Abstract
In this report, we explored the mechanisms underlying keratinocyte-specific and differentiation-specific gene expression in the skin. We have identified five keratinocyte-specific, open chromatin regions that exist within the 6 kb of 5′ upstream regulatory sequence known to faithfully recapitulate the strong endogenous keratin 5 (K5) promoter and/or enhancer activity. One of these, DNase I-hypersensitive site (HSs) 4, was unique in that it acted independently to drive abundant and keratinocyte-specific reporter gene activity in culture and in transgenic mice, despite the fact that it was not essential for K5 enhancer activity. We have identified evolutionarily conserved regulatory elements and a number of their associated proteins that bind to this compact and complex enhancer element. The 125-bp 3′ half of this element (referred to as 4.2) is by far the smallest known strong enhancer element possessing keratinocyte-specific activity in vivo. Interestingly, its activity is restricted to a subset of progeny of K5-expressing cells located within the sebaceous gland. The other half of HSs 4 (termed 4.1) possesses activity to suppress sebocyte-specific expression and induce expression in the channel (inner root sheath) cells surrounding the hair shaft. Our findings lead us to a view of keratinocyte gene expression which is determined by multiple regulatory modules, many of which contain AP-2 and/or Sp1/Sp3 binding sites for enhancing expression in skin epithelium, but which also harbor one or more unique sites for the binding of factors which determine specificity. Through mixing and matching of these modules, additional levels of specificity are obtained, indicating that both transcriptional repressors and activators govern the specificity.
Keratinocytes are the constituents of self-renewing stratified squamous epithelia, which in the skin include the epidermis and its notable appendages, including the hair follicles, sebaceous glands, and sweat glands. In the innermost, basal layer of the epidermis, mitotically active keratinocytes possess unique biochemical and morphological characteristics that change as they withdraw from the cell cycle and terminally differentiate through the spinous, granular, and cornified layers before being sloughed from the skin's surface. The basal epidermal layer is continuous with the hair follicle's outer root sheath, which both houses the stem cell compartment of the skin and buds the peripheral layer of the sebaceous gland (for reviews, see references 15 and 25).
All of these basal cells have in common their expression of K5 and K14, which assemble into an extensive cytoskeletal network of 10-nm intermediate filaments that protects the keratinocytes from mechanical stress. K5 and K14, which constitute ≈25% of total cellular protein and approximately 10% of total cell transcription, typify the mitotically active keratinocyte. The keratinocyte is particularly amenable to exploring cell type-specific gene regulation because the morphological and biochemical features of the keratinocyte, including K5 and K14 gene transcription, can be maintained in culture out of context of the normal cell environment. As keratinocytes differentiate in vivo or in vitro, they switch off K5 and K14 gene transcription and induce expression of other keratins that specify differentiation-specific routes while still maintaining the mechanical integrity of the differentiating cells (14).
Analysis of epidermal-specific promoters has implicated a number of transcription factors in orchestrating keratinocyte-specific and differentiation-specific gene expression in the epidermis (1, 5, 6, 9, 11, 13, 22, 33, 45, 46). The promoters of many basally expressed genes, including those encoding K14 and K5, contain functional AP-2 and Sp1 sites that contribute to keratinocyte-specific gene expression in vitro and in transgenic mice in vivo (5, 22, 26, 27, 39, 48). Regions of open chromatin containing enhancer elements within the K14 gene have recently been characterized, and they too possess functional AP-2 and Sp1 sites but, in addition, Ets and/or AP-1 sites (45, 46). Thus, while compelling evidence implicates a common set of transcription factors in governing keratinocyte-specific gene expression, the mechanisms that control differentiation-specific gene expression remain obscure.
One possibility is that specialized basal layer-specific or differentiation-specific factors act in concert with these common transcription factors to govern gene expression at each differentiation stage in skin keratinocytes. Evidence in support of this notion stems from the emergence of a number of additional transcription factors whose role in epidermal gene expression has not yet been elucidated. These factors include Klf4 (43), C/EBP (8, 23, 33), POU domain proteins (11, 13, 49), Dlx3 (35), and steroid hormone receptors (12, 30, 31, 40). Alternatively, differentiation specificity may be achieved through regulation of specific members for the common transcription factor families (or their interacting partners) (2, 33, 39, 45, 46).
To probe further into the regulation of keratinocyte-specific and differentiation-specific gene expression in the skin, we have identified and characterized one of the major enhancer elements of the K5 gene. Previous studies have shown that 6,000 bp of sequence upstream from the human K5 coding region is sufficient to faithfully and consistently target expression of a β-galactosidase reporter gene to the basal layer of the epidermis, the outer root sheath and stem cell compartment of the hair follicle, and the sebaceous gland (5, 6). In contrast, the minimal K5 promoter of 120 bp (−90 to +30) preferentially targeted reporter gene expression to keratinocytes, but this resulted in decreased expression levels, fewer expressing transgenic lines, loss of differentiation specificity (suprabasal rather than basal expression), and ectopic expression.
To identify key enhancer elements embedded within the region between bp +30 and −6000, we identified those regions that exhibit open chromatin structure only in the transcriptionally active native locus, based on DNase I-hypersensitive site (HSs) mapping. We found five such regions in the human K5 promoter, and in this report, we have concentrated our analysis on the regulatory region that exhibits both strong activity and cell type specificity in keratinocytes in culture. We have identified functionally important transcription factor binding sites within each of two subdomains within this element, and these include both well-characterized factors and unknown factors as well as at least one possible keratinocyte-specific factor. Finally, we have examined the enhancer activity of this region in vivo by using transgenic mice and unveiled a level of complexity not previously appreciated in keratinocyte gene regulation. Our evidence supports a model of K5 gene regulation which relies on the combinatorial input of multiple enhancer elements, which utilize both cell type-specific and common transcription factors in conferring specificity.
MATERIALS AND METHODS
DNase I-hypersensitive site mapping.
DNase I-hypersensitive site mapping was performed as previously described (3). For DNase I digestion, 90 μl of resuspended nuclei from primary human keratinocytes, human fibroblasts, or mouse nuclei was treated with 10 μl of 0-, 1-, 2-, 4-, or 6-U/μl DNase I solution (0-, 1-, 2-, or 4-U/μl in the case of mouse keratinocytes) for 10 min at 37°C. Additional procedures were as described before (45).
Cell culture and transient transfections.
HepG2 and HeLa cells were grown as previously described (5, 27). Epidermal keratinocytes were derived from human foreskin and early-passage (one to three) cells were grown in low-Ca2+ keratinocyte growth medium (KGM; from Clonetics) or EpiLife (Cascade Biologicals). A spontaneously immortalized mouse keratinocyte line (UG-1 [16]) was grown in a low-Ca2+ medium comprised of a 3:1 mixture of Ham's F12 and Dulbecco's modified Eagle's media.
Cells were split into six-well plates (38.5-cm2 per well; Falcon), grown to 20 to 30% confluence (estimated ≈2 × 105 to 3 × 105 cells), and fed just prior to transfection. Transfections were performed by using the Fugene 6 reagent (Roche) per the manufacturer's instructions. One microgram of luciferase construct DNA was transfected per well plus 0.1 μg of plasmid CMV-β-gal DNA to serve as an internal control for transfection efficiency. Cells were cultured for 24 to 48 h after transfections to 80 to 100% confluence (estimated ≈0.9 × 106 to 1 × 106), washed twice with phosphate-buffered saline, and then lysed in 300 μl of luciferase cell culture lysis reagent (Promega) per well of cells. Insoluble debris was removed by centrifugation, and 10 μl of the cell extract supernatant was assayed for luciferase activity by using 100 μl of luciferase assay buffer plus substrate (Promega). Then 2 μl of supernatant was assayed by using the Galactolight Plus kit (Tropix) as per the manufacturer's instructions. Chemiluminescence values for luciferase were normalized to those determined for β-galactosidase. Transfections were performed in duplicate, and values were then averaged. The transfections were repeated independently two to three times, and averages plus/minus standard deviations were determined and are reported here.
Generation of DNA constructs.
Standard cloning methods were used throughout, and all constructs were verified by sequencing. Mutations were introduced by using a PCR-based method (51). Luciferase constructs were derived from pGL3Basic (Promega). For these constructs, the minimal human K5 (hK5) promoter was cloned into the XhoI and HindIII and region 4.1 and region 4.2 enhancer fragments (wild type and mutants) were cloned into the KpnI and XhoI or the KpnI and NheI sites.
HSs 4 (from the ApaI at −2600 to the SmaI at −2030) was removed from the full hK5 promoter by using a PCR-based approach and verified by sequencing. Constructs used for transgenics were derived from a modified version of pNASSβ (Clontech) (45). The thymidine kinase (TK) promoter was introduced at the NheI and XhoI sites. Multimerized constructs [4×125 K5, 4×125 TK, 4×125(5′) TK, and 4×125(3′) TK] were generated from PCR-amplified segments by using primers with KpnI and BamHI restriction sites in the forward primer and BglII and NheI restriction sites in the reverse primer. The fragments were first cloned into the KpnI and NheI restriction sites of pBlueScript (Stratagene), and a direct repeat was generated by reinserting a BamHI/NheI fragment into the vector cut with BglII and NheI. The BamHI/BglII hybrid site is destroyed, and the process is repeated to generate four direct repeats. The multimerized enhancer was then cloned into a modified pNASSβ containing the minimal hK5 promoter or TK promoter at the KpnI and NheI restriction sites (45).
Gel shift analysis.
Gel shift analysis and nuclear extract isolation were performed as previously described with some modifications (42). From 1 to 2 μl of nuclear extracts was used per reaction along with 3 × 104 to 10 × 104 cpm of end-labeled oligonucleotides. Competitor oligonucleotides were added in ≈40- and 200-fold excess. One microliter of antibody was added where indicated (1 or 2 μl in the case of anti-NF-I antibody). Reaction mixtures were incubated with competitor oligonucleotide or antibody for 10 min at room temperature prior to the addition of labeled oligonucleotides. Reaction mixtures were then incubated for 30 min on ice before loading on either a 4.5% or 5% polyacrylamide gel. Gels were run for 3 h at 100 V, dried, and visualized by using autoradiography. Antibodies used for supershifts included the following: Sp3 (Santa Cruz, D-20), Sp1 (Geneka, NuShift rabbit polyclonal, catalog number 1615314), AP-2α (Santa Cruz, C-18), AP-2gamma (Santa Cruz, H-77), and NF-I/CTF (gift from N. Tanese, serum 8199).
Sp1 and AP-2 consensus oligonucleotides were purchased from Promega. Other oligonucleotides used in gel shifts presented here are shown in Table 1.
TABLE 1.
