Abstract
HMGB1 (also called HMG-1) is a DNA-bending protein that augments the affinity of diverse regulatory proteins for their DNA sites. Previous studies have argued for a specific interaction between HMGB1 and target proteins, which leads to cooperative binding of the complex to DNA. Here we propose a different model that emerged from studying how HMGB1 stimulates enhanceosome formation by the Epstein-Barr viral activator Rta on a target gene, BHLF-1. HMGB1 stimulates binding of individual Rta dimers to multiple sites in the enhancer. DNase I and hydroxyl radical footprinting, electrophoretic mobility shift assays, and immobilized template assays failed to reveal stable binding of HMGB1 within the complex. Furthermore, mutational analysis failed to identify a specific HMGB1 target sequence. The effect of HMGB1 on Rta could be reproduced by individual HMG domains, yeast HMO1, or bacterial HU. These results, combined with the effects of single-amino-acid substitutions within the DNA-binding surface of HMGB1 domain A, argue for a mechanism whereby DNA-binding and bending by HMGB1 stimulate Rta-DNA complex formation in the absence of direct interaction with Rta or a specific HMGB1 target sequence. The data contrast with our analysis of HMGB1 action on another BHLF-1 regulatory protein called ZEBRA. We discuss the two distinct modes of HMGB1 action on a single regulatory region and propose how HMGB1 can function in diverse contexts.
The assembly of nucleoprotein complexes frequently requires architectural proteins to facilitate interactions of nearby proteins and to create DNA conformations central to transcription, replication, recombination, and repair (7, 53). In transcription, a complex of gene activators and bending proteins is called an enhanceosome (7, 39). Most abundant architectural proteins interact with DNA nonspecifically, and it is not well understood how these factors augment specific DNA-binding reactions. This issue has been addressed in our laboratories by studying how two Epstein-Barr virus (EBV) activators, ZEBRA and Rta, assemble into enhanceosomes.
ZEBRA and Rta are sequence-specific viral activators that bind to and activate genes that control EBV DNA replication during the early phase of the lytic cycle (19, 20). Rta and ZEBRA together stimulate maximal levels of lytic gene expression and combinatorially activate a subset of the early genes (12, 49). Genetic studies have confirmed the requirement for both activators in lytic cycle transcription (11, 20, 48, 60). BHLF-1 and BHRF-1 are divergent genes controlled combinatorially by ZEBRA and Rta through a single intergenic regulatory region (32). BHRF-1 encodes a viral protein homologous to the BCL-2 proto-oncogene, while the BHLF-1 gene product localizes within the nucleoli of lytically infected cells (32). We have focused our efforts on BHLF-1.
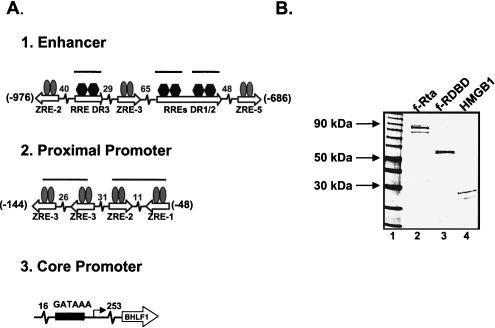
Figure 1A summarizes the organization of the intergenic regulatory region with respect to the BHLF-1 gene. The core promoter is positioned 26 bp upstream of the transcription start site and is recognized by the general factor TFIID (16). The proximal promoter region spans nucleotides −48 to −144 and contains four 7-bp ZEBRA-responsive elements (ZREs), which bind dimers of ZEBRA (16, 31, 32). Previous studies have shown that the architectural protein HMGB1 stabilizes binding of ZEBRA, a b-ZIP family member, to two pairs of sites on the BHLF-1 proximal promoter (16). For one pair of ZREs, which we examined in detail, this effect is dependent on the presence of a specific DNA sequence between the sites. The enhancer region is located between positions −686 and −976 and contains three ZREs (31-33) and three 17-bp Rta-responsive elements (RREs) (23). Studies presented below indicate that HMGB1 plays a role in the assembly of Rta complexes on the enhancer.
FIG. 1.
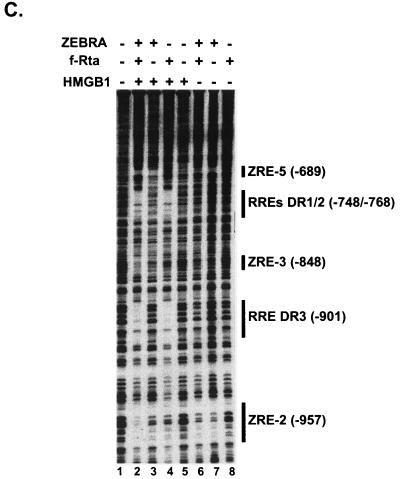
HMGB1 promotes the assembly of Rta enhanceosomes on the BHLF-1 enhancer. (A) Schematic representation of the architecture of the BHLF-1 regulatory region from −976 to +1. The start site of transcription is at position 52783 in the viral genome (32, 51). The gray ovals represent ZEBRA dimers bound to 7-bp responsive elements (ZREs). The black hexagons represent Rta dimers bound to 17-bp responsive elements (RREs). The thick lines over pairs of ZREs in the proximal promoter and single RREs in the enhancer indicate sites where HMGB1 facilitates activator binding (see text). The ZEBRA sites in the proximal promoter (from −48 to −144) are the ZRE-1, ZRE-2, and two ZRE-3 elements initially identified by Lieberman and colleagues (31, 32). The two adjacent Rta-responsive elements in the enhancer (RRE-DR1 and RRE-DR2) were mapped by Gruffat and colleagues (23). The ZREs in the enhancer region (ZRE-5, ZRE-3, and ZRE-2, from −686 to −961) were mapped by Lieberman and colleagues (32). (B) Coomassie blue-stained SDS-polyacrylamide gel of the recombinant activators. Flag-tagged Rta (f-Rta, lane 2) and its DNA-binding domain (f-RDBD, lane 3) were overexpressed and purified as described in Materials and Methods. Recombinant HMGB1 (lane 4) was purified as previously described (17); 600 ng of each protein was resolved on an SDS-4 to 20% polyacrylamide gel. (C) HMGB1 facilitates binding of intact Rta to its sites on the BHLF-1 enhancer. A 32P end-labeled restriction fragment bearing the 1,050-bp BHLF-1 regulatory region (5 fmol) was subjected to DNase I footprinting analysis in the presence of 4 ng (11 nM) of ZEBRA, 5 ng (4.5 nM) of f-Rta, 316 ng (0.85 μM) of HMGB1, and 7.7 μg of poly(dI-dC) per ml. After a 30-min incubation at 30°C, the reactions were subjected to cleavage with DNase I for 1 min, and the products were resolved on a 6% polyacrylamide-7 M urea sequencing gel. The positions of the sites relative to the BHLF-1 start site of transcription are shown on the right and were verified by electrophoresing a dideoxy sequencing ladder alongside the cleavage products (data not shown).
HMGB1 and HMGB2 are the founding members of a DNA architectural protein family characterized by the conserved HMG domain (box), which interacts with the DNA through the minor groove (6, 53). The interaction can be sequence specific, as in the case of LEF-1 and SRY, or sequence neutral, as illustrated by HMGB1 and HMGB2 (22), although, as discussed above, HMGB1 does require a specific site to function in at least one context (16). Structural studies of individual HMG boxes have shown that the 80-amino-acid domain folds into three α-helices packed together into an L-shaped structure, which bends DNA by intercalating hydrophobic amino acids between the base pairs (41, 54). The structures indicate that sequence-neutral HMG domains can bend DNA by greater than 60° (2, 42, 44). HMGB1 and -2 contain two copies of the HMG domain, referred to as the A and B boxes.
HMGB1 and -2 have been shown to stimulate the binding of a variety of sequence-specific DNA-binding proteins in vitro (reviewed in reference 53). These include p53, the progesterone receptor (PR), octamer binding factors (Oct1/2), ZEBRA, Hox domain proteins (HoxD9), the RAG1/2 recombinase, and the Rel protein Dorsal (1, 5, 13, 16, 17, 27, 61, 62). The issue has arisen as to how HMGB1 can influence such diverse nucleoprotein complexes with little sequence or structural conservation. In the case of the EBV ZEBRA protein, HMGB1 binds cooperatively with ZEBRA, but there is no evidence for a direct contact (17). This mode of action resembles in some respects the sequence-specific binding of LEF-1 and its effect on enhanceosome formation on the T-cell receptor alpha (TCR-α) enhancer (21). In all other cited cases, direct interactions between HMGB1 and the sequence-specific binding protein have been detected in solution. These interactions were hypothesized to aid DNA binding via cooperativity. p53, Oct1/2, PR, and RAG-1 all introduce bends into their sites, and HMGB1 may facilitate binding through a bending mechanism. HMGB1 containing complexes have been detected by electrophoretic mobility shift assays (EMSAs) in the case of PR, HoxD9, RAG-1/2, and ZEBRA (1, 5, 17, 61).
The present study describes our analysis of the effect of HMGB1 on Rta binding to the BHLF-1 enhancer. The findings suggest that HMGB1 can stimulate Rta binding to DNA but makes no stable protein contacts with the Rta-DNA complex. Indeed, the sequence-neutral DNA-bending protein HU can functionally substitute for HMGB1 in stimulating Rta binding. Because Rta bends DNA, we hypothesize that HMGB1 acts nonspecifically by helping to create a DNA conformation favorable for Rta binding. Despite the apparent dissimilarity with the action of HMGB1 on ZEBRA, a common theme emerges of how generalized bending activity rather than specific protein interfaces allows HMGB1 to augment binding.
MATERIALS AND METHODS
Cloning of expression constructs.
