Abstract
mtCLIC/CLIC4 (referred to here as mtCLIC) is a p53- and tumor necrosis factor alpha-regulated cytoplasmic and mitochondrial protein that belongs to the CLIC family of intracellular chloride channels. mtCLIC associates with the inner mitochondrial membrane. Dual regulation of mtCLIC by two stress response pathways suggested that this chloride channel protein might contribute to the cellular response to cytotoxic stimuli. DNA damage or overexpression of p53 upregulates mtCLIC and induces apoptosis. Overexpression of mtCLIC by transient transfection reduces mitochondrial membrane potential, releases cytochrome c into the cytoplasm, activates caspases, and induces apoptosis. mtCLIC is additive with Bax in inducing apoptosis without a physical association of the two proteins. Antisense mtCLIC prevents the increase in mtCLIC levels and reduces apoptosis induced by p53 but not apoptosis induced by Bax, suggesting that the two proapoptotic proteins function through independent pathways. Our studies indicate that mtCLIC, like Bax, Noxa, p53AIP1, and PUMA, participates in a stress-induced death pathway converging on mitochondria and should be considered a target for cancer therapy through genetic or pharmacologic approaches.
Apoptosis is a form of programmed cell death that enables organisms to eliminate unwanted cells through a tightly controlled process. Acquisition of resistance to apoptosis participates in the development of neoplasia (20), since cells with damaged DNA, karyotypic abnormalities, or an aberrant cell cycle (1, 29) are not eliminated. Furthermore, alterations in genes involved in the initiation or execution of apoptosis will render tumor cells resistant to drug treatment (46). The tumor suppressor protein p53 plays a central role in the elimination of cells with unregulated mitotic potential or DNA damage (9, 30, 42). p53 contributes to genomic stability by sensing DNA damage in cells and subsequently inducing growth arrest or apoptosis (5, 38). Missense mutations in the p53 gene found in tumors are mainly localized to its DNA-binding domain, resulting in a protein that fails to bind DNA in a sequence-specific manner (32). This finding suggests an important role for p53 transcriptional activity in its tumor suppressor function. The proapoptotic protein Bax is a target for p53 transcription (33), and recently several other p53-regulated genes involved in apoptosis were reported (3, 41, 47, 50). p53-regulated genes localized to mitochondria, i.e., those for Noxa (36), p53AIP1 (37), and PUMA (34, 58), were recently implicated in the apoptotic response. p53 can also induce apoptosis in a transcription-independent manner, as exemplified by apoptosis induced by p53 mutants that cannot transactivate or by wild-type p53 in the presence of inhibitors of RNA and protein synthesis (5).
Mitochondria are key organelles that integrate apoptotic signals in damaged cells (18). Apoptotic signals cause selective mitochondrial membrane permeabilization (29); consequent changes in pH; generation of reactive oxygen species; release of caspase activators, procaspases, Smac/Diablo, and apoptosis-inducing factor; and depletion of ADP and ATP (7, 24, 29). Apoptosis mediated by p53 involves mitochondrial changes (48), and specific effector proteins engaged in the process are currently being recognized. mtCLIC/CLIC4 (referred to here as mtCLIC), a p53- and tumor necrosis factor alpha-regulated intracellular chloride channel protein that localizes to the cytoplasm and the mitochondria in skin keratinocytes, was previously characterized (15). The subcellular localization of mtCLIC is variable in other cell types (6) and, to date, no biological function has been identified for mtCLIC. We now report that mtCLIC is upregulated in the apoptotic responses to p53 and DNA damage. Direct overexpression of mtCLIC induces apoptosis, and mtCLIC cooperates with Bax in the induction of cell death. Furthermore, suppression of mtCLIC upregulation prevents the apoptotic response to elevated p53 levels. Thus, mtCLIC is a newly identified effector of apoptosis that is capable of altering mitochondrial function, leading to caspase activation and cell death, and that may also be involved in p53 function as a tumor suppressor.
MATERIALS AND METHODS
Cell cultures.
Keratinocytes were isolated from newborn BALB/c, p53+/+, and p53−/− mice by using established methods (10) and plated at a density of 3 × 106 cells per 60-mm dish, 7 × 106 cells per 100-mm dish, and 15 × 106 cells per 150-mm dish, respectively. Cultures were maintained in Eagle's minimum essential medium without Ca2+ (Bio-Whittaker) but with 8% Chelex-treated fetal bovine serum (Gemini Bio-Products), 0.05 mM Ca2+, and 20 U of penicillin-streptomycin (Gibco) ml−1. Cell line SP1, derived from a chemically induced mouse skin papilloma (52), was cultured in the medium described above. The nontumorigenic S1 cell line was derived from normal mouse keratinocytes and maintained in the medium described above but supplemented with keratinocyte growth factor. p53-null keratinocytes (line AK1b) (4) were maintained in medium consisting of 16% fibroblast-conditioned Eagle's minimum essential medium with 8% Chelex-treated serum and 0.05 mM Ca2+. The p53 Tet-On Saos-2 cell line (45) and the Bax Tet-On Saos-2 cell line (40) were generous gifts from Kevin Ryan and Karen Vousden, National Cancer Institute. The cells were maintained in Dulbecco's modified Eagle's medium (Bio-Whittaker) with 8% fetal bovine serum (Gemini) and 20 U of penicillin-streptomycin ml−1. Induction of the regulated genes was achieved by treating the cells with doxycycline at a final concentration of 800 ng/ml. The Bax sequence is tagged with a p53 epitope recognized by antibody DO-1 (see below). Cells were treated with etoposide or adriamycin (both from Sigma) in culture medium at various concentrations and times.
Plasmids.
The cloning of mtCLIC and the construction of the pEGFP-N1 (GFP-mtCLIC) and pEGFP-C1 (mtCLIC-GFP) fusion vectors have been described elsewhere (15). The mtCLIC open reading frame was also cloned in the pCR3.0 vector (Invitrogen) in the sense and antisense orientations and sequenced. The plasmid expressing the green fluorescent protein (GFP) spectrum was obtained from Clontech, Palo Alto, Calif., and used as a transfection control and cotransfection marker for fluorescence-activated cell sorter (FACS) analysis.
Antibody generation, immunoprecipitation, and Western blot analysis.
Polyclonal antibodies generated against the N-terminal and C-terminal peptides of mtCLIC have been described elsewhere (15). The polyclonal sera were purified through a protein A column (Pharmacia) following manufacturer specifications and dialyzed in borate buffer. The polyclonal sera were also affinity purified against the immunogenic peptides at the Core Facility of the Frederick Cancer Research and Development Center.
Protein expression was analyzed by Western blotting. Cells were washed and then gently scraped into radioimmunoprecipitation lysis buffer.Thirty micrograms of protein was separated by 10 or 12% polyacrylamide gel electrophoresis-sodium dodecyl sulfate and transferred to nitrocellulose membranes. In some experiments, subcellular fractions were isolated as described previously (15). Antibodies against the N terminus and the C terminus of mtCLIC were used at 1:1,000 and 1:4,000 dilutions, respectively. A goat anti-rabbit horseradish peroxidase conjugate (Bio-Rad) was used as a secondary antibody. Monoclonal antibodies directed to murine p53 were raised as culture supernatants from the mouse hybridoma cell line PAb122 (19). Antibody DO-1 to a p53 sequence tag on Bax was from Oncogene, anti-β-actin mouse polyclonal antibody was from Boehringer Mannheim, and anti-cytochrome c antibody was from BD PharMingen. Blots were developed with enhanced chemiluminescence and SuperSignal chemiluminescence substrates (Pierce).
Immunoprecipitation was performed as follows. Cells were washed with cold phosphate-buffered saline (PBS) and lysed in 50 mM Tris buffer containing 150 mM NaCl, 1.5 mM MgCl2 10% glycerol, 1% Triton X-100, 5 mM EGTA, 20 μM leupeptin, 10 μg of aprotinin/ml, 1 mM phenylmethylsulfonyl fluoride, 200 μM NaVO3, and 10 mM NaF. Lysates were precleared with protein G/A PLUS-agarose beads and incubated with the desired primary antibody at 4°C for 2 h before overnight incubation with protein G/A PLUS-agarose beads at 4°C. Beads were washed in radioimmunoprecipitation buffer, centrifuged, resuspended, and boiled prior to electrophoresis.
Electron microscopy.
