Abstract
To discover and study intracellular signals that regulate proteolysis in muscle, we have employed transgenic strains of Caenorhabditis elegans that produce a soluble LacZ reporter protein limited to body-wall and vulval muscles. This reporter protein is stable in well-fed wild-type animals, but its degradation is triggered upon a shift to 25°C in a strain carrying a temperature-sensitive activating mutation in the Ras oncogene homologue let-60. These mutants are not physiologically starved, inasmuch as growth rates are normal at 25°C. Ras-induced degradation is not prevented by the presence of cycloheximide added at or before the temperature shift and thus uses preexisting proteolytic systems and signaling components. Furthermore, degradation is triggered when adult animals are shifted to conditions of 25°C, confirming that Ras acutely promotes protein degradation in muscles whose developmental history is normal. Reduction-of-function mutations in the downstream protein kinase Raf (lin-45), MEK (mek-2), or mitogen-activated protein kinase (MAPK) (mpk-1) prevent Ras-induced protein degradation, whereas activated MPK-1 is sufficient to trigger degradation, indicating that this kinase cascade is the principal route by which Ras signaling triggers protein degradation in muscle. This pathway is activated in hypodermal cells by the LET-23 epidermal growth factor receptor homologue, but an activating mutation in let-23 does not promote proteolysis in muscle. Starvation-induced LacZ reporter degradation is unaffected by reduction-of-function mutations in Ras, Raf, MEK, or MAPK, implying that Ras activation and starvation trigger proteolysis by mechanisms that are at least partially independent. This is the first evidence that Ras-Raf-MEK-MAPK signaling activates protein degradation in differentiated muscle.
The regulation of proteolysis in muscle is a clinically significant problem because of its relevance to muscle wasting and atrophy in skeletal muscle and pathological hypertrophy and atrophy in cardiac muscle. Muscle proteolysis is promoted by fasting or chronic starvation (18, 29), by disuse or denervation (19, 31), in sepsis (10), and in cancer cachexia (53). Catastrophic loss of muscle protein is associated with increased mortality in patients with cancer, diabetes, sepsis, heart failure, or AIDS (30, 52), and extreme muscle wasting can itself be the proximal cause of death.
The molecular mechanisms of proteolysis in muscle have been extensively studied (3, 4, 15, 31-33, 44, 45, 51), although there is considerable uncertainty remaining about the specific roles of the proteolytic systems involved. Moreover, several external signals that regulate muscle proteolysis have been identified but we know little about how intramuscular signal-transduction mechanisms might couple external stimuli to the regulation of protein degradation.
We have been developing a genetically tractable model for studying the regulation of proteolysis in muscle, using strains of the nematode Caenorhabditis elegans that express a transgene-coded LacZ reporter protein specifically in body-wall and vulval muscles. This protein is completely stable in well-fed wild-type animals, but its degradation can be triggered by starvation (59) or by loss of cholinergic neural inputs (50). In both instances, the activation of preexisting proteolytic systems is sufficient to account for the observed protein degradation. The reporter protein is ubiquitinated in starved animals (59), and inhibitor data indicate that proteasome activity is required for its degradation (50).
Ras signaling is known to be important in a number of disease states associated with muscle wasting. We report here that proteolysis in muscle is triggered acutely by activation of the Ras homologue LET-60, that signaling occurs by the Raf-MEK-MAPK cascade of protein kinases, and that the resultant protein degradation uses a preexisting proteolytic system. We also show that reduction-of-function mutations in this pathway do not affect starvation-induced or denervation-induced protein degradation, implying that the various mechanisms of stimulating proteolysis in muscle are at least partly distinct.
MATERIALS AND METHODS
Nematode strains were maintained, grown, and roughly age synchronized as described previously (59), except that strains containing let-60(ga89ts) were routinely kept at 16°C. The genetic markers used in this study were as follows: on linkage group LG I, mek-2(ku114); on LG II, let-23(sa62); on LG III, mpk-1(n2521); on (LG) IV, let-60(ga89ts), let-60(n1046), let-60(n2021), lin-45(sy96), him-8(e1489), cha-1(p1182ts), dpy-13(e184), and unc-24(e138); on LG V, ccIs55(unc-54::lacZ); and on LG X, gap-1(n1329), gap-2(pe103), unc-2(e55), and unc-18(e81). A strain containing the integrated transgene gaIs37 [EF1α-D-mek(gf) hs-mpk-1(wt)] IV was obtained from S. Kim; at 25°C, the constitutively active Drosophila MEK homologue activates wild-type C. elegans MPK-1 expressed from a heat shock promoter (28). Strains containing unlinked mutations and/or the integrated transgene (59) ccIs55(unc-54::lacZ) on linkage group V or the transgene iwIs16(act-4::lacZ) on linkage group X (49) were constructed by conventional genetic methods. A double-mutant strain containing let-60(ga89ts) and lin-45(sy96) on LG IV was obtained by constructing a lin-45/unc-24 let-60 heterozygote, selecting (at 25°C) their nonuncoordinated multivulva (MuV) progeny (presumed lin-45 let-60/unc-24 let-60 recombinants arising by crossing over between let-60 and unc-24), and then selecting their vulvaless (Vul) progeny (at 20°C) to obtain lin-45 homozygotes. The presence of suppressed let-60(ga89ts) in this strain was confirmed by the production of MuV individuals at 25°C after outcrossing.
Methods for histochemical staining of β-galactosidase with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) were as described previously (59). Staining times were 1 to 2 h at room temperature, governed by visual examination of stained control animals (wild type or mutant at permissive temperature) included with every experiment. Stained animals were photographed on Kodak Ektachrome EH-160 transparency film under bright-field illumination with a red suppression filter or, in some later experiments, with a Nikon 990 digital camera. The automatically controlled exposure is dominated by the bright-field background and depends little on the intensity of staining of the animals being photographed and is thus approximately constant from experiment to experiment.
Methods for gel electrophoresis, fluorometric assay of β-galactosidase activity, and immunoblotting and quantitation were as described previously (59). Movement rates were measured as previously described (6), except that rates were measured with animals suspended in liquid (70 mM potassium phosphate, 70 mM NaCl [pH 7]). Optical measurement of worm growth was as described previously (6).
RESULTS
Ras activation promotes proteolysis in muscle.
We followed proteolysis in muscle by using an UNC-54::LacZ reporter fusion protein (17) that forms tetramers with β-galactosidase activity (59). The fusion protein contains only part of the N-terminal UNC-54 (myosin heavy chain) ATPase domain (17) and therefore does not associate with myofibrils but remains soluble in the cytosol.
