Abstract
Regulation of mast cell degranulation is dependent on the subtle interplay of cellular signaling proteins. The Src homology 2 (SH2) domain-containing inositol-5′-phosphatase (SHIP), which acts as the gatekeeper of degranulation, binds via both its SH2 domain and its phosphorylated NPXY motifs to the adapter protein Shc via the latter's phosphorylated tyrosines and phosphotyrosine-binding domain, respectively. This theoretically leaves Shc's SH2 domain available to bind proteins, which might be part of the SHIP/Shc complex. In a search for such proteins, protein kinase C-δ (PKC-δ) was found to coprecipitate in mast cells with Shc and to interact with Shc's SH2 domain following antigen or pervanadate stimulation. Phosphorylation of PKC-δ's Y332, most likely by Lyn, was found to be responsible for PKC-δ's binding to Shc's SH2 domain. Using PKC-δ−/− bone marrow-derived mast cells (BMMCs), we found that the antigen-induced tyrosine phosphorylation of Shc was similar to that in wild-type (WT) BMMCs while that of SHIP was significantly increased. Moreover, increased translocation of PKC-δ to the membrane, as well as phosphorylation at T505, was observed in SHIP−/− BMMCs, demonstrating that while PKC-δ regulates SHIP phosphorylation, SHIP regulates PKC-δ localization and activation. Interestingly, stimulation of PKC-δ−/− BMMCs with suboptimal doses of antigen yielded a more sustained calcium mobilization and a significantly higher level of degranulation than that of WT cells. Altogether, our data suggest that PKC-δ is a negative regulator of antigen-induced mast cell degranulation.
Mast cell degranulation is induced by multivalent allergens which cross-link immunoglobulin E (IgE) molecules that are bound to high-affinity IgE receptors (FcɛR1) at the cell surface (3). Receptor aggregation leads subsequently to the activation of phosphatidylinositol 3-kinase (PI3K), generating phosphatidylinositol-3,4,5-trisphosphate (PIP3) at the plasma membrane. PIP3 then attracts various intracellular proteins with pleckstrin homology (PH) domains that play critical roles in triggering degranulation. These include phospholipase C-γ (PLC-γ) (12) and the tyrosine kinase Btk (44). PLC-γ hydrolyzes PI-4,5-biophosphate, thereby generating diacylglycerol (DAG) and inositol-1,4,5-trisphosphate (the latter triggering the release of intracellular calcium from the endoplasmic reticulum). The tyrosine kinase Btk (44) phosphorylates and activates PLC-γ, thereby sustaining calcium release from the endoplasmic reticulum and promoting the influx of extracellular calcium through ICRAC channels in the plasma membrane (45). The Src homology 2 (SH2) domain-containing inositol-5′-phosphatase (SHIP) acts as a gatekeeper of antigen-induced degranulation by hydrolyzing PIP3 (18), and there is considerable interest in identifying molecules that interact with SHIP in the hope that some of these molecules might modulate its activity and, in turn, regulate mast cell degranulation. The predominant SHIP-interacting protein in hemopoietic cells is the adapter protein Shc, which consists of an N-terminal phosphotyrosine-binding (PTB) domain; a central collagen homology (CH) domain containing three tyrosines, Y239, Y240, and Y317, that become phosphorylated and targets of SH2-containing proteins upon cell stimulation; and a C-terminal SH2 domain. SHIP appears to interact with Shc via different intermolecular mechanisms, depending on the cell type involved. In myeloid cells, for example, SHIP appears to interact with Shc in a bidentate manner, with SHIP's SH2 domain binding to one of the three phosphorylated tyrosines within Shc's CH domain while SHIP's two phosphorylated NPXY motifs bind to Shc's PTB domain (34). In B lymphocytes, on the other hand, the adapter protein Grb2 appears to be required for an efficient association between Shc and SHIP, and a ternary complex of SHIP, Shc, and Grb2 is formed (16). Furthermore, studies with T cells suggest that Shc interacts solely via its PTB domain with one of SHIP's two phosphorylated NPXY motifs (29). Common to all three models, however, the SH2 domain of Shc is not involved in the Shc-SHIP interaction and therefore might be available to recruit one or more regulatory proteins into the Shc-SHIP complex.
To date, very few proteins have been shown to bind to the SH2 domain of Shc. These include the signal-transducing subunits of the B-cell receptor (2) and the β-chain of FcɛR1 (23), following receptor activation. Interestingly, unlike the B-cell receptor system, where both signaling components, Ig-α and Ig-β, interact with Shc, only the β-subunit of FcɛR1 appears to associate with this adapter protein. In addition, the Shc SH2 domain has been shown to bind to the adapter protein Gab2 (4) and mPAL, a protein whose expression is restricted to tissues containing actively dividing cells (47).
The present study was aimed at identifying proteins in bone marrow-derived mast cells (BMMCs) that were capable of binding to Shc via the latter's SH2 domain. Interestingly, protein kinase C-δ (PKC-δ) was found to be such a protein and to exist in a complex, following antigen stimulation, with both Shc and SHIP. Studies with both PKC-δ−/− and SHIP−/− BMMCs suggest that PKC-δ and SHIP regulate each other's activation and/or phosphorylation states. Moreover, using BMMCs from wild-type (WT) and PKC-δ−/− mice, we found that PKC-δ reduced antigen-induced mast cell degranulation.
MATERIALS AND METHODS
Cell culture.
RBL-2H3 cells were maintained (37°C; 5% CO2) in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine, 50 U of penicillin/ml, 50 mg of streptomycin/ml, and 50 μM 2-mercaptoethanol. For the generation of BMMCs, bone marrow cells (106/ml) from 4- to 8-week-old WT and PKC-δ−/− littermates (129/Sv) (32) were cultured (37°C; 5% CO2) in a single cell suspension in Iscove's modified Dulbecco's medium (IMDM) containing 20% FCS, 1% X63Ag8-653-conditioned medium as a source of interleukin 3 (IL-3) (22), 2 mM l-glutamine, 10−5 M 2-mercaptoethanol, 50 U of penicillin/ml, and 50 mg of streptomycin/ml. At weekly intervals, the nonadherent cells were reseeded at 106 cells/ml in fresh medium. By 5 to 6 weeks in culture, >99% of the cells were c-kit and FcɛR1 positive as assessed by fluorescence-activated cell sorter (FACS) analysis using phycoerythrin-labeled anti-c-kit antibodies (Pharmingen, Mississauga, Canada) and fluorescein isothiocyanate-labeled IgE (anti-erythropoietin 26 [17]), respectively. Adherent bone marrow cells were used for experiments after 5 to 7 days in culture. SHIP−/− BMMCs, as well as the respective WT cells, were generated and kept as previously described (17). Drosophila S2 cells were maintained (27°C; atmospheric CO2 levels) in Schneider's Drosophila medium (Gibco Life Technologies, Karlsruhe, Germany) containing 5% FCS and 50 μg of gentamicin (Gibco)/ml.
Construct generation and S2 cell transfection.
PKC-δ of rat origin was cloned into the expression vector pRmHa-3 containing a metallothionein promoter to yield pD-rPKC-δ. The vectors pD-hLyn and pD-hSyk were kindly provided by V. Rolli (Freiburg, Germany). The PKC-δ Y-to-F mutations were introduced using the QuickChange site-directed mutagenesis kit (Stratagene, Amsterdam, The Netherlands) according to the manufacturer's instructions. The following primer pairs were used: PKC-δ Y52F sense (5′-G GTA CAG AAG AAG CCC ACC ATG TTC CCT GAG TGG AAG TCG ACA TTC G-3′) and antisense (5′-C GAA TGT CGA CTT CCA CTC AGG GAA CAT GGT GGG CTT CTT CTG TAC C-3′); PKC-δ Y64F sense (5′-CGA CGC CCA CAT CTT TGG AGG CCG TGT CAT-3′) and antisense (5′-ATG ACA CGG CCT CCA AAG ATG TGG GCG TCG-3′); PKC-δ Y187F sense (5′-GCC TCA ACA AGC AAG GCT TCA AGT GCA GGC AAT GC-3′) and antisense (5′-GCA TTG CCT GCA CTT GAA GCC TTG CTT GTT GAG GC-3′); PKC-δ Y332F sense (5′-CCA GAC AAC AAC GGG ACG TTT GGC AAG ATC TGG G-3′) and antisense (5′-CCC AGATCT TGC CAA ACG TCC CGT TGT TGT CTG G-3′); PKC-δ Y372F sense (5′-GAA GGG CAA GGA AAG GTT CTT TGC AAT CAA GTA CCT G-3′) and antisense (5′-CTG GTA CTT GAT TGC AAA GAA CCT TTC CTT GCC CTT C-3′); and PKC-δ Y565F sense (5′-GGG TGG ACA CAC CGC ACT TCC CGC GCT GG-3′) and antisense (5′-CCA GCG CGG GAA GTG CGG TGT GTC CAC CC-3′). cDNA constructs were verified by sequencing to ensure that no unwanted mutation had been introduced in the process of their generation (data not shown). S2 cells were transfected using the calcium-phosphate method. Expression of the exogenous proteins was induced from the metallothionein promoter by addition of 1 mM CuSO4; 12 to 24 h later, the cells were harvested and used for the respective experiments.
