Abstract
Receptor tyrosine kinases (RTKs) play distinct roles in multiple biological systems. Many RTKs transmit similar signals, raising questions about how specificity is achieved. One potential mechanism for RTK specificity is control of the magnitude and kinetics of activation of downstream pathways. We have found that the protein tyrosine phosphatase Shp2 regulates the strength and duration of phosphatidylinositol 3′-kinase (PI3K) activation in the epidermal growth factor (EGF) receptor signaling pathway. Shp2 mutant fibroblasts exhibit increased association of the p85 subunit of PI3K with the scaffolding adapter Gab1 compared to that for wild-type (WT) fibroblasts or Shp2 mutant cells reconstituted with WT Shp2. Far-Western analysis suggests increased phosphorylation of p85 binding sites on Gab1. Gab1-associated PI3K activity is increased and PI3K-dependent downstream signals are enhanced in Shp2 mutant cells following EGF stimulation. Analogous results are obtained in fibroblasts inducibly expressing dominant-negative Shp2. Our results suggest that, in addition to its role as a positive component of the Ras-Erk pathway, Shp2 negatively regulates EGF-dependent PI3K activation by dephosphorylating Gab1 p85 binding sites, thereby terminating a previously proposed Gab1-PI3K positive feedback loop. Activation of PI3K-dependent pathways following stimulation by other growth factors is unaffected or decreased in Shp2 mutant cells. Thus, Shp2 regulates the kinetics and magnitude of RTK signaling in a receptor-specific manner.
Receptor tyrosine kinases (RTKs) play critical roles in the regulation of cell growth, motility, differentiation, and death (12, 37). There are a large number of RTKs, and genetic analyses reveal that they have profoundly different biological effects. However, RTK signaling mechanisms are remarkably similar (28, 37). Ligand binding causes receptor dimerization, kinase activation, and trans-phosphorylation of the RTK on multiple tyrosyl residues. These phosphotyrosines serve as docking sites for the recruitment of a variety of signal relay molecules that contain Src homology 2 (SH2) or phosphotyrosine binding (PTB) domains, leading to the activation of multiple downstream signaling pathways. Some receptors also employ scaffolding adapter molecules, which appear to amplify receptor signals (28). Typically, scaffolding adapters are comprised of a membrane-targeting motif (typically a pleckstrin homology [PH] and often a PTB domain) and multiple additional binding sites for SH2- and/or PTB domain-containing proteins. Some differences in RTK biological effects may be explained by differential utilization of signal relay molecules. However, many RTKs utilize the same (or a similar) panoply of signal relay molecules-scaffolding adapters. Understanding how signal specificity is achieved by these receptors remains a major challenge. An important mechanism of receptor specificity may be control of the kinetics and/or the magnitude of downstream signal responses (21). Yet how these are regulated differentially by RTKs remains unclear.
Gab1 (Grb2-associated binder 1) is a member of a small group of scaffolding adapters that includes Drosophila melanogaster Dos (Daughter of Sevenless) and mammalian Gab2 and Gab3 (8-10, 25, 30, 47; H. Keilhack, H. Gu, and B. G. Neel, unpublished data). These proteins contain an amino-terminal PH domain, several proline-rich sequences, and multiple binding sites for SH2 domain-containing proteins. Upon stimulation of appropriate cells with any of a number of RTK ligands, including epidermal growth factor (EGF), hepatocyte growth factor (HGF), platelet-derived growth factor (PDGF), nerve growth factor (NGF), and insulin or insulin-like growth factor 1 (IGF-1), Gab1 rapidly becomes tyrosyl phosphorylated (10, 11, 25, 44). Tyrosyl-phosphorylated Gab1 binds multiple signal relay molecules, including the p85 subunit of phosphatidylinositol 3′-kinase (PI3K) (p85), Shc, Grb2, and the protein tyrosine phosphatase (PTP) Shp2 (10, 11, 25, 41, 44). Gab1 is required for signaling by several RTKs, as Gab1-deficient mice die in utero (at E12.5 to E17.5) with phenotypes similar to those observed for mice defective in signaling by HGF, PDGF, or EGF (14, 34). Moreover, primary fibroblasts from Gab1−/− embryos exhibit decreased activation of the Erk mitogen-activated protein kinase pathway in response to EGF, PDGF, and HGF.
To understand how Gab1 participates in RTK signaling, the functions of individual Gab1-signal relay molecule interactions and how these interactions affect each other must be elucidated. Several lines of evidence indicate that Gab1 acts via Shp2 to control Erk activation. Mutants of Gab1 (20) or receptor-Gab1 chimeras (36) that lack Shp2 binding sites are unable to cause either Erk activation or morphogenesis in MDCK cells. In addition, overexpression of deletion or point mutants of Gab1 lacking Shp2 binding blocks EGF-stimulated Erk activation in transient-transfection systems (2, 3). Furthermore, dominant-negative (PTP-inactive) mutants of Shp2 block Erk activation in response to stimulation by a wide variety of growth factors (4, 24), many of which signal through Gab1, and Shp2 mutant fibroblasts exhibit defective Erk activation in response to most of these growth factors (35, 38, 39). Although Shp2 appears to act upstream of Ras in regulating Erk activation (26, 39), its precise target in this pathway remains unknown. Conceivably, Shp2 might regulate the phosphorylation of Gab1 or a Gab1 binding protein. However, total Gab1 tyrosyl phosphorylation is unaffected in Shp2 mutant fibroblasts (39), arguing that, if Shp2 dephosphorylates Gab1, it must target specific (and a limited number of) sites.
