Abstract
The stable maintenance of the 2μm circle plasmid depends on its ability to overcome intrinsic maternal inheritance bias, which in yeast normally results in the failure to transmit DNA molecules efficiently to daughter cells. In addition to the plasmid proteins Rep1 and Rep2 acting on the plasmid DNA locus STB, it is likely that other chromosomally encoded yeast proteins are required. We have isolated mutants of yeast unable to maintain 2μm and found that RSC2 is essential for 2μm to overcome maternal inheritance bias. Rsc2 is part of a multisubunit RSC chromatin remodeling complex, and we show that in the absence of Rsc2 the chromatin structure of the STB region is significantly altered and the Rep1 protein loses its normal localization to subnuclear foci. Rsc1, a closely related homolog of Rsc2 present in an alternative form of the RSC complex, is not required for 2μm maintenance and does not replace the requirement for Rsc2 when overexpressed. This represents the first specific role for Rsc2 that has been related to a change in chromatin structure, as well as the first direct evidence linking chromatin structure to 2μm segregation.
The 2μm circle is a high-copy-number plasmid widespread in laboratory and industrial strains of Saccharomyces cerevisiae that has been used as a molecular model for aspects of cellular function such as DNA replication, gene transcription, and recombination, as well as the basis of yeast cloning vectors. High-copy-number plasmids carrying only replication origins show maternal inheritance bias (MIB) and are poorly segregated to daughter cells (31), whereas 2μm and its derivatives are effectively segregated between mother and daughter cells at division (22).
However, the mechanism by which 2μm escapes MIB resulting in equal segregation is not fully understood, nor have all of the host components involved been identified. Of the four genes carried by the plasmid, REP1 and REP2 are necessary for MIB to be overcome, and their products act at the plasmid locus STB to confer plasmid stability (2, 10, 18, 22, 33, 36). Within the nucleus, Rep1 and Rep2 proteins are localized into discrete foci (39) in which the plasmid is also colocalized (39, 52). These plasmid and protein foci are segregated in a manner similar to that of the chromosomes themselves, a finding consistent with the observation that the 2μm plasmid is packaged into nucleosomes (50). Thus, the plasmid may be exploiting the chromosomal segregation machinery indirectly by associating with specific or nonspecific chromosomal sites. It has been therefore suggested that the segregation of 2μm, and hence its overcoming of MIB, likely requires host (non-plasmid-encoded) genes (18). One candidate is the product of the SHF1 (CST6) gene, which has been shown to bind to STB in vivo and in vitro (51).
Nucleosomes regulate and modify the ability of proteins to interact with DNA, and the remodeling of chromatin and repositioning of nucleosomes is important in gene regulation and other functions of DNA. There is evidence that specific chromatin structures are important in 2μm functions, since the STB region has been reported to be relatively free of nucleosomes (15, 25), and changes occur in nucleosome positioning in the presence of 2μm gene products (50). One class of chromatin remodeling complex is typified by the SWI/SNF and RSC complexes, which contain 8 to 15 subunits (6). SWI/SNF is required for the regulated expression of about 200 yeast genes (35, 44) but is nonessential and of relatively low abundance.
Components of the RSC complex were first isolated as homologs of the SWI/SNF chromatin remodeling complex but, unlike those of the SWI/SNF complex, most of the components of RSC are essential for growth (7, 9, 24, 47). The complex is 10-fold more abundant than the SWI/SNF complex (7) and comprises at least 15 subunits. Sth1 is an essential ATPase in RSC that is required for alteration of the chromatin structure at the centromeres (13, 48), repression of the CHA1 gene (30), and maximal expression of early meiotic genes (54). In addition, both Sth1 and another component, Sfh1, are required for mitotic cell cycle progression (9, 24, 49).
At least two forms of the RSC complex have been identified that have both shared core components and unique subunits. Both Sth1 and Sfh1 are core components, but two proteins found in separate complexes are Rsc1 and Rsc2 (8). Rsc1 and Rsc2 are 45% identical and share the same domain structure, and both contain two bromodomains. Although the rsc1Δ rsc2Δ double null mutant is nonviable, the single nulls have relatively mild growth phenotypes, suggesting that there is a high level of redundancy between the two complexes as well as potential unique roles (8).
We have used a genetic screen to isolate mutants deficient in the maintenance of the 2μm plasmid. We show that, surprisingly, five of six mutants isolated have mutations in RSC2. Loss of RSC2 function results in a failure to effectively overcome MIB, and hence cells are defective in 2μm plasmid maintenance. This plasmid instability correlates with significant changes in the chromatin structure at the STB locus and a loss of the normal localization of the Rep proteins. In contrast, loss of the RSC2 homolog RSC1 does not affect 2μm plasmid maintenance. This identifies the 2μm plasmid as uniquely requiring Rsc2 and provides the first evidence for a specific molecular target for the Rsc2-containing complex.
MATERIALS AND METHODS
Strains, growth procedures, and mutagenesis.
S. cerevisiae GY59 MATα ade1 his3 leu2 trp1 ura3 [cir0] and GY18 MATα ade1-101 leu2-3,112 his3-11,15 [cir+] (derived from LL20 [16]), A363A MATa ade1 ade2 ura1 his7 lys2 tyr1 gal1 [cir+] (19), W303 MATa ade2 his3 leu2 trp1 ura3 can1-100 (45), and mutant derivatives were grown as previously described at 30°C unless otherwise stated (39). We found only a marginal growth disadvantage for rsc2 mutants at this temperature. YPD medium (1% yeast extract, 2% Bacto Peptone, and 2% d-glucose) was supplemented with Geneticin G418 (Gibco) at 200 μg/ml for selection of KanMX gene disruptants (1). YPGal has glucose replaced by 2% d-galactose. GY59 carrying plasmid 2μm-ADE1 was mutagenized with ethyl methanesulfonate as described previously (46). Mutants were screened for plasmid loss by the qualitative colony sectoring assay described below.
Plasmids.
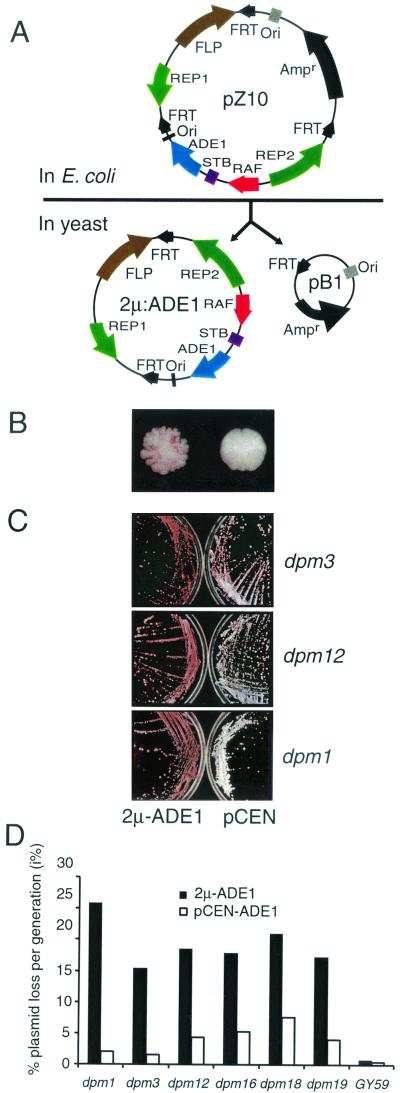
Plasmid pZ10 carries one complete copy of the 2μm genome, plus a duplication of one of the inverted repeat sequences, and a 1.4-kb fragment spanning the ADE1 gene inserted within the unique 2μm sequences at the SnaBI site which does not disrupt 2μm plasmid maintenance significantly (4, 11). In addition, pZ10 carries sequences for propagation in Escherichia coli, positioned within the plasmid such that the action of the 2μm Flp recombinase is to excise the bacterial sequences, leaving the plasmid 2μm-ADE1. 2μm-ADE1 is identical to wild-type 2μm plasmid except for the ADE1 insertion and carries no bacterium-derived or other duplicated sequences (Fig. 1A ). pCEN-ADE1 carries a 1.6-kb fragment spanning ADE1 cloned between the XhoI-HindIII sites of pRS313 (41). pASF60 (43) contains multiple copies of the lac operator cloned into the KpnI-SphI sites of plasmid YEplac181a, and pASF135 (43) carries the Lacr lac repressor encoding gene fused to green fluorescent protein (GFP) inserted between the NotI-XhoI sites of pRS303. The series of centromeric plasmids expressing RSC2 with truncated or mutated domains were a gift of B. R. Cairns (8). pCM5 and pCM13 carry RSC2 and RSC1 inserted in the galactose-controlled expression vector pYX143 (R&D Systems, Abingdon, United Kingdom).
