Abstract
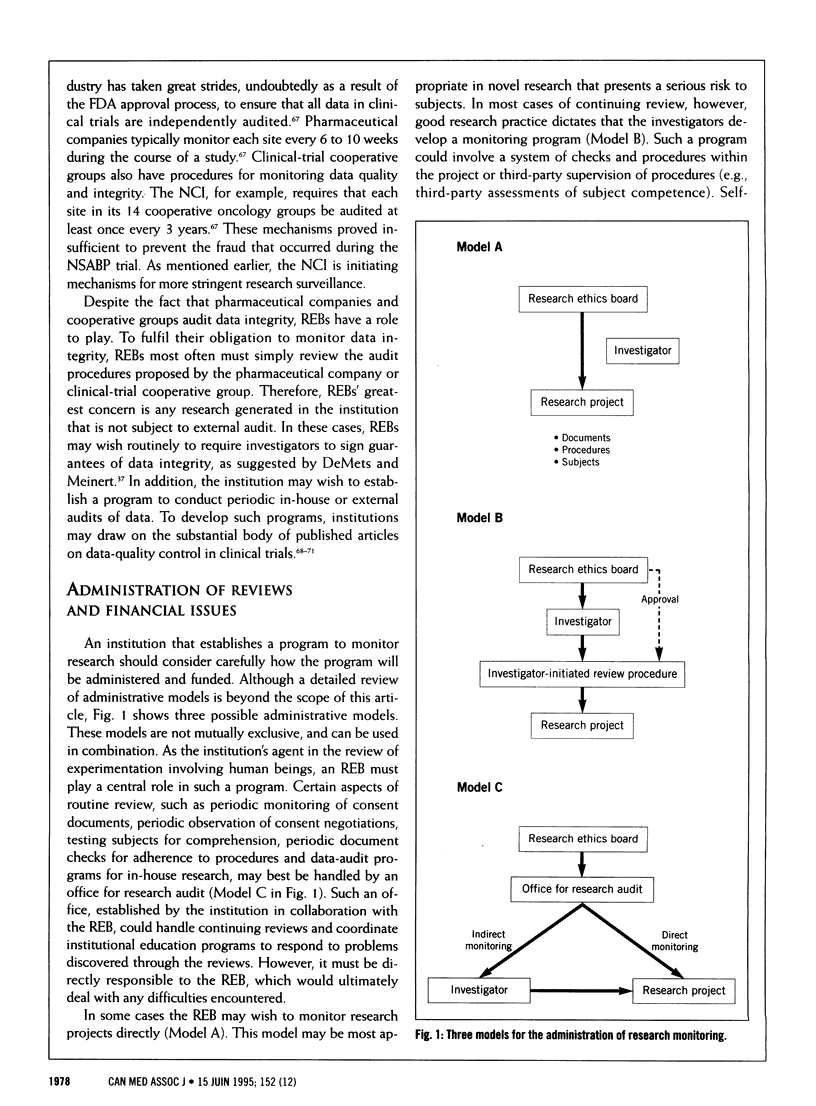
The revelation that data obtained for the US-based National Surgical Adjuvant Breast and Bowel Project (NSABP) from subjects enrolled at Hôpital Saint-Luc in Montreal was falsified has eroded public trust in research. Institutions can educate researchers and help prevent unethical research practices by establishing procedures to monitor research involving human subjects. Research monitoring encompasses four categories of activity: annual reviews of continuing research, monitoring of informed consent, monitoring of adherence to approved protocols and monitoring of the integrity of data. The authors describe characteristics of research projects that may call for monitoring procedures in each category. The form taken by such monitoring depends on the nature of the protocol. Although appropriate research monitoring requires substantial investment of personnel and financial resources, it is required under guidelines regulating research involving human subjects in Canada. Research monitoring is a step forward in re-establishing public confidence in medical research.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angell M., Kassirer J. P. Setting the record straight in the breast-cancer trials. N Engl J Med. 1994 May 19;330(20):1448–1450. doi: 10.1056/NEJM199405193302010. [DOI] [PubMed] [Google Scholar]
- Angell M., Relman A. S. Fraud in biomedical research: a time for congressional restraint. N Engl J Med. 1988 Jun 2;318(22):1462–1463. doi: 10.1056/NEJM198806023182210. [DOI] [PubMed] [Google Scholar]
- Bailey K. R. Detecting fabrication of data in a multicenter collaborative animal study. Control Clin Trials. 1991 Dec;12(6):741–752. doi: 10.1016/0197-2456(91)90037-m. [DOI] [PubMed] [Google Scholar]
- Barbour G. L., Blumenkrantz M. J. Videotape aids informed consent decision. JAMA. 1978 Dec 15;240(25):2741–2742. [PubMed] [Google Scholar]
- Barendregt W. B., de Boer H. H., Kubat K. Autopsy analysis in surgical patients: a basis for clinical audit. Br J Surg. 1992 Dec;79(12):1297–1299. doi: 10.1002/bjs.1800791218. [DOI] [PubMed] [Google Scholar]
- Canner P. L., Krol W. F., Forman S. A. The Coronary Drug Project. External quality control programs. Control Clin Trials. 1983 Dec;4(4):441–466. doi: 10.1016/0197-2456(83)90028-4. [DOI] [PubMed] [Google Scholar]
- Cassileth B. R., Heiberger R. M., March V., Sutton-Smith K. Effect of audiovisual cancer programs on patients and families. J Med Educ. 1982 Jan;57(1):54–59. doi: 10.1097/00001888-198201000-00012. [DOI] [PubMed] [Google Scholar]