S1 cells transfected with mtCLIC-GFP or GFP were fixed with 2.5% glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.2), postfixed with 1% osmium tetroxide in the same buffer, treated with 2% aqueous uranyl acetate, dehydrated in a graded ethanol series, and embedded in EMbed 812 epoxy resin (Electron Microscopy Sciences). Sections (60 to 80 nm) were stained with uranyl acetate and lead citrate. All grids, including those with the immunolabeled specimens described below, were examined with a Zeiss EM902 microscope at 80 kV.
Immunogold labeling.
Cryosections were prepared and labeled by a modification of the Tokuyasu technique (39). Briefly, HACAT cells were fixed with 2% formaldehyde-0.2% glutaraldehyde, infiltrated with 2.3 M sucrose, and frozen in liquid nitrogen. Frozen sections were cut, mounted on Formvar-carbon-coated grids, blocked on drops of 1% bovine serum albumin in PBS, and then incubated with the antibody against the C-terminal domain of mtCLIC. Labeling was visualized with 6-nm gold particles coupled to protein A (Aurion) diluted to an A520 of 0.05. After extensive washing with PBS, sections were fixed with 1% glutaraldehyde and embedded in 1.8% methylcellulose-0.4% uranyl acetate. Each labeling experiment was accompanied by a control in which an unrelated rabbit polyclonal antibody (51) was used instead of the anti-mtCLIC antibody. The dilution of the control antibody was such that it produced robust labeling when the corresponding antigen was present.
RNA isolation, Northern blot hybridization, and reverse transcription (RT)-PCR.
Total RNA was isolated from cultured cells by Trizol extraction. RNA was resolved by formaldehyde-agarose gel electrophoresis and blotted as previously described (57). A 300-bp DNA fragment for mtCLIC was amplified from the pCRII vector by using M13 amplification primers. cDNA probes were radiolabeled with 32P (Lofstrand) and hybridized to the blots as described previously (57). Bands were quantified by using a PhosphorImager (Molecular Dynamics). Loading equivalence was assessed on the basis of the 28S band or by reprobing blots for glyceraldehyde-3-phosphate dehydrogenase.
p53 Tet-On Saos-2 cells were grown to mid-log phase, treated with doxycycline (800 ng/ml), and collected at different time points. Approximately 0.5 μg of purified RNA was used to generate first-strand cDNA by RT with Superscript II (Gibco). Aliquots of the synthesized cDNA were used as a template in a PCR with Supermix (Gibco). The mtCLIC-specific primer set (5′-TTCCCCTTCATTTAAACACCTTT-3′ and 5′-TGCTATCTACATGCAACTCTGGA-3′) and the 18S gene-specific and Competimer primer set (Ambion) were mixed at a 2:8 ratio. PCR was performed for 30 cycles, and the PCR-amplified set (18S internal control, 550 bp; mtCLIC, 450 bp) of DNA fragments from each time point was analyzed on 4% agarose gels containing ethidium bromide.
Construction of a SEAP reporter vector containing the human mtCLIC promoter.
The human mtCLIC cDNA sequence was used as a query to search the public human genome database (National Center for Biotechnology Information, Bethesda, Md.), and the 5′ region upstream from the known 5′ untranslated region of mtCLIC was identified in the human contiguous AL445648.10 segment. From the identified sequence, the putative transcription start site was determined by using web-based bioinformation programs (Pedro's Biomolecular Research Tools). Specific primers and purified genomic DNA from cultured human foreskin keratinocytes were used to amplify by PCR a 3.5-kb DNA fragment that corresponded to the promoter sequence upstream from the putative transcription start site. Two separate sets of primers and the 3.5-kb DNA fragment were used to amplify by PCR 1.5-kb (PmtCLIC A/B) and 1-kb (PmtCLIC C) DNA fragments containing the putative p53-binding sites (TF-Bind and TF-Factor). These two fragments were cloned into the pGEM-T Easy vector (Promega, Madison, Wis.) and then further subcloned into the pSEAP-basic reporter vector (Clontech) by ligating the EcoRI-digested DNA fragment from the pGEM-T Easy plasmid to the EcoRI-digested reporter plasmid. The orientation of the promoters was determined by restriction and sequencing analyses. A positive control vector was constructed by ligating a BamHI/SalI-digested secreted embryonic alkaline phosphatase (SEAP) insert from the pSEAP-basic reporter vector to the BamHI/SalI-digested pp53-TA-Luc vector and was confirmed by restriction and sequencing analyses. Reporter vectors were transfected into p53 Tet-On Saos-2 cells, and expression in transfectants was determined in the presence or absence of doxycycline. The expression of SEAP driven by PmtCLIC A/B and PmtCLIC C promoter activity was measured with a Great EscAPe SEAP chemiluminescence detection kit (Clontech) as described by the manufacturer.
Transfection.
Transfection of the GFP-mtCLIC fusion vectors or the pCR3.0 plasmids into primary BALB/c keratinocytes and SP1 and S1 cell lines was performed by using Lipofectamine Plus reagent (Gibco). Briefly, cell lines were plated 2 days before transfection at a density of 3 × 105 cells per 60-mm dish, and primary cells were plated 2 to 3 days before transfection at 3 × 106 cells per 60-mm dish. Cells were transfected with 4 μg of plasmid DNA (per dish) in serum-free medium that was replaced after 3 h with culture medium. Transfected cells were visualized with an inverted fluorescence microscope (Zeiss). With this approach, transfection efficiencies were 60 to 70% in cell lines and 20 to 30% in primary cultures. p53 Tet-On Saos-2 cells were transfected with Lipofectamine Plus reagent by using an empty adenovirus carrier (49).
Apoptosis assays and FACS analysis.
Primary keratinocytes and SP1 and S1 cells transfected with the GFP fusion constructs mtCLIC-GFP and GFP-mtCLIC and with GFP plasmids or cotransfected with GFP and mtCLIC plasmids were analyzed by flow cytometry on a FACSCalibur instrument (Becton Dickinson). At various times after transfection, cells were trypsinized and collected in 1 ml of medium. All samples were assayed in the presence or absence of propidium iodide (PI) at a final concentration of 0.5 μg/ml. In a live-cell suspension, only cells with damaged membranes (dying cells) will take up PI. Double-color analysis was carried out on all samples. Similar results were obtained with cotransfection or transfection of fusion plasmids.
Z-VAD-FMK.
Keratinocytes transfected with the fusion proteins described above were treated with Z-VAD-FMK (Enzyme Systems Products) at a concentration of 40 μM. In one group, the caspase inhibitor was added immediately after the transfection medium was removed; in a second group, Z-VAD-FMK was added 24 h after removal of the transfection medium. Cells were analyzed by flow cytometry 48 h after transfection.
Annexin V.
Transfected cultures were analyzed for the presence of cell surface annexin V as a measure of apoptotic death. Briefly, cells were trypsinized 24 and 48 h after transfection, centrifuged, and incubated with a biotin-conjugated antibody against annexin V (Genzyme). Cells were then washed and fixed in 10% neutral buffered formalin for 10 min. Fixed cells were incubated with allophycocyanin-streptavidin (Becton Dickinson) diluted in 1× binding buffer and analyzed by flow cytometry.
Mitochondrial membrane potential.
Transfected cells were trypsinized, resuspended in medium, and incubated for 30 min with Mitotracker. Samples were read immediately in the red and green channels in the flow cytometer. Analysis was performed by gating GFP-transfected cells.
PI staining.
Cells transfected with mtCLIC sense and antisense plasmids together with the GFP spectrum plasmid were trypsinized and fixed in cold 70% ethanol. Samples were kept at −20°C for at least 18 h. Cells were then pelleted, and 1 ml of PBS-Tween was added. After a second centrifugation, cells were treated with RNase A for 30 min, and 500 μl of PI (50 μg/ml) was added before flow cytometric analysis. The presence of the sub-G1 peak in gated green cells was used as a measure of apoptosis.
MTT assay.
Cell viability was assessed by the methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay (Promega) following manufacturer instructions.
RESULTS
DNA damage in keratinocytes increases mtCLIC protein expression.
