Abstract
While transport of RNA-binding protein HuR from nucleus to cytoplasm is emerging as a key regulatory step for HuR function, the mechanisms underlying this process remain poorly understood. Here, we report that the AMP-activated kinase (AMPK), an enzyme involved in responding to metabolic stresses, potently regulates the levels of cytoplasmic HuR. Inhibition of AMPK, accomplished either through cell treatment or by adenovirus infection to express dominant-negative AMPK, was found to increase the level of HuR in the cytoplasm and to enhance the binding of HuR to p21, cyclin B1, and cyclin A mRNA transcripts and elevate their expression and half-lives. Conversely, AMPK activation, achieved by means including infection to express constitutively active AMPK, resulted in reduced cytoplasmic HuR; decreased levels and half-lives of mRNAs encoding p21, cyclin A, and cyclin B1; and diminished HuR association with the corresponding transcripts. We therefore propose a novel function for AMPK as a regulator of cytoplasmic HuR levels, which in turn influences the mRNA-stabilizing function of HuR and the expression of HuR target transcripts.
In response to stimuli of internal or external origin, mammalian cells implement a series of adaptive modifications, which culminate in altering the pattern of expressed genes. Posttranscriptional mechanisms of gene regulation, particularly those affecting mRNA stability, are emerging as critical effectors of gene expression changes during immune cell activation, cellular proliferation, and the stress response (49, 50). Even small differences in half-life provide a highly effective means of radically altering the abundance of a given mRNA and consequently the amount of protein expressed. Although the mechanisms determining mRNA turnover are poorly understood, they are generally believed to involve RNA-binding proteins recognizing specific RNA sequences. Best characterized among the RNA sequences that influence mRNA stability are AU-rich elements (AREs), usually found in the 3′ untranslated regions (UTR) of short-lived mRNAs (7, 61), such as those encoding cytokines (interferon, interleukins [IL], tumor necrosis factor alpha [TNF-α]), cell cycle regulatory genes (the cyclin-dependent kinase p21, cyclin A, cyclin B1, and cdc25 genes), growth factors (granulocyte-macrophage colony-stimulating factor and vascular endothelial growth factor [VEGF]), and proto-oncogenes (c-fos and c-myc). Likewise, many RNA-binding proteins that selectively recognize and bind to AREs of labile mRNAs, including AU-A, AU-B, AU-C, adenosine-uridine binding factor, AUF1 (hnRNP D), Hel-N1, HuC, HuD, hnRNP A0, hnRNP A1, hnRNP C, HuR (HuA), tristetraprolin, and TIAR, have been described (2, 5, 8, 40, 21, 22, 43, 64); those whose influence on mRNA turnover has been studied the most extensively are HuR and AUF1 (4, 35, 37).
Beyond the identification of RNA recognition sites and RNA-binding proteins, relatively little is known about the mechanisms that govern mRNA turnover. Recently, however, several studies have provided increasing support for the notion that mRNA stability is regulated through mechanisms akin to those controlling gene transcription, i.e., signal transduction pathways involving phosphorylation events. Early reports described the altered turnover of ARE-containing mRNAs in response to extracellular as well as internally generated signals, such as phorbol esters, antibodies recognizing CD3/CD28 surface receptors, and TNF-α (17, 36, 44). Protein kinase C (PKC) was specifically implicated in the enhanced stability of many labile mRNAs, such as those encoding p21 and IL-1 (17, 46); similarly, PKC activity was shown to influence the binding of adenosine-uridine binding factor to target labile mRNAs, leading to their enhanced stability (40). Several mitogen-activated protein kinases (MAPK) have also been implicated in regulating mRNA turnover. The MAPK c-Jun N-terminal kinase (JNK) pathway was found to participate in the stabilization of ARE-containing IL-3 and IL-2 mRNAs (6, 41). The MAPK extracellular signal-regulated protein kinase (ERK) pathway was implicated in the stabilization of mRNAs encoding nucleolin and p21 and the destabilization of the mRNA encoding β-amyloid precursor protein (11, 56, 57). Another MAPK, p38, was shown to participate in the stabilization of mRNAs encoding IL-8, c-fos, granulocyte-macrophage colony-stimulating factor, TNF-α, VEGF, and cyclo-oxygenase 2 (10, 32, 45, 58). Other stress-triggered events have also been shown to influence mRNA stability. Heat shock, for example, regulates the turnover of ARE-containing mRNAs through a process that involves AUF1 and the ubiquitin/proteasome system (34). More recently, heat shock was shown to suppress the binding of HuR to target mRNAs in the cytoplasm and to increase it in the nucleus. However, heat shock specifically enhanced HuR-mediated export of hsp70 mRNA to the cytoplasm, purportedly through HuR's association with protein ligands pp32 and APRIL (14, 15). Finally, hypoxia has been shown to regulate the expression and stability of mRNAs encoding VEGF, tyrosine hydroxylase, and erythropoietin (47).
HuR is a ubiquitously expressed member of the elav (embryonic-lethal abnormal visual in Drosophila melanogaster) family of RNA-binding proteins, which also comprises the neuron-specific proteins HuD, HuC, and Hel-N1 (16, 38). The protein products of all four elav genes bind with high affinity and specificity to AREs in a variety of mRNAs, such as those encoding VEGF, p21, cyclin A, cyclin B1, GLUT-1, and c-fos, and are believed to increase mRNA stability, mRNA translation, or both (12, 29, 30, 39, 52). While the precise mechanisms regulating HuR function in mRNA stabilization remain largely unknown, it is becoming increasingly apparent that HuR's subcellular localization is intimately linked to its function. Since HuR is predominantly (>90%) localized in the nuclei of unstimulated cells, it has been proposed that the mRNA-stabilizing influence of HuR requires its translocation to the cytoplasm (1, 12, 13, 31, 48, 52). The HuR-dependent increase in the half-life of p21 mRNA following exposure to short-wavelength UV light (UVC) was shown to be associated with increased cytoplasmic presence of HuR (52). It was subsequently reported that the levels of cytoplasmic HuR varied throughout the cell division cycle, being highest during S and G2, the period of greatest stability of the ARE-containing HuR target mRNAs encoding cyclins A and B1 (53).
In light of the observation that HuR translocation to the cytoplasm is closely linked to its ability to stabilize target mRNAs, we sought to elucidate the signaling events controlling HuR subcellular localization. An initial screen, performed using a wide variety of inhibitors and activators of signaling pathways, revealed the lack of involvement of classical stress-regulated kinases (ERK, JNK, p38, PKC, PKA, etc.) and instead uncovered the critical participation of the AMP-activated kinase (AMPK), also known as a cellular sensor of metabolic stress, in this process. AMPK activation decreased cytoplasmic HuR and consequently decreased the binding of HuR to target transcripts (cyclin A, cyclin B1, and p21 mRNAs) and diminished the expression and half-lives of such HuR target mRNAs. Conversely, inhibition of AMPK led to a dramatic increase in cytoplasmic HuR, which in turn enhanced the binding of HuR to target transcripts and heightened their expression and half-lives.
MATERIALS AND METHODS
Cell culture, treatments, infection, and cell cycle distribution.
Human colorectal carcinoma RKO cells were cultured as described previously (19). Ionomycin, actinomycin D, suramin, calyculin A, genistein, herbimycin A, tetradecanoyl phorbol acetate, staurosporine, N-acetyl cysteine, wortmannin, rapamycin, antimycin A, sodium azide, 5-amino-imidazole-4-carboxamide riboside (AICAR), 5′-AMP, vanadate, and lithium acetate were from Sigma (St. Louis, Mo.). Bisindolylmaleimide I, H7, SB-203580, and PD-098059 were from Calbiochem (San Diego, Calif.). Phosphorothioate oligonucleotides targeting JNK expression were used as described previously (3). Adenoviruses expressing either the control green fluorescent protein (GFP) (AdGFP), a dominant-negative isoform of the α1 subunit of AMPK [Ad(DN)AMPK], or a constitutively active isoform of the AMPK α1 subunit [Ad(CA)AMPK] (59) were amplified and titered in 293 cells by standard methodologies. Infections were carried out in serum-free Dulbecco's modified Eagle's medium for 4 h. Infection efficiency of RKO cells was determined by infection with AdGFP at various numbers of PFU per cell and assessment of the percentage of GFP-expressing cells 48 h later. For >90% infection, a level of 100 PFU/cell was required, in accordance with the low infection rates of RKO cells (19); this level was used in all infections. Fluorescence-activated cell sorter (FACS) analysis was performed as described previously (53).
Northern and Western blot analyses and subcellular fractionation.
Northern blot analysis and 18S rRNA assessments were performed as described previously (20). For detection of mRNAs encoding p21, cyclin A, cyclin B1, and β-actin, PCR fragments encompassing the respective coding regions were random-primer labeled with [α-32P]dATP; detection of cyclin D1 mRNA was carried out as previously described (35). Northern blotting signals were quantitated with a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.). For Western blotting, whole-cell (20 μg), cytoplasmic (40 μg), and nuclear (10 μg) lysates were size fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes. HuR was detected with monoclonal antibody 19F12 (30, 52), β-actin was detected with a monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.), and BAF57c was detected with a polyclonal antibody (55). Following secondary-antibody incubations, signals were detected by enhanced chemiluminescence. Cytoplasmic, nuclear, and whole-cell fractions were prepared as described previously (52).
