Abstract
We have characterized Drosophila melanogaster ACK (DACK), one of two members of the ACK family of nonreceptor tyrosine kinases in Drosophila. The ACKs are likely effectors for the small GTPase Cdc42, but signaling by these proteins remains poorly defined. ACK family tyrosine kinase activity functions downstream of Drosophila Cdc42 during dorsal closure of the embryo, as overexpression of DACK can rescue the dorsal closure defects caused by dominant-negative Dcdc42. Similar to known participants in dorsal closure, DACK is enriched in the leading edge cells of the advancing epidermis, but it does not signal through activation of the Jun amino-terminal kinase cascade operating in these cells. Transcription of DACK is responsive to changes in Dcdc42 signaling specifically at the leading edge and in the amnioserosa, two tissues involved in dorsal closure. Unlike other members of the ACK family, DACK does not contain a conserved Cdc42-binding motif, and transcriptional regulation may be one route by which Dcdc42 can affect DACK function. Expression of wild-type and kinase-dead DACK transgenes in embryos, and in the developing wing and eye, reveals that ACK family tyrosine kinase activity is involved in a range of developmental events similar to that of Dcdc42.
Cdc42 is a member of the Rho family of Ras-related small GTPases originally identified through a mutation in Saccharomyces cerevisiae that affects formation of the bud site. The Cdc42 protein is required for the assembly of a ring of F-actin filaments in the neck of the bud (1). Subsequent work in mammalian fibroblasts demonstrated that Cdc42 drives the formation of F-actin-rich filopodia (40, 50), and numerous later studies have confirmed that Cdc42 regulates the actin cytoskeleton and, as a consequence, cell shape (65). Cdc42 participates in a diverse range of cellular processes including membrane trafficking, transcription, cell growth, and Ras-mediated transformation (65). The various effects of Cdc42 are presumed to be mediated through the interaction of the activated, GTP-bound form of the protein with downstream effectors.
Given the important events controlled by Cdc42, intensive efforts have been made to elucidate the signaling pathways activated by this GTPase. This work has largely focused on identifying proteins that interact with GTP-bound Cdc42. Two such proteins are ACK-1 and ACK-2, closely related mammalian nonreceptor tyrosine kinases that bind GTP-bound Cdc42 and not its inactive GDP-bound form (44, 67). ACK-1 and ACK-2 cannot bind either version of the closely related Rho family GTPases Rac1 and RhoA, and these kinases represent likely effectors in Cdc42-specific signaling.
To date, much of what is known about Rho family signaling has come from biochemical and cell biological work, but it is now being studied with genetic approaches in a number of model organisms, including Drosophila melanogaster. The Drosophila homolog of Cdc42, Dcdc42, has been studied by using dominantly acting mutant transgenes and loss-of-function mutations. This work has indicated that Dcdc42 participates in a wide range of developmental events including neurite outgrowth (25, 43), actin filament assembly and follicle cell morphogenesis during oogenesis (26, 48), and various aspects of wing development including cell elongation, planar polarity, cell fate choice, and apposition of the wing surfaces (5, 19, 20, 26). Dcdc42 is also required for germband retraction and dorsal closure of the epidermis during embryogenesis (26, 29, 57).
In the interest of further exploring Dcdc42 signaling in Drosophila development, we have characterized a Drosophila member of the ACK family of nonreceptor tyrosine kinases, DACK. DACK is one of two ACK family members in Drosophila, the other being DPR2. Through the expression of wild-type and kinase-dead DACK transgenes, we show that alterations in ACK family tyrosine kinase activity produce phenotypes similar to those resulting from perturbation of Dcdc42 signaling. We present evidence that ACK family tyrosine kinase activity occurs downstream of Dcdc42 during dorsal closure.
MATERIALS AND METHODS
Standard molecular biology procedures were performed as described elsewhere (61).
PCR amplification of a DACK genomic fragment.
In a screen originally intended to identify Polo-like kinases, PCR was performed on Drosophila genomic DNA using the degenerate oligonucleotides 5′-AAGAT(T/C/A)GG(T/C/G)GA(T/C)TT(T/C)GG(N)(C/G)T-3′ (forward primer) and 5′-(C/G)(T/A)(G/A)TA(G/A)TC(G/A)ACCCA(T/C)TT-3′ (reverse primer) corresponding to the likely conserved amino acid sequences KIGDFGL/V and KWVDYS. Amplified fragments were treated with Klenow polymerase, cloned into EcoRV-digested pBluescript, and sequenced. Among the PCR products identified was a 0.4-kb fragment, with the forward PCR primer at both ends, that encoded a predicted amino acid sequence with significant homology to mammalian ACK proteins.
Whole-mount in situ hybridization to embryos.
In situ mRNA hybridizations using digoxigenin-labeled RNA probes were performed essentially as described previously (66).
Preparation of dsRNA for RNA-mediated interference (RNAi) studies.
A PstI/PvuII restriction fragment encompassing nucleotides 2512 to 3285 of the DACK cDNA sequenced by the Berkeley Drosophila Genome Project (BDGP) was subcloned into PstI/SmaI-cut pKS-ds-T7, a modified pBluescript SK(+) vector with two T7 promoter sites (11). The DACK fragment was released with T7 promoters on both ends by AscI digestion, and transcription was performed using the RiboMAX kit (Promega) according to the manufacturer's directions. The integrity of the double-stranded RNA (dsRNA) was checked by agarose gel electrophoresis prior to injection. Injection of dsRNA was performed as described by Kennerdell and Carthew (39).
Construction of transgenic lines.
A kinase-dead mutant version of a DACK cDNA was made using the QuikChange site-directed mutagenesis kit (Stratagene). The oligonucleotide 5′ CCCGGTGGCCGTCAGGGTGCTGAAGTCGG 3′ was used to convert amino acid residue 156 from Lys to Arg. The base change altering the codon is in bold. Mutant and wild-type DACK cDNAs were subcloned into the pUAST vector (7) and injected into yw embryos, and transgenic lines were established (54).
Fly stocks and transgene expression.
Standard Drosophila procedures were followed. Unless otherwise stated, all flies were raised and crossed at 25°C. Transgenes under upstream activation sequence (UAS) control were expressed using GAL4 (7). Females from GAL4 lines were crossed to males from the pUAST transgenic lines and the progeny were examined as embryos or adults. For heat shock induction of transgenes, embryos were collected and aged at 25°C until 6 to 12 h after egg laying. They were then placed in vials and heat shocked in a water bath set at 37°C. Following heat shock, embryos were aged at 21°C for at least 48 h and subjected to cuticle preparation, or aged for 7 h at 21°C and fixed for RNA in situ hybridization.
Antibodies.
A glutathione S-transferase (GST) fusion protein containing sequences from the predicted DACK protein was used to immunize rabbits. Antibodies were affinity purified by the low-pH method (61). A monoclonal antiphosphotyrosine antibody was obtained from Upstate Biotechnology Inc.
Immunohistochemistry.
Fixing and antibody staining of embryos were as described previously (4). Peroxidase-conjugated goat secondary antibodies (Jackson ImmunoResearch Laboratories) were detected by using the glucose oxidase-diaminobenzidine-nickel method (34).
In situ hybridization to polytene chromosomes.
Polytene chromosomes were prepared and hybridized with a biotinylated DNA probe as described elsewhere (4). Peroxidase detection of signals was done with a Detek-1-HRP kit (Enzo Biochemicals).
In vitro binding assay.
The full-length DACK open reading frame was transcribed and translated into [35S]methionine-labeled protein using the Promega TNT Quick Coupled Transcription/Translation kit. This protein was then tested for binding to GST-Dcdc42 fusion protein that had been loaded with GTPγS or GDP, using the protocol described by Lu and Settleman (42). Briefly, GST-Dcdc42 was bound to glutathione-Sepharose beads and loaded with GTPγS or GDP. The GST-Dcdc42 samples were then incubated with in vitro-translated DACK, washed, and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Signals were detected by autoradiography.
Cuticle preparations and wing mounts.
Cuticles were prepared as described previously (4), but with the fixation step removed. At least 300 embryos were examined in each experiment. Wings were mounted in Aquamount (BDH).
Mobilization and plasmid rescue study of the P-element insertion l(3)S137212.
The P-element in the l(3)S137212 lethal insertion line was mobilized by mating to flies carrying the Sb P[Δ2-3](99B) element and excision lines established previously (59). Plasmid rescue of sequences flanking the l(3)S137212 insertion was performed as described elsewhere (55).
RESULTS
There are two members of the ACK family of tyrosine kinases in Drosophila.
