Abstract
The major inducible heat shock protein Hsp72 has been shown to protect cells from certain apoptotic stimuli. Here we investigated the mechanism of Hsp72-mediated protection from tumor necrosis factor (TNF)-induced apoptosis of primary culture of IMR90 human fibroblasts. Hsp72 temporarily blocked apoptosis in response to TNF and permanently protected cells from heat shock. An Hsp72 mutant (Hsp72ΔEEVD) with a deletion of the four C-terminal amino acids, which are essential for the chaperone function, blocked TNF-induced apoptosis in a manner similar to that of normal Hsp72 but did not inhibit heat shock-induced death. Therefore, the chaperone activity of Hsp72 is dispensable for suppression of TNF-induced apoptosis but is required for protection from heat shock. In fibroblasts derived from Bid knockout mice, similar temporal inhibition of TNF-induced apoptosis was seen. In these cells neither normal Hsp72 nor Hsp72ΔEEVD conferred additional protection from apoptosis, suggesting that Hsp72 specifically affects Bid-dependent but not Bid-independent apoptotic pathways. Furthermore, both normal Hsp72 and ΔHsp72EEVD inhibited Bid activation and downstream events, including release of cytochrome c, activation of caspase 3, and cleavage of poly-ADP-ribose polymerase. Both Hsp72 and ΔHsp72EEVD blocked activation of the stress kinase c-jun N-terminal kinase (JNK) by TNF, and specific inhibition of JNK similarly temporarily blocked Bid activation and the downstream apoptotic events. These data strongly suggest that in TNF-induced apoptosis, Hsp72 specifically interferes with the Bid-dependent apoptotic pathway via inhibition of JNK.
The major inducible heat shock protein Hsp72 protects cells from a variety of stressful conditions, including heat shock, ischemia, UV radiation, tumor necrosis factor (TNF), and anticancer drugs (for reviews see references 1, 13, and 17). A number of studies have recently demonstrated that in the protection of cells Hsp72 suppresses a stress-induced apoptotic program, but the mechanism of Hsp72 action in apoptotic signal transduction is still a matter of debate. The key element of the apoptotic program is the efflux of cytochrome c from mitochondria to cytosol, where it subsequently forms a complex (apoptosome) with Apaf-1 and caspase 9, leading to activation of the latter (3). In turn, active caspase 9 cleaves and activates the major effector caspase, caspase 3, leading to the execution of apoptosis. A number of studies from several labs have demonstrated that Hsp72 blocks stress-induced activation of the caspase cascade (2, 5, 12, 25, 27, 30) (although it should be noted that there was a report that Hsp72 can also regulate apoptosis downstream of caspases [18]).
Experiments with cell lysates showed that Hsp72 may directly prevent apoptosome formation (2, 30) and caspase 3 activation (19). Other studies, however, indicated that in vivo Hsp72 functions at an early step, upstream of mitochondria and caspase activation. For instance, Hsp72 overexpression was shown to prevent cytochrome c release after heat shock (27) or hydrogen peroxide (7), although the mechanism of this effect of Hsp72 is unknown. It appears that Hsp72 can preserve mitochondrial integrity during activation of the apoptotic program by suppression of a signaling pathway that leads to mitochondrial damage (e.g., release of cytochrome c). Indeed, Hsp72 can block activation of a stress kinase, c-jun N-terminal kinase (JNK) (12, 25), an indispensable early element in stress-induced apoptotic program which controls the release of cytochrome c (34). In fact, it has recently been demonstrated that in murine embryonal fibroblasts derived from a JNK knockout mouse (a mutant with deletion of the two major isoforms JNK1 and JNK2), cytochrome c cannot be released from mitochondria after exposure to UV irradiation and some other stressful treatments (8, 35). Hsp72 and JNK also appear to play a general role in the control of various caspase-independent types of cell death (14, 15).
Hsp72 is a molecular chaperone involved in refolding and degradation of stress-damaged proteins (see reference 9 for a review). It binds to denatured polypeptides via its peptide-binding domain and promotes their refolding in an ATP-dependent process (16). Deletion of the ATPase domain locks Hsp72 in a substrate-bound form which inhibits the refolding reaction (4). Whether the chaperone function of Hsp72 is required for its antiapoptotic activity has been a controversy. Several studies demonstrated that the protein refolding function of Hsp72 is dispensable for suppression of JNK activation and apoptosis, since the ATPase deletion mutant of Hsp72, CTF (C-terminal fragment), was fully active in protection of fibroblasts from heat- or UV-induced cell death (19, 20, 28, 37, 39). On the other hand, a recent study with lymphoid cells demonstrated that the chaperone function of Hsp72 is necessary for protection (27). In this work it was shown that normal Hsp72 or the CTF mutant could efficiently block activation of JNK by heat shock, but only normal Hsp72 could protect cells from heat-induced apoptosis (27). Especially interesting were the data with a mutant form of Hsp72 in which four critical C-terminal amino acids, EEVD, that are necessary for the chaperone function of Hsp72 were lacking or were replaced with AAAA (11). In contrast to normal Hsp72, this Hsp72 mutant could not prevent cytochrome c-induced caspase 3 activation in vitro (2) and heat-induced apoptosis in vivo (27); however, it efficiently inhibited JNK activity (27). Since specific inhibition of JNK was demonstrated to be sufficient to block heat-induced apoptosis in several cellular models (15, 36, 41), the data with these mutants appeared puzzling. These data could either suggest that Hsp72 can block apoptosis in a JNK-independent manner or that a latent JNK-independent pathway could be activated by heat shock when cells express Hsp72 mutants but not normal Hsp72. In other words, according to the latter interpretation, when the mutant form of Hsp72 is expressed, protein damage in cells exposed to heat shock is so severe that cells start to die in a JNK-independent manner. On the other hand, when normal Hsp72 is expressed, JNK is inhibited and proteins become repaired, leading to suppression of apoptosis.
There were two goals of this study: (i) to elucidate whether the chaperone function of Hsp72 is necessary for inhibition of the apoptotic program initiated by agents that do not damage proteins and (ii) to clarify the effect of Hsp72 on the apoptotic program.
MATERIALS AND METHODS
Cell cultures.
IMR90 human lung fibroblasts (American Type Culture Collection) that underwent 10 to 20 population doublings were grown in minimal essential medium with 15% fetal bovine serum and 2 mM glutamine. Bid−/− and Bid+/+ simian virus 40-transformed murine embryonal fibroblasts (MEF), kindly provided by S. Korsmeyer (Dana-Farber Cancer Institute), were grown in Dulbecco's modified minimal essential medium with 10% serum. Cells were used for experiments at 50 to 80% confluence. Experiments with TNF (plus emetine) were performed in medium containing 0.5% serum to increase apoptotic sensitivity. A combination of TNF and emetine was essential for all the apoptotic experiments, since neither TNF nor emetine alone caused cell toxicity for at least 24 h.
Adenoviral-based expression of Hsp72, ΔHsp72, and SEK (K/R).
