Abstract
BRCA1 plays an important role in mechanisms of response to double-strand breaks, participating in genome surveillance, DNA repair, and cell cycle checkpoint arrests. Here, we identify a constitutive BRCA1-c-Abl complex and provide evidence for a direct interaction between the PXXP motif in the C terminus of BRCA1 and the SH3 domain of c-Abl. Following exposure to ionizing radiation (IR), the BRCA1-c-Abl complex is disrupted in an ATM-dependent manner, which correlates temporally with ATM-dependent phosphorylation of BRCA1 and ATM-dependent enhancement of the tyrosine kinase activity of c-Abl. The BRCA1-c-Abl interaction is affected by radiation-induced modification to both BRCA1 and c-Abl. We show that the C terminus of BRCA1 is phosphorylated by c-Abl in vitro. In vivo, BRCA1 is phosphorylated at tyrosine residues in an ATM-dependent, radiation-dependent manner. Tyrosine phosphorylation of BRCA1, however, is not required for the disruption of the BRCA1-c-Abl complex. BRCA1-mutated cells exhibit constitutively high c-Abl kinase activity that is not further increased on exposure to IR. We suggest a model in which BRCA1 acts in concert with ATM to regulate c-Abl tyrosine kinase activity.
Germ line mutations in BRCA1 are responsible for the majority of hereditary breast cancers and cause almost all the familial cases involving both breast and ovarian cancers (15). The patients are heterozygous, and loss of heterozygosity is observed in the primary breast and ovarian tumors, providing clues that the BRCA1 protein is encoded by a tumor suppressor gene which functions as a genome caretaker (25). However, although there is now a plethora of data suggesting that BRCA1 is involved in DNA repair, the biological role of BRCA1 remains unclear (14, 50).
BRCA1 is a nuclear phosphoprotein with an N-terminal zinc ring finger domain which mediates protein-protein interactions (6) and a C-terminal tandem BRCT domain (47). BRCT motifs are encountered in several DNA repair and cell cycle control proteins (7, 47). Treatment with ionizing radiation (IR) or other DNA-damaging agents triggers phosphorylation of BRCA1 and its cellular relocalization as discrete nuclear dots (33). BRCA1 interacts with RAD51, a human homolog of RecA (34), and with the hRAD50-hMRE11-NBS complex (52). The homologous complex in Saccharomyces cerevisiae, Rad50-Mre11-Xrs2, has been reported to be required for nonhomologous end joining, homologous recombination, and telomere maintenance (5). The ATM and ATR kinases, both implicated in responses to genotoxic stress, are also required for the radiation-induced phosphorylation of BRCA1 (12, 38). More recently, a group of complexed BRCA1 protein partners forming the BRCA1-associated genome surveillance complex (BASC) has been identified; all the BASC components are involved in DNA damage recognition and/or DNA replication-associated repair (45). Lastly, BRCA1 was also shown to be associated with Fanconi anemia protein D2 (16) and BACH1 helicase (8).
ATM and RAD51, two of the protein partners of BRCA1, are also complexed with the nonreceptor tyrosine kinase c-Abl (35, 49). c-Abl is the cellular homolog of the Abelson murine leukemia virus oncogene, v-Abl, which encodes a ubiquitously expressed protein (36). Homozygous null mutation of c-Abl confers neonatal lethality in mutant mice despite normal fetal development (40). Although no human genetic disease has yet been specifically associated with mutations in c-Abl, c-Abl is activated as an oncogene through its fusion with BCR in chronic myelogenous leukemia (13, 36, 37, 44). The c-Abl protein has a number of specific domains, notably Src homolog binding sites (SH domains), including a tyrosine kinase domain, SH2 (which has a high affinity for phosphorylated tyrosine residues), and SH3 (which preferentially binds to proline-rich domains) (2, 28, 29, 36, 48). The c-Abl tyrosine kinase is activated in response to radiation through an ATM-dependent pathway (4, 35).
Here, our findings show that BRCA1 and c-Abl interact constitutively. Following exposure to IR, the complex is disrupted in an ATM-dependent manner, which correlates temporally with the phosphorylation of BRCA1 and the increase of the tyrosine kinase activity of c-Abl. Loss of BRCA1 results in constitutively elevated c-Abl tyrosine kinase activity. Our findings suggest that one function of BRCA1 is to control c-Abl activity in an ATM-dependent manner.
MATERIALS AND METHODS
Cell culture.
The 293 cell line is an immortalized line derived from a primary human embryonal kidney line transformed by human adenovirus 5. The HCC1937 (BRCA1-mutated) tumor cell line is derived from a human ductal carcinoma bearing a BRCA1 5382insC mutation in one allele and a deletion of the second allele. The BRCA1 protein in HCC1937 lacks the BRCA1 C terminus, and extracts show very low levels of BRCA1 protein (9, 39). AT5BIVA (ATM−/−) and MRC5VI (control) are simian virus 40-transformed human fibroblasts. The cells were cultured in Dulbecco's modified Eagle's medium supplemented with antibiotics and 20% fetal calf serum.
Recombinant adenoviruses and viral infection.
The construction of the adenoviruses and the infection procedures have been described previously (31). Briefly, AdBRCA1 and AdCo1 are nonreplicative E1/E3-defective recombinant adenoviruses carrying a wild-type BRCA1 cDNA or an empty cassette, respectively. The Ad1025 mutational change arose spontaneously and results in expression of the first 1,321 residues of BRCA1 but loss of the C terminus. The optimal multiplicity of infection was determined after infection with control viruses carrying the Escherichia coli LacZ transgene by scoring LacZ-positive blue cells.
GST fusion protein constructs.
The pGST-BRCA1#1 to -#6 plasmids were constructed from human BRCA1 cDNA. The GST-BRCA1#1 to -#6 fusion proteins contain residues 1 to 324, 260 to 553, 502 to 802, 758 to 1064, 1005 to 1313, and 1314 to 1863 of BRCA1, respectively (34). The PXXP motif from residue 1856 in the GST-BRCA1#6 constructs has been mutated by separately replacing prolines with glycines using the primers 5′-CTGGACACCTACCTGATTGCGCAGATCCCCC-3′ for #6-P1856G and 5′-CCTGATACCCCAGATCGCGCACAGCCACTACC-3′ for #6-P1859G with the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.). The mutated constructs were less stably expressed in E. coli than the wild-type constructs, and a 20-fold-greater volume of bacteria was needed to get similar protein levels. The pGST-BRCT tandem plasmid contains a portion of BRCA1 encoding residues 1640 to 1863. All the pGST-cAbl plasmids were constructed from mouse c-Abl type IV cDNA. The SH domains of murine c-Abl type IV are 99% identical to those of human c-Abl (36). The pGST-NSH, pGST-SH3+SH2+kinase, and pGST-SH3 plasmids encode glutathione S-transferase (GST) fusions to c-Abl residues 489 to 543, 15 to 607, and 15 to 138, respectively. The GST-SH3 plasmid was constructed by insertion of a BamHI-HincII fragment of the pGST-SH3+SH2+kinase plasmid into a BamHI-SmaI fragment of pGEX-4T-1 vector (Pharmacia, Bucks, United Kingdom). The GST-CTD fusion protein expressing the C-terminal domain of PolII, which is a substrate for c-Abl kinase (3), was kindly provided by M. Bensaude (Ecole Normale Supérieure, Paris, France).
Irradiation.
Irradiations were performed on ice using a γ-ray 137Cs source (20 Gy at 4 Gy · min−1). Postirradiation incubations were carried out at 37°C.
Use of EA.
Methyl 2,5-dihydroxycinnamate (erbstatin analog [EA]) (Calbiochem, Darmstadt, Germany) is about four times more stable than erbstatin in calf serum (41). Cells treated with EA were incubated in Dulbecco's modified Eagle's medium supplemented with antibiotics, 20% fetal calf serum, and 0.15 μg of EA · ml−1 4 h before irradiation and renewed every hour up to the end of the repair period.
Nuclear extracts.
Nuclear extracts were prepared using a standard protocol with minor modifications (30). Briefly, cells were collected by scraping and rinsed by centrifugation with cold phosphate-buffered saline (1,500 rpm; 200 × g; 4°C; 5 min). The pellet was suspended in hypotonic buffer (10 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride) supplemented with 50 mM NaF, 2 mM sodium orthovanadate, and protease inhibitors (Calbiochem) for 10 min at 4°C. Nuclei were collected by centrifugation (4,000 rpm; 800 × g; 4°C; 10 min) and resuspended in hypertonic buffer (20 mM HEPES [pH 7.9], 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride, 10% glycerol) supplemented with 0.6% NP-40, 50 mM NaF, 2 mM sodium orthovanadate, and protease inhibitors. After being stirred for 15 min at 4°C, the supernatant was collected by centrifugation (10,000 rpm; 1,500 × g; 4°C; 5 min). Aliquots were kept at −70°C. Protein concentrations were estimated using the Bradford assay (Bio-Rad, Hercules, Calif.). Treatment of nuclear extracts with λ-phosphatase (Pharmacia) was performed at 37°C for 20 min as recommended by the manufacturer.
Immunoprecipitation and immunoblotting.
