Abstract
In primary mammalian cells, oncogenic ras induces premature senescence, depending on an active MEK-extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK) pathway. It has been unclear how activation of the mitogenic MEK-ERK pathway by ras can confer growth inhibition. In this study, we have found that the stress-activated MAPK, p38, is also activated during the onset of ras-induced senescence in primary human fibroblasts. Constitutive activation of p38 by active MKK3 or MKK6 induces senescence. Oncogenic ras fails to provoke senescence when p38 activity is inhibited, suggesting that p38 activation is essential for ras-induced senescence. Furthermore, we have demonstrated that p38 activity is stimulated by ras as a result of an activated MEK-ERK pathway. Following activation of MEK and ERK, expression of oncogenic ras leads to the accumulation of active MKK3/6 and p38 activation in a MEK-dependent fashion and subsequently induces senescence. Active MEK1 induces the same set of changes and provokes senescence relying on active p38. Therefore, oncogenic ras provokes premature senescence by sequentially activating the MEK-ERK and MKK3/6-p38 pathways in normal, primary cells. These studies have defined the molecular events within the ras signaling cascade that lead to premature senescence and, thus, have provided new insights into how ras confers oncogenic transformation in primary cells.
The ras proto-oncogene family encodes small GTP binding proteins that transduce growth signals from cell surface receptors in response to extracellular stimuli (1, 6, 37). Previous studies have suggested that aberrant activation of ras is a crucial step during tumorigenesis. Constitutive activation of ras genes is found associated with a wide variety of human tumors at high frequency (3, 4). In both cell culture models and animal models, activated ras cooperates with other oncogenic genetic alterations to induce transformation (13, 19, 25, 49, 57, 61).
The transforming activity of activated ras depends on at least three downstream effectors, including Raf-1/mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase, and Ral-GDS (29, 48, 53, 56), which mediate different aspects of oncogenic transformation. It is believed that activation of the MAPK pathway provides cells with constitutive mitogenic signals independent of extracellular stimuli (7). Interaction between Ras and Raf-1 leads to the sequential activation of the MAP kinase kinases (MAPKKs) MEK1 and MEK2, and the MAPKs extracellular signal-regulated kinase 1 (ERK1) and ERK2. Activated ERK1 and ERK2 promote cell proliferation. For example, it has been demonstrated that active ERK stimulates DNA synthesis (18), inactivates cell cycle inhibitor kinase MYT1 (45), and enhances the activity of AP-1 transcription factor, which induces the expression of growth-promoting genes such as that for cyclin D1 (33, 55).
In contrast to its mitogenic activity, expression of oncogenic ras in normal primary cells induces premature senescence, a permanent growth arrest that is morphologically indistinguishable from replicative senescence observed in aged primary cells (51). This senescence-like growth arrest induced by ras is associated with accumulation of growth inhibitors such as p53 and p16INK4A (51). Interestingly, the ability of oncogenic ras to induce premature senescence depends on the Raf-MEK-ERK pathway that mediates cell proliferation (36). Constitutive activation of this pathway induces p53, p16, and p21 and leads to premature senescence. In addition, ras fails to induce senescence when the activation of the MEK-ERK pathway is specifically inhibited. It remains unclear how activation of the mitogenic Raf-MEK-ERK pathway by ras can induce premature senescence and how this negative growth impact of ras is bypassed in tumors.
Besides the Raf-MEK-ERK cascade, oncogenic ras also activates the Jun amino-terminal kinase (JNK) and p38 MAPK pathways in several different cell lines (8, 31, 38, 62). Like ERK, JNK also enhances the activity of AP-1 and promotes cyclin D1 transcription when activated by its upstream kinases, MKK4 and MKK7, and thus is likely to be involved in the ability of ras to regulate cell proliferation (7, 30, 31, 44). The p38 MAPK is phosphorylated and activated by its upstream MAPKKs MKK4, MKK3, and MKK6, usually in response to nonmitogenic signals such as proinflammatory cytokines and environmental stress (43). However, the biological significance of p38 activation by oncogenic ras remains unclear. It has been reported that under certain biological conditions p38 can negatively regulate cell growth. Microinjection of a p38-encoding plasmid into NIH 3T3 fibroblasts led to down-regulation of cyclin D1 expression and cell cycle arrest at G1 (40). Ectopic expression of MEKK3, a MAPKKK that activates p38, induced G1 arrest and reversed ras-mediated transformation in NIH 3T3 cells, again through down-regulation of cyclin D1 (14). In addition, the MKK6-p38γ cascade mediated γ-irradiation-induced G2 cell cycle arrest (58).
The negative role of p38 in proliferation led us to investigate the involvement of this pathway in oncogenic ras-induced premature senescence. In the present study, we have demonstrated that oncogenic ras induces premature senescence through sequential activation of the MEK-ERK pathway and the MKK3/6-p38 pathway in primary human fibroblasts. The MEK-ERK pathway, when activated by ras, stimulates the activity of p38, which in turn leads to senescence. Therefore, besides transducing mitogenic signals, the ras-activated MEK-ERK pathway has an additional biological consequence, induction of premature senescence through the p38 pathway. These results have important implications for our understanding of the mechanisms by which ras transforms cells.
MATERIALS AND METHODS
Cell culture.
BJ human foreskin fibroblasts were obtained from J. Smith (Baylor College of Medicine) maintained in minimum essential medium supplemented with 10% fetal calf serum, nonessential amino acids, glutamine, and antibiotics. LinX-A retroviral packaging cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, glutamine, and antibiotics.
Plasmids and reagents.
BabeHygro-hTERT, BabePuro-Ha-rasV12, and BabePuro-MEK1Q56P were obtained from R. A. Weinberg, D. Beach, and S. Lowe, respectively. BabePuro-MKK3E, -MKK6E, -MKK7D, and -p38αWT were constructed by cloning the respective cDNA fragment from an adenovirus vector (23, 59) into BabePuro vector. BabeHygro-MKK3A and -MKK6A were constructed by cloning the respective cDNA fragment from an adenovirus vector (23) into BabeHygro vector. Dominant-negative mutant alleles of p38α, p38β, p38γ, and p38δ were obtained from Jiahui Han and were excised from pcDNA3 vectors (20, 26, 27, 35) and subcloned into the BabeHygro vector. SB203580 and U0126 were purchased from Calbiochem. Tumor necrosis factor alpha (TNF-α) was purchased from Chemicon International, Inc.
Retroviral gene transduction.
Retroviral gene transduction was carried out as previously described, using an amphotropic packaging cell line (LinX-A) (54). Treatments with SB203580 (8 μM), U0126 (5 μM), or vehicle control usually started at the time of initial retroviral infection. Cells transduced with retroviruses were purified with 100 μg of hygromycin B/ml, 400 μg of G418/ml, and/or 1 μg of puromycin/ml, starting 1 to 2 days after infection. We typically achieve 30 to 50% infection rates in BJ cells before selection and 90 to 100% after selection.
Analysis of senescence.
Cells infected with appropriate retroviruses were selected with puromycin for 4 days to eliminate the uninfected cells, after which assays were performed to analyze premature senescence. The day when drug selection was completed (day 5 after infection) was defined as day 0.
For growth curves, 104 cells were plated into each well in 12-well plates in duplicates or triplicates. Every 3 to 4 days, cells were trypsinized from plates and cell numbers were counted. At each split, 104 cells were reseeded to each well in fresh plates and allowed to grown until the next split. Population doublings (PD) were calculated with the formula PD = log(n2/n1)/log2, where n1 is the number of cells seeded and n2 is the number of cells recovered (52). Cells with senescence-associated β-galactosidase (SA-β-gal) activity were detected as previously described (51). At least 200 cells were counted in randomly chosen fields from each culture well.
Western blotting.
Lysates for Western blot analysis were normally prepared 8 to 10 days after the initial retroviral gene transduction. Cells at 30 to 50% confluence were lysed in NP-40 lysis buffer (50 mM Tris-HCl [pH 8.0], 120 mM NaCl, 0.5% NP-40, and Complete protease inhibitors [Roche]) or RIPA buffer (phosphate-buffered saline containing 1% Triton X-100, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 1 mM Na3VO4, and Complete protease inhibitors [Roche]). After the lysates were cleared by centrifugation, protein concentrations were determined by Bradford assays. Twenty to 80 μg of proteins was separated on an SDS-10% polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to Trans-Blot nitrocellulose membranes (Bio-Rad). The primary antibodies used were for Ha-Ras (C-20; Santa Cruz), phospho-ERK (Thr202/Tyr204; Cell Signaling), ERK (Cell Signaling), ERK2 (C-14; Santa Cruz), actin (Sigma), p16INK4A (DCS-50; Novocastra), p53 (CM1 from Novocastra and FL-393 from Santa Cruz), phospho-p38 (Thr180/Tyr182; Cell Signaling), p38 (Cell Signaling), phospho-MEK1/2 (Ser217/221; Cell Signaling), phospho-MKK3/6 (Ser189/207; Cell Signaling), MKK3 (C-19; Santa Cruz), MKK6 (V-20; Santa Cruz), phospho-MKK4 (Thr261; Cell Signaling), MKK4 (C-20; Santa Cruz), phospho-JNK (Thr183/Tyr185; Cell Signaling), JNK (Cell Signaling), and JNK1 (C-17; Santa Cruz). Horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) (Santa Cruz) or goat anti-mouse IgG (Jackson Laboratories) antibodies were used as secondary antibodies. Reactive proteins were visualized using enhanced chemiluminescence (SuperSignal; Pierce).
Immunoprecipitation and protein kinase assays.
