Abstract
Ligand activation of the fibroblast growth factor receptor (FGFR) represses myogenesis and promotes activation of extracellular signal-regulated kinases 1 and 2 (Erks). The precise mechanism through which the FGFR transmits both of these signals in myoblasts remains unclear. The SH2 domain-containing protein tyrosine phosphatase, SHP-2, has been shown to participate in the regulation of FGFR signaling. However, no role for SHP-2 in FGFR myogenic signaling is known. In this study, we show that stimulation of C2C12 myoblasts with FGF-2 induces SHP-2 complex formation with tyrosyl-phosphorylated FGFR substrate 2α (FRS-2α). Both the catalytic activity and, to a much lesser extent, the Grb2 binding-tyrosyl phosphorylation sites of SHP-2 are required for maximal FGF-2-induced Erk activity and Elk-1 transactivation. When overexpressed in C2C12 myoblasts, wild-type SHP-2, but not a catalytically inactive SHP-2 mutant, potentiates the suppressive effects of FGF-2 on muscle-specific gene expression. In addition, expression of a constitutively active mutant of SHP-2 is sufficient to prevent myogenesis. The constitutively active mutant of SHP-2 induces hyper-tyrosyl phosphorylation of FRS-2α but fails to stimulate or potentiate either FGF-2-induced Erk activation or Elk-1 transactivation. These data suggest that in myoblasts, SHP-2 represses myogenesis via a pathway that is independent of the Erks. We propose that SHP-2 plays a pivotal role in FGFR signaling in myoblasts via both Erk-dependent and Erk-independent pathways.
Cell lines of the myogenic lineage provide an excellent model for studying signaling pathways that control the decision between growth and differentiation. In culture, myogenic cell lines are inhibited in their ability to differentiate when exposed to serum, fibroblast growth factors (FGFs), and transforming growth factor β (8, 29, 57, 63, 73). It is now believed that mitogens prevent myogenesis by maintaining myoblasts in the proliferative phase, thereby suppressing muscle-specific gene expression and terminal differentiation (28, 38, 56). Upon mitogen withdrawal, cultured myoblasts exit the cell cycle and coordinately activate muscle regulatory factors (MRFs) in the basic helix-loop-helix (bHLH) family. These MRFs, such as MyoD, myogenin, Myf5, and MRF4, promote skeletal muscle differentiation by activating muscle-specific genes that include the myosin light and heavy chains, desmin, and troponin T (11, 43, 67).
The intracellular signaling cascades that regulate entry into myogenesis have been the focus of several laboratories. Some of these studies suggest that extracellular signal-regulated kinases 1 and 2 (Erks) negatively regulate the initiation of myogenesis. A combination of pharmacological inhibitors, overexpression of dominant-interfering mutants of the Erk pathway, and overexpression of the mitogen-activated protein kinase (MAPK) phosphatase 1 all demonstrate that the Erks negatively regulate myogenesis (5, 9, 69, 70). One report indicates that the Erks positively regulate myogenesis (16), while others suggest that mitogen-mediated inhibition of myogenesis occurs in an Erk-independent manner (22, 26, 27, 50, 51, 57, 58, 69). It has also been reported that FGF-2 mediates myoblast proliferation independently of its repressive effects on myogenesis (22, 26, 27, 57). Thus, it appears that the decision between the proliferative and myogenic signaling pathways in cultured myoblasts may be governed by both Erk-dependent and Erk-independent mechanisms.
Despite the fact that in myoblasts ligand engagement of the FGF receptor (FGFR) leads to activation of the Erks, MAPK-dependent activation of transcription factors such as Elk-1, and repression of myogenesis (7, 22, 27), it is clear that understanding the molecules involved in propagating these early signaling events remains to be established. Activation of the FGFR leads to tyrosyl phosphorylation of the SNTs (Suc-associated neurotrophic factor-induced tyrosine-phosphorylated targets) (48), also known as FGFR substrate 2 (FRS-2) (25). The FRS-2 family of proteins consists of FRS-2α (SNT1) and FRS-2β (SNT2), which are lipid-anchored multisubstrate adaptor molecules that recruit the SH2 domain-containing protein Grb2 and the SH2-containing protein tyrosine phosphatase (PTP) SHP-2 (17, 25). Tyrosyl phosphorylation of FRS-2α is critical for the initiation of FGFR signaling (17, 45). It has been shown that FRS-2α and the FGFR participate in the promotion of neurite outgrowth in PC12 cells (17, 34, 49). Stimulation of PC12 cells with FGF-2 induces differentiation via sustained activation of the Erks, which is mediated by recruitment of SHP-2 to FRS-2α (17). In contrast, the FGFR signaling pathway engaged by myoblasts suppresses myogenesis (8, 29, 57, 63, 73). It remains to be established whether SHP-2 participates downstream of the FGFR in myoblasts to mediate entry into myogenesis.
SHP-2 is a ubiquitously expressed tyrosine-specific protein phosphatase that contains two SH2 domains (1, 2, 14, 15, 65). The catalytic activity of SHP-2 is required for activation of the Ras/Raf/Erk pathway downstream of several growth factor receptors and cytokines (13, 39-41, 62). The direct targets of tyrosyl dephosphorylation by SHP-2 that regulate the Ras/Raf/Erk pathway, however, remain to be identified. SHP-2 becomes tyrosyl phosphorylated in its C terminus on residues Y542 and Y580 in response to growth factor receptor activation (4, 14, 30, 65, 66). Tyrosyl phosphorylation of these residues creates a binding site for Grb2 (4, 32, 60, 61, 68), and in some cases this adaptor mechanism serves to assemble the Grb2-SOS complex, thereby linking receptor activation to the Erk pathway (6). Thus, SHP-2 has the capacity to signal both via its PTP domain and through an adaptor mechanism with Grb2.
Binding of the NH2-terminal SH2 domain (N-SH2) of SHP-2 with its cognate phosphotyrosyl peptide is necessary and sufficient to activate the PTP (31). Resolution of the crystal structure of SHP-2 has revealed the molecular mechanisms by which engagement of the N-SH2 domains by a phosphotyrosyl peptide leads to activation of the PTP (20). In the inactive state, the N-SH2 domain exhibits an intramolecular interaction with the catalytic site of the PTP domain. Engagement of the N-SH2 domain with a phosphotyrosyl peptide disrupts the N-SH2-PTP domain interaction, resulting in the ability of the PTP domain to bind its substrate (20). These structural features of SHP-2 were harnessed to generate a form of the phosphatase that is constitutively active but retains N-SH2 phosphotyrosyl binding activity (46). Activated mutants of SHP-2 were used to demonstrate that the catalytic activity of the enzyme was sufficient to induce Xenopus mesoderm (46). This activated mutant of SHP-2 is therefore useful for determining the sufficiency of SHP-2 in propagating downstream signaling pathways.
Although work from many laboratories supports a critical role for SHP-2 in a variety of biological systems, its function in myogenic signaling has yet to be determined. In this study, we have utilized the C2C12 myoblast cell line to investigate the role of SHP-2 in FGFR signaling. The results indicate that SHP-2 is a component of the FGFR/FRS-2α signaling complex and functions to mediate activation of the Erk/Elk-1 pathway in myoblasts by FGF-2. We also show that a constitutively active mutant of SHP-2 is sufficient to repress myogenesis. Interestingly, the constitutively active mutant of SHP-2 represses myogenesis without any appreciable activation of the Erk/Elk-1 pathway. These data demonstrate that SHP-2 is necessary, but not sufficient, for activation of the Erk pathway in myoblasts and suggest that SHP-2 mediates the repressive effects of the FGFR on myogenesis via a pathway that is parallel to the activation of the Erks.
MATERIALS AND METHODS
Cell culture and reagents.
C2C12 myoblasts were purchased from the American Type Culture Collection (Manassas, Va.). C2C12 and 293 cells were cultured at 37°C and 5% CO2 in growth medium (GM) containing Dulbecco's modified Eagle medium (GIBCO, Grand Island, N.Y.) supplemented with 10% fetal bovine serum (FBS) (Sigma, St. Louis, Mo.), 1% sodium pyruvate, and 1% penicillin-streptomycin. C2C12 cells were induced to differentiate by transfer to differentiation medium (DM) containing Dulbecco's modified Eagle medium supplemented with 0.1% FBS, 1% sodium pyruvate, 1% penicillin-streptomycin, and 5 μg each of insulin (Sigma-Aldrich, St. Louis, Mo.) and transferrin (Sigma)/ml. FGF-2 and insulin-like growth factor I (IGF-I) were obtained from Calbiochem (San Diego, Calif.). Antiphosphotyrosine antibodies (4G10) were purchased from Upstate Biotechnology Inc. (UBI; Lake Placid, N.Y.). FRS-2α rabbit polyclonal antibodies used for immunoprecipitation were either purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, Calif.) or obtained from either Mitchel Goldfarb (Albert Einstein College of Medicine, New York, N.Y.) or Joseph Schlessinger (Yale University School of Medicine, New Haven, Conn.). Polyclonal antibodies used for immunoprecipitating SHP-2 were purchased from Santa Cruz Biotechnology, and for immunoblotting, a mouse monoclonal SHP-2 antibody was obtained from Becton Dickinson Transduction Laboratories (Lexington, Ky.). Anti-Myc (9E10) monoclonal antibodies and rabbit polyclonal IRS-1 antibodies were purchased from Santa Cruz Biotechnology. For FRS-2α affinity precipitation, p13-Suc-1 agarose beads were purchased from UBI or a glutathione S-transferase (GST) fusion protein of Suc-1 (p13-Suc-1 cDNA was provided by William Dunphy, California Institute of Technology) was used. Anti-phospho-Erk1/2 antibodies were purchased from Cell Signaling Technology (Beverly, Mass.). The anti-Erk1/2 antibody C1 was kindly provided by John Blenis (Harvard Medical School, Boston, Mass.). Myosin heavy chain (MHC) was purchased from the Developmental Studies Hybridoma Bank (Iowa City, Iowa). Anti-FGFR antibodies were provided by Joseph Schlessinger. Horseradish peroxidase-conjugated anti-mouse and anti-rabbit secondary antibodies were purchased from Amersham-Pharmacia Biotechnology (Arlington Heights, Ill.) and detected by using enhanced chemiluminescence.
Plasmid and adenovirus generation.
Wild-type (WT) SHP-2, a catalytically inactive mutant (Cys459 to Ser459) (CS), and a tyrosyl-to-phenylalanine mutant form (Tyr542 to Phe542 and Tyr580 to Phe580) (Y2F) of SHP-2 were transiently expressed in C2C12 myoblasts by using the pJ3 vector as described previously (3). SHP-2 expression was also established by subcloning WT SHP-2, the CS mutant, and the constitutively active mutant of SHP-2 (Glu76 to Ala76) (E76A) into the pIRES-green fluorescent protein (GFP) vector (Invitrogen, Carlsbad, Calif.). To generate the constitutively active SHP-2 mutant, Glu76 was mutated to Ala in the SHP-2 cDNA (20, 46). A Myc epitope-tagged wild-type SHP-2 cDNA subcloned into pJ3 (37) was subjected to site-directed mutagenesis using the QuikChange mutagenesis kit (Stratagene, La Jolla, Calif.) according to the manufacturer's instructions. Glu76 of SHP-2 was mutated to Ala76 by PCR using the forward primer 5′-GCCACTTTGGCTGCGTTGGTCCAGTATTAC-3′ and the reverse primer 5′-GTAATACTGGACCAACGCAGCCAAAGTGGC-3′. The sequence of the resultant construct was confirmed by automated sequencing to generate pJ3-SHP2-E76A. The E76A cDNA was then subcloned into pIRES-GFP to generate pIRES-GFP-E76A (pIRES-E76A).
Recombinant WT and constitutively active (E76A) SHP-2 adenoviruses were generated by the pAdEasy method (19). pJ3-SHP-2-WT and pJ3-SHP2-E76A were subcloned into pAdTrack by restriction digestion with XhoI and XbaI to generate pAdTrack-GFP-WT and pAdTrack-GFP-E76A. The pAdTrack plasmid encodes GFP, which facilitates visualization of the recombinant adenovirus when it is transduced into myoblasts (see Fig. 5B). Recombinant adenoviruses were generated by cotransformation of pAdTrack-GFP-WT and pAdTrack-GFP-E76A with the adenovirus backbone plasmid pAdEasy-1 into BJ5183. Positive recombinant pAd-GFP-WT (pAd-WT) and pAd-GFP-E76A (pAd-E76A) adenoviruses were screened for resistance to kanamycin. Recombinant pAd-WT and pAd-E76A adenoviruses and the control pAd-GFP adenovirus were amplified by using 293 cells and purified as described previously (19).
