Abstract
Leukemia-associated Rho guanine-nucleotide exchange factor (LARG) belongs to the subfamily of Dbl homology RhoGEF proteins (including p115 RhoGEF and PDZ-RhoGEF) that possess amino-terminal regulator of G protein signaling (RGS) boxes also found within GTPase-accelerating proteins (GAPs) for heterotrimeric G protein α subunits. p115 RhoGEF stimulates the intrinsic GTP hydrolysis activity of Gα12/13 subunits and acts as an effector for G13-coupled receptors by linking receptor activation to RhoA activation. The presence of RGS box and Dbl homology domains within LARG suggests this protein may also function as a GAP toward specific Gα subunits and couple Gα activation to RhoA-mediating signaling pathways. Unlike the RGS box of p115 RhoGEF, the RGS box of LARG interacts not only with Gα12 and Gα13 but also with Gαq. In cellular coimmunoprecipitation studies, the LARG RGS box formed stable complexes with the transition state mimetic forms of Gαq, Gα12, and Gα13. Expression of the LARG RGS box diminished the transforming activity of oncogenic G protein-coupled receptors (Mas, G2A, and m1-muscarinic cholinergic) coupled to Gαq and Gα13. Activated Gαq, as well as Gα12 and Gα13, cooperated with LARG and caused synergistic activation of RhoA, suggesting that all three Gα subunits stimulate LARG-mediated activation of RhoA. Our findings suggest that the RhoA exchange factor LARG, unlike the related p115 RhoGEF and PDZ-RhoGEF proteins, can serve as an effector for Gq-coupled receptors, mediating their functional linkage to RhoA-dependent signaling pathways.
Four classes of heterotrimeric G alpha proteins, Gs, Gi, Gq, and G12, couple heptahelical G protein-coupled receptors (GPCRs) to effectors to relay extracellular signals into eukaryotic cells (14). Each of these heterotrimeric G proteins is composed of an α, β, and γ subunit. The GDP-bound heterotrimer is an inactive form of the G protein. Ligand-bound, activated receptors catalyze the exchange of GDP by GTP on the Gα subunit, leading to heterotrimer dissociation and activation of signaling pathways by the separated G protein subunits (Gα and Gβγ).
An important mechanism used to control the duration and sensitivity of G protein-mediated signaling is alteration of the intrinsic GTPase activity of Gα subunits. Regulator of G protein signaling (RGS) proteins are a newly described superfamily of G protein signaling modulators that each contains a conserved domain (the RGS box) that interacts specifically with activated Gα subunits. Biochemical studies have demonstrated that the RGS box functions primarily as a GTPase-accelerating protein (GAP) for Gα subunits, accelerating their intrinsic GTPase activities (32, 36). Most of the characterized RGS proteins inhibit signaling pathways that use Gαi and Gαq heterotrimers as signal transducers. However, p115 RhoGEF/Lsc, a guanine nucleotide exchange factor (GEF) for the small GTPase RhoA (11, 16), has recently been shown to have an RGS box with GAP activity for Gα12 and Gα13 (24) that can block the signaling and/or transforming activity of GPCRs coupled to Gα12/13 (28, 35, 45).
Similar to the Gα subunits, the Rho family of small GTPases are guanine nucleotide binding proteins that function as molecular switches that cycle between active GTP- and inactive GDP-bound states (3, 40). The best-characterized members of this family are RhoA, Rac1, and Cdc42. Rho family proteins mediate a wide range of cellular activities that include actin cytoskeletal reorganization as well as cell growth and transformation (13, 47). Two major classes of regulatory proteins modulate cellular control of the GDP/GTP cycle of this family of proteins. Dbl family proteins act as GEFs to promote formation of the active GTP-bound protein, whereas Rho GTPase-activating proteins stimulate the intrinsic GTPase activity to convert these small GTPases to their inactive GDP-bound states (22).
A large body of research suggests that certain cellular phenotypes elicited by GPCRs are dependent upon the activation of Rho GTPase-controlled signaling pathways. For example, various ligands that stimulate GPCRs cause activation of Rho GTPase-dependent changes in actin organization (3, 40). Moreover, various GPCRs transform NIH 3T3 cells by activation of specific Rho family members, including Mas (coupled to Gαq and Gαi), G2A (coupled to Gα13 and Gαi), PAR-1 (coupled to Gαi, Gαq, and the G12 family) and KSHV-GPCR (primarily to Gα13, also Gαq and Gαi) (20, 28, 35, 45; I. E. Zohn, M. Symons, C. J. Der, and J. Boyer, unpublished data). In addition, Gαq, Gα12, and Gα13 induce the formation of stress fibers, activation of the serum response factor (SRF), and apoptosis in fibroblast cells through the small GTPase RhoA (1, 4, 30, 45).
The mechanism by which GPCRs cause activation of RhoA has been determined for Gα13-coupled receptors. Hart et al. and Kozasa et al. reported that activated Gα13 binds to and stimulates the GEF activity of p115 RhoGEF in vitro, a Dbl homology (DH) domain-containing protein that also possesses an amino-terminal RGS box capable of GAP activity towards both Gα12 and Gα13. Thus, p115 RhoGEF serves as an effector molecule for GTP-bound Gα13, which activates the GEF function of the DH domain by a Gα13-RGS box interaction. Importantly, Gα12 did not stimulate the GEF activity of p115 RhoGEF in vitro (15, 24).
Subsequent to these studies, Fukuhara et al. and Jackson et al. reported that PDZ-RhoGEF/KIAA0380/GTRAP48, another RGS box-containing Dbl family protein and RhoA-specific GEF, also interacts with Gα12 and Gα13 (10, 19). Furthermore, coexpression of Gα12 and Gα13 enhanced PDZ-RhoGEF-mediated SRF activation. However, Wells et al. recently determined that the rat ortholog of PDZ-RhoGEF, GTRAP48, while capable of binding Gα13, neither exhibits significant GAP activity toward Gα12/13 nor is stimulated by Gα13 to increase its guanine nucleotide exchange activity in vitro (41). Thus, there is currently no clear evidence that PDZ-RhoGEF/GTRAP48 serves as an effector of Gα subunits and the Dbl family protein(s) that promotes RhoA activation by Gα subunits i, q, and 12 has not been identified.
Leukemia-associated Rho GEF (LARG) (KIAA0382) is a Dbl family member similar to PDZ-RhoGEF and p115 RhoGEF that was identified recently as a fusion partner with mixed-lineage leukemia protein in a patient with acute myeloid leukemia (23). Like all Dbl family proteins, LARG possesses a DH domain followed by a carboxy-terminal pleckstrin homology (PH) domain (5, 43). It has previously been shown that the LARG DH domain serves as a GEF for RhoA but not for Rac1 or Cdc42 (31). In addition to the tandem DH-PH domain, LARG also contains a PDZ domain likely to promote protein-protein interactions (37) and an RGS box that may function as a GTPase-activating protein for Gα subunits. In this study, we determined that the LARG RGS box is a negative regulator of Gαq, Gα12, and Gα13 in vivo and, when coexpressed with activated Gα12, Gα13, or Gαq, it synergistically enhanced LARG formation of GTP-bound RhoA and stimulation of SRF activity. These observations suggest that LARG may be an effector of Gαq in cells, linking Gq-coupled as well as Gα12- and Gα13-coupled GPCRs to RhoA activation.
MATERIALS AND METHODS
Molecular constructs.