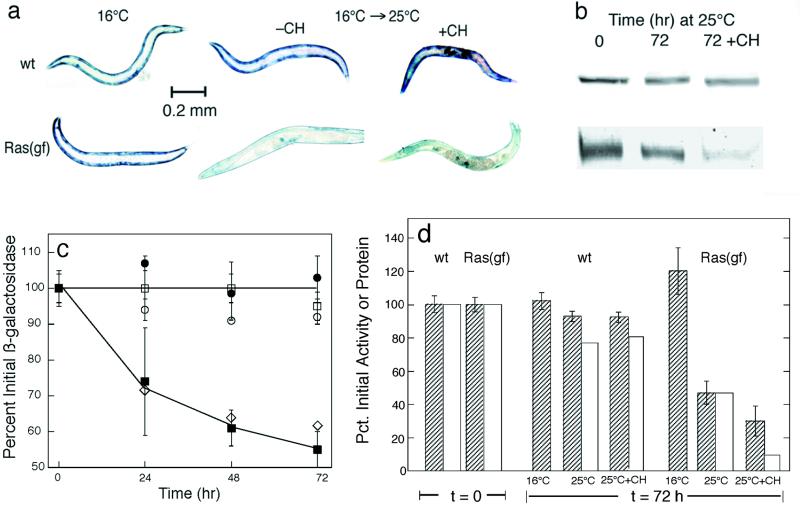
To determine whether Ras signaling regulates muscle protein degradation, we constructed strains containing the unc-54::lacZ transgene in combination with the let-60 Ras mutant allele ga89ts. The ga89ts mutant Ras has temperature-sensitive GTPase activity (16), such that Ras signaling is activated at the nonpermissive temperature (25°C). Well-fed ga89ts homozygotes expressed approximately wild-type levels of LacZ fusion protein when grown at 16°C. To measure protein degradation without regard to any potential effects of Ras on transcription or translation of the unc-54::lacZ transgene, we grew animals to mid-adulthood at 16°C, such that lacZ expression was essentially completed. Studies have shown previously (59) that the fusion protein is completely stable in well-fed wild-type animals (Fig. 1c), so that any subsequent decline in LacZ levels (as measured by either β-galactosidase activity or immunoreactive protein) in a mutant strain must represent net degradation of the preexisting LacZ fusion protein. We found that activated-Ras (ga89ts) homozygotes, when shifted to 25°C as adults, showed a time-dependent loss of preexisting reporter protein that did not occur in similarly treated wild-type animals (Fig. 1). In these and all subsequent experiments below, the amounts of 146-kDa reporter protein (Fig. 1b, c, and d) and the levels of β-galactosidase activity (Fig. 1c and d) declined in parallel.
FIG. 1.
Ras activation promotes proteolysis in muscle. (a) Animals stained for β-galactosidase activity (blue) after growth at 16°C (left) or 72 h after young adults grown at 16°C were shifted to 25°C (right) either with (+CH) or without (−CH) cycloheximide at 400 μg/ml. (b) Immunoblot analysis of 146-kDa fusion protein in 30-worm lysates. Each band shown from left to right in the two rows of bands is the 146-kDa band from the corresponding animal shown from left to right in panel a. (c) Kinetics of loss of β-galactosidase activity at 16°C (open circles and squares) or at 25°C (filled circles and squares) in wild-type (circles) or let-60(ga89ts) (squares) homozygotes. Diamonds show decline of protein in ga89ts after a shift to 25°C, measured by Western blot analysis similar to that described for panel b. (d) Quantitation of 146-kDa fusion protein (open bars) by integration of the bands shown in panel b for the 72-h group and of β-galactosidase activity (striped bars) by fluorometric assay of 10-worm lysates. Data are means ± standard deviations of triplicate samples.
In a strain containing activated-Ras (ga89ts) and ccIs56, an unc-54::lacZ fusion construct identical to ccIs55 but with the addition of a simian virus 40 nuclear localization signal (17), we observed β-galactosidase initially in both muscle cytosol and nuclei. At 72 h after a shift to 25°C, there was no histochemically detectable β-galactosidase activity in cytosol but some was still present in the nuclei (R. Harper and L. Jacobson, unpublished data). It was not possible to tell if any nuclear fusion protein had been degraded, but evidently nuclear fusion protein is at least somewhat protected from degradation relative to the same protein in the muscle cytosol.
We have also examined in a more limited way the effect of activated Ras on the stability of an ACT-4::LacZ fusion encoded by the integrated transgene iwIs16 (49). In contrast to the much larger N-terminal moiety on the UNC-54::LacZ fusion protein, the ACT-4::LacZ fusion protein adds only the first 14 amino acid residues of ACT-4 (actin) to the N terminus of Escherichia coli LacZ and is expressed from the act-4 promoter in 95 body-wall muscle cells, the eight vulval muscles, and the spermathecae. The ACT-4::LacZ fusion was also completely stable in wild-type animals and was degraded (in muscles but not the spermathecae) in activated-Ras (ga89ts) homozygotes after a shift to 25°C, with kinetics approximately the same as those we observed for the UNC-54::LacZ fusion. This suggests that the in vivo stabilities of the fusion proteins are determined primarily by the large LacZ regions (1,015 residues) rather than by the smaller N-terminal fusion regions. Furthermore, we examined a strain expressing soluble, cytosolic green fluorescent protein from an unc-54 promoter (but with no added amino acid residues) and, after activation of ga89ts Ras at 25°C, observed time-dependent loss of green fluorescent protein fluorescence in body-wall muscle over 72 h. Thus, these various reporter proteins provided us with information about a Ras-induced proteolytic process in body-wall muscle cells that is not specific for LacZ.
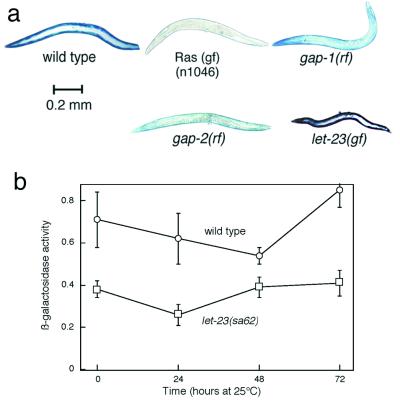
We infer that protein catabolism is provoked by activation of Ras signaling rather than by some other peculiar property of ga89ts mutant Ras, because protein is also degraded in other strains with Ras activation. We observed (Fig. 2) protein degradation in strains homozygous for a constitutively active (47) let-60 allele (n1046) and in strains with increased Ras activation as the result of reduction-of-function mutations in the Ras GTPase-activating protein gene (20, 21) gap-1 or gap-2. Additionally, the gap-1 reduction-of-function mutation enhanced protein degradation at 25°C in a strain carrying let-60(ga89ts). It is notable that the gap-1 and gap-2 single mutants do not show the MuV phenotype (20, 21) characteristic of strong Ras activation (47).