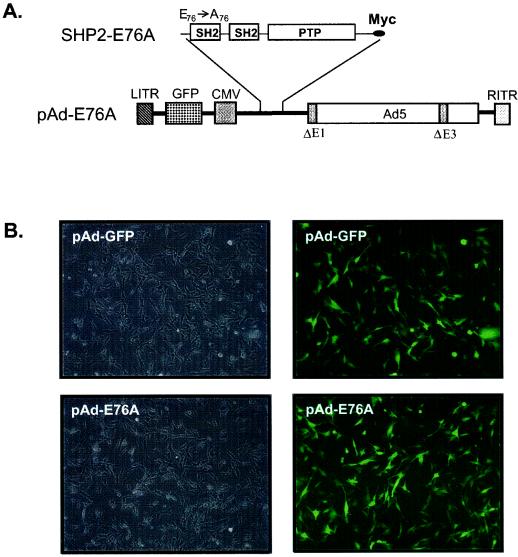
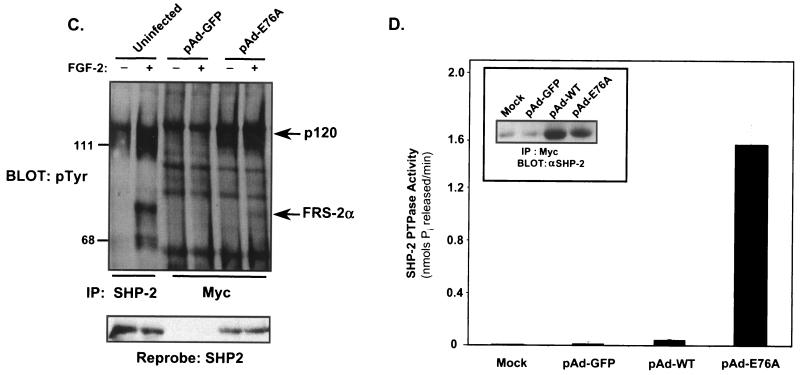
FIG. 5.
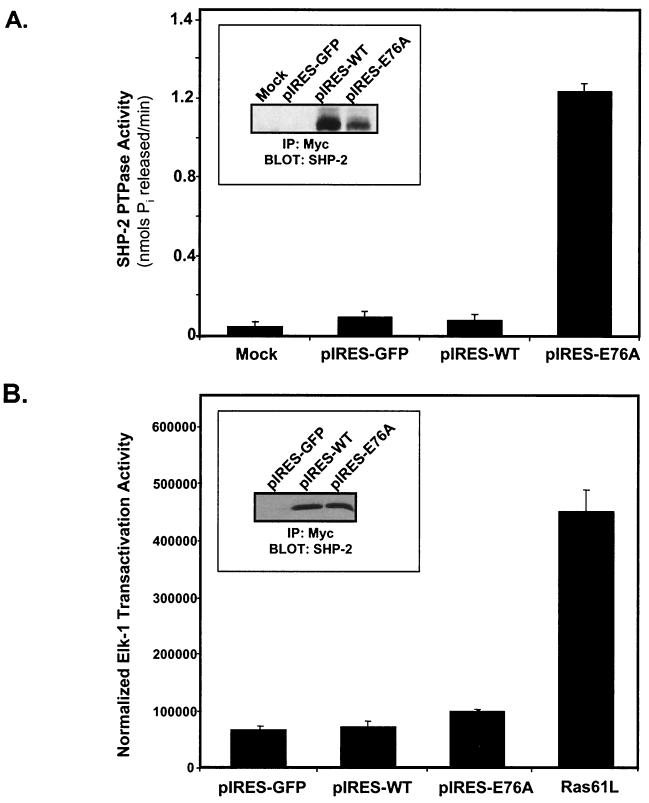
Constitutive activation and association of SHP-2 E76A with FRS-2α and p120 tyrosyl-phosphorylated proteins in C2C12 myoblasts. (A) Schematic representation of the adenovirus construct expressing GFP and the constitutively active mutant of SHP-2 in which E76 within theN-SH2 domain was mutated to A76. (B) C2C12 myoblasts were either left uninfected or infected with either pAd-GFP or pAd-GFP-SHP-2-E76A (pAd-E76A) for 24 h. Representative photomicrographs (magnification, ×100) are phase-contrast images (left) and corresponding indirect-immunofluorescence images (right) and show ∼100% of the adenovirus-infected myoblasts expressing GFP. (C) C2C12 myoblasts were either left uninfected or infected with pAd-GFP or pAd-E76A for 24 h. Myoblasts were then serum starved for an additional 18 h and either left unstimulated or restimulated with FGF-2 (10 ng/ml) for 10 min. Lysates from these C2C12 myoblasts were subjected to immunoprecipitation (IP) with either anti-SHP-2 antibodies (for uninfected myoblasts) or anti-Myc (9E10) antibodies (for infected myoblasts). These immune complexes were resolved by SDS-PAGE and immunoblotted with antiphosphotyrosine (pTyr) antibodies. The lower panel represents a reprobe of the membrane with anti-SHP-2 antibodies. (D) 293 cells were left untransduced or were transduced with pAd-GFP, pAd-WT, or pAd-E76A for 24 h. SHP-2 was immunoprecipitated with anti-Myc antibodies, and immune complexes were used to perform a phosphatase assay using pNPP as a substrate. Results shown are representative of two independent experiments, where SHP-2 E76A gives an ∼25-fold-higher level of pNPP hydrolysis than WT SHP-2. The inset shows that equivalent levels of SHP-2 protein were expressed in these immune complex phosphatase assays.
Immunoprecipitation and immunoblotting.
C2C12 myoblasts either were left unstimulated or were stimulated with FGF-2 at 10 ng/ml for 10 min. Cells were washed twice with ice-cold phosphate-buffered saline (PBS) and lysed in 1 ml of NP-40 lysis buffer (1.0% NP-40, 50 mM Tris · HCl [pH 7.4], 150 mM NaCl, 5 mM EDTA, 5 μg of leupeptin/ml, 5 μg of aprotinin/ml, 1 μg of pepstatin A/ml, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, and 2 mM sodium orthovanadate [NaVO3]) or 1 ml of radioimmunoprecipitation assay lysis buffer (1.0% NP-40, 0.1% sodium dodecyl sulfate [SDS], 0.5% sodium deoxycholate, 25 mM Tris · HCl [pH 7.4], 150 mM NaCl, 5 μg of leupeptin/ml, 5 μg of aprotinin/ml, 1 μg of pepstatin A/ml, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, and 2 mM NaVO3). Cell lysates were cleared by centrifugation at 4°C at 20,800 × g for 15 min. Protein concentrations were measured by using the Coomassie protein reagent (Pierce, Rockford, Ill.) according to the manufacturer's instructions. Approximately 0.5 to 1.0 mg of cell lysates was incubated with protein A- or protein G-Sepharose (Amersham Pharmacia Biotechnology) and antibodies or affinity reagents for 3 h to overnight at 4°C. For immunodepletion experiments, lysates were incubated with the anti-FRS-2α polyclonal antibodies and protein A for 3 h at 4°C. The supernatant was recovered and then incubated with anti-SHP-2 polyclonal antibodies and protein A for a further 3 h at 4°C. In all cases, after the final immunoprecipitation, the immunoprecipitates were washed four times with ice-cold NP-40 lysis buffer supplemented with 2 mM NaVO3 and once with ice-cold ST (150 mM NaCl-50 mM Tris · HCl [pH 8]) buffer supplemented with 2 mM NaVO3. Proteins resolved by SDS-polyacrylamide gel electrophoresis (PAGE) were transferred to an Immobilon-P membrane (Millipore, Bedford, Mass.), immunoblotted with the indicated antibodies, and detected by using peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (Amersham Pharmacia Biotechnology). Immunoblots were controlled for protein loading by Ponceau S staining of the transferred proteins on the Immobilon membrane. Densitometric analyses on immunoblots were performed by using LabWorks 4.0 Image Analysis software (UVP Inc., Upland, Calif.).
Erk kinase assays.
C2C12 myoblasts either were left uninfected or were infected with pAd-GFP, pAd-WT, or pAd-E76A. For transient transfection experiments, 10 μg of a pJ3 expression construct for WT or mutant SHP-2 plus 2 μg of pCG-HA-Erk2 were transfected into C2C12 myoblasts. Both infected and transfected C2C12 myoblasts were rendered quiescent by serum deprivation in DM for approximately 16 to 18 h and were then stimulated with FGF-2 (10 ng/ml) for the indicated times. Unstimulated and FGF-2-stimulated C2C12 myoblasts were washed twice in ice-cold PBS and lysed in 1% NP-40 lysis buffer. Erk2 was immunoprecipitated overnight by using either anti-Erk2 antibodies (SC-154; Santa Cruz) or antihemagglutinin (anti-HA) antibodies (Roche Boehringer Mannheim, Indianapolis, Ind.). Immune complexes were collected by using protein A-Sepharose and were washed three times in 1% NP-40 plus 1 mM sodium orthovanadate and then once in myelin basic protein (MBP) kinase buffer (20 mM HEPES [pH 7.4], 1 mM EGTA, 1 mM dithiothreitol, and 10 mM MgCl2) without MBP. Immune complexes were incubated with MBP kinase buffer plus MBP (0.25 mg/ml) for 5 min at 30°C prior to initiation of the reaction with 50 μM ATP containing 5 μCi of [γ-32P]ATP for a further 15 min at 30°C. Immune complex kinase assays were stopped by addition of 0.5 M EDTA, and 20-μl aliquots of the kinase mix were spotted onto P81 phosphocellulose paper. Filters were washed four times in 0.5% phosphoric acid, dried, and Cerenkov counted. Additionally, the immune complexes were resolved by SDS-PAGE and immunoblotted for Erk2 in order to control for the levels of Erk2 present in the kinase assay.
Photomicroscopy and indirect immunofluorescence.
Adenovirus-infected C2C12 myoblasts that were differentiated on plastic dishes were fixed in methanol for 20 min, washed three times in PBS, and visualized with Wright-Giemsa stain (Sigma). Cells were photographed by use of bright-field microscopy. Adenovirus-infected C2C12 myoblasts were also differentiated on coverslips. These cultures were washed three times in PBS, fixed with 3.7% paraformaldehyde, and permeabilized with 0.1% Triton X-100 in PBS (PBST). Coverslips were then rinsed three times in PBST and incubated with Texas red phalloidin (Molecular Probes, Eugene, Oreg.) diluted in PBST for 30 min. Finally, the coverslips were washed first in PBST and then in PBS and were mounted onto glass slides with anti-fade mixture containing 30% glycerol and 2.5% 1,4-diazabicyclo-(2,2)-octane. Bright-field, GFP, and Texas red phalloidin images were viewed by using a Zeiss Axiovert 100 epifluorescence microscope with a 60× immersion oil objective. Images were collected on a SPOT charge-coupled device (CCD) camera (Diagnostic Instruments Inc., Sterling Heights, Mich.) and processed by using Adobe Photoshop 6.0 (San Jose, Calif.).
Transient transfection and luciferase reporter assays.
Mammalian expression plasmids were transfected into C2C12 myoblasts by using either calcium phosphate precipitation, Lipofectamine 2000, or FuGENE 6 transfection reagent (Roche Molecular Biochemicals, Indianapolis, Ind.) according to the manufacturer's instructions. Luciferase activities were measured by using the Luciferase Assay System Kit from Promega (Madison, Wis.). C2C12 myoblasts cultured in 60-mm dishes in GM were transfected at 70 to 80% confluence with 0.5 μg of 5XGAL4-luciferase, 0.5 μg of Elkc-GAL4, 0.5 μg of simian virus 40 β-galactosidase, and 2.5 μg of WT or mutant SHP-2 expression plasmids as indicated. Twenty-four hours following transfection, C2C12 myoblasts were rendered quiescent by serum deprivation in DM for approximately 16 to 18 h. C2C12 myoblasts either were left unstimulated or were stimulated with FGF-2 (10 ng/ml) for 4 h, lysates were prepared, and β-galactosidase and luciferase activities were determined. For assessment of muscle-specific gene expression, C2C12 myoblasts cultured in the presence of either GM or FGF-2 (10 ng/ml) were transfected by using FuGENE 6 with the M isozyme of the creatine kinase (MCK) promoter from rabbit skeletal muscle fused to luciferase (MCK-Luc) (64) and WT or mutant SHP-2 expression plasmids as indicated.
Immune complex phosphatase assay.
To verify that both transiently transfected and adenovirus-transduced SHP-2 E76A exhibited higher basal phosphatase activity than WT SHP-2, immune complex phosphatase assays were performed. The rates of hydrolysis of para-nitrophenyl phosphate (pNPP; Sigma) by WT SHP-2 and SHP-2 E76A were determined. WT SHP-2 and SHP-2 E76A were expressed by either calcium phosphate transfection or adenovirus transduction into 293 cells. Forty-eight hours following either transfection or transduction, lysates were prepared and SHP-2 proteins were immunoprecipitated by using anti-Myc (9E10) antibodies coupled to protein G-Sepharose. Anti-Myc-SHP-2 immune complexes were washed three times in 1% NP-40 lysis buffer (without sodium orthovanadate) and once in wash buffer (30 mM HEPES [pH 7.4]-120 mM NaCl without pNPP). Immune complex phosphatase assays were performed in duplicate at 37°C in 50 μl of phosphatase assay buffer (30 mM HEPES [pH 7.4], 120 mM NaCl, 5 mM dithiothreitol, and 10 mM pNPP); addition of 1.45 ml of 0.2 N NaOH stopped the reactions. The amount of phosphate released was determined by measuring the absorbance at 410 nm per min. Additionally, the immune complexes were boiled in 2× sample buffer, separated by SDS-PAGE, and immunoblotted to detect the presence of Myc-tagged SHP-2.