A PCR fragment encoding a predicted amino acid sequence with strong homology to ACK-1 and ACK-2 (44, 67) was identified in a PCR screen for Polo-like kinases. This fragment was used to screen a plasmid embryonic 4- to 8-h cDNA library (9), and a single cDNA clone of 3.9 kb was isolated. This cDNA contained an open reading frame encoding a predicted 1,073-amino-acid protein with a molecular mass of 118 kDa. Subsequently, the BDGP reported the sequence of a 4.4-kb cDNA from the same gene (60). The BDGP sequence (GenBank accession no. AF181642) is identical to ours, except that their cDNA is 0.5 kb longer at the 5′ end. A BLASTP search of the GenBank database revealed that the predicted protein encoded by these cDNAs was most similar to murine ACK (GenBank accession no. NP_058068), with the two proteins showing 68% identity in their tyrosine kinase domains (Fig. 1). The next closest matches were with human ACK-1 (44) and bovine ACK-2 (67), with their tyrosine kinase domains showing 67% identity to DACK. Due to these strong homologies to the ACKs, we named our protein DACK. The same name has been independently chosen by another group, who used dsRNA interference in Drosophila cell lines to demonstrate that DACK is a component of signaling by the adaptor protein Dock (14). Another ACK-like tyrosine kinase has been described in Drosophila (2, 36). DPR2 encodes predicted proteins of 1,274 (GenBank accession no. AAF58423) and 1,356 (GenBank accession no. AAG22275) amino acids which differ in their N termini but have identical tyrosine kinase domains. The DPR2 tyrosine kinase domain has 44% identity with that of DACK, and it is significantly more divergent from the mammalian ACKs than DACK, showing only 44% identity with ACK-1 in the tyrosine kinase domain. The tyrosine kinase domain of DPR2 is most similar to that of ARK-1, a Caenorhabditis elegans member of the ACK family (33). Figure 1A shows an alignment of the amino acid sequences of the DACK and DPR2 tyrosine kinase domains with other members of the ACK family. Members of the ACK family share conserved motifs in addition to their tyrosine kinase domains (Fig. 1B). All have a conserved stretch of sequence N terminal to the tyrosine kinase domain and all have an SH3 domain (49) on the C-terminal side. With the exception of DACK and the human protein TNK1 (32), all members of the family shown in Fig. 1B have a CRIB (Cdc42/Rac interactive binding) domain next to the SH3 domain. The CRIB domain has been found in a wide range of proteins and mediates binding to the Rho family members Cdc42 and Rac (10). With regard to the ACK family, the CRIB domains of ACK-1 and DPR2 have been shown to bind Cdc42 (10, 44, 47). Finally, all members of the family in Fig. 1 have proline-rich C termini containing copies of the minimal SH3-binding motif PXXP (3).
FIG. 1.
ACK family tyrosine kinase domains. (A) Alignment of the tyrosine kinase domain of DACK (accession no. AF181642) with the tyrosine kinase domains of murine ACK (accession no. NP_058068), human ACK-1 (accession no. NP_005772), bovine ACK-2 (accession no. AAC05310), C. elegans B0302.1 (accession no. T15316), human TNK1 (accession no. NP_003976), Drosophila DPR2 (accession no. AAF58423), and C. elegans ARK-1 (accession no. CAB65957). Sequences are arranged in order of degree of identity with DACK. (B) Schematic diagram of domains found in ACK family members. Listed on the left are the percent identities between the tyrosine kinase domains of each family member and DACK.
DACK transcripts and protein are enriched at the leading edge of the epidermis during dorsal closure.
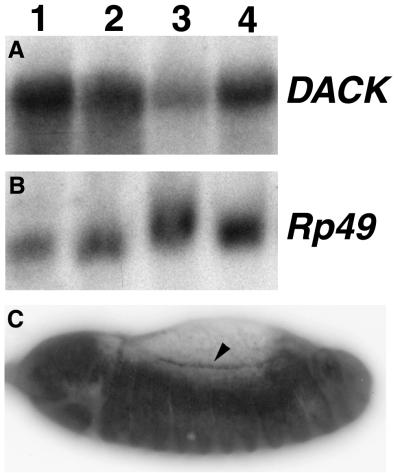
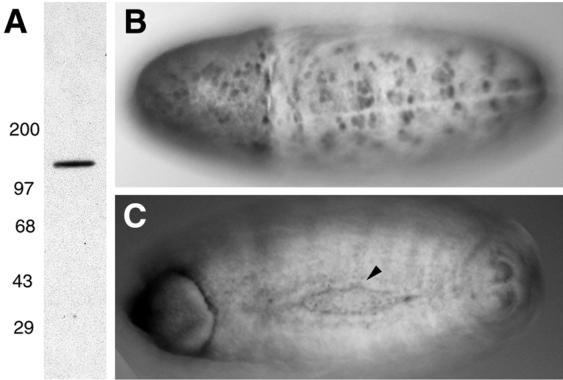
Northern analysis of total RNA from adult heads and bodies revealed a single DACK transcript of about 4.75 kb that was present at higher levels in the head (Fig. 2A). We looked at DACK transcript distribution during embryogenesis by using whole-mount in situ hybridization with a DACK RNA probe. DACK transcripts were widely distributed during embryogenesis and, early in dorsal closure, DACK transcript levels were elevated at the leading edge of the advancing epidermis (Fig. 2C). We raised polyclonal antibodies against a GST fusion protein containing amino acids 873 to 979 from the C-terminal, nonconserved end of DACK. Western analysis using our affinity-purified antiserum detected a single band of an estimated 130 kDa in head extracts (Fig. 3A). During the course of this work, an antiserum raised against the full-length DACK protein was reported (14). We performed whole-mount immunostainings on embryos with either our affinity-purified anti-DACK antiserum or that raised by the other group. For both antisera, we saw strong staining in early embryos in cells corresponding to the mitotic domains of synchronized cell division (Fig. 3B) (23). During dorsal closure, there was elevated DACK staining along the leading edge that was similar to what was seen with DACK RNA in situ hybridizations, but the protein persisted at the leading edge until a later stage of dorsal closure than the transcript (Fig. 3C).
FIG. 2.
Analysis of DACK transcripts by Northern blotting and RNA in situ hybridization. (A) About 30 μg of total RNA from Canton S adult head (lane 1), Canton S adult body (lane 2), Df(3L)C175/TM3Sb whole adults heterozygous for a deficiency predicted to remove the DACK locus (lane 3), or Canton S whole adults (lane 4) was hybridized with a DACK cDNA probe and showed a single transcript of about 4.75 kb. (B) The same blot shown in panel A was probed with an Rp49 cDNA as a loading control (53), demonstrating that DACK transcript levels are higher in the head than the body and are reduced in Df(3L)C175/TM3Sb flies relative to Canton S flies. (C) Lateral view of stage 13 wild-type embryo at the beginning of dorsal closure, showing enrichment of DACK transcripts at the leading edge of the epidermis (arrowhead). The head is to the left in this and all subsequent embryo figures.
FIG. 3.
Anti-DACK antibody stainings. (A) Western blot of adult head lysate incubated with affinity-purified anti-DACK antiserum, showing a single band of about 130 kDa. (B and C) Whole-mount stainings of embryos with anti-DACK antiserum. (B) Dorsal view of stage 9 embryo showing strong staining in mitotic domains. (C) Dorsal view of stage 15 embryo showing enrichment of DACK at the leading edge of the epidermis late in dorsal closure (arrowhead).
DACK transcript levels in tissues participating in dorsal closure are affected by the level of Dcdc42 function.
The lack of a CRIB domain in DACK is surprising, given that the Drosophila tyrosine kinase domain most similar to those of the mammalian ACKs is found in DACK. Despite repeated attempts, we have not been able to detect binding of DACK to either GDP-bound or GTP-bound Dcdc42 (data not shown). Our existing data, therefore, indicate that DACK is not a Dcdc42-binding protein. Unlike the Rac/Cdc42-binding serine/threonine kinase PAK, which is activated by binding of GTP-bound Rac or Cdc42 (45), ACK function has not been shown to be directly affected by Cdc42 binding (44, 67).
It is feasible that Cdc42 signaling could regulate DACK and other ACK family members by other means. One possibility, given the known involvement of the Rho family in signaling to the nucleus, would be by regulation of transcription of ACK-encoding genes. Elevated expression at the leading edge, similar to what we see for DACK, has been described for a number of genes participating in dorsal closure, including decapentaplegic (dpp) and puckered (puc) (52). The leading edge expression of dpp and puc is dependent on a Jun amino-terminal kinase (JNK) cascade that appears to be triggered by the Rho family small GTPase Drac1 and possibly Dcdc42. Further support for the idea that Rho family small GTPases could regulate their effector kinases transcriptionally comes from our work on the Drac1/Dcdc42-binding kinase DPAK, a member of the PAK/STE20 family (28). We noticed that excessive Drac1 signaling or impairment of Dcdc42 signaling in the embryo leads to a dramatic increase in DPAK transcript levels in the amnioserosa. In wild-type embryos, DPAK transcript levels in the amnioserosa are no higher than in the surrounding epidermis, but following expression of either UAS-Drac1V12, a constitutively active Drac1 transgene (43), or UAS-Dcdc42N17, a dominant-negative Dcdc42 transgene (43), by heat shock using the ubiquitously expressed heat shock-inducible Hs-GAL4M-4driver, DPAK transcript levels increased in the amnioserosa (Fig. 4A and data not shown).