The full-length, ΔN308, and ΔN754 LARG expression constructs have been described previously (31). Full-length LARG and p115 RhoGEF cDNA (the latter was a kind gift from Gideon Bollag, Onyx) (16) were used as templates for PCR to generate cDNA sequences encoding the isolated RGS boxes (residues 1120 to 1491 and 1 to 246, respectively) with flanking BamHI sites for subcloning into pCGN-hygro, a eukaryotic expression vector which provides an in-frame, amino-terminal hemagglutinin (HA) epitope tag. The cDNA sequence encoding full-length muscarinic acetylcholine receptor 1 (m1-mAChR; a kind gift of Ernest Peralta, Harvard) was modified by PCR to include 5′ BamHI and 3′ EcoRI restriction sites for subcloning into the BamHI and EcoRI sites of pBabe-puro eukaryotic expression vector (29).
The following pcDNA3 eukaryotic expression plasmids containing cDNA sequences encoding wild-type (wt) or GTPase-deficient constitutively activated Gα subunits were kindly provided by Henry Bourne (University of California at San Francisco): Gαq (wt), Gαq (Q229L), Gα13 (wt), Gα13 (Q226L), Gα12 (wt), and Gα12 (Q229L). All cDNA sequences were sequenced to verify the identity and accuracy of the coding sequence.
Cell culture and transformation assay.
NIH 3T3 mouse fibroblasts were cultured in Dulbecco's modified Eagle's medium supplemented with 10% calf serum (designated growth medium). DNA transfections for primary focus formation transformation assays were performed with calcium phosphate precipitation as described previously (6). For secondary focus formation transformation assays, NIH 3T3 cells stably expressing m1-mAChR alone or together with either the LARG- or the p115-RGS box were established by cotransfection of pBabe-m1-mAChR (puromycin resistant) with pCGN-LARG-RGS or pCGN-p115-RGS constructs (hygromycin resistant) and isolation of drug-resistant colonies after growth in medium supplemented with hygromycin (400 μg/μl) and puromycin (1 mg/ml). Multiple drug-resistant colonies (>100) were pooled together to establish the stable cell lines. For each transformation assay, parallel cell lines were established that contained the cognate empty vector and used as controls. Transfected cultures were maintained in growth medium for 14 days, fixed, and stained with crystal violet (0.5%), and the number of foci of transformed cells was quantitated.
Coimmunoprecipitations.
NIH 3T3 cells were cotransfected with plasmids expressing HA epitope-tagged LARG- or p115-RGS box together with wt Gα12, Gα13, and Gαq by using the Lipofectamine Plus reagent as described by the manufacturer (Life Technologies). After culture for 48 h, cells were lysed for 20 min on ice in 0.7 ml of buffer A (20 mM Tris [pH 7.5], 1 mM dithiothreitol, 100 mM NaCl, 2.5 mM MgCl2, 1 mM EGTA, 1% Triton X-100, 1 mM NaVO4, 10 μg of aprotinin/ml, and 10 μg of leupeptin/ml) or buffer A containing 20 mM NaF and 20 μM AlCl3 to activate the alpha subunits (i.e., in the presence of AlF4−). Lysates were clarified by centrifugation at 16,000 × g for 30 min and precleared for 30 min at 4°C with 30 μl of a 50% slurry of protein A-agarose beads. HA-LARG or HA-p115-RGS boxes were immunoprecipitated with anti-HA mouse monoclonal antibody (12CA5; Roche Biochemicals). Immunoprecipitates were captured on protein A-agarose beads (Life Technologies, Inc.), washed three times in their respective lysis buffers, resolved on a 4 to 20% gradient gel, and analyzed for coimmunoprecipitating proteins by immunoblotting with anti-Gαq (a gift from T. Kendall Harden, University of North Carolina at Chapel Hill), -Gα12, or -Gα13 rabbit polyclonal antiserum (Calbiochem).
Measurement of Rho protein activation in vivo.
The activation of Rho proteins in vivo was determined by using a modification of the assay originally described by Taylor and Shalloway (38). A glutathione S-transferase (GST) fusion protein containing the RhoA effector binding domain (RBD) of rhotekin (amino acids 7 to 89) (rhotekin RBD) (25) (provided by Keith Burridge, University of North Carolina at Chapel Hill) was expressed and purified by a procedure described previously (25). Seventy percent confluent cultures (100-mm-diameter dishes) of NIH 3T3 cells were transfected with LARG and Gα expression plasmids by using Lipofectamine Plus according to the manufacturer's protocol. Three hours after transfection, cells were placed in complete growth medium for 24 h and then placed in starvation medium containing 0.5% fetal bovine serum for a subsequent 24 h. After starvation, cells were rinsed once with 20 mM HEPES (pH 7.4) and 150 mM NaCl and then lysed in buffer A (50 mM Tris [pH 7], 500 mM NaCl, 0.5 mM MgCl2, 0.01% sodium dodecyl sulfate [SDS], 0.5% deoxycholate, 1% Triton X-100, 10 μg of aprotinin/ml, and 10 μg of leupeptin/ml) on ice for 10 min. Lysates were clarified by centrifugation at 16,000 × g for 4 min. Thirty micrograms of GST-rhotekin RBD fusion protein immobilized on glutathione-Sepharose 4B beads (Amersham Pharmacia) was incubated with 1 mg of cell lysate in a final volume of 0.5 ml for 30 min at 4°C. The beads were washed three times with lysis buffer A and three times with buffer B (50 mM Tris [pH 7.4], 150 mM NaCl, 1% Triton X-100, 0.5 mM MgCl2, 10 μg of aprotinin/ml, and 10 μg of leupeptin/ml), and bound proteins were eluted in protein sample buffer and analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to filters, and used for Western blot analyses. Thirty micrograms of total protein was used to confirm equal amounts of RhoA for each transfection condition. The following antibodies were used for Western blot analyses to verify expression of proteins: anti-HA (16B12; Covance), anti-RhoA (Transduction Labs), anti-Gαq (T. Kendall Harden, University of North Carolina), anti-Gα12 (Calbiochem), and anti-Gα13 (Calbiochem).
Transient expression reporter gene assays.
NIH 3T3 cells were transfected by using Lipofectamine Plus. Three hours posttransfection, cells were placed in starvation medium containing 0.5% bovine calf serum for 24 h. After starvation, cells were rinsed once with ice-cold phosphate-buffered saline and then lysed in 300 μl of 1× luciferase cell lysis buffer (Becton Dickinson Co.) on ice for 10 min. The analysis of cell lysates was performed by using enhanced chemiluminescent reagents and a Monolight 2010 luminometer (Analytical Luminescence). The (SREm)2-Luc reporter plasmid contains a luciferase gene whose expression is under the control of an SRF-responsive promoter as described previously (42). Thirty micrograms of total protein was resolved on a 4 to 20% gradient gel (Bio-Rad), and Western blot analyses were performed to verify the expression of transfected genes. All assays were performed at least in triplicate.
RESULTS
RGS box of LARG associates with Gα subunits q, 12, and 13 in vivo.