RESULTS
FGF-2-dependent complex formation of SHP-2 with FRS-2α in C2C12 myoblasts.
In order to investigate the role of SHP-2 in FGF-2-mediated myogenic signaling, we first determined whether SHP-2 interacts with tyrosyl-phosphorylated proteins in response to FGF-2 in the C2C12 myoblast cell line. C2C12 myoblasts rendered quiescent by serum deprivation either were left unstimulated or were stimulated with FGF-2 (10 ng/ml) for 10 min. Upon immunoprecipitation of SHP-2 from unstimulated C2C12 myoblasts, we detected SHP-2-associated tyrosyl-phosphorylated proteins that migrated with apparent molecular masses of 70 and 120 kDa (Fig. 1A). In response to FGF-2, an additional SHP-2-associated tyrosyl-phosphorylated protein of 90 kDa was detected. The 70-kDa protein, which we identified as SHP-2, showed an increase in its level of tyrosyl phosphorylation following FGF-2 stimulation (Fig. 1A). In contrast, no change in the level of tyrosyl phosphorylation of the 120-kDa protein was observed in response to FGF-2 (Fig. 1A). As a control, we subjected these lysates to immunoprecipitation using normal rabbit serum (NRS); as shown, NRS did not result in immunoprecipitation of the SHP-2/p90/p120 complex (Fig. 1A). Previously, we determined that this 120-kDa SHP-2-interacting tyrosyl-phosphorylated protein in C2C12 myoblasts is a complex that contains Grb2-associated binder 1 (Gab-1) and SHP-2 substrate 1 (SHPS-1) (24). Consistent with the inability of FGF-2 to induce tyrosyl phosphorylation of the SHP-2-associated 120-kDa protein, we found that FGF-2 did not induce tyrosyl phosphorylation of either SHPS-1 or Gab-1 in C2C12 myoblasts (data not shown). Thus, FGF-2 stimulation of C2C12 myoblasts results in the tyrosyl phosphorylation of SHP-2, as well as that of a 90-kDa protein which is complexed with SHP-2.
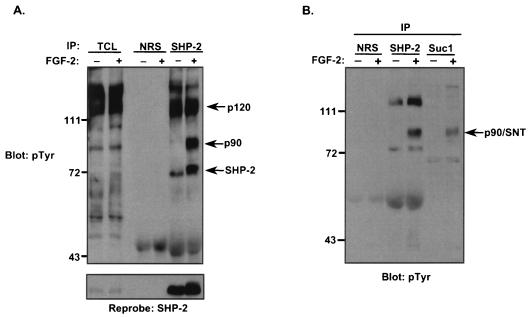
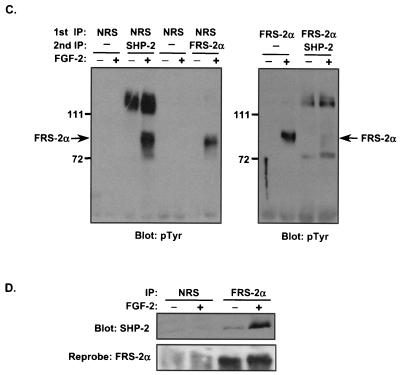
FIG. 1.
FGF-2 induces FRS-2α tyrosyl phosphorylation and complex formation with SHP-2 in C2C12 myoblasts. (A) C2C12 myoblasts were serum starved overnight and then either left untreated (−) or treated (+) with FGF-2 (10 ng/ml) for 10 min. Lysates from unstimulated and stimulated myoblasts were immunoprecipitated either with NRS or with anti-SHP-2 antibodies. Immune complexes and total-cell lysates (TCL) from unstimulated and FGF-2-stimulated C2C12 myoblasts were resolved by SDS-8% PAGE and analyzed by immunoblotting with antiphosphotyrosine (4G10) antibodies (pTyr). The lower panel represents a reprobe of the membrane with anti-SHP-2 antibodies. (B) Lysates prepared from either unstimulated (−) or FGF-2-stimulated (+) C2C12 myoblasts were prepared as described above. Lysates were subjected either to affinity precipitation with p13-Suc-1 agarose beads (Suc1) or to immunoprecipitation with anti-SHP-2 antibodies. p13-Suc-1 agarose affinity complexes or anti-SHP-2 antibody immune complexes were resolved by SDS-PAGE, and tyrosyl-phosphorylated proteins were visualized by immunoblotting with 4G10 antibodies. (C) Lysates prepared as described in the legend to panel B were subjected to immunoprecipitation (IP) either with NRS or with anti-FRS-2α antibodies (1st IP). The supernatants from the first IP were either left alone or subsequently immunoprecipitated with anti-SHP-2 antibodies or anti-FRS-2α antibodies (2nd IP). Immune complexes were analyzed by immunoblotting with 4G10 antibodies. (D) Lysates were prepared from C2C12 myoblasts that were either left untreated (−) or treated (+) with FGF-2 (10 ng/ml) for 10 min. Lysates were immunoprecipitated either with NRS or with anti-FRS-2α antibodies, and immune complexes were resolved and immunoblotted with anti-SHP-2 antibodies. The lower panel represents a reprobe of the membrane with anti-FRS-2α antibodies.
Since FGF-2 stimulation of C2C12 myoblasts resulted in tyrosyl phosphorylation of a 90-kDa protein that complexed with SHP-2, we focused on the identification of this protein. FRS-2α is a 90-kDa FGF-2-inducible tyrosyl-phosphorylated protein that belongs to the SNT/FRS-2 family of adaptor proteins. It was likely that the 90-kDa tyrosyl-phosphorylated protein identified in these SHP-2 immune complexes in response to FGF-2 was FRS-2α, since it is the major 90-kDa FGF-inducible tyrosyl-phosphorylated protein (see the introduction). The yeast cell cycle protein p13-Suc-1 interacts specifically with the SNT family of proteins (48). Using p13-Suc-1 coupled to agarose as an affinity reagent, we were able to precipitate a 90-kDa tyrosyl-phosphorylated protein from lysates prepared from FGF-2-treated C2C12 myoblasts (Fig. 1B). The 90-kDa tyrosyl-phosphorylated protein which interacted with p13-Suc-1 also comigrated with the 90-kDa tyrosyl-phosphorylated protein identified in SHP-2 immune complexes (Fig. 1B). These data suggest that the 90-kDa associated protein found in SHP-2 immune complexes was likely to be an SNT family member.
To test directly the possibility that SHP-2 complexes with FRS-2α in myoblasts, we asked whether FRS-2α-specific antibodies could deplete the SHP-2 complex of the 90-kDa tyrosyl-phosphorylated protein. First, as a control, we tested whether NRS could affect the ability of either anti-SHP-2 or anti-FRS-2α antibodies to immunoprecipitate their respective protein complexes. As expected (Fig. 1C, left panel), control NRS immunoprecipitation followed by either anti-SHP-2 or anti-FRS-2α immunoprecipitation resulted in formation of the appropriate protein complex with SHP-2 and the precipitation of tyrosyl-phosphorylated FRS-2α from FGF-2-stimulated myoblasts. Next, lysates prepared from either unstimulated or FGF-2-stimulated C2C12 myoblasts were either subjected to immunoprecipitation with anti-FRS-2α antibodies alone or subjected to immunoprecipitation first with anti-FRS-2α antibodies and then with anti-SHP-2 antibodies. As shown in Fig. 1C (right panel), FRS-2α became appropriately tyrosyl phosphorylated in response to FGF-2. However, immunodepletion of FRS-2α followed by anti-SHP-2 immunoprecipitation resulted in the complete loss of tyrosyl-phosphorylated FRS-2α from these SHP-2 complexes (Fig. 1C, right panel). In the absence of growth factors, immunoprecipitation of FRS-2α revealed that SHP-2 associated basally with FRS-2α (Fig. 1D). This low but detectable basal association of SHP-2 with FRS-2α is likely due to the autocrine production of FGF in C2C12 myoblasts (18). Nevertheless, SHP-2/FRS-2α complex formation was substantially enhanced upon addition of exogenous FGF-2 (Fig. 1D). Together, these data demonstrate that SHP-2 undergoes tyrosyl phosphorylation in response to FGF-2 in myoblasts and that the major 90-kDa tyrosyl-phosphorylated protein complexed with SHP-2 in myoblasts following FGF-2 stimulation is FRS-2α.
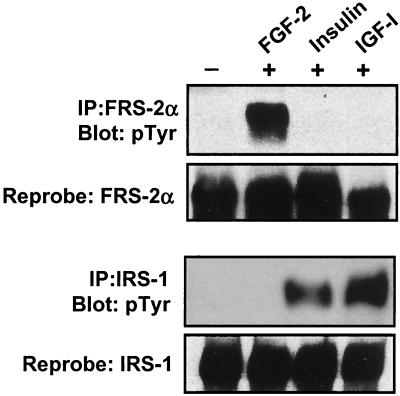
FRS-2α is tyrosyl phosphorylated in response to FGF-2 but not insulin or IGF-I.
The phosphotyrosine-binding (PTB) domain of FRS-2α and that of IRS-1 share approximately 29% sequence identity (25). Moreover, it has been shown that the PTB domain of FRS-2α competes for the same NPXpY motif on the TrkA receptor as Shc (34). Thus, it is formally possible that FRS-2α could become tyrosyl phosphorylated in response to insulin via an interaction with the NPXpY(960) motif on the insulin receptor in myoblasts. Indeed, insulin has been shown to induce FRS-2α tyrosyl phosphorylation in PC12 cells that overexpress the insulin receptor (10). To determine if FRS-2α becomes tyrosyl phosphorylated in response to either insulin or IGF-I in myoblasts, FRS-2α and IRS-1 were immunoprecipitated from C2C12 myoblasts that were stimulated with either FGF-2, insulin, or IGF-I. Following immunoprecipitation, these immune complexes were subjected to antiphosphotyrosine immunoblotting to detect tyrosyl-phosphorylated FRS-2α and IRS-1. As shown in Fig. 2, FRS-2α becomes inducibly tyrosyl phosphorylated in response to FGF-2 but not in response to insulin or IGF-I. However, IRS-1 does not become tyrosyl phosphorylated by FGF-2 under these conditions but does so in response to both insulin and IGF-1 (Fig. 2). These data indicate that the FGF-2/FRS-2α and insulin/IGF/IRS-1 signaling pathways are mutually exclusive in myoblasts, supporting the antagonistic biological effects of these mitogens on muscle differentiation.
FIG. 2.
FGF-2 induces tyrosyl phosphorylation of FRS-2α but not IRS-1 in C2C12 myoblasts. C2C12 myoblasts were serum deprived for 18 h and then either left unstimulated (−) or stimulated (+) with either FGF-2 (10 ng/ml), insulin (100 nM), or IGF-I (100 ng/ml) for 10 min. These cell lysates were immunoprecipitated (IP) with either anti-FRS-2α or anti-IRS-1 antibodies; immune complexes were resolved by SDS-PAGE and immunoblotted by using 4G10 antiphosphotyrosine antibodies. The same membranes were then reprobed with either anti-FRS-2α or anti-IRS-1 antibodies.
SHP-2's catalytic activity is required for FGF-2-mediated transactivation of Elk-1 and Erk activation in C2C12 myoblasts.