The Gab1-p85 interaction appears to play a distinct but nonetheless important role in RTK signaling, as it is critical for PI3K activation in response to stimulation of the NGF receptor TrkA (11) and the EGF receptor (EGFR) (31). Schlessinger and colleagues proposed a positive feedback loop model involving Gab1 and PI3K in EGFR signaling (31). Initial recruitment of Gab1 by the EGFR is mediated by two EGFR tyrosyl residues (Y1068 and Y1086) and the proline-rich Met binding domain on Gab1. This results in Gab1 tyrosyl phosphorylation and PI3K association which, in turn, catalyze local production of PI3,4,5P3 (PIP3). PIP3 binds to the Gab1 PH domain, increasing the recruitment of Gab1 to the plasma membrane and leading to a further increase in Gab1 tyrosyl phosphorylation and PI3K activation. This positive feedback loop may be required to generate a PI3K signal that is sustained sufficiently to elicit biological effects. Analogous pathways could exist for other RTKs that signal via Gab1.
This positive feedback model raises the obvious question of how PI3K signaling is terminated. Here, we show that, in addition to its role in Gab1-mediated Erk activation, Shp2 attenuates PI3K activation by controlling the phosphorylation of the p85 binding sites on Gab1 in response to EGF. Interestingly, this regulatory mechanism is RTK specific: PI3K activation in response to other growth factors is unaffected or potentiated by Shp2. Our results show that scaffolding adapters, such as Gab1, in concert with PTPs such as Shp2, can control the kinetics, extent, and location of PI3K activation in response to specific growth factors.
MATERIALS AND METHODS
Antibodies and reagents.
Recombinant human EGF was purchased from Upstate Biotechnology Inc. (Lake Placid, N.Y.). Recombinant human PDGF BB and IGF-1 were purchased from Life Technologies (Carlsbad, Calif.). Rabbit polyclonal anti-Gab1 antibodies were raised against a glutathione S-transferase (GST)-Gab1(238-379) fusion protein. Anti-Shc and anti-Grb2 monoclonal antibodies (MAbs) were purchased from Transduction Laboratories (Lexington, Ky.). Rabbit polyclonal anti-p85 antibodies were a gift of Lewis C. Cantley (Beth Israel-Deaconess Medical Center, Boston, Mass.). Anti-phospho-Akt, anti-phospho-Erk, anti-phospho-glycogen synthase kinase 3β (anti-phospho-GSK-3β), and anti-GSK-3β antibodies were purchased from Cell Signaling Technology (Beverly, Mass.). Anti-EGFR, anti-Shp2, anti-Akt, and anti-GST antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). Antiphosphotyrosine MAb (4G10) was from Upstate Biotechnology. A bacterial expression vector for GST-p85-N-SH2 was kindly provided by Brian S. Schaffhausen (Tufts University Medical School, Boston, Mass.). The green fluorescent protein (GFP)-Gab1, hemagglutinin (HA)-tagged wild-type (WT) Gab1, and HA-tagged Gab1ΔShp2 mammalian expression constructs were a gift from Christiane R. Maroun and Morag Park (McGill University, Montreal, Quebec, Canada). Protein A and protein G-Sepharose were purchased from Pharmacia Biotech (Uppsala, Sweden).
Cell lines and culture.
3T3-immortalized fibroblast lines from Shp2 exon 3−/− (Shp2Δ46-110) and littermate WT mice were described previously (27) and were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. To stably restore Shp2 expression to the Shp2 mutant cells, full-length Shp2 cDNA (6) was inserted into the retroviral plasmid pBabe-puro (22). Phoenix-Ecotropic packaging cells (a gift of Garry P. Nolan, Stanford University School of Medicine) were transiently transfected with the Shp2 retroviral construct. After 24 h, viral supernatants were collected and used to infect Shp2−/− cells in the presence of Polybrene (4 μg/ml) for 2 h. Cells were allowed to recover in normal medium (see above) for 24 h and then selected with puromycin (2 μg/ml). Puromycin-resistant clones were pooled, and Shp2 expression was assessed by immunoblotting.
A tetracycline-inducible NIH 3T3 cell line that expresses catalytically inactive, HA-tagged Shp2 was established in this laboratory by transfecting parental NIH 3T3 cells stably expressing the tetracycline transactivator tTA (40) with HA-tagged Shp2 C459S (C/S) in the vector pTet-Splice (Life Technologies, Inc.). Cells were maintained in the same growth medium as above plus tetracycline (2 μg/ml) and puromycin (2 μg/ml). Expression of Shp2 (C/S) protein was very low in the presence of tetracycline; removal of tetracycline from the growth medium resulted in expression of Shp2 (C/S) protein at about twice the level of the endogenous WT Shp2 (see Fig. 3).
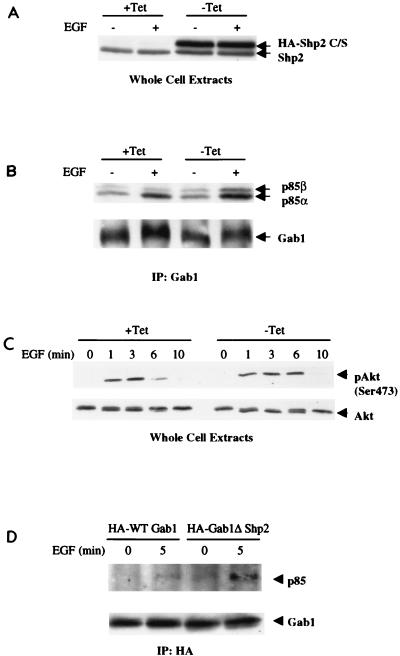
FIG. 3.