Reagents and antibodies.
The glutathione S-transferase (GST) fusion protein consisting of the 27-kDa N-terminal GST linked to the human Shc SH2 domain (amino acid residues 488 to 583) was expressed in Escherichia coli in a pGEX-2T plasmid (Pharmacia), and the fusion protein was purified from lysates of sonicated bacteria with glutathione-Sepharose beads (Pharmacia) as described previously (9). Pervanadate (PV) solution was prepared according to the method of Baumann et al. (2). Polyclonal anti-Shc antibodies were from Transduction Laboratories (Hamburg, Germany). Polyclonal anti-PKC-δ antibodies (C-17), polyclonal anti-Lyn antibodies (44), and monoclonal anti-SHIP antibodies (P1C1) were obtained from Santa Cruz (Heidelberg, Germany). Monoclonal anti-phosphotyrosine antibody (4G10) was purchased from Biozol (Eching, Germany), and polyclonal anti-GST antibodies were from Molecular Probes (Leiden, The Netherlands). Polyclonal antibodies specific for PKC-δ phosphorylated at T505 were purchased from Cell Signaling Technology (Frankfurt am Main, Germany). Polyclonal anti-SHIP antibodies (anti-N and anti-M) have been described (8). Monoclonal anti-β-chain antibodies were a kind gift from R. Siraganian (Bethesda, Md.). Dinitrophenol-human serum albumin (DNP-HSA) containing 30 to 40 mol of DNP per mol of albumin was purchased from Sigma (Deisenhofen, Germany), and phorbol myristate acetate (PMA), as well as thapsigargin, was purchased from Calbiochem (Bad Soden, Germany).
Immunoprecipitation and Western blotting.
Cells were either preloaded with 0.5 μg of IgE (anti-DNP; clone SPE-7; Sigma)/ml overnight or left untreated and then washed in phosphate-buffered saline and resuspended (2 × 107/ml) in Tyrode's buffer (130 mM NaCl, 5 mM KCl, 1.4 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, and 0.1% bovine serum albumin in 10 mM HEPES, pH 7.4). The cells were adapted to 37°C for 20 min and stimulated with various concentrations of PV or DNP-HSA. After stimulation, the cells were pelleted and solubilized with 0.5% NP-40 in 4°C phosphorylation solubilization buffer (PSB; 500 μl) (34). Thirty microliters of the total cell lysates (TCL) was directly separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subjected to Western blot analysis. The remaining lysate was subjected to immunoprecipitation with three subsequent washing steps using PSB containing 0.1% NP-40. The precipitate was separated by SDS-PAGE and analyzed by Western blotting.
λ-PPase treatment and membrane preparation.
Anti-PKC-δ immunoprecipitates were washed three times with 1 ml of PSB containing 0.1% NP-40 followed by two washes with 100 μl of λ-phosphatase (λ-PPase) buffer (0.1 mM Na2EDTA, 5 mM dithiothreitol, and 0.01% Brij 35 in 50 mM Tris-HCl, pH 7.5). The washed precipitates were incubated for 1 h at 30°C in 50 μl of λ-PPase buffer containing 2 mM MnCl2 and 200 U of λ-PPase (New England Biolabs, Frankfurt am Main, Germany). The reaction was stopped by adding 12 μl of 5× sample buffer and boiling the mixture for 5 min. The phosphatase-treated precipitates were then resolved on SDS-8% polyacrylamide gels. Membrane preparation was carried out as previously described (20).
Degranulation assay.
For degranulation studies, cells were preloaded with 0.5 μg of IgE anti-DNP/ml overnight. The cells were then washed and resuspended in Tyrode's buffer, adapted to 37°C for 30 min, and treated for 25 min at 37°C as described above. The degree of degranulation was determined by measuring release of β-hexosaminidase (19).
Calcium measurement.
IgE-preloaded BMMCs were washed with IMDM; resuspended at 5 × 106/ml in IMDM containing 1% FCS, 30 μM Indo-1 AM (Molecular Probes), and 0.045% pluronic F-127 (Molecular Probes); and incubated for 45 min at 37°C. The cells were then pelleted, resuspended in IMDM containing 1% FCS, and analyzed in a FACStar plus (Becton Dickinson, Franklin Lakes, N.J.) after stimulation procedures. The FACS profiles were converted to line graph data using the FCS Assistant version 1.3.1a application.
RESULTS
Shc binds via its SH2 domain to the tyrosine-phosphorylated form of PKC-δ.
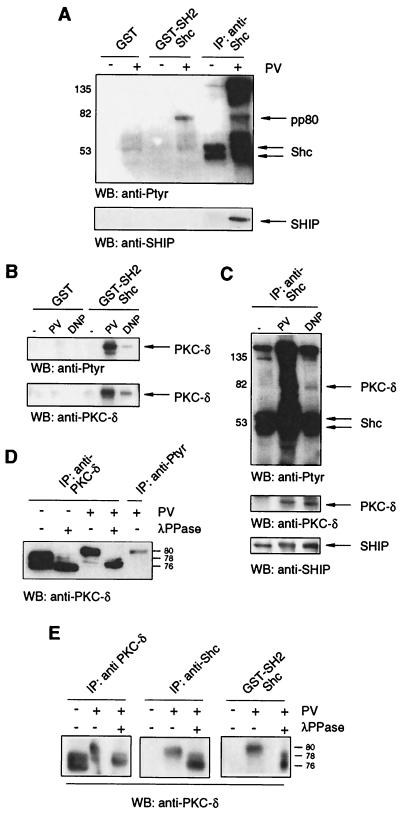
The goal of this study was to identify binding partners for Shc via Shc's SH2 domain, reasoning that these proteins might be present in the Shc-SHIP complex that forms following antigen-induced activation. An 80-kDa tyrosine-phosphorylated protein (pp80) bound to beads bearing a GST-Shc-SH2 fusion protein but neither to beads bearing GST alone (Fig. 1A, top) nor to a GST-Shc-PTB fusion protein (data not shown) following PV stimulation of RBL-2H3 mast cells. An 80-kDa tyrosine-phosphorylated protein also coprecipitated with Shc following PV stimulation of these cells. SHIP, as expected, coprecipitated with anti-Shc antibodies but did not bind to GST-SH2-bearing beads (Fig. 1A, bottom), suggesting that pp80 binds to Shc rather than to SHIP. An exhaustive probing of this 80-kDa band with antibodies to candidate proteins revealed that it was the tyrosine-phosphorylated form of PKC-δ (Fig. 1B). Accordingly, Shc and SHIP could be detected in anti-PKC-δ immunoprecipitates from PV-treated RBL-2H3 mast cells (data not shown). We further demonstrated that the interaction of Shc's SH2 domain with PKC-δ could also be detected in lysates of antigen-stimulated RBL-2H3 cells (Fig. 1B), as well as untransformed IL-3-dependent CFTL12 mast cells (data not shown). Moreover, though overall stimulation was less efficient with antigen than with PV, PKC-δ and SHIP were copurified with anti-Shc antibodies after FcɛR1 engagement on RBL-2H3 mast cells (Fig. 1C). Using the raft-disrupting detergent n-dodecyl-α-d-maltoside, we ruled out the possibility that this copurification of Shc, SHIP, and PKC-δ was due to cocompartmentalization within lipid rafts (data not shown).
FIG. 1.
Both PV treatment and FcɛRI engagement stimulate the tyrosine phosphorylation of PKC-δ and its association with Shc via the latter's SH2 domain. (A) RBL-2H3 cells were untreated (−) or treated (+) with 75 μM PV for 3 min, and NP-40 cell lysates were incubated with Sepharose beads bearing GST or GST-Shc-SH2 or with anti-Shc antibodies. The precipitates were analyzed by anti-Ptyr (top) and anti-SHIP (bottom) immunoblotting. IP, immunoprecipitation; WB, Western blotting. (B) IgE-preloaded RBL-2H3 cells were untreated or treated with 75 μM PV for 2 min or 200 ng of DNP-HSA/ml (DNP) for 3 min, and the lysates were incubated with beads bearing GST or GST-Shc-SH2. The precipitates were analyzed by anti-Ptyr (top) and anti-PKC-δ (bottom) immunoblotting. (C) IgE-preloaded RBL-2H3 cells were treated with 75 μM PV for 2 min, 200 ng of DNP-HSA/ml (DNP) for 3 min, or left unstimulated (−), and the lysates were incubated with anti-Shc antibodies. The precipitates were analyzed by anti-Ptyr (top), anti-PKC-δ (middle), and anti-SHIP (bottom) immunoblotting. (D) RBL-2H3 cells were untreated or treated with 75 μM PV for 3 min, and cell lysates were incubated with antibodies specific for PKC-δ or Ptyr. The precipitates were incubated with λ-PPase (+)or λ-PPase buffer only (−) and were analyzed by anti-PKC-δ immunoblotting. (E) Lysates of unstimulated and PV-stimulated (75 μM; 3 min) RBL-2H3 cells were incubated with anti-PKC-δ antibodies (left), anti-Shc antibodies (middle), or GST-Shc-SH2 (right). The precipitates were incubated with λ-PPase (+) or λ-PPase buffer only (−) and analyzed by anti-PKC-δ immunoblotting. Valves to right or left of gels are in kilodaltons.