Oligonucleotides
| Figure | Oligonucleotide | Sequence |
|---|---|---|
| 5 | A upper | AGTGAAATTCCAGGCTGGGGTGGGACTCTCCCGAGCTGC |
| A lower | ACCACTGCAGCTCGGGAGAGTCCCACCCCAGCCTGGAAT | |
| B upper | TGAGGGAGAAGCTGAAGCAACATGTCCCAC | |
| B lower | TTGGTGGGACATGTTGCTTCAGCTTCTCCC | |
| C upper | GTACAATCCCTCCCTTCCTGCCACAGTGAAATT | |
| C lower | TGGAATTTCACTGTGGCAGGAAGGGAGGGATTG | |
| Cm upper | GTACAATTTTTCCCTTCCTGCCACAGTGAAATT | |
| Cm lower | TGGAATTTCACTGTGGCAGGAAGGGAAAAATTG | |
| 7 | A upper | CTGTGTCTCAGGAGGGGTGGGGCTTCCCTGAGGGCAGTGAGG |
| A lower | CTGCCTCACTGCCCTCAGGGAAGCCCCACCCCTCCTGAGACA | |
| B upper | TGGGGCTTCCCTGAGGGCAGTGAGGTCT | |
| B lower | TACAGACCTCACTGCCCTCAGGGAAGCC | |
| NF-I upper | AGAACGCATTCCTGCTCTGCCAAGTCCTCTTT | |
| NF-I lower | AAGAAAGAGGACTTGGCAGAGCAGGAATGCGT | |
| NF-Im upper | AGAACGCATTCCTGCTCTGTTAAGTCCTCTTT | |
| NF-Im lower | AAGAAAGAGGACTTAACAGAGCAGGAATGCGT | |
| G/C2 upper | TGCCAAGTCCTCTTTCTTCACAACACAGCTGGGAGGAGAA | |
| G/C2 lower | TGATTCTCCTCCCAGCTGTGTTGTGAAGAAAGAGGACTTG | |
| G/C2m upper | TGCCAAGTCCTCTTTCTTCACAACACAGCTAAAAGGAGAA | |
| G/C2m lower | TGATTCTCCTTTTAGCTGTGTTGTGAAGAAAGAGGACTTG | |
| AP-2m1 upper | AGGGGTGGGGCTTCCCTGGAATTCGTGAGGTCTGTACCCG | |
| AP-2m1 lower | TCCCGGGTACAGACCTCACGAATTCCAGGGAAGCCCCACC |
Transgenic mice.
Transgenic mice were generated as previously described (52). Genomic DNA was isolated from toe samples, and mice were genotyped for the presence of the lacZ gene by using the following primers: forward, 5′-CTTCTAGGCCTGTACGGAAGTGTTAC; reverse, 5′-GGCGCATCGTAACCGTGCATCTGCC. Tail samples from genotypically positive mice, along with control littermates, were collected and assayed for the expression of lacZ (see below).
Histology and immunofluorescence.
Tissue samples were embedded in optimal cutting temperature compound, and 10-μm sections were cut by using a cryostat. For assessment of lacZ expression, sections were fixed for 1.5 min in 0.1% glutaraldehyde, washed six times in phosphate-buffered saline, and then placed in 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining solution in the dark at 37°C. Blue staining was evident within 1 h, and some samples were allowed to incubate for up to 24 h to examine for weak staining. A stock solution of Oil Red O was prepared with 0.25 g of powdered Oil Red O (Sigma) in 50 ml of 98% isopropanol, and a filtered working solution (24 ml of stock plus 16 ml of water) was added to X-Gal-stained sections for 8 to 10 min, followed by multiple washes in deionized water. Counterstaining was performed by using hematoxylin (1 min of incubation) followed by multiple washes in deionized water. In some cases, sections were incubated in eosin working solution for 5 min and then washed.
Immunofluorescence was performed by using standard procedures on 10-μm-thick OCT sections of tail samples from control and transgenic mice. Sections were fixed in 4% paraformaldehyde for 10 min prior to staining. SuperBlock (Pierce) was used in the K5/β-galactosidase costaining, and the M.O.M. kit (Vector Laboratories) was used for the AP-2α/β-galactosidase costaining. Antibodies to K5 (used at 1:200 dilution) were generated in our laboratory. Antibodies to AP-2α were a gift from T. Williams (used at 1:5 dilution), and antibodies to β-galactosidase were purchased (Harlan; used at 1:300 dilution). Fluorescein isothiocyanate- or Texas Red-conjugated secondary antibodies (Jackson Laboratories; used at 1:100 dilution) were detected on a laser scanning confocal microscope (Zeiss 410).
RESULTS
Identification of DNase I HSs in the promoter and/or enhancer of the murine and human K5 genes.
Six thousand base pairs of sequence directly upstream of the transcriptional start site of the human K5 gene contain sufficient regulatory information to recapitulate K5 gene expression in transgenic mice (5, 6). To identify the regions within this sequence which are likely to be important for regulating K5 gene expression in epidermal keratinocytes, we used DNase I HSs mapping to identify keratinocyte-specific alterations in the canonical nucleosome structure within this segment of the gene. Alterations in chromatin structure, detected by hypersensitivity to DNase I endonuclease, have often been useful markers for locating sites at which relevant transcription factors are bound (20).
Nuclei from primary human keratinocytes (K5-expressing cells) and fibroblasts (non-K5-expressing cells) were treated with increasing amounts of DNase I, which preferentially cleaves in regions of open or altered chromatin structure. Following treatment, genomic DNA was isolated and digested with specific restriction endonucleases. Southern blot analysis of the DNA fragments hybridized with appropriate radiolabeled probes revealed five DNase I-hypersensitive sites present within the 6,000 bp of the regulatory region of the K5 gene in nuclei from keratinocytes but not fibroblasts (Fig. 1).
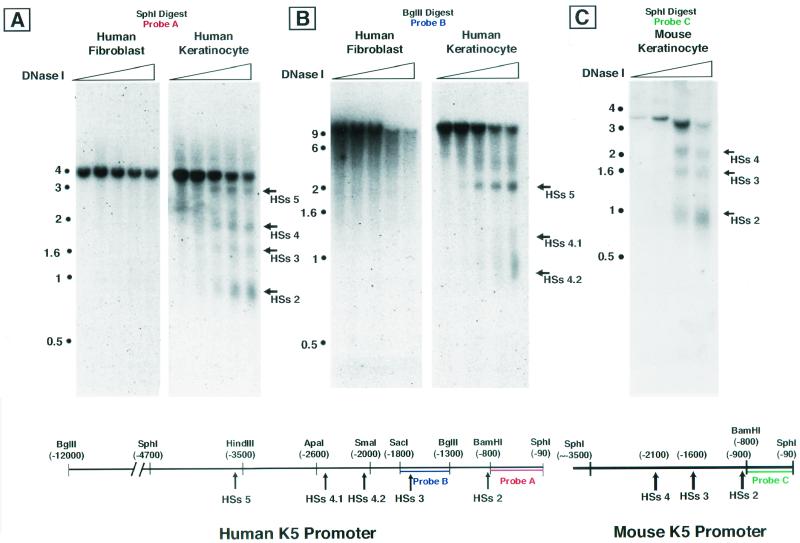
FIG. 1.
DNase I-hypersensitive site mapping of human and murine keratin 5 promoter and/or enhancer. (A) Shown here are four HSs which are present in keratinocytes but absent in fibroblasts. The approximate locations of HSs 2 through HSs 5 are indicated on the map, along with restriction sites. Nucleotide positions are numbered relative to the transcriptional start site (+1), around which HSs 1 is located (not shown here). Nuclei from human fibroblasts or keratinocytes were treated with increasing amounts of DNase I. The DNA was digested with SphI restriction enzyme, and a Southern blot was performed with probe A. (B) Finer mapping revealed that HSs 4 contains two closely spaced HSs, indicated here as HSs 4.1 and HSs 4.2. Mapping was performed as for panel A, but DNA was digested with BglII. Southern blotting was performed with probe B. (C) The murine K5 promoter contains at least three HSs (HSs 2 to 4) which are located analogously to HSs 2 to 4 in the human K5 promoter. Mouse nuclei were isolated and treated with increasing amounts of DNase I, digested with SphI, and probed on a Southern blot with probe C.
HSs 1 localized to the transcriptional start site region (data not shown), which we have analyzed extensively in previous studies (5, 6). HSs 2 to 5 were located within the first 3.5 kb of the promoter and/or enhancer (Fig. 1A). Finer analysis of HSs 4 revealed that it actually contains two closely spaced HSs, which we have referred to as HSs 4.1 and HSs 4.2 (Fig. 1B). Additional probes were used to assay for HSs beyond the SphI site at 6-kb, but none were identified out to ≈12 kb (data not shown).
To estimate the potential importance of these HSs, we used a PCR-based strategy (Genome Walker Kit; Clontech) to isolate and sequence the murine K5 (mK5) upstream regulatory region corresponding to HSs 1 to 4 (from bp +1 to ca. −3300). We subsequently used this information to design appropriate probes to identify the DNase I-hypersensitive sites of the native K5 promoter and/or enhancer in mouse keratinocytes and to compare these with the human HSs. Within the mK5 gene, three keratinocyte-specific HSs were identified that are positioned analogously to HSs 2 through HSs 4 of the hK5 gene (Fig. 1C); the presence of a nonconserved SphI restriction site at approximately −3500 precluded a simple comparison of the upstream regulatory region encompassing HSs 5.
Assessment of the transcriptional activity of individual hK5 HSs revealed a strong, keratinocyte-specific activity contained within HSs 4 .
To concentrate on the enhancer region(s) which might be responsible for keratinocyte-specific expression of K5, we assayed for the ability of each HSs to activate transcription of a luciferase (luc) reporter gene in transient-transfection assays. We linked sequences corresponding to each of the other four HSs to the minimal hK5 promoter encompassing HSs 1 (positions −90 to +30 as defined by Byrne et al. [5]). These regulatory sequences were then tested for their ability to activate transcription over the minimal hK5 promoter when transfected into the mouse keratinocyte line UG1 (Fig. 2).
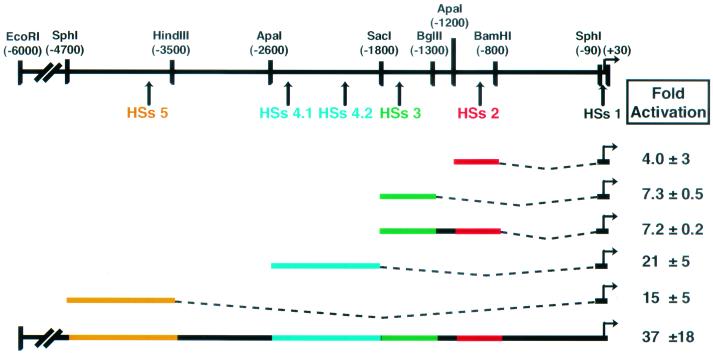
FIG. 2.