Immunoprecipitation of p38 protein and subsequent protein kinase assays using [γ-32P]ATP were carried out as described previously (23, 28). Cells at 30 to 50% confluence were lysed in RIPA buffer (phosphate-buffered saline containing 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS, 1 mM Na3VO4, and Complete protease inhibitors [Roche]). p38 protein was purified from equal amounts of lysates using an antibody against p38 (Cell Signaling) (23) or actin (Sigma) and protein A-Sepharose CL-4B (Amersham Pharmacia Biotech). Protein kinase assays were performed at 37°C for 30 min in a 50-μl volume with 7 μg of glutathione S-transferase (GST)-ATF2 (residues 1 to 109), 10 μM ATP, 10 μCi of [γ-32P]ATP, 20 mM HEPES (pH 7.6), 20 mM MgCl2, 25 mM β-glycerophosphate, 0.1 mM Na3VO4, and 2 mM dithiothreitol. The reactions were terminated with Laemmli sample buffer, and the products were separated on SDS-PAGE and visualized and quantitated with a phosphorimager. Nonradioactive p38 kinase assays were carried out using the p38 MAP Kinase Assay Kit from Cell Signaling, following the manufacturer's instructions.
ERK and JNK kinase activities were measured using the p44/42 MAP Kinase Assay Kit and the SAPK/JNK MAP Kinase Assay Kit from Cell Signaling, following the manufacturer's instructions.
Cell cycle analysis.
After 2 days of selection with puromycin (3 days postinfection), cells transduced with Ha-rasV12 or a vector control in the presence of SB203580 or vehicle control were grown in medium containing 0.2% fetal bovine serum for 21 h before being labeled with 30 μM bromodeoxyuridine (BrdU) (Sigma) for 3 h. Cells were removed from plates with trypsin and fixed in 70% ethanol at 4°C overnight. Cells were then treated with 2 N HCl-0.5% Triton X-100 for 30 min at room temperature, followed by neutralization with 0.1 M Na2B4O7 and subsequent incubation with fluorescein isothiocyanate (FITC)-conjugated anti-BrdU antibody (Becton Dickinson) for 1 h at room temperature. Finally, cells were washed with PBS-1% bovine serum albumin-0.5% Tween 20, resuspended in PBS containing 5 μg of propidium iodide/ml, and analyzed by two-dimensional flow cytometry to detect both fluorescein and propidium iodide.
Northern blotting.
Total RNA was isolated from cells using TRIzol reagent (Gibco BRL) according to the manufacturer's instructions. Eight micrograms of RNA was separated on a 1% agarose gel containing 3.7% formaldehyde in 1× morpholinepropanesulfonic acid (MOPS) buffer (20 mM MOPS, 5 mM NaOAc, and 1 mM EDTA; pH 7.0), transferred to Hybond N+ nylon membranes in 10× SSC (1.5 M NaCl and 150 mM sodium citrate; pH 7.0), and hybridized at 65°C in Church-Gilbert buffer (1% bovine serum albumin, 400 mM NaPO4 [pH 7.0], 15% formamide, 1 mM EDTA, and 7% SDS) to an 800-bp human p16 cDNA probe labeled with [α-32P]dATP and [α-32P]dCTP by random priming. After extensive washing with 0.2× SSC-0.1% SDS buffer at 65°C, the signals were visualized and quantitated with a phosphorimager.
RESULTS
p38 is activated during ras-induced premature senescence.
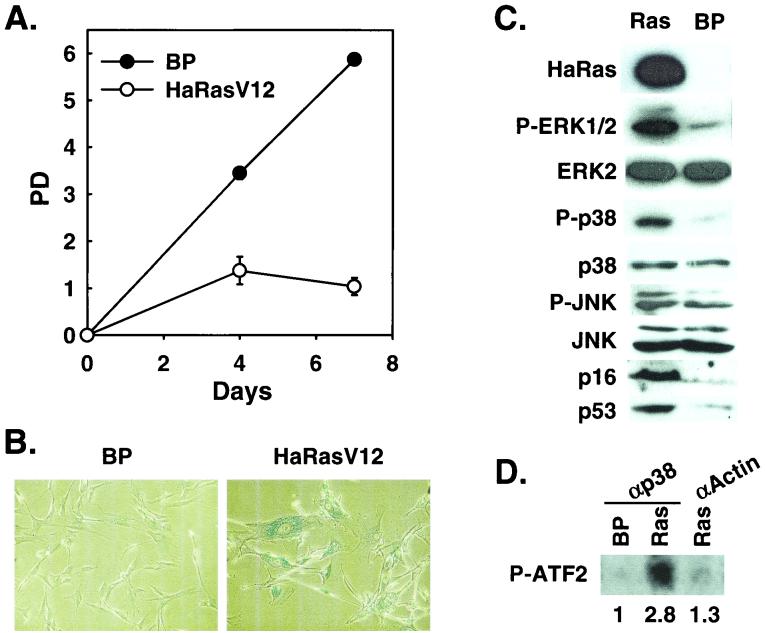
Activation of p38 by oncogenic ras was previously observed in immortalized murine NIH 3T3 fibroblasts (8, 38, 62). While oncogenic ras induces premature senescence in primary cells, immortalized murine cells are refractory to premature senescence (51). To explore the possible involvement of the p38 pathway in oncogenic ras-induced senescence, we examined whether Ha-rasV12, an oncogenic ras mutant allele found in human tumors, activated p38 during the onset of senescence in BJ primary human foreskin fibroblasts. Ha-RasV12 was transduced into BJ cells via retroviral infection. Consistent with previous findings in other primary human and murine fibroblasts, Ha-RasV12 induced premature senescence in early-passaged BJ cells (Fig. 1). Cells stopped proliferating 7 to 10 days after the initial transduction of Ha-RasV12 (Fig. 1A). The growth-arrested cells acquired the enlarged and flattened morphology that was characteristic of cellular senescence. In addition, these cells accumulated SA-β-gal (Fig. 1B), a biomarker for senescent cells (12).
FIG. 1.
Induction of premature senescence by oncogenic ras correlates with p38 activation. (A) PD of BJ cells transduced at PD 20 with Ha-RasV12 or a vector control. Values are means ± standard deviations for duplicates. (B) Morphology of BJ cell populations transduced with Ha-RasV12 or a vector control, after staining for SA-β-gal (pH 6.0) at day 7 postselection. (C) Western blot analysis of BJ cells transduced with Ha-RasV12 (Ras) or a vector control (BP), showing the levels of Ha-Ras, phospho-ERK (P-ERK1/2), ERK2, phospho-p38 (P-p38), p38, phospho-JNK (P-JNK), JNK, p16INK4A, and p53. (D) p38 protein kinase activity in BJ cells transduced with Ha-RasV12 (Ras) or a vector control (BP), determined at day 4 postselection in a kinase assay by using [γ-32P]ATP and GST-ATF2 as substrates, following immunoprecipitation of p38 with an anti-p38 antibody (αp38) or an antiactin antibody (αActin). Shown are the relative amounts of phosphorylated ATF2 substrate after normalization to background. The signals were visualized and quantitated with a phosphorimager.
In BJ cells undergoing premature senescence, we detected increased phosphorylation of p38 on Thr180 and Tyr182 in Western blot analysis by using an antibody that specifically recognizes p38 phosphorylated on these sites (Fig. 1C). The phosphorylation of p38 on Thr180 and Tyr182 is known to lead to the activation of p38 (10, 21, 22, 46, 47). To confirm the activation of p38, p38 protein was isolated from Ha-RasV12-expressing cells or control cells by immunoprecipitation using an anti-p38 antibody, and it was tested for its ability to phosphorylate a downstream substrate, ATF2 (10, 46). Indeed, p38 purified from ras-expressing cells had an increased (nearly threefold higher) activity in phosphorylation of ATF2, compared to that from cells expressing a vector control (Fig. 1D). The immune complex precipitated from ras-expressing cells by using a control antibody (antiactin) displayed no kinase activity. Therefore, in BJ primary human fibroblasts, oncogenic ras-induced premature senescence correlated with p38 activation. In contrast, although we observed a slight increase in the level of phospho-JNK when Ha-rasV12 was expressed in BJ fibroblasts (Fig. 1C), the JNK activity was not detected from the ras-expressing cells in an in vitro kinase assay using c-Jun as substrate (Fig. 4B).
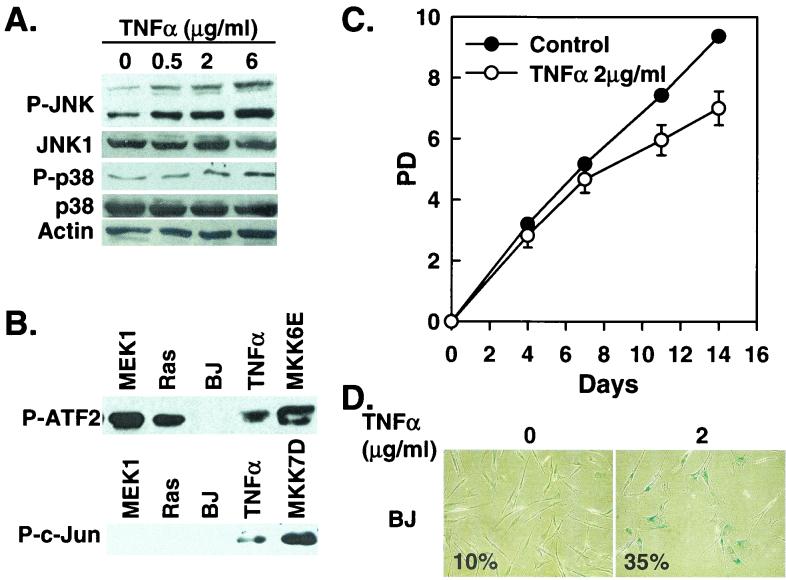
FIG. 4.
TNF-α induces senescence in BJ cells. (A) Western blot analysis of BJ cells (PD 24) treated with the indicated concentrations of TNF-α, showing levels of phospho-JNK (P-JNK), JNK1, phospho-p38 (P-p38), p38, and actin. (B) p38 (P-ATF2) and JNK (P-c-Jun) kinase activities were determined in BJ cells 8 days after being transduced at PD 32 with MEK1Q56P (MEK1), Ha-RasV12 (Ras), a vector control (BP), or MKK6E or after being treated with 2 μg of TNF-α/ml. p38 and JNK were isolated and assayed from 200 μg (p38) or 250 μg (JNK) of cell lysates by using the p38 MAP Kinase Assay Kit and the SAPK/JNK MAP Kinase Assay Kit (Cell Signaling), respectively. The phosphorylated substrates, ATF2 for p38 and c-Jun for JNK, were visualized by Western blot analysis by using antibodies against phospho-ATF2 or phospho-c-Jun. (C) PD of BJ cells (PD 29) treated with 2 μg of TNF-α/ml or vehicle control. Values are means ± standard deviations for triplicates. (D) Morphology of BJ cells treated with 2 μg of TNF-α/ml (2) or vehicle control (0), after staining for SA-β-gal (pH 6.0) at day 11 of the treatment. Numbers represent percentages of cells positive for SA-β-gal in each population.