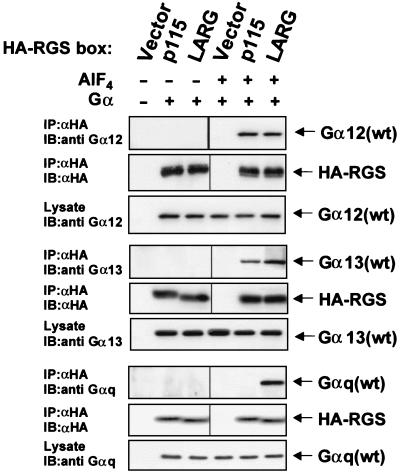
We first investigated the ability of the isolated RGS box of LARG (Fig. 1) to associate with members of the G12, Gi, and Gq family of Gα subunits. The isolated RGS box of p115 RhoGEF, which can associate with Gα12 and Gα13 but not with Gαq (24), was used as a control for these experiments. For these analyses, we transiently overexpressed the isolated RGS boxes of LARG or p115 RhoGEF (designated LARG-RGS and p115-RGS, respectively) together with different Gα subunits. An anti-HA antibody was used to immunoprecipitate the HA epitope-tagged LARG-RGS or p115-RGS from NIH 3T3 cells also coexpressing wt Gα12, Gα13, Gαq, or Gαi1. Both Gα12 and Gα13 coimmunoprecipitated with LARG-RGS and p115-RGS (Fig. 2). These interactions were observed only in the presence of aluminum tetrafluoride (AlF4−), a planar ion that binds Gα to form a transition-state mimetic to which RGS boxes have the highest affinity for Gα subunits (39, 44). Surprisingly, we found that LARG-RGS, but not p115-RGS, also interacted with Gαq in an AlF4-dependent manner. We did not observe any association of LARG-RGS or p115-RGS with Gαi1 (data not shown). Furthermore, Gα12, Gα13, or Gαq did not coprecipitate with an amino-terminal deletion mutant of LARG (ΔN754-LARG) that lacks the RGS domain, but a LARG truncation mutant lacking solely the PDZ domain (ΔN308-LARG) (Fig. 1) did bind all three Gα subtypes in an AlF4-dependent manner (data not shown). These results suggest that LARG, unlike p115 RhoGEF, binds to Gαq as well as to Gα12 and Gα13, and this interaction is mediated through the RGS box.
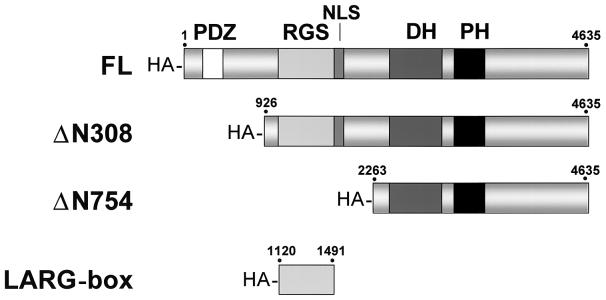
FIG. 1.
Functional domains and structural mutants of LARG. Schematic representations of full-length (FL), amino-terminal truncation mutants (ΔN308 and ΔN754), and the isolated RGS box (LARG-box) proteins are shown. Numbers correspond to amino acids. HA, HA epitope tag; PDZ, PSD-95/Discs-large/ZO-1 domain; NLS, putative nuclear localization signal; DH, DH domain; PH, PH domain.
FIG. 2.
LARG RGS box complexes with activated Gα12, Gα13, and Gαq subunits in vivo. NIH 3T3 cells were cotransfected with vectors that express HA epitope-tagged versions of the isolated RGS box of p115 RhoGEF or LARG (1 μg) and expression vectors encoding wt Gα12, Gα13, or Gαq (1 μg). Whole-cell lysates were prepared in the presence or absence of AlF4−, which activates Gα subunits to adopt a conformation mimicking the transition-state for GTP hydrolysis (44). HA-tagged RGS box proteins were immunoprecipitated (IP) from whole cell lysates (800 μg of total protein) with an anti-HA (αHA) monoclonal antibody and resolved by SDS-PAGE. Immunoprecipitated p115-RGS and LARG-RGS protein and coimmunoprecipitated Gα12, Gα13, and Gαq subunits were detected by immunoblot analyses (IB). To control for expression levels, separate aliquots of each lysate (20 μg) were taken before immunoprecipitation and resolved by SDS-PAGE and amounts of Gα12, Gα13, or Gαq were detected by immunoblot analyses.
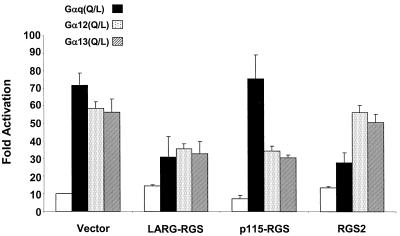
Our coimmunoprecipitation analyses suggest that the LARG RGS box associates with Gαq as well as Gα12 and Gα13 in vivo. We therefore also examined the possibility that expressing LARG-RGS could block Gαq-, Gα12-, and Gα13-mediated signaling. In addition to their capacity to promote stress fiber formation and mediate focus formation in NIH 3T3 cells, activated mutants of Gα12, Gα13, and Gαq also induce transcriptional responses characteristic of RhoA (18), such as activation of SRF and concomitant expression from the c-fos serum response element (7, 26). We performed transient transfection assays to determine if LARG-RGS could impair the ability of activated mutants of Gαq, Gα12, and Gα13 to stimulate an SRF-responsive luciferase reporter (Fig. 3). For these analyses, we included an expression vector encoding RGS2, a Gαq-specific GAP (17). NIH 3T3 cells were transiently transfected with expression vectors encoding GTPase-deficient (Q→L), constitutively activated mutants of each Gα subunit, either alone or together with expression plasmids for RGS2, p115-RGS, or LARG-RGS, and an SRF-responsive reporter gene plasmid (42). Expression of constitutively activated Gαq, Gα12, or Gα13 alone greatly enhanced SRF activity (five- to sevenfold). As expected, the SRF activities induced by Gα12 and Gα13 were suppressed by coexpression with p115-RGS but not RGS2. Conversely, Gαq-induced SRF activation was significantly decreased by RGS2 expression but not by p115 RhoGEF expression. These results are wholly consistent with the known Gα specificities of these two RGS proteins (17, 24). Consistent with our coprecipitation data, we found that coexpression of LARG-RGS caused an approximately 50% reduction in the ability of all three Gα subunits to stimulate SRF activity. These observations, when taken together with the coprecipitation analyses, support the ability of the LARG, but not the p115 RhoGEF, RGS box to interact with Gαq in vivo.
FIG. 3.
LARG RGS box attenuates Gα12-, Gα13-, and Gαq-mediated SRF activation in NIH 3T3 cells. NIH 3T3 cells were transiently cotransfected with expression plasmids (1 μg) encoding LARG RGS, p115 RGS, RGS2, and GTPase-deficient Gα12 (Q229L), Gα13 (Q226L), or Gαq (Q229L) proteins and a luciferase reporter plasmid (0.5 μg) that is responsive to activation of SRF [(SREm)2-Luc]. After transfection, cells were placed in 0.1% calf serum for 24 h. Cell lysates were then analyzed for luciferase activity and normalized to cells transfected with empty vectors (fold activation). All reporter assays were performed in six-well plates in duplicate. Total cell lysates (30 μg) were analyzed for protein expression by Western blot analysis with anti-Gα12, -Gα13, -Gαq, or -HA antibodies (data not shown). The data shown represent the averages of three experiments (± standard errors of the means).
The LARG RGS box abolishes signaling and focus formation mediated by Gαq- and Gα12-coupled GPCRs.