For immunoprecipitation, immune complexes were first precleared by stirring nuclear extracts, protein A-Sepharose beads (Pharmacia), and preimmune immunoglobulin G (IgG) for 30 min at 4°C in NET-N buffer (50 mM Tris [pH 8], 1 mM EDTA, 120 mM NaCl, 0.5% NP-40) supplemented with phosphatase and protease inhibitors (see above). After centrifugation, immunoprecipitation was performed with fresh protein A-Sepharose beads, 300 μg of precleared lysates, and 0.5 to 1 μg of specific monoclonal antibody for 4 h at 4°C. After four washes in NET-N, immunoprecipitates were collected by centrifugation and kept in loading buffer before being separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (see below). Preimmune rabbit (DAKO, Glostrup, Denmark) and mouse (Jackson Immunoresearch, West Grove, Pa.) IgGs were used as controls.
For immunoblotting, 100 μg of cell extracts (unless otherwise stated) was boiled in loading buffer and separated by SDS-PAGE. The protein transfer to nitrocellulose membrane (Schleicher and Schuell, Dassel, Germany) was performed for 4 h with a semidry transfer system (Bio-Rad), and the membrane was blocked overnight in 5% milk TBS-T solution (100 mM Tris [pH 8], 150 mM NaCl, 1% Tween 20) supplemented with 0.1% sodium azide. Primary antibody was applied for 6 h in 2% milk TBS-T solution. Anti-BRCA1 and anti-c-Abl antibodies were from Oncogene Research (Darmstadt, Germany). Anti-p-Tyr (Y-99) antibody was from Santa Cruz Biotechnology (Santa Cruz, Calif.). After the membrane was washed for 1 h in TBS-T, anti-rabbit (DAKO) or anti-mouse (Jackson Immunoresearch) secondary antibody was applied in 2% milk TBS-T solution for 40 min, and the membrane was rewashed against TBS-T for 1 h. Blotting was revealed using an ECL kit (Amersham, Little Chalfont, United Kingdom).
GST fusion protein pull-down assay.
Synthesis of GST fusion proteins was performed as previously described, and protein loading was estimated following electrophoresis and Coomassie blue staining (22). Briefly, bacterial lysates were incubated in GST-Sepharose beads (Pharmacia). After being washed, purified GST fusion proteins (5 to 10 μg) were incubated in nuclear extracts of 293 cells (100 μg) and in NET-N buffer for 1 h at 4°C. GST-bound protein complexes were washed and separated by SDS-PAGE. To examine a direct interaction, the GST fusion proteins were eluted with glutathione as recommended by the manufacturers. The presence of the GST fusion protein was examined by using the anti-GST antibody provided by Pharmacia.
c-Abl tyrosine kinase activity assay.
One hundred micrograms of lysates was subjected to immunoprecipitation with anti-c-Abl antibody (Ab-3) (Oncogene Research) for 2 h at 4°C. Kinase assays were performed by incubating the immunoprecipitates with 2 μCi of [γ-32P]ATP and 20 μM substrate peptide EAIYAAPFAKKK (New England Biolabs, Beverly, Mass.) in c-Abl kinase buffer (50 mM Tris [pH 7.5], 10 mM MnCl2, 1 mM DTT) for 20 min at 30°C (23). Protein A-Sepharose beads bound to 32P-labeled substrates were collected, and 32P activity was quantified by liquid scintillation counting. In all these experiments, the assay was verified by using purified c-Abl protein expressing only the SH2 kinase domain or by immunoprecipitation with the anti-c-Abl (Ab-1; Oncogene Research) antibody that inhibits kinase activity.
RESULTS
BRCA1 and c-Abl interact constitutively.
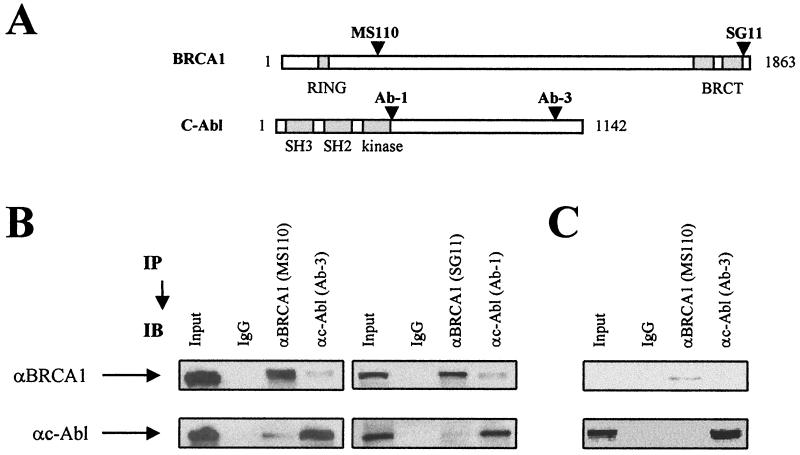
The BRCA1-c-Abl complex was identified by coimmunoprecipitation in nuclear extracts from the BRCA1- and c-Abl-positive cell line 293. Using two anti-BRCA1 antibodies with N-terminal and C-terminal epitopes and two anti-c-Abl antibodies with kinase-adjacent and C-terminal epitopes (Fig. 1A), the presence of c-Abl protein was detected in immunoprecipitated BRCA1 and vice versa (Fig. 1B). HCC1937 (BRCA1-mutated) cells show low levels of mutated BRCA1 but normal levels of c-Abl (Fig. 1C). In these cells, no coimmunoprecipitation was observed, most likely due to the low levels of BRCA1. This demonstrates that coimmunoprecipitation depends upon the presence of BRCA1 and is not due to nonspecific binding of the antibodies (Fig. 1C). Additionally, in column chromatography, BRCA1 and c-Abl coeluted in the 0.5 M KCl residual fraction, consistent with an association between the two proteins (data not shown).
FIG. 1.
Coimmunoprecipitation of BRCA1 and c-Abl. (A) Positions of the epitope domains of the anti-BRCA1 and the anti-c-Abl antibodies used in this study. The MS110 and SG11 antibodies recognize the N-terminal and the C-terminal domain of BRCA1, respectively; the Ab-1 and Ab-3 antibodies recognize the kinase-adjacent domain and the C-terminal domain of c-Abl, respectively. (B) Nuclear extracts from exponentially growing 293 cells were subjected to BRCA1 and c-Abl immunoprecipitation (IP). The immunoprecipitates were analyzed by immunoblotting (IB). The masses of endogenous BRCA1 and c-Abl are 220 and 140 kDa, respectively. αBRCA1, anti-BRCA1 antibody; αc-Abl, anti-c-Abl antibody. (C) Nuclear extracts from exponentially growing HCC1937 cells were subjected to BRCA1 and c-Abl immunoprecipitation.
c-Abl binds to the C-terminal domain of BRCA1.
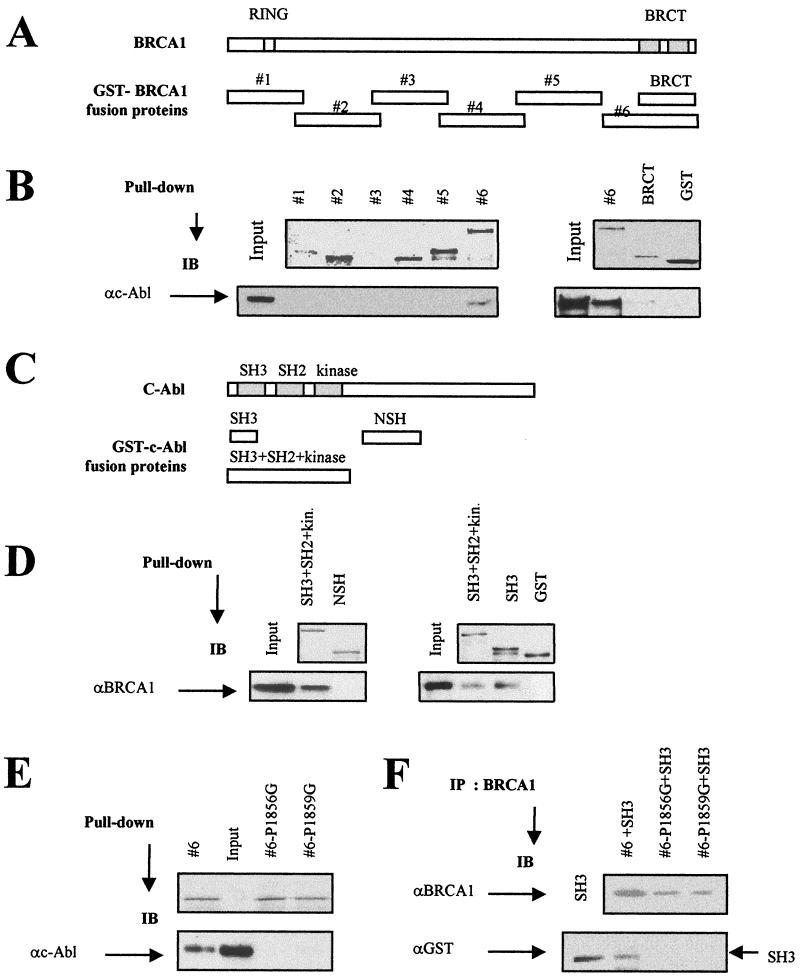
To identify the region of BRCA1 that mediates the interaction with c-Abl, the abilities of GST-tagged deletion constructs of BRCA1 to bind endogenous c-Abl were examined. Synthesis of the GST-BRCA1 fusion proteins was verified using a Coomassie blue-stained SDS-PAGE gel, and the size of each product was as anticipated (Fig. 2A and B). The GST-BRCA1#3 fusion protein was not stably expressed, as found previously (34). Following mixing of the GST-BRCA1 fusion proteins with nuclear extracts from 293 cells, c-Abl protein was detected among proteins interacting with the GST-BRCA1#6 fusion product, suggesting that c-Abl binds specifically to the C-terminal region of BRCA1 (residues 1314 to 1863) (Fig. 2B). The amount of c-Abl protein bound to the BRCT tandem repeat is significantly reduced relative to the BRCA1#6 fusion construct (Fig. 2B), suggesting that sequences adjacent to the BRCT motifs are required for efficient interaction.
