Abstract
The tumor suppressor gene CHK2 encodes a versatile effector serine/threonine kinase involved in responses to DNA damage. Chk2 has an amino-terminal SQ/TQ cluster domain (SCD), followed by a forkhead-associated (FHA) domain and a carboxyl-terminal kinase catalytic domain. Mutations in the SCD or FHA domain impair Chk2 checkpoint function. We show here that autophosphorylation of Chk2 produced in a cell-free system requires trans phosphorylation by a wortmannin-sensitive kinase, probably ATM or ATR. Both SQ/TQ sites and non-SQ/TQ sites within the Chk2 SCD can be phosphorylated by active Chk2. Amino acid substitutions in the SCD and the FHA domain impair auto- and trans-kinase activities of Chk2. Chk2 forms oligomers that minimally require the FHA domain of one Chk2 molecule and the SCD within another Chk2 molecule. Chk2 oligomerization in vivo increases after DNA damage, and when damage is induced by gamma irradiation, this increase requires ATM. Chk2 oligomerization is phosphorylation dependent and can occur in the absence of other eukaryotic proteins. Chk2 can cross-phosphorylate another Chk2 molecule in an oligomeric complex. Induced oligomerization of a Chk2 chimera in vivo concomitant with limited DNA damage augments Chk2 kinase activity. These results suggest that Chk2 oligomerization regulates Chk2 activation, signal amplification, and transduction in DNA damage checkpoint pathways.
DNA damage activates response pathways that halt the cell cycle, induce the transcription of genes that facilitate DNA repair and DNA replication, alter telomeres, and induce apoptosis if damage cannot be repaired (65). Checkpoint defects may result in genomic instability, a mutagenic condition that predisposes organisms to cancer. On the other hand, DNA-damaging agents, in the form of gamma irradiation and genotoxic drugs, are mainstays of current cancer treatment regimens. Manipulation of checkpoint genes may ultimately benefit chemo- and radiotherapy (23).
Checkpoint pathways are analogous to growth factor-regulated signal transduction pathways, in which DNA damage initiates a signal that is transduced and amplified to generate checkpoint responses. Although the precise nature of the initial step of signal transduction is poorly understood, damaged DNA activates a cascade of protein kinases. In mammals, these kinases include the phosphoinositide kinase-related kinases (PIKKs) Atm (ataxia telangiectasia mutated) and Atr (Atm and Rad3 related) and the downstream serine/threonine checkpoint kinases Chk1 and Chk2. Orthologs of these genes have been identified in yeasts, with Saccharomyces cerevisiae Mec1 and Tel1 serving Atm- or Atr-like functions and Chk1 and Rad53 resembling mammalian Chk1 and Chk2, respectively. Effectors that execute the functions of the DNA damage responses include substrates of both PIKKs and checkpoint kinases.
Atm is a central signaling protein in the response to ionizing radiation and other sources of double-strand DNA breaks. Homozygous mutations in ATM are responsible for the pleiotropic ataxia-telangiectasia syndrome, which includes cancer predisposition and sensitivity to ionizing radiation along with progressive cerebellar defects (29). Chk2 is a major effector of Atm (6, 7, 10, 40). Both the breast cancer susceptibility gene product Brca1 (16, 33) and p53 (3, 8, 11, 12, 25, 52) are substrates of Atm and Chk2. Li-Fraumeni syndrome is a hereditary disorder predisposing to multiple neoplasms and is generally associated with a constitutional TP53 mutation. CHK2 mutations have been identified in some Li-Fraumeni syndrome kindreds that do not have p53 mutations (5, 58), in myelodysplastic syndromes and acute myeloid leukemias (27), in lung cancer (24, 44), in osteosarcoma (44), and in ovarian cancer (44). CHK2, therefore, is a regulator of tumor suppressor gene products and is itself a likely tumor suppressor gene.
Chk2 activation in response to ionizing irradiation is ATM dependent (6, 7, 10, 40). Activated Chk2 in turn phosphorylates p53 at serine-20 (11, 25, 52), Cdc25A at serine-123 (21), and Cdc25C at serine-216 (6, 7, 10, 40), contributing to the G1/S, S, and G2/M checkpoints, respectively.
Atm-like PIKKs show a strong sequence preference for phosphorylation of SQ/TQ sites (2, 30). The SQ/TQ cluster domain (SCD), near the amino terminus of Chk2, includes seven SQ/TQ sites, including known ATM- and ATR-dependent phosphorylation targets (1, 41, 42, 64). The SCD is followed by a forkhead-associated (FHA) domain and a carboxyl-terminal kinase catalytic domain. Activation of Chk2 occurs through a series of steps, including trans phosphorylation by Atm or Atr at sites within the SCD, including T68 (1, 41, 42, 64). PIKK-dependent phosphorylation is required for autophosphorylation within the activation loop of the catalytic domain at T383 and/or T387 (32).
The intact FHA domain is required for damage-dependent activation of Chk2 (11, 32). FHA protein homology domains were first identified in a subset of forkhead transcription factors (26). They are present in a wide variety of proteins in prokaryotes and eukaryotes (34). Many eukaryotic FHA domain-containing proteins are found in the nucleus and are involved in DNA repair and cell cycle arrest (34). Recent work shows that FHA domains are phosphopeptide recognition domains (18, 19, 36, 37, 55, 57), and structurally they have some similarity to the MH2 domains of R-Smads (19), signal transducers and transcriptional comodulators of transforming growth factor beta (TGF-β) signaling (39). However, only a small number of protein-protein interactions that are mediated by FHA domains have been identified. They include association of an Arabidopsis thaliana phosphatase FHA domain with a phosphorylated receptor-like kinase (35, 55) and interaction of S. cerevisiae Rad53 with phosphorylated Rad9 (57), which operates upstream from Rad53 in the damage-dependent signaling cascade.
The FHA domains of the S. cerevisiae Chk2 homolog Rad53 couple Rad53 to damage-dependent signals through direct binding to a second damage response protein, Rad9 (57). DNA damage induces PIKK-dependent phosphorylation of Rad9 (20, 47, 56, 59). Once phosphorylated, Rad9 binds tightly to the two FHA domains of Rad53 (18, 36, 37, 57). Disruption of this interaction either through mutations of Rad53 FHA domains (57) or mutations in the Rad9 sites that bind Rad53 FHA domains (49a) prevents activation of Rad53. Since Rad9 and Rad53 both require Mec1 for activating phosphorylations, these results suggested that phospho-Rad9 acts as an adaptor that recruits Rad53 to an activating complex containing Mec1. Alternatively, it has been proposed that phosphorylated Rad9 dimer functions as a scaffold to bring Rad53 molecules into close proximity to each other, facilitating cross-phosphorylation between Rad53 molecules and subsequent release of activated Rad53 (22).
Activation of protein kinases through regulated oligomerization has been demonstrated for both tyrosine kinases and serine/threonine kinases (28, 38, 50). We report here that Chk2 undergoes oligomerization in response to DNA damage. This process is mediated by the phosphorylated SCD in an activated Chk2 molecule and the FHA domain in another Chk2 molecule. With limited DNA damage, oligomerization of Chk2 modulates phosphorylation and kinase activity. We propose that Chk2 oligomerization is central to regulation of Chk2 activation, signal transduction, and signal amplification.
MATERIALS AND METHODS
Antibodies.
Rabbit polyclonal anti-Chk2 T26/S28 was a kind gift from Yi Tan (Cell Signaling Technology). This antibody recognizes Chk2 after gamma irradiation, but not when T26 and S28 have been replaced with alanine (X. Xu and D. F. Stern, unpublished data). Rabbit anti-phospho-T68 was produced by immunization with keyhole limpet hemocyanin coupled to KSSLETVSpTQELYSI, where pT is phosphothreonine. This antibody recognizes Chk2 after gamma irradiation, but not when T68 has been replaced with alanine (Fig. 7E and data not shown). Mouse monoclonal anti-HA antibody (16B12) was purchased from Covance; mouse monoclonal anti-Flag, rabbit anti-glutathione-S-transferase (GST), and mouse immunoglobulin G (IgG) were purchased from Sigma; horseradish peroxidase-conjugated mouse antihemagglutinin (HA) (12CA5) and rat anti-HA (3F10) monoclonal antibodies were from Roche; and goat anti-Chk2 (N-17) was from Santa Cruz. Antigen-antibody complexes were recovered with protein G plus protein A-agarose (CalBiochem). Horseradish peroxidase-conjugated secondary antibodies and chemiluminescence reagents were from Pierce.
FIG. 7.
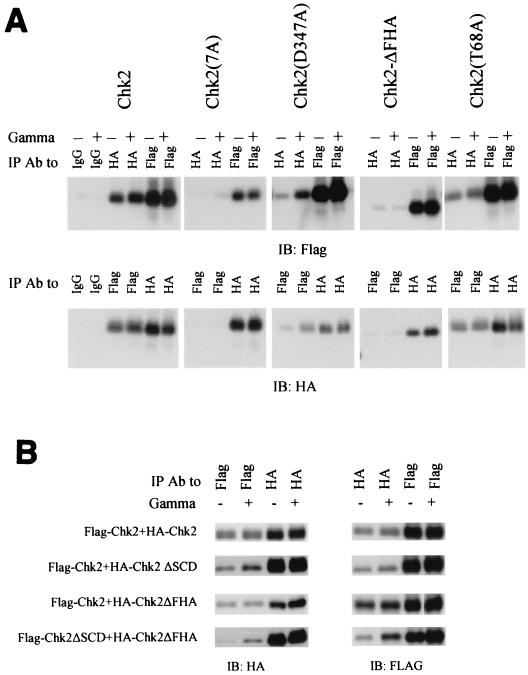
Chk2 oligomerization domains. (A) Requirements for oligomerization in 293 cells. HA-tagged and Flag-tagged versions of individual Chk2 mutants were expressed by transient transfection in 293 cells. Transfectants were exposed to 20 Gy of gamma irradiation 48 h after transfection. Cell lysates were harvested 2 h after irradiation, and equal amounts were used for immunoprecipitation with anti-HA antibody or anti-Flag antibody as indicated and immunoblotted with anti-Flag (top panel) or anti-HA (bottom panel). Because homologous immunoprecipitations (e.g., IP anti-Flag, blot anti-Flag) were more efficient than heterologous coimmunoprecipitations (e.g., IP anti-Flag, blot anti-HA), only a one-fifth equivalent of homologous immunoprecipitation samples was analyzed relative to the cross-immunoprecipitation samples. (B) Oligomerization of FHA domain and SCD in vivo. Performed as in A except that Flag-tagged and HA-tagged Chk2 molecules were evaluated in the pairwise combinations listed at left. (C) GST-FHA binds to activated Chk2. Various Chk2-GST fusion proteins expressed in bacteria were used to isolate HA-Chk2 stably expressed in 293 cells. Experiments were performed as described in the legend to A. Equal portions of lysate from nonirradiated and irradiated cells were incubated with GST-Chk2 or its mutants. Pulldowns were blotted for HA-Chk2 with anti-HA antibody and for input of GST fusion protein with anti-GST antibodies. Different sizes of GST fusions on the bottom panel were cropped and realigned from one autoradiograph. Total lysate used for anti-HA immunoprecipitation was one-fifth of that for the GST pulldown. (D) Bacterially produced FHA domain of Chk2 binds to SCD in HA-Chk2 and its mutants expressed in 293 cells after gamma irradiation. The strategy described for Fig. 6C was used. Only one representative immunoblot for input of GST fusions (bottom panel) is shown. Different sizes of GST fusions on the bottom panel were cropped from one autoradiograph. (E) T68 phosphorylation of Chk2 and its mutants in vivo. Cells were handled essentially as described in A. Cell lysates were immunoprecipitated with anti-HA and detected by immunoblotting with anti-phospho-T68 or anti-HA. IP, immunoprecipitation; IB, immunoblotting; Ab, antibody.
