Abstract
Human Polo-like kinase 3 (Plk3, previously termed Prk or Fnk) is involved in regulation of cell cycle progression through the M phase (B. Ouyang, H. Pan, L. Lu, J. Li, P. Stambrook, B. Li, and W. Dai, J. Biol. Chem. 272:28646-28651, 1997). Here we report that in most interphase cells endogenous Plk3 was predominantly localized around the nuclear membrane. Double labeling with Plk3 and γ-tubulin, the latter a major component of pericentriole materials, revealed that Plk3 was closely associated with centrosomes and that its localization to centrosomes was dependent on the integrity of microtubules. Throughout mitosis, Plk3 appeared to be localized to mitotic apparatus such as spindle poles and mitotic spindles. During telophase, a significant amount of Plk3 was also detected in the midbody. Ectopic expression of Plk3 mutants dramatically changed cell morphology primarily due to their effects on microtubule dynamics. Expression of a constitutively active Plk3 (Plk3-A) resulted in rapid cell shrinkage, which led to formation of cells with an elongated, unsevered, and taxol-sensitive midbody. In contrast, cells expressing a kinase-defective Plk3 (Plk3K52R) mutant exhibited extended, deformed cytoplasmic structures, the phenotype of which was somewhat refractory to taxol treatment. Expression of both Plk3-A and Plk3K52R induced apparent G2/M arrest followed by apoptosis, although the kinase-defective mutant was less effective. Taken together, our studies strongly suggest that Plk3 plays an important role in the regulation of microtubule dynamics and centrosomal function in the cell and that deregulated expression of Plk3 results in cell cycle arrest and apoptosis.
Members of the Polo family of protein kinases, conserved through evolution, have been described in yeast (15), Caenorhabditis elegans (25), Drosophila melanogaster (8), Xenopus laevis (16) mouse (7, 27), and human (12, 18). The founding member of this family, Polo, was originally identified in the fruit fly and was shown to be a serine-threonine kinase that is required for mitosis (8). Mutations in the Polo gene result in abnormal mitotic and meiotic division (10). The kinase activity of Polo peaks cyclically at anaphase-telophase, and the protein also undergoes translocations during the cell cycle progression (9). Whereas it is located predominantly in the cytoplasm during interphase, at M phase it becomes associated with condensed chromosomes and other components of the mitotic apparatus, including centrosomes, kinetochores, and the spindle midzone (9). In addition to the conserved kinase domain, Polo family proteins all share a short amino acid sequence termed the Polo box (18). Mutations in the Polo box of the Plk homologue in budding yeast (Cdc5) that do not affect kinase activity abolish the ability of this protein to complement functionally temperature-sensitive mutants of budding yeast (30), suggesting that the Polo box is essential for its biological activity.
Mammalian cells contain at least three Polo family proteins (human Polo-like kinase 1 [Plk1], Plk2, and Plk3) that exhibit marked sequence homology to Drosophila Polo (7, 13, 18, 27). As cells progress through the cell cycle, Plk proteins undergo substantial changes in abundance, kinase activity, or subcellular localization. In human cells, the levels of Plk1 protein and its kinase activity peak at mitosis (13). During mitosis, Plk1 transiently associates with mitotic structures such as the spindles, kinetochores, and centrosomes (1, 11). Recent studies have shown that Plk1 contributes to a variety of mitotic (or meiotic) events, including activation of cyclin B-Cdc2 (CDK1), breakdown of the nuclear membrane, centrosome maturation, and formation of the bipolar spindle at the onset of mitosis (4, 22, 26).
Plk3, originally cloned and characterized in our laboratory, appears to function differently from Plk1. The abundance of Plk3 remains relatively constant during the cell cycle, and its kinase activity peaks during late S and G2 phases (24). Furthermore, Plk3 phosphorylates Cdc25C on serine-216, resulting in inhibition of the activity of this protein (23), whereas phosphorylation of cyclin B by Plk1 results in its translocation from the cytosol to the nucleus, thus activating Cdc2 kinase (31). In addition, the amount of Plk3 mRNA but not of Plk1 mRNA is rapidly and transiently increased in response to mitogenic stimulation (18). Plk2 (also known as Snk) was originally identified as the product of an immediate-early gene and is less characterized than are Plk1 and Plk3. Recent studies have shown that both Snk and Fnk associate with CIB, a calmodulin-related protein (14). Both Snk and Fnk have been implicated in long-term synaptic plasticity and thus may perform postmitotic functions (14).
The present study was designed to reveal the subcellular localization of Plk3 during the cell cycle, describe its potential role in regulating microtubule dynamics and the microtubule organization center (MTOC), and evaluate its role in the maintenance of cell morphology, viability, and malignant transformation.
MATERIALS AND METHODS
Cell culture and treatment.
The GM00637 cell line (human fibroblast) was originally obtained from the Coriell Institute for Medical Research. A549 (lung carcinoma), HeLa (cervical carcinoma), Daudi (lymphoblastic leukemia), HEL (erythroleukemia), HL-60 (myelogenous leukemia), PC-3 (prostate carcinoma), LNCaP (prostate carcinoma), and DU145 (prostate carcinoma) cell lines were obtained from the American Type Culture Collection. Cells were cultured in culture dishes or on Lab-Tek II chamber slides (Fisher Scientific) in appropriate media supplemented with 10% fetal bovine serum and antibiotics (100 μg of penicillin and 50 μg of streptomycin sulfate per ml) with 5% CO2. For some experiments, A549 cells at about 80% confluence were treated with a low temperature (4°C) for 5 min or taxol (5 μM) for 4 h before analysis (unless specified otherwise).
Transfection.
A549 cells were transfected, using the Lipofectamine method, with pCR259-Plk3-A, pCR259-Plk3K52R, or the empty vector (GIBCO/Invitrogen). The constitutively active Plk3 cDNA was as described previously (18). The Plk3K52R was obtained by replacing the conserved lysine-52 residue with arginine; the resulting protein was kinase defective (23).
Immunoblotting and pull-down assays.