Increased Gab1-p85 association and elevated Akt activity in NIH 3T3 cells inducibly overexpressing catalytically inactive (C/S) Shp2. (A) Inducible Shp2 C/S cells were serum starved overnight in the presence (+Tet) or absence (−Tet) of tetracycline before stimulation with EGF (50 ng/ml) for 5 min. Total cellular proteins (50 μg) were resolved by SDS-PAGE and immunoblotted with anti-Shp2. Note that, following induction, expression of HA-Shp2 (C/S) was about twice that of endogenous Shp2. (B) Inducible Shp2 C/S cells were serum starved and stimulated as described for panel A. Gab1 immunoprecipitates were analyzed by SDS-PAGE and immunoblotting with anti-p85 antibodies. The blot was then stripped and reprobed with anti-Gab1. Note that Gab1 associates with both p85α and p85β in these cells. (C) Inducible Shp2 C/S cells were serum starved and stimulated as for panel A. Akt phosphorylation was examined by immunoblotting with anti-phospho-Akt (Ser-473) antibodies, after which the membrane was stripped and reprobed with anti-total Akt. (D) 293 cells were transiently transfected with HA-WT Gab1 or HA-Gab1ΔShp2 constructs. After 24 h, cells were serum starved overnight and then stimulated with EGF (50 ng/ml) for 5 min. Cell lysates were subjected to immunoprecipitation with anti-HA antibodies followed by SDS-PAGE and immunoblotting with anti-p85 antibodies. The blot was then stripped and reprobed with anti-HA antibodies to ensure comparable loading. IP, immunoprecipitation.
293 cells were maintained in DMEM plus 10% fetal bovine serum and antibiotic as described above. Transient transfections of HA-tagged WT Gab1 or Gab1ΔShp2 expression constructs (10 μg) were carried out by the calcium phosphate method.
For all growth factor stimulation experiments, cells were first starved for 24 h in DMEM containing 0.2% fetal calf serum and then either exposed to the appropriate growth factor (50 ng of EGF/ml, 50 ng of PDGF/ml, or 40 ng of IGF-1/ml) for various times or left unstimulated.
Immunoprecipitation and immunoblotting.
Control or growth factor-stimulated cells were lysed for 40 min at 4°C with Triton lysis buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 10 mM NaF, 20 mM β-glycerophosphate, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml, 0.5 μg of antipain/ml, and 0.5 μg of pepstatin/ml). Cellular debris was removed by centrifugation at 10,000 × g for 5 min at 4°C, and lysates (1 mg of total protein) were incubated with the indicated antibodies for 2 h at 4°C; collected on protein A or protein G-Sepharose; and washed twice with 1 ml of lysis buffer, twice with 1 ml of high-salt (0.5 M NaCl) lysis buffer, and twice more with lysis buffer. Immunoprecipitates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride (PVDF) membranes, and probed with the appropriate primary antibodies, followed by secondary sheep anti-mouse, donkey anti-rabbit, or protein A conjugated to horseradish peroxidase (1:4,000; Amersham, Little Chalfont, United Kingdom), as indicated. Commercial antibodies were used at the concentrations recommended by their manufacturers. Anti-Gab1 immunoprecipitations utilized 2 μg of antibody/1 mg of total cell extracts. For p85 immunoblotting, anti-p85 antibodies were used at 1:1,000. Immunoreactive bands were visualized by enhanced chemiluminescence (ECL) (Amersham).
Far-Western blots.
GST-p85-N-SH2 was purified on glutathione-agarose beads, as described previously (5). Gab1 immunoprecipitates from control or EGF-stimulated cell lysates were resolved by SDS-PAGE, transferred to PVDF membranes, and incubated for 3 h with GST-p85-N-SH2 (1 μg/ml), followed by anti-GST antibodies (1:1,000). Blots were washed and incubated for 2 h with horseradish peroxidase-conjugated donkey anti-rabbit antibodies (1:4,000), and signals were detected by ECL.
Akt assays.
Lysates from EGF-stimulated WT or Shp2−/− cells were subjected to immunoprecipitation with anti-total Akt antibodies (2 μg), as described above, followed by two additional washes with 1 ml of kinase buffer (20 mM HEPES [pH 7.6], 2 mM dithiothreitol, 20 mM MgCl2, 20 mM MnCl2, 1 mM EDTA, 1 mM NaF, 20 mM β-glycerophosphate, and 0.1 mM Na3VO4). Kinase reactions were performed at 30°C for 45 min in 20 μl of kinase buffer supplemented with 10 μCi of [γ-32P]ATP, 50 μM ATP, and 1 μg of purified GST-GSK-3β as substrate. Reactions were terminated by the addition of SDS-PAGE sample buffer (20 μl), and reaction mixtures were boiled for 5 min, resolved by SDS-PAGE, and visualized by autoradiography.
PI3K assays.