FIG. 2.
Ras activation, but not LET-23 EGFR activation, promotes degradation. (a) Adult animals grown at 20°C were stained for β-galactosidase activity. (b) β-Galactosidase activity as a function of age of wild-type and let-23(sa62)-activated EGFR animals. Age-synchronized L1 larvae were grown for 84 h at 16°C and then shifted to 25°C at the 0 time point. Data represent means ± standard deviations of fluorometric assays of triplicate 10-worm samples.
In the course of this and other studies in our laboratory, we have examined a wide variety of C. elegans mutants for expression and degradation of this UNC-54::LacZ fusion protein. It was previously reported (50) that LacZ degradation (in starved animals only) is promoted by mutations affecting the presynaptic synthesis or release of acetylcholine or the action of postsynaptic acetylcholine receptors; however, many other mutations that affect morphology (e.g., dumpy, blister, roller, etc.) or behavior (e.g., uncoordinated, chemotaxis defective, egg laying defective, etc.) have little or no effect on LacZ expression or stability as assessed by histochemical staining.
Ras activation induces changes in gene expression through the action of various downstream effectors, which in many cases act to promote the transcription of specific genes. To determine if Ras activation stimulates proteolysis by inducing de novo gene expression, we followed protein degradation in ga89ts mutant animals treated with cycloheximide. Studies have previously shown that cycloheximide at this concentration blocks protein synthesis within a few minutes at most but does not affect the rate of protein degradation in muscle (59). If Ras-induced proteolysis required the participation of newly synthesized proteins (e.g., signaling proteins, proteases, etc.), then cycloheximide should block the Ras effect and the LacZ reporter protein should remain stable after a shift to 25°C. To the contrary, we observed that onset of Ras-induced protein degradation was not blocked by cycloheximide added at the time of a shift to 25°C (Fig. 1), implying that stimulation of proteolysis does not require the synthesis of new proteins but is mediated by preexisting signaling proteins and proteases. Pretreatment with cycloheximide for 6 h prior to a shift to 25°C did not alter this result (data not shown), mitigating against the possibility that relevant Ras-induced gene expression takes place in a (hypothetical) brief period before protein synthesis is blocked by cycloheximide. Cycloheximide-treated wild-type controls showed little or no LacZ degradation over the same time interval (Fig. 1) (59).
The Ras effect is physiological rather than developmental.
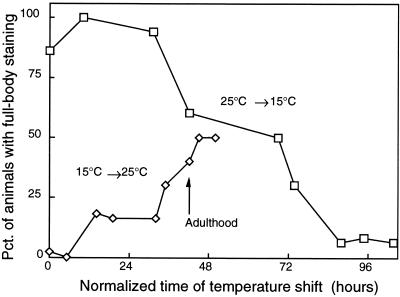
Ras activation is widely known to affect the developmental fates of specific cells in C. elegans (47) as well as in other organisms (8, 11, 39). To distinguish developmental effects of Ras on the muscle cells from acute physiological effects on protein degradation in those cells, we performed temperature upshift and downshift experiments with let-60(ga89ts) homozygotes. Figure 3 shows that animals shifted from 25 to 15°C conditions at or prior to adulthood retained full β-galactosidase activity and that animals shifted from 15 to 25°C conditions well after adulthood still lost significant activity. In other words, early Ras activation at 25°C does not provoke later protein degradation if the mutant animals are returned to the permissive temperature (15°C) prior to reaching adulthood. Conversely, mutant animals raised to adulthood at 15°C, and thus having a near-wild-type experience during development, can still be provoked after reaching adulthood to degrade muscle protein when Ras is activated by a shift to 25°C. These observations imply that Ras activation promotes protein degradation by acutely stimulating proteolysis rather than by altering some developmental process in the muscles. This is entirely consistent with the inference from the cycloheximide experiments (Fig. 1) that Ras activation causes signal to pass via preexisting proteins to a preexisting proteolytic system.
FIG. 3.
Ras activation effects on β-galactosidase in muscle are acute rather than developmental. Ras mutant [let-60(ga89ts)] animals were grown at 15°C or 25°C, and L1-L2 animals at each temperature were then transferred to nine new plates. At 0, 8, 24, 32, 53, 57, 68, 72, and 80 h after transfer, one plate was moved from 15°C to 25°C and one was moved from 25°C to 15°C. At 119 h after transfer, 50 animals from each plate were stained for β-galactosidase activity and the stained animals were examined to score the fraction showing full-body staining (ordinate). Times of temperature shift were normalized to growth at 20°C (7) to generate the abscissa.
The data in Fig. 3 show that there is some variability between individual animals in the rate or extent of protein degradation, and ga89ts individuals also show only a moderate incidence of the MuV phenotype resulting from ectopic differentiation of hypodermal cells (16) in response to Ras activation. That is, both the MuV and protein-degradation phenotypes display variable expressivity and perhaps variable penetrance. However, we found that those ga89ts individuals that are MuV did not degrade the LacZ reporter any more than their non-MuV siblings and, conversely, those ga89ts individuals with the most pronounced LacZ degradation were not more likely to be MuV. We interpret this lack of correlation between activated-Ras phenotypes in hypodermis and muscle to mean that individuals differ not only in organism-wide level of Ras activation but also in the efficiency with which Ras signal affects the ultimate phenotypic output. Animals showing the MuV phenotype differ from each other in their numbers of ectopic differentiation events, which is indicative of cell-to-cell variability. In contrast, we did not observe much variation between individual muscle cells in the same activated-Ras animal in the extent of protein degradation (although there was variation between individual animals), suggesting a systemic rather than cell-to-cell variation in downstream execution of events leading to the phenotype.
