Abstract
In the past few years, many nuclear receptor coactivators have been identified and shown to be an integral part of receptor action. The most frequently studied of these coactivators are members of the steroid receptor coactivator (SRC) family, SRC-1, TIF2/GRIP1/SRC-2, and pCIP/ACTR/AIB-1/RAC-3/TRAM-1/SRC-3. In this report, we describe the biochemical purification of SRC-1 and SRC-3 protein complexes and the subsequent identification of their associated proteins by mass spectrometry. Surprisingly, we found association of SRC-3, but not SRC-1, with the IκB kinase (IKK). IKK is known to be responsible for the degradation of IκB and the subsequent activation of NF-κB. Since NF-κB plays a key role in host immunity and inflammatory responses, we therefore investigated the significance of the SRC-3-IKK complex. We demonstrated that SRC-3 was able to enhance NF-κB-mediated gene expression in concert with IKK. In addition, we showed that SRC-3 was phosphorylated by the IKK complex in vitro. Furthermore, elevated SRC-3 phosphorylation in vivo and translocation of SRC-3 from cytoplasm to nucleus in response to tumor necrosis factor alpha occurred in cells, suggesting control of subcellular localization of SRC-3 by phosphorylation. Finally, the hypothesis that SRC-3 is involved in NF-κB-mediated gene expression is further supported by the reduced expression of interferon regulatory factor 1, a well-known NF-κB target gene, in the spleens of SRC-3 null mutant mice. Taken together, our results not only reveal the IKK-mediated phosphorylation of SRC-3 to be a regulated event that plays an important role but also substantiate the role of SRC-3 in multiple signaling pathways.
The nuclear receptor (NR) superfamily is a large class of ligand-dependent transcription factors that play pivotal roles in a wide spectrum of biological processes such as development, reproduction, and homeostasis (32, 51). In the past few years, many nuclear receptor coactivators have been identified, including the steroid receptor coactivator (SRC) family. Members of the SRC family, which include SRC-1, SRC-2/GRIP1/TIF2, and SRC-3/ACTR/AIB-1/pCIP/RAC3/TRAM-1, interact with nuclear receptors and enhance their transactivation in a ligand-dependent manner (3, 11, 21, 25, 27, 29, 38, 49, 50, 52). Two members of the SRC family, SRC-1 and SRC-3, are also known to contain histone acetyltransferase activity (11, 47), and all three members contain an intrinsic transcriptional activation function when tethered to the GAL4 DNA-binding domain (29, 37, 52). Additionally, it has recently been reported that SRC-1 and SRC-3 are phosphoproteins (18, 45) and that the activity of SRC-3 is attenuated by acetylation (12). However, it is unclear how these posttranslational modifications might be regulated and what their actual roles are.
Nuclear factor κB (NF-κB) is a family of signal-inducible transcription factors whose members, including p50, p52, p65 (RelA), c-Rel, and RelB, play an essential role in the regulation of genes involved in inflammatory responses and cell survival (4, 5, 39). All NF-κB family members share a region known as the Rel homology domain that is responsible for DNA binding, nuclear translocation, and dimerization. In most cells, NF-κB exists in an inactive state in which it is sequestered in the cytoplasm by binding to an inhibitory protein, IκB. In response to many activating signals such as tumor necrosis factor alpha (TNF-α) and interleukin-1, IκB is phosphorylated by a multisubunit IκB kinase (IKK) complex and marked for rapid degradation by the 26S proteasome (1, 6, 13, 14, 16). Degradation of IκB results in the release of NF-κB. Once released from the inhibitory IκB complex, NF-κB translocates to the nucleus, where it activates expression of target genes. Although NF-κB activation requires multiple steps, IκB phosphorylation by IKK is the primary regulatory step of this process. Since NF-κB is the key activator of genes involved in the host immune and inflammatory responses, IKK has been suggested to be the master regulator of host defense system because of its ability to regulate NF-κB activity (24, 57).
Given the critical roles of NR and NF-κB in gene regulation, it is interesting that cross talk between these two pathways through sharing of some common coactivator proteins has been reported (46). More specifically, it was shown that SRC-1, SRC-3, and CBP can each interact with NF-κB and enhance its transcriptional activity (20, 23, 35, 40, 46, 55, 63). These results suggest that the functions of these coactivators are not limited to NRs but are involved in diverse biological processes.
Herein we describe the purification of the native complexes of two SRC family members, SRC-1 and SRC-3, and the subsequent identification of their associated proteins. We found that IKK was associated exclusively with SRC-3 and not SRC-1 complexes. As a result, we found that SRC-3 is able to greatly enhance NF-κB-mediated gene expression in concert with IKK. In addition, we demonstrated the in vitro phosphorylation of SRC-3 by the two catalytic subunits of the IKK complex, IKKα and IKKβ. Additionally, elevated phosphorylation and increased nuclear translocation of SRC-3 were also detected in vivo in response to treatment with TNF-α, a well-known activator of IKK activity. Finally, we showed an increased expression of proinflammatory caspases (10) in SRC-3-overexpressing cells and a reduced expression level of interferon regulatory factor 1 (IRF-1) in the spleens of SRC-3 null mice, supporting the hypothesis of a physiological role of SRC-3 in immune response and a functional association of SRC-3 with the NF-κB pathway. Taken together, our results suggest that phosphorylation of SRC-3 by IKK might modulate SRC-3 activity and that SRC-3 plays an important role in diverse signaling pathways.
MATERIALS AND METHODS
Cell line and nuclear extract preparation.
For transfection, HeLa cells were routinely maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal bovine serum (FBS) and grown at 37°C in the presence of 5% CO2. For crude nuclear extract preparation, a large quantity (40 liters) of spinner HeLa S3 cells was purchased from the National Culture Center (Minneapolis, Minn.). Fractionation of HeLa cells grown in DMEM supplemented with 0.5% FBS was performed with NE-PER reagents (Pierce). Nuclear extract was prepared essentially as described previously (17).
LNCaP cells (obtained from the American Type Culture Collection) were grown in RPMI 1640 supplemented with 10% FBS and 1% penicillin and streptomycin. The regulator and target gene expression vectors were constructed by using a two-vector inducible transcription system as described previously (7). Briefly, the regulator, which consists of a GAL4 DNA binding domain, a progesterone receptor ligand binding domain with a 19-amino-acid deletion at the C terminal, and a p65 activation domain, was under the control of the cytomegalovirus promoter. The target gene expression vector (SRC-3) was under the control of the upstream activator sequence (UAS). LNCaP cells were transfected by using the expression vectors with lipofectamine (Gibco BRL and Life Technologies). Following transfection, cells were grown in medium containing G418 (400 mg/ml) and hygromycin B (200 mg/ml) to select for stable clones that expressed SRC-3 only in the presence of RU486 (mifepristone).
Purification of recombinant proteins and generation of antiserum.
The maltose-binding protein fusion proteins used for antibody production were expressed in bacteria and purified according to the manufacturer's instructions (New England Biolabs). All rabbit polyclonal antibodies were generated at the Josman Laboratory by immunizing the rabbits with the corresponding purified maltose-binding protein fusion proteins as antigens. The regions used to raise antibodies contained amino acids 1067 to 1441 and 582 to 842 for SRC-1 and SRC-3, respectively. The antibodies were purified over affinity columns with the corresponding recombinant protein as antigen.
Gel filtration, ion-exchange chromatography, and antibody affinity purification.
Gel filtration of HeLa nuclear extracts was performed as previously described (53). Trichloroacetic acid protein precipitation was performed following fractionation, and the protein samples were separated by sodium dodecyl sulfate-8% polyacrylamide gel electrophoresis (SDS-8% PAGE) and analyzed by Western blotting with the indicated antibodies. The Q Sepharose (Pharmacia) fractionation of HeLa nuclear extracts is illustrated in Fig. 1B. All antibody affinity resins were prepared by the binding and cross-linking of affinity-purified antibodies to protein A Sepharose beads (Sigma) as described previously (22). Affinity purification was carried out with the 0.3 M KCl Q Sepharose eluate. The samples were resolved by SDS-10% PAGE (Novex, San Diego, Calif.). Protein identification by mass spectrometry (MS) was performed as described previously (42).
FIG. 1.