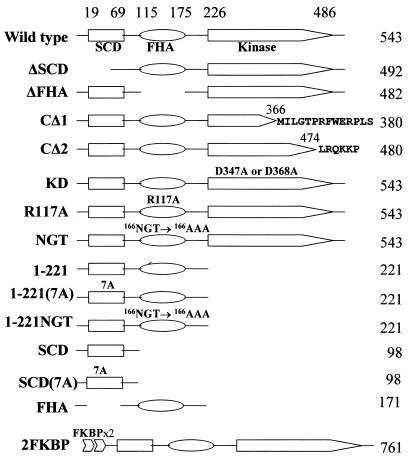
Plasmids (Fig. 1).
FIG. 1.
Schematic diagrams of Chk2 and Chk2 mutants. SCD, FHA, and kinase catalytic domains are marked, with amino acid coordinates above. CΔ1 corresponds to a spontaneous variant of CHK2 from Li-Fraumeni syndrome, with a frameshift mutation causing readthrough into alternate reading frames as indicated. CΔ2, a similar frameshift mutation thought to be a spontaneous variant of CHK2 from Li-Fraumeni syndrome, was found to be a polymorphism in the homologous fragment present on chromosome 15 (53). KD is kinase defective owing to substitutions of conserved residues in the catalytic domain. R117A and NGT/AAA are substitutions of conserved FHA domain residues. 7A has alanine substituted for each S or T of all seven SQ and TQ sites within the SCD. 2FKBP contains two copies of the FKBP mutant (F36V) fused to the amino terminus of Chk2.
A clone within the expressed sequence tag database (GenBank accession no. AA285249) containing the entire coding sequence of Chk2 was obtained from Thanos D. Halazonetis (Wistar Institute). For expression in mammalian cells, Chk2-coding sequences were amplified by PCR and cloned into the pcDNA3xHA-Neo and pcDNA3xFlag-Neo vectors, resulting in pcDNA-HAChk2 and pcDNA-FlagChk2, respectively. Point and internal deletion mutants were generated from pcDNA-HAChk2 by PCR-based site-directed mutagenesis (63).
For expression in Escherichia coli, Chk2 sequences (Fig. 1) were cloned into pGEX4T vectors (Amersham Pharmacia Biotech) for GST fusions and pTrcHis vectors (Invitrogen) for His-tagged fusions. Human Cdc25C (amino acids 200 to 256) and Cdc25A (amino acids 101 to 140) were isolated by PCR from expressed sequence tag clones (GenBank accession no. AW401554 and BE743496, respectively). Two copies of the FK506-binding protein (FKBP) mutant were isolated by PCR from pC4M-Fv2E (Ariad Pharmaceuticals, Inc., Cambridge, Mass.), digested with ApoI and EcoRI, and subcloned into the EcoRI site of the pcDNA3xHA and pcDNA3xFlag vectors. The resulting constructs were designated pcDNAHA2FKBP and pcDNAFlag2FKBP, respectively. Chk2 and its mutants were subcloned into these vectors.
Plasmid constructs were verified by sequence analysis. Primer sequences and detailed cloning strategies are available upon request. Wild-type and kinase-defective (D2870A and N2875K, respectively) Flag-ATM constructs (8) were kind gifts from Michael Kastan (St. Jude Children's Hospital). pGEX-Chk2(1-222) and pGEX-Chk2(57-222) (9) were obtained from Susan Lees-Miller (University of Calgary). pC4M-Fv2E, containing two copies of mutated FKBP, was provided by Ariad Pharmaceuticals, Inc. (Cambridge, Mass.).www.ariad.com/regulationkits
Recombinant proteins.
Expression of GST fusions or His fusions in E. coli strain DH5α was induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 3 to 4 h at 37°C. For GST fusion proteins, cell lysates were harvested in PBS (Dulbecco's phosphate-buffered saline lacking Ca2+ and Mg2+) in the presence of 1 mg of lysozyme and 10 U of DNase I per ml. For His fusion proteins, cell lysates were collected in 50 mM NaH2PO4-300 mM NaCl-10 mM imidazole, pH 8.0, in the presence of 1 mg of lysozyme and 10 U of DNase I per ml. Cell lysates then went through 10 cycles of freezing and thawing. GST and His fusion proteins were batch purified with glutathione-Sepharose beads (Amersham Pharmacia Biotech) or Ni-nitrilotriacetic acid (NTA) beads (Qiagen), respectively, according to the manufacturers' procedures. GST fusion proteins were eluted with 50 mM Tris-10 mM glutathione, pH 8.0. His fusion proteins were eluted with 50 mM NaH2PO4-300 mM NaCl-250 mM imidazole, pH 8.0, and then dialyzed against PBS.
In vitro coupled transcription-translation assays.
Chk2 constructs (pcDNA series) were used as templates for in vitro transcription-translation of Chk2 in the absence or presence of [35S]Met-Cys labeling mix (Amersham Pharmacia Biotech). Promega TNT T7 quick coupled transcription-translation reticulocyte lysate system and T7 coupled transcription-translation wheat germ extract system were used in a standard 50-μl reaction according to procedures recommended by the manufacturer.
Cell culture and transfection.
ATM-deficient (GM5849C) simian virus 40-transformed human fibroblasts were obtained from the Coriell Institute for Medical Research, Camden, N.J. Other cell lines were obtained from the American Type Culture Collection. Cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 50 U of penicillin/ml, and 50 mg of streptomycin/ml. Transfection was performed with Fugene 6 (Roche) at a ratio of 1 μg of plasmid to 2 μl of Fugene. Stable transfectants were selected in medium containing G418 (Life Technologies) at 700 μg/ml. Cells were treated with 1 mM hydroxyurea (Sigma) 24 h after transfection for 24 h. Cells were irradiated in a Mark I 137Cs irradiator (Shepherd) at a dose rate of 1.8 Gy/min 48 h after transfection. Cells were UV irradiated at a dose of 50 J/m2 with a Stratagene Cross-linker 48 h after transfection.
Immunoprecipitation and immunoblotting.
Cell lysate was harvested 2 h after irradiation or 24 h after hydroxyurea treatment in TSD buffer (20 mM Tris [pH 7.5], 100 mM NaCl, 0.1% sodium deoxycholate, 0.1% Triton X-100, and protease inhibitor cocktail [Roche]). Two micrograms of antibodies was used for immunoprecipitation from 400 to 500 μg of total lysate at 4°C overnight. Precipitates were washed with TSD buffer lacking protease inhibitors. In vitro translation product was mixed with 300 μl of NETN (20 mM Tris-HCl [pH 8.0], 0.1 M NaCl, 1 mM EDTA, 0.5% NP-40, and protease inhibitor cocktail) for immunoprecipitation. Precipitates were washed with NETN buffer lacking protease inhibitors. Immunoblots on nitrocellulose were blocked with 5% nonfat milk in PBST (PBS with 0.5% Tween 20) and washed in PBST.
GST pulldown experiments.
Two micrograms of soluble GST fusion proteins and 20 μl of glutathione-Sepharose beads were incubated with 500 μg of total lysate in TSD buffer derived from HEK 293 cells expressing HA-tagged Chk2 and mutants (Fig. 6C, 7C, and 7D) or with 0.5 μg of soluble wild-type or kinase-defective His-Flag-Chk2 in the presence of 300 μl of NETN buffer (Fig. 6A, 6B, and 8) at 4°C overnight. The beads were washed in NETN buffer lacking protease inhibitors.
FIG. 6.