FIG. 1.
Characterization of mutants unable to stably maintain 2μm-type plasmids. (A) pZ10 carries the complete 2μm plasmid genome plus a duplication of one inverted repeat containing the Flp recognition target (FRT) site inserted in the E. coli vector pUC7. The ADE1 gene is inserted in 2μm sequences between STB and Ori at the unique SnaBI site. FLP-mediated recombination in yeast excises 2μm- ADE1 carrying a wild-type 2μm plasmid except for the ADE1 insertion. (B) Colony phenotypes of dpm19 (left) and wild-type (right) GY59 colonies containing 2μm-ADE1. (C) dpm mutants show a much higher frequency of pink (ade−) sectors and colonies with 2μm-ADE1 (left) than with pCEN-ADE1, as shown by the overall pink color developed on plates. (D) Plasmid stability assay of dpm mutants carrying 2μm-ADE1 and the centromeric plasmid pCEN-ADE1.
Colony-sectoring (plasmid-loss) assay.
The loss of 2μm-ADE1 was monitored in an ade1 genetic background by accumulation of red pigment due to the absence of ADE1 in plasmid-free cells. Yeast cells were grown on YPD, and the red color was allowed to develop at 4°C for at least 1 week. Qualitative assays (as used for mutant screening) were based on examination of the frequency of sectoring. For quantitative analysis of loss rates, half-sector analysis of loss rate of 2μm-ADE1 was carried out as described previously (20). This measures the loss of plasmid in one generation, since sectors encompassing half of a complete colony arise from loss in the first division that occurs in the formation of a colony from a single cell.
Loss of native 2μm plasmid was monitored by PCR. Plasmid 2μm was detected by the REP1 amplifying primers (forward primer, ACAGCGCTGATATACAATG; reverse primer, CTGTCGGCTATTATCTCCG) and the presence of the disruption (KanMX) sequence by using internal primers (forward, TAGCCCATACATCCCCATGT; reverse, ACAGGAATCGAATGCAACCG). Single colonies were picked as a template for the amplification reactions, resuspended in a PCR mixture containing 2.5 U of Taq DNA polymerase (Roche), Taq buffer 1, 0.2 μM concentrations of each deoxynucleoside triphosphate, and a 1 μM concentration of each primer, and then cycled as follows: 94°C (10 min), followed by 30 cycles of 94°C (30 s), 55°C (1 min), and 72°C (5 min), with a final 7 min at 72°C.
Complementation of the dpm19 mutant.
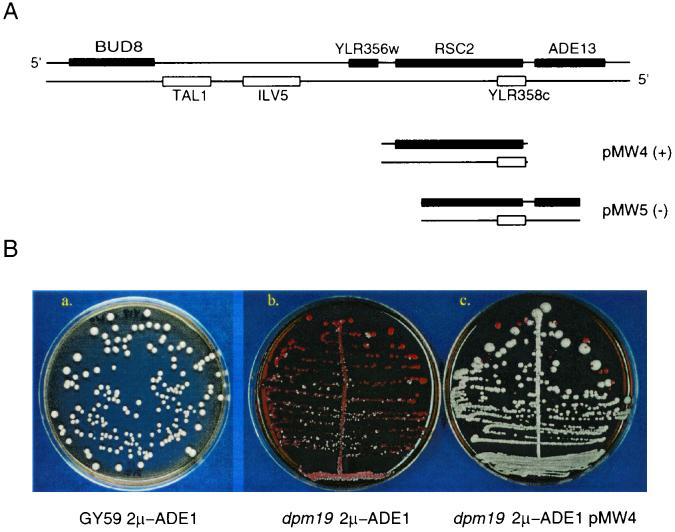
The dpm19 mutant was complemented with a yeast genomic library in the centromere-containing plasmid YCp50 (37). Since dpm19 loses 2μm-ADE1 rapidly, pZ10 and the library plasmid were used to cotransform dpm19 as described previously (17). Transformants were plated on minimal medium lacking adenine and uracil to select for both 2μm-ADE1 and YCp50. A complementing fragment of 9.3 kb was isolated and designated pMW1. Deletion derivatives identified the complementing gene as RSC2 (Fig. 2A).
FIG. 2.
Characterization of cloned genomic library fragments. (A) Plasmids pMW4 and pMW5 were derived from the complementing clone pMW1, which carries the 9.3-kb fragment (shown at top). The “+” indicates subclones complementing the dpm19 mutant phenotype; the “−” indicates subclones unable to complement dpm19. (B) The subclones derived from pMW1 were tested for their ability to reestablish plasmid stability in dpm19. Subpanels: a, wild-type GY59; b, dpm19 mutant; c, dpm19 mutant with complementing pMW1 clone (all carrying 2μm-ADE1).
PCR-mediated gene disruption.
PCR-mediated gene disruption was as described previously (3). RSC2 was disrupted with the KanMX gene (conferring Geneticin resistance) or TRP1. Details of primers and PCR conditions used can be obtained upon request. PCR fragments were used to generate yeast transformants, which were selected on YPD containing Geneticin or synthetic dextrose dropout (1) medium lacking tryptophan. Disruptants were confirmed with primers external and internal to the disruption cassette and both boundaries.
Chromatin analysis.
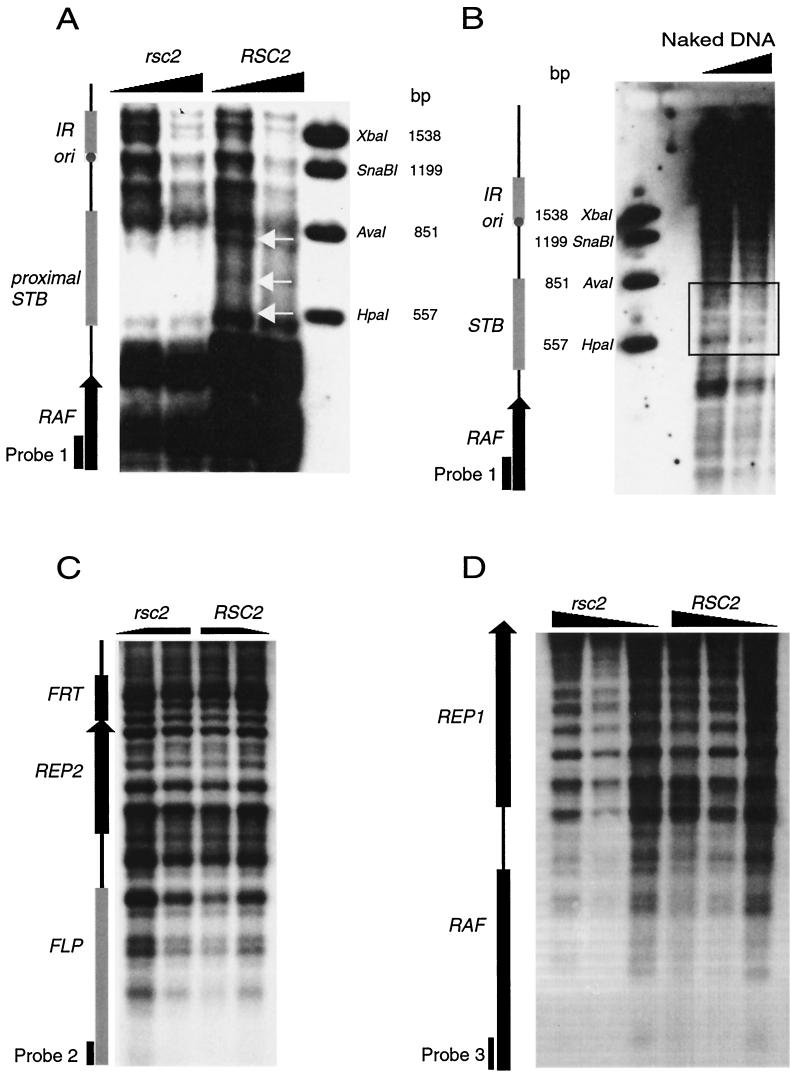
Micrococcal nuclease (MNase) analysis of plasmid DNA was carried out as described previously (29, 50) with minor modifications. Yeast cells were washed once with cold water, resuspended in 5 ml of lysis buffer (1 M sorbitol plus 5 mM 2-mercaptoethanol containing 2 mg of 100T Zymolyase [Seikagaku Corp., Tokyo, Japan]) per 1 g (wet weight) of cells, and incubated at 30°C for 20 min. Pelleted cells were washed and resuspended in 7 ml of Ficoll solution (18 [wt/vol] Ficoll type 400 [Sigma], 20 mM KH2PO4 [pH 6.8], 1 mM MgCl2, 0.25 mM EGTA, 0.25 mM EDTA) per 1 g (wet weight) of cells. Cells were centrifuged at 16,000 rpm in a Sorvall SS34 rotor (30 min at 5°C). The nuclear pellet was digested in 1.2 ml of digestion buffer (15 mM Tris-HCl [pH 7.5], 75 mM NaCl, 3 mM MgCl2, 1.5 mM CaCl2, 1 mM 2-mercaptoethanol). Then, 200-μl aliquots were transferred to microfuge tubes, digested with 0.125 to 1 U of MNase (Worthington Biochemical Corp. catalog no. 4797; stock solution dissolved in MNase buffer [50 mM Tris-HCl, pH 8.0; 0.5 mM CaCl2; 20% glycerol] to a concentration of 100 U/μl), and digested for 10 min at 37°C. Digestion was terminated with 30 μl of stop solution (1% sodium dodecyl sulfate [SDS] and 5 mM EDTA, final concentration) and 30 μl of proteinase K (0.5 mg/ml) at 55°C for 2 h. Purified DNA was resuspended in 100 to 200 μl of Tris-EDTA buffer and digested to completion with an appropriate restriction enzyme that cleaves DNA at a site adjacent to the region to be mapped. DNA was electrophoresed on a 1.5% SeaKem LE agarose at 45 V overnight in Tris-borate-EDTA buffer. DNA was transferred to Genescreen (Du Pont) by using the method of Sambrook et al. (38). DNA probes immediately proximal to the chosen restriction enzyme site were used (see Fig. 5 legend). Then, 25 ng of probe was radiolabeled by using [α-32P]dCTP with the Rediprime II kit (Amersham Pharmacia Biotech, Amersham, United Kingdom). Filters were hybridized in 0.5 M NaH2PO4-0.5 M NaH2PO4-7% SDS (pH 7.2) (12) for 48 h at 60°C and washed twice with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at 60°C for 5 min and once with 2× SSC-0.1% SDS at 60°C for 30 min.