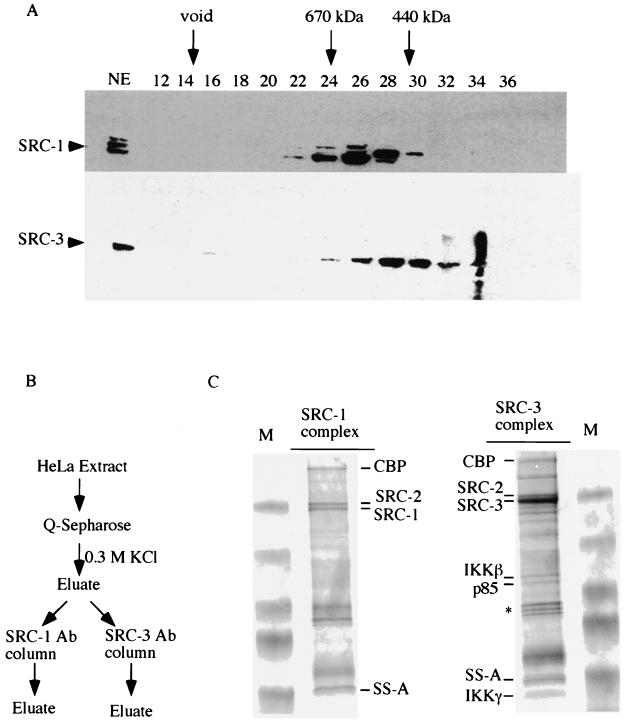
Purification of SRC complexes and analysis of associated proteins by MS. (A) HeLa cell nuclear extracts (NE) were fractionated on a Superose 6 sizing column. The presence of SRC-1 and SRC-3 in the indicated fractions was detected by Western blot analysis. Arrows indicate the positions of standard proteins of known molecular weights. Numbers at the top of the panel indicate the fraction number collected. (B) Schematic diagram of protein purification. Ab, antibody. (C) The immunocomplexes resulting from the purification process diagrammed in panel B were resolved by SDS-PAGE and stained with Coomassie blue. Both SRC-1 and SRC-3 complexes are shown. The identities of the indicated proteins from each complex were determined by MS. An asterisk indicates the contaminated heat shock proteins. M, molecular weight markers.
Identification of proteins by MS.
Protein sequencing using MS was carried out as described previously (36). Briefly, the Coomassie blue-stained protein band was digested in gel with trypsin and the recovered peptides were analyzed by using an electrospray ion trap mass spectrometer (LCQ Finnigan MAT; Thermo Finnigan, San Jose, Calif.) coupled on-line with a capillary high performance liquid chromatography apparatus (Magic 2002; Michrom BioResources, Auburn, Calif.) to acquire mass spectrometry-mass spectrometry spectra. Data derived from the mass spectrometry-mass spectrometry spectra were used to search a complied protein database that was composed of the protein database NR and a six-reading-frame translated expressed sequence tag database to identify the protein by using the PROWL program, which is publicly available on the World Wide Web (http://prowl.rockefeller.edu/).
Transfection and luciferase reporter gene assay.
Transfection of HeLa cells with lipofectamine was carried out according to the manufacturer's instructions (Life Technologies). Typically, all the cells were transfected with 1.1 μg of total plasmid DNA (a combination of 0.1 μg of NF-κB-luciferase and 0.5 μg of indicated expression vector or empty vector as filler DNA). Five hours after transfection, transfected cells were washed twice with phosphate-buffered saline and fresh DMEM was added for further incubation. Where indicated, the transfected cells were treated with recombinant human TNF-α (20 ng/ml; Roche Molecular Biochemicals) 24 h after transfection for 4 h. Cotransfection of HeLa cells with 5× UAS TATA luciferase reporter (0.1 μg) along with either GAL4 (0.1 μg) or GAL4-VP16 (0.1 μg) expression plasmid was carried out as described above. Whole cell lysates were prepared and assayed for luciferase activity as instructed by the manufacturer, and the activity was normalized against total protein (Promega, Madison, Wis.).
Translation and interaction in vitro .
Cotranslation of SRC-3 and IKK was performed in the presence of [35S]methionine by using a TNT kit (Promega) according to the manufacturer's instructions. In vitro pull-down assays were carried out by incubating the in vitro translated products with anti-HA antibody at 4°C for 1 h. After extensive washing, the products were analyzed by SDS-8% PAGE and the presence of proteins was detected by autoradiography.
Immunoprecipitation and Western blotting analysis.
Transfections were carried out essentially as described previously. The transfected cells were lysed in lysis buffer (20 mM Tris-HCl [pH 8.0], 125 mM NaCl, 0.5% NP-40, 2 mM EDTA, 0.2 mM NaF, 0.2 mM Na3VO4, protease inhibitor cocktail) for 30 min and the debris was cleared by centrifugation at 13,400 × g for 20 min at 4°C. For immunoprecipitation experiments, the lysate was incubated with 0.5 μg of antihemagglutinin (anti-HA; Roche Molecular Biochemicals) or anti-Flag (Sigma) antibody for 2 h on ice. The antibody was allowed to bind to protein A and G beads for 1 h and then washed extensively with lysis buffer. For Western blot analysis, the samples were resolved by SDS-8% PAGE and transferred to nitrocellulose membranes (Bio-Rad). The indicated antibodies were diluted in TBST buffer (50 mM Tris-HCl, 150 mM NaCl [pH 7.5], 0.1% Tween 20) and added to the membranes for 1 h at room temperature (RT) or overnight at 4°C followed by incubation with the appropriate horseradish peroxidase-conjugated secondary antibodies for 1 h at RT. All blots were developed with Supersignal substrate (Pierce) and visualized by chemiluminescence. Subsequent probing with different antibodies was made possible by stripping the membranes with buffer (62.5 mM Tris-HCl [pH 6.8], 2% SDS, 100 mM β-mercaptoethanol) at 55°C for 30 min.
In vitro protein kinase assay and in vivo labeling.
After extensive washing with protein lysis buffer, the immunoprecipitates were washed twice with kinase buffer (10 mM Tris-HCl [pH 7.5], 5 mM MgCl2, 1 mM dithiothreitol) prior to performing the kinase reaction. Flag-SRC-3 (30) was expressed in Xenopus oocytes by microinjecting the mRNA encoding Flag-SRC-3. The expressed Flag-SRC-3 protein was purified from the oocytes with anti-Flag (M2) beads according to the manufacturer's instructions (Sigma). The in vitro kinase reaction was performed by using purified Flag-SRC-3 as the substrate. The reaction was carried out in a final volume of 20 μl in the presence of [γ-32P]ATP (3.3 μCi) at RT for 30 min. The reaction with [γ-32P]ATP was stopped by adding 5× loading buffer, and the reaction mixture was boiled at 100°C and loaded onto SDS-10% PAGE. The gel was then dried for autoradiography. For labeling in vivo, HeLa cells were grown in phosphate-free DMEM supplemented with 5% dialyzed calf serum and metabolically labeled with orthophosphate (32Pi) according to established protocols. Briefly, the cells were labeled with 0.5 mCi of orthophosphate per ml for 6 h and TNF-α was added for the last hour of labeling. Afterward, the cells were washed with Tris-buffered saline (25 mM Tris-HCl [pH 7.4], 136.8 mM NaCl, 5 mM KCl, 0.9 mM CaCl2, 0.5 mM MgCl2, 0.7 mM Na2HPO4) and lysed with lysis buffer and immunoprecipitated with anti-SRC-3 or anti-SRC-1. The immunoprecipitates were resolved by SDS-PAGE, dried, and exposed to X-ray film.
RNA preparation and RNase protection assay.
Total RNA was isolated from the spleens of 4-week-old littermates or LNCaP cells with TRIzol reagent (Life Technologies) according to the manufacturer's instructions. Ten micrograms of total RNA was used in the RNase protection assay performed using the RNase protection assay (RPA) kit (Pharmingen). hAPO-1c multiprobe template (Pharmingen) and riboprobes for IRF-1 and cyclophilin were transcribed in vitro with T7 RNA polymerase.
RESULTS
Both SRC-1 and SRC-3 exist in protein complexes.
In order to gain more insight into the nature of NR-coactivator complexes, we used biochemical fractionation to ascertain the existence of such complexes. For this purpose, HeLa nuclear extracts were fractionated on a Superose 6 sizing column and the distribution of SRC-1 and SRC-3 in each fraction was analyzed by Western blotting. In agreement with previous results from our laboratory, SRC-1 was eluted in fractions with estimated sizes of 440 to 700 kDa (Fig. 1A). We found that the majority of SRC-3 was eluted in fractions overlapping with SRC-1 (28, 33), but the peak concentrations did not coincide (fractions 28 and 30). Additionally, a minor pool of SRC-3 existed in fractions with an estimated size of 1.5 MDa (Fig. 1A, lane 16). Since members of the SRC family of coactivators are about 160 kDa in size, these results indicate that both SRC-1 and SRC-3 proteins exist in large protein complexes.
We next used a combination of conventional gel filtration and antibody affinity chromatography to further purify these SRC-1- and SRC-3-containing complexes (Fig. 1B). After fractionation of HeLa nuclear extracts on a Q Sepharose column (Pharmacia), both SRC-1 and SRC-3 proteins were found to be present and enriched in the 0.3 M KCl eluate as determined by Western blot analysis (data not shown). This 0.3 M KCl fraction was subjected to further purification by SRC-1 and SRC-3 antibody affinity columns. Following purification on the antibody affinity columns, the resulting SRC-1 and SRC-3 protein complexes were resolved by SDS-10% PAGE and the identities of the associated proteins were determined by MS (42). As expected, SRC-1 and SRC-3 were eluted from the antibody columns, as confirmed by MS (Fig. 1C). In addition, the presence of SRC-2 in both SRC-1 and SRC-3 complexes is somewhat interesting, supporting the possibility of heterodimerization of SRC family members, as had been suggested previously (33). However, the authenticity of this dimerization awaits further verification, as the possible presence of small amounts of antibody cross-reactivity cannot be completely ruled out.