Cultured cells were lysed as described earlier (24). Equal amounts (50 μg) of protein lysates and purified Plk1 and Plk3 were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by immunoblotting with antibodies to human Plk3 (Pharmingen [23]), BUBR1, α-tubulin (Sigma), Plk1 (Zymed), or cyclin B (DAKO). Recombinant His6-Plk3 expressed with the use of a baculoviral expression system as described earlier (23) was affinity purified with and subsequently conjugated to Ni-nitrilotriacetic acid resin (Qiagen). Flag-tagged Plk1 was a gift from John Cogswell (GlaxoSmithKline). Specific signals were detected with horseradish peroxidase-conjugated goat secondary antibodies (Sigma) and enhanced chemiluminescence reagents (Amersham Pharmacia Biotech).
Fluorescence microscopy.
Localization of Plk3 was determined by double immunofluorescence analysis of a centrosomal marker. Cells were quickly washed with phosphate-buffered saline (PBS) and fixed in methanol for 5 min at room temperature. Fixed cells were treated with 0.1% Triton X-100 in PBS for 5 min and were then washed three times with ice-cold PBS. After blocking with 2.0% bovine serum albumin (BSA) in PBS for 15 min on ice, cells were incubated for 1 h with mouse monoclonal Plk3 immunoglobulin G (IgG, 4 μg/ml) and/or rabbit anti-γ-tubulin IgG (T3559, 1:250 dilution; Sigma) in 2% BSA solution, washed with PBS, and then incubated with rhodamine red X-conjugated anti-mouse IgGs and/or fluorescein isothiocyanate-conjugated anti-rabbit IgGs (Jackson ImmunoResearch) at 4°C for 1 h in the dark. Cells were finally stained with 4′,6′-diamidino-2-phenylindole (DAPI) (1 μg/ml; Fluka) for 5 min. Fluorescence microscopy was performed on a Nikon microscope, and images were captured using a digital camera (Optronics) using Optronics MagFire and Image-Pro Plus softwares.
Flow cytometry.
A549 cells transfected with or without Plk3 mutants for 14 h were analyzed for cell cycle stage and apoptosis. Briefly, cells were fixed with methanol, treated with Triton X-100 (0.25% in PBS) for 5 min, washed with PBS with 1% BSA, and incubated for 1 h with (or without as a control) an antibody to a caspase-cleaved poly(ADP-ribose) polymerase fragment (PARP p85) (Promega). Cells were rinsed with PBS and then incubated with a second antibody conjugated with fluorescein isothiocyanate for 1 h, rinsed with PBS containing 1% BSA, and stained with DAPI (1 μg/ml). The fluorescence of cells processed for flow cytometry was measured by the ELITE.ESP cytometer/cell sorter (Coulter) as described previously (19). Each experiment was repeated at least three times.
RESULTS
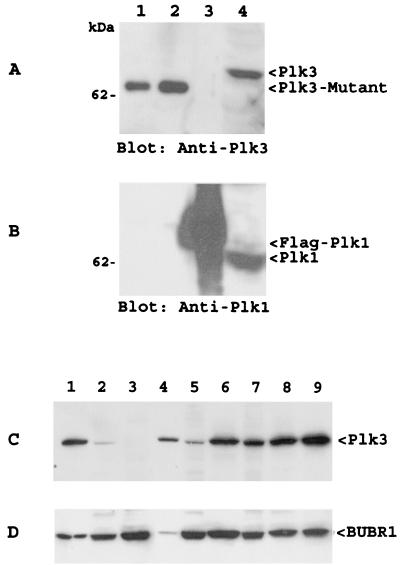
Although early experiments have shown that our Plk3 antibody does not cross-react with Plk1 (32), we further determined the specificity of the Plk3 antibody via Western blotting. Two sets of purified recombinant Plk1 and Plk3 samples, as well as A549 cell lysates, were blotted with antibodies to Plk1 and Plk3, respectively. Figure 1 shows that neither Plk1 (Fig. 1A) nor Plk3 (Fig. 1B) antibody cross-reacted with each other. To further test the antibody, we examined expression of Plk3 in various cell lines via Western blotting. Figure 1C showed that the Plk3 antibody detected a single antigen that migrated at the position predicted for full-length Plk3. No additional prominent band(s) was detected by the antibody in these cell lines, indicating that our Plk3 antibody did not cross-react with Plk1. As a loading control, the same blot was stripped and reprobed with an antibody to BUBR1 (Fig. 1D), a spindle checkpoint gene product.
FIG. 1.
Plk3 antibody did not recognize Plk1. (A) Purified recombinant His6-Plk3-A (lanes 1 and 2) and Flag-Plk1 (lane 3), as well as A549 cell lysates (lane 4), were blotted with the anti-Plk3 antibody. Recombinant Plk3 migrated faster than the cellular form because of a short deletion at the amino terminus (20). (B) A duplicate blot as shown in panel A was blotted for Plk1. Recombinant Plk1 migrated slightly more slowly than the cellular form because of an addition of the Flag tag. (C and D) Protein lysates (50 μg/lane) from A549, DAMI, Daudi, DU145, GM00637, HEL, HeLa, LNCaP, and PC-3 were blotted with the Plk3 antibody (C) or BUBR1 antibody (D).
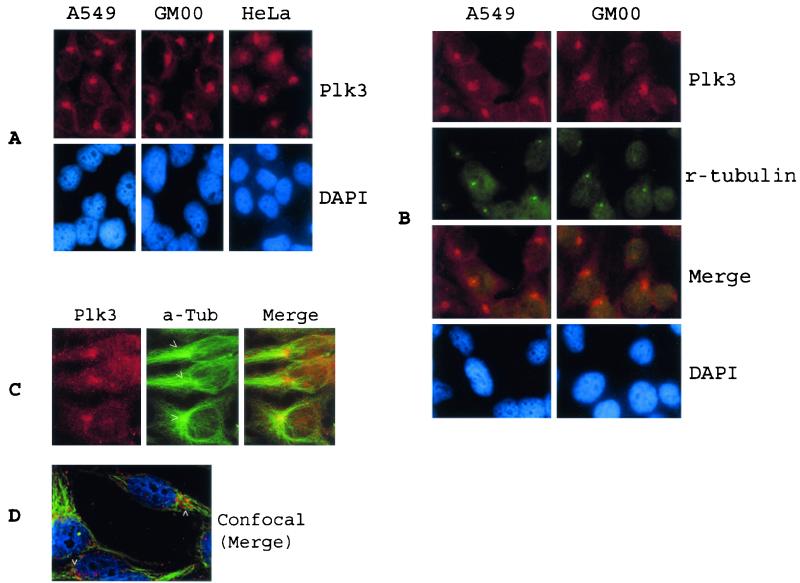
In an attempt to identify potential physiological targets of Plk3, we examined the subcellular localization of Plk3 in cultured cell lines via indirect immunofluorescence microscopy. We detected (Fig. 2A) strong Plk3-specific staining as spots around the nuclear membrane of A549 cells, although diffuse, much weaker staining was also detected in the cytoplasm. Perinuclear, condensed spots were detected in over 90% of the cells. When the spot was detected in cells, those cells usually contained one spot (69%). This Plk3 subcellular localization pattern was also observed in the other two cell lines (GM00637 and HeLa), indicating a common cellular function associated with Plk3 subcellular localization. Double-labeling experiments using an antibody to γ-tubulin, a major component of pericentriole materials, showed that Plk3 localized around centrosomes in both A549 and GM00637 cells (Fig. 2B). However, the distribution of Plk3 staining was slightly more diffuse than that of γ-tubulin. The same colocalization of Plk3 with centrosomes was observed in several other cell lines, including HeLa cells (data not shown). Double staining using an antibody to α-tubulin confirmed that Plk3 was tightly associated with MTOC (Fig. 2C and D, arrowheads).