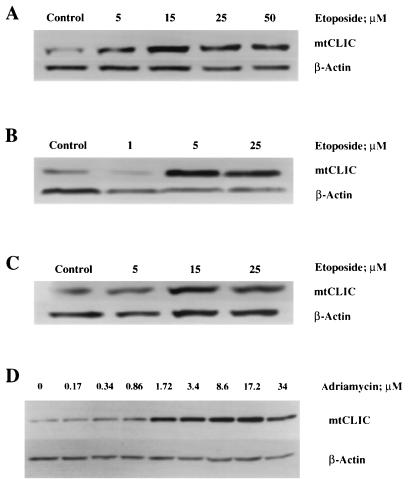
Treatment of normal and neoplastic keratinocytes with the DNA-damaging agent etoposide induces apoptosis. Morphological changes can be observed 24 h after treatment with 5 to 15 μM, with the full onset of apoptotic characteristics (bleb formation, nuclear condensation, and detachment) being visible 48 h after treatment. At higher doses, apoptosis occurred earlier. mtCLIC protein expression was upregulated following treatment with etoposide in normal (Fig. 1A), tumor-derived (Fig. 1B), and p53-null (Fig. 1C) keratinocytes. Upregulation occurred before apoptotic bodies could be detected, suggesting a role for mtCLIC in the induction of apoptosis. Similar results were obtained with adriamycin treatment (Fig. 1D), which both induced mtCLIC and caused apoptosis in a dose-dependent pattern. Treatment with etoposide or adriamycin at all concentrations and times tested (6, 18, and 30 h) reduced mtCLIC mRNA levels (data not shown), suggesting that upregulation of mtCLIC after etoposide damage is posttranscriptional and not dependent on p53 induction.
FIG. 1.
mtCLIC protein is upregulated during etoposide- or adriamycin-induced apoptosis. Keratinocytes were treated with different concentrations of etoposide or adriamycin for 24 h. Protein samples were collected for immunoblot analysis for mCLIC, and β-actin was used as a loading control. Data are representative of three separate experiments for etoposide and one experiment for adriamycin. (A and D) Normal keratinocytes (S1 cell line). (B) Neoplastic keratinocytes (SP1 papilloma cell line). (C) p53-null keratinocytes (AK1b cell line).
Overexpression of mtCLIC causes keratinocyte apoptosis that is inhibited by Z-VAD-FMK.
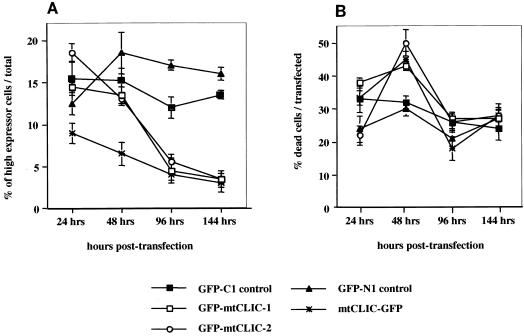
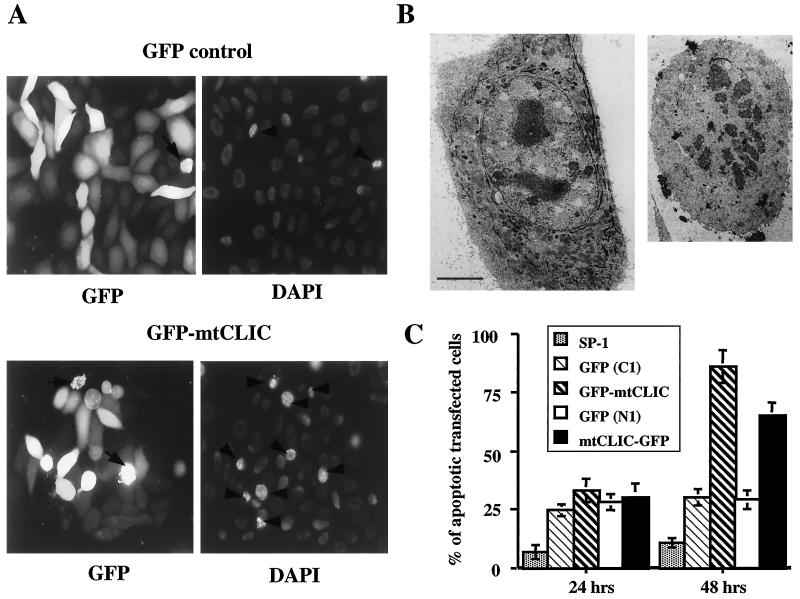
To determine whether an increase in mtCLIC expression in keratinocytes produced a biological response, primary keratinocytes or SP1 cells were transfected with mtCLIC-GFP, GFP-mtCLIC, or GFP control constructs or cotransfected with parental plasmids as described in Materials and Methods. The fate of fluorescing cells was monitored over time by flow cytometry. The results were identical for either cell type. Cells expressing the highest levels of the fusion proteins (highest fluorescence intensity) were eliminated over 44 to 144 h, while the number of transfected cells expressing a high level of GFP did not change (Fig. 2A). This large decrease in the number of mtCLIC-transfected cells showing the highest level of expression correlates with an increase in the number of dying cells, as detected by PI uptake in unfixed cells at 48 h (Fig. 2B). Thus, as cells accumulate GFP-mtCLIC, viability decreases and the cells are lost from the population. Furthermore, mtCLIC-GFP-transfected cells expressing high levels of mtCLIC showed cell rounding, detachment, fragmentation, and nuclear condensation upon 4′,6′-diamidino-2-phenylindole (DAPI) staining, while most cells transfected with GFP only, with the same level of fluorescence intensity, displayed normal nuclear staining and remained attached to the substrate (Fig. 3A). At the electron microscopic level (Fig. 3B), mtCLIC-GFP-transfected cells exhibited typical morphological markers for apoptosis (2). Fragmented nuclei, ruffling of the plasma membrane, and loss of tonofilaments were frequently observed in cells, while the mitochondria remained largely intact. These preparations also contained postapoptotic cells (data not shown) in a more advanced state of degeneration, with degraded nuclei and deteriorating organelles.
FIG. 2.
Overexpression of mtCLIC in cultured keratinocytes is lethal. Plasmid constructs encoding GFP, GFP-mtCLIC, or mtCLIC-GFP were transiently transfected into SP1 cells. Attached cells were trypsinized 24, 48, 96, and 144 h after transfection; combined with cells that had detached during these times; and incubated with PI. The level of green and red fluorescence was quantitated by FACS analysis. At least 10,000 transfected cells were collected for each quantitation and analyzed without fixation. Values are for duplicate dishes at each time point. Data are representative of two independent experiments. Error bars show standard deviations. GFP-mtCLIC-1 and GFP-mtCLIC-2 were independently constructed plasmids encoding identical sequences. (A) High-expression cells. Cells were classified as high expressors when the amount of green fluorescence was 2 log units higher than that of the cells with the lowest level of expression. Each population shown was quantitated at the indicated times. (B) Dead transfected cells. The percentage of transfected cells that incorporated PI was quantitated by gating the transfected cell population and analyzing green and red fluorescence. PI was added to the suspension of unfixed cells analyzed over 144 h after transfection of GFP-only or GFP fusion plasmids.
FIG. 3.
mtCLIC overexpression induces apoptosis in keratinocytes. (A) SP1 keratinocytes were transfected with GFP or GFP-mtCLIC; 48 h later, cells were fixed in neutral buffered formalin and stained with DAPI. Samples were analyzed in a fluorescence microscope. Many cells expressing high levels of mtCLIC displayed morphological features characteristic of apoptotic cells, including cell rounding, condensed nuclei (arrowheads), and bleb formation from the membrane (arrows). A few condensed nuclei were also detected in GFP-transfected cells (GFP control). (B) Transmission electron micrographs of thin sections through a normal S1 keratinocyte transfected with the GFP vector (left panel) or a typical apoptotic S1 cell transfected with mtCLIC-GFP (right panel) and fixed after 48 h. Apoptotic cells are smaller and have condensed and fragmented nuclei, reduced or absent tonofilaments, and ruffling of the plasma membrane. Bar = 5 μm. (C) Apoptotic cells were detected by the presence of annexin V on their membranes. SP1 keratinocytes transfected with GFP control plasmids or mtCLIC-GFP or GFP-mtCLIC fusion proteins were collected 24 and 48 h after transfection. Cells were labeled with annexin V antibody as described in Materials and Methods and analyzed by FACS analysis. A minimum of 20,000 transfected cells were collected for each sample analyzed. The green/transfected population was gated, and the percentage of annexin V-positive cells was calculated. Duplicate dishes were analyzed. The data are representative of two independent experiments; error bars show standard deviations.