The simian virus 40 (SV40)-HMGB1 expression vector pSV40HMGB1 has been described previously (17). The SV40-Rta expression vector (pSV40Rta) contains a 2.1-kb NcoI/DraI fragment bearing the Rta cDNA ligated into the pBXG0 backbone containing the SV40 enhancer/promoter and poly(A) site (18). pSV40f-Rta (f-Rta, Flag-tagged Rta) was generated by cloning an oligonucleotide encoding the Flag epitope (N-AspTyrLysAspAspAspAspLys-C) followed by a valine to preserve the reading frame into NcoI-digested pSV40Rta. pET11df-Rta was constructed by ligating an NcoI/XbaI f-Rta fragment from pSV40f-Rta into NcoI- and BamHI-digested pET11d (Novagen). The pET11df-RtaDBD plasmid was constructed by cloning the ApaI/NcoI fragment bearing the 1,082-bp Flag-tagged Rta DNA-binding domain (f-RtaDBD) fragment (amino acids 1 to 356) (37) into pET11d cleaved with NcoI and BamHI.
Cloning of reporter and binding constructs.
The pHLCAT construct has been described previously (16). The pHLlux construct was generated by PCR amplification of the 1,050-bp BHLF-1 regulatory region from pHLCAT and insertion into the BglII site of pGL3 basic (Promega). pR2E4T was constructed by cloning a 110-nucleotide PCR fragment spanning positions 53495 to 53605 in the B95-8 EBV genome (51) into BamHI- and HindIII-digested pE4T (34). The DR2 Rta binding site in pHLlux was mutated by two-step PCR to generate pΔDR2 lux. This mutant contains base pair substitutions in the 17-bp DR2 element. pCY4DR1, used to generate the fragments for the circular permutation experiments, was constructed by cloning a 32-bp double-stranded oligonucleotide containing the DR1 Rta binding site into BglII-digested pCY4 (47).
Generation of wild-type and mutant EMSA templates.
EMSAs were performed on 27-bp double-stranded oligonucleotides containing either the wild-type 17-bp DR1 sequence (WT) or mutant sequences bearing 2-bp substitutions in the central region of DR1 (C1 and C2) or in the 5′ or 3′ 5-bp flanking sequences (5′ and 3′). The sequences of the oligonucleotides are shown below, with the DR1 site underlined and the mutated bases shown in bold: wild-type top strand, 5′-ACATTGTCCACGGGACAAGGCACAGGC-3′; wild-type bottom strand, 5′-GCCTGTGCCTTGTCCCGTGGACAATGT-3′; C1 mutant top strand, 5′-ACATTGTCCACGCCACAAGGCACAGGC-3′; C1 mutant bottom strand, 5′-GCCTGTGCCTTGTGGCGTGGACAATGT-3′; C2 mutant top strand, 5′-ACATTGTCCACGGGGGAAGGCACAGGC-3′; C2 mutant bottom strand, 5′-GCCTGTGCCTTCCCCCGTGGACAATGT-3′; 5′ mutant top strand, 5′-CATCAGTCCACGGGACAAGGCACAGGC-3′; 5′ mutant bottom strand, 5′-GCCTGTGCCTTGTCCCGTGGACTGATG-3′; 3′ mutant top strand, 5′-ACATTGTCCACGGGACAAGGCAACAAG-3′; 3′ mutant bottom strand, 5′-CTTGTTGCCTTGTCCCGTGGACAATGT-3′.
Transcription factor purification.
Recombinant ZEBRA was expressed and purified as described (10). f-Rta and Flag-tagged Rta DNA-binding domain (f-RDBD) were expressed and purified as follows. The pET11df-Rta and pET11df-RDBD expression constructs were transformed into a strain of Escherichia coli BL21(DE3) that contains a plasmid bearing the genes for four tRNAs with humanized anticodons (proline, isoleucine, and two different anticodons for arginine) under chloramphenicol selection (12.5 μg per ml of medium; a generous gift of Ren Sun). For each protein, one liter of E. coli was grown at 37°C (for f-RDBD) or 25°C (for f-Rta) until an optical density at 600 nm (OD600) of 0.6.
Protein expression was induced for 30 min by the addition of isopropylβ-d-thiogalactopyranoside. The cell pellets were resuspended in 40 ml of buffer D (20 mM HEPES [pH 7.9], 0.5 mM EDTA, 20% glycerol, 10 mM β-mercaptoethanol, 1 mM phenymethylsulfonyl fluoride, 10 μg each of leupeptin, pepstatin, and benzamidine per ml, 0.05% Nonidet P-40) containing 0.3 M KCl. The cells were lysed by sonication, insoluble debris was removed by centrifugation, and the supernatant was mixed with αFlag M2-agarose affinity beads (Sigma). After 2 h at 4°C, the beads were washed with buffer D containing 1 M KCl. The bound proteins were eluted in buffer D containing 0.3 M KCl and 0.4 mg of Flag peptide per ml (Sigma). Recombinant HMGB1 and HMGB1 deletion derivatives were expressed and purified as described (17). HMGB2 was purified from calf thymus as described (45). HU (25), HMO1 (35), and NHP6A (59) were expressed and purified as described.
Purification of HMGB1 box A proteins.
Cloning of mutant HMGB1 box A genes into the pET11a vector was based on a two-step PCR method used in reference 2. Wild-type, F38A, and R24E HMGB1 box A proteins were overexpressed in E. coli strains RJ5137, RJ5592 and RJ5591, respectively, and purified according to reference 17 with modifications. Prior to 0.3% polyethyleneimine precipitation, cell extracts were supplemented with NaCl to a final concentration of 0.75 M. Following dialysis against buffer A (50 mM NaCl), cell extracts were chromatographed through a 4-ml phosphocellulose column with a 30-ml linear gradient of buffer A containing 50 mM NaCl to buffer A containing 1 M NaCl. Fractions containing peak HMG box proteins were pooled and subjected to sequential 2% and 10% trichloroacetic acid precipitation. Pellets were resuspended in 1 ml of buffer A (50 mM NaCl) and loaded onto a fast protein liquid chromatography (FPLC) Superdex 75 sizing column to obtain a homogeneous preparation of HMG box proteins. Protein concentrations were calculated by the Bradford assay using lysozyme as the standard and confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis.
EMSAs.
EMSAs were performed on the wild-type and mutant DR1 oligonucleotides using previously described binding conditions (17). Quantitation was performed with a Phosphorimager and ImageQuant software, and the percent bound complex was calculated. The antibody EMSAs contained 1.6 μg of polyclonal, affinity-purified αHMGB1 antibody (Pharmingen).
Circular-permutation EMSAs.
A set of five 407-bp fragments in which the position of DR1 is circularly permuted with respect to the ends was generated by digesting pCY4DR1 with BamHI, NheI, EcoRI, EcoRV, or HindIII. These fragments were 32P labeled with T4 polynucleotide kinase and [γ-32P]ATP and gel purified. The reaction mixtures for EMSA were as described above and fractionated on a 5% native polyacrylamide (60:1, acrylamide-bisacrylamide) gel in 0.5× Tris-borate-EDTA (TBE).
DNase I footprinting.
Intact Rta, ZEBRA, and HMGB1 were footprinted with DNase I on the BHLF-1 enhancer as previously described (9). HMGB1, its deletion derivatives, and f-RDBD were footprinted on a 110-bp fragment containing the DR1 and DR2 Rta binding or a mutant fragment containing base substitutions in the 17-bp DR2 site (ΔDR2).
Hydroxyl radical footprinting.
Hydroxyl radical footprinting was performed as described previously (14) with protein preparations lacking glycerol and EDTA. DNase I footprinting reactions were performed in parallel under the hydroxyl radical footprinting conditions to ensure that f-RDBD was binding and that the helping effect of HMGB1 was observed. The hydroxyl radical protections were quantitated essentially as described (14, 56).
Immobilized template assays and immunoblotting.
The 85-bp biotinylated template containing the DR1 and DR2 sites was prepared by PCR amplification of pHLlux with primers MCR2Down and 5′ biotinylated MCR2Up (operon). The amplification product was conjugated to M-280-streptavidin magnetic beads (Dynal) according to the manufacturer's specifications. For each binding reaction, 1,420 fmol of the immobilized fragment were blocked for 30 min with rotation at room temperature in 100 μl of reaction buffer [13 mM HEPES (pH 7.9), 65 mM KCl, 0.32 mM EDTA, 13% glycerol, 6 mM MgCl2, 0.5 mg of bovine serum albumin per ml, 0.5 mM phenylmethylsulfonyl fluoride, 0.05% Nonidet P-40, 0.5 mM dithiothreitrol, and 0.25 mg poly(dI-dC) per ml]. After blocking, the buffer was changed, and f-RDBD and HMGB1 were added in the amounts indicated in the figure legends. After a 20-min incubation, the beads were washed twice with 200 μl of reaction buffer, and the bound proteins were eluted in 5 μl of protein loading dye. The bound proteins were resolved on 4 to 20% polyacrylamide gels (Bio-Rad) and blotted onto a polyvinylidene difluoride membrane (Immobilon-P; Millipore), in 3-(cyclohexylamino)-1-propanesulfonic acid-20% methanol. The membranes were probed with αFlag M2 monoclonal antibody (Sigma) and αHMGB1 affinity-purified polyclonal antibody (Pharmingen).
Transient transfections.
Transfections were performed in triplicate as described (17). Effector plasmids expressing Rta and HMGB1 driven by the SV40 promoter were cotransfected in the amounts indicated in the figure legends, along with 50 ng of the pHLlux reporter plasmid. Carrier DNA consisting of pBXG0 (SV40 promoter alone) was added to normalize the total DNA amounts to 1 μg. The cells were harvested and processed as described (17).
RESULTS
Enhanceosome assembly on the BHLF-1 enhancer.
To characterize nucleoprotein complex formation on the BHLF-1 enhancer, we expressed Flag-tagged Rta (f-Rta) in E. coli and purified the intact protein and its DNA-binding domain (f-RDBD) to near homogeneity by Flag antibody affinity chromatography. A Coomassie-stained gel of the recombinant activators is shown in Fig. 1B. Subsaturating concentrations of Rta and ZEBRA alone bind very weakly to their sites on the BHLF-1 enhancer in a DNase I footprinting assay (Fig. 1C, lanes 7 and 8). The presence of both activators does not further stimulate binding (Fig. 1C, lane 6). In agreement with our previous studies, HMGB1 has no effect on the occupancy of single ZREs (Fig. 1C, lane 3). In contrast, it strongly facilitates the binding of Rta to the two adjacent RREs (RRE-DR1 and RRE-DR2) and to a single site, which we designated RRE-DR3 (Fig. 1C, lane 4). Rta, in turn, stimulates the binding of ZEBRA to the ZRE-2 and ZRE-3 elements (Fig. 1C, lane 2). We will present a detailed description of the mechanism of combinatorial synergy of BHLF-1 transcription in another report. In the present study we focus on the action of HMGB1 on Rta binding to the enhancer.