In addition, we also identified CBP and SS-A/Ro autoantigens as common components of both SRC-1 and SRC-3 complexes. CBP had been shown to interact with SRC family members (50), and the presence of CBP thus supports the validity of the use of antibody affinity purification as a means for identifying associated proteins.
SS-A/Ro contains putative zinc finger domains and a leucine zipper motif at its N-terminal half and has been shown to bind to DNA (9, 19). Its presence is of specific clinical interest, as antibodies to this protein are common in patients with rheumatic diseases, including systemic lupus erythematosus and Sjogren's syndrome (8, 41). The function of SS-A/Ro in the complexes is not yet known.
Most interestingly, two components of the IKK complex, IKKβ and IKKγ, were identified exclusively from the SRC-3, but not the SRC-1, protein complex. The IKK complex, composed of the two catalytic subunits IKKα and IKKβ and one regulatory IKKγ subunit (15, 34, 56, 62), has been shown to phosphorylate IκB in response to proinflammatory stimuli, such as TNF-α, bacterial lipopolysaccharides, or interleukin-1 (15, 34, 43, 56, 61, 62). Although the third component of the IKK complex, IKKα, was not initially identified by MS from the immunocomplex, a protein corresponding to the molecular mass of IKKα (p85) was visualized by Coomassie blue staining and was subsequently identified as IKKα by Western blot analysis. Taken together, our results identified the complete IKK enzyme as a component of the SRC-3 protein complex.
Association of SRC-3 and IKK.
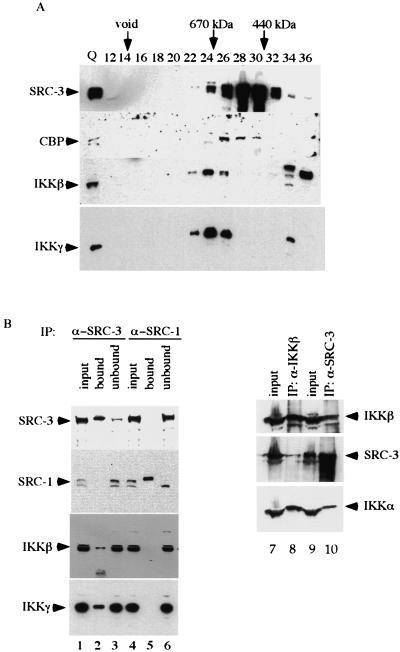
After determining the identities of these associated proteins, we wished to confirm the capacity of SRC-3 to associate with the identified proteins. Since cofractionation of proteins on a sizing column indicates possible protein association, we fractionated HeLa nuclear extracts on a Superose 6 gel column and analyzed the candidate proteins for coelution with SRC-3 by using Western blotting. As shown in Fig. 2A, CBP, a known SRC-3-interacting protein that was identified here as an SRC-3-associated protein by MS, was found also to cofractionate with the major SRC-3 fractions (fractions 26, 28, and 30) of SRC-3. In addition, the IKKβ and IKKγ subunits of the IKK complex cofractionated with the larger SRC-3 complex, suggesting an association of these proteins in a common complex. However, since the peaks of IKKβ and IKKγ (fraction 24) did not coincide with the main SRC-3 peak, our results indicate that the SRC-3 complex is heterogeneous in nature.
FIG. 2.
Confirmation of intracellular association of SRC-3 and IKK subunits. (A) HeLa cell nuclear extracts were fractionated as described in the legend for Fig. 1. The presence of the indicated proteins was determined by Western blot analysis. Numbers at the top of the panel indicate the fraction number collected. Q, Q Sepharose eluate. (B) HeLa cell nuclear extracts were immunoprecipitated with anti-SRC-1 or anti-SRC-3 antibodies. The immunoprecipitates were assayed by Western blot analysis with the indicated antibodies (left panel). For reciprocal coimmunoprecipitation, anti-IKKβ antibody was used in parallel with anti-SRC-3 antibody and the immunoprecipitates were assayed by Western blot analysis with the indicated antibodies (right panel). The input lanes represent 30% of the actual amount for IP. (C) HeLa cells were transfected with the indicated expression vectors. The cell lysates were prepared and subjected to immunoprecipitation with anti-Flag (M2) antibody. The immunoprecipitates were used for Western blot analysis with anti-HA antibody. (D) The cytoplasmic and nuclear extracts from HeLa cells were used for immunoprecipitation with anti-SRC-3 antibody. The presence of the IKK subunits in the immunoprecipitates was confirmed by Western blot analysis with specific antibodies. As a control for immunoprecipitation, the rabbit preimmune serum was used in parallel with anti-SRC-3 antibody and the immunoprecipitates were assayed by Western blot analysis with the indicated antibodies. The input lanes represent 30% of the actual amount for IP.
To further confirm the association of IKK with SRC-3, we analyzed the presence of IKKβ and IKKγ in the purified SRC-3 immunocomplex by using Western blot analysis. As expected, SRC-1 and SRC-3 were highly and specifically enriched by the immunopurification (Fig. 2B, lane 2 for SRC-3 and lane 5 for SRC-1). More importantly, we found that the SRC-3-specific antibody could coimmunoprecipitate (Co-IP) IKKβ and IKKγ, whereas the SRC-1-specific antibody failed to do so (Fig. 2B, left panel; compare lanes 2 and 5). In support of this finding, the IKKβ-specific antibody not only precipitated IKKβ and IKKα but also SRC-3 in a reciprocal Co-IP experiment (Fig. 2B, right panel, lane 8). As a control, rabbit preimmune serum was used and was found to be unable to precipitate SRC-3, IKKα, or IKKβ under similar conditions (Fig. 2D, lanes 6 and 7).
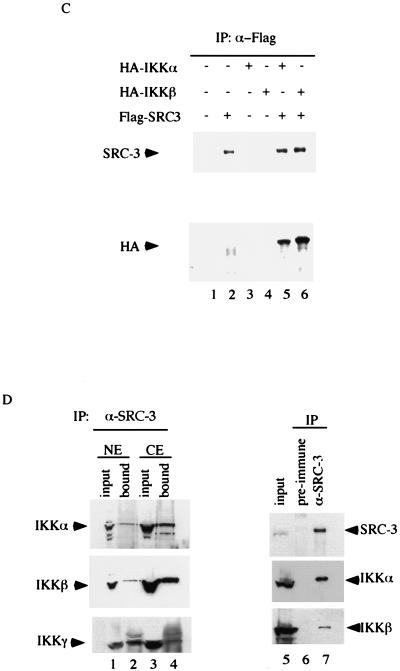
To further substantiate the association of SRC-3 with the IKK subunits in vivo, we tested their interactions by transiently coexpressing these proteins in HeLa cells. Following coexpression of Flag-tagged SRC-3 and HA-tagged IKKβ in HeLa cells, an immunoprecipitation (IP)-Western blot analysis was performed. As shown in Fig. 2C, cotransfection of Flag-SRC-3 and HA-IKKβ, followed by IP with anti-Flag antibody, resulted in the Co-IP of IKKβ with SRC-3, as demonstrated by Western blot analysis using anti-HA antibody (Fig. 2C, lane 6), whereas IP from control cells or cells which received transfection of either SRC-3 or IKKβ alone failed to do so (Fig. 2C, lanes 1, 2, and 4). We confirmed the presence of IKKα in a similar IP-Western blot experiment. Clearly, both SRC-3 and IKKα were found to Co-IP by Flag antibody when Flag-SRC-3 and HA-IKKα were coexpressed in the same cell but not in cells transfected with either one alone (Fig. 2C, lanes 2, 3, and 5).
To ensure that the association of IKK and SRC-3 interaction is physiological, we examined their association in HeLa cells where the SRC-3-IKK complex was initially identified. For this purpose, we performed a Co-IP with anti-SRC-3 antibody by using both cytoplasmic and nuclear extracts from HeLa cells. We found association of SRC-3 with all three subunits of IKK in both nuclear and cytoplasmic extracts (Fig. 2D, lanes 2 and 4). Taken together, our results indicate that IKK is a genuine component of the SRC-3 complex.
SRC-3 and IKK act in concert to activate NF-κB-mediated transactivation.