FIG. 5.
Alterations in STB chromatin structure but not in other 2μm plasmid regions in rsc2 cells as analyzed by MNase digestion and indirect end labeling. Chromatin was isolated from GY59 (right lanes) and GY59 rsc2Δ (left lanes), digested with MNase, deproteinized, blotted, and probed as indicated (see Materials and Methods). (A) Chromatin structure of the STB region. Main differences between wild-type and rsc2 cells are indicated by white arrows. Chromatin was digested to completion with EcoRI and probed with the EcoRI/PstI fragment from the RAF gene (positions 2407 to 2652 as numbered from the EcoRI site in the FLP gene of the 2μm-A form) (probe 1). The concentrations of MNase in both rsc2 and RSC2 were 0.125 U (left lane of each) and 0.25 U (right lanes). (B) Purified naked control DNA subjected to MNase digestion and analyzed with probe 1. Left lane, 0.125 U of MNase; right lane, 0.25 U of MNase. (C) Chromatin structure of FLP/REP2 promoter (intergenic) region in rsc2 and RSC2 cells. Chromatin was digested with HincII and probed with a PCR product from bases 2651 to 2960 at the end of RAF (probe 2). The left lane of rsc2 and the right lane of RSC2 samples were digested with 0.125 U of MNase, and the right lane of rsc2 and the left lane of RSC2 were digested with 0.5 U. (D) Chromatin structure of the RAF/REP1 promoter (intergenic) region. Chromatin was digested with EcoRI and probed with a PCR product from positions 5910 to 6308 of FLP (probe 3). The concentrations of MNase from left to right of both rsc2 and RSC2 lanes were 0.5, 0.25, and 0.125 U. Marker lanes were derived from double digestion of 2μm DNA with EcoRI and the enzymes indicated. Marker sizes are shown in base pairs.
Immunostaining and GFP localization of plasmids.
Strains were grown in selective SD media (1) with 6.5 g of sodium citrate/liter for yeast strains expressing GFP. Strains containing pAFS60 and pAFS135 were induced with 15 mM 3-amino-1,2,4-triazole added to the medium to a final concentration of 10 mM for 15 min prior to live imaging or fixation in 3.7% (vol/vol) formaldehyde in 0.1 M KH2PO4 solution.
Immunostaining for Rep1 and Rep2 protein was as described previously (39). Images were captured by using a Nikon Optiphot2 with a Hamamatsu Orca2 digital camera and then processed by using Openlab software (Improvision, Ltd., Warwick, United Kingdom).
RESULTS
Identification of chromosomal mutants causing 2μm plasmid loss.
Replicating plasmids in yeast are normally subject to strong MIB, resulting in frequent production of plasmid-free cells. The 2μm plasmid is able to overcome MIB by a mechanism that requires the plasmid proteins Rep1 and Rep2 and the DNA sequence STB, together with unknown host cell functions. A colony-sectoring assay was devised for identification of mutations in host genes, resulting in the loss of plasmid stability and MIB of 2μm plasmids. pZ10 (see Materials and Methods) disintegrates in yeast, resulting in the plasmid 2μm-ADE1, which is identical to wild-type 2μm plasmid except for insertion of the ADE1 gene into a nonessential region. Between the STB and ORI loci (Fig. 1A), 2μm-ADE1 exhibits stability characteristics similar to that of native 2μm plasmid. Since ade1 cells accumulate a pink pigment, the loss of 2μm-ADE1 from the ade1 strain GY59 can be readily observed as pink ade1 sectors in otherwise white (i.e., 2μm-ADE1-containing) colonies (Fig. 1B).
A total of 225,000 colonies were examined after ethyl methanesulfonate mutagenesis of strain GY59 (2μm-ADE1), and six independent colonies exhibited consistent frequent sectoring. 2μm-ADE1 was reintroduced into plasmid-free segregants of each putative mutant, and formation of sectored colonies was observed with all six mutants after the fresh transformation, confirming that a genomic mutation was responsible for the defect in plasmid maintenance (Fig. 1C). These mutants were named dpm (defective in plasmid maintenance) mutants, i.e., the dpm1, dpm3, dpm12, dpm16, dpm18, and dpm19 mutants.
dpm mutants are defective in escaping MIB.
Plasmid loss from dpm mutant cells could be caused either by defective plasmid replication or by plasmid missegregation as a consequence of failure to escape MIB. In order to distinguish between these possibilities, we introduced into the dpm mutants the minichromosome (centromeric) plasmid pCEN-ADE1, which replicates by using a 2μm plasmid replication origin but is stably maintained at one to two copies per cell as a consequence of carrying the centromere of chromosome IV. If dpm mutants were affected in plasmid replication, a more severe loss phenotype would be predicted for pCEN-ADE1 than 2μm-ADE1 due to the lower copy number of pCEN-ADE1.
Introduction of pCEN-ADE1 into all six dpm mutants resulted in a mild plasmid loss phenotype with reduced sectoring of colonies (Fig. 1C). A quantitative plasmid stability assay comparing the stability of the centromeric and 2μm plasmids in dpm and wild-type cells showed that all dpm mutants lose 2μm-ADE1 at frequencies of between 15 and 26% per cell per generation (Fig. 1D). In contrast, the loss rate of the centromeric plasmid pCEN-ADE1 was ca. 5% or less per cell per generation. The parent strain GY59 loses both plasmids at ca. 1% per generation.
The results indicate that the stability of a low-copy-number centromeric minichromosome was mildly affected, whereas the stability of the high-copy-number 2μm-ADE1 plasmid carrying the same replication origin was severely affected. Therefore, elevated loss of 2μm-ADE1 in dpm mutants was likely due to a failure of normal 2μm plasmid segregation.
RSC2 complements the mutant phenotype of dpm19.
