-1 from the cytosol to the cell membrane, where activation takes place (reviewed in references 4, 18, 21, 22, and 26). Activation involves phosphorylation on serine 338 (17, 23) and tyrosine 341 (10, 23) as well as other yet-unknown modifications. It has been extremely difficult to identify the activating modifications, in part because presumably only a small fraction of Raf-1 becomes activated (14). Hence, despite receiving intensive attention during the last 10 years, several facets of Raf-1 regulation have evaded elucidation. This also pertains to the cross talk between the Raf-MEK-ERK and cyclic AMP (cAMP) signaling systems; and the mechanism of Raf-1 regulation by cAMP has not been completely clarified (15).
In resting cells Raf-1 is phosphorylated on serines 43, 259, and 621 (27). Serines 43 and 621 have been previously described as target sites for the cAMP-regulated protein kinase, PKA, which inhibits Raf-1 (13, 25, 38). Phosphorylation of serine 43 was reported to diminish the affinity of Raf-1 for Ras (38) and thereby to interfere with Raf-1 activation (30, 38), although recent results dispute this (34). The role of serine 621 phosphorylation is more difficult to study, because mutation of this residue is incompatible with kinase function (25, 27). This observation was taken as an indication that serine 621 phosphorylation is essential for Raf-1 function. Biochemical experiments confirmed that the integrity of serine 621 was indeed required but also correlated its phosphorylation with the inhibition of catalytic activity (25). The latter experiments were done with the isolated Raf-1 kinase domain, and the role of serine 621 phosphorylation in the context of full-length Raf-1 is less clear. Recent experiments have shown that in macrophages the dephosphorylation of serines 621 and especially 259 is required for efficient Raf-1 activation (1). Blocking this dephosphorylation with concentrations of okadaic acid which selectively inhibited the serine/threonine phosphatase PP2A severely blunted the CSF-1-induced Raf-1 activation. Mutation of serine 259 rendered Raf-1 largely resistant to inhibition by okadaic acid. PP2A was found to associate with Raf-1 in the membrane compartment, suggesting that in macrophages Raf-1 membrane translocation is accompanied by PP2A binding and that PP2A enables Raf-1 activation by dephosphorylating serines 259 and 621 (1). Dephosphorylation of serine 259 was recently shown to be a key step in Raf-1 activation, regulating its binding to upstream activators as well as to its substrate, MEK (8). Serine 259 was also described as a target site for Raf-1 inhibition mediated by Akt/protein kinase B (PKB) (40), although this cross talk appears to occur only in certain cell types (31).
The present report highlights the importance of serine 259 for the regulation of Raf-1. It shows that serine 259 is a target site for phosphorylation by PKA and that it is the main site mediating PKA-induced Raf-1 inhibition. Using RafS259 mutants we also present evidence that PKA uses Raf-1 inhibition as main interface for the negative control of ERK activity and ERK-induced gene transcription.
MATERIALS AND METHODS
Reagents and expression vectors.
The Raf-1 cDNA and mutants, in which serine 43 or 259 was changed by site-directed mutagenesis, were cloned into the EcoRI-BamHI sites of pCMV5 (2). The double-mutant pCMV5-RafSS43/259AA was made by site-directed mutagenesis of pCMV-RafS43A.The FLAG-tagged Raf-1 plasmid was a generous gift from Debbie Morrison. The pCEVα expression vector for the catalytic subunit of PKA was kindly provided by Stanley McKnight. All other expression vectors and baculoviruses have been described previously (13, 25). For Raf-1 immunoprecipitation and Western blotting crafVI, an antiserum raised against the 12 C-terminal amino acids of Raf-1, was used (13). The Raf-1 anti-phosphoserine 338 and anti-phosphoserine 259 antibodies were purchased from UBI and New England Biolabs, respectively. The Raf-1 anti-phosphoserine 43 antibody was a generous gift from Manuela Baccarini. The 9E10 myc and anti-hemagglutinin (HA) tag antibodies were from Roche Diagnostics; the anti-FLAG antibody was from Sigma.
Cell culture and transfection.
NIH 3T3 and COS-1 cells were cultivated in Dulbecco's modified Eagle's medium (DMEM) supplemented with 2 mM glutamine and 10% fetal calf serum. COS-1 cells were transfected in six-well plates with 1.5 μg of each DNA/well using DEAE dextrane and chloroquine as described previously (25) or Effectene according to the manufacturer's instructions. NIH 3T3 cells were transfected as described previously (19). Cells were serum starved overnight prior to treatment with mitogens. Protein expression in Sf-9 insect cells was done as described previously (25).
Raf phosphorylation and two-dimensional phosphopeptide mapping.
The phosphorylation of recombinant Raf-1 proteins with PKA in vitro was done as described previously (13) except that the Raf proteins were produced in COS-1 cells. Metabolic labeling with [32P]orthophosphoric acid and forskolin treatment of NIH 3T3 cells were done as previously described (13). Raf-1 was immunoprecipitated, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiographed. Raf bands were cut out and subjected to tryptic digestion and two-dimensional peptide mapping as described earlier (14). A pH 8.9 buffer was used for the first dimension, and phosphochromatography buffer was used for the second dimension. Phosphopeptides were quantified with a Fuji phosphorimager.
Kinase assays and Western blotting.
Kinase assays and Western blotting were done as described earlier (25). Raf-1 activity was determined by a linked assay, where Raf-1 immunoprecipitates were incubated with recombinant MEK-1 and kinase-dead ERK-2 proteins. This assay measures the ability of Raf-1 to phosphorylate and activate MEK. MEK activation is detected by the phosphorylation of kinase-negative ERK. ERK activation was assayed with myelin basic protein as a substrate. In some experiments kinase activity was measured in a more sensitive two-step kinase assay exactly as described earlier (3). In this assay Raf proteins were immunoprecipitated with the crafVI antibody and used in the first step to activate recombinant MEK and ERK. In the second step an aliquot of the reaction is incubated with myelin basic protein in the presence of [γ-32P]ATP to measure the activity of ERK. Western blots were developed by using the enhanced chemiluminescence kit from Roche Diagnostics according to the manufacturer's instructions. Densitometric evaluation was done with NIH Image.
Reporter gene assays.
Reporter gene assays were done as described previously (19).
Ras pulldown assays.
Ras pulldown assays were done as described previously (28), except that the Ha-Ras protein was bound to beads before loading with guanylimido diphosphate.
RESULTS
PKA induces Raf-1 hyperphosphorylation on three major sites.