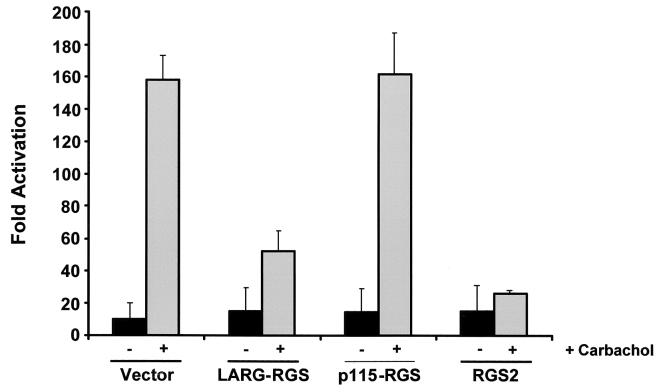
We further evaluated the ability of the LARG RGS box to modulate Gαq-mediated SRF activation by using a Gαq-coupled muscarinic cholinergic receptor (m1-mAChR) system. Since the natural agonist for m1-mAChR (acetylcholine) is not present in serum, receptor activity can be induced by the addition of an exogenous agonist such as carbachol to the growth medium. NIH 3T3 cells stably expressing the m1-mAChR alone or together with either p115 RhoGEF or the LARG RGS box were supertransfected with the SRF reporter plasmid and, where indicated, RGS2. m1-mAChR-expressing NIH 3T3 cells induced SRF activation in response to carbachol stimulation (Fig. 4). Coexpression of LARG-RGS or RGS2 attenuated SRF activation induced by carbachol (Fig. 4). In contrast, coexpression of the p115-RGS had no effect on SRF activation elicited by the m1 muscarinic receptor. These findings further suggest that the RGS box of LARG, unlike that of p115 RhoGEF, is uniquely capable of modulating Gαq- as well as Gα12/13-mediated signal transduction events.
FIG. 4.
LARG RGS box attenuates agonist-dependent m1-mAChR-mediated SRF activation in NIH 3T3 cells. NIH 3T3 cell lines stably expressing the Gq-coupled m1-mAChR and the indicated RGS box proteins were transiently transfected with the (SREm)2-Luc SRF-responsive reporter plasmid (0.5 μg). NIH 3T3 cells stably expressing m1-mAChR alone were supertransfected with (SREm)2-Luc (0.5 μg) and RGS2 (1 μg). After transfection, cells were placed in growth medium supplemented with 0.1% calf serum for 24 h. Cell lysates were than analyzed for luciferase activity, and fold activation was determined by the number of relative luciferase units relative to the number of units seen with the empty vector control. All reporter assays were performed in six-well plates in duplicate. The data shown are representative of at least three independent assays performed on duplicate plates.
Our analyses above indicated that the RGS box of LARG, but not that of p115 RhoGEF, interacts with and regulates the function of Gαq in vivo. To further assess this difference in Gα specificity, we evaluated the ability of LARG-RGS and p115-RGS to modulate the transforming activity of GPCRs that are coupled to Gα13 or Gαq. For these analyses, we determined if coexpression of each RGS box could inhibit the ability of a GPCR to cause focus formation in NIH 3T3 cells.
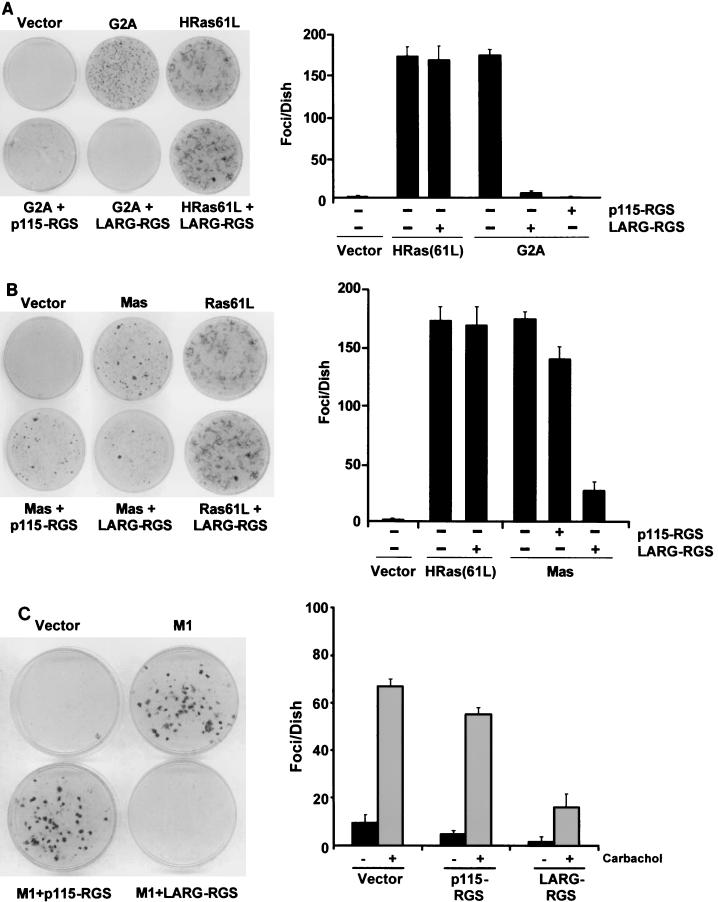
G2A was identified in a screen for novel oncogenes and is a GPCR that causes Gα13-dependent activation of RhoA and RhoA-dependent transformation of NIH 3T3 cells (20, 45). It was previously shown that coexpression of the amino-terminal RGS box of Lsc, the mouse ortholog of p115 RhoGEF, resulted in complete reversion of G2A-expressing cells to a nontransformed morphology (45). We therefore tested whether the RGS box of LARG might also function to inhibit Gα13-mediated signaling and transformation in G2A-transformed NIH 3T3 cells. Expression of the isolated RGS box of LARG or p115 RhoGEF greatly inhibited the transformation of NIH 3T3 cells induced by G2A overexpression (Fig. 5A). We also found that expression of either RGS box completely abolished the transforming activity of activated mutants of Gα12 and Gα13 (data not shown) while neither blocked transformation induced by activated Ras (Fig. 5A). These results suggest that the LARG RGS box can specifically interdict signaling from GPCR-mediated activation of Gα13.
FIG. 5.
LARG RGS box abolishes focus formation mediated by Gαq- and Gα13-coupled GPCRs. (A) LARG RGS box blocks G2A-mediated transformation of NIH 3T3 cells. G2A is an oncogenic GPCR that couples to Gα13 (20). NIH 3T3 cells were cotransfected with expression vectors encoding transforming oncoproteins G2A (0.5 μg) or activated HRas(61L) (25 ng) along with vectors (1 μg) that express the RGS box of p115 RhoGEF (p115-RGS) or LARG (LARG-RGS). Focus-forming activity was determined 14 days after transfection. The data shown represent the average number of foci of three experiments performed in duplicate. (B) LARG RGS box blocks Mas-mediated transformation of NIH 3T3 cells. Mas is an oncogenic GPCR that couples to Gαq and Gαi. NIH 3T3 cells were cotransfected with plasmid vectors encoding Mas (0.5 μg) or HRas(61L) (25 ng) either alone or together with vectors (1 μg) encoding p115-RGS or LARG-RGS. Focus-forming activity was determined 14 days after transfection. The data shown represent the average number of foci of three experiments performed in duplicate. (C) LARG RGS box blocks agonist-dependent m1-mAChR-mediated transformation of NIH 3T3 cells. Stable NIH 3T3 cell lines were established expressing the Gq-coupled m1-mAChR and the indicated RGS box proteins. Cells were maintained in growth medium supplemented with either 100 μM carbachol (an m1-mAChR agonist) or vehicle, and focus-forming activity was determined after 12 days. The data shown represent the average number of foci of three individual experiments performed in duplicate.