PKC-δ has been reported to be a doublet of 76 and 78 kDa (37). We detected this doublet in unstimulated RBL-2H3 cells and found that λ-PPase treatment resulted in the disappearance of the 78-kDa species (Fig. 1D), in agreement with this species being a phosphorylated form of the 76-kDa species (37). A shift of almost all PKC-δ molecules to approximately 80 kDa was observed in PV-stimulated cells, and the 80-kDa species could be reverted to 76 kDa by λ-PPase treatment (Fig. 1D). The same 80-kDa PKC-δ species was observed in anti-Ptyr precipitates of PV-treated cells (Fig. 1D, extreme right lane), indicating that the 80-kDa fraction of total PKC-δ is composed, at least in part, of tyrosine-phosphorylated PKC-δ. Correlating with this observation, as shown in Fig. 1E, PKC-δ precipitating with anti-Shc antibodies or the Shc GST-SH2 fusion protein is represented by the 80-kDa species, which can be dephosphorylated by λ-PPase to yield the 76-kDa protein.
PKC-δ is the only PKC isoform that complexes with Shc and SHIP.
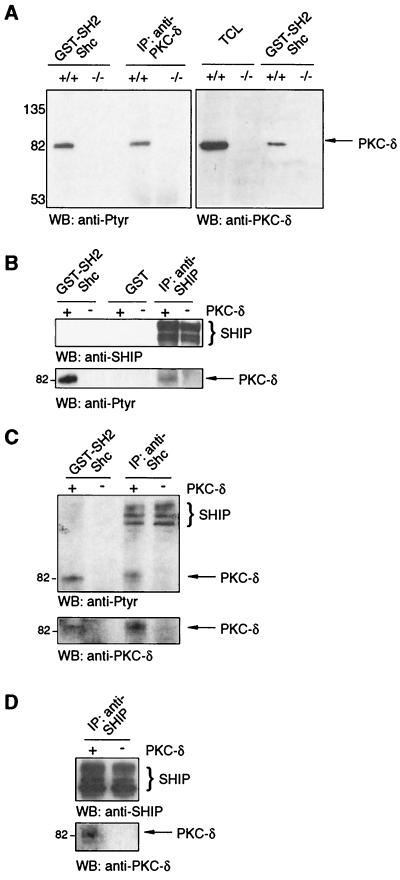
To address the question of whether other tyrosine-phosphorylated proteins, especially PKCs, are able to substitute for PKC-δ binding to Shc's SH2 domain, we used PKC-δ-deficient cells derived from PKC-δ knockout mice (32) (material requests concerning the PKC-δ knockout mice should be addressed to michael.leitges@mpihan.mpg.de). Adherent bone marrow cells from WT and PKC-δ−/− mice were stimulated with PV, and the lysates were incubated with beads containing GST-Shc-SH2 fusion protein or with anti-PKC-δ antibodies. As in RBL-2H3 cells, an Shc SH2 domain-interacting 80-kDa tyrosine-phosphorylated protein was detected in adherent bone marrow cell lysates from WT cells but was missing in lysates from PKC-δ−/− cells (Fig. 2A, left), indicating that no other tyrosine-phosphorylated proteins in the size range compatible with other PKC isotypes are substituting for PKC-δ. Western blot analysis of the GST-Shc-SH2 bead-bound protein with PKC-δ-specific antibodies confirmed that the protein was PKC-δ (Fig. 2A, right). To determine if SHIP was present within the PKC-δ/Shc complex, lysates of PV-stimulated adherent bone marrow cells were incubated with beads containing GST-Shc-SH2 or GST alone or with SHIP-specific antibodies. As shown in Fig. 2B, tyrosine-phosphorylated PKC-δ bound to GST-Shc-SH2 but not to GST beads alone. Anti-SHIP antibodies also precipitated PKC-δ, suggesting that SHIP is present in the Shc/PKC-δ complex (Fig. 2B).
FIG. 2.
PKC-δ is the only PKC isoform that associates with Shc and is present in Shc/SHIP complexes. (A) Adherent bone marrow cells from PKC-δ+/+ and PKC-δ−/− mice were stimulated with 75 μM PV for 3 min, and the lysates were subjected directly to SDS-PAGE (TCL) or incubated with GST-Shc-SH2 beads or PKC-δ-specific antibodies and subjected to Western analysis (WB) with anti-Ptyr antibodies (left) and anti-PKC-δ antibodies (right). (B) Adherent bone marrow cells from PKC-δ+/+ (+) and PKC-δ−/− (−) mice were PV stimulated (75 μM; 3 min), and the lysates were incubated with GST or GST-Shc-SH2 beads or anti-SHIP antibodies. The precipitates were analyzed by anti-SHIP (top) and anti-Ptyr (bottom) immunoblotting. (C) PKC-δ+/+ (+) and−/− (−) BMMCs were PV stimulated (75 μM; 3 min), and the lysates were incubated with GST-Shc-SH2 beads or anti-Shc antibodies. The precipitates were subjected to Western analysis with anti-Ptyr (top) and anti-PKC-δ (bottom) antibodies. (D) Total cell lysates from PV-stimulated PKC-δ+/+ and PKC-δ−/− BMMCs were incubated with anti-SHIP antibodies, and the immunoprecipitates were subjected to Western analysis with anti-SHIP (top) and anti-PKC-δ (bottom) antibodies. Values to right or left of gels are in kilodaltons.
To verify these findings in mast cells, we generated PKC-δ+/+ and PKC-δ−/− BMMCs by in vitro differentiation of bone marrow from WT and PKC-δ−/− littermates, stimulated these with PV, and compared the tyrosine-phosphorylated proteins that bound to GST-Shc-SH2 beads and to anti-Shc antibodies. As shown in Fig. 2C (top), SHIP from both cell types was found to coprecipitate with Shc, while only in the WT lysates, an 80-kDa tyrosine-phosphorylated protein was purified and subsequently shown to be PKC-δ (Fig. 2C, bottom). Interestingly, as was observed in adherent bone marrow cells, no other tyrosine-phosphorylated proteins in the size range compatible with other PKC isotypes were detected, again indicating the δ-isotype specificity of the Shc interaction. Furthermore, PKC-δ was also detected in anti-SHIP immunoprecipitates of stimulated PKC-δ+/+ BMMCs (Fig. 2D). These data suggested that PKC-δ/Shc/SHIP complexes formed after stimulation of mast cells via FcɛR1.
Shc binds via its SH2 domain to phosphorylated Y332 within PKC-δ.
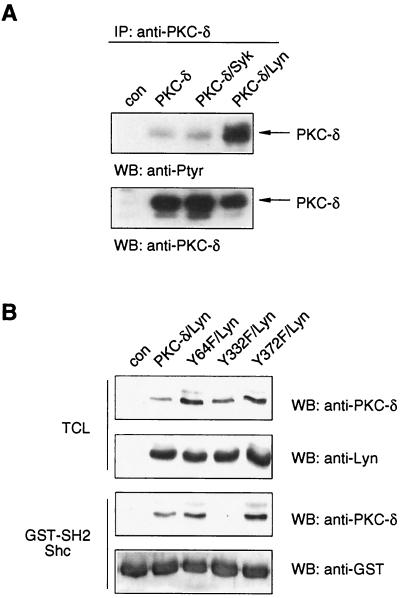
Having established that tyrosine-phosphorylated PKC-δ binds to the SH2 domain of Shc, we sought to determine the tyrosine residue(s) within PKC-δ protein responsible for this binding. Using Drosophila S2 cells, we first assessed which FcɛR1-proximal tyrosine kinase was capable of phosphorylating PKC-δ in this cell system. As shown in Fig. 3A, PKC-δ was only weakly tyrosine phosphorylated in S2 cells, and coexpression of the tyrosine kinase Syk did not alter this level of phosphorylation. The coexpression of Lyn, however, dramatically increased the tyrosine phosphorylation of PKC-δ (Fig. 3A) and thereby induced binding to beads bearing Shc's SH2 domain, as shown in Fig. 3B.
FIG. 3.
PKC-δ binds via its phosphorylated Y332 to Shc's SH2 domain. (A) Drosophila S2 cells were left untransfected (con) or transfected with expression plasmids encoding rat PKC-δ, rat PKC-δ and human Syk, or rat PKC-δ and human Lyn. Expression of exogenous proteins was induced by CuSO4, and 24 h later, total cell lysates were subjected to anti-PKC-δ immunoprecipitation (IP) and Western analysis (WB) with anti-Ptyr (top) and anti-PKC-δ (bottom) antibodies. (B) Plasmids encoding rat PKC-δ WT, Y64F, Y332F, or Y372F were cotransfected with a human Lyn-containing plasmid into S2 cells. Protein expression was induced, and TCL were subjected directly to Western analysis with anti-PKC-δ and anti-Lyn antibodies (upper two blots) or were first incubated with GST-Shc-SH2 beads and the precipitates then subjected to Western analysis with anti-PKC-δ and anti-GST antibodies (lower two blots).