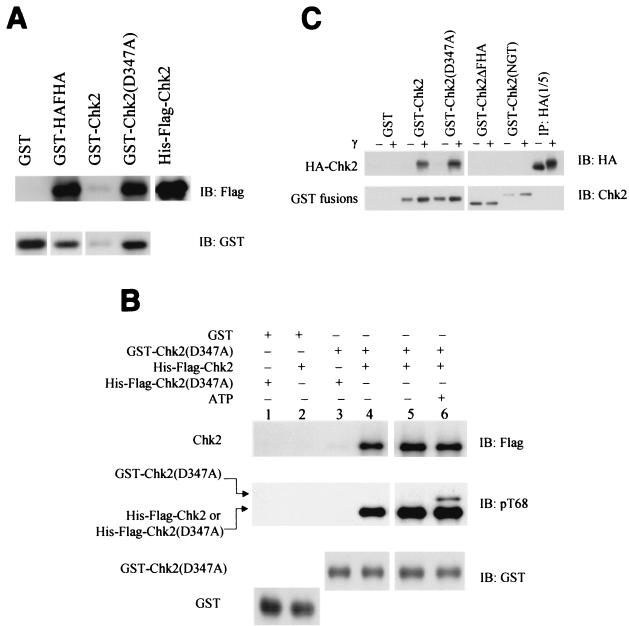
Oligomerization of Chk2 produced in bacteria and 293 cells. (A) Bacterial expression. GST fusion protein pulldown experiment with bacterially produced soluble GST-HA-FHA, GST-Chk2, or kinase-defective GST-Chk2(D347A) incubated with His-Flag-Chk2. The pulldowns were blotted for wild-type His-Flag-Chk2 with anti-Flag antibody (top panel) and for total input of GST fusions with anti-GST antibodies (bottom panel). Images of different-sized GST fusion proteins in the bottom panel were cropped from one autoradiograph and aligned with one another to save space. (B) Phosphorylation of Chk2D347A by Chk2. Soluble GST-Chk2D347A produced in bacteria was used to pull down soluble His-Flag-Chk2 (lanes 4, 5, and 6) or kinase-defective His-Flag-Chk2(D347A) (lane 3). The affinity complexes were incubated in 1× kinase buffer in the absence (lane 5) or in the presence (lane 6) of ATP. Chk2 phosphorylation was evaluated by immunoblotting with anti-phospho-T68. GST-Chk2D347A is significantly larger than His-Flag-Chk2. (C) Mammalian expression. 293 cells stably expressing HA-Chk2 were exposed to 20 Gy of gamma irradiation. Cell lysates were harvested 2 h after irradiation. Lysates were normalized for protein concentration and used for pulldown with GST-Chk2 and its mutants. The GST pulldowns were blotted for HA-Chk2 with anti-HA antibody and for GST fusion protein with anti-Chk2 antibodies (N-17). Different sizes of GST fusions on the bottom panel were cropped and realigned from a single autoradiograph. IP, immunoprecipitation; IB, immunoblotting.
In vitro kinase assays.
Kinases (prepared as soluble GST or His fusion proteins, immune complexes, or GST affinity isolates) were incubated with substrates at 30°C for 5 to 10 min in 1× kinase buffer (20 mM Tris [pH 7.5], 10 mM MgCl2, 10 mM MnCl2, 1 mM dithiothreitol) supplemented with either 2 μM nonradioactive ATP or 2 μM nonlabeled ATP and 10 μCi of [γ-32P]ATP (>5,000 Ci/mmol; AA0018, Amersham Pharmacia Biotech).
Phosphatase treatment.
Immunoprecipitates of HA-Chk2 produced by translation in the coupled reticulocyte lysate system were incubated with calf intestinal phosphatase (New England Biolabs) for 1 h at 37°C. Soluble wild-type His-Flag-Chk2 (0.5 μg) was incubated with 1,000 U of λ phosphatase (New England Biolabs) in the presence of 2 mM MnCl2 for 1 to 2 h at 30°C in a 50-μl reaction volume.
Induced oligomerization of Chk2 chimeras.
HEK 293 cells were transiently transfected with pcDNAHA2FKBP-Chk2 (or its kinase-defective mutant) and/or pcDNAFlag2FKBP-Chk2 (or its kinase-defective mutant). Forty-eight hours later, transfectants were either mock-treated with 0.1% ethanol or treated with a 10 nM concentration of the bivalent ligand AP20187 (Ariad Pharmaceuticals, Inc.) for the FKBP mutant. Where indicated, transfectants were exposed to 10-Gy or 2.5-Gy gamma irradiation immediately before adding ligand. Lysates were harvested between 2 and 6 h after treatment. All solutions for immunoprecipitation, washing, and kinase assays for the AP20187-treated samples contained 10 μM AP20187.
RESULTS
Cell-free system for activation of Chk2.
In S. cerevisiae, DNA damage induces a stable modification of the Chk2 homolog Rad53 that results in elevated activity detectable by in-gel kinase assays after denaturing gel electrophoresis and subsequent renaturation (45). This modification, probably phosphorylation, depends upon the Atm/Atr homolog Mec1. However, efforts to activate mammalian Chk2 in vitro by phosphorylation with Atm (41) or DNA-dependent protein kinase (DNA-PK) (X. Xu and D. F. Stern, unpublished data) have been unsuccessful.
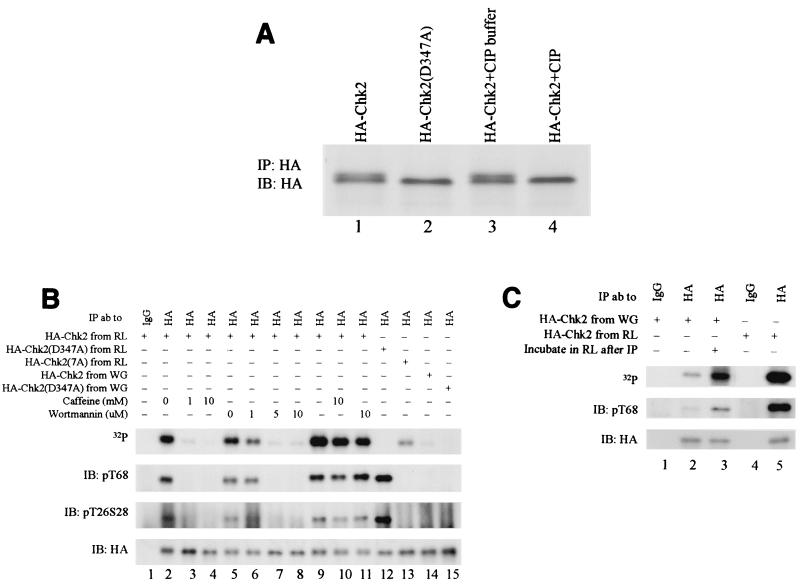
Chk2 produced by coupled in vitro transcription-translation in rabbit reticulocyte lysates migrates heterogeneously in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels (Fig. 2A, lane 1). The slower-migrating form was eliminated by phosphatase treatment (Fig. 2A, lanes 3 and 4). Expression of the kinase-defective D347A allele in rabbit reticulocyte lysates yielded only the hypophosphorylated form (Fig. 2A, lane 2), indicating that autophosphorylation is required for the mobility shift. Phosphorylation of immunoreactive T26/S28 and T68 in the SCD was evident in both wild-type and kinase-defective Chk2 derived from rabbit reticulocyte lysates (Fig. 2B, lane 12). Thus, one or more protein kinases that can phosphorylate Chk2 at these sites are present in the rabbit reticulocyte lysates. Since the kinase-defective form does not undergo an extreme mobility shift (Fig. 2A, lane 2), phosphorylation at T26/S28 and/or T68 is not sufficient to retard the mobility of Chk2.
FIG. 2.
Cell-free phosphorylation and activation of Chk2. HA-Chk2 or kinase-defective HA-Chk2(D347A) was produced by coupled transcription-translation in reticulocyte lysate (RL) or wheat germ lysate (WG) and isolated by immunoprecipitation with anti-HA. (A) Phosphorylation of HA-Chk2 produced in vitro. HA-Chk2 or HA-Chk2(D347A) produced in rabbit reticulocyte lysates was immunoprecipitated and detected by immunoblotting with anti-HA. For phosphatase experiments (right lanes), immunoprecipitated HA-Chk2 was incubated in calf intestinal phosphatase (CIP) buffer or calf intestinal phosphatase buffer plus calf intestinal phosphatase (1). HA-Chk2 produced in the reticulocyte lysate in vitro translation system is phosphorylated. Both HA-Chk2 and HA-Chk2(D347A) were in vitro transcribed-translated in the reticulocyte lysate with [35S]Met-Cys for labeling and precipitated with anti-HA antibody. The immunoprecipitates of HA-Chk2 were treated with alkaline phosphatase buffer alone or plus alkaline phosphatase at 37°C for 1 h. IP, immunoprecipitation; IB, immunoblotting; ab, antibody. (B) Chk2 phosphorylation and kinase activity. Forms of HA-Chk2 produced by cell-free transcription-translation were immunoprecipitated and incubated with [γ-32P]ATP for in vitro kinase assays. Recovery of Chk2 was monitored by immunoblotting with anti-HA (bottom). Phosphorylation at T68 or T26/S28 was measured by immunoblotting with the appropriate phosphospecific antibody; in vitro kinase activity was monitored by incorporation of [γ-32P]ATP (top). The 32P, phospho-T68, and HA panels are all from the same filter probed with anti-phospho-T68, stripped, and reprobed with anti-HA. Phospho-T26 (phospho-T68) and S28 are from an independent blot of portions of the same samples. In lanes 2 through 8, forms of HA-Chk2 were produced in the rabbit reticulocyte lysate system after incubation of the lysate with the designated concentration of vehicle control, caffeine, or wortmannin. Both caffeine and wortmannin inhibit Chk2 trans-phosphorylation and autophosphorylation. HA-Chk2 and its mutants were produced in either the in vitro translation wheat germ extract system or the in vitro translation reticulocyte lysate system, in which reticulocyte lysates were either mock treated or incubated with the indicated concentrations of caffeine or wortmannin. In lanes 9, 10, and 11, in vitro kinase assays were performed on the anti-HA immunoprecipitates in the presence of [γ-32P]ATP only (lane 9) or in the presence of [γ-32P]ATP and caffeine (10 mM, lane 10) or wortmannin (10 μM, lane 11) (top panel). IP, immunoprecipitation; IB, immunoblotting; ab, antibody. (C) Activation of Chk2 produced in wheat germ lysates. Phosphorylation of T68 and T26S28 was examined with Chk2 phospho-specific antibodies. HA-Chk2 was produced in the in vitro translation wheat germ extract system (lanes 1 to 3) or reticulocyte lysate system (lanes 4 and 5). HA-Chk2 was then precipitated with anti-HA antibody (lanes 2, 3, and 5) or mouse IgG (lanes 1 and 4). One Chk2 precipitate from the wheat germ extract system (lane 3) was incubated with reticulocyte lysate at 30°C for 30 min prior to the kinase assay. All the immunocomplexes were incubated with [γ-32P]ATP for in vitro kinase assays. Recovery of Chk2 was monitored by immunoblotting with anti-HA (bottom panel) or with anti-phospho-T68 (middle panel) or by incorporation of [γ-32P]ATP (top panel). IP, immunoprecipitation; IB, immunoblotting; ab, antibody.