Recombinant adenovirus vectors expressing Hsp72 and an EEVD deletion mutant of Hsp72 (Hsp72ΔEEVD) together with green fluorescent protein (GFP) in a dicistronic transcription unit were described previously (23, 26). This transcription unit is controlled by the tetracycline-regulated transactivator protein tTA, which was expressed from a separate recombinant adenovirus (AdCMVtTA) (23). Administration of 3 × 107 PFU of each virus per 35-mm-diameter dish was sufficient to infect almost 100% of cells. This was confirmed each time by observation under a fluorescence microscope of a proportion of the cells expressing GFP. As a control, adenovirus expressing GFP under the regulation of tTA was used. Adenovirus expressing dominant-negative SEK-1 (K/R) is a kinase-inactive mutant of SEK-1 tagged with M2 FLAG epitope at its amino terminus (6). Adenoviruses were propagated in 293 cells, and high-titer stocks were obtained and purified by CsCl2 density gradient centrifugation.
Apoptosis quantification.
Apoptosis was measured by fluorescence microscopy with Hoechst-33342 (5 μM) staining. Rounded cells with condensed or fragmented nuclei were considered apoptotic. About 300 to 500 cells were counted in each experiment, which was repeated at least twice. Apoptotic caspase activation was assayed either by immunoblotting of total cell lysates with antibodies to caspase 3 (H-277; Santa Cruz, Santa Cruz, Calif.); a caspase 3 substrate, poly-ADP-ribose polymerase (PARP) (Ab-2; Oncogene, Boston, Mass.); or caspase 8 (C15; a gift from P. Krammer). Secondary antibodies conjugated with peroxidase were visualized with ECL substrates (Amersham, Piscataway, N.J.), and the intensity of the bands was quantified by densitometry.
Measurement of JNK activity.
Cells were washed twice with phosphate-buffered saline (PBS) on a dish, aspirated, and lysed by being thoroughly scraped with a plastic scraper in 200 μl of lysis buffer per 35-mm-diameter dish (40 mM HEPES [pH 7.5], 50 mM KCl, 1% Triton X-100, 2 mM dithiothreitol, 1 mM Na3VO4, 50 mM β-glycerophosphate, 50 mM NaF, 5 mM EDTA, 5 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamedine, and 5-μg/ml concentrations of each leupeptine, pepstatine A, and aprotinin). The lysates were clarified by centrifugation in a microcentrifuge at 12,000 × g for 5 min. Total protein concentration was measured in the supernatants by Bio-Rad protein assay reagent, after which they were diluted with the lysis buffer to achieve equal protein concentrations in all samples. All procedures were performed at 4°C. After separation of samples by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transfer to nitrocellulose membranes, activities of two major isoforms of JNK (46 and 54 kDa) were measured with a JNK antibody specific to the activated (phosphorylated) form of JNK (Promega, Madison, Wis.).
Immunolocalization of cytochrome c.
For immunostaining, cells on plastic culture dishes were fixed with 4% formalin for 15 min at room temperature, washed with PBS, frozen in liquid nitrogen, and stored at −80°C. The cells were thawed in PBS-0.05% Tween 20, postfixed in 2% formalin and 0.5% Triton X-100 in PBS for 5 min at room temperature, and rinsed in PBS-Tween 20 three times before being reacted with primary antibodies to cytochrome c (BD PharMingen, San Diego, Calif.) overnight. All antibodies were in a buffer containing 2% bovine serum albumin, 0.1% acetylated bovine serum albumin, 0.05% gelatin, and 0.03% histone in PBS, and goat immunoglobulin G (50 μg/ml) was added to the buffer during incubation with the primary antibodies. After overnight incubation with primary antibodies, cells were washed with PBS-Tween 20 three times and then the bound primary antibodies were detected with a AlexorFluor 594 Signal-Amplification kit (Molecular Probes, Eugene, Oreg.). Images were obtained with a Carl Zeiss microscope Axiovert 200 M and AxioVision software.
RESULTS
Chaperone function of Hsp72 is dispensable for protection from TNF-induced apoptosis.
Here we tested the hypothesis that the chaperone activity of Hsp72, while being essential specifically for suppression of cell death induced by protein damage (e.g., by heat shock), is not necessary for the effect of Hsp72 on the apoptotic machinery in general. To test this possibility, we studied effects of the Hsp72ΔEEVD mutant on apoptosis of IMR90 fibroblasts caused by heat shock and TNF, a cytokine that initiates an apoptotic signal while apparently not causing protein damage. As we reported previously, heat-induced apoptosis in IMR90 cells was dependent on activation of JNK and was inhibited by Hsp72 and by survival kinases ERK and Akt (15). Interestingly, heat-induced apoptosis in these cells, although morphologically indistinguishable from TNF-induced apoptosis, was caspase independent, while TNF-induced death required caspase activation (15 and see below).
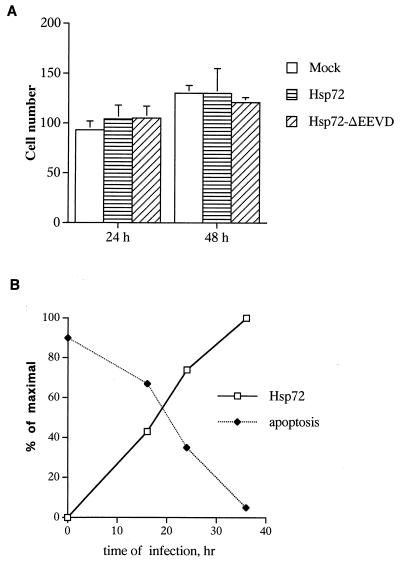
Hsp72ΔEEVD and normal Hsp72 were expressed in IMR90 cells under the control of a tetracycline-regulated promoter by using adenovirus vectors described previously (15, 27). With this model, neither Hsp72 nor Hsp72ΔEEVD affected growth or were toxic to IMR90 cells until 48 h of expression (Fig. 1A). It should be noted, however, that at the later time points Hsp72, but not Hsp72ΔEEVD, caused some toxicity. To find the optimal conditions of Hsp72-mediated protection, cells were infected with adenovirus encoding Hsp72. After various time periods infected cells were exposed to TNF (10 ng/ml), and expressions of Hsp72 and apoptosis were measured. In this experiment the time-dependent increase of Hsp72 expression correlated with the progressive inhibition of apoptosis (Fig. 1B). Maximal protection was seen at 36 h of Hsp72 expression, and these conditions were used in all further experiments.
FIG. 1.
Effects of adenovirus-mediated Hsp72 expression on cell growth and TNF-induced apoptosis. IMR90 human fibroblasts were infected with adenoviruses expressing normal Hsp72, Hsp72ΔEEVD, or GFP (as a control) for various time periods. Cells were exposed to TNF (10 ng/ml) in the presence of the protein synthesis inhibitor emetine (10 μM). (A) The number of live cells per square millimeter (means ± standard deviations) was counted after 24 and 48 h following infection with various adenoviruses and induction of GFP, Hsp72, or Hsp72ΔEEVD. (B) Dependence of inhibition of TNF-induced apoptosis on Hsp72 expression. Different levels of Hsp72 expression were achieved by infection for various time periods. Apoptosis was measured by Hoechst-33342 staining, and the levels of Hsp72 were assessed by immunoblotting with anti-Hsp72 antibody.