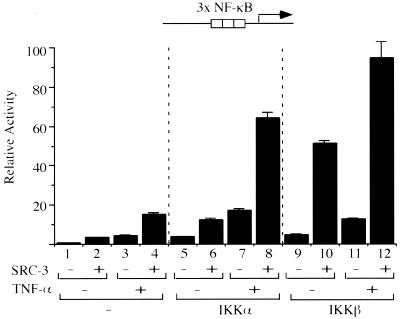
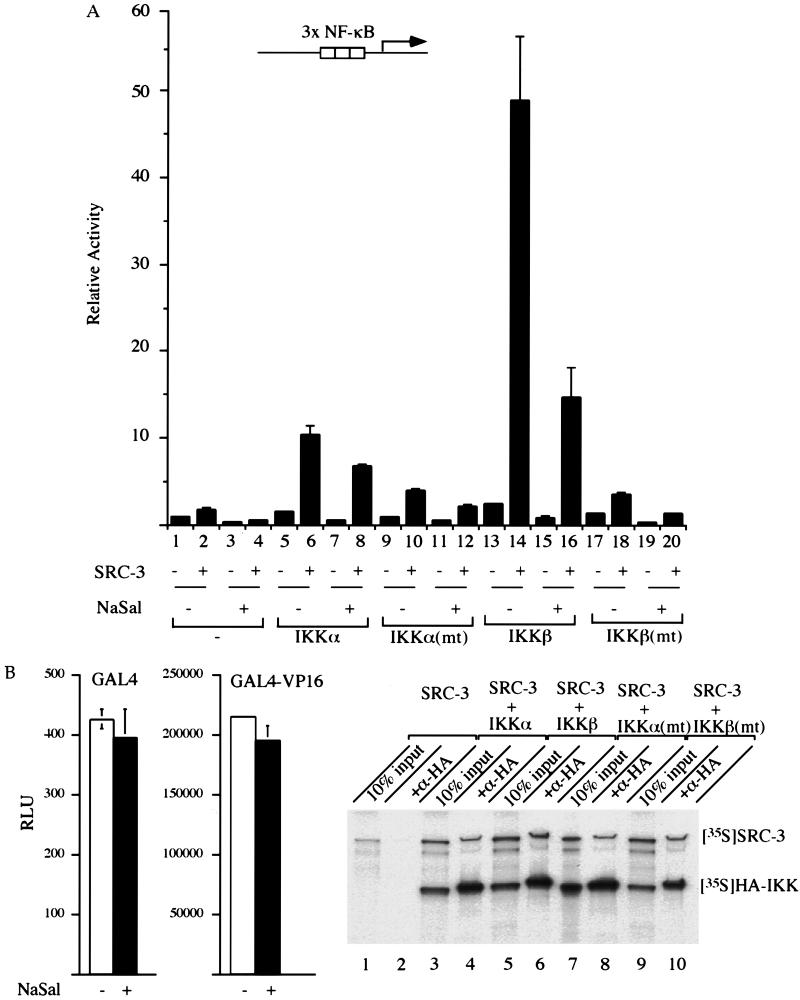
We questioned whether NF-κB-mediated gene expression can be synergistically modulated by SRC-3 and IKK. For this purpose, a reporter gene harboring three copies of the NF-κB responsive element was cotransfected with individual expression vectors for SRC-3 and IKK, either alone or in combination. Our results showed that transfection of IKKα, IKKβ, or SRC-3 alone resulted in a three- to sixfold increase in activation of the promoter (Fig. 3, compare lanes 2, 5, and 9 to lane 1). Importantly, cotransfection of SRC-3 with either IKKα or IKKβ resulted in 17- or 50-fold activation, respectively (Fig. 3, lanes 6 and 10). These results show that IKK and SRC-3 can synergistically activate NF-κB-dependent transcription.
FIG. 3.
Synergistic activation of an NF-κB-responsive promoter by SRC-3 and IKK. HeLa cells were transfected with either SRC-3 or IKK expression vector alone or with the two combined. Where indicated, TNF-α (+) was added 24 h after transfection for 4 h. Thereafter, the luciferase activity derived from the transfected κB-responsive promoter was measured and normalized by total input proteins. The y axis represents relative activities by comparison to those of samples transfected with the NF-κB-responsive promoter alone, whose activity was assigned a value of 1. The bars represent the means and standard deviations of the results from three independent experiments with triplicate samples.
Since TNF-α activates NF-κB-dependent transcription through modulation of IKK activity, we tested the effects of TNF-α in our system. We found that treatment with TNF-α alone can activate the promoter threefold (Fig. 3, lanes 1 and 3). In the presence of either SRC-3 or IKK alone, TNF-α was able to moderately enhance promoter activity (Fig. 3, lanes 2 and 4, 5 and 7, and 9 and 11). In contrast, a much greater enhancement by TNF-α of promoter activity was achieved in the presence of both SRC-3 and IKK (Fig. 3, compare lanes 7 and 8 and 11 and 12).
IKK kinase activity is required for synergistic activation with SRC-3.
To assess how IKK synergizes with SRC-3 to activate the NF-κB-responsive promoter, we asked whether IKK kinase activity is essential for the activation process. For this purpose, we first tested the ability of an inhibitor of IKK, sodium salicylate (NaSal), to block promoter activation (2). This inhibitor has been shown to specifically inhibit IKKβ activity but not that of other kinases tested (60). The results showed that on the addition of NaSal, the activation of the promoter was significantly reduced even in the presence of both SRC-3 and IKK, although it was not completely abolished (Fig. 4A, lanes 6, 8, 14, and 16). As a control, we tested this inhibitor on a promoter that does not contain the NF-κB-responsive element (5× UAS TATA), and the results showed that its activity was not affected (Fig. 4B, left panel).
FIG. 4.
Kinase activity of IKK is required for optimum NF-κB activation by SRC-3. (A) HeLa cells were transfected as described in the legend for Fig. 3. The IKK inhibitor, NaSal, was added to a final concentration of 5 mM after transfection and incubated for another 24 h. Thereafter, the luciferase activity was determined and expressed as described earlier. (B) As a control, HeLa cells were transfected with a reporter gene (5× UAS TATA luciferase) along with either GAL4 or GAL4-VP16 expression plasmid, and the results showed that neither the basal (GAL4) (bottom left panel) nor the activated (GAL4-VP16) (bottom right panel) activity of this promoter was affected. RLU, relative light units. SRC-3 and the indicated IKK were cotranslated and labeled with [35S]methionine in vitro. The products were then immunoprecipitated with anti-HA antibody, resolved by SDS-PAGE, and visualized by autoradiography.
To demonstrate further the importance of kinase activity, we next compared the abilities of the wild-type IKK and a kinase-defective mutant of IKK (62) to activate the NF-κB promoter. As shown in Fig. 4A, cotransfection of SRC-3 together with the wild-type IKK resulted in greater activation of the promoter than with the mutant IKK counterpart, even in the presence of SRC-3 (Fig. 4A, lanes 9 to 12 and 17 to 20), suggesting that kinase activity is important for activation.
To rule out that the possibility that the defective kinase is simply unable to interact with SRC-3, we tested their interactions in an in vitro pull-down assay. Our results showed that wild-type and mutant kinases interacted equally well with SRC-3 in vitro, suggesting that the inability of the mutants to activate the promoter is due to a decrease in kinase activity and not to an inability to interact with SRC-3 (Fig. 4B, lanes 4, 6, 8, and 10). Taken together, these results strongly suggest that IKK kinase activity is important for SRC-3-enhanced NF-κB gene activation.
Nuclear translocation-stabilization of SRC-3 is induced by TNF-α.
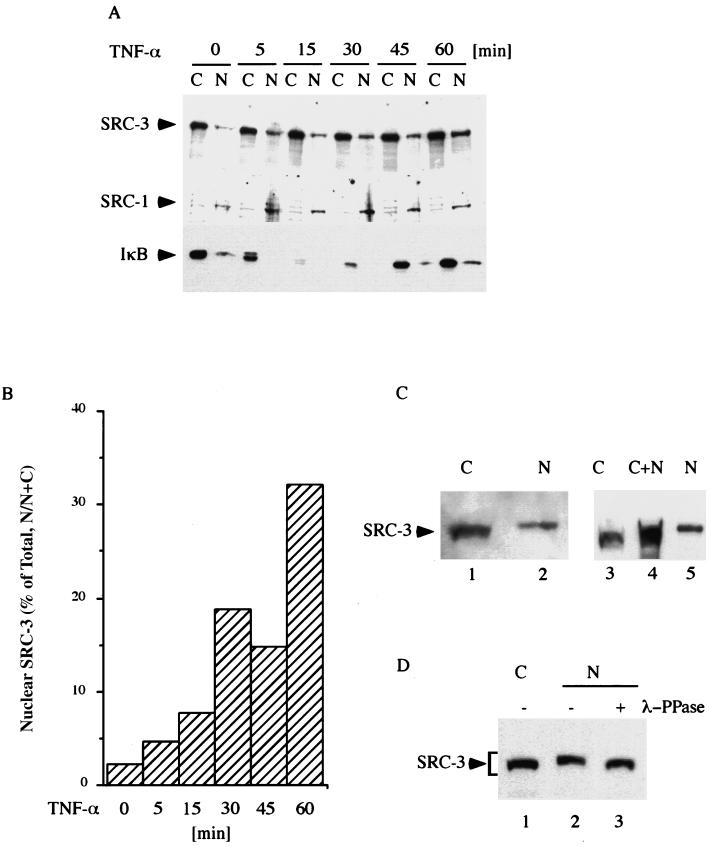
It is known that activation of IKK in response to TNF-α results in nuclear translocation of NF-κB. We showed that TNF-α potentiated SRC-3 activity in NF-κB-mediated gene expression. We therefore investigated the effects of TNF-α on the subcellular distribution of SRC-3. Vehicle-treated cells and cells treated with TNF-α were lysed and fractionated into cytoplasmic and nuclear fractions. These fractions were then subjected to Western blot analysis to determine the distribution of SRC-3. In line with previous reports (54), our results showed that the majority of SRC-3 was present in the cytoplasmic fractions prior to TNF-α treatment (Fig. 5A, top panel). Interestingly, we found that SRC-3 showed time-dependent translocation to the nucleus from cytoplasm in response to TNF-α. Compared to the vehicle-treated control (2%), up to 33% of SRC-3 was found in the nucleus 1 h after TNF-α treatment (Fig. 5B). In contrast, TNF-α did not have the same effect on SRC-1, which exists in the nucleus most of the time, suggesting that the translocation is limited to SRC-3 (Fig. 5A, middle panel). As a control for TNF-α action, the degradation of IκB from these cells was monitored. We observed a typical rapid degradation and reappearance pattern (Fig. 5A, bottom panel), which was consistent with earlier reports (16, 62).