Anti-Gab1 immunoprecipitates, prepared as described above, were washed an additional two times with 10 mM HEPES (pH 7.0)-0.5 M LiCl and twice with 10 mM HEPES (pH 7.0). Washed immunoprecipitates were subjected to a PI3K assay using crude brain lipids as substrate as described previously (33). Products were resolved by thin-layer chromatography. Phosphatidylserine and PI4,5P2 were phosphorylated by active p110 produced in Sf9 cells (a gift of Lewis C. Cantley) in the presence of [γ-32P]ATP and used as markers.
Immunofluorescence microscopy.
Cells grown on glass coverslips were transfected with GFP-Gab1 expression vector with Lipofectamine (Gibco BRL), according to the manufacturer's instructions. After 24 h, cells were starved overnight in DMEM containing 0.2% fetal calf serum. Forty-eight hours posttransfection, cells were left untreated or stimulated with EGF and were fixed in 2% paraformaldehyde in phosphate-buffered saline for 30 min at room temperature. GFP fusion proteins were visualized by conventional fluorescence microscopy.
RESULTS
Shp2 regulates Gab1-p85 association in response to EGF.
Previous studies suggested that Gab1 (3, 25), as well as its relative Gab2 (7, 8), might be an Shp2 substrate. However, overall Gab1 tyrosyl phosphorylation is unaffected in immortalized fibroblasts expressing a mutant version of Shp2 (39). Thus, if Shp2 regulates Gab1 tyrosyl phosphorylation, it must act on a limited number of Gab1 tyrosyl phosphorylation sites. To begin to test this possibility, we assessed the association of Gab1 with known binding partners in 3T3-immortalized fibroblasts from mice bearing a targeted mutation in Shp2 exon 3 (Shp2Δ46-110) and WT controls. Exon 3-deletion (hereafter, Shp2−/−) fibroblasts express small amounts of a truncated protein that lacks the N-terminal SH2 domain of Shp2. Although this protein has increased catalytic activity (29), it does not localize appropriately to activated receptors (35, 39) and, in all cases examined thus far, appears to behave as a hypomorphic mutant (27, 35, 38, 39, 46; W. Yang, L. Klaman, B. Chen, S. M. Thomas, E. George, and B. G. Neel, unpublished data).
WT and Shp2−/− cells were starved and then stimulated with EGF or left unstimulated, and Gab1 immunoprecipitates were resolved by SDS-PAGE and immunoblotted with anti-Shc, anti-Grb2, and anti-p85 antibodies, respectively. As expected, there was an increase in the association of Gab1 with each of these signal relay molecules following EGF stimulation. Compared to WT cells, there was no difference in the amount of Gab1-associated Shc and possibly a small decrease in Gab1-associated Grb2 in Shp2−/− cells (Fig. 1A); notably, reprobing the same membrane with anti-Gab1 antibodies showed that similar amounts of Gab1 were recovered in each of these lanes. In contrast, EGF-induced association of p85 with Gab1 was enhanced markedly in Shp2−/− cells (Fig. 1B). Consistent with previous studies (39), there was no effect of the Shp2 mutation on either total EGFR or total Gab1 tyrosyl phosphorylation in response to EGF stimulation (Fig. 1C). Taken together, these results suggest that Shp2 negatively regulates EGF-induced Gab1-p85 association.
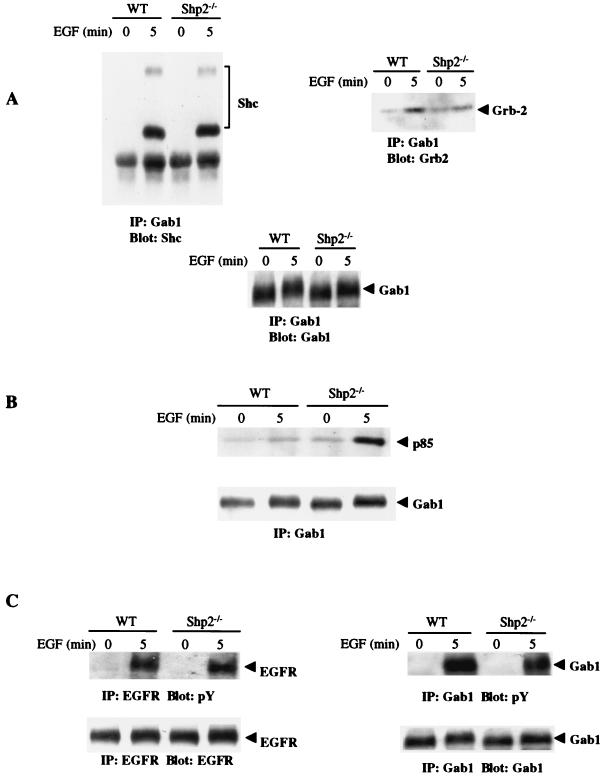
FIG. 1.
EGF-induced association of p85 with Gab1 is enhanced in Shp2−/− cells. (A and B) WT and Shp2−/− cells were serum starved for 24 h and subsequently stimulated with EGF (50 ng/ml) for 5 min or left unstimulated, as indicated. Cell lysates were subjected to immunoprecipitation with anti-Gab1 antibodies followed by SDS-PAGE and immunoblotting with anti-Shc, anti-Grb2, or anti-p85 antibodies. The same membrane was stripped and reprobed with anti-Gab1 antibodies to determine the amount of Gab1 protein precipitated from each sample. (C) Unstimulated or EGF-stimulated cell lysates were subjected to immunoprecipitation with anti-EGFR or anti-Gab1 antibodies followed by SDS-PAGE and immunoblotting with antiphosphotyrosine (pY) antibodies. IP, immunoprecipitation.