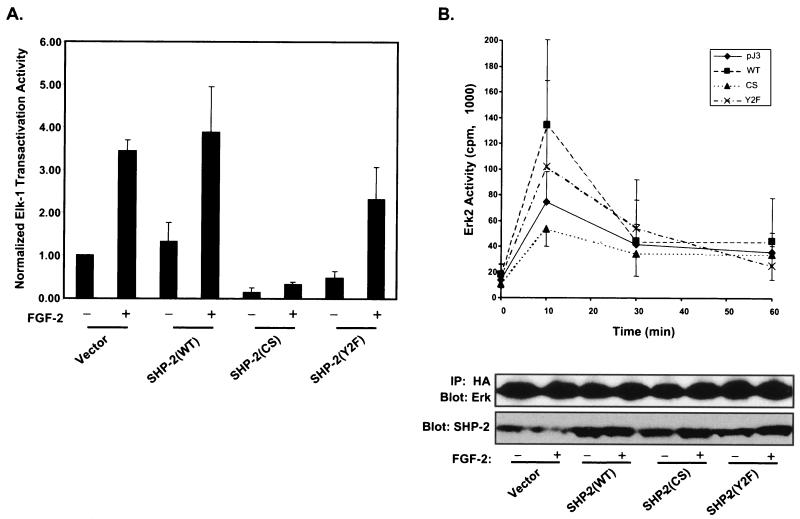
We next sought to determine whether SHP-2 regulates the early FGF-2 signaling events in myoblasts. Stimulation of myoblasts with FGF-2 activates the MAPKs, but predominantly it activates the Erks (7, 22, 27, 33). Therefore, we assessed the requirement for SHP-2 in FGF-2-mediated activation of the MAPK pathway in C2C12 myoblasts. The ETS domain transcription factor Elk-1 is transactivated by the Erks, as well as the c-Jun amino-terminal kinase (JNK) and p38 subfamilies of the MAPKs (72). By measuring the ability of FGF-2 to transactivate Elk-1, the requirement of SHP-2 for regulation of the MAPK pathway can be assessed. A chimera between the transactivation domain of Elk-1 and the DNA-binding domain of GAL4 (Elkc-GAL4) was used to measure Elk-1 transactivation. Upon activation by the MAPKs, Elkc-GAL4 transactivation can be monitored by measuring its ability to drive luciferase expression via a GAL4 DNA binding element. When transiently overexpressed in C2C12 myoblasts, the catalytically inactive mutant of SHP-2 inhibited both basal and FGF-2-induced Elk-1 transactivation relative to that with the vector control and WT SHP-2 (Fig. 3A). Since we had noted that FGF-2 stimulation causes SHP-2 to become tyrosyl phosphorylated in C2C12 myoblasts (Fig. 1), we asked whether tyrosyl phosphorylation of SHP-2 was required for FGF-2-induced Elk-1 transactivation. SHP-2 has been shown previously to become tyrosyl phosphorylated within its C terminus on Y542 and Y580, and these sites can serve to bind Grb2, thereby linking SHP-2 to the MAPK pathway (4, 32, 60, 61, 66, 68). To test for a potential role of these Grb2 binding sites in FGF-2-induced Elk-1 transactivation, a mutant of SHP-2 in which both tyrosyl residues 542 and 580 were mutated to phenylalanine (Y2F) was overexpressed in C2C12 myoblasts. We observed a modest (approximately 25 to 30%) but consistent attenuation of FGF-2-induced Elkc-GAL4 transactivation relative to that with overexpressed WT SHP-2 (Fig. 3A). These data demonstrate that the PTP domain of SHP-2 contributes the predominant signal for Elk-1 transactivation following activation of the FGFR in myoblasts, while the Grb2 binding- tyrosyl phosphorylation sites appear to contribute only a minor component of the maximal Elk-1 transactivation response.
FIG. 3.
SHP-2's catalytic activity is required for FGF-2-mediated activation of Elk-1 and Erk2 in C2C12 myoblasts. (A) C2C12 myoblasts were transfected with 0.5 μg of 5XGAL4-luciferase, 0.5 μg of Elkc-GAL4, 0.5 μg of β-galactosidase, and 2.5 μg of either vector alone, WT SHP-2, the catalytically inactive cysteine-to-serine (CS) mutant of SHP-2, or the Y542F/Y580F (Y2F) double tyrosyl phosphorylation site mutant of SHP-2. Twenty-four hours after transfection, myoblasts were transferred to serum deprivation medium for a further 24 h and were either left unstimulated (−) or stimulated (+) with FGF-2 (10 ng/ml) for 4 h. Luciferase and β-galactosidase activity assays were performed as described in Materials and Methods, and the raw luciferase values were normalized to β-galactosidase activity. Transfections were performed in triplicate for each condition, and the normalized (relative to the unstimulated vector control) data are means ± standard errors of the means from at least three separate experiments. Immunoblotting of C2C12 myoblasts with anti-Myc antibodies confirmed similar expression levels of transfected SHP-2 (data not shown). (B) C2C12 myoblasts were transfected with 2 μg of HA-Erk2 and 10 μg of either vector alone, WT SHP-2, or the CS or Y2F mutant of SHP-2 into serum deprivation medium. Twenty-four hours after transfection, the cells were either left unstimulated or stimulated with FGF-2 (10 ng/ml) for 10, 30, or 60 min. Myoblasts were then harvested, and HA-tagged Erk2 was immunoprecipitated (IP) with anti-HA antibodies. These HA-Erk2 immune complexes were assayed for Erk2 activity by using MBP as a substrate. Lower panels show results of representative control experiments with the above lysates in which the HA-Erk2 immune complexes were resolved by SDS-PAGE and immunoblotted with anti-Erk2 antibodies, and the cell lysates were examined for SHP-2 expression levels by immunoblotting.
The results shown in Fig. 3A suggested that the catalytic activity of SHP-2 is required for FGF-2-induced Elk-1 transactivation. The Erks, as well as the JNK and p38 MAPK subfamilies, can transactivate Elk-1 by phosphorylation. Therefore, in order to determine whether the catalytic activity of SHP-2 and its Grb2 binding-tyrosyl phosphorylation sites were required directly for FGF-2-induced activation of the Erks, we performed Erk immune complex kinase assays. C2C12 myoblasts were transiently transfected with HA-Erk2 and either WT SHP-2 or mutants of SHP-2. Transfected C2C12 myoblasts were rendered quiescent and then restimulated with FGF-2 for various times. HA-Erk2 immune complexes prepared from these transfected C2C12 lysates were assayed for activity by using MBP as a substrate. We observed that overexpression of WT SHP-2 potentiated the activation of Erk2 in response to FGF-2 after 10 min relative to that with the vector-alone control. However, overexpression of the catalytically inactive mutant of SHP-2 not only failed to potentiate FGF-2-induced-Erk2 activation relative to that of WT SHP-2, but it also resulted in the attenuation of FGF-2-induced Erk2 activation compared to that of the vector control (Fig. 3B). The Grb2 binding-tyrosyl phosphorylation site mutant of SHP-2 also potentiated FGF-2-induced Erk activation, but to a lesser extent than WT SHP-2 (Fig. 3B). Immunoblotting of these transfected C2C12 myoblasts for SHP-2 confirmed that it was overexpressed approximately fivefold relative to expression of endogenous SHP-2 in untransfected myoblasts (Fig. 3B). A representative HA-Erk2 immune complex from these assays, which was immunoblotted for Erk2, is shown in the lower panel of Fig. 3B, confirming that equal amounts of HA-Erk2 were used in these kinase assays. Taken together, these data demonstrate that the catalytic activity of SHP-2 is required for the activation of the Erks by FGF-2 in C2C12 myoblasts.
SHP-2's catalytic activity is required to potentiate FGF-2-induced inhibition of myogenic gene expression.
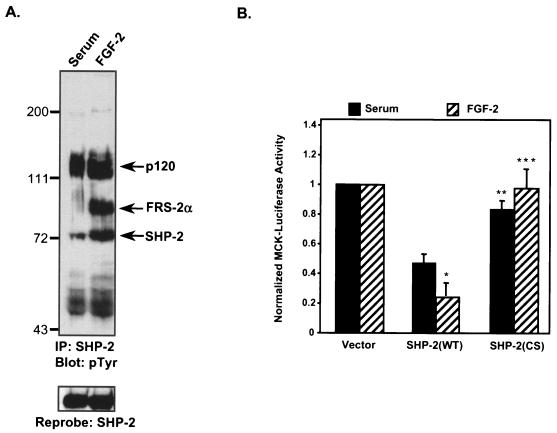
We hypothesized that when overexpressed, SHP-2 may drive the suppressive effects of FGF-2 on muscle-specific gene activity more effectively than in the presence of serum, where SHP-2 does not form a complex with FRS-2α. In addition, if the PTP activity of SHP-2 is responsible for transmitting the suppressive effects of FGF-2, the catalytically inactive SHP-2 mutant should ameliorate the inhibitory effects of FGF-2 on myogenesis relative to that of WT SHP-2. To test these hypotheses, SHP-2 was immunoprecipitated from lysates prepared from C2C12 myoblasts in the presence of either serum or FGF-2. When immunoprecipitated from myoblasts cultured in the presence of serum, SHP-2 did not form a complex with tyrosyl-phosphorylated FRS-2α, but it did so in the presence of FGF-2 (Fig. 4A). Next, C2C12 myoblasts were transfected with either WT SHP-2 or a catalytically inactive mutant of SHP-2 in the presence of either serum or FGF-2 for 24 h. Muscle-specific gene expression was assessed by using the MCK promoter fused to the luciferase gene (MCK-Luc). These experiments revealed that, relative to vector control transfectants, overexpression of WT SHP-2 potentiated the inhibitory effects of both serum and FGF-2 on MCK-mediated gene expression (Fig. 4B). When WT SHP-2 was overexpressed in C2C12 myoblasts cultured in the presence of FGF-2, the suppressive effects on MCK-mediated gene expression were significantly greater than those observed when WT SHP-2 was overexpressed in the presence of serum (P = 0.01) (Fig. 4B). Importantly, overexpression of the catalytically inactive mutant of SHP-2, compared to that of WT SHP-2, failed to potentiate the inhibitory effects on muscle-specific gene expression mediated by either serum or FGF-2 (P = 0.03) (Fig. 4B). These data support the notion that the catalytic activity of SHP-2 is required to potentiate the inhibitory actions of both serum and FGF-2 on early muscle-specific gene expression. Finally, the observation that SHP-2 inhibits MCK-mediated gene expression more effectively when complexed with tyrosyl-phosphorylated FRS-2α suggests that SHP-2 participates in FGF-2-mediated inhibition of muscle-specific genes.
FIG. 4.
SHP-2 potentiates the inhibition of FGF-2 on myogenic gene expression. (A) C2C12 myoblasts were cultured either in the presence of serum (10% FBS) or in the presence of FGF-2 (10 ng/ml), and SHP-2 was immunoprecipitated (IP) as described in the text. Anti-SHP-2 immune complexes were resolved by SDS-PAGE and immunoblotted with antiphosphotyrosine (4G10) antibodies (pTyr). The lower panel represents a reprobe of this membrane with anti-SHP-2 antibodies. (B) C2C12 myoblasts cultured in the presence of either serum or FGF-2 were transfected with 0.5 μg of MCK-luciferase (MCK-Luc), 0.5 μg of β-galactosidase, and 2.5 μg of either the pJ3 vector control, WT SHP-2, or the CS mutant of SHP-2. Twenty-four hours after transfection, lysates were prepared for analysis of MCK-luciferase and β-galactosidase activities. Transfections were performed in triplicate and normalized to the vector control. Results are means ± standard errors of the means from three separate experiments, and data were analyzed by using a one-tailed paired t test. ∗, P = 0.01 for WT SHP-2 in serum versus WT SHP-2 in FGF-2; ∗∗, P = 0.03 for WT SHP-2 in serum versus the SHP-2 CS mutant in serum; ∗∗∗, P = 0.03 for WT SHP-2 in FGF-2 versus the SHP-2 CS mutant in FGF-2.
A constitutively active mutant of SHP-2 (E76A) does not stimulate Erk activity in C2C12 myoblasts.
Our experiments presented in Fig. 3 demonstrate a requirement for the PTP domain of SHP-2 in FGF-induced activation of the Erk pathway in myoblasts. Recent work has shown that an activated mutant of SHP-2 (E76A) is sufficient to induce mesoderm without any discernible level of Erk activation (46). To test the sufficiency of SHP-2 to activate Erk in C2C12 myoblasts, we generated a constitutively active mutant of SHP-2 (E76A) in the context of an adenovirus (Fig. 5A). As a control, we also generated a WT SHP-2 adenovirus (pAd-WT). When transduced into C2C12 myoblasts, both the control GFP adenovirus (pAd-GFP) and the SHP-2 E76A adenovirus (pAd-E76A) are expressed in as many as 100% of cultured myoblasts, as indicated by GFP expression (Fig. 5B). When pAd-E76A was transduced into unstimulated C2C12 myoblasts, SHP-2 E76A interacted appropriately with the p120 tyrosyl-phosphorylated complex and subsequently with tyrosyl-phosphorylated FRS-2α following FGF-2 stimulation (Fig. 5C). These results confirm that the SHP-2 E76A mutant retains its SH2 domain phosphotyrosyl binding capacity, as reported previously (46). To confirm that the pAd-E76A mutant of SHP-2 exhibited constitutive tyrosine phosphatase activity, we performed immune complex phosphatase assays. pAd-WT and pAd-E76A were transduced into 293 cells for 24 h, and exogenously expressed SHP-2 was recovered by immunoprecipitation with anti-Myc antibodies. The rates of hydrolysis of pNPP in these immune complexes were then determined. Figure 5D shows results of an immune complex phosphatase assay for SHP-2 in which the constitutively active E76A mutant exhibited an approximately 25-fold-higher level of pNPP hydrolysis than WT SHP-2. Under these conditions, the immune complexes contained equivalent levels of both WT and SHP-2 E76A protein (Fig. 5D, inset). These data confirm that the pAd-E76A adenovirus expresses a constitutively active mutant of SHP-2 relative to WT SHP-2.
