Abstract
Axam has been identified as a novel Axin-binding protein that inhibits the Wnt signaling pathway. We studied the molecular mechanism by which Axam stimulates the downregulation of β-catenin. The C-terminal region of Axam has an amino acid sequence similar to that of the catalytic region of SENP1, a SUMO-specific protease (desumoylation enzyme). Indeed, Axam exhibited activity to remove SUMO from sumoylated proteins in vitro and in intact cells. The Axin-binding domain is located in the central region of Axam, which is different from the catalytic domain. Neither the Axin-binding domain nor the catalytic domain alone was sufficient for the downregulation of β-catenin. An Axam fragment which contains both domains was able to decrease the level of β-catenin. On substitution of Ser for Cys547 in the catalytic domain, Axam lost its desumoylation activity. Further, this Axam mutant decreased the activity to downregulate β-catenin. Although Axam strongly inhibited axis formation and expression of siamois, a Wnt-response gene, in Xenopus embryos, AxamC547S showed weak activities. These results demonstrate that Axam functions as a desumoylation enzyme to downregulate β-catenin and suggest that sumoylation is involved in the regulation of the Wnt signaling pathway.
Axin was originally identified as a product of the mouse Fused locus (69). The mouse mutant Fused is recessive lethal; mutants have a duplication of the embryonic axis. Expression of Axin in Xenopus embryos causes strong defects in the axis, and coexpression of Axin inhibits the Wnt-dependent axis duplication. Thus, Axin is a negative regulator of the Wnt signaling pathway and inhibits axis formation. We have identified rAxin (for rat Axin) and its homolog, Axil (for Axin-like), as proteins interacting with glycogen synthase kinase 3β (GSK-3β) (21, 65) and proposed that Axin is a key molecule in the Wnt signaling pathway (27).
It is well known that during animal development, the Wnt signaling pathway plays crucial roles in cell adhesion and cell fate determination (7, 63). Defects in this pathway result in abnormalities of physiological events ranging from early developmental processes to oncogenesis. Wnt proteins constitute a large family of cysteine-rich secreted ligands. In unstimulated cells, free cytoplasmic β-catenin is destabilized by a multiprotein complex containing Axin, GSK-3β, and adenomatous polyposis coli (APC) protein (21, 27, 31). Axin functions as a scaffold protein in this complex by directly binding to GSK-3β, β-catenin, and APC. The interaction of GSK-3β with Axin in the complex facilitates efficient phosphorylation of β-catenin by GSK-3β. Phosphorylated β-catenin forms a complex with Fbw1 (βTrCP/FWD1), a member of the F-box protein family, resulting in the degradation of β-catenin in the ubiquitin and proteasome pathways (13, 32). Indeed, Axin inhibits Wnt-dependent accumulation of β-catenin (29). In addition, APC and Axin are also phosphorylated by GSK-3β in the Axin complex. Phosphorylation of APC enhances its binding to β-catenin (52), whereas phosphorylation of Axin stabilizes it, in contrast to phosphorylation of β-catenin (64).
When cells are stimulated by Wnt, Dvl, a cytoplasmic protein, antagonizes the action of GSK-3β (6, 9). Dvl binds to the Axin complex and inhibits GSK-3β-dependent phosphorylation of β-catenin (30). Once the phosphorylation of β-catenin is reduced, it dissociates from the Axin complex, and β-catenin is no longer degraded, resulting in its accumulation in the cytoplasm. Stabilized β-catenin is translocated into the nucleus, where it binds to T-cell factor (Tcf)/lymphoid-enhancer factor (Lef), a transcription factor (2, 40), and serves as a coactivator of Tcf to stimulate transcription of the Wnt target genes, including c-myc, fra, jun, cyclin D1, peroxisome proliferator-activated receptor δ (PPARδ), and matrilysin (3, 63). Thus, the Wnt signal stabilizes β-catenin by inhibiting its phosphorylation and ubiquitination, thereby regulating the expression of various genes.
The small ubiquitin-related modifier (SUMO) modification (sumoylation) pathway resembles the ubiquitin conjugation pathway, but the enzymes involved in the two processes are distinct (18, 43, 66). There are three mammalian SUMOs, SUMO-1, SUMO-2, and SUMO-3, and one budding yeast homolog, Smt3. SUMO-1 has been most extensively studied. SUMO-1 is activated for conjugation by the E1 enzyme AOS/Uba2, subsequently transferred to the E2 conjugation enzyme Ubc9, and finally conjugated to target proteins by the E3 ligase PIAS (protein inhibitor of activated STAT) (22, 24, 53, 54). The genes encoding all key proteins of the modification process are essential in budding yeast, and the conjugation machinery is well conserved. Sumoylation is likely to be an important protein modification, as well as phosphorylation and ubiquitination. Sumoylation plays roles in (i) protein localization, (ii) protein stabilization, and (iii) transcriptional activation. Conjugation to RanGAP1 targets the cytoplasmic protein to the nuclear pore complex (37, 39), and modification of PML by SUMO-1 directs it to subnuclear structures termed PML bodies (44, 59). Sumoylation of IκBα or Mdm2 prevents its ubiquitination and proteasomal degradation (5, 8). Modification of p53 by SUMO-1 enhances its transcriptional activity (12, 50). In contrast, the target proteins of SUMO-2 and SUMO-3 have not yet been identified, and the physiological roles of modification with SUMO-2 and SUMO-3 are not known.
Sumoylation is reversible, and there are several SUMO-specific proteases in yeast and mammals (18, 43, 66). A single and essential Saccharomyces cerevisiae gene product, ubiquitin-like protein-specific protease 1 (Ulp1), catalyzes two critical functions via an encoded cysteinyl protease activity (35). Ulp1 processes the Smt3 C-terminal sequence (-GGATY) to its mature form (-GG) and deconjugates Smt3 from the lysine ɛ-amino group of the target protein. These functions are required for G2/M cell cycle progression in yeast (35). Three mammalian SUMO-specific proteases, SENP1, SUSP1, and SMT3IP1, have been identified, and all have a conserved C-terminal region, even though their sizes differ (11, 28, 47). The similarity between yeast Ulp1 and mammalian SUMO-specific proteases is confined to the C-terminal region of ∼200 amino acids, with an ∼90-residue segment forming a core structure which is common to these cysteine proteases (42, 66). However, the physiological roles of SUMO-specific proteases in mammalian cells are not known.
We have recently identified a novel Axin-binding protein and designated it Axam (for Axin-associating molecule) (23). Axam induces the degradation of β-catenin in SW480 cells (human colon cancer cells) and inhibits axis formation in Xenopus embryos. Therefore, Axam functions as a negative regulator of the Wnt signaling pathway. However, how Axam inhibits the Wnt signaling pathway is not clear. Here, we show that Axam has the catalytic activity to remove SUMO-1 from sumoylated proteins and that its mutant without this activity is less able to downregulate β-catenin and to inhibit axis formation of Xenopus embryos. These results demonstrate that Axam functions as a desumoylation enzyme to downregulate β-catenin in mammalian cells and suggest that sumoylation is involved in the regulation of the Wnt signaling pathway.
MATERIALS AND METHODS
Materials and chemicals.
Glutathione S-transferase (GST)-AOS1 and six-histidine-tagged (His6) Uba2 purified from Spodoptera frugiperda SF9 cells and pAcGHLT/p53 were supplied by H. Yasuda (Tokyo University of Pharmacy and Life Science, Tokyo, Japan) (19, 48). pcDNA3-Flag/SUMO-1(GG) and pUC/EF-1α/β-cateninSA were provided by M. Nakao and A. Nagafuchi (Kumamoto University, Kumamoto, Japan), respectively. Wnt-3a-producing L cells were provided by S. Takada (Kyoto University, Kyoto, Japan) (57). The anti-GST and anti-importin α-P1 antibodies were supplied by M. Nakata (Sumitomo Electronics, Yokohama, Japan) and Y. Yoneda (Osaka University, Suita, Japan), respectively. Leptomycin B was provided by M. Yoshida (University of Tokyo, Tokyo, Japan) (34). GST-Ulp-1 was purified as described previously (28). Other GST fusion proteins and His6-tagged proteins were purified from Escherichia coli according to the manufacturer's instructions (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom, and Invitrogen, Carlsbad, Calif.). L cells stably expressing wild-type hemagglutinin (HA)-Axam (L/Axam) were generated by selection with G418 as described previously (29). The anti-Myc antibody was prepared from 9E10 cells. The anti-GSK-3β and anti-β-catenin antibodies, anti-green fluorescent protein (GFP) antibody, anti-SUMO-1 antibody, anti-Flag (M2) antibody, anti-p53 antibody (DO-7), and anti-histone H1 antibody were purchased from Transduction Laboratories, Molecular Probes, Inc., Zymed, Novocastra Laboratories Ltd. (Newcastle, United Kingdom), Sigma-Aldrich (Steinheim, Germany), and Santa Cruz Biotechnology, respectively. Lipofectamine and Lipofectamine 2000 (Life Technologies Inc.) were used for transfection of L cells and of COS and SW480 cells, respectively. Lactacystin was purchased from PEPTIDE Institute, Inc. (Osaka, Japan). Other materials were from commercial sources.
Plasmid construction.
pBJ-Myc/rAxin, pCGN/Dvl-1, pGEX-4T/Axam-(72-588), pEGFP-C2/Axam-(1-113), pEGFP-C2/Axam-(72-588), pGEX-2T/Axam-(1-113), pGEX-2T/Axam, pEGFP-C1/Axam, pEGFP-C2/Axam-(72-400), and pSP64T/Myc-Axam were constructed as described previously (23). Standard recombinant DNA techniques were used to construct the following plasmids: pEGFP-C3/Axam-(381-588), pEGFP-C2/AxamC547S, pEGFP-C2/Axam-(72-588)C547S, pGEX-KG/Axam-(82-382), pGEX-KG/Axam-(381-588), pGEX-2T/AxamC547S, pGEX-4T/Axam-(72-588)C547S, pSP64T/Myc-AxamC547S, pSP64T/Myc-Axam-(72-400), pEF-BOS-HA/Axam, pGEX-2TK/SUMO-1(GG), pRSETA/Ubc-9, pGEX-6P-1/SUMO-1-Myc, and pAcGHLT/p53. In these plasmids, some constructs were made by digesting the original plasmids with restriction enzymes and inserting the fragments into the vectors. The other constructs were obtained by inserting fragments generated by the Expand High Fidelity PCR system (Roche Diagnostics GmbH, Mannheim, Germany) into the vectors. The entire PCR products were sequenced, and the structures of all plasmids were confirmed by restriction analysis.
Immunofluorescence study.