Constitutively active MKK3 and MKK6 induce premature senescence.
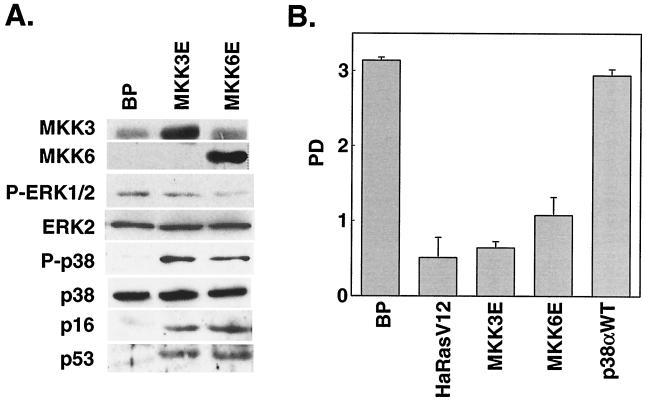
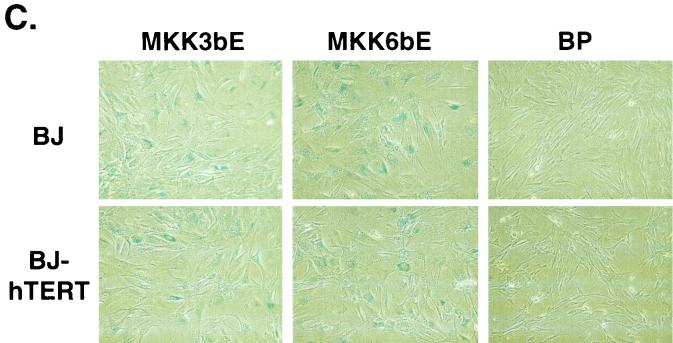
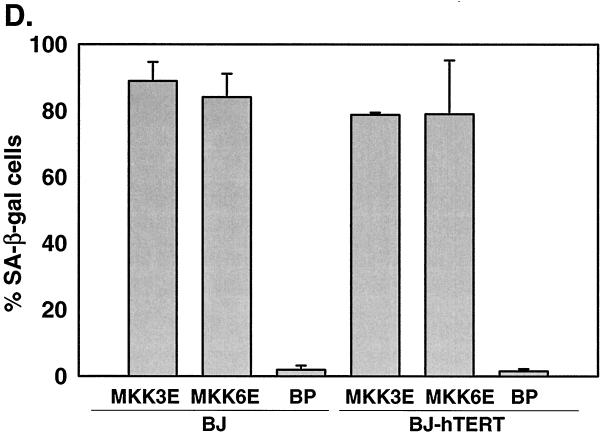
p38 MAPK can be activated through phosphorylation by two upstream MAPKKs, MKK3 and MKK6, in response to extracellular stimuli (10, 21, 22, 46, 47). MKK3 and MKK6 are activated by dual phosphorylation on Ser207/Thr211 and Ser189/Thr193, respectively. Substitution of these serine/threonine residues with glutamic acid (E) results in constitutively active forms of MKK3 and MKK6 (MKK3E and MKK6E) that can phosphorylate and activate p38 independently of upstream signals (22, 47). We therefore examined whether activation of p38 by these two mutant MKKs would lead to senescence. MKK3E and MKK6E were expressed in early-passaged BJ fibroblasts via retroviral gene transduction (Fig. 2A). While Ha-RasV12 led to the phosphorylation of both p38 and ERK, MKK3E and MKK6E only increased activating phosphorylation of p38 but not that of ERK (Fig. 2A). MKK3E and MKK6E also stimulated p38 kinase activity (see Fig. 8C). BJ cells transduced with a vector control (BP) or a wild-type p38α gene that was not activated displayed normal growth rates (Fig. 2B). In contrast, expression of MKK3E or MKK6E led to growth arrest in BJ cells (Fig. 2B). The arrested cells acquired a senescence-like morphology (Fig. 2C). In addition, we estimated that more than 80% of MKK3E- or MKK6E-expressing cells had accumulated SA-β-gal, while very few of the vector control cells displayed this senescence marker (Fig. 2C and D). These results indicated that constitutive activation of p38 could induce premature senescence.
FIG. 2.
Activation of p38 by active MKK3 or MKK6 induces premature senescence. (A) Western blot analysis of BJ cells transduced at PD 18 with a vector control (BP) or constitutively active MKK3 (MKK3E) or MKK6 (MKK6E). Levels of MKK3, MKK6, phospho-ERK (P-ERK1/2), ERK2, phospho-p38 (P-p38), p38, p16INK4A, and p53 were determined 10 days postinfection. (B) PD of BJ cells between day 7 and day 11 after infection at PD 18 with a vector control (BP), Ha-RasV12 (HaRasV12), a constitutively active MKK3 (MKK3E) or MKK6 (MKK6E), or wild-type p38α (p38αWT). Values are means ± standard deviations (SD) for triplicates. (C) Morphology of BJ cells (BJ) or BJ cells immortalized with hTERT (BJ-hTERT) that had been transduced with a vector control (BP) or constitutively active MKK3 (MKK3E) or MKK6 (MKK6E), after staining for SA-β-gal (pH 6.0) at day 12 postinfection. (D) Quantification of percentages of SA-β-gal-positive cells within cell populations shown in panel C. Values are means ± SD for two separate wells. At least 200 cells were counted for each sample.
FIG. 8.
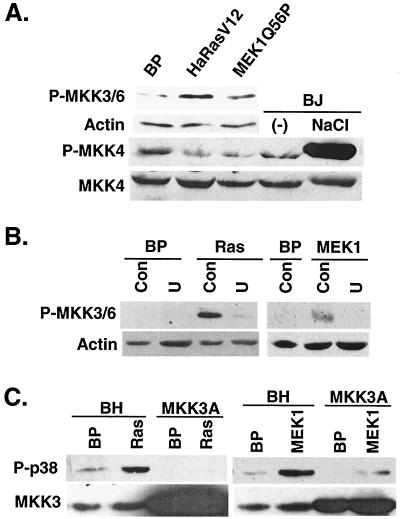
p38 activation by ras is mediated by an active MEK-ERK pathway. (A) Constitutively active MEK1 increases p38 phosphorylation. Shown are the results of Western blot analysis of BJ cells transduced with a vector control (BP), Ha-RasV12 (HaRasV12), or MEK1Q56P (MEK1Q56P) at day 6 postselection, indicating the protein levels of MEK1 (MEK1), phospho-ERK (P-ERK1/2), ERK2 (ERK2), phospho-p38 (P-p38), p38 (p38), p53 (p53), and p16INK4A (p16). (B) Inhibition of MEK-ERK activity prevented ras-induced p38 phosphorylation. Shown are the results of Western blot analysis of BJ cells transduced with a vector control (BP), Ha-RasV12 (HaRasV12), MKK3E (MKK3E), or MKK6E (MKK6E) in the presence of vehicle control (Con), U0126 (U), or SB203580 (SB) at day 10 postinfection, with the levels of phospho-p38 and p38 indicated. (C) Protein kinase activity of p38 immunoprecipitated from BJ cells transduced at PD 30 with a vector control (BP), Ha-RasV12 (HaRasV12) in the presence of vehicle control (Con), U0126 (U), or SB203580 (SB), constitutively active MKK3 (MKK3E) or MKK6 (MKK6E), or active MEK1 (MEK1Q56P) at day 10 postinfection. [γ-32P]ATP and GST-ATF2 were used as substrates. The numbers represent the relative amounts of phosphorylated ATF2 substrate after normalization to background. The signals were visualized and quantitated with a phosphorimager.
Oncogenic ras-induced premature senescence differs from replicative senescence in that it is telomere independent. Although the human telomerase catalytic subunit hTERT prevents replicative senescence when ectopically expressed (2), it does not rescue oncogenic ras-induced premature senescence (60). MKK3E and MKK6E also induced senescence in BJ cells that had been immortalized with hTERT (Fig. 2C and D). When not transduced with MKK3/6, these hTERT-expressing cells proliferated well beyond PD 150, while the control BJ cells underwent replicative senescence at PD 80 to 90 (data not shown). Thus, senescence induced by p38 activation was not rescued by forced expression of hTERT either, suggesting that both oncogenic ras and activated p38 induced senescence via a pathway that was distinct from that mediating replicative senescence.
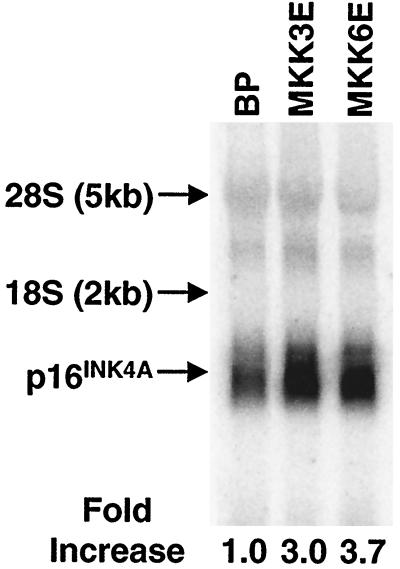
Like Ha-RasV12, MKK3E and MKK6E induced the expression p53 and p16INK4A (Fig. 2A). While the role of p53 in premature senescence is still controversial, p16 INK4A was previously shown to be involved in ras-induced senescence (64) and thus may also mediate senescence induced by activated p38 in our study. Therefore, we further explored the mechanism of p16INK4A accumulation following p38 activation. p16INK4A mRNA was up-regulated by three- to fourfold following p38 activation by active MKK3 or MKK6, as determined in a Northern blotting analysis using a p16INK4A cDNA as probe (Fig. 3). Therefore, although we cannot rule out that MKK3/6 may also regulate p16 protein levels, at least part of p16 accumulation is due to the up-regulation of its mRNA.