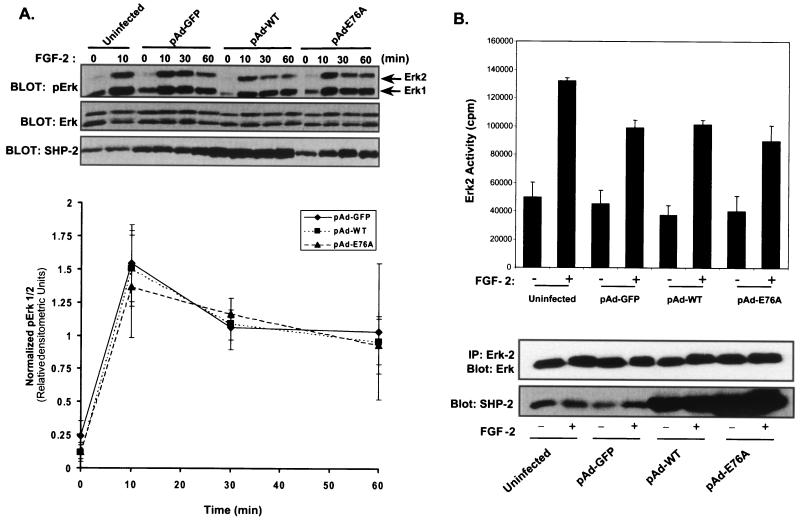
We next determined whether the constitutively active mutant of SHP-2 had the capacity to either induce Erk, independently of FGF-2 stimulation, or potentiate FGF-2-induced Erk activity. pAd-GFP, pAd-WT, and pAd-E76A were transduced into C2C12 myoblasts rendered quiescent by serum deprivation. Levels of Erk activity were assessed in unstimulated myoblasts and in an FGF-2-stimulated time course in C2C12 myoblasts using a phosphospecific Erk antibody. Following FGF-2 stimulation, phospho-Erk immunoreactivity increased dramatically by 10 min in uninfected and pAd-GFP-, pAd-WT-, and pAd-E76A-infected C2C12 myoblasts (Fig. 6A). In the absence of FGF-2, SHP-2 E76A overexpression did not result in the activation of Erk (Fig. 6A). Moreover, no increase in either the magnitude or the duration of phospho-Erk was detected when SHP-2 E76A was overexpressed in FGF-2-stimulated C2C12 myoblasts compared to uninfected, pAd-GFP-infected, or pAd-WT-infected myoblasts (Fig. 6A). These observations were subsequently supported by quantitative densitometric analysis of pErk/Erk ratios from these experiments (Fig. 6A, graph). It was conceivable that the phospho-Erk immunoblots were not sensitive enough to detect small changes in the basal levels of Erk activity in pAd-E76A-infected myoblasts. Therefore, we performed direct Erk2 kinase assays on lysates from unstimulated and FGF-2-stimulated C2C12 myoblasts that were infected with either the pAd-GFP, pAd-WT, or pAd-E76A adenovirus. These experiments revealed that neither basal nor FGF-2-induced Erk2 kinase activity was affected when C2C12 myoblasts overexpressed the activated mutant of SHP-2 compared to activity with either pAd-GFP control or pAd-WT-infected myoblasts (Fig. 6B). Immunoblotting of HA-Erk2 immune complexes with Erk2 antibodies confirmed that Erk2 was present at equivalent levels in these kinase assays. In addition, immunoblotting of cell lysates indicated that both WT SHP-2 and SHP-2 E76A were overexpressed (Fig. 6B, bottom panel). Thus, we conclude that the activated mutant of SHP-2 does not affect basal Erk2 kinase activity.
FIG. 6.
A constitutively active mutant of SHP-2 fails to either activate or potentiate FGF-2-induced Erk. (A) C2C12 myoblasts that were left uninfected or infected with pAd-GFP, pAd-WT, or pAd-E76A were serum starved and either left untreated (−) or treated (+) with FGF-2 (10 ng/ml) for the indicated lengths of time. Activation of the Erks was assessed by immunoblotting of lysates prepared from the indicated conditions with anti-phospho-Erk antibodies (pErk). Immunoblots were reprobed for Erk and SHP-2 expression levels. Arrows to the right indicate the positions of Erk1 and Erk2. The graph shows results of densitometric analysis of pErk1/2 levels from experiments performed as described above. Normalized pErk1/2 represents the ratio of the densitometric units of pErk1/2 to the corresponding densitometric units of Erk1/2. Data shown are means ± standard errors of the means from four to five independent experiments. (B) C2C12 myoblasts either were left uninfected or were infected with pAd-GFP, pAd-WT, or pAd-E76A. Twenty-four hours after infection, the cells were serum starved for an additional 24 h and then were either left unstimulated or restimulated with FGF-2 (10 ng/ml) for 10 min. Myoblasts were then harvested, and lysates were immunoprecipitated (IP) with anti-Erk2 antibodies. These immune complexes were assayed for Erk2 activity by using MBP as a substrate. Lower panels show results of representative control experiments from the same lysates in which the Erk2 immune complexes were resolved by SDS-PAGE and immunoblotted with anti-Erk2 antibodies, and cell lysates were examined for SHP-2 expression levels by immunoblotting.
We also tested the ability of SHP-2 E76A to sustain elevated levels of basal Erk activation by using the more sensitive Elk-1 transactivation assay. For these experiments, pIRES-WT, pIRES-E76A, and a vector-alone control were transiently transfected into 293 cells and immune complex phosphatase assays were again performed in order to confirm that this expression vector generated the expected constitutively active PTP mutant of SHP-2. When transiently expressed, SHP-2 E76A exhibited an approximately 15-fold-higher level of phosphatase activity than WT SHP-2 (Fig. 7A). As shown in Fig. 7B, however, when SHP-2 E76A was transiently transfected into C2C12 myoblasts which were rendered quiescent by serum deprivation, SHP-2 E76A failed to appreciably increase the basal levels of Elk-1 transactivation relative to those with either the vector control or WT SHP-2. Under these conditions, equivalent levels of SHP-2 were expressed (Fig. 7B, inset). The failure of SHP-2 E76A to drive Elk-1 transcription was further exemplified when it was compared to a constitutively active Ras61(Leu), which produced robust transactivation of Elk-1. Collectively, these experiments support the data obtained with pAd-E76A, indicating that overexpression of SHP-2 E76A does not appear to be sufficient to appreciably activate the Erks or transactivate Elk-1 in myoblasts.
FIG. 7.
Effect of the constitutively active SHP-2 mutant on Elk-1 transactivation. (A) Myc-tagged WT SHP-2 and SHP-2 E76A were subcloned into the pIRES-GFP vector and were transfected, along with the pIRES-GFP control, into 293 cells. Anti-Myc-SHP-2 immune complexes were measured for phosphatase activity as described in Materials and Methods. Results shown are representative of two independent experiments, where E76A gives an ∼15-fold-higher level of pNPP hydrolysis than WT SHP-2. (Inset) Levels of SHP-2 protein assayed for phosphatase activity in these immune complexes were determined by immunoblotting of the resolved immune complexes with anti-SHP-2 antibodies. (B) C2C12 myoblasts were transfected with either pIRES-GFP, WT SHP-2 (pIRES-WT), or the constitutively active mutant of SHP-2 (pIRES-E76A). As a positive control, activated Ras [Ras61(Leu)] was also transfected into C2C12 myoblasts. After 24 h, the cultures were transferred to serum-free medium for a further 24 h, after which Elk-1 luciferase and β-galactosidase activities were determined. The experiments were performed in triplicate, and results are means ± standard errors of the means from three separate experiments, with Elk-1 luciferase activity normalized to β-galactosidase activity. (Inset) Equivalent expression levels of WT SHP-2 and SHP-2 E76A were confirmed following transfection of C2C12 myoblasts with pIRES-GFP, pIRES-WT, or pIRES-E76A. Transfectants were cultured for 48 h, and lysates were prepared and immunoprecipitated with anti-Myc (9E10) antibodies. IP, immunoprecipitation.
The constitutively active mutant of SHP-2 prevents entry into myogenesis.
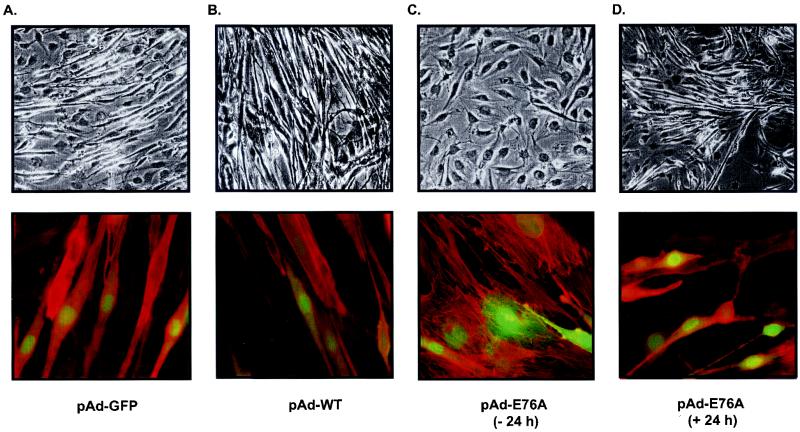
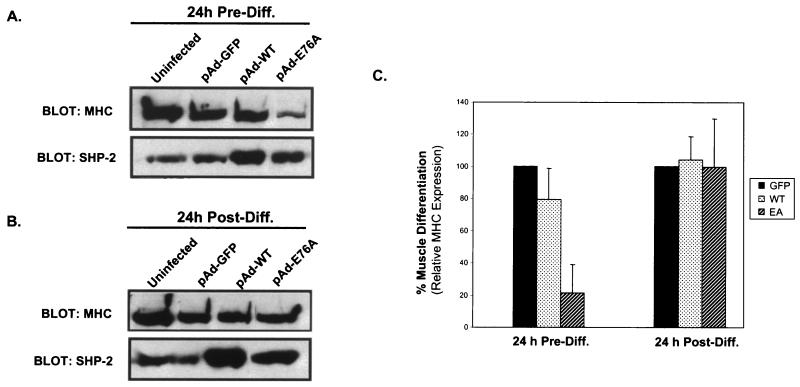
The FGFR has been implicated in regulating myogenesis in both Erk-dependent and -independent pathways (see the introduction). We therefore determined whether SHP-2 E76A, which appears to have little effect on either Elk-1 transactivation or Erk activity (Fig. 6 and 7), could inhibit myogenesis. To test whether the constitutively active PTP mutant of SHP-2 is sufficient to prevent myogenesis in the absence of growth factors, C2C12 myoblasts were infected with either pAd-GFP, pAd-WT, or pAd-E76A 24 h prior to initiation of myogenesis. Twenty-four hours later, differentiation of pAd-GFP-, pAd-WT-, and pAd-E76A-infected C2C12 myoblasts was induced for a further 72 h. pAd-GFP- and pAd-WT-infected C2C12 myoblasts underwent myogenesis appropriately and formed elongated myotubes (Fig. 8A and B). However, expression of pAd-E76A 24 h prior to the initiation of myogenesis inhibited C2C12 myoblast differentiation, as indicated by the absence of multinucleated myotubes (Fig. 8C). These morphological observations were supported by biochemical analysis of myogenesis. Uninfected, pAd-GFP-infected, and pAd-WT-infected C2C12 myoblasts expressed the terminal differentiation marker MHC, while myoblasts into which pAd-E76A was transduced prior to the initiation of myogenesis expressed MHC at significantly lower levels (Fig. 9A). Quantitative densitometric analyses of several experiments show that overexpression of SHP-2 E76A before differentiation results in approximately 80% inhibition of MHC expression relative to that with pAd-GFP alone (Fig. 9C).
FIG. 8.
The constitutively active mutant of SHP-2 is sufficient to block myogenesis. C2C12 myoblasts were infected either with pAd-GFP (A), pAd-WT (B), or pAd-E76A (C) 24 h prior to the initiation of differentiation or with pAd-E76A 24 h postdifferentiation (D). Shown are representative photomicrographs of Wright-Giemsa-stained C2C12 myoblasts (top row) and coimmunofluorescence images of adenovirus-infected myoblasts expressing GFP that were stained with Texas red phalloidin (bottom row). Infection of C2C12 myoblasts with the pAd-E76A adenovirus 24 h prior to the initiation of differentiation prevents multinucleated myotube formation as compared to pAd-GFP- or pAd-WT-infected myoblasts. When C2C12 myoblasts are infected with pAd-E76A 24 h after the initiation of differentiation, multinucleated myotube formation proceeds normally.
FIG. 9.