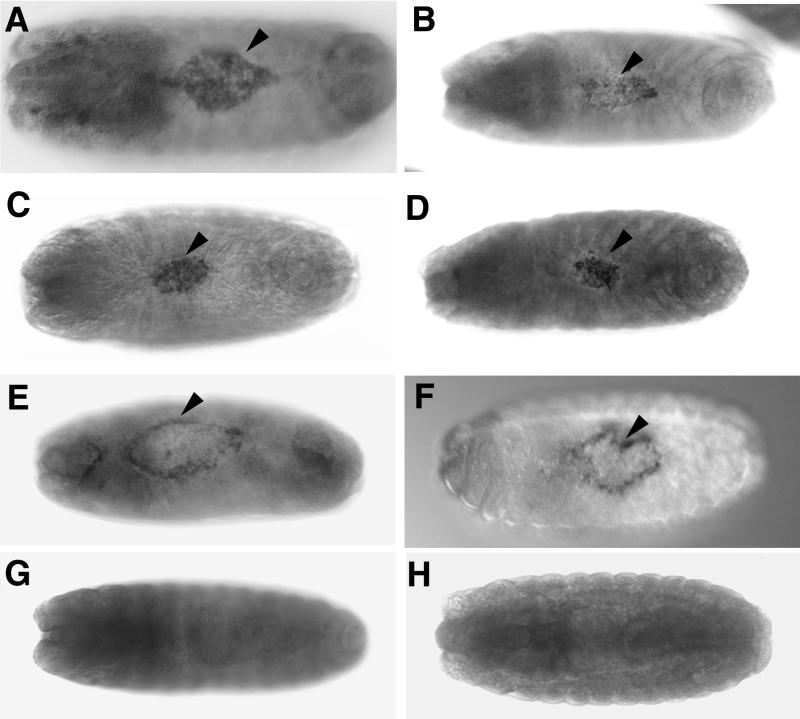
FIG. 4.
RNA in situ hybridizations of DPAK (A) or DACK (B to H) riboprobes to embryos expressing Dcdc42 transgenes reveal that alteration of Dcdc42 signaling affects DPAK and DACK expression in tissues involved in dorsal closure. Embryos were staged based on the degree of head involution. (A) Dorsal view of early stage 15 UAS-Dcdc42N17; Hs-GAL4M-4 embryo that had been heat shocked for 1 h at 37°C, showing DPAK transcript accumulation in the amnioserosa (arrowhead). (B to D) Dorsal views of stage 15 UAS-Dcdc42V12/Hs-GAL4M-4 embryos that had been heat shocked for 1 h at 37°C, showing DACK transcript accumulation in the amnioserosa (arrowheads). (E and F) Dorsal views of stage 15 (E) and stage 16 (F) UAS-Dcdc42N17; Hs-GAL4M-4 embryos that had been heat shocked for 1 h at 37°C, showing DACK transcript accumulation at the leading edge at later stages than seen in wild-type embryos (arrowheads). The distorted dorsal hole in panel F is typical of the dorsal closure failures seen following Dcdc42N17 expression. (G) Dorsal view of stage 15 UAS-Dcdc42V12/Hs-GAL4M-4 embryo that had been maintained at 21°C, showing no areas of elevated DACK transcription on the dorsal surface. (H) Dorsal view of stage 15 UAS-Dcdc42N17; Hs-GAL4M-4 embryo that had been maintained at 21°C, showing no areas of elevated DACK transcription on the dorsal surface.
We determined if alterations in Dcdc42 signaling had any effect on DACK expression in embryos by expressing UAS-Dcdc42V12 and UAS-Dcdc42N17 transgenes by heat shock using Hs-GAL4M-4 and performing RNA in situ hybridizations with a DACK cDNA probe. As controls, we hybridized wild-type embryos, transgenic embryos not exposed to heat shock, and heat-shocked Hs-GAL4M-4 embryos. A strong staining for DACK transcripts was seen in the amnioserosa late in dorsal closure in UAS-Dcdc42V12/Hs-GAL4M-4 embryos that had been exposed to a 1-h heat shock (Fig. 4B to D). Wild-type embryos, heat-shocked Hs-GAL4M-4 embryos, and UAS-Dcdc42V12/Hs-GAL4M-4 embryos not exposed to heat shock showed no enrichment for DACK transcripts in the amnioserosa (Fig. 4G and data not shown). Accumulation of DACK transcripts at the leading edge of the epidermis persisting until late in dorsal closure was seen following Dcdc42N17 expression with a 1-h heat shock (Fig. 4E and F). Wild-type embryos, heat-shocked Hs-GAL4M-4 embryos, and UAS-Dcdc42N17; Hs-GAL4M-4 embryos not exposed to heat shock only showed DACK transcript accumulation at the leading edge at the beginning of dorsal closure and not later (Fig. 2C and 4H and data not shown).
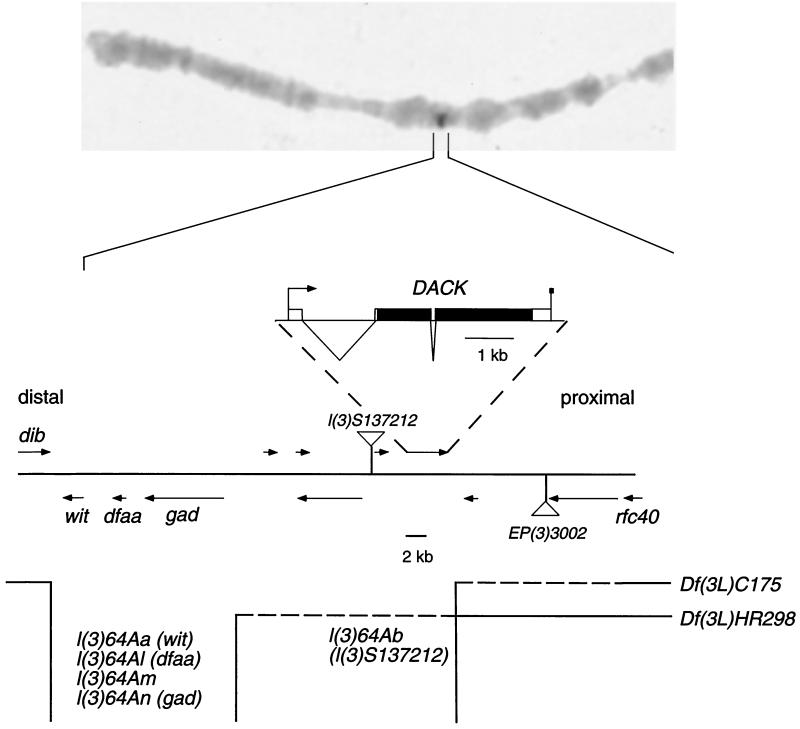
Analysis of the DACK genomic region.
The cytological position of the DACK locus has been estimated by the BDGP to be 64A8-64A9. A subsequent chromosome in situ hybridization (Fig. 5) and Northern blot analysis (Fig. 2A) revealed that DACK is removed by the deficiency Df(3L)C175, mapping it to 64A3-64A5. The 64A region has been well characterized genetically and it is likely that most of the essential genes have been found (30, 41). Five lethal complementation groups have been identified which are removed by Df(3L)C175, with all being represented by at least six alleles, except for l(3)64Am, which has one allele (Fig. 5). Three of these complementation groups, l(3)64Aa, l(3)64Al, and l(3)64An, have been assigned to the known genes wit, dfaa, and gad, respectively (13, 22). We found that a lethal P-element insertion, l(3)S137212, which had been localized to this region (16), failed to complement an allele of l(3)64Ab and therefore belongs to this complementation group. We performed plasmid rescue on l(3)S137212 and obtained sequences flanking the insertion. When these sequences were aligned against the BDGP genomic sequence, we found that the P-element in l(3)S137212 was inserted 72 bp upstream of the predicted initiator methionine codon of the predicted gene CG14991 (2), which is the closest neighbor to DACK on the distal side (Fig. 5). l(3)S137212 is more likely to be affecting the function of CG14991 than DACK, and we have been unable to rescue the lethality caused by this insertion through overexpression of a DACK transgene. We have confirmed that the lethality in the l(3)S137212 line is caused by the P-element insertion by mobilizing the element and recovering viable excisions. Northern analysis of alleles from the five lethal complementation groups uncovered by Df(3L)C175 revealed no effects of any of these on the size or quantity of the DACK transcript (data not shown). Taken together, our existing data on these complementation groups do not support any of them corresponding to DACK, and it may be that DACK does not mutate to zygotic lethality.
FIG. 5.
Position of the DACK gene. (Top) Chromosomal in situ hybridization with DACK genomic probe done on a Df(3L)C175/+ larva, showing signal at 64AB on only one chromosome. (Bottom) Diagram of DACK genomic region. Characterized transcription units and those predicted by the BDGP are shown as arrows. The positions of two P-element insertions are indicated by triangles. The position of the l(3)S137212 insertion was determined in this study, and the lethality associated with this insertion was assigned to the l(3)64Ab complementation group. An expanded view of the DACK transcription unit is shown at the top of the diagram. Exons are shown as boxes, introns are shown as triangles, and the open reading frame is in black. The bottom of the figure shows the breakpoints of two deficiencies, Df(3L)C175 and Df(3L)H298, with the sequences removed by the deficiencies indicated by open spaces. Dashed horizontal lines indicate uncertainty in the breakpoints. The five lethal complementation groups uncovered by Df(3L)C175 are listed, together with the genes to which they have been assigned.
ACK family tyrosine kinase activity is involved in a set of developmental events similar to that of Dcdc42.
We attempted to address DACK function using RNAi, an approach that has been successfully used to study DACK in Drosophila cell lines (14). We injected embryos with a 0.8-kb dsRNA transcribed from a DACK cDNA. Other than those embryos that leaked cytoplasm upon injection, embryos injected with DACK dsRNA hatched into morphologically normal larvae and went on to develop into fertile, morphologically normal adults.