The simplest explanation for these results is that an endogenous Chk2 activating kinase is present in this system, with likely candidates being endogenous PIKKs and/or Chk2 (see below). Preincubation of rabbit reticulocyte lysates with 1 mM caffeine or 5 μM wortmannin inhibited phosphorylation at T26/S28 and at T68 (Fig. 2B, lanes 3 and 7). At these concentrations, caffeine inhibits human Atm and, partially, Atr but not DNA-PK, and wortmannin inhibits both Atm and DNA-PK but not Atr (48, 49). Loss of phosphorylation at these sites was accompanied by loss of Chk2 autophosphorylation activity, assayed by 32P incorporation (Fig. 2B, lanes 3 and 7). We conclude that Chk2 derived from the rabbit reticulocyte lysates is phosphorylated by an Atm-like kinase and that this phosphorylation is required for strong Chk2 kinase activity.
In contrast to material produced in rabbit reticulocyte lysates, HA-Chk2 derived by translation in wheat germ extracts was not hyperphosphorylated, lacked T26/S28 and T68 phosphorylation, and had minimal autophosphorylation activity (Fig. 2B, lane 14, and Fig. 2C, lane 2). This system probably lacks an endogenous kinase that is capable of activating mammalian Chk2. Incubation of this material in rabbit reticulocyte lysates enhanced T68 phosphorylation and Chk2 autophosphorylation (Fig. 2C, lane 3; compare to Chk2 produced in rabbit reticulocyte lysates in lane 5). To our knowledge, this is the first mammalian cell-free system to enable catalytic activation of Chk2.
SCD is phosphorylated by Chk2.
In the rabbit reticulocyte lysate system and in intact cells exposed to ionizing radiation, kinase-defective Chk2 is phosphorylated in trans at T26/S28 and T68 (Fig. 2B and data not shown). However, the full phosphorylation of Chk2 resulting in electrophoretic mobility shift requires a functional Chk2 kinase domain (Fig. 2B, lanes 2 and 12, and data not shown). This suggests that, at minimum, Chk2 electrophoretic mobility shift requires successive trans phosphorylation and autophosphorylation. Phosphorylation of Chk2 within the activation loop of the kinase domain is required for catalytic activation of Chk2 (32), and other autophosphorylation sites may also contribute to the mobility shift.
We analyzed a series of bacterially expressed GST-Chk2 alleles in order to identify domains in Chk2 that are required for kinase activity and domains that are subject to autophosphorylation (Fig. 3). In these assays, both autophosphorylation of Chk2 and trans phosphorylation of a GST-Cdc25C substrate peptide were monitored. Both of the kinase-defective alleles, GST-Chk2(D347A) and GST-Chk2(D368A), lacked kinase activity in this assay. Within the SCD, the T68A substitution slightly reduced autophosphorylation and Cdc25C trans-phosphorylation activity. A more substantial decrease was observed with GST-Chk2(7A), in which all seven SQ/TQ sites within the SCD were replaced with AQ. Deletion of the entire SCD (GST-Chk2-ΔSCD) eliminated autophosphorylation, but only moderately diminished Cdc25 peptide phosphorylation. Similar results were observed with Chk2 and Chk2 mutants produced in the rabbit reticulocyte lysate system (data not shown). These data indicate that the SCD is required for maximal Chk2 kinase activity.
FIG. 3.
Mutations in either SCD or FHA domain impair Chk2 kinase activities. GST fusion proteins were produced in bacteria, purified on glutathione beads, and released in soluble form with reduced glutathione. In vitro kinase assays were performed in the presence of Chk2 substrate GST-Cdc25C (amino acids 200 to 256) and [γ-32P]ATP. Upper panel, [γ-32P]ATP incorporation into GST-Chk2 and mutants; middle panel, [γ-32P]ATP incorporation into GST-Cdc25C. Bottom panel, GST-Chk2 fusion proteins were quantified by immunoblotting with anti-Chk2 antibody (N-17). Coomassie brilliant blue staining showed substrate GST-Cdc25C. IB, immunoblotting.
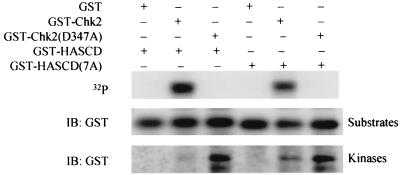
The stronger effects on autophosphorylation than on trans phosphorylation suggested that the SCD contains Chk2 autophosphorylation sites, including T68. Potential phosphorylation sites within the SCD include the seven SQ/TQ sites, as well as an additional 17 serines and 4 threonines. GST-Chk2 phosphorylated GST-SCD (Fig. 4, lane 3) and SCD(7A), lacking the SQ/TQ sites, to a lesser extent (Fig. 4, lane 5), but not GST (data not shown). A GST fusion protein of the Chk2 FHA domain was not phosphorylated (data not shown). Hence, the SCD is evidently a target for Chk2 autophosphorylation.
FIG. 4.
Chk2 phosphorylates the SCD in vitro. In vitro kinase assays were performed on soluble GST-Chk2 and mutants in the presence of the substrate GST-HA-SCD or GST-HA-SCD(7A) and [γ−32P]ATP. The top panel shows [γ-32P]ATP incorporation into GST-HA-SCD or GST-HA-SCD(7A). GST-HA-SCD or GST-HA-SCD(7A) (middle panel). GST-Chk2 and its mutants (bottom panel) were detected by immunoblotting with anti-GST. IB, immunoblotting.
FHA domain is required for autophosphorylation of Chk2.
FHA domains are phosphopeptide-binding modules (18, 19, 36, 37, 55, 57). Deletion of the core FHA domain of Chk2 resulted in a significantly lower autokinase activity and diminished trans-kinase activity (Fig. 3, lane 5). Similarly, triple mutation of the conserved FHA domain residues NGT (GST-Chk2-NGT) abrogated kinase activity (Fig. 3, lane 6). However, substitution of conserved R117 did not affect kinase activity. Similar results were obtained in the rabbit reticulocyte lysate system (data not shown). These data suggested that the FHA domain regulates Chk2 kinase activity, consistent with earlier work showing that mutations in the FHA domain prevent Chk2 activation in vivo (11, 32, 62).
Ionizing radiation enhances ATM-dependent oligomerization of Chk2.
A yeast two-hybrid screen with yeast Rad53 as the bait identified Rad53 as an interacting protein (Z. Sun and D. F. Stern, unpublished results). Since this suggested that Rad53 forms dimers or oligomers, we determined whether Chk2 oligomers are produced in mammalian cells. In 293 cells expressing Flag-Chk2 and HA-Chk2, the tagged proteins coimmunoprecipitated, and the coimmunoprecipitation was enhanced by exposure to ionizing radiation (Fig. 5A and 5B). In ATM-deficient fibroblasts originating in a patient with ataxia-telangiectasia, enhancement of Chk2 oligomerization after gamma irradiation occurred with coexpression of wild-type Atm but not kinase-defective Atm (Fig. 5B). The higher baseline oligomerization compared to that in Fig. 5A probably reflects higher total expression of Chk2 with transient expression of both tagged proteins. These results demonstrated that at least two Chk2 molecules are components of an oligomer in vivo, that oligomerization is enhanced in response to DNA damage, and that this enhancement is ATM dependent. Thus, Chk2 oligomerization may be a regulated process that is linked to Chk2 activation.
FIG. 5.
Chk2 oligomerization. (A) 293 cells stably expressing Flag-Chk2 were transiently transfected with HA-Chk2. Transfectants were treated with 1 mM hydroxyurea for 24 h, beginning 24 h after transfection, or exposed to gamma irradiation (20 Gy) or UV (50 J/m2) 48 h after transfection. Cell lysates were harvested 24 h after hydroxyurea treatment or 2 h after irradiation. Upper panel, expression and mobility shift of HA-Chk2 were determined by immunoblotting lysates with anti-HA antibody. Lower panel, coimmunoprecipitation with anti-Flag antibody was performed with equal portions of cell lysates and detected with anti-HA. (B) ATM-dependent increase in Chk2 coimmunoprecipitation. GM5849C ataxia-telangiectasia cells were transiently transfected with HA-Chk2 and Flag-Chk2, plus vector (pcDNA3), wild-type Flag-ATM, or kinase-defective Flag-ATMkd. Transfectants were exposed to 20 Gy of gamma irradiation 48 h after transfection. Cell lysates were harvested 2 h after irradiation. Equal amounts of lysates were immunoprecipitated with anti-HA (top and bottom panels) or anti-Flag (middle panel) antibodies. Precipitates were blotted for Flag-Chk2 (top two panels) or HA-Chk2 (bottom panel). IP, immunoprecipitation; IB, immunoblotting; Ab, antibody.
Direct interactions of Chk2.
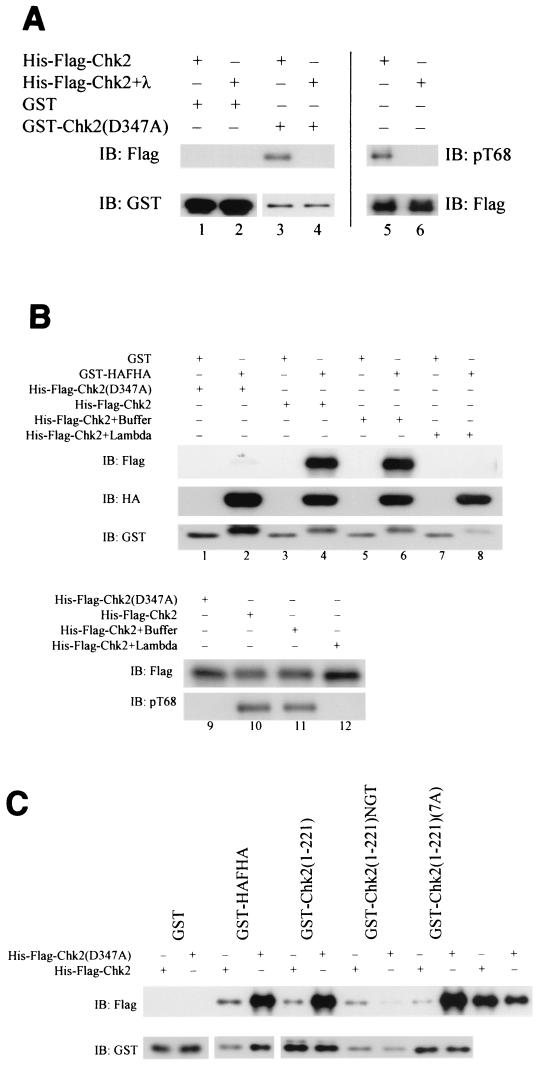
Coimmunoprecipitation of two tagged Chk2 molecules may occur if they form homodimers or participate in a larger complex containing additional proteins. Bacterially expressed GST-Chk2 and kinase-defective GST-Chk2(D347A) interacted with His-Flag-Chk2 and His-Flag-Chk2(D347A) in GST pulldown experiments even though no other eukaryotic proteins were present (Fig. 6A and 6B). However, His-Flag-Chk2(D347A) does not interact well with GST-Chk2(D347A) (Fig. 6B). Hence, Chk2 homomers can form, provided that at least one molecule has catalytic activity.