Immunofluorescence.
Cells were seeded on coverslips and treated. At the end of the treatment period, cells were fixed for 15 min in phosphate-buffered saline (PBS) containing 4% paraformaldehyde and permeabilized for 15 min in PBS containing 0.4% Triton X-100. After incubation for 16 h in blocking buffer (PBS containing 2% bovine serum albumin and 0.1% Tween 20), coverslips were incubated for 1 h in a 1:500 dilution of mouse anti-HuR (Santa Cruz Biotechnology) prepared in blocking buffer. Following washes with blocking buffer, samples were incubated for 1 h with a mixture of horse anti-mouse Texas red (1:200; Jackson Laboratories) and Hoechst 33342 (1:5,000; Molecular Probes). After washes with blocking buffer, coverslips were mounted in Vectashield (Vector Laboratories) and visualized with an Axiovert 200 M microscope (Zeiss; 63× lens) by using separate channels for the analysis of phase-contrast images, red fluorescence, and blue fluorescence. Images were then processed with the AxioVision, version 3.0, program (Zeiss). Representative photographs from three independent experiments are shown.
Synthesis of radiolabeled transcripts.
cDNA, prepared from RKO cell RNA, was used as a template for PCR amplification of DNA encoding the 3′UTRs of p21, cyclin B1, cyclin A, and cyclin E as described previously (52, 53). All 5′ oligonucleotides contained the T7 RNA polymerase promoter sequence CCAAGCTTCTAATACGACTCACTATAGGGAGA(T7). To prepare the 3′ UTR template for p21, oligonucleotides (T7)CCAAGAGGAAGCCCTAATCC and GAAAAGGAGAACACGGGATG (region encoded by nucleotides 554 to 851) were used. To prepare the 3′ UTR template for cyclin A, oligonucleotides (T7)CCAGAGACACTAAATCTGTAAC and GGTAACAAATTTCTGGTTTATTTC (region encoded by nucleotides 1499 to 2718) were used. To prepare the 3′ UTR template for cyclin B1, oligonucleotides (T7)GTCAAGAACAAGTATGCCA and CTGAAGTGGGAGCGGAAAAG (region encoded by nucleotides 1369 to 1702) were used. To prepare the 3′ UTR template for cyclin E, oligonucleotides (T7)CACAGAGCGGTAAGAAGCAG and GGATAGATATAGCAGCACTTAC (region encoded by nucleotides 1169 to 1714) were used. PCR fragments served as templates for the synthesis of corresponding RNAs (18), which were used at a specific activity of 100,000 cpm/μl (2 to 10 fmol/μl).
RNA-protein binding reactions and supershift assays.
Reaction mixtures (10 μl) containing 1 μg of tRNA, 2 to 10 fmol of RNA, and 5 μg of protein in reaction buffer (15 mM HEPES [pH 7.9], 10 mM KCl, 10% glycerol, 0.2 mM dithiothreitol [DTT], 5 mM MgCl2) were incubated for 30 min at 25°C and digested with RNase T1 (100 U/reaction) for 15 min at 37°C. Complexes were resolved by electrophoresis through native gels (7% acrylamide in 0.25× Tris-borate-EDTA buffer). Gels were subsequently dried, and radioactivity was visualized with a PhosphorImager. For supershifts, 4 μg of antibody was incubated with lysates for 1 h on ice before addition of radiolabeled RNA; all subsequent steps were as described for gel shift. The anti-p38 antibody used in the supershift assays was from Pharmingen (San Diego, Calif.).
Pull-down assay.
PCR fragments encompassing the 3′ UTRs of p21, cyclin A, cyclin B1, and cyclin E genes, which were synthesized bearing T7 on the 5′ ends, served as templates for in vitro transcription using biotinylated CTP (Sigma; 1/10 of total CTP), as described previously (53). The binding of proteins to biotinylated transcripts was performed with 140 μg of cytoplasmic lysate supplemented with RNase inhibitor (5′→3′, Boulder, Colo.), a protease inhibitor cocktail (Sigma), and 2 μg of biotinylated transcript for 30 min at room temperature. Complexes were isolated with paramagnetic streptavidin Dynabeads (Dynal, Oslo, Norway), washed with PBS, and subjected to Western blot analysis to detect HuR.
AMPK assay.
AMPK was assayed as described previously (24). Briefly, AMPK was immunoprecipitated from 5 μg of cell lysate using 1 μg of anti-α1 and 1 μg of anti-α2 polyclonal antibodies in AMPK immunoprecipitation (IP) buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 50 mM NaF, 5 mM sodium pyrophosphate, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.1 mM benzamidine, 0.1 mM phenylmethylsulfonyl fluoride [PMSF], 5 μg of soybean trypsin inhibitor/ml) for 2 h at 4°C. Immunocomplexes were washed with IP buffer plus 1 M NaCl and then with a buffer containing 62.5 mM HEPES, pH 7.0, 62.5 mM NaCl, 62.5 mM NaF, 6.25 mM sodium pyrophosphate, 1.25 mM EDTA, 1.25 mM EGTA, 1 mM DTT, 1 mM benzamidine, 1 mM PMSF, and 5 μg of soybean trypsin inhibitor/ml. AMPK activity in immunocomplexes was determined by phosphorylation of peptide HMRSAMSGLHLVKRR (SAMS) (24) in reaction buffer (50 mM HEPES [pH 7.4], 1 mM DTT, 0.02% Brij 35, 0.25 mM SAMS, 0.25 mM AMP, 5 mM MgCl2, 10 μCi of [γ-32P]ATP) for 10 min at 30°C. Assay mixtures were spotted onto P81 filter paper and rinsed in 1% (vol/vol) phosphoric acid with gentle stirring to remove free ATP. The phosphorylated substrate was measured by scintillation counting.
RESULTS
Signaling events regulating the cytoplasmic localization of HuR.
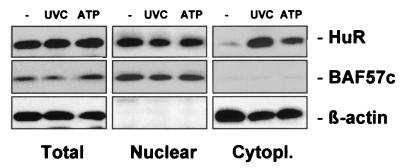
As shown in Fig. 1, following exposure of RKO cells to UVC, the level of HuR in the cytoplasm increased markedly, in agreement with earlier observations (52). Treatment with ATP also moderately elevated the HuR level in the cytoplasm (Fig. 1). These alterations in HuR's subcellular localization did not result from overall increased expression of HuR, since the total cellular HuR did not change. Instead, they likely result from elevated transport of HuR to the cytoplasm, as previously reported (1, 12, 13, 31, 48, 52). The absence of a correspondingly marked reduction in nuclear HuR is explained by the relative abundance of HuR in each cellular compartment: in untreated RKO cells, HuR is about 20-fold more abundant in the nucleus, so even substantial elevations in cytoplasmic HuR produce little change in the nuclear HuR. Despite its relatively low cytoplasmic abundance, HuR is readily detectable on Western blots, where 40 μg of cytoplasmic lysates is routinely assayed. Hybridization of the same membranes with antibodies recognizing nucleus- and cytoplasm-specific proteins (BAF57c and β-actin, respectively) verified that nuclear proteins did not leak into the cytoplasmic fractions during cell treatment or fractionation and further revealed evenness in loading and transfer of the samples (Fig. 1). Analysis of BAF57c and β-actin was routinely performed on all Western blots in which subcellular fractions were assessed.
FIG. 1.
Subcellular localization of HuR in RKO cells. Three hours after exposure of RKO cells to either 20-J/m2 UVC (lane UVC) or 1 mM ATP (lane ATP), whole-cell (20 μg), nuclear (10 μg), and cytoplasmic (40 μg) lysates were subjected to Western blot analysis to monitor the expression of HuR. Sequential hybridizations using antibodies against BAF57c and β-actin were carried out to assess the quality of the fractionation process and the uniformity in loading and transfer of nuclear and cytoplasmic samples, respectively. Lane −, untreated lysates.