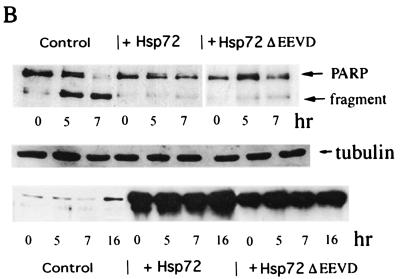
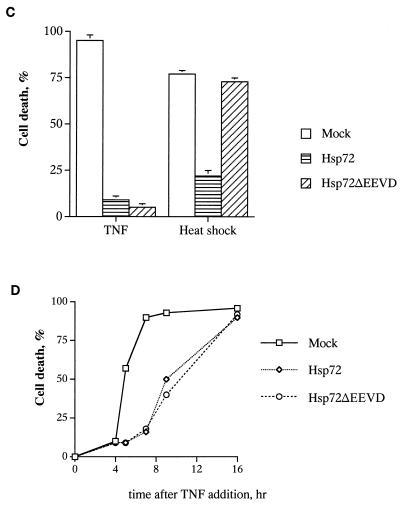
Cells were infected with either Hsp72 or Hsp72ΔEEVD adenoviruses, and after 36 h they were exposed to either heat shock (45°C for 90 min) or TNF (10 ng/ml). Activation of apoptosis was determined at different time points after the challenges. The effects of Hsp72ΔEEVD and Hsp72 on TNF- and heat-induced apoptosis differed dramatically. In fact, both Hsp72ΔEEVD and Hsp72 efficiently inhibited TNF-induced apoptosis, as judged by counting cells with apoptotic morphology (rounded cells) (Fig. 2A) and Hoechst-33342 staining (Fig. 2A and C) and by measuring cleavage of PARP, which is dependent on the activation of caspase 3 (Fig. 2B). By contrast, no protection from heat-induced cell killing was seen with Hsp72ΔEEVD, while cells expressing normal Hsp72 were resistant to heat shock (Fig. 2C). The Hsp72-AAAA mutant affected apoptosis in a manner similar to that of Hsp72ΔEEVD (data not shown). Therefore, in line with a previous report (27), the chaperone function of Hsp72 appeared necessary for suppression of apoptosis induced by heat shock (a protein-damaging stress). However, the chaperone activity was dispensable for protection from stresses that do not cause protein damage (e.g., TNF).
FIG.2.
Chaperone function of Hsp72 is dispensable for suppression of TNF-induced apoptosis but is required for protection from heat shock. (A to C) Hsp72 and Hsp72ΔEEVD inhibit TNF-induced apoptosis. IMR90 cells were infected with adenoviruses expressing either normal Hsp72, Hsp72ΔEEVD, or GFP (control) for 36 h and then were exposed to TNF and emetine for various time periods. (A) Phase-contrast microscopy (upper panel objective, 10×) and Hoechst-33342 staining (lower panel objective, 40×) of cells exposed to TNF for 8 h. Expression of Hsp72 or Hsp72ΔEEVD prevents the appearance of apoptotic cells. (B) Effects of Hsp72 or Hsp72ΔEEVD on PARP cleavage in cells exposed to TNF for 5 or 7 h. The middle panel shows a loading control (expression of tubulin). The lower panel demonstrates expression of Hsp72 or Hsp72ΔEEVD over the course of the experiment as measured by immunoblotting. (C) Hsp72, but not Hsp72ΔEEVD, can prevent heat-induced cell death. Control fibroblasts or cells expressing Hsp72 or Hsp72ΔEEVD were treated with TNF or heat shock (45°C for 90 min), and their deaths (means ± standard deviations) were assessed by Hoechst-33342 staining after 7 (TNF) or 24 h (heat shock). Hsp72ΔEEVD did not affect heat-induced death at any time point. (D) Protection against TNF-induced apoptosis by Hsp72 or Hsp72ΔEEVD is temporal. Apoptosis was assessed by PARP cleavage.
Hsp72 suppresses the Bid-dependent apoptotic pathway.
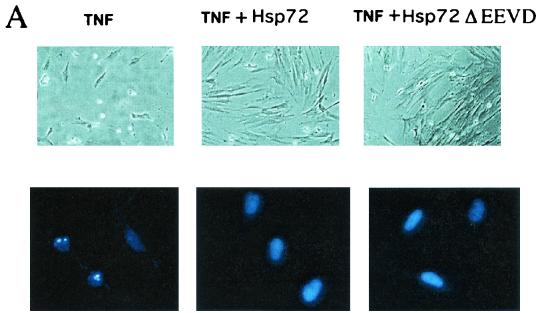
Protection of IMR90 cells by Hsp72 or Hsp72ΔEEVD from TNF toxicity was temporal. Indeed, although at 5 to 8 h after TNF addition protection by Hsp72 or Hsp72ΔEEVD was very remarkable, it diminished at later time points and disappeared after 16 h of incubation (Fig. 2D). Of note, time courses of death in cells expressing normal Hsp72 and Hsp72ΔEEVD were similar (Fig. 2D). The temporal protection of cells by Hsp72 was in sharp contrast to the protective effect of caspase inhibition, since no cell killing was seen in the presence of the irreversible pan-caspase inhibitor z-VAD-fmk (20 μM), even after 16 to 24 h (data not shown; see Discussion). (Of note, 20 μM was the minimal concentration of z-VAD-fmk that caused complete inhibition of caspase 3 in IMR90 cells, and decreasing the concentration led to progressive reduction of the effects on both caspase 3 and apoptosis.) These data suggest that the effect of Hsp72 was not directly related to inhibition of caspases.