FIG. 2.
Plk3 colocalized with centrosomes. (A) A549, GM00637, and HeLa cells cultured on chamber slides were incubated with an antibody to Plk3, and DNA was stained with DAPI. Plk3 signals were detected by indirect immunofluorescence microscopy. (B) A549 and GM00637 cells were double stained with antibodies to Plk3 (red) and γ-tubulin (green), a centrosome-specific marker. (C) A549 cells were double stained with antibodies to Plk3 (red) and α-tubulin (green). (D) A549 cells were triple stained with Plk3 (red), α-tubulin (green), and DAPI (blue). Specific signals were detected by confocal microscopy. Arrowheads indicate the location of MTOCs.
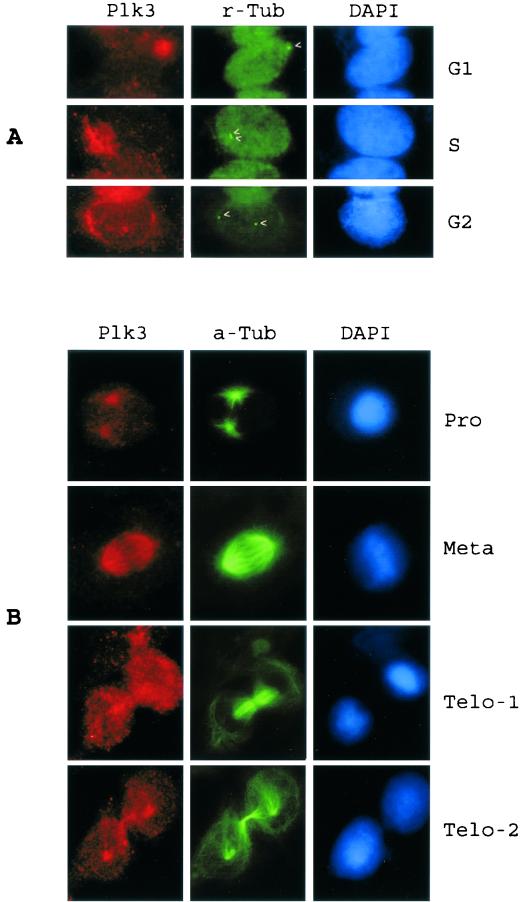
Interestingly, the expression of Plk3 in some cells was more extended and diffuse than were the discrete spots as described above (Fig. 3A). A close examination revealed that these cells contained duplicated centrosomes as shown by γ-tubulin staining (Fig. 3A, arrowheads). Plk3 thus appeared to migrate with the duplicated centrosomes. During prophase, a significant amount of Plk3 was detected around spindle pole regions (Fig. 3B). As mitosis progressed, Plk3 colocalized with mitotic spindles during metaphase. By telophase, concentrated Plk3 was found at the midbody region as well as at spindle poles (Fig. 3B). It should be noted that low levels of Plk3 were also detected in the other regions of the cell during telophase.
FIG. 3.
Plk3's association with centrosomes/spindle poles was dependent on cell cycle status. (A) A549 cells were stained with antibodies to Plk3 (red) and γ-tubulin (r-tubulin) (green). Various cell cycle stages were determined by DNA staining, as well as the number and position of centrosomes (arrowheads) in the cells. G1 phase with unduplicated centrosome; S phase with duplicated centrosomes; G2 phase with duplicated and physically separated centrosomes and with intact nuclear membrane. (B) A549 cells were stained with antibodies to Plk3 (red) or α-tubulin (a-Tub) (green). Cells of various mitotic stages as shown by the DNA staining were presented.
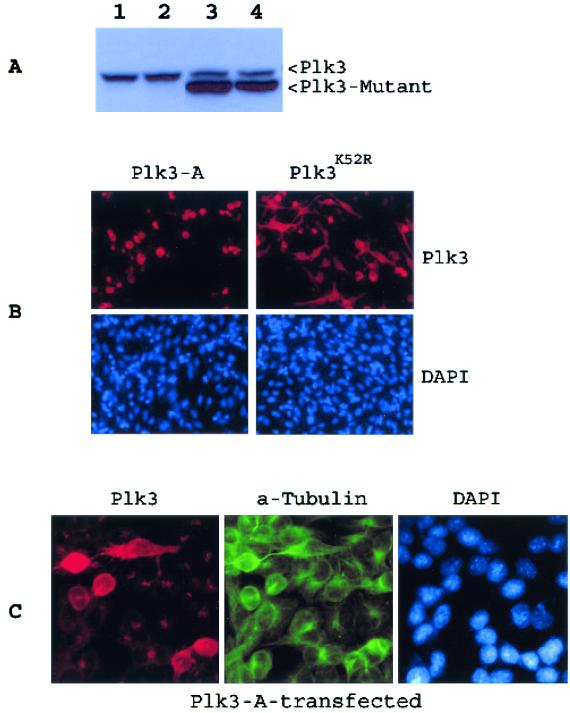
Subcellular localization patterns suggest that Plk3 may be involved in regulating the activity of MOTC and/or microtubule dynamics. To determine whether disruption of Plk3 function would affect microtubule integrity and cell viability, we transfected A549 cells with a plasmid construct expressing constitutively active Plk3 (Plk3-A) (23). As a control, a kinase-defective Plk3 mutant (Plk3K52R) expression construct was also transfected. Twenty hours posttransfection, cells were first analyzed for expression of transfected Plk3 mutants. Figure 4A shows that both Plk3K52R and Plk3-A were expressed at similar levels (Fig. 4A, lanes 3 and 4, arrowhead Plk3-Mutant). The transfected Plk3 mutant proteins were highly expressed compared with the endogenous Plk3 (arrow Plk3). The faster mobility of transfected Plk3 mutant proteins was due to a short deletion at the amino terminus (18). Fluorescence microscopy revealed (Fig. 4B) that a significant fraction (about 20%) of the transfected cells expressed high levels of Plk3-A or Plk3K52R. Cells expressing Plk3-A appeared shrunken and spherical (Fig. 4B and Table 1). On the other hand, most cells expressing Plk3K52R remained attached, exhibiting a variety of extended, irregular shapes. Endogenous Plk3 signals were usually overwhelmed by the strong signals of ectopically expressed Plk3 mutant proteins, although sometimes bright dots of endogenous Plk3 at MTOC could also be recorded along with ectopically expressed Plk3-A during imaging (Fig. 4C).