The constitutively active mutant of SHP-2 is sufficient to block muscle-specific gene expression. Lysates were prepared from C2C12 myoblasts that were either uninfected or infected 24 h prior to (A) or 24 h after (B) differentiation with pAd-GFP, pAd-WT, or pAd-E76A. Lysates were immunoblotted for the expression of SHP-2 and MHC. (C) Densitometric analysis of MHC expression in C2C12 myoblasts infected with either pAd-GFP, pAd-WT, or pAd-E76A 24 h prior to or 24 h following the initiation of differentiation. Data are means ± standard errors of the means from four to six separate experiments.
We next determined whether SHP-2 E76A could inhibit myogenesis after the process had been initiated. We infected C2C12 myoblasts with either pAd-GFP, pAd-WT, or pAd-E76A 24 h after the initiation of differentiation by serum withdrawal. We found that expression of SHP-2 E76A did not affect myogenic progression following the initiation of differentiation, as indicated by both the appearance of multinucleated myotubes (Fig. 8D) and the expression of MHC at levels similar to those observed in uninfected, pAd-GFP-infected, and pAd-WT-infected C2C12 myoblasts (Fig. 9B and C). Importantly, the inability of SHP-2 E76A to block the differentiation of C2C12 myoblasts once the process has been initiated shows that overexpression of SHP-2 E76A is not likely to be a nonspecific inhibitor of myogenic progression. These data demonstrate that the expression of a constitutively active mutant of SHP-2 is sufficient to prevent entry into myogenesis. However, once myogenesis has been initiated and myoblasts are irrevocably committed to terminal differentiation, the expression of SHP-2 E76A neither reverses nor retards this process. Taken together with the experiments for which results are presented in Fig. 4, our data suggest that the catalytic activity of SHP-2 negatively regulates myogenesis.
We have shown previously that in the absence of growth factors, SHP-2 in C2C12 myoblasts associates with a p120 complex that consists of both Gab-1 and SHPS-1 (24). SHP-2 also associates basally with FRS-2α in the absence of exogenous FGF-2 (Fig. 1D), presumably due to low levels of autocrine FGF-2 production (12, 18). Therefore, it is reasonable to propose that the inhibitory myogenic effects of SHP-2 E76A are mediated through SHPS-1/Gab-1 and/or FRS-2α complexes.
The activated mutant of SHP-2 induces hyper- and sustained tyrosyl phosphorylation of FRS-2α.
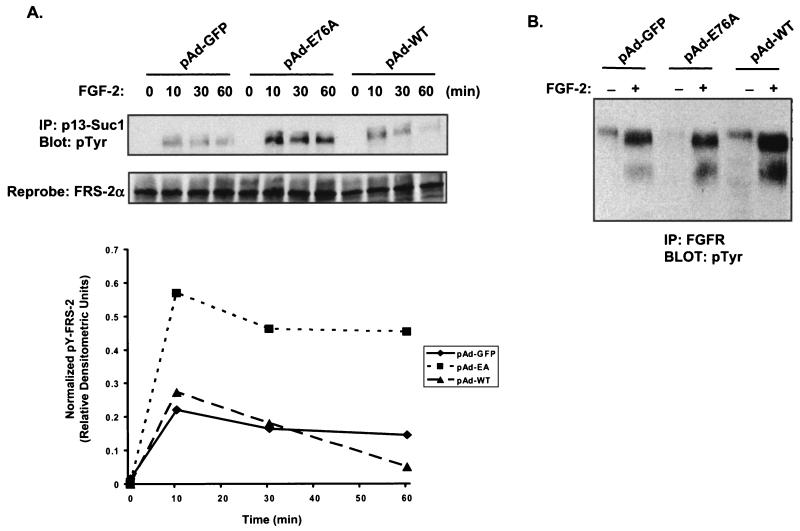
In order to investigate the mechanism by which the activated mutant of SHP-2 suppresses myogenesis, we asked whether this mutant affects tyrosyl phosphorylation of FRS-2α and/or activation of the FGFR. To test this, C2C12 myoblasts were infected with either pAd-GFP, pAd-E76A, or pAd-WT. Myoblasts were rendered quiescent by serum deprivation and then were either left unstimulated or stimulated with FGF-2 for various times. Surprisingly, we found that FGF-2 stimulation resulted in a sustained level of hyper-tyrosyl phosphorylation on FRS-2α in SHP-2 E76A-expressing myoblasts, relative to those in pAd-GFP- and pAd-WT-infected myoblasts, as determined by p13-Suc-1 affinity precipitation (Fig. 10A). These observations were confirmed following quantitative densitometric analysis of tyrosyl-phosphorylated FRS-2α immunoblots that were normalized to levels of immunoprecipitated FRS-2α (Fig. 10A). Similar results were also obtained with anti-FRS-2α antibodies (data not shown). Next, we tested whether the SHP-2 E76A-induced hyper-tyrosyl phosphorylation of FRS-2α was due to the ability of SHP-2 E76A to enhance the activity of the FGFR. Levels of FGFR activity in C2C12 myoblasts infected with pAd-GFP, pAd-WT, or pAd-E76A were determined by assessing the levels of FGFR auto-tyrosyl phosphorylation following FGF-2 stimulation. These experiments showed that expression of SHP-2 E76A in C2C12 myoblasts resulted in levels of FGFR auto-tyrosyl phosphorylation equivalent to those in GFP control- and WT SHP-2-infected myoblasts in response to FGF-2 stimulation (Fig. 10B). The results of these experiments reveal an unexpected finding, that the activated mutant of SHP-2 augments FGF-2-induced FRS-2α tyrosyl phosphorylation in myoblasts without affecting the activation of the FGFR. These data raise the possibility that the activated mutant of SHP-2 mimics the inhibitory myogenic effects of the FGFR in C2C12 myoblasts by potentiating FRS-2α tyrosyl phosphorylation.
FIG. 10.
The activated mutant of SHP-2 induces hyper- and sustained tyrosyl phosphorylation of FRS-2α. C2C12 myoblasts were infected with pAd-GFP, pAd-E76A, or pAd-WT for 24 h prior to serum deprivation. Twenty-four hours later, the cells were stimulated with 10 ng of FGF-2/ml for the indicated times. (A) Cells were harvested, and lysates were subjected to affinity precipitation with p13-Suc-1 agarose beads. This complex was resolved by SDS-PAGE and immunoblotted with antiphosphotyrosine (4G10) antibodies (pTyr) or anti-FRS-2α antibodies for the reprobe. The graph shows the results of densitometric analysis of the above experiment represented as arbitrary phosphotyrosyl FRS-2α levels normalized to total FRS-2α levels. (B) C2C12 myoblasts were infected with either pAd-GFP, pAd-E76A, or pAd-WT for 24 h prior to serum deprivation. Twenty-four hours later, the cells were stimulated with 10 ng of FGF-2/ml for 10 min. Cells were harvested, and lysates were subjected to immunoprecipitation with anti-FGFR antibodies, resolved by SDS-PAGE, and immunoblotted with anti-pTyr (4G10) antibodies. IP, immunoprecipitation.
DISCUSSION
The FGFR is responsible for mediating a diverse array of effects in different biological systems including cell proliferation, angiogenesis, mesoderm induction, patterning, and neurite outgrowth. In skeletal muscle, ligand activation of the FGFR stimulates Erk activation, myoblast proliferation, and repression of differentiation (22, 26, 27, 36, 52, 73). In this study, we have examined the role of SHP-2 in FGFR signaling in myoblasts. We have found that SHP-2 is recruited to a complex containing the multisubstrate adaptor protein FRS-2α following FGFR activation. Furthermore, the catalytic activity of SHP-2 is required for activation of the Erks and, subsequently, for the transactivation of Elk-1 in response to FGF-2 in myoblasts. Using a constitutively active mutant of SHP-2, we have also found that SHP-2 mediates the suppressive effects of the FGFR on myogenesis. Interestingly, the suppressive effect of the activated mutant of SHP-2 on myogenesis appears to occur via an Erk-independent pathway. Finally, our data suggest that the ability of the activated mutant of SHP-2 to recapitulate some of the FGFR signaling pathways is due to its ability to induce hyper-tyrosyl phosphorylation of FRS-2α in response to FGF-2. Thus, SHP-2 may participate in the early regulation of signaling pathways downstream of the FGFR that are responsible for myogenic regulation.
Activation of the FGFR leads to tyrosyl phosphorylation of a 90-kDa protein termed FRS-2α, a lipid-anchored, multisubstrate adaptor protein that contains a PTB domain (25, 48, 71). Using C2C12 myoblasts, we show that FRS-2α is expressed, becomes tyrosyl phosphorylated, and complexes with SHP-2 following FGFR activation (Fig. 1). Both FRS-2α and IRS-1 belong to the multisubstrate adaptor protein family. However, unlike the FGFs, which suppress myogenesis, the IGFs promote myogenesis via IRS-1 tyrosyl phosphorylation. Thus, it was formally possible that the IGFs could induce tyrosyl phosphorylation of FRS-2α, especially since ligands other than the FGFs, such as nerve growth factor, have been shown to induce FRS-2α tyrosyl phosphorylation (34). Also, there is the potential for the PTB domain of FRS-2α to compete for the same Shc binding site on the insulin receptor, allowing FRS-2α to bind and become tyrosyl phosphorylated by insulin (34). A recent report has suggested that insulin can induce FRS-2α tyrosyl phosphorylation in PC12 cells overexpressing the insulin receptor (10). This issue is of particular importance because the ability of the IGFs to initiate signaling via FRS-2α could have significant implications for our understanding of how these ligands antagonistically regulate skeletal muscle function. Our experiments indicate that under conditions where either insulin or IGF-I induces tyrosyl phosphorylation of IRS-1, FRS-2α does not become tyrosyl phosphorylated (Fig. 2). Conversely, FGF-2 stimulation of myoblasts does not result in tyrosyl phosphorylation of IRS-1 (Fig. 2). These data demonstrate that the signaling pathways for the IGFs and FGFs in skeletal muscle are indeed distinct.
The PTP domain of SHP-2 has been shown to be required for signaling to the Erks following FGF stimulation of fibroblasts (53), PC12 (17), and Xenopus ectodermal explants (59). However, a role for SHP-2 in FGF-2 signaling in myoblasts has yet to be demonstrated. We now show that the PTP activity of SHP-2 is required for FGF-2 stimulation of MAPK-mediated Elk-1 transactivation (Fig. 3). Following FGF-2 stimulation of myoblasts, we noted that SHP-2 becomes tyrosyl phosphorylated (Fig. 1), prompting us to test the requirement for the C-terminal Grb2 binding-tyrosyl phosphorylation sites in this pathway. We observed a modest but consistent inhibition of FGF-2-induced Elk-1 transactivation when the two C-terminal tyrosyl residues (Y542 and Y580) were mutated (Fig. 3). When Erk2 kinase activity was assessed following expression of the Grb2 binding-tyrosyl phosphorylation site mutant, maximal potentiation of Erk2 activity was not achieved following FGF-2 stimulation. Thus, as is the case in other systems, our data demonstrate that in myoblasts, the catalytic activity of SHP-2 is required for transmission of the predominant signal to the Erks and subsequently to Elk-1 following FGF-2 stimulation.
In this report we demonstrate that overexpression of wild-type SHP-2 potentiates both serum- and FGF-2-induced inhibition of myogenesis (Fig. 4). However, overexpression of a catalytically inactive mutant of SHP-2 abrogates both serum- and FGF-2-induced inhibition of muscle-specific gene expression. Therefore, these data reveal that the catalytic activity of SHP-2 participates in a pathway that contributes to the inhibitory actions of both serum and FGF-2 on myogenesis. When SHP-2 was overexpressed, its inhibitory effects on muscle-specific gene expression were significantly greater in the presence of FGF-2 than in the presence of serum. Previously, we have shown that SHP-2 complexes with both Gab-1 and SHPS-1 in C2C12 myoblasts in the presence of serum (24). However, in the presence of FGF-2, SHPS-1, Gab-1, and FRS-2α all complex with SHP-2 (Fig. 4A). It is conceivable that the ability of SHP-2 to further suppress muscle-specific gene expression in the presence of FGF-2, compared to serum, is reflected by its ability to form an additional complex with tyrosyl-phosphorylated FRS-2α. Given that SHP-2 has been shown to bind tyrosyl-phosphorylated FRS-2α directly through phosphotyrosyl residues pY436 and pY471 (17), we hypothesize that the interaction of SHP-2 with these residues plays a critical role in mediating the inhibitory myogenic effects by the FGFR. FRS-2α also interacts directly with Grb2 (17, 25), which could also participate in transmitting the inhibitory myogenic signals from the FGFR. Further work is required to determine the significance of the FRS-2α tyrosyl phosphorylation sites in FGF-2-mediated inhibition of myogenesis.