Rta activates transcription in cell-based assays.
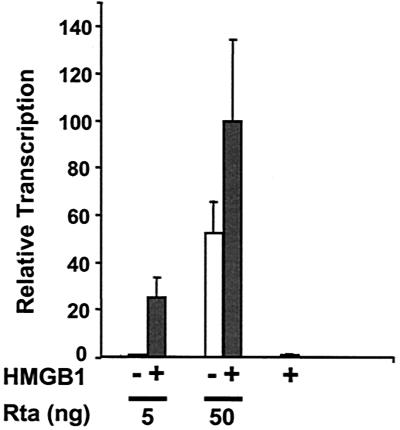
HMGB1 potentiates Rta-mediated transcription of BHLF-1 in a transient transfection assay (Fig. 2). The intact BHLF-1 regulatory region was fused upstream of the luciferase reporter gene and transfected into BHK cells in the presence of effector constructs expressing Rta or HMGB1. Low concentrations of Rta effector do not elicit BHLF-1 activation (Fig. 2, white bars). The addition of HMGB1 results in a stimulatory effect of 25-fold (Fig. 2, gray bars). At a 10-fold-higher concentration of Rta effector, the level of BHLF-1 luciferase transcription increases 52-fold, but the presence of HMGB1 further increases activation by less than twofold. This result is consistent with a mechanism by which HMGB1 facilitates binding of Rta to the BHLF-1 enhancer at low concentrations.
FIG. 2.
HMGB1 potentiates Rta-mediated activation of BHLF-1 in a cell-based assay; 50 ng of BHLF-1 luciferase reporter (pHLlux) was transfected into BHK-21 cells, along with 5 or 50 ng of Rta effector plasmid (pSV40Rta) in the absence (white bars) or presence of 1 μg of HMGB1 expressor (pSV40HMGB1; grey bars). After 24 h the cells were lysed, and luciferase activity was measured. Transfections were performed in triplicate, and the standard deviation is indicated by error bars. The highest luciferase value (1.5 million light units on average) was assigned an arbitrary value of 100%, and all other values were normalized to that.
Parameters of the stimulatory effect: protein and DNA requirements.
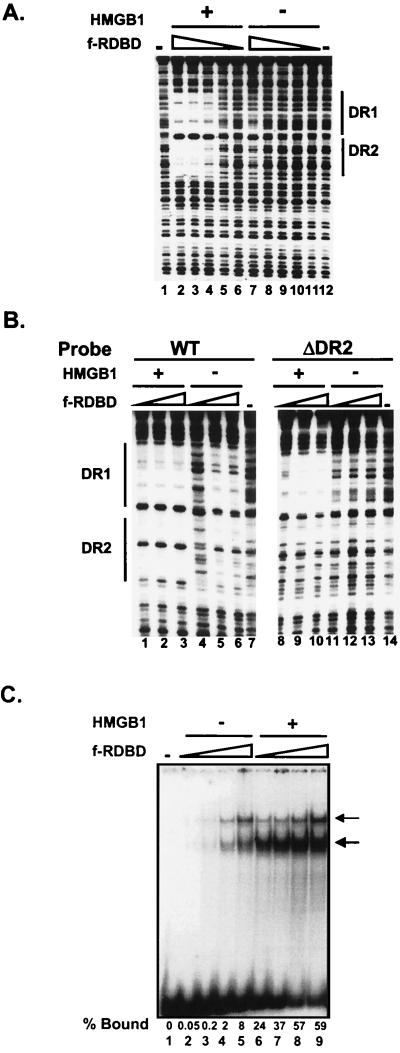
We next asked whether HMGB1 could promote DNA binding by an Rta derivative containing only its DNA-binding and dimerization domain (f-RDBD). Figure 3 compares HMGB1 stimulation of f-RDBD binding to a DNA fragment containing the two adjacent Rta sites from the BHLF-1 enhancer (RREs DR1 and DR2). HMGB1 stimulates f-RDBD binding 27-fold (Fig. 3A), analogous to the effect observed with intact f-Rta. Thus, the C-terminal transcription activation domain, is not required for HMGB1-mediated cooperative binding of Rta dimers. We note that the footprints of f-Rta and f-RDBD on the BHLF-1 enhancer are identical (data not shown).
FIG. 3.
HMGB1 facilitates binding of Rta to a single site. (A) The Rta DNA-binding domain is sufficient for facilitated binding to a pair of sites. A 32P-end-labeled 110-bp restriction fragment encompassing DR1 and DR2 (5 fmol) was incubated in the presence of decreasing amounts of f-RDBD (30, 9.9, 3.3, 1.1, and 0.6 ng or 42, 13.8, 4.5, 1.5, and 0.51 nM; lanes 2 to 6 and 7 to 11) and 7.7 μg of poly(dI-dC) per ml. Then 316 ng of recombinant HMGB1 (0.85 μM) was added to lanes 2 to 6. The lines indicate the positions of the Rta binding sites. (B) DR2 is not required for the helping effect of HMGB1 on f-RDBD binding; 5 fmol of the 85-bp radiolabeled wild-type (left panel) or ΔDR2 mutant (right panel) enhancer fragments was incubated in the presence of increasing amounts of f-RDBD (15, 30, and 60 ng or 21, 42, and 84 nM; lanes 1 to 3, 4 to 6, 8 to 10, and 11 to 13) and 7.7 μg of poly(dI-dC) per ml. Then 316 ng (0.85 μM) of HMGB1 was added to lanes 1 to 3 and 8 to 10. The panels correspond to sections of the same gel. Note the absence of protection over the remaining DR1 site even at the highest amount of f-RDBD used (compare lanes 6 and 13). (C) HMGB1 promotes binding of f-RDBD to an isolated site; 1 fmol of the radiolabeled wild-type DR1 oligonucleotide was incubated in the presence of increasing concentrations of f-RDBD (0.4, 1.2, 3.6, and 11.1 ng or 0.6, 1.7, 5, or 15 nM; lanes 2 to 5 and 6 to 9) and 7.7 μg of poly(dI-dC) per ml. Then 158 ng of recombinant HMGB1 (0.4 μM) was added to lanes 6 to 9. The bold arrow indicates the predominant f-RDBD complex that is preferentially enhanced by HMGB1. The thin arrow points to a second f-RDBD complex of lower mobility. The percentage of the probe bound by f-RDBD in each lane is also shown. Both f-RDBD complexes were included in the calculation.
Figure 3B shows that the stimulatory effect of HMGB1 occurs on a single isolated site. Deletion of DR2 reduced the affinity of RDBD for its site by fourfold, implying that Rta dimers bind DR1 and DR2 cooperatively (compare lanes 5 and 12). However, HMGB1 still strongly enhanced RDBD binding to DR1 (compare lanes 8 to 10 and 11 to 13). Notably, no additional protections were observed in the presence of HMGB1 on the single or double site probes beyond those generated by high concentrations of Rta alone (see also Fig. 3A).
Taken collectively, the footprinting results argue for a complex relationship between Rta and HMGB1, whereby HMGB1 amplifies cooperative binding by Rta to the BHLF-1 enhancer. When superimposed on the cooperative effect of Rta on ZEBRA binding, these effects delineate a network of interactions that likely provide stability to the enhanceosome. Although the biological rationale is unclear, the BHLF-1/BHRF-1 intergenic enhancer drives the strongest levels of transcription among almost three dozen early lytic genes (40).
HMGB1-mediated stimulation of a single Rta dimer could also be recapitulated in an EMSA on an oligonucleotide bearing the DR1 site (Fig. 3C). The stimulatory effect ranged up to 500-fold at low RDBD concentrations. F-RDBD generates two complexes that are resolvable by EMSA. We suspect that these complexes represent isomers of a complex containing Rta and bent DNA (see below). HMGB1 did not decrease the mobility of either of the two complexes, thus raising the possibility that it is not present within the final complex. Under similar experimental conditions, the presence of HMGB1 did result in supershifted complexes with ZEBRA (17). We note that stimulation of binding was observed with two different carrier DNAs, poly(dI-dC) and poly(dG-dC) (data not shown).
HMGB1 does not form a stable ternary complex with Rta and DNA.
We considered the following possibilities to explain the absence of an HMGB1 footprint or a supershift of f-RDBD:DNA complexes. First, HMGB1 was binding to a position within the Rta site that was undetectable by DNase I footprinting, and second, changes in mobility resulting from the presence of HMGB1 were too small to detect by EMSA. To further probe for the presence of HMGB1 in the Rta-DNA complexes, we employed hydroxyl radical footprinting, immobilized template, and antibody supershift assays. None of these different assays yielded any evidence for the presence of HMGB1 in the final Rta-DNA complex.
Hydroxyl radical footprinting.
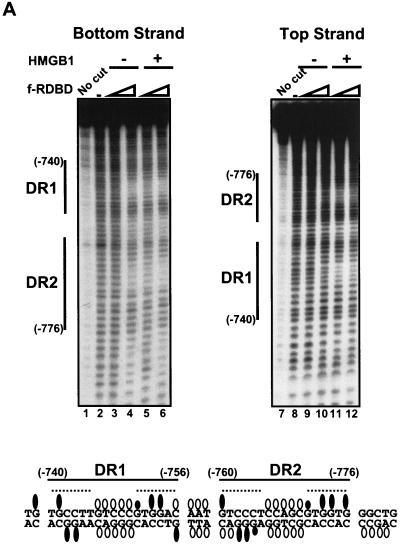
Figure 4A shows the results of hydroxyl radical footprint analysis used to identify sugars within the minor groove of the DR1 and DR2 region that are in close proximity to Rta in the presence and absence of HMGB1. HMGB1 is a minor-groove-binding protein that has been shown to protect DNA against hydroxyl radical cleavage in other contexts (17, 44). A fragment from the BHLF-1 enhancer containing the DR1 and DR2 sites was end labeled on either the bottom (left panel) or the top strand (right panel), incubated in the presence of f-RDBD and HMGB1, and subjected to hydroxyl radical attack. F-RDBD alone generates a distinct set of weak protections on both strands (Fig. 4A, lanes 4 and 10). Consistent with the stimulatory effect by HMGB1 on f-RDBD binding, the presence of HMGB1 elicited hydroxyl radical protections at lower f-RDBD concentrations (compare lanes 3 and 5 and lanes 9 and 11). However, the set of protections generated in the presence of HMGB1 were nearly identical to those generated in reactions only containing high concentrations of f-RDBD.