FIG.4.
Ectopic expression of Plk3 mutants. (A) Protein lysates from the cells transfected for 20 h with Plk3-A (lane 3) and Plk3K52R (lane 4) expression constructs, as well as the vector (lane 2), were blotted for Plk3. Protein lysates from untransfected cells were also used as a control (lane 1). The endogenous (arrowhead Plk3) protein and transfected (arrowhead Plk3-Mutant) Plk3 mutant protein are shown. (B) A549 cells transfected with Plk3-A or Plk3 K52R were stained with the Plk3 antibody (red) and DAPI (blue). (C) A549 cells transfected with Plk3-A were stained with antibodies to Plk3 (red) and α-tubulin (green). DNA was stained with DAPI (blue). Besides six cells expressing high levels of transfected Plk3-A, the endogenous Plk3 concentrated at the centrosomal regions was also seen.
TABLE 1.
Percentage of round cells expressing Plk3-A or Plk3K52R at various times after transfectiona
| Treatment | % At different times
|
||
|---|---|---|---|
| 14 h | 24 h | 36 h | |
| Plk3-A | 90 | 90 | 97 |
| Plk3K52R | 34 | 26 | 27 |
Transfected A549 cells were labeled with the antibody to Plk3 and examined by fluorescence microscopy. At least 200 cells expressing transfected Plk3 mutant proteins were counted for each time point, and data were summarized from two independent experiments.
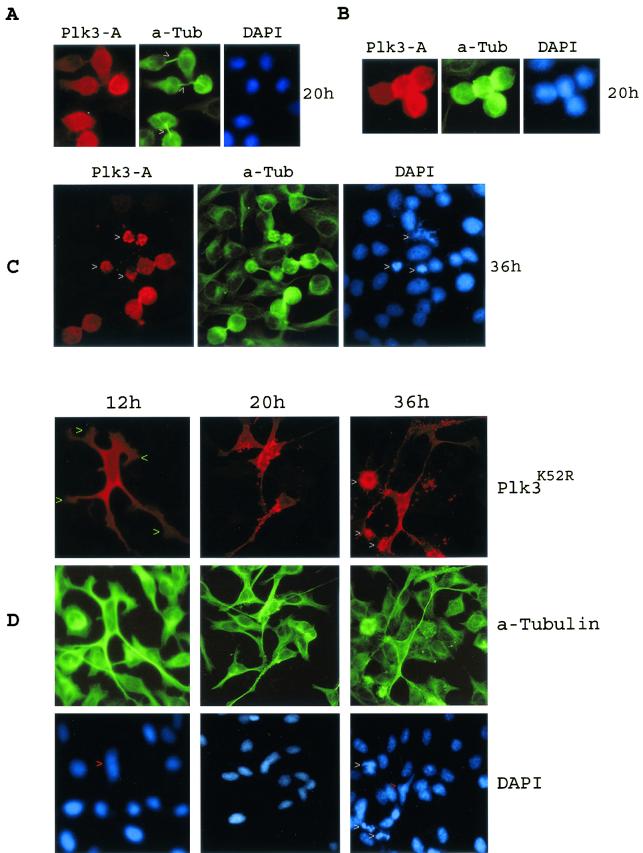
Frequently, two cells that expressed Plk3-A were found together often attached to each other (Fig. 5A). In fact, over 80% of the cells expressing Plk3-A either formed doublets or were near each other (Table 2). Occasionally, four Plk3-A-expressing cells were stuck together (Fig. 5B). These cells were spherical and loosely attached to the culture plates and resembled mitotic cells. Double-labeling experiments with an antibody to α-tubulin revealed that these cells were connected by an elongated and unsevered midbody (Fig. 5A, arrowheads), suggesting that they were incapable of completion of cytokinesis. The apparent defect in cytokinesis of those Plk3-A-expressing cells eventually resulted in apoptosis, as evidenced by chromatin condensation and nuclear fragmentation (Fig. 5C, arrowheads).
FIG. 5.
Expression of Plk3 mutants resulted in a dramatic change in cell shape. (A) A549 cells transfected with Plk3-A for 20 h were stained with antibodies to Plk3 (red) and α-tubulin (green). Arrowheads indicate the position of midbodies. (B) An example of four cells stuck together that were transfected with Plk3-A for 20 h. (C) A549 cells transfected with Plk3-A expression construct for 36 h were stained with antibodies to Plk3 (red) and α-tubulin (green). Arrowheads indicate Plk3-A-expressing cells undergoing apoptosis. (D) A549 cells transfected with Plk3K52R expression construct for various times were stained with antibodies to Plk3 (red) and α-tubulin (green). Green arrowheads indicate the cytoplasmic bodies. White arrowheads indicate the apoptotic cells, and red arrowheads show the deformed nuclei.
TABLE 2.