The consensus sequence recognized by Shc's SH2 domain has been reported as Y-I/E/Y/L-X-I/L/M (50). In the mouse, rat, and human PKC-δ proteins, there are only two tyrosine residues (out of 19, 21, and 20, respectively) possessing this consensus sequence, with an isoleucine at the +3 position (Y332 and Y372 in the rat sequence). Both tyrosines, as well as their surrounding sequences, are conserved among rat, mouse, and human PKC-δ proteins (data not shown). Furthermore, N-terminal to both tyrosines are acidic amino acids thought to be important for substrate recognition by Lyn (55). To test this, the WT and Y332F and Y372F PKC-δ mutants were coexpressed with Lyn in S2 cells, and binding to GST-Shc-SH2 beads was analyzed. The Y64F mutant of PKC-δ was included as a putative negative control, since the +3 position of Y64 is theoretically not compatible with binding to Shc's SH2 domain. Figure 3B shows that the proteins were comparably expressed and the Y332F mutant could not bind the Shc GST-SH2 fusion protein. Interestingly, the Y332F mutant was tyrosine phosphorylated to almost the same extent as WT PKC-δ by Lyn (data not shown), suggesting that even though Y332 is responsible for binding Shc, it is not a major tyrosine phosphorylation site within PKC-δ. The Y372F and Y64F mutants bound as well as WT PKC-δ to Shc's SH2 domain (Fig. 3B). Since Y52 and Y187 had been previously reported to be major tyrosine phosphorylation sites of PKC-δ (33, 51), we also assayed them for Shc SH2 domain binding. Similar to the Y64F, the Y372F, and the additionally generated Y565F mutants, the Y52F and Y187F mutants still interacted with the Shc SH2 domain (data not shown), indicating that Y332 of rat PKC-δ (corresponding to Y358 and Y334 of mouse and human PKC-δ proteins, respectively) is the tyrosine mediating the interaction with Shc.
Functional interactions between Shc, SHIP, and PKC-δ in mast cells.
To examine the role of PKC-δ in the Shc/SHIP/PKC-δ complex following antigen-induced BMMC stimulation, we first compared the tyrosine phosphorylation levels of Shc and SHIP in WT and PKC-δ−/− BMMCs. These two cell types showed no developmental differences and expressed equal amounts of FcɛR1 and c-kit, as assessed by FACS analysis (reference 31 and data not shown). Proliferation and survival in response to different doses of IL-3 or cytokine withdrawal did not seem to be significantly altered (as determined by viable-cell counting and (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assays) (data not shown). Furthermore, IL-3-induced phosphorylation of the survival kinase PKB was the same in WT and PKC-δ−/− BMMCs (data not shown).
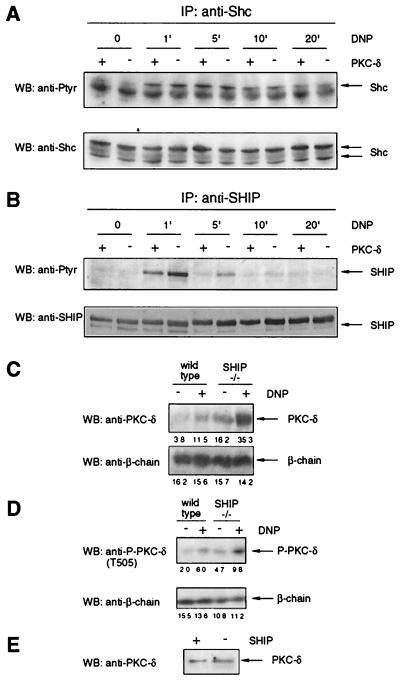
While Shc tyrosine phosphorylation was similar in WT and PKC-δ−/− BMMCs following FcɛR1 engagement (Fig. 4A), SHIP tyrosine phosphorylation was significantly increased and prolonged in PKC-δ-deficient cells (Fig. 4B). A comparable result, though with altered phosphorylation kinetics, was obtained when WT and PKC-δ−/− BMMCs were generated from the bone marrow of mice from a different strain (129/Sv × C57BL/6; data not shown). Also, SHIP from stimulated WT BMMCs was found to consistently migrate slightly more slowly in SDS gels than SHIP from stimulated PKC-δ−/− BMMCs (Fig. 4B [5 min of stimulation; WB: anti-SHIP] and data not shown). This suggested that SHIP might be a substrate for PKC-δ and that Shc might be functioning as a scaffold, bringing together SHIP and PKC-δ. Having established that PKC-δ affected SHIP tyrosine phosphorylation levels (Fig. 4A), we then asked whether SHIP, in turn, might affect PKC-δ activity. This was considered feasible, since SHIP has been shown to be a key regulator of PIP3 levels in BMMCs (10) and PKC-δ is activated via phosphorylation within its activation loop (T505) by the PIP3-dependent kinase PDK1 (30). To address this question, WT and SHIP−/− BMMCs were stimulated via FcɛR1, and membrane fractions were prepared. As shown in Fig. 4C (top), membrane recruitment of PKC-δ in response to FcɛR1 engagement was strongly enhanced in SHIP−/− cells. Accordingly, PKC-δ levels were significantly reduced in the cytosol fraction of stimulated SHIP−/− BMMCs (data not shown). Moreover, basal levels of PKC-δ in the membrane preparations of SHIP−/− BMMCs were already higher than stimulated levels in WT cells (Fig. 4C, top), and membrane translocation of PKC-δ in response to antigen stimulation was abrogated by the use of the PI3K inhibitor LY294002 in WT and SHIP−/− BMMCs (data not shown), indicating strict control of PKC-δ localization by SHIP. Consistent with this, phosphorylation of PKC-δ at the activation loop residue T505 in response to antigen was increased in SHIP−/− BMMCs compared to WT cells (Fig. 4D). Total cell lysates from SHIP+/+ and SHIP−/− BMMCs were probed with anti-PKC-δ antibodies to demonstrate equal expression levels of PKC-δ (Fig. 4E). These results indicate substantial functional interactions between SHIP and PKC-δ.
FIG. 4.
PKC-δ and SHIP influence each other in BMMCs. (A) IgE-preloaded PKC-δ+/+ (+) and PKC-δ−/− (−) BMMCs were stimulated with 200 ng of DNP-HSA/ml (DNP) for the indicated times, and the lysates were subjected to anti-Shc immunoprecipitation (IP) and Western analysis (WB) with anti-Ptyr (top) and anti-Shc (bottom) antibodies. (B) WT (+) and PKC-δ−/− (−) BMMCs were treated as for panel A, and the lysates were subjected to anti-SHIP immunoprecipitation and Western analysis with anti-Ptyr antibodies (top). The blot was reprobed with anti-SHIP antibodies (bottom) to demonstrate equal loading. (C) IgE-preloaded SHIP+/+ and SHIP−/− BMMCs were stimulated with 20 ng of DNP-HSA/ml (DNP) for 3 min, and membranes were prepared as described in Materials and Methods. The membrane preparations were analyzed for the presence of PKC-δ (top), as well as for expression of the FcɛR1 β-subunit, to prove comparable loading (bottom). Densitometry was performed, and relative expression levels are indicated under each band. (D) IgE-preloaded WT and SHIP−/− BMMCs were stimulated with 20 ng of antigen/ml (DNP) for 2 min. Total cell lysates were analyzed by anti-P-PKC-δ (top), as well as by anti-FcɛR1 β-subunit immunoblotting (bottom). Densitometry was performed, and relative expression levels are indicated under each band. (E) Total cell lysates from WT (+) and SHIP−/− (−) BMMCs were subjected to Western analysis with anti-PKC-δ antibodies.
PKC-δ negatively regulates mast cell calcium mobilization and degranulation.
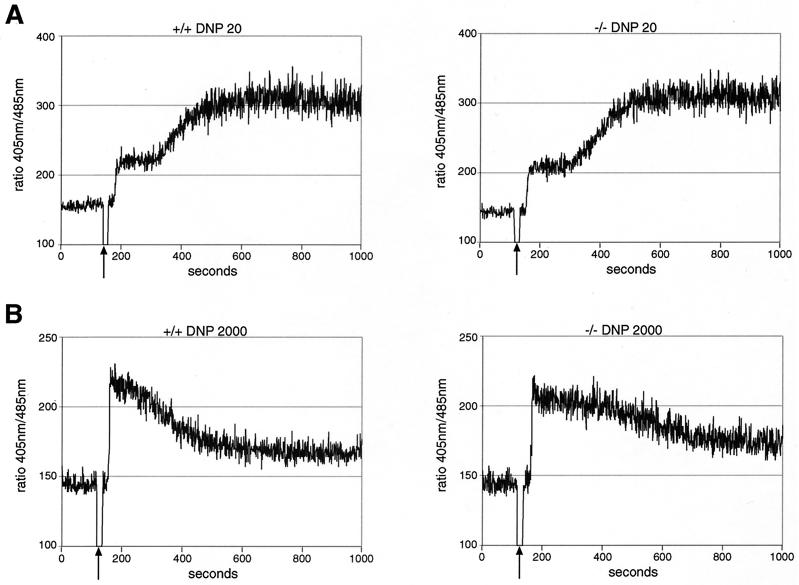
We have shown previously that SHIP is a critical negative regulator of mast cell degranulation and that it acts, at least in part, by attenuating the influx of extracellular calcium via ICRAC channels (17, 19). Since SHIP tyrosine phosphorylation was enhanced under conditions of PKC-δ deficiency (Fig. 4B), we compared the induction of calcium fluxes in response to FcɛR1 engagement under optimal (20 ng of DNP-HSA/ml) (Fig. 5A) and suboptimal (2,000 ng of DNP-HSA/ml) (Fig. 5B) conditions in WT and PKC-δ−/− BMMCs. No effect of PKC-δ deficiency was detected in optimally stimulated cells (20 ng of DNP-HSA/ml) (Fig. 5A). However, the measured calcium response after suboptimal antigen stimulation was significantly sustained in PKC-δ−/− (Fig. 5B, right) compared to WT (Fig. 5B, left) BMMCs. These results suggest that PKC-δ's action leads to an abbreviated FcɛR1-triggered calcium mobilization under suboptimal cross-linking conditions (2,000 ng of DNP-HSA/ml).