FIG. 3.
Active MKK3 and MKK6 up-regulate 16INK4A mRNA levels. Total RNA was isolated from BJ cells 8 days after being transduced with a vector control (BP) or retroviruses encoding active MKK3 (MKK3E) or MKK6 (MKK6E). Eight micrograms of RNA was separated on an agarose gel, transferred to nylon membrane, and hybridized to a p16INK4A cDNA probe labeled by random priming. The signals were visualized and quantitated with a phosphorimager. The numbers represent the relative intensities of p16INK4A signals after normalization to those of background and GAPDH signals (data not shown).
TNF-α-induced senescence in BJ fibroblasts.
Because activation of p38 by oncogenic ras or active MKK3 and MKK6 can lead to premature senescence in primary BJ fibroblasts, we explored the possibility that other p38 activators may also induce senescence in these cells. TNF-α is an inflammatory cytokine that has been shown to activate p38 (32, 50). In BJ fibroblasts, TNF-α treatment induced the accumulation of phospho-p38 (Fig. 4A) and an increase in p38 activity (Fig. 4B). When treated continuously with TNF-α, BJ cells displayed a slower growth rate than the control cells (Fig. 4C). More than 30% of the cells within the TNF-α-treated population were positive for SA-β-gal (Fig. 4D), indicating that TNF-α-induced growth inhibition was at least partially due to premature senescence. Compared with Ha-RasV12- or MEK1Q56P-induced premature senescence, the senescent phenotypes induced by TNF-α were less severe. This might be attributed to the fact that Ha-RasV12 or MEK1Q56P led to stronger activation of p38 than did TNF-α (Fig. 4B). Overall, these data suggest that activation of p38 by TNF-α can also lead to premature senescence in primary BJ fibroblasts.
While the JNK activity was undetectable in cells expressing oncogenic ras or active MEK1 (Fig. 4B), TNF-α led to increases in the phosphorylation of JNK in a dose-dependent manner (Fig. 4A) and induced JNK activity toward phosphorylation of c-Jun (Fig. 4A and B). TNF-α did not induce the activation of ERK in BJ cells (data not shown).
Activation of JNK by active MKK7 does not induce senescence.
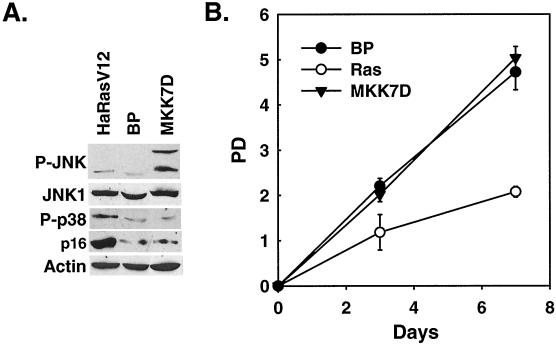
Overexpression of oncogenic ras led to an increase in the levels of phospho-JNK (Fig. 1C). In a kinase assay in which the JNK activity induced by TNF-α or active MKK7 was well detected, the JNK kinase activity was undetectable from ras- or MEK1-expressing cells (Fig. 4B). However, it was possible that ras still stimulated JNK activity, but only to a level that was imperceptible by the kinase assay. In addition, TNF-α, which induced senescence in BJ cells, also activated JNK (Fig. 4A and B). Therefore, we further investigated the role of JNK activation in premature senescence. Early-passaged BJ cells were infected with a recombinant retrovirus encoding a constitutively active mutant of MKK7 (MKK7D), in which Ser271 and Thr275 had been mutated to Asp (41, 59). MKK7D led to a marked increase in the phosphorylation of JNK1 and JNK2 without affecting the phosphorylation status of p38 (Fig. 5A) and greatly stimulated the ability of JNK to phosphorylate c-Jun (Fig. 4B). However, BJ cells expressing MKK7D did not undergo premature senescence. These cells proliferated continuously, as did the cells infected with a vector control (Fig. 5B), and they showed no senescent phenotypes. Active MKK7 did not induce the accumulation of p16INK4A, either (Fig. 5A). Therefore, activation of the JNK pathway is not sufficient to cause premature senescence. Taken together with the observation that Ha-RasV12 did not significantly induce JNK activation, these data suggest that activation of JNK is unlikely to be the major mediator of premature senescence induced by oncogenic ras.
FIG. 5.
Activation of JNK by active MKK7 does not induce senescence. (A) Levels of phospho-JNK (P-JNK), JNK1, phospho-p38 (P-p38), p16INK4A, and actin were determined by Western blot analysis 10 days after transduction of BJ cells at PD 24 with Ha-RasV12 (HaRasV12), constitutively active MKK7 (MKK7D), or a vector control (BP). (B) PD of BJ cells transduced at PD 24 with Ha-RasV12 (Ras), constitutively active MKK7 (MKK7D), or a vector control (BP). Day 0 represents the fifth day postinfection. Values are means ± standard deviations for triplicates.
p38 activation is required for ras-induced senescence.
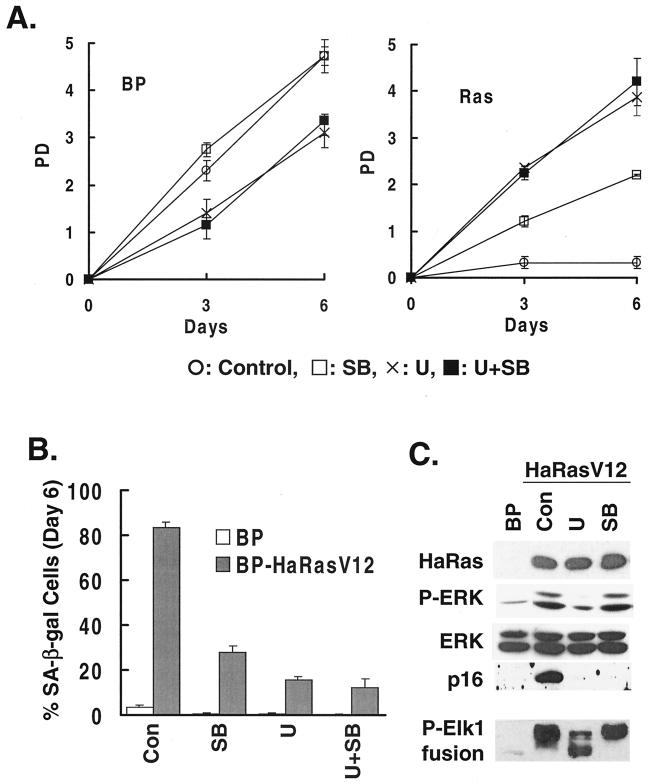
Although activation of p38 by active MKK3 or MKK6 is sufficient to cause premature senescence by itself, the possibility still exists that ras-induced senescence is not mediated by p38. As a result, p38 activation may not be required for the ability of ras to induce senescence. Alternatively, activation of p38 may be an essential step in the pathway leading from ras activation to premature senescence. To differentiate these possibilities, we took advantage of SB203580, a pyridinyl imidazole derivative that could specifically inhibit the activation of p38 but not that of the other MAPK pathways. SB203580 blocks the ability of active p38α and p38β to phosphorylate and activate their downstream targets (9, 15, 16). Treatment of ras-expressing cells with SB203580 reduced the ability of p38 to phosphorylate ATF2 (see Fig. 8C), while having no effect on the phosphorylation of ERK1 and ERK2 induced by ras (Fig. 6C, top panels). In addition, treatment with SB203580 did not alter the ras-induced activity of ERK toward phosphorylation of one of its natural substrates, Elk1, as determined in an in vitro kinase assay following the isolation of ERK from cells by immunoprecipitation (Fig. 6C, bottom panel). As a control, U0126, a specific inhibitor of the MAPKKs MEK1 and MEK2, was used to block the activation of the MEK-ERK pathway by oncogenic ras (11, 17). In BJ cells, U0126 inhibited the ras-induced phosphorylation of ERK1 and ERK2 (Fig. 4C, upper panels) and reduced the activity of ERK toward phosphorylation of Elk1 in an in vitro kinase assay (Fig. 6C, bottom panel).
FIG. 6.
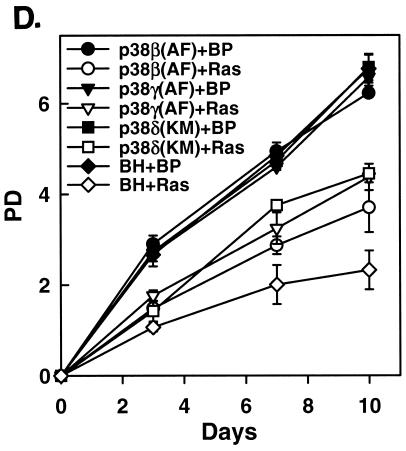
Specific inhibition of p38 activation by ras rescues ras-induced premature senescence. (A) PD of BJ cells transduced at PD 18 with a vector control (BP) or Ha-RasV12 (Ras) in the presence of a vehicle control, SB203580, U0126, or U0126 and SB203580. Values are means ± standard deviations (SD) for triplicates. (B) Percentage of SA-β-gal-positive cells within cell populations transduced at PD 18 with a vector control or Ha-RasV12 in the presence of a vehicle control (Con), SB203580 (SB), U0126 (U), or U0126 and SB203580 (U+SB). Values are means ± SD for triplicates. (C) Western blot analysis of BJ cells transduced at PD 18 with a vector control (BP) or Ha-RasV12 (HaRasV12) in the presence of a vehicle control (Con), U0126 (U), or SB203580 (SB), showing the levels of Ras, phospho-ERK (P-ERK), ERK, and p16INK4A. The bottom panel (P-Elk1 fusion) shows the kinase activity of ERK immunoprecipitated from the same cells, as determined in an in vitro kinase assay using Elk1 as substrate. The phosphorylated substrate was visualized by Western blot analysis with an antibody against phospho-Elk1. (D) Dominant-negative alleles of p38β, p38γ, and p38δ rescued ras-induced senescence. BJ cells expressing a dominant-negative allele of p38β [p38β(AF)], p38γ [p38γ(AF)], p38δ [p38δ(AF)], or a vector control (BH) were transduced with Ha-RasV12 (Ras; open symbols) or a vector control (BP; filled symbols) at PD 34. The PD of these cells were followed starting from day 5 postinfection (designated as day 0). Values are means ± SD for triplicates.