The dpm19 mutant was cotransformed with a yeast genomic library in the centromeric vector YCp50 (37) and plasmid pZ10, which disintegrates to produce 2μm-ADE1 in yeast cells. Transformants were plated on medium lacking adenine and uracil to select for the presence of both 2μm-ADE1 and YCp50. From about 500 transformed colonies, eight nonsectoring white colonies were obtained. The library plasmids from two of these colonies were found on retransformation to confer a stable ADE+ phenotype on the dpm19 mutant only in the presence of 2μm-ADE1, confirming that these clones reestablished plasmid stability rather than carrying ADE1 genomic sequences. Restriction mapping indicated that both plasmids carried the same fragment of ∼10 kb, and they were designated pMW1. We note that the frequency of rescuing library clones is high (2/500), which may reflect the extreme instability of 2μm-ADE1 in dpm19 cells, resulting in a low frequency of colony development of noncomplemented transformants.
Partial sequencing of the genomic insert in pMW1 showed that it comprises 9.3 kb of genomic sequence from the right arm of chromosome XII, encompassing five complete (TAL1, ILV5, YLR356W, RSC2, and YLR358C) and two incomplete (BUD8 and ADE13) open reading frames. Deletion and subcloning analysis showed that plasmid pMW4, containing RSC2 and YLR358c complemented the mutant phenotype, whereas pMW5, which contains YLR358c and a partial deletion of RSC2, failed to complement (Fig. 2). Sequencing of the rsc2 allele of the dpm19 mutant showed a single nucleotide deletion at position 871, causing a frameshift mutation. These results indicate that RSC2 is required for normal segregation of 2μm-ADE1.
All dpm mutants except the dpm1 mutant carry alleles of rsc2.
Plasmid pMW4, carrying RSC2, was introduced into the five other dpm mutants, together with pZ10. pMW4 was found to complement the mutant phenotype of all of the dpm mutants except the dpm1 mutant, resulting in stable maintenance of the 2μm-ADE1 plasmid. Sequencing of the RSC2 alleles from other dpm mutants confirmed that all mutants except the dpm1 mutant have mutations in RSC2. The dpm16 and dpm19 mutants both have a single base deletion at position 871, but the dpm16 mutant carries an additional A-G base substitution at position 260. The dpm3 mutant has a single base substitution from G to A at position 1402, causing a glutamate residue to be replaced by lysine. The dpm12 mutant has a single C-T base substitution at position 235, converting a glutamine codon to a premature stop. The dpm18 mutant has a single G-to-A base substitution at position 1802, changing the glycine residue to a glutamate.
These results indicate that RSC2 plays a significant role in overcoming MIB in 2μm plasmid maintenance, since five independent rsc2 alleles were identified in our screen. dpm1 was not complemented by RSC2, indicating that at least one other genomic gene is required for 2μm plasmid maintenance.
RSC2 is essential for native 2μm plasmid maintenance.
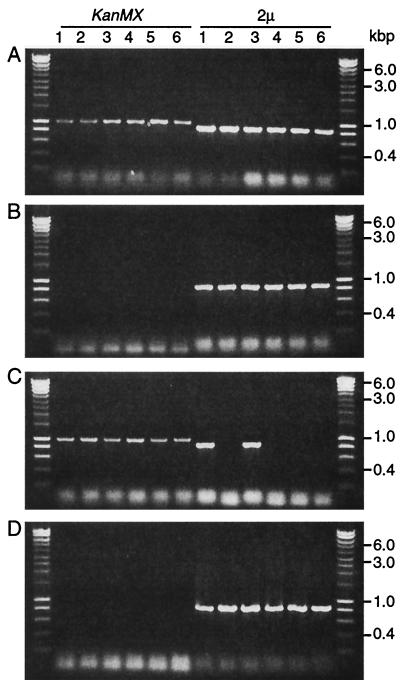
To confirm that RSC2 is involved in plasmid maintenance, RSC2 was deleted in the haploid strain GY59 carrying 2μm-ADE1 and in strains A364A [cir+] and GY18 [cir+], which harbor the native 2μm plasmid. The GY59 rsc2Δ (2μm-ADE1) null mutant formed highly sectored colonies identical to dpm19 colonies, indicating high-frequency loss of 2μm-ADE1 (not shown). Since native 2μm plasmid does not confer a phenotype, colonies of A364A rsc2Δ [cir+] and GY18 rsc2Δ [cir+] mutants were screened for the presence of native 2μm plasmid by PCR. Immediately after deletion of RSC2, most colonies still contained cells carrying 2μm, a finding consistent with a defect in segregation. After several subcultures, four of six rsc2Δ colonies contained no plasmid-bearing cells, while all of the colonies of the control RSC2 strain maintained plasmid (Fig. 3). Similar results were obtained with W303 rsc2Δ and with spores germinated from a diploid GY59 rsc2Δ × W303 cross (not shown). We conclude that RSC2 is essential for normal 2μm plasmid maintenance.
FIG. 3.
RSC2 is required for wild-type 2μm plasmid maintenance. RSC2 disruption and the presence of the 2μm plasmid was tested in six colonies each of GY18 rsc2Δ (A and C) and GY18 RSC2 (B and D). Colonies were analyzed by using KanMX primers (specific for rsc2Δ) and 2μm primers (detecting 2μm plasmid). Panels A and B show analysis directly after the detection of rsc2Δ; panels C and D show the results six subcultures later. An amplification product with the KanMX primers confirms the presence of the rsc2Δ allele, and product with 2μm primers indicates the presence of plasmid in that colony.
RSC2 is required for escape from MIB and for normal Rep protein localization.
DNA molecules carrying lac operator-binding sites can be visualized in yeast in the presence of a GFP-Lacr protein fusion (43, 52). To confirm the involvement of RSC2 in the escape from MIB, we observed the behavior of a 2μm plasmid derivative carrying the lac operator repeats in RSC2 and rsc2Δ strains.
In GY59 (RSC2), the plasmids were detected in ca. 60 to 80% of cells, and the fluorescent intensity indicated relatively even distribution of the copy number between cells. Plasmids were observed to be concentrated in clusters within the nucleus, and plasmid segregation into daughter buds was generally observed concomitantly with bulk chromatin, as previously reported (Fig. 4A and B) (39, 52). In contrast, in GY59 rsc2Δ plasmids were found in ca. 5% of cells, which predominantly showed intense fluorescent signals (Fig. 4C and D). Some mother-bud pairs enter mitosis failing to transmit plasmids to the daughter bud (Fig. 4E and F). This is indicative of a high plasmid copy number accumulating in a subset of cells, a finding consistent with the appearance of MIB in rsc2Δ cells. These observations confirm that RSC2 is required for 2μm to overcome MIB.
FIG. 4.