The ectopic differentiation of hypodermal cells to produce the MuV phenotype is efficiently signaled by activation of the LET-23 receptor (2, 48), a homologue of mammalian epidermal growth factor receptor (EGFR) which signals via Ras (47). To test whether LET-23 also signals to Ras to induce muscle proteolysis and to further examine the lack of correlation between the Ras-induced protein-degradation and MuV phenotypes, we constructed a strain carrying the unc-54::lacZ transgene and an activating mutation in the extracellular domain of LET-23 (25). This mutant strain [homozygous for let-23(sa62)] showed high penetrance and expressivity of the MuV phenotype (Fig. 2a) but on the basis of histochemical staining did not obviously degrade the LacZ reporter. Quantitative fluorometric assaying of β-galactosidase activity (Fig. 2b) showed that the β-galactosidase levels in the activated-LET-23 mutants were about 50 to 70% of those in wild-type animals of similar chronological age; this is approximately proportionate to the reduced body size of the mutants (Fig. 2a), so the lower activity on a per-animal basis is likely to reflect only generally reduced growth and protein synthesis rather than increased protein degradation as a result of LET-23 activation. Furthermore, there was no loss of LacZ reporter protein as assayed over time by Western blotting and reporter activity did not disappear in let-23(sa62) animals treated with cycloheximide. We conclude that the LET-23 EGFR does not signal to cause Ras activation in muscle, consistent with the fact that no LET-23 expression has been reported in muscle (23).
Raf, MEK, and MAPK are downstream Ras effectors.
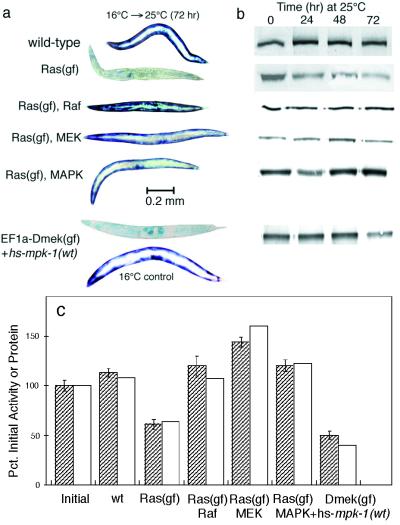
Ras effects may be mediated by a wide variety of downstream effectors (8); the effect on hypodermal development in C. elegans involves signal transduction by the Raf-MEK-MAPK cascade of protein kinases (47). To determine whether Ras signals protein degradation in muscle by the same pathway, we constructed strains containing the unc-54::lacZ transgene and temperature-activated let-60(ga89ts) in combination with mutant alleles that produce reduction of function in the protein kinases Raf [lin-45(sy96)], MEK [mek-2(ku114)], or MAPK [mpk-1(n2521)]. In each case, a mutation in Raf, MEK, or MAPK efficiently suppressed Ras-induced protein degradation after temperature upshift (Fig. 4). Ras-induced protein degradation in ga89ts mutant animals was also prevented by 1 μM PD-98059, a pharmacological inhibitor of MEK activity (1, 14). Conversely, mutational activation of a MAPK transgene [EF1α-D-mek(gf) + hs-mpk-1(wt)] (28) was sufficient to provoke protein degradation (Fig. 4). Note that in this case there was an obvious lag between the shift to 25°C and the onset of reporter protein degradation, as would be expected if degradation were triggered only after MPK-1 had been expressed de novo from the heat shock promoter.
FIG. 4.
Reduction-of-function mutations in downstream effectors suppress Ras-induced protein degradation. (a) Histochemical stain for β-galactosidase activity in muscle. (b) Immunoblot analysis of 146-kDa fusion protein in 30-worm lysates. Each row of bands shows the 146-kDa lacZ fusion bands from the corresponding double-mutant animal shown in panel a. (c) Quantitation of 146-kDa fusion protein (open bars) by integration of the bands at 72 h (gel in panel b) and of β-galactosidase activity (filled bars) by fluorometric assay of 10-worm lysates made 72 h after a shift to 25°C. Data represent means ± standard deviations of triplicate samples.
We infer that the Raf-MEK-MAPK cascade is the principal route by which Ras signaling promotes protein degradation in muscle. The relevant downstream target of MAPK action is presently unknown (the LacZ fusion protein itself contains no recognizable MAPK phosphorylation site), but the cycloheximide data (Fig. 1) imply that the known outputs involving activation of transcription factors (47) are insufficient to explain how Ras induces proteolysis.
Ras activation does not cause starvation or loss of neural function.
Five lines of evidence indicate that protein degradation in activated-Ras mutants is not the result of starvation. First, ga89ts animals raised at 25°C grew at a rate indistinguishable from that of wild-type animals (Fig. 5), whereas starved animals show arrested development or markedly decreased growth rate. Second, ga89ts animals raised at 25°C do not enter the dauer larva state associated with poor nutritional status (9). Third, ga89ts animals did not catabolize lipid stored in the intestinal cells after a shift to 25°C (as judged by staining with Sudan Black [reference 27 and data not shown]), whereas these stores are completely depleted after about 8 h of acute starvation (S. J. Barmada and L. A. Jacobson, unpublished data). Fourth, ga89ts animals degraded LacZ protein in all muscles at 25°C (Fig. 1), whereas in starved wild-type animals (or starved ga89ts mutants at 16°C) the protein is degraded in the posterior body-wall muscles but preserved in head and vulval muscles (50). Fifth, Ras-induced reporter degradation was significantly slower (50% degradation in about 72 h at 25°C; Fig. 1) than is starvation-induced degradation (50% degradation in 16 h at 20°C [59]). This kinetic discrepancy is large enough to preclude experiments to determine whether Ras-induced and starvation-induced protein degradation would be quantitatively additive.
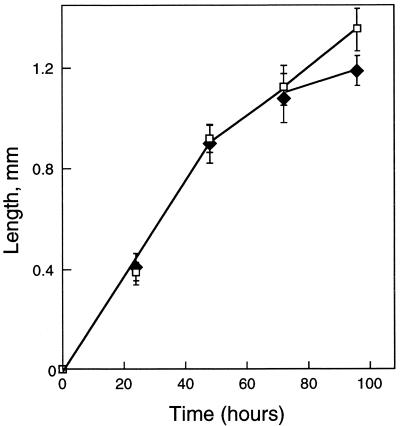
FIG. 5.
Growth rates of wild-type (filled diamonds) and Ras mutants [let-60(ga89ts); open squares] at 25°C. Eggs were laid on plates for 2 h; the 0 time point represents the end of this period. Data show means ± standard deviations of results from 6 animals at each time point.