A total of 70 to 85% of SP1 cells expressing the GFP-mtCLIC construct, but not GFP alone, became annexin V positive by 48 h after transfection (Fig. 3C). Both groups of cells demonstrated increased annexin V staining compared to nontransfected controls at 24 h, suggesting that the transfection procedure results in a finite level of apoptosis. To determine whether apoptosis occurs through a defined pathway, the caspase inhibitor Z-VAD-FMK was added to the culture medium either immediately after transfection of the fusion protein (prior to expression) or 24 h later (when the fusion protein is accumulating). The addition of Z-VAD-FMK at either time prevented the loss of the population expressing a high level of GFP-mtCLIC (Table 1), suggesting that the overexpression of mtCLIC leads to caspase activation.
TABLE 1.
Cell death induced by overexpression of mtCLIC-GFP fusion proteins is blocked by caspase inhibitor Z-VAD-FMKa
| Construct | Fluorescence intensity determined by FACS analysis (mean ± SD % of transfected cells) for the following expressors:
|
||
|---|---|---|---|
| Low | Medium | High | |
| GFP-C1 | 38 ± 1 | 35 ± 3 | 27 ± 2 |
| GFP-mtCLIC | 48 ± 5 | 42 ± 5 | 10 ± 2 |
| GFP-C1/ZVAD | 40 ± 4 | 27 ± 1 | 33 ± 3 |
| GFP-mtCLIC/ZVAD | 33 ± 5 | 32 ± 4 | 35 ± 2 |
| GFP-C1/ZVAD-24 | 47 ± 3 | 24 ± 2 | 29 ± 3 |
| GFP-mtCLIC/ZVAD-24 | 37 ± 7 | 38 ± 6 | 25 ± 4 |
| GFP-N1 | 33 ± 2 | 29 ± 3 | 38 ± 2 |
| mtCLIC-GFP | 43 ± 4 | 45 ± 4 | 12 ± 2 |
| GFP-N1/ZVAD | 31 ± 3 | 25 ± 4 | 44 ± 4 |
| mtCLIC-GFP/ZVAD | 24 ± 3 | 41 ± 4 | 35 ± 4 |
| GFP-N1/ZVAD-24 | 34 ± 3 | 21 ± 4 | 45 ± 3 |
| mtCLIC-GFP/ZVAD-24 | 27 ± 4 | 43 ± 4 | 30 ± 5 |
SP1 keratinocytes at 60% confluence were transfected for 4 h with the specified plasmid constructs. After transfection, two sets of dishes were cultured in complete medium, and one set (GFP-C1/ZVAD) was cultured in complete medium containing 40 μM Z-VAD-FMK. After 24 h, another set (GFP-C1/ZVAD-24) was refed with Z-VAD-FMK-containing medium. Fluorescence was analyzed 48 h after transfection by FACS analysis, and cells were quantitated on a logarithmic scale according to the intensity of the GFP signal. Low, medium, and high each represent 1 log difference in fluorescence intensity. GFP-C1 and GFP-N1 are the parental plasmids (pEGFP-C1 and pEGFP-N1) that produce GFP.
mtCLIC-induced apoptosis is associated with alterations in mitochondrial membrane potential and release of cytochrome c.
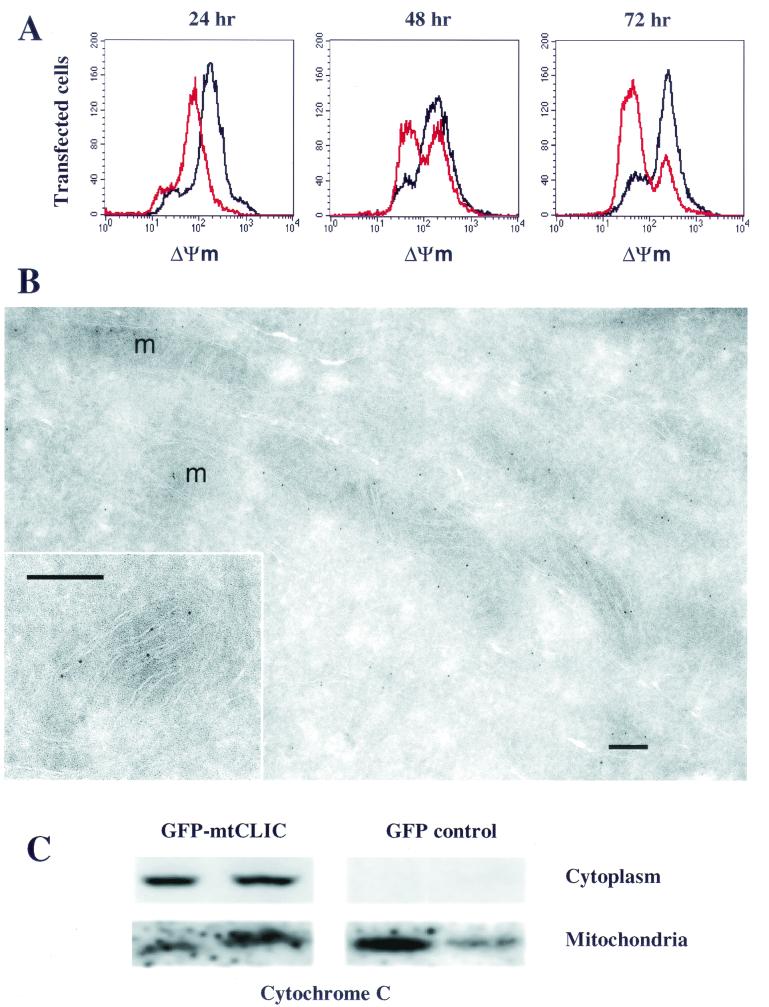
Loss of mitochondrial membrane potential precedes the opening of the permeability transition pore complex, which is considered central to apoptosis. To determine whether the overexpression of mtCLIC caused mitochondrial alterations, mitochondrial membrane potential was measured after transfection of GFP or GFP-mtCLIC plasmids into SP1 cells (Fig. 4A) and correlated with annexin V staining in the same cells (Fig. 3C). A reduction in membrane potential could be detected by 24 h in the GFP-mtCLIC transfectants, a time preceding annexin V staining (Fig. 3C). Further reduction was seen by 48 h, and by 72 h most of the transfected cells had lost mitochondrial membrane potential and were undergoing apoptosis. These changes in mitochondria may contribute to the opening of the permeability transition pore complex and the release of cytochrome c that were detected at 48 h in the cytoplasm of SP1 cells transfected with the fusion protein (Fig. 4C).
FIG. 4.
mtCLIC induces apoptosis through dissipation of mitochondrial membrane potential (ΔΨm) and release of cytochrome c. (A) SP1 keratinocytes were transfected with plasmids encoding GFP (black line) or GFP-mtCLIC (red line); 24, 48, and 72 h after transfection, cells were trypsinized and stained with Mitotracker to measure mitochondrial membrane potential. A minimum of 30,000 cells were analyzed by flow cytometry. Data were analyzed by gating the transfected cell population and plotting red fluorescence. Graphs are representative of four separate transfections. (B) Cryosections of HACAT cells were labeled with an affinity-purified antibody against the C-terminal domain of mtCLIC and visualized with protein A-gold. Label is concentrated inside mitochondria. At a higher magnification (inset), gold particles are seen in close association with the inner membrane and cristae. m, mitochondrion. Bar = 0.2 μm. (C) SP1 keratinocytes transfected with GFP or GFP-mtCLIC were collected 48 h after transfection. Cells were fractionated into mitochondrial and cytoplasmic fractions as described previously (15). Samples were analyzed for cytochrome c localization by Western blotting. Each lane represents results from an independent transfection and fractionation experiment.
To localize mtCLIC protein within the mitochondria, we performed immunoelectron microscopy on cryosections of human keratinocytes with an antibody that recognizes the C-terminal domain of this protein (Fig. 4B). Positive labeling of mitochondria was obtained, whereas control sections incubated with an unrelated antibody (see Materials and Methods) showed a complete absence of labeling. Almost all of the gold particles labeling mitochondria were in close association with cristae or the peripheral inner membrane. A few gold particles appeared to be in the matrix, without a membrane nearby; however, in such instances, the antigen could well have been in a membrane that was cut at an oblique angle and therefore was not visible in the section. There was no significant labeling of the mitochondrial outer membrane. Outside mitochondria, labeling was much more sparse (by at least 1 order of magnitude), appeared to be cytosolic, and was not associated with any membrane-bound compartment.
Overexpression of p53 causes apoptosis involving mtCLIC upregulation.