Percentage of doublets with midbody that express Plk3-A at various times after transfection in presence or absence of taxola
| % Found at different times with and without taxol | |||||
|---|---|---|---|---|---|
| 14 h (no taxol) | 24 h (no taxol) | 36 h (no taxol) | 14 h (taxol) | 24 h (taxol) | 36 h (taxol) |
| 83 | 79 | 64 | 3.8 | 2.8 | ∗ |
Transfected A549 cells were labeled with the antibody to Plk3 and examined by fluorescence microscopy. At least 200 cells expressing transfected Plk3-A were counted for each time point, and data were summarized from two independent experiments. *, cells became apoptotic.
In contrast, cells expressing Plk3K52R typically formed extended cytoplasmic protrusions or branches (Fig. 5D, 12 h), which in turn formed cytoplasmic bodies (arrowheads). These protrusions/bodies also contained microtubule (α-tubulin staining) structures. As Plk3K52R continued to exert its effect, most cells contained granules that were brightly stained with the Plk3 antibody. Eventually, some Plk3K52R-expressing cells appeared to fragment their cytoplasmic extensions and underwent apoptosis as shown by highly condensed, hyperchromatic chromatin and fragmented nuclei (Fig. 5D, 36 h, arrowheads). The cytoplasmic distortion in the Plk3K52R-overexpressing cells also exerted apparent stress on nuclei, resulting in their elongated or deformed shape (DAPI staining, red arrowheads).
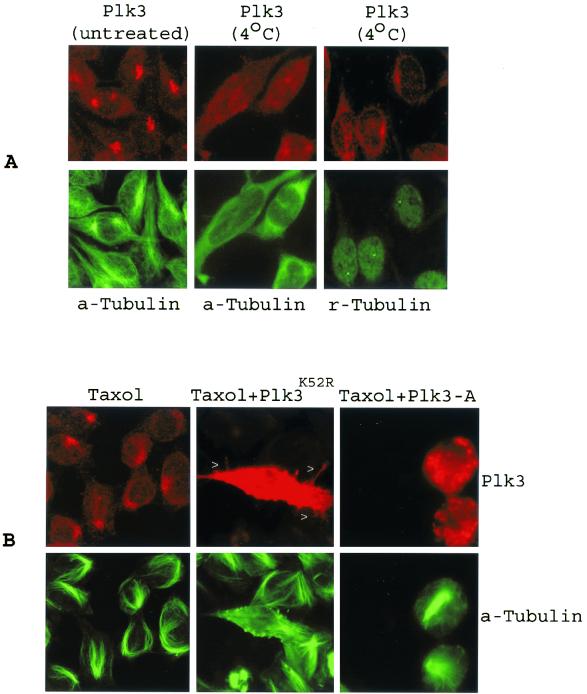
To further link Plk3 to microtubule dynamics, we next tested whether centrosomal localization of endogenous Plk3 was affected by microtubule depolymerization. Compared with the untreated controls, exposure of A549 cells to a low temperature (4°C) resulted in diffusion of Plk3 (Fig 6A). Plk3 spread throughout the cell, whereas centrosome integrity (γ-tubulin staining) was largely unaffected. A subsequent analysis of cells treated with nocodazole confirmed that, when microtubule integrity was disrupted, Plk3 was no longer localized around centrosomes as the discrete spots (data not shown). Interestingly, taxol, which causes stabilization of the microtubule, did not significantly affect the localization of endogenous Plk3 to MTOC. Whereas control cells formed microtubule bundles upon taxol treatment, cells expressing transfected Plk3K52R were capable of forming protrusions (spikes) (Fig. 6B, arrowheads), although they were not as extended as in the non-taxol-treated ones (Fig. 5D). In contrast, cells expressing Plk3-A formed thick microtubule bundles. Midbody-like structures were observed rarely, if at all, in Plk3-A-expressing cells treated with taxol (Fig. 6B and Table 2). The mechanism by which taxol induces disappearance of the midbody by taxol is unclear at present.
FIG. 6.
Plk3's localization to centrosomes is sensitive to cold but not to taxol. (A) A549 cells treated with or without cold were stained with antibodies to Plk3 (red) and α-tubulin (a-Tubulin) (green) or γ-tubulin (r-Tubulin) (green). (B) Parental A549 cells or cells transfected with Plk3-A or Plk3K52R in the presence of taxol (5 μM, 16 h) were stained with Plk3 (red) and α-tubulin (green). Arrowheads indicate the cytoplasmic protrusions in a cell expressing high levels of Plk3K52R.
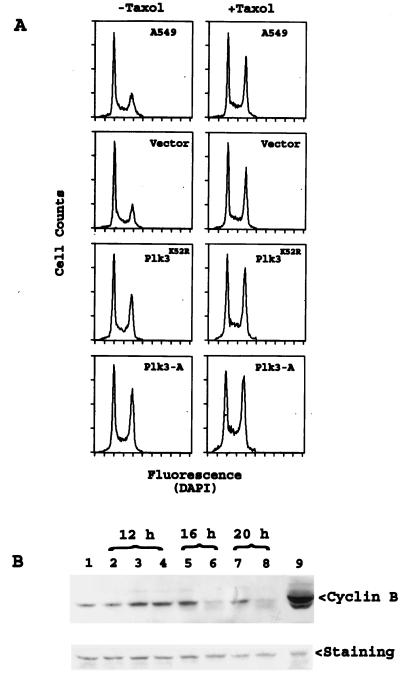
To examine the effect of Plk3 mutant expression on cell cycle status, A549 cells transfected with Plk3K52R, Plk3-A, or vector alone for 12 h were stained with DAPI and their DNA content was analyzed by flow cytometry. Figure 7 shows that transfection of A549 cells with the vector alone did not alter the cell cycle distribution compared with that of parental cells. However, expression of Plk3-A for 12 h significantly elevated the proportion of G2/M cells. Expression of Plk3K52R also increased the frequency of G2/M cells, albeit to a lesser degree than that of Plk3-A (Fig. 7A and Table 3). Taxol treatment also resulted in a mitotic arrest (Fig. 7A and Table 3). Compared with that of taxol alone, a significant reduction in the G1 population coupled with an increase in the S and G2/M populations was observed in cells transfected with Plk3-A or Plk3K52R in the presence of taxol (Table 3), indicating that the effect of taxol and Plk3 on cell cycle arrest was additive.
FIG. 7.