Activity of the PI3K pathway is enhanced in Shp2−/− cells.
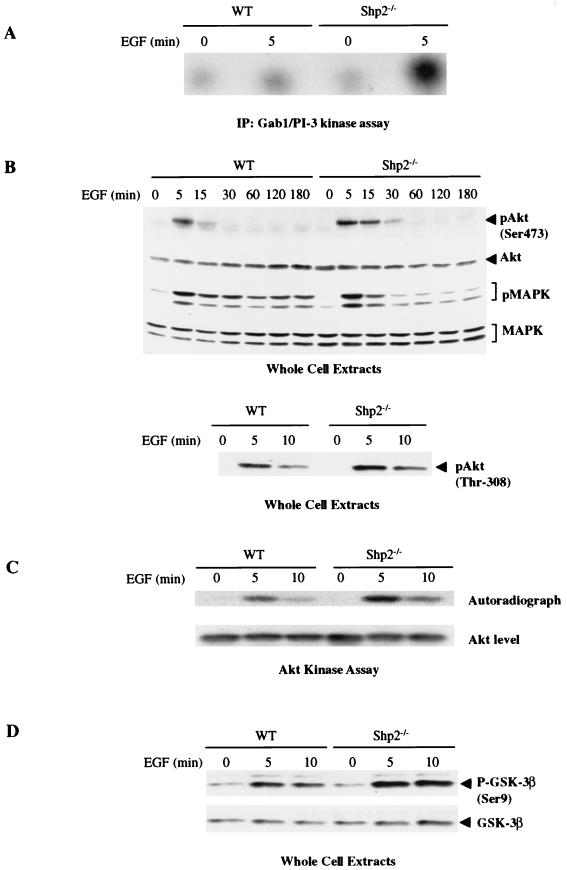
To begin to assess the downstream consequences of enhanced Gab1-p85 association, we measured Gab1-associated PI3K activity in WT and Shp2−/− cells. These experiments revealed markedly increased PI3K activity associated with Gab1 in the absence of normal Shp2 (Fig. 2A). Most of the phosphotyrosine-associated PI3K activity (which is believed to represent the activated pool of PI3K) in EGF-stimulated cells is associated with Gab1 (31). Thus, our data suggested that downstream components of the PI3K pathway might also be increased in Shp2−/− cells. Indeed, EGF-induced activation of Akt, as measured by immunoblotting using antibodies against the two activating phosphorylation sites (Ser-473 and Thr-308) in Akt (Fig. 2B) or by Akt kinase assays (Fig. 2C), was increased and sustained in Shp2−/− cells compared to WT cells. Importantly, as expected from earlier studies (35, 38, 39), EGF-induced Erk activation was impaired in the same cells in which Akt activation was enhanced (Fig. 2B). Phosphorylation of Ser-9 in GSK-3β, a downstream target of Akt, also was enhanced in the absence of normal Shp2 (Fig. 2D). Collectively, these data show that the enhanced association of p85 with Gab1 in response to EGF stimulation of Shp2−/− cells results in increased activity of the PI3K pathway in these cells.
FIG. 2.
Increased activity of the PI3K pathway in response to EGF stimulation of Shp2−/− cells. (A) Serum-starved cells were treated for 5 min with EGF (50 ng/ml), and cell lysates were subjected to immunoprecipitations with anti-Gab1 antibodies, followed by immune-complex PI3K activity assays. (B) WT and Shp2−/− cells were left unstimulated or stimulated with EGF for the indicated times. Total cellular proteins (50 μg) were resolved by SDS-PAGE and immunoblotted with anti-phospho-Erk or anti-phospho-Akt antibodies that recognize pSer473 or pThr308, as indicated. The same blot was stripped and reprobed with anti-total Akt and anti-total Erk1/2. (C) Lysates from control or EGF-stimulated cells were immunoprecipitated with anti-Akt antibodies, and kinase activities were determined with recombinant GST-GSK-3β as a substrate. The amount of Akt in the immune complexes was assessed by immunoblotting with anti-Akt. (D) The indicated cells were stimulated with EGF as described for panel C. Total cellular protein (50 μg) was subjected to immunoblotting with anti-phospho-GSK-3β antibodies. The blot was then stripped and reprobed with anti-total GSK-3β. IP, immunoprecipitation; MAPK, mitogen-activated protein kinase.
Inducible overexpression of catalytically inactive Shp2 protein also increases Gab1-p85 association.
Shp2−/− cells have been found to act as hypomorphs in all cases studied in detail thus far (see above). However, these cells express a truncated, catalytically activated version of Shp2, and so it remains possible that the increase in EGF-stimulated Gab1-p85 association in these cells reflected some neomorphic effect of truncated Shp2. To exclude this possibility and to assess the role of the catalytic domain of Shp2 in regulating Gab1-p85 association in response to EGF, we made use of a stable NIH 3T3 cell line that expresses an HA-tagged catalytically inactive (C459S) Shp2 mutant under tetracycline control. Upon removal of tetracycline from the medium (maximal induction), the Shp2 C/S mutant is expressed at about twice the level of endogenous Shp2 (Fig. 3A) and competes with it for binding to Gab1 upon EGF stimulation. Consistent with our findings with Shp2−/− cells, expression of catalytically inactive Shp2 enhanced EGF-stimulated association of p85 with Gab1 (Fig. 3B). As in Shp2−/− cells, Akt activation also was increased upon expression of the Shp2 C/S mutant (Fig. 3C).