We next determined if bacterially produced GST-Chk2 or kinase-defective GST-Chk2(D347A) would pull down HA-tagged Chk2 stably expressed in 293 cells. Both GST-Chk2 and GST-Chk2(D347A) preferentially bound to HA-Chk2 after gamma irradiation (Fig. 6C). Similar results were obtained with both GST pulldown and coimmunoprecipitation of wild-type and kinase-effective Chk2 in the rabbit reticulocyte lysate system (data not shown). In these experiments, with Chk2 produced in bacteria, rabbit reticulocyte lysates, or in mammalian cells, kinase-defective Chk2 associated more strongly with Chk2 than did wild-type Chk2 (Fig. 6A and B and data not shown).
Chk2 oligomerization requires SCD and FHA domains.
Domains required for Chk2 oligomerization were localized by mutational analysis. We first mapped the Chk2-interacting domain(s) by coimmunoprecipitation from 293 cells transiently expressing various forms of HA-tagged and Flag-tagged Chk2. We note that, at most, heterooligomers potentially represent only one-third of the total oligomerized Chk2 molecules (homooligomers of HA-tagged or Flag-tagged Chk2 or heterooligomers of HA-tagged and Flag-tagged Chk2). Deletion of the FHA domain virtually eliminated coimmunoprecipitation of Chk2 molecules.
Phosphorylation at T68 is induced by Atm and possibly Atr after DNA damage and is required for quantitative activation of Chk2 by ionizing radiation (1, 42, 64). Substitution of T68 with alanine only slightly reduced oligomerization both before and after gamma irradiation (Fig. 7A). Point substitution of other SQ/TQ sites (S19, T26/S28, S33/S35, and S50) did not significantly affect oligomerization (data not shown). However, mutation of all seven SQ/TQ sites, including T68, within the SCD significantly diminished coimmunoprecipitation (Fig. 7A). This suggests that Chk2 oligomerization involves one or more intact SQ/TQ sites within the SCD, but we cannot rule out a global structural effect of multiple substitutions. Deletion and substitution mutants of bacterially produced GST-Chk2 fusion proteins were used to determine the minimal fragment of Chk2 required for binding wild-type Chk2 produced in irradiated 293 cells (Fig. 7C). GST-HA-FHA was sufficient to preferentially bind wild-type Chk2 after DNA damage.
Since coimmunoprecipitation of two tagged Chk2 molecules required both the FHA domain and phosphorylation within the SCD, and since GST-FHA is sufficient to isolate Chk2 after DNA damage, we hypothesized that oligomerization of Chk2 is mediated by FHA/phospho-SCD interactions. Consistent with this hypothesis, GST-HA-FHA failed to pull down HA-Chk2ΔSCD in 293 cells after gamma irradiation. GST-HA-FHA did bind to HA-Chk2-SCD(7A) expressed in 293 cells after gamma irradiation (Fig. 7D), perhaps reflecting Chk2 autophosphorylation at non-SQ/TQ sites within the SCD (Fig. 4).
If an FHA domain in one Chk2 molecule associates with the phospho-SCD in another Chk2 molecule, then it should be possible to form a heterodimer containing one molecule with an intact phospho-SCD but deleted FHA domain, and another with a deleted SCD but intact FHA domain. Indeed, Flag-Chk2ΔSCD coimmunoprecipitated with HA-Chk2-ΔFHA, and the association was enhanced by ionizing radiation (Fig. 7B).
Chk2 oligomerization is phosphorylation dependent.
In order to directly test the significance of phosphorylation on the putative FHA/phospho-SCD interaction, we determined whether the interaction is affected by phosphatase treatment. The ability of bacterially expressed GST-Chk2(D347A) to pull down bacterially produced His-Flag-Chk2 (Fig. 8A ) was prevented by prior dephosphorylation of His-Flag-Chk2 with λ phosphatase (Fig. 8B). Similar results were obtained with the GST-FHA domain rather than full-length Chk2 as a pulldown reagent (Fig. 8B). In order to verify that this binding is mediated by the FHA domain rather than other sequences present in the fusion protein, we used GST fusion proteins containing and lacking the FHA domain and also GST1-221NGT, with alanine substitutions in a tripeptide important for FHA function. GST-Chk2 fusion proteins containing an intact FHA domain strongly bound to His-Flag-Chk2 but not His-Flag-Chk2(D347A). However, GST1-221NGT did not bind (Fig. 8C).
FIG.8.
Phosphorylation-dependent oligomerization of Chk2. (A) Bacterially expressed His-Flag-Chk2 was incubated in the absence (lanes 3 and 5) or presence (lanes 4 and 6) of λ phosphatase and either blotted directly with anti-phospho-T68 or anti-Flag (lanes 5 and 6) or pulled down with either GST or GST-Chk2(D347A) expressed in bacteria (lanes 1, 2, 3, and 4). Affinity-purified Chk2 was detected by blotting with anti-Flag, and the loading of GST proteins was monitored with anti-GST. (B) Phosphorylation-dependent binding of the Chk2 FHA domain. Bacterially expressed His-Flag-Chk2 or His-Flag-Chk2(D347A) was incubated in the absence (lanes 5, 6, and 11) or presence (lanes 7, 8, and 12) of λ phosphatase and immunoblotted with anti-Flag or anti-phospho-T68 (lanes 9, 10, 11, and 12). Bacterially produced GST or GST-HA-FHA was used to pull down additional portions of phosphatase-treated or nontreated kinase-defective His-Flag-Chk2(D347A) or His-Flag-Chk2. Wild-type and kinase-defective His-Flag-Chk2 were detected with anti-Flag antibody (upper panel), for input of GST-HA-FHA with anti-HA antibody (second panel) and for GST with anti-GST antibody (lower panel). (C) Effect of FHA domain mutations on binding to His-Flag-Chk2. GST fusion proteins expressed in bacteria were used to pull down bacterially produced wild-type and kinase-defective His-Flag-Chk2. His-Flag-tagged Chk2 was detected with anti-Flag antibody, and GST fusions were detected with anti-GST. Differently sized GST fusion proteins on the bottom panel were cropped and realigned from one autoradiograph. IP, immunoprecipitation; IB, immunoblotting.
trans phosphorylation in the Chk2-Chk2 complex in vitro.
An important function of Chk2 oligomerization may be that it enables cross-phosphorylation of Chk2 molecules, which in turn enhances Chk2 activation. Hence, we determined whether Chk2 could cross-phosphorylate a kinase-defective Chk2 molecule in a heterodimer. In vitro kinase assays with bacterially produced GST-Chk2(D347A) and His-Flag-Chk2 revealed that cross-phosphorylation of kinase-defective GST-Chk2(D347A) by His-Flag-Chk2 at least occurs at T68 (Fig. 6B).
Forced oligomerization of Chk2.
We examined the functional consequences of Chk2 oligomerization in vivo by the regulated induction of dimerization. This system is based on the fact that the immunosuppressive drugs FK506 and rapamycin bind with high affinity to the cellular receptor FKBP12, which is an abundant, cytoplasmic 108-amino-acid protein (54). The synthetic ligand AP20187 binds with subnanomolar affinity to FKBPs with a single amino acid substitution, F36V (Fv), while binding with 1,000-fold lower affinity to the wild-type protein www.ariad.com/regulationkits(14). Introduction of the FKBP (F36V) module into a heterologous protein allows ligand-dependent homo- and heterodimerization of the target proteins.
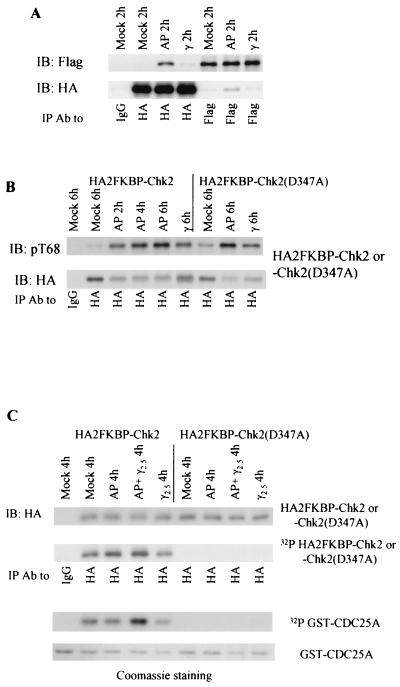
We introduced two tandem FKBP(F36V) modules into the amino terminus of wild-type or kinase-defective Chk2 (Fig. 1). When HEK 293 cells were cotransfected with both HA- and Flag-tagged 2FKBP-Chk2, approximately one-third of Flag-tagged 2FKBP-Chk2 coimmunoprecipitated with HA-tagged 2FKBP-Chk2 2 h after addition of the synthetic ligand (Fig. 9A). (Note that heterooligomerization between HA-tagged and Flag-tagged 2FKBP-Chk2 potentially represents only one-third of the total amount of oligomerized 2FKBP-Chk2.) Ligand-induced oligomerization of Chk2 was significantly higher than that induced by ionizing irradiation (Fig. 9A). Induced oligomerization in vivo significantly increased T68 phosphorylation of wild-type Chk2 (Fig. 9B), reinforcing the conclusion that oligomerization facilitates Chk2 phosphorylation.
FIG. 9.