SW480, COS, and L cells (3.5-cm-diameter dish) transfected with pEGFP-derived and pBJ-Myc-derived plasmids and pUC/EF-1α/β-cateninSA were grown on coverslips and fixed for 20 min in phosphate-buffered saline (PBS) containing 4% paraformaldehyde. The cells were washed three times with PBS and then permeabilized with PBS containing 0.2% Triton X-100 and 2 mg of bovine serum albumin/ml for 2 to 12 h. They were washed and incubated for 1 h with the anti-SUMO-1, anti-β-catenin, anti-Axam, anti-histone H1, anti-Myc, or anti-GFP antibody. To examine the specificity of the anti-Axam antibody, 3 μg of anti-Axam antibody was incubated with 25 pmol of GST-Axam-(72-588) or GST immobilized on glutathione-Sepharose 4B in 200 μl of PBS at 4°C for 1 h. After incubation, the mixture was centrifuged, and the supernatant was used as the antibody source. After being washed with PBS, the cells were further incubated for 1 h with Alexa 488-labeled goat anti-rabbit immunoglobulin G or Alexa 594-labeled goat anti-mouse immunoglobulin G. The nuclei were counterstained with 4′,6-diamidine-2-phenylindole (DAPI). The coverslips were washed with PBS, mounted on glass slides, and viewed with a confocal laser scanning microscope (LSM510; Carl-Zeiss, Jena, Germany). In some experiments, the cells were directly viewed with the confocal microscope to detect GFP-Axam and its mutants. For multichannel imaging, each fluorescence was imaged sequentially to eliminate cross talk between the channels. When necessary, COS cells expressing GFP-Axam-(72-400) or GFP-Axam-(381-588) were grown on coverslips and treated with leptomycin B (20 ng/ml) or 0.2% ethanol (a solvent control) for 30 min. The cells were fixed and incubated with primary and secondary antibodies. To inhibit the proteasomal degradation of β-catenin, the cells were treated with 10 μM lactacystin for 10 h.
Enzyme assay.
To examine the hydrolase activity of Axam, 7 μg of GST-SUMO-1-Myc was incubated with 3 μg of GST-Axam, GST-Axam-(1-113), or GST-Ulp-1 in 30 μl of reaction mixture (100 mM Tris-HCl [pH 8.0], 2 mM dithiothreitol [DTT], 1 mM EDTA, and 5% glycerol) at 30°C for 1 or 3 h. After incubation, the mixtures were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Coomassie brilliant blue staining. To prepare sumoylated GST-p53 (GST-SUMO-1-GST-p53), 0.5 μg of GST-AOS/His6-Uba2 (E1), 2.5 μg of His6-Ubc9 (E2), 10 μg of GST-SUMO-1(GG), and 2.5 μg of GST-p53 were incubated in 50 μl of reaction mixture (50 mM Tris-HCl [pH 7.5], 2 mM DTT, 1 mM MgCl2, and 5 mM ATP) at 25°C for 3 h. After incubation, the mixtures were probed with the anti-p53 or anti-SUMO-1 antibody. For analysis of cleavage of GST-SUMO-1-GST-p53 by Axam, GST-SUMO-1-GST-p53 was incubated with 100 ng of GST-Axam or its mutants in 50 μl of reaction mixture (50 mM Tris-HCl [pH 7.5], 1 mM DTT, 20 mM EDTA, and 150 mM NaCl) for 30 min at 30°C. After incubation, the mixtures were probed with the anti-p53 antibody. To show desumoylation activity of Axam in intact cells, Flag-SUMO-1(GG) was expressed with GFP-Axam or GFP-AxamC547S in COS cells (3.5-cm-diameter dish). After the cells were lysed in 200 μl of Laemmli's sample buffer (65 mM Tris-HCl [pH 6.8], 3.3% SDS, 5.3% glycerol, and 2% 2-mercaptoethanol), the lysates were probed with the anti-Flag and anti-GFP antibodies.
Wnt-3a treatment.
To collect Wnt-3a-conditioned medium from cultures of Wnt-3a-producing L cells, the cells were seeded at a density of 106 in a 10-cm-diameter dish containing a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham F-12 medium supplemented with 10% fetal calf serum and cultured for 4 days (57). Then the conditioned medium was harvested, centrifuged at 1,000 × g for 10 min, and filtered through a nitrocellulose membrane. As a control, conditioned medium was prepared from L cells that had been transfected with only pGKneo and cultured under the same conditions described above. L cells (3.5-cm-diameter dish) grown on coverslips for 24 h were transfected with pEGFP-derived plasmids and incubated for an additional 12 h. After removal of the culture medium, Wnt-3a-conditioned medium was added (29). After a further 12-h incubation, the cells were fixed and stained with the anti-GFP or anti-β-catenin antibody.
Xenopus injections and analysis of phenotypes.
Myc-tagged Axam mutants were subcloned into pSP64T (33). Sense mRNA was obtained by in vitro transcription of linearized templates using an SP6-mMESSAGE mMACHINE kit (Ambion, Austin, Tex.). Fertilized eggs were dejellied using 4.5% l-cysteine hydrochloride monohydrate, and mRNAs were injected into the equatorial region of dorsal or ventral blastomeres at the four-cell stage. After injection, the embryos were cultured for 3 days and scored with the dorso-anterior index (DAI) (26). To carry out reverse transcription (RT)-PCR, total RNAs of embryos at stage 10.5 were isolated. Oligo(dT)-primed cDNAs were synthesized using 10 μg of total RNA from five embryos. PCR analyses (29 cycles) were performed with ExTaq DNA polymerase (TAKARA). The primers for PCR of the genes were as follows: siamois (5′-AAG ATA ACT GGC ATT CCT GAG C-3′ and 5′-GGT AGG GCT GTG TAT TTG AAG G-3′) and ornithine decarboxylase (5′-GCC ATT GTG AAG ACT CTC TCC ATT C-3′ and 5′-TTC GGG TGA TTC CTT GCC AC-3′).
Other.
Protein concentrations were determined with bovine serum albumin as a standard (4). Detection of downregulation of β-catenin in SW480 and L cells by immunoblotting was carried out as described previously (23, 29). The phosphorylation status of Axin and complex formation of Axam with Axin were examined as described previously (23).
RESULTS
Structural characterization of Axam.
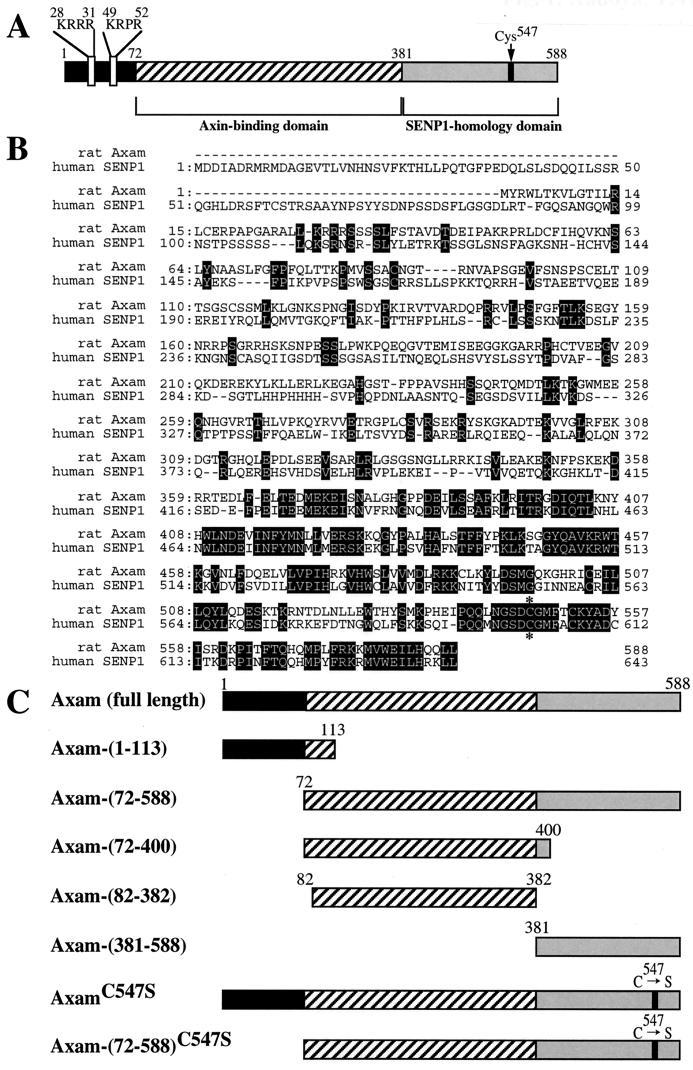
In a prior study (23), we found that ectopically expressed Axam is mainly localized to the nucleus and that Axam-(72-588), from which 71 N-terminal amino acids have been removed, is present in both the cytoplasm and the nucleus. These results suggest that the N-terminal region is important for the nuclear localization of Axam. Indeed, the basic amino acid region (KRRR31 and KRPR52), which is suggested to be a nuclear localization signal (NLS), is present at the N terminus (Fig. 1A). Although the PEST sequence lies between the putative NLSs (23), it is unknown whether this sequence regulates the stability of Axam.
FIG. 1.
Structural characterization of Axam. (A) Schematic representation of Axam showing its functional domains. (B) Sequence alignment of rat Axam and human SENP1. Sequences of rAxam and human SENP1 are aligned using the GENETYX-MAC program. Amino acid identity is highlighted in black. The asterisks indicate the catalytic cysteine residues. (C) Axam constructs used in this study.
After we had reported Axam (23), we found that it has high homology with SENP1, a SUMO-specific protease (11) (Fig. 1A and B). About 200 C-terminal amino acids of Axam have 59% identity and 89% similarity with 200 C-terminal amino acids of SENP1, which is its catalytic region. SENP1 removed SUMO from sumoylated PML in vitro and in intact cells. The SENP family consists of seven proteins (SENP1 to -7) (66). All of them share the C-terminal region that could function as a catalytic domain, while their N-terminal regions are variable and may regulate their subcellular localization and substrate specificities. SUSP1 and SMT3IP1 are the same as SENP6 and SENP3, respectively (28, 47). Rat Axam is 87% identical with and 94% similar to human SENP2 (DDBJ-EMBL-GenBank accession number AF15169). However, no biochemical characterization concerning the desumoylation activity in Axam (SENP2) had been performed. On the basis of these structural characteristics, we examined whether the distinct regions of Axam have specific functions. Various Axam constructs used in this study are shown in Fig. 1C.
Subcellular localization of Axam.