We also examined the effect of mutating the Shp2 binding sites on Gab1 on Gab1-p85 association. For these studies, 293 cells were transfected transiently with constructs that direct expression of either HA-tagged WT Gab1 or an HA-tagged Gab1 mutant in which the Shp2 binding sites have been mutated (HA-Gab1ΔShp2). As shown in Fig. 3D, loss of Shp2 binding led to increased association of p85 with Gab1 following EGF stimulation. Thus, our results show that Shp2 and, in particular, Shp2 catalytic activity negatively regulate p85 binding to Gab1 and thereby PI3K activation in response to EGF. Moreover, this regulation appears to be mediated by Shp2 that is bound to Gab1.
Shp2 regulates p85 binding sites on Gab1 in response to EGF.
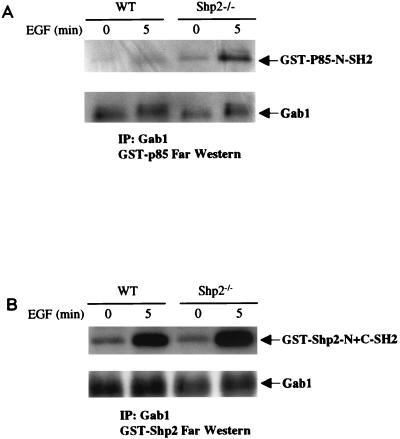
The increase in p85 binding to Gab1 in Shp2−/− cells could be due to an effect of Shp2 directly on Gab1 binding sites for p85 or an indirect effect of Shp2 on p85 (or conceivably some other protein that regulates p85 association with Gab1). There are three potential binding sites for p85 on Gab1 (Tyr-447, -472, and -589) (11). If Shp2 specifically dephosphorylates Gab1 p85 binding sites, then the phosphorylation of one or more of these sites should be increased in Shp2−/− cells compared to that in WT cells following EGF stimulation. Phosphospecific antibodies to these sites are not available. Instead, we used a GST fusion protein to the N-SH2 domain of p85 (GST-p85-N-SH2) in far-Western blots to indirectly assess their phosphorylation status. Gab1 immunoprecipitates prepared from EGF-stimulated WT and Shp2−/− cells were resolved by SDS-PAGE, transferred to a PVDF membrane, incubated with GST-p85-N-SH2, and probed with anti-GST antibodies. As expected, there was an increase in GST-p85-N-SH2 binding to immunoprecipitated Gab1 following EGF stimulation. Moreover this interaction was enhanced markedly in Gab1 isolated from Shp2−/− cells (Fig. 4A). These data indicate that the ability of purified GST-p85-N-SH2 to bind directly to Gab1 is increased in EGF-stimulated cells lacking Shp2. Since GST-p85-N-SH2 binding to Gab1 is dependent on the tyrosyl phosphorylation of p85 binding sites on Gab1, the most likely explanation for these findings is that phosphorylation of p85 binding sites on Gab1 is increased in the absence of Shp2. We also assessed direct binding of the SH2 domains of Shp2 (GST-Shp2-N+C-SH2) to Gab1 immunoprecipitates isolated from WT and Shp2−/− cells after EGF stimulation. Only a minimal increase in binding was observed, consistent with selective dephosphorylation of the p85 binding sites of Gab1 by Shp2.
FIG. 4.
Far-Western analysis of Gab1-p85 interaction in WT and Shp2−/− cells. Gab1 immunoprecipitates, prepared from WT and Shp2−/− cells stimulated with EGF or left unstimulated as indicated, were resolved by SDS-PAGE and transferred to PVDF membranes. The membranes were incubated with GST-p85-N-SH2 (A) or GST-Shp2-N+C-SH2 (B), followed by anti-GST and secondary antibodies. Total Gab1 levels in the Gab1 immune complexes are shown at the bottom. IP, immunoprecipitation.
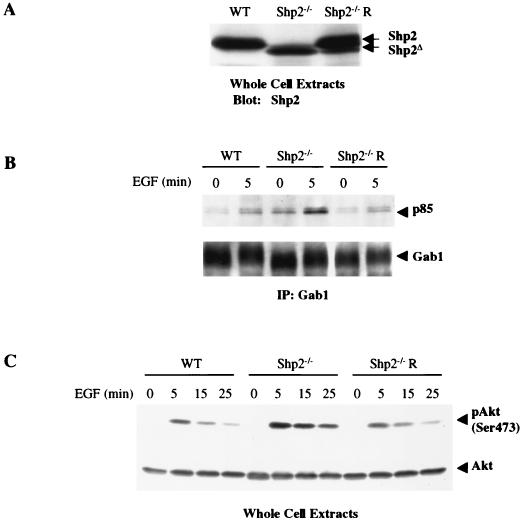
Effect of restoring WT Shp2 expression to Shp2−/− cells.
We next asked whether reintroduction of WT Shp2 could rescue the effects of Shp2 deficiency on EGF signaling. Shp2 was restored to Shp2−/− cells by using retroviral gene transduction (see Materials and Methods). Pooled drug-resistant colonies were tested for Shp2 expression by immunoblotting analysis. As expected from previous studies (see above), Shp2−/− cells expressed a truncated Shp2 protein at lower levels than did WT cells. Importantly, the pooled clones reexpressed WT Shp2 at levels comparable to that found in the WT fibroblasts (Fig. 5A). Reintroduction of Shp2 markedly decreased the amount of p85 that associates with Gab1 (Fig. 5B) and also reduced EGF-induced Akt activation (Fig. 5C) compared to that in Shp2−/− cells. These results, together with those obtained by inducible overexpression of catalytically inactive Shp2, argue strongly that the increase in EGF-evoked Gab1-p85 association and activation of PI3K and its downstream targets in Shp2−/− cells is due to the absence of WT Shp2, rather than to some indirect effect of clonal selection.