FIG. 4.
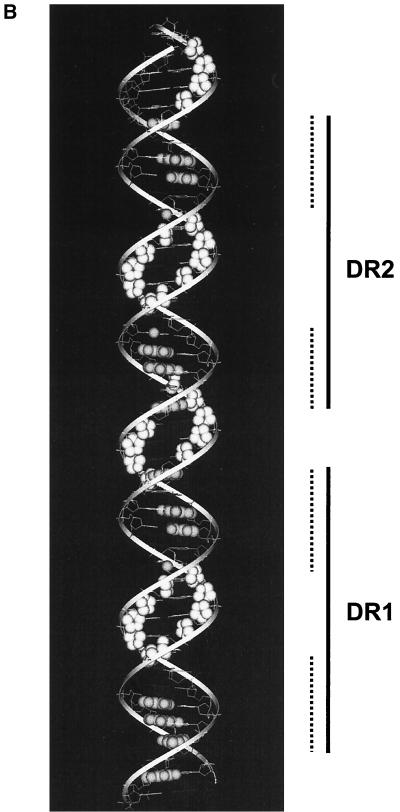
HMGB1 does not form a stable ternary complex with f-RDBD and DNA. (A) Hydroxyl radical footprinting reveals the interactions of f-RDBD with the minor groove, but no HMGB1 specific interactions; 7 fmol of an 85-bp fragment containing DR1 and DR2 was 32P end labeled on the bottom (left panel) or the top strand (right panel) and incubated in the presence of increasing amounts of f-RDBD (9.7 ng or 13.5 nM, lanes 3, 5, 9, and 11; 132 ng or 185 nM, lanes 4, 6, 10, and 12) and 7.7 μg of poly(dI-dC) per ml. Then 712 ng (1.8 μM) of HMGB1 was added to lanes 5 to 6 and 11 to 12. The positions of the sites relative to the start site of transcription are also shown. The bands protected by f-RDBD from hydroxyl radical cleavage were quantitated and identified as described in Materials and Methods. DNase I footprinting reactions were performed in parallel under the hydroxyl radical binding conditions to ensure f-RDBD binding and that the helping effect of HMGB1 was observed (data not shown). The bottom panel summarizes the hydroxyl radical protections (open circles) identified above. Guanines in DR1 and DR2 that interfere with Rta binding in methylation experiments by Gruffat and Sergeant (24) are shown as black ovals. The size of the ovals corresponds to the degree of interference. The dashed lines indicate the DR1 and DR2 half-sites, which are presumably contacted by Rta monomers within the dimer (24). (B) Modeling of the hydroxyl radical and DMS interference results summarized in the previous panel. A helical projection of the region of the BHLF-1 enhancer encompassing DR1 and DR2 was generated using InsightII. The sugars attacked by hydroxyl radical cleavage are highlighted in white. Guanines shown to interfere with Rta binding by Gruffat and Sergeant (24) are highlighted in gray. Guanines that interfere to a lesser extent are only highlighted on N7. The dashed lines indicate the DR1 and DR2 half sites.
A summary of the hydroxyl radical protections, together with the previously determined methylation interference data on the major groove N7 position of guanines (24), is presented below the footprints. Upon examination of this schematic, it becomes apparent that every guanine within the major groove of the DR1 and DR2 half-sites is contacted by the bound Rta dimers. In addition, a subset of the hydroxyl radical protections are located in the center of both DR1 and DR2. These protections are each 5 bp in length, symmetric on each strand, and centered on the ninth nucleotide or center of pseudosymmetry of each site. Sequences in the center of Rta binding sites are very loosely conserved in binding site selection assays relative to those in the individual half-sites and are likely not directly contacted by the protein (24). Furthermore, the sugars in the 3-bp spacer between DR1 and DR2 as well as in the sequence flanking DR2 are also protected from hydroxyl radical cleavage.
When the data summarized in the above schematic are superimposed on a schematic B-DNA helix, a model emerges for how Rta binds its sites (Fig. 4B). DR1 and DR2 are bound by adjacent Rta dimers, with each monomer contacting a single half-site (24). Within each site, adjacent major grooves along one face of the helix appear to bind each monomer of Rta. The central minor groove of each site displays a pattern of continuous hydroxyl radical protections on both strands and could represent the region over which Rta dimerization occurs. The protections in the 3-bp spacer between the sites could be a result of cooperative interactions between the adjacent dimers or due to DNA interactions by the flanking Rta monomers. The central minor grooves in DR1 and DR2 represent potential docking sites for HMGB1. Because these regions are protected from hydroxyl radical cleavage even in the absence of HMGB1, we might be unable to detect additional protections even if HMGB1 were a stable component of the complex. Alternatively, the fact that these minor grooves are already protected by Rta alone could suggest that there is a steric block that precludes HMGB1 from stably associating with Rta-DNA complexes.
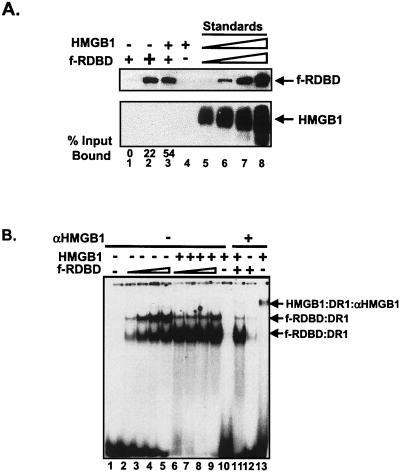
Immobilized template assays.
Figure 5A shows the results of an immobilized template assay in which a biotinylated DNA fragment bearing DR1 and DR2 was incubated with f-RDBD in the presence and absence of HMGB1. Bound proteins remaining after the washes were detected by immunoblotting and probing with α-Flag or α-HMGB1 antibody. The amounts of bound proteins were quantified by densitometry in comparison with a standard curve produced from known amounts of protein electrophoresed on the same gel. HMGB1 elicited a large stimulation of f-RDBD binding by this assay (compare lanes 1 and 3). Based on calculations from the standard curve, we estimate that 47 fmol of f-RDBD bound to 1,420 fmol of template in the presence of HMGB1. Assuming a 1:1 ratio of HMGB1 to RDBD, one would expect 47 fmol of HMGB1 to be bound. Since 47 fmol of HMGB1 is easily detectable (lane 5), we conclude from the absence of any detectable HMGB1 that much less than a 1:1 ratio of HMGB1 to RDBD exists in the final complex. We also constructed an affinity matrix composed of f-RDBD bound to Flag antibody beads. At a 1:1 molar ratio of f-RDBD to HMGB1, no specific HMGB1 binding was observed (data not shown).
FIG. 5.
HMGB1 is not a component of the Rta-DNA complex. (A) Immobilized template assays fail to reveal the presence of a ternary complex. An 85-bp biotinylated fragment containing DR1 and DR2 was immobilized on streptavidin magnetic beads, and 1,420 fmol was incubated with increasing amounts of f-RDBD (11.1 ng or 6.7 nM, lanes 1 and 3, 33.3 ng or 20.1 nM, lane 2) and 250 μg of poly(dI-dC) per ml. Then 75 ng (0.13 μM) of HMGB1 was added to lanes 3 and 4. After a 20-min incubation, the binding reactions were washed twice with buffer containing 250 μg of poly(dI-dC) per ml as described in Materials and Methods, and the bound proteins were eluted in protein loading dye and resolved by SDS-PAGE. Lanes 5 to 8 represent a standard curve of f-RDBD and HMGB1. The following amounts of each protein were loaded: lane 5, 1.38 ng (47 fmol) of HMGB1 and 1.5 ng (11.8 fmol) of f-RDBD; lane 6, 2.8 ng (94 fmol) of HMGB1 and 3 ng (23.5 fmol) of f-RDBD; lane 7, 5.5 ng (188 fmol) of HMGB1 and 6 ng (47 fmol) of f-RDBD; lane 8, 11 ng (376 fmol) of HMGB1 and 12 ng (94 fmol) of f-RDBD. Bound proteins were detected by immunoblotting and probing with αFlag and αHMGB1 antibodies. The amount of protein bound in lanes 1 to 4 was calculated by densitometry using lanes 5 to 8 as standards. The percentage of input f-RDBD bound was subsequently calculated. The two panels represent strips of the same membrane. The arrows indicate the positions of the bound proteins. (B) HMGB1 cannot be detected in a ternary complex with f-RDBD by antibody supershift; 1 fmol of radiolabeled DR1 oligonucleotide was incubated with increasing amounts of f-RDBD (3.6 ng or 5 nM, lanes 2, 6, 11, and 12; 11.1 ng or 15 nM, lanes 3 and 7; 33.3 ng or 45 nM, lanes 4 and 8; 100 ng or 135 nM, lanes 5 and 9) and 7.7 μg of poly(dI-dC) per ml. Then 158 ng of HMGB1 (0.4 μM) was added to lanes 6 to 11 and 13. A 1.6-μg sample of αHMGB1 antibody was added to the reaction mixtures in lanes 11 to 13. The upper arrow indicates the αHMGB1/HMGB1/DR1 ternary complex. The two lower arrows correspond to f-RDBD/DNA complexes.
Antibody supershift assays.