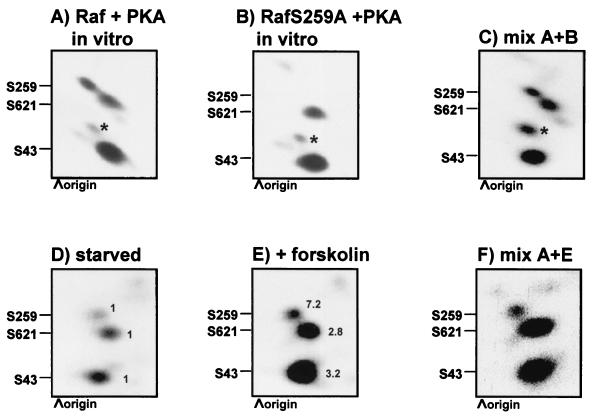
cAMP agonists have been previously shown to induce the hyperphosphorylation of Raf-1 on serines 43 and 621 (5, 25, 38). However, based on two-dimensional tryptic phosphopeptide maps, PKA phosphorylated Raf-1 in vitro on three prominent and three minor sites (Fig. 1A). Therefore, we set out to analyze the remaining phosphorylation sites. The intensity of the minor sites varied between experiments, and since they were phosphorylated only in vitro, their identification was not further pursued. The three major in vitro sites were also found to be phosphorylated in resting NIH 3T3 cells (Fig. 1D). They were hyperinduced in response to forskolin and PKA activation (Fig. 1E). Mixing experiments confirmed that the three prominent in vitro phosphorylation sites correspond to the three forskolin-induced sites (Fig. 1F). Using point mutants, two of these sites were previously identified as serines 43 and 621 (13, 25). The third site corresponds to serine 259, since it is absent in a Raf-1 mutant in which this serine was changed to alanine (Fig. 1B and C). Remarkably, although serine 259 was not the most prominent phosphorylation site, it exhibited the strongest induction in response to forskolin (Fig. 1E).
FIG. 1.
Raf-1 is phosphorylated by PKA on serine 259. Shown are tryptic phosphopeptide maps of recombinant Raf-1 phosphorylated by PKA (Raf + PKA) in vitro (A), recombinant RafS259A phosphorylated by PKA (RafS259A + PKA) in vitro (B), a mixture of Raf + PKA and RafS259A + PKA (C), endogenous Raf-1 immunoprecipitated from serum-starved NIH 3T3 cells labeled with [32P]orthophosphoric acid (D), endogenous Raf-1 from forskolin-treated cells (40 μM, 30 min) (E), and a mixture of panels A and E (F). Phosphorylation sites are indicated. Asterisks indicate spurious in vitro phosphorylation sites that do not occur in cells. In panels D and E the spots were quantified with a phosphorimager and the relative changes are indicated. Equal amounts of Raf-1 protein were loaded.
These results indicate that PKA suppresses Raf-1 activity through enhancing the phosphorylation of the three basal Raf-1 phosphorylation sites. They further raise the question of which of these sites are necessary for PKA-mediated inhibition. We have previously shown in a series of biochemical in vitro experiments that the phosphorylation of serine 621 by PKA downregulates the activity of the isolated Raf-1 kinase domain, BXB, which lacks serines 43 and 259 (25). PKA also leads to the inactivation of BXB or v-raf in vitro and in vivo (13, 25). The mutation of serine 621 to a variety of other amino acids inactivates the catalytic activity of both full-length Raf-1 and the isolated kinase domain (7, 25), hampering functional studies with serine 621 mutants. In contrast, serines 43 and 259 are readily amenable to mutational analysis.
Serine 43 does not mediate Raf-1 inhibition in COS-1 cells.
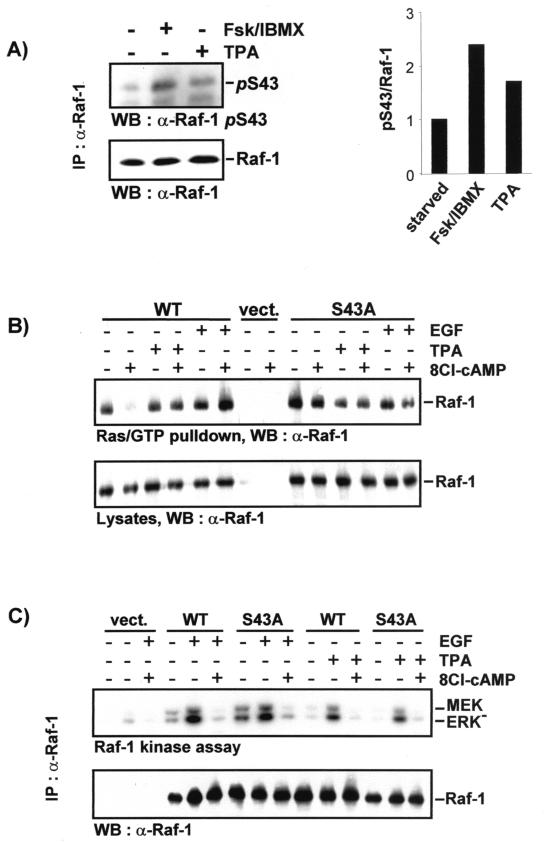
The phosphorylation of serine 43 has been reported to prevent Raf-1 activation by Ras, because it lowers the affinity of Raf-1 towards activated Ras/GTP (5, 35, 38). However, a recent study challenges this conclusion by showing that the mutation of serine 43 to alanine does not prevent the inhibition of Raf-1 by cAMP (34). Using an antibody specific for phosphoserine 43, we first confirmed that treatment with cAMP agonists (forskolin plus IBMX) induced serine 43 phosphorylation (Fig. 2A). Phosphorylation of serine 43 was also induced by the potent Raf-1 activator TPA, which argues against a purely negative role of serine 43 phosphorylation. We then tested Raf-1 and the RafS43A mutant for Ras binding and kinase activity in response to stimulation with cAMP agonists and mitogens (Fig. 2). In serum-starved COS-1 cells 8Cl-cAMP treatment almost completely abolished the binding of Raf-1 to recombinant Ras/GTP immobilized on beads (Fig. 2B). In contrast, the Ras association of a RafS43A mutant was not affected. Surprisingly, when cells were treated simultaneously with 8Cl-cAMP plus epidermal growth factor (EGF) or 8Cl-cAMP plus tetradecanoylphorbol-13-acetate (TPA), the binding of Raf-1 to Ras/GTP was fully rescued. TPA or EGF alone did not influence the binding of Raf-1 or RafS43A to Ras/GTP, indicating that the mitogens selectively counteract the loss of binding caused by serine 43 phosphorylation rather than enhancing the affinity of Raf-1 towards Ras/GTP in general. Aliquots of the same cell lysates were used to immunoprecipitate and measure the kinase activities of Raf-1 and RafS43A (Fig. 2C). Here, no differences could be detected. RafS43A was consistently activated and inhibited to extents similar to those of wild-type Raf-1. These results confirm the initial observations that serine 43 phosphorylation diminishes the binding of Raf-1 to Ras (5, 38) but further suggest that this effect is counteracted by mitogen stimulation. Importantly, RafS43A was still fully susceptible to inhibition by PKA, demonstrating that phosphorylation on this residue is not required for the inhibitory effect.