Whereas G2A transformation involves the activation of Gα13, Mas transformation appears to be mediated by Gαi and Gαq activation (45, 46). When coexpressed with LARG-RGS, the transforming activity of Mas was almost completely abolished, whereas coexpression of p115-RGS had no effect (Fig. 5B). In our transformation assays, we found that expression of activated Gαq or activated Gαi alone was unable to induce focus formation in NIH 3T3 cells, and instead, coexpression of both activated subunits was required to cause transformation (I. E. Zohn and C. J. Der, unpublished observations). Therefore, we were unable to determine if the LARG RGS box was suppressing Gαi-dependent transformation by performing coexpression studies. However, since we have not detected Gαi association with LARG-RGS (data not shown), we suspect that LARG-RGS inhibition of Mas transformation is mediated by inhibition of Gαq.
To further investigate the possibility that the LARG RGS box can suppress Gαq-induced transformation, we used a Gαq-coupled mAChR. Certain mAChR subtypes are known to transform NIH 3T3 cells in a strictly agonist-dependent manner (12). Thus, transforming activity is induced only in the presence of exogenous carbachol. Gutkind and colleagues employed this approach to establish that Gq-coupled mAChRs (m1, m3, and m5) are highly transforming, whereas Gi-coupled mAChRs (m2 and m4) do not elicit focus-forming activity (12). Therefore, we performed secondary focus formation transformation assays in NIH 3T3 cells stably expressing the Gαq-coupled m1-mAChR to evaluate the effect of the LARG RGS box on Gαq-mediated transforming activity. In the absence of carbachol, cells stably transfected with m1-mAChR alone or with m1-mAChR combined with the RGS box of p115 RhoGEF or LARG appeared morphologically indistinguishable from control (empty vector transfected) cells. However, when maintained for 14 days in growth medium supplemented with carbachol, the m1-mAChR-expressing cells and m1-mAChR-p115 RhoGEF RGS box-cotransfected cells readily formed foci in transformed cells (Fig. 5C). In contrast, carbachol-induced focus formation mediated by the m1 muscarinic receptor was blocked by coexpression of LARG-RGS but not p115-RGS. These results confirm the specificity of the LARG RGS box for suppressing Gαq-dependent transformation.
LARG enhances Gαq-, Gα12-, and Gα13-mediated accumulation of RhoA-GTP.
Previous studies showed that activated Gα13, but not Gα12 or Gαq, stimulated the GEF activity of p115 RhoGEF in vitro (15) and p115 RhoGEF activation of SRF in vivo (27). Therefore, other Dbl family proteins must mediate Gα12 and Gαq activation of RhoA, and our present analyses support such a role for LARG. Thus, we next investigated this possibility and evaluated the ability of Gα subunits to cooperate with LARG to cause activation of RhoA in vivo.
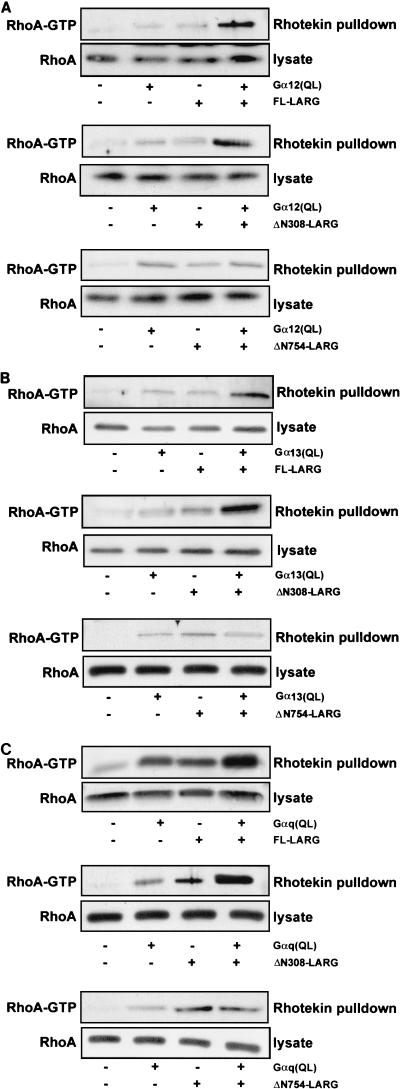
For these analyses we generated expression vectors encoding full-length LARG and two amino-terminally truncated versions (Fig. 1). The first truncation mutant, designated ΔN308 LARG, lacks the amino-terminal sequences of LARG that are deleted as a consequence of the formation of leukemia-associated mixed-lineage leukemia-LARG fusion protein (i.e., lacking the PDZ domain but encoding the RGS box) (Fig. 1). The second truncation mutant, designated ΔN754 LARG, additionally lacks the RGS box. Each LARG protein was expressed either alone or together with constitutively activated mutants of Gαq, Gα12, or Gα13 in NIH 3T3 cells, and RhoA activation was assayed by affinity precipitation with GST-rhotekin RBD. Following precipitation, Western blot analyses with a RhoA-specific antibody provided an assessment of the formation of activated, GTP-bound RhoA, and blot analyses of total cell lysates were done to verify the equivalent total RhoA protein expression in each condition.
We found that the expression of each constitutively activated Gα subunit alone, or of wt or truncated LARG protein alone, caused a limited, but significant, increase in the amount of activated GTP-bound RhoA (Fig. 6). Importantly, we found that coexpression of activated Gαq, Gα12, or Gα13 with full-length or ΔN308 LARG, but not ΔN754 LARG, caused an enhanced formation of GTP-bound RhoA. These data suggest that Gαq, Gα12, or Gα13 activation may result in enhanced LARG DH domain catalytic activity in vivo and this coupling of Gαq, Gα12, or Gα13 to RhoA activation depends on the presence of the RGS box but not the PDZ domain.
FIG. 6.
Coexpression of activated Gα12, Gα13, and Gαq enhances LARG-mediated RhoA activation. NIH 3T3 cells were transiently transfected with expression plasmids (1 μg) encoding activated mutants of Gα12 (A), Gα13 (B), Gαq (C); full-length (2 μg), ΔN308, or ΔN754 LARG; or the indicated combinations of activated Gα subunits and LARG. After transfection, cells were cultured for 24 h in growth medium supplemented with low serum (0.1%) and lysed, and the lysates (800 μg total protein) were used in GST pull-down assays using GST-rhotekin RBD immobilized on glutathione-agarose beads (20 μg). Bound proteins and total cell lysates were analyzed by Western blot analyses with anti-RhoA antibodies. The expression of Gα subunits and LARG was analyzed by Western blot analyses of total cell lysates using antibodies to Gα12, Gα13, Gαq, or HA (data not shown).