Each human HSs alone shows enhancer-like activity in transient-transfection assays. Mouse keratinocytes were transiently transfected with constructs containing the indicated HSs region linked to the hK5 minimal promoter driving a luciferase reporter. Plasmid CMV-β-gal was cotransfected to control for transfection efficiency. Values are averages with standard deviations of two to three independent duplicate experiments. Fold activation = activity of HSs/luciferase activity of hK5 minimal promoter alone.
The previously described 6-kb upstream sequence, referred to here as the full hK5, activated transcription strongly in mouse keratinocytes. Relative to this activity, HSs 3 and HSs 2 on their own displayed only weak activity, even when combined as a single element. However, HSs 4 and HSs 5 each activated transcription quite strongly, at ≈50% of the activity of the full hK5. Of these, the enhancer activity of HSs 5 did not behave as a cell type-specific element in vitro; moreover, this activity could not be readily dissected into a smaller region without losing the bulk of the activity (data not shown). In contrast, when assayed in primary human keratinocytes, HSs 4 activated transcription comparably to the full hK5 region (Fig. 3A). The enhancer activity also appeared to be largely specific for keratinocytes, as judged from the very weak activation in human HepG2 (liver) and HeLa (cervical carcinoma) cells. Consequently, we focused on characterizing HSs 4 as a major cell type-specific enhancer element within the K5 gene.
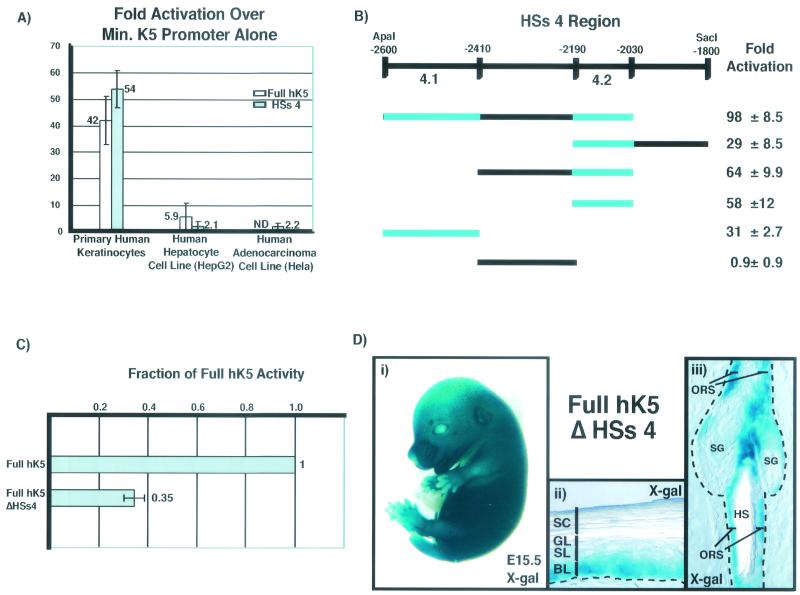
FIG. 3.
HSs 4 contains cell type-specific enhancer activity which can be localized to two small regions (4.1 and 4.2). Deletion of HSs 4 from the full 6-kb HK5 promoter substantially decreases promoter activity in transient transfections, but does not affect the pattern of transgene expression in vivo in transgenic mice. (A) Epidermal (primary human keratinocytes) and nonepidermal (HepG2 and HeLa) cell lines were transiently transfected with the full 6-kb hK5 promoter or HSs 4 plus the minimal (Min.) hK5 promoter driving the luciferase gene. Notice the strong activation in keratinocytes, which is not seen in HepG2 and HeLa cells. Transfections were performed as described in the legend to Fig. 2. ND, not determined. (B) Segments of the HSs 4 region were linked to the minimal hK5 promoter driving the luciferase gene, and enhancer activities were determined as in the legend to Fig. 2. Note that regions 4.1 and 4.2 activated transcription strongly in contrast to the intervening segment (−2410 to −2190). (C) Primary human keratinocytes were transiently transfected as for panel A with the full hK5 promoter or with the full hK5 promoter lacking HSs 4 (full HK5 ΔHSs4). Values are given as a fraction of full hK5 promoter activation over the minimal K5 promoter. Note that removing HSs 4 decreases transcriptional activation to 35% ± 4% of the full hK5. (D) Transgenic mice were generated with the full hK5 promoter lacking HSs 4 (ΔHSs4) driving the lacZ reporter gene. Eight out of nine genotypically positive founder lines showed transgene expression in the epidermis. (i) Shown is an example of a whole-mount F1 transgenic embryonic day 15.5 (E15.5) embryo stained with X-Gal. (ii and iii) The transgene is expressed in endogenous K5-expressing cells, i.e., the basal layer (BL) of the epidermis, the outer root sheath (ORS) of the hair follicle, and the sebaceous gland (SG). These 10-μm sections of tail samples from a ≈1-month-old F0 transgenic mouse were stained with X-Gal. HS, hair shaft.
Further testing by gene transfection assays revealed that the 570-bp HSs 4 region (−2600 to −2030) is a composite of two smaller enhancer elements of 190 bp (region 4.1) and 160 bp (region 4.2) (Fig. 3B). These elements are separated by a 220-bp intervening segment (−2410 to −2190) which did not contribute to transcriptional activation of the luciferase reporter gene. Closer inspection by DNase I HSs mapping confirmed the existence of two closely spaced, keratinocyte-specific HSs within HSs 4 (Fig. 1B), suggesting that these elements are the targets for transcription factors that bind to and alter chromatin structure within the region.
When the 570-bp HSs 4 segment (from −2600 to −2030) was removed from the full 6-kb hK5 promoter and tested in transient-transfection assays, a significant decrease in enhancer activity was observed, revealing a special importance of this element for robust gene expression in keratinocytes (full hK5 ΔHSs 4 activation decreased to 35% ± 4% of full hK5 activation, Fig. 3C). When full hK5 ΔHSs 4 was tested in transgenic mice, eight of nine genotypically positive founder transgenic lines possessed detectable lacZ reporter expression, as assayed by whole-mount X-Gal staining of embryos or X-Gal staining of tail sections. As illustrated in the representative examples shown in Fig. 3D, the removal of HSs 4 did not appreciably alter the expression pattern of the lacZ reporter gene (basal layer of epidermis, outer root sheath of hair follicle, and sebaceous gland) from that previously reported for the full hK5 promoter (5, 6). Overall, these findings suggest HSs 4 possesses appreciable enhancer activity, but it is not essential for proper cell type- or differentiation-specific targeting in vivo when the other enhancer elements are left intact.
Alignment of murine and human K5 promoter and/or enhancer sequences.
The similar positions of HSs 2 to 4 in the mK5 and hK5 genes were suggestive of a potentially conserved mechanism for keratinocyte-specific transcription of the K5 gene. To explore this possibility, we sequenced the murine K5 promoter out to approximately position −3300 and obtained additional upstream sequence (from approximately −3300 to −6000) from the Celera Mouse Genome database. We also acquired human K5 promoter sequence upstream of position −2.5 kb from the public human genome draft sequence (24). We then used the Pustell algorithm (MacVector; Oxford Molecular Group) to align the sequences of the mK5 and hK5 genes and to identify conserved stretches of sequence within the 5′ upstream regions (Fig. 4A).
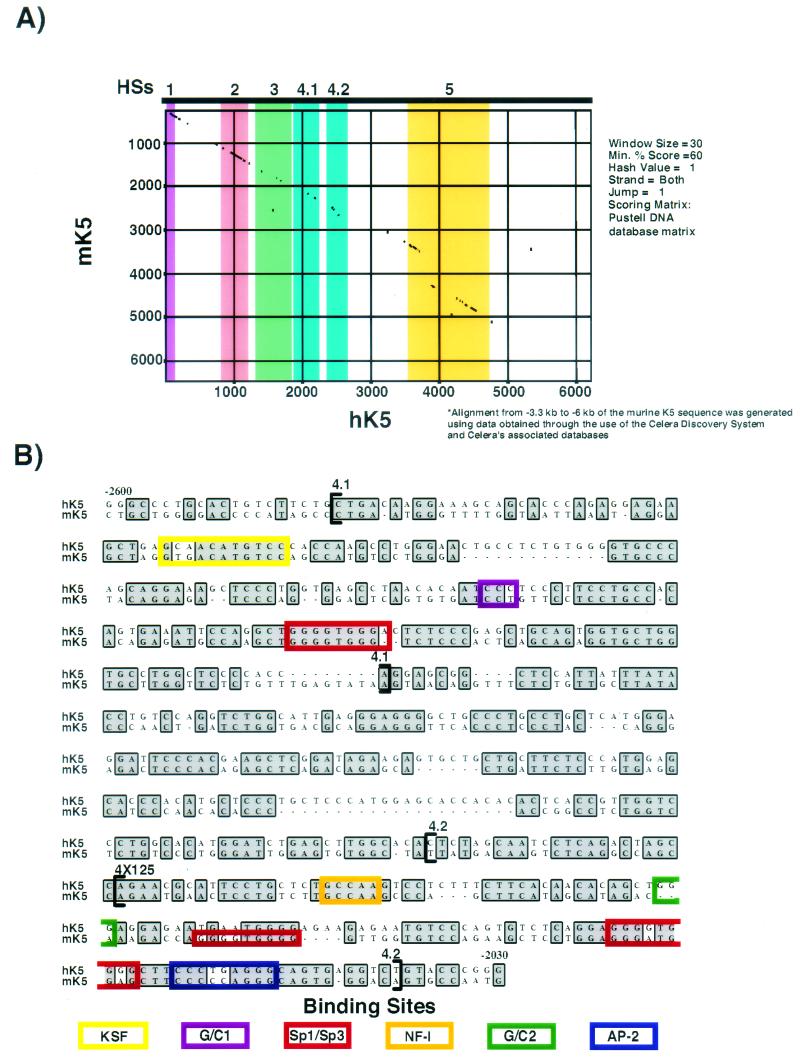
FIG.4.