FIG. 5.
Nuclear translocation of SRC-3 is induced by TNF-α. (A) The proteins from untreated cells and cells treated with TNF-α for the times indicated (all grown in DMEM containing 0.5% FBS) were separated into cytoplasmic and nuclear fractions. The membrane containing the fractions was subjected to sequential Western blot analysis by stripping and reprobing the membrane with the indicated antibodies (left panel). The degradation of IκB as evidenced by Western blot analysis was used to monitor the effect of TNF-α on these cells. The results shown are representative of two independent experiments with similar results. (B) Bar chart showing data from experiments as described for panel A. Values corresponding to the y axis represent the percentages of SRC-3 from nuclei (nuclear/nuclear + cytoplasmic [N/N+C]), whereas those corresponding to the x axis represent durations of TNF-α treatment. (C) The proteins from TNF-α-treated HeLa cells were fractionated as described for panel A and assayed by Western blot analysis (left panel). Furthermore, the cytoplasmic fraction was mixed with the nuclear fraction and run side by side with each separated fraction to compare levels of mobility (right panel). (D) Where indicated (+), the nuclear extract was treated with λ-phosphatase (λ-PPase) prior to Western blot analysis. The differences in mobility are indicated by a bracket. N, nucleus; C, cytoplasm.
Phosphorylation of SRC-3 by IKK.
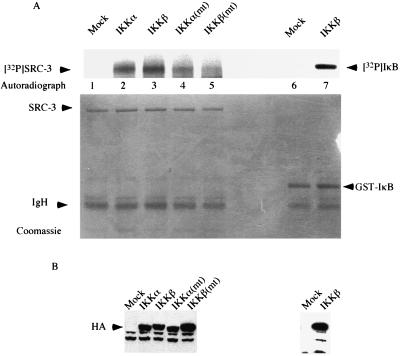
Careful analysis of the data showed that the nuclear SRC-3 displayed slower mobility than the cytoplasmic counterpart (Fig. 5C, lanes 1 and 2). The mobility difference was observed again when a mixture of cytoplasmic and nuclear extracts was run side by side with each separated extract (Fig. 5C, lanes 3, 4, and 5). Furthermore, treatment of nuclear extract with λ-phosphatase resulted in faster migration of SRC-3, which is similar to the mobility obtained for cytoplasmic SRC-3 (Fig. 5D, lanes 1, 2, and 3), suggesting that nuclear SRC-3 was phosphorylated. To substantiate this hypothesis, we asked if SRC-3 is a substrate of IKK. For this purpose, we employed an in vitro kinase assay using purified SRC-3 as substrate. Figure 6A shows that SRC-3 was able to be phosphorylated by both IKKα and IKKβ in vitro, with IKKβ phosphorylating SRC-3 more efficiently than IKKα (Fig. 6A, lanes 2 and 3). In addition, we also tested the phosphorylation abilities of the kinase mutants in parallel. Our results showed that the mutants still contained some residual activity and were able to phosphorylate SRC-3 but only at much lower levels (Fig. 6A, lanes 4 and 5). The expected residual kinase activity could explain the slight activation we observed, as shown in Fig. 4A (lanes 10 and 18). The differential phosphorylation of SRC-3 was not due to the inability of mutant IKK to interact with SRC-3 (Fig. 4B) or to lack of expression, as Western blot analysis showed that similar levels of protein were expressed (Fig. 6B).
FIG. 6.
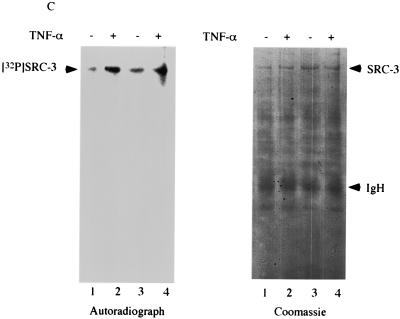
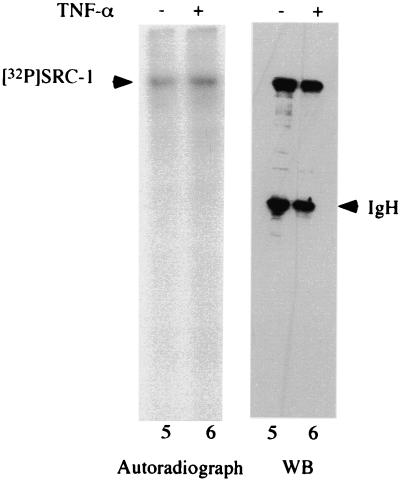
Phosphorylation of SRC-3 by IKK. (A) HeLa cells were subjected to transfection with the indicated IKK expression vectors, which was followed by immunoprecipitation with anti-HA antibody. The immunoprecipitates were assayed for kinase activity in vitro with purified SRC-3 or IκB as a substrate in the presence of [γ-32P]ATP. The top panel shows the phosphorylated SRC-3 and IκB, and the bottom panel shows the same gel stained with Coomassie blue, indicating the presence of equal amounts of SRC-3 and IκB. Mock, mock transfected. (B) A portion of the immunoprecipitates from the transfections described for panel A was used for Western blot analysis with anti-HA antibody. The results indicated similar amounts of HA-tagged proteins were being precipitated. Mock, mock transfected. (C) HeLa cells were labeled with 32Pi in vivo in the presence (+) or absence (−) of TNF-α. The lysates were prepared and subjected to immunoprecipitation by anti-SRC-3 antibody. Shown on the left is the extent of SRC-3 phosphorylation, and on the right is the same gel stained with Coomassie blue, which shows that similar amounts of SRC-3 were immunoprecipitated. The experiment was done in duplicate. The phosphorylation of SRC-1 was also determined in a similar manner by using anti-SRC-1 antibody. The presence of TNF-α is indicated by a plus sign. Western blot analysis (WB) was used to determine the SRC-1 protein amount.
Since SRC-3 is phosphorylated by IKK in vitro, we next asked whether TNF-α, a physiological inducer of IKK activity, affects the phosphorylation of SRC-3 in vivo. To this end, HeLa cells were labeled with orthophosphate (32Pi) in the presence or absence of TNF-α and then subjected to IP with an anti-SRC-3 antibody to determine whether SRC-3 phosphorylation can be regulated by TNF-α. Our results showed that SRC-3 was a phosphoprotein in vivo and, more importantly, that phosphorylation of SRC-3 was enhanced by TNF-α (Fig. 6C, compare lanes 2 and 4 to 1 and 3). As a control, the levels of SRC-3 expression were left unaltered in the presence or absence of TNF-α (right panel, Coomassie staining). In contrast to SRC-3, our results also showed that phosphorylation of SRC-1 was not affected by TNF-α (Fig. 6C, lanes 5 and 6). Since TNF-α treatment activates IKK, our results support the conclusion that SRC-3 is a target for IKK.
Involvement of SRC-3 in NF-κB-mediated gene expression.