DISCUSSION
A wide variety of GPCRs have been shown to cause activation of RhoA (9, 34). Extracellular signal-mediated activation of Rho family GTPases typically involves activation of a Dbl family protein. p115 RhoGEF/Lsc and PDZ-RhoGEF/GTRAP48, together with LARG, represent novel members of the Dbl family that contain amino-terminal RGS boxes and are RhoA-specific GEFs (8, 10, 11, 16, 31, 33). Since RGS boxes can interact with specific Gα subunits, this group of Dbl family proteins represents strong candidates for mediators of GPCR activation of RhoA. p115 RhoGEF has been shown to enhance the intrinsic GTPase activity of the Gα12/13 subfamily (24) and to directly link Gα13, but not Gα12, to RhoA activation in vitro (15) or to p115 RhoGEF signaling in vivo (27). However, no RhoA GEF has yet been identified that directly links the Gα subunits Gα12, Gαq, or Gαi with the activation of RhoA. In the present study we evaluated the specificity of the LARG RGS box towards Gα subunits that stimulate RhoA activation in vivo. Unexpectedly, we found that the isolated RGS box of LARG associates not only with Gα12 and Gα13, but also with Gαq in vivo and blocked the activity of Gα12- and Gαq-coupled GPCRs. Additionally, we determined that activated Gαq, as well as Gα12 and Gα13, synergistically enhanced LARG stimulation of RhoA GTP formation in vivo. We suggest LARG is functionally distinct from the other RGS box-containing Dbl family proteins and can function as a critical mediator of RhoA activation by GPCRs that couple with Gαq and Gα12 as well as Gα13.
Our coimmunoprecipitation assays revealed that the LARG RGS box associated with Gαq as well as Gα12 and Gα13 in vivo. This is in contrast to what has been described for the previously studied RGS box-containing RhoA GEFs (p115 RhoGEF and PDZ-RhoGEF), which interact only with Gα12 and Gα13. Using the assay conditions with which we detected LARG-RGS interaction with Gαq, we verified that the RGS box of p115 RhoGEF did not interact with Gαq in vitro or in vivo. During the course of our studies, Fukuhara et al. reported that the LARG RGS box associated with GTPase-deficient mutants of Gα12 (Q229L) and Gα13 (Q226L) but failed to associate with the analogous mutant of Gαq (Q229L) in vitro (8). What is the basis for this apparent discrepancy in the observations made in our study? Fukuhara et al. utilized mammalian cell-expressed constitutively activated (Q to L) Gα subunits in their binding analyses with LARG (8). In our initial coprecipitation analyses with Q-to-L mutants of Gα proteins, we also observed no interaction between the LARG RGS box and constitutively activated Gαq but we also failed to find association with Gα12 or Gα13 (data not shown). However, previous studies indicated that RGS proteins bind with highest affinity to Gα subunits in the transition state for GTP hydrolysis (2, 39). When cells are lysed in the presence of aluminum tetrafluoride, the conformation of GDP-bound, AlF4−-activated Gα subunit mimics the transition state of GTP hydrolysis. Only in the presence of AlF4− did we observe an interaction between the LARG RGS box and Gαq, Gα12, and Gα13. Hart et al. and Wells et al. also observed an AlF4−-dependent association between Gα13 and p115 RhoGEF (15, 41). Furthermore, in their initial characterization of PDZ-Rho-GEF, Fukuhara et al. (10) also performed coprecipitation analyses utilizing the Q-to-L mutants of Gα12 and Gα13 in the presence of aluminum fluoride in the lysis buffer. Thus, we do not believe that our observed interaction between the LARG RGS box and Gαq is an artifact of our coimmunoprecipitation conditions since, in this same assay, the RGS box of p115 RhoGEF did not associate with Gαq and neither the p115 RhoGEF nor the LARG RGS box was seen to bind Gαi1. Finally, LARG-RGS, but not p115-RGS, blocked Gαq-stimulated activation of SRF. Thus, in contrast to the RGS box of p115 RhoGEF, the RGS box of LARG can interact with Gαq. A demonstration that endogenous LARG can form a stable complex with endogenously activated Gα subunits would be one approach to verify the physiological significance of our observations. However, in light of the low affinity of RGS boxes for Gα subunits, we are not aware that any endogenous RGS box-containing protein has been shown to complex with activated Gα subunits. Instead, we are currently determining whether the LARG RGS box exhibits higher affinity for Gαq, Gα12, or Gα13 and GAP activity for Gαq. These analyses may provide some indication regarding how LARG may regulate the specificity of signaling from GPCRs that are coupled to different Gα subunits.
Further support for the ability of the LARG RGS box to interact with Gα13 and Gαq comes from our demonstration that the isolated RGS box of LARG blocked the transforming activity of GPCRs that are coupled to these Gα subunits. G2A causes p115 RhoGEF/Lsc- and RhoA-dependent transformation (45). G2A also efficiently induces stress fiber formation in mouse fibroblasts lacking Gα12 or Gαq, but not Gα13, indicating that Gα13 is the key mediator of G2A activation of RhoA (20). Although a recent report suggested that this receptor can couple to Gαi (21), pertussis toxin failed to inhibit the ability of G2A to induce NIH 3T3 cell transformation, indicating that G2A is not mediating transformation through Gαi/o subunits (20, 45). This exclusion of Gα12 and Gαq as candidate mediators of G2A signaling, coupled with our finding that LARG fails to associate with Gαi after AlF4 treatment, suggests that inhibition of G2A transforming activity by the LARG RGS box most likely occurs via interdiction of signal transduction from Gα13 to RhoA.
Our additional finding that the RGS box of LARG, but not p115 RhoGEF, inhibited m1 muscarinic receptor signaling and transformation, a strictly Gαq-linked receptor and activator of RhoA (7), suggests that the LARG RGS box specifically interacts with Gαq. Similarly, the ability of the RGS box of LARG to suppress the transforming activity of Mas, a GPCR that is coupled to Gαi and Gαq, is also likely to be due to inhibition of activated Gαq-mediated signaling pathways. While these cellular assays suggest that the LARG RGS box has GAP activity toward Gαq, Gα12, and Gα13, we are currently evaluating this possibility more directly with purified recombinant Gα and LARG proteins in single-turnover GTPase assays. Interestingly, the RGS box of the closely related GTRAP48 was recently found to possess only poor GAP activity towards Gα12/13 proteins relative to p115 RhoGEF (41).
Various indirect analyses suggest that chronically activated mutants Gα12, Gα13, and Gαq may activate RhoA (4, 26, 45). Our data showing that activated versions of these Gα subunits cause an increase in RhoA GTP verify this possibility. While p115 RhoGEF has been shown to connect Gα13 activation with RhoA-specific exchange activity (15), the link that mediates RhoA activation by Gα12 and Gαq has not been determined. Although the ability of LARG to activate RhoA by coexpressing activated forms of Gα12 or Gα13 could not be augmented in previous preliminary observations (31), the present study showed that LARG can mediate Gα12 and Gαq, as well as Gα13, activation of RhoA. The different observations made in the present study are most likely due to different cell types used in the analyses (NIH 3T3 versus 293T) as well as differences in the protocols used for transfection and cell lysis. Using the same cell type in which our transformation assays were done, namely NIH 3T3 cells, we determined that coexpression of constitutively activated versions of all three Gα subunits synergistically enhanced (two- to threefold above additive) the ability of LARG to promote the formation of active, GTP-bound RhoA in vivo and this cooperative activity was seen only with full-length or a truncation variant of LARG that contained the RGS box. We are further evaluating the ability of LARG to link Gαq to RhoA activation by using purified recombinant Gα subunits, LARG, and RhoA in in vitro exchange assays. Recent analyses determined that Gα13 binding to p115 RhoGEF involves sequences outside the RGS box and may involve the DH and PH domains (41). Our Gα binding studies utilized a fragment of LARG corresponding to the RGS box alone, suggesting that the RGS box alone is sufficient for Gα interaction but leaving unanswered whether additional Gα contacts with other LARG domain(s) are required for Gα specificity or Gα-mediated GEF activation. However, preliminary studies suggest that the PDZ domain does not decouple the LARG protein's ability to mediate signaling events through activated Gα subunits. We are currently perusing structure-function studies to determine if other signaling domains present in LARG mediate interactions with specific Gα subunits.