Mitotic arrest induced by Plk3-A and Plk3K52R. (A) A549 cells transfected with vector (pCR259), Plk3K52R, or Plk3-A expression constructs for 12 h were treated with or without taxol (25 μM) for an additional 4 h. The parental and transfected A549 cells were stained with DAPI, and their cell cycle status was analyzed by flow cytometry. (B) A549 cells transfected with vector (lane 2), Plk3K52R (lanes 3, 5, and 7), or Plk3-A (lanes 4, 6, and 8) for various times as indicated were lysed, and equal amounts of proteins were blotted for cyclin B expression. A549 cells treated with (lane 9) or without (lane 1) nocodazole were used as controls.
TABLE 3.
Cell cycle distribution (%) of A549 transfected with or without various expression constructsa
| Treatment | % Found at different phases
|
||
|---|---|---|---|
| G1 | S | G2/M | |
| A549 | 43 | 38 | 19 |
| Vector | 47 | 36 | 18 |
| Plk3-A | 33 | 36 | 32 |
| Plk3K52R | 38 | 35 | 28 |
| A549 + taxol | 35 | 39 | 26 |
| Vector + taxol | 34 | 35 | 30 |
| Plk3-A + taxol | 26 | 40 | 33 |
| Plk3K52R + taxol | 29 | 38 | 33 |
Parental A549 cells or A549 cells transfected with Plk3-A, Plk3K52R, or the vector alone for 12 h were treated with or without taxol for an additional 4 h. The cells were then processed and stained with propidium iodide. Cell cycle distributions were analyzed by flow cytometry. Data were summarized from three independent experiments.
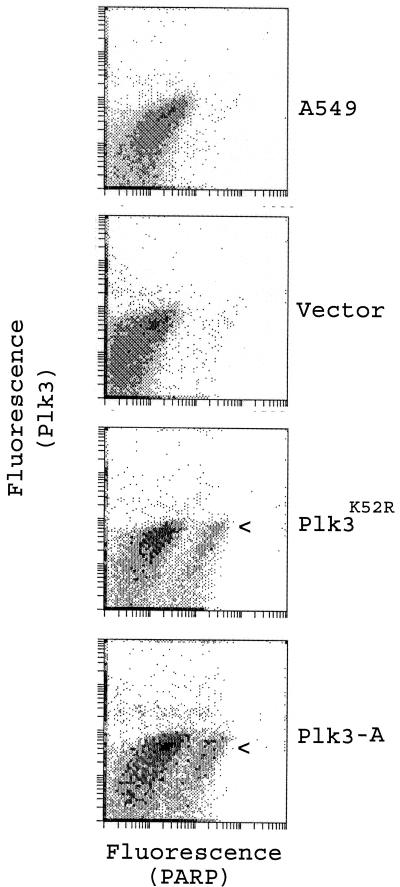
As an alternative method to confirm mitotic arrest after expression of Plk3-A and Plk3K52R, we measured cyclin B levels. Western blotting analysis showed (Fig. 7B) that significantly more cyclin B was detected in cells expressing Plk3-A and Plk3K52R than in control cells 12 h posttransfection. Interestingly, by 16 h (or longer) posttransfection, cyclin B was rapidly decreased in cells transfected with Plk3-A (Fig. 7B). On the other hand, no significant cyclin B degradation was observed in cells expressing Plk3K52R until at least 16 h posttransfection. The disappearance of cyclin B suggested that cells either exited from mitosis or underwent apoptosis. Further analysis revealed (Fig. 8) that, whereas few parental A549 or vector-transfected cells contained cleaved PARP p85 fragment, an early apoptotic marker, a significant fraction of Plk3-A-transfected cells were PARP p85 positive (Fig. 8, arrowhead), indicating that enforced expression of Plk3-A initiated programmed cell death following mitotic arrest. Consistent with cell cycle arrest and cyclin B levels (Fig. 7), expression of Plk3K52R also resulted in a significant increase in the number of cells expressing PARP p85 (Fig. 8).
FIG. 8.
Apoptosis induced by Plk3-A and Plk3K52R. A549 cells transfected with vector (pCR259), Plk3K52R, or Plk3-A expression constructs for 16 h were stained with antibodies to Plk3 and to caspase-cleaved PARP p85 fragment, an early apoptotic marker. The fluorescence of the cells was then analyzed by flow cytometry. Parental A549 cells were processed and analyzed in the same manner as a control.
DISCUSSION
Polo-like kinases play important roles in the regulation of the G2/M transition, mitotic progression, and DNA damage checkpoint response (22, 28, 32). To date, much effort has been focused on the role of Plk1 during cell cycle control in vertebrates. We have been studying the biological role of human Plk3, and our early studies have indicated that Plk3 functions differently from Plk1 (23, 32). Here we report that Plk3 colocalizes with centrosomes or the spindle poles. Several previous studies show that Plk1 also localizes to centrosomes (11) and the kinetochore/centromere (1). Thus, Plk3 shares a subcellular localization pattern (Fig. 2 and 3) similar to that of Plk1 during interphase and mitotic prophase (11). However, Plk3 colocalizes with the mitotic spindle (Fig. 3), whereas Plk1 is confined to the spindle poles during metaphase (11). Plk3 also differed from Plk1 (1, 11) in that no significant Plk3 signals were detected on kinetochores. In all stages of the cell cycle, Plk3 is apparently associated with the centrosome(s) or the spindle poles, strongly suggesting a role for Plk3 in the regulation of MTOC as well as mitosis. Plk3 is not a core component of centrioles, because its subcellular localization is much more diffuse than that of γ-tubulin (Fig. 2). In addition, its association with MTOC is microtubule dependent, as depolymerization of microtubules with cold treatment significantly compromised the localization of Plk3 to the centrosomal regions (Fig. 6).