FIG. 5.
Reexpression of WT Shp2 in Shp2−/− cells. (A) WT Shp2 expression was restored to Shp2−/− cells by retroviral gene transduction (see Materials and Methods). Pooled puromycin-resistant clones were assessed for Shp2 expression by immunoblotting. The level of Shp2 expression in WT cells also is shown. Note the N-terminally truncated Shp2 protein in Shp2−/− cells and the near-WT levels of WT Shp2 in the reconstituted cells. (B) The indicated cell lines were serum starved and then stimulated with EGF (50 ng/ml) for 5 min or left unstimulated. Gab1 immunoprecipitates were subjected to SDS-PAGE and anti-p85 immunoblotting. The levels of Gab1 in the immunoprecipitates were detected by stripping the membrane and reprobing with anti-Gab1 antibodies. (C) The indicated cell lines were serum starved and stimulated with EGF for the indicated times or left unstimulated. Phospho-Akt and total Akt were detected by immunoblotting of total cell lysates. IP, immunoprecipitation.
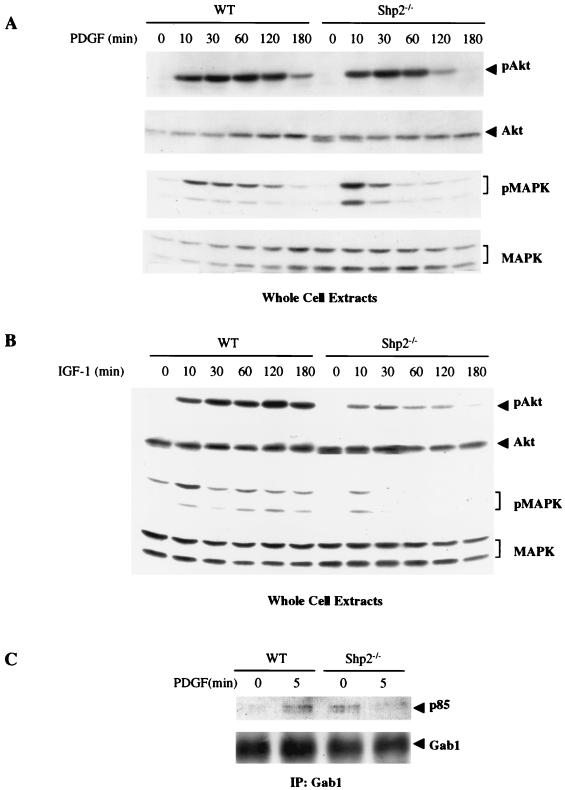
Akt activation in response to other RTKs is not increased in Shp2−/− cells.
Gab1 participates in signaling by a variety of growth factors, as does Shp2. To investigate whether Shp2 negatively regulates PI3K activation in response to other growth factors, WT and Shp2−/− cells were treated with PDGF or IGF-1, and Akt activation was assessed by immunoblotting with activation-specific antibodies. Surprisingly, compared to WT cells, there was no enhancement of PDGF- or IGF-1-evoked Akt activation in Shp2−/− cells (Fig. 6A and B). Indeed, Akt activation in response to these growth factors actually was lower in Shp2−/− cells. Notably, in the same cells (and consistent with previous reports; see above), PDGF- and IGF-1-induced Erk activation levels were substantially diminished in Shp2−/− cells compared to those in WT cells. Similar results were obtained with fibroblast growth factor stimulation (data not shown). In contrast to our observations for EGF-stimulated cells, association of p85 with Gab1 in response to PDGF stimulation in Shp2−/− cells was not increased; in fact, it appeared to decrease (Fig. 6C). Thus, Shp2 appears to selectively attenuate PI3K activation in the EGFR pathway.
FIG. 6.
PDGF- and IGF-1-evoked Akt activity is not enhanced in Shp2−/− cells. (A and B) Starved WT and Shp2−/− cells were stimulated with 50 ng of PDGF/ml (A) or 40 ng of IGF-1/ml (B) for the indicated times. Cell lysates were resolved by SDS-PAGE and subjected to immunoblotting with pSer473-specific Akt or pErk antibodies, as indicated. The membranes were then stripped and reprobed with anti-total Akt or anti-total Erk1/2 antibodies. (C) WT and Shp2−/− cells were serum starved for 24 h and subsequently stimulated with PDGF (50 ng/ml) for 5 min or left unstimulated, as indicated. Cell lysates were subjected to immunoprecipitation with anti-Gab1 antibodies, followed by SDS-PAGE and immunoblotting with anti-p85 antibodies. Total Gab1 levels in the Gab1 immune complexes are shown at the bottom. IP, immunoprecipitation; MAPK, mitogen-activated protein kinase.