In summary, our observations show that, unlike other RGS box RhoGEF family members, LARG associates with and negatively regulates Gαq in addition to the Gα12 subfamily of Gα subunits. Our studies further suggest that the binding of activated GTP-bound Gαq, as well as Gα12 and Gα13, to the RGS box of LARG enhances RhoA activation in cells. LARG transcripts are expressed ubiquitously. Hence, we suggest that LARG may serve as a mediator of RhoA activation by GPCRs that are coupled to Gαq as well as Gα12 and Gα13 in a wide variety of cells.
Acknowledgments
We thank members of the Der, Harden, Siderovski, and Sondek laboratories for technical assistance and helpful comments. We also thank Michael Caligiuri and Gary Reuther for the LARG plasmids.
Our research was supported by grants from the National Institutes of Health to C.J.D. (CA63071 and CA92240) and D.P.S. (GM62338), and M.A.B was supported by a Leukemia and Lymphoma Society Postdoctoral Fellowship (5394-02). D.P.S. is also a Year 2000 Scholar of the EJLB Foundation (Montreal, Canada) and recipient of the Burroughs-Wellcome New Investigator Award in the Pharmacological Sciences.
REFERENCES
- 1.Althoefer, H., P. Eversole-Cire, and M. I. Simon. 1997. Constitutively active Gαq and Gα13 trigger apoptosis through different pathways. J. Biol. Chem. 272:24380-24386. [DOI] [PubMed] [Google Scholar]
- 2.Berman, D. M., T. Kozasa, and A. G. Gilman. 1996. The GTPase-activating protein RGS4 stabilizes the transition state for nucleotide hydrolysis. J. Biol. Chem. 271:27209-27212. [DOI] [PubMed] [Google Scholar]
- 3.Bishop, A. L., and A. Hall. 2000. Rho GTPases and their effector proteins. Biochem. J. 348(Pt 2):241-255. [PMC free article] [PubMed] [Google Scholar]
- 4.Buhl, A. M., N. L. Johnson, N. Dhanasekaran, and G. L. Johnson. 1995. Gα12 and Gα13 stimulate Rho-dependent stress fiber formation and focal adhesion assembly. J. Biol. Chem. 270:24631-24634. [DOI] [PubMed] [Google Scholar]
- 5.Cerione, R. A., and Y. Zheng. 1996. The Dbl family of oncogenes. Curr. Opin. Cell Biol. 8:216-222. [DOI] [PubMed] [Google Scholar]
- 6.Clark, G. J., A. D. Cox, S. M. Graham, and C. J. Der. 1995. Biological assays for Ras transformation. Methods Enzymol. 255:395-412. [DOI] [PubMed] [Google Scholar]
- 7.Fromm, C., O. A. Coso, S. Montaner, N. Xu, and J. S. Gutkind. 1997. The small GTP-binding protein Rho links G protein-coupled receptors and Gα12 to the serum response element and to cellular transformation. Proc. Natl. Acad. Sci. USA 94:10098-10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuhara, S., H. Chikumi, and J. S. Gutkind. 2000. Leukemia-associated Rho guanine nucleotide exchange factor (LARG) links heterotrimeric G proteins of the G(12) family to Rho. FEBS Lett. 485:183-188. [DOI] [PubMed] [Google Scholar]
- 9.Fukuhara, S., H. Chikumi, and J. S. Gutkind. 2001. RGS-containing RhoGEFs: the missing link between transforming G proteins and Rho? Oncogene 20:1661-1668. [DOI] [PubMed] [Google Scholar]
- 10.Fukuhara, S., C. Murga, M. Zohar, T. Igishi, and J. S. Gutkind. 1999. A novel PDZ domain containing guanine nucleotide exchange factor links heterotrimeric G proteins to Rho. J. Biol. Chem. 274:5868-5879. [DOI] [PubMed] [Google Scholar]
- 11.Glaven, J. A., I. P. Whitehead, T. Nomanbhoy, R. Kay, and R. A. Cerione. 1996. Lfc and Lsc oncoproteins represent two new guanine nucleotide exchange factors for the Rho GTP-binding protein. J. Biol. Chem. 271:27374-27381. [DOI] [PubMed] [Google Scholar]
- 12.Gutkind, J. S., E. A. Novotny, M. R. Brann, and K. C. Robbins. 1991. Muscarinic acetylcholine receptor subtypes as agonist-dependent oncogenes. Proc. Natl. Acad. Sci. USA 88:4703-4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509-514. [DOI] [PubMed] [Google Scholar]
- 14.Hamm, H. E., and A. Gilchrist. 1996. Heterotrimeric G proteins. Curr. Opin. Cell Biol. 8:189-196. [DOI] [PubMed] [Google Scholar]
- 15.Hart, M. J., X. Jiang, T. Kozasa, W. Roscoe, W. D. Singer, A. G. Gilman, P. C. Sternweis, and G. Bollag. 1998. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science 280:2112-2114. [DOI] [PubMed] [Google Scholar]
- 16.Hart, M. J., S. Sharma, N. elMasry, R.-G. Qui, P. McCabe, P. Polakis, and G. Bollag. 1996. Identification of a novel guanine nucleotide exchange factor for the Rho GTPase. J. Biol. Chem. 271:25452-25458. [DOI] [PubMed] [Google Scholar]
- 17.Heximer, S. P., N. Watson, M. E. Linder, K. J. Blumer, and J. R. Hepler. 1997. RGS2/G0S8 is a selective inhibitor of Gqalpha function. Proc. Natl. Acad. Sci. USA 94:14389-14393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill, C. S., J. Wynne, and R. Treisman. 1995. The Rho family GTPases RhoA, Rac1 and Cdc42Hs regulate transcriptional activation by SRF. Cell 81:1159-1170. [DOI] [PubMed] [Google Scholar]
- 19.Jackson, M., W. Song, M. Y. Liu, L. Jin, M. Dykes-Hoberg, C. I. Lin, W. J. Bowers, H. J. Federoff, P. C. Sternweis, and J. D. Rothstein. 2001. Modulation of the neuronal glutamate transporter EAAT4 by two interacting proteins. Nature 410:89-93. [DOI] [PubMed] [Google Scholar]
- 20.Kabarowski, J., J. Feramisco, L. Q. Le, J. Gu, S. Luoh, M. Simon, and O. N. Witte. 2000. Direct genetic demonstration of Gα13 coupling to the orphan G protein-coupled receptor G2A leading to RhoA-dependent actin rearrangement. Proc. Natl. Acad. Sci. USA 97:12109-12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabarowski, J. H., K. Zhu, L. Q. Le, O. N. Witte, and Y. Xu. 2001. Lysophosphatidylcholine as a ligand for the immunoregulatory receptor G2A. Science 293:702-705. [DOI] [PubMed] [Google Scholar]
- 22.Khosravi-Far, R., S. Campbell, K. L. Rossman, and C. J. Der. 1998. Increasing complexity of Ras signal transduction: involvement of Rho family proteins. Adv. Cancer Res. 72:57-107. [DOI] [PubMed] [Google Scholar]