Figure 5B shows the result of Rta-DNA EMSAs probed with HMGB1 antibody. Lanes 2 to 9 recapitulate the stimulatory effect of HMGB1 on f-RDBD binding. Unstable binding by HMGB1 is reflected by the trailing of the DNA above the unbound probe, as seen in lane 10. Incubation of HMGB1 with an antibody against HMGB1 resulted in the appearance of a small amount of a slower-migrating supershifted complex (lane 13). No evidence, however, of an f-RDBD/HMGB1/DNA ternary complex was present in lane 11 (the wells of the gel where a supershifted complex might be expected to migrate are also shown), even though a trace amount of the slower-migrating complex corresponding to the HMGB1:α-HMGB1:DR1 complex was observed. As expected, incubation with the HMGB1 antibody did not generate any additional shifted complexes in reactions containing only f-RDBD and DNA (lane 12) or DNA alone (data not shown). Our inability to detect HMGB1 by antibody supershift assays within Rta-DNA complexes whose assembly was promoted by HMGB1 suggests that HMGB1 is not present in the final complex, but also could be attributed to occlusion of the HMGB1 epitope in the context of a ternary complex.
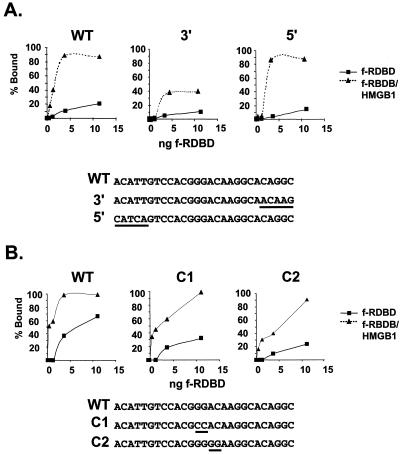
Sequence requirements for HMGB1 stimulation of Rta binding.
Mutations were introduced into nucleotides encompassing the binding site of the Rta subunits to determine if the nature of the DNA sequences influences HMGB1 stimulation of binding. Two classes of mutants were analyzed. The first group contains 5-bp substitutions in the 5′ or 3′ flank of DR1 (5′ and 3′ mutants, respectively) (Fig. 6A). The second group bears 2-bp substitutions in the nonconserved center of the site (C1 and C2 mutants, Fig. 6B). These sequences encompass either the minor grooves flanking DR1 (5′ and 3′ mutants) or the central minor groove of the site (C1 and C2 mutants), which represent potential docking sites for HMGB1. Note that this central region appears to represent the region over which Rta dimerizes (also see Fig. 4B). The mutants were assayed by EMSA, and the percent shift by f-RDBD in the presence (triangles) or absence (rectangles) of HMGB1 was plotted against the concentration of f-RDBD.
FIG. 6.
Sequence requirements for the stimulatory effect of HMGB1. (A) Mutations in the 5′ and 3′ DR1 flanking sequences do not abolish the stimulatory effect of HMGB1; 1 fmol of radiolabeled wild-type (WT) or 5′ or 3′ mutant oligonucleotides was incubated in the presence of increasing concentrations of f-RDBD (0.4, 1.2, 3.6, and 11.1 ng or 0.6, 1.7, 5, and 15 nM) and 7.7 μg of poly(dI-dC) per ml, in the presence or absence of 158 ng (0.4 μM) of recombinant HMGB1. The reaction mixtures were then subjected to EMSA. The percentages of probe bound by f-RDBD in the absence (rectangles) and presence (triangles) of HMGB1 were calculated and plotted against the amount of f-RDBD used. The graphs represent data points from the same gel. The sequences of the oligonucleotides are shown below the graphs. The 5-bp mutations are underlined. (B) Mutations in the central, nonconserved region of DR1 do not affect HMGB1-mediated cooperativity. Reactions were performed as described above using the WT or the C1 or C2 mutant oligonucleotide. The reactions were quantitated and plotted as described above. The sequences of the oligonucleotides are shown below the graphs. The 2-bp mutations are underlined.
The experiments shown in Fig. 6A and B were performed separately, and the amount of shift on the WT probe in the absence of HMGB1 differed slightly in the two experiments. However, by comparison to the WT sequence, the mutations influenced binding of f-RDBD alone on the 3′ mutant and on C1 and C2. Nevertheless, the cooperative effect of HMGB1 persisted in each case although the final magnitude was lower on the 3′ mutant. We attribute this to the lower affinity of f-RDBD to the 3′ mutant. Taken together, the data presented in Fig. 4 to 6 argue against the stable association of HMGB1 with Rta:DR1 complexes. These findings contrast directly with our study of HMGB1-mediated stimulation of ZEBRA binding within the promoter proximal region. Data from footprinting, mutational analysis and EMSA supported a sequence-specific mode for binding of HMGB1 and stimulation of ZEBRA.
Rta bends DNA.
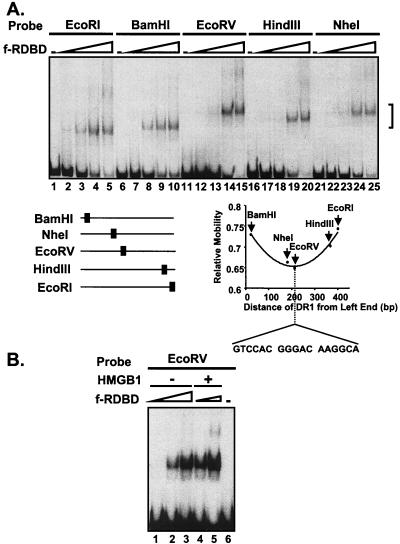
One mechanism explaining the transient role for HMGB1 in Rta binding would involve bending of DNA to generate a conformation favorable for Rta binding. In support of this hypothesis, circular permutation analysis of the mobility of Rta-DNA complexes provides evidence that the DNA within an Rta complex is bent. A 17-bp oligonucleotide representing the DR1 subsite of RRE1 was cloned into the circular permutation vector pCY4. Cleavage by different restriction endonucleases generates a series of DNA fragments of equal lengths but containing the Rta binding site positioned at different locations within the fragment (Fig. 7A). Modification of the DNA curvature by Rta would lead to distinct mobilities of Rta-DNA complexes based on the position of the binding site. The lowest mobility complex is generated when the bend center is located at or near the middle of the fragment (58). f-RDBD complexes assembled on the EcoRV-generated fragments (lanes 14 and 15) displayed the slowest mobility whereas complexes assembled on the EcoRI-generated fragments (lanes 4 and 5) displayed the fastest mobility. As shown in Fig. 7A, this analysis localizes the bend site to the cloned DR1 sequence. The similar migration of the unbound fragment in the circular permuted set (Fig. 7A) indicates that the DR1 sequence does not introduce an intrinsic DNA bend.
FIG. 7.
Rta DNA-binding domain bends its site upon binding. (A) Circular permutation analysis of DR1; 2 fmol of each of the radiolabeled, circularly permuted fragments, shown in the bottom left panel, was incubated in the presence of increasing amounts of f-RDBD (0.4 ng or 0.56 nM, lanes 2, 7, 12, 17, and 22; 1.2 ng or 1.7 nM, lanes 3, 8, 13, 18, and 23; 3.6 ng or 5 nM, lanes 4, 9, 14, 19, and 24; 11.1 ng or 15 nM, lanes 5, 10, 15, 20, and 25) and 3.5 μg of poly(dI-dC) per ml. The location of the complexes is indicated by a bracket. The relative mobility of each complex with respect to the free probe was calculated and plotted against the distance of DR1 from the end of the particular fragment. Note that the slowest migrating complex corresponds to the probe containing DR1 in the center. (B) Circular permutation analysis of DR1 in the presence of HMGB1; 2 fmol of the EcoRV radiolabeled probe was incubated in the presence of increasing amounts of f-RDBD (0.4 ng or 0.56 nM, lanes 1 and 4, 1.2 ng or 1.7 nM, lanes 2 and 5, and 3.6 ng or 5 nM, lane 3) and 3.5 μg of poly(dI-dC) per ml. Then 20 ng (0.05 μM) of HMGB1 was added to lanes 4 and 5.
A similar approach was employed to examine whether the DNA curvature present in Rta complexes assembled in the presence of HMGB1 was different from that of those assembled in its absence. The autoradiogram in Fig. 7B illustrates the electrophoretic mobility of complexes formed in the presence or absence of HMGB1 on the EcoRV probe, where differences in DNA curvature would be most easily detected. Rta-DNA complexes formed in the presence of HMGB1 (lanes 4 and 5) displayed the same mobility as the complexes formed in its absence (lanes 2 and 3). This result suggests that even though HMGB1 facilitates the assembly of Rta complexes containing bent DNA, it does not alter the curvature of the final Rta-DNA complex. These results are also consistent with the model that HMGB1 is not present within the final Rta-DNA complex but rather transiently assists in its assembly.
Individual HMGB1 DNA-binding domains are sufficient to promote Rta-DNA complex formation.
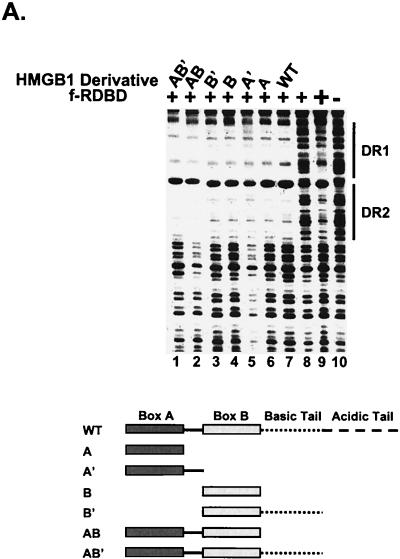
HMGB1 contains two DNA-binding domains, box A and box B, followed by basic and acidic C-terminal regions. A set of deletion mutants of HMGB1 was tested for the ability to promote f-RDBD binding to the DR1 and DR2 RREs in a DNase I footprinting assay. Figure 8A shows that each of the isolated DNA-binding domains can promote f-RDBD binding to DR1 and DR2 to the extent observed with intact HMGB1. The same results were obtained in EMSAs (data not shown). This result contrasts with stimulation of ZEBRA binding to the BHLF-1 proximal promoter by HMGB1 where both HMG boxes were required (17). The ability of either domain to function independently to facilitate Rta binding implies that DNA-bending ability alone may be sufficient.
FIG.8.