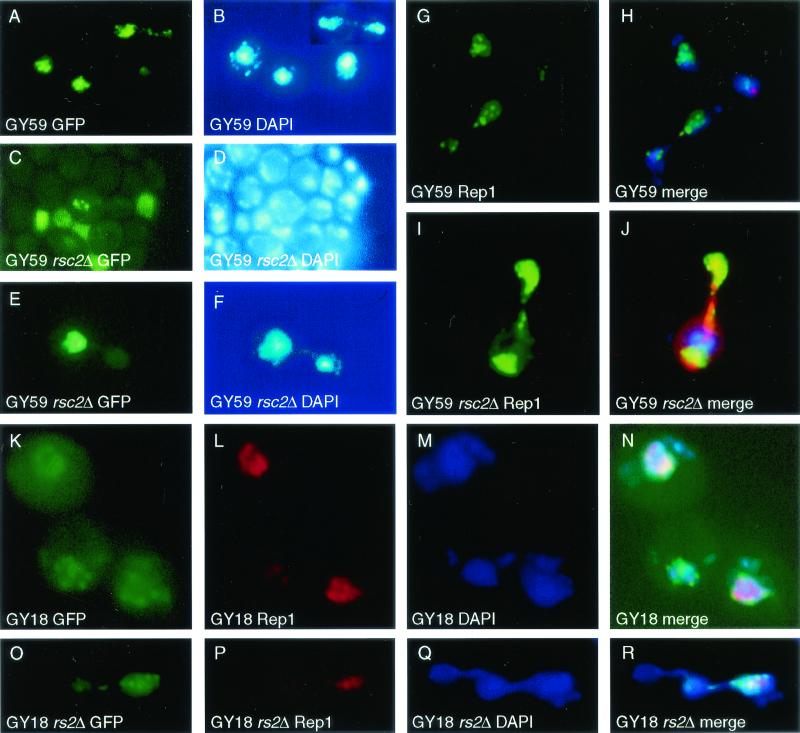
MIB is associated with loss of normal Rep1 localization in rsc2Δ mutants. (A) Localization of 2μm-based plasmid pAFS60, which carries lac operator repeats that bind GFP-Lacr fusion protein. Discrete plasmid clusters (A) that bind coincident with bulk nuclear DNA (B) are observed. (C) 2μm is found at a high copy number in a small subset of GY59 rsc2Δ cells. (D) Same as for panel C, but visualized with DAPI. (E) GY59 rsc2Δ mother-daughter cell pair exhibiting MIB (failure to transmit plasmid to daughter bud). (F) Same as for panel E, but visualized with DAPI, showing nuclear DNA in both mother and daughter cells. (G) Immunolocalization of Rep1 in GY59 showing discrete foci of plasmid proteins. (H) Merged image as in panel G, showing Rep1 (green), DAPI staining of nuclear DNA (blue), and spindle microtubules detected by using Texas red-conjugated anti-tubulin (red). Note that the Rep1 protein lies entirely within the domain of DAPI staining. (I) Immunolocalization of Rep1 in GY59 rsc2Δ showing the absence of foci of plasmid proteins and the presence of Rep1 throughout the nucleus. Note in merged image J (same colors as for panel H) that Rep1 immunostaining lies substantially outside the region of DAPI staining, indicating that Rep1 has lost its association with chromatin. (K) Wild-type GY18 shows multiple plasmid foci (green). (L) Immunolocalization of Rep1 shows nuclear foci. (N) Merged image of panels K, L, and M (DAPI stained) shows that the majority of 2μm is colocalized with the Rep1 protein and the chromatin. (O) Image shows that GY18 rsc2Δ 2μm plasmid is subject to MIB. (P) Immunolocalization of Rep1 shows some loss of nuclear localization. (R) Merged images of O, P, and Q (DAPI) show that Rep1 and some 2μm plasmid lose association. (A, C, E, K, and O) Localization of 2μm-based plasmids by using GFP-Lacr bound to pAFS60; (B, D, F, M, and Q) bulk nuclear DNA visualized by using DAPI; (G and I) indirect immunofluorescent detection of Rep1 (FITC); (L and P) indirect immunofluorescent detection of Rep1 (Texas red); (H and J) merged images of Rep1 (green), bulk nuclear DNA (blue), and spindle microtubules (red); (N and R) merged images of 2μm-based plasmid pAFS60 made with GFP-Lacr (green), Rep1 (red), and DNA (blue).
The 2μm plasmid segregation proteins Rep1 and Rep2 have previously been shown to localize to discrete nuclear foci (39). These foci are believed to contain plasmid-protein complex assembly sites essential to allow 2μm plasmid to overcome MIB (39a, 52).
Formation of Rep foci was examined in GY59 rsc2Δ by indirect immunofluorescence (39). In wild-type GY59 cells, Rep1 immunofluorescence was observed in the majority of cells and is clearly present in discrete foci (Fig. 4G), which in merged images overlies the bulk of DAPI (4′,6′-diamidino-2-phenylindole)-stained chromatin (Fig. 4H). In GY59 rsc2Δ, Rep1 and Rep2 immunofluorescence was observed in ca. 5% of cells, a finding consistent with the number previously found to contain plasmid, indicating that the loss of segregation of 2μm plasmids in rsc2 mutants is not due to a failure to express the REP genes. However, Rep1 was no longer predominantly observed in foci (Fig. 4I), and the distribution of Rep1 is no longer coincident with the bulk DAPI-stained chromatin (Fig. 4J). Similar observations were made for Rep2 (not shown).
The extreme instability of 2μm-ADE1 in GY59 rsc2Δ makes it technically difficult to assess colocalization of GFP-labeled plasmid with Rep protein foci. Strain GY18 rsc2Δ has a less-severe loss rate of native 2μm plasmid than GY59 rsc2Δ (see above). As expected from previously published data (39, 52), the majority of GFP foci colocalize with Rep1 protein foci in GY18 cells (Fig. 4K to N). In GY18 rsc2Δ the proportion of cells carrying plasmid is 25% lower than in GY18 and, although the Rep protein has a similar overall staining intensity to GY18 cells, it appears to be less concentrated in foci. In addition, MIB can be observed in some division events (Fig. 4O to R).
The MIB and lack of Rep foci seen in rsc2Δ mutant cells suggest that Rep proteins may be unable to form productive complexes with STB in the absence of Rsc2. This could be explained by a direct interaction of Rsc2 with the Rep proteins. Two-hybrid analysis between Rep1, Rep2, and Rsc2 confirmed the Rep1-Rep2 interactions previously reported (39, 51), but no interaction between Rsc2 and Rep1 or Rep2 was detected in this assay (data not shown).
Altered STB chromatin structure in rsc2 mutants.
Our previous results have shown that Rep protein focus formation requires the presence of plasmids carrying the STB locus (39). An alternative explanation to the MIB and failure to form normal Rep complexes is therefore that Rsc2 is required to create a chromatin structure amenable to Rep protein interaction with plasmid DNA.
The RSC complex perturbs nucleosome structure in vitro (7), and mutation of the RSC component Nps1/Sth1 alters the chromatin structure of centromeres (48), suggesting a possible role for Rsc2 in establishing specific chromatin structures. The chromatin structure of the STB locus, Flp target sites (FRT), and the promoter regions of FLP, REP1, REP2, and RAF were examined by MNase digestion and indirect end labeling in wild-type and rsc2Δ mutant GY18 [cir+] cells. No changes in the chromatin structure in the promoter regions of the genes (Fig. 5C and D) or the FRT sites (not shown) were observed, demonstrating that Rsc2 is not required for the general nucleosomal structure of 2μm plasmid.
In contrast, a significant difference in the chromatin pattern at the STB region was observed (Fig. 5A). The essential region of STB consists of two repeats of 124 bp superimposed on five repeats of 62 bp located between the plasmid HpaI and AvaI sites (32). In wild-type cells, strong MNase cleavage sites are observed at the borders of STB and a weaker site is seen at the center, together with a general background smear over STB, indicative of partial protection by two protein complexes. In contrast, in rsc2Δ cells no bands were observed over the STB region, and the central and border cleavage sites were absent or very strongly diminished. The cleavage pattern in rsc2Δ cells was distinct from that observed with naked DNA (Fig. 5B), indicating that STB continues to interact with proteins in rsc2Δ cells. We conclude that RSC2 is needed for normal protein-DNA interactions at STB but not for the general chromatin structure of the remainder of the 2μm plasmid.
RSC1 is not required for 2μm plasmid segregation.
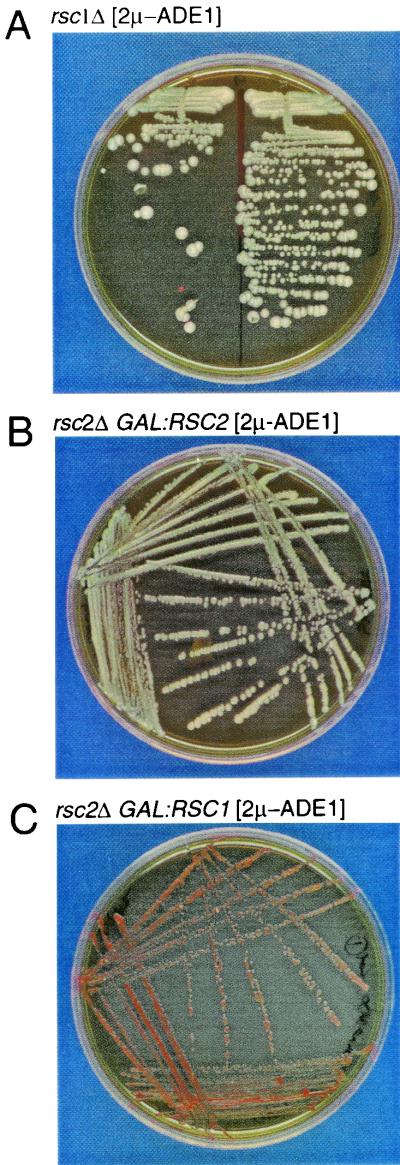
RSC2 has previously been shown to be a nonessential component of the RSC complex due to the existence of RSC1, a closely related RSC complex member with 45% identity (62% similarity) to RSC2. Deletion of either RSC1 or RSC2 creates only mild growth phenotypes under specific stress conditions, whereas the deletion of both is lethal. Rsc1 and Rsc2 have been shown to be present in separate subcomplexes that have separate and common targets (7, 8). RSC1 was disrupted in strain GY59 (2μm-ADE1), and the stability of 2μm-ADE1 was assessed in rsc1Δ and rsc2Δ strains. 2μm-ADE1 MIB was only observed in the rsc2Δ strain and not in the rsc1Δ mutant (Fig. 6A). GAL1-directed expression of RSC2 was able to complement the rsc2Δ plasmid instability phenotype (Fig. 6B), but GAL1-directed expression of RSC1, which resulted in its accumulation to the same level as that observed with GAL1-RSC2 expression, was unable to complement the MIB phenotype of rsc2Δ and stabilize 2μm-ADE1. We conclude that Rsc1 is not required for 2μm plasmid segregation, and thus Rsc2 has a unique role in this process.