When Ha-RasV12 was transduced into BJ cells in the presence of SB203580, premature senescence was prevented compared to when the vehicle control was present (Fig. 6A). Treatment with U0126 also rescued premature senescence in BJ cells expressing Ha-RasV12 (Fig. 6A), confirming the essential role of MEK-ERK activation in ras-induced senescence (36, 64). In addition, SB203580 and U0126 also significantly reduced the percentage of Ha-RasV12-expressing cells that were positive for SA-β-gal activity from more than 80% to 10 to 30% (Fig. 6B), and these compounds blocked the induction of p16INK4A by ras (Fig. 6C), suggesting that the onset of the senescence process was blocked when activation of the p38 or MEK-ERK pathway by ras was prevented in these cells. These results demonstrated that the activation of p38 is essential for oncogenic ras to induce premature senescence in BJ primary fibroblasts. When the ability of ras to activate p38 was specifically blocked, premature senescence did not occur, even though the MEK-ERK pathway was still active.
To confirm the essential role of p38, ras-induced premature senescence was analyzed in early-passaged BJ cells that had been transduced with dominant-negative mutants of different p38 isoforms, p38α(FA), p38β(FA), p38γ(FA), and p38δ(KM). These mutant alleles of p38 harbor mutations within the dual phosphorylation sites (TGY to FGA for α, β, and γ, or to KGM for δ) and have been shown to inhibit the activities of endogenous p38 in cells in a dominant-negative fashion (20, 26, 27, 35). Expression of p38β(FA), p38γ(FA), or p38δ(KM) partially rescued the growth inhibition provoked by oncogenic ras (Fig. 6D), indicating that these three isoforms of p38 were involved in ras-induced senescence. Unlike the other p38 isoforms, p38α(FA) had no apparent effect on the negative growth regulation by ras (data not shown). These results with dominant interfering mutant alleles of p38 further confirmed that p38 activity was essential for oncogenic ras to induce premature senescence in BJ fibroblasts.
p38 is not required for the mitogenic activity of ras.
Since oncogenic ras induces both proliferation and premature senescence in normal diploid human fibroblasts, we further investigated whether p38 was also essential for the mitogenic activity of ras. In primary fibroblasts, premature senescence is a late response to oncogenic ras expression, while the immediate consequence of ras activation is growth stimulation (36). Indeed, the growth arrest was not obvious until 7 to 10 days after the initial transduction of the Ha-rasV12 gene in BJ cells (day 1 to 4 in growth curve assays; see Materials and Methods) (Fig. 1A and data not shown).
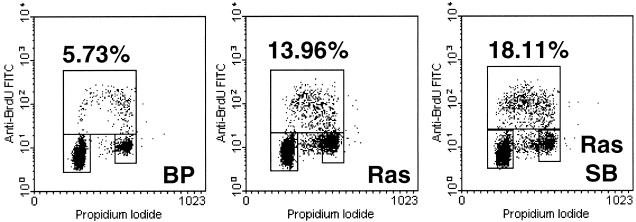
To examine the mitogenic activity of ras, BrdU incorporation was measured in BJ cell populations shortly after ras transduction. Four days postinfection and immediately after the infected cells had been purified with puromycin, oncogenic ras expression resulted in a two- to threefold increase in BrdU incorporation when the cells were grown in a low serum concentration (Fig. 7), confirming the previous findings in IMR90 human fibroblasts (36). This assay revealed the role of oncogenic ras in mitogenic stimulation before premature senescence occurred in BJ cells. The ras-induced increase in BrdU incorporation was not prevented when p38 was inhibited by SB203580 (Fig. 7), indicating that the mitogenic activity of oncogenic ras does not rely on active p38. These results suggest that while p38 activation is essential for ras-induced premature senescence, the mitogenic activity of ras seems to be independent of p38.
FIG. 7.
p38 is not required for the mitogenic activity of ras. Three days postinfection, early-passaged BJ cells transduced at PD 18 with a vector control (BP) or Ha-RasV12 (Ras) in the presence of vehicle control or SB203580 (SB) were placed in low-serum medium for 20 h and labeled with BrdU for 3 h. Cells were harvested, stained with FITC-conjugated anti-BrdU antibody and propidium iodide, and analyzed for BrdU incorporation and DNA content by two-color flow cytometry. The upper box represents cells incorporating BrdU (in S phase), the lower-left box represents G1 cells, and the lower-right box represents G2/M cells. The percentage of BrdU-positive cells is indicated for each cell population.
p38 is activated as a result of sustained MEK-ERK activation by ras.
Results from this study and previous studies have demonstrated that both the MEK-ERK and MKK3/6-p38 MAPK pathways are necessary for oncogenic ras-induced senescence. In addition, activation of ERK or p38 alone by their respective upstream kinases induced premature senescence. These observations suggest that MEK-ERK and MKK3/6-p38 may act in a linear pathway in mediating ras-induced senescence. Supporting this hypothesis, inhibition of both MEK-ERK and MKK3/6-p38 pathways simultaneously by U0126 and SB203580 did not have additive effects in preventing ras-induced senescence (Fig. 6A and B). Although MEK-ERK and MKK3/6-p38 constitute two separate MAPK pathways that mediate responses to different extracellular signals, our results suggest a possibility that sustained stimulation of one pathway by ras expression may lead to the activation of the other.
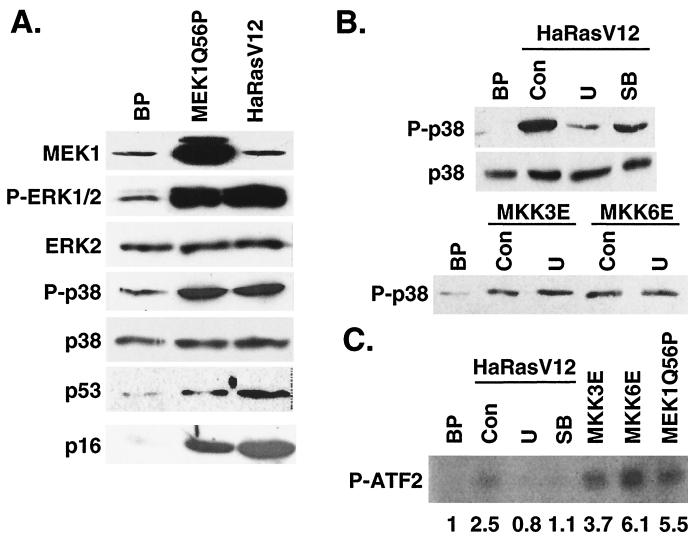
Since activation of p38 by MKK3 or MKK6 did not increase ERK phosphorylation (Fig. 2A), we examined the effects of an active MEK-ERK pathway on the activity of p38. To activate the ERK pathway, a constitutively active mutant of MEK1 (MEK1Q56P) (5) was transduced into BJ cells. The active MEK1 increased the activating phosphorylation of ERK (Fig. 8A) and led to premature senescence in primary BJ fibroblasts (see Fig. 11), consistent with previous findings in other primary fibroblasts (36, 64). Expression of the constitutively active MEK1 also induced the phosphorylation of p38 on Thr180 and Tyr182 (Fig. 8A) and increased the kinase activity of p38 toward phosphorylation of ATF2 (Fig. 8C). Therefore, constitutive activation of the MEK-ERK pathway led to activation of p38.
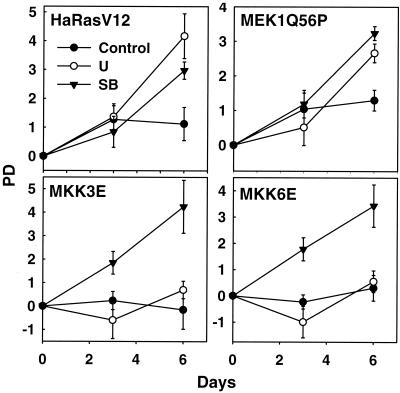
FIG. 11.
Both Ha-RasV12- and MEK1Q56P-induced senescence requires active p38. Shown are the growth curves of BJ cells transduced at PD 17 with Ha-rasV12 (Ha-RasV12) or constitutively active MEK1 (MEKQ56P), MKK3 (MKK3E), or MKK6 (MKK6E) in the presence of vehicle control, U0126 (U), or SB203580 (SB). Values are means ± standard deviations as determined for triplicates at the indicated days postselection.
Results from previous studies and those presented here (Fig. 1C) have demonstrated that oncogenic ras activates both ERK and p38 pathways. Our observation that activated MEK1 could lead to p38 activation raised a possibility that ras might stimulate p38 activity through activation of the MEK-ERK pathway. Indeed, inhibition of MEK activity by U0126 treatment greatly reduced the ability of Ha-RasV12 to induce p38 phosphorylation (Fig. 8B) and p38 activity toward phosphorylation of ATF2 (Fig. 8C). U0126 did not reduce the p38 phosphorylation induced by active MKK3 or MKK6 (Fig. 8B), demonstrating that U0126 blocked ras-induced p38 activation by inhibiting MEK activity rather than by inhibiting MKK3/6 directly. Thus, the activation of p38 by oncogenic ras requires a functional MEK-ERK pathway. This finding suggests that in primary human fibroblasts p38 is not activated by oncogenic ras directly, but rather as a result of the ras-induced activation of the MEK-ERK pathway.
MKK3/6 mediates p38 activation by oncogenic ras and active MEK1.
We have demonstrated the stimulation of p38 activity as a result of MEK-ERK activation by oncogenic ras. p38 is activated through phosphorylation by its upstream kinases, MKK4, MKK3, and MKK6 (10, 21, 22, 32, 46, 47). We therefore investigated whether MKK4, MKK3, and MKK6 were involved in the activation of p38 by oncogenic ras and active MEK1.