FIG. 2.
Localization of the domains involved in the BRCA1-c-Abl complex. (A) Positions of the GST-BRCA1 fusion proteins. (B) Equal amounts (10 μg) of GST fusion proteins (except for GST controls [15 μg]) were analyzed by Coomassie blue-stained gels (top) and incubated with 293 cell nuclear extracts, and the resulting GST adsorbates were subjected to immunoblotting (IB) using anti-c-Abl (αc-Abl; Ab-3) antibody (bottom). The GST-BRCA1#3 protein was found to be unstable, as shown previously (34). (C) Positions of the GST-c-Abl fusion proteins. (D) Equal amounts (5 μg) of GST fusion proteins shown by Coomassie blue-stained gels (top) were incubated with 293 cell nuclear extracts, and the resulting GST adsorbates were subjected to immunoblotting using anti-BRCA1 (αBRCA1; MS110) antibody. (E) Equal amounts (5 μg) of GST fusion proteins shown by Coomassie blue-stained gels (top; the second lane is empty) were incubated with 293 cell nuclear extracts, and the resulting GST adsorbates were subjected to immunoblotting using anti-c-Abl (Ab-3) antibody (bottom). (F) Eluted wild-type and mutated GST-BRCA1#6 (P1856G and P1859G) proteins were mixed with eluted GST-SH3 proteins and subjected to BRCA1 immunoprecipitation using anti-BRCA1 (Ab-5 and Ab-3) antibodies. The presence of the GST-SH3 proteins in the adsorbates was examined by immunoblotting using anti-GST (αGST) antibody.
BRCA1 binds to the N-terminal domain of c-Abl.
The abilities of GST-tagged deletion contructs of c-Abl to bind BRCA1 were investigated to identify the region of c-Abl that mediates interaction with BRCA1. Synthesis of the GST-c-Abl fusion proteins was verified using a Coomassie blue-stained SDS-PAGE gel, and the size of each product was as anticipated (Fig. 2C and D). The GST-c-Abl fusion proteins were incubated with nuclear extracts from 293 cells, and the adsorbates were subjected to BRCA1 immunoblotting. BRCA1 was detected among proteins bound to the SH3 domain and to a fragment encompassing the SH2, SH3, and kinase domains (Fig. 2C and D).
Mutation of the PXXP site in the BRCA1 C terminus abrogates the BRCA1-c-Abl interaction.
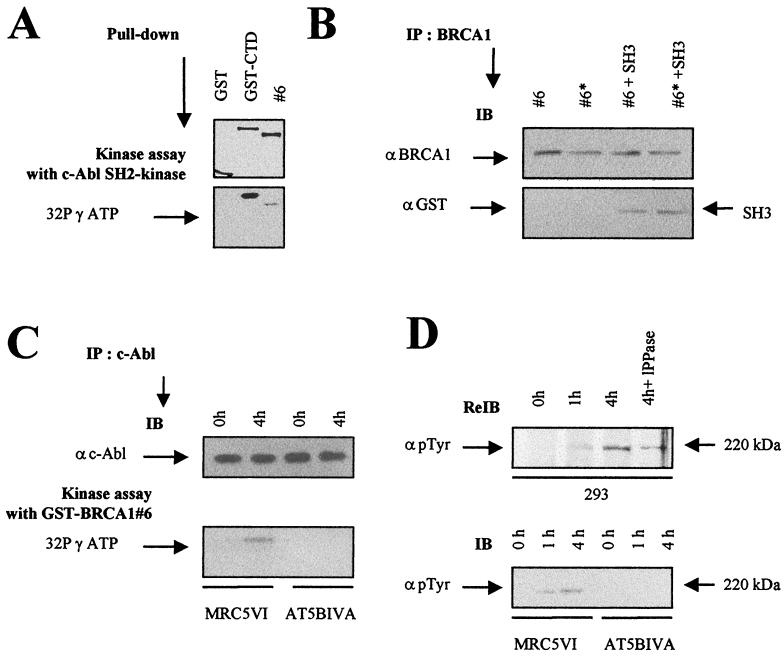
The SH3 domain of c-Abl has a high affinity for proline-rich PXXP sites (28, 36), and significantly, the C terminus of BRCA1 encompasses such a motif at residue 1853. To address whether the PXXP site is essential for the BRCA1-c-Abl interaction, we replaced the first or second proline in this motif in the GST-BRCA1#6 construct with a glycine, producing GST-BRCA1#6-P1856G and -P1859G, respectively. Following mixing of the wild-type or mutant GST-BRCA1#6 fusion protein with nuclear extracts from 293 cells, c-Abl protein was not detected among proteins interacting with either P1856G or P1859G, in contrast to the results obtained with the wild-type fusion protein (Fig. 2E). These findings show that the PXXP site in the C terminus of BRCA1 is essential for the c-Abl-BRCA1 interaction involving the SH3 motif. To investigate whether this represents a direct interaction, we mixed the eluted GST-SH3 fusion protein with either the wild-type or the mutated GST-BRCA1#6 protein. GST-SH3 was present only in the BRCA1 immunoprecipitates using the GST-BRCA1#6 but not the PXXP-mutated form, providing strong evidence that the SH3 domain of c-Abl interacts directly with the PXXP domain in the C terminus of BRCA1 (Fig. 2F).
The BRCA1-c-Abl complex is disrupted after irradiation.
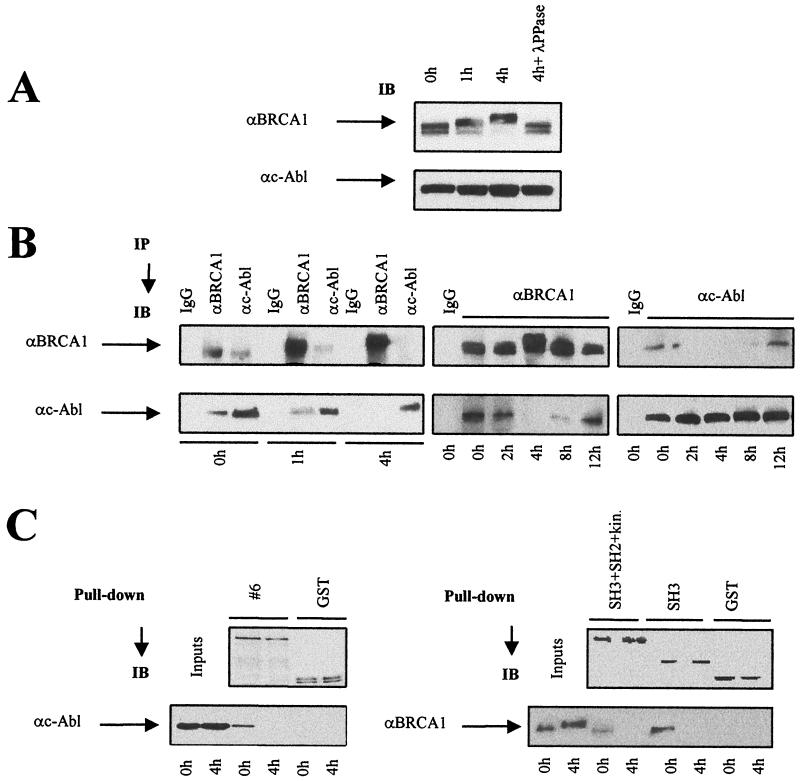
IR induces ATM-dependent BRCA1 phosphorylation (12). From immunoblotting performed with nuclear extracts from irradiated 293 cells, partial phosphorylation of BRCA1 was observed 1 h after irradiation with 20 Gy, and a large shift, representing further phosphorylation event(s), was observed after 4 h (Fig. 3A). The BRCA1 phosphorylation was progressively lost after 8 h, returning to basal levels at 24 h (data not shown). These shifts represent phosphorylation events, since they are not observed following treatment with λ-phosphatase (Fig. 3A). These findings are consistent with previous observations that BRCA1 undergoes phosphorylation at multiple sites following exposure to IR (17, 18).
FIG. 3.
Disruption of the BRCA1-c-Abl complex after irradiation. Control and irradiated (20 Gy) 293 cells were collected at the indicated times. (A) Nuclear extracts were subjected to BRCA1 and c-Abl immunoblotting (IB) with MS110 and Ab-3 antibodies, respectively. λ-PPase, λ-phosphatase. (B) The same batch of extracts was subjected to BRCA1 and c-Abl immunoprecipitation (IP) with MS110 and Ab-3 antibodies, respectively. Preimmune mouse IgGs were used as controls. αc-Abl, anti-c-Abl; αBRCA1, anti-BRCA1. (C) As described in the legend to Fig. 2, purified GST-BRCA1#6, GST-SH3+SH2+kinase, GST-SH3, and GST fusion proteins were incubated with nuclear extracts from irradiated 293 cells; GST adsorbates were collected and subjected to BRCA1 or c-Abl immunoblotting using the same antibodies used for Fig. 2.