We have so far found no evidence that relates Ras-Raf-MEK-MAPK signaling to starvation-induced degradation of protein in muscle. Reduction-of-function mutations in Ras [let-60(n2021)], Raf, MEK, or MAPK affected neither the rate nor the anterior-posterior cellular specificity of starvation-induced LacZ degradation (Fig. 6). Thus, activation of the Ras-MAPK pathway is not necessary for starvation-induced protein degradation.
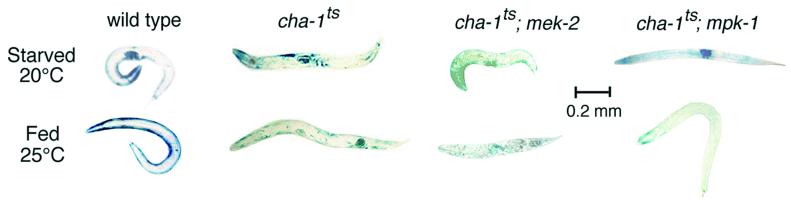
FIG. 6.
Reduction-of-function mutations in mek-2 or mpk-1 do not block starvation- or denervation-induced proteolysis. Histochemical stains for β-galactosidase activity in muscle are shown. Starvation of adult animals was for 24 h at 20°C as described previously (59). At 25°C the cha-1(p1182ts) mutation blocks acetylcholine synthesis; these animals were essentially wild-type at 20°C in both movement and protein degradation. The mek-2 mutants appear short because of a dpy-13 mutation in the background.
There was a rough correlation between Ras-induced protein degradation and a progressive loss of mobility (Table 1) in the mutant animals after temperature shift. This does not appear to result from proteolysis of myofilament proteins leading to major structural defects, since myofibrillar structure appeared grossly normal after staining with Oregon Green phalloidin (data not shown). Soluble proteins (including the LacZ fusion) and myofilament proteins are likely to be degraded by distinct routes (44); thus, the loss of movement may reflect degradation of soluble muscle enzymes needed to power contractions. Alternatively, the mobility defect may be unrelated to protein degradation in muscle.
TABLE 1.
Ras activation leads to time-dependent loss of mobilitya
| Genotype | Temp (°C) | Movement rate (waves/min) (h)
|
|||
|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | ||
| wt | 16 | 95 ± 12 | 101 ± 7 | 103 ± 15 | 100 ± 9 |
| 16 → 25 | 92 ± 8 | 95 ± 13 | 100 ± 11 | ||
| let-60(ga89ts) | 16 | 109 ± 20 | 115 ± 13 | 106 ± 15 | 98 ± 16 |
| 16 → 25 | 85 ± 14 | 57 ± 16 | 51 ± 13 | ||
Animals at t = 0 were grown from L1 larvae for 72 h at 16°C. Movement rates (mean ± standard deviation of 10 animals) were measured as described in Materials and Methods. wt, wild type.
It seems unlikely that Ras activation affects muscle simply by provoking functional denervation (50), since Ras-induced proteolysis was not prevented by the nicotinic agonist levamisole, which does prevent denervation-induced proteolysis (50). Conversely, neither mek-2 nor mpk-1 reduction-of-function mutations prevented the protein degradation induced by disruption of cholinergic signaling in a cha-1(p1182ts) mutant at nonpermissive temperature (Fig. 6), implying that MEK-MAPK signaling is not required for denervation-induced proteolysis. However, these observations do not exclude the possibility that signals from denervation and Ras activation converge at some downstream effector.
We presently believe that starvation and Ras activation trigger different proteolytic mechanisms. We have previously shown that in starved or denervated animals, the LacZ reporter protein becomes conjugated to ubiquitin (59) and that its subsequent inactivation is prevented by inhibitors of the proteasome (50). By contrast, the protein degradation provoked by Ras activation was not prevented by proteasome inhibitors Z-leu3-CHO (Fig. 7) or clasto-lactacystin-β-lactone (not shown), implying that Ras-induced degradation occurs by a mechanism independent of proteasome activity. Furthermore, in starvation-induced degradation it is characteristic that 146-kDa protein (as detected on Western blots) is lost more rapidly than is β-galactosidase activity, because proteolytic fragments remain noncovalently associated in active tetramers (59). By contrast, our data indicate that during Ras-induced reporter degradation there was a very good correlation between the disappearance of 146-kDa protein and that of activity (Fig. 1), perhaps implying that early proteolytic cleavages result in loss of activity. Taken together, these observations thus suggest that Ras-induced reporter degradation occurs by a mechanism distinct from that provoked by starvation or denervation.
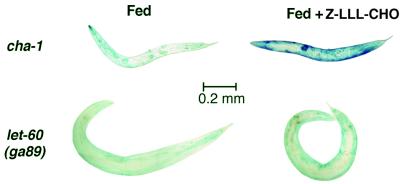
FIG. 7.
Proteasome inhibitor Z-leu3-CHO does not prevent Ras-induced protein degradation. Mutant strains [cha-1(p1182ts) or let-60(ga89ts)] were grown at 16°C and shifted as young adults to 25°C either with or without 200 μM Z-leu3-CHO (MG-132; BIOMOL Research Laboratories) and then stained with X-Gal 68 h later. The cha-1ts mutants were unable to synthesize acetylcholine at 25°C and were both genetically denervated and starved because they ceased feeding.
DISCUSSION
We have shown that Ras activation acutely provokes the degradation of reporter proteins in muscle by signaling to the Raf-MEK-MAPK cascade and that Ras-induced proteolysis is largely distinct from starvation-induced or denervation-induced proteolysis in signal transduction and probably in the proteolytic system(s) that is triggered.
There is certainly no reason to suppose that the degradation of the UNC-54::LacZ fusion protein in any way represents the catabolism of normal UNC-54 myosin heavy chains. The N-terminal UNC-54 fragment represents only a portion of the myosin ATPase domain (263 residues of the 1,966 present in full-length UNC-54) and is evidently insufficient to support assembly of the fusion protein into myofilaments. It was reported (59) that early in starvation-induced degradation, this region may be separated from the LacZ domain by a set of proteolytic cleavages. Our phalloidin staining results indicate that myofibrils are largely resistant to the proteolytic processes that degrade the reporter proteins. We believe that these soluble reporter proteins are instead reporting on the processes that are most likely to affect the soluble proteins of the muscle cytosol. There are few reliable identifications of endogenous muscle-specific, soluble cytosolic proteins, and few tools are available for studying them. Experiments to identify other endogenous substrates among soluble muscle proteins are presently in progress in our laboratory.