In early-passaged BJ cells, MEK1Q56P and Ha-RasV12 induced the activating phosphorylation of MKK3/6 (on Ser189/Thr193 of MKK3 and on Ser207/Thr211 of MKK6, respectively) (21, 46, 47) (Fig. 9A). On the other hand, while treatment of BJ cells with NaCl led to strong activation of MKK4, Ha-RasV12 and MEK1Q56P did not stimulate the activating phosphorylation of MKK4 (Fig. 9A). The ras-induced increases in the levels of active MKK3/6 depended on active MEK, because inhibition of MEK activity with U0126 abolished the ability of Ha-RasV12 to induce the accumulation of phospho-MKK3/6 (Fig. 9B). Consistent with the role of MEK in MKK3/6 activation, U0126 also blocked MKK3/6 phosphorylation induced by MEK1Q56P (Fig. 9B). Therefore, MKK3/6 can be activated by ras in a MEK-dependent fashion during the onset of premature senescence. Our data suggested that MKK3/6 could be important in mediating p38 activation in response to oncogenic ras and active MEK and that MKK4 was probably not involved in this pathway.
FIG. 9.
MKK3/6 mediates the activation of p38 by oncogenic ras and active MEK1. (A) Ha-RasV12 and MEK1Q56P induced the accumulation of active MKK3/6 but had no effect on the levels of active MKK4. The levels of phospho-MKK3/6 (P-MKK3/6), actin, phospho-MKK4 (P-MKK4), and MKK4 were determined by Western blotting 10 days after the transduction of BJ cells at PD 33 with a vector control (BP), oncogenic Ras (HaRasV12), or constitutively active MEK1 (MEK1Q56P). The levels of phospho-MKK4 (P-MKK4) and MKK4 were also determined in BJ cells (PD 26) treated with 700 mM NaCl for 15 min (NaCl) or left untreated (−). (B) Up-regulation of phospho-MKK3/6 by Ha-RasV12 and MEK1Q56P relied on MEK activity. The levels of phospho-MKK3/6 (P-MKK3/6) and actin were determined by Western blotting 7 days after the transduction of BJ cells at PD 39 with a vector control (BP), Ha-RasV12 (Ras), or MEK1Q56P (MEK1) in the presence of vehicle control (Con) or U0126 (U). (C) MKK3 is required for p38 activation by oncogenic ras and active MEK1. The levels of phospho-p38 (P-p38) and MKK3 were determined by Western blot analysis in BJ cells expressing a dominant-negative allele of MKK3 (MKK3A) or a vector control (BH) 8 days after transduction at PD 33 with Ha-RasV12 (Ras), MEK1Q56P (MEK1), or a vector control.
The involvement of MKK3/6 in ras- and MEK-mediated p38 activation was further demonstrated using dominant-negative mutants of MKK3 and MKK6. These inactive mutants (MKK3A and MKK6A) were constructed by substitution of the two serine/threonine residues in the dual phosphorylation sites with alanine (23). In BJ cells expressing the dominant-negative allele of MKK3, MKK3A, the ability of Ha-RasV12 and MEK1Q56P to induce the activating phosphorylation of p38 was significantly reduced (Fig. 9C). Similar results were obtained in BJ cells transduced with MKK6A (data not shown). These studies confirmed the essential roles of MKK3 and MKK6 in mediating ras- and MEK-induced activation of p38. Taken together, our findings argue that MEK, when turned on by oncogenic Ras, may stimulate p38 activity by activating MKK3/MKK6 in BJ human fibroblasts.
Activation of MEK-ERK by ras precedes the activation of MKK3/6-p38 and senescence.
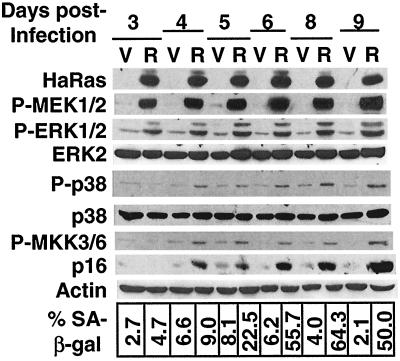
The finding that the MKK3/6-p38 pathway is activated as a result of the activation of the MEK-ERK pathway by oncogenic ras suggests that activation of the MEK-ERK pathway may precede that of the MKK3/6-p38 pathway following the overexpression of ras. Such a temporal difference will be more obvious if the activation of the MKK3/6-p38 pathway by MEK is not achieved through direct signaling but instead involves indirect, slow routes such as transcriptional regulation or other mechanisms that require novel protein synthesis. To determine the timing of the activation of the MEK-ERK and MKK3/6-p38 pathways, we followed the time course of the status of these two pathways, starting from day 3 through day 9 after the initial transduction of BJ cells with Ha-RasV12 (Fig. 10). By day 3, MEK and ERK had already been strongly activated. Since it minimally took 3 days to obtain a pure cell population transduced with ras, we were unable to monitor the status of MEK and ERK before day 3. However, because phospho-MEK and phospho-ERK were already at their maximum levels by day 3, it was likely that the MEK-ERK pathway was activated by ras well before day 3, which would be consistent with the direct activation of MEK-ERK by ras. On the contrary, phosphorylation of MKK3/6 and that of p38 were not induced by ras until day 4. We did not observe any time gap between the induction of phospho-MKK3/6 and that of phospho-p38, suggesting that p38 was activated by MKK3/6 directly. The p16 protein was induced moderately at day 4 and then to higher levels after day 6. Cells displaying the SA-β-gal senescence marker started to accumulate at day 5 and reached the maximum percentage within the population at day 6.
FIG. 10.
Activation of MEK-ERK precedes the activation of MKK3/6-p38 and premature senescence. The levels of Ha-Ras, phospho-MEK1 and 2 (P-MEK1/2), phospho-ERK1 and 2 (P-ERK1/2), ERK2, phospho-p38 (P-p38), p38, phospho-MKK3 and 6 (P-MKK3/6), p16INK4A, and actin were determined by Western blotting after different days (days 3 to 9) following the transduction of BJ cells at PD 35 with a vector control (V) or Ha-RasV12 (R). The percentages of cells positive for SA-β-gal within these same cell populations are shown at the bottom.
These data demonstrated that the MEK-ERK pathway was activated earlier by ras than the MKK3/6-p38 pathway was and that the accumulation of p16 and the appearance of the senescence phenotype occurred even later than the activation of p38. The results support our conclusion that p38, when activated by ras as a result of sustained MEK-ERK activation, triggers premature senescence.
Senescence induced by ras and MEK1 both require active p38.
The dependence of p38 activation by ras on active MEK-ERK, together with the finding that active p38 leads to senescence, suggests that the sequential activation of MEK-ERK and MKK3/6-p38 by ras may be responsible for the ability of oncogenic ras to induce premature senescence in primary cells. To explore this possibility, we analyzed premature senescence induced by Ha-RasV12, MEK1Q56P, MKK3E, or MKK6E under conditions when MEK or p38 was specifically blocked. Specific inhibition of the p38 activity with SB203580 prevented premature senescence induced by Ha-RasV12, MEK1Q56P, MKK3E, and MKK6E. In contrast, treatment with U0126, which blocks activation of the MEK-ERK pathway, only rescued senescence caused by expression of Ha-RasV12 and MEK1Q56P but not that by constitutively active MKK3 or MKK6 (Fig. 11). Therefore, both oncogenic ras-induced and active MEK1-induced senescence relies on an active p38 pathway, while constitutively activated p38 can cause premature senescence without the activity of MEK-ERK.
These results have placed MKK3/6-p38 downstream of MEK-ERK concerning the execution of senescence elicited by ras, and thus they have defined a pathway leading from oncogenic ras expression to premature senescence. Oncogenic ras activates the MEK-ERK pathway, which in turn induces the phosphorylation and activation of MKK3/6 and p38. Activated p38 then provokes premature senescence by inducing the accumulation of growth inhibitors such as p16INK4A. Confirming this notion, senescence induced by active MKK3 and MKK6 consistently occurred earlier than that induced by Ha-RasV12 or active MEK1. In growth curve assays, Ras- or MEK1-expressing cells retained substantial levels of growth during the first 3 days (day 5 to 8 postinfection) compared to control cells, and they underwent massive growth arrest afterwards (Fig. 1A and 11). In contrast, cells transduced with MKK3E or MKK6E almost completely ceased to proliferate within the first 3 days of the assays. In fact, the growth inhibition was already noticeable in MKK3E- and MKK6E-expressing cells during puromycin selection (1 to 2 day postinfection), even before the growth curve assays began. The direct activation of p38 by exogenously expressed, active MMK3 or MKK6 might have bypassed the need for the activation of endogenous MKK3/6 proteins by MEK or ras.
DISCUSSION
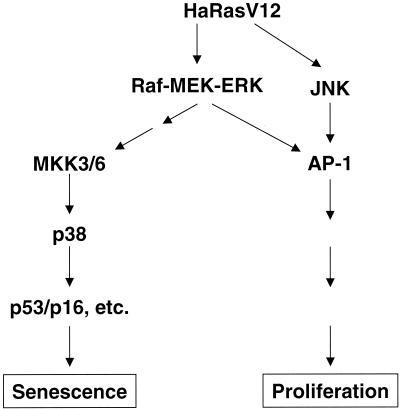
Oncogenic ras-induced activation of the MEK-ERK pathway, which usually confers growth stimulation, leads to premature senescence that serves as a tumor-suppressing response in normal primary cells (36). In tumor cells or cell lines where premature senescence has been compromised, oncogenic ras or a constitutively active MEK-ERK pathway only promotes cell growth and leads to transformation (36). It has been unclear how activation of the mitogenic MEK-ERK pathway by oncogenic ras can lead to senescence. In this study, we have demonstrated that activation of the MEK-ERK pathway by ras leads to the activation of another MAPK, p38, and that activated p38 in turn induces the accumulation of growth inhibitors and premature senescence. Thus, while the ras-activated MEK-ERK cascade may transduce mitogenic signals through transcription factor AP-1 (31), it provokes premature senescence by activating the p38 pathway in normal, primary cells (Fig. 12). These findings have identified within the ras signaling cascade a branch that mediates premature senescence, and thus they have provided an explanation to how ras achieves both positive and negative growth regulation. Our data also suggest that one possible way for a tumor to bypass ras-induced premature senescence and achieve oncogenic transformation is to acquire genetic mutations that nullify the activation of the p38 pathway.