We used a transgenic approach to study ACK family kinase function by expressing wild-type and kinase-dead versions of DACK. An invariant lysine involved in ATP binding in the tyrosine kinases can be mutated to create a protein devoid of tyrosine kinase activity (35, 63). Such kinase-dead mutants have been frequently used as dominant-negative proteins to study signaling by nonreceptor tyrosine kinases such as Src, and they have been shown to block tyrosine phosphorylation by their wild-type counterparts (for an example, see reference 69). If DACK and the other ACK family kinase, DPR2, have shared targets during development, the presence of DPR2 may be sufficient to rescue the effects of loss of DACK, and this may explain the lack of phenotypes in our RNAi experiment. Expression of kinase-dead DACK might be expected to block phosphorylation of such shared targets by DPR2 and reveal roles for these ACK family proteins.
To check that our transgenes were expressing DACK protein, we induced them with an engrailed-GAL4 (en-GAL4) driver that drives transgene expression in the en expression pattern (A. Brand, personal communication to FlyBase [http://flybase.bio.indiana.edu/]). Staining of the resulting embryos with our anti-DACK antiserum revealed elevated DACK protein in en stripes (17) for both wild-type and kinase-dead DACK (KD-DACK) transgenes (Fig. 6A and C). Wild-type embryos do not show this striped DACK staining pattern (Fig. 6E). To see if DACK transgene expression had a visible effect on the distribution of phosphotyrosine in embryos, we stained en-GAL4; UAS-DACK and en-GAL4; UAS-KD-DACK embryos with antiphosphotyrosine antibodies. In en-GAL4; UAS-DACK embryos, there was a dramatic increase in phosphotyrosine staining in en stripes (Fig. 6B). This result indicated that the wild-type DACK protein overexpressed from the transgene had tyrosine kinase activity in the absence of a concomitant increase in Dcdc42 activity. The antiphosphotyrosine staining pattern of en-GAL4; UAS-KD-DACK embryos was similar to that of wild type (Fig. 6D and F). We also expressed the DACK transgenes using GAL4 driven by the patched (ptc) promoter (GAL4559.1 [31]) and stained them with antiphosphotyrosine antibodies. In late embryos, ptc expression is limited to two narrow stripes per segment, and in GAL4559.1; UAS-DACK embryos phosphotyrosine levels are elevated in this pattern (Fig. 6G). We saw a low frequency of mild defects in dorsal closure in GAL4559.1; UAS-DACK embryos, with some embryos showing slight “bunching” of the dorsal hole and delayed closure relative to wild-type embryos of similar age (compare Fig. 6G and H). GAL4559.1; UAS-KD-DACK embryos were highly disorganized and specific phenotypes could not be interpreted (data not shown).
FIG. 6.
Expression of DACK transgenes with the en-GAL4 and GAL4559.1 drivers to demonstrate effects on phosphotyrosine levels in embryos. (A and C) Lateral views of embryos in which either a UAS-DACK transgene (A) or KD-DACK transgene (C) had been expressed with en-GAL4, stained with anti-DACK antiserum to show overexpression of DACK proteins in en stripes. (B) Lateral view of en-GAL4; UAS-DACK embryo stained with antiphosphotyrosine antibodies to show elevated phosphotyrosine levels in en stripes. (D) Lateral view of en-GAL4; UAS-KD-DACK embryo stained with antiphosphotyrosine antibodies to show a staining pattern similar to that of the wild type (compare to panel F). (E) Wild-type embryo stained with anti-DACK antiserum. (F) Wild-type embryo stained with antiphosphotyrosine. (G) Confocal fluorescent micrograph of dorsal view of GAL4559.1; UAS-DACK embryo stained with antiphosphotyrosine antibodies to show elevated phosphotyrosine levels in ptc stripes. Although head involution is advanced, the dorsal surface is still open and segments are slightly bunched around the dorsal hole (arrowhead). (H) Confocal fluorescent micrograph of dorsal view of wild-type embryo at similar stage of development as that in panel G, stained with antiphosphotyrosine antibodies.
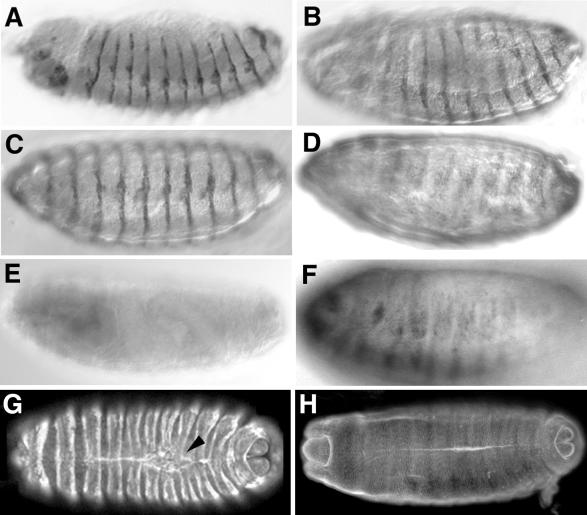
Heat shock inductions of either constitutively active or dominant-negative Dcdc42 transgenes cause a range of defects in embryonic epithelial morphogenesis (26; N. Harden, unpublished observations). We expressed our DACK transgenes in a similar fashion and evaluated embryonic morphology using cuticle preparations. Expression of UAS-DACK in 6- to 12-h-old embryos with a 1-h heat shock using Hs-GAL4M-4 resulted in about half of the embryos failing to survive to the first instar larval stage. Of these dead embryos, 45% showed germband retraction failures (Fig. 7C) and 20% had holes in the dorsal cuticle, indicating failures of dorsal closure. A further 25% of the dead embryos did not secrete any cuticle. Control inductions of a UAS-lacZ transgene with Hs-GAL4M-4 did not result in any of these phenotypic effects. A collection of phenotypic effects similar to those caused by UAS-DACK was seen following expression of UAS-KD-DACK with the Hs-GAL4M-4 driver. Forty percent of Hs-GAL4M-4; UAS-KD-DACK embryos died before hatching into larvae. Of the embryos that failed to hatch, 34% had germband retraction failures, 15% had dorsal holes (Fig. 7E), and 35% failed to secrete cuticle. Most of the Hs-GAL4M-4; UAS-KD-DACK embryos with dorsal holes also had head defects (data not shown). Head defects, defective dorsal closure, and germband retraction failures have all been seen following expression of either dominant-negative or constitutively active Dcdc42 transgenes and in embryos with reduced maternal Dcdc42 function (23, 26, 53; N. Harden, unpublished observations).
FIG. 7.
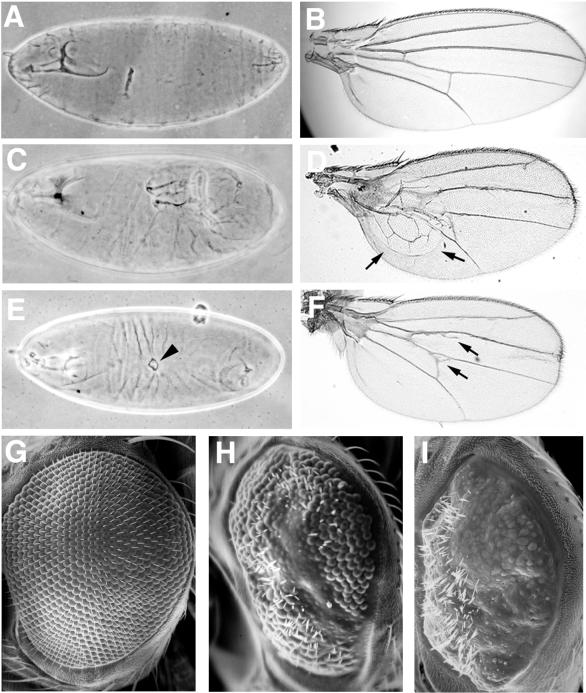
Developmental defects resulting from expression of DACK transgenes with GAL4 drivers. (A) Lateral view of wild-type embryonic cuticle; (B) wild-type wing; (C) lateral view of embryonic cuticle showing failure of germband retraction following expression of UAS-DACK by heat shock using the Hs-GAL4M-4 driver. (D and F) Wings of flies in which UAS-KD-DACK had been expressed with the 71B-GAL4 driver. Arrows in panel D show a large blister; arrows in panel F indicate ectopic vein tissue. (E) Dorsal view of embryonic cuticle showing dorsal hole (arrowhead) following expression of UAS-KD-DACK by heat shock using the Hs-GAL4M-4 driver. (G to I) Scanning electron micrographs of eyes of wild-type (G), GMR-GAL4; UAS-DACK (H), and GMR-GAL4; UAS-KD-DACK (I) flies.
Given that loss of Dcdc42 signaling can affect a range of developmental events in the wing (5, 19, 20, 26), we looked for wing phenotypes following expression of KD-DACK in the developing wing. KD-DACK was expressed in the central portion of the wing pouch of the wing imaginal disk by using the 71B-GAL4 driver (12). 71B-GAL4; UAS-KD-DACK flies had rounded, spread-out wings with frequent blisters and ectopic veins (Fig. 7D and F). Both wing blisters and ectopic veins have been seen in flies bearing partial loss-of-function Dcdc42 mutations and following expression of dominant-negative Dcdc42 in the developing wing (5, 19, 26). Expression of the UAS-DACK transgene with the 71B-GAL4 driver resulted in lethality at the pharate adult stage. We have not attempted an examination of the wings of these flies.