- Christakis Nicholas A. Should IRBs monitor research more strictly? IRB. 1988 Mar-Apr;10(2):8–10. [PubMed] [Google Scholar]
- Cohen J. Clinical trial monitoring: hit or miss? Science. 1994 Jun 10;264(5165):1534–1537. doi: 10.1126/science.8202707. [DOI] [PubMed] [Google Scholar]
- Darsee J. R., Heymsfield S. B., Nutter D. O. Hypertrophic cardiomyopathy and human leukocyte antigen linkage: differentiation of two forms of hypertrophic cardiomyopathy. N Engl J Med. 1979 Apr 19;300(16):877–882. doi: 10.1056/NEJM197904193001602. [DOI] [PubMed] [Google Scholar]
- DeMets D. L., Meinert C. L. Data integrity. Control Clin Trials. 1991 Dec;12(6):727–730. doi: 10.1016/0197-2456(91)90035-k. [DOI] [PubMed] [Google Scholar]
- Faden Ruth R., Lewis Carol, Rimer Barbara. Monitoring informed consent procedures: an exploratory record review. IRB. 1980 Oct;2(8):9–10. [PubMed] [Google Scholar]
- Fleming T. R. Data monitoring committees and capturing relevant information of high quality. Stat Med. 1993 Mar;12(5-6):565–573. doi: 10.1002/sim.4780120524. [DOI] [PubMed] [Google Scholar]
- Freedman B. Equipoise and the ethics of clinical research. N Engl J Med. 1987 Jul 16;317(3):141–145. doi: 10.1056/NEJM198707163170304. [DOI] [PubMed] [Google Scholar]
- Freedman B., Fuks A., Weijer C. Demarcating research and treatment: a systematic approach for the analysis of the ethics of clinical research. Clin Res. 1992 Dec;40(4):653–660. [PubMed] [Google Scholar]
- Gavaghan H. Cancer institute to tighten control of trials. Nature. 1994 Apr 21;368(6473):679–679. doi: 10.1038/368679a0. [DOI] [PubMed] [Google Scholar]
- Gray B. H. An assessment of institutional review committees in human experimentation. Med Care. 1975 Apr;13(4):318–328. doi: 10.1097/00005650-197504000-00004. [DOI] [PubMed] [Google Scholar]
- Gray B. H., Cooke R. A., Tannenbaum A. S. Research involving human subjects. Science. 1978 Sep 22;201(4361):1094–1101. doi: 10.1126/science.356268. [DOI] [PubMed] [Google Scholar]
- Greenberg D. S. Dingell and the breast cancer trials. Lancet. 1994 Apr 30;343(8905):1089–1089. doi: 10.1016/s0140-6736(94)90189-9. [DOI] [PubMed] [Google Scholar]
- Gunsalus C. K. Institutional structure to ensure research integrity. Acad Med. 1993 Sep;68(9 Suppl):S33–S38. doi: 10.1097/00001888-199309000-00031. [DOI] [PubMed] [Google Scholar]
- Hilner J. E., McDonald A., Van Horn L., Bragg C., Caan B., Slattery M. L., Birch R., Smoak C. G., Wittes J. Quality control of dietary data collection in the CARDIA study. Control Clin Trials. 1992 Apr;13(2):156–169. doi: 10.1016/0197-2456(92)90021-q. [DOI] [PubMed] [Google Scholar]