Antibody injection studies suggest that Plk1 may be involved in centrosome separation and maturation (17). However, Plk1 is rapidly degraded upon exit from mitosis by the antigen-presenting-cell-mediated proteolytic pathway (21). As a result, the Plk1 protein level remains low during interphase. Its level increases rapidly at the onset of mitosis, indicating that Plk1 functions primarily during mitosis (6, 22). It appears that no rigorous tests were performed in these studies to assure that the antibodies used did not cross-react with Plk3; it is possible that the proposed Plk1 functions in centrosome separation and that maturation may be partly the result of Plk3 activity. This notion is consistent with the observations that Plk3 protein levels remain rather constant during the cell cycle and that Plk3 shares extensive structural homology with Plk1 (24). Therefore, during interphase, Plk3, rather than Plk1, may play a major role in the regulation of MTOC, including its structural integrity and maturation of duplicated centrosomes. The present studies suggest that Plk3 may also have a role during mitosis because it is associated with the mitotic apparatus (Fig. 3). In addition, cells expressing Plk3-A are unable to complete cytokinesis, indicating its role in regulating mitotic exit. These observations are also consistent with early reports that Polo and CDC5 are required for completion of cytokinesis as well as for mitotic progression (29). However, compared to CDC5 (29), the interference of cytokinesis by Plk3-A does not appear to rely on the Polo domain, because Plk3-A lacks only a short sequence at the amino terminus (24). Clearly, it is necessary to study the subcellular localization of Plk1 and Plk3 in the same cell system in order to understand the respective roles of these two homologues in cell cycle regulation.
Disruption of Plk3 function by enforced expression of Plk3K52R apparently causes disorganization of microtubule structures. This is manifested by the formation of long cytoplasmic extensions or branches (Fig. 5D), reminiscent of neuronal cell differentiation. In fact, an early study has shown that Plk3 expression level is high in brain tissues and that it is involved in the maintenance of long-term synaptic plasticity (14). We propose that Plk3 may regulate microtubule dynamics by upregulation of the minus end activity, resulting in accelerated shortening of microtubules because of its close association with MTOC. Disruption (inhibition) of Plk3 activity upon Plk3K52R expression would thus lead to stabilization of microtubules. This notion is supported by the observation that expression of Plk3-A results in rapid cell shrinkage (Fig. 4 and 5) accompanied by cell cycle arrest (Fig. 7). Moreover, Plk3K52R-expressing cells continue to form cell surface protrusions and granules even in the presence of taxol (Fig. 6B).
Although less pronouced than that of Plk3-A, Plk3K52R also induces apparent G2 and/or mitotic arrest followed by apoptosis (Fig. 7 and 8). One explanation is that Plk3K52R retains a weak kinase activity (24). Alternatively, the cell cycle arrest and apoptosis induced by Plk3K52R and Plk3-A may be mediated by different mechanisms. The latter scenario is supported by the observations that Plk3-A arrests cells at the M/G1 junction, whereas Plk3K52R primarily induces cytoplasmic membrane extensions followed by membrane shedding or fragmentation. Moreover, whereas Plk3K52R expression induced a persistent increase in cyclin B levels (Fig. 7B, lanes 3, 5, and 7), Plk3-A-induced increase in cyclin B expression was short lived (Fig. 7B, lanes 6 and 8), consistent with the notion that cells expressing Plk3-A are G1-like before undergoing apoptosis. Further studies are needed to determine the underlying mechanisms of these mutants on cell cycle status.
Recent studies have indicated that the phosphorylation of microtubule-associated proteins plays a critical role in the regulation of microtubule stability (2, 5). Several Drosophila proteins, such as DMAP-85 (Drosophila 85-kDa microtubule-associated protein) and Asp (abnormal spindle), interact with microtubules and are phosphorylated in vitro by Polo (2, 5). Given that both Asp and DMAP-85 associate also with centrosomes or microtubules and that their interactions are regulated by phosphorylation (2, 5) it is tempting to speculate that these human homologues are potential in vivo targets of Plk3.
Centrosome abnormalities are implicated in chromosomal instability and in the development of cancer, and in fact, many cancer cells display multiple centrosomes or enhanced centrosomal activity (20). We have previously shown that Plk3 phosphorylates the oncogene product Cdc25C on serine-216 (23), a site phosphorylation of which should result in downregulation of its activity. Recently, we have also demonstrated that Plk3 phosphorylates p53 on serine-20, an activating phosphorylation site of the tumor suppressor protein (32). Given that Plk3 is downregulated in several types of cancer (3, 18), it is possible that deregulated centrosome or MTOC activities as a result of Plk3 deficiency may contribute to the development and/or progression of these cancers.
Acknowledgments
We are grateful to John Cogswell and Intisar Husain for Plk3 mutant-expressing constructs and recombinant Plk1 protein. We also thank Sansar Sharma and Jane Lin for their assistance in confocal microscopy.
This work was supported in part by a Public Health Service Award (RO1-74229) and a Department of Defense award to W.D.
REFERENCES
- 1.Arnaud, L., J. Pines, and E. A. Nigg. 1998. GFP tagging reveals human Polo-like kinase 1 at the kinetochore/centromere region of mitotic chromosomes. Chromosoma 107:424-429. [DOI] [PubMed] [Google Scholar]
- 2.Cambiazo, V., E. Logarinho, H. Pottstock, and C. E. Sunkel. 2000. Microtubule binding of the drosophila DMAP-85 protein is regulated by phosphorylation in vitro. FEBS Lett. 483:37-42. [DOI] [PubMed] [Google Scholar]
- 3.Dai, W., Y. Li, B. Ouyang, H. Pan, P. Reissmann, J. Li, J. Wiest, P. Stambrook, J. L. Gluckman, A. Noffsinger, and P. Bejarano. 2000. PRK, a cell cycle gene localized to 8p21, is downregulated in head and neck cancer. Genes Chromosomes Cancer 27:332-336. [DOI] [PubMed] [Google Scholar]