FIG. 2.
The role of Raf-1 serine 43. (A) cAMP agonists and TPA induce phosphorylation of S43. COS-1 cells were transfected with pCMV5-Raf-1, serum starved overnight, and treated with 40 μM forskolin plus 100 μM IBMX for 30 min or 100 ng of TPA per ml for 15 min. Raf-1 immunoprecipitates were sequentially immunoblotted with phospho-Raf-1 S43 and Raf-1 antibodies. (B) COS-1 cells transfected with pCMV5-Raf-1 or pCMV5-RafS43A were serum starved overnight and treated with 100 μM 8Cl-cAMP, 100 ng of TPA per ml, or 20 ng of EGF per ml for 30 min as indicated. For cotreatments 8Cl-cAMP was administered first (20 min). Cell lysates were incubated with Ras/GTP beads for 1 h, and the pulldowns were analyzed by immunoblotting with a Raf-1 antibody. In the lower panel cell lysates were examined for the expression of transfected Raf proteins. (B) An aliquot of the cell lysates was immunoprecipitated with crafVI antibody to assay kinase activity by using a linked assay with MEK and kinase-negative ERK (ERK−) as substrates. The kinase assays were blotted, autoradiographed (upper panel), and stained with crafVI (lower panel). vect., vector-transfected cells; S43A, pCMV5-RafS43A-transfected cells.
Serine 259 is critical for PKA inhibition of Raf-1.
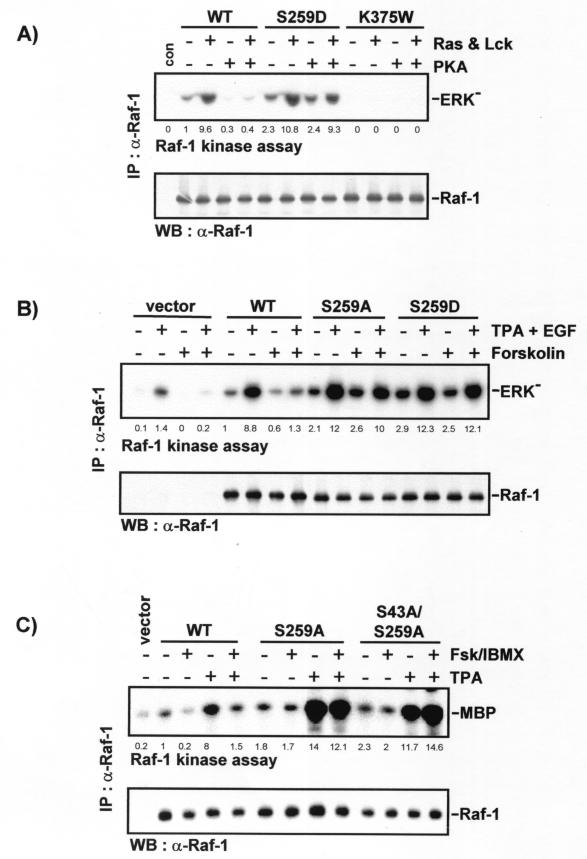
The phosphorylation of serine 259 has been previously implicated in the negative regulation of Raf-1 (24, 27, 37, 40). Exchanging this residue for alanine resulted in a modest activation of Raf-1 expressed in Sf-9 insect cells (27). Surprisingly, when serine 259 was replaced by aspartic acid in order to mimic phosphorylation, the RafS259D mutant also showed a similar small, about twofold, enhancement of basal kinase activity (Fig. 3A), demonstrating that a negative charge is not an adequate substitute for the phosphate group in this case. Also, mutation of serine 259 seems to affect mainly the basal kinase activity, because the activity induced by coexpression of Ras and Lck was similar to that of wild-type Raf-1. However, mutation of serine 259 altered the susceptibility of Raf-1 to PKA inhibition. Coexpression of the catalytic subunit of PKA efficiently suppressed both the basal activity and the induced activity of wild-type Raf-1. In contrast, the elevated basal activity of RafS259D was completely refractory to PKA, and the induced activity was only modestly inhibited. This residual inhibition may be due to phosphorylation of serine 621. Consistent results were obtained when Raf was activated by coexpression of protein kinase C α (data not shown). No kinase activity was observed in the kinase-dead RafK375W mutant, which was used as a control to exclude the possibility that any of the coexpressed kinases phosphorylates MEK.
FIG. 3.