We next examined coimmunoprecipitation of BRCA1 and c-Abl in the nuclear extracts of irradiated 293 cells (Fig. 3B). While the BRCA1-c-Abl complex was observed constitutively in unirradiated 293 cells (Fig. 1B) and in 293 cells collected immediately after irradiation on ice (Fig. 3B), the amount of c-Abl in immunoprecipitated BRCA1 and the amount of BRCA1 in the c-Abl immunoprecipitated material decreased from 2 to 8 h after irradiation (20 Gy), indicating that disruption of the BRCA1-c-Abl complex occurs postirradiation (Fig. 3B). This disruption is transient, since reassociation of BRCA1 and c-Abl was observed 12 h postirradiation (Fig. 3B). Our findings demonstrate that the BRCA1-c-Abl complex is transiently disrupted after treatment with 20 Gy of IR.
To investigate further the disruption of the BRCA1-c-Abl complex, pull-down assays were performed by incubating either the GST-BRCA1#6, GST-SH3, or GST-SH3+SH2+kinase fusion protein with extracts from unirradiated and irradiated 293 cells. The interaction of c-Abl with GST-BRCA1#6 was greater in unirradiated cells than in irradiated cells, suggesting a lower affinity of purified C-terminal BRCA1 for c-Abl activated by IR (Fig. 3C). In parallel, the amount of BRCA1 protein bound to GST-SH3+SH2+kinase and GST-SH3 adsorbates was greater in unirradiated cells than in irradiated cells subjected to 4 h of incubation (Fig. 3C).
Finally, to examine whether the BRCA1-c-Abl association is DNA dependent, immunoprecipitations were performed with DNase I-pretreated extracts in the presence of ethidium bromide. DNase I treatment had no effect on the association between BRCA1 and c-Abl, suggesting that formation of the BRCA1-c-Abl complex is not mediated by DNA (data not shown).
The radiation-induced disruption of the BRCA1-c-Abl complex is ATM dependent.
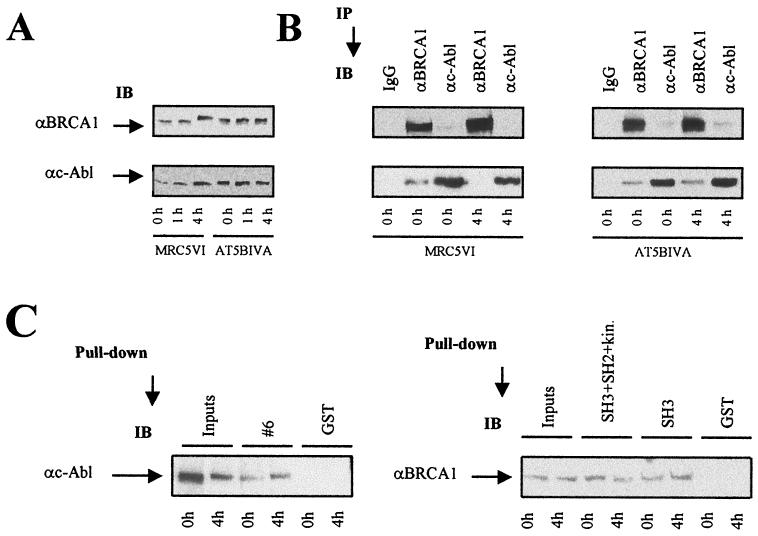
The phosphorylation of BRCA1 and the activation of c-Abl kinase is impaired in ATM−/− cells (4, 12). We therefore investigated BRCA1-c-Abl association and disruption in the ATM−/− AT5BIVA and control MRC5VI simian virus 40-transformed human fibroblast cell lines. BRCA1 phosphorylation was clearly evident in MRC5VI cells following irradiation, although the kinetics were slightly different from those seen in 293 cells (Fig. 3A versus 4A). As anticipated, no phosphorylation of BRCA1 was seen in AT5BIVA fibroblasts following exposure to 20 Gy (Fig. 4A), consistent with previous findings (12, 17). Unlike MRC5VI cells, the radiation-induced disruption of the BRCA1-c-Abl complex was not observed in ATM−/− cells, raising the possibility that radiation-induced phosphorylation of BRCA1 and/or the activation of c-Abl, which is ATM dependent, is required to disrupt the BRCA1-c-Abl complex (Fig. 4B).
FIG. 4.
Disruption of the BRCA1-c-Abl complex is ATM dependent. Control and irradiated (20 Gy) MRC5VI and AT5BIVA fibroblast cells were collected at the indicated times. (A) Nuclear extracts were subjected to BRCA1 and c-Abl immunoblotting (IB) with MS110 and Ab-3 antibodies, respectively. αc-Abl, anti-c-Abl; αBRCA1, anti-BRCA1. (B) The same batch of extracts was subjected to BRCA1 and c-Abl immunoprecipitation (IP) with the same antibodies and preimmune IgGs used for Fig. 3. (C) Equal amounts (10 μg) of purified GST and GST-BRCA1#6 fusion proteins (see the Coomassie blue-stained gel in Fig. 2) were incubated with nuclear extracts from irradiated AT5BIVA cells subjected or not to 4 h of incubation. The resulting GST adsorbates were analyzed by immunoblotting using the anti-c-Abl (Ab-3) antibody. Equal amounts (5 μg) of purified GST, GST-SH3+SH2+kinase, and GST-SH3 fusion protein (see the Coomassie blue-stained gel in Fig. 2) were incubated with nuclear extracts from irradiated AT5BIVA cells subjected or not to 4 h of incubation. The resulting adsorbates were analyzed by immunoblotting with the anti-BRCA1 (MS110) antibody.
To investigate further the disruption of the BRCA1-c-Abl complex in ATM−/− cells, pull-down assays were performed by incubating either GST-BRCA1#6, GST-SH3, or GST-SH3+SH2+kinase fusion protein with extracts from unirradiated and irradiated ATM−/− cells. Using the same fusion protein preparations used with 293 cells (Fig. 2 and 3C), GST-BRCA1#6 interacted with endogenous c-Abl from ATM−/− cells independently of whether the cells were irradiated (Fig. 4C). Similarly, using the GST-SH3+SH2+kinase and GST-SH3 fusion proteins, the interaction with BRCA1 from ATM−/− cells was maintained following irradiation (Fig. 4C).
We also observed a constitutive BRCA1-c-Abl complex and its radiation-induced disruption in HeLa and MCF7 (BRCA1- and c-Abl-positive) cells and in MO59J (DNA-PKcs−/−) and SaOS2 (Rb−/−) tumor cells (data not shown). Moreover, in BRCA2−/− Capan1 tumor cells, the amount of BRCA1-c-Abl complex is considerably reduced, and its radiation-induced disruption occurs earlier than 4 h, raising the possibility that BRCA2 may also participate in the BRCA1-c-Abl association (data not shown).
The radiation-induced disruption of BRCA1-c-Abl complex correlates temporally with the increase of c-Abl tyrosine kinase activity.
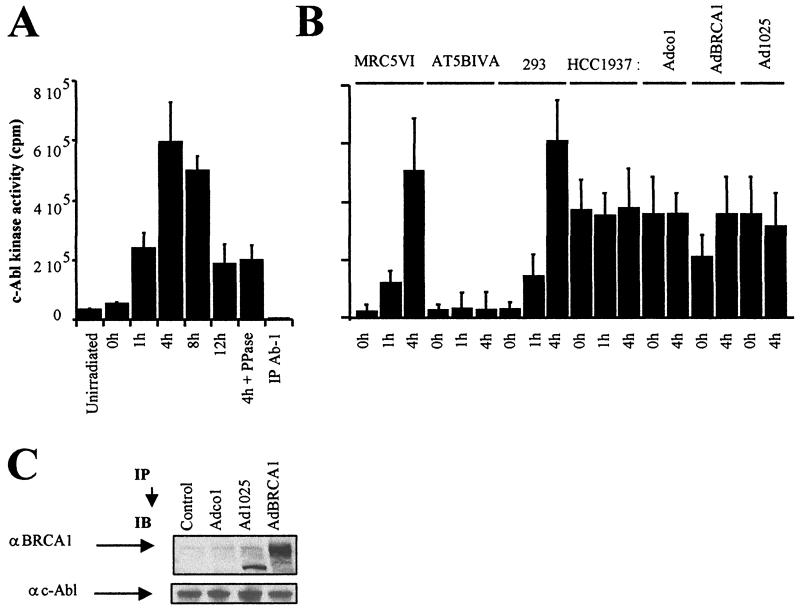
To quantify the endogenous c-Abl tyrosine kinase activity, we immunoprecipitated c-Abl and carried out a kinase assay using a c-Abl autophosphorylation peptide as a substrate (23). Following irradiation, the c-Abl tyrosine kinase activity of 293 cells increased slightly at 1 h, reached a maximum at 4 h, and then decreased after 8 h, declining up to 12 h (Fig. 5A). This correlates temporally with the phosphorylation of BRCA1 observed by immunoblotting and the disruption of the complex (Fig. 3A and B).
FIG. 5.