Given the growing body of evidence showing that HuR translocation to the cytoplasm is linked to its ability to stabilize target mRNAs, we sought to identify the signaling pathway(s) influencing the subcellular localization of HuR. We first tested a large panel of modulators of signaling pathways for their influence on both basal and treatment-induced cytoplasmic HuR levels. This assessment was carried out by Western blot analysis of HuR abundance in the cytoplasmic and nuclear fractions such as that shown in Fig. 2. Our results showed that the majority of such activators and inhibitors of signaling cascades had no influence on the subcellular localization of HuR (Table 1). For example, none of the MAPKs, ERK, JNK, and p38, were found to be involved in regulating cytoplasmic HuR levels. We had anticipated their potential participation in this process, since MAPKs are activated by the same agents (UVC, actinomycin D [ActD], methyl methanesulfonate, prostaglandin A2, etc.) that have been reported to increase cytoplasmic HuR (52). Other classical signaling molecules, such as PKC, PKA, phosphatidylinositol 3-kinase (PI 3-kinase), S6 kinase, and additional proteins involved in growth factor- and stress-activated cascades, also appeared not to be involved in regulating the abundance of cytoplasmic HuR (Table 1). By contrast, several treatments tested did influence the cytoplasmic presence of HuR (Table 1 and Fig. 2). Several of these agents modulated the activity of AMPK, an enzyme considered to be a cellular sensor of metabolic stress. AMPK activation by either antimycin A, sodium azide, AICAR (currently the most widely used pharmacological activator of AMPK [24]), or 5′-AMP led to HuR's reduced cytoplasmic localization (Table 1), both in unstimulated cells and in cells treated with UVC (as shown in Fig. 2). On the other hand, treatment with ATP, an inhibitor of AMPK, produced a significant increase in cytoplasmic HuR (Table 1; Fig. 1).
FIG. 2.
Methodology used to assess the effect of the various treatments on the cytoplasmic HuR levels. Shown is a Western blot analysis of HuR abundance in whole-cell, nuclear, and cytoplasmic lysates prepared as described in the legend for Fig. 1 from RKO cells that were either left untreated or treated with AICAR. Lanes −, unirradiated cells (control); lanes UVC, treatment with UVC.
TABLE 1.
Survey of modulators of signaling pathways: effect on subcellular localization of HuR
| Treatmenta | Target | Dose | Effect on cytoplasmic HuRb |
|---|---|---|---|
| Suramin | Growth factor receptor inhibitor | 300 μM | — |
| Genistein | PTKe inhibitor | 20 μM | — |
| Herbimycin A | PTK inhibitor | 10 μM | — |
| Bisindolylmaleimide I | PKC inhibitor | 1 μM | — |
| TPAc | PKC activator | 1 μM | — |
| H7 | PKA/PKC inhibitor | 50 nM | — |
| Staurosporine | PKA, PKC, PKG, and CAM kinase inhibitor | 40 nM | — |
| N-Acetyl cysteine | Oxidants | 20 mM | — |
| SB-203580 | p38MAPK inhibitor | 20 μM | — |
| PD-098059 | MEK inhibitor | 20 μM | — |
| ASJNKd oligonucleotides | Inhibitor of JNKMAPK expression | 400 nM | — |
| Wortmannin | PI 3-kinase inhibitor | 50 nM | — |
| Rapamycin | S6 kinase inhibitor | 100 nM | — |
| Antimycin A | AMPK activator | 1 μM | ↓ |
| Sodium azide | AMPK activator | 2 mM | ↓ |
| AICAR | AMPK activator | 2 mM | ↓ |
| 5′-AMP | AMPK activator | 2 mM | ↓ |
| Calyculin A | PP1, PP2A, and PP2B inhibitor | 30 nM | ↑ |
| ATP | AMPK inhibitor | 1 mM | ↑ |
| Ionomycin | Ca2+ ionophore | 2 μM | ↓ |
| Vanadate | PTK phosphatase inhibitor | 60 μM | ↓ |
| Lithium acetate | Inositol phosphate inhibitor | 2 mM | ↓ |
A list of positive controls serving to assess the effectiveness of drugs is available upon request.
—, no effect; ↓, reduction; ↑, elevation.
TPA, tetradecanoyl phorbol acetate.
ASJNK, antisense JNK oligonucleotides.
PTK, protein tyrosine kinase.
Role of AMPK in regulating cytoplasmic HuR.
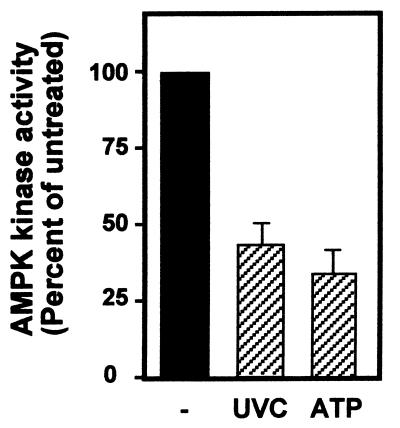
To further explore the potential involvement of AMPK in regulating HuR's presence in the cytoplasm, we directly examined the influence of AMPK activators and inhibitors on the subcellular localization of HuR. We began by examining AMPK activity in RKO cells following treatments known to activate cytoplasmic HuR (Fig. 1). Following IP of AMPK using a combination of polyclonal antibodies recognizing either the α1 or the α2 subunit (catalytic) of AMPK, the kinase activity associated with the immunoprecipitates was measured with a synthetic peptide, as previously reported (24). As shown (Fig. 3), both treatments led to a sizeable inhibition of AMPK activity. It should be noted that AMPK activity changes only moderately (at best approximately four- to fivefold) in the presence of even strong activators or inhibitors, but these small alterations in activity are capable of eliciting potent changes in downstream target effectors (23, 25). The moderate alterations in AMPK activity contrast with the dramatic changes seen in the activity of other kinases (notably MAPKs) in response to stress and mitogenic stimulation.
FIG. 3.
Inhibition of AMPK kinase activity in RKO cells by treatments that enhance HuR presence in the cytoplasm. Three hours after exposure of RKO cells to either 20-J/m2 UVC (lane UVC) or 1 mM ATP (lane ATP), whole-cell lysates were prepared and AMPK kinase activity was tested after IP with polyclonal antibodies recognizing the AMPK α1 and α2 subunits and with synthetic peptide SAMS as the substrate. −, untreated lysates.
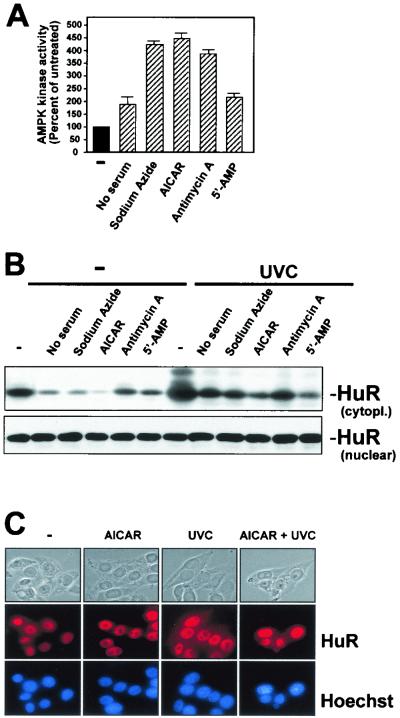
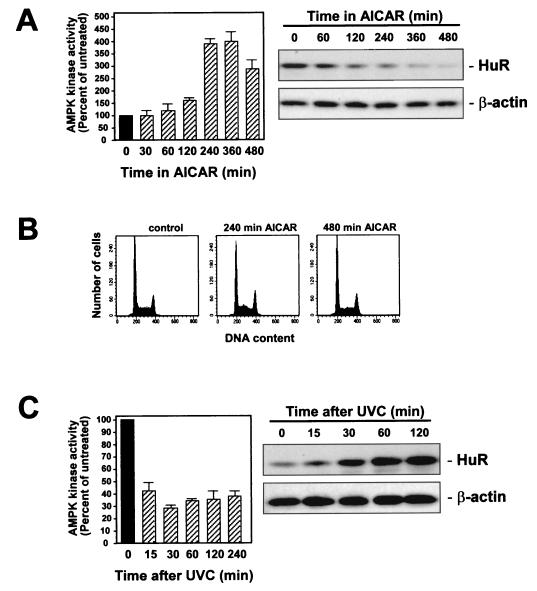
Next, we investigated the influence of AMPK activators on the cytoplasmic HuR levels. All of the treatments examined (sodium azide, AICAR, antimycin A, 5′-AMP, and serum starvation) elevated AMPK activity in RKO cells (Fig. 4A). Similarly treated cells were fractionated into nuclear and cytoplasmic components, and HuR expression was assayed by Western blotting. As shown, basal cytoplasmic HuR levels were substantially reduced in the presence of each of the AMPK activators. Moreover, UVC-enhanced cytoplasmic HuR was greatly diminished when cells were preincubated with AMPK activators before UVC irradiation (Fig. 4B). Nuclear HuR, by contrast, did not change noticeably (Fig. 4B). Confirmation that UVC and AICAR were capable of eliciting changes in HuR's subcellular localization was obtained by in situ HuR detection using immunofluorescence (Fig. 4C). HuR was mostly nuclear in all treatment groups, and the low cytoplasmic signal observed in untreated cells (Fig. 4C, images labeled −) was effectively eliminated in the AICAR-treated populations; similarly, while UVC triggered a substantial increase in cytoplasmic HuR, pretreatment with AICAR (images labeled AICAR + UVC) greatly reduced this cytoplasmic elevation. However, given the relatively low HuR abundance in RKO cells, the detection of cytoplasmic HuR by immunofluorescence was rather poor. We therefore conducted all subsequent assessments of HuR subcellular localization by Western blotting, which allowed use of sufficient cytoplasmic material for reliable analysis and quantitation. As shown in Fig. 5A, the increase in AMPK activity mirrored the reduction of HuR in the cytoplasm; importantly, the change in subcellular localization of HuR did not occur as a consequence of altered cell cycle distribution, as the cell cycle profiles remained essentially unaltered throughout the treatment (Fig. 5B). Inhibition of AMPK by UVC also correlated with the rates of increased cytoplasmic HuR (Fig. 5C). The rough correlation between the two processes (Fig. 4 and 5) likely indicates that the effect of AMPK on HuR is indirect.