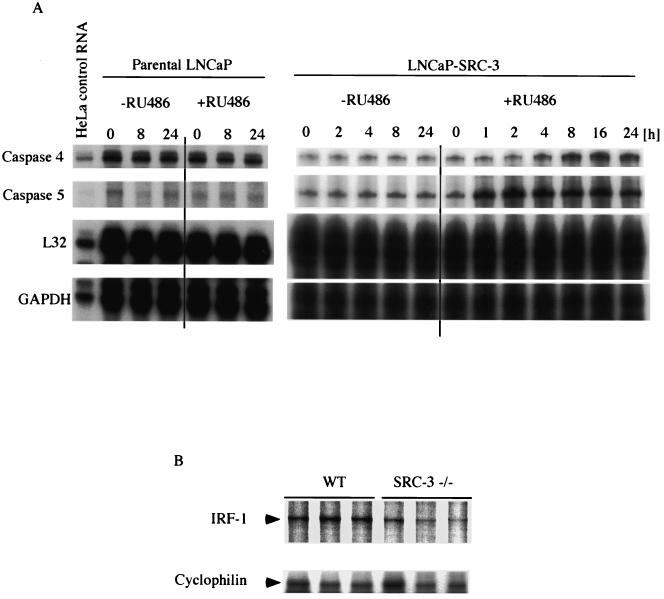
Our data suggested that SRC-3 plays an important role in NF-κB-mediated gene expression (Fig. 3) and that its activity is subjected to regulation by phosphorylation. To support the physiological importance of these findings, we investigated the possible role of SRC-3 in immune response. We first examined the expression of the proinflammatory caspases in LNCaP cells, which overexpress SRC-3 in a strictly RU486-inducible manner (7). The expression of SRC-3 in these cells has been shown to be induced very rapidly by RU486 and reaches maximum levels 2 to 4 h after exposure to the inducing agent (Y. Hashimoto, S. Y. Tsai, and M.-J. Tsai, unpublished result). As shown in Fig. 7A (right panel), induction of SRC-3 by RU486 resulted in the increased expression of two proinflammatory caspases, caspase 4 and caspase 5, as detected by RPA. The induction of caspase expression is rapid and can be detected within the first 4 h upon addition of RU486 and can be maintained for up to 24 h in the continuous presence of RU486. In contrast, no change in the expression of these caspases was detected from the parental LNCaP cells as determined by RPA (left panel).
FIG. 7.
NF-κB target gene expression in SRC-3 null animals. (A) Inducible LNCaP cells were grown in the presence (+) or absence (−) of RU486 (100 nM) for the times indicated to induce expression of SRC-3. Afterward, total RNA was prepared and the expression levels of caspase 4 and caspase 5 were determined by RPA (right panel). The expression of these two caspases from the parental LNCaP cells was also determined by RPA and was shown not to change even in the presence of RU486 (left panel). The levels of L32 and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) are shown as a loading control. (B) Total RNA from spleens was analyzed by RPA to determine the expression level of IRF-1. The RPA results for three mice from each group are shown. The level of cyclophilin expression was determined in the same reaction and used as an RNA loading control.
Recently, eye and skin infections have been noted in our SRC-3 null mice, suggesting a possible impairment of the immune system in these animals (58). This result is consistent with a role for SRC-3 in inflammatory responses. To investigate this defect further, we examined the status of IRF-1, a known NF-κB target gene (31), in SRC-3 null mice. Total RNA prepared from the spleens of control and SRC-3 null mouse littermates was analyzed by RPA to determine whether the levels of IRF-1 mRNA are affected by the genetic deletion of SRC-3. As might be predicted, we found reduced but not abolished expression of IRF-1 in the SRC-3 null mice (Fig. 7B). In total, we analyzed five mice from each group. Among these, four out of five SRC-3 null mice exhibited significantly reduced basal expression of IRF-1 (Fig. 7B and data not shown). The animal results are also compatible with those for SRC-3 involvement in the regulation of NF-κB-responsive genes in vivo.
DISCUSSION
Despite sequence and functional similarities, several lines of evidence have suggested that members of the SRC family of coactivators play roles in distinct biological processes, since SRC-3 null mice exhibit quite distinct phenotypes from those reported for SRC-1 null mice (54, 58, 59). Furthermore, the differences in the subcellular localizations of SRC-1 and SRC-3 suggest that the activity of SRCs could be subject to further regulation. This notion applies to SRC-3 in particular, as it resides predominantly in the cytoplasm, away from its functional site of nuclear transcription (54).
In the present study, we described the biochemical purification of SRC complexes and the subsequent identification of IKK as part of the SRC-3 but not the SRC-1 complex. IKK is considered to be the master regulator of host immune response by virtue of its ability to control NF-κB activation. Since NF-κB is a central mediator of human immune-inflammatory response, the differential association of SRC-3 and not SRC-1 with IKK is of great interest.
In support of the significance of the SRC-3-IKK complex, we showed that SRC-3 was able to enhance NF-κB transactivation synergistically in concert with IKK in a transient transfection. To elucidate the underlying mechanism of this transactivation, our results showed that SRC-3 is phosphorylated by IKK despite the fact that, to our knowledge, IκB is the only reported substrate of IKK (Fig. 6A). Consistent with the results of a previous report (18), we showed that SRC-3 is a phosphoprotein in vivo; we went on to show that phosphorylation of SRC-3 is induced in response to TNF-α, a stimulator of IKK activity. At present, however, we cannot exclude the possibility that SRC-3 is phosphorylated by some TNF-α activated kinase(s) other than IKK in intact cells. In further support of the importance of SRC-3 phosphorylation, our experiments demonstrated that IKK phosphorylation of SRC-3 in vitro correlates with its ability to activate promoters in a transient transfection. Therefore, our results suggest that the coactivator activity of SRC-3 can be subject to regulation by a chain of events in response to TNF-α, including IKK activation, inducible SRC-3 phosphorylation, nuclear translocation, and target gene activation.
At present the exact mechanism whereby phosphorylation results in SRC-3 activation is not clear, but it has been postulated that phosphorylation of SRC-3 might activate its own intrinsic acetyltransferase activity or serve as the platform for the recruitment of other coactivators, such as CBP/p300 (18). However, as suggested by our results, another mechanism by which phosphorylation can regulate SRC-3 activity is through the control of its subcellular localization. In agreement with an earlier report (54), we also found that SRC-3 frequently resides predominantly in the cytoplasm, whereas SRC-1 is almost exclusively a nuclear protein. Our results showed that the nuclear translocation of SRC-3 is subject to regulation by TNF-α, as evidenced by its accumulation in the nucleus upon addition of TNF-α. This accumulation could result either from active translocation or from a shift in compartmental equilibrium whereby nuclear export is decreased; which of the two events is responsible remains to be determined. The importance of SRC-3 phosphorylation is supported by the fact that phosphorylated forms of SRC-3 are the predominant forms that are found in the nucleus. Although nuclear translocation may be important in IKK-mediated activation of SRC-3, other mechanisms must also play a role in IKK-induced SRC-3 activity. Taken together, our results indicate that the mechanism by which TNF-α activates SRC-3 is twofold and includes increased phosphorylation and nuclear translocation-stabilization.
Although the results of transfection studies have implicated SRC-1 in the enhancement of NF-κB-mediated transactivation (35) and SRC-1 has been reported to be a phosphoprotein (44, 45), it is notable that phosphorylation of SRC-1 was not affected by TNF-α in our study (Fig. 6C). We reason that this anomaly might be attributed to the differential association capacities of IKK with SRC-3 and SRC-1, a conjecture further supported by our finding that unlike that of SRC-3, the SRC-1 complex does not contain IKK subunits.
The preferential ability of IKKβ to more efficiently activate NF-κB-mediated transactivation than IKKα is also of interest. Although IKKα and IKKβ share a high degree of sequence identity (62), our results are consistent with the observation that IKKβ is more important than IKKα for the activation of NF-κB in response to TNF-α stimulation (26, 48).
Finally, we observed an increased expression of proinflammatory caspases (10) in SRC-3-overexpressing cells and a reduced level of IRF-1, an NF-κB target gene (31), in the spleens of SRC-3 null mice. Since IRF-1 is one of the major mediators of NF-κB's action in the immune response, it is not unexpected that SRC-3 null mice should have increased skin and eye infections (58). More importantly, this result lends further support to the physiological significance of our observation that SRC-3 plays a role in maintaining the NF-κB response.