Tyrosine kinase activity of c-Abl after irradiation. (A) Tyrosine kinase activity of c-Abl measured from c-Abl immunoprecipitates (IP) of nuclear extracts from 293 cells exposed to 20 Gy and collected after irradiation at the indicated times. (B) Tyrosine kinase activity of c-Abl in nuclear extracts from 293, MRC5VI, AT5BIVA (ATM−/−), and HCC1937 (BRCA1-mutated) cells exposed to 20 Gy and incubated for the times indicated. HCC1937 cells were infected by empty cassette (Adco1), wild-type BRCA1 (AdBRCA1) adenoviruses, or adenoviruses expressing only the first 1,321 residues of BRCA1 (Ad1025). All the data presented are the mean plus standard error of triplicate experiments. The assay was controlled, with each sample using an anti-c-Abl (Ab-1) antibody that inhibits kinase activity, as recommended by the manufacturers (see Materials and Methods). We verified that the levels of c-Abl protein immunoprecipitated in all samples were similar (data not shown). (C) As a control for adenovirus expression, nuclear extracts from exponentially growing adenovirus-infected HCC1937 cells were subjected to immunoblotting (IB) using anti-BRCA1 (αBRCA1; MS110) and anti-c-Abl (αc-Abl; Ab-3) antibodies. It is noteworthy that the Ad1025 adenovirus produces a 156-kDa truncated BRCA1 protein.
BRCA1-mutated cells show constitutively elevated c-Abl activity.
If the radiation-induced activation of c-Abl is causally related to the disruption of the BRCA1-c-Abl complex, a prediction is that cells with impaired BRCA1 function should have constitutively high c-Abl tyrosine kinase activity. We therefore examined c-Abl activity in unirradiated and irradiated 293 (BRCA1-positive), HCC1937 (BRCA1-mutated), AT5BIVA (ATM−/−), and control MRC5VI cell extracts. The c-Abl kinase activity measured in ATM−/− cells was low and did not increase significantly following radiation treatment, in agreement with previous reports (Fig. 5B) (4). Significantly, we observed elevated tyrosine kinase activity in HCC1937 cells and no further elevation postirradiation (Fig. 5B).
To verify that this is due to lack of BRCA1 function, HCC1937 cells were infected with adenovirus containing wild-type or mutant BRCA1. In the cells infected with wild-type BRCA1, the spontaneous c-Abl tyrosine kinase activity was decreased, although the activity did not return to the level seen in control cells (Fig. 5B). Although HCC1937 cells are efficiently infected, we have only observed partial correction in all parameters monitored, including cellular radiosensitivity and double-strand break repair (data not shown). As controls, no change in tyrosine kinase activity was observed following infection with an empty cassette or with a mutant Ad1025 adenovirus expressing only the first 1,321 residues of BRCA1 (Fig. 5B). Immunoblotting was carried out to verify expression of BRCA1 in infected cells. The adenovirus infection did not significantly affect the expression of c-Abl (Fig. 5C). Together, these data suggest that BRCA1 expression serves to prevent c-Abl acting as a tyrosine kinase.
The C-terminal domain of BRCA1 is a substrate for c-Abl tyrosine kinase.
It has been reported previously that BRCA1 is modified at tyrosine residues (32, 43, 51). The association of c-Abl with BRCA1 raises the possibility that c-Abl might modify BRCA1 and that this modification might influence the interaction. We, therefore examined whether an SH2 kinase fragment of c-Abl that is sufficient for tyrosine kinase activity (23) is able to phosphorylate the GST-BRCA1#6 fusion protein. A GST-CTD fusion fragment was used as a control substrate. Although the phosphorylation of GST-CTD is stronger, c-Abl efficiently phosphorylated GST-BRCA1#6, suggesting that the C terminus of BRCA1 is an in vitro substrate for the c-Abl kinase activity (Fig. 6A). No significant phosphorylation has been observed for the other GST-BRCA1 fusion proteins used in this study (data not shown). As a first step to investigate whether this modification might cause the disruption of the complex, we also examined whether the tyrosine-phosphorylated GST-BRCA1#6 could interact with GST-SH3. No effect on the interaction was observed (Fig. 6B).
FIG. 6.
C-terminal BRCA1 is a substrate for c-Abl tyrosine kinase. (A) c-Abl tyrosine kinase assay of GST-BRCA1#6 using c-Abl SH2 kinase. Purified c-Abl SH2 kinase (1 μg) was incubated with equal amounts (5 μg) of GST, GST-CTD, and GST-BRCA1#6 fusion proteins as indicated in Materials and Methods (top). The resulting GST adsorbates were subjected to SDS-PAGE and phosphorimager analysis (bottom). (B) SH3 binding of c-Abl-phosphorylated GST-BRCA1#6 substrates. Equal amounts of GST-BRCA1#6, whether tyrosine phosphorylated (#6*) or not (#6) as described below, were subjected to BRCA1 immunoprecipitation (IP) using anti-BRCA1 (αBRCA1; Ab-5 and Ab-3) antibodies. The substrates were mixed with equal amounts of eluted GST-SH3 proteins. The presence of the GST-SH3 proteins in the adsorbates was examined by immunoblotting (IB) using anti-GST (αGST) antibody. (C) c-Abl tyrosine kinase assay of GST-BRCA1#6 using endogenous c-Abl. c-Abl immunoprecipitates of nuclear extracts from MRC5VI and AT5BIVA (ATM−/−) cells exposed to 20 Gy were collected after irradiation at the indicated times (top) in a kinase assay with the GST-BRCA1#6 fusion protein (5 μg) as a substrate. The resulting GST adsorbates were subjected to SDS-PAGE and phosphorimager analysis (bottom). (D) The nitrocellulose membrane used for BRCA1 immunoblotting of 293 cells extracts shown in Fig. 3A was rehybridized by anti-p-Tyr (α p Tyr) tyrosine antibody (top). Nuclear extracts from unirradiated and irradiated MRC5VI and AT5BIVA cells were subjected to p-Tyr immunoblotting (bottom).
The phosphorylation reaction described above utilizes a truncated form of c-Abl. We also examined the ability of endogenous c-Abl to phosphorylate the BRCA1 protein by carrying out a c-Abl kinase assay using GST-BRCA1#6 as a substrate and immunoprecipitated c-Abl derived from unirradiated or irradiated MRC5VI (controls) and AT5BIVA (ATM−/−) fibroblasts as the source of c-Abl (Fig. 6C). Only the c-Abl obtained from irradiated MRC5VI cells was able to phosphorylate GST-BRCA1#6; no significant phosphorylation was observed with unirradiated controls or with either irradiated or unirradiated AT5BIVA cells (Fig. 6C). Thus, c-Abl kinase activated endogenously can also phosphorylate the C terminus of BRCA1. To investigate further the possibility that BRCA1 is phosphorylated by c-Abl tyrosine kinase in vivo, we rehybridized the same membrane used in Fig. 3A with phosphotyrosine-specific antibodies. We observed no constitutive tyrosine phosphorylation of BRCA1 but clear radiation-induced phosphorylation (Fig. 6D, top). Moreover, no tyrosine-phosphorylated BRCA1 was observed in AT5BIVA fibroblasts whether irradiated or not (Fig. 6D, bottom). This result shows that BRCA1 is phosphorylated at tyrosine residues in a radiation- and ATM-dependent manner. Since this correlates with the activation of c-Abl, it strongly implicates c-Abl as the responsible kinase.
Radiation-induced disruption of the BRCA1-c-Abl complex is not dependent upon c-Abl activation.
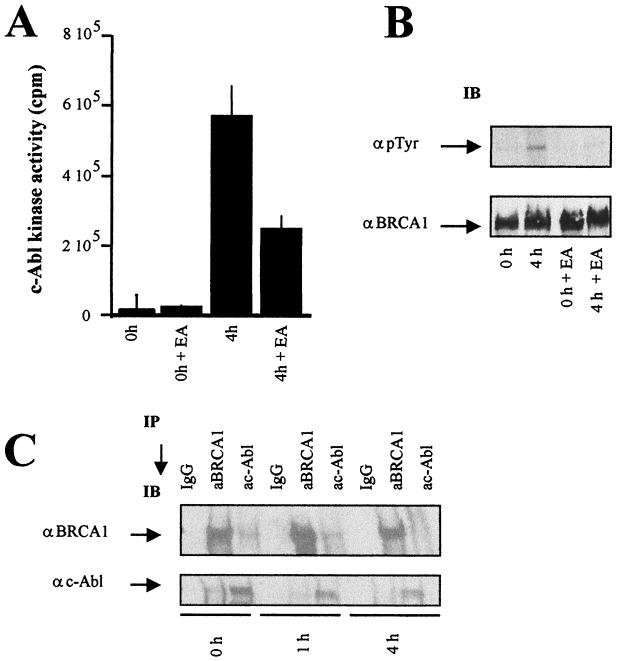
Two phosphorylation events involving BRCA1 may underlie the radiation-induced disruption of the BRCA1-c-Abl complex: ATM-dependent phosphorylation (at serine and threonine residues) or c-Abl-dependent tyrosine phosphorylation that we demonstrated also occurs (see above). The observation that the disruption is not observed in ATM−/− cells is not informative in this context, since neither ATM-dependent BRCA1 phosphorylation nor c-Abl activation occurs in ATM−/− cells. Our findings described above suggest that tyrosine phosphorylation of GST-BRCA1#6 does not affect interaction with the GST-SH3 construct. To address further the role of c-Abl phosphorylation of BRCA1 in the radiation-induced disruption of the BRCA1-c-Abl complex, we exploited the EA to inhibit c-Abl tyrosine kinase activity in 293 cells. EA treatment resulted in a threefold decrease in c-Abl kinase activity 4 h postirradiation and prevented the radiation-induced tyrosine phosphorylation of BRCA1 (Fig. 7A and B). (It is noteworthy that both in the absence and presence of EA, the majority of BRCA1 migrates with a slower mobility postirradiation. However, a small fraction that maintains the mobility of BRCA1 seen in unirradiated cells is visible when analyzed using low-percentage polyacrylamide gels (Fig. 3A). We attribute the mobility shift to multiple ATM-dependent serine and threonine phosphorylation events. The tyrosine-phosphorylated form of BRCA1 identified using the phosphotyrosine-specific antibodies has the mobility of BRCA1 seen in unirradiated cells, suggesting that this represents a distinct, minor fraction of BRCA1 (see Discussion). We next investigated whether EA inhibition of BRCA1 tyrosine phosphorylation affects the radiation-induced disruption of the BRCA1-cAbl complex by examining the presence of c-Abl or BRCA1 in immunoprecipitated BRCA1 or c-Abl material as shown in Fig. 3B. The amount of c-Abl in immunoprecipitated BRCA1 and the amount of BRCA1 in the immunoprecipitated c-Abl material decreased 4 h postirradiation (20 Gy) in the EA-treated cells as efficiently as that seen in cells not exposed to EA (Fig. 7C versus 3B). Hence, we conclude that neither c-Abl activation nor tyrosine phosphorylation of BRCA1 is required for the radiation-induced disruption of the BRCA1-c-Abl complex.