Perturbations of Cdc42 signaling also affect development of the adult eye. Overexpression of Dcdc42 in the developing eye under control of the synthetic GMR promoter results in a rough eye phenotype characterized by missing photoreceptors and disruption of ommatidial morphology (51). Flies homozygous for partial loss-of-function Dcdc42 mutations or carrying combinations of weak and strong Dcdc42 alleles exhibit ommatidial fusions and loss or duplication of bristles (26). We expressed our DACK transgenes in the developing eye using a GMR-GAL4 driver (24). Eyes in which wild-type DACK had been overexpressed were rough and smaller than wild type. Scanning electron micrographs revealed that ommatidia were disorganized and consistently missing in a band running dorsoventrally across the middle of the eye (Fig. 7H). There was also serious disruption of the pattern of bristles, with bristles missing in some areas and excessive in number in others. Expression of KD-DACK also resulted in small, rough eyes, but scanning electron micrographs revealed an eye phenotype more severe than that generated by overexpression of wild-type DACK. Eyes were disorganized and almost completely devoid of ommatidia (Fig. 7I). Bristles were missing in the posterior two-thirds of each eye, while there were clusters of bristles at the anterior end.
Overexpression of DACK can rescue the dorsal closure defects caused by expression of dominant-negative Dcdc42.
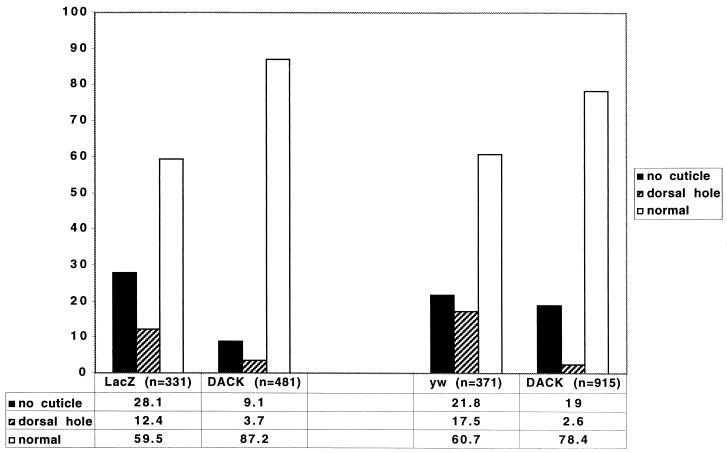
The dorsal closure failures caused by DACK transgene expression are an indication of a role for ACK family kinases in dorsal closure. We wondered if ACK family tyrosine kinase activity was operating downstream of Dcdc42 during dorsal closure and we checked to see if overexpression of DACK could rescue the dorsal closure defects caused by UAS-Dcdc42N17. Our results with the en-GAL4-driven expression of DACK suggested that DACK has some degree of tyrosine kinase activity even in the absence of Dcdc42 signaling. Thus, even if DACK is dependent on Dcdc42 for its full activity, overexpression of the protein may be able to bypass a requirement for Dcdc42. Heat shock transgene inductions in this experiment were kept to 30 min to minimize the phenotypic effects of DACK overexpression. Flies heterozygous for a chromosome bearing both UAS-Dcdc42N17 and the heat shock-inducible GAL4 driver Hs-GAL42207were mated to either a control line or flies homozygous for a UAS-DACK transgene. The progeny were heat shocked as embryos 6 to 12 h after egg laying and examined with cuticle preparations. For each experiment in which the UAS-DACK transgene was coexpressed with UAS-Dcdc42N17, a control cross was performed in parallel, with the two crosses and heat shocks being handled simultaneously and identically. We consistently found that coexpression of UAS-Dcdc42N17 with UAS-DACK produced significantly lower dorsal hole frequencies than when UAS-Dcdc42N17 was expressed without UAS-DACK. The results of two such experiments are given in Fig. 8. Half the progeny in any cross will have transgene expression, and thus the actual frequencies of phenotypic effects in transgene-expressing embryos are estimated to be twice the values shown. In the first experiment shown in Fig. 8, the control cross was designed to control for a possible general effect of UAS transgene coexpression on the UAS-Dcdc42N17-induced phenotypes. For this, UAS-Dcdc42N17 was coexpressed with a UAS-LacZ transgene (which has no phenotypic effects when expressed alone). A total of 12.4% of the progeny had dorsal holes. When the UAS-Ddc42N17 transgene was coexpressed with UAS-DACK, the frequency of dorsal holes was 3.7%. In the second experiment shown in Fig. 8, the control cross was designed to control for possible genetic background effects of crossing to the UAS-DACK line. For this, the Hs-GAL42207, UAS-Dcdc42N17 line was crossed to the yw strain that had been used to make the UAS-DACK line. Following induction of UAS-Dcdc42N17, 17.5% of the progeny from this cross had dorsal holes. In the parallel cross in which UAS-DACK was coexpressed with UAS-Dcdc42N17, the frequency of dorsal holes was 2.6%.
FIG. 8.
Rescue of Dcdc42N17-induced dorsal closure defects by overexpression of DACK. The graph and table show frequencies of cuticle phenotypes (in percent) for two independent experiments, each of which consisted of one control expression of UAS-Dcdc42N17 and one coexpression of UAS-Dcdc42N17 with UAS-DACK. no cuticle, embryo failed to secrete cuticle; dorsal hole, failure of cuticle secretion in a portion of the dorsal surface; normal, dorsal surface indistinguishable from wild type; LacZ, progeny of the cross Hs-GAL42207, UAS-Dcdc42N17/CyO × UAS-LacZ/UAS-LacZ; DACK, progeny of the cross Hs-GAL42207, UAS-Dcdc42N17/CyO × UAS-DACK/UAS-DACK; yw, progeny of the cross Hs-GAL42207, UAS-Dcdc42N17/CyO × yw.
DACK does not participate in activation of the JNK cascade, and the JNK cascade is not required for the leading edge expression of DACK.
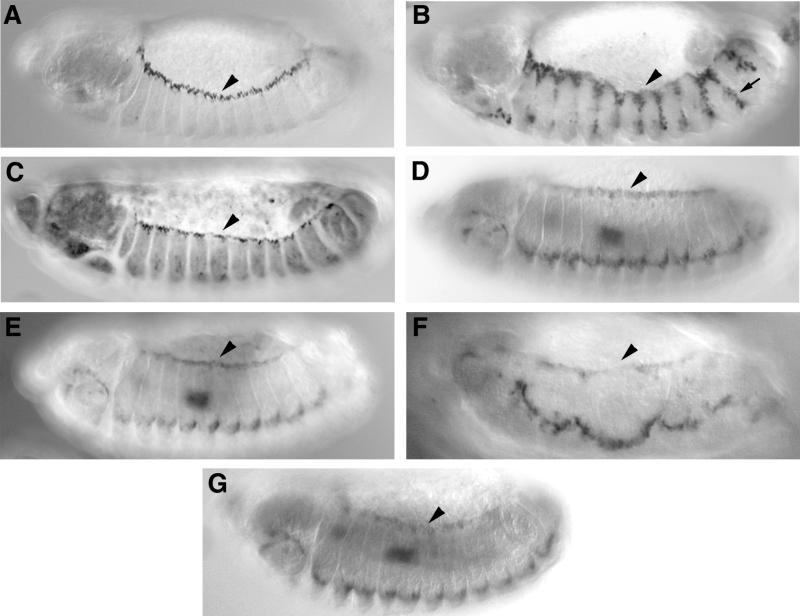
The rescue of Dcdc42N17-induced dorsal closure defects by overexpression of DACK indicates that ACK family tyrosine kinase activity is a major component of Dcdc42 signaling during dorsal closure. A potential route of Dcdc42 signaling during dorsal closure is activation of the JNK cascade at the leading edge, leading to transcription of dpp and puc (52). Expression of either a UAS-Dcdc42V12 transgene or a UAS-Drac1V12 transgene with the en-GAL4 driver causes ectopic activation of the JNK cascade in en stripes (27). However, this may not be physiologically relevant, as Dcdc42 loss-of-function mutants do not show disruption of the JNK cascade, and there is evidence for Dcdc42 acting downstream of JNK in the Dpp pathway (26, 56). We tested to see if overexpression of DACK could cause ectopic activation of the JNK cascade, using the same assay as was used for Dcdc42V12. UAS-DACK was expressed with en-GAL4 in the presence of the puc-lacZ enhancer-trap insertion pucE69, which allows transcriptional control of puc gene expression to be visualized by staining for β-galactosidase (58). This approach has also been used to assess the effects on the JNK cascade of overexpressing the kinase dTAK, a Drosophila member of the mitogen-activated protein kinase kinase kinase family. Overexpression of dTAK causes ectopic activation of puc transcription similar to that seen with Dcdc42V12 (46, 64). As a positive control for our experiment, we repeated the en-GAL4 induction of Dcdc42V12 and observed ectopic puc transcription in en stripes (Fig. 9B). Overexpression of DACK, however, did not lead to ectopic puc expression (Fig. 9C). We have also tested the effects of DACK overexpression on the JNK-dependent transcription of dpp at the leading edge. Embryos in which DACK had been overexpressed in the epidermis using the GAL4559.1 driver (31) were hybridized with a dpp riboprobe. There was no evidence of ectopic dpp expression, and the distribution of dpp transcripts in these embryos was indistinguishable from that of the wild type (Fig. 9E). We looked at the effects of impairment of DACK function on the JNK cascade by examining the leading edge transcription of dpp in embryos homozygous for Df(3L)C175, which are devoid of zygotic DACK, and in embryos expressing KD-DACK. In both cases, dpp transcription was maintained at the leading edge (Fig. 9F and G).