- Lichter P. R. Our system, our responsibility: research and the public trust. Ophthalmology. 1994 Jul;101(7):1163–1164. doi: 10.1016/s0161-6420(94)31193-6. [DOI] [PubMed] [Google Scholar]
- Marshall E. San Diego's tough stand on research fraud. Science. 1986 Oct 31;234(4776):534–535. doi: 10.1126/science.3764425. [DOI] [PubMed] [Google Scholar]
- Mauer J. K., Hoth D. F., Macfarlane D. K., Hammershaimb L. D., Wittes R. E. Site visit monitoring program of the clinical cooperative groups: results of the first 3 years. Cancer Treat Rep. 1985 Oct;69(10):1177–1187. [PubMed] [Google Scholar]
- McNeill P. M., Berglund C. A., Webster I. W. Do Australian researchers accept committee review and conduct ethical research? Soc Sci Med. 1992 Aug;35(3):317–322. doi: 10.1016/0277-9536(92)90028-o. [DOI] [PubMed] [Google Scholar]
- McNeill P. M., Berglund C. A., Webster I. W. Reviewing the reviewers: a survey of institutional ethics committees in Australia. Med J Aust. 1990 Mar 19;152(6):289–296. doi: 10.5694/j.1326-5377.1990.tb120948.x. [DOI] [PubMed] [Google Scholar]
- Meisel Alan, Roth Loren H. Toward an informed discussion of informed consent: a review and critique of the empirical studies. Ariz Law Rev. 1983;25(2):265–346. [PubMed] [Google Scholar]
- Miller Judith N. Ethics review in Canada: highlights from a national workshop, part 1. Ann R Coll Physicians Surg Can. 1989 Nov;22(7):515–523. [PubMed] [Google Scholar]
- Neaton J. D., Bartsch G. E., Broste S. K., Cohen J. D., Simon N. M. A case of data alteration in the Multiple Risk Factor Intervention Trial (MRFIT). The MRFIT Research Group. Control Clin Trials. 1991 Dec;12(6):731–740. doi: 10.1016/0197-2456(91)90036-l. [DOI] [PubMed] [Google Scholar]
- Nutter D. O., Heymsfield S. B., Glenn J. F. Retraction. Darsee JR, Heymsfield SB, Nutter DO. Hypertrophic cardiomyopathy and human leukocyte antigen linkage: differentiation of two forms of hypertrophic cardiomyopathy. N Engl J Med 1979;300:877-82,. N Engl J Med. 1983 Jun 9;308(23):1400–1400. doi: 10.1056/nejm198306093082307. [DOI] [PubMed] [Google Scholar]
- Petersdorf R. G. The pathogenesis of fraud in medical science. Ann Intern Med. 1986 Feb;104(2):252–254. doi: 10.7326/0003-4819-104-2-252. [DOI] [PubMed] [Google Scholar]
- Prud'homme G. J., Canner P. L., Cutler J. A. Quality assurance and monitoring in the Hypertension Prevention Trial. Hypertension Prevention Trial Research Group. Control Clin Trials. 1989 Sep;10(3 Suppl):84S–94S. doi: 10.1016/0197-2456(89)90044-5. [DOI] [PubMed] [Google Scholar]
- Relman A. S. Lessons from the Darsee affair. N Engl J Med. 1983 Jun 9;308(23):1415–1417. doi: 10.1056/NEJM198306093082311. [DOI] [PubMed] [Google Scholar]