FIG. 5.
PKC-δ attenuates suboptimal FcɛR1-mediated calcium mobilization in BMMCs. (A) IgE-preloaded PKC-δ+/+ (left) and PKC-δ−/− (right) BMMCs were given optimal levels of cross-linker (20 ng of DNP-HSA/ml) at the time indicated by the arrow, and cytosolic calcium levels were measured. Similar results were obtained in three separate experiments with different BMMC preparations. (B) PKC-δ+/+ (left) and PKC-δ−/− (right) BMMCs were sensitized with IgE, and cytosolic calcium levels were measured in response to suboptimal stimulation of FcɛR1 (2,000 ng of DNP-HSA/ml). Similar results were obtained in three separate experiments with different BMMC preparations.
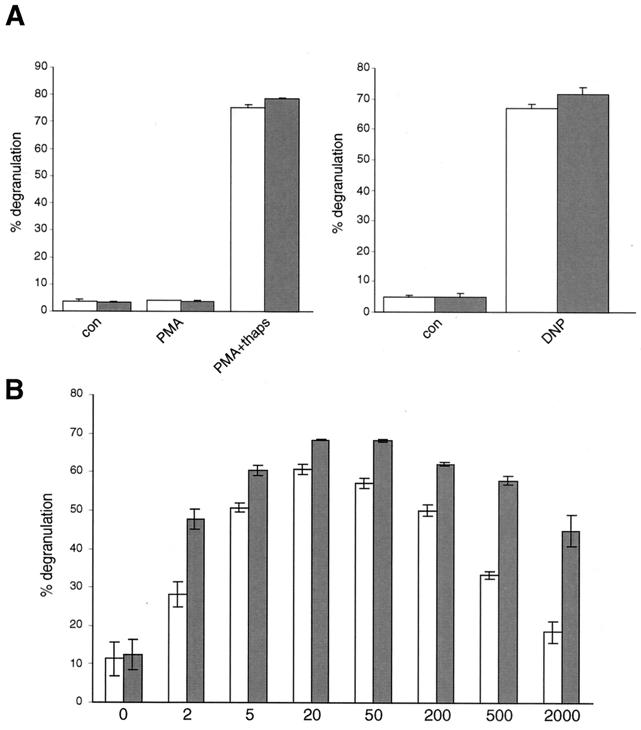
To investigate whether the sustained calcium mobilization in PKC-δ−/− BMMCs might translate into a different secretory behavior, degranulation of WT and PKC-δ−/− BMMCs was assessed. As shown in Fig. 6A (left), there were no significant differences in degranulation of WT and PKC-δ−/− BMMCs in response to PMA or PMA plus thapsigargin. Moreover, release of β-hexosaminidase was comparable when an optimal dose of antigen (20 ng of DNP-HSA/ml) was used (Fig. 6A, right). Also, the kinetics of degranulation were comparable and the total amounts of β-hexosaminidase per cell did not differ significantly between the two cell types (data not shown). However, when the cells were challenged with increasing antigen concentrations (2 to 2,000 ng of DNP-HSA/ml), it was evident that with suboptimal antigen doses (2, 500, and 2,000 ng of DNP-HSA/ml), PKC-δ−/− BMMCs were much more prone to degranulation than their WT counterparts (Fig. 6B). This suggested that PKC-δ was a negative regulator of antigen-induced mast cell degranulation.
FIG. 6.
PKC-δ reduces suboptimal FcɛRI-induced BMMC degranulation. (A) IgE-preloaded PKC-δ+/+ (open bars) and PKC-δ−/− (shaded bars) BMMCs were left unstimulated (con) or were stimulated with 100 ng of PMA/ml or 100 ng of PMA/ml plus 0.5 μg of thapsigargin/ml (PMA+thaps) for 25 min (left) or were activated by FcɛR1 engagement with 20 ng of DNP-HSA/ml (DNP) for 25 min (right). Degranulation was assessed by performing β-hexosaminidase assays. Each bar is the mean of duplicates plus standard deviation (SD). Similar results were obtained in three separate experiments with different BMMC preparations. (B) IgE-preloaded PKC-δ+/+ (open bars) and PKC-δ−/− (shaded bars) BMMCs were stimulated with the indicated concentrations of DNP-HSA (in nanograms per milliliter), and degranulation was determined. Each bar is the mean of duplicates ± SD. Similar results were obtained in three separate experiments with different BMMC preparations.
DISCUSSION
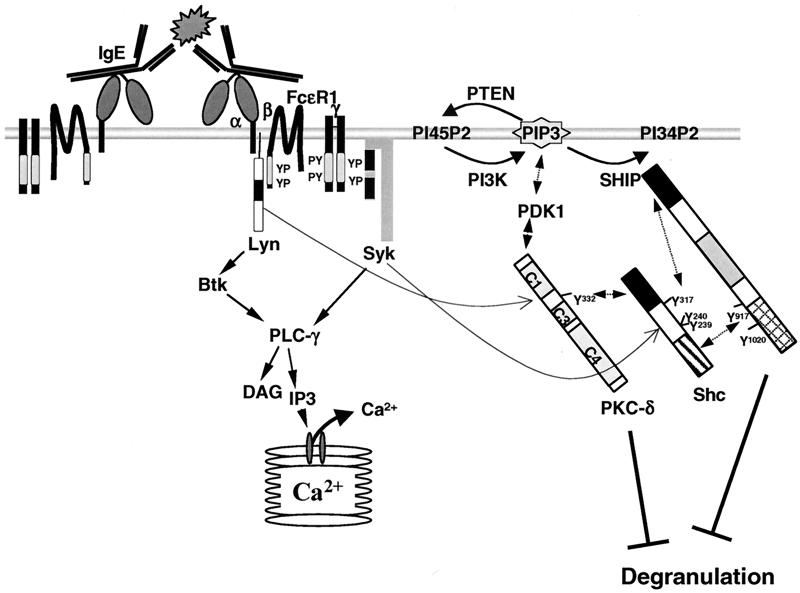
Taken together with previously published data, our results suggest a model (Fig. 7) in which Lyn phosphorylates the β- and γ-subunits of FcɛRI after receptor engagement and phosphorylation of the γ-subunits attracts Syk, which then becomes tyrosine phosphorylated and activated by Lyn. PI3K also becomes attracted to the plasma membrane, via Gab2-p85 interactions (14), and catalyzes the conversion of PI-4,5-biphosphate into the potent second messenger PIP3, which attracts a plethora of PH-containing signaling proteins, including PLC-γ and PDK1. Also, SHIP is attracted via its SH2 domain and proline-rich regions (10, 34) to the plasma membrane, perhaps directly to the phosphorylated β- and γ-subunits of FcɛRI (24, 38), and becomes tyrosine phosphorylated, most likely by Lyn (29, 35, 43; M. D. Ware and G. Krystal, unpublished results). Phosphorylation of SHIP on its NPXY motifs attracts Shc via the latter's PTB domain, and Shc then becomes a target of Syk-mediated tyrosine phosphorylation. Support for this comes from previous reports that FcɛR1-mediated Shc phosphorylation is totally dependent on the presence of SHIP (17) and that the tyrosine phosphorylation of Shc is dependent on Syk (21). As suggested by Tridandapani et al., Shc phosphorylation then enables Shc to bind to SHIP's SH2 domain and wrest SHIP away from the membrane, limiting SHIP's ability to degrade PIP3 (53). The Lyn-mediated tyrosine phosphorylation of PKC-δ on several tyrosines, including Y332, enables it to bind to the SHIP/Shc complex. Our finding that SHIP migrates slightly faster and is more tyrosine phosphorylated in PKC-δ−/− BMMCs suggests that PKC-δ enhances the serine/threonine phosphorylation of SHIP (either directly or indirectly), and this may restrict its tyrosine phosphorylation in WT cells. Experiments unraveling SHIP's phosphorylation patterns under WT and PKC-δ-deficient conditions are under way. At the same time, SHIP restricts the plasma membrane localization and activation of PKC-δ by hydrolyzing PIP3, which will be discussed in detail below.
FIG. 7.
Model of antigen-induced SHIP/Shc/PKC-δ interactions in BMMCs. The immunoreceptor tyrosine-based activation motifs are depicted as lightly shaded bars in the β- and γ-subunits of FcɛR1; the SH2 domains of Lyn, Syk, Shc, and SHIP are shown as solid; the PTB domain of Shc is shown as a hatched bar; and the proline-rich region of SHIP is shown as a cross-hatched bar. In order not to overload the figure, the PKC-δ-Lyn interaction and the binding of PKC-δ to DAG in the plasma membrane via its C1 domain (both mentioned in Discussion) are not depicted.