FIG. 9.
JNK cascade-dependent transcription of genes at the leading edge is not affected by gains or losses of DACK function. Lateral views of embryos stained with anti-β-galactosidase antibody (A to C) or hybridized with a dpp riboprobe (D to G). Arrowheads in each panel denote the leading edge. (A) pucE69/+ embryo showing puc expression at the leading edge; (B) en-GAL4/+; pucE69, UAS-Dcdc42V12/+ embryo showing ectopic puc expression in en stripes (arrow); (C) en-GAL4/+; pucE69, UAS-DACK/+ embryo showing puc expression at the leading edge; (D) wild-type embryo showing dpp expression at the leading edge, in the lateral epidermis and in the midgut. (E) GAL4559.1/+; UAS-DACK/+ embryo showing wild-type distribution of dpp transcripts; (F) Df(3L)C175/Df(3L)C175 embryo showing dpp transcripts at the leading edge and in the lateral epidermis; (G) wild-type distribution of dpp transcripts following expression of UAS-KD-DACK by heat shock using the Hs-GAL4M-4 driver.
Given that DACK is itself enriched at the leading edge during dorsal closure, it is possible that DACK transcription could be under control of the JNK cascade. We looked at DACK transcript distribution in basket mutant embryos bearing a loss-of-function mutation in the gene encoding Drosophila JNK (57, 62) and found that the leading-edge expression of DACK was intact (data not shown). We also ectopically activated the JNK cascade in en stripes by expressing UAS-Drac1V12 with en-GAL4 and looked at DACK transcripts in embryos. There was no increase in DACK transcript levels in en stripes (data not shown).
DISCUSSION
We have characterized a nonreceptor tyrosine kinase, DACK, which is the closest Drosophila homolog to the mammalian ACKs. DACK is one of two known ACK family members that lacks the GTPase-binding CRIB domain, and we have not been able to demonstrate in vitro binding to GTP-bound Cdc42. This is in contrast to the mammalian ACK family members ACK-1 and ACK-2, which bind GTP-bound Cdc42 in vitro through the CRIB domain (44, 67). The relevance of Cdc42 binding to the function of ACK-1 and ACK-2 remains uncertain. The association of ACK-1 with GTP-bound Cdc42 inhibits both the intrinsic and GAPase-activating protein-stimulated GTPase activity of Cdc42 and may therefore be used to sustain Cdc42 in the active state (44). In in vitro kinase assays, ACK-2 is not activated by binding to Cdc42, but ACK-2 kinase activity is increased when ACK-2 is cotransfected into COS-7 cells with wild-type or constitutively active Cdc42 (67). Various results indicate roles for ACK-1 and ACK-2 in Cdc42 signaling. Melanoma chondroitin sulfate proteoglycan-induced spreading of melanoma cells is dependent on Cdc42 and ACK-1, and activation of ACK-2 by cell adhesion is Cdc42 dependent (21, 68). In response to epidermal growth factor, ACK-1 can tyrosine phosphorylate and activate Dbl, a guanine nucleotide exchange factor for the Rho family, in a Cdc42-dependent manner (37, 38).
An attempt to inhibit DACK function by using RNAi yielded no obvious phenotypic effects, suggesting that loss of DACK is nonlethal. A likely possibility is that DACK shares target proteins with the other ACK family tyrosine kinase in Drosophila, DPR2. Expression of DACK transgenes during development did produce phenotypic effects, presumably by affecting DACK and/or DPR2 signaling pathways. Expression of KD-DACK during embryogenesis, wing development, and eye development resulted in a range of phenotypic effects similar to those caused by loss-of-function mutations in Dcdc42 or by expression of Dcdc42N17. More importantly, overexpression of wild-type DACK can suppress dorsal closure defects caused by Dcdc42N17 expression. The extensive rescue of Dcdc42N17-induced dorsal closure failures by DACK overexpression indicates that ACK family tyrosine kinase activity is a major route for Cdc42 signaling during dorsal closure. We have demonstrated that overexpression of DACK does not trigger ectopic activation of the JNK cascade, in contrast to the previous finding that constitutive activation of Cdc42 signaling using Dcdc42V12 induces this pathway (27). Furthermore, we show that the JNK cascade is not disrupted by either impairment of ACK family tyrosine kinase function through expression of KD-DACK or by loss of zygotic DACK through a deficiency removing the DACK gene. Our results suggest that the JNK cascade does not lie downstream of ACK family tyrosine kinase activity in Dcdc42 signaling. We have also shown that the JNK cascade does not drive expression of DACK. Our work is consistent with analysis of loss-of-function alleles of Dcdc42, which indicates that the JNK cascade is not a major component of Dcdc42 signaling (26). Dcdc42 may normally make a minor contribution to the activation of the JNK cascade that could be greatly amplified by expression of Dcdc42V12.
We cannot exclude the possibility that the ACK family tyrosine kinase activity acting downstream of Dcdc42 during dorsal closure is provided entirely by DPR2. However, the leading-edge enrichment of DACK and the alterations in DACK transcription in the leading edge and amnioserosa in response to Dcdc42 transgene expression are indications that DACK has a role in Dcdc42 signaling during dorsal closure. The transcriptional regulation of DACK does not appear to be a simple homeostatic response, as it is tissue specific and works in opposite directions in two tissues, i.e., dominant-negative Cdc42 causes upregulation of DACK transcripts at the leading edge, whereas constitutively active Dcdc42 causes upregulation of transcription in the amnioserosa. The relevance of this transcriptional regulation of DACK remains unknown, but it may provide a route for Dcdc42 to regulate DACK function during dorsal closure. The serine/threonine kinase DPAK, a likely downstream effector for Drac1 and Dcdc42, also responds transcriptionally to a change in Dcdc42 signaling in the amnioserosa but, interestingly, in the opposite direction from DACK, in that it is dominant-negative Dcdc42 that induces upregulation of DPAK transcription in this tissue.
Dcdc42 might also regulate DACK through its GTPase activity. Although Dcdc42 does not appear to bind DACK directly, it could possibly influence DACK function indirectly in a signaling complex. An indirect mode of activation of ACK proteins by Cdc42 proteins is consistent with the finding that constitutively active Cdc42 fails to activate ACK-2 in vitro but can promote activation when cotransfected with ACK-2 in vivo (67).
The high level of DACK protein seen in mitotic domains is of interest, as Cdc42 is involved in yeast budding and cytokinesis in Xenopus laevis embryos (1, 18). To date, no defects in Drosophila cytokinesis have been seen with impaired Dcdc42 function, although constitutively active Dcdc42 disrupts cellularization of the embryo, a specialized form of cytokinesis (15, 26).
The wing blisters induced by expression of KD-DACK are reminiscent of those found in wings bearing clones homozygous for loss-of-function mutations in the genes encoding the Drosophila integrins αPS1, αPS2, and βPS (8). There is evidence that the mammalian ACKs function in integrin signaling (21, 68), and the Drosophila wing may provide a useful model to genetically dissect this role for the ACK family.
Despite being among the first-described potential effectors for Cdc42, the ACKs remain poorly characterized in terms of the signaling they participate in. The strong eye phenotypes generated by DACK transgene expression should provide a particularly good system for investigating signaling pathways involving the Drosophila ACK family proteins. The rough eye phenotypes generated by Rho family transgene expression in Drosophila have been used to identify second site mutations in genes encoding components of Rho family signal transduction (6, 51), and we have recently identified deficiencies suppressing the rough eye phenotype induced by overexpression of wild-type ACK.
This genetic analysis of ACK function in Drosophila using eye development, combined with genetic approaches using other well-defined processes such as wing development and dorsal closure, should aid greatly in elucidating ACK-mediated pathways.
Acknowledgments
We thank F. R. Jackson, L. Luo, M. Krasnow, A. Martinez-Arias, M. Nakamura, J. Roote, and the Bloomington and Szeged Stock Centers for fly stocks; J. Clemens and N. Simonson-Leff for anti-DACK antibodies; Ong Chin Tong for help with fly injections; J. Sanny for technical assistance; Sheila MacLean for help with RNA in situ; members of the Harden and Lim laboratories for advice and discussions; and E. Verheyen for critically reading the manuscript.
This work was supported by grants from the British Columbia Health Research Foundation and the Canadian Institutes of Health Research to N.H. and from the Glaxo Singapore Research Fund to L.L.