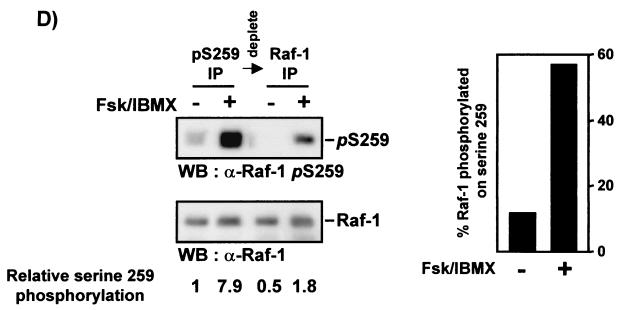
Mutation of Raf-1 serine 259 impedes the downregulation of Raf kinase activity by PKA. (A) Sf-9 cells were coinfected with baculoviruses encoding Raf-1, the RafS259D, or the kinase-negative RafK375 M mutant in combination with Ras plus Lck and PKA as indicated. The kinase activity of Raf-1 immunoprecipitates was measured in a linked assay using MEK and kinase-negative ERK (ERK−) as substrates. con, no Raf added. (B) COS-1 cells were transiently transfected with Raf-1, RafS259A, or RafS259D cloned into pCMV5. On day 3 posttransfection serum-starved cells were preincubated with 25 μg of forskolin per ml for 30 min and then treated with TPA (100 ng/ml) plus EGF (20 ng/ml) for 15 min as indicated. Raf-1 was immunoprecipitated and subjected to a linked kinase assay using recombinant MEK and kinase-negative ERK as substrates. The data shown represent three independent experiments. The kinase reactions were blotted, autoradiographed, and subsequently stained with crafVI antiserum. (C) Kinase activity of a RafS43A/S259A double mutant. COS cells were transfected with the indicated expression plasmids. Cells were treated with 20 μM forskolin (Fsk) plus 100 μM IBMX for 30 min followed by 100 ng of TPA per ml for 15 min as indicated. Raf proteins were immunoprecipitated with crafVI and examined for kinase activity by the two-step kinase assay. An anti-Raf Western blot of the immunoprecipitates is shown in the lower panel. (D) Endogenous Raf-1 was immunoprecipitated by using the phosphoserine 259-specific antiserum from COS cells treated as indicated for panel C. The supernatants were subsequently immunoprecipitated with a non-phosphorylation-sensitive Raf-1 antiserum. The precipitates were immunoblotted with anti-phosphoserine 259 and the anti-Raf antisera. In the blots shown the loading of the anti-phosphoserine 259 immunoprecipitates was adjusted to equal levels of total Raf-1 protein, and the fold induction of serine 259 phosphorylation is given below. The graph shows a quantitation of the unadjusted immunoprecipitates. The fraction of Raf-1 immunodepleted by the phosphoserine 259 antiserum is plotted against the amount of total Raf-1 according to the following formula: (Raf immunodepleted by α-p259)/(Raf immunodepleted by α-p259 + Raf subsequently immunoprecipitated with α-Raf-1 antiserum). Quantification of blots was done with PDI scan.
While these results point to an important regulatory role for serine 259 phosphorylation, they were obtained in Sf-9 insect cells, and we wished to authenticate these results in mammalian cells. Like in Sf-9 cells, in COS-1 cells (Fig. 3B) both the RafS259A and RafS259D mutants displayed an increase in basal kinase activity. The kinase activities of both mutants could be further induced by mitogen treatment. A combination of EGF and TPA was used in order to achieve maximal Raf-1 kinase activation. However, similar results were obtained when EGF and TPA were used individually. Importantly, the activation of PKA with forskolin (Fig. 3B) or forskolin plus IBMX (data not shown) almost completely abolished the mitogen-induced activity of Raf-1 but only modestly inhibited the RafS259A and RafS259D mutants. The basal kinase activities of these mutants appeared completely resistant to PKA inhibition. These results are very similar to those obtained with Sf-9 cells, demonstrating that the role of serine 259 seems conserved between insect and mammalian cells.
These results pinpoint serine 259 as the major site for PKA-mediated Raf-1 inhibition. In order to test whether serine 43 can further modulate the activity and response to PKA of a RafS259 mutant, we generated a double mutant, RafS43A/S259A (Fig. 3C). In serum-starved cells the kinase activity of the double mutant behaved in a manner very similar to that of the RafS259A single mutant: it was constitutively elevated and refractory to inhibition by the cAMP agonists forskolin plus IBMX. Similarly, forskolin plus IBMX failed to suppress the TPA stimulation of both RafS259A and the RafS43A/S259A double mutant.
We used the phosphoserine 259-specific antiserum to estimate the stoichiometry of phosphorylation in response to cAMP agonists (Fig. 3D). Raf-1 was immunoprecipitated by using the phosphoserine 259-specific antiserum, and supernatants were subsequently immunoprecipitated with a non-phosphorylation-sensitive Raf-1 antiserum. For ease of comparison the gels were adjusted to equal loadings of total Raf-1. The induction of serine 259 phosphorylation was more than sevenfold in two independent experiments. This probably is an underestimation, since the immunodepletion of serine 259-phosphorylated Raf-1 was not complete. Importantly, the fraction of Raf-1 that is phosphorylated on serine 259 rises to almost 60% in response to cAMP agonists. This stoichiometry is consistent with the substantial inhibition of Raf-1 activity, which is of a similar extent (Fig. 3B).
In summary, these results indicate that in the context of full-length Raf-1 serine 259 represents the main target for inhibitory phosphorylation by PKA. They, however, do not strictly rule out a modulatory role for serine 43 which may fine-tune the exact extent of Raf-1 activation. The mutation of serine 259 enhances the affinity of Raf-1 towards Ras (8) and could override small modulatory effects of serine 43 phosphorylation in the RafS43A/S259A double mutant. The results further imply that the phosphorylation of serine 259 downregulates Raf-1 by preventing the transition from the inactive into a partially activated state.
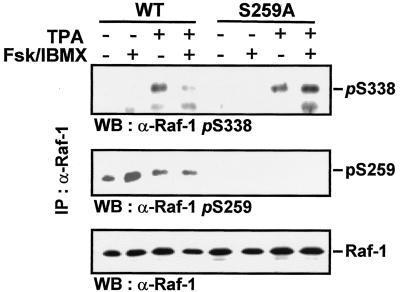
Serine 259 modulates the phosphorylation of serine 338.
In order to test the above hypotheses, the phosphorylation of serine 338 was examined. Serine 338 phosphorylation is required but not fully sufficient for Raf-1 activation (17, 23). Therefore, we assayed the phosphorylation status of serine 338 in Raf-1 and RafS259A (Fig. 4). Both mutants were poorly phosphorylated on serine 338 in starved cells. TPA increased serine 338 phosphorylation of both Raf-1 and RafS259A. In wild-type Raf-1 there appeared to be an inverse relationship between serine 259 and serine 338 phosphorylations: PKA activation decreased the phosphorylation of serine 338 in wild-type Raf-1, but not in RafS259A. These data suggest that serine 259 can at least in part control the phosphorylation of serine 338 and thus the transition from a state primed for activation into full activation.
FIG. 4.
cAMP agonists inhibit the phosphorylation of Raf-1 on serine 338. COS-1 cells were transfected with pCMV5Raf-1 or pCMV5-RafS259A, serum starved overnight, and treated with 40 μM forskolin plus 50 μM IBMX for 20 min and 100 ng of TPA per ml for 15 min as indicated. (A) The Raf-1 proteins were immunoprecipitated with the crafVI antibody and immunoblotted with anti-phosphoserine 338 and anti-serine 259 antibodies. After being stripped, the blots were stained with crafVI antiserum.