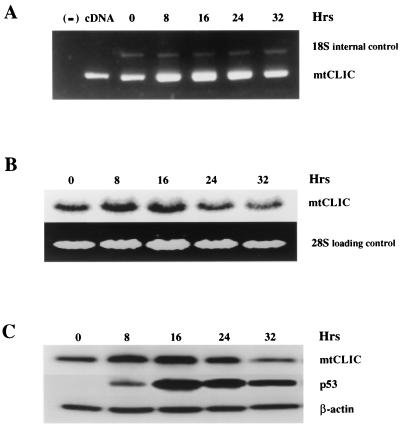
It was previously shown that overexpression of p53 in primary mouse keratinocytes by an adenovirus vector induces mtCLIC mRNA and protein (15). This activity was associated with apoptosis (data not shown). To further analyze the relationship of p53, mtCLIC, and apoptosis, we used the Saos-2 cell line, in which p53 is regulated by a tetracycline-inducible promoter (45). This strategy provides a controlled model, since the addition of doxycycline upregulates p53 and extensive apoptosis ensues. Figure 5 indicates that mtCLIC mRNA and protein were induced by doxycycline in a time course consistent with p53 induction (first detected at 8 h).
FIG. 5.
Overexpression of p53 upregulates mtCLIC and induces apoptosis. p53 Tet-On Saos-2 cells were treated with doxycycline, and the time course of mtCLIC mRNA induction was determined by RT-PCR detection with primers for mtCLIC RNA and mouse 18S RNA as an internal control (A) or by Northern blotting (B). The negative control (lane −) in panel A contained no DNA, and the positive control (lane cDNA) contained mtCLIC cDNA. (C) p53 Tet-On Saos-2 cells were treated with doxycycline to induce p53 expression and apoptosis. Protein samples were collected at different times after induction and analyzed by Western blotting with β-actin as a loading control.
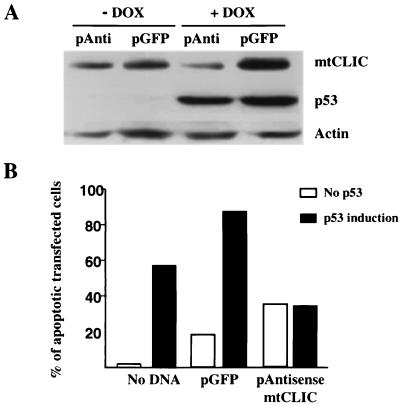
In order to determine whether mtCLIC is required for p53-induced apoptosis, we generated a plasmid in which the mtCLIC open reading frame was cloned in the antisense orientation. Transfection of antisense mtCLIC into p53 Tet-On Saos-2 cells suppressed doxycycline-induced mtCLIC without altering p53 induction (Fig. 6A). Under these conditions, apoptosis was inhibited after the induction of p53 by doxycycline (Fig. 6B). These data suggest that the upregulation of mtCLIC is involved in p53-induced apoptosis. Transfection of antisense mtCLIC is lethal over time, and we have been unable to generate stable cell lines expressing this construct (data not shown). These data suggest that mtCLIC has an essential function in cells and that deprivation of mtCLIC is incompatible with cell survival.
FIG. 6.
mtCLIC activity is an important component of p53-induced apoptosis. (A) Western blot of mtCLIC, p53, and β-actin from lysates of p53 Tet-On Saos-2 cells transfected with the GFP spectrum plasmid (pGFP) or the antisense plasmid (pAnti) for mtCLIC and treated with doxycycline (DOX) to induce p53 expression. Upregulation of mtCLIC but not p53 was blocked by the antisense plasmid. (B) p53 Tet-On Saos-2 cells were transfected with the GFP spectrum plasmid alone or in combination with the antisense mtCLIC plasmid. After 24 h, transfected cells were treated with doxycycline. Cells were collected 24 h later by trypsinization, fixed overnight in 70% ethanol, and stained with PI. DNA content was analyzed by flow cytometry, and sub-G1 cells were quantified by gating green fluorescent cells. A minimum of 10,000 transfected cells were analyzed for each sample. Results shown are representative of three independent experiments.
mtCLIC cooperates with Bax to induce apoptosis.
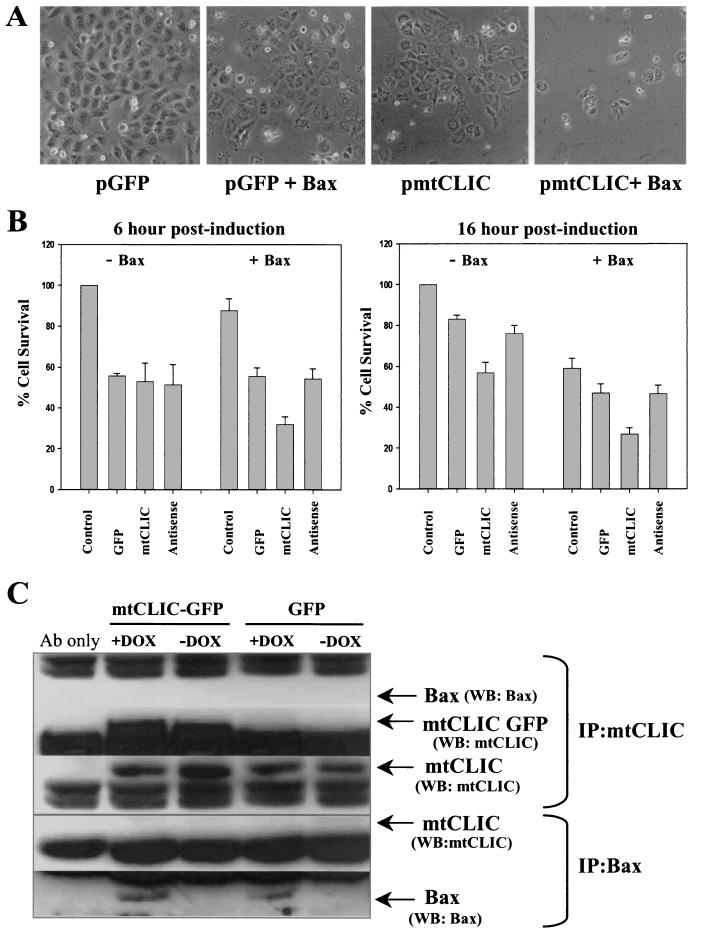
Bax and mtCLIC are both regulated by p53 and share other properties, such as cytoplasmic and mitochondrial localization, putative anion channel activity, and proapoptotic function. The Bax Tet-On Saos-2 cell line (40) was transfected with plasmids encoding mtCLIC in the sense and antisense orientations. Bax expression was induced by doxycycline, and viability was assessed by morphological analysis and by the MTT assay. Bax and mtCLIC cooperated in the induction of apoptosis. As shown in Fig. 7A, the overexpression of Bax and mtCLIC together for 22 h induced extensive cell death compared with the results obtained for controls expressing GFP and Bax or mtCLIC alone. Analysis of cell viability by the MTT assay showed that the coexpression of Bax and mtCLIC caused substantial cell death after only 6 h (Fig. 7B), while cell death above that associated with transfection was not detected in the controls. Bax (45) and mtCLIC were lethal individually by 16 h, but the combination was more lethal. Downregulation of mtCLIC by transfection of the antisense plasmid did not prevent Bax-induced apoptosis, suggesting that mtCLIC and Bax function through separate pathways and that their proapoptotic activities are additive. Similarly, these results indicate that mtCLIC levels do not influence a potential independent biological effect of doxycycline. Immunoprecipitation of lysates from cells overexpressing mtCLIC and in which Bax expression was induced failed to coprecipitate both proteins, while endogenous mtCLIC and exogenous mtCLIC were precipitated without Bax and Bax was precipitated without mtCLIC (Fig. 7C). These studies indicated that mtCLIC and Bax cooperate in the induction of apoptosis without a physical association detectable by coimmunoprecipitation.