REFERENCES
- 1.Adams, A. E., D. I. Johnson, R. M. Longnecker, B. F. Sloat, and J. R. Pringle. 1990. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J. Cell Biol. 111:131-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams, M. D., S. E. Celniker, R. A. Holt, C. A. Evans, J. D. Gocayne, P. G. Amanatides, S. E. Scherer, P. W. Li, R. A. Hoskins, R. F. Galle, R. A. George, S. E. Lewis, S. Richards, M. Ashburner, S. N. Henderson, G. G. Sutton, J. R. Wortman, M. D. Yandell, Q. Zhang, L. X. Chen, R. C. Brandon, Y. H. Rogers, R. G. Blazej, M. Champe, B. D. Pfeiffer, K. H. Wan, C. Doyle, E. G. Baxter, G. Helt, C. R. Nelson, G. L. Gabor, J. F. Abril, A. Agbayani, H. J. An, C. Andrews-Pfannkoch, D. Baldwin, R. M. Ballew, A. Basu, J. Baxendale, L. Bayraktaroglu, E. M. Beasley, K. Y. Beeson, P. V. Benos, B. P. Berman, D. Bhandari, S. Bolshakov, D. Borkova, M. R. Botchan, J. Bouck, P. Brokstein, P. Brottier, K. C. Burtis, D. A. Busam, H. Butler, E. Cadieu, A. Center, I. Chandra, J. M. Cherry, S. Cawley, C. Dahlke, L. B. Davenport, P. Davies, B. de Pablos, A. Delcher, Z. Deng, A. D. Mays, I. Dew, S. M. Dietz, K. Dodson, L. E. Doup, M. Downes, S. Dugan-Rocha, B. C. Dunkov, P. Dunn, K. J. Durbin, C. C. Evangelista, C. Ferraz, S. Ferriera, W. Fleischmann, C. Fosler, A. E. Gabrielian, N. S. Garg, W. M. Gelbart, K. Glasser, A. Glodek, F. Gong, J. H. Gorrell, Z. Gu, P. Guan, M. Harris, N. L. Harris, D. Harvey, T. J. Heiman, J. R. Hernandez, J. Houck, D. Hostin, K. A. Houston, T. J. Howland, M. H. Wei, C. Ibegwam, et al. 2000. The genome sequence of Drosophila melanogaster. Science 287:2185-2195. [DOI] [PubMed] [Google Scholar]
- 3.Alexandropoulos, K., G. Cheng, and D. Baltimore. 1995. Proline-rich sequences that bind to Src homology 3 domains with individual specificities. Proc. Natl. Acad. Sci. USA 92:3110-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashburner, M. 1989. Drosophila: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 5.Baron, M., V. O'Leary, D. A. Evans, M. Hicks, and K. Hudson. 2000. Multiple roles of the Dcdc42 GTPase during wing development in Drosophila melanogaster. Mol. Gen. Genet. 264:98-104. [DOI] [PubMed] [Google Scholar]
- 6.Barrett, K., M. Leptin, and J. Settleman. 1997. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell 91:905-915. [DOI] [PubMed] [Google Scholar]
- 7.Brand, A. H., and N. Perrimon. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118:401-415. [DOI] [PubMed] [Google Scholar]
- 8.Brown, N. H., S. L. Gregory, and M. D. Martin-Bermudo. 2000. Integrins as mediators of morphogenesis in Drosophila. Dev. Biol. 223:1-16. [DOI] [PubMed] [Google Scholar]
- 9.Brown, N. H., and F. C. Kafatos. 1988. Functional cDNA libraries from Drosophila embryos. J. Mol. Biol. 203:425-437. [DOI] [PubMed] [Google Scholar]
- 10.Burbelo, P. D., D. Drechsel, and A. Hall. 1995. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J. Biol. Chem. 270:29071-29074. [DOI] [PubMed] [Google Scholar]
- 11.Cai, Y., W. Chia, and X. Yang. 2001. A family of snail-related zinc finger proteins regulates two distinct and parallel mechanisms that mediate Drosophila neuroblast asymmetric divisions. EMBO J. 20:1704-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capdevila, J., and I. Guerrero. 1994. Targeted expression of the signaling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. EMBO J. 13:4459-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chavez, V. M., G. Marques, J. P. Delbecque, K. Kobayashi, M. Hollingsworth, J. Burr, J. E. Natzle, and M. B. O'Connor. 2000. The Drosophila disembodied gene controls late embryonic morphogenesis and codes for a cytochrome P450 enzyme that regulates embryonic ecdysone levels. Development 127:4115-4126. [DOI] [PubMed] [Google Scholar]
- 14.Clemens, J. C., C. A. Worby, N. Simonson-Leff, M. Muda, T. Maehama, B. A. Hemmings, and J. E. Dixon. 2000. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc. Natl. Acad. Sci. USA 97:6499-6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crawford, J. M., N. Harden, T. Leung, L. Lim, and D. P. Kiehart. 1998. Cellularization in Drosophila melanogaster is disrupted by the inhibition of rho activity and the activation of Cdc42 function. Dev. Biol. 204:151-164. [DOI] [PubMed] [Google Scholar]
- 16.Deak, P., M. M. Omar, R. D. Saunders, M. Pal, O. Komonyi, J. Szidonya, P. Maroy, Y. Zhang, M. Ashburner, P. Benos, C. Savakis, I. Siden-Kiamos, C. Louis, V. N. Bolshakov, F. C. Kafatos, E. Madueno, J. Modolell, and D. M. Glover. 1997. P-element insertion alleles of essential genes on the third chromosome of Drosophila melanogaster: correlation of physical and cytogenetic maps in chromosomal region 86E-87F. Genetics 147:1697-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiNardo, S., J. M. Kuner, J. Theis, and P. H. O'Farrell. 1985. Development of embryonic pattern in D. melanogaster as revealed by accumulation of the nuclear engrailed protein. Cell 43:59-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drechsel, D. N., A. A. Hyman, A. Hall, and M. Glotzer. 1997. A requirement for Rho and Cdc42 during cytokinesis in Xenopus embryos. Curr. Biol. 7:12-23. [DOI] [PubMed] [Google Scholar]
- 19.Eaton, S., P. Auvinen, L. Luo, Y. N. Jan, and K. Simons. 1995. CDC42 and Rac1 control different actin-dependent processes in the Drosophila wing disc epithelium. J. Cell Biol. 131:151-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eaton, S., R. Wepf, and K. Simons. 1996. Roles for Rac1 and Cdc42 in planar polarization and hair outgrowth in the wing of Drosophila. J. Cell Biol. 135:1277-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenmann, K. M., J. B. McCarthy, M. A. Simpson, P. J. Keely, J. L. Guan, K. Tachibana, L. Lim, E. Manser, L. T. Furcht, and J. Iida. 1999. Melanoma chondroitin sulphate proteoglycan regulates cell spreading through Cdc42, Ack-1 and p130cas. Nat. Cell Biol. 1:507-513. [DOI] [PubMed] [Google Scholar]
- 22.Featherstone, D. E., E. M. Rushton, M. Hilderbrand-Chae, A. M. Phillips, F. R. Jackson, and K. Broadie. 2000. Presynaptic glutamic acid decarboxylase is required for induction of the postsynaptic receptor field at a glutamatergic synapse. Neuron 27:71-84. [DOI] [PubMed] [Google Scholar]
- 23.Foe, V. E. 1989. Mitotic domains reveal early commitment of cells in Drosophila embryos. Development 107:1-22. [PubMed] [Google Scholar]
- 24.Freeman, M. 1996. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell 87:651-660. [DOI] [PubMed] [Google Scholar]
- 25.Gao, F. B., J. E. Brenman, L. Y. Jan, and Y. N. Jan. 1999. Genes regulating dendritic outgrowth, branching, and routing in Drosophila. Genes Dev. 13:2549-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Genova, J. L., S. Jong, J. T. Camp, and R. G. Fehon. 2000. Functional analysis of Cdc42 in actin filament assembly, epithelial morphogenesis, and cell signaling during Drosophila development. Dev. Biol. 221:181-194. [DOI] [PubMed] [Google Scholar]
- 27.Glise, B., and S. Noselli. 1997. Coupling of Jun amino-terminal kinase and decapentaplegic signaling pathways in Drosophila morphogenesis. Genes Dev. 11:1738-1747. [DOI] [PubMed] [Google Scholar]
- 28.Harden, N., J. Lee, H. Y. Loh, Y. M. Ong, I. Tan, T. Leung, E. Manser, and L. Lim. 1996. A Drosophila homolog of the Rac- and Cdc42-activated serine/threonine kinase PAK is a potential focal adhesion and focal complex protein that colocalizes with dynamic actin structures. Mol. Cell. Biol. 16:1896-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harden, N., M. Ricos, Y. M. Ong, W. Chia, and L. Lim. 1999. Participation of small GTPases in dorsal closure of the Drosophila embryo: distinct roles for Rho subfamily proteins in epithelial morphogenesis. J. Cell Sci. 112:273-284. [DOI] [PubMed] [Google Scholar]
- 30.Harrison, S. D., N. Solomon, and G. M. Rubin. 1995. A genetic analysis of the 63E-64A genomic region of Drosophila melanogaster: identification of mutations in a replication factor C subunit. Genetics 139:1701-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinz, U., B. Giebel, and J. A. Campos-Ortega. 1994. The basic-helix-loop-helix domain of Drosophila lethal of scute protein is sufficient for proneural function and activates neurogenic genes. Cell 76:77-87. [DOI] [PubMed] [Google Scholar]