Raf-1-induced ERK activation and transcription are suppressed by cAMP and rescued by mutation of serine 259.
The fact that the RafS259 mutants were resistant to inhibition by cAMP allowed us to test whether cAMP impinges on other components of the pathway downstream of Raf-1 (Fig. 5). This important experiment has not been possible previously, because of the lack of a Raf-1 mutant resistant to cAMP downregulation.
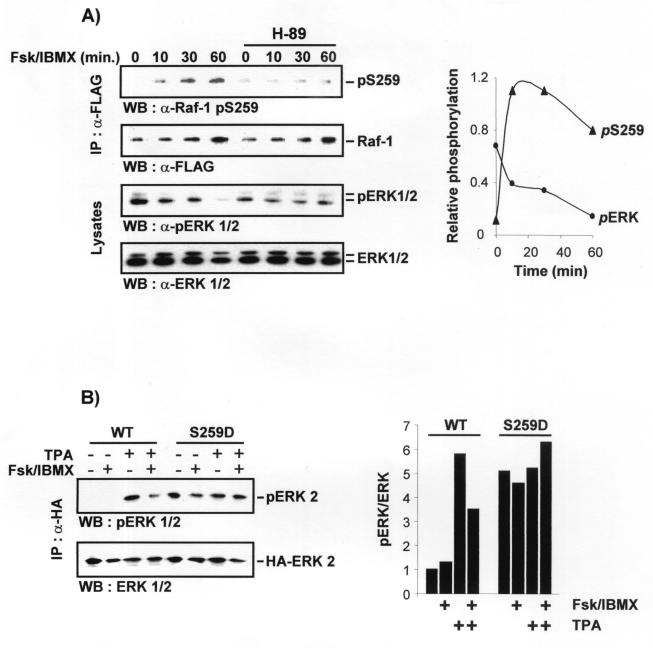
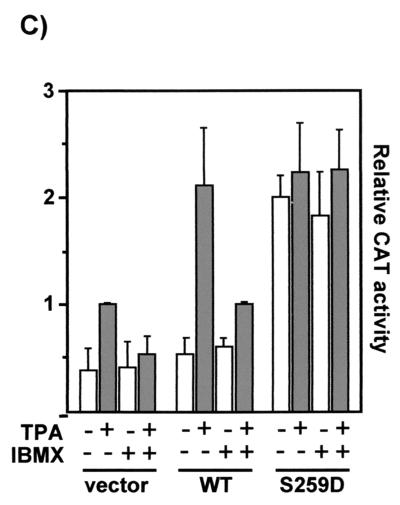
FIG. 5.
cAMP inhibits the ERK pathway at the level of Raf-1 through the induction of the phosphorylation of serine 259 in Raf-1. (A) RafS259-stimulated ERK activity is resistant to inhibition by cAMP. COS-1 cells were transfected with HA-ERK-2 plus pCMV5Raf-1 or pCMV5RafS259D. Cells were serum starved and treated with forskolin and IBMX as in Fig. 4. HA-ERK-2 was immunoprecipitated with an HA antibody, and activated ERK was detected by immunoblotting with a phosphospecific ERK antibody (upper panel). The blot was stripped and subsequently stained with an anti-ERK antiserum. (B) The cAMP-induced increase in Raf-1 serine 259 phosphorylation is followed by inhibition of ERK. COS-1 cells were transfected with a small amount (0.4 μg) of FLAG-tagged Raf-1 in order to facilitate the use of the phosphoserine 259-specific antibody. Growing cells were treated with 10 μM H-89 for 30 min followed by 40 μM forskolin plus 100 μM IBMX for the indicated time points (Fsk/IBMX). Cells were lysed, and FLAG-Raf-1 was immunoprecipitated with anti-FLAG antibodies and stained with a phosphoserine 259-specific antibody (upper panel). The blots were stripped and subsequently stained with a FLAG antibody (lower panel). ERK phosphorylation (upper panel) and expression (lower panel) were examined by blotting whole-cell lysates. (C) RafS259-induced reporter gene expression is refractory to inhibition by cAMP. NIH 3T3 cells were cotransfected with a GAL4-Elk-1 transcription factor, a CAT reporter gene under the control of GAL-4 DNA binding sites and Raf-1 or RafS259D. Cells were treated as indicated, and CAT expression was measured 8 h posttreatment.
First, we examined ERK activation (Fig. 5A). COS-1 cells were cotransfected with HA-ERK-2 and Raf-1 or RafS259D, respectively. Serum-starved cells were treated with cAMP agonists and TPA alone or in combination as indicated, and HA-ERK-2 immunoprecipitates were examined for kinase activity. The activity of HA-ERK-2 in Raf-1-transfected cells was induced by TPA and downregulated by cAMP agonists. In contrast, RafS259D induced constitutive ERK activation, which was completely refractory to cAMP inhibition. These results show that cAMP does not affect MEK or ERK activity but that the inhibition of the ERK pathway by PKA occurs on the level of Raf-1. They further predict that the inhibition of ERK activity should correlate with a hyperphosphorylation of Raf-1 on serine 259.
In order to test this hypothesis we examined the phosphorylation of Raf-1 on serine 259 and the phosphorylation of ERK on the activating sites over a time course of stimulation with forskolin plus IBMX (Fig. 5B). Forskolin plus IBMX treatment of growing cells induced the phosphorylation of Raf-1 on serine 259 within 10 min. In parallel, ERK activity declined steadily, although it took up to 1 h to see a marked inhibition. The delayed kinetics of ERK inactivation is consistent with the downregulation of an upstream activator, such as Raf-1, rather than with a direct inhibition of ERK by cAMP signaling. The induction of serine 259 phosphorylation and the inhibition of ERK were efficiently antagonized by H89, a chemical inhibitor of PKA.
To examine signaling downstream of ERK, we used a transcriptional readout that relies on the phosphorylation of Elk-1, a member of the ternary complex transcription factor family. Elk-1 is a well-characterized substrate for ERK, which enhances Elk-1-mediated transcription by phosphorylating the transactivation domain (12). If the Elk-1 transactivation domain is fused to a GAL4 DNA binding domain, the transcription of a reporter gene under the control of GAL4 binding sites becomes strongly dependent on the activity of the ERK pathway. The expression of the reporter gene is induced by TPA or growth factors that stimulate Raf-1 and can be suppressed by dominant negative Raf-1 mutants (data not shown). Transfection of Raf-1 enhanced the TPA-induced activation of the GAL4-Elk-1 fusion protein (Fig. 5C). This increase was thoroughly suppressed when cAMP degradation was inhibited by IBMX. The expression of RafS259D stimulated the basal activity to levels comparable to those obtained with TPA induction. This activation was completely insensitive to IBMX, demonstrating that cAMP blocks the pathway at the level of Raf-1 and does not affect downstream components. Likewise, RafS259A or RafS259E induced the constitutive activation of the GAL4-Elk-1 reporter, whereas RafS43A behaved like wild-type Raf-1 and RafS621A behaved like a dominant negative mutant (data not shown).