FIG. 7.
Effect of c-Abl kinase inhibition on the BRCA1-c-Abl complex. (A) c-Abl tyrosine kinase activity measured following c-Abl immunoprecipitation of nuclear extracts from untreated and EA-treated 293 cells exposed to 20 Gy and collected at the indicated times postirradiation. The error bars indicate standard deviations. (B) Nuclear extracts from irradiated control or EA-treated 293 cells were subjected to p-Tyr and BRCA1 immunoblotting (IB). α p Tyr, anti-p-Tyr; αBRCA1, anti-BRCA1. (C) Nuclear extracts from control and EA-treated cells were subjected to BRCA1 and c-Abl immunoprecipitation (IP) for BRCA1-c-Abl interaction using MS110 and Ab-3 antibodies, respectively. Preimmune mouse IgGs were used as controls. αc-Abl, anti-c-Abl. The experiments shown in panels B and C were carried out using 4% polyacrylamide gels in contrast to the experiment shown in Fig. 3A, where higher-percentage polyacrylamide gels were used. The difference in mobility shift postirradiation is therefore less pronounced in panels B and C.
DISCUSSION
Multiprotein repair complexes.
BRCA1 appears to be a central component of a large multiprotein complex with binding demonstrated to BRCA2, RAD51 (10, 34), ATM (12), ATR (38), RAD50-MRE11-NBS (52), and a BASC protein component (45). Additionally, the redistribution of BRCA1 in complexes appears to change following replication blockage (11). However, while there is increasing evidence that such complexes are essential for genome surveillance, the biological role and dynamic release of each participant remains to be investigated. Here, a BRCA1-c-Abl complex is identified. Following irradiation and incubation, the BRCA1-c-Abl complex was disrupted in an ATM-dependent manner, which occurred concomitantly with an ATM-dependent increase of c-Abl tyrosine kinase activity and an ATM-dependent phosphorylation of BRCA1.
Specificity of the BRCA1-c-Abl interaction.
The BRCA1-c-Abl interaction has been shown to be mediated by the C terminus of BRCA1 and the N terminus of c-Abl. The C-terminal domain of BRCA1 is suggested to function in transactivation and mediates DNA and protein interactions via the BRCT motifs (20, 47). However, the interaction of c-Abl with the BRCT domain alone appears to be weaker than that observed with a longer C-terminal fragment of BRCA1. Additionally, c-Abl does not interact with the second BRCT domain alone (data not shown). We therefore conclude that the BRCT domains alone are insufficient for optimal c-Abl binding (see below). Following irradiation, c-Abl undergoes ATM-dependent modification such that it is no longer able to bind the C-terminal fragment of BRCA1. We also observed a constitutive interaction between endogenous BRCA1 and the SH3 domain of c-Abl that disappears postirradiation. Thus, the binding of BRCA1 to the SH3 domain of c-Abl correlates with the in vivo interaction between BRCA1 and c-Abl, suggesting that this domain is the mediator of complex formation with BRCA1. The SH3 domain of c-Abl has a high affinity for proline-rich PXXP sites (36), and notably, there is such a motif at residue 1853 in the C terminus of BRCA1 which lies outside the BRCT domain but is present in GST-BRCA1#6. Mutation of either of the two prolines prevents the SH3-BRCA1 interaction (Fig. 2E and F). Taken together, our findings provide evidence for a direct interaction between the PXXP site of BRCA1 and the SH3 domain of c-Abl.
We have shown that c-Abl can phosphorylate the C-terminal domain of BRCA1 in vitro, and we provide evidence that BRCA1 is an in vivo c-Abl substrate, since BRCA1 is tyrosine phosphorylated after radiation treatment in an ATM-dependent manner, which correlates temporally with c-Abl activation. Furthermore, treatment with EA, a specific c-Abl inhibitor (41), prevents this phosphorylation. It is noteworthy that, whereas the major proportion of BRCA1 undergoes a marked mobility shift postirradiation, most likely due to multiple serine and threonine phosphorylation events (Fig. 3A), the phosphotyrosine-modified BRCA1 retains the mobility of BRCA1 present in unirradiated cells. This is consistent with a previous report that BRCA1 tyrosine modifications are less common than serine and threonine phosphorylations and suggests additionally that only a minor fraction of BRCA1 undergoes tyrosine phosphorylation and that this represents a fraction distinct from that receiving serine and threonine phosphorylation (32).
Radiation-induced disruption of the BRCA1-c-Abl complex.
At 4 h following irradiation with 20 Gy, a number of events have taken place in 293 cells: BRCA1 is phosphorylated, the tyrosine activity of c-Abl is activated, and the BRCA1-c-Abl complex is disrupted. None of these events are detected in ATM−/− cells. Our binding studies with the GST fusion proteins suggests that ATM-dependent, radiation-induced modifications to both BRCA1 and c-Abl influence the BRCA1-c-Abl interaction. Previous studies have shown that ATM phosphorylates serine residues of BRCA1 in response to DNA damage (12) and that ATM influences the activation of c-Abl tyrosine kinase after irradiation (4). Our results raise the question whether BRCA1-c-Abl dissociation precedes and causes c-Abl activation or, conversely, c-Abl tyrosine kinase activation causes BRCA1-c-Abl disruption, possibly by tyrosine phosphorylation of BRCA1. In this context, three key observations suggesting that c-Abl activation is a consequence of the disruption of the complex rather than inducing the disruption are as follows. (i) Cells lacking BRCA1 have constitutively elevated c-Abl tyrosine kinase activity. Assuming that c-Abl is not constitutively phosphorylated in BRCA1-mutated cells, this shows that c-Abl activation arises as a consequence of the lack of a BRCA1-c-Abl complex. (ii) Abrogation of c-Abl tyrosine kinase activity by EA does not impact upon BRCA1-c-Abl disruption. (iii) Tyrosine phosphorylation of GST-BRCA1#6 did not affect its interaction with GST-SH3. The observation that only a minor subset of BRCA1 undergoes phosphotyrosine modification further supports this notion.
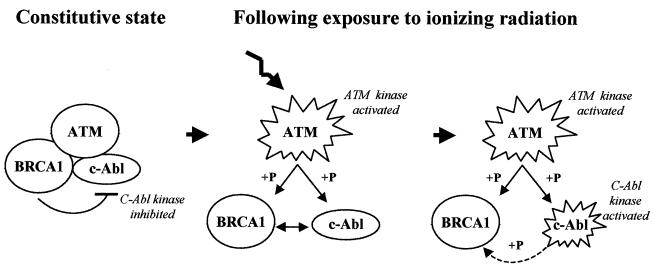
We therefore propose a model in which BRCA1 together with ATM functions to control the tyrosine kinase activity of c-Abl (Fig. 8). In unirradiated cells, BRCA1 and c-Abl are associated in a complex which acts to suppress c-Abl tyrosine kinase activity. Following irradiation, both BRCA1 and c-Abl are phosphorylated at serine residues directly or indirectly by ATM. One or both of these events drives the disruption of the BRCA1-c-Abl complex, which results in activation of c-Abl tyrosine kinase activity. This activity then modifies BRCA1 at tyrosine residues, but this may represent only a minor fraction of BRCA1; its functional significance is unclear, but it is not a driving force in disrupting the BRCA1-c-Abl complex (Fig. 8).
FIG. 8.
Model for the association and disruption of the BRCA1-c-Abl complex and its role. BRCA1 and c-Abl interact constitutively, and c-Abl tyrosine kinase activity is inhibited. After irradiation, ATM kinase is activated, phosphorylating BRCA1 and activating c-Abl, causing disruption of the complex. Consequently, the c-Abl tyrosine kinase activity is stimulated, leading to phosphorylation of a minor subset of BRCA1.