Alignment of the human and murine HSs 4 regions reveals considerable sequence conservation. (A) Pustell alignment of 6 kb of the human and murine K5 promoter and enhancer sequences. The HSs regions used in functional assays are highlighted. Note the marked coincidence of conserved sequence and HSs location in most cases throughout the promoter. For this comparison, murine sequence upstream of approximately bp −3300 was obtained from the Celera database. (B) A ClustalW alignment was performed with the human and murine K5 sequences. Shown are the sequences corresponding to HSs 4 obtained in their entirety at the University of Chicago from the cloned human and murine K5 genes. Functional transcription factor binding sites (as determined by gel shift and mutational analyses, see below) are indicated by the colored boxes. Intriguingly, the core recognition sites for Sp1/Sp3, AP-2, and NF-I are conserved, as well as the putative binding sites for KSF and G/C1. A nonconserved Sp1/Sp3 binding site is indicated in the murine HSs 4.2 region.
Most notably for this study, blocks of homology were present in the regions corresponding to HSs 2 through HSs 5, and the homology decreased in the intervening regions devoid of visible alterations in chromatin conformation. We then performed an alignment with the ClustalW algorithm (MacVector) to screen for conserved sequences which might contain putative transcription factor binding sites. Using both the Matinspector and Alibaba 2.1 algorithms to search the Transfac database, a number of transcription factor binding sites were predicted for transcription factors known to be expressed in skin epithelium (17, 54). These included GC boxes, which are recognized by Sp1 and Sp3 and possibly by Kruppel-like factors, including Klf4, Klf5, Klf13, and Klf9 (32, 36, 43); AP-2 family members (21, 43); and NF-I family members (Fig. 4B) (19). In addition, sequences were also conserved that encompassed the regions marked KSF and G/C1 in Fig. 4B. We later show that all of these sites bind factors that are present in keratinocyte nuclei.
Identification of functional transcription factor binding sites in region 4.1 and region 4.2.
To identify the potential transcription factors that might be governing the enhancer activities of regions 4.1 and 4.2, we first used overlapping radiolabeled oligonucleotides and electrophoretic mobility shift assays with nuclear extracts from both human and mouse keratinocytes and from nonepidermal cells. To assess the specificity of the protein complexes and to define more precisely their binding sites, we used unlabeled oligonucleotides in competitive binding electrophoretic mobility shift assays and also used oligonucleotides harboring point mutations in putative factor binding sites. Whenever possible, we employed antibodies against known transcription factor family members to assign an identity to the proteins that bound specifically to an oligonucleotide. By concomitantly testing the functionality of these key sites in transfection assays, we subsequently assessed the extent to which each site contributed to keratinocyte-specific transcriptional activity.
Region 4.1.
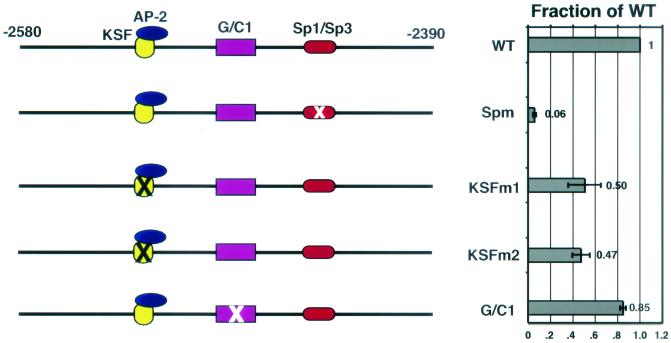
Our analysis of region 4.1 is shown in Fig. 5A and revealed a complex enhancer element encompassing the binding of at least three distinct protein complexes. A radiolabeled oligonucleotide spanning the GC-box (Sp1/Sp3 binding site) (29, 38) was found to bind to a set of proteins present in keratinocyte nuclei. In electrophoretic mobility shift assays, this complex was inhibited by an unlabeled oligonucleotide containing a GC-box. The complex appeared to be predominantly composed of the Sp1 and Sp3 proteins, as judged by the ability of antibodies specific for Sp1 and Sp3 to cause a supershift of the complex (Fig. 5A). Mutation of this site within region 4.1 (Spm, GGG GTG GGG → GGT TGG GGG) abrogated the ability of Sp3 and Sp1 complexes to bind to the site (data not shown) and also resulted in a severe reduction in the transactivating activity of region 4.1 (reduced to ≈6% of the wild-type level) (Fig. 6).
FIG. 5.
Gel shift analysis reveals the presence of three transcription factor binding sites in region 4.1. Nuclear extracts (NE) from primary human keratinocytes (hK) were used in electrophoretic mobility shift assays performed by using overlapping oligonucleotides spanning the entire region 4.1. Data for oligonucleotides which bound specific protein complexes are shown. The identities of these proteins are indicated at the left, and proteins which bound antibodies (Ab) to supershift a complex(es) are denoted by an asterisk (∗). (A) Sp1/Sp3 binding. The unlabeled competing oligonucleotides (comp. oligonucleotide) used were from Promega Biotechnology Corporation and contained a consensus (Sp) or a mutated (Spm) Sp1/Sp3 binding site. The complexes were also supershifted with antibodies to Sp1 or Sp3. (B) KSF-AP-2 complexes. A complex (referred to here as KSF) containing AP-2α (note supershift) bound to oligonucleotide B. KSF did not appear to bind to a consensus AP-2 binding site, as it was not inhibited by unlabeled oligonucleotides containing a consensus AP-2 site. This complex was only seen in keratinocytes. HDF, human diploid fibroblasts. (C) G/C1 complexes. A mutation (oligonucleotide Cm, CCC → TTT) prevented formation of the complex referred to here as G/C1. NS, nonspecific complex.
FIG. 6.
Protein binding sites that are functionally important for region 4.1 enhancer activity. Mutations which prevent binding of the proteins identified in Fig. 5 were introduced into region 4.1 and linked to the minimal human K5 promoter driving the luciferase reporter gene. Transient transfections of primary human keratinocytes and luciferase assays were performed in duplicate, and the activities were determined and recorded relative to the wild-type (WT) value of 1.0. Note that mutation of the Sp1/Sp3 binding site (Spm, GGG GTG GGG → GGT TGG GGG) nearly abrogated enhancer activity, whereas two different mutations of the putative KSF binding site (M1, AGCAAC → AGCTTC, and M2, AGCAAC → ATACCC) decreased the region's activity by approximately 50%. Mutation of G/C1 (CCC → TTT) had a minimal effect.
While Sp1 and Sp3 binding sites are often features of housekeeping genes, bona fide Sp1 and Sp3 sites have also been identified in the minimal hK5 promoter and enhancer (5, 45) as well as in other epidermal promoters, including K14, involucrin, SPRR2A, and transglutaminase 3 (9, 13, 26-28). Sp3 null mice die postnatally and display defects in lung and tooth development (4). We examined the skin of late-stage Sp3 null embryos but did not observe any obvious abnormalities (data not shown). Functional redundancy with Sp1 cannot at present be ruled out, given that genetic ablation of Sp1 in mice results in death by embryonic day 9.5, i.e., prior to skin differentiation (32).
A second DNA-protein complex binding to region 4.1 was only detected with nuclear extracts from keratinocytes and not from other human cell types, including hepatocytes (HepG2 cells), T lymphocytes (Jurkat cells), or diploid fibroblasts (dermal cells), leading us to name this complex keratinocyte-specific factor (KSF) (Fig. 5B). This complex was supershifted by antibodies to AP-2α (Fig. 5B) and AP-2α (data not shown), the two major AP-2 family members of keratinocytes (data for AP-2α are shown). Surprisingly, however, the radiolabeled oligonucleotide used in the electrophoretic mobility shift assay did not contain a consensus AP-2 binding site, nor was AP-2 involved in this complex inhibited by an unlabeled oligonucleotide harboring a bona fide AP-2 site (Fig. 5B).
Two mutations (M2, AGCAAC → AGCTTC, and M3, AGCAAC → ATACCC) prevented binding of KSF (data not shown) and led to a reduction in the enhancer activity of region 4.1 (50 and 47% of the wild-type level, respectively; Fig. 6). These residues were contained within a novel sequence not resembling any consensus sequence for known DNA-binding proteins. Taken together, these data suggested that AP-2 proteins are a part of a keratinocyte-specific protein complex that can bind to a unique sequence motif in region 4.1. An alternative explanation, still untested, is that the observed anti-AP-2 reactivity of this complex stemmed from cross-reactivity with a novel keratinocyte protein.
A third specific protein complex bound to a conserved, GC-rich element (G/C1) within region 4.1. Interestingly, G/C1 contains a perfect Ets core consensus sequence (ATCC). This said, the G/C1 complex was not inhibited by a consensus Ets oligonucleotide (data not shown), and thus we have no evidence to implicate Ets proteins in G/C1-mediated regulation. A 3-bp mutation (G/C1m, CCC → TTT) prevented formation of the G/C1 protein complex (Fig. 5C) and had a modest effect on the enhancer activity of region 4.1 (reduced to 85% of the wild-type level; Fig. 6). In addition to its relatively weak impact on transcriptional activity, this protein complex was seen in electrophoretic mobility shift assays involving nuclear extracts from all cell types examined. Thus, relative to the other two complexes, this complex did not appear to be a major contributor to the enhancer activity of region 4.1, and we did not pursue it further.
Region 4.2.
Dissection of region 4.2 revealed an enhancer element of even greater complexity than region 4.1. Electrophoretic mobility shift assays involving a series of radiolabeled oligonucleotides spanning this region uncovered the existence of at least five different keratinocyte nuclear protein complexes. Closely positioned putative binding sites for Sp1/Sp3 and AP-2 transcription factors reside near the 3′ end of this region (Fig. 7). A nearly perfect GC-box motif bound Sp1 and Sp3, as judged by electrophoretic mobility shift assays and supershifts with the appropriate antibodies (Fig. 7A). Thus, as for the 4.1 element, these sequences appeared to be occupied primarily by Sp1/Sp3 proteins rather than Klf family members, at least in nuclear extracts from cultured keratinocytes. The putative AP-2 binding site, CCCTGAGGG, differed somewhat from the canonical AP-2 sequence motif (GCCN3GGC) (21). However, this site appeared to bind AP-2α and AP-2γ, as judged by electrophoretic mobility shift assays and supershifts with the corresponding AP-2 antibodies (Fig. 7B).
FIG. 7.