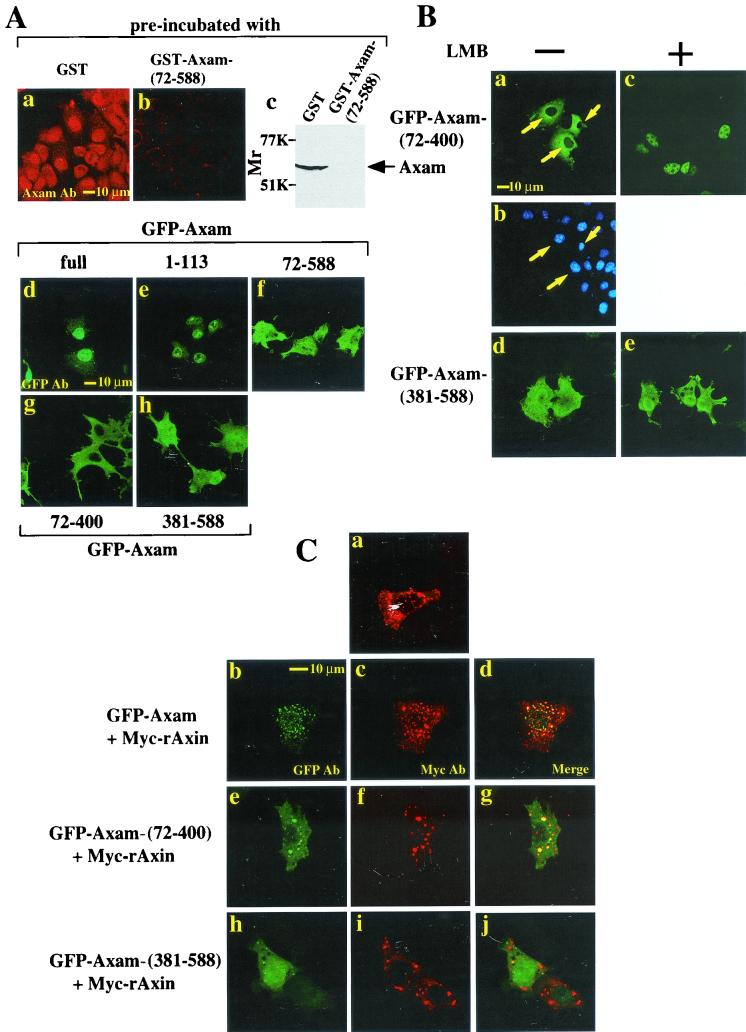
To clarify the subcellular localization of Axam, DLD-1 cells, human colon cancer cells, were stained with the anti-Axam antibody, because DLD-1 cells showed a high level of Axam expression by immunoblot analysis among various cell lines, including COS, SW480, L, 293, PC12, F9, RCN9, and DLD-1 cells (23). Endogenous Axam was found mainly in the nucleus and partly in the cytoplasm with the anti-Axam antibody but not with the antibody incubated with GST-Axam-(72-588) (Fig. 2A, a and b). The specificity of this Axam antibody was also confirmed by immunoblot analysis (Fig. 2A, c). When GFP-tagged Axam was expressed in SW480 cells (human colon cancer cells), it was also observed mainly in the nucleus and partly in the cytoplasm (Fig. 2A, d). GFP-Axam-(1-113) was localized in the nucleus, while GFP-Axam-(72-588) and GFP-Axam-(381-588) were present throughout the cytoplasm and nucleus (Fig. 2A, e, f, and h). The region containing amino acids 1 to 113 has the basic amino acid clusters KRRR31 and KRPR52. These are typical NLSs which bind to importin α (20). Importin α recognizes the NLS and mediates the selective transport of karyophilic proteins to the nuclei (67). Indeed, Axam-(1-113) but not Axam-(72-588) formed a complex with endogenous importin α in COS cells (data not shown). Therefore, these results suggest that the N-terminal region of Axam is mainly responsible for its nuclear localization by binding to importin α. GFP-Axam-(72-400) was clearly found in the cytoplasm (Fig. 2A, g). To examine whether the region containing amino acids 72 to 400 is localized in the cytoplasm in a Crm1-dependent manner, COS cells expressing GFP-Axam-(72-400) were treated with leptomycin B, which is known to be a specific inhibitor of Crm1-mediated export (34). Leptomycin B suppressed the cytoplasmic localization of GFP-Axam-(72-400) but had no effect on the subcellular distribution of GFP-Axam-(381-588) (Fig. 2B). As it is unknown whether Axam has nuclear export signal, these results suggest that the subcellular localization of Axam-(72-400) is regulated by Crm1 directly or indirectly. Taken together, it is likely that importin α and Crm1 regulate the subcellular localization of Axam.
FIG. 2.
Subcellular localization of Axam. (A) Subcellular localization of Axam mutants. DLD-1 cells were stained with the anti-Axam antibody incubated with GST (a) or GST-Axam-(72-588) (b). The lysates (20 μg of protein) of DLD-1 cells were probed with the indicated anti-Axam antibodies (Ab) (c). SW480 cells expressing GFP-Axam mutants were stained with the anti-GFP antibody (d to h). (B) Nuclear accumulation ofAxam by leptomycin B treatment. After COS cells expressing GFP-Axam-(72-400) (a to c) or GFP-Axam-(381-588) (d and e) had been treated with (+; c and e) or without (−; a, b, and d) 20 ng of leptomycin B/ml for 30 min, the cells were visualized under a confocal laser scanning microscope. The nuclei in image a were visualized by DAPI (b). LMB, leptomycin B. Arrows indicate the cells expressing GFP-Axam-(72-400). (C) Colocalization of Axam and Axin. SW480 cells expressing Myc-rAxin alone (a), Myc-rAxin and GFP-Axam (b to d), Myc-rAxin and GFP-Axam-(72-400) (e to g), or Myc-rAxin and GFP-Axam-(381-588) (h to j) were stained with the anti-Myc antibody to detect Myc-rAxin (a, c, f, and i) and the anti-GFP antibody to detect GFP-Axam and its mutants (b, e, and h). The merged image shows colocalization of Myc-rAxin with GFP-Axam or GFP-Axam-(72-400) but not with GFP-Axam-(381-588) (d, g, and j).
Since it has been shown that Axin is predominantly located in the cytoplasm (62), we asked whether Axin and Axam colocalized in the cytoplasm or nucleus. As shown previously (23), Myc-rAxin was observed as small particles in SW480 cells (Fig. 2C, a). When GFP-Axam was coexpressed with Myc-rAxin, GFP-Axam was detected on the small particles in the cytoplasm where Myc-rAxin was observed (Fig. 2C, b to d). GFP-Axam-(72-400), but not GFP-Axam-(381-588), was colocalized with Myc-rAxin in the cytoplasm (Fig. 2C, e to j). These results indicate that Axam is able to colocalize with Axin in the cytoplasm through its central region.
Enzymatic activity of Axam.
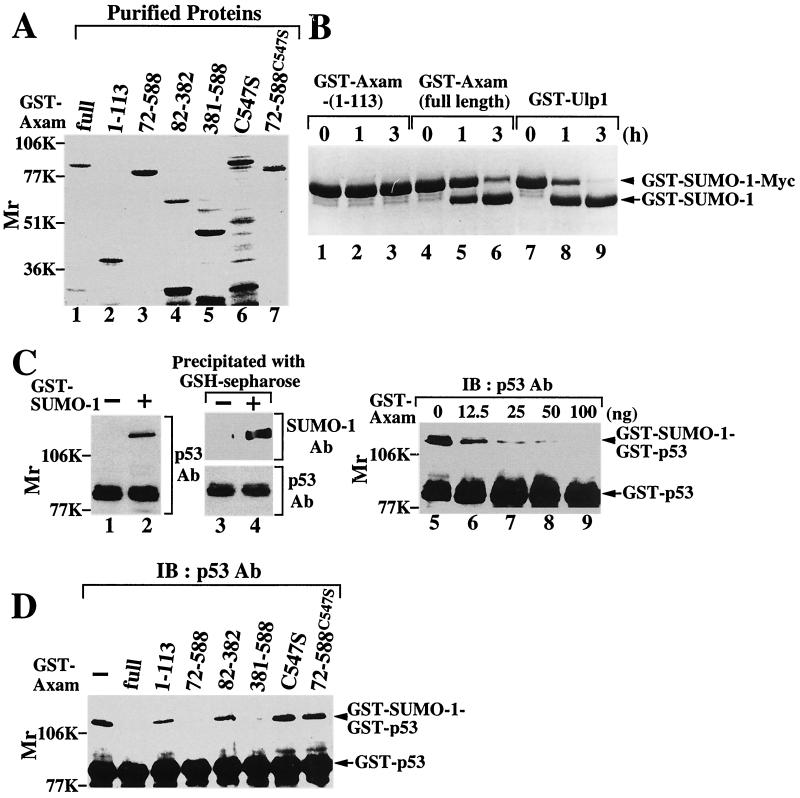
Yeast Ulp1 and human SUSP1 have two enzymatic activities (28, 35). One is the hydrolase activity to cleave the carboxyl side of the C-terminal Gly-Gly residues of SUMO-1 precursor to produce the mature form, and the other is the isopeptidase activity to deconjugate SUMO-1 from the lysine ɛ-amino group of the target protein. To examine whether Axam has these activities, various deletion mutants of Axam were purified as GST fusion proteins (Fig. 3A). GST-Axam digested GST-SUMO-1-Myc in a time-dependent manner, as did yeast Ulp1 (Fig. 3B, lanes 4 to 9). However, GST-Axam-(1-113) did not digest GST-SUMO-1-Myc (Fig. 3B, lanes 1 to 3). We then examined whether Axam might be capable of releasing SUMO-1 from sumoylated proteins. To this end, a SUMO-1-modified p53 was prepared. We used GST-SUMO-1(GG), which is the mature form, in this experiment. When GST-p53 and GST-SUMO-1(GG) were incubated with GST-AOS/His6-Uba2 and His6-Ubc9, a 120-kDa protein recognized by the anti-p53 antibody appeared (Fig. 3C, lane 2). In the absence of GST-SUMO-1(GG), this band was not observed (Fig. 3C, lane 1). The 120-kDa protein was also detected by the anti-SUMO-1 antibody (Fig. 3C, lanes 3 and 4), suggesting that this higher-molecular-mass form of p53 is sumoylated. GST-Axam removed SUMO-1 from sumoylated p53 in a dose-dependent manner (Fig. 3C, lanes 5 to 9). These results indicate that Axam has a desumoylation activity. Among the deletion mutants, GST-Axam-(72-588) and GST-Axam-(381-588) removed SUMO-1 from sumoylated p53, but GST-Axam-(1-113) and GST-Axam-(82-382) did not (Fig. 3D). Axam has a catalytic triad (His, Asp, and Cys) that is conserved in other desumoylation enzymes (42, 66). On substitution of Ser for Cys547 in GST-Axam and GST-Axam-(72-588), the SUMO-1 protease activity was lost (Fig. 3D). Altogether, Axam possesses SUMO-1 C-terminal hydrolase activity and isopeptidase activity capable of releasing SUMO-1 from SUMO-1 conjugates. Furthermore, the C-terminal 200 amino acids of Axam possess the desumoylation activity.
FIG. 3.
Enzyme activity of Axam in vitro. (A) Purified proteins (0.5 μg of each) used in this experiment were subjected to SDS-PAGE followed by Coomassie brilliant blue staining. (B) Hydrolase activity of Axam. After 7 μg of GST-SUMO-1-Myc had been incubated with 3 μg of GST-Axam-(1-113) (lanes 1 to 3), GST-Axam (lanes 4 to 6), or GST-Ulp1 (lanes 7 to 9) for the times indicated at 30°C, the mixtures were subjected to SDS-PAGE followed by Coomassie brilliant blue staining. (C) Cleavage of SUMO-1-p53 by Axam. After 2.5 μg of GST-p53 had been incubated with (+; lanes 2 and 4) or without (−; lanes 1 and 3) GST-SUMO-1(GG) for 3 h at 25°C, the mixtures were probed with the anti-p53 antibody (Ab; lanes 1 and 2). GST-SUMO-1-GST-p53 was precipitated with glutathione-Sepharose 4B, and the precipitates were probed with the anti-p53 and anti-SUMO-1 antibodies (lanes 3 and 4). After GST-SUMO-1-GST-p53 had been incubated with the indicated amounts of GST-Axam for 10 min at 30°C, the mixtures were probed with the anti-p53 antibody (lanes 5 to 9). IB, immunoblotting. (D) Cleavage of SUMO-1-p53 by Axam mutants. After GST-SUMO-1-GST-p53 had been incubated with 100 ng of GST-Axam or its mutants for 30 min at 30°C, the mixtures were probed with the anti-p53 antibody. The results shown are representative of three independent experiments.