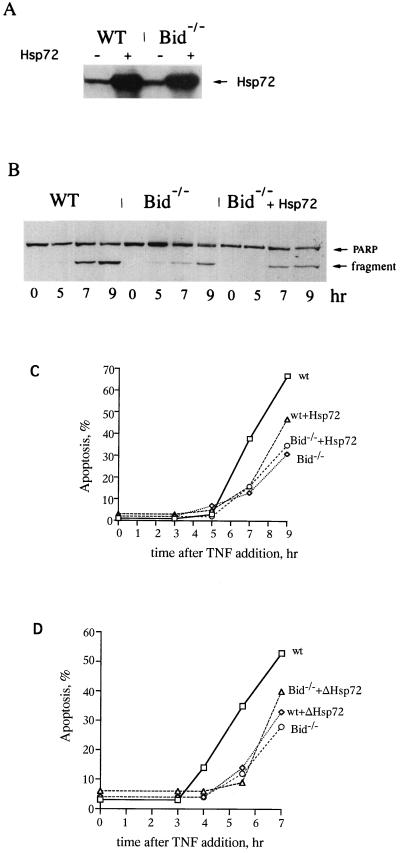
The temporal suppression of apoptosis by Hsp72 and Hsp72ΔEEVD was strikingly similar to the effect of a knockout of Bid on TNF-induced apoptosis in mouse fibroblasts (40). Bid is a proapoptotic member of the Bcl-2 protein family whose activation via cleavage is induced when cells are treated with TNF or FAS (21, 22). The truncated form of Bid translocates to mitochondria causing cytochrome c efflux and activation of the caspase 9/caspase 3 cascade (21, 22). As reported previously, lack of Bid did not completely prevent cell death in response to TNF or FAS but delayed apoptosis by several hours (40), indicating that a Bid-independent apoptotic pathway was activated at later time points and destroyed cells. Because of the similarities in time courses of cell death, we addressed the question of whether the effect of Hsp72 on TNF-induced apoptosis was related to the Bid-dependent apoptotic pathway. Accordingly, we compared time courses of TNF-induced apoptosis in Bid−/− and Bid+/+ murine embryonal fibroblasts expressing Hsp72 and Hsp72ΔEEVD. MEF cells were more sensitive to expression of Hsp72 than IMR90 cells, but no inhibition of growth or toxicity was seen until at least 40 h after infection. Hsp72 and Hsp72ΔEEVD were expressed in infected cells for 30 h (Fig. 3A), and then cells were incubated with TNF for various time periods and the degree of PARP cleavage was measured by immunoblotting with an anti-PARP antibody (Fig. 3B). In line with what was previously reported (40), TNF-induced apoptosis in Bid−/− cells was delayed compared to that of Bid+/+ cells (Fig. 3C and D). Importantly, the time course of apoptosis in Bid−/− cells was almost identical to that of Bid+/+ cells expressing Hsp72 or Hsp72ΔEEVD (Fig. 3C and D). Moreover, when Hsp72 or Hsp72ΔEEVD was expressed in Bid−/− cells, no additional protective effect was seen (Fig. 3C and D). Similar data were obtained with Hoechst-33342 staining (data not shown). In other words, the protective effects of Hsp72 and knocking out of Bid were not additive. These data strongly suggest that Hsp72 specifically suppresses a Bid-dependent but not a Bid-independent pathway in TNF-induced apoptosis.
FIG. 3.
Suppression of the Bid-dependent apoptotic pathway by Hsp72 or Hsp72ΔEEVD. (A) Expression of Hsp72 in MEF cells. MEF cells were infected with adenovirus expressing Hsp72 for 30 h, and the levels of expression were measured by immunoblotting with anti-Hsp72 antibody. (B) Effects of Bid knockout and Hsp72 on PARP cleavage induced by TNF. (C and D) Hsp72 or Hsp72ΔEEVD delay TNF-induced apoptosis in wild-type (WT), but not in Bid−/− knockout, fibroblasts. Wild-type (Bid+/+) and knockout (Bid−/−) MEF were infected with adenoviruses expressing Hsp72 or Hsp72ΔEEVD for 30 h and were exposed to TNF as for the experiment depicted in Fig. 1, and apoptosis at different time points was assessed by PARP cleavage. As a mock infection we used adenovirus expressing GFP only in tetracycline-regulated systems. The experiment was repeated four times. Data of typical experiments are presented.
Hsp72 suppresses cleavage of Bid and release of cytochrome c from mitochondria.
To further investigate the mechanism of suppression of TNF-induced apoptosis by Hsp72, we addressed the question of what step of the Bid-dependent pathway is inhibited by Hsp72. As shown above (Fig. 2B), both normal Hsp72 and Hsp72ΔEEVD inhibited cleavage of PARP, indicating that Hsp72 acts at the level of or upstream of procaspase 3 activation. Indeed, in Hsp72-expressing IMR90 cells caspase 3 could not be activated upon addition of TNF (Fig. 4A). In this experiment we studied the disappearance of procaspase 3 by immunoblotting with an anti-caspase 3 antibody. The inhibition of caspase 3 activation was temporal and had a time course similar to that of PARP cleavage or appearance of cells with apoptotic morphology (data not shown).
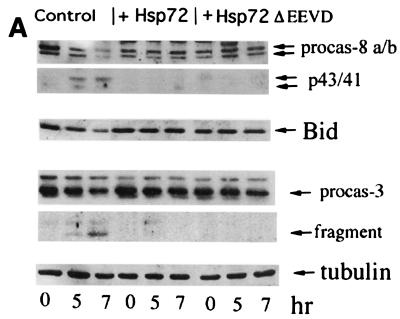
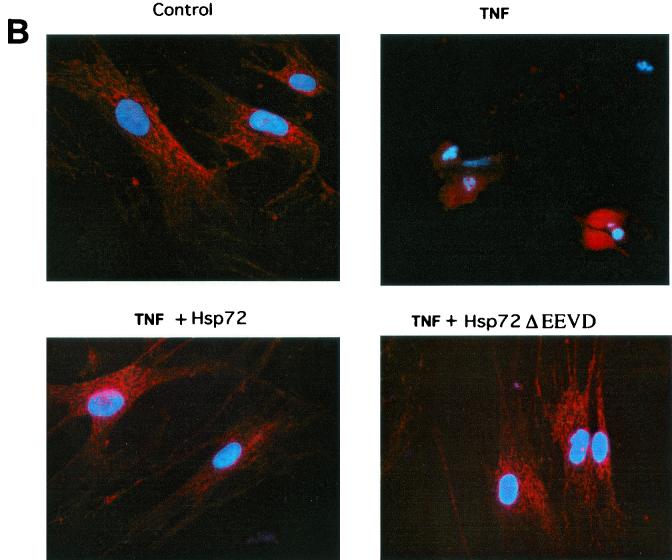
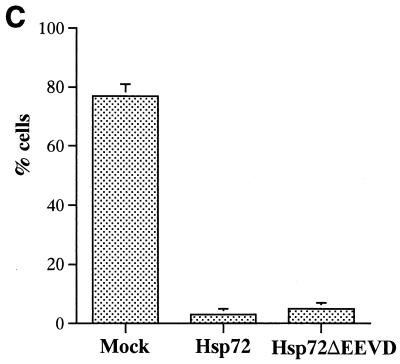
FIG. 4.
Hsp72 or Hsp72ΔEEVD block TNF-induced cleavage of Bid, translocation of cytochrome c, and activation of caspase 8 and caspase 3. IMR90 fibroblasts were infected with adenoviruses and exposed to TNF as described for Fig. 2. (A) Effects of Hsp72 and Hsp72ΔEEVD on cleavage of procaspase 8, Bid, and procaspase 3 were assessed by immunoblotting. (B) Effects of Hsp72 and Hsp72ΔEEVD on translocation of cytochrome c were assessed by immunofluorescence. Cells were double stained with anti-cytochrome c antibody and Hoechst-33342. The appearance of distribution of cytochrome c in cells is shown (magnification, ×40). (C) Quantification of fractions of cells (means ± standard deviations) with released cytochrome c at 7 h after TNF addition.
Next we studied whether Hsp72 can inhibit a more upstream event in the apoptotic pathway, the release of cytochrome c from mitochondria. The release of cytochrome c was measured by immunofluorescence with an antibody against the native form of cytochrome c. In unstressed IMR90 cells cytochrome c was localized to mitochondria, while after the TNF addition it redistributed to the cytosol and the degree of fluorescence increased (Fig. 4B and C). Upon expression of Hsp72 or Hsp72ΔEEVD the redistribution of cytochrome c to the cytosol of TNF-treated cells was significantly delayed (Fig. 4B and C). These data indicate that Hsp72 somehow regulates the release of cytochrome c from mitochondria.