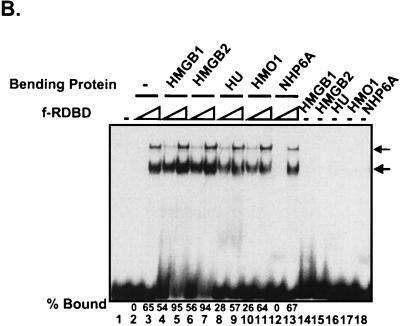
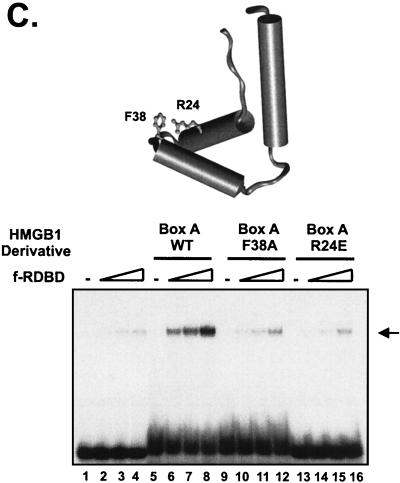
Variety of DNA-bending proteins can facilitate Rta binding. (A) Deletion of the individual DNA-binding domains of HMGB1 has no effect on the stimulation of f-RDBD binding; 5 fmol of a 110-bp radiolabeled restriction fragment containing the DR1 and DR2 binding sites was incubated in the presence of saturating (60 ng or 84 nM, lane 9) and subsaturating (15 ng or 21 nM, lanes 1 to 8) amounts of f-RDBD and 7.7 μg of poly(dI-dC) per ml. The following deletion derivatives of HMGB1 were added: 316 ng (0.84 μM, lane 7) of wild-type HMGB1, 333 ng (lane 6) of A box, 333 ng (lane 5) of A′ box, 712 ng (lane 4) of B box, 333 ng (lane 3) of B′ box, 712 ng (lane 2) of AB box, and 712 ng (lane 1) of AB′ box. The schematic in the bottom panel shows the domain organization of the various deletion derivatives. Each deletion mutant was initially titrated with f-RDBD, and the amount eliciting the greatest response was chosen for subsequent experiments. (B) Eukaryotic and prokaryotic DNA-bending proteins promote f-RDBD binding to DR1; 1 fmol of the wild type DR1 oligonucleotide was incubated in the presence of increasing amounts of f-RDBD (0.4 and 11.1 ng or 0.56 and 15 nM, respectively; lanes 2 and 3, 4 and 5, 6 and 7, 8 and 9, 10 and 11, and 12 and 13) and 7.7 μg of poly(dI-dC) per ml. The following DNA-bending proteins were added: 158 ng of HMGB1 (0.4 μM, lanes 4, 5, and 14), 333 ng of HMGB2 (0.85 μM, lanes 6, 7, and 15), 110 ng of HU (0.47 μM, lanes 8, 9, and 16), 53 ng of HMO1 (0.16 μM, lanes 10, 11, and 17), and 21 ng of NHP6A (0.15 μM, lanes 12, 13, and 18). The percent probe bound by f-RDBD was calculated as described for Fig. 3C. The bending proteins were initially titrated with f-RDBD, and the concentrations that elicited the maximal helping effect were chosen for subsequent experiments. (C) Mutations in the HMGB1 A box that affect DNA bending and DNA binding abolish the helping effect on f-RDBD binding. The schematic illustration of the box A structure (44) indicates the position of the three α-helices in box A and the positions of phenylalanine 38, which was mutated to an alanine, and arginine 24, which was replaced with a glutamic acid. The proteins were expressed and purified as described in Materials and Methods. The wild-type and mutant proteins were assayed by EMSA for their ability to facilitate f-RDBD binding (bottom panel); 1 fmol of radiolabeled DR1 oligonucleotide was incubated with increasing amounts of f-RDBD (0.2, 0.4, and 1.2 ng or 0.3, 0.6, and 1.7 nM) and 7.7 μg of poly(dI-dC) per ml. Then 150 ng (0.18 μM) of box A wild-type or mutants was added to the reaction mixtures as indicated. Note that addition of the DNA-bending mutant (F38A) causes a slight upward smear of the probe in a manner similar to wild-type box A, which reflects their ability to form unstable complexes with DNA. The DNA-binding mutant R24E does not smear the probe.
Other DNA architectural proteins promote Rta binding.
Several other sequence-neutral DNA bending proteins were tested by EMSA for their ability to promote f-RDBD binding to the DR1 site (Fig. 8B). As expected, HMGB2, which is highly related to HMGB1, displays activity similar to that of HMGB1. HMO1 is a distantly related two-domain HMGB protein from Saccharomyces cerevisiae, whose DNA-binding and -bending properties are similar to those of HMGB1 (35; Y.-M. Yen and R. C. Johnson, unpublished). HMO1 promotes f-RDBD binding (lanes 10 and 11), although its activity is not quite as strong as that of HMGB1. Most significantly, a completely unrelated prokaryotic protein HU (15) is also active in facilitating f-RDBD binding (lanes 8 and 9). The magnitude of the stimulation at low f-RDBD ranged from 54- and 56-fold for HMGB1 and -2, respectively, to 26-fold for HMO1 and 28-fold for HU. The S. cerevisiae HMGB protein NHP6A failed to stimulate f-RDBD binding (compare lanes 2 and 12). This finding might be explained by the fact that NHP6A binds DNA with considerably higher affinity than the other tested proteins (46), an effect that may potentially interfere with the interaction of f-RDBD with its site during the formation of a stable f-RDBD:DNA complex. The activities of the unrelated DNA architectural proteins argue against the existence of specific protein-protein interactions with Rta that operate to promote binding.
Mutants of HMGB1 are defective in promoting Rta binding.
In order to directly establish the importance of HMGB-induced DNA bending in promotion of Rta binding, we tested two mutants of HMGB1 box A containing single-amino-acid substitutions of the residues critical for DNA bending and DNA binding (Fig. 8C). Phenylalanine 38 of box A (see Fig. 8C, top panel) has been shown by X-ray crystallography to intercalate into the kinked base pair step induced by cisplatination (44). Moreover, the analogous phenylalanine in NHP6A has been found to be the most important residue for DNA bending by nuclear magnetic resonance and ligation-mediated circularization assays (2). The side chain of arginine 24 of box A directly contacts the DNA backbone (44). Substitutions within the analogous residues of box B (arginine 110) and NHP6A (arginine 36) result in mutants that are defective in DNA binding (2, 50). Both the F38A and R24E mutants fail to stimulate bending by a ligation-mediated circularization assay under conditions where the wild type displayed robust activity (data not shown). As shown in the EMSA experiment of Fig. 8C, box A F38A and R24E only weakly, if at all, stimulate Rta binding to the RRE probe. These results reinforce the notion that DNA binding and bending by the HMGB proteins are a critical determinant in the stimulation of Rta binding.
DISCUSSION
HMGB1 assembles enhanceosome complexes on the BHLF-1 regulatory region through two distinct modes of action.
On the BHLF-1 enhancer, HMGB1 stimulates binding of individual Rta dimers to their cognate sites. The results of transfection experiments indicate that this event augments transcription. HMGB1-stimulated Rta binding in vitro requires only the DNA-binding domain of Rta and occurs on a single site. However, despite the use of high-resolution footprinting, EMSA, affinity resins, and immobilized template assays, HMGB1 was not found to be a stable component of the Rta-DNA complex. Moreover, the mutation of sequences within and around the Rta site also failed to reveal an HMGB1 target sequence. HMGB2 and the individual A and B DNA-binding domains of HMGB1 substitute for intact HMGB1. Furthermore, the distantly related HMO1 protein of yeast, which also contains two HMG domains, and the completely unrelated HU protein of bacteria also functionally substitute for HMGB1 to promote Rta binding. Finally, a mutant box A protein, containing an F38A substitution at a critical bending residue, failed to stimulate RDBD binding, as did a DNA-binding mutant, R24E.
These findings, taken collectively, imply that the ability of a protein to bind and bend DNA is the primary determinant of the stimulatory effect, rather than a particular feature of the primary sequence or structure of the bending protein. The data argue strongly against the existence of direct protein interactions between the bending protein and Rta. The effect is not entirely nonspecific, because the box A mutants, NHP6A, bovine serum albumin, and DNA condensation agents like spermidine were inactive.
The results with Rta contrast with those previously observed with ZEBRA (16, 17). HMGB1 stimulates ZEBRA-mediated transcription in transient transfection assays, and promotes the binding of pairs of ZEBRA dimers to their sites in the proximal promoter; HMGB1 did not display a binding effect on individual ZREs. Also unlike Rta, HMGB1 joins the complex with pairs of ZEBRA dimers and generates a distinct DNase I and hydroxyl radical footprint between the sites. HMGB1 binding was sequence dependent because mutation of the DNA protected by HMGB1 abolished its ability to bind and stimulate ZEBRA but did not influence the intrinsic affinity of ZEBRA for its sites. Furthermore, insertion of helical increments abolished binding. Finally, only intact HMGB1, which contains two HMG domains, could mediate cooperative binding of ZEBRA. The individual A and B boxes of HMGB1, NHP6A, and HMO1 all failed to stimulate. Importantly, HMGB1 interaction with ZEBRA was not observed in either a glutathione S-transferase-ZEBRA pulldown assay nor by EMSA with ZEBRA bound at individual ZREs.
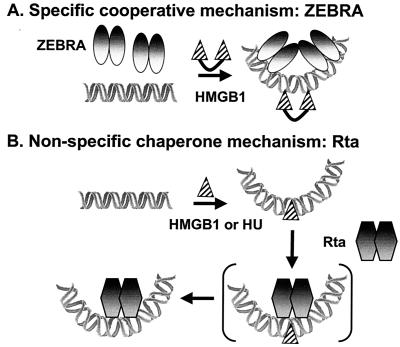
Taken together, the results of the two studies indicate that HMGB1 has two modes of action at BHLF-1 (Fig. 9): it can function as a stable component of a nucleoprotein complex, where it displays elements of sequence dependence, and it can function in an unstable mode, with no apparent specificity. In this unstable mode, there may be a transient intermediate where the bending protein is at or near the site, thereby facilitating Rta binding, but the bending protein does not stably associate nor does it alter the intrinsic bend elicited by Rta. In neither the case of ZEBRA at Z3 and Z4 or Rta at an RRE is an interaction between the activator and HMGB1 observed. This dual functionality enables HMGB1 to facilitate the assembly of complexes containing diverse activators throughout the BHLF-1 regulatory region.