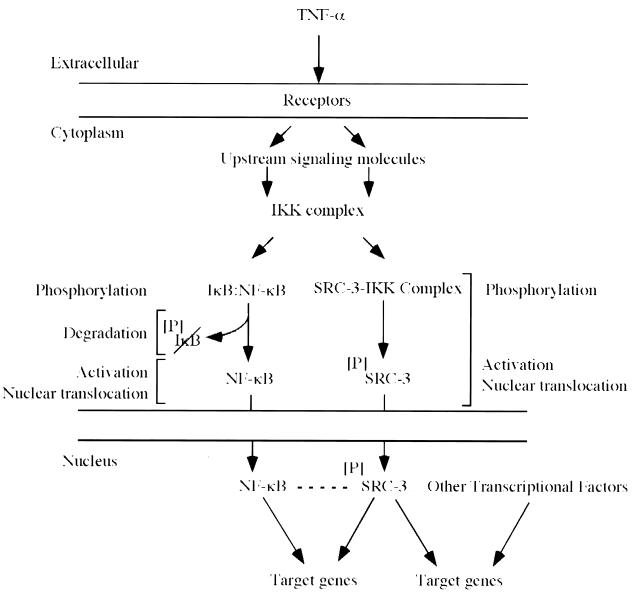
Our results and those of others have shown that steroid receptors as well as NF-κB can use SRC-3 as a coactivator and that IKK plays an important role in SRC-3 functions (Fig. 8). Consequently, cytokine stimulation (e.g., TNF-α) activates IKK and leads to phosphorylation and degradation of IκB as well as to nuclear localization of SRC-3. In this sense, cytokines can enhance the activities of both NF-κB and steroid receptor pathways. Under normal conditions, when the concentration of SRC-3 is limiting, nuclear competition for SRC-3 could lead to squelching and interpathway inhibition (23, 35, 55). Nuclear competition therefore represents a mechanism whereby steroid receptor complexes could exert their well-known antiinflammatory influences, the composite result of which would be dependent upon the cell-specific concentrations of SRC-3. In the case of endocrine tumors, however, where SRC-3 (AIB1) is usually overexpressed (3), the local concentrations of coactivator may be sufficient for full activation of these and other SRC-3-dependent pathways—a potentially dangerous situation.
FIG. 8.
Model for SRC-3 regulation by phosphorylation. In response to the presence of TNF-α or ligands, increased phosphorylation of SRC-3 led to the activation and nuclear translocation of SRC-3. The effects of phosphorylation were similar for NF-κB. The association of SRC-3 with IKK was observed in both the cytoplasm and nucleus but is omitted for the nucleus for clarity. The dashed line indicates the putative interaction between SRC-3 and NF-κB (55).
Acknowledgments
We thank Ebrahim Zandi for the IKK expression vectors and Li-Yuan Yu-Lee for the IRF-1 and cyclophilin DNA templates and for helpful suggestions regarding RPA. We also thank Zheng Liu and Zhi-Qing Ma for their help with experiments and David Moore and Neil McKenna for critical reading of the manuscript.
This work was supported by a grant from the National Institute of Health-National Institute of Child Health and Human Development (to B.W.O.). R.-C.W. is a recipient of a postdoctoral fellowship from the U.S. Department of Defense Breast Cancer Research Program.
REFERENCES
- 1.Alkalay, I., A. Yaron, A. Hatzubai, S. Jung, A. Avraham, O. Gerlitz, I. Pashut-Lavon, and Y. Ben-Neriah. 1995. In vivo stimulation of IκB phosphorylation is not sufficient to activate NF-κB. Mol. Cell. Biol. 15:1294-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpert, D., and J. Vilcek. 2000. Inhibition of IkappaB kinase activity by sodium salicylate in vitro does not reflect its inhibitory mechanism in intact cells. J. Biol. Chem. 275:10925-10929. [DOI] [PubMed] [Google Scholar]
- 3.Anzick, S. L., J. Kononen, R. L. Walker, D. O. Azorsa, M. M. Tanner, X. Y. Guan, G. Sauter, O. P. Kallioniemi, J. M. Trent, and P. S. Meltzer. 1997. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277:965-968. [DOI] [PubMed] [Google Scholar]
- 4.Baeuerle, P. A., and D. Baltimore. 1996. NF-kappa B: ten years after. Cell 87:13-20. [DOI] [PubMed] [Google Scholar]
- 5.Baldwin, A. S. 1996. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649-683. [DOI] [PubMed] [Google Scholar]
- 6.Brown, K., S. Gerstberger, L. Carlson, G. Franzoso, and U. Siebenlist. 1995. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science 267:1485-1488. [DOI] [PubMed] [Google Scholar]
- 7.Burcin, M. M., G. Schiedner, S. Kochanek, S. Y. Tsai, and B. W. O'Malley. 1999. Adenovirus-mediated regulable target gene expression in vivo. Proc. Natl. Acad. Sci. USA 96:355-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buyon, J. P., and R. Winchester. 1990. Congenital complete heart block. A human model of passively acquired autoimmune injury. Arthritis Rheum. 33:609-614. [DOI] [PubMed] [Google Scholar]
- 9.Chan, E. K., J. C. Hamel, C. L. Peebles, J. P. Buyon, and E. M. Tan. 1990. Molecular characterization and cloning of the 52 kDa SS-A/Ro protein. Mol. Biol. Rep. 14:53.. [DOI] [PubMed] [Google Scholar]
- 10.Chang, H. Y., and X. Yang. 2000. Proteases for cell suicide: functions and regulation of caspases. Microbiol. Mol. Biol. Rev. 64:821-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, H., R. J. Lin, R. L. Schiltz, D. Chakravarti, A. Nash, L. Nagy, M. L. Privalsky, Y. Nakatani, and R. M. Evans. 1997. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 90:569-580. [DOI] [PubMed] [Google Scholar]
- 12.Chen, H., R. J. Lin, W. Xie, D. Wilpitz, and R. M. Evans. 1999. Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase. Cell 98:675-686. [DOI] [PubMed] [Google Scholar]
- 13.Chen, Z., J. Hagler, V. J. Palombella, F. Melandri, D. Scherer, D. Ballard, and T. Maniatis. 1995. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 9:1586-1597. [DOI] [PubMed] [Google Scholar]
- 14.DiDonato, J., F. Mercurio, C. Rosette, J. Wu-Li, H. Suyang, S. Ghosh, and M. Karin. 1996. Mapping of the inducible IκB phosphorylation sites that signal its ubiquitination and degradation. Mol. Cell. Biol. 16:1295-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiDonato, J. A., M. Hayakawa, D. M. Rothwarf, E. Zandi, and M. Karin. 1997. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature 388:548-554. [DOI] [PubMed] [Google Scholar]
- 16.DiDonato, J. A., F. Mercurio, and M. Karin. 1995. Phosphorylation of I kappa B alpha precedes but is not sufficient for its dissociation from NF-kappa B. Mol. Cell. Biol. 15:1302-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dignam, J. D. 1990. Preparation of extracts from higher eukaryotes. Methods Enzymol. 182:194-203. [DOI] [PubMed] [Google Scholar]
- 18.Font de Mora, J., and M. Brown. 2000. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol. Cell. Biol. 20:5041-5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frank, M. B. 1999. Characterization of DNA binding properties and sequence specificity of the human 52 kDa Ro/SS-A (Ro52) zinc finger protein. Biochem. Biophys. Res. Commun. 259:665-670. [DOI] [PubMed] [Google Scholar]
- 20.Gerritsen, M. E., A. J. Williams, A. S. Neish, S. Moore, Y. Shi, and T. Collins. 1997. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc. Natl. Acad. Sci. USA 94:2927-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halachmi, S., E. Marden, G. Martin, H. MacKay, C. Abbondanza, and M. Brown. 1994. Estrogen receptor-associated proteins: possible mediators of hormone-induced transcription. Science 264:1455-1458. [DOI] [PubMed] [Google Scholar]
- 22.Harlow, E., and D. Lane (ed.). 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 23.Harnish, D. C., M. S. Scicchitano, S. J. Adelman, C. R. Lyttle, and S. K. Karathanasis. 2000. The role of CBP in estrogen receptor cross-talk with nuclear factor-kappaB in HepG2 cells. Endocrinology 141:3403-3411. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann, J. A., F. C. Kafatos, C. A. Janeway, and R. A. Ezekowitz. 1999. Phylogenetic perspectives in innate immunity. Science 284:1313-1318. [DOI] [PubMed] [Google Scholar]
- 25.Hong, H., K. Kohli, A. Trivedi, D. L. Johnson, and M. R. Stallcup. 1996. GRIP1, a novel mouse protein that serves as a transcriptional coactivator in yeast for the hormone binding domains of steroid receptors. Proc. Natl. Acad. Sci. USA 93:4948-4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu, Y., V. Baud, M. Delhase, P. Zhang, T. Deerinck, M. Ellisman, R. Johnson, and M. Karin. 1999. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKalpha subunit of IkappaB kinase. Science 284:316-320. [DOI] [PubMed] [Google Scholar]
- 27.Kamei, Y., L. Xu, T. Heinzel, J. Torchia, R. Kurokawa, B. Gloss, S. C. Lin, R. A. Heyman, D. W. Rose, C. K. Glass, and M. G. Rosenfeld. 1996. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell 85:403-414. [DOI] [PubMed] [Google Scholar]
- 28.Lanz, R. B., N. J. McKenna, S. A. Onate, U. Albrecht, J. Wong, S. Y. Tsai, M.-J. Tsai, and B. W. O'Malley. 1999. A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell 97:17-27. [DOI] [PubMed] [Google Scholar]