FIG. 6.
Overexpression of RSC1 cannot compensate for loss of RSC2 function. (A) GY59 rsc1Δ maintains 2μm-ADE1 with no loss of stability. (B) GY59 rsc2Δ stably maintain 2μm-ADE1 on galactose medium when cells are cotransformed with pCM5 (GAL1:RSC2). (C) GY59 rsc2Δ cells do not maintain 2μm-ADE1 on galactose medium when cells are cotransformed with pCM13 (GAL1:RSC1). All plates contain YPGal.
The bromodomains and the C-terminal (CT) domain of Rsc2 are essential for 2μm plasmid maintenance.
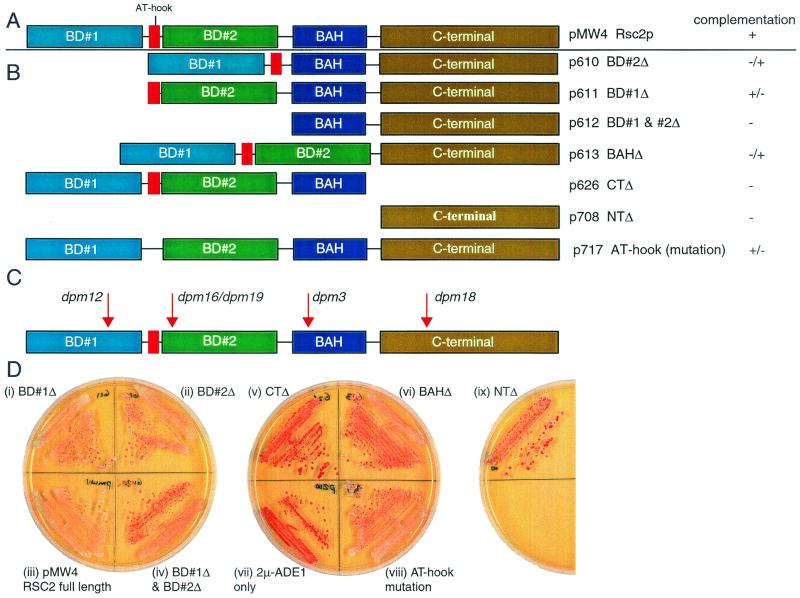
The region of similarity between Rsc1 and Rsc2 is divided into five sections (8), bromodomains BD#1 and BD#2, the AT-hook motif, a bromodomain-adjacent homology (BAH), and a CT domain (Fig. 7A). BD#2, BAH, and a CT domain were previously found to be absolutely required for any of the tested functions in both Rsc1 and Rsc2, but BD#1 was required only for certain Rsc2 functions (8). The AT-hook motif of Rsc2 was found to be required only for growth in an rsc1 rsc2 background. As discussed above, two of the originally isolated dpm mutants have substitution mutations in RSC2. dpm3 causes an E467K mutation in the BAH, and dpm18 results in the substitution G600E within the CT domain (Fig. 7C). Both E467 and G600 are conserved in Rsc1 and Rsc2 and lie within regions of very high identity between the two proteins. Their phenotype in dpm3 and dpm18 strains suggests that these residues are essential for Rsc2 function. We tested the ability of the domain deletions of Rsc2 to complement 2μm-ADE1 plasmid stability in GY59 rsc2Δ by using plasmids described by Cairns et al. (8), which have previously been shown to result in the approximately equal accumulation of each protein. We found that all domains of Rsc2 are important in its function in 2μm maintenance. Rsc2 derivatives lacking both bromodomains or the CT domain were nonfunctional, indicating that the presence of a BD and the CT domain is essential. Loss of either BD#2 or the BAH had severe effects but the protein retained limited function, whereas Rsc2 with a mutated AT-hook motif or BD#1 resulted in intermediate stability of 2μm-ADE1 (Fig. 7B).
FIG. 7.
Domain analysis of Rsc2 function in 2μm maintenance. (A) Five domains of Rsc2: BD#1, AT-hook, BD#2, BAH, and CT as defined previously (8). (B) Centromeric plasmids expressing truncated or deleted Rsc2 domains as indicated were assessed for complementation of 2μm-ADE1 stability in rsc2Δ cells. Complementation is indicated from full (+) to none (−) as shown in panel B. (C) Positions of dpm mutations within RSC2. (D) Plates illustrating the phenotypes in the complementation experiment described for panels B and C.
DISCUSSION
Replicating extrachromosomal DNA circles are inherently lost at a high rate from yeast cells. This is due to MIB that results in the frequent failure to transmit such molecules to daughter cells (31, 42). The molecular explanation of MIB is unclear, since the nuclear volume is shared approximately equally between mother cell and daughter bud. However, it may result from the failure of DNA circles to be liberated from replication sites, since linear plasmids do not exhibit MIB (31) and since a mutation that reduces MIB of some plasmids was found to lie in DNA polymerase δ (21). Mechanisms that allow circular DNA molecules to be efficiently transmitted generally involve their association with segregated nuclear structures (5, 14, 23, 27, 28).
The 2μm plasmid of S. cerevisiae is a rare example of a widespread high-copy-number eukaryotic nuclear extrachromosomal element. In order to be maintained in yeast populations, 2μm must therefore possess a mechanism allowing it to overcome MIB. Although the 2μm plasmid contains only four open reading frames, the molecular details of how this is achieved are not clear. The plasmid proteins Rep1 and Rep2 associate with the STB locus (18, 51) and result in the localization of plasmid and Rep proteins to discrete subnuclear foci which are segregated at mitosis and appear to be associated with bulk nuclear chromatin (39, 52).
Here we show that chromatin structure is central to plasmid stability and demonstrate that 2μm plasmid is a novel target for Rsc2, which contains two bromodomains (34, 53) and is a component of the RSC complex (7, 8). The related protein Rsc1 is unable to substitute for Rsc2. This is the first example of a phenotype attributed to RSC2 that is correlated with specific changes in chromatin structure in vivo.
Six chromosomal mutants were isolated that maintain the 2μm-like plasmid 2μm-ADE1 poorly and, by complementation with a genomic library, RSC2 was found to rescue the mutant phenotype of the dpm19 mutant and four other dpm mutants. RSC2 disruption in other strains revealed a phenotype similar to that of the dpm19 mutant, affecting not only 2μm-ADE1 but also the native 2μm plasmid. A failure of RSC2 to complement dpm1 indicates that there is at least one other gene that is required for 2μm plasmid maintenance.
RSC1, a homologue of RSC2, has 45% identity (62% similarity), and both RSC1 and RSC2 have been shown to be present in different subcomplexes that have both separate and common targets (8). The combined loss of both RSC1 and RSC2 is lethal, but loss of either results in mild growth defects (8). We have shown that the role of RSC2 in 2μm plasmid stability is unique, since loss of RSC1 has no effect on 2μm maintenance.
A high plasmid loss rate could be due to a failure either in plasmid replication or in the segregation of the replicated molecules. Plasmid replication failure would be predicted to produce a low average plasmid copy number across the cell population, whereas segregation failure would lead to a high plasmid copy number in a small population of cells. We believe that it is unlikely that the failure of plasmid maintenance in rsc2 mutants is due to a failure in DNA replication, since the loss rate of the high-copy-number 2μm-ADE1 is substantially higher than that of a low-copy-number centromere-containing plasmid, pCEN-ADE1. This is supported by the visualization of plasmids with a GFP-Lacr fusion that shows accumulation of plasmid at a high copy number in a small proportion of rsc2 cells. In certain instances plasmids were observed failing to segregate, despite the presence of a significant plasmid signal. This demonstrates that plasmid segregation rather than replication is primarily affected in rsc2 mutants.