FIG. 12.
Schematic representation of a model indicating the role of p38 in oncogenic ras-induced premature senescence in primary fibroblasts. Activation of the Raf-MEK-ERK pathway by oncogenic ras confers cell proliferation through transcription factor AP-1, as suggested in previously published literature, and concurrently leads to premature senescence by stimulating the activity of the p38 pathway, whose activation is sufficient to induce the accumulation of p53 and p16INK4A.
The p38 pathway mediates responses to environmental stresses, including DNA-damaging agents such as UV and γ-irradiation (43, 46, 58). A recent study reported that Ha-RasV12 induced premature senescence by increasing the intracellular levels of reactive oxygen species (34). Since reactive oxygen species induce DNA damage, it is possible that in primary BJ fibroblasts p38 is activated as a result of DNA damage due to prolonged oxidative stress induced by oncogenic ras. If this notion is confirmed, premature senescence may serve as a DNA damage check point that is enforced by the activated p38 pathway in primary fibroblasts.
MEK-ERK and MKK3/6-p38 constitute two independent MAPK pathways that mediate responses to different extracellular stimuli (6). Our results have revealed an interaction between these two pathways, concerning the effects of oncogenic ras in primary cells. Like oncogenic Ras, constitutively active MEK1 induced the accumulation of active MKK3/6, the activation of p38 and, subsequently, the occurrence of premature senescence. Activation of MKK3/6 and p38 by Ha-rasV12 requires an activated MEK-ERK pathway. In addition, the ability of active MEK1 to mediate ras-induced premature senescence relies on the activation of p38. These observations have defined a linear pathway involving the sequential activation of MEK-ERK and MKK3/6-p38 by ras, which mediates premature senescence in primary human fibroblasts.
Several lines of evidence suggest that oncogenic ras and active MEK-ERK do not activate the MKK3/6-p38 pathway through direct signaling. First of all, the activation of MKK3/6 and p38 occurred after 4 days following oncogenic ras overexpression, lagging that of MEK and ERK by at least 1 to 2 days (Fig. 10). This observation is inconsistent with a mechanism of direct activation, whose effects would be achieved within hours if not minutes after the initiation of the signal. Instead, MKK3/6 and p38 may be activated as a result of the sustained presence of an active MEK-ERK cascade. Confirming the direct activation of p38 by MKK3/6, increases in the phosphorylation of MKK3/6 and p38 were observed simultaneously following ras gene transduction. In addition, premature senescence is a late response of normal cells to oncogenic activation of ras. Growth arrest induced by Ha-rasV12 or active MEK1 was not apparent until 7 to 10 days after the initial transduction of these genes (Fig. 1A and 11). This agrees with a model in which the trigger of ras- or MEK-induced senescence is activated through an indirect, slow route. Also supporting the indirect activation model, active MKK3 or MKK6 induced senescence much more rapidly than oncogenic ras or active MEK1 (Fig. 11). Growth inhibition was already noticeable 1 to 2 days after the transduction of active MKK3 or MKK6. The ectopic expression of active MKK3 or MKK6 might have bypassed the slow route that was required for MEK to activate MKK3/6 and thus may have speeded up the senescence process.
A similar reliance of p38 activation by ras on MEK-ERK has been observed in immortalized NIH 3T3 fibroblasts with another oncogenic ras gene, Ha-rasL61, although oncogenic ras does not induce senescence in these cells (8). The expression of an active MEK also activated p38 in PC12 cells, where p38 activation is required for neuronal differentiation induced by nerve growth factor (42). Therefore, the sequential activation of the MEK-ERK and MKK3/6-p38 pathways may also operate in other cell types and may mediate cellular processes other than premature senescence.
Premature senescence is accompanied, and thus may be mediated by, the accumulation of growth inhibitors such as p16INK4A and p53. We found that activation of p38 is sufficient to induce increases in the protein levels of these genes. In the case of p16INK4A, activation of p38 by MKK3 or MKK6 led to increases in its mRNA levels. Activated p38 is known to regulate gene expression by increasing mRNA stability (24, 39, 63). Therefore, it is conceivable that activation of p38 by ras may result in the stabilization of p16INK4A and p53 mRNA, leading to the accumulation of p16INK4A and p53 proteins and, eventually, premature senescence.
Acknowledgments
We are grateful to Peter Wright and Richard Lerner for support. We thank R. A. Weinberg, D. Beach, S. Lowe, J. Smith, and J. Han for providing reagents and Kathrina Pestano for administrative assistance.
P.S. is supported by a start-up fund from the Scripps Research Institute and is a New Scholar of the Ellison Medical Foundation. S.H. is supported by grants from the Department of Defense and California Cancer Research Program.
Footnotes
This is Scripps manuscript no. 14467-MB.
REFERENCES
- 1.Barbacid, M. 1987. ras genes. Annu. Rev. Biochem. 56:779-827. [DOI] [PubMed] [Google Scholar]
- 2.Bodnar, A. G., M. Ouellette, M. Frolkis, S. E. Holt, C. P. Chiu, G. B. Morin, C. B. Harley, J. W. Shay, S. Lichtsteiner, and W. E. Wright. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279:349-352. [DOI] [PubMed] [Google Scholar]
- 3.Bos, J. L. 1988. The ras gene family and human carcinogenesis. Mutat. Res. 195:255-271. [DOI] [PubMed] [Google Scholar]
- 4.Bos, J. L. 1989. ras oncogenes in human cancer: a review. Cancer Res. 49:4682-4689. [PubMed] [Google Scholar]
- 5.Bottorff, D., S. Stang, S. Agellon, and J. C. Stone. 1995. RAS signaling is abnormal in a c-raf1 MEK1 double mutant. Mol. Cell. Biol. 15:5113-5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cahill, M. A., R. Janknecht, and A. Nordheim. 1996. Signalling pathways: jack of all cascades. Curr. Biol. 6:16-19. [DOI] [PubMed] [Google Scholar]
- 7.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. [DOI] [PubMed] [Google Scholar]
- 8.Chen, G., M. Hitomi, J. Han, and D. W. Stacey. 2000. The p38 pathway provides negative feedback for Ras proliferative signaling. J. Biol. Chem. 275:38973-38980. [DOI] [PubMed] [Google Scholar]
- 9.Cuenda, A., J. Rouse, Y. N. Doza, R. Meier, P. Cohen, T. F. Gallagher, P. R. Young, and J. C. Lee. 1995. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 364:229-233. [DOI] [PubMed] [Google Scholar]
- 10.Derijard, B., J. Raingeaud, T. Barrett, I. H. Wu, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science 267:682-685. [DOI] [PubMed] [Google Scholar]
- 11.DeSilva, D. R., E. A. Jones, M. F. Favata, B. D. Jaffee, R. L. Magolda, J. M. Trzaskos, and P. A. Scherle. 1998. Inhibition of mitogen-activated protein kinase kinase blocks T cell proliferation but does not induce or prevent anergy. J. Immunol. 160:4175-4181. [PubMed] [Google Scholar]
- 12.Dimri, G. P., X. Lee, G. Basile, M. Acosta, G. Scott, C. Roskelley, E. E. Medrano, M. Linskens, I. Rubelj, O. Pereira-Smith, et al. 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 92:9363-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elenbaas, B., L. Spirio, F. Koerner, M. D. Fleming, D. B. Zimonjic, J. L. Donaher, N. C. Popescu, W. C. Hahn, and R. A. Weinberg. 2001. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 15:50-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellinger-Ziegelbauer, H., K. Kelly, and U. Siebenlist. 1999. Cell cycle arrest and reversion of Ras-induced transformation by a conditionally activated form of mitogen-activated protein kinase kinase kinase 3. Mol. Cell. Biol. 19:3857-3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enslen, H., J. Raingeaud, and R. J. Davis. 1998. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J. Biol. Chem. 273:1741-1748. [DOI] [PubMed] [Google Scholar]
- 16.Eyers, P. A., M. Craxton, N. Morrice, P. Cohen, and M. Goedert. 1998. Conversion of SB 203580-insensitive MAP kinase family members to drug-sensitive forms by a single amino-acid substitution. Chem. Biol. 5:321-328. [DOI] [PubMed] [Google Scholar]
- 17.Favata, M. F., K. Y. Horiuchi, E. J. Manos, A. J. Daulerio, D. A. Stradley, W. S. Feeser, D. E. Van Dyk, W. J. Pitts, R. A. Earl, F. Hobbs, R. A. Copeland, R. L. Magolda, P. A. Scherle, and J. M. Trzaskos. 1998. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273:18623-18632. [DOI] [PubMed] [Google Scholar]
- 18.Graves, L. M., H. I. Guy, P. Kozlowski, M. Huang, E. Lazarowski, R. M. Pope, M. A. Collins, E. N. Dahlstrand, H. S. Earp III, and D. R. Evans. 2000. Regulation of carbamoyl phosphate synthetase by MAP kinase. Nature 403:328-332. [DOI] [PubMed] [Google Scholar]
- 19.Hahn, W. C., C. M. Counter, A. S. Lundberg, R. L. Beijersbergen, M. W. Brooks, and R. A. Weinberg. 1999. Creation of human tumour cells with defined genetic elements. Nature 400:464-468. [DOI] [PubMed] [Google Scholar]
- 20.Han, J., J. D. Lee, L. Bibbs, and R. J. Ulevitch. 1994. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265:808-811. [DOI] [PubMed] [Google Scholar]
- 21.Han, J., J. D. Lee, Y. Jiang, Z. Li, L. Feng, and R. J. Ulevitch. 1996. Characterization of the structure and function of a novel MAP kinase kinase (MKK6). J. Biol. Chem. 271:2886-2891. [DOI] [PubMed] [Google Scholar]