- 4.Descombes, P., and E. A. Nigg. 1998. The polo-like kinase Plx1 is required for M phase exit and destruction of mitotic regulators in Xenopus egg extracts. EMBO J. 17:1328-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.do Carmo, A. M., A. Tavares, and D. M. Glover. 2001. Polo kinase and Asp are needed to promote the mitotic organizing activity of centrosomes. Nat. Cell Biol. 3:421-424. [DOI] [PubMed] [Google Scholar]
- 6.Donaldson, M. M., A. A. Tavares, I. M. Hagan, E. A. Nigg, and D. M. Glover. 2001. The mitotic roles of Polo-like kinase. J. Cell Sci. 114:2357-2358. [DOI] [PubMed] [Google Scholar]
- 7.Donohue, P. J., G. F. Alberts, Y. Guo, and J. A. Winkles. 1995. Identification by targeted differential display of an immediate early gene encoding a putative serine/threonine kinase. J. Biol. Chem. 270:10351-10357. [DOI] [PubMed] [Google Scholar]
- 8.Fenton, B., and D. M. Glover. 1993. A conserved mitotic kinase active at late anaphase-telophase in syncytial Drosophila embryos. Nature 363:637-640. [DOI] [PubMed] [Google Scholar]
- 9.Glover, D. M., I. M. Hagan, and A. A. Tavares. 1998. Polo-like kinases: a team that plays throughout mitosis. Genes Dev. 12:3777-3787. [DOI] [PubMed] [Google Scholar]
- 10.Glover, D. M., H. Ohkura, and A. Tavares. 1996. Polo kinase: the choreographer of the mitotic stage? J. Cell Biol. 135:1681-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golsteyn, R. M., K. E. Mundt, A. M. Fry, and E. A. Nigg. 1995. Cell cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J. Cell Biol. 129:1617-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golsteyn, R. M., S. J. Schultz, J. Bartek, A. Ziemiecki, T. Ried, and E. A. Nigg. 1994. Cell cycle analysis and chromosomal localization of human Plk1, a putative homologue of the mitotic kinases Drosophila polo and Saccharomyces cerevisiae Cdc5. J. Cell Sci. 107:1509-1517. [DOI] [PubMed] [Google Scholar]
- 13.Hamanaka, R., M. R. Smith, P. M. O'Connor, S. Maloid, K. Mihalic, J. L. Spivak, D. L. Longo, and D. K. Ferris. 1995. Polo-like kinase is a cell cycle-regulated kinase activated during mitosis. J. Biol. Chem. 270:21086-21091. [DOI] [PubMed] [Google Scholar]
- 14.Kauselmann, G., M. Weiler, P. Wulff, S. Jessberger, U. Konietzko, J. Scafidi, U. Staubli, J. Bereiter-Hahn, K. Strebhardt, and D. Kuhl. 1999. The polo-like protein kinases Fnk and Snk associate with a Ca(2+)- and integrin-binding protein and are regulated dynamically with synaptic plasticity. EMBO J. 18:5528-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitada, K., A. L. Johnson, L. H. Johnston, and A. Sugino. 1993. A multicopy suppressor gene of the Saccharomyces cerevisiae G1 cell cycle mutant gene dbf4 encodes a protein kinase and is identified as CDC5. Mol. Cell. Biol. 13:4445-4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumagai, A., and W. G. Dunphy. 1996. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science 273:1377-1380. [DOI] [PubMed] [Google Scholar]
- 17.Lane, H. A., and E. A. Nigg. 1996. Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J. Cell Biol. 135:1701-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, B., B. Ouyang, H. Pan, P. T. Reissmann, D. J. Slamon, R. Arceci, L. Lu, and W. Dai. 1996. Prk, a cytokine-inducible human protein serine/threonine kinase whose expression appears to be down-regulated in lung carcinomas. J. Biol. Chem. 271:19402-19408. [DOI] [PubMed] [Google Scholar]
- 19.Li, X., and Z. Darzynkiewicz. 2001. Cleavage of poly(ADP-ribose) polymerase measured in situ in individual cells: relationship to DNA fragmentation and cell cycle position during apoptosis. Exp. Cell Res. 255:125-132. [DOI] [PubMed] [Google Scholar]
- 20.Marx, J. 2001. Cell biology. Do centrosome abnormalities lead to cancer? Science 292:426-429. [DOI] [PubMed] [Google Scholar]
- 21.Mussman, J. G., H. F. Horn, P. E. Carroll, M. Okuda, P. Tarapore, L. A. Donehower, and K. Fukasawa. 2000. Synergistic induction of centrosome hyperamplification by loss of p53 and cyclin E overexpression. Oncogene 19:1635-1646. [DOI] [PubMed] [Google Scholar]
- 22.Nigg, E. A. 1998. Polo-like kinases: positive regulators of cell division from start to finish. Curr. Opin. Cell Biol. 10:776-783. [DOI] [PubMed] [Google Scholar]
- 23.Ouyang, B., W. Li, H. Pan, J. Meadows, I. Hoffmann, and W. Dai. 1999. The physical association and phosphorylation of Cdc25C protein phosphatase by Prk. Oncogene 18:6029-6036. [DOI] [PubMed] [Google Scholar]
- 24.Ouyang, B., H. Pan, L. Lu, J. Li, P. Stambrook, B. Li, and W. Dai. 1997. Human Prk is a conserved protein serine/threonine kinase involved in regulating M phase functions. J. Biol. Chem. 272:28646-28651. [DOI] [PubMed] [Google Scholar]
- 25.Ouyang, B., Y. Wang, and D. Wei. 1999. Caenorhabditis elegans contains structural homologs of human prk and plk. DNA Sequence 10:109-113. [DOI] [PubMed] [Google Scholar]
- 26.Qian, Y. W., E. Erikson, C. Li, and J. L. Maller. 1998. Activated polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus laevis. Mol. Cell. Biol. 18:4262-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simmons, D. L., B. G. Neel, R. Stevens, G. Evett, and R. L. Erikson. 1992. Identification of an early-growth-response gene encoding a novel putative protein kinase. Mol. Cell. Biol. 12:4164-4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smits, V. A., R. Klompmaker, L. Arnaud, G. Rijksen, E. A. Nigg, and R. H. Medema. 2000. Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat. Cell Biol. 2:672-676. [DOI] [PubMed] [Google Scholar]
- 29.Song, S., T. Z. Grenfell, S. Garfield, R. L. Erikson, and K. S. Lee. 2000. Essential function of the polo box of Cdc5 in subcellular localization and induction of cytokinetic structures. Mol. Cell. Biol. 20:286-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song, S., and K. S. Lee. 2001. A novel function of Saccharomyces cerevisiae CDC5 in cytokinesis. J. Cell Biol. 152:451-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toyoshima-Morimoto, F., E. Taniguchi, N. Shinya, A. Iwamatsu, and E. Nishida. 2001. Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Nature 410:215-220. [DOI] [PubMed] [Google Scholar]
- 32.Xie, S., H. Wu, Q. Wang, J. P. Cogswell, I. Husain, C. W. Conn, P. Stambrook, M. Jhanwar-Uniyal, and W. Dai. 2001. Plk3 links DNA damage to cell cycle arrest and apoptosis at least in part via the p53 pathway. J. Biol. Chem. 276:43305-43312. [DOI] [PubMed] [Google Scholar]