It is worthy of note that while PKC-δ was the predominant tyrosine-phosphorylated protein that bound to GST-Shc-SH2 beads in our studies, longer radiographic exposures revealed the presence of the FcɛR1 β-chain from mast cell lysates and Ig-α from B-cell lysates (data not shown), confirming earlier reports (2, 23) and demonstrating the integrity of our fusion protein.
It is also worthy of note that although PKC-δ has been shown previously to be phosphorylated on several tyrosine residues in response to different extracellular stimuli (25, 28, 33, 51), none of these appeared to be involved in PKC-δ's binding to Shc's SH2 domain. Instead, Y332, one of the two tyrosines with an isoleucine at the +3 position and thus fitting with the Shc SH2 domain consensus binding site (50), was found to be essential. Since the Y332F mutant was still capable of being immunoprecipitated with anti-PKC-δ antibodies and associating with Lyn, like all the other mutants tested, this suggested proper folding of the mutant (data not shown). Moreover, since the overall tyrosine phosphorylation of the Y332F mutant was similar to that of WT PKC-δ (data not shown), Y332 is likely not a major tyrosine phosphorylation site. Related to this, Konishi et al. reported that Y332 was phosphorylated in response to H2O2 and that mutating this Y332 to a phenylalanine had only marginal effects on PKC-δ activity and total tyrosine phosphorylation levels (26). This corroborates our observations and suggests a scaffold function rather than an activation function for this tyrosine. Our finding that PKC-δ binds to a GST-SH2 (Shc) fusion protein after FcɛR1 engagement on RBL-2H3 (Fig. 1B) and nontransformed IL-3-dependent CFTL12 mast cells (data not shown) indicates that Y332 is phosphorylated after antigen stimulation of mast cells.
PKC-δ and Lyn have been shown previously to interact with and phosphorylate each other, resulting in decreased Lyn activity (49). Our finding that the tyrosine phosphorylation of SHIP was enhanced and prolonged in PKC-δ−/− BMMCs after FcɛR1 engagement is consistent with a scenario in which PKC-δ controls SHIP tyrosine phosphorylation via Lyn. Intriguingly, though we measured slightly enhanced Lyn activity in PKC-δ−/− BMMCs after antigen stimulation (data not shown), total tyrosine phosphorylation of cellular proteins in WT and PKC-δ−/− BMMCs upon antigen treatment did not show significant overall differences (data not shown). However, these two findings are not necessarily contradictory. It has been suggested that Lyn can phosphorylate and activate SHP-1 (15), and Somani et al. have demonstrated that SHP-1 can dephosphorylate the Lyn autophosphorylation site, thereby reducing Lyn's activation state (48). In agreement with this, overexpression of SHP-1 in mast cells results in decreased FcɛR1-mediated tyrosine phosphorylation of the receptor's β- and γ-chains, as well as the tyrosine kinase Syk, which are well-characterized Lyn-dependent events (54). Thus, Lyn and SHP-1 have the potential to establish a self-regulating activation-deactivation cycle. For the PKC-δ−/− BMMCs, this could mean (i) there is no general enhancement of substrate tyrosine phosphorylation, since slightly more active Lyn results in slightly more active SHP-1, which prevents the system from overreacting, and (ii) inhibition of SHP-1 should result in enhanced substrate tyrosine phosphorylation in PKC-δ−/− BMMCs. In agreement with the latter, we found enhanced substrate tyrosine phosphorylation in PKC-δ−/− BMMCs compared to WT cells after tyrosine phosphatases were inhibited by PV treatment (31).
PKC-δ is a member of the novel PKC subfamily and thus is a DAG-dependent, calcium-independent PKC isoform (36). One current model of PKC-δ activation suggests that PKC-δ is recruited to the membrane via its C1 domain after receptor-triggered DAG generation and that this allows PDK1 to phosphorylate T505 in the activation loop of PKC-δ (41). PKC-δ is able to form a complex with PDK1 (30), providing the basis for additional PKC-δ recruitment to the membrane via PIP3-bound PDK1. Next, the C-terminal hydrophobic site, serine 662, is phosphorylated by an aPKC-ζ-containing complex (56), and this site is dephosphorylated by an mTOR-controlled phosphatase (40). Interestingly, all these steps can be subject to regulation by PIP3 and thus by SHIP. Another model of PKC-δ activation, based on biochemical studies with the conventional PKC-βII (11), suggests a different order of events. Here, the PKC precursor is modified by priming phosphorylations, resulting in a catalytically competent but inactive enzyme, which resides in the cytosol. This already-phosphorylated PKC is then attracted to the membrane by DAG, resulting in its activation (11). Though we cannot provide proof for one or the other model, we can speculate on the impact of SHIP deficiency on PKC-δ localization and phosphorylation based on our results. Enhanced PKC-δ membrane translocation in SHIP−/− BMMCs might be due to more DAG generation after FcɛR1 engagement and/or to more PDK1 at the membrane and thus more PDK1/PKC-δ complexes in SHIP−/− BMMCs. Since we could demonstrate recently that PKB membrane translocation, as well as activation, is drastically increased in SHIP−/− BMMCs compared to WT BMMCs (46), it is conceivable that more PDK1 is present in the membrane fraction of these cells. Concerning DAG generation, comparable PLC-γ activation has been demonstrated in both activated WT and SHIP−/− BMMCs (19) and platelets (42). However, there still may be higher DAG levels in SHIP−/− BMMCs via the sequential action of phospholipase D (PLD) and phosphatidic acid phosphohydrolase. In fact, PLD is expressed in mast cells, and regulation of PLD by PI3K has been suggested previously (6, 27). However, PLD activation has not been compared so far in WT and SHIP−/− BMMCs. As far as enhanced PKC-δ phosphorylation on T505 in SHIP−/− BMMCs is concerned, it is highly likely that the dramatically increased activation of the PI3K pathway in these cells (17, 19, 20, 46) directly translates into enhanced PDK1-mediated phosphorylation of T505.
As far as the impact of tyrosine phosphorylation on SHIP's function is concerned, an early report suggested that SHIP tyrosine phosphorylation by Lck reduced its phosphatase activity (38). However, another study suggested that SHIP's activity was not altered via tyrosine phosphorylation but by subcellular localization (7). To get a handle on SHIP activity in PKC-δ+/+ and PKC-δ−/− BMMCs after antigen stimulation, we have measured phosphorylation/activation of PKB, which is a PIP3-dependent event (1). No significant difference in PKB phosphorylation was observed between WT and PKC-δ−/− BMMCs after stimulation with different concentrations of antigen, suggesting that SHIP's activity is not affected by PKC-δ. However, since SHIP's tyrosine phosphorylation status is affected by PKC-δ deficiency, SHIP's task as an adapter protein (52) might be affected by PKC-δ. SHIP's role as an adapter in the course of FcɛR1 signaling is presently under investigation. Nevertheless, it is intriguing that a complex of two attenuators of degranulation (SHIP and PKC-δ) can be observed after FcɛR1 stimulation and that they seem to be influencing each other.
Although previous work with RBL-2H3 cells using overexpression of PKC-δ and PKC-δ reconstitution of permeabilized cells has suggested that PKC-δ either has no impact on degranulation (5) or is proexocytotic (39), respectively, our finding that stimulation of PKC-δ−/− BMMCs with suboptimal doses of antigen results in enhanced secretion suggests that PKC-δ performs a gatekeeping function for mast cell degranulation. The prolonged SHIP tyrosine phosphorylation and calcium mobilization we observe in PKC-δ−/− BMMCs could suggest that PKC-δ's role in antigen-induced degranulation is to terminate secretion-driving signals. Related to this, PKC-δ has been reported to threonine phosphorylate the FcɛR1 γ-chain to trigger efficient FcɛR1 endocytosis after receptor engagement (13). Thus, the absence of PKC-δ might delay receptor endocytosis and thereby sustain the antigen-induced secretory response.
In conclusion, our results suggest that PKC-δ acts as a negative regulator of antigen-mediated degranulation of mast cells. PKC-δ might thus represent an attenuating element for mast cell degranulation in vivo, especially in cases of low-affinity antigens or low levels of high-affinity antigens.
Acknowledgments
We thank R. Siraganian for providing β-chain-specific antibodies and F. Melchers for providing X63Ag8-653 cells. Furthermore, we thank V. Rolli for pD-hLyn and pD-hSyk and A. Wuerch for help with calcium measurements. We sincerely thank Michael Reth for his advice and his generous support.
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) through grant SFB 388 (M.H.) and the Leibniz prize to Michael Reth. G.K. is a Terry Fox Cancer Research Scientist of the NCI-C, supported by funds from the Canadian Cancer Society and the Terry Fox Run. M.L. was supported by the DFG (Sta314/2-1 and KE246/7-2).