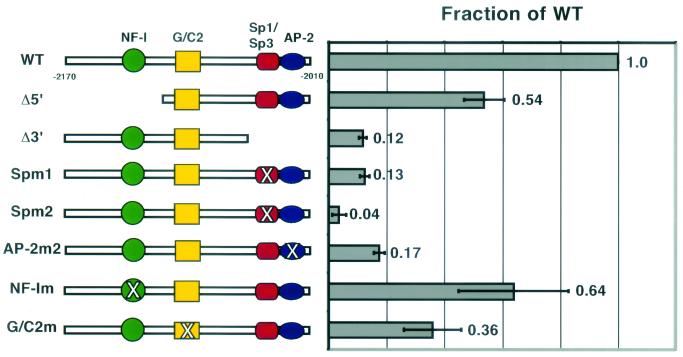
Gel shift analysis reveals four transcription factor binding sites in region 4.2. Gel shift analyses with nuclear extract (NE) from primary human keratinocytes (hK) were performed by using overlapping oligonucleotides spanning the entire region 4.2. Oligonucleotides which bound specific complexes are shown. The identities of the proteins which bound are indicated at the left, and an asterisk (∗) denotes larger complexes (supershifted complexes) that formed with the addition of the antibodies (Ab) indicated. Additional details are outlined in the legend to Fig. 5. Shown are data indicating protein-DNA complexes for (A) Sp1/Sp3 and oligonucleotide A; (B) AP-2 family members and oligonucleotide B; (C) NF-I family members and oligonucleotide NF-I; and (D) G/C2 and oligonucleotide G/C2. NS, nonspecific complex. Competitor oligonucleotides: AP-2, consensus AP-2 binding site (Promega); AP-2m1, oligonucleotide containing the sequences in oligonucleotide B but with a mutated AP-2 site (CCC TGA GGG → CCC TGG AAT); G/C2m, GGG → AAA mutation as described in the text; NF-Im, a mutation in the core consensus sequence of NF-I (GCCAA → GTTAA). Antibodies used are described in Materials and Methods.
Mutations of residues within the consensus sequence motif for Sp1/3 and AP-2 binding prevented complex formation in an electrophoretic mobility shift assay (data not shown), and importantly, mutation of either the Sp1/Sp3 (Sp1m1 and Spm2, respectively) or the AP-2 (AP-2m) site strongly reduced enhancer activity within region 4.2 (Fig. 8). A 5-bp mutation of the Sp1/Sp3 binding site (GGG GTG GG → GTC TAGAGG) decreased the enhancer activity to 13% ± 2% of the wild-type level, while a 3-bp mutation of the Sp1/Sp3 binding site (GGG GTG GGG → GGT TGG GGG) decreased activity to only 4% ± 2% of the wild-type level (Fig. 8). Similarly, a targeted 2-bp mutation of the AP-2 binding site (CCC TGA GGG → CAA TGA GGG) severely diminished the enhancer activity to 17% ± 2% of the wild-type level (Fig. 8). Thus, both Sp1/Sp3 and AP-2 family members appeared to function in regulating 4.2 enhancer activity through direct binding to canonical core DNA-binding sites.
FIG. 8.
Protein binding sites that are functionally important for region 4.2 enhancer activity. Mutations of the consensus binding sites (Sp1/Sp3, AP-2, and NF-I) and mutations which prevent complex formation in gel shift analysis (G/C2) were introduced into region 4.2, coupled to the minimal hK5 promoter. Enhancer activity was determined by using a luciferase reporter as described in the legend to Fig. 2, and these values are reported relative to the wild-type (WT) value of 1.0. Mutations are as follows (wild-type binding site → mutated site): Spm1 (GGG GTG GG → GTC TAG AGG), Spm2 (GGG GTG GGG → GGT TGG GGG), AP-2m2 (CCC TGA GGG → CAA TGA GGG), NF-Im (GCCAA → GTTAA), and GGGm (GGG → AAA).
Our discovery of AP-2 function in the K5 enhancer is in good agreement with the existence of functional AP-2 binding sites in many epidermal promoters and enhancers (5, 26, 27, 45, 47) and with the importance of AP-2 in keratinocyte gene expression in vitro (6). This said, definitive evidence of a role for AP-2 in skin development is still lacking (41, 53) and will not be resolved until conditional targeting studies are conducted to remove the redundancy of AP-2 factor expression in skin.
Our analysis of region 4.2 also revealed a half-site for NF-I/CTF family transcription factors. A targeted 2-bp mutation of the consensus NF-I half-site (NF-Im, GCCAA → GTTAA) prevented this complex from binding in an electrophoretic mobility shift assay, and addition of an antibody which recognizes the C terminus of NF-I family transcription factors resulted in a supershift of this complex (Fig. 7C). Introduction of this mutation into region 4.2 had a more modest effect on the enhancer activity in vitro (reduced to 64% ± 19% of the wild-type level; Fig. 8).
Two additional DNA-protein complexes were identified within region 4.2, and both bound to the same oligonucleotide in an electrophoretic mobility shift assay (Fig. 7D). A single mutation (G/C2m, GGG → AAA) abolished the binding of both complexes (Fig. 7D), indicating that a group of proteins may be part of a single complex that binds to this site. Despite the G/C-rich nature of the binding site, neither complex was supershifted with antibodies against Sp1/Sp3 or against AP-2 factors (data not shown). While the site contributed modestly to the overall enhancer activity of region 4.2, we did not pursue the identity of the complex(es) further because it did not appear to be keratinocyte specific (data not shown), nor was the core binding site conserved between the mouse and human sequences.
In transgenic mice, the 4.1-4.2 enhancer directs inner root sheath-specific gene expression.
As assayed in cell culture, regions 4.1 and 4.2 together constitute an enhancer element that in vitro accounts for a major portion of the keratinocyte-specific transcriptional activity within the 6,000 bp 5′ upstream of the K5 gene (Fig. 3A). Although our earlier in vivo studies did not reveal an essential role for the HSs 4 enhancer element in the keratinocyte-specific activity of the full-length K5 promoter and/or enhancer, we wondered whether the HSs 4 element alone might nevertheless possess keratinocyte-specific enhancer activity in vivo.
Previous studies showed that the minimal hK5 promoter displayed weak activity in the suprabasal layer of the epidermis of two of twenty transgenic lines derived, whereas the 6,000-bp hK5 promoter and/or enhancer exhibited strong activity within the basal layer of the epidermis and in the sebaceous gland and follicle outer root sheath in 75% of all transgenic lines engineered (5, 6). Therefore, we tested the two major HSs 4 regulatory elements for activity in vivo by coupling them to the minimal hK5 promoter.
Of 14 independently engineered animals harboring the transgene, two exhibited β-galactosidase activity. These two displayed identical expression patterns, suggesting that the K5 promoter and/or enhancer elements were responsible for this pattern (Fig. 9A). Interestingly, β-galactosidase expression appeared to be largely if not solely restricted to the skin epithelium. Although skin contains over 20 different cell types (including fibroblasts, keratinocytes, neurons, muscle cells, endothelial cells, chondrocytes, adipocytes, osteoblasts, T lymphocytes, macrophages, melanocytes, and Leydig cells), lacZ expression was detected only in keratinocytes.
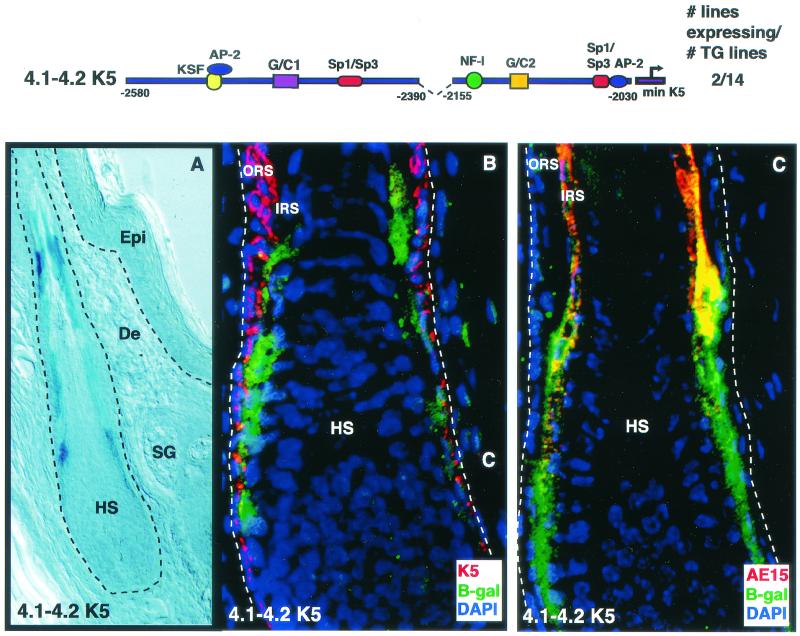
FIG. 9.
Regions 4.1 and 4.2 together target transgene expression to the inner root sheath of the hair follicle. Regions 4.1 and 4.2 were linked to the minimal hK5 promoter driving the lacZ gene, and the gene was used to engineer transgenic mice. Frozen sections (10 μm) from tails of founders were stained for β-galactosidase expression (X-Gal; frame A) or processed for indirect immunofluorescence (B and C). Antibodies used are indicated in the color-coded boxes at the lower right of each frame. K5 is an outer root sheath (ORS) marker, while AE15 is a marker of the inner root sheath (IRS). Note X-Gal staining in a portion of the hair follicle in A. Dashed line indicates dermal-epithelial boundaries. Note that the images shown in B and C are magnified over that shown in A to illustrate the lower portion of the hair follicle. Epi, epidermis; De, dermis; HS, hair shaft; IRS, inner root sheath; SG, sebaceous gland.
Immunofluorescence analysis revealed that the β-galactosidase-expressing cells were restricted to a specific subset of keratinocytes that were distinct from the basal cells of the epidermis and the outer root sheath (Fig. 9B). Thus, rather than being expressed in the mitotically active keratinocytes, which naturally possess abundant K5 promoter and/or enhancer activity (Fig. 9B), the transgene-expressing cells colabeled with antibody AE15 against trichohyalin, a protein specifically expressed in the inner root sheath channel that encases the hair shaft (Fig. 9C). Like the other differentiating keratinocytes of the skin, the inner root sheath is derived from K5-positive stem cells in the bulge of the hair follicle (15, 37, 50). Thus, despite strong activity in the K5-expressing keratinocytes cultured in vitro, the 4.1-4.2 enhancer element conferred in vivo expression to the inner root sheath, a differentiating compartment that does not express the endogenous K5 gene.
In transgenic mice, enhancer region 4.2 targets sebaceous gland-specific expression.
Both regions 4.1 and 4.2 contained elements which exhibited keratinocyte specificity in vitro . This led us to wonder which of these two regions might be responsible for the keratinocyte-specific expression pattern observed in our transgenic mice. To explore this further, we tested the elements on their own. Since the individual segments possessed only weak activity in vitro, it was necessary to multimerize individual segments, verify that they behaved analogously to but more robustly than the single copy in vitro, and then use these elements as enhancers to drive β-galactosidase expression in mice. Of the two, only the multimerized 4.2 element possessed sufficiently high activity in vitro to render it suitable for analysis in vivo.