We have utilized a constitutively active mutant of SHP-2 to assess the sufficiency of the PTP activity of SHP-2 to inhibit myogenesis. These data demonstrate that the catalytic activity of SHP-2 is sufficient to promote myogenic inhibition prior to the initiation of differentiation. However, the activated mutant of SHP-2 no longer suppresses differentiation once the myogenic process has been initiated (Fig. 8 and 9). This is consistent with the notion that once myogenesis has been initiated following growth factor removal, myoblasts become irrevocably committed to terminal differentiation (8, 21, 44, 47). These observations indicate that the suppressive effects of the activated SHP-2 mutant are likely restricted to myoblasts that have not yet begun the process of myogenesis. The inhibitory effects of the activated SHP-2 mutant on myogenesis provide a potential mechanism whereby the mitogenic and the inhibitory myogenic effects of the FGFR can be separated (22, 26, 27, 57). SHP-2 may represent the bifurcation in the signaling pathway downstream of the FGFR that mediates both the Erk-dependent proliferative and the Erk-independent suppressive myogenic effects of FGF-2.
Our data do not support the interpretation that activation of Erk is necessary to mediate the suppressive effects of FGFR signaling on myogenesis. Although overexpression of an activated mutant of SHP-2 suppresses myogenesis, no appreciable increase in either Erk activity or Elk-1 transactivation is observed (Fig. 6 and 7). Importantly, the suppressive effects of the activated SHP-2 mutant are not likely due to its mislocalization, since this mutant interacts appropriately with both FRS-2α and the p120 complex (containing Gab-1 and SHPS-1) (Fig. 5B). These data raise two possibilities for how SHP-2 is involved in mediating the repressive effects of FGF-2 on myogenesis. SHP-2 may either signal in a completely distinct pathway, independent of the Erks, to repress myogenesis, or it may activate the Erks to levels just sufficient to suppress myogenesis. We favor the former explanation because direct Erk2 kinase assays of lysates from SHP-2 E76A-transduced myoblasts showed that SHP-2 did not alter the level of basal Erk2 activity (Fig. 6B). Although there is evidence to indicate that Erk is sufficient to repress myogenesis, there is also evidence for Erk-independent pathways that participate in suppressing myogenesis (22, 26, 27). In MM14 cells, a skeletal muscle satellite cell line that is strictly dependent on the FGFs, proliferation and repression of differentiation are separate events (26). Moreover, Erk activity is dispensable for myogenic repression by FGF-2 in these MM14 cells (22). It is conceivable that our data implicate SHP-2 as a component of the Erk-independent signaling pathway that represses differentiation.
In many regards, the results presented here are similar to those reported by O'Reilly et al., who found that the activated mutant of SHP-2 was sufficient for mesoderm induction to levels similar to those with FGF stimulation (46). However, unlike FGF, which induces a robust activation of Erk in Xenopus explants, the activated mutant of SHP-2 fails to significantly induce Erk activity (46). The activated SHP-2 mutant may retain the capacity to engage the Ras/Raf/Erk pathway weakly but may signal more robustly to other pathways, presumably those that lead to the control of differentiation and/or other morphogenic processes. SHP-2 has been shown to be upstream of Ras (42, 46, 55) and has recently been implicated as a regulator of other small GTP-binding proteins such as Rho (23, 46, 54). Interestingly, activated mutants of RhoG, Rac1, and cdc42Hs have been shown to prevent myogenesis (35). These observations raise the possibility that the activated mutant of SHP-2 could potentially inhibit myogenesis by engaging a Rho GTP-binding protein.
Our data have revealed an intriguing and unexpected finding; they indicate that the activated mutant of SHP-2 promotes and subsequently potentiates FGF-2-induced FRS-2α tyrosyl phosphorylation (Fig. 10A). These observations may explain why the activated mutant of SHP-2 recapitulates the actions of FGF-2 to inhibit myogenesis. It is conceivable that under low concentrations of FGF-2, FRS-2α is not stoichiometrically tyrosyl phosphorylated and FGF-2-mediated signaling is silent. However, expression of the activated mutant of SHP-2 in the absence of exogenous FGF-2 may promote tyrosyl phosphorylation of FRS-2α to levels that are sufficient to propagate the FGFR signal. In fact, the activated mutant of SHP-2 can synergize with subthreshold levels of exogenous FGF-2, resulting in the full restoration of animal cap elongation (46). In the absence of growth factors, autocrine production of FGF-2 in myoblasts is insufficient to repress myogenesis. The activated mutant of SHP-2 may cooperate, either additively or synergistically, with these low levels of FGF-2 to induce an inhibitory myogenic signal by increasing FRS-2α tyrosyl phosphorylation. In agreement with this hypothesis, we did observe a slightly higher level of FRS-2α tyrosyl phosphorylation (data not shown), and SHP-2 is basally associated with FRS-2α in the absence of growth factors (Fig. 1D). Recent evidence suggests that Ras is responsible for the autocrine production of FGF-2 in the MM14 skeletal muscle satellite cell line (12). One possibility is that the activated mutant of SHP-2 may promote FGF-2 autocrine production via a Ras, or another small GTP-binding protein-dependent pathway, in addition to regulating tyrosyl phosphorylation of FRS-2α. In combination, these two mechanisms could culminate in an FGFR/FRS-2α signal that would be sufficient to suppress myogenesis.
Although the activated mutant of SHP-2 did not cause activation of the Erks, it did induce hyper-tyrosyl phosphorylation of FRS-2α (Fig. 10). One might have anticipated that hyper-tyrosyl phosphorylation of FRS-2α would be sufficient to induce Erk activation. One interpretation of these observations is that there are tyrosyl phosphorylation sites that reside on FRS-2α which do not couple to the Erk pathway but rather couple the FGFR to the myogenic pathway. These data are supported by the longstanding observation that FGFR-mediated proliferation and inhibition of myogenesis are separable events (22, 26, 27, 57). The precise mechanisms by which the activated mutant of SHP-2 causes hyper- and sustained tyrosyl phosphorylation of FRS-2α in response to FGF-2 remain to be elucidated. However, one could speculate that this mutant of SHP-2 leads to the activation of a tyrosine kinase that subsequently phosphorylates FRS-2α. One obvious candidate is the FGFR itself; however, we find no difference in the levels of FGFR auto-tyrosyl phosphorylation when the activated mutant of SHP-2 is expressed (Fig. 10B). An alternative mechanism could involve inhibition of a PTP by the activated SHP-2 mutant that negatively regulates FRS-2α tyrosyl phosphorylation. Clearly, much work is needed to resolve the precise mechanistic basis of these effects of the activated SHP-2 mutant on FRS-2α tyrosyl phosphorylation. In summary, our results reveal a pathway in which SHP-2 functions to mediate both the proliferative Erk signaling pathway and the inhibitory myogenic signals. Further investigation into the targets of SHP-2 in myogenic signaling is required to elucidate the details of this apparent dual signaling role for SHP-2 in myoblasts.
Acknowledgments
We thank Mitchel Goldfarb for providing the FRS-2α antibodies and Joseph Schlessinger for providing FRS-2α and FGFR antibodies. We thank the members of the Bennett laboratory and P. Lombroso, J. Howe, and M. Stern for comments and help on this work.
This work was supported by Public Health Service grant R01-AR46504 and a Burroughs-Wellcome New Investigators Award in the Pharmacological Sciences to A.M.B. M.I.K. and X.L. were supported by NIH training grants T32-GM07324 and T32-NS07136, respectively.
REFERENCES
- 1.Adachi, M., M. Sekiya, T. Miyachi, K. Matsuno, Y. Hinoda, K. Imai, and A. Yachi. 1992. Molecular cloning of a novel protein-tyrosine phosphatase, SH-PTP3, with sequence similarity to the src-homology region 2. FEBS Lett. 314:335-339. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad, S., D. Banville, Z. Zhao, E. H. Fischer, and S.-H. Shen. 1993. A widely expressed human protein tyrosine phosphatase contains src homology 2 domains. Proc. Natl. Acad. Sci. USA 90:2197-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett, A. M., S. F. Hausdorff, A. M. O'Reilly, R. M. Freeman, and B. G. Neel. 1996. Multiple requirements for SHPTP2 in epidermal growth factor-mediated cell cycle progression. Mol. Cell. Biol. 16:1189-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett, A. M., T. L. Tang, S. Sugimoto, C. T. Walsh, and B. G. Neel. 1994. Protein-tyrosine-phosphatase SHPTP2 couples platelet-derived growth factor receptor β to Ras. Proc. Natl. Acad. Sci. USA 91:7335-7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett, A. M., and N. K. Tonks. 1997. Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science 278:1288-1291. [DOI] [PubMed] [Google Scholar]
- 6.Bjorbak, C., R. M. Buchholz, S. M. Davis, S. H. Bates, D. D. Pierroz, H. Gu, B. G. Neel, M. G. Myers, Jr., and J. S. Flier. 2001. Divergent roles of SHP-2 in ERK activation by leptin receptors. J. Biol. Chem. 276:4747-4755. [DOI] [PubMed] [Google Scholar]
- 7.Campbell, J. S., M. P. Wenderoth, S. D. Hauschka, and E. G. Krebs. 1995. Differential activation of mitogen-activated protein kinase in response to basic fibroblast growth factor in skeletal muscle. Proc. Natl. Acad. Sci. USA 92:870-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clegg, C., T. Linkhart, B. Olwin, and S. Hauschka. 1987. Growth factor control of skeletal muscle differentiation: commitment to terminal differentiation occurs in G1 phase and is repressed by fibroblast growth factor. J. Cell Biol. 105:949-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coolican, S. A., D. S. Samuel, D. Z. Ewton, F. J. McWade, and J. R. Florini. 1997. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J. Biol. Chem. 272:6653-6662. [DOI] [PubMed] [Google Scholar]
- 10.Delahaye, L., S. Rocchi, and E. Van Obberghen. 2000. Potential involvement of FRS2 in insulin signaling. Endocrinology 141:621-628. [DOI] [PubMed] [Google Scholar]
- 11.Dias, P., M. Dilling, and P. Houghton. 1994. The molecular basis of skeletal muscle differentiation. Semin. Diagn. Pathol. 11:3-14. [PubMed] [Google Scholar]
- 12.Fedorov, Y. V., R. S. Rosenthal, and B. B. Olwin. 2001. Oncogenic Ras-induced proliferation requires autocrine fibroblast growth factor 2 signaling in skeletal muscle cells. J. Cell Biol. 152:1301-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng, G. S. 1999. Shp-2 tyrosine phosphatase: signaling one cell or many. Exp. Cell Res. 253:47-54. [DOI] [PubMed] [Google Scholar]
- 14.Feng, G.-S., C.-C. Hui, and T. Pawson. 1993. SH2-containing phosphotyrosine phosphatase as a target of protein-tyrosine kinases. Science 259:1607-1611. [DOI] [PubMed] [Google Scholar]
- 15.Freeman, R. M., J. Plutzky, and B. G. Neel. 1992. Identification of a human src homology 2-containing protein tyrosine-phosphatase: a putative homolog of Drosophila corkscrew. Proc. Natl. Acad. Sci. USA 89:11239-11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gredinger, E., A. N. Gerber, Y. Tamir, S. J. Tapscott, and E. Bengal. 1998. Mitogen-activated protein kinase pathway is involved in the differentiation of muscle cells. J. Biol. Chem. 273:10436-10444. [DOI] [PubMed] [Google Scholar]
- 17.Hadari, Y. R., H. Kouhara, I. Lax, and J. Schlessinger. 1998. Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol. Cell. Biol. 18:3966-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hannon, K., A. J. Kudla, M. J. McAvoy, K. L. Clase, and B. B. Olwin. 1996. Differentially expressed fibroblast growth factors regulate skeletal muscle development through autocrine and paracrine mechanisms. J. Cell Biol. 132:1151-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hof, P., S. Pluskey, S. Dhe-Pagganon, M. J. Eck, and S. E. Shoelson. 1998. Crystal structure of the tyrosine phosphatase SHP-2. Cell 92:441-450. [DOI] [PubMed] [Google Scholar]
- 21.Itoh, N., T. Mima, and T. Mikawa. 1996. Loss of fibroblast growth factor receptors is necessary for terminal differentiation of embryonic limb muscle. Development 122:291-300. [DOI] [PubMed] [Google Scholar]