- 23.Kourlas, P. J., M. P. Strout, B. Becknell, M. L. Veronese, C. M. Croce, K. S. Theil, R. Krahe, T. Ruutu, S. Knuutila, C. D. Bloomfield, and M. A. Caligiuri. 2000. Identification of a gene at 11q23 encoding a guanine nucleotide exchange factor: evidence for its fusion with MLL in acute myeloid leukemia. Proc. Natl. Acad. Sci. USA 97:2145-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozasa, T., X. Jiang, M. J. Hart, P. M. Sternweis, W. D. Singer, A. G. Gilman, G. Bollag, and P. C. Sternweis. 1998. p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science 280:2109-2111. [DOI] [PubMed] [Google Scholar]
- 25.Liu, B. P., and K. Burridge. 2000. Vav2 activates Rac1, Cdc42, and RhoA downstream from growth factor receptors but not β1 integrins. Mol. Cell. Biol. 20:7160-7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mao, J., H. Yuan, W. Xie, M. I. Simon, and D. Wu. 1998. Specific involvement of G proteins in regulation of serum response factor-mediated gene transcription by different receptors. J. Biol. Chem. 273:27118-27123. [DOI] [PubMed] [Google Scholar]
- 27.Mao, J., H. Yuan, W. Xie, and D. Wu. 1998. Guanine nucleotide exchange factor GEF115 specifically mediates activation of Rho and serum response factor by the G protein alpha subunit Galpha13. Proc. Natl. Acad. Sci. USA 95:12973-12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin, C. B., G. M. Mahon, M. B. Klinger, R. J. Kay, M. Symons, C. J. Der, and I. P. Whitehead. 2001. The thrombin receptor, PAR-1, causes transformation by activation of Rho-mediated signaling pathways. Oncogene 20:1953-1963. [DOI] [PubMed] [Google Scholar]
- 29.Morgenstern, J. P., and H. Land. 1990. A series of mammalian expression vectors and characterisation of their expression of a reporter gene in stably and transiently transfected cells. Nucleic Acids Res. 18:1068.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponimaskin, E., H. Behn, V. Adarichev, T. A. Voyno-Yasenetskaya, S. Offermanns, and M. F. Schmidt. 2000. Acylation of Galpha(13) is important for its interaction with thrombin receptor, transforming activity and actin stress fiber formation. FEBS Lett. 478:173-177. [DOI] [PubMed] [Google Scholar]
- 31.Reuther, G. W., Q. T. Lambert, M. A. Booden, K. Wennerberg, B. Becknell, G. Marcucci, J. Sondek, M. A. Caligiuri, and C. J. Der. 2001. Leukemia-associated Rho guanine nucleotide exchange factor, a Dbl family protein found mutated in leukemia, causes transformation by activation of RhoA. J. Biol. Chem. 276:27145-27151. [DOI] [PubMed] [Google Scholar]
- 32.Ross, E. M., and T. M. Wilkie. 2000. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu. Rev. Biochem. 69:795-827. [DOI] [PubMed] [Google Scholar]
- 33.Rumenapp, U., A. Blomquist, G. Schworer, H. Schablowski, A. Psoma, and K. H. Jakobs. 1999. Rho-specific binding and guanine nucleotide exchange catalysis by KIAA0380, a dbl family member. FEBS Lett. 459:313-318. [DOI] [PubMed] [Google Scholar]
- 34.Seasholtz, T. M., M. Majumdar, and J. H. Brown. 1999. Rho as a mediator of G protein-coupled receptor signaling. Mol. Pharmacol. 55:949-956. [DOI] [PubMed] [Google Scholar]
- 35.Shepard, L. W., M. Yang, P. Xie, D. D. Browning, T. Voyno-Yasenetskaya, T. Kozasa, and R. D. Ye. 2001. Constitutive activation of NF-kappa B and secretion of interleukin-8 induced by the G protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus involve G alpha(13) and RhoA. J. Biol. Chem. 276:45979-45987. [DOI] [PubMed] [Google Scholar]
- 36.Siderovski, D. P., B. Strockbine, and C. I. Behe. 1999. Whither goest the RGS proteins? Crit. Rev. Biochem. Mol. Biol 34:215-251. [DOI] [PubMed] [Google Scholar]
- 37.Taya, S., N. Inagaki, H. Sengiku, H. Makino, A. Iwamatsu, I. Urakawa, K. Nagao, S. Kataoka, and K. Kaibuchi. 2001. Direct interaction of insulin-like growth factor-1 receptor with leukemia-associated RhoGEF. J. Cell Biol. 155:809-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor, S. J., and D. Shalloway. 1996. Cell cycle-dependent activation of Ras. Curr. Biol. 6:1621-1627. [DOI] [PubMed] [Google Scholar]
- 39.Tesmer, J. J., D. M. Berman, A. G. Gilman, and S. R. Sprang. 1997. Structure of RGS4 bound to AlF4-activated G(i alpha1): stabilization of the transition state for GTP hydrolysis. Cell 89:251-261. [DOI] [PubMed] [Google Scholar]
- 40.Van Aelst, L., and C. D'Souza-Schorey. 1997. Rho GTPases and signaling networks. Genes Dev. 11:2295-2322. [DOI] [PubMed] [Google Scholar]
- 41.Wells, C. D., M. Y. Liu, M. Jackson, S. Gutowski, P. M. Sternweis, J. D. Rothstein, T. Kozasa, and P. C. Sternweis. 2002. Mechanisms for reversible regulation between G13 and Rho exchange factors. J. Biol. Chem. 277:1174-1181. [DOI] [PubMed] [Google Scholar]
- 42.Westwick, J. K., Q. T. Lambert, G. J. Clark, M. Symons, L. Van Aelst, R. G. Pestell, and C. J. Der. 1997. Rac regulation of transformation, gene expression, and actin organization by multiple, PAK-independent pathways. Mol. Cell. Biol. 17:1324-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitehead, I. P., S. Campbell, K. L. Rossman, and C. J. Der. 1997. Dbl family proteins. Biochem. Biophys. Acta 1332:F1-F23. [DOI] [PubMed]
- 44.Wittinghofer, A. 1997. Signaling mechanistics: aluminum fluoride for molecule of the year. Curr. Biol. 7:R682-R685. [DOI] [PubMed]
- 45.Zohn, I. E., M. Klinger, X. Karp, H. Kirk, M. Symons, M. Chrzanowska-Wodnicka, C. J. Der, and R. J. Kay. 2000. G2A is an oncogenic G protein-coupled receptor. Oncogene 19:3866-3877. [DOI] [PubMed] [Google Scholar]
- 46.Zohn, I. E., M. Symons, M. Chrzanowska-Wodnicka, J. K. Westwick, and C. J. Der. 1998. Mas oncogene signaling and transformation require the small GTP-binding protein Rac. Mol. Cell. Biol. 18:1225-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zohn, I. M., S. L. Campbell, R. Khosravi-Far, K. L. Rossman, and C. J. Der. 1998. Rho family proteins and Ras transformation: the RHOad less traveled gets congested. Oncogene 17:1415-1438. [DOI] [PubMed] [Google Scholar]