Five independently derived transgenic mice harbored the lacZ gene controlled by the multimerized 4.2 enhancer element and the minimal hK5 promoter. Three of these lines expressed the transgene, and all displayed an identical and conspicuous pattern limited to keratinocytes (Fig. 10A, A′, and B). The 4.2 enhancer specifically and strongly targeted expression to a highly restricted subset of keratinocytes within the sebaceous gland (Fig. 10A and A′). The identity of the transgene-expressing cells was confirmed by costaining with Oil Red O, a histochemical dye which stains the neutral lipids present in sebocytes. Those cells that expressed β-galactosidase as well as the neutral lipids appeared dark blue or black in the X-Gal/Oil Red O double stain (Fig. 10B).
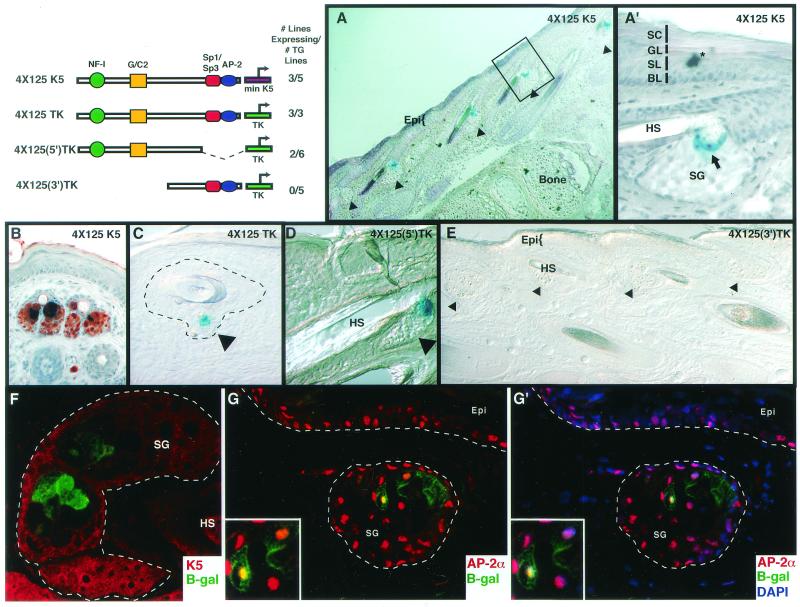
FIG. 10.
Region 4.2 targets transgene expression to a specific subset of differentiated cells of the sebaceous gland, a K5-expressing appendage of the epidermis. The diagrammed segments of region 4.2 were multimerized (four direct repeats) and linked to either the minimal hK5 promoter or the thymidine kinase promoter (TK) to drive expression of β-galactosidase in transgenic mice. At the upper left of the figure are depictions of the constructs used and the fraction of total independently derived lines that expressed each transgene. For each construct, all gave nearly identical patterns of expression. Frozen backskin samples from representative newborn animals were sectioned (10 μm). The construct expressed in each skin sample is indicated in the upper right corner of each frame, except F to G′, which are from 4×125 K5 skin. Frames A to E were subjected to X-Gal staining to identify transgene expression. The asterisk in A denotes a piece of debris related to the fixation and staining process. The hair shafts in frames A and E have a slight blue tone which is due to photographing but does not correspond to specific X-Gal staining, as can be seen in the higher magnification in frame A′. Frame B was also subjected to Oil Red O staining for lipids within differentiating sebaceous gland cells (B). The boxed area in A is shown at higher magnification in A′. Arrowheads denote sebaceous glands (SG), and arrows denote lacZ-expressing cells within sebaceous glands. The dotted line in C encompasses the outer root sheath, inner root sheath, and sebaceous gland surrounding the hair; only a sebaceous gland cell stained positively for lacZ in this section. Also, only the 4×125 K5, 4×125 TK, and 4×125(5′) TK skin samples gave positive X-Gal staining in the sebaceous gland, and this was never seen in the wild-type skin samples (not shown) or in 4×125(3′) TK skin samples (E). Frames F to G′ were subjected to indirect immunofluorescence to examine transgene expression (lacZ) in the sebaceous glands relative to keratin 5 and AP-2α expression (F to G′). Frames shown in G and G′ are identical except that G′ also shows 4′,6′-diamidino-2-phenylindole (DAPI) staining to show that AP-2α is nuclear. The antibodies used are indicated in the lower right of frames F to G′, and lettering is color-coded to the secondary antibody used. Dotted lines in F to G′ encase the sebaceous glands and demarcate the dermal-epidermal boundary. Note the presence of K5 in the peripheral cells that surround the lipid-filled, differentiating sebocytes of the sebaceous gland; these are not the lacZ-expressing sebaceous gland cells. The region in the lower left boxes of G and G′ are magnified views of sebaceous gland cells within the main frame. Epi, epidermis; HS, hair shaft; SG, sebaceous gland; BL, basal layer; SL, spinous layer; GL, granular layer; CL, cornified layer.
To evaluate whether the enhancer activity of these segments was dependent upon the presence of the native hK5 minimal promoter, which possesses weak activity in suprabasal epidermal cells (5), we coupled the heterologous thymidine kinase promoter to the 4.2 enhancer element and generated additional transgenic lines. All three independently derived transgenic mice that we made exhibited an expression pattern which was indistinguishable from that of the 4×125 K5 transgenic animals (Fig. 10C). Thus, the highly restricted, sebaceous gland-specific expression pattern appeared to be a feature of the 4.2 enhancer element and did not appear to require other tissue-specific elements for its activity. Moreover, given the high frequency with which the element was able to impart sebaceous gland-specific gene expression, the element appeared to be relatively insensitive to the integration site of the transgene.
To perform a more extensive analysis of tissue-specific expression patterns imparted by the 4.2 enhancer element, we bred the transgenic founder animals and maintained two independently derived 4×125 TK lacZ lines from their F1 offspring. Sagittal frozen sections of whole newborn mice were stained, and we found that strong β-galactosidase activity was limited to the sebaceous glands (data not shown). After extended development of the β-galactosidase activity assay (24 h), very faint blue staining was observed in a few cells of the bone marrow (data not shown). These findings underscored the high degree of cell type- and differentiation-specific transcriptional regulation governed by the 125-bp 4.2 enhancer element.
From additional mutation and transgenic analyses, we learned that it is the 5′ domain of the 4.2 enhancer that contains the regulatory elements essential for sebaceous gland-specific expression (Fig. 10D and E). Our in vitro studies (above) had shown that the 5′ segment contains the NF-I binding site and G/C2 motif but lacks the Sp1/Sp3 and AP-2 binding sites. The result was somewhat surprising given that Sp1 and Sp3 are broadly expressed genes, we detected AP-2α in the nuclei of lacZ-expressing sebocytes (Fig. 10G and G′), and we had anticipated an involvement of Sp1/Sp3 and AP-2 from our studies on epidermally expressed genes. This said, the sebaceous gland activity of the 5′ segment of region 4.2 was novel, as was our identification of key regulatory roles for the NF-I and G/C2 sequences.
Although future studies will be necessary to fully elucidate the molecular mechanism by which this small and highly specific enhancer element functions, it is important to note that throughout the 6,000 bp of the K5 enhancer, there are 47 NF-I half-sites and a number of G/C-rich sequence stretches, providing some insight as to why sebaceous gland activity was still observed when HSs 4 was removed from the full-length enhancer.
DISCUSSION
Conservation and overall structure of K5 promoter.
In this study, we discovered a marked evolutionary conservation of sequence and location of the open chromatin regions that govern keratinocyte-specific activity within the keratin 5 promoter and/or enhancer. This analysis has uncovered novel molecular insights into the earlier observation that the 6,000 bp of 5′ upstream sequence of human K5 is able to effectively and faithfully target the expression of foreign genes in mice (5, 6).
Our in vitro studies led us to a hitherto unexplored enhancer element contained within one of five keratinocyte-specific DNase I-hypersensitive sites (HSs 4) of the K5 gene. HSs 4 was distinguished from the others in that in vitro it exhibited strong, cell type-specific activity, and this activity could be localized to two compact domains (regions 4.1 and 4.2). Analyses of this HSs region revealed a level of complexity in keratinocyte-specific gene regulation that is substantially greater than that which was known previously. This complexity is reflected not only in the large number of regulatory sites within this single enhancer element, but also in the diversity and importance of the proteins that bind and contribute to keratinocyte-specific gene expression in vivo.
Differentiation specificity embedded within the K5 HSs 4 enhancer.
Cultured keratinocyte populations harbor K5-expressing skin epithelial stem cells, which are thought to be multipotent and which in vivo are able to give rise to all of the epithelial compartments of the skin (15, 37, 50). Without analysis of promoter and enhancer elements in vivo, it is impossible to assess whether the keratinocyte specificity observed in vitro reflects proper differentiation specificity. The paucity of in vivo data in this area prompted us to employ a multidimensional approach to tackle this intriguing problem.
Our in vitro studies underscored the significant contribution of HSs 4 and its subelements to the transcriptional activity of the full 6-kb hK5 promoter and/or enhancer. The ability of a 6-kb K5 promoter and/or enhancer lacking the HSs 4 element to function in a proper tissue-specific fashion reveals the existence of multiple and likely redundant regulatory elements within the 6-kb sequence. In support of this notion, an analysis of the 6-kb hK5 promoter by using the default settings of Matinspector predicts the following putative transcription factor binding sites: 14 AP-2 sites, 14 Sp1/3 sites, and 47 NF-I half-sites (data not shown). The magnitude of the complexity we have uncovered leaves it beyond the scope of the present study to identify in complete detail the mechanism by which even one of these enhancer elements functions. However, it was particularly interesting that despite the dispensability of HSs 4 to tissue-specific K5 enhancer activity, the element HSs 4 on its own was able to drive highly restricted patterns of keratinocyte-specific lacZ gene expression.
HSs 4 encompasses two domains which together bind at least five different protein complexes, two of which are keratinocyte specific. Although the specific differentiating keratinocytes targeted by 4.1-4.2 and 4.2 enhancer activity are not natural targets for K5 and K14 promoter and/or enhancer activity, they are derived from progeny of the K5-expressing multipotent skin epithelial stem cells. However, not only did the 4.1-4.2 and 4.2 enhancers fail to target reporter gene expression to the stem cell compartment, they also failed to turn off reporter gene expression in the differentiating keratinocytes of the inner root sheath (4.1-4.2) and sebaceous gland (4.2). Based upon these studies, we surmise that the elements on their own lack some positive and negative regulators present within the larger 6 kb of the K5 promoter and/or enhancer. Finally, since the 4.2 subelement on its own gave activity that was distinct from the 4.1-4.2 element, we must further conclude that the 4.1 element contains sequences that suppress sebaceous gland activity as well as sequences that promote inner root sheath activity.