- 22.Jones, N. C., Y. V. Fedorov, R. S. Rosenthal, and B. B. Olwin. 2001. ERK1/2 is required for myoblast proliferation but is dispensable for muscle gene expression and cell fusion. J. Cell. Physiol. 186:104-115. [DOI] [PubMed] [Google Scholar]
- 23.Kodama, A., T. Matozaki, A. Fukuhara, M. Kikyo, M. Ichihashi, and Y. Takai. 2000. Involvement of an SHP-2-Rho small G protein pathway in hepatocyte growth factor/scatter factor-induced cell scattering. Mol. Biol. Cell 11:2565-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kontaridis, M. I., X. Liu, L. Zhang, and A. M. Bennett. 2001. SHP-2 complex formation with the SHP-2 substrate-1 during C2C12 myogenesis. J. Cell Sci. 114:2187-2198. [DOI] [PubMed] [Google Scholar]
- 25.Kouhara, H., Y. R. Hadari, T. Spivak-Kroizman, J. Schilling, D. Bar-Sagi, I. Lax, and J. Schlessinger. 1997. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 89:693-702. [DOI] [PubMed] [Google Scholar]
- 26.Kudla, A., N. Jones, R. Rosenthal, K. Arthur, K. Clase, and B. Olwin. 1998. The FGF receptor-1 tyrosine kinase domain regulates myogenesis but is not sufficient to stimulate proliferation. J. Cell Biol. 142:241-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kudla, A. J., M. L. John, D. F. Bowen-Pope, B. Rainish, and B. B. Olwin. 1995. A requirement for fibroblast growth factor in regulation of skeletal muscle growth and differentiation cannot be replaced by activation of platelet-derived growth factor signaling pathways. J. Cell Biol. 15:3238-3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lassar, A. B., S. X. Skapek, and N. Bennett. 1994. Regulatory mechanisms that coordinate skeletal muscle differentiation and cell cycle withdrawal. Curr. Opin. Cell Biol. 6:788-794. [DOI] [PubMed] [Google Scholar]
- 29.Lathrop, B., K. Thomas, and L. Glaser. 1985. Control of myogenic differentiation by fibroblast growth factor is mediated by position in the G1 phase of the cell cycle. J. Cell Biol. 101:2194-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lechleider, R. J., R. M. Freeman, Jr., and B. G. Neel. 1993. Tyrosyl phosphorylation and growth factor receptor association of the human corkscrew homologue, SH-PTP2. J. Biol. Chem. 268:13434-13438. [PubMed] [Google Scholar]
- 31.Lechleider, R. J., S. Sugimoto, A. M. Bennett, A. Kashishian, J. A. Cooper, S. E. Shoelson, C. T. Walsh, and B. G. Neel. 1993. Activation of the SH2-containing phosphotyrosine phosphatase SH-PTP2 by its binding site, phosphotyrosine 1009, on the human platelet-derived growth factor receptor β. J. Biol. Chem. 268:21478-21481. [PubMed] [Google Scholar]
- 32.Li, W., R. Nishimura, A. Kashishian, A. G. Batzer, W. J. H. Kim, J. A. Cooper, and J. Schlessinger. 1994. A new function for a phosphotyrosine phosphatase: linking GRB2-Sos to a receptor tyrosine kinase. Mol. Cell. Biol. 14:509-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maher, P. 1999. p38 mitogen-activated protein kinase activation is required for fibroblast growth factor-2-stimulated proliferation but not differentiation. J. Biol. Chem. 274:17491-17498. [DOI] [PubMed] [Google Scholar]
- 34.Meakin, S. O., J. I. MacDonald, E. A. Gryz, C. J. Kubu, and J. M. Verdi. 1999. The signaling adapter FRS-2 competes with Shc for binding to the nerve growth factor receptor TrkA. A model for discriminating proliferation and differentiation. J. Biol. Chem. 274:9861-9870. [DOI] [PubMed] [Google Scholar]
- 35.Meriane, M., P. Roux, M. Primig, P. Fort, and C. Gauthier-Rouviere. 2000. Critical activities of Rac1 and Cdc42Hs in skeletal myogenesis: antagonistic effects of JNK and p38 pathways. Mol. Biol. Cell 11:2513-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milasincic, D. J., M. R. Calera, S. R. Farmer, and P. F. Pilch. 1996. Stimulation of C2C12 myoblast growth by basic fibroblast growth factor and insulin-like growth factor 1 can occur via mitogen-activated protein kinase-dependent and -independent pathways. Mol. Cell. Biol. 16:5964-5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgenstern, J. P., and H. Land. 1990. A series of mammalian expression vectors and characterisation of their expression of a reporter gene in stably and transiently transfected cells. Nucleic Acids Res. 18:1068.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naya, F. S., and E. Olson. 1999. MEF2: a transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation. Curr. Opin. Cell Biol. 11:683-688. [DOI] [PubMed] [Google Scholar]
- 39.Neel, B. G. 1997. Role of phosphatases in lymphocyte activation. Curr. Opin. Immunol. 9:405-420. [DOI] [PubMed] [Google Scholar]
- 40.Neel, B. G. 1993. Structure and function of SH2-domain-containing tyrosine phosphatases. Semin. Cell Biol. 4:419-432. [DOI] [PubMed] [Google Scholar]
- 41.Neel, B. G., and N. K. Tonks. 1997. Protein tyrosine phosphatases in signal transduction. Curr. Opin. Cell Biol. 9:193-204. [DOI] [PubMed] [Google Scholar]
- 42.Noguchi, T., T. Matozaki, K. Horita, Y. Fujioka, and M. Kasuga. 1994. Role of SH-PTP2, a protein-tyrosine phosphatase with src homology 2 domains, in insulin-stimulated ras activation. Mol. Cell. Biol. 14:6674-6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olson, E., and W. Klein. 1994. bHLH factors in muscle development: dead lines and commitments, what to leave in and what to leave out. Genes Dev. 8:1-8. [DOI] [PubMed] [Google Scholar]
- 44.Olwin, B. B., and S. D. Hauschka. 1988. Cell surface fibroblast growth factor and epidermal growth factor receptors are permanently lost during skeletal muscle terminal differentiation in culture. J. Cell Biol. 107:761-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ong, S. H., G. R. Guy, Y. R. Hadari, S. Laks, N. Gotoh, J. Schlessinger, and I. Lax. 2000. FRS2 proteins recruit intracellular signaling pathways by binding to diverse targets on fibroblast growth factor and nerve growth factor receptors. Mol. Cell. Biol. 20:979-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Reilly, A., S. Pluskey, S. E. Shoelson, and B. G. Neel. 2000. Activated mutants of SHP-2 preferentially induce elongation of Xenopus animal caps. Mol. Cell. Biol. 20:299-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel, S. G., P. E. Funk, and J. X. DiMario. 1999. Regulation of avian fibroblast growth factor receptor 1 (FGFR-1) gene expression during skeletal muscle differentiation. Gene 237:265-276. [DOI] [PubMed] [Google Scholar]
- 48.Rabin, S. J., V. Cleghon, and D. R. Kaplan. 1993. SNT, a differentiation-specific target of neurotrophic factor-induced tyrosine kinase activity in neurons and PC12 cells. Mol. Cell. Biol. 13:2203-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raffioni, S., D. Thomas, E. D. Foehr, L. M. Thompson, and R. A. Bradshaw. 1999. Comparison of the intracellular signaling responses by three chimeric fibroblast growth factor receptors in PC12 cells. Proc. Natl. Acad. Sci. USA 96:7178-7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramocki, M., S. Johnson, M. White, C. Ashendel, S. Konieczny, and E. Taparowsky. 1997. Signaling through mitogen-activated protein kinase and Rac/Rho does not duplicate the effects of activated Ras on skeletal myogenesis. Mol. Cell. Biol. 17:3547-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramocki, M. B., M. A. White, S. F. Konieczny, and E. J. Taparowsky. 1998. A role for RalGDS and a novel Ras effector in the Ras-mediated inhibition of skeletal myogenesis. J. Biol. Chem. 273:17696-17701. [DOI] [PubMed] [Google Scholar]
- 52.Rapraeger, A. C., A. Krufka, and B. B. Olwin. 1991. Requirement of heparan sulfate for bFGF-mediated fibroblast growth factor and myoblast differentiation. Science 252:1705-1708. [DOI] [PubMed] [Google Scholar]
- 53.Saxton, T. M., M. Henkemeyer, S. Gasca, R. Shen, D. J. Rossi, F. Shalaby, G. S. Feng, and T. Pawson. 1997. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 16:2352-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schoenwaelder, S. M., L. A. Petch, D. Williamson, R. Shen, G. Feng, and K. Burridge. 2000. The protein tyrosine phosphatase shp-2 regulates RhoA activity. Curr. Biol. 10:1523-1526. [DOI] [PubMed] [Google Scholar]
- 55.Shi, Z. Q., D. H. Yu, M. Park, M. Marshall, and G. S. Feng. 2000. Molecular mechanism for the Shp-2 tyrosine phosphatase function in promoting growth factor stimulation of Erk activity. Mol. Cell. Biol. 20:1526-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skapek, S., J. Rhee, D. Spicer, and A. Lassar. 1995. Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science 267:1022-1024. [DOI] [PubMed] [Google Scholar]
- 57.Spizz, G., D. Roman, A. Strauss, and E. Olson. 1986. Serum and fibroblast growth factor inhibit myogenic differentiation through a mechanism dependent on protein synthesis and independent of cell proliferation. J. Biol. Chem. 261:9483-9488. [PubMed] [Google Scholar]
- 58.Takano, H., I. Komuro, T. Oka, I. Shiojima, Y. Hiroi, T. Mizuno, and Y. Yazaki. 1998. The Rho family G proteins play a critical role in muscle differentiation. Mol. Cell. Biol. 18:1580-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang, T. L., R. M. Freeman, Jr., A. M. O'Reilly, B. G. Neel, and S. Y. Sokol. 1995. The SH2-containing protein tyrosine phosphatase SH-PTP2 is required upstream of MAP kinase for early Xenopus development. Cell 80:473-483. [DOI] [PubMed] [Google Scholar]
- 60.Tauchi, T., G.-S. Feng, M. S. Marshall, R. Shen, C. Mantel, T. Pawson, and H. E. Broxmeyer. 1994. The ubiquitously expressed Syp phosphatase interacts with c-kit and Grb2 in hematopoietic cells. J. Biol. Chem. 269:25206-25211. [PubMed] [Google Scholar]
- 61.Tauchi, T., G.-S. Feng, R. Shen, M. Hoatlin, G. C. Bagby, D. Kabat, and H. E. Broxmeyer. 1995. Involvement of SH2-containing phosphotyrosine phosphatase Syp in erythropoietin receptor signal transduction pathways. J. Biol. Chem. 270:5631-5635. [DOI] [PubMed] [Google Scholar]
- 62.Tonks, N. K., and B. G. Neel. 1996. From form to function: signaling by protein tyrosine phosphatases. Cell 87:365-368. [DOI] [PubMed] [Google Scholar]
- 63.Vaidya, T. B., S. J. Rhodes, E. J. Taparowsky, and S. F. Konieczny. 1989. Fibroblast growth factor and transforming growth factor β repress transcription of the myogenic regulatory gene MyoD1. Mol. Cell. Biol. 9:3576-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vincent, C. K., A. Gualberto, C. V. Patel, and K. Walsh. 1993. Different regulatory sequences control creatine kinase-M gene expression in directly injected skeletal and cardiac muscle. Mol. Cell. Biol. 13:1264-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vogel, W., R. Lammers, J. Huang, and A. Ullrich. 1993. Activation of a phosphotyrosine phosphatase by tyrosine phosphorylation. Science 259:1611-1614. [DOI] [PubMed] [Google Scholar]
- 66.Vogel, W., and A. Ullrich. 1996. Multiple in vivo phosphorylated tyrosine phosphatase SHP-2 engages binding to Grb2 via tyrosine 584. Cell Growth Differ. 7:1589-1597. [PubMed] [Google Scholar]
- 67.Weintraub, H. 1993. The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell 75:1241-1244. [DOI] [PubMed] [Google Scholar]
- 68.Welham, M. J., U. Dechert, K. B. Leslie, F. Jirik, and J. W. Schrader. 1994. Interleukin (IL)-3 and granulocyte/macrophage colony-stimulating factor, but not IL-4, induce tyrosine phosphorylation, activation, and association of SHPTP2 with Grb2 and phosphatidylinositol 3′-kinase. J. Biol. Chem. 269:23764-23768. [PubMed] [Google Scholar]
- 69.Weyman, C. M., and A. Wolfman. 1998. Mitogen-activated protein kinase kinase (MEK) activity is required for inhibition of skeletal muscle differentiation by insulin-like growth factor 1 or fibroblast growth factor 2. Endocrinology 139:1794-1800. [DOI] [PubMed] [Google Scholar]
- 70.Wu, Z., P. J. Woodring, K. S. Bhakta, K. Tamura, F. Wen, J. R. Feramisco, M. Karin, J. Y. Wang, and P. L. Puri. 2000. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol. Cell. Biol. 20:3951-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu, H., K. W. Lee, and M. Goldfarb. 1998. Novel recognition motif on fibroblast growth factor receptor mediates direct association and activation of SNT adapter proteins. J. Biol. Chem. 273:17987-17990. [DOI] [PubMed] [Google Scholar]
- 72.Yang, S. H., A. J. Whitmarsh, R. J. Davis, and A. D. Sharrocks. 1998. Differential targeting of MAP kinases to the ETS-domain transcription factor Elk-1. EMBO J. 17:1740-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoshida, S., A. Fujisawa-Sehara, T. Taki, and Y. Nabeshima. 1996. Lysophosphatidic acid and bFGF control different modes in proliferating myoblasts. J. Cell Biol. 132:181-193. [DOI] [PMC free article] [PubMed] [Google Scholar]