- 32.Hoehn, G. T., T. Stokland, S. Amin, M. Ramirez, A. L. Hawkins, C. A. Griffin, D. Small, and C. I. Civin. 1996. Tnk1: a novel intracellular tyrosine kinase gene isolated from human umbilical cord blood CD34+/Lin−/CD38− stem/progenitor cells. Oncogene 12:903-913. [PubMed] [Google Scholar]
- 33.Hopper, N. A., J. Lee, and P. W. Sternberg. 2000. ARK-1 inhibits EGFR signaling in C. elegans. Mol. Cell 6:65-75. [PubMed] [Google Scholar]
- 34.Hsu, S., G. Ju, and L. Fan. 1988. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci. Lett. 85:169-171. [DOI] [PubMed] [Google Scholar]
- 35.Hunter, T., and J. A. Cooper. 1985. Protein-tyrosine kinases. Annu. Rev. Biochem. 54:897-930. [DOI] [PubMed] [Google Scholar]
- 36.Ito, M., T. Matsui, T. Taniguchi, and K. Chihara. 1994. Alternative splicing generates two distinct transcripts for the Drosophila melanogaster fibroblast growth factor receptor. Gene 139:215-218. [DOI] [PubMed] [Google Scholar]
- 37.Kato, J., Y. Kaziro, and T. Satoh. 2000. Activation of the guanine nucleotide exchange factor Dbl following ACK1-dependent tyrosine phosphorylation. Biochem. Biophys. Res. Commun. 268:141-147. [DOI] [PubMed] [Google Scholar]
- 38.Kato-Stankiewicz, J., S. Ueda, T. Kataoka, Y. Kaziro, and T. Satoh. 2001. Epidermal growth factor stimulation of the ACK1/Dbl pathway in a Cdc42 and Grb2-dependent manner. Biochem. Biophys. Res. Commun. 284:470-477. [DOI] [PubMed] [Google Scholar]
- 39.Kennerdell, J. R., and R. W. Carthew. 1998. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell 95:1017-1026. [DOI] [PubMed] [Google Scholar]
- 40.Kozma, R., S. Ahmed, A. Best, and L. Lim. 1995. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol. Cell. Biol. 15:1942-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kulkarni, S. J., L. M. Newby, and F. R. Jackson. 1994. Drosophila GABAergic systems. II. Mutational analysis of chromosomal segment 64AB, a region containing the glutamic acid decarboxylase gene. Mol. Gen. Genet. 243:555-564. [DOI] [PubMed] [Google Scholar]
- 42.Lu, Y., and J. Settleman. 1999. The Drosophila Pkn protein kinase is a Rho/Rac effector target required for dorsal closure during embryogenesis. Genes Dev. 13:1168-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo, L., Y. J. Liao, L. Y. Jan, and Y. N. Jan. 1994. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 8:1787-1802. [DOI] [PubMed] [Google Scholar]
- 44.Manser, E., T. Leung, H. Salihuddin, L. Tan, and L. Lim. 1993. A non-receptor tyrosine kinase that inhibits the GTPase activity of p21cdc42. Nature 363:364-367. [DOI] [PubMed] [Google Scholar]
- 45.Manser, E., T. Leung, H. Salihuddin, Z. S. Zhao, and L. Lim. 1994. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367:40-46. [DOI] [PubMed] [Google Scholar]
- 46.Mihaly, J., L. Kockel, K. Gaengel, U. Weber, D. Bohmann, and M. Mlodzik. 2001. The role of the Drosophila TAK homologue dTAK during development. Mech. Dev. 102:67-79. [DOI] [PubMed] [Google Scholar]
- 47.Mott, H. R., D. Owen, D. Nietlispach, P. N. Lowe, E. Manser, L. Lim, and E. D. Laue. 1999. Structure of the small G protein Cdc42 bound to the GTPase-binding domain of ACK. Nature 399:384-388. [DOI] [PubMed] [Google Scholar]
- 48.Murphy, A. M., and D. J. Montell. 1996. Cell type-specific roles for Cdc42, Rac, and RhoL in Drosophila oogenesis. J. Cell Biol. 133:617-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Musacchio, A., T. Gibson, V. P. Lehto, and M. Saraste. 1992. SH3—an abundant protein domain in search of a function. FEBS Lett. 307:55-61. [DOI] [PubMed] [Google Scholar]
- 50.Nobes, C. D., and A. Hall. 1995. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81:53-62. [DOI] [PubMed] [Google Scholar]
- 51.Nolan, K. M., K. Barrett, Y. Lu, K. Q. Hu, S. Vincent, and J. Settleman. 1998. Myoblast city, the Drosophila homolog of DOCK180/CED-5, is required in a Rac signaling pathway utilized for multiple developmental processes. Genes Dev. 12:3337-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noselli, S., and F. Agnes. 1999. Roles of the JNK signaling pathway in Drosophila morphogenesis. Curr. Opin. Genet. Dev. 9:466-472. [DOI] [PubMed] [Google Scholar]
- 53.O'Connell, P. O., and M. Rosbash. 1984. Sequence, structure, and codon preference of the Drosophila ribosomal protein 49 gene. Nucleic Acids Res. 12:5495-5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Connor, M., and W. Chia. 1993. P element-mediated germ-line transformation of Drosophila, p. 75-85. In D. Murphy and D. A. Carter (ed.), Transgenesis techniques: principles and protocols, vol. 18. Humana Press, Totowa, N.J. [DOI] [PubMed] [Google Scholar]
- 55.O'Kane, C. J. 1998. Enhancer traps, p. 131-178. In D. B. Roberts (ed.), Drosophila, a practical approach. Oxford University Press, New York, N.Y.
- 56.Ricos, M. G., N. Harden, K. P. Sem, L. Lim, and W. Chia. 1999. Dcdc42 acts in TGF-β signaling during Drosophila morphogenesis: distinct roles for the Drac1/JNK and Dcdc42/TGF-β cascades in cytoskeletal regulation. J. Cell Sci. 112:1225-1235. [DOI] [PubMed] [Google Scholar]
- 57.Riesgo-Escovar, J. R., M. Jenni, A. Fritz, and E. Hafen. 1996. The Drosophila Jun-N-terminal kinase is required for cell morphogenesis but not for DJun-dependent cell fate specification in the eye. Genes Dev. 10:2759-2768. [DOI] [PubMed] [Google Scholar]
- 58.Ring, J. M., and A. Martinez Arias. 1993. puckered, a gene involved in position-specific cell differentiation in the dorsal epidermis of the Drosophila larva. Dev. Suppl. 1993:251-259. [PubMed] [Google Scholar]
- 59.Robertson, H. M., C. R. Preston, R. W. Phillis, D. M. Johnson-Schlitz, W. K. Benz, and W. R. Engels. 1988. A stable genomic source of P element transposase in Drosophila melanogaster. Genetics 118:461-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rubin, G. M., L. Hong, P. Brokstein, M. Evans-Holm, E. Frise, M. Stapleton, and D. A. Harvey. 2000. A Drosophila complementary DNA resource. Science 287:2222-2224. [DOI] [PubMed] [Google Scholar]
- 61.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 62.Sluss, H. K., Z. Han, T. Barrett, R. J. Davis, and Y. T. Ip. 1996. A JNK signal transduction pathway that mediates morphogenesis and an immune response in Drosophila. Genes Dev. 10:2745-2758. [DOI] [PubMed] [Google Scholar]
- 63.Snyder, M. A., J. M. Bishop, J. P. McGrath, and A. D. Levinson. 1985. A mutation at the ATP-binding site of pp60v-src abolishes kinase activity, transformation, and tumorigenicity. Mol. Cell. Biol. 5:1772-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takatsu, Y., M. Nakamura, M. Stapleton, M. C. Danos, K. Matsumoto, M. B. O'Connor, H. Shibuya, and N. Ueno. 2000. TAK1 participates in c-Jun N-terminal kinase signaling during Drosophila development. Mol. Cell. Biol. 20:3015-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Aelst, L., and C. D'Souza-Schorey. 1997. Rho GTPases and signaling networks. Genes Dev. 11:2295-2322. [DOI] [PubMed] [Google Scholar]
- 66.Van Vactor, D., and C. Kopczynski. 1999. Anatomical techniques for analysis of nervous system development in the Drosophila embryo, p. 490-513. In J. Richter (ed.), A comparative methods approach to the study of oocytes and embryos. Oxford University Press, New York, N.Y.
- 67.Yang, W., and R. A. Cerione. 1997. Cloning and characterization of a novel Cdc42-associated tyrosine kinase, ACK-2, from bovine brain. J. Biol. Chem. 272:24819-24824. [DOI] [PubMed] [Google Scholar]
- 68.Yang, W., Q. Lin, J. L. Guan, and R. A. Cerione. 1999. Activation of the Cdc42-associated tyrosine kinase-2 (ACK-2) by cell adhesion via integrin β1. J. Biol. Chem. 274:8524-8530. [DOI] [PubMed] [Google Scholar]
- 69.Zhang, Z., R. Baron, and W. C. Horne. 2000. Integrin engagement, the actin cytoskeleton, and c-Src are required for the calcitonin-induced tyrosine phosphorylation of paxillin and HEF1, but not for calcitonin-induced Erk1/2 phosphorylation. J. Biol. Chem. 275:37219-37223. [DOI] [PubMed] [Google Scholar]