Achieving differentiation as well as keratinocyte specificity.
To assess how these individual elements maintain keratinocyte specificity, achieve their unique differentiation specificity, and yet act combinatorially to provide distinct differentiation specificities, it is necessary to know which transcription factors are involved in orchestrating the expression patterns of each element. AP-2 and Sp1/Sp3 sites have been found in the K14 and K5 promoters as well as in many genes expressed specifically in keratinocytes (5, 26, 27, 48). Our finding that AP-2 and Sp1/Sp3 sites also exist in the K5 4.2 enhancer element is particularly interesting in light of the recent discovery that these sites also exist in several of the keratinocyte-specific HSs of the K14 gene (45, 46). It is also intriguing that the K5 4.1 element possesses a functional Sp1/Sp3 binding site, and although the 4.1 enhancer does not seem to bind AP-2 directly, it appears to do so indirectly, through KSF.
Are these elements necessary and sufficient for keratinocyte specificity? Our in vitro observations indicated that of all the elements within the HSs 4 region, the AP-2 and Sp1/Sp3 sites contributed most strongly to the overall levels of keratinocyte-specific gene expression in vitro. This finding lends strong support to the view that enhancer activity in actively dividing keratinocytes in vitro relies heavily on the coordinate involvement of AP-2 and Sp1 factors. However, our in vivo data revealed that AP-2 and Sp1/Sp3 sites could be removed from region 4.2 without compromising its exquisite specificity for the differentiating keratinocytes of the sebaceous gland. Conversely, we failed to detect any keratinocyte-specific expression in vivo with the segment containing the AP-2 and Sp1/Sp3 sites on their own [4×125(3′) TK transgenics]. While these findings do not rule out a role for AP-2 and Sp1/Sp3 in keratinocyte-specific gene expression in vivo, they do illustrate that the sites alone or in concert are not sufficient for targeting keratinocyte specificity.
Our findings imply that differentiation specificity in vivo is orchestrated by a constellation of additional factors, which, for the sebaceous gland, include the keratinocyte proteins that bind to the G/C2 and NF-I sites (19, 34) within region 4.2. The G/C2 element and its binding partners await a more detailed characterization, although the associated complex identified from electrophoretic mobility shift assays of cultured cell nuclear extracts did not appear to be cell type specific. In contrast, even though NF-I family members are expressed widely, their levels in cells vary considerably (7). The importance we have assigned to NF-I in controlling keratinocyte-specific gene expression in the sebaceous gland is interesting in that NF-I sites have been identified as regulatory elements of genes expressed in the adipocyte, a cell type which also develops large lipid stores (18, 44). At present, functional studies on NF-I family members are largely lacking, although the few NF-IA null mice that survive after birth appear to develop normal whiskers and hair (10). Whether sebaceous gland differentiation requires this or other family members awaits future investigation and is beyond the scope of the present study.
The HSs 4 enhancer was remarkable not only for its sebaceous gland-specific region 4.2, but also for the fact that its two regulatory elements combined (4.2 and 4.1) suppressed sebaceous gland-specific gene expression and promoted inner root sheath-targeted gene expression. The features responsible for this redirection of differentiation-specific gene expression are still not clear, and we did not analyze the 4.1 element to the extent that we did the 4.2 enhancer. This said, three functional elements were identified within region 4.1 (Sp1/3, KSF/AP-2, and G/C1), as might be expected for an element that can display both repressive and activator functions. While at first glance the task of dissecting the 4.1 enhancer might seem daunting, it is interesting that another inner root sheath enhancer was recently discovered embedded in a 150-bp DNase I-hypersensitive element within the K14 enhancer (45). The K14 HSs contains sites for the binding of CACCC box proteins, Sp1/Sp3 family members, Ets proteins, and Skn-1 factors, revealing some potential parallels between the two inner root sheath-specific elements. As importantly, these studies begin to unveil clues that the regulatory elements within these two coordinately expressed genes may have been evolutionary conserved, despite the lack of overall sequence similarities between the 5′ upstream sequences of the K5 and K14 genes.
In summary, the K5 HSs 4.2 enhancer element represents the first small (125-bp) element taken out of context of a keratinocyte-expressed gene and shown to function as a strong enhancer in a keratinocyte-specific manner. Our characterization of this element has provided us with the clearest picture to date of how differentiation specificity may be achieved within the skin epithelium. We are faced with the notion that keratinocyte-specific gene expression is dependent on the combinatorial control of multiple transcription factors. The HSs appear to represent modules which together are necessary for the complete, tight, and coordinate regulation of the K5 and K14 genes. Of the three HSs we have analyzed thus far (regions 4.1 and 4.2 in this report and region 1 in a previous study [6]), each contains unique transcription factor binding sites as well as elements which recruit AP-2 and/or Sp1/Sp3. The unique sites appear to impart to the element its ability to direct expression to a subset of keratinocytes within the skin. This modular architecture allows a particularly fine tuning of keratinocyte-specific and differentiation-specific gene expression, which may be important in manifesting the diverse and critical functions of the different epithelial stem cell lineages in the skin.
Acknowledgments
We thank Naoko Tanese at New York University School of Medicine for the generous gift of antibodies to NF-I/CTF (serum 8199), Trevor Williams for the gift of the antibodies to AP-2α, and Guntram Suske for providing us with the opportunity to examine Sp3−/− embryos.
C.K. and D.B. are recipients of Medical Scientist Training Program fellowships (Medical Scientist National Research Service Award, NIH/National Institute of General Medical Sciences grant 5 T32 GM07281). S.S. is the recipient of a National Institutes of Health postdoctoral fellowship. E.F. is an Investigator of the Howard Hughes Medical Institute. This work was supported in part by a grant from the National Institutes of Health (R01-AR31737). The transgenic work was conducted in the University of Chicago Cancer Center mouse facility.
The first two authors contributed equally to this work.
REFERENCES
- 1.Andersen, B., W. C. Weinberg, O. Rennekampff, R. J. McEvilly, J. R. Bermingham, Jr., F. Hooshmand, V. Vasilyev, J. F. Hansbrough, M. R. Pittelkow, S. H. Yuspa, and M. G. Rosenfeld. 1997. Functions of the POU domain genes Skn-1a/i and Tst-1/Oct-6/SCIP in epidermal differentiation. Genes Dev. 11:1873-1884. [DOI] [PubMed] [Google Scholar]
- 2.Andreoli, J. M., S. I. Jang, E. Chung, C. M. Coticchia, P. M. Steinert, and N. G. Markova. 1997. The expression of a novel, epithelium-specific ets transcription factor is restricted to the most differentiated layers in the epidermis. Nucleic Acids Res. 25:4287-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellard, M., G. Dretzen, A. Giangrande, and P. Ramain. 1989. Nuclease digestion of transcriptionally active chromatin. Methods Enzymol. 170:317-346. [DOI] [PubMed] [Google Scholar]
- 4.Bouwman, P., H. Gollner, H. P. Elsasser, G. Eckhoff, A. Karis, F. Grosveld, S. Philipsen, and G. Suske. 2000. Transcription factor Sp3 is essential for postnatal survival and late tooth development. EMBO J. 19:655-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrne, C., and E. Fuchs. 1993. Probing keratinocyte and differentiation specificity of the human K5 promoter in vitro and in transgenic mice. Mol. Cell. Biol. 13:3176-3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne, C., M. Tainsky, and E. Fuchs. 1994. Programming gene expression in developing epidermis. Development 120:2369-2383. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhry, A. Z., G. E. Lyons, and R. M. Gronostajski. 1997. Expression patterns of the four nuclear factor I genes during mouse embryogenesis indicate a potential role in development. Dev. Dyn. 208:313-325. [DOI] [PubMed] [Google Scholar]
- 8.Corbi, A. L., U. B. Jensen, and F. M. Watt. 2000. The alpha2 and alpha5 integrin genes: identification of transcription factors that regulate promoter activity in epidermal keratinocytes. FEBS Lett. 474:201-207. [DOI] [PubMed] [Google Scholar]
- 9.Crish, J. F., T. M. Zaim, and R. L. Eckert. 1998. The distal regulatory region of the human involucrin promoter is required for expression in epidermis. J. Biol. Chem. 273:30460-30465. [DOI] [PubMed] [Google Scholar]
- 10.das Neves, L., C. S. Duchala, F. Godinho, M. A. Haxhiu, C. Colmenares, W. B. Macklin, C. E. Campbell, K. G. Butz, and R. M. Gronostajski. 1999. Disruption of the murine nuclear factor I-A gene (Nfia) results in perinatal lethality, hydrocephalus, and agenesis of the corpus callosum. Proc. Natl. Acad. Sci. USA 96:11946-11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faus, I., H. J. Hsu, and E. Fuchs. 1994. Oct-6, a regulator of keratinocyte gene expression in stratified squamous epithelia. Mol. Cell. Biol. 14:3263-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng, X., Z. H. Peng, W. Di, X. Y. Li, C. Rochette-Egly, P. Chambon, J. J. Voorhees, and J. H. Xiao. 1997. Suprabasal expression of a dominant-negative RXR alpha mutant in transgenic mouse epidermis impairs regulation of gene transcription and basal keratinocyte proliferation by RAR-selective retinoids. Genes Dev. 11:59-71. [DOI] [PubMed] [Google Scholar]