2μm plasmid is condensed into 21 to 34 nucleosomes per plasmid that closely resemble those of chromosomal DNA (26). The protection of 2μm chromatin from nuclease digestion is not random within the plasmid (25), and in particular the STB region has an abnormal structure (15, 50). Our analysis of the 2μm chromatin structure in rsc2 mutants suggests the following. First, the chromatin structure is only altered within STB, and no changes were observed in the promoter regions of the four open reading frames. This indicates that loss of RSC2 does not perturb the overall chromatin structure of 2μm plasmid but rather has a specific effect on STB. Second, RSC2 does not seem to form a stable complex with the STB region, since there is no increase in sensitivity at STB when RSC2 is absent. Finally, we found no interaction between either Rep1 or Rep2 and Rsc2 in two-hybrid assays (not shown).
STB has been divided into two domains, lying on either side of the unique HpaI site. STB-distal is the region further from the replication origin (Ori) and corresponds to the transcription termination region of the RAF gene. STB-proximal is made up of five direct tandem repeats of an AT-rich 62-bp consensus sequence which can also be aligned as two tandem repeats of 124 bp (32) and is the binding site of Rep1-Rep2 complexes (51). Our results suggest that, in wild-type cells, STB-proximal is protected by two protein complexes that bind to the tandem repeat sequences and are separated by a nuclease-sensitive spacer sequence and flanked by hypersensitive sites. These may represent complexes on each of the 124-bp repeats. In the absence of RSC2, a more extensive complex is formed that is able to bind the spacer region and protects the whole of STB-proximal from nuclease digestion. The two sensitive sites at the border of the STB-proximal are displaced and have reduced nuclease sensitivity. This change in chromatin structure is coincident with the failure of plasmid segregation.
rsc2 mutant cells therefore exhibit MIB of 2μm despite the presence of Rep1 and Rep2. We propose that the altered STB chromatin structure observed in rsc2 mutants is due to the failure of productive Rep protein-STB complexes to form. In rsc2 mutant cells, Rep1 is nuclear but is less distinctly localized into discrete foci. We have previously shown that the ability of Rep1 to form normal subnuclear foci requires the presence of plasmids carrying STB (39). The absence of such Rep1 foci in rsc2 mutants is therefore consistent with a failure of Rep1 to interact correctly with STB. We therefore propose that in wild-type cells RSC2 functions to maintain the correct nucleosome positioning at the STB so that a productive segregation protein complex can bind the STB and effect plasmid segregation. In the absence of Rsc2, STB chromatin is not maintained in the correct form, and thus the protein complexes may bind, randomly producing an unproductive complex. Alternatively, a different complex may be able to bind to the STB sequence, preventing efficient plasmid segregation.
Recently, Sengupta et al. (40) have shown that specific domains of Rep1 and Rep2 mediate dimerization, interaction, and DNA binding. They proposed that competition between Rep1 and Rep2 for association with Rep1 determines the formation or disassembly of the segregation complex. Changes in the chromatin structure at STB may influence this competition, promoting disassembly or otherwise changing the segregation complex and leading to segregation failure.
Surprisingly, five of six of the dpm mutants that we generated contain a mutation in RSC2. dpm12, dpm16, and dpm19 mutants have mutations which cause frameshifts of premature termination and hence abolish the function of the C terminus of the RSC2 gene and produce a phenotype similar to that of an rsc2 null mutant. dpm3 and dpm18 mutants have point mutations within the BAH and C terminus, respectively, and identify E467 and G600 as key residues in Rsc2 function.
Overall, the structure of 2μm-derived plasmid constructs has long been suspected to be important in influencing their stability. For example, Futcher and Cox (16) found that plasmids that carry very similar regions of the 2μm genome, but in different DNA contexts, can differ substantially in stability. Moreover, the plasmid chromatin structure is dependent on the plasmid proteins present (50). Here we present strong evidence that the chromatin structure of STB is of key importance in allowing the plasmid to escape MIB and show that Rsc2 is essential for maintaining that chromatin structure. Remaining challenges include the characterization of the complexes that bind STB in both wild-type and rsc2 backgrounds.
ADDENDUM IN PROOF
In contrast to the results we report here indicating a specific role for Rsc2 in 2μm plasmid maintenance, Ng et al. (Genes Dev. 16:806-819, 2002) have recently shown that the global binding profiles of the Rsc1 and Rsc2 isoforms of the RSC complex are indistinguishable.
Acknowledgments
This work was funded by Biotechnology and Biological Sciences Research Council (BBSRC) grant 8/G10977. M.J.H. was supported by a BBSRC studentship, and M.C.V.L.W. was supported by a Ph.D. studentship from the Ministry of Science Technology and Environment of Malaysia.
REFERENCES
- 1.Adams, A., D. E. Gottschling, C. A. Kaiser, and T. Stearns. 1997. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 2.Ahn, Y. T., X. L. Wu, S. Biswal, S. Velmurugan, F. C. Volkert, and M. Jayaram. 1997. The 2μm plasmid-encoded Rep1 and Rep2 proteins interact with each other and colocalize to the Saccharomyces cerevisiae nucleus. J. Bacteriol. 179:7497-7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baudin, A., O. Ozier-Kalogeropoulos, A. Denouel, F. Lacroute, and C. Cullin. 1993. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 21:3329-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bijvoet, J. F. M., A. L. Van der Zanden, N. Goosen, J. Brouwer, and P. Van de Putte. 1991. DNA insertions in the “silent” regions of the 2μ plasmid of Saccharomyces cerevisiae influence plasmid stability. Yeast 7:347-356. [DOI] [PubMed] [Google Scholar]
- 5.Brand, A. H., G. Micklem, and K. Nasmyth. 1987. A yeast silencer contains sequences that can promote autonomous plasmid replication and transcriptional activation. Cell 51:709-719. [DOI] [PubMed] [Google Scholar]
- 6.Cairns, B. R. 1998. Chromatin remodeling machines: similar motors, ulterior motives. Trends Biochem. Sci. 23:20-25. [DOI] [PubMed] [Google Scholar]
- 7.Cairns, B. R., Y. Lorch, Y. Li, M. Zhang, L. Lacomis, H. Erdjument-Bromage, P. Tempst, J. Du, B. Laurent, and R. D. Kornberg. 1996. RSC, an essential, abundant chromatin-remodeling complex. Cell 87:1249-1260. [DOI] [PubMed] [Google Scholar]
- 8.Cairns, B. R., A. Schlichter, H. Erdjument-Bromage, P. Tempst, R. D. Kornberg, and F. Winston. 1999. Two functionally distinct forms of the RSC nucleosome-remodeling complex, containing essential AT hook, BAH, and bromodomains. Mol. Cell 4:715-723. [DOI] [PubMed] [Google Scholar]
- 9.Cao, Y., B. R. Cairns, R. D. Kornberg, and B. C. Laurent. 1997. Sfh1p, a component of a novel chromatin-remodeling complex, is required for cell cycle progression. Mol. Cell. Biol. 17:3323-3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cashmore, A. M., M. S. Albury, C. Hadfield, and P. A. Meacock. 1986. Genetic analysis of partitioning functions encoded by the 2μ circle of Saccharomyces cerevisiae. Mol. Gen. Genet. 203:154-162. [Google Scholar]
- 11.Chinery, S. A., and E. Hinchliffe. 1989. A novel class of vector for yeast transformation. Curr. Genet. 16:21-25. [DOI] [PubMed] [Google Scholar]
- 12.Church, G. M., and W. Gilbert. 1984. Genomic sequencing. Proc. Natl. Acad. Sci. USA 81:1991-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du, J., I. Nasir, B. K. Benton, M. P. Kladde, and B. C. Laurent. 1998. Sth1p, a Saccharomyces cerevisiae Snf2p/Swi2p homolog, is an essential ATPase in RSC and differs from Snf/Swi in its interactions with histones and chromatin-associated proteins. Genetics 150:987-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enomoto, S., M. S. Longtine, and J. Berman. 1994. Enhancement of telomere-plasmid segregation by the X-telomere associated sequence in Saccharomyces cerevisiae involves SIR2, SIR3, SIR4 and ABF1. Genetics 136:757-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fagrelius, T. J., and D. M. Livingston. 1984. Location of DNase I-sensitive cleavage sites in the yeast 2μ plasmid DNA chromosome. J. Mol. Biol. 173:1-13. [DOI] [PubMed] [Google Scholar]