REFERENCES
- 1.Aman, M. J., T. D. Lamkin, H. Okada, T. Kurosaki, and K. S. Ravichandran. 1998. The inositol phosphatase SHIP inhibits Akt/PKB activation in B cells. J. Biol. Chem. 273:33922-33928. [DOI] [PubMed] [Google Scholar]
- 2.Baumann, G., D. Maier, F. Freuler, C. Tschopp, K. Baudisch, and J. Wienands. 1994. In vitro characterization of major ligands for Src homology 2 domains derived from protein tyrosine kinases, from the adaptor protein SHC and from GTPase-activating protein in Ramos B cells. Eur. J. Immunol. 24:1799-1807. [DOI] [PubMed] [Google Scholar]
- 3.Beaven, M. A., and H. Metzger. 1993. Signal transduction by Fc receptors: the Fc epsilon RI case. Immunol. Today 14:222-226. [DOI] [PubMed] [Google Scholar]
- 4.Bone, H., and M. J. Welham. 2000. Shc associates with the IL-3 receptor beta subunit, SHIP and Gab2 following IL-3 stimulation: contribution of Shc PTB and SH2 domains. Cell. Signal. 12:183-194. [DOI] [PubMed] [Google Scholar]
- 5.Chang, E. Y., Z. Szallasi, P. Acs, V. Raizada, P. C. Wolfe, C. Fewtrell, P. M. Blumberg, and J. Rivera. 1997. Functional effects of overexpression of protein kinase C-alpha, -beta, -delta, -epsilon, and -eta in the mast cell line RBL-2H3. J. Immunol. 159:2624-2632. [PubMed] [Google Scholar]
- 6.Cissel, D. S., P. F. Fraundorfer, and M. A. Beaven. 1998. Thapsigargin-induced secretion is dependent on activation of a cholera toxin-sensitive and phosphatidylinositol-3-kinase-regulated phospholipase D in a mast cell line. J. Pharmacol. Exp. Ther. 285:110-118. [PubMed] [Google Scholar]
- 7.Damen, J. E., L. Liu, P. Rosten, R. K. Humphries, A. B. Jefferson, P. W. Majerus, and G. Krystal. 1996. The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-triphosphate 5-phosphatase. Proc. Natl. Acad. Sci. USA 93:1689-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damen, J. E., L. Liu, M. D. Ware, M. Ermolaeva, P. W. Majerus, and G. Krystal. 1998. Multiple forms of the SH2-containing inositol phosphatase, SHIP, are generated by C-terminal truncation. Blood 92:1199-1205. [PubMed] [Google Scholar]
- 9.Damen, J. E., A. L. Mui, L. Puil, T. Pawson, and G. Krystal. 1993. Phosphatidylinositol 3-kinase associates, via its Src homology 2 domains, with the activated erythropoietin receptor. Blood 81:3204-3210. [PubMed] [Google Scholar]
- 10.Damen, J. E., M. D. Ware, J. Kalesnikoff, M. R. Hughes, and G. Krystal. 2001. SHIP's C-terminus is essential for its hydrolysis of PIP3 and inhibition of mast cell degranulation. Blood 97:1343-1351. [DOI] [PubMed] [Google Scholar]
- 11.Dutil, E. M., and A. C. Newton. 2000. Dual role of pseudosubstrate in the coordinated regulation of protein kinase C by phosphorylation and diacylglycerol. J. Biol. Chem. 275:10697-10701. [DOI] [PubMed] [Google Scholar]
- 12.Falasca, M., S. K. Logan, V. P. Lehto, G. Baccante, M. A. Lemmon, and J. Schlessinger. 1998. Activation of phospholipase C gamma by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 17:414-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Germano, P., J. Gomez, M. G. Kazanietz, P. M. Blumberg, and J. Rivera. 1994. Phosphorylation of the gamma chain of the high affinity receptor for immunoglobulin E by receptor-associated protein kinase C-delta. J. Biol. Chem. 269:23102-23107. [PubMed] [Google Scholar]
- 14.Gu, H., K. Saito, L. D. Klaman, J. Shen, T. Fleming, Y. Wang, J. C. Pratt, G. Lin, B. Lim, J. P. Kinet, and B. G. Neel. 2001. Essential role for Gab2 in the allergic response. Nature 412:186-190. [DOI] [PubMed] [Google Scholar]
- 15.Harder, K. W., L. M. Parsons, J. Armes, N. Evans, N. Kountouri, R. Clark, C. Quilici, D. Grail, G. S. Hodgson, A. R. Dunn, and M. L. Hibbs. 2001. Gain- and loss-of-function Lyn mutant mice define a critical inhibitory role of Lyn in the myeloid lineage. Immunity 15:603-615. [DOI] [PubMed] [Google Scholar]
- 16.Harmer, S. L., and A. L. DeFranco. 1999. The Src homology domain 2-containing inositol phosphatase SHIP forms a ternary complex with Shc and Grb2 in antigen receptor-stimulated B lymphocytes. J. Biol. Chem. 274:12183-12191. [DOI] [PubMed] [Google Scholar]
- 17.Huber, M., C. D. Helgason, J. E. Damen, L. Liu, R. K. Humphries, and G. Krystal. 1998. The Src Homology 2-containing inositol phosphatase (SHIP) is the gatekeeper of mast cell degranulation. Proc. Natl. Acad. Sci. USA 95:11330-11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huber, M., C. D. Helgason, J. E. Damen, M. P. Scheid, V. Duronio, V. Lam, R. K. Humphries, and G. Krystal. 1999. The role of the SRC homology 2-containing inositol 5′-phosphatase in Fc epsilon R1-induced signaling. Curr. Top. Microbiol. Immunol. 244:29-41. [PubMed] [Google Scholar]
- 19.Huber, M., C. D. Helgason, M. P. Scheid, V. Duronio, R. K. Humphries, and G. Krystal. 1998. Targeted disruption of SHIP leads to Steel factor-induced degranulation of mast cells. EMBO J. 17:7311-7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huber, M., M. R. Hughes, and G. Krystal. 2000. Thapsigargin-induced degranulation of mast cells is dependent on transient activation of phosphatidylinositol-3 kinase. J. Immunol. 165:124-133. [DOI] [PubMed] [Google Scholar]
- 21.Jabril-Cuenod, B., C. Zhang, A. M. Scharenberg, R. Paolini, R. Numerof, M. A. Beaven, and J. P. Kinet. 1996. Syk-dependent phosphorylation of Shc. A potential link between FcɛRI and the Ras/mitogen-activated protein kinase signaling pathway through SOS and Grb2. J. Biol. Chem. 271:16268-16272. [DOI] [PubMed] [Google Scholar]
- 22.Karasuyama, H., and F. Melchers. 1988. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur. J. Immunol. 18:97-104. [DOI] [PubMed] [Google Scholar]
- 23.Kimura, T., H. Kihara, S. Bhattacharyya, H. Sakamoto, E. Appella, and R. P. Siraganian. 1996. Downstream signaling molecules bind to different phosphorylated immunoreceptor tyrosine-based activation motif (ITAM) peptides of the high affinity IgE receptor. J. Biol. Chem. 271:27962-27968. [DOI] [PubMed] [Google Scholar]
- 24.Kimura, T., H. Sakamoto, E. Appella, and R. P. Siraganian. 1997. The negative signaling molecule SH2 domain-containing inositol-polyphosphate 5-phosphatase (SHIP) binds to the tyrosine-phosphorylated beta subunit of the high affinity IgE receptor. J. Biol. Chem. 272:13991-13996. [DOI] [PubMed] [Google Scholar]
- 25.Konishi, H., M. Tanaka, Y. Takemura, H. Matsuzaki, Y. Ono, U. Kikkawa, and Y. Nishizuka. 1997. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc. Natl. Acad. Sci. USA 94:11233-11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konishi, H., E. Yamauchi, H. Taniguchi, T. Yamamoto, H. Matsuzaki, Y. Takemura, K. Ohmae, U. Kikkawa, and Y. Nishizuka. 2001. Phosphorylation sites of protein kinase C δ in H2O2-treated cells and its activation by tyrosine kinases in vitro. Proc. Natl. Acad. Sci. USA 98:6587-6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozawa, O., P. Blume-Jensen, C. H. Heldin, and L. Ronnstrand. 1997. Involvement of phosphatidylinositol 3′-kinase in stem-cell-factor-induced phospholipase D activation and arachidonic acid release. Eur. J. Biochem. 248:149-155. [DOI] [PubMed] [Google Scholar]
- 28.Kronfeld, I., G. Kazimirsky, P. S. Lorenzo, S. H. Garfield, P. M. Blumberg, and C. Brodie. 2000. Phosphorylation of protein kinase C delta on distinct tyrosine residues regulates specific cellular functions. J. Biol. Chem. 275:35491-35498. [DOI] [PubMed] [Google Scholar]
- 29.Lamkin, T. D., S. F. Walk, L. Liu, J. E. Damen, G. Krystal, and K. S. Ravichandran. 1997. Shc interaction with Src homology 2 domain containing inositol phosphatase (SHIP) in vivo requires the Shc-phosphotyrosine binding domain and two specific phosphotyrosines on SHIP. J. Biol. Chem. 272:10396-10401. [DOI] [PubMed] [Google Scholar]