Forced oligomerization and Chk2 activation. (A) AP20187 induced Chk2 oligomerization in vivo. HEK 293 cells were transiently cotransfected with both HA- and Flag-tagged 2FKBP-Chk2. Transfectants were either mock treated, treated with 10 μM AP20187, or irradiated with 10 Gy of gamma irradiation 48 h after transfection. Lysates were harvested 2 h after treatment and used for immunoprecipitation followed by immunoblotting. (B) Induced oligomerization resulted in T68 phosphorylation. Performed as in A, except that only HA-tagged wild-type or kinase-defective 2FKBP-Chk2 was transiently expressed. Lysates were harvested at each time point as indicated after treatment and used for immunoprecipitation with anti-HA antibody followed by immunoblotting for anti-phospho-T68 (top panel) or anti-HA (bottom panel, duplicate blot) antibodies. (C) Induced oligomerization after exposure to low-dose ionizing irradiation activated Chk2. Performed as in B, except that the dose of γ irradiation was 2.5 Gy and AP20187 was added to one set of 293 cells immediately after exposure to 2.5 Gy of irradiation. Immunocomplexes were incubated with [γ-32P]ATP in the presence of bacterially produced GST-Cdc25A (amino acids 101 to 140). Recovery of Chk2 was monitored by immunoblotting with anti-HA (top panel) or incorporation of [γ-32P]ATP (second panel). Recovery of GST-Cdc25A was detected by incorporation of [γ-32P]ATP (third panel) or Coomassie staining (bottom panel). IP, immunoprecipitation; IB, immunoblotting; Ab, antibody.
Surprisingly, kinase-defective Chk2 also showed greater T68 phosphorylation in the presence of ligand (Fig. 9B). Possible explanations include trans autophosphorylation in an oligomerized complex, in which kinase-defective Chk2 may associate with and be phosphorylated by the endogenous wild-type Chk2. Alternatively, the oligomerized Chk2 complex preferentially binds to and is phosphorylated by ATM/ATR kinases independent of Chk2 kinase activity. T68 phosphorylation in the ligand-induced oligomerized Chk2 immune complex was associated with increased Chk2 autokinase activity (Fig. 9C). However, this phosphorylation did not increase Chk2 trans phosphorylation of the Cdc25A polypeptide substrate (Fig. 9C), nor was it associated with measurable effects on cell cycle regulation or p53 stability (data not shown).
When Chk2 oligomerization was induced by ligand after exposure to a low dose of ionizing irradiation (2.5 Gy), both Chk2 auto- and trans-kinase activities increased (Fig. 9C) to levels comparable to that induced by 10-Gy irradiation (data not shown). This suggests that oligomerization plus a second DNA damage-dependent event, such as priming phosphorylation by ATM/ATR kinases, association with soluble activating or target proteins, or appropriate geometry of Chk2 oligomers induced by association with scaffolding molecules, is required for Chk2 activation and checkpoint pathway regulation.
DISCUSSION
We show here that Chk2 itself can phosphorylate Chk2 at T68 and other sites. Since T68 has already been identified as a trans-regulatory site, the Chk2-dependent phosphorylation of Chk2 has important implications for Chk2 regulation. Chk2 forms homomeric complexes in which the Chk2 FHA domain interacts with a second phosphorylated molecule of Chk2. Artificial dimerization of Chk2 in vivo concomitant with limited DNA damage augments Chk2 kinase activity. Finally, we provide evidence that Chk2 participates in DNA damage-dependent oligomeric complexes in vivo that have the same domain requirements as Chk2 homomers. These data suggest that the regulation of Chk2 by trans and autophosphorylation is more complicated than hitherto appreciated and involves a cascade of phosphorylation events that lead to the production of Chk2 homomeric complexes.
FHA domains are phosphopeptide interaction domains (18, 19, 36, 37, 55, 57). The Chk2 FHA domain is required for DNA damage-dependent Chk2 activation (11, 32, 62), and FHA domain mutations have been identified in alleles of Chk2 associated with variant TP53+/+ Li-Fraumeni syndrome (5, 58) and a variety of tumors (27, 44). In the budding yeast DNA damage response, Rad53 FHA domains are required for DNA damage-dependent phosphorylation and activation of Rad53, which also requires the Atr homolog Mec1 and/or Tel1 (20, 47, 56, 57). A current model is that Mec1 is localized to sites of DNA damage and subsequently recruits and phosphorylates a second protein, Rad9, which is also required for damage-dependent activation of Rad53 (20, 47, 56, 57). Rad53 is recruited to the complex through interactions between Rad53 FHA domains and Rad9 phosphopeptides created by Mec1-mediated phosphorylation (M. F. Schwartz and D. F. Stern, submitted for publication). Since Mec1 is required for Rad53 activation and Rad53 has a cluster of potential PIKK phosphorylation sites similar to the Chk2 SCD, known to be a target for mammalian PIKKs, the relocalization of Rad53 mediated by FHA domains may be important for connecting Rad53 with the upstream activating PIKK Mec1. Another model, which is not incompatible with the first, is that dimerization of Rad53 through binding to dimeric Rad9 promotes Rad53 cross-phosphorylation and activation (22).
No mammalian Rad9 ortholog has been identified, nor has the binding partner for the Chk2 FHA domain. However, mutational analysis of Chk2 reveals the same dependencies of Chk2 function on the FHA domain (11, 32, 62), so that the same mechanisms probably operate in mammalian cells. With DNA damage, the initial function for the FHA domain will be coupling of Chk2 to upstream regulatory pathways, by bringing Chk2 to Atm/Atr and/or by adaptor- or scaffold-dependent activation. Our findings suggest that the FHA domain is required for an additional process involving intra- and/or intermolecular binding of the FHA domain to one or more phosphorylated sites within Chk2.
Similar to the Rad53 FHA domains, the Chk2 FHA domain is required for its PIKK-dependent phosphorylation. Point mutation (R145W) (33, 62) or deletion mutation (21 and 42 amino acids surrounding R145) (33) of the FHA domain abolished T68 phosphorylation. This suggests that the FHA domain couples Chk2 to an Atm- or Atr-activated complex. Nevertheless, we observed that deletion of the core FHA domain (amino acids 115 to 175) of Chk2 spared T68 phosphorylation after gamma irradiation (Fig. 7E) and binding to the bacterially produced FHA domain (Fig. 7D, second panel). This suggests that an additional mechanism of T68 phosphorylation bypassing the requirement for the Chk2 FHA domain can operate under some circumstances.
T68 is phosphorylated during damage-dependent activation of Chk2 in vivo and is likely to be a target for both Atm and Atr (1, 41, 42, 64). Phosphorylation at T68 is permissive for further Chk2 autophosphorylation at two sites in the activation loop, which is thought to be required for full activation of Chk2 (33). The mechanism by which T68 phosphorylation promotes Chk2 activation has not been determined. It may promote allosteric changes that are permissive for activation loop phosphorylation or may regulate intra- or intermolecular Chk2 interactions. Our finding that deletion of the FHA domain impairs the kinase activity of bacterially produced Chk2 suggests that this domain has a positive regulatory influence. However, we cannot rule out the possibility that this is a result of nonspecific effects on Chk2 folding.
An important implication of the finding that Chk2 can phosphorylate itself at a known regulatory site is that phospho-Chk2 may be able to activate other molecules of Chk2. In this scenario, DNA damage-dependent activation of PIKKs would result in phosphorylation of Chk2 at T68 and other SQ/TQ sites within the SCD (1, 41, 42, 64). Phosphorylation of T68 would enable Chk2 autophosphorylation at the activation loop (32) and additional sites within the SCD. Nonphosphorylated Chk2 molecules would then be recruited and activated through FHA-SCD interactions and cross-phosphorylation. Hence, after priming phosphorylation by PIKKs, additional molecules could be activated independent of PIKK activity, thereby latching on Chk2 activation. This model is consistent with the intriguing finding that Rad9 can activate Rad53 in an ATP-dependent manner in the absence of Mec1 (22). Scaffolding of a Rad53 dimer by Rad9 could promote priming activation of Rad53, which could then activate additional molecules of Rad53. The catalytic function of Rad53 would be the source of the ATP dependence of the activation.
The ability of the Chk2 FHA domain to bind phospho-SCD suggests that FHA/phospho-SCD interactions may be important in intra- or intermolecular regulation of Chk2. Phosphorylation of the Chk2 SCD may enable an intramolecular interaction with the FHA domain that would either disturb a basal occupation of the kinase domain by the FHA domain or induce a structural change in the kinase domain, which is directly adjacent to the FHA domain. Alternatively, FHA/phospho-SCD interactions may enable recruitment of a second molecule of Chk2 through its FHA domain to a complex, thereby facilitating catalytic activation through cross-phosphorylation.
In other signaling systems involving phosphopeptide binding interactions, dynamic changes in association and localization of signaling proteins are mediated by a cascade of phosphopeptide/binding domain interactions. STATs are recruited to activated receptor tyrosine kinases by interaction of their SH2 domains with phosphopeptides on the phosphorylated receptors. Once recruited, the receptors phosphorylate the STATs, creating new STAT SH2 binding sites on the STATs themselves. An exchange of STAT-SH2/phosphoreceptor interactions with STAT-SH2/phospho-STAT interactions is an important step in releasing STATs from the sites of activation at the cell surface for transit to the nucleus, where they function as transcription factors (4, 13, 17).
Similarly, the R-Smad MH2 domain, a phosphoserine-binding motif structurally related to FHA domains, binds to phosphopeptides on the TGF-β-activated TGF-β type I receptor. Phosphorylation at the C-terminal serine residues of R-Smad by TGF-β type I receptor promotes homooligomerization by binding to the MH2 domain of the second R-Smad molecule and then dissociation from TGF-β type I receptor (46, 51, 61). It is noteworthy that unphosphorylatable co-Smad Smad4 competes with a phospho-R-Smad homooligomeric complex to form a more stable Smad4/phospho-R-Smad heterooligomeric complex (46, 61).
The ability of the Chk2 FHA domain to bind phospho-Chk2 as well as the likelihood that it binds to putative upstream activating proteins suggest a similar series of phosphopeptide binding site exchanges in Chk2 activation. An overall model is that DNA checkpoint activation results in direct recruitment of PIKKs (ATR and ATM) and, independently, the Rad1-Rad9 (unrelated to budding yeast Rad9)-Hus1PCNA-related complex to sites of DNA damage (15, 31, 43, 66). DNA damage promotes relocalization of Chk2 phosphorylated at T68 (60). Recruitment of Chk2 would depend upon an interaction between its FHA domain and a phosphorylated constituent of the DNA damage complex. Once recruited, Chk2 would be activated by PIKK-dependent phosphorylation and/or by scaffolding-induced oligomerization. Additional Chk2 molecules would be activated similarly and by FHA domain-dependent binding of additional Chk2 molecules to already active and phosphorylated Chk2. Head-to-tail polymerization of phospho-Chk2 could potentially result in assembly of large active complexes.