FIG. 4.
Effect of AMPK activators on the subcellular localization of HuR. Shown is an assessment of AMPK activity (A) and HuR levels (B) in cytoplasmic (40 μg) and nuclear (10 μg) lysates prepared from RKO cells that were serum starved for 36 h or were treated for 4 h with either 2 mM sodium azide, 2 mM AICAR, 1μM antimycin A, or 1 mM ATP. For Western blot analysis, cells were either mock irradiated but otherwise exposed to AMPK-activating treatments continuously for 4 h (−) or were exposed to 20-J/m2 UVC 6 h after addition of AMPK activators and then cultured for an additional 3 h. (C) Immunofluorescence detection of HuR in RKO cells that were treated with AICAR, UVC, or both AICAR and UVC as explained in the legend for panel B. Top, phase-contrast images; middle, HuR immunofluorescence; bottom, Hoechst staining to visualize nuclei.
FIG. 5.
Kinetics of AMPK kinase activity and HuR subcellular localization. (A) Assessment of AMPK activity (left) and HuR presence in the cytoplasm (right) at the indicated times following treatment of RKO cells with 2 mM AICAR. (B) FACS analysis after treatment of RKO cells with AICAR for the times indicated. (C) Assessment of AMPK activity (left) and HuR presence in the cytoplasm (right) at the indicated times following treatment of RKO cells with 20-J/m2 UVC. Western blots (A and C) were stripped and rehybridized to assess β-actin levels in order to control for sample loading and transfer.
Cytoplasmic HuR levels are influenced by adenoviruses expressing mutant AMPK.
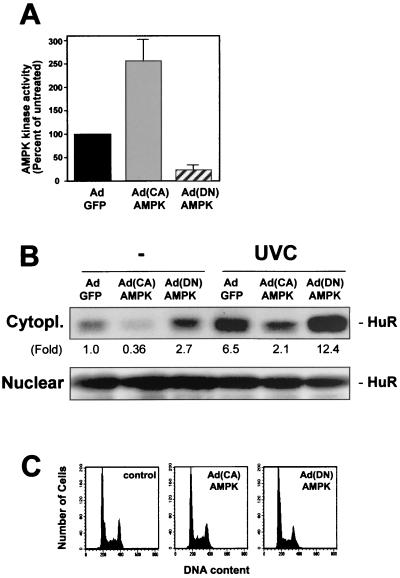
To gain direct evidence for the potential role of AMPK in regulating the levels of cytoplasmic HuR, adenovirus vectors were used to express each of two mutant forms of the AMPK α (catalytic) subunit; the vectors were Ad(CA)AMPK, carrying α1312 (a constitutively active α1 mutant), and Ad(DN)AMPK, which carries a dominant-negative α1 mutant (59). Compared with infections using a control adenovirus which expresses the GFP protein (AdGFP), infection with Ad(CA)AMPK led to a 2.5-fold increase in AMPK activity in RKO cells, with >90% cells infected at 100 PFU/cell (Fig. 6A). Importantly, such intervention led to a marked decrease in cytoplasmic HuR (Fig. 6B). Conversely, infection with Ad(DN)AMPK led to an approximately fourfold reduction in AMPK activity and markedly elevated cytoplasmic HuR (Fig. 6A and B). Similar results were obtained with a virus expressing a dominant-negative α2 isoform (not shown). The reduction or enhancement in HuR levels in the cytoplasm following infections to either activate or reduce the activity of AMPK was seen both for basal and for UVC-induced cytoplasmic HuR (Fig. 6B). Neither nuclear (Fig. 6B) nor total cellular HuR (not shown) was altered with the infections. As shown in Fig. 6C, the changes in cytoplasmic HuR did not arise as a consequence of altered cell cycle distribution, as the cell cycle profiles were not significantly altered by 48 h following adenovirus infection.
FIG. 6.
Influence of adenoviruses expressing mutant AMPK catalytic subunits on AMPK kinase activity and cytoplasmic HuR levels. Forty-eight hours after infection of RKO cells with 100 PFU of either AdGFP, Ad(CA)AMPK, or Ad(DN)AMPK/cell, AMPK activity (A) and HuR levels (B) were measured in the cytoplasmic (40 μg) and nuclear (10 μg) fractions of cells that were either left unirradiated or exposed to UVC (20 J/m2) and collected 3 h later. Fold, difference in HuR signal between indicated population and AdGFP-infected, unirradiated population. In panel A, data represent the means ± standard errors of the means from four independent experiments. (C) FACS analysis of RKO populations 48 h after infection.
AMPK-regulated binding of cytoplasmic HuR to target transcripts.
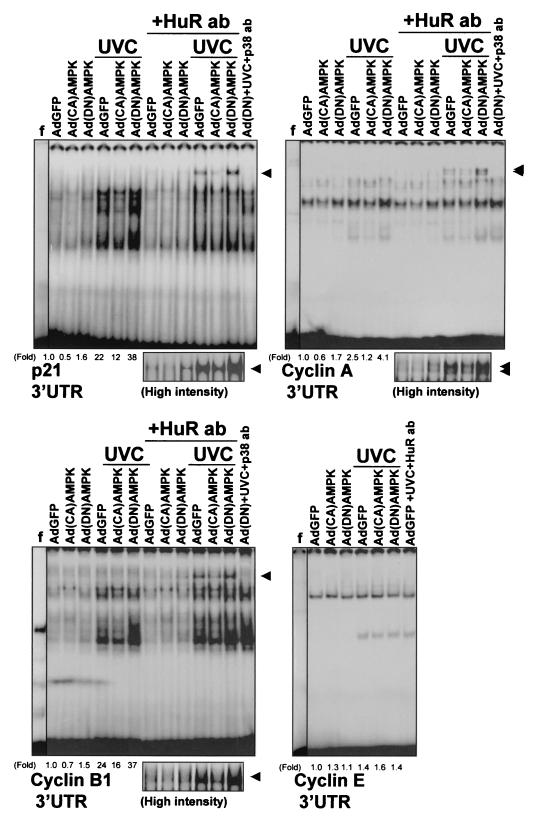
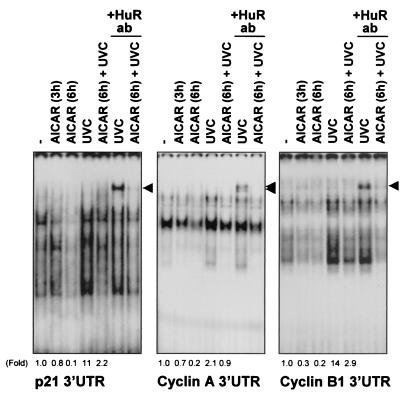
To assess the functional consequences of the AMPK-regulated presence of HuR in the cytoplasm, we set out to examine the influence of AMPK-triggered events on the expression of HuR target transcripts. First, we investigated the ability of HuR to bind to mRNAs encoding p21, cyclin A, and cyclin B1, previously reported to be targets of HuR binding and HuR-mediated stabilization (52-54). Depicted in Fig. 7 are electrophoretic mobility shift assays (EMSA) to study HuR-associated RNA-binding activity in cytoplasmic lysates from RKO cells 48 h after infection with either AdGFP, Ad(CA)AMPK, or Ad(DN)AMPK. When radiolabeled transcripts corresponding to the 3′UTRs of mRNAs encoding cyclin A, cyclin B1, and p21 were used, RNA-protein complexes formed readily, as previously reported (52, 53). However, complex formation was lower in Ad(CA)AMPK-infected cells than in AdGFP-infected cells, for both UVC-irradiated (UVC) and untreated (mock-irradiated) cells. Conversely, Ad(DN)AMPK-infected cells exhibited more-abundant binding to target transcripts than did AdGFP-infected cells. In each case, the presence of HuR in complexes containing either cyclin A, cyclin B1, or p21 transcripts was detected by supershift EMSA using an anti-HuR antibody (Fig. 7, lanes +HuR ab and lane AdGFP+UVC+HuR ab). The HuR-containing supershifted bands in both unirradiated and UVC-irradiated cells are also depicted at a higher intensity of signals (Fig. 7). A control antibody recognizing the MAPK p38 did not supershift any of the complexes [lanes Ad(DN)+UVC+p38 ab]. A control cyclin E transcript, which is not a target of HuR (53), did not form supershifts in the presence of the anti-HuR antibody (Fig. 7). Further characterization of HuR binding to these mRNAs, including EMSA using transcripts corresponding to the coding regions of mRNA for p21; cyclins A, B1, and E; and nuclear proteins and unlabeled competitor RNAs, is not included here, as it was reported previously (52, 53). AICAR treatment was capable of reducing the association of HuR with target mRNAs, both in cells treated with UVC and in control, mock-irradiated cells (Fig. 8). Again, direct evidence of HuR's involvement in these complexes came from supershift EMSA using an anti-HuR antibody (Fig. 8, lanes +HuR ab). In Fig. 7 and 8, the relative intensities of the shifted complexes are indicated (fold).