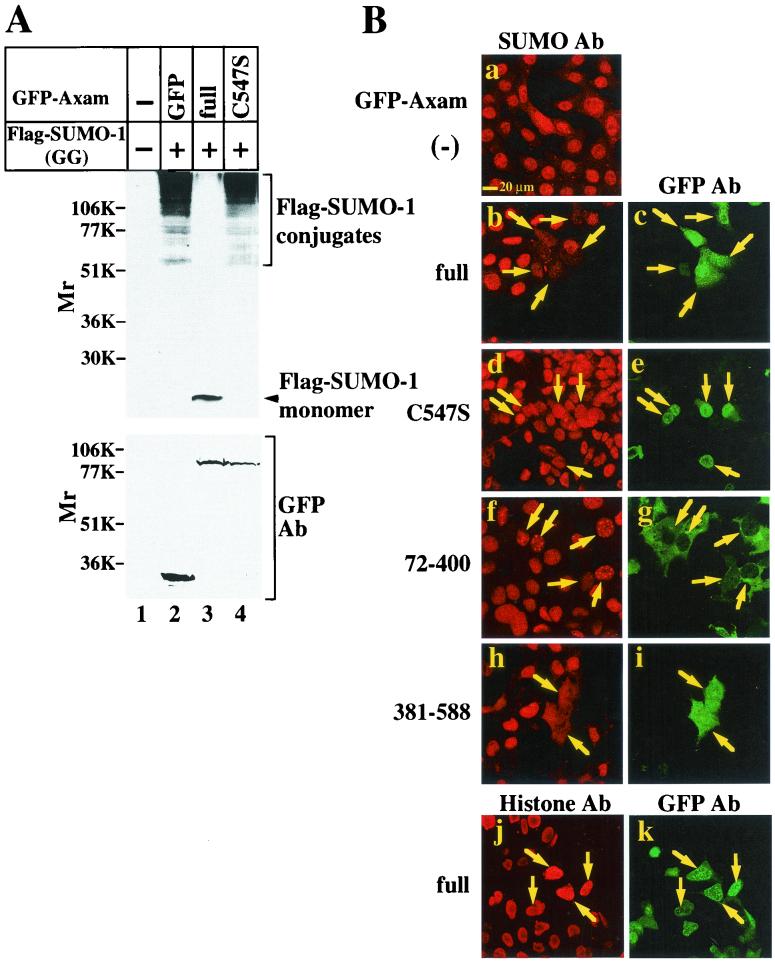
To examine whether Axam deconjugates SUMO-1 from the target proteins in intact cells, Flag-SUMO-1(GG) was expressed in COS cells with or without GFP-Axam. When the lysates of COS cells were probed with the anti-Flag antibody, Flag-SUMO-1 was conjugated to cellular target proteins and free Flag-SUMO-1 was not observed (Fig. 4A, lanes 1 and 2). These results are consistent with the previous observations that in most cells SUMO-1 is found in conjugation with target proteins and that the pool of free SUMO-1 is limiting (8). By expression of GFP-Axam, most of the conjugates disappeared and free Flag-SUMO-1 was detected (Fig. 4A, lane 3). However, expression of GFP-AxamC547S did not affect the Flag-SUMO-1-conjugated proteins (Fig. 4A, lane 4). Ectopically expressed GFP-Axam seems to remove SUMO-1 from almost all sumoylated proteins in intact cells. As expression of SENP1 also resulted in desumoylation of many endogenously sumoylated proteins (11), it would be due to overexpression of desumoylation enzymes.
FIG. 4.
Desumoylation activity of Axam in intact cells. (A) Biochemical analysis. COS cells expressing Flag-SUMO-1(GG) with (+) GFP (lane 2), GFP-Axam (lane 3), or GFP-AxamC547S (lane 4) were lysed in Laemmli's sample buffer. The lysates were probed with the anti-Flag (top) and anti-GFP (bottom) antibodies (Ab). COS cells transfected with empty vectors were used as a control (lane 1). (B) Immunohistochemical analysis. SW480 cells expressing GFP-Axam (full-length) (b and c), GFP-AxamC547S (d and e), GFP-Axam-(72-400) (f and g), or GFP-Axam-(381-588) (h and i) were stained with the anti-SUMO-1 (a, b, d, f, and h) and anti-GFP (c, e, g, i, and k) antibodies. The cells transfected with empty vector (−) were used as a control (a). SW480 cells expressing GFP-Axam (full length) were also stained with the anti-histone H1 antibody (j). The arrows indicate the cells expressing GFP-Axam or its mutants.
To confirm the desumoylation activity of Axam in intact cells by a different method, SW480 cells were stained with the anti-SUMO-1 antibody. Consistent with previous observations (11), SUMO-1 was predominantly localized to the nucleus (Fig. 4B, a). The intracellular signal was largely homogenous. However, SUMO-1 was also concentrated in dots that varied widely in number and in intensity from cell to cell. Since cell fractionation analysis indicated that 80 to 90% of endogenous SUMO-1-conjugated proteins show a nuclear distribution (25, 59), this SUMO-1 staining pattern in SW480 cells would show the presence of SUMO-1-conjugated proteins. When GFP-Axam was expressed in SW480 cells, the staining of SUMO-1 was markedly reduced (Fig. 4B, b and c). On the whole, expression of GFP-AxamC547S did not affect the staining of SUMO-1 (Fig. 4B, d and e). Since GFP-Axam did not affect nuclear staining with the anti-histone H1 antibody (Fig. 4B, j and k), the decrease in the staining of SUMO-1 would not be nonspecific. Furthermore, GFP-Axam-(381-588) and GFP-Axam-(72-588) reduced the staining of SUMO-1, but GFP-Axam-(72-400) and GFP-Axam-(72-588)C547S did not (Fig. 4B, f to i, and data not shown). The same immunohistochemical experiments were done with mouse fibroblast L cells, and essentially similar results were obtained (data not shown). Taken together, these results demonstrate that Axam functions as a desumoylation enzyme in intact cells.
Involvement of desumoylation activity of Axam in downregulation of β-catenin.
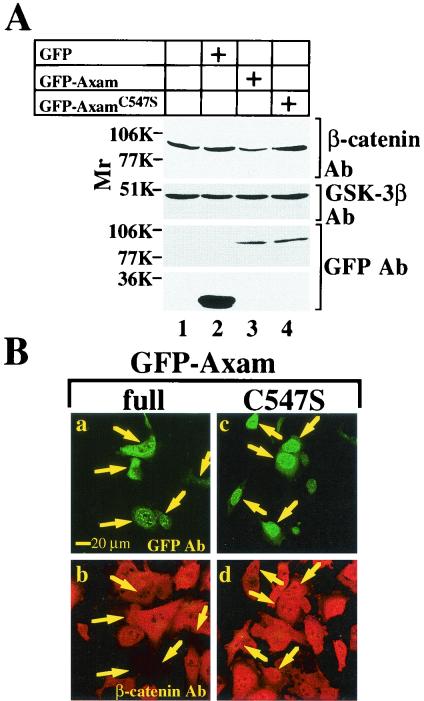
Although it became clear that Axam has desumoylation activity, we do not know the specificity of Axam on the target proteins. To clarify the specific function of Axam as a desumoylation enzyme, we asked whether the desumoylation activity of Axam is involved in the downregulation of β-catenin, because Axam induces the downregulation of β-catenin (23). β-Catenin was accumulated in both the cytoplasm and nucleus of SW480 cells due to the C-terminal truncation of APC. Immunoblot analysis showed that expression of GFP-Axam in SW480 cells reduces the amount of β-catenin protein and that GFP-AxamC547S does not (Fig. 5A). Immunohistochemical analysis also showed that GFP-Axam reduces the β-catenin staining (23) but that GFP-AxamC547S does not (Fig. 5B). These results suggest that the desumoylation activity of Axam is involved in downregulation of β-catenin in SW480 cells.
FIG. 5.
Involvement of desumoylation activity of Axam in downregulation of β-catenin in SW480 cells. (A) Biochemical analysis. The lysates (5 μg of protein) of SW480 cells expressing (+) GFP (lane 2), GFP-Axam (lane 3), or GFP-AxamC547S (lane 4) were probed with the anti-β-catenin, anti-GFP, and anti-GSK-3β antibodies (Ab). SW480 cells transfected with empty vectors were used as a control (lane 1). (B) Immunohistochemical analysis. SW480 cells expressing GFP-Axam (full length) (a and b) or GFP-AxamC547S (c and d) were stained with the anti-GFP (a and c) and anti-β-catenin (b and d) antibodies. Arrows indicate the cells expressing GFP-Axam or GFP-AxamC547S.