DISCUSSION
Since the discovery that cAMP elevation can inhibit the ERK pathway, several models have been proposed. All feature Raf-1 as a target for inhibition but differ in regard to explaining the mechanism of inhibition. The first model suggested that the PKA-mediated phosphorylation of serine 43 diminishes the affinity of Raf-1 towards Ras and may contribute to preventing Raf-1 activation (38). A recent paper suggested that PKA inhibits Raf-1 by inactivating PAK, a kinase that is crucial for the activation of Raf-1 by phosphorylating serine 338 (16). Other studies suggested that PKA can directly inhibit Raf-1 catalytic activity by the phosphorylation of serine 621 (25). These studies concentrated on the mechanism of inhibition of the isolated Raf-1 kinase domain which lacks the regulatory domain and hence serine 259. In this report we have further characterized PKA-induced phosphorylation of Raf-1. We identified serine 259 as an in vivo phosphorylation site and provide evidence that this site is the main mediator of PKA-induced Raf-1 inhibition. Together with serines 43 and 621, serine 259 is one of the three constitutive phosphorylation sites found on Raf-1 in resting cells (27). These sites are phosphorylated by purified PKA in vitro and induced by treatment of cells with activators of PKA and thus appear to be genuine PKA phosphorylation sites (this study and references 13, 25, 33, and 38). Serine 259 has also been recently reported to be a target site for Akt/PKB-induced inhibitory phosphorylation (40).
Serine 43 can be phosphorylated by PKA (5, 30, 38) and the cyclic GMP-dependent kinase, PKG (35), and was reported to prevent Raf-1 activation in these studies by interfering with the binding of Raf-1 to Ras. These observations have been challenged by a recent report that dissociated Raf-1 inhibition by PKA from the phosphorylation of serine 43 (34). Our data suggest that the effects of serine 43 phosphorylation are context dependent as the inhibition of Ras binding can be abolished by TPA or EGF. These agents induce serine 43 phosphorylation on their own (Fig. 2A) (8), making it unlikely that they rescue Ras binding by preventing serine 43 phosphorylation. However, serine 43 phosphorylation could serve as a timing device which determines the duration of the Ras-Raf interaction. Given that Ras interacts with multiple effectors (reviewed in reference 4), which all compete for binding to a single shared effector domain, there must be mechanisms to coordinate the timely interaction with the different effectors.
Serine 621 also appears to have a complex role. Unfortunately, this residue is not readily amenable to mutational analysis. Changing it to a number of other amino acids resulted in the loss of catalytic function (25, 27). This observation demonstrates that serine 621 is essential for kinase activity and has further been used to argue that the phosphorylation of this residue is necessary for kinase activity (36). Biochemical experiments have confirmed that serine 621 is essential but also indicated that the phosphorylation of this residue inhibits catalytic activity (25). These experiments were performed with the isolated Raf-1 kinase domain expressed in Sf-9 insect cells, but it has also been previously shown that the activity of v-Raf, which consists of the Raf-1 kinase domain fused to retroviral Gag sequences, is also downregulated by PKA (13). It has been shown that the Raf-1 regulatory domain restrains catalytic activity through physical interactions with the kinase domain (6). Thus, it is possible that serine 621 becomes fully accessible for phosphorylation only once the regulatory domain is removed. This could explain why the inhibitory effects of serine 621 are not evident in full-length Raf-1 but readily become apparent in the context of the isolated kinase domain. This hypothesis is supported by the observation that in vitro serine 621 in the isolated Raf-1 kinase domain is a much better substrate for PKA than serine 621 in full-length Raf-1 (13).
A possible explanation for the dual role of serine 621 may be related to 14-3-3 binding. 14-3-3 proteins are ubiquitously expressed adapter proteins that require phosphoserine for binding (11, 24, 29). Both serines 259 and 621 in Raf-1 are docking sites for 14-3-3 proteins, and 14-3-3 proteins have been implicated in the activation of Raf-1 and the regulation of serine 621 phosphorylation (36, 39) as well as in stabilizing both active and inactive Raf-1 conformations (37). The displacement of 14-3-3 from Raf-1 by a synthetic peptide encompassing phosphoserine 621 in vitro led to a complete loss of Raf-1 catalytic activity, which could be restored by the addition of recombinant 14-3-3 protein. Interestingly, a RafS259A mutant also was susceptible to inhibition by this phosphopeptide (37). Since the RafS259A mutant still has an intact 14-3-3 docking site on phosphoserine 621, this experiment suggests that the inhibitory effect of serine 621 phosphorylation can be reversed by 14-3-3 binding. This hypothesis is currently being investigated.
Several studies have observed that the mutation of serine 259 modestly increases the catalytic activity of Raf-1 (8, 24, 27, 32, 37). The same activated phenotype is produced by replacing serine 259 with either alanine, aspartic acid, or glutamic acid. Due to their negative charge, aspartic and glutamic acids often act as phosphomimetic substitutes. But our results indicate that the physical presence of a phosphate group at serine 259 is necessary for inhibition. It has been suggested that 14-3-3 binding to phosphoserine 259 (32, 37) inhibits Raf-1. As 14-3-3 docking requires a phosphoserine (24, 29), the activated phenotype of RafS259 mutants could be due to the loss of 14-3-3 binding. To test the contribution of 14-3-3 binding, we have mutated both 14-3-3 docking sites, but unfortunately the serine 259/621 double mutant is devoid of kinase activity (data not shown), precluding a conclusive investigation. The present study demonstrates that both the basal and the stimulated kinase activities are enhanced by mutation of serine 259. Raf S259 mutants are largely refractory to the inhibitory effects of PKA, demonstrating that serine 259 phosphorylation is sufficient for the inhibition of full-length Raf-1 by PKA.