- Schwarz R. P., Jr Maintaining integrity and credibility in industry-sponsored clinical research. Control Clin Trials. 1991 Dec;12(6):753–760. doi: 10.1016/0197-2456(91)90038-n. [DOI] [PubMed] [Google Scholar]
- Shapiro M. F., Charrow R. P. Scientific misconduct in investigational drug trials. N Engl J Med. 1985 Mar 14;312(11):731–736. doi: 10.1056/NEJM198503143121128. [DOI] [PubMed] [Google Scholar]
- Shapiro M. F., Charrow R. P. The role of data audits in detecting scientific misconduct. Results of the FDA program. JAMA. 1989 May 5;261(17):2505–2511. [PubMed] [Google Scholar]
- Sondik E. J. Reanalyses of NSABP studies. National Surgical Adjuvant Breast and Bowel Project. J Natl Cancer Inst. 1994 May 4;86(9):655–655. doi: 10.1093/jnci/86.9.655. [DOI] [PubMed] [Google Scholar]
- Taub H. A., Baker M. T. A reevaluation of informed consent in the elderly: a method for improving comprehension through direct testing. Clin Res. 1984 Feb;32(1):17–21. [PubMed] [Google Scholar]
- Taub H. A., Baker M. T., Kline G. E., Sturr J. F. Comprehension of informed consent information by young-old through old-old volunteers. Exp Aging Res. 1987 Winter;13(4):173–178. doi: 10.1080/03610738708259321. [DOI] [PubMed] [Google Scholar]
- Taub H. A., Baker M. T., Sturr J. F. Informed consent for research. Effects of readability, patient age, and education. J Am Geriatr Soc. 1986 Aug;34(8):601–606. doi: 10.1111/j.1532-5415.1986.tb05766.x. [DOI] [PubMed] [Google Scholar]
- Taub H. A., Baker M. T. The effect of repeated testing upon comprehension of informed consent materials by elderly volunteers. Exp Aging Res. 1983 Fall;9(3):135–138. doi: 10.1080/03610738308258441. [DOI] [PubMed] [Google Scholar]
- Taub Harvey A. Comprehension of informed consent for research: issues and directions for future study. IRB. 1986 Nov-Dec;8(6):7–10. [PubMed] [Google Scholar]
- Thompson I. E., French K., Melia K. M., Boyd K. M., Templeton A. A., Potter B. Research ethical committees in Scotland. Br Med J (Clin Res Ed) 1981 Feb 28;282(6265):718–720. doi: 10.1136/bmj.282.6265.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. B., Vogelzang N. J., Peterson B. A., Panasci L. C., Carpenter J. T., Gavigan M., Sartell K., Frei E., 3rd, McIntyre O. R. A successful system of scientific data audits for clinical trials. A report from the Cancer and Leukemia Group B. JAMA. 1993 Jul 28;270(4):459–464. [PubMed] [Google Scholar]
- Woolley F. R. Ethical issues in the implantation of the total artificial heart. N Engl J Med. 1984 Feb 2;310(5):292–296. doi: 10.1056/NEJM198402023100505. [DOI] [PubMed] [Google Scholar]