FIG. 7.
Effect of AMPK-expressing adenoviruses on the association of HuR with target transcripts. EMSA were performed using radiolabeled RNAs encoding the 3′ UTRs of p21, cyclin A, and cyclin B1 genes (see Materials and Methods) and proteins present in cytoplasmic lysates of RKO cells that were infected with either AdGFP, Ad(CA)AMPK, or Ad(DN)AMPK and then were either exposed to 20-J/m2 UVC or left unirradiated and collected 3 h later. The presence of HuR in RNA-protein complexes was assayed by monitoring the formation of supershifted bands in the presence of anti-HuR antibodies (+HuR ab) or control antibodies (+p38 ab). Arrowheads, supershifted complexes. Fold, difference in total signals of radiolabeled complexes between indicated cells and AdGFP-infected, unirradiated cells. Fold differences were not calculated for supershift lanes. f, free probe, not incubated with cytoplasmic lysate; high intensity, supershifted bands developed at greater intensity.
FIG. 8.
Influence of AMPK modulators on the association of HuR with target transcripts. EMSA was performed using radiolabeled transcripts encoding the 3′ UTRs of p21, cyclin A, and cyclin B1 genes and proteins present in cytoplasmic lysates of RKO cells that had either been treated with 2 mM AICAR for the times indicated and then left unirradiated or exposed to 20-J/m2 UVC (+UVC). The presence of HuR in the RNA-protein complexes was assayed by monitoring the formation of supershifted bands in the presence of anti-HuR antibodies (+HuR ab). Arrowheads, supershifted complexes. Fold, relative signals of radiolabeled complexes compared with those measured in untreated (−), unirradiated cells. Fold differences were not calculated for supershift lanes.
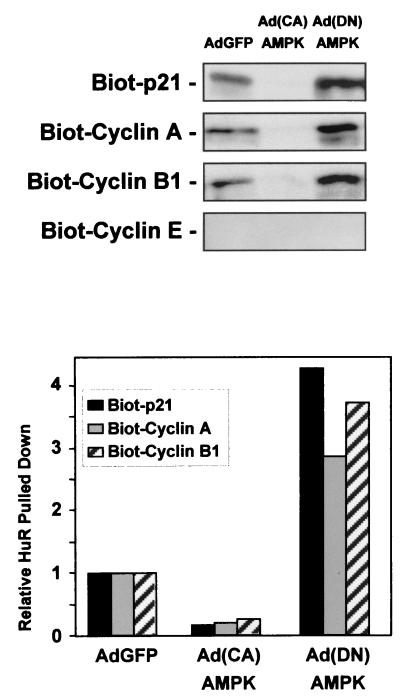
To obtain independent evidence for the association between HuR and its target transcripts, lysates from RKO cells in the three infection groups were incubated with biotinylated RNAs that encompassed the 3′UTRs of p21, cyclin A, and cyclin B1 mRNAs. As shown in Fig. 9, these biotinylated transcripts effectively pulled down HuR (described in Materials and Methods), as detected by Western blot analysis. A control biotinylated transcript encompassing the cyclin E mRNA, which is not a target of HuR, did not pull down HuR. In agreement with the gel shift and supershift data, reduced association between target biotinylated transcripts and HuR was seen in lysates from Ad(CA)AMPK-infected cells, while the most-abundant associations were seen in lysates from the Ad(DN)AMPK-infected populations (Fig. 9).
FIG. 9.
HuR forms complexes with biotinylated target transcripts. Cytoplasmic lysates prepared 48 h after infection of RKO cells with either AdGFP, Ad(CA)AMPK, or Ad(DN)AMPK were incubated with biotinylated transcripts corresponding to the 3′ UTRs of the genes encoding the proteins indicated. Biotinylated (Biot) RNA-protein complexes were pulled down with streptavidin-conjugated magnetic beads and analyzed by Western blotting to assess HuR levels. The pull-down shown is representative of two independent experiments. The graph depicts quantitation of the signals on the Western blots.
In summary, AMPK-activating treatments such as AICAR and infection with Ad(CA)AMPK, which consequently reduced cytoplasmic HuR, were capable of greatly reducing HuR's binding to target transcripts encoding p21, cyclin B1, and cyclin A. Conversely, AMPK-inhibitory treatments such as UVC and infection with Ad(DN)AMPK enhanced binding of HuR to target mRNAs. Similarly, the UVC-mediated increase in HuR association with target RNAs was blocked by AMPK activators [AICAR and Ad(CA)AMPK] and it was potentiated by AMPK inhibition after Ad(DN)AMPK infection.
AMPK regulates the expression and half-lives of HuR target mRNAs.
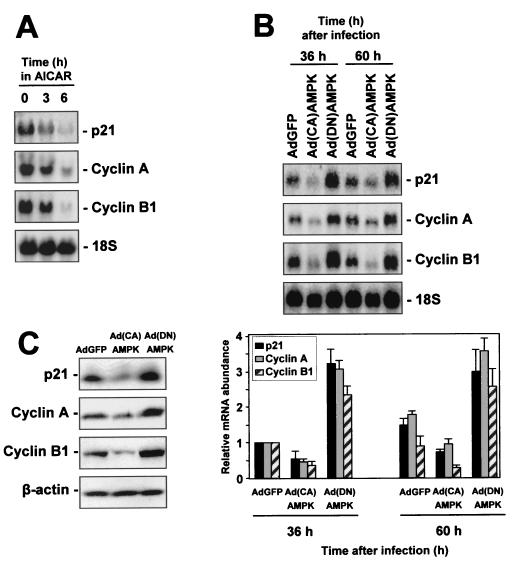
To investigate how AMPK-regulated events influencing cytoplasmic HuR in turn affect the expression of HuR target transcripts encoding p21, cyclin A, and cyclin B1, Northern blot analysis was performed. Levels of the respective mRNAs were assessed following various interventions that modulate AMPK activity (Fig. 10). Exposure to AICAR for increasing lengths of time progressively reduced the expression of mRNAs encoding p21, cyclin A, and cyclin B1 (Fig. 10A). Such reductions in mRNA levels were in keeping with AICAR-mediated increase in AMPK activity, reduction in cytoplasmic HuR (Fig. 5), and reduction in binding of HuR to target mRNAs (Fig. 8).
FIG. 10.
AMPK-modulatory interventions influence the expression of targets of HuR-mediated mRNA stabilization. (A) RKO cells were either left untreated or were treated with AICAR (2 mM) for 3 or 6 h, whereupon RNA was prepared and subjected to Northern blot analysis to assess the expression levels of mRNAs encoding p21, cyclin A, and cyclin B1. (B) Top, measurement of the levels of mRNAs encoding p21, cyclin A, and cyclin B1 in RKO cells at the indicated times following adenovirus infection. Bottom, quantitation of mRNA levels, with data representing the means ± standard errors of the means of three independent experiments. 18S rRNA signals served to monitor the equality of loading and transfer among samples. (C) Western blot analysis of protein expression 48 h after infection.
Infection with either AdGFP, Ad(CA)AMPK, or Ad(DN)AMPK further established that expression of mRNAs that are stabilized by HuR was influenced by AMPK activity. As shown in Fig. 10B, 36 and 60 h after Ad(CA)AMPK infection, the basal levels of p21, cyclin A, and cyclin B1 mRNAs were substantially reduced over those seen in AdGFP-infected cells. By contrast, Ad(DN)AMPK-infected populations exhibited heightened mRNA levels relative to what was seen in AdGFP-infected cells (Fig. 10B). Western blot analysis of p21, cyclin A, and cyclin B1 revealed that the changes in mRNA levels led to changes in protein expression (Fig. 10C).