It is important to note that this conclusion does not discount a role for the other PKA phosphorylation sites serines 43 and 621. They could serve as conditional modulators. As outlined above, serine 43 phosphorylation may be part of an intricate regulatory mechanism that modulates Raf-1 activation by Ras, while serine 621 phosphorylation may regulate the Raf-1 catalytic activity in a 14-3-3-dependent manner. This regulation may apply to different pools of Raf-1. Compartmentalization of signaling molecules is increasingly being recognized as an important regulatory mechanism, in particular in the ERK and cAMP signaling systems (reviewed in references 15 and 18). It has been recently shown that serine 259 phosphorylation affects the subcellular localization of Raf-1 (8, 20). In a broader sense compartmentalization can also be perceived in terms of functional separation because of differential modifications. As the phosphopeptide maps show, the cAMP-induced increase in serine 43, 259, and 621 phosphorylation is not uniform, implicating that subpopulations of Raf-1 which bear different combinations of phosphorylations exist. It will be a challenge to sort out whether PKA inhibits Raf-1 solely via the serine 259 master site, as this study implies, or whether there is a subtle interplay between the three PKA target sites.
In addition to inhibiting Raf-1 by direct phosphorylation, PKA may also use an indirect route to prevent Raf-1 activation. The suppression of Raf-1 activation in detached cells caused by the loss of integrin engagement was also recently shown to result from the PKA-mediated inhibition of PAK (16). PAK has been described as the kinase which phosphorylates Raf-1 on serine 338 (17). Work with mutants has suggested that serine 338 phosphorylation is essential but not sufficient for the full activation of Raf-1 (9, 17, 23) and that S338 phosphorylation is indicative of, but does not quantitatively correlate with, Raf-1 activity (23). As shown in Fig. 4, we found that cAMP elevation reduced Raf-1 phosphorylation on serine 338, which conceivably could contribute to Raf-1 inhibition. Mutation of serine 259 completely prevented the downregulation of serine 338 phosphorylation by cAMP. These observations suggest that, rather than suppressing a serine 338 kinase or inducing a serine 338 phosphatase, the effect of PKA on serine 338 phosphorylation is regulated by the phosphorylation of serine 259. Since serine 259 regulates Raf-1 membrane localization and its interaction with Ras (8, 20), a plausible mechanism whereby phosphorylation of serine 259 reduces serine 338 phosphorylation is by diminishing the accessibility of Raf-1 to membrane-bound serine 338 kinases. These results also raise the possibility that in adherent cells PKA influences serine 338 phosphorylation through the modulation of serine 259 phosphorylation rather than through the inhibition of PAK. In summary, the inhibition of Raf-1 by PKA seems to utilize multiple layers of regulation and begins to unfold a complexity similar to that of Raf-1 activation itself.
Acknowledgments
We thank Deborah Morrison, Stanley McKnight, and Richard Marais for reagents and John Wyke, Vaughn Cleghon, and Richard Marais for critical reading of the manuscript and comments.
This work was supported by grants from the Cancer Research Campaign and the DFG.
REFERENCES
- 1.Abraham, D., K. Podar, M. Pacher, M. Kubicek, N. Welzel, B. A. Hemmings, S. M. Dilworth, H. Mischak, W. Kolch, and M. Baccarini. 2000. Raf-1-associated protein phosphatase 2A as a positive regulator of kinase activation. J. Biol. Chem. 275:22300-22304. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, S., D. L. Davis, H. Dahlback, H. Jornvall, and D. W. Russell. 1989. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J. Biol. Chem. 264:8222-8229. [PubMed] [Google Scholar]
- 3.Barnard, D., B. Diaz, D. Clawson, and M. Marshall. 1998. Oncogenes, growth factors and phorbol esters regulate Raf-1 through common mechanisms. Oncogene 17:1539-1547. [DOI] [PubMed] [Google Scholar]
- 4.Campbell, S. L., R. Khosravi-Far, K. L. Rossman, G. J. Clark, and C. J. Der. 1998. Increasing complexity of Ras signaling. Oncogene 17:1395-1413. [DOI] [PubMed] [Google Scholar]
- 5.Chuang, E., D. Barnard, L. Hettich, X. F. Zhang, J. Avruch, and M. S. Marshall. 1994. Critical binding and regulatory interactions between Ras and Raf occur through a small, stable N-terminal domain of Raf and specific Ras effector residues. Mol. Cell. Biol. 14:5318-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cutler, R. E., Jr., R. M. Stephens, M. R. Saracino, and D. K. Morrison. 1998. Autoregulation of the Raf-1 serine/threonine kinase. Proc. Natl. Acad. Sci. USA 95:9214-9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dent, P., D. B. Reardon, D. K. Morrison, and T. W. Sturgill. 1995. Regulation of Raf-1 and Raf-1 mutants by Ras-dependent and Ras-independent mechanisms in vitro. Mol. Cell. Biol. 15:4125-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhillon, A. S., S. Meikle, Z. Yazici, M. Eulitz, and W. Kolch. 2002. Regulation of Raf-1 activation and signalling by dephosphorylation. EMBO J. 21:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz, B., D. Barnard, A. Filson, S. MacDonald, A. King, and M. Marshall. 1997. Phosphorylation of Raf-1 serine 338-serine 339 is an essential regulatory event for Ras-dependent activation and biological signaling. Mol. Cell. Biol. 17:4509-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fabian, J. R., I. O. Daar, and D. K. Morrison. 1993. Critical tyrosine residues regulate the enzymatic and biological activity of Raf-1 kinase. Mol. Cell. Biol. 13:7170-7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu, H., R. R. Subramanian, and S. C. Masters. 2000. 14-3-3 proteins: structure, function, and regulation. Annu. Rev. Pharmacol. Toxicol. 40:617-647. [DOI] [PubMed] [Google Scholar]