The critical question raised at this point was whether Hsp72 can directly or indirectly inhibit activation of Bid. IMR90 cells expressing either normal Hsp72 or mutant Hsp72 were exposed to TNF, and Bid cleavage (indicating its activation) was studied by immunoblotting with an anti-Bid antibody. In line with previously reports (21, 22), Bid cleavage (as well as the cytochrome c release and PARP cleavage) in response to TNF was partially dependent on caspase 8, since it was inhibited by about 50% by the specific caspase 8 inhibitor z-IETD-fmk (data not shown; see Discussion). Cleavage of Bid in IMR90 cells was initiated within 5 h after the TNF addition and was almost completed by 7 h (Fig. 4A). Importantly, expression of Hsp72 or Hsp72ΔEEVD in these cells strongly delayed Bid cleavage and activation of caspase 8 (Fig. 4A). These data indicated that Hsp72 temporally inhibited Bid activation (probably indirectly; see Discussion), thus delaying cytochrome c release and activation of the downstream caspase cascade.
Hsp72 suppresses cleavage of Bid via inhibition of JNK activity.
Hsp72-mediated suppression of the Bid-dependent pathway in TNF-induced apoptosis may be related to the inhibition of JNK. In fact, it has been recently shown that JNK activity is necessary for cytochrome c release and Bid cleavage upon UV-induced apoptosis of fibroblasts (34) (although it should be emphasized that activation of Bid is probably irrelevant to UV-induced apoptosis, since this apoptosis was not suppressed in Bid−/− fibroblasts [38]). Since Hsp72 prevented activation of JNK by various stressful treatments, we suggested that Hsp72-mediated inhibition of Bid cleavage might be associated with suppression of JNK.
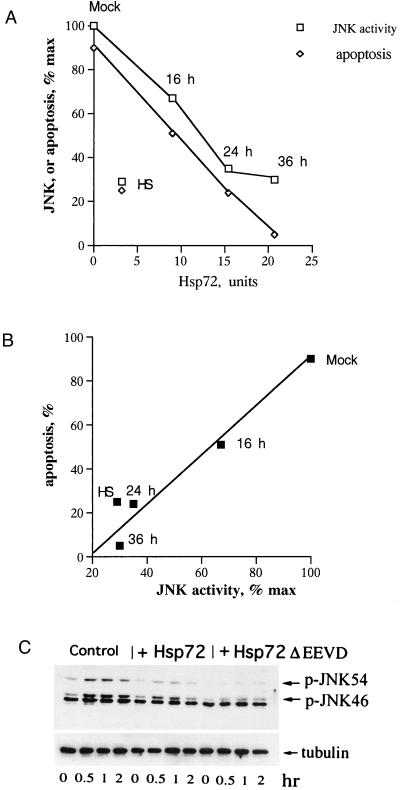
To address this question, we first tested whether Hsp72ΔEEVD and normal Hsp72 suppress TNF-induced JNK activation. Cells infected with adenovirus encoding Hsp72 for various time periods were exposed to TNF (10 ng/ml), and expression of Hsp72 (prior to TNF addition), activation of JNK (1 h after TNF addition), and apoptosis (7 h after TNF addition) were measured. The progressive suppression of TNF-induced JNK activation upon increase of Hsp72 expression correlated with the progressive inhibition of apoptosis (Fig. 5A). Notably, pretreatment of cells with mild heat shock to induce Hsp72 in a more physiologically relevant way also led to suppression of the TNF-induced activation of JNK and apoptosis. Effects of mild heat shock pretreatment on TNF-induced activation of JNK and apoptosis were similar to that of Hsp72 expressed via adenovirus infection for 24 h (Fig. 5A), although the level of Hsp72 induced by mild heat shock was lower (Fig. 5A). This difference most likely is due to induction by mild heat shock of Hsp40 and other cofactors that may potentiate Hsp72-mediated protection. Importantly, as we have shown previously, the effect of mild heat shock pretreatment on activation of JNK by TNF was absolutely dependent on induction of Hsp72, since it was suppressed by Hsp72 antisense RNA (15). The presentation of data shown in Fig. 5A in a graph of apoptosis versus JNK activation shows that these two parameters closely correlate with each other (Fig. 5B).
FIG. 5.
Hsp72 and Hsp72DEEVD suppress TNF-induced JNK activation. (A) TNF-induced activation of JNK and apoptosis in cells expressing different amounts of Hsp72. Either cells were infected with Hsp72-expressing adenovirus for various time periods or Hsp72 was induced by priming cells with heat shock (45°C for 30 min) followed by 16 h of recovery at 37°C. Activation of JNK was measured by immunoblotting with an antibody to the phosphorylated (active) form of JNK. Apoptosis was measured by PARP cleavage. Data of a typical experiment are shown. (B) Presentation of data from panel A in a graph of apoptosis versus JNK activation. (C) Effects of Hsp72 and Hsp72ΔEEVD on TNF-induced activation of JNK. Activation of JNK was measured by immunoblotting with an antibody to the phosphorylated (active) form of JNK. Although various splice isoforms of both JNK1 and JNK2 are present in both 46- and 54-kDa bands, the majority of JNK1 is present in the 46-kDa band while the majority of JNK2 is present in the 54-kDa band (34).
Cells infected with either Hsp72 or Hsp72ΔEEVD adenoviruses for 36 h were exposed to TNF, and activation of JNK was determined at different time points after the challenge. Hsp72ΔEEVD, similar to normal Hsp72, markedly reduced TNF-induced activation of JNK (Fig. 5C). Of note, neither Hsp72 nor Hsp72ΔEEVD inhibited TNF-induced degradation of I-κB (data not shown), indicating that the TNF receptor is not affected. Therefore, Hsp72 and Hsp72ΔEEVD can suppress TNF-induced JNK activation.
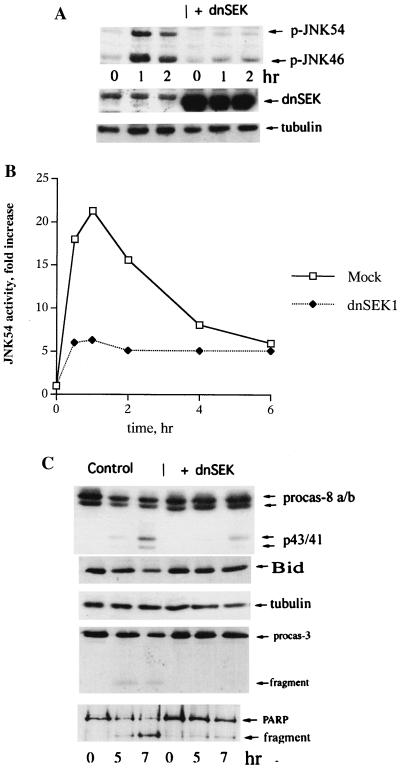
To investigate whether these effects on JNK are sufficient to suppress TNF-induced apoptosis, we tested if inhibition of JNK by an independent method can prevent Bid cleavage and death of IMR90 cells upon exposure to TNF. To suppress JNK activation, we employed a dominant-negative mutant of SEK1/MKK4 (dnSEK1), an immediate upstream component of the JNK signaling cascade. We have used this method to study the JNK dependence of heat-induced apoptosis in IMR90 cells (15). Cells were infected with an adenovirus encoding dnSEK1 for 72 h (which did not affect cell viability measured either by PARP cleavage or Hoechst-33342 staining), and then TNF was added and activation of JNK and the extent of apoptosis were measured. Expression of dnSEK1 markedly reduced JNK activation after TNF treatment (Fig. 6A and B). Furthermore, expression of dnSEK1 significantly delayed Bid cleavage, caspase 8 and caspase 3 activation (Fig. 6C), PARP cleavage (Fig. 6C), cytochrome c efflux, and cell death measured by Hoechst-33342 staining (data not shown). The time courses of all these parameters were similar to those in cells expressing Hsp72 or ΔHsp72EEVD (data not shown). Of note, inhibition of p38 activity by SB203580 (10 μM) did not affect TNF-induced apoptosis (data not shown). Thus, it appeared that JNK activation was required for TNF-induced activation of Bid at the initial time period. Since Hsp72 efficiently inhibited activation of JNK by TNF, these data indicated that in cells exposed to TNF, Hsp72 most likely inhibited cleavage and activation of Bid by suppression of JNK (see Fig. 5 and 6). Furthermore, the chaperone activity of Hsp72 was not critical for these effects.