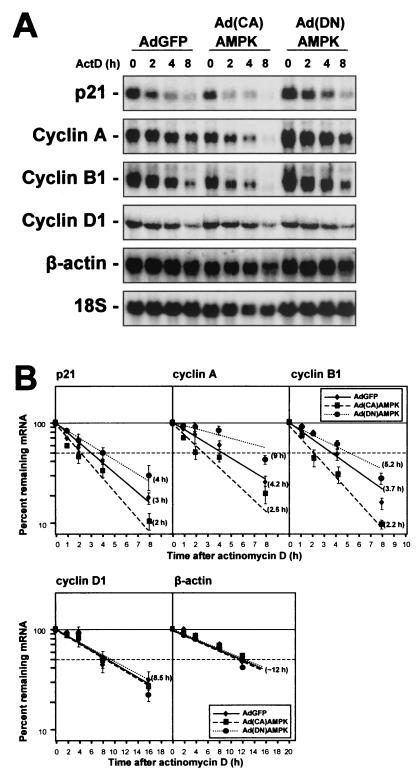
In addition, the half-life of each transcript was assessed by an ActD-based approach (Fig. 11). Briefly, addition of ActD (at time zero) to block new mRNA transcription was followed by RNA extraction at the indicated times thereafter and Northern blot analysis to monitor the rate of clearance of the transcript of interest (Fig. 11A). While this methodology has drawbacks and cannot be applied to the study of certain genes, it has been useful in determining the apparent half-lives of many mRNAs, including those that encode p21, cyclin A, and cyclin B1 (52-54). By this approach, the half-life of p21 mRNA was approximately 3 h in AdGFP-infected cells but decreased to 2 h in Ad(CA)AMPK-infected cells and increased to about 4 h in Ad(DN)AMPK-infected cells. Similarly, the half-life of the cyclin A mRNA varied from 4.2 h in AdGFP-infected cells to 2.5 and ∼9 h in Ad(CA)AMPK- and Ad(DN)AMPK-infected populations, respectively. In AdGFP-infected cells, the cyclin B1 mRNA exhibited a half-life of 3.7 h, but the half-life was reduced to 2.2 h in Ad(CA)AMPK-infected cells and elevated to 5.2 h in Ad(DN)AMPK-infected cells (Fig. 11B). Hybridizations were also carried out to monitor the half-lives of control mRNAs encoding cyclin D1 (a labile, ARE-containing mRNA that is not a target of HuR) and β-actin. Assessment of the stability of these mRNAs revealed that the effects of AMPK modulators were specific for HuR target mRNAs, as their half-lives remained essentially unchanged (∼8.5 and ∼12 h, respectively) in all three infection groups (Fig. 11B).
FIG. 11.
AMPK-modulatory interventions influence the half-lives of mRNAs that are stabilized by HuR. (A) Assessment of the stability of mRNAs encoding p21, cyclin A, cyclin B1, cyclin D1, and β-actin in RKO cells 48 h after infection with the adenoviruses indicated. (B) mRNA half-lives were calculated after treating cells with 2 μg of ActD/ml, preparing RNA at the times indicated, and measuring p21, cyclin A, and cyclin B1 mRNA Northern blot signals, normalizing them to 18S rRNA signals, and plotting them on a logarithmic scale (bottom). Note that the time scales for the cyclin D1 and β-actin mRNA plots are different. Horizontal dashed lines, 50% of level for untreated cells. Data represent the means ± standard errors of the means for three independent experiments.
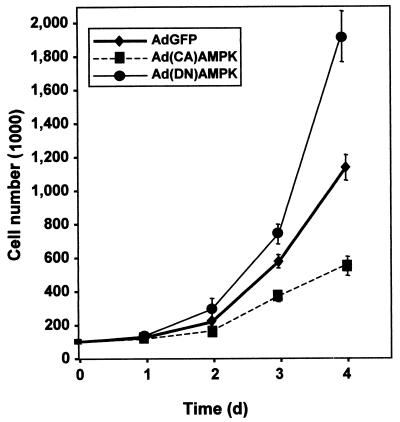
Finally, the impact of altering AMPK activity on cell proliferation was assessed by monitoring the growth rates of RKO populations. Compared with control infections (AdGFP), cells infected with Ad(CA)AMPK exhibited reduced growth rates, while Ad(DN)AMPK-infected cells proliferated more rapidly (Fig. 12). We propose that AMPK may influence the proliferative status of the cell, at least in part, through its regulation of HuR, which in turn affects the expression of cycle regulatory genes.
FIG. 12.
Influence of AMPK on cell proliferation. RKO cells (100,000 cells per dish) were infected with 100 PFU/cell, and cell numbers were monitored every 24 h thereafter with a hemacytometer. The experiment was done in triplicate, and data are means ± standard errors of the means.
DISCUSSION
In this study, we report that the levels of cytoplasmic HuR are potently influenced by the activity of AMPK, an enzyme that plays a key physiological role in the response to cellular stresses causing ATP depletion, including metabolic stresses (25, 27). Contrary to our expectations, none of the major stress-activated signaling cascades (ERK, JNK, and p38 pathways) or other more general signaling molecules (PI-3 kinase and PKC) appeared to influence HuR cytoplasmic levels (Table 1). By contrast, activators of AMPK, including various cell treatments (AICAR, antimycin A, etc.) and infection with an adenovirus that expresses a constitutively active form of the α (catalytic) subunit of AMPK reduced the cytoplasmic levels of HuR. On the other hand, inhibition of AMPK activity either through cell treatment (UVC, ATP, etc.) or by infection with an adenovirus expressing a dominant-negative α subunit of AMPK caused elevations in the cytoplasmic HuR levels. Expression of HuR target mRNAs encoding cyclin A, cyclin B1, and p21 varied according to the AMPK-modulated cytoplasmic HuR. AMPK activation led to decreased binding of cytoplasmic HuR to target transcripts and consequently led to reduced steady-state levels and half-lives of such mRNAs. Conversely, decreased AMPK activity resulted in increased binding of cytoplasmic HuR to target transcripts and elevated the expression and half-lives of such target mRNAs.
AMPK was discovered almost 3 decades ago, but details of its regulation have only recently begun to be elucidated. Highly conserved throughout evolution, AMPK is believed to function as a cellular metabolic sensor or a “low fuel warning system” of the cell (25). Structurally, mammalian AMPK is a heterotrimer of three subunits: one catalytic subunit (α) and two regulatory subunits (β and γ). AMPK activity is ubiquitous, although different isoforms of AMPK subunits display tissue-specific and preferential subcellular localizations (e.g., α2 subunits are partly nuclear, while α1 subunits are only found in the cytoplasm). The kinase is activated directly by elevations in 5′-AMP and inhibited by high concentrations of ATP. Cellular stresses that elevate the AMP/ATP ratio in the cell, such as arsenite, azide, hypoglycemia, serum/growth factor depletion, and treatment with the pharmacological agent AICAR (which mimics the effect of AMP), can cause activation of AMPK (26, 27). In turn, AMPK inhibits biosynthetic pathways, thus conserving energy, while it activates catabolic pathways, thus generating more ATP. In addition, AMPK can regulate gene expression, as shown both in Saccharomyces cerevisiae, where AMPK homologue SNF1 plays a pivotal role in regulating glucose-related genes, and in mammalian cells, where AMPK has been proposed to regulate the expression of genes important for energy conservation at a time of low fuel availability (25). Furthermore, AMPK has been postulated to modulate cell proliferation and to aid in the implementation of cellular growth arrest under adverse growth conditions. For example, AICAR-mediated AMPK activation was recently shown to suppress cell growth, and this effect was proposed to be mediated, at least in part, by AICAR-triggered phosphorylation of tumor suppressor p53 (28). Our findings reported here indicate that AMPK-mediated suppression of cell cycle-regulatory genes may play a pivotal role in AMPK-triggered growth inhibition (Fig. 10 to 12). Elevated AMPK activity potently decreased cytoplasmic HuR levels, thus preventing HuR-mediated stabilization and enhanced expression of HuR target mRNAs encoding p21, cyclin A, and cyclin B1. By contrast, conditions that inhibit AMPK activity induced cytoplasmic HuR, which in turn led to the stabilization and increased abundance of p21, cyclin A, and cyclin B1. We postulate that such changes in proliferation-associated genes contribute to the altered growth of cells in which AMPK activity is modulated (Fig. 12).
Elevated cyclin A and cyclin B1 expression clearly contributes to enhancing cell proliferation. For p21, however, the situation is less straightforward. While at first it might seem counterintuitive that the cell simultaneously elevates the expression of proliferative genes encoding cyclin A and cyclin B1 and the gene encoding the growth inhibitor p21, we believe this coordinate expression suits well the AMPK-dictated growth requirements of the cell. Even though robustly overexpressed p21 functions as a universal cdk inhibitor (60), moderate elevations in the levels of p21 such as those described here may have a more complex function in cell cycle progression. First, p21 is an integral part of active cdk/cyclin complexes, and its presence within the complex is generally believed to be required for cdk activity (62, 63). Second, at low concentrations, p21 promotes the assembly of active cdk-containing complexes (33). The cdk-inhibitory function of p21 is exerted when multiple copies of p21 per cdk/cyclin complex exist. Thus, concomitant downregulation of cyclin A, cyclin B1, and p21 genes under conditions of activated AMPK activity may represent a valuable coordinate effort to lower the proliferative status of the cell under adverse growth conditions (low nutrient availability, for example). Conversely, joint upregulation of all three genes in response to lower AMPK activity may contribute to an optimal proliferative state when growth conditions are adequate. Finally, the possibility remains that AMPK-modulated p21 gene upregulation does indeed inhibit cyclin/cdk complexes and could perhaps serve to ensure that the cell's proliferative potential is kept in check and that no excessive proliferation ensues from the heightened abundance of cyclins A and B1.