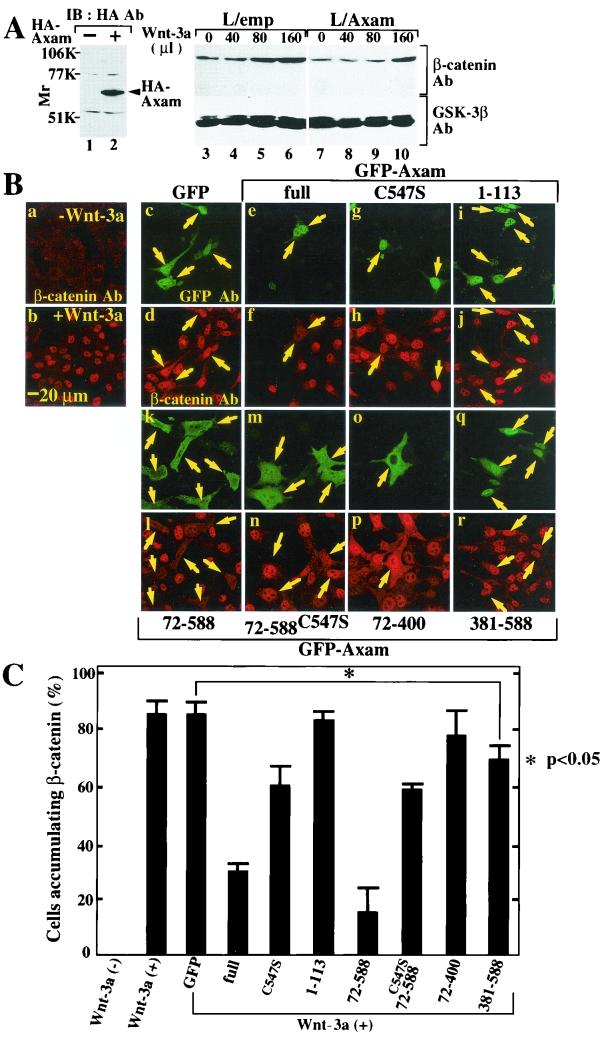
Although β-catenin is abnormally accumulated in SW480 cells, the protein levels of cytoplasmic and nuclear β-catenin are physiologically low in the normal cells, and Wnt induces the nuclear accumulation of β-catenin. We examined effects of desumoylation activity of Axam on the Wnt-dependent accumulation of β-catenin in L cells. To this end, we generated L cells stably expressing HA-Axam (L/Axam cells) and L cells transfected with empty vector (L/emp cells) (Fig. 6A, lanes 1 and 2). The levels of β-catenin protein accumulated in response to Wnt-3a were reduced in L/Axam cells compared with L/emp cells (Fig. 6A, lanes 3 to 10). Similar results were obtained with three independent clones of L/Axam cells. Since we did not succeed in generating L cells stably expressing AxamC547S, we examined the effects of AxamC547S on Wnt-3a-dependent accumulation of β-catenin by transient-expression assays. Immunohistochemical study revealed that the level of cytoplasmic β-catenin in L cells is low in the absence of Wnt-3a (Fig. 6B, a, and C). Wnt-3a-conditioned medium induced the nuclear accumulation of β-catenin in more than 80% of the cells (Fig. 6B, b, and C). When GFP was transiently expressed in L cells, more than 80% of the cells expressing GFP showed nuclear accumulation of β-catenin in response to Wnt-3a (Fig. 6B, c and d, and C). Therefore, the numbers of cells accumulating β-catenin among the cells expressing GFP-Axam or GFP-AxamC547S were counted. Expression of GFP-Axam reduced the numbers of cells accumulating nuclear β-catenin to 30% (Fig. 6B, e and f, and C). GFP-AxamC547S, which lost desumoylation activity, exhibited less activity to reduce the numbers of cells that accumulated β-catenin (Fig. 6B, g and h, and C). These are consistent with the observations using SW480 cells and suggest that desumoylation activity of Axam is involved in the downregulation of β-catenin.
FIG. 6.
Involvement of desumoylation activity of Axam in inhibition of Wnt-3a-dependent accumulation of β-catenin in L cells. (A) Biochemical analysis. The lysates (20 μg of protein) of L/emp cells (lane 1) or L/Axam cells (lane 2) were probed with the anti-HA antibody (Ab). After the L/emp cells (lanes 3 to 6) or L/Axam cells (lanes 7 to 10) were treated with the indicated amounts of Wnt-3a-conditioned medium for 1 h, thecells were lysed and probed with the anti-β-catenin and anti-GSK-3β antibodies. (B) Immunohistochemical analysis. Wild-type L cells were treated with control-conditioned medium (a) or Wnt-3a-conditioned medium (b). L cells expressing GFP (c and d), GFP-Axam (full length) (e and f), GFP-AxamC547S (g and h), GFP-Axam-(1-113) (i and j), GFP-Axam-(72-588) (k and l), GFP-Axam-(72-588)C547S (m and n), GFP-Axam-(72-400) (o and p), or GFP-Axam-(381-588) (q and r) were incubated with Wnt-3a-conditioned medium for 12 h. The cells were stained with the anti-β-catenin (a, b, d, f, h, j, l, n, p, and r) and anti-GFP (c, e, g, i, k, m, o, and q) antibodies. (C) Frequency of suppression of Wnt-3a-dependent β-catenin accumulation by Axam mutants. The histograms show the percentages of the cells with nuclear accumulation of β-catenin in the cells expressing GFP-Axam mutants (>100 cells) in panel B. The results shown are the mean ± standard error of four independent experiments (∗, P < 0.05 versus the cells expressing GFP; Mann-Whitney U test).
Using this immunohistochemical assay, we further examined the effects of deletion mutants of Axam on the downregulation of β-catenin. GFP-Axam-(1-113) and GFP-Axam-(72-400) did not affect the Wnt-3a-dependent accumulation of β-catenin (Fig. 6B, i, j, o, and p, and C). GFP-Axam-(72-588) suppressed the accumulation to an extent similar to that with GFP-Axam (Fig. 6B, k and l, and C). GFP-Axam-(72-588)C547S showed weak activity to downregulate β-catenin (Fig. 6B, m and n, and C). GFP-Axam-(381-588) possessing desumoylation activity decreased the activity to suppress nuclear accumulation of β-catenin compared with GFP-Axam and GFP-Axam-(72-588), but it still had statistically significant activity (Fig. 6B, q and r, and C). These results suggest that neither the Axin-binding domain nor the catalytic domain is sufficient for the activity of Axam to downregulate β-catenin and that both domains are required.
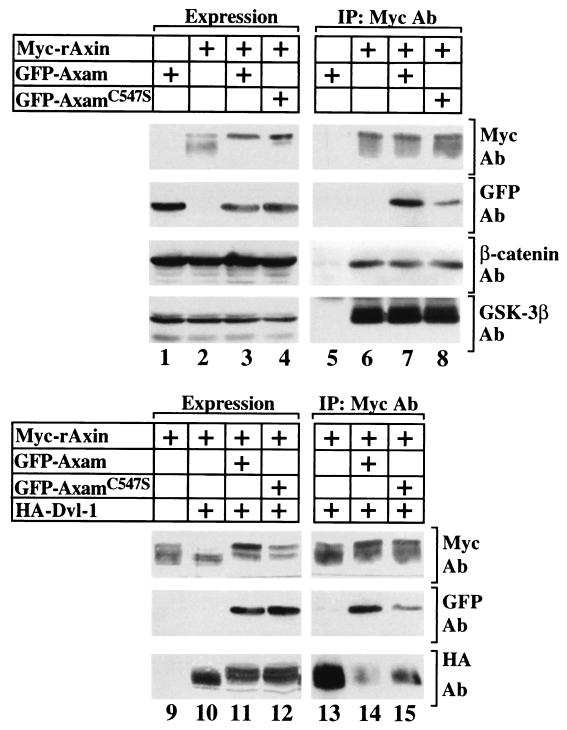
We previously showed that Axam does not affect the binding of β-catenin and GSK-3β to Axin but inhibits that of Dvl to Axin (23). These activities of AxamC547S were examined. The binding activity of GFP-AxamC547S to Myc-rAxin was less than that of GFP-Axam to Myc-rAxin (Fig. 7, lanes 1 to 8). Although AxamC547S did not affect the complex formation of β-catenin and GSK-3β with Myc-rAxin (Fig. 7, lanes 1 to 8), the activity of GFP-AxamC547S to inhibit the interaction of HA-Dvl-1 with Myc-rAxin was less than that of GFP-Axam (Fig. 7, lanes 9 to 15). These results suggest that sumoylation may inhibit the interaction of Axam with Axin. Alternatively, conformational change by the mutation of Cys547 to Ser may affect the binding activity of Axam to Axin. When the phosphorylation status of Axin was examined on an SDS gel, Myc-rAxin migrated faster by expression of HA-Dvl-1 (23) (Fig. 7, lanes 9 and 10). GFP-Axam and GFP-AxamC547S reversed the mobility shift of Myc-rAxin induced by HA-Dvl-1 with similar efficiencies (Fig. 7, lanes 11 and 12). At present, we do not know the relationship between the phosphorylation of Axin induced by Axam and a role of Axam in the downregulation of β-catenin.
FIG. 7.
Interaction of AxamC547S with Axin. The lysates (20 μg of protein) of COS cells expressing (+) the indicated proteins (lanes 1 to 4 and 9 to 12) were probed with the anti-Myc, anti-GFP, anti-β-catenin, anti-GSK-3β, and anti-HA antibodies (Ab). The same lysates (150 μg of protein) were immunoprecipitated (IP) with the anti-Myc antibody, and the immunoprecipitates were probed with the indicated antibodies (lanes 5 to 8 and 13 to 15). The results shown are representative of three independent experiments.
Axam-dependent downregulation of β-catenin via the proteasome.
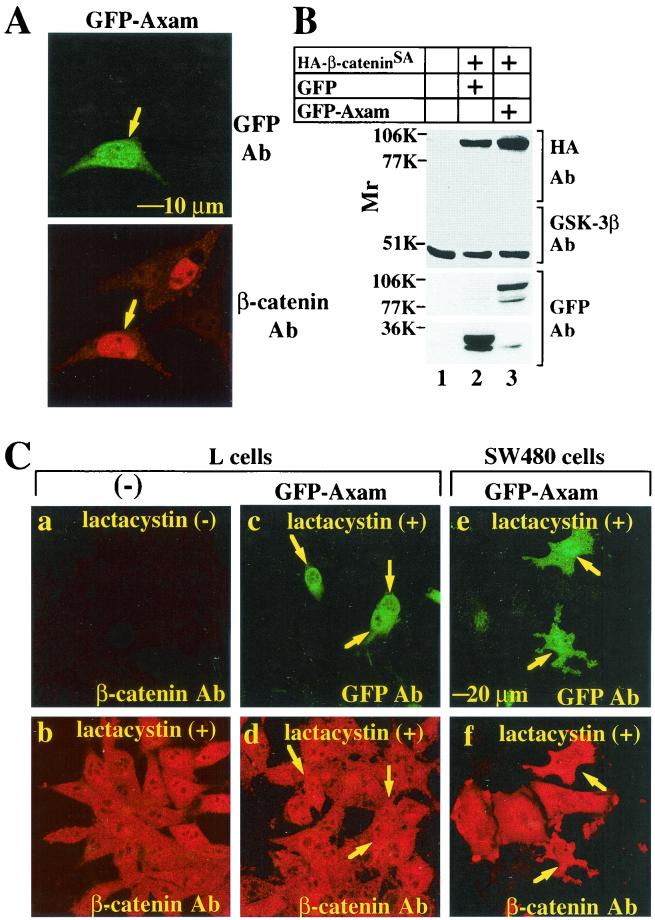
The phosphorylation of β-catenin by GSK-3β in the Axin complex triggers its degradation via the ubiquitin and proteasome system (1, 21). We examined whether Axam-dependent downregulation is mediated by this system. Ser33, Ser37, Thr41, and Ser45 in β-catenin are amino acids phosphorylated by GSK-3β, and mutations in these amino acids stabilize β-catenin and activate Tcf (49). β-CateninSA, in which Ser33, Ser37, Thr41, and Ser45 are all replaced with Ala, was accumulated in the nuclei of L cells (Fig. 8A). Ectopically, expression of wild-type β-catenin was not observed in L cells, probably due to rapid degradation by the proteasome (data not shown). Expression of GFP-Axam did not decrease the level of β-cateninSA (Fig. 8A). Consistent with these immunohistochemical results, immunoblot analysis revealed that expression of GFP-Axam in SW480 cells does not reduce the level of β-cateninSA protein (Fig. 8B). By treatment of L cells with lactacystin, a proteasome inhibitor, β-catenin was accumulated in the cytoplasm and nucleus (Fig. 8C, a and b). GFP-Axam did not inhibit the lactacystin-induced accumulation of β-catenin (Fig. 8C, c and d). Furthermore, lactacystin prevented Axam from downregulating β-catenin in SW480 cells (Fig. 8C, e and f). These results indicate that Axam induces the downregulation of β-catenin via the proteasome and suggest that Axam promotes GSK-3β-dependent phosphorylation and ubiquitination of β-catenin.