FIG. 7.
mtCLIC cooperates with Bax in the induction of apoptosis without a physical association. The Bax Tet-On Saos-2 cell line was transfected with plasmids encoding the GFP spectrum alone or in combination with either sense mtCLIC or antisense mtCLIC. At 24 h after transfection, cells were treated with doxycycline to induce Bax expression. Cells were analyzed at different time points after Bax induction. (A) Bax or mtCLIC individually induced similar levels of apoptosis, but viable cells were rare in cultures coexpressing Bax and mtCLIC. Photographs shown were taken 22 h after Bax induction and are representative of three independent transfections. (B) Cell survival in the transfected cells was assessed by the MTT assay at 6 h (left panel) and 16 h (right panel) after Bax induction; survival for untransfected-uninduced samples was set at 100%. Data are the means of two independent experiments performed in duplicate. Error bars indicate standard deviations. (C) Immunoprecipitation (IP) and Western blotting (WB) were carried out with transfected cells and affinity-purified antibody (Ab) to mtCLIC or antibody DO-1, which recognizes a p53 sequence tag in the induced Bax protein. mtCLIC and Bax were immunoprecipitated independently, even in the samples overexpressing both proteins, but coimmunoprecipitation was not detected. DOX, doxycycline.
The region upstream of the mtCLIC gene contains functional p53-binding sites.
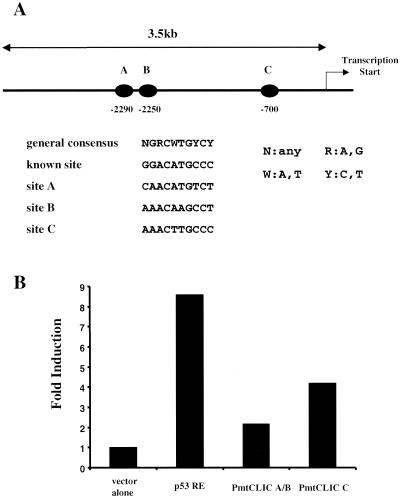
An examination of the public human genome database for sequences in the region upstream of the human mtCLIC gene on chromosome 1 revealed three dexamers highly homologous to consensus p53-binding sites lying 2,290, 2,250, and 700 bp upstream from the transcription start site (Fig. 8). A 3.5-kb region was cloned from human genomic DNA, and inserts from subclones containing the two distal elements (A and B in Fig. 8A) and the proximal element (C in Fig. 8A) were ligated into a reporter vector encoding SEAP. Reporter plasmids were transfected into p53 Tet-On Saos-2 cells, and p53 was induced by doxycycline. Figure 8B indicates that the reporters containing distal elements A and B and proximal element C were responsive to p53 induction. The level of response was below that of the positive control vector encoding two different p53 response elements (28). However, the increase was consistent with that detected for mtCLIC transcripts in response to doxycycline treatment of p53 Tet-On Saos-2 cells. These results indicate that mtCLIC transcription is directly responsive to p53 induction, although determination of the precise contribution of each of the three elements and adjacent sequences will require additional experimentation.
FIG. 8.
The region upstream of the human mtCLIC gene contains functional p53-binding sites. (A) Schematic localization of putative p53-binding sites (solid ovals) in a 3.5-kb region upstream of the human mtCLIC transcription start site. Sequence analysis indicates that these sites are more than 90% homologous to known p53-binding consensus sequences. (B) Fragments encompassing elements A and B (PmtCLIC A/B) and C (PmtCLIC C) were cloned into the pSEAP-basic reporter vector system along with a p53 response element (p53 RE) (positive control) (28) and transfected into p53 Tet-On Saos-2 cells. After 12 h, doxycycline was added to the culture medium, and medium was collected 24 h later and assayed for SEAP activity. Results represent the fold increase over the results obtained with the vector alone after subtraction of values from duplicate transfected cultures not induced by doxycycline. All values are corrected for cell numbers. Comparable results were obtained in three separate transfection experiments.
DISCUSSION
Induction of apoptosis and removal of genetically damaged cells are likely to be major components of p53 tumor suppressor activity, and mtCLIC appears to be involved in these processes. mtCLIC upregulation in programmed cell death occurs through at least two mechanisms. The upregulation of mtCLIC detected after etoposide-induced DNA damage in keratinocytes is posttranscriptional and can be independent of p53, since it occurs in p53-null keratinocytes. Adriamycin also increases mtCLIC protein expression without upregulating mtCLIC mRNA. Our studies with primary keratinocytes and normal and neoplastic keratinocyte cell lines suggest that the upregulation of mtCLIC contributes to the initiation of apoptosis, since mtCLIC expression is increased in response to DNA damage before the onset of apoptotic markers. Alternatively, apoptosis resulting directly from the upregulation of p53 involves increases in both mtCLIC mRNA and mtCLIC protein expression coincident with the upregulation of p53 and is inhibited by prevention of mtCLIC upregulation with antisense suppression. In this scenario, mtCLIC appears to be a direct transcriptional target for p53, as we have identified several functional p53-binding consensus elements in the mtCLIC promoter sequences. Of likely relevance to the posttranslational upregulation of mtCLIC after etoposide-induced DNA damage is the recent report indicating that the elevation of p53 expression in the context of blocked DNA synthesis impairs the transcriptional activity of the p53 protein (17). This is an expected consequence of etoposide treatment (53).
Mitochondria are likely to be at least one of the critical targets for mtCLIC action, as the overexpression of mtCLIC causes the loss of mitochondrial membrane potential, the release of cytochrome c, and caspase activation. In addition, mtCLIC associates with the inner mitochondrial membrane and may complement the action of Bax on the outer membrane to enhance the apoptotic response. Together, these data imply that mtCLIC is a new component in the multifaceted pathways through which cells regulate life and death in response to environmental stress.
The mtCLIC gene belongs to a diverse set of p53-regulated genes involved in apoptosis or growth control.
Several genes regulated by p53 mediate cell cycle arrest following DNA damage or stress (27). For example, p21/WAF1 (14, 21) and GADD45 (25) participate in G1 cell cycle arrest. A G2/M checkpoint may be mediated in part by 14-3-3 σ (23). Likewise, p53-dependent apoptosis may involve Bax (33); PIG proteins related to oxidative stress (41); mitochondrial proteins, such as Noxa (36), p53AIP1 (37), PUMA (34, 58), and mtCLIC; or a membrane protein, PERP (3). Induction of a combination of these proteins by p53 may result in effective induction of cell death, and each one probably can account for only part of the full apoptotic response to p53 activation.
mtCLIC-mediated cell death is associated with the dissipation of mitochondrial membrane potential and the release of cytochrome c and is inhibited by Z-VAD-FMK, suggesting the involvement of caspases downstream of mtCLIC. Similarly, Bax (33) and the recently reported BH3-only p53-regulated Noxa (36) induce the loss of mitochondrial membrane potential and the release of cytochrome c. In Bax Tet-On Saos-2 cells, Bax and mtCLIC cooperate in the induction of apoptosis without a direct physical association. In addition, coimmunoprecipitation of Bax and mtCLIC was not detected in keratinocytes (data not shown), nor was Noxa coprecipitated with Bax in HeLa cells (36). However, Noxa could interact with antiapoptotic members of the Bcl-2 family (36). It remains to be seen whether mtCLIC interacts with other Bcl-2 family members, but its association with the inner mitochondrial membrane suggests that it has a mechanism of action different from that of the BH-domain family. Since p53-mediated apoptosis may occur when Bax is genetically deleted (26) and Bax is not always detected during p53-mediated apoptosis (41), mtCLIC may serve as an alternate regulator of ion flux and volume in mitochondria or other organelles. Induction of mtCLIC by etoposide and by tumor necrosis factor alpha (15) in p53-null keratinocytes suggests that mtCLIC may have a broader role in cell death pathways or stress responses through multiple independent regulatory mechanisms.
mtCLIC supports a relationship for ion regulation and apoptosis.
The organelle location, apparent membrane association, and putative pore-forming and ion transport activities of mtCLIC are consistent with a role for the opening of channels in mitochondrial or other intracellular membranes in the apoptotic response (18, 29). mtCLIC belongs to a growing family of chloride channels, the CLIC family, with seven known members: p64 (31), CLIC-1 (55), CLIC-2 (22), CLIC-3 (43), CLIC-4/mtCLIC (8, 12, 15), CLIC-5 (6), and parchorin (35). All the members share extensive homology in their pore-forming portion but diverge in the N and C termini, and each displays a unique intracellular distribution. Intracellular localization signals at the N and C termini have been described for p64 (44), but the sequence of mtCLIC fails to yield a definitive target signal (15). p64H1, the rat homologue of mtCLIC in the endoplasmic reticulum, is reported to participate in chloride channel regulation when endoplasmic reticulum vesicles are incorporated into lipid bilayers (11). Recombinant CLIC-1 forms chloride channels in artificial membranes (54). Intracellular chloride channels can act in concert with the electrogenic proton pump, regulating the pH of organelles and influencing critical functions (8). These data suggest a role for mtCLIC in mitochondrial electron transport, i.e., interaction with the proton pump to regulate pH and mitochondrial function, a role consistent with the lethal consequences of a substantial reduction of mtCLIC protein levels by antisense expression. A similar mechanism may underlie the induction of apoptosis by elevated mtCLIC levels. Vander Heiden and collaborators (56) have shown alterations in mitochondrial activity during apoptosis that lead to a defect in mitochondrial ADP-ATP exchange. In addition, changes in pH occur early during apoptosis and may play a role in driving the subsequent biochemical events (24).