Dissociation of Chk2 from the activated complexes could result from competition among FHA domain binding sites. We have found that phospho-Rad9 complexes with kinase-defective Rad53 are more abundant than those between phospho-Rad9 and kinase-active Rad53 (M. F. Schwartz and D. F. Stern, unpublished data). These data may be explained by a phosphorylation-dependent mechanism for dissociation of Chk2 FHA domain complexes that have already formed. If, hypothetically, the Chk2 FHA domain interacts more strongly with the Chk2 phospho-SCD than with the Rad9-like binding target in DNA damage complexes, then progressive activation of Chk2 would favor production of Chk2 homodimers over heteromers with the activation complex. This would result in release of active Chk2 homodimers, in much the same way that phosphorylated STATs and R-Smads are released from the activating receptors. Since heteromeric complexes between kinase-active Chk2 and inactive Chk2 are more readily isolated than those between two kinase-active molecules, even with bacterially expressed proteins, it is possible that the dual phosphorylated dimer is itself unstable and dissociates to release active monomers.
ADDENDUM IN PROOF
Related work is reported by Ahn et al. (J. Y. Ahn, X. Li, H. L. Davis, and C. E. Canman, J. Biol. Chem., in press).
Acknowledgments
We thank Michael Kastan and Susan Lees-Miller for plasmids and JoAnn Falato for secretarial assistance. We thank other members of the Stern laboratory for helpful comments, particularly Soo-Jung Lee, Jonathan McMenamin-Balano, and Marc F. Schwartz.
This work was supported by USAMRMC DAMD 17-98-1-8272, USPHS R01CA82257, and USAMRMC DAMD 17-01-1-0465 (X.X.), a Leslie H. Warner fellowship from the Yale Cancer Center (X.X.), an Anna Fuller fellowship in molecular oncology (L.T.), Susan G. Komen Breast Cancer Foundation fellowship PDF2000 719 (L.T.), and USAMRMC DAMD 17-01-1-0464 (L.T.).
REFERENCES
- 1.Ahn, J. Y., J. K. Schwarz, H. Piwnica-Worms, and C. E. Canman. 2000. Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res. 60:5934-5936. [PubMed]
- 2.Anderson, C. W., and S. P. Lees-Miller. 1992. The nuclear serine/threonine protein kinase DNA-PK. Crit. Rev. Eukaryot. Gene Expr. 2:283-314. [PubMed] [Google Scholar]
- 3.Banin, S., L. Moyal, S. Shieh, Y. Taya, C. W. Anderson, L. Chessa, N. I. Smorodinsky, C. Prives, Y. Reiss, Y. Shiloh, and Y. Ziv. 1998. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281:1674-1677. [DOI] [PubMed] [Google Scholar]
- 4.Becker, S., B. Groner, and C. W. Muller. 1998. Three-dimensional structure of the Stat3beta homodimer bound to DNA. Nature 394:145-151. [DOI] [PubMed] [Google Scholar]
- 5.Bell, D. W., J. M. Varley, T. E. Szydlo, D. H. Kang, D. C. Wahrer, K. E. Shannon, M. Lubratovich, S. J. Verselis, K. J. Isselbacher, J. F. Fraumeni, J. M. Birch, F. P. Li, J. E. Garber, and D. A. Haber. 1999. Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science 286:2528-2531. [DOI] [PubMed] [Google Scholar]
- 6.Blasina, A., I. V. de Weyer, M. C. Laus, W. H. Luyten, A. E. Parker, and C. H. McGowan. 1999. A human homologue of the checkpoint kinase Cds1 directly inhibits Cdc25 phosphatase. Curr. Biol. 9:1-10. [DOI] [PubMed] [Google Scholar]
- 7.Brown, A. L., C. H. Lee, J. K. Schwarz, N. Mitiku, H. Piwnica-Worms, and J. H. Chung. 1999. A human Cds1-related kinase that functions downstream of ATM protein in the cellular response to DNA damage. Proc. Natl. Acad. Sci. USA 96:3745-3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canman, C. E., D. S. Lim, K. A. Cimprich, Y. Taya, K. Tamai, K. Sakaguchi, E. Appella, M. B. Kastan, and J. D. Siliciano. 1998. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 281:1677-1679. [DOI] [PubMed] [Google Scholar]
- 9.Chan, D. W., S. C. Son, W. Block, R. Ye, K. K. Khanna, M. S. Wold, P. Douglas, A. A. Goodarzi, J. Pelley, Y. Taya, M. F. Lavin, and S. P. Lees-Miller. 2000. Purification and characterization of ATM from human placenta. A manganese-dependent, wortmannin-sensitive serine/threonine protein kinase. J. Biol. Chem. 275:7803-7810. [DOI] [PubMed] [Google Scholar]
- 10.Chaturvedi, P., W. K. Eng, Y. Zhu, M. R. Mattern, R. Mishra, M. R. Hurle, X. Zhang, R. S. Annan, Q. Lu, L. F. Faucette, G. F. Scott, X. Li, S. A. Carr, R. K. Johnson, J. D. Winkler, and B. B. Zhou. 1999. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene 18:4047-4054. [DOI] [PubMed] [Google Scholar]
- 11.Chehab, N. H., A. Malikzay, M. Appel, and T. D. Halazonetis. 2000. Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev. 14:278-288. [PMC free article] [PubMed] [Google Scholar]
- 12.Chehab, N. H., A. Malikzay, E. S. Stavridi, and T. D. Halazonetis. 1999. Phosphorylation of Ser-20 mediates stabilization of human p53 in response to DNA damage. Proc. Natl. Acad. Sci. USA 96:13777-13782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, X., U. Vinkemeier, Y. Zhao, D. Jeruzalmi, J. E. Darnell, Jr., and J. Kuriyan. 1998. Crystal structure of a tyrosine phosphorylated STAT-1 dimer bound to DNA. Cell 93:827-839. [DOI] [PubMed] [Google Scholar]
- 14.Clackson, T., W. Yang, L. W. Rozamus, M. Hatada, J. F. Amara, C. T. Rollins, L. F. Stevenson, S. R. Magari, S. A. Wood, N. L. Courage, X. Lu, F. Cerasoli, Jr., M. Gilman, and D. A. Holt. 1998. Redesigning an FKBP-ligand interface to generate chemical dimerizers with novel specificity. Proc. Natl. Acad. Sci. USA 95:10437-10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortez, D., S. Guntuku, J. Qin, and S. J. Elledge. 2001. ATR and ATRIP: partners in checkpoint signaling. Science 294:1713-1716. [DOI] [PubMed] [Google Scholar]
- 16.Cortez, D., Y. Wang, J. Qin, and S. J. Elledge. 1999. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science 286:1162-1166. [DOI] [PubMed] [Google Scholar]
- 17.Darnell, J. E., Jr. 1997. STATs and gene regulation. Science 277:1630-1635. [DOI] [PubMed] [Google Scholar]
- 18.Durocher, D., J. Henckel, A. R. Fersht, and S. P. Jackson. 1999. The FHA domain is a modular phosphopeptide recognition motif. Mol. Cell 4:387-394. [DOI] [PubMed] [Google Scholar]
- 19.Durocher, D., I. A. Taylor, D. Sarbassova, L. F. Haire, S. L. Westcott, S. P. Jackson, S. J. Smerdon, and M. B. Yaffe. 2000. The molecular basis of FHA domain:phosphopeptide binding specificity and implications for phospho-dependent signaling mechanisms. Mol. Cell 6:1169-1182. [DOI] [PubMed] [Google Scholar]
- 20.Emili, A. 1998. MEC1-dependent phosphorylation of Rad9p in response to DNA damage. Mol. Cell 2:183-189. [DOI] [PubMed] [Google Scholar]
- 21.Falck, J., N. Mailand, R. G. Syljuasen, J. Bartek, and J. Lukas. 2001. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature 410:842-847. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert, C. S., C. M. Green, and N. F. Lowndes. 2001. Budding yeast Rad9 is an ATP-dependent Rad53 activating machine. Mol. Cell 8:129-136. [DOI] [PubMed] [Google Scholar]
- 23.Hartwell, L. H., and M. B. Kastan. 1994. Cell cycle control and cancer. Science 266:1821-1828. [DOI] [PubMed] [Google Scholar]
- 24.Haruki, N., H. Saito, Y. Tatematsu, H. Konishi, T. Harano, A. Masuda, H. Osada, Y. Fujii, and T. Takahashi. 2000. Histological type-selective, tumor-predominant expression of a novel CHK1 isoform and infrequent in vivo somatic CHK2 mutation in small cell lung cancer. Cancer Res. 60:4689-4692. [PubMed] [Google Scholar]
- 25.Hirao, A., Y. Y. Kong, S. Matsuoka, A. Wakeham, J. Ruland, H. Yoshida, D. Liu, S. J. Elledge, and T. W. Mak. 2000. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science 287:1824-1827. [DOI] [PubMed] [Google Scholar]
- 26.Hofmann, K., and P. Bucher. 1995. The FHA domain: a putative nuclear signalling domain found in protein kinases and transcription factors. Trends Biochem. Sci. 20:347-349. [DOI] [PubMed] [Google Scholar]
- 27.Hofmann, W. K., C. W. Miller, K. Tsukasaki, S. Tavor, T. Ikezoe, D. Hoelzer, S. Takeuchi, and H. P. Koeffler. 2001. Mutation analysis of the DNA-damage checkpoint gene CHK2 in myelodysplastic syndromes and acute myeloid leukemias. Leukoc. Res. 25:333-338. [DOI] [PubMed] [Google Scholar]
- 28.Inbal, B., G. Shani, O. Cohen, J. L. Kissil, and A. Kimchi. 2000. Death-associated protein kinase-related protein 1, a novel serine/threonine kinase involved in apoptosis. Mol. Cell. Biol. 20:1044-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khanna, K. K. 2000. Cancer risk and the ATM gene: a continuing debate. J. Natl. Cancer Inst. 92:795-802. [DOI] [PubMed] [Google Scholar]