- 22.Han, J., X. Wang, Y. Jiang, R. J. Ulevitch, and S. Lin. 1997. Identification and characterization of a predominant isoform of human MKK3. FEBS Lett. 403:19-22. [DOI] [PubMed] [Google Scholar]
- 23.Huang, S., Y. Jiang, Z. Li, E. Nishida, P. Mathias, S. Lin, R. J. Ulevitch, G. R. Nemerow, and J. Han. 1997. Apoptosis signaling pathway in T cells is composed of ICE/Ced-3 family proteases and MAP kinase kinase 6b. Immunity 6:739-749. [DOI] [PubMed] [Google Scholar]
- 24.Huang, S., L. New, Z. Pan, J. Han, and G. R. Nemerow. 2000. Urokinase plasminogen activator/urokinase-specific surface receptor expression and matrix invasion by breast cancer cells requires constitutive p38α mitogen-activated protein kinase activity. J. Biol. Chem. 275:12266-12272. [DOI] [PubMed] [Google Scholar]
- 25.Hunter, T. 1991. Cooperation between oncogenes. Cell 64:249-270. [DOI] [PubMed] [Google Scholar]
- 26.Jiang, Y., C. Chen, Z. Li, W. Guo, J. A. Gegner, S. Lin, and J. Han. 1996. Characterization of the structure and function of a new mitogen-activated protein kinase (p38β). J. Biol. Chem. 271:17920-17926. [DOI] [PubMed] [Google Scholar]
- 27.Jiang, Y., H. Gram, M. Zhao, L. New, J. Gu, L. Feng, F. Di Padova, R. J. Ulevitch, J. Han, J. Han, J. D. Lee, L. Bibbs, and R. J. Ulevitch. 1997. Characterization of the structure and function of the fourth member of p38 group mitogen-activated protein kinases, p38δA MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. J. Biol. Chem. 272:30122-30128. [DOI] [PubMed] [Google Scholar]
- 28.Jiang, Y., Z. Li, E. M. Schwarz, A. Lin, K. Guan, R. J. Ulevitch, and J. Han. 1997. Structure-function studies of p38 mitogen-activated protein kinase. Loop 12 influences substrate specificity and autophosphorylation, but not upstream kinase selection. J. Biol. Chem. 272:11096-11102. [DOI] [PubMed] [Google Scholar]
- 29.Joneson, T., M. A. White, M. H. Wigler, and D. Bar-Sagi. 1996. Stimulation of membrane ruffling and MAP kinase activation by distinct effectors of RAS. Science 271:810-812. [DOI] [PubMed] [Google Scholar]
- 30.Kallunki, T., T. Deng, M. Hibi, and M. Karin. 1996. c-Jun can recruit JNK to phosphorylate dimerization partners via specific docking interactions. Cell 87:929-939. [DOI] [PubMed] [Google Scholar]
- 31.Karin, M. 1995. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 270:16483-16486. [DOI] [PubMed] [Google Scholar]
- 32.Kyriakis, J. M., and J. Avruch. 1996. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays 18:567-577. [DOI] [PubMed] [Google Scholar]
- 33.Lavoie, J. N., G. L'Allemain, A. Brunet, R. Muller, and J. Pouyssegur. 1996. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J. Biol. Chem. 271:20608-20616. [DOI] [PubMed] [Google Scholar]
- 34.Lee, A. C., B. E. Fenster, H. Ito, K. Takeda, N. S. Bae, T. Hirai, Z. X. Yu, V. J. Ferrans, B. H. Howard, and T. Finkel. 1999. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J. Biol. Chem. 274:7936-7940. [DOI] [PubMed] [Google Scholar]
- 35.Li, Z., Y. Jiang, R. J. Ulevitch, and J. Han. 1996. The primary structure of p38 gamma: a new member of p38 group of MAP kinases. Biochem. Biophys. Res. Commun. 228:334-340. [DOI] [PubMed] [Google Scholar]
- 36.Lin, A. W., M. Barradas, J. C. Stone, L. Van Aelst, M. Serrano, and S. W. Lowe. 1998. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 12:3008-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medema, R. H., and J. L. Bos. 1993. The role of p21ras in receptor tyrosine kinase signaling. Crit. Rev. Oncog. 4:615-661. [PubMed] [Google Scholar]
- 38.Minden, A., A. Lin, M. McMahon, C. Lange-Carter, B. Derijard, R. J. Davis, G. L. Johnson, and M. Karin. 1994. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science 266:1719-1723. [DOI] [PubMed] [Google Scholar]
- 39.Miyazawa, K., A. Mori, H. Miyata, M. Akahane, Y. Ajisawa, and H. Okudaira. 1998. Regulation of interleukin-1β-induced interleukin-6 gene expression in human fibroblast-like synoviocytes by p38 mitogen-activated protein kinase. J. Biol. Chem. 273:24832-24838. [DOI] [PubMed] [Google Scholar]
- 40.Molnar, A., A. M. Theodoras, L. I. Zon, and J. M. Kyriakis. 1997. Cdc42Hs, but not Rac1, inhibits serum-stimulated cell cycle progression at G1/S through a mechanism requiring p38/RK. J. Biol. Chem. 272:13229-13235. [DOI] [PubMed] [Google Scholar]
- 41.Moriguchi, T., F. Toyoshima, N. Masuyama, H. Hanafusa, Y. Gotoh, and E. Nishida. 1997. A novel SAPK/JNK kinase, MKK7, stimulated by TNFα and cellular stresses. EMBO J. 16:7045-7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morooka, T., and E. Nishida. 1998. Requirement of p38 mitogen-activated protein kinase for neuronal differentiation in PC12 cells. J. Biol. Chem. 273:24285-24288. [DOI] [PubMed] [Google Scholar]
- 43.Nebreda, A. R., and A. Porras. 2000. p38 MAP kinases: beyond the stress response. Trends Biochem. Sci. 25:257-260. [DOI] [PubMed] [Google Scholar]
- 44.Olson, M. F., A. Ashworth, and A. Hall. 1995. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science 269:1270-1272. [DOI] [PubMed] [Google Scholar]
- 45.Palmer, A., A. C. Gavin, and A. R. Nebreda. 1998. A link between MAP kinase and p34(cdc2)/cyclin B during oocyte maturation: p90(rsk) phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1. EMBO J. 17:5037-5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raingeaud, J., S. Gupta, J. S. Rogers, M. Dickens, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 270:7420-7426. [DOI] [PubMed] [Google Scholar]
- 47.Raingeaud, J., A. J. Whitmarsh, T. Barrett, B. Derijard, and R. J. Davis. 1996. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol. Cell. Biol. 16:1247-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez-Viciana, P., P. H. Warne, R. Dhand, B. Vanhaesebroeck, I. Gout, M. J. Fry, M. D. Waterfield, and J. Downward. 1994. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 370:527-532. [DOI] [PubMed] [Google Scholar]
- 49.Ruley, H. E. 1990. Transforming collaborations between ras and nuclear oncogenes. Cancer Cells 2:258-268. [PubMed] [Google Scholar]
- 50.Saklatvala, J., W. Davis, and F. Guesdon. 1996. Interleukin 1 (IL1) and tumour necrosis factor (TNF) signal transduction. Philos. Trans. R. Soc. London B 351:151-157. [DOI] [PubMed] [Google Scholar]
- 51.Serrano, M., A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593-602. [DOI] [PubMed] [Google Scholar]
- 52.Shay, J. W., and W. E. Wright. 1989. Quantitation of the frequency of immortalization of normal human diploid fibroblasts by SV40 large T-antigen. Exp. Cell Res. 184:109-118. [DOI] [PubMed] [Google Scholar]
- 53.Spaargaren, M., and J. R. Bischoff. 1994. Identification of the guanine nucleotide dissociation stimulator for Ral as a putative effector molecule of R-ras, H-ras, K-ras, and Rap. Proc. Natl. Acad. Sci. USA 91:12609-12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun, P., P. Dong, K. Dai, G. J. Hannon, and D. Beach. 1998. p53-independent role of MDM2 in TGF-β1 resistance. Science 282:2270-2272. [DOI] [PubMed] [Google Scholar]
- 55.Treinies, I., H. F. Paterson, S. Hooper, R. Wilson, and C. J. Marshall. 1999. Activated MEK stimulates expression of AP-1 components independently of phosphatidylinositol 3-kinase (PI3-kinase) but requires a PI3-kinase signal to stimulate DNA synthesis. Mol. Cell. Biol. 19:321-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Aelst, L., M. Barr, S. Marcus, A. Polverino, and M. Wigler. 1993. Complex formation between RAS and RAF and other protein kinases. Proc. Natl. Acad. Sci. USA 90:6213-6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogelstein, B., and K. W. Kinzler. 1993. The multistep nature of cancer. Trends Genet. 9:138-141. [DOI] [PubMed] [Google Scholar]
- 58.Wang, X., C. H. McGowan, M. Zhao, L. He, J. S. Downey, C. Fearns, Y. Wang, S. Huang, and J. Han. 2000. Involvement of the MKK6-p38γ cascade in γ-radiation-induced cell cycle arrest. Mol. Cell. Biol. 20:4543-4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang, Y., S. Huang, V. P. Sah, J. Ross, Jr., J. H. Brown, J. Han, and K. R. Chien. 1998. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J. Biol. Chem. 273:2161-2168. [DOI] [PubMed] [Google Scholar]
- 60.Wei, S., S. Wei, and J. M. Sedivy. 1999. Expression of catalytically active telomerase does not prevent premature senescence caused by overexpression of oncogenic Ha-Ras in normal human fibroblasts. Cancer Res. 59:1539-1543. [PubMed] [Google Scholar]
- 61.Weinberg, R. A. 1989. Oncogenes, antioncogenes, and the molecular bases of multistep carcinogenesis. Cancer Res. 49:3713-3721. [PubMed] [Google Scholar]
- 62.Whitmarsh, A. J., S. H. Yang, M. S. Su, A. D. Sharrocks, and R. J. Davis. 1997. Role of p38 and JNK mitogen-activated protein kinases in the activation of ternary complex factors. Mol. Cell. Biol. 17:2360-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winzen, R., M. Kracht, B. Ritter, A. Wilhelm, C. Y. Chen, A. B. Shyu, M. Muller, M. Gaestel, K. Resch, and H. Holtmann. 1999. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 18:4969-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu, J., D. Woods, M. McMahon, and J. M. Bishop. 1998. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 12:2997-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]