FIG. 6.
Suppression of TNF-induced JNK activation prevents cleavage of Bid and caspase activation. IMR90 fibroblasts were infected with dnSEK1 or control adenoviruses for 72 h and exposed to TNF as for Fig. 2. (A and B) Expression of dnSEK1 inhibits TNF-induced JNK activation. JNK activity depicted in panels A and B was measured by immunoblotting as described in the legend to Fig. 5A. Expression of SEK1 was assessed by immunoblotting with an SEK1-specific antibody. Infection of cells with dnSEK1 virus was not toxic for cells for at least 4 days. (C) Expression of dnSEK1 blocks cleavage of Bid, procaspase 8, procaspase 3, and PARP as assessed by immunoblotting.
DISCUSSION
Protection of cells from various stresses by Hsp72 was discovered long ago, and more recently this protection was attributed to inhibitory effects of Hsp72 on apoptosis and other types of regulated cell death (1, 13, 17, 31). So far, the exact mechanism of the inhibitory effect of Hsp72 on the apoptotic pathway has not been established, although it appears that there are multiple mechanisms, including inhibition of activation of the stress kinase JNK and inhibition of apoptosome assembly (1, 13). Here we investigated the effects of Hsp72 on cell death by using TNF-induced apoptosis of primary human fibroblast culture IMR90 as a model.
Two lines of evidence indicated that, in the case of TNF-induced apoptosis, Hsp72 regulates cleavage and, thus, activation of a proapoptotic Bcl-2 family member, Bid. These include (i) Hsp72 inhibited activation of Bid in response to TNF and (ii) Hsp72 inhibited apoptotic events downstream of Bid activation, i.e., cytochrome c efflux from mitochondria, activation of caspase 3, and cleavage of PARP. Although in fibroblasts TNF induced both Bid-dependent and Bid-independent apoptotic pathways, Hsp72 could only inhibit the Bid-dependent pathway. Indeed, eliminating Bid by gene knockout led to a delay of apoptosis similar to that seen in cells expressing Hsp72 (see Fig. 3). Furthermore, the effects of Bid knockout and expression of Hsp72 were not additive, indicating that Hsp72 was unable to affect a Bid-independent pathway activated at later time points.
Most likely, Hsp72 affected Bid activation indirectly via suppression of activation of JNK (Fig. 5 and 6). Indeed, JNK activation was critical for activation of Bid and other downstream events of the apoptotic pathway induced by TNF, since inhibition of JNK by expression of dnSEK1 temporarily blocked all these events; i.e., it had effects similar to those of expression of Hsp72. Therefore, inhibition of JNK was sufficient to cause an apoptotic delay. Since Hsp72 efficiently suppressed JNK activation, we argue that Hsp72 affects this apoptotic pathway through inhibition of JNK. In line with this suggestion was the fact that the time courses of inhibition of Bid cleavage, activation of caspase 8, cytochrome c efflux, and activation of caspase 3 were similar in cells expressing dnSEK1 and cells expressing Hsp72.
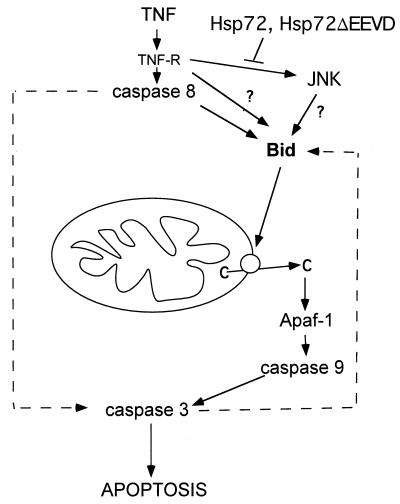
How can JNK, and thus Hsp72, regulate Bid cleavage? A known effect of TNF on Bid involves procaspase 8, which becomes associated with the TNF receptor and is autoactivated in response to TNF binding and can then directly cleave Bid, thus activating the mitochondrial pathway (3). These data were obtained with so-called type I cells, in which caspase 8 associated with FAS and TNF receptors is activated by addition of TNF and then can directly cleave and activate caspase 3 (32). This process does not require activation of the mitochondrial pathway, and therefore Bid cleavage in these cells is a side effect of TNF or FAS addition. In contrast, in the majority of other cells (type II cells) the mitochondrial pathway is critical for activation of caspase 3 by TNF (32). In these cells, the major fraction of caspase 8 appears to associate with mitochondria rather than with the TNF receptor (29). Bid cleavage and activation of the mitochondrial apoptotic pathway in these cells may be mediated not by caspase 8 but by other caspases or even proteases other than caspases (34), e.g., cathepsin B (10). TNF-induced cleavage of Bid, cytochrome c release, and activation of caspase 3 in IMR90 fibroblasts was partially (by 40 to 50%) inhibited by a specific inhibitor of caspase 8, suggesting that in fibroblasts caspase 8 may directly cleave Bid. However, it is clear that other proteolytic enzymes also contribute to Bid cleavage, and the nature of these proteases has yet to be identified. Accordingly, JNK either could regulate cleavage of Bid by active caspase 8 and other proteolytic enzymes or could regulate activation of these proteases (Fig. 7). In IMR90 cells expressing dnSEK1 or Hsp72, activation of caspase 8 by TNF was delayed (Fig. 4 and 6). On the other hand, these data are difficult to interpret, since the major fraction of caspase 8 in these cells is associated with mitochondria, and it has been technically impossible to specifically study the fraction of caspase 8 in these cells that is associated with the TNF receptor. It should also be noted that JNK was reported to regulate a caspase 8-independent Bid cleavage in UV-irradiated MEF cells (34). Therefore, the exact mechanism of regulation of Bid cleavage by JNK and Hsp72 remains to be studied.
FIG. 7.
Proposed mechanism of Hsp72-mediated inhibition of TNF-induced apoptosis.