In addition to the radiation-induced activation of c-Abl, c-Abl activity is also activated in S phase. In S phase, Rb is hyperphosphorylated and dissociates from c-Abl (46). BRCA1 has been shown to interact physically with the A-B pocket site of Rb and to release Rb in S phase (1, 42). Our findings raise the possibility that a BRCA1-Rb-c-Abl complex participates in cell cycle regulation and is also disrupted following irradiation. Other multiprotein complexes involving BRCA1 protein have been shown to be disrupted after irradiation. For example, BRCA1 and hCds/Chk2 and BRCA1 and CtIP interact constitutively and dissociate after irradiation in an ATM-dependent manner (26, 27). Together, these findings suggest that BRCA1 and ATM may act to coordinate the regulation of cell cycle control, DNA repair, and apoptosis via the regulation of protein complexes.
Biological function of the BRCA1-c-Abl complex and its disruption.
Our findings show that BRCA1 contributes to the regulation of c-Abl activity. Activation of c-Abl has been reported to have several distinct and potentially disparate effects. First, it has been linked to the onset of apoptosis via the JNK/SAPK pathways (19, 24, 36). Conversely, and probably by a distinct mechanism, activation of c-Abl, for example, in BCR/ABL translocations, results in elevated tumor incidence (13). Additionally, previous observations have linked c-Abl to both of the double-strand break repair pathways, homologous recombination and nonhomologous end joining. On the one hand, c-Abl has been reported to inhibit in vitro the DNA strand exchange properties mediated by RAD51 and to contribute to the disruption of the DNA-protein kinase complex (21, 49). More recently, however, it has been reported that activation of c-Abl upregulates RAD51 expression and homologous recombination activity (37). Either effect could influence the nature and/or fidelity of DNA repair and thereby influence the response to DNA damage. Which of these disparate events takes place may depend upon the tissue or the level of activated c-Abl.
In conclusion, our findings demonstrate that BRCA1 and c-Abl coassociate constitutively and that exposure to IR triggers an ATM-dependent disruption of this BRCA1-c-Abl complex, which coincides with the activation of c-Abl kinase activity. Loss of BRCA1 results in constitutively elevated c-Abl kinase activity, suggesting that BRCA1 is involved in the control of c-Abl kinase activity. This suggests a route by which BRCA1 impacts upon cellular responses to DNA damage distinct from a direct role in DNA repair or a role in cell cycle checkpoint control.
Acknowledgments
We thank J. Feunteun (Institut Gustave-Roussy, Villejuif, France) for stimulating discussions in the initial steps of this work. We thank R. Scully and D. Livingston (Dana Farber Institute, Boston, Mass.) and P.-A. Briand for kindly providing GST-BRCA1 and GST-c-Abl fusion constructs, respectively. We are grateful to A. Chompret and B. Bressac (Institut Gustave-Roussy) for fruitful discussions and Jeannine Cabannes (Institut Gustave-Roussy), F. Mégnin-Chanet, N. Giocanti, M. Fernet, and C. Reis (Institut Curie, Orsay, France) for skillful help.
N.F. is the recipient of a Fondation de la Recherche Médicale and an A.R.C. postdoctoral fellowship. D.P. was supported by the Swiss National Science Foundation and the Canton de Genève. This work was supported by the Fondation de la Recherche Médicale, Electricité de France (Comité de Radioprotection), the Ligue Nationale Contre le Cancer and the A.R.C. Work in the P.J. laboratory contributing to this study was supported by the Leukemia Research Fund.
REFERENCES
- 1.Aprelikova, O. N., B. S. Fang, E. G. Meissner, S. Cotter, M. Campbell, A. Kuthiala, M. Bessho, and R. A. Jensen. 1999. BRCA1-associated growth arrest is RB-dependent. Proc. Natl. Acad. Sci. USA 96:11866-11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barila, D., and G. Superti-Furga. 1998. An intermolecular SH3-domain interaction regulates c-Abl activity. Nat. Genet. 18:280-282. [DOI] [PubMed] [Google Scholar]
- 3.Baskaran, R., M. E. Dahmus, and J. Y. Wang. 1993. Tyrosine phosphorylation of mammalian RNA polymerase II carboxyl-terminal domain. Proc. Natl. Acad. Sci. USA 90:11167-11171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baskaran, R., L. D. Wood, L. L. Whitaker, C. E. Canman, S. E. Morgan, Y. Xu, C. Barlow, D. Baltimore, A. Wynshaw-Boris, M. B. Kastan, and J. Y. J. Wang. 1997. Ataxia telangiectasia mutant protein activates c-Abl tyrosine kinase in response to ionizing radiation. Nature 387:516-519. [DOI] [PubMed] [Google Scholar]
- 5.Bressan, D. A., B. K. Baxter, and J. H. Petrini. 1999. The Mre11-Rad50-Xrs2 protein complex facilitates homologous recombination-based double-strand break repair in Saccharomyces cerevisiae. Mol. Cell. Biol. 11:7681-7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brzovic, P. S., P. Rajagopal, D. W. Hoyt, M. C. King, and R. E. Klevit. 2001. Structure of a BRCA1-BARD1 heterodimeric RING-RING complex. Nat. Struct. Biol. 8:833-837. [DOI] [PubMed] [Google Scholar]
- 7.Callebaut, I., and J. P. Mornon. 1997. From BRCA1 to RAP1: a widespread BRCT module closely associated with DNA repair. FEBS Lett. 400:25-30. [DOI] [PubMed] [Google Scholar]
- 8.Cantor, S. B., D. W. Bell, S. Ganesan, E. M. Kass, R. Drapkin, S. Grossman, D. C. R. Wharer, D. C. Sgroi, W. S. Lane, and D. A. Haber. 2001. BACH1, a novel helicase-like protein, interacts directly with BRCA1 and contributes to its DNA repair function. Cell 105:149-160. [DOI] [PubMed] [Google Scholar]
- 9.Chen, J., D. P. Silver, D. Walpita, S. B. Cantor, A. F. Gazdar, G. Tomlinson, F. J. Couch, T. Ashley, D. M. Livingston, and R. Scully. 1998. Stable interaction between the products of the BRCA1 and BRCA2 tumor suppressor genes in mitotic and meiotic cells. Mol. Cell 2:317-328. [DOI] [PubMed] [Google Scholar]
- 10.Chen, J. J., D. Silver, S. Cantor, D. M. Livingston, and R. Scully. 1999. BRCA1, BRCA2 and Rad51 operate in a common DNA damage response pathway. Cancer Res. 59:1752s-1756s. [PubMed]
- 11.Chiba, N., and J. D. Parvin. 2001. Redistribution of BRCA1 among four different protein complexes following replication blockage. J. Biol. Chem. 276:38459-38554. [DOI] [PubMed] [Google Scholar]
- 12.Cortez, D., Y. Wang, and J. Qin. 1999. Requirement of ATM-dependent phosphorylation of Brca1 in the DNA damage response to DNA double-strand breaks. Science 286:1162-1166. [DOI] [PubMed] [Google Scholar]
- 13.Daley, G. Q., and Y. Ben-Neriah. 1991. Implicating the brc/abl gene in the pathogenesis of Philadelphia chromosome-positive human leukemia. Adv. Cancer Res. 57:151-184. [DOI] [PubMed] [Google Scholar]
- 14.Deng, C.-X., and S. G. Brodie. 2000. Roles of BRCA1 and its interacting proteins. Bioessays 22:726-737. [DOI] [PubMed] [Google Scholar]
- 15.Easton, D. F., D. T. Bishop, D. Ford, G. P. Crockford, and The Breast Cancer Consortium. 1993. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. Am. J. Hum. Genet. 52:678-701. [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Higuera, I., T. Taniguchi, S. Ganesan, M. S. Meyn, C. Timmers, J. Hejna, A. Grompe, and M. D'Andrea. 2001. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol. Cell 7:249-262. [DOI] [PubMed] [Google Scholar]
- 17.Gatei, M., S. P. Scott, I. Filippovitch, N. Soronika, M. F. Lavin, B. Weber, and K. K. Khanna. 2000. Role for ATM in DNA damage-induced phosphorylation of BRCA1. Cancer Res. 60:3299-3304. [PubMed] [Google Scholar]
- 18.Gatei, M., B. B. Zhou, K. Hobson, S. Scott, D. Young, and K. K. Khanna. 2001. Ataxia telangiectasia mutated (ATM) kinase and ATM and Rad3 related kinase mediate phosphorylation of Brca1 at distinct and overlapping sites. In vivo assessment using phospho-specific antibodies. J. Biol. Chem. 276:17276-17280. [DOI] [PubMed] [Google Scholar]