The precise mechanisms whereby AMPK regulates the relative subcellular localization of HuR remain to be elucidated. Experiments aimed at assessing whether HuR is a direct phosphorylation target of AMPK are under way. However, comparison of the kinetics of HuR translocation and AMPK activity changes (Fig. 5) suggests the alternative, more plausible hypothesis that AMPK-triggered events regulate HuR abundance in the cytoplasm through indirect mechanisms involving the phosphorylation of additional, as yet unidentified targets. Support for this possibility came from reports that PP2A can dephosphorylate and inactivate AMPK in cell-free assay mixtures (9, 42, 51). Intriguingly, HuR-associated proteins SETα, SETβ, and PP32 function as inhibitors of PP2A. It is therefore possible, at least in theory, that SETα and -β and/or PP32 could help retain HuR in the nucleus by inhibiting PP2A, which in turn would allow active AMPK to elicit downstream effects. Nucleopore component CRM1 was recently shown to be involved in the nuclear export of HuR (15). However, CRM1 does not appear to be required for regulating AMPK-modulated cytoplasmic HuR levels, as treatment with CRM1 inhibitor leptomycin B actually caused a moderate increase in cytoplasmic HuR, both in unstimulated cells and in cells treated with UVC (our unpublished observations). The recent surge in interest in AMPK research will likely lead to the identification of downstream targets of this kinase, including those regulating HuR presence in the cytoplasm.
In summary, we have described a novel activity for AMPK as a regulator of the subcellular localization of HuR, which consequently influences the expression of HuR target mRNAs. Given the profound effect of AMPK on cell proliferation, and the clear growth-regulatory potential of many HuR target transcripts (cyclin A, cyclin B1, p21, c-fos, VEGF, etc.), it will be of great interest to elucidate the precise mechanisms whereby AMPK regulates HuR function. The present study provides additional support for the increasing role that HuR plays as a regulator of gene expression during stress, including genotoxic and oxidative stress (52) and also possibly hypoxic stress (47) and heat stress (14, 15). Our findings further implicate HuR in the cellular response to metabolic stress, underscoring HuR's function within the global adaptive response to changes in environmental conditions.
Acknowledgments
We thank M. B. Kastan for the RKO cells, W. Wang for the BAF57c antibody, and H. Furneaux for the 19F12 antibody. We thank O. Potapova for assistance with the JNK antisense transfections and R. Wersto, C. Morris, and F. J. Chrest for the flow cytometry analysis. We thank our colleague J. Martindale for critical reading of the manuscript.
REFERENCES
- 1.Atasoy, U., J. Watson, D. Patel, and J. D. Keene. 1998. ELAV protein HuA (HuR) can redistribute between nucleus and cytoplasm and is upregulated during serum stimulation and T cell activation. J. Cell Sci. 111:3145-3156. [DOI] [PubMed] [Google Scholar]
- 2.Bohjanen, P. R., B. Petryniak, C. H. June, C. B. Thompson, and T. Lindsten. 1992. AU RNA-binding factors differ in their binding specificities and affinities. J. Biol. Chem. 267:6302-6309. [PubMed] [Google Scholar]
- 3.Bost, F., N. Dean, R. McKay, and D. Mercola. 1997. The JUN kinase/stress-activated protein kinase pathway is required for epidermal growth factor stimulation of growth of human A549 lung carcinoma cells. J. Biol. Chem. 272:33422-33429. [DOI] [PubMed] [Google Scholar]
- 4.Brennan, C. M., and J. A. Steitz. 2001. HuR and mRNA stability. Cell. Mol. Life Sci. 58:266-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carballo, E., W. S. Lai, and P. J. Blackshear. 1998. Feedback inhibition of macrophage tumor necrosis factor-α production by tristetraprolin. Science 281:1001-1005. [DOI] [PubMed] [Google Scholar]
- 6.Chen, C. Y., F. Del Gatto-Konczak, Z. Wu, and M. Karin. 1998. Stabilization of interleukin-2 mRNA by the c-Jun NH2-terminal kinase pathway. Science 280:1945-1949. [DOI] [PubMed] [Google Scholar]
- 7.Chen, C. Y., and A. B. Shyu. 1995. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem. Sci. 20:465-470. [DOI] [PubMed] [Google Scholar]
- 8.Choi, Y. D., and G. Dreyfuss. 1984. Monoclonal antibody characterization of the C proteins of heterogeneous nuclear ribonucleoprotein complexes in vertebrate cells. J. Cell Biol. 99:1197-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies, S. P., N. R. Helps, P. T. Cohen, and D. G. Hardie. 1995. 5′-AMP inhibits dephosphorylation, as well as promotes phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C α and native bovine protein phosphatase-2AC. FEBS Lett. 377:421-425. [DOI] [PubMed] [Google Scholar]
- 10.Dean, J. L., M. Brook, A. R. Clark, and J. Saklatvala. 1999. p38 mitogen-activated protein kinase regulates cyclooxygenase-2 mRNA stability and transcription in lipopolysaccharide-treated human monocytes. J. Biol. Chem. 274:264-269. [DOI] [PubMed] [Google Scholar]
- 11.Esposito, F., F. Cuccovillo, M. Vanoni, F. Cimino, C. W. Anderson, E. Appella, and T. Russo. 1997. Redox-mediated regulation of p21(waf1/cip1) expression involves a posttranscriptional mechanism and activation of the mitogen-activated protein kinase pathway. Eur. J. Biochem. 245:730-737. [DOI] [PubMed] [Google Scholar]
- 12.Fan, X. C., and J. A. Steitz. 1998. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J. 17:3448-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan, X. C., and J. A. Steitz. 1998. HNS, a nuclear-cytoplasmic shuttling sequence in HuR. Proc. Natl. Acad. Sci. USA 95:15293-15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallouzi, I.-E., C. M. Brennan, M. G. Stenberg, M. S. Swanson, A. Eversole, N. Maizels, and J. A. Steitz. 2000. HuR binding to cytoplasmic mRNA is perturbed by heat shock. Proc. Natl. Acad. Sci. USA 97:3073-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallouzi, I.-E., C. M. Brennan, and J. A. Steitz. 2001. Protein ligands mediate the CRM1-dependent export of HuR in response to heat shock. RNA 7:1348-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Good, P. J. 1995. A conserved family of elav-like genes in vertebrates. Proc. Natl. Acad. Sci. USA 92:4557-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorospe, M., S. Kumar, and C. Baglioni. 1993. Tumor necrosis factor increases stability of interleukin-1 mRNA by activating protein kinase C. J. Biol. Chem. 268:6214-6220. [PubMed] [Google Scholar]
- 18.Gorospe, M., and C. Baglioni. 1994. Degradation of unstable interleukin-1α mRNA in a rabbit reticulocyte cell-free system. Localization of an instability determinant to a cluster of AUUUA motifs. J. Biol. Chem. 269:11845-11851. [PubMed] [Google Scholar]
- 19.Gorospe, M., X. Wang, K. Z. Guyton, and N. J. Holbrook. 1996. Protective role of p21Waf1/Cip1 against prostaglandin A2-mediated apoptosis of human colorectal carcinoma cells. Mol. Cell. Biol. 16:6654-6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorospe, M., X. Wang, and N. J. Holbrook. 1998. p53-dependent elevation of p21Waf1 expression by UV light is mediated through mRNA stabilization and involves a vanadate-sensitive regulatory system. Mol. Cell. Biol. 18:1400-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gueydan, C., L. Droogmans, P. Chalon, G. Huez, D. Caput, and V. Kruys. 1999. Identification of TIAR as a protein binding to the translational regulatory AU-rich element of tumor necrosis factor α mRNA. J. Biol. Chem. 274:2322-2326. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton, B. J., E. Nagy, J. S. Malter, B. A. Arrick, and W. F. Rigby. 1993. Association of heterogeneous nuclear ribonucleoprotein A1 and C proteins with reiterated AUUUA sequences. J. Biol. Chem. 268:8881-8887. [PubMed] [Google Scholar]
- 23.Hardie, D. G. 1999. Roles of the AMP-activated/SNF1 protein kinase family in the response to cellular stress. Biochem. Soc. Symp. 64:13-27. [PubMed] [Google Scholar]
- 24.Hardie, D. G., I. P. Salt, and S. P. Davies. 2000. Analysis of the role of the AMP-activated protein kinase in the response to cellular stress. Methods Mol. Biol. 99:63-74. [DOI] [PubMed] [Google Scholar]
- 25.Hardie, D. G., and D. Carling. 1997. The AMP-activated protein kinase. Fuel gauge of the mammalian cell? Eur. J. Biochem. 246:259-273. [DOI] [PubMed] [Google Scholar]