There are some caveats to the use of fusion proteins as reporters, one of which is that if misfolded, they may enter a misfolded-protein degradation pathway (37). However, it is very unlikely that the reporter proteins we have studied are degraded simply because they are recognized as misfolded. There is no direct way to assess the conformation of the N-terminal UNC-54 fragment, but we infer that the C-terminal 1,015-residue portion of the UNC-54::LacZ reporter protein achieves a native or near-native β-galactosidase conformation because the protein-protein subunit contacts are properly formed to promote association into tetramers (59) and the assembled tetramers have β-galactosidase activity. The crucial point is that in the context of well-fed wild-type animals, this fusion protein is completely stable (59) and thus does not by default enter a misfolded-protein degradation pathway by virtue of its structure alone. Even after Ras activation its degradation is not rapid, proceeding at a rate four- to fivefold lower than in starved animals.
Neither is it plausible that these fusion proteins are somehow identified as foreign and consequently singled out for degradation by a proteolytic system that has no endogenous protein substrates. It is extremely difficult to imagine how such discrimination might be achieved at the molecular level; indeed, even the best-known examples of enzymes that selectively degrade foreign molecules, the DNA restriction endonucleases, do not differentially identify foreign DNA molecules at all but instead ignore resident DNA because its recognition sites have been methylated. Thus, the transgene-coded proteins in our studies are probably reporting on the activity of a protein-degradation system that has naturally occurring protein substrates in muscle cells. In many of the most familiar instances of Ras-Raf-MEK-MAPK signaling, downstream effectors are phosphorylated by MAPK to trigger an increase in transcription leading to either a developmental decision (e.g., the differentiation of hypodermal precursor cells to form the C. elegans vulva [47]) or a more general increase in biosynthetic activity (e.g., increased cell proliferation after transformation). We have consequently become accustomed to thinking of Ras signaling as having fundamentally anabolic consequences, and it thus seems paradoxical at first that Ras-MAPK signaling should also promote the catabolism of existing proteins.
There are some cases in mammals where Ras-MAPK signaling has been implicated in promoting proteolysis of specific proteins, such as cell cycle regulators cyclin D1 (42), cyclin-dependent kinase inhibitor p27Kip1 (26), and myc (41). It has also been reported that matrix metalloprotease (22) and cathepsin (43) activities are upregulated by Ras signaling. However, this is the first report that Ras-MAPK signaling promotes protein degradation in differentiated muscle.
The body-wall muscle of C. elegans is most nearly similar to the striated skeletal muscle of vertebrates (55). A signaling process in mammalian skeletal muscle analogous to that we have uncovered in C. elegans muscle is suggested by the observations (38) in cultured mouse myotubes that expression of activated Raf promotes atrophy and, conversely, that expression of dominant-negative Raf promotes hypertrophy. It is important to note, however, that these cellular responses were not shown directly to reflect Raf-responsive change in the rate of proteolysis.
Signaling by the Ras-MAPK pathway opposes the conversion of committed mammalian myoblasts into terminally differentiated skeletal muscle (13, 35, 54), and Ras activation is classically associated with transformation or dedifferentiation (11), although it is not clear that the same Ras effectors mediate all these effects (56). On the other hand, it is evident that in order to lose its differentiated phenotype(s) and structures, a cell not only must synthesize new proteins but also must degrade at least some key proteins characteristic of the differentiated state (e.g., myoD in muscle [34, 46]). A similar argument may apply to remodeling of differentiated tissue. Protein degradation in skeletal muscle is increased by heavy exercise (5, 12, 24, 36), which also promotes an increase in MAPK activation (40, 57, 58). Thus, our results raise the intriguing possibility that the stimulation of proteolysis by Ras-MAPK signaling is an important feature of transformation and/or remodeling in mammalian muscle.
It is also worth considering more generally whether the temporal sequence of stimulation of protein synthesis and of protein degradation, and/or the balance between these processes, determines whether Ras signaling results in catabolic, anabolic, or remodeling effects. It may be significant in this context that Ras-stimulated protein degradation appears to involve the activation of a preexisting proteolytic system rather than to depend upon the synthesis of new proteins. This implies that the earliest response to Ras activation might be an increase in degradation of existing proteins and that a lag would likely intervene before Ras-promoted stimulation of transcription resulted in the eventual synthesis and accumulation of new proteins. A temporal separation of this kind would be well suited to facilitating a change in differentiated state or significant remodeling of a relatively plastic tissue like muscle.
Acknowledgments
This work was supported by NSF grants MCB-9630841 and MCB-0090734.
We are grateful to A. Fire, D. Eisenmann, S. Kim, A. Golden, J. Shaw, and M. Han for generously providing nematode strains, M. Sundaram for useful discussions, and R. Harper for technical assistance. Many strains were obtained from the Caenorhabditis Genetics Center, which is supported by the NIH National Center for Research Resources.