FIG. 9.
Two models for HMGB1 action. (A) Two ZEBRA dimers and HMGB1 bind cooperatively. Binding of HMGB1 is specific but only occurs in the context of two pairs of ZEBRA dimers. (B) The chaperone mechanism proposed for Rta, where a stable HMGB1-containing intermediate is not detected prior to or after Rta binding. It is plausible that an unstable intermediate exists where Rta and HMGB1 are transiently bound (brackets). At this point, however, it is not known whether Rta or HMGB1 binds first and stimulates the other. However, we find no evidence for a stable interaction of HMGB1 with Rta in either the presence or absence of DNA. The figure is schematic and not drawn to scale. The placement of the proteins on the schematic DNA is not intended to indicate their positions in the grooves.
The term DNA chaperone has been applied to proteins that induce and stabilize a DNA conformation, such as a bend, that is favorable for nucleoprotein complex assembly (55). The prokaryotic architectural factor HU functions as a DNA chaperone. HU is a highly abundant, dimeric protein that displays little sequence preference and, like eukaryotic HMGB1/2, bends its target DNA through interactions with the minor groove (43). HU has been shown to participate in DNA replication, recombination, transcription and repair (3, 4, 25, 26, 29, 30). Ironically, HMGB1 and -2 were identified as nucleoprotein assembly factors by virtue of their ability to functionally substitute for HU in the DNA inversion reaction by the prokaryotic Hin recombinase (45). Although there have been a number of examples where HMGB1 has successfully substituted for HU and integration host factor (IHF) in prokaryotic reactions, HU stimulation of BHLF-1 enhanceosome assembly is the first case we are aware of where the reverse is true. The ability of unrelated DNA-bending proteins to substitute for one another is strong evidence for a chaperone function where no specific protein-protein interactions are involved.
It is difficult to craft a specific hypothesis as to how HMGB1 functions with Rta. In order to do so requires that we understand when and where HMGB1 acts. Perhaps Rta binds DNA weakly and the subsequent transient binding of HMGB1 or HU alters the DNA conformation. Alternatively, the architectural proteins could create conformations that facilitate Rta binding. Detailed kinetic analyses would be required to address this issue.
In an attempt to understand where HMGB1 acts, we modeled Rta-DNA complexes using the chemical probe data. The binding of Rta to its site resembles in some respects the binding of GAL4 and lambda repressor (8, 28, 38, 57). All three proteins bind 17-bp pseudosymmetric sites as homodimers. Although the structures of the lambda repressor and GAL4 DNA-binding domains are distinct, their contacts with DNA and general mode of binding bear some resemblance (discussed in reference 8). In both cases the two monomers insert recognition α-helices into major grooves in each half site and dimerize over the intervening minor groove in the center of pseudosymmetry. The structural data indicate that the guanines protected from DMS in each half site represent binding of individual monomers of GAL4 and lambda repressor. The hydroxyl radical protections in the center of the sites represent the region below the dimerization interface of the two proteins. The hydroxyl radical footprinting performed on Rta here (Fig. 4B), taken together with the dimethyl sulfate interference data generated by Gruffat and colleagues (24), reveals a similar overall pattern. The methylated guanines that interfere with Rta binding lie in adjacent major grooves and the sugars in the central minor groove of each site are protected from hydroxyl radical cleavage. In conclusion, we predict that Rta is binding as a homodimer with monomers contacting guanines in each half-site as the dimerization occurs above the intervening minor groove.
This model, however, precludes one obvious hypothesis for how HMGB1 stimulates Rta binding, through bending of DNA at the center of the site. HMGB1 is a minor-groove-binding protein, and the center of the RRE likely coincides with the Rta dimerization interface. It is therefore unlikely that HMGB1 binds there in the presence of a stable Rta dimer. It is plausible, however, that individual Rta monomers bind weakly to the half sites and that transient bending by HMGB1, followed by its dissociation, promotes Rta dimerization and stable binding. If this model is correct, the directionality of the bend must not matter. HU stimulates Rta binding but bends the DNA in a completely different direction than HMGB1.
How HMGB1 functions in other cases.
Steroid receptor and RAG1/2 bind DNA as dimers to 15- and 38-bp sites, respectively, and both proteins introduce bends into the DNA. However, the precise mechanism by which bending facilitates binding of these factors remains to be elucidated. In both cases HMGB1 contacts the target protein and appears to join the complex on DNA by EMSA. Members of the steroid receptor family have been crystallized and suggest a mode of binding similar to that proposed for Rta, where the center is occupied by the dimerization interface (36). Thus, it is unlikely that HMGB1 binds there stably. In contrast, RAG1 and -2 bind to conserved nonamer and heptamer sites separated by 12 and 23 bp. HMGB1 stimulates binding of RAG1 and -2 to the 23-bp RSS, presumably through interactions with the RAG1 homeodomain. There is a significant distance between the bound RAG proteins, suggesting that HMGB1 could bind at different positions between the sites. This supposition was supported by recent chemical and nuclease probing of the RAG1/2-HMGB1/2-23-bp RSS complex, which suggested HMGB1 binding 5′ to the nonamer (52). The ZEBRA sites in BHLF-1 are 26 bp apart, and HMGB1-stimulated RAG1/2 binding may be occurring in a similar fashion, with the caveat that the sequence divergence of the RSS argues against a specific HMGB1 interaction.
Our studies demonstrate that HMGB1 appears to stimulate protein binding to DNA simply through bending. In some cases HMGB1 may stably join the complex with one or more proteins, whereas in other cases the association may be transient. This will ultimately depend on the geometry and energetics of complex assembly. Our results underscore the potential versatility of HMGB1 in promoting DNA transactions in gene regulation and favor the notion that a direct HMGB1 interaction with a target protein is not necessarily required.
Acknowledgments
We thank Yi-Meng Yen for providing us with recombinant NHP6A and HMO1, Sarah McLeod for advice with circular permutation assays, Janet Treger for help with immunoblotting, and Mike Haykinson for assistance with InsightII.
This study was supported by grants GM057283 (M.C.) and GM38509 (R.C.J.) from the National Institutes of Health.
REFERENCES
- 1.Aidinis, V., T. Bonaldi, M. Beltrame, S. Santagata, M. E. Bianchi, and E. Spanopoulou. 1999. The RAG1 homeodomain recruits HMG1 and HMG2 to facilitate recombination signal sequence binding and to enhance the intrinsic DNA-bending activity of RAG1-RAG2. Mol. Cell. Biol. 19:6532-6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allain, F. H., Y. M. Yen, J. E. Masse, P. Schultze, T. Dieckmann, R. C. Johnson, and J. Feigon. 1999. Solution structure of the HMG protein NHP6A and its interaction with DNA reveals the structural determinants for non-sequence-specific binding. EMBO J. 18:2563-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahloul, A., F. Boubrik, and J. Rouviere-Yaniv. 2001. Roles of Escherichia coli histone-like protein HU in DNA replication: HU-beta suppresses the thermosensitivity of dnaA46ts. Biochimie 83:219-229. [DOI] [PubMed] [Google Scholar]
- 4.Bonnefoy, E., and J. Rouviere-Yaniv. 1992. HU, the major histone-like protein of E. coli, modulates the binding of IHF to oriC. EMBO J. 11:4489-4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boonyaratanakornkit, V., V. Melvin, P. Prendergast, M. Altmann, L. Ronfani, M. E. Bianchi, L. Taraseviciene, S. K. Nordeen, E. A. Allegretto, and D. P. Edwards. 1998. High-mobility group chromatin proteins 1 and 2 functionally interact with steroid hormone receptors to enhance their DNA-binding in vitro and transcriptional activity in mammalian cells. Mol. Cell. Biol. 18:4471-4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bustin, M. 1999. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol. Cell. Biol. 19:5237-5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carey, M. 1998. The enhanceosome and transcriptional synergy. Cell 92:5-8. [DOI] [PubMed] [Google Scholar]
- 8.Carey, M., H. Kakidani, J. Leatherwood, F. Mostashari, and M. Ptashne. 1989. An amino-terminal fragment of GAL4 binds DNA as a dimer. J. Mol. Biol. 209:423-432. [DOI] [PubMed] [Google Scholar]
- 9.Carey, M., J. Kolman, D. A. Katz, L. Gradoville, L. Barberis, and G. Miller. 1992. Transcriptional synergy by the Epstein-Barr virus transactivator ZEBRA. J. Virol. 66:4803-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chi, T., and M. Carey. 1993. The ZEBRA activation domain: modular organization and mechanism of action. Mol. Cell. Biol. 13:7045-7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Countryman, J., and G. Miller. 1985. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc. Natl. Acad. Sci. USA 82:4085-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox, M. A., J. Leahy, and J. M. Hardwick. 1990. An enhancer within the divergent promoter of Epstein-Barr virus responds synergistically to the R and Z transactivators. J. Virol. 64:313-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decoville, M., M. J. Giraud-Panis, C. Mosrin-Huaman, M. Leng, and D. Locker. 2000. HMG boxes of DSP1 protein interact with the rel homology domain of transcription factors. Nucleic Acids Res. 28:454-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dixon, W. J., J. J. Hayes, J. R. Levin, M. F. Weidner, B. A. Dombroski, and T. D. Tullius. 1991. Hydroxyl radical footprinting. Methods Enzymol. 208:380-413. [DOI] [PubMed] [Google Scholar]
- 15.Drlica, K., and J. Rouviere-Yaniv. 1987. Histonelike proteins of bacteria. Microbiol. Rev. 51:301-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellwood, K., W. Huang, R. Johnson, and M. Carey. 1999. Multiple layers of cooperativity regulate enhanceosome-responsive RNA polymerase II transcription complex assembly. Mol. Cell. Biol. 19:2613-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellwood, K. B., Y. M. Yen, R. C. Johnson, and M. Carey. 2000. Mechanism for specificity by HMG-1 in enhanceosome assembly. Mol. Cell. Biol. 20:4359-4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emami, K. H., and M. Carey. 1992. A synergistic increase in potency of a multimerized VP16 transcriptional activation domain. EMBO J. 11:5005-5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feederle, R., M. Kost, M. Baumann, A. Janz, E. Drouet, W. Hammerschmidt, and H. J. Delecluse. 2000. The Epstein-Barr virus lytic program is controlled by the cooperative functions of two transactivators. EMBO J. 19:3080-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fields, B. N., D. M. Knipe, and P. M. Howley (ed.). 1996. Fields virology, 3rd ed. Lippincott-Raven Press, New York, N.Y.