FIG. 8.
Axam-dependent downregulation of β-catenin via the proteasome. (A) Effects of Axam on the stability of β-cateninSA in L cells. β-CateninSA was expressed with GFP-Axam in L cells. The cells were stained with the anti-GFP (top) and anti-β-catenin (bottom) antibodies (Ab). (B) Effects of Axam on the stability of β-cateninSA in SW480 cells. The lysates (5 μg) of SW480 cells expressing (+) HA-β-cateninSA with GFP (lane 2) or GFP-Axam (lane 3) were probed with the anti-HA, anti-GSK-3β, and anti-GFP antibodies. SW480 cells transfected with empty vectors were used as a control (lane 1). (C) Effects of lactacystin on Axam-dependent downregulation of β-catenin. After L cells had been treated with (+; b) or without (−; a) 10 μM lactacystin for 10 h, the cells were stained with the anti-β-catenin antibody. After L cells (c and d) and SW480 cells (e and f) expressing GFP-Axam had been treated with lactacystin, the cells were stained with the anti-GFP (c and e) and anti-β-catenin (d and f) antibodies. Arrows in panels A and C indicate the cells expressing GFP-Axam.
Effects of Axam mutants on axis formation in Xenopus embryos.
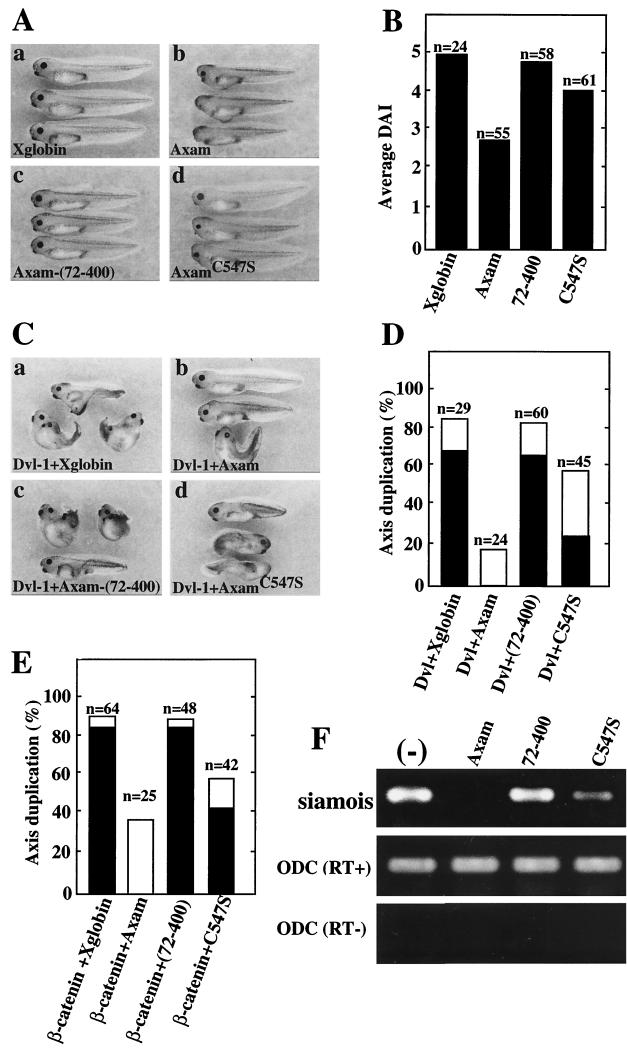
Xenopus embryos are useful tools for analyzing the functions of the components of the Wnt signaling pathway (41). Ventral expression of positive regulators of the Wnt signaling pathway, including Wnt-8, Dvl, and β-catenin, has been shown to induce the formation of a secondary dorsal axis (10, 41, 58, 61). Dorsal expression of negative regulators, including GSK-3β, Axin, Duplin, ICAT, and Idax, inhibits axis formation (14, 16, 55, 60, 68, 69). Previously, we showed that expression of Axam results in ventralization phenotypes and inhibits Dvl-induced formation of axis duplication (23). Furthermore, Axam inhibited β-catenin strongly but inhibited β-cateninSA-dependent axis duplication weakly (23). These results indicate that Axam functions as a negative regulator of the Wnt signaling pathway, and they are consistent with the results showing that Axam has the ability to downregulate β-catenin. Finally, using Xenopus embryo assays, we examined the involvement of desumoylation activity of Axam in the Wnt signaling pathway. Dorsal expression of Axam-(72-400) did not affect axis formation, and that of AxamC547S showed a weak ventralizing activity compared with Axam (Fig. 9A and B). Although ventral ex pression of Axam inhibited Dvl-dependent formation of axis duplication, Axam-(72-400) did not affect it (Fig. 9C and D). The activity of AxamC547S to suppress Dvl-dependent formation of a secondary axis was less than that of Axam (Fig. 9C and D). Ventral expression of β-catenin induced axis duplication (Fig. 9E). Axam inhibited β-catenin-dependent formation of axis duplication, but Axam-(72-400) did not (Fig. 9E). The activity of AxamC547S to suppress β-catenin-dependent formation of a secondary axis was less than that of Axam (Fig. 9E). Furthermore, expression of siamois, a Wnt target gene, was suppressed by Axam but not by Axam-(72-400) (Fig. 9F). The activity of AxamC547S to suppress siamois expression was less than that of Axam (Fig. 9F). These results indicate that the desumoylation activity of Axam is important for the negative regulation of axis formation and of the Wnt signaling pathway in Xenopus embryos.
FIG. 9.
Involvement of desumoylation activity of Axam in the axis formation of Xenopus embryos. (A) Phenotypes of embryos induced by Axam mutants. Embryos were injected dorsally with mRNA (500 pg each) of Xenopus globin (Xglobin) (a), Axam (b), Axam-(72-400) (c), or AxamC547S(d). (B) Average DAI of Xenopus embryos expressing Axam mutants in panel A. The indicated mRNAs were injected into dorsal blastomeres. The average DAI is not an accurate concept but is used simply for illustrative purposes. (C) Effects of Axam mutants on Dvl-induced secondary axis formation. Embryos were injected ventrally with mRNAs of Dvl-1 (1 ng) and Xglobin (500 pg) (a), Dvl-1 (1 ng) and Axam (500 pg) (b), Dvl-1 (1 ng) and Axam-(72-400) (500 pg) (c), and Dvl-1 (1 ng) and AxamC547S (500 pg) (d). (D) The results shown in panel C were expressed as the percentage of species with axis duplication. The solid bars show complete axis duplication, which includes eyes and cement glands. The open bars indicate incomplete axis duplication characterized by lack of head structures but with a distinct branched axis. (E) Effects of Axam mutants on β-catenin-induced secondary axis formation. Embryos were injected ventrally with mRNAs (500 pg each) of β-catenin and Xglobin, β-catenin and Axam, β-catenin and Axam-(72-400), and β-catenin and AxamC547S. The results were expressed as the percentage of species with axis duplication. The solid and open bars indicate the same phenotypes described for panel D. (F) Inhibition of siamois expression by Axam mutants. Expression of siamois was detected by RT-PCR analysis in embryos dorsally injected with mRNA (500 pg each) of Axam, Axam-(72-400), or AxamC547S. Embryos without injection (−) were used as a control. The amounts of cDNA were standardized with ornithine decarboxylase. The experiments without RT were carried out to rule out the possibility of contamination.
DISCUSSION
Here, we have shown that Axam has a desumoylation activity and that sumoylation is involved in the regulation of the stability of β-catenin. Several lines of evidence support the idea that Axam is a desumoylation enzyme. (i) The C-terminal 200 amino acids of Axam have high homology with the catalytic domains of the mammalian desumoylation enzymes SUSP1, SENP1, and SMT3IP1; (ii) Axam shows hydrolase activity to cleave the carboxyl side of the C-terminal Gly-Gly residues of SUMO-1 and to remove SUMO-1 from sumoylated p53 in vitro; (iii) expression of Axam in COS, L, and SW480 cells deconjugates SUMO-1 from the target proteins; and (iv) these activities are completely lost on the substitution of Ser for Cys547, an amino acid essential for the catalytic activity of other desumoylation enzymes. These results clearly demonstrate that Axam is a desumoylation enzyme.
Although the functions of mammalian desumoylation enzymes are not known, budding yeast Ulp1 is essential for G2/M transition, and the same phenotype is observed for the Ubc9 mutation in yeast (56). These observations suggest that the modification and removal of Smt3 are required for the dynamic regulation of cell cycle progression in yeast. Although it has been shown that sumoylation regulates the intracellular localization, stability, and function of the target proteins in mammals (18, 43, 66), whether desumoylation enzymes affect these cellular functions has not yet been clarified. We have demonstrated for the first time that desumoylation is involved in the degradation of β-catenin in SW480 cells and L cells. Although expression of Axam in these cells leads to a decrease in the protein level of β-catenin, removal of the catalytic domain from Axam [Axam-(72-400), the Axin-binding domain alone] removes its activity to downregulate β-catenin, and substitution of Ser for Cys547 (a catalytic inactive mutant) reduces the activity. These results are shown by biochemical and immunohistochemical assays. In addition, we have shown by three different assays using Xenopus embryos that the desumoylation activity of Axam is involved in axis formation regulated by the Wnt signaling pathway. First, AxamC547S shows weak ventralizing activity in comparison with Axam, and Axam-(72-400) does not induce ventralization. Second, AxamC547S also exhibits less activity to suppress Dvl-1- and β-catenin-dependent axis duplication than Axam, and Axam-(72-400) loses the activity. Third, although Axam suppresses expression of siamois, AxamC547S shows less activity, and Axam-(72-400) does not affect expression of siamois. Taken together, these results indicate that the desumoylation activity of Axam is important for the downregulation of β-catenin and for the negative regulation of the Wnt signaling pathway. However, the desumoylation activity is not sufficient for the downregulation of β-catenin, because the catalytic domain alone shows weak activity to degrade β-catenin. In a prior study (23), we showed that Axam competes with Dvl for binding to Axin and suggested that this competition may be the mechanism of β-catenin downregulation. The activity of AxamC547S to inhibit the interaction of Dvl with Axin is less than that of Axam. Therefore, loss of desumoylation activity of Axam leads to sumoylation of the target proteins, and the enhancement of sumoylation may inhibit the interaction of Axam with Axin. Alternatively, conformational change by the mutation of Cys547 to Ser may affect the binding activity of Axam to Axin. Therefore, two activities of Axam, to inhibit the binding of Dvl to Axin and to remove SUMO, are important for the activity to degrade β-catenin. Members of the family of desumoylation enzymes share the C-terminal catalytic domain but differ in the N-terminal region. Therefore, the N-terminal region may regulate subcellular localization and substrate specificity. It is intriguing to speculate that Axam recognizes the substrates by binding to Axin.