- 12.Gille, H., M. Kortenjann, O. Thomae, C. Moomaw, C. Slaughter, M. H. Cobb, and P. E. Shaw. 1995. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J. 14:951-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Häfner, S., H. S. Adler, H. Mischak, P. Janosch, G. Heidecker, A. Wolfman, S. Pippig, M. Lohse, M. Ueffing, and W. Kolch. 1994. Mechanism of inhibition of Raf-1 by protein kinase A. Mol. Cell. Biol. 14:6696-6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hallberg, B., S. I. Rayter, and J. Downward. 1994. Interaction of Ras and Raf in intact mammalian cells upon extracellular stimulation. J. Biol. Chem. 269:3913-3916. [PubMed] [Google Scholar]
- 15.Houslay, M. D., and W. Kolch. 2000. Cell-type specific integration of cross-talk between extracellular signal-regulated kinase and cAMP signaling. Mol. Pharmacol. 58:659-668. [PubMed] [Google Scholar]
- 16.Howe, A. K., and R. L. Juliano. 2000. Regulation of anchorage-dependent signal transduction by protein kinase A and p21-activated kinase. Nat. Cell Biol. 2:593-600. [DOI] [PubMed] [Google Scholar]
- 17.King, A. J., H. Sun, B. Diaz, D. Barnard, W. Miao, S. Bagrodia, and M. S. Marshall. 1998. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature 396:180-183. [DOI] [PubMed] [Google Scholar]
- 18.Kolch, W. 2000. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J. 351:289-305. [PMC free article] [PubMed] [Google Scholar]
- 19.Kortenjann, M., O. Thomae, and P. E. Shaw. 1994. Inhibition of v-raf-dependent c-fos expression and transformation by a kinase-defective mutant of the mitogen-activated protein kinase Erk2. Mol. Cell. Biol. 14:4815-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kubicek, M., M. Pacher, D. Abraham, K. Podar, M. Eulitz, and M. Baccarini. 2001. Dephosphorylation of Ser-259 regulates Raf-1 membrane association. J. Biol. Chem. 277:7913-7919. [DOI] [PubMed] [Google Scholar]
- 21.Lewis, T. S., P. S. Shapiro, and N. G. Ahn. 1998. Signal transduction through MAP kinase cascades. Adv. Cancer Res. 74:49-139. [DOI] [PubMed] [Google Scholar]
- 22.Marais, R., and C. J. Marshall. 1996. Control of the ERK MAP kinase cascade by Ras and Raf. Cancer Surv. 27:101-125. [PubMed] [Google Scholar]
- 23.Mason, C. S., C. J. Springer, R. G. Cooper, G. Superti-Furga, C. J. Marshall, and R. Marais. 1999. Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation. EMBO J. 18:2137-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michaud, N. R., J. R. Fabian, K. D. Mathes, and D. K. Morrison. 1995. 14-3-3 is not essential for Raf-1 function: identification of Raf-1 proteins that are biologically activated in a 14-3-3- and Ras-independent manner. Mol. Cell. Biol. 15:3390-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mischak, H., T. Seitz, P. Janosch, M. Eulitz, H. Steen, M. Schellerer, A. Philipp, and W. Kolch. 1996. Negative regulation of Raf-1 by phosphorylation of serine 621. Mol. Cell. Biol. 16:5409-5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrison, D. K., and R. E. Cutler. 1997. The complexity of Raf-1 regulation. Curr. Opin. Cell Biol. 9:174-179. [DOI] [PubMed] [Google Scholar]
- 27.Morrison, D. K., G. Heidecker, U. R. Rapp, and T. D. Copeland. 1993. Identification of the major phosphorylation sites of the Raf-1 kinase. J. Biol. Chem. 268:17309-17316. [PubMed] [Google Scholar]
- 28.Muller, G., P. Storz, S. Bourteele, H. Doppler, K. Pfizenmaier, H. Mischak, A. Philipp, C. Kaiser, and W. Kolch. 1998. Regulation of Raf-1 kinase by TNF via its second messenger ceramide and cross-talk with mitogenic signalling. EMBO J. 17:732-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muslin, A. J., J. W. Tanner, P. M. Allen, and A. S. Shaw. 1996. Interaction of 14-3-3 with signalling proteins is mediated by the recognition of phosphoserine. Cell 84:889-897. [DOI] [PubMed] [Google Scholar]
- 30.Ramstad, C., V. Sundvold, H. K. Johansen, and T. Lea. 2000. cAMP-dependent protein kinase (PKA) inhibits T cell activation by phosphorylating ser-43 of raf-1 in the MAPK/ERK pathway. Cell. Signal. 12:557-563. [DOI] [PubMed] [Google Scholar]
- 31.Rommel, C., B. A. Clarke, S. Zimmermann, L. Nunez, R. Rossman, K. Reid, K. Moelling, G. D. Yancopoulos, and D. J. Glass. 1999. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science 286:1738-1741. [DOI] [PubMed] [Google Scholar]
- 32.Rommel, C., G. Radziwill, K. Moelling, and E. Hafen. 1997. Negative regulation of Raf activity by binding of 14-3-3 to the amino terminus of Raf in vivo. Mech. Dev. 64:95-104. [DOI] [PubMed] [Google Scholar]
- 33.Schramm, K., M. Niehof, G. Radziwill, C. Rommel, and K. Moelling. 1994. Phosphorylation of c-Raf-1 by protein kinase A interferes with activation. Biochem. Biophys. Res. Commun. 201:740-747. [DOI] [PubMed] [Google Scholar]
- 34.Sidovar, M. F., P. Kozlowski, J. W. Lee, M. A. Collins, Y. He, and L. M. Graves. 2000. Phosphorylation of serine 43 is not required for inhibition of c-Raf kinase by the cAMP-dependent protein kinase. J. Biol. Chem. 275:28688-28694. [DOI] [PubMed] [Google Scholar]
- 35.Suhasini, M., H. Li, S. M. Lohmann, G. R. Boss, and R. B. Pilz. 1998. Cyclic-GMP-dependent protein kinase inhibits the Ras/Mitogen-activated protein kinase pathway. Mol. Cell. Biol. 18:6983-6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thorson, J. A., L. W. K. Yu, A. L. Hsu, N. Y. Shih, P. R. Graves, J. W. Tanner, P. M. Allen, H. Piwnica-Worms, and A. S. Shaw. 1998. 14-3-3 proteins are required for maintenance of Raf-1 phosphorylation and kinase activity. Mol. Cell. Biol. 18:5229-5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tzivion, G., Z. Luo, and J. Avruch. 1998. A dimeric 14-3-3 protein is an essential cofactor for Raf kinase activity. Nature 394:88-92. [DOI] [PubMed] [Google Scholar]
- 38.Wu, J., P. Dent, T. Jelinek, A. Wolfman, M. J. Weber, and T. W. Sturgill. 1993. Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3′,5′-monophosphate. Science 262:1065-1069. [DOI] [PubMed] [Google Scholar]
- 39.Yip-Schneider, M. T., W. Miao, A. Lin, D. S. Barnard, G. Tzivion, and M. S. Marshall. 2000. Regulation of the Raf-1 kinase domain by phosphorylation and 14-3-3 association. Biochem. J. 351:151-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmermann, S., and K. Moelling. 1999. Phosphorylation and regulation of Raf by Akt (protein kinase B). Science 286:1741-1744. [DOI] [PubMed] [Google Scholar]