- 21.Giese, K., C. Kingsley, J. R. Kirshner, and R. Grosschedl. 1995. Assembly and function of a TCR alpha enhancer complex is dependent on LEF-1-induced DNA bending and multiple protein-protein interactions. Genes Dev. 9:995-1008. [DOI] [PubMed] [Google Scholar]
- 22.Grosschedl, R., K. Giese, and J. Pagel. 1994. HMG domain proteins: architectural elements in the assembly of nucleoprotein structures. Trends Genet. 10:94-100. [DOI] [PubMed] [Google Scholar]
- 23.Gruffat, H., E. Manet, A. Rigolet, and A. Sergeant. 1990. The enhancer factor R of Epstein-Barr virus (EBV) is a sequence-specific DNA-binding protein. Nucleic Acids Res. 18:6835-6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gruffat, H., and A. Sergeant. 1994. Characterization of the DNA-binding site repertoire for the Epstein-Barr virus transcription factor R. Nucleic Acids Res. 22:1172-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haykinson, M. J., and R. C. Johnson. 1993. DNA looping and the helical repeat in vitro and in vivo: effect of HU protein and enhancer location on Hin invertasome assembly. EMBO J. 12:2503-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaffe, A., D. Vinella, and R. D'Ari. 1997. The Escherichia coli histone-like protein HU affects DNA initiation, chromosome partitioning via MukB, and cell division via MinCDE. J. Bacteriol. 179:3494-3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jayaraman, L., N. C. Moorthy, K. G. Murthy, J. L. Manley, M. Bustin, and C. Prives. 1998. High mobility group protein-1 (HMG-1) is a unique activator of p53. Genes Dev. 12:462-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordan, S. R., and C. O. Pabo. 1988. Structure of the lambda complex at 2.5 Å resolution: details of the repressor-operator interactions. Science 242:893-899. [DOI] [PubMed] [Google Scholar]
- 29.Kamashev, D., and J. Rouviere-Yaniv. 2000. The histone-like protein HU binds specifically to DNA recombination and repair intermediates. EMBO J. 19:6527-6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis, D. E., M. Geanacopoulos, and S. Adhya. 1999. Role of HU and DNA supercoiling in transcription repression: specialized nucleoprotein repression complex at gal promoters in Escherichia coli. Mol. Microbiol. 31:451-461. [DOI] [PubMed] [Google Scholar]
- 31.Lieberman, P. M., and A. J. Berk. 1990. In vitro transcriptional activation, dimerization, and DNA-binding specificity of the Epstein-Barr virus Zta protein. J. Virol. 64:2560-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieberman, P. M., J. M. Hardwick, and S. D. Hayward. 1989. Responsiveness of the Epstein-Barr virus NotI repeat promoter to the Z transactivator is mediated in a cell-type-specific manner by two independent signal regions. J. Virol. 63:3040-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lieberman, P. M., J. M. Hardwick, J. Sample, G. S. Hayward, and S. D. Hayward. 1990. The Zta transactivator involved in induction of lytic cycle gene expression in Epstein-Barr virus-infected lymphocytes binds to both AP-1 and ZRE sites in target promoter and enhancer regions. J. Virol. 64:1143-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu, F., and M. R. Green. 1990. A specific member of the ATF transcription factor family can mediate transcription activation by the adenovirus E1a protein. Cell 61:1217-1224. [DOI] [PubMed] [Google Scholar]
- 35.Lu, J., R. Kobayashi, and S. J. Brill. 1996. Characterization of a high mobility group 1/2 homolog in yeast. J. Biol. Chem. 271:33678-33685. [DOI] [PubMed] [Google Scholar]
- 36.Luisi, B. F., W. X. Xu, Z. Otwinowski, L. P. Freedman, K. R. Yamamoto, and P. B. Sigler. 1991. Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature 352:497-505. [DOI] [PubMed] [Google Scholar]
- 37.Manet, E., A. Rigolet, H. Gruffat, J. F. Giot, and A. Sergeant. 1991. Domains of the Epstein-Barr virus (EBV) transcription factor R required for dimerization, DNA-binding and activation. Nucleic Acids Res. 19:2661-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marmorstein, R., M. Carey, M. Ptashne, and S. C. Harrison. 1992. DNA recognition by GAL4: structure of a protein-DNA complex. Nature 356:408-414. [DOI] [PubMed] [Google Scholar]
- 39.Merika, M., and D. Thanos. 2001. Enhanceosomes. Curr. Opin. Genet. Dev. 11:205-208. [DOI] [PubMed] [Google Scholar]
- 40.Metzenberg, S. 1989. Relative rates of RNA synthesis across the genome of Epstein-Barr virus are highest near oriP and oriLyt. J. Virol. 63:4938-4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy, F. V., IV, and M. E. Churchill. 2000. Nonsequence-specific DNA recognition: a structural perspective. Structure Fold Des. 8:R83-R89. [DOI] [PubMed] [Google Scholar]
- 42.Murphy, F. V., IV, R. M. Sweet, and M. E. Churchill. 1999. The structure of a chromosomal high mobility group protein-DNA complex reveals sequence-neutral mechanisms important for non-sequence-specific DNA recognition. EMBO J. 18:6610-6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nash, H. A. 1996. The E. coli HU and IHF proteins: accessory factors for complex protein-DNA assemblies, p. 149-179. In A. S. Lynch and E. E. C. Lin (ed.), Regulation of gene expression in Escherichia coli. R. G. Landes Co., Austin, Tex.
- 44.Ohndorf, U. M., M. A. Rould, Q. He, C. O. Pabo, and S. J. Lippard. 1999. Basis for recognition of cisplatin-modified DNA by high-mobility-group proteins. Nature 399:708-712. [DOI] [PubMed] [Google Scholar]
- 45.Paull, T. T., M. J. Haykinson, and R. C. Johnson. 1993. The nonspecific DNA-binding and -bending proteins HMG1 and HMG2 promote the assembly of complex nucleoprotein structures. Genes Dev. 7:1521-1534. [DOI] [PubMed] [Google Scholar]
- 46.Paull, T. T., and R. C. Johnson. 1995. DNA looping by Saccharomyces cerevisiae high mobility group proteins NHP6A/B. Consequences for nucleoprotein complex assembly and chromatin condensation. J. Biol. Chem. 270:8744-8754. [DOI] [PubMed] [Google Scholar]
- 47.Prentki, P., M. H. Pham, and D. J. Galas. 1987. Plasmid permutation vectors to monitor DNA bending. Nucleic Acids Res. 15:10060.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ragoczy, T., L. Heston, and G. Miller. 1998. The Epstein-Barr virus Rta protein activates lytic cycle genes and can disrupt latency in B lymphocytes. J. Virol. 72:7978-7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ragoczy, T., and G. Miller. 1999. Role of the Epstein-Barr virus Rta protein in activation of distinct classes of viral lytic cycle genes. J. Virol. 73:9858-9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stros, M., and E. Muselikova. 2000. A role of basic residues and the putative intercalating phenylalanine of the HMG-1 box B in DNA supercoiling and binding to four-way DNA junctions. J. Biol. Chem. 275:35699-35707. [DOI] [PubMed] [Google Scholar]
- 51.Sugden, B., J. Yates, and W. Mark. 1984. Transforming functions associated with Epstein-Barr virus. J. Investig. Dermatol. 83:82s-87s. [DOI] [PubMed]
- 52.Swanson, P. C. 2002. Fine structure and activity of discrete RAG-HMG complexes on V(D)J recombination signals. Mol. Cell. Biol. 22:1340-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas, J. O., and A. A. Travers. 2001. HMG1 and 2, and related "architectural' DNA-binding proteins. Trends Biochem. Sci. 26:167-174. [DOI] [PubMed] [Google Scholar]
- 54.Travers, A. 2000. Recognition of distorted DNA structures by HMG domains. Curr. Opin. Struct. Biol. 10:102-109. [DOI] [PubMed] [Google Scholar]
- 55.Travers, A. A., S. S. Ner, and M. E. Churchill. 1994. DNA chaperones: a solution to a persistence problem? Cell 77:167-169. [DOI] [PubMed] [Google Scholar]
- 56.Tullius, T. D. 1988. DNA footprinting with hydroxyl radical. Nature 332:663-664. [DOI] [PubMed] [Google Scholar]
- 57.Tullius, T. D., and B. A. Dombroski. 1986. Hydroxyl radical “footprinting”: high-resolution information about DNA-protein contacts and application to lambda repressor and Cro protein. Proc. Natl. Acad. Sci. USA 83:5469-5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu, H. M., and D. M. Crothers. 1984. The locus of sequence-directed and protein-induced DNA bending. Nature 308:509-513. [DOI] [PubMed] [Google Scholar]
- 59.Yen, Y. M., B. Wong, and R. C. Johnson. 1998. Determinants of DNA-binding and bending by the Saccharomyces cerevisiae high mobility group protein NHP6A that are important for its biological activities. Role of the unique N terminus and putative intercalating methionine. J. Biol. Chem. 273:4424-4435. [DOI] [PubMed] [Google Scholar]
- 60.Zalani, S., E. Holley-Guthrie, and S. Kenney. 1996. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc. Natl. Acad. Sci. USA 93:9194-9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zappavigna, V., L. Falciola, M. Helmer-Citterich, F. Mavilio, and M. E. Bianchi. 1996. HMG1 interacts with HOX proteins and enhances their DNA-binding and transcriptional activation. EMBO J. 15:4981-4991. [PMC free article] [PubMed] [Google Scholar]
- 62.Zwilling, S., H. Konig, and T. Wirth. 1995. High mobility group protein 2 functionally interacts with the POU domains of octamer transcription factors. EMBO J. 14:1198-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]