Our study has shown that expression of Axam deconjugates SUMO-1 from most of its target proteins. Since Axam is present not only in the nucleus but also in the cytoplasm, it may have broad substrate specificity. However, Axam could be specifically directed to components of the Wnt signaling pathway under physiological conditions, because the catalytic domain of Axam alone is not sufficient for the downregulation of β-catenin. What is the substrate of sumoylation among the components of Wnt signaling? Axam induces the downregulation of β-catenin in SW480 cells, where the accumulated β-catenin is neither phosphorylated nor ubiquitinated due to the C-terminal truncation of APC (17). Therefore, Axam may stimulate the phosphorylation and ubiquitination of β-catenin. In that case, removal of SUMO-1 from the molecule(s) in the Axin complex would enhance the phosphorylation and ubiquitination of β-catenin. This is supported by our previous observation that the activity of Axam to suppress β-cateninSA-induced duplication of the axis in Xenopus embryos is less than that to suppress wild-type β-catenin-induced duplication (23). Furthermore, we have shown in this study that Axam does not downregulate β-cateninSA. In our preliminary experiments, β-catenin and Axin were sumoylated in vitro (data not shown). It has been shown that (I/L)K(Q/T)E is a consensus sequence for sumoylation (66), and β-catenin and Axin indeed possess the sequence. It is intriguing to speculate that β-catenin is sumoylated and that the sumoylation blocks the phosphorylation or ubiquitination of β-catenin. This is the case for the degradation of IκBα and Mdm2 (5, 8). Ubiquitin and SUMO-1 share the same conjugation site on IκBα and Mdm2, and sumoylation blocks their ubiquitin-dependent degradation. Axin enhances the GSK-3β-dependent phosphorylation of β-catenin (21). If sumoylation of Axin inhibits this activity, the desumoylation of Axin would promote the phosphorylation of β-catenin by GSK-3β. However, since it is hard to detect the sumoylation of β-catenin and Axin in intact cells, other molecules may be sumoylated. Axin forms a complex with GSK-3β, Dvl, APC, and protein phosphatase 2A in addition to β-catenin. We are now examining whether they are sumoylated. It has recently been reported that Siah-1 induces the degradation of β-catenin through APC in response to p53 (36, 38). This new mechanism of β-catenin degradation requires the formation of a complex of Siah-1 with the C-terminal region of APC. Therefore, it is unlikely that desumoylation by Axam is involved in this pathway, because Axam is able to downregulate β-catenin in SW480 cells, where the C-terminal half of APC is truncated. The target protein(s) of sumoylation might be a protein other than those directly involved in the regulation of the stability of β-catenin in the Wnt signaling pathway. It has been shown that Lef-1 is sumoylated and that PIASy stimulates the sumoylation (53). Furthermore, PIASy inhibits β-catenin-dependent Lef-1 activation, suggesting that sumoylation regulates the Wnt signaling pathway negatively (53). Therefore, it is intriguing to speculate that sumoylation and desumoylation regulate this signaling pathway at multiple steps.
Axin is present in the cytoplasm, and β-catenin is degraded in the Axin complex (45, 62). Although Axam is mainly localized to the nucleus, it is also present in the cytoplasm. We have shown that Axam forms a complex with Axin in the cytoplasm. As it is conceivable that nuclear β-catenin is exported to the cytoplasm, where it is degraded, the mode of action of Axam may be to stimulate the export of β-catenin and make it accessible to Axin. Therefore, the subcellular localization of Axam may be functionally regulated. β-Catenin is exported in APC-dependent and -independent manners (15, 51, 62). APC has NLS and nuclear export signal, and its subcellular localization determines the stability of β-catenin (45). The molecule regulating the nuclear export of β-catenin and Axam may be also a candidate for sumoylation.
During the preparation of the manuscript, it was reported that Axam2, an Axam homolog, is still capable of downregulating β-catenin without desumoylation activity, and it was concluded that the desumoylation activity of Axam2 is not involved in the downregulation of β-catenin (46). This result is consistent with our observations that AxamC547S still has the activity to downregulate β-catenin, although the activity is less than that of Axam. But, it is opposite to our conclusion that the desumoylation activity of Axam is important for inducing the degradation of β-catenin. Although we do not know the reasons for this discrepancy, Axam2 may have different substrate specificity than Axam as a desumoylation enzyme, because Axam2 is lacking the N-terminal region in Axam and is mainly present in the cytoplasm. Further experiments will be necessary to clarify the physiological significance of sumoylation in the Wnt signaling pathway and to understand the functions of Axam.
Acknowledgments
We thank H. Yasuda and M. Nakao for helpful discussion. We are also grateful to B. Dallapiccola, G. Novelli, M. Nakao, A. Nagafuchi, S. Takada, M. Nakata, Y. Yoneda, and M. Yoshida for plasmids, antibodies, and reagents.
This work was supported by grants-in-aid for scientific research (B) and for scientific research on priority areas (C) from the Ministry of Education, Science, and Culture, Japan (2000 and 2001), and by grants from the Yamanouchi Foundation for Research on Metabolic Disorders (2000 and 2001).
REFERENCES
- 1.Aberle, H., A. Bauer, J. Stappert, A. Kispert, and R. Kemler. 1997. β-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 16:3797-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behrens, J., J. P. von Kries, M. Kühl, L. Bruhn, D. Wedlich, R. Grosschedl, and W. Birchmeier. 1996. Functional interaction of β-catenin with the transcription factor LEF-1. Nature 382:638-642. [DOI] [PubMed] [Google Scholar]
- 3.Bienz, M., and H. Clevers. 2000. Linking colorectal cancer to Wnt signaling. Cell 103:311-320. [DOI] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Buschmann, T., S. Y. Fuchs, C. G. Lee, Z. Q. Pan, and Z. Ronai. 2000. SUMO-1 modification of Mdm2 prevents its self-ubiquitination and increases Mdm2 ability to ubiquitinate p53. Cell 101:753-762. [DOI] [PubMed] [Google Scholar]
- 6.Cook, D., M. J. Fry, K. Hughes, R. Sumathipala, J. R. Woodgett, and T. C. Dale. 1996. Wingless inactivates glycogen synthase kinase-3 via an intracellular signalling pathway which involves a protein kinase C. EMBO J. 15:4526-4536. [PMC free article] [PubMed] [Google Scholar]
- 7.Dale, T. C. 1998. Signal transduction by the Wnt family of ligands. Biochem. J. 329:209-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desterro, J. M., M. S. Rodriguez, and R. T. Hay. 1998. SUMO-1 modification of IκBα inhibits NF-κB activation. Mol. Cell 2:233-239. [DOI] [PubMed] [Google Scholar]
- 9.Ding, V. W., R. H. Chen, and F. McCormick. 2000. Differential regulation of glycogen synthase kinase 3β by insulin and Wnt signaling. J. Biol. Chem. 275:32475-32481. [DOI] [PubMed] [Google Scholar]
- 10.Funayama, N., F. Fagotto, P. McCrea, and B. M. Gumbiner. 1995. Embryonic axis induction by the armadillo repeat domain of β-catenin: evidence for intracellular signaling. J. Cell Biol. 128:959-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong, L., S. Millas, G. G. Maul, and E. T. Yeh. 2000. Differential regulation of sentrinized proteins by a novel sentrin-specific protease. J. Biol. Chem. 275:3355-3359. [DOI] [PubMed] [Google Scholar]
- 12.Gostissa, M., A. Hengstermann, V. Fogal, P. Sandy, S. E. Schwarz, M. Scheffner, and G. Del Sal. 1999. Activation of p53 by conjugation to the ubiquitin-like protein SUMO-1. EMBO J. 18:6462-6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hart, M., J.-P. Concordet, I. Lassot, I. Albert, R. de los Santos, H. Durand, C. Perret, B. Rubinfeld, F. Margottin, R. Benarous, and P. Polakis. 1999. The F-box protein β-TrCP associates with phosphorylated β-catenin and regulates its activity in the cell. Curr. Biol. 9:207-210. [DOI] [PubMed] [Google Scholar]
- 14.He, X., J.-P. Saint-Jeannet, J. R. Woodgett, H. E. Varmus, and I. B. Dawid. 1995. Glycogen synthase kinase-3 and dorsoventral patterning in Xenopus embryos. Nature 374:617-622. [DOI] [PubMed] [Google Scholar]
- 15.Henderson, B. R. 2000. Nuclear-cytoplasmic shuttling of APC regulates β-catenin subcellular localization and turnover. Nat. Cell Biol. 2:653-660. [DOI] [PubMed] [Google Scholar]
- 16.Hino, S.-I., S. Kishida, T. Michiue, A. Fukui, I. Sakamoto, S. Takada, M. Asashima, and A. Kikuchi. 2001. Inhibition of the Wnt signaling pathway by Idax, a novel Dvl-binding protein. Mol. Cell. Biol. 21:330-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinoi, T., H. Yamamoto, M. Kishida, S. Takada, S. Kishida, and A. Kikuchi. 2000. Complex formation of adenomatous polyposis coli gene product and Axin facilitates glycogen synthase kinase-3β-dependent phosphorylation of β-catenin and downregulates β-catenin. J. Biol. Chem. 275:34399-34406. [DOI] [PubMed] [Google Scholar]
- 18.Hochstrasser, M. 2000. Evolution and function of ubiquitin-like protein-conjugation systems. Nat. Cell Biol. 2:E153-E157. [DOI] [PubMed] [Google Scholar]
- 19.Honda, R., and H. Yasuda. 1999. Association of p19ARF with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53. EMBO J. 18:22-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurt, E. C. 1993. The nuclear pore complex. FEBS Lett. 325:76-80. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda, S., S. Kishida, H. Yamamoto, H. Murai, S. Koyama, and A. Kikuchi. 1998. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3β and β-catenin and promotes GSK-3β-dependent phosphorylation of β-catenin. EMBO J. 17:1371-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson, E. S., I. Schwienhorst, R. J. Dohmen, and G. Blobel. 1997. The ubiquitin-like protein Smt3p is activated for conjugation to other proteins by an Aos1p/Uba2p heterodimer. EMBO J. 16:5509-5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadoya, T., S. Kishida, A. Fukui, T. Hinoi, T. Michiue, M. Asashima, and A. Kikuchi. 2000. Inhibition of Wnt signaling pathway by a novel Axin-binding protein. J. Biol. Chem. 275:37030-37037. [DOI] [PubMed] [Google Scholar]