REFERENCES
- 1.Alessi, D. R., A. Cuenda, P. Cohen, D. T. Dudley, and A. R. Saltiel. 1995. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase in vitro and in vivo. J. Biol. Chem. 270:27489-27494. [DOI] [PubMed] [Google Scholar]
- 2.Aroian, R. V., and P. W. Sternberg. 1991. Multiple functions of let-23, a Caenorhabditis elegans receptor tyrosine kinase gene required for vulval induction. Genetics 128:251-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attaix, D., E. Aurousseau, L. Combaret, A. Kee, D. Larbaud, C. Ralliere, B. Souweine, D. Taillandier, and T. Tilignac. 1998. Ubiquitin-proteasome-dependent proteolysis in skeletal muscle. Reprod. Nutr. Dev. 38:153-165. [DOI] [PubMed] [Google Scholar]
- 4.Attaix, D., L. Combaret, M. N. Pouch, and D. Taillandier. 2001. Regulation of proteolysis. Curr. Opin. Clin. Nutr. Metab. Care 4:45-49. [DOI] [PubMed]
- 5.Biolo, G., S. P. Maggi, B. D. Williams, K. D. Tipton, and R. R. Wolfe. 1995. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am. J. Physiol. 268:E514-E520. [DOI] [PubMed]
- 6.Bolanowski, M. A., R. L. Russell, and L. A. Jacobson. 1981. Quantitative measures of aging in the nematode Caenorhabditis elegans. I. Population and longitudinal studies of two behavioral parameters. Mech. Ageing Dev. 15:279-295. [DOI] [PubMed] [Google Scholar]
- 7.Byerly, L., R. C. Cassada, and R. L. Russell. 1976. The life cycle of the nematode Caenorhabditis elegans. I. Wild-type growth and reproduction. Dev. Biol. 51:23-33. [DOI] [PubMed] [Google Scholar]
- 8.Campbell, S. L., R. Khosravi-Far, K. L. Rossman, G. J. Clark, and C. J. Der. 1998. Increasing complexity of Ras signaling. Oncogene 17:1395-1413. [DOI] [PubMed] [Google Scholar]
- 9.Cassada, R. C., and R. L. Russell. 1975. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 46:326-342. [DOI] [PubMed] [Google Scholar]
- 10.Cooney, R. N., S. R. Kimball, and T. C. Vary. 1997. Regulation of skeletal muscle protein turnover during sepsis: mechanisms and mediators. Shock 7:1-16. [DOI] [PubMed] [Google Scholar]
- 11.Crespo, P., and J. Leon. 2000. Ras proteins in the control of the cell cycle and cell differentiation. Cell. Mol. Life Sci. 57:1613-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dohm, G. L., E. B. Tapscott, and G. J. Kasperek. 1987. Protein degradation during endurance exercise and recovery. Med. Sci. Sports Exerc. 19:S166-S171. [PubMed]
- 13.Dorman, C. M., and S. E. Johnson. 1999. Activated Raf inhibits avian myogenesis through a MAPK-dependent mechanism. Oncogene 18:5167-5176. [DOI] [PubMed] [Google Scholar]
- 14.Dudley, D. T., L. Pang, S. J. Decker, A. J. Bridges, and A. R. Saltiel. 1995. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. USA 92:7686-7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eble, D. M., M. L. Spragia, A. G. Ferguson, and A. M. Samarel. 1999. Sarcomeric myosin heavy chain is degraded by the proteasome. Cell Tissue Res. 296:541-548. [DOI] [PubMed] [Google Scholar]
- 16.Eisenmann, D. M., and S. K. Kim. 1997. Mechanism of activation of the Caenorhabditis elegans ras homologue let-60 by a novel, temperature-sensitive, gain-of-function mutation. Genetics 146:553-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fire, A., and R. H. Waterston. 1989. Proper expression of myosin genes in transgenic nematodes. EMBO J. 8:3419-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fulks, R. M., J. B. Li, and A. L. Goldberg. 1975. Effects of insulin, glucose and amino acids on protein turnover in rat diaphragm. J. Biol. Chem. 250:290-298. [PubMed] [Google Scholar]
- 19.Furuno, K., M. N. Goodman, and A. L. Goldberg. 1990. Role of different proteolytic systems in the degradation of muscle proteins during denervation atrophy. J. Biol. Chem. 265:8550-8557. [PubMed] [Google Scholar]
- 20.Hajnal, A., C. W. Whitfield, and S. K. Kim. 1997. Inhibition of Caenorhabditis elegans vulval induction by gap-1 and by let-23 receptor tyrosine kinase. Genes Dev. 11:2715-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashizaki, S., Y. Iino, and M. Yamamoto. 1998. Characterization of the C. elegans gap-2 gene encoding a novel Ras-GTPase activating protein and its possible role in larval development. Genes Cells 3:189-202. [DOI] [PubMed] [Google Scholar]
- 22.Himelstein, B. P., E. J. Lee, H. Sato, M. Seiki, and R. J. Muschel. 1997. Transcriptional activation of the matrix metalloproteinase-9 gene in an H-ras and v-myc transformed rat embryo cell line. Oncogene 14:1995-1998. [DOI] [PubMed] [Google Scholar]
- 23.Kaech, S. M., C. W. Whitfield, and S. K. Kim. 1998. The LIN-2/LIN-7/LIN-10 complex mediates basolateral membrane localization of the C. elegans EGF receptor LET-23 in vulval epithelial cells. Cell 94:761-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasperek, G. J., and R. D. Snider. 1985. Increased protein degradation after eccentric exercise. Eur J. Appl. Physiol. Occup. Physiol. 54:30-34. [DOI] [PubMed] [Google Scholar]
- 25.Katz, W. S., G. M. Lesa, D. Yannoukakos, T. R. Clandinin, J. Schlessinger, and P. W. Sternberg. 1996. A point mutation in the extracellular domain activates LET-23, the Caenorhabditis elegans epidermal growth factor receptor homolog. Mol. Cell. Biol. 16:529-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawada, M., S. Yamagoe, Y. Murakami, K. Suzuki, S. Mizuno, and Y. Uehara. 1997. Induction of p27Kip1 degradation and anchorage independence by Ras through the MAP kinase signaling pathway. Oncogene 15:629-637. [DOI] [PubMed] [Google Scholar]
- 27.Kimura, K. D., H. A. Tissenbaum, Y. Liu, and G. Ruvkun. 1997. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277:942-946. [DOI] [PubMed] [Google Scholar]
- 28.Lackner, M. R., and S. K. Kim. 1998. Genetic analysis of the Caenorhabditis elegans MAP kinase gene mpk-1. Genetics 150:103-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, J. B., and A. L. Goldberg. 1976. Effects of food deprivation on protein synthesis and degradation in rat skeletal muscles. Am. J. Physiol. 231:441-448. [DOI] [PubMed] [Google Scholar]
- 30.Llovera, M., C. Garcia-Martinez, N. Agell, F. J. Lopez-Soriano, F. J. Authier, R. K. Gherardi, and J. M. Argiles. 1998. Ubiquitin and proteasome gene expression is increased in skeletal muscle of slim AIDS patients. Int. J. Mol. Med. 2:69-73. [PubMed] [Google Scholar]