- 19.Harkin, D. P., J. M. Bean, D. Miklos, Y. H. Song, V. B. Truong, C. Englert, F. C. Christians, L. W. Ellisen, S. Maheswaran, J. D. Oliner, and D. A. Haber. 1999. Induction of GADD45 and JNK/SAPK-dependent apoptosis following inducible expression of BRCA1. Cell 97:575-586. [DOI] [PubMed] [Google Scholar]
- 20.Huyton, T., P. A. Bates, X. Zhang, M. J. E. Sterberg, and P. S. Freemont. 2000. The BRCA1 C-terminal domain: structure and function. Mutat. Res. 460:319-332. [DOI] [PubMed] [Google Scholar]
- 21.Jin, S., S. Kharbanda, B. Mayer, D. Kufe, and D. T. Weaver. 1997. Binding of Ku and c-Abl at the kinase homology region of DNA-dependent protein kinase catalytic subunit. J. Biol. Chem. 272:24763-24766. [DOI] [PubMed] [Google Scholar]
- 22.Kaelin, W. G. J., W. Krek, W. R. Sellers, J. A. DeCaprio, F. Ajchenbaum, C. S. Fuchs, T. Chittenden, Y. Li, P. J. Farnham, et al. 1992. Expression cloning of a cDNA encoding a retinoblastoma-binding protein with E2F-like properties. Cell 70:351-364. [DOI] [PubMed] [Google Scholar]
- 23.Kharbanda, S., R. Ren, P. Pandey, T. D. Shafman, S. M. Feller, R. R. Weichselbaum, and D. W. N. Kufe. 1995. Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature 376:785-788. [DOI] [PubMed] [Google Scholar]
- 24.Kharbanda, S., Z. M. Yuan, R. Weichselbaum, and D. Kufe. 1998. Determination of cell fate by c-Abl activation in the response to DNA damage. Oncogene 17:3309-3318. [DOI] [PubMed] [Google Scholar]
- 25.Kinzler, K. W., and B. Vogelstein. 1997. Cancer-susceptibility genes: gatekeepers and caretakers. Nature 386:761-763. [DOI] [PubMed] [Google Scholar]
- 26.Lee, J. S., K. M. Collins, A. L. Brown, C. H. Lee, and J. H. Chung. 2000. hCds1-mediated phosphorylation of BRCA1 regulates the DNA damage response. Nature 404:201-204. [DOI] [PubMed] [Google Scholar]
- 27.Li, S., N. S. Ting, L. Zheng, P. L. Chen, Y. Ziv, Y. Shiloh, E. Y. Lee, and W. H. Lee. 2000. Functional link of BRCA1 and ataxia telangiectasia gene product in DNA damage response. Nature 406:210-215. [DOI] [PubMed] [Google Scholar]
- 28.Mayer, B. J., and D. Baltimore. 1994. Mutagenic analysis of the roles of SH2 and SH3 domains in regulation of the Abl tyrosine kinase. Mol. Cell. Biol. 14:2883-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayer, B. J., P. K. Jackson, and D. Baltimore. 1991. The noncatalytic src homology region 2 segment of abl tyrosine kinase binds to tyrosine-phosphorylated cellular proteins with high affinity. Proc. Natl. Acad. Sci. USA 88:627-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olnes, M. I., and R. N. Kurl. 1994. Isolation of nuclear extracts from fragile cells: a simplified procedure applied to thymocytes. BioTechniques 17:828-829. [PubMed] [Google Scholar]
- 31.Randrianarison, V., D. Marot, N. Foray, J. Cabannes, V. Meret, E. Connault, N. Vitrat, P. Opolon, M. Perricaudet, and J. Feunteun. 2001. BRCA1 carries tumor suppressor activity distinct from that of p53 and p21. Cancer Gene Ther. 8:759-770. [DOI] [PubMed] [Google Scholar]
- 32.Ruffner, H., and I. M. Verma. 1997. BRCA1 is a cell cycle-regulated nuclear phosphoprotein. Proc. Natl. Acad. Sci. USA 94:7138-7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scully, R., J. Chen, R. L. Ochs, K. Keegan, M. Hoekstra, J. Feunteun, and D. M. Livingston. 1997. Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell 90:425-435. [DOI] [PubMed] [Google Scholar]
- 34.Scully, R., J. Chen, A. Plug, Y. Xiao, D. Weaver, J. Feunteun, T. Ashley, and D. M. Livingston. 1997. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell 88:1-20. [DOI] [PubMed] [Google Scholar]
- 35.Shafman, T., K. K. Khanna, P. Kedar, K. Spring, S. Kozlov, T. Yen, K. Hobson, M. Gatei, N. Zhang, D. Watters, M. Egerton, Y. Shiloh, S. Kharbanda, D. Kufe, and M. F. Lavin. 1997. Interaction between ATM protein and c-Abl in response to DNA damage. Nature 387:520-522. [DOI] [PubMed] [Google Scholar]
- 36.Shaul, Y. 2000. C-Abl: activation and nuclear targets. Cell Death Differ. 7:10-16. [DOI] [PubMed] [Google Scholar]
- 37.Slupianek, A., C. Schmutte, G. Tombline, M. Nieborowska-Skorska, G. Hoser, M. O. Nowicki, A. J. Pierce, R. Fishel, and T. Skroski. 2001. BCR/ABL regulates mammalian RecA homologs, resulting in drug resistance. Mol. Cell 8:795-806. [DOI] [PubMed] [Google Scholar]
- 38.Tibbetts, R. S., D. Cortez, K. M. Brumbaugh, R. Scully, D. Livingston, S. J. Elledge, and R. T. Abraham. 2000. Functional interactions between BRCA1 and the checkpoint kinase ATR during genotoxic stress. Genes Dev. 14:2989-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomlinson, G. E., T. T. Chen, V. A. Stastny, M. A. Spillman, V. Tonk, J. L. Blum, N. R. Schneider, I. I. Wistuba, J. W. Shay, J. D. Minna, and A. F. Gazdar. 1999. Characterization of a breast cancer cell line derived from a germ-line BRCA1 mutation carrier. Cancer Res. 58:3237-3242. [PubMed] [Google Scholar]
- 40.Tybulewicz, V. L., C. E. Crawford, P. K. Jackson, R. T. Bronson, and R. C. Mulligan. 1991. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-Abl proto-oncogene. Cell 65:1153-1163. [DOI] [PubMed] [Google Scholar]
- 41.Umezawa, K., and M. Imoto. 1991. Use of erbstatin as protein-tyrosine kinase inhibitor. Methods Enzymol. 201:379-385. [DOI] [PubMed] [Google Scholar]
- 42.Wang, A., R. Schneider-Broussard, A. P. Kumar, M. C. MacLeod, and D. G. Johnson. 2000. Regulation of BRCA1 expression by the Rb-E2F pathway. J. Biol. Chem. 275:4532-4536. [DOI] [PubMed] [Google Scholar]
- 43.Wang, H., N. Shao, Q. Ming Ding, J.-Q. Cui, E. Shyam, P. Reddy, and V. N. Rao. 1997. BRCA1 proteins are transported to the nucleus in the absence of serum and splice variants BRCA1a, BRCA1b are tyrosine phosphoproteins that associate with E2F, cyclins and cyclin dependent kinases. Oncogene 15:143-157. [DOI] [PubMed] [Google Scholar]
- 44.Wang, J. Y. 2001. Regulation of cell death by the Abl tyrosine kinase. Oncogene 19:5643-5650. [DOI] [PubMed] [Google Scholar]
- 45.Wang, T., D. Cortez, P. Yazdi, N. Neff, S. J. Elledge, and J. Qin. 2000. BASC, a super complex of BRCA1-associated proteins involved in the recognition and repair of aberrant DNA structures. Genes Dev. 14:927-939. [PMC free article] [PubMed] [Google Scholar]
- 46.Welch, P. J., and Y. J. Wang. 1993. A C-terminal protein-binding domain in the retinoblastoma protein regulates nuclear c-Abl tyrosine kinase in the cell cycle. Cell 75:779-790. [DOI] [PubMed] [Google Scholar]
- 47.Williams, R. S., R. Green, and J. N. Glover. 2001. Crystal structure of the BRCT repeat region from the breast cancer-associated protein BRCA1. Nat. Struct. Biol. 8:838-842. [DOI] [PubMed] [Google Scholar]
- 48.Young, M. A., S. Gonfloni, G. Superti-Furga, B. Roux, and J. Kuriyan. 2001. Dynamic coupling between the SH2 and SH3 domains of c-Src and Hck underlies their inactivation by C-terminal tyrosine phosphorylation. Cell 105:115-126. [DOI] [PubMed] [Google Scholar]
- 49.Yuan, Z.-M., Y. Huang, T. Ishiko, S. Nakada, T. Utsugisawa, S. Kharbanda, R. Wang, P. Sung, A. Shinohara, R. Weichselbaum, and D. Kufe. 1998. Regulation of Rad51 function by c-Abl in response to DNA damage. J. Biol. Chem. 13:3799-3802. [DOI] [PubMed] [Google Scholar]
- 50.Zhang, H., G. Tombline, and B. L. Weber. 1998. BRCA1, BRCA2 and DNA damage response: collision or collusion? Cell 92:433-436. [DOI] [PubMed] [Google Scholar]
- 51.Zhang, H. T., X. Zhang, H. Z. Zhao, Y. Kajino, B. L. Weber, J. G. Davis, Q. Wang, D. M. O'Rourke, H. B. Zhang, K. Kajino, and M. I. Greene. 1997. Relationship of p215 BRCA1 to tyrosine kinase signaling pathways and the cell cycle in normal and transformed cells. Oncogene 14:2863-2869. [DOI] [PubMed] [Google Scholar]
- 52.Zhong, Q., C. F. Chen, S. Li, Y. Chen, C. C. Wang, J. Xiao, P. L. Chen, Z. D. Sharp, and W. H. Lee. 1999. Association of BRCA1 with the hRad50-hMre11-p95 complex and the DNA damage response. Science 285:747-750. [DOI] [PubMed] [Google Scholar]