- 13.Fischer, D. F., S. Gibbs, P. van De Putte, and C. Backendorf. 1996. Interdependent transcription control elements regulate the expression of the SPRR2A gene during keratinocyte terminal differentiation. Mol. Cell. Biol. 16:5365-5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuchs, E., and C. Byrne. 1994. The epidermis: rising to the surface. Curr. Opin. Genet. Dev. 4:725-736. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs, E., and J. A. Segre. 2000. Stem cells: a new lease on life. Cell 100:143-155. [DOI] [PubMed] [Google Scholar]
- 16.Gat, U., R. DasGupta, L. Degenstein, and E. Fuchs. 1998. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell 95:605-614. [DOI] [PubMed] [Google Scholar]
- 17.Grabe, N. 2002. AliBaba2: context specific identification of transcription factor binding sites. In Silico Biol. 2:S1-15. [PubMed] [Google Scholar]
- 18.Graves, R. A., P. Tontonoz, S. R. Ross, and B. M. Spiegelman. 1991. Identification of a potent adipocyte-specific enhancer: involvement of an NF-1-like factor. Genes Dev. 5:428-437. [DOI] [PubMed] [Google Scholar]
- 19.Gronostajski, R. M. 2000. Roles of the NFI/CTF gene family in transcription and development. Gene 249:31-45. [DOI] [PubMed] [Google Scholar]
- 20.Hardison, R., J. L. Slightom, D. L. Gumucio, M. Goodman, N. Stojanovic, and W. Miller. 1997. Locus control regions of mammalian beta-globin gene clusters: combining. Gene 205:73-94. [DOI] [PubMed] [Google Scholar]
- 21.Hilger-Eversheim, K., M. Moser, H. Schorle, and R. Buettner. 2000. Regulatory roles of AP-2 transcription factors in vertebrate development. Gene 260:1-12. [DOI] [PubMed] [Google Scholar]
- 22.Jonas, E. A., A. M. Snape, and T. D. Sargent. 1989. Transcriptional regulation of a Xenopus embryonic epidermal keratin gene. Development 106:399-405. [DOI] [PubMed] [Google Scholar]
- 23.Komine, M., L. S. Rao, I. M. Freedberg, M. Simon, V. Milisavljevic, and M. Blumenberg. 2001. Interleukin-1 induces transcription of keratin K6 in human epidermal keratinocytes. J. Investig. Dermatol. 116:330-338. [DOI] [PubMed] [Google Scholar]
- 24.Lander, E. S., L. M. Linton, B. Birren, C. Nusbaum, M. C. Zody, J. Baldwin, K. Devon, K. Dewar, M. Doyle, W. FitzHugh, R. Funke, D. Gage, K. Harris, A. Heaford, J. Howland, L. Kann, J. Lehoczky, R. LeVine, P. McEwan, K. McKernan, J. Meldrim, J. P. Mesirov, C. Miranda, W. Morris, J. Naylor, C. Raymond, M. Rosetti, R. Santos, A. Sheridan, C. Sougnez, N. Stange-Thomann, N. Stojanovic, A. Subramanian, D. Wyman, J. Rogers, J. Sulston, R. Ainscough, S. Beck, D. Bentley, J. Burton, C. Clee, N. Carter, A. Coulson, R. Deadman, P. Deloukas, A. Dunham, I. Dunham, R. Durbin, L. French, D. Grafham, S. Gregory, T. Hubbard, S. Humphray, A. Hunt, M. Jones, C. Lloyd, A. McMurray, L. Matthews, S. Mercer, S. Milne, J. C. Mullikin, A. Mungall, R. Plumb, M. Ross, R. Shownkeen, S. Sims, R. H. Waterston, R. K. Wilson, L. W. Hillier, J. D. McPherson, M. A. Marra, E. R. Mardis, L. A. Fulton, A. T. Chinwalla, K. H. Pepin, W. R. Gish, S. L. Chissoe, M. C. Wendl, K. D. Delehaunty, T. L. Miner, A. Delehaunty, J. B. Kramer, L. L. Cook, R. S. Fulton, D. L. Johnson, P. J. Minx, S. W. Clifton, T. Hawkins, E. Branscomb, P. Predki, P. Richardson, S. Wenning, T. Slezak, N. Doggett, J. F. Cheng, A. Olsen, S. Lucas, C. Elkin, E. Uberbacher, M. Frazier, et al. 2001. Initial sequencing and analysis of the human genome. Nature 409:860-921. [DOI] [PubMed] [Google Scholar]
- 25.Lavker, R. M., and T. T. Sun. 2000. Epidermal stem cells: properties, markers, and location. Proc. Natl. Acad. Sci. USA 97:13473-13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leask, A., C. Byrne, and E. Fuchs. 1991. Transcription factor AP2 and its role in epidermal-specific gene expression. Proc. Natl. Acad. Sci. USA 88:7948-7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leask, A., M. Rosenberg, R. Vassar, and E. Fuchs. 1990. Regulation of a human epidermal keratin gene: sequences and nuclear. Genes Dev. 4:1985-1998. [DOI] [PubMed] [Google Scholar]
- 28.Lee, J. H., S. I. Jang, J. M. Yang, N. G. Markova, and P. M. Steinert. 1996. The proximal promoter of the human transglutaminase 3 gene. Stratified squamous epithelial-specific expression in cultured cells is mediated by binding of Sp1 and ets transcription factors to a proximal promoter element. J. Biol. Chem. 271:4561-4568. [PubMed] [Google Scholar]
- 29.Letovsky, J., and W. S. Dynan. 1989. Measurement of the binding of transcription factor Sp1 to a single GC box recognition sequence. Nucleic Acids Res. 17:2639-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, M., H. Chiba, X. Warot, N. Messaddeq, C. Gerard, P. Chambon, and D. Metzger. 2001. RXR-alpha ablation in skin keratinocytes results in alopecia and epidermal alterations. Development 128:675-688. [DOI] [PubMed] [Google Scholar]
- 31.Li, M., A. K. Indra, X. Warot, J. Brocard, N. Messaddeq, S. Kato, D. Metzger, and P. Chambon. 2000. Skin abnormalities generated by temporally controlled RXRalpha mutations in mouse epidermis. Nature 407:633-636. [DOI] [PubMed] [Google Scholar]
- 32.Marin, M., A. Karis, P. Visser, F. Grosveld, and S. Philipsen. 1997. Transcription factor Sp1 is essential for early embryonic development but dispensable for cell growth and differentiation. Cell 89:619-628. [DOI] [PubMed] [Google Scholar]
- 33.Maytin, E. V., J. C. Lin, R. Krishnamurthy, N. Batchvarova, D. Ron, P. J. Mitchell, and J. F. Habener. 1999. Keratin 10 gene expression during differentiation of mouse epidermis requires transcription factors C/EBP and AP-2. Dev. Biol. 216:164-181. [DOI] [PubMed] [Google Scholar]
- 34.Meisterernst, M., I. Gander, L. Rogge, and E. L. Winnacker. 1988. A quantitative analysis of nuclear factor I/DNA interactions. Nucleic Acids Res. 16:4419-4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morasso, M. I., N. G. Markova, and T. D. Sargent. 1996. Regulation of epidermal differentiation by a Distal-less homeodomain gene. J. Cell Biol. 135:1879-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohnishi, S., F. Laub, N. Matsumoto, M. Asaka, F. Ramirez, T. Yoshida, and M. Terada. 2000. Developmental expression of the mouse gene coding for the Kruppel-like transcription factor KLF5. Dev. Dyn. 217:421-429. [DOI] [PubMed] [Google Scholar]
- 37.Oshima, H., A. Rochat, C. Kedzia, K. Kobayashi, and Y. Barrandon. 2001. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 104:233-245. [DOI] [PubMed] [Google Scholar]
- 38.Philipsen, S., and G. Suske. 1999. A tale of three fingers: the family of mammalian Sp/XKLF transcription factors. Nucleic Acids Res. 27:2991-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prowse, D. M., L. Bolgan, A. Molnar, and G. P. Dotto. 1997. Involvement of the Sp3 transcription factor in induction of p21Cip1/WAF1 in keratinocyte differentiation. J. Biol. Chem. 272:1308-1314. [DOI] [PubMed] [Google Scholar]
- 40.Radoja, N., M. Komine, S. H. Jho, M. Blumenberg, and M. Tomic-Canic. 2000. Novel mechanism of steroid action in skin through glucocorticoid receptor monomers. Mol. Cell. Biol. 20:4328-4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schorle, H., P. Meier, M. Buchert, R. Jaenisch, and P. J. Mitchell. 1996. Transcription factor AP-2 essential for cranial closure and craniofacial development. Nature 381:235-238. [DOI] [PubMed] [Google Scholar]
- 42.Schreiber, E., P. Matthias, M. M. Muller, and W. Schaffner. 1989. Rapid detection of octamer binding proteins with mini-extracts, prepared from a small number of cells. Nucleic Acids Res. 17:6419.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Segre, J. A., C. Bauer, and E. Fuchs. 1999. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat. Genet. 22:356-360. [DOI] [PubMed] [Google Scholar]
- 44.Singh, M. V., and J. M. Ntambi. 1998. Nuclear factor 1 is essential for the expression of stearoyl-CoA desaturase 1 gene during preadipocyte differentiation. Biochim. Biophys. Acta 1398:148-156. [DOI] [PubMed] [Google Scholar]
- 45.Sinha, S., L. Degenstein, C. Copenhaver, and E. Fuchs. 2000. Defining the regulatory factors required for epidermal gene expression. Mol. Cell. Biol. 20:2543-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sinha, S., and E. Fuchs. 2001. Identification and dissection of an enhancer controlling epithelial gene expression in skin. Proc. Natl. Acad. Sci. USA 98:2455-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snape, A. M., E. A. Jonas, and T. D. Sargent. 1990. KTF-1, a transcriptional activator of Xenopus embryonic keratin expression. Development 109:157-165. [DOI] [PubMed] [Google Scholar]
- 48.Snape, A. M., R. S. Winning, and T. D. Sargent. 1991. Transcription factor AP-2 is tissue-specific in Xenopus and is closely related or identical to keratin transcription factor 1 (KTF-1). Development 113:283-293. [DOI] [PubMed] [Google Scholar]
- 49.Sugihara, T. M., E. I. Kudryavtseva, V. Kumar, J. J. Horridge, and B. Andersen. 2001. The POU domain factor Skin-1a represses the keratin 14 promoter independent of DNA binding: possible role for interactions between Skn-1a and CBP/p300. J. Biol. Chem. 276:33036-33044. [DOI] [PubMed]
- 50.Taylor, G., M. S. Lehrer, P. J. Jensen, T. T. Sun, and R. M. Lavker. 2000. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 102:451-461. [DOI] [PubMed] [Google Scholar]
- 51.Vallette, F., E. Mege, A. Reiss, and M. Adesnik. 1989. Construction of mutant and chimeric genes by using the polymerase chain reaction. Nucleic Acids Res. 17:723-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vassar, R., M. Rosenberg, S. Ross, A. Tyner, and E. Fuchs. 1989. Tissue-specific and differentiation-specific expression of a human K14 keratin gene in transgenic mice. Proc. Natl. Acad. Sci. USA 86:1563-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.West-Mays, J. A., J. Zhang, T. Nottoli, S. Hagopian-Donaldson, D. Libby, K. J. Strissel, and T. Williams. 1999. AP-2alpha transcription factor is required for early morphogenesis of the lens vesicle. Dev. Biol. 206:46-62. [DOI] [PubMed] [Google Scholar]
- 54.Wingender, E., X. Chen, R. Hehl, H. Karas, I. Liebich, V. Matys, T. Meinhardt, M. Pruss, I. Reuter, and F. Schacherer. 2000. TRANSFAC: an integrated system for gene expression regulation. Nucleic Acids Res. 28:316-319. [DOI] [PMC free article] [PubMed] [Google Scholar]