What is the mechanism of TNF-induced Bid-independent apoptosis that takes over at later time points? This apoptosis may be associated with activation of caspase 3 via direct cleavage by caspase 8 (3) (or some other proteolytic enzyme) (Fig. 7). It is conceivable that in type II cells this process may take place, although it takes longer than it does in type I cells. In such a scenario, activation of Bid and release of cytochrome c at later time points was probably due to the activation of a caspase 3-dependent positive feedback loop (Bid cleavage by caspase 3 was reported previously [33]). Indeed, both Bid cleavage and cytochrome c efflux in TNF-treated IMR90 cells at later time points were blocked by a caspase 3 inhibitor, DEVD-fmk (data not shown). Importantly, this late caspase 3-dependent Bid cleavage and cytochrome c release were not regulated by JNK and Hsp72, since expression of neither dnSEK1 nor Hsp72 could affect these processes at later time points after TNF addition.
Here we also demonstrated that Hsp72ΔEEVD, a mutant that lacks the four C-terminal amino acids EEVD, can efficiently inhibit TNF-induced apoptosis. Effects of Hsp72ΔEEVD were indistinguishable from the effects of normal Hsp72; i.e., both blocked activation of JNK and caused similar delays in Bid cleavage, release of cytochrome c, activation of caspase 3, cleavage of PARP, and morphological apoptotic changes in cells. Since the Hsp72ΔEEVD mutant has no chaperone activity, these data indicate that this activity of Hsp72 is not critical for inhibition of TNF-induced apoptosis. Furthermore, since a similar Hsp72 mutant was unable to inhibit apoptosome assembly (2), we conclude that in TNF-induced apoptosis in IMR90 cells (i) effects of Hsp72 on apoptosome assembly are not important and (ii) the effect of Hsp72 on JNK-dependent Bid cleavage plays a major role in cell protection.
Interestingly, in line with results of a previous report (27), the Hsp72ΔEEVD mutant was unable to protect cells from heat-induced apoptosis. The strong inhibition of heat-induced JNK activation by Hsp72ΔEEVD (27 and data not shown) indicated that in the presence of Hsp72ΔEEVD a JNK-independent pathway of cell death was activated. This pathway, however, was dormant in cells that did not express Hsp72ΔEEVD, since inhibition of JNK without expression of Hsp72ΔEEVD (i.e., by dnSEK1) was sufficient to inhibit heat-induced apoptosis in IMR90 cells (15). It is possible that the activity of an endogenous constitutively expressed Hsp72 homolog, Hsc73, is critical for resistance to heat-induced apoptosis, and Hsp72ΔEEVD may have a dominant-inhibitory effect on Hsc73. Under these conditions more protein damage may occur in heat-shocked cells, which may trigger a normally silent JNK-independent apoptotic pathway.
As described previously, inhibition of JNK activity by Hsp72 acts mostly through facilitation of JNK dephosphorylation (24), although there seems to be another target of Hsp72 in a JNK-activating kinase cascade. In contrast to normal Hsp72, Hsp72ΔEEVD was unable to facilitate JNK dephosphorylation (27), since the peptide-binding domain which is involved in this regulation (39) is not functional in this mutant (11). However, Hsp72ΔEEVD inhibited the JNK-activating kinase cascade (unpublished data), and these effects were stronger than effects of normal Hsp72. The exact mechanisms of regulation of the JNK-activating cascade by Hsp72 and Hsp72ΔEEVD have yet to be resolved.
In conclusion, this work demonstrated that Hsp72 downregulates TNF-induced apoptosis via inhibition of JNK, which in turn inhibits activation of Bid and subsequent events of the mitochondrial apoptotic pathway. In other types of cell death, especially in Bid-independent processes (e.g., UV-induced apoptosis), other mechanisms of Hsp72-mediated protection should be involved. Some of these mechanisms are related to inhibition of JNK. In other cases, effects of Hsp72 on apoptosome assembly may be critical.
Acknowledgments
This study was supported by an RO1 grant from the National Institutes of Health to M.Y.S.
We thank S. Korsmeyer for providing Bid knockout cells and P. Krammer for C15 antibody.
REFERENCES
- 1.Beere, H. M., and D. R. Green. 2001. Stress management heat shock protein-70 and the regulation of apoptosis. Trends Cell Biol. 11:6-10. [DOI] [PubMed] [Google Scholar]
- 2.Beere, H. M., B. B. Wolf, K. Cain, D. D. Mosser, A. Mahboubi, T. Kuwana, P. Tailor, R. I. Morimoto, G. M. Cohen, and D. R. Green. 2000. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2:469-475. [DOI] [PubMed] [Google Scholar]
- 3.Budihardjo, I., H. Oliver, M. Lutter, X. Luo, and X. Wang. 1999. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 15:269-290. [DOI] [PubMed] [Google Scholar]
- 4.Burkholder, W. F., X. Zhao, X. Zhu, W. A. Hendrickson, A. Gragerov, and M. E. Gottesman. 1996. Mutations in the C-terminal fragment of DnaK affecting peptide binding. Proc. Natl. Acad. Sci. USA 93:10632-10637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buzzard, K. A., A. J. Giaccia, M. Killender, and R. L. Anderson. 1998. Heat shock protein 72 modulates pathways of stress-induced apoptosis. J. Biol. Chem. 273:17147-17153. [DOI] [PubMed] [Google Scholar]
- 6.Choukroun, G., R. Hajjar, J. M. Kyriakis, J. V. Bonventre, A. Rosenzweig, and T. Force. 1998. Role of stress-activated protein kinases in endothelin-induced cardiomyocyte hyperthrophy. J. Clin. Investig. 102:1311-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Creagh, E. M., R. J. Carmody, and T. G. Cotter. 2000. Heat shock protein 70 inhibits caspase-dependent and-independent apoptosis in Jurkat T cells. Exp. Cell Res. 257:58-66. [DOI] [PubMed] [Google Scholar]
- 8.Davis, R. J. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103:239-252. [DOI] [PubMed] [Google Scholar]
- 9.Feige, U., R. Morimoto, I. Yahara, and B. Polla. 1996. Stress-inducible cellular responses. Birkhauser Verlag, Basel, Switzerland.