Stabilization of mtCLIC may contribute to the apoptotic response.
During etoposide- or adriamycin-induced apoptosis in keratinocytes, the level of mtCLIC protein increases while that of mtCLIC mRNA decreases, implying that mtCLIC protein is stabilized after DNA damage. Posttranslational modifications of mtCLIC may contribute to protein stabilization, subcellular distribution, and function. For example, phosphorylated Bad is sequestered in the cytosol by 14-3-3 proteins and, upon desphosphorylation, translocates to mitochondria to induce cell death (1). For some members of the CLIC family, activity is regulated by phosphorylation. p64-associated chloride channel activity is enhanced by the coexpression of p59fyn, a Src family tyrosine kinase (13). CLIC-3 interacts with ERK-7, a mitogen-activated protein kinase (43). mtCLIC has several consensus phosphorylation sites (15), including those for protein kinases A and C, casein kinase 2, and tyrosine kinase. Two potential N myristoylation sites are also present. Furthermore, the diverse locations of CLIC family members in specific cell types (6) and their potential to translocate suggest that interactions with chaperone proteins, such as A kinase-anchoring proteins, could influence intracellular location and function (6, 16).
mtCLIC appears to be a direct transcriptional target for p53 and required for the induction of apoptosis by p53, since antisense mtCLIC prevents the upregulation of mtCLIC and blocks p53-induced apoptosis in p53 Tet-On Saos-2 cells. Several genes that are involved in the induction of apoptosis by p53 have now been described, and these are likely to lead to an understanding of the molecular mechanism involved in p53-induced apoptosis. It is likely that p53 utilizes a combination of transcription-dependent and independent mechanisms to efficiently induce apoptosis. The effector genes downstream of p53 may vary among cell types and stimuli. None of the genes by themselves can account for the full apoptotic response, and their products could be shared in pathways that are independent of p53. mtCLIC is now defined as an important effector of apoptosis with a known biochemical function involved in p53-dependent and independent pathways.
Acknowledgments
We thank Karen Vousden and Kevin Ryan of the National Cancer Institute for generously supplying the Saos-2 cell lines, Adam Glick for critical review of the manuscript, and Bettie Sugar and Mary Velthuis for editorial assistance.
REFERENCES
- 1.Adams, J. M., and S. Cory. 1998. The Bcl-2 protein family: arbiters of cell survival. Science 281:1322-1326. [DOI] [PubMed] [Google Scholar]
- 2.Allen, R. T., W. J. Hunter, I. I. I., and D. K. Agrawal. 1997. Morphological and biochemical characterization and analysis of apoptosis. J. Pharmacol. Toxicol. Methods 37:215-228. [DOI] [PubMed] [Google Scholar]
- 3.Attardi, L. D., E. E. Reczek, C. Cosmas, E. G. Demicco, M. E. McCurrach, S. W. Lowe, and T. Jacks. 2000. PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes Dev. 14:704-718. [PMC free article] [PubMed] [Google Scholar]
- 4.Azzoli, C. G., M. Sagar, A. Wu, D. Lowry, H. Hennings, D. L. Morgan, and W. C. Weinberg. 1998. Cooperation of p53 loss of function and v-Ha-ras in transformation of mouse keratinocyte cell lines. Mol. Carcinog. 21:50-61. [DOI] [PubMed] [Google Scholar]
- 5.Bates, S., and K. H. Vousden. 1996. p53 in signaling checkpoint arrest or apoptosis. Curr. Opin. Genet. Dev. 6:12-18. [DOI] [PubMed] [Google Scholar]
- 6.Berryman, M., and A. Bretscher. 2000. Identification of a novel member of the chloride intracellular channel gene family (CLIC5) that associates with the actin cytoskeleton of placental microvilli. Mol. Biol. Cell 11:1509-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner, C., and G. Kroemer. 2000. Apoptosis. Mitochondria-the death signal integrators. Science 289:1150-1151. [DOI] [PubMed] [Google Scholar]
- 8.Chuang, J. Z., T. A. Milner, M. Zhu, and C. H. Sung. 1999. A 29 kDa intracellular chloride channel p64H1 is associated with large dense-core vesicles in rat hippocampal neurons. J. Neurosci. 19:2919-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colman, M. S., C. A. Afshari, and J. C. Barrett. 2000. Regulation of p53 stability and activity in response to genotoxic stress. Mutat. Res. 462:179-188. [DOI] [PubMed] [Google Scholar]
- 10.Dlugosz, A. A., A. B. Glick, T. Tennenbaum, W. C. Weinberg, and S. H. Yuspa. 1995. Isolation and utilization of epidermal keratinocytes for oncogene research. Methods Enzymol. 254:3-20. [DOI] [PubMed] [Google Scholar]
- 11.Duncan, R. R., P. K. Westwood, A. Boyd, and R. H. Ashley. 1997. Rat brain p64H1, expression of a new member of the p64 chloride channel protein family in endoplasmic reticulum. J. Biol. Chem. 272:23880-23886. [DOI] [PubMed] [Google Scholar]
- 12.Edwards, J. C. 1999. A novel p64-related Cl-channel: subcellular distribution and nephron segment-specific expression. Am. J. Physiol. 276:F398-F408. [DOI] [PubMed] [Google Scholar]
- 13.Edwards, J. C., and S. Kapadia. 2000. Regulation of the bovine kidney microsomal chloride channel p64 by p59fyn, a Src family tyrosine kinase. J. Biol. Chem. 275:31826-31832. [DOI] [PubMed] [Google Scholar]
- 14.El-Deiry, W. S., W. S. Tokino, V. E. Velculescu, D. B. Levy, R. Parsons, J. M. Trent, D. Lin, W. E. Mercer, K. W. Kinzler, and B. Vogelstein. 1993. WAF1, a potential mediator of p53 tumor suppression. Cell 75:817-825. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Salas, E., M. Sagar, C. Cheng, S. H. Yuspa, and W. C. Weinberg. 1999. p53 and tumor necrosis factor α regulate the expression of a mitochondrial chloride channel protein. J. Biol. Chem. 274:36488-36497. [DOI] [PubMed] [Google Scholar]
- 16.Fraser, I. D., M. Cong, J. Kim, E. N. Rollins, Y. Daaka, R. J. Lefkowitz, and J. D. Scott. 2000. Assembly of an A kinase-anchoring protein-beta(2)-adrenergic receptor complex facilitates receptor phosphorylation and signaling. Curr. Biol. 10:409-412. [DOI] [PubMed] [Google Scholar]
- 17.Gottifredi, V., S. Shieh, Y. Taya, and C. Prives. 2001. From the Cover: p53 accumulates but is functionally impaired when DNA synthesis is blocked. Proc. Natl. Acad. Sci. USA 98:1036-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green, D. R., and J. C. Reed. 1998. Mitochondria and apoptosis. Science 281:1309-1312. [DOI] [PubMed] [Google Scholar]
- 19.Gurney, E. G., R. O. Harrison, and J. Fenno. 1980. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct subclasses of large T antigen and for similarities among nonviral T antigens. J. Virol. 34:752-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 100:57-70. [DOI] [PubMed] [Google Scholar]
- 21.Harper, J. W., G. R. Adami, N. Wei, K. Keyomarsi, and S. J. Elledge. 1993. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75:805-816. [DOI] [PubMed] [Google Scholar]
- 22.Heiss, N. S., and A. Poustka. 1997. Genomic structure of a novel chloride channel gene, CLIC2, in Xq28. Genomics 45:224-228. [DOI] [PubMed] [Google Scholar]
- 23.Hermeking, H., C. Lengauer, K. Polyak, T. C. He, L. Zhang, S. Thiagalingam, K. W. Kinzler, and B. Vogelstein. 1997. 14-3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol. Cell 1:3-11. [DOI] [PubMed] [Google Scholar]