- 31.Medina, R., S. W. Wing, A. Haas, and A. L. Goldberg. 1991. Activation of the ubiquitin-ATP-dependent proteolytic system in skeletal muscle during fasting and denervation atrophy. Biomed. Biochim. Acta 50:347-356. [PubMed] [Google Scholar]
- 32.Mitch, W. E., and A. L. Goldberg. 1996. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N. Engl. J. Med. 335:1897-1905. [DOI] [PubMed] [Google Scholar]
- 33.Newby, F. D., and S. R. Price. 1998. Determinants of protein turnover in health and disease. Miner. Electrolyte Metab. 24:6-12. [DOI] [PubMed] [Google Scholar]
- 34.Odelberg, S. J., A. Kollhoff, and M. T. Keating. 2000. Dedifferentiation of mammalian myotubes induced by msx1. Cell 103:1099-1109. [DOI] [PubMed] [Google Scholar]
- 35.Olson, E. N., G. Spizz, and M. A. Tainsky. 1987. The oncogenic forms of N-ras or H-ras prevent skeletal myoblast differentiation. Mol. Cell. Biol. 7:2104-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ordway, G. A., P. D. Neufer, E. R. Chin, and G. N. DeMartino. 2000. Chronic contractile activity upregulates the proteasome system in rabbit skeletal muscle. J. Appl. Physiol. 88:1134-1141. [DOI] [PubMed] [Google Scholar]
- 37.Patil, C., and P. Walter. 2001. Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr. Opin. Cell Biol. 13:349-355. [DOI] [PubMed] [Google Scholar]
- 38.Rommel, C., B. A. Clarke, S. Zimmermann, L. Nunez, R. Rossman, K. Reid, K. Moelling, G. D. Yancopoulos, and D. J. Glass. 1999. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science 286:1738-1741. [DOI] [PubMed] [Google Scholar]
- 39.Rommel, C., and E. Hafen. 1998. Ras—a versatile cellular switch. Curr. Opin. Genet. Dev. 8:412-418. [DOI] [PubMed] [Google Scholar]
- 40.Ryder, J. W., R. Fahlman, H. Wallberg Henriksson, D. R. Alessi, A. Krook, and J. R. Zierath. 2000. Effect of contraction on mitogen-activated protein kinase signal transduction in skeletal muscle. Involvement of the mitogen- and stress-activated protein kinase 1. J. Biol. Chem. 275:1457-1462. [DOI] [PubMed] [Google Scholar]
- 41.Sears, R., F. Nuckolls, E. Haura, Y. Taya, K. Tamai, and J. R. Nevins. 2000. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 14:2501-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shao, J., H. Sheng, R. N. DuBois, and R. D. Beauchamp. 2000. Oncogenic Ras-mediated cell growth arrest and apoptosis are associated with increased ubiquitin-dependent cyclin D1 degradation. J. Biol. Chem. 275:22916-22924. [DOI] [PubMed] [Google Scholar]
- 43.Silberman, S., M. Janulis, and R. M. Schultz. 1997. Characterization of downstream Ras signals that induce alternative protease-dependent invasive phenotypes. J. Biol. Chem. 272:5927-5935. [DOI] [PubMed] [Google Scholar]
- 44.Solomon, V., and A. L. Goldberg. 1996. Importance of the ATP-ubiquitin-proteasome pathway in the degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J. Biol. Chem. 271:26690-26697. [DOI] [PubMed] [Google Scholar]
- 45.Solomon, V., S. H. Lecker, and A. L. Goldberg. 1998. The N-end rule pathway catalyzes a major fraction of the protein degradation in skeletal muscle. J. Biol. Chem. 273:25216-25222. [DOI] [PubMed] [Google Scholar]
- 46.Song, A., Q. Wang, M. G. Goebl, and M. A. Harrington. 1998. Phosphorylation of nuclear MyoD is required for its rapid degradation. Mol. Cell. Biol. 18:4994-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sternberg, P. W., and M. Han. 1998. Genetics of RAS signaling in C. elegans. Trends Genet. 14:466-472. [DOI] [PubMed] [Google Scholar]
- 48.Sternberg, P. W., G. Lesa, J. Lee, W. S. Katz, C. Yoon, T. R. Clandinin, L. S. Huang, H. M. Chamberlin, and G. Jongeward. 1995. LET-23-mediated signal transduction during Caenorhabditis elegans development. Mol. Reprod. Dev. 42:523-528. [DOI] [PubMed] [Google Scholar]
- 49.Stone, S., and J. E. Shaw. 1993. A Caenorhabditis elegans act-4::lacZ fusion: use as a transformation marker and analysis of tissue-specific expression. Gene 131:167-173. [DOI] [PubMed] [Google Scholar]
- 50.Szewczyk, N. J., J. J. Hartman, S. J. Barmada, and L. A. Jacobson. 2000. Genetic defects in acetylcholine signalling promote protein degradation in muscle cells of Caenorhabditis elegans. J. Cell Sci. 113:2003-2010. [DOI] [PubMed] [Google Scholar]
- 51.Tawa, N. E., Jr., R. Odessey, and A. L. Goldberg. 1997. Inhibitors of the proteasome reduce the accelerated proteolysis in atrophying rat skeletal muscles. J. Clin. Investig. 100:197-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tisdale, M. J. 2001. Loss of skeletal muscle in cancer: biochemical mechanisms. Front. Biosci. 6:D164-D174. [DOI] [PubMed]
- 53.Toomey, D., H. P. Redmond, and D. Bouchier Hayes. 1995. Mechanisms mediating cancer cachexia. Cancer 76:2418-2426. [DOI] [PubMed] [Google Scholar]
- 54.Vaidya, T. B., C. M. Weyman, D. Teegarden, C. L. Ashendel, and E. J. Taparowsky. 1991. Inhibition of myogenesis by the H-ras oncogene: implication of a role for protein kinase C. J. Cell Biol. 114:809-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waterston, R. H. 1988. Muscle, p. 281-335. In W. Wood and the Community of C. elegans Researchers (ed.), The nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 56.Weyman, C. M., M. B. Ramocki, E. J. Taparowsky, and A. Wolfman. 1997. Distinct signaling pathways regulate transformation and inhibition of skeletal muscle differentiation by oncogenic Ras. Oncogene 14:697-704. [DOI] [PubMed] [Google Scholar]
- 57.Widegren, U., J. W. Ryder, and J. R. Zierath. 2001. Mitogen-activated protein kinase signal transduction in skeletal muscle: effects of exercise and muscle contraction. Acta Physiol. Scand. 172:227-238. [DOI] [PubMed] [Google Scholar]
- 58.Widegren, U., C. Wretman, A. Lionikas, G. Hedin, and J. Henriksson. 2000. Influence of exercise intensity on ERK/MAP kinase signalling in human skeletal muscle. Pflueg. Arch. 441:317-322. [DOI] [PubMed] [Google Scholar]
- 59.Zdinak, L. A., I. B. Greenberg, N. J. Szewczyk, S. J. Barmada, M. Cardamone Rayner, J. J. Hartman, and L. A. Jacobson. 1997. Transgene-coded chimeric proteins as reporters of intracellular proteolysis: starvation-induced catabolism of a lacZ fusion protein in muscle cells of Caenorhabditis elegans. J. Cell. Biochem. 67:143-153. [PubMed] [Google Scholar]