- 24.Kahyo, T., T. Nishida, and H. Yasuda. 2001. Involvement of PIAS1 in the sumoylation of tumor suppressor p53. Mol. Cell 8:713-718. [DOI] [PubMed] [Google Scholar]
- 25.Kamitani, T., H. P. Nguyen, and E. T. Yeh. 1997. Preferential modification of nuclear proteins by a novel ubiquitin-like molecule. J. Biol. Chem. 272:14001-14004. [DOI] [PubMed] [Google Scholar]
- 26.Kao, K. R., and R. P. Elinson. 1988. The entire mesodermal mantle behaves as Spemann's organizer in dorsoanterior enhanced Xenopus laevis embryos. Dev. Biol. 127:64-77. [DOI] [PubMed] [Google Scholar]
- 27.Kikuchi, A. 1999. Roles of Axin in the Wnt signalling pathway. Cell. Signal. 11:777-788. [DOI] [PubMed] [Google Scholar]
- 28.Kim, K. I., S. H. Baek, Y. J. Jeon, S. Nishimori, T. Suzuki, S. Uchida, N. Shimbara, H. Saitoh, K. Tanaka, and C. H. Chung. 2000. A new SUMO-1-specific protease, SUSP1, that is highly expressed in reproductive organs. J. Biol. Chem. 275:14102-14106. [DOI] [PubMed] [Google Scholar]
- 29.Kishida, M., S. Koyama, S. Kishida, K. Matsubara, S. Nakashima, K. Higano, R. Takada, S. Takada, and A. Kikuchi. 1999. Axin prevents Wnt-3a-induced accumulation of β-catenin. Oncogene 18:979-985. [DOI] [PubMed] [Google Scholar]
- 30.Kishida, S., H. Yamamoto, S.-I. Hino, S. Ikeda, M. Kishida, and A. Kikuchi. 1999. DIX domains of Dvl and Axin are necessary for protein interactions and their ability to regulate β-catenin stability. Mol. Cell. Biol. 19:4414-4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kishida, S., H. Yamamoto, S. Ikeda, M. Kishida, I. Sakamoto, S. Koyama, and A. Kikuchi. 1998. Axin, a negative regulator of the Wnt signaling pathway, directly interacts with Adenomatous Polyposis Coli and regulates the stabilization of β-catenin. J. Biol. Chem. 273:10823-10826. [DOI] [PubMed] [Google Scholar]
- 32.Kitagawa, M., S. Hatakeyama, M. Shirane, M. Matsumoto, N. Ishida, K. Hattori, I. Nakamichi, A. Kikuchi, K.-I. Nakayama, and K. Nakayama. 1999. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of β-catenin. EMBO J. 18:2401-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreig, P. A., and D. A. Melton. 1984. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 12:7057-7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kudo, N., B. Wolff, T. Sekimoto, E. P. Schreiner, Y. Yoneda, M. Yanagida, S. Horinouchi, and M. Yoshida. 1998. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp. Cell Res. 242:540-547. [DOI] [PubMed] [Google Scholar]
- 35.Li, S. J., and M. Hochstrasser. 1999. A new protease required for cell-cycle progression in yeast. Nature 398:246-251. [DOI] [PubMed] [Google Scholar]
- 36.Liu, J., J. Stevens, C. A. Rote, H. J. Yost, Y. L. Hu, R. L. White, and N. Matsunami. 2001. Siah-1 mediates a novel β-catenin degradation pathway linking p53 to the adenomatous polyposis coli protein. Mol. Cell 7:927-936. [DOI] [PubMed] [Google Scholar]
- 37.Mahajan, R., C. Delphin, T. Guan, L. Gerace, and F. Melchior. 1997. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell 88:97-107. [DOI] [PubMed] [Google Scholar]
- 38.Matsuzawa, S.-I., and J. C. Reed. 2001. Siah-1, SIP, and Ebi collaborate in a novel pathway for β-catenin degradation linked to p53 responses. Mol. Cell 7:915-926. [DOI] [PubMed] [Google Scholar]
- 39.Matunis, M. J., E. Coutavas, and G. Blobel. 1996. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J. Cell Biol. 135:1457-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molenaar, M., M. van de Wetering, M. Oosterwegel, J. Peterson-Maduro, S. Godsave, V. Korinek, J. Roose, O. Destrée, and H. Clevers. 1996. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell 86:391-399. [DOI] [PubMed] [Google Scholar]
- 41.Moon, R. T., and D. Kimelman. 1998. From cortical rotation to organizer gene expression: toward a molecular explanation of axis specification in Xenopus. Bioessays 20:536-545. [DOI] [PubMed] [Google Scholar]
- 42.Mossessova, E., and C. D. Lima. 2000. Ulp1-SUMO crystal structure and genetic analysis reveal conserved interactions and a regulatory element essential for cell growth in yeast. Mol. Cell 5:865-876. [DOI] [PubMed] [Google Scholar]
- 43.Müller, S., C. Hoege, G. Pyrowolakis, and S. Jentsch. 2001. SUMO, ubiquitin's mysterious cousin. Nat. Rev. Mol. Cell. Biol. 2:202-210. [DOI] [PubMed] [Google Scholar]
- 44.Müller, S., M. J. Matunis, and A. Dejean. 1998. Conjugation with the ubiquitin-related modifier SUMO-1 regulates the partitioning of PML within the nucleus. EMBO J. 17:61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neufeld, K. L., F. Zhang, B. R. Cullen, and R. L. White. 2000. APC-mediated downregulation of β-catenin activity involves nuclear sequestration and nuclear export. EMBO Rep. 1:519-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishida, T., F. Kaneko, M. Kitagawa, and H. Yasuda. 2001. Characterization of a novel mammalian SUMO-1/Smt3-specific isopeptidase, a homologue of rat Axam, which is an Axin-binding protein promoting β-catenin degradation. J. Biol. Chem. 276:39060-39066. [DOI] [PubMed] [Google Scholar]
- 47.Nishida, T., H. Tanaka, and H. Yasuda. 2000. A novel mammalian Smt3-specific isopeptidase 1 (SMT3IP1) localized in the nucleolus at interphase. Eur. J. Biochem. 267:6423-6427. [DOI] [PubMed] [Google Scholar]
- 48.Okuma, T., R. Honda, G. Ichikawa, N. Tsumagari, and H. Yasuda. 1999. In vitro SUMO-1 modification requires two enzymatic steps, E1 and E2. Biochem. Biophys. Res. Commun. 254:693-698. [DOI] [PubMed] [Google Scholar]
- 49.Polakis, P. 1999. The oncogenic activation of β-catenin. Curr. Opin. Genet. Dev. 9:15-21. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez, M. S., J. M. Desterro, S. Lain, C. A. Midgley, D. P. Lane, and R. T. Hay. 1999. SUMO-1 modification activates the transcriptional response of p53. EMBO J. 18:6455-6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosin-Arbesfeld, R., F. Townsley, and M. Bienz. 2000. The APC tumour suppressor has a nuclear export function. Nature 406:1009-1012. [DOI] [PubMed] [Google Scholar]
- 52.Rubinfeld, B., I. Albert, E. Porfiri, C. Fiol, S. Munemitsu, and P. Polakis. 1996. Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science 272:1023-1026. [DOI] [PubMed] [Google Scholar]
- 53.Sachdev, S., L. Bruhn, H. Sieber, A. Pichler, F. Melchior, and R. Grosschedl. 2001. PIASy, a nuclear matrix-associated SUMO E3 ligase, represses LEF1 activity by sequestration into nuclear bodies. Genes Dev. 15:3088-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saitoh, H., D. B. Sparrow, T. Shiomi, R. T. Pu, T. Nishimoto, T. J. Mohun, and M. Dasso. 1998. Ubc9p and the conjugation of SUMO-1 to RanGAP1 and RanBP2. Curr. Biol. 8:121-124. [DOI] [PubMed] [Google Scholar]
- 55.Sakamoto, I., S. Kishida, A. Fukui, M. Kishida, H. Yamamoto, S.-I. Hino, T. Michiue, S. Takada, M. Asashima, and A. Kikuchi. 2000. A novel β-catenin-binding protein inhibits β-catenin-dependent Tcf activation and axis formation. J. Biol. Chem. 275:32871-32878. [DOI] [PubMed] [Google Scholar]
- 56.Seufert, W., B. Futcher, and S. Jentsch. 1995. Role of a ubiquitin-conjugating enzyme in degradation of S- and M-phase cyclins. Nature 373:78-81. [DOI] [PubMed] [Google Scholar]
- 57.Shibamoto, S., K. Higano, R. Takada, F. Ito, M. Takeichi, and S. Takada. 1998. Cytoskeletal reorganization by soluble Wnt-3a protein signalling. Genes Cells 3:659-670. [DOI] [PubMed] [Google Scholar]
- 58.Sokol, S. Y. 1996. Analysis of Dishevelled signalling pathways during Xenopus development. Curr. Biol. 6:1456-1467. [DOI] [PubMed] [Google Scholar]
- 59.Sternsdorf, T., K. Jensen, and H. Will. 1997. Evidence for covalent modification of the nuclear dot-associated proteins PML and Sp100 by PIC1/SUMO-1. J. Cell Biol. 139:1621-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tago, K.-I., T. Nakamura, M. Nishita, J. Hyodo, S.-I. Nagai, Y. Murata, S. Adachi, S. Ohwada, Y. Morishita, H. Shibuya, and T. Akiyama. 2000. Inhibition of Wnt signaling by ICAT, a novel β-catenin-interacting protein. Genes Dev. 14:1741-1749. [PMC free article] [PubMed] [Google Scholar]
- 61.Tian, Q., T. Nakayama, M. P. Dixon, and J. L. Christian. 1999. Post-transcriptional regulation of Xwnt-8 expression is required for normal myogenesis during vertebrate embryonic development. Development 126:3371-3380. [DOI] [PubMed] [Google Scholar]
- 62.Wiechens, N., and F. Fagotto. 2001. CRM1- and Ran-independent nuclear export of β-catenin. Curr. Biol. 11:18-27. [DOI] [PubMed] [Google Scholar]
- 63.Wodarz, A., and R. Nusse. 1998. Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 14:59-88. [DOI] [PubMed] [Google Scholar]
- 64.Yamamoto, H., S. Kishida, M. Kishida, S. Ikeda, S. Takada, and A. Kikuchi. 1999. Phosphorylation of Axin, a Wnt signal negative regulator, by glycogen synthase kinase-3β regulates its stability. J. Biol. Chem. 274:10681-10684. [DOI] [PubMed] [Google Scholar]
- 65.Yamamoto, H., S. Kishida, T. Uochi, S. Ikeda, S. Koyama, M. Asashima, and A. Kikuchi. 1998. Axil, a member of the Axin family, interacts with both glycogen synthase kinase-3β and β-catenin and inhibits axis formation of Xenopus embryos. Mol. Cell. Biol. 18:2867-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yeh, E. T., L. Gong, and T. Kamitani. 2000. Ubiquitin-like proteins: new wines in new bottles. Gene 248:1-14. [DOI] [PubMed] [Google Scholar]
- 67.Yoneda, Y. 2000. Nucleocytoplasmic protein traffic and its significance to cell function. Genes Cells 5:777-787. [DOI] [PubMed] [Google Scholar]
- 68.Yost, C., M. Torres, J. R. Miller, E. Huang, D. Kimelman, and R. T. Moon. 1996. The axis-inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 10:1443-1454. [DOI] [PubMed] [Google Scholar]
- 69.Zeng, L., F. Fagotto, T. Zhang, W. Hsu, T. J. Vasicek, W. L. Perry III, J. J. Lee, S. M. Tilghman, B. M. Gumbiner, and F. Costantini. 1997. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 90:181-192. [DOI] [PubMed] [Google Scholar]