- 10.Foghsgaard, L., D. Wissing, D. Mauch, U. Lademann, L. Bastholm, M. Boes, F. Elling, M. Leist, and M. Jaattela. 2001. Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J. Cell Biol. 153:999-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman, B. C., M. P. Myers, R. Schumacher, and R. I. Morimoto. 1995. Identification of a regulatory motif in Hsp70 that affects ATPase activity, substrate binding and interaction with HDJ-1. EMBO J. 14:2281-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabai, V. L., A. B. Meriin, D. D. Mosser, A. W. Caron, S. Rits, V. I. Shifrin, and M. Y. Sherman. 1997. Hsp70 prevents activation of stress kinases. A novel pathway of cellular thermotolerance. J. Biol. Chem. 272:18033-18037. [DOI] [PubMed] [Google Scholar]
- 13.Gabai, V. L., A. B. Meriin, J. A. Yaglom, V. Z. Volloch, and M. Y. Sherman. 1998. Role of Hsp70 in regulation of stress-kinase JNK: implication in apoptosis and aging. FEBS Lett. 438:1-4. [DOI] [PubMed] [Google Scholar]
- 14.Gabai, V. L., A. B. Meriin, J. A. Yaglom, J. Y. Wei, D. D. Mosser, and M. Y. Sherman. 2000. Suppression of stress kinase JNK is involved in HSP72-mediated protection of myogenic cells from transient energy deprivation: HSP72 alleviates the stress-induced inhibition of JNK dephosphorylation. J. Biol. Chem. 275:38088-38094. [DOI] [PubMed] [Google Scholar]
- 15.Gabai, V. L., J. A. Yaglom, V. Volloch, A. B. Meriin, T. Force, M. Koutroumanis, B. Massie, D. D. Mosser, and M. Y. Sherman. 2000. Hsp72-mediated suppression of c-Jun N-terminal kinase is implicated in development of tolerance to caspase-independent cell death. Mol. Cell. Biol. 20:6826-6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendrick, J. P., and F. U. Hartl. 1995. The role of molecular chaperones in protein folding. FASEB J. 9:1559-1569. [DOI] [PubMed] [Google Scholar]
- 17.Jaattela, M. 1999. Escaping cell death: survival proteins in cancer. Exp. Cell Res. 248:30-43. [DOI] [PubMed] [Google Scholar]
- 18.Jaattela, M., D. Wissing, K. Kokholm, T. Kallunki, and M. Egeblad. 1998. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J. 17:6124-6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, C. Y., J. S. Lee, Y. G. Ko, J. I. Kim, and J. S. Seo. 2000. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J. Biol. Chem. 275:25665-25671. [DOI] [PubMed] [Google Scholar]
- 20.Li, G. C., L. Li, R. Y. Liu, M. Rehman, and W. M. F. Lee. 1992. Heat shock protein hsp70 protect cells from thermal stress even after deletion of its ATP-binding domain. Proc. Natl. Acad. Sci. USA 89:2036-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, H., H. Zhu, C. J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491-501. [DOI] [PubMed] [Google Scholar]
- 22.Luo, X., I. Budihardjo, H. Zou, C. Slaughter, and X. Wang. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481-490. [DOI] [PubMed] [Google Scholar]
- 23.Massie, B., F. Couture, L. Lamoureux, D. D. Mosser, C. Guilbault, P. Jolicoeur, F. Belanger, and Y. Langelier. 1998. Inducible overexpression of a toxic protein by an adenovirus vector with a tetracycline-regulatable expression cassette. J. Virol. 72:2289-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meriin, A. B., J. A. Yaglom, V. L. Gabai, D. D. Mosser, L. Zon, and M. Y. Sherman. 1999. Protein damaging stresses activate JNK via inhibition of its phosphatase: a novel pathway controlled by Hsp72. Mol. Cell. Biol. 19:2547-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosser, D. D., A. W. Caron, L. Bourget, C. Denis-Larose, and B. Massie. 1997. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol. Cell. Biol. 17:5317-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosser, D. D., A. W. Caron, L. Bourget, P. Jolicoeur, and B. Massie. 1997. Use of a dicistronic expression cassette encoding the green fluorescent protein for the screening and selection of cells expressing inducible gene products. BioTechniques 22:150-161. [DOI] [PubMed] [Google Scholar]
- 27.Mosser, D. D., A. W. Caron, L. Bourget, A. B. Meriin, M. Y. Sherman, R. I. Morimoto, and B. Massie. 2000. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol. Cell. Biol. 20:7146-7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park, H. S., J. S. Lee, S. H. Huh, J. S. Seo, and E. J. Choi. 2001. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J. 20:446-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin, Z. H., Y. Wang, K. K. Kikly, E. Sapp, K. B. Kegel, N. Aronin, and M. DiFiglia. 2001. Pro-caspase-8 is predominantly localized in mitochondria and released into cytoplasm upon apoptotic stimulation. J. Biol. Chem. 276:8079-8086. [DOI] [PubMed] [Google Scholar]
- 30.Saleh, A., S. M. Srinivasula, L. Balkir, P. D. Robbins, and E. S. Alnemri. 2000. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat. Cell Biol. 2:476-483. [DOI] [PubMed] [Google Scholar]
- 31.Samali, A., and S. Orrenius. 1998. Heat shock proteins: regulators of stress response and apoptosis. Cell Stress Chaperones 3:228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scaffidi, C., S. Fulda, A. Srinivasan, C. Friesen, F. Li, K. J. Tomaselli, K. M. Debatin, P. H. Krammer, and M. E. Peter. 1998. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17:1675-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slee, E. A., S. A. Keogh, and S. J. Martin. 2000. Cleavage of BID during cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3: a potential feedback loop for amplification of apoptosis-associated mitochondrial cytochrome c release. Cell Death Differ. 7:556-565. [DOI] [PubMed] [Google Scholar]
- 34.Tournier, C., P. Hess, D. D. Yang, J. Xu, T. K. Turner, A. Nimnual, D. Bar-Sagi, S. N. Jones, R. A. Flavell, and R. J. Davis. 2000. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science 288:870-874. [DOI] [PubMed] [Google Scholar]
- 35.Tournier, C., A. J. Whitmarsh, J. Cavanagh, T. Barrett, and R. J. Davis. 1997. Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase. Proc. Natl. Acad. Sci. USA 94:7337-7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verheij, M., R. Bose, X. H. Lin, B. Yao, W. D. Jarvis, S. Grant, M. J. Birrer, E. Szabo, L. I. Zon, J. M. Kyriakis, A. Haimovitz-Friedman, Z. Fuks, and R. N. Kolesnick. 1996. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature 380:75-79. [DOI] [PubMed] [Google Scholar]
- 37.Volloch, V., V. L. Gabai, S. Rits, and M. Y. Sherman. 1999. ATPase activity of the heat shock protein hsp72 is dispensable for its effects on dephosphorylation of stress kinase JNK and on heat-induced apoptosis. FEBS Lett. 461:73-76. [DOI] [PubMed] [Google Scholar]
- 38.Wei, M. C., W. X. Zong, E. H. Cheng, T. Lindsten, V. Panoutsakopoulou, A. J. Ross, K. A. Roth, G. R. MacGregor, C. B. Thompson, and S. J. Korsmeyer. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yaglom, J. A., V. L. Gabai, A. B. Meriin, D. D. Mosser, and M. Y. Sherman. 1999. The function of HSP72 in suppression of c-Jun N-terminal kinase activation can be dissociated from its role in prevention of protein damage. J. Biol. Chem. 274:20223-20228. [DOI] [PubMed] [Google Scholar]
- 40.Yin, X. M., K. Wang, A. Gross, Y. Zhao, S. Zinkel, B. Klocke, K. A. Roth, and S. J. Korsmeyer. 1999. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400:886-891. [DOI] [PubMed] [Google Scholar]
- 41.Zanke, B. W., K. Boudreau, E. Rubie, E. Winnett, L. A. Tibbles, L. Zon, J. Kyriakis, F. F. Liu, and J. R. Woodgett. 1996. The stress-activated protein kinase pathway mediates cell death following injury induced by cis-platinum, UV irradiation or heat. Curr. Biol. 6:606-613. [DOI] [PubMed] [Google Scholar]