- 30.Le Good, J. A., W. H. Ziegler, D. B. Parekh, D. R. Alessi, P. Cohen, and P. J. Parker. 1998. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 281:2042-2045. [DOI] [PubMed] [Google Scholar]
- 31.Leitges, M., W. Elis, K. Gimborn, and M. Huber. 2001. Rottlerin-independent attenuation of pervanadate-induced tyrosine phosphorylation events by PKC-δ in hemopoietic cells. Lab. Investig. 81:1087-1095. [DOI] [PubMed] [Google Scholar]
- 32.Leitges, M., M. Mayr, U. Braun, U. Mayr, C. H. Li, G. Pfister, N. Ghaffari-Tabrizi, G. Baier, Y. H. Hu, and Q. B. Xu. 2001. Exacerbated vein graft arteriosclerosis in protein kinase C delta-null mice. J. Clin. Investig. 108:1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, W., X. H. Chen, C. A. Kelley, M. Alimandi, J. Zhang, Q. Chen, D. P. Bottaro, and J. H. Pierce. 1996. Identification of tyrosine 187 as a protein kinase C-delta phosphorylation site. J. Biol. Chem. 271:26404-26409. [DOI] [PubMed] [Google Scholar]
- 34.Liu, L., J. E. Damen, M. R. Hughes, I. Babic, F. R. Jirik, and G. Krystal. 1997. The Src homology 2 (SH2) domain of SH2-containing inositol phosphatase (SHIP) is essential for tyrosine phosphorylation of SHIP, its association with Shc, and its induction of apoptosis. J. Biol. Chem. 272:8983-8988. [DOI] [PubMed] [Google Scholar]
- 35.Mikhalap, S. V., L. M. Shlapatska, A. G. Berdova, C. L. Law, E. A. Clark, and S. P. Sidorenko. 1999. CDw150 associates with src-homology 2-containing inositol phosphatase and modulates CD95-mediated apoptosis. J. Immunol. 162:5719-5727. [PubMed] [Google Scholar]
- 36.Mochly-Rosen, D., and A. S. Gordon. 1998. Anchoring proteins for protein kinase C: a means for isozyme selectivity. FASEB J. 12:35-42. [PubMed] [Google Scholar]
- 37.Ogita, K., S. Miyamoto, K. Yamaguchi, H. Koide, N. Fujisawa, U. Kikkawa, S. Sahara, Y. Fukami, and Y. Nishizuka. 1992. Isolation and characterization of delta-subspecies of protein kinase C from rat brain. Proc. Natl. Acad. Sci. USA 89:1592-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osborne, M. A., G. Zenner, M. Lubinus, X. Zhang, Z. Songyang, L. C. Cantley, P. Majerus, P. Burn, and J. P. Kochan. 1996. The inositol 5′-phosphatase SHIP binds to immunoreceptor signaling motifs and responds to high affinity IgE receptor aggregation. J. Biol. Chem. 271:29271-29278. [DOI] [PubMed] [Google Scholar]
- 39.Ozawa, K., Z. Szallasi, M. G. Kazanietz, P. M. Blumberg, H. Mischak, J. F. Mushinski, and M. A. Beaven. 1993. Ca(2+)-dependent and Ca(2+)-independent isozymes of protein kinase C mediate exocytosis in antigen-stimulated rat basophilic RBL-2H3 cells. Reconstitution of secretory responses with Ca(2+) and purified isozymes in washed permeabilized cells. J. Biol. Chem. 268:1749-1756. [PubMed] [Google Scholar]
- 40.Parekh, D., W. Ziegler, K. Yonezawa, K. Hara, and P. J. Parker. 1999. Mammalian TOR controls one of two kinase pathways acting upon nPKC-δ and nPKC-ɛ. J. Biol. Chem. 274:34758-34764. [DOI] [PubMed] [Google Scholar]
- 41.Parekh, D. B., W. Ziegler, and P. J. Parker. 2000. Multiple pathways control protein kinase C phosphorylation. EMBO J. 19:496-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasquet, J. M., L. Quek, C. Stevens, R. Bobe, M. Huber, V. Duronio, G. Krystal, and S. P. Watson. 2000. Phosphatidylinositol 3,4,5-trisphosphate regulates Ca2+ entry via Btk in platelets and megakaryocytes without increasing phospholipase C activity. EMBO J. 19:2793-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phee, H., A. Jacob, and K. M. Coggeshall. 2000. Enzymatic activity of the Src homology 2 domain-containing inositol phosphatase is regulated by a plasma membrane location. J. Biol. Chem. 275:19090-19097. [DOI] [PubMed] [Google Scholar]
- 44.Salim, K., M. J. Bottomley, E. Querfurth, M. J. Zvelebil, I. Gout, R. Scaife, R. L. Margolis, R. Gigg, C. I. Smith, P. C. Driscoll, M. D. Waterfield, and G. Panayotou. 1996. Distinct specificity in the recognition of phosphoinositides by the pleckstrin homology domains of dynamin and Bruton's tyrosine kinase. EMBO J. 15:6241-6250. [PMC free article] [PubMed] [Google Scholar]
- 45.Scharenberg, A. M., O. El-Hillal, D. A. Fruman, L. O. Beitz, Z. Li, S. Lin, I. Gout, L. C. Cantley, D. J. Rawlings, and J. P. Kinet. 1998. Phosphatidylinositol-3,4,5-trisphosphate (PtdIns-3,4,5-P3)/Tec kinase-dependent calcium signaling pathway: a target for SHIP-mediated inhibitory signals. EMBO J. 17:1961-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scheid, M. P., M. Huber, J. E. Damen, M. Hughes, V. Kang, P. Neilson, G. D. Prestwich, G. Krystal, and V. Duronio. 2002. Phosphatidylinositol(3,4,5)P3 is essential but not sufficient for PKB activation: phosphatidylinositol(3,4)P2 is required for PKB phosphorylation at Ser473. Studies using cells from SHIP knockout mice. J. Biol. Chem. 277:9027-9035. [DOI] [PubMed] [Google Scholar]
- 47.Schmandt, R., S. K. Liu, and C. J. McGlade. 1999. Cloning and characterization of mPAL, a novel Shc SH2 domain-binding protein expressed in proliferating cells. Oncogene. 18:1867-1879. [DOI] [PubMed] [Google Scholar]
- 48.Somani, A. F., K. Yuen, F. H. Xu, J. Y. Zhang, D. R. Branch, and K. A. Siminovitch. 2001. The SH2 domain containing tyrosine phosphatase-1 down-regulates activation of Lyn and Lyn-induced tyrosine phosphorylation of the CD19 receptor in B cells. J. Biol. Chem. 276:1938-1944. [DOI] [PubMed] [Google Scholar]
- 49.Song, J. S., P. G. Swann, Z. Szallasi, U. Blank, P. M. Blumberg, and J. Rivera. 1998. Tyrosine phosphorylation-dependent and -independent associations of protein kinase C-delta with Src family kinases in the RBL-2H3 mast cell line: regulation of Src family kinase activity by protein kinase C-delta. Oncogene 16:3357-3368. [DOI] [PubMed] [Google Scholar]
- 50.Songyang, Z., S. E. Shoelson, J. McGlade, P. Olivier, T. Pawson, X. R. Bustelo, M. Barbacid, H. Sabe, H. Hanafusa, T. Yi, et al. 1994. Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol. Cell. Biol. 14:2777-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szallasi, Z., M. F. Denning, E. Y. Chang, J. Rivera, S. H. Yuspa, C. Lehel, Z. Olah, W. B. Anderson, and P. M. Blumberg. 1995. Development of a rapid approach to identification of tyrosine phosphorylation sites: application to PKC-delta phosphorylated upon activation of the high affinity receptor for IgE in rat basophilic leukemia cells. Biochem. Biophys. Res. Commun. 214:888-894. [DOI] [PubMed] [Google Scholar]
- 52.Tamir, I., J. C. Stolpa, C. D. Helgason, K. Nakamura, P. Bruhns, M. Daeron, and J. C. Cambier. 2000. The RasGAP-binding protein p62dok is a mediator of inhibitory FcγRIIB signals in B cells. Immunity 12:347-358. [DOI] [PubMed] [Google Scholar]
- 53.Tridandapani, S., M. Pradhan, J. R. LaDine, S. Garber, C. L. Anderson, and K. M. Coggeshall. 1999. Protein interactions of Src homology 2 (SH2) domain-containing inositol phosphatase (SHIP): association with Shc displaces SHIP from Fc gamma RIIb in B cells. J. Immunol. 162:1408-1414. [PubMed] [Google Scholar]
- 54.Xie, Z. H., J. Zhang, and R. P. Siraganian. 2000. Positive regulation of c-Jun N-terminal kinase and TNF-α production but not histamine release by SHP-1 in RBL-2H3 mast cells. J. Immunol. 164:1521-1528. [DOI] [PubMed] [Google Scholar]
- 55.Zhou, S., K. L. Carraway III, M. J. Eck, S. C. Harrison, R. A. Feldman, M. Mohammadi, J. Schlessinger, S. R. Hubbard, D. P. Smith, C. Eng, et al. 1995. Catalytic specificity of protein-tyrosine kinases is critical for selective signalling. Nature 373:536-539. [DOI] [PubMed] [Google Scholar]
- 56.Ziegler, W. H., D. B. Parekh, J. A. Le Good, R. D. Whelan, J. J. Kelly, M. Frech, B. A. Hemmings, and P. J. Parker. 1999. Rapamycin-sensitive phosphorylation of PKC on a carboxy-terminal site by an atypical PKC complex. Curr. Biol. 9:522-529. [DOI] [PubMed] [Google Scholar]