- 30.Kim, S. T., D. S. Lim, C. E. Canman, and M. B. Kastan. 1999. Substrate specificities and identification of putative substrates of ATM kinase family members. J. Biol. Chem. 274:37538-37543. [DOI] [PubMed] [Google Scholar]
- 31.Kondo, T., T. Wakayama, T. Naiki, K. Matsumoto, and K. Sugimoto. 2001. Recruitment of Mec1 and Ddc1 checkpoint proteins to double-strand breaks through distinct mechanisms. Science 294:867-870. [DOI] [PubMed] [Google Scholar]
- 32.Lee, C. H., and J. H. Chung. 2001. The hCds1 (Chk2)-FHA domain is essential for a chain of phosphorylation events on hCds1 that is induced by ionizing radiation. J. Biol. Chem. 276:30537-30541. [DOI] [PubMed] [Google Scholar]
- 33.Lee, J. S., K. M. Collins, A. L. Brown, C. H. Lee, and J. H. Chung. 2000. hCds1-mediated phosphorylation of BRCA1 regulates the DNA damage response. Nature 404:201-204. [DOI] [PubMed] [Google Scholar]
- 34.Li, J., G. I. Lee, S. R. Van Doren, and J. C. Walker. 2000. The FHA domain mediates phosphoprotein interactions. J. Cell Sci. 113:4143-4149. [DOI] [PubMed] [Google Scholar]
- 35.Li, J., G. P. Smith, and J. C. Walker. 1999. Kinase interaction domain of kinase-associated protein phosphatase, a phosphoprotein-binding domain. Proc. Natl. Acad. Sci. USA 96:7821-7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao, H., I. J. Byeon, and M. D. Tsai. 1999. Structure and function of a new phosphopeptide-binding domain containing the FHA2 of Rad53. J. Mol. Biol. 294:1041-1049. [DOI] [PubMed] [Google Scholar]
- 37.Liao, H., C. Yuan, M. I. Su, S. Yongkiettrakul, D. Qin, H. Li, I. J. Byeon, D. Pei, and M. D. Tsai. 2000. Structure of the FHA1 domain of yeast Rad53 and identification of binding sites for both FHA1 and its target protein Rad9. J. Mol. Biol. 304:941-951. [DOI] [PubMed] [Google Scholar]
- 38.Madhani, H. D. 2001. Accounting for specificity in receptor tyrosine kinase signaling. Cell 106:9-11. [DOI] [PubMed] [Google Scholar]
- 39.Massague, J., and Y. G. Chen. 2000. Controlling TGF-beta signaling. Genes Dev. 14:627-644. [PubMed] [Google Scholar]
- 40.Matsuoka, S., M. Huang, and S. J. Elledge. 1998. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 282:1893-1897. [DOI] [PubMed] [Google Scholar]
- 41.Matsuoka, S., G. Rotman, A. Ogawa, Y. Shiloh, K. Tamai, and S. J. Elledge. 2000. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc. Natl. Acad. Sci. USA 97:10389-10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melchionna, R., X. B. Chen, A. Blasina, and C. H. McGowan. 2000. Threonine 68 is required for radiation-induced phosphorylation and activation of Cds1. Nat. Cell Biol. 2:762-765. [DOI] [PubMed] [Google Scholar]
- 43.Melo, J. A., J. Cohen, and D. P. Toczyski. 2001. Two checkpoint complexes are independently recruited to sites of DNA damage in vivo. Genes Dev. 15:2809-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller, C. W., T. Ikezoe, U. Krug, W. K. Hofmann, S. Tavor, V. Vegesna, K. Tsukasaki, S. Takeuchi, and H. P. Koeffler. 2002. Mutations of the CHK2 gene are found in some osteosarcomas, but are rare in breast, lung, and ovarian tumors. Genes Chromosomes Cancer 33:17-21. [DOI] [PubMed] [Google Scholar]
- 45.Pellicioli, A., C. Lucca, G. Liberi, F. Marini, M. Lopes, P. Plevani, A. Romano, P. P. Di Fiore, and M. Foiani. 1999. Activation of Rad53 kinase in response to DNA damage and its effect in modulating phosphorylation of the lagging strand DNA polymerase. EMBO J. 18:6561-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin, B. Y., B. M. Chacko, S. S. Lam, M. P. de Caestecker, J. J. Correia, and K. Lin. 2001. Structural basis of Smad1 activation by receptor kinase phosphorylation. Mol. Cell 8:1303-1312. [DOI] [PubMed] [Google Scholar]
- 47.Sanchez, Y., B. A. Desany, W. J. Jones, Q. Liu, B. Wang, and S. J. Elledge. 1996. Regulation of RAD53 by the ATM-like kinases MEC1 and TEL1 in yeast cell cycle checkpoint pathways. Science 271:357-360. [DOI] [PubMed] [Google Scholar]
- 48.Sarkaria, J. N., E. C. Busby, R. S. Tibbetts, P. Roos, Y. Taya, L. M. Karnitz, and R. T. Abraham. 1999. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 59:4375-4382. [PubMed] [Google Scholar]
- 49.Sarkaria, J. N., R. S. Tibbetts, E. C. Busby, A. P. Kennedy, D. E. Hill, and R. T. Abraham. 1998. Inhibition of phosphoinositide 3-kinase related kinases by the radiosensitizing agent wortmannin. Cancer Res. 58:4375-4382. [PubMed] [Google Scholar]
- 49a.Schwartz, M. F., J. K. Duong, Z. Sun, J. S. Morrow, D. Pradhan, and D. F. Stern. Rad9 phosphorylation sites couple Rad53 to the Saccharomyces cerevisiae DNA damage checkpoint. Mol. Cell, in press. [DOI] [PubMed]
- 50.Shani, G., S. Henis-Korenblit, G. Jona, O. Gileadi, M. Eisenstein, T. Ziv, A. Admon, and A. Kimchi. 2001. Autophosphorylation restrains the apoptotic activity of DRP-1 kinase by controlling dimerization and calmodulin binding. EMBO J. 20:1099-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi, Y. 2001. Structural insights on Smad function in TGFbeta signaling. Bioessays 23:223-232. [DOI] [PubMed] [Google Scholar]
- 52.Shieh, S. Y., J. Ahn, K. Tamai, Y. Taya, and C. Prives. 2000. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 14:289-300. [PMC free article] [PubMed] [Google Scholar]
- 53.Sodha, N., R. Williams, J. Mangion, S. L. Bullock, M. R. Yuille, and R. A. Eeles. 2000. Screening hCHK2 for mutations. Science 289:359.. [DOI] [PubMed] [Google Scholar]
- 54.Spencer, D. M., T. J. Wandless, S. L. Schreiber, and G. R. Crabtree. 1993. Controlling signal transduction with synthetic ligands. Science 262:1019-1024. [DOI] [PubMed] [Google Scholar]
- 55.Stone, J. M., M. A. Collinge, R. D. Smith, M. A. Horn, and J. C. Walker. 1994. Interaction of a protein phosphatase with an Arabidopsis serine-threonine receptor kinase. Science 266:793-795. [DOI] [PubMed] [Google Scholar]
- 56.Sun, Z., D. S. Fay, F. Marini, M. Foiani, and D. F. Stern. 1996. Spk1/Rad53 is regulated by Mec1-dependent protein phosphorylation in DNA replication and damage checkpoint pathways. Genes Dev. 10:395-406. [DOI] [PubMed] [Google Scholar]
- 57.Sun, Z., J. Hsiao, D. S. Fay, and D. F. Stern. 1998. Rad53 FHA domain associated with phosphorylated Rad9 in the DNA damage checkpoint. Science 281:272-274. [DOI] [PubMed] [Google Scholar]
- 58.Vahteristo, P., A. Tamminen, P. Karvinen, H. Eerola, C. Eklund, L. A. Aaltonen, C. Blomqvist, K. Aittomaki, and H. Nevanlinna. 2001. p53, CHK2, and CHK1 genes in Finnish families with Li-Fraumeni syndrome: further evidence of CHK2 in inherited cancer predisposition. Cancer Res. 61:5718-5722. [PubMed] [Google Scholar]
- 59.Vialard, J. E., C. S. Gilbert, C. M. Green, and N. F. Lowndes. 1998. The budding yeast Rad9 checkpoint protein is subjected to Mec1/Tel1-dependent hyperphosphorylation and interacts with Rad53 after DNA damage. EMBO J. 17:5679-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ward, I. M., X. Wu, and J. Chen. 2001. Threonine 68 of Chk2 is phosphorylated at sites of DNA strand breaks. J. Biol. Chem. 276:47755-47758. [DOI] [PubMed] [Google Scholar]
- 61.Wu, J. W., M. Hu, J. Chai, J. Seoane, M. Huse, C. Li, D. J. Rigotti, S. Kyin, T. W. Muir, R. Fairman, J. Massague, and Y. Shi. 2001. Crystal structure of a phosphorylated Smad2. Recognition of phosphoserine by the MH2 domain and insights on Smad function in TGF-beta signaling. Mol. Cell 8:1277-1289. [DOI] [PubMed] [Google Scholar]
- 62.Wu, X., S. R. Webster, and J. Chen. 2001. Characterization of tumor-associated Chk2 mutations. J. Biol. Chem. 276:2971-2974. [DOI] [PubMed] [Google Scholar]
- 63.Xu, X., J. Liao, K. E. Creek, and L. Pirisi. 1999. Human keratinocytes and tumor-derived cell lines express alternatively spliced forms of transforming growth factor-alpha mRNA, encoding precursors lacking carboxyl-terminal valine residues. Oncogene 18:5554-5562. [DOI] [PubMed] [Google Scholar]
- 64.Zhou, B. B., P. Chaturvedi, K. Spring, S. P. Scott, R. A. Johanson, R. Mishra, M. R. Mattern, J. D. Winkler, and K. K. Khanna. 2000. Caffeine abolishes the mammalian G2/M DNA damage checkpoint by inhibiting ataxia-telangiectasia-mutated kinase activity. J. Biol. Chem. 275:10342-10348. [DOI] [PubMed] [Google Scholar]
- 65.Zhou, B. B., and S. J. Elledge. 2000. The DNA damage response: putting checkpoints in perspective. Nature 408:433-439. [DOI] [PubMed] [Google Scholar]
- 66.Zou, L., D. Cortez, and S. J. Elledge. 2002. Regulation of ATR substrate selection by Rad17-dependent loading of Rad9 complexes onto chromatin. Genes Dev. 16:198-208. [DOI] [PMC free article] [PubMed] [Google Scholar]