- 26.Hardie, D. G., D. Carling, and M. Carlson. 1998. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 67:821-855. [DOI] [PubMed] [Google Scholar]
- 27.Hardie, D. G., and S. A. Hawley. 2001. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays 23:1112-1119. [DOI] [PubMed] [Google Scholar]
- 28.Imamura, K., T. Ogura, A. Kishimoto, M. Kaminishi, and H. Esumi. 2001. Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem. Biophys. Res. Commun. 287:562-567. [DOI] [PubMed] [Google Scholar]
- 29.Jain, R. G., L. G. Andrews, K. M. McGowan, P. H. Pekala, and J. D. Keene. 1997. Ectopic expression of Hel-N1, an RNA-binding protein, increases glucose transporter (GLUT1) expression in 3T3-L1 adipocytes. Mol. Cell. Biol. 17:954-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joseph, B., M. Orlian, and H. Furneaux. 1998. p21waf1 mRNA contains a conserved element in its 3′-untranslated region that is bound by the Elav-like mRNA-stabilizing proteins. J. Biol. Chem. 273:20511-20516. [DOI] [PubMed] [Google Scholar]
- 31.Keene, J. D. 1999. Why is Hu where? Shuttling of early-response-gene messenger RNA subsets. Proc. Natl. Acad. Sci. USA 96:5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kotlyarov, A., A. Neininger, C. Schubert, R. Eckert, C. Birchmeier, H. D. Volk, and M. Gaestel. 1999. MAPKAP kinase 2 is essential for LPS-induced TNF-α biosynthesis. Nat. Cell. Biol. 1:94-97. [DOI] [PubMed] [Google Scholar]
- 33.LaBaer, J., M. D. Garrett, L. F. Stevenson, J. M. Slingerland, C. Sandhu, H. S. Chou, A. Fattaey, and E. Harlow. 1997. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11:847-862. [DOI] [PubMed] [Google Scholar]
- 34.Laroia, G., R. Cuesta, G. Brewer, and R. J. Schneider. 1999. Control of mRNA decay by heat shock-ubiquitin-proteasome pathway. Science 284:499-502. [DOI] [PubMed] [Google Scholar]
- 35.Lin, S., W. Wang, G. M. Wilson, X. Yang, G. Brewer, N. J. Holbrook, and M. Gorospe. 2000. Downregulation of cyclin D1 expression by prostaglandin A2 is mediated by enhanced cyclin D1 mRNA turnover. Mol. Cell. Biol. 20:7903-7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindstein, T., C. H. June, J. A. Ledbetter, G. Stella, and C. B. Thompson. 1989. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science 244:339-343. [DOI] [PubMed] [Google Scholar]
- 37.Loflin, P., C. Y. Chen, and A.-B. Shyu. 1999. Unraveling a cytoplasmic role for hnRNP D in the in vivo mRNA destabilization directed by the AU-rich element. Genes Dev. 13:1884-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma, W. J., S. Cheng, C. Campbell, A. Wright, and H. Furneaux. 1996. Cloning and characterization of HuR, a ubiquitously expressed Elav-like protein. J. Biol. Chem. 271:8144-8151. [DOI] [PubMed] [Google Scholar]
- 39.Ma, W. J., S. Chung, and H. Furneaux. 1997. The Elav-like proteins bind to AU-rich elements and to the poly(A) tail of mRNA. Nucleic Acids Res. 25:3564-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malter, J. S., and Y. Hong. 1991. A redox switch and phosphorylation are involved in the post-translational up-regulation of the adenosine-uridine binding factor by phorbol ester and ionophore. J. Biol. Chem. 266:3167-3171. [PubMed] [Google Scholar]
- 41.Ming, X. F., M. Kaiser, and C. Moroni. 1998. c-jun N-terminal kinase is involved in AUUUA-mediated interleukin-3 mRNA turnover in mast cells. EMBO J. 17:6039-6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore, F., J. Weekes, and D. G. Hardie. 1991. Evidence that AMP triggers phosphorylation as well as direct allosteric activation of rat liver AMP-activated protein kinase. A sensitive mechanism to protect the cell against ATP depletion. Eur. J. Biochem. 199:691-697. [DOI] [PubMed] [Google Scholar]
- 43.Myer, V. E., and J. A. Steitz. 1995. Isolation and characterization of a novel, low abundance hnRNP protein: A0. RNA 1:171-182. [PMC free article] [PubMed] [Google Scholar]
- 44.Nair, A. P., S. Hahn, R. Banholzer, H. H. Hirsch, and C. Moroni. 1994. Cyclosporin A inhibits growth of autocrine tumour cell lines by destabilizing interleukin-3 mRNA. Nature 369:239-242. [DOI] [PubMed] [Google Scholar]
- 45.Pages, G., E. Berra, J. Milanini, A. P. Levy, and J. Pouyssegur. 2000. Stress-activated protein kinases (JNK and p38/HOG) are essential for vascular endothelial growth factor mRNA stability. J. Biol. Chem. 275:26484-26491. [DOI] [PubMed] [Google Scholar]
- 46.Park, J. W., M. A. Jang, Y. H. Lee, A. Passaniti, and T. K. Kwon. 2001. p53-independent elevation of p21 expression by PMA results from PKC-mediated mRNA stabilization. Biochem. Biophys. Res. Commun. 280:244-248. [DOI] [PubMed] [Google Scholar]
- 47.Paulding, W. R., and M. F. Czyzyk-Krzeska. 2000. Hypoxia-induced regulation of mRNA stability. Adv. Exp. Med. Biol. 475:111-121. [DOI] [PubMed] [Google Scholar]
- 48.Peng, S. S., C. Y. Chen, N. Xu, and A.-B. Shyu. 1998. RNA stabilization by the AU-rich element binding protein HuR, an ELAV protein. EMBO J. 17:3461-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross, J. 1995. mRNA stability in mammalian cells. Microbiol. Rev. 59:423-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sachs, A.-B. 1993. Messenger RNA degradation in eukaryotes. Cell 74:413-421. [DOI] [PubMed] [Google Scholar]
- 51.Salt, I. P., G. Johnson, S. J. Ashcroft, and D. G. Hardie. 1998. AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic beta cells, and may regulate insulin release. Biochem. J. 335:533-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang, W., H. Furneaux, H. Cheng, M. C. Caldwell, D. Hutter, Y. Liu, N. J. Holbrook, and M. Gorospe. 2000. HuR regulates p21 mRNA stabilization by UV light. Mol. Cell. Biol. 20:760-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang, W., S. Lin, M. C. Caldwell, H. Furneaux, and M. Gorospe. 2000. HuR regulates cyclin A and cyclin B1 mRNA stability during the cell division cycle. EMBO J. 19:2340-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, W., X. Yang, V. J. Cristofalo, N. J. Holbrook, and M. Gorospe. 2001. Loss of HuR is linked to reduced expression of proliferative genes during replicative senescence. Mol. Cell. Biol. 21:5889-5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang, W., T. Chi, Y. Xue, A. Kuo, S. Zhou, and G. Crabtree. 1998. Architectural DNA binding by a high-mobility-group/kinesin-like subunit in mammalian SWI/SNF-related complexes. Proc. Natl. Acad. Sci. USA 95:492-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Westmark, C. J., and J. S. Malter. 2001. Up-regulation of nucleolin mRNA and protein in peripheral blood mononuclear cells by extracellular-regulated kinase. J. Biol. Chem. 276:1119-1126. [DOI] [PubMed] [Google Scholar]
- 57.Westmark, C. J., and J. S. Malter. 2001. Extracellular-regulated kinase controls beta-amyloid precursor protein mRNA decay. Brain Res. Mol. Brain Res. 90:193-201. [DOI] [PubMed] [Google Scholar]
- 58.Winzen, R., M. Kracht, B. Ritter, A. Wilhelm, C. Y. Chen, A.-B. Shyu, M. Muller, M. Gaestel, K. Resch, and H. Holtmann. 1999. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 18:4969-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woods, A., D. Azzout-Marniche, M. Foretz, S. C. Stein, P. Lemarchand, P. Ferré, F. Foufelle, and D. Carling. 2000. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol. Cell. Biol. 20:6704-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiong, Y., G. J. Hannon, H. Zhang, D. Casso, R. Kobayashi, and D. Beach. 1993. p21 is a universal inhibitor of cyclin kinases. Nature 366:701-704. [DOI] [PubMed] [Google Scholar]
- 61.Xu, N., C. Y. Chen, and A.-B. Shyu. 1997. Modulation of the fate of cytoplasmic mRNA by AU-rich elements: key sequence features controlling mRNA deadenylation and decay. Mol. Cell. Biol. 17:4611-4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, H., G. J. Hannon, and D. Beach. 1994. p21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 8:1750-1758. [DOI] [PubMed] [Google Scholar]
- 63.Zhang, H., G. J. Hannon, D. Casso, and D. Beach. 1994b. p21 is a component of active cell cycle kinases. Cold Spring Harbor Symp. Quant. Biol. 59:21-29. [DOI] [PubMed]
- 64.Zhang, W., B. J. Wagner, K. Ehrenman, A. W. Schaefer, C. T. DeMaria, D. Crater, K. DeHaven, L. Long, and G. Brewer. 1993. Purification, characterization, and cDNA cloning of an AU-rich element RNA-binding protein, AUF1. Mol. Cell. Biol. 13:7652-7665. [DOI] [PMC free article] [PubMed] [Google Scholar]