- 24.Johnson, D. E. 2000. Programmed cell death regulation: basic mechanisms and therapeutic opportunities. Leukemia 14:1340-1344. [DOI] [PubMed] [Google Scholar]
- 25.Kastan, M. B., Q. Zhan, W. S. El-Deiry, F. Carrier, T. Jacks, W. V. Walsh, B. S. Plunkett, B. Vogelstein, and A. J. Fornace, Jr. 1992. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 71:587-597. [DOI] [PubMed] [Google Scholar]
- 26.Knudson, C. M., K. S. Tung, W. G. Tourtellotte, G. A. Brown, and S. J. Korsmeyer. 1995. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 270:96-99. [DOI] [PubMed] [Google Scholar]
- 27.Ko, L. J., and C. Prives. 1996. p53: puzzle and paradigm. Genes Dev. 10:1054-1072. [DOI] [PubMed] [Google Scholar]
- 28.Komarova, E. A., M. V. Chernov, R. Franks, K. Wang, G. Armin, C. R. Zelnick, D. M. Chin, S. S. Bacus, G. R. Stark, and A. V. Gudkov. 1997. Transgenic mice with p53-responsive lacZ: p53 activity varies dramatically during normal development and determines radiation and drug sensitivity in vivo. EMBO J. 16:1391-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroemer, G., and J. C. Reed. 2000. Mitochondrial control of cell death. Nat. Med. 6:513-519. [DOI] [PubMed] [Google Scholar]
- 30.Lakin, N. D., and S. P. Jackson. 1999. Regulation of p53 in response to DNA damage. Oncogene 18:7644-7655. [DOI] [PubMed] [Google Scholar]
- 31.Landry, D., S. Sullivan, M. Nicolaides, C. Redhead, A. Edelman, M. Field, Q. al Awqati, and J. Edwards. 1993. Molecular cloning and characterization of p64, a chloride channel protein from kidney microsomes. J. Biol. Chem. 268:14948-14955. [PubMed] [Google Scholar]
- 32.Levine, A. J. 1997. p53, the cellular gatekeeper for growth and division. Cell 88:323-331. [DOI] [PubMed] [Google Scholar]
- 33.Miyashita, T., and J. C. Reed. 1995. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80:293-299. [DOI] [PubMed] [Google Scholar]
- 34.Nakano, K., and K. H. Vousden. 2001. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell 7:683-694. [DOI] [PubMed] [Google Scholar]
- 35.Nishizawa, T., T. Nagao, T. Iwatsubo, J. G. Forte, and T. Urushidani. 2000. Molecular cloning and characterization of a novel chloride intracellular channel-related protein, parchorin, expressed in water-secreting cells. J. Biol. Chem. 275:11164-11173. [DOI] [PubMed] [Google Scholar]
- 36.Oda, E., R. Ohki, H. Murasawa, J. Nemoto, T. Shibue, T. Yamashita, T. Tokino, T. Taniguchi, and N. Tanaka. 2000. Noxa, a BH3-only member of the bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288:1053-1058. [DOI] [PubMed] [Google Scholar]
- 37.Oda, K., H. Arakawa, T. Tanaka, K. Matsuda, C. Tanikawa, T. Mori, H. Nishimori, K. Tamai, T. Tokino, Y. Nakamura, and Y. Taya. 2000. p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell 102:849-862. [DOI] [PubMed] [Google Scholar]
- 38.Oren, M. 1994. Relationship of p53 to the control of apoptotic cell death. Semin. Cancer Biol. 5:221-227. [PubMed] [Google Scholar]
- 39.Peters, P. J., and W. Hunziker. 2001. Subcellular localization of Rab17 by cryo-immunogold electron microscopy in epithelial cells grown on polycarbonate filters. Methods Enzymol. 329:210-225. [DOI] [PubMed] [Google Scholar]
- 40.Phillips, A. C., S. Bates, K. M. Ryan, K. Helin, and K. H. Vousden. 1997. Induction of DNA synthesis and apoptosis are separable functions of E2F-1. Genes Dev. 11:1853-1863. [DOI] [PubMed] [Google Scholar]
- 41.Polyak, K., Y. Xia, J. L. Zweier, K. W. Kinzler, and B. Vogelstein. 1997. A model for p53-induced apoptosis. Nature 389:300-305. [DOI] [PubMed] [Google Scholar]
- 42.Prives, C., and P. A. Hall. 1999. The p53 pathway. J. Pathol. 187:112-126. [DOI] [PubMed] [Google Scholar]
- 43.Qian, Z., D. Okuhara, M. K. Abe, and M. R. Rosner. 1999. Molecular cloning and characterization of a mitogen-activated protein kinase-associated intracellular chloride channel. J. Biol. Chem. 274:1621-1627. [DOI] [PubMed] [Google Scholar]
- 44.Redhead, C., S. K. Sullivan, C. Koseki, K. Fujiwara, and J. C. Edwards. 1997. Subcellular distribution and targeting of the intracellular chloride channel p64. Mol. Biol. Cell 8:691-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryan, K. M., M. K. Ernst, N. R. Rice, and K. H. Vousden. 2000. Role of NF-kappaB in p53-mediated programmed cell death. Nature 404:892-897. [DOI] [PubMed] [Google Scholar]
- 46.Schmitt, C. A., and S. W. Lowe. 1999. Apoptosis and therapy. J. Pathol. 187:127-137. [DOI] [PubMed] [Google Scholar]
- 47.Scholl, F. A., P. McLoughlin, E. Ehler, C. de Giovanni, and B. W. Schafer. 2000. DRAL is a p53-responsive gene whose four and a half LIM domain protein product induces apoptosis. J. Cell Biol. 151:495-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schuler, M., E. Bossy-Wetzel, J. C. Goldstein, P. Fitzgerald, and D. R. Green. 2000. p53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. J. Biol. Chem. 275:7337-7342. [DOI] [PubMed] [Google Scholar]
- 49.Seth, P., U. Brinkmann, G. N. Schwartz, D. Katayose, R. Gress, I. Pastan, and K. Cowan. 1996. Adenovirus-mediated gene transfer to human breast tumor cells: an approach for cancer gene therapy and bone marrow purging. Cancer Res. 56:1346-1351. [PubMed] [Google Scholar]
- 50.Sheikh, M. S., and A. J. Fornace, Jr. 2000. Role of p53 family members in apoptosis. J. Cell Physiol. 182:171-181. [DOI] [PubMed] [Google Scholar]
- 51.Speransky, V. V., K. L. Taylor, H. K. Edskes, R. B. Wickner, and A. C. Steven. 2001. Prion filament networks in [URE3] cells of Saccharomyces cerevisiae. J. Cell Biol. 153:1327-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strickland, J. E., D. A. Greenhalgh, A. Koceva-Chyla, H. Hennings, C. Restrepo, M. Balaschak, and S. H. Yuspa. 1988. Development of murine epidermal cell lines which contain an activated rasHa oncogene and form papillomas in skin grafts on athymic nude mouse hosts. Cancer Res. 48:165-169. [PubMed] [Google Scholar]
- 53.Takimoto, R., and W. S. el Deiry. 2001. DNA replication blockade impairs p53-transactivation. Proc. Natl. Acad. Sci. USA 98:781-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tulk, B. M., P. H. Schlesinger, S. A. Kapadia, and J. C. Edwards. 2000. CLIC-1 functions as a chloride channel when expressed and purified from bacteria. J. Biol. Chem. 275:26986-26993. [DOI] [PubMed] [Google Scholar]
- 55.Valenzuela, S. M., D. K. Martin, S. B. Por, J. M. Robbins, K. Warton, M. R. Bootcov, P. R. Schofield, T. J. Campbell, and S. N. Breit. 1997. Molecular cloning and expression of a chloride ion channel of cell nuclei. J. Biol. Chem. 272:12575-12582. [DOI] [PubMed] [Google Scholar]
- 56.Vander Heiden, M. G., N. S. Chandel, P. T. Schumacker, and C. B. Thompson. 1999. Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol. Cell 3:159-167. [DOI] [PubMed] [Google Scholar]
- 57.Weinberg, W. C., C. G. Azzoli, N. Kadiwar, and S. H. Yuspa. 1994. p53 gene dosage modifies growth and malignant progression of keratinocytes expressing the v-rasHa oncogene. Cancer Res. 54:5584-5592. [PubMed] [Google Scholar]
- 58.Yu, J., L. Zhang, P. M. Hwang, K. W. Kinzler, and B. Vogelstein. 2001. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol. Cell 7:673-682. [DOI] [PubMed] [Google Scholar]