- 16.Futcher, A. B., and B. S. Cox. 1983. Maintenance of the 2μ circle plasmid in populations of Saccharomyces cerevisiae. J. Bacteriol. 154:612-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gietz, R. D., and R. H. Schiestl. 1995. Transforming yeast with DNA. Methods Mol. Cell. Biol. 5:255-269. [Google Scholar]
- 18.Hadfield, C., R. C. Mount, and A. M. Cashmore. 1995. Protein binding interactions at the STB locus of the yeast 2μ plasmid. Nucleic Acids Res. 23:995-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartley, J. L., and J. E. Donelson. 1980. Nucleotide sequence of the yeast plasmid. Nature 286:860-864. [DOI] [PubMed] [Google Scholar]
- 20.Hieter, P., D. Pridmore, J. H. Hegemann, M. Thomas, R. W. Davis, and P. Philippsen. 1985. Functional selection and analysis of yeast centromeric DNA. Cell 42:913-921. [DOI] [PubMed] [Google Scholar]
- 21.Houtteman, S. W., and R. T. Elder. 1993. A DNA polymerase mutation that suppresses the segregation bias of an ARS plasmid in Saccharomyces cerevisiae. Mol. Cell. Biol. 13:1489-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kikuchi, Y. 1983. Yeast plasmid requires a cis-acting locus and two plasmid proteins for its stable maintenance. Cell 35:487-493. [DOI] [PubMed] [Google Scholar]
- 23.Kimmerly, W. J., and J. Rine. 1987. Replication and segregation of plasmids containing cis-acting regulatory sites of silent mating-type genes in Saccharomyces cerevisiae are controlled by the SIR genes. Mol. Cell. Biol. 7:4225-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laurent, B. C., X. Yang, and M. Carlson. 1992. An essential Saccharomyces cerevisiae gene homologous to SNF2 encodes a helicase-related protein in a new family. Mol. Cell. Biol. 12:1893-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livingston, D. M. 1982. A sequence of the yeast 2μm DNA plasmid chromosome near the origin of replication is exposed to restriction endonuclease digestion. J. Mol. Biol. 160:397-410. [DOI] [PubMed] [Google Scholar]
- 26.Livingston, D. M., and S. Hahne. 1979. Isolation of a condensed, intracellular form of the 2μ DNA plasmid of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 76:3727-3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longtine, M. S., S. Enomoto, S. L. Finstad, and J. Berman. 1992. Yeast telomere repeat sequence (TRS) improves circular plasmid segregation, and TRS plasmid segregation involves the RAP1 gene product. Mol. Cell. Biol. 12:1997-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Longtine, M. S., S. Enomoto, S. L. Finstad, and J. Berman. 1993. Telomere-mediated plasmid segregation in Saccharomyces cerevisiae involves gene products required for transcriptional repression at silencers and telomeres. Genetics 133:171-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreira, J. M., and S. Holmberg. 1998. Nucleosome structure of the yeast CHA1 promoter: analysis of activation-dependent chromatin remodeling of an RNA-polymerase-II-transcribed gene in TBP and RNA pol II mutants defective in vivo in response to acidic activators. EMBO J. 17:6028-6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreira, J. M., and S. Holmberg. 1999. Transcriptional repression of the yeast CHA1 gene requires the chromatin-remodeling complex RSC. EMBO J. 18:2836-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray, A. W., and J. W. Szostak. 1983. Pedigree analysis of plasmid segregation in yeast. Cell 34:961-970. [DOI] [PubMed] [Google Scholar]
- 32.Murray, J. A. H., and G. Cesareni. 1986. Functional analysis of the yeast 2μ plasmid partition locus STB. EMBO J. 5:3391-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray, J. A. H., M. Scarpa, N. Rossi, and G. Cesareni. 1987. Antagonistic controls regulate copy number of the yeast 2μ plasmid. EMBO J. 6:4205-4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ornaghi, P., P. Ballario, A. M. Lena, A. Gonzalez, and P. Filetici. 1999. The bromodomain of Gcn5p interacts in vitro with specific residues in the N terminus of histone H4. J. Mol. Biol. 287:1-7. [DOI] [PubMed] [Google Scholar]
- 35.Peterson, C. L., and J. L. Workman. 2000. Promoter targeting and chromatin remodeling by the SWI/SNF complex. Curr. Opin. Genet. Dev. 10:187-192. [DOI] [PubMed] [Google Scholar]
- 36.Reynolds, A. E., A. W. Murray, and J. W. Szostak. 1987. Roles of the 2μm gene products in stable maintenance of the 2μm plasmid of Saccharomyces cerevisiae. Mol. Cell. Biol. 7:3566-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose, M. D., P. Novick, J. H. Thomas, D. Botstein, and G. R. Fink. 1989. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene 60:237-243. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Scott-Drew, S. R. S., and J. A. H. Murray. 1998. Localization and interaction of the protein components of the yeast 2μ circle plasmid partitioning system suggest a mechanism for plasmid inheritance. J. Cell Sci. 111:1779-1789. [DOI] [PubMed] [Google Scholar]
- 39a.Scott-Drew, S. R. S., M. V. L. Wong, and J. A. H. Murray. DNA plasmid transmission in yeast is associated with specific subnuclear localization during cell division. Cell Biol. Int., in press. [DOI] [PubMed]
- 40.Sengupta, A., K. Blomqvist, A. J. Pickett, Y. Zhang, J. S. Chew, and M. J. Dobson. 2001. Functional domains of yeast plasmid-encoded Rep proteins. J. Bacteriol. 183:2306-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stinchcomb, D. T., K. Struhl, and R. W. Davis. 1979. Isolation and characterisation of a yeast chromosomal replicator. Nature 282:39-43. [DOI] [PubMed] [Google Scholar]
- 43.Straight, A. F., A. S. Belmont, C. C. Robinett, and A. W. Murray. 1996. GFP tagging of budding yeast chromosomes reveals that protein-protein interactions can mediate sister chromatid cohesion. Curr. Biol. 6:1599-1608. [DOI] [PubMed] [Google Scholar]
- 44.Sudarsanam, P., V. R. Iyer, P. O. Brown, and F. Winston. 2000. Whole-genome expression analysis of snf/swi mutants of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 97:3364-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas, B. J., and R. Rothstein. 1989. The genetic control of direct-repeat recombination in Saccharomyces: the effect of rad52 and rad1 on mitotic recombination at GAL10, a transcriptionally regulated gene. Genetics 123:725-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thrash-Bingham, C., and W. L. Fangman. 1989. A yeast mutation that stabilizes a plasmid bearing a mutated ARS1 element. Mol. Cell. Biol. 9:809-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Treich, I., and M. Carlson. 1997. Interaction of a Swi3 homolog with Sth1 provides evidence for a Swi/Snf-related complex with an essential function in Saccharomyces cerevisiae. Mol. Cell. Biol. 17:1768-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsuchiya, E., T. Hosotani, and T. Miyakawa. 1998. A mutation in NPS1/STH1, an essential gene encoding a component of a novel chromatin-remodeling complex RSC, alters the chromatin structure of Saccharomyces cerevisiae centromeres. Nucleic Acids Res. 26:3286-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsuchiya, E., M. Uno, A. Kiguchi, K. Masuoka, Y. Kanemori, S. Okabe, and T. Mikayawa. 1992. The Saccharomyces cerevisiae NPS1 gene, a novel CDC gene which encodes a 160-kDa nuclear protein involved in G2 phase control. EMBO J. 11:4017-4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Veit, B. E., and W. L. Fangman. 1985. Chromatin organization of the Saccharomyces cerevisiae 2μm plasmid depends on plasmid-encoded products. Mol. Cell. Biol. 5:2190-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Velmurugan, S., Y. T. Ahn, X. M. Yang, X. L. Wu, and M. Jayaram. 1998. The 2μm plasmid stability system: analyses of the interactions among plasmid- and host-encoded components. Mol. Cell. Biol. 18:7466-7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Velmurugan, S., X. M. Yang, C. S. Chan, M. Dobson, and M. Jayaram. 2000. Partitioning of the 2μ circle plasmid of Saccharomyces cerevisiae: functional coordination with chromosome segregation and plasmid-encoded Rep protein distribution. J. Cell Biol. 149:553-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winston, F., and C. D. Allis. 1999. The bromodomain: a chromatin-targeting module? Nat. Struct. Biol. 6:601-604. [DOI] [PubMed] [Google Scholar]
- 54.Yukawa, M., S. Katoh, T. Miyakawa, and E. Tsuchiya. 1999. Nps1/Sth1p, a component of an essential chromatin-remodeling complex of Saccharomyces cerevisiae, is required for the maximal expression of early meiotic genes. Genes Cells 4:99-110. [DOI] [PubMed] [Google Scholar]