- 29.Li, H., P. J. Gomes, and J. D. Chen. 1997. RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proc. Natl. Acad. Sci. USA 94:8479-8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, J., B. W. O'Malley, and J. Wong. 2000. p300 requires its histone acetyltransferase activity and SRC-1 interaction domain to facilitate thyroid hormone receptor activation in chromatin. Mol. Cell. Biol. 20:2031-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo, G., and L. Yu-Lee. 2000. Stat5b inhibits NFkappaB-mediated signaling. Mol. Endocrinol. 14:114-123. [DOI] [PubMed] [Google Scholar]
- 32.Mangelsdorf, D. J., C. Thummel, M. Beato, P. Herrlich, G. Schutz, K. Umesono, B. Blumberg, P. Kastner, M. Mark, P. Chambon, et al. 1995. The nuclear receptor superfamily: the second decade. Cell 83:835-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKenna, N. J., Z. Nawaz, S. Y. Tsai, M.-J. Tsai, and B. W. O'Malley. 1998. Distinct steady-state nuclear receptor coregulator complexes exist in vivo. Proc. Natl. Acad. Sci. USA 95:11697-11702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mercurio, F., H. Zhu, B. W. Murray, A. Shevchenko, B. L. Bennett, J. Li, D. B. Young, M. Barbosa, M. Mann, A. Manning, and A. Rao. 1997. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science 278:860-866. [DOI] [PubMed] [Google Scholar]
- 35.Na, S. Y., S. K. Lee, S. J. Han, H. S. Choi, S. Y. Im, and J. W. Lee. 1998. Steroid receptor coactivator-1 interacts with the p50 subunit and coactivates nuclear factor kappaB-mediated transactivations. J. Biol. Chem. 273:10831-10834. [DOI] [PubMed] [Google Scholar]
- 36.Ogryzko, V. V., T. Kotani, X. Zhang, R. L. Schlitz, T. Howard, X. J. Yang, B. H. Howard, J. Qin, and Y. Nakatani. 1998. Histone-like TAFs within the PCAF histone acetylase complex. Cell 94:35-44. [DOI] [PubMed] [Google Scholar]
- 37.Onate, S. A., V. Boonyaratanakornkit, T. E. Spencer, S. Y. Tsai, M.-J. Tsai, D. P. Edwards, and B. W. O'Malley. 1998. The steroid receptor coactivator-1 contains multiple receptor interacting and activation domains that cooperatively enhance the activation function 1 (AF1) and AF2 domains of steroid receptors. J. Biol. Chem. 273:12101-12108. [DOI] [PubMed] [Google Scholar]
- 38.Onate, S. A., S. Y. Tsai, M.-J. Tsai, and B. W. O'Malley. 1995. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270:1354-1357. [DOI] [PubMed] [Google Scholar]
- 39.Pennington, K. N., J. A. Taylor, G. D. Bren, and C. V. Paya. 2001. IB kinase-dependent chronic activation of NF-B is necessary for p21WAF1/Cip1 inhibition of differentiation-induced apoptosis of monocytes. Mol. Cell. Biol. 21:1930-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perkins, N. D., L. K. Felzien, J. C. Betts, K. Leung, D. H. Beach, and G. J. Nabel. 1997. Regulation of NF-kappaB by cyclin-dependent kinases associated with the p300 coactivator. Science 275:523-527. [DOI] [PubMed] [Google Scholar]
- 41.Provost, T. T., and M. Reichlin. 1988. Immunopathologic studies of cutaneous lupus erythematosus. J. Clin. Immunol. 8:223-233. [DOI] [PubMed] [Google Scholar]
- 42.Qin, J., D. Fenyo, Y. Zhao, W. W. Hall, D. M. Chao, C. J. Wilson, R. A. Young, and B. T. Chait. 1997. A strategy for rapid, high-confidence protein identification. Anal. Chem. 69:3995-4001. [DOI] [PubMed] [Google Scholar]
- 43.Regnier, C. H., H. Y. Song, X. Gao, D. V. Goeddel, Z. Cao, and M. Rothe. 1997. Identification and characterization of an IkappaB kinase. Cell 90:373-383. [DOI] [PubMed] [Google Scholar]
- 44.Rowan, B. G., N. Garrison, N. L. Weigel, and B. W. O'Malley. 2000. 8-Bromo-cyclic AMP induces phosphorylation of two sites in SRC-1 that facilitate ligand-independent activation of the chicken progesterone receptor and are critical for functional cooperation between SRC-1 and CREB binding protein. Mol. Cell. Biol. 20:8720-8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rowan, B. G., N. L. Weigel, and B. W. O'Malley. 2000. Phosphorylation of steroid receptor coactivator-1. Identification of the phosphorylation sites and phosphorylation through the mitogen-activated protein kinase pathway. J. Biol. Chem. 275:4475-4483. [DOI] [PubMed] [Google Scholar]
- 46.Sheppard, K. A., D. W. Rose, Z. K. Haque, R. Kurokawa, E. McInerney, S. Westin, D. Thanos, M. G. Rosenfeld, C. K. Glass, and T. Collins. 1999. Transcriptional activation by NF-kappaB requires multiple coactivators. Mol. Cell. Biol. 19:6367-6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spencer, T. E., G. Jenster, M. M. Burcin, C. D. Allis, J. Zhou, C. A. Mizzen, N. J. McKenna, S. A. Onate, S. Y. Tsai, M.-J. Tsai, and B. W. O'Malley. 1997. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature 389:194-198. [DOI] [PubMed] [Google Scholar]
- 48.Takeda, K., O. Takeuchi, T. Tsujimura, S. Itami, O. Adachi, T. Kawai, H. Sanjo, K. Yoshikawa, N. Terada, and S. Akira. 1999. Limb and skin abnormalities in mice lacking IKKalpha. Science 284:313-316. [DOI] [PubMed] [Google Scholar]
- 49.Takeshita, A., G. R. Cardona, N. Koibuchi, C. S. Suen, and W. W. Chin. 1997. TRAM-1, A novel 160-kDa thyroid hormone receptor activator molecule, exhibits distinct properties from steroid receptor coactivator-1. J. Biol. Chem. 272:27629-27634. [DOI] [PubMed] [Google Scholar]
- 50.Torchia, J., D. W. Rose, J. Inostroza, Y. Kamei, S. Westin, C. K. Glass, and M. G. Rosenfeld. 1997. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature 387:677-684. [DOI] [PubMed] [Google Scholar]
- 51.Tsai, M.-J., and B. W. O'Malley. 1994. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 63:451-486. [DOI] [PubMed] [Google Scholar]
- 52.Voegel, J. J., M. J. Heine, C. Zechel, P. Chambon, and H. Gronemeyer. 1996. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 15:3667-3675. [PMC free article] [PubMed] [Google Scholar]
- 53.Wang, W., J. Cote, Y. Xue, S. Zhou, P. A. Khavari, S. R. Biggar, C. Muchardt, G. V. Kalpana, S. P. Goff, M. Yaniv, J. L. Workman, and G. R. Crabtree. 1996. Purification and biochemical heterogeneity of the mammalian SWI-SNF complex. EMBO J. 15:5370-5382. [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, Z., D. W. Rose, O. Hermanson, F. Liu, T. Herman, W. Wu, D. Szeto, A. Gleiberman, A. Krones, K. Pratt, R. Rosenfeld, C. K. Glass, and M. G. Rosenfeld. 2000. Regulation of somatic growth by the p160 coactivator p/CIP. Proc. Natl. Acad. Sci. USA 97:13549-13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Werbajh, S., I. Nojek, R. Lanz, and M. A. Costas. 2000. RAC-3 is a NF-kappaB coactivator. FEBS Lett. 485:195-199. [DOI] [PubMed] [Google Scholar]
- 56.Woronicz, J. D., X. Gao, Z. Cao, M. Rothe, and D. V. Goeddel. 1997. IkappaB kinase-beta: NF-kappaB activation and complex formation with IkappaB kinase-alpha and NIK. Science 278:866-869. [DOI] [PubMed] [Google Scholar]
- 57.Wright, S. D. 1999. Toll, a new piece in the puzzle of innate immunity. J. Exp. Med. 189:605-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu, J., L. Liao, G. Ning, H. Yoshida-Komiya, C. Deng, and B. W. O'Malley. 2000. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc. Natl. Acad. Sci. USA 97:6379-6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu, J., Y. Qiu, F. J. DeMayo, S. Y. Tsai, M.-J. Tsai, and B. W. O'Malley. 1998. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science 279:1922-1925. [DOI] [PubMed] [Google Scholar]
- 60.Yin, M. J., Y. Yamamoto, and R. B. Gaynor. 1998. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature 396:77-80. [DOI] [PubMed] [Google Scholar]
- 61.Zandi, E., and M. Karin. 1999. Bridging the gap: composition, regulation, and physiological function of the IkappaB kinase complex. Mol. Cell. Biol. 19:4547-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zandi, E., D. M. Rothwarf, M. Delhase, M. Hayakawa, and M. Karin. 1997. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell 91:243-252. [DOI] [PubMed] [Google Scholar]
- 63.Zhong, H., R. E. Voll, and S. Ghosh. 1998. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol. Cell 1:661-671. [DOI] [PubMed] [Google Scholar]