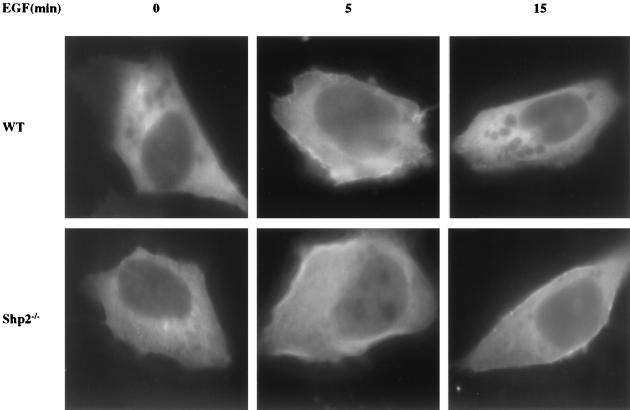
EGF induces sustained translocation of Gab1 to the plasma membrane in Shp2−/− cells.
By binding to PI3,4,5P3, the PH domain of Gab1 targets the Gab1 protein to the plasma membrane in a PI3K-dependent manner in response to growth factor stimulation (18, 19, 31). To investigate whether the enhanced Gab1-associated p85 and PI3K activity causes increased recruitment of Gab1 to the plasma membrane following EGF stimulation, we transiently expressed a Gab1-GFP fusion protein in WT and Shp2−/− cells and monitored its localization in response to EGF treatment. In unstimulated cells, Gab1-GFP was distributed diffusely in the cytoplasm. Upon EGF stimulation, there was a significant increase in the amount of Gab1-GFP at the plasma membrane. Compared to WT cells, in Shp2−/− cells, there was more Gab1-GFP translocated to the plasma membrane at 5 min after EGF treatment, and plasma membrane localization was sustained for longer times (Fig. 7).
FIG. 7.
Sustained EGF-induced relocalization of GFP-Gab1 to the plasma membrane in Shp2−/− cells. WT and Shp2−/− cells were transfected with a GFP-Gab1 expression vector. Forty-eight hours posttransfection, cells were stimulated with EGF (50 ng/ml) for the indicated times, fixed, and analyzed by fluorescence microscopy.
DISCUSSION
The scaffolding adapter Gab1 is critical for signaling by a number of RTKs (10, 11, 25, 44) and possibly for some cytokine and antigen receptors (13, 25). Upon cell stimulation, Gab1 becomes tyrosyl phosphorylated on multiple sites and recruits several signal relay proteins (10, 11, 25, 41, 44). The precise ways in which these proteins then transduce signals further downstream remain unclear. Previous studies implicated Gab1-Shp2 association in signaling to the Ras-Raf-Mek-Erk pathway, whereas interaction of Gab1 with p85 is implicated in PI3K activation. Our work has revealed further complexity in the interaction of Gab1 with p85 and Shp2. Specifically, we have found that Shp2 regulates the amount of p85 that is bound to Gab1 following EGF stimulation and thereby determines the kinetics and extent of activation of PI3K and its downstream targets Akt and GSK-3β. Surprisingly, this regulation appears to be RTK specific, such that PI3K activation following PDGF and IGF-1 stimulation is not increased—indeed, appears to be decreased—in the absence of Shp2. Thus, the Gab1-Shp2 interaction has distinct functions in cell signaling in response to different growth factors and may help to determine the specificity of signals delivered by RTKs.
Previous studies suggested that Gab1 and Gab2 might be Shp2 substrates (3, 7, 8, 25). Consistent with previous work (39), however, we observed no difference in total Gab1 tyrosyl phosphorylation in response to EGF (Fig. 1C) or other growth factors (data not shown) in the absence of Shp2. Moreover, Shc association with Gab1 was similar in WT and Shp2−/− cells (Fig. 1A). These findings left open the possibility that Shp2 might act on specific tyrosyl-phosphorylation sites on Gab1. Indeed, Gab1-associated p85 is increased dramatically in Shp2−/− cells (Fig. 1B). Accordingly, Gab1-associated PI3K activity is enhanced (Fig. 2A) and PI3K-dependent downstream targets (Fig. 2B to D) exhibit increased activation in response to EGF stimulation of these cells. Analogous results were obtained by using a cell line that inducibly overexpresses catalytically inactive Shp2 (Fig. 3). The truncated protein that is expressed in Shp2−/− cells lacks its N-terminal SH2 domain (35) and cannot bind to Gab1 (39). Furthermore, a Gab1 mutant unable to bind Shp2 exhibits increased p85 binding compared to that for WT Gab1 following EGF stimulation.
Taken together, these results imply that Shp2 must be recruited to Gab1 and be catalytically active to regulate Gab1-p85 association. The most likely explanation for these findings is that, upon recruitment to Gab1, Shp2 is activated by engagement of its N-terminal SH2 domain (1) and then dephosphorylates one or more of the PI3K binding sites on Gab1, thereby regulating Gab1-p85 interaction. Consistent with a direct effect of Shp2 deficiency on the tyrosyl phosphorylation of p85 sites on Gab1, far-Western analysis revealed a marked enhancement in the ability of p85 to bind to Gab1 isolated from Shp2−/− cells (Fig. 4A), whereas binding of Shp2 was only minimally affected (Fig. 4B). Our results clearly exclude the possibility that enhanced p85 association with Gab1 in the absence of functional Shp2 reflects an indirect effect of Shp2 deficiency on p85 or another protein that affects p85 binding to Gab1. However, we cannot exclude the possibility that Shp2 deficiency indirectly affects the ability of Gab1 to bind p85, e.g., by leading to another type of modification on Gab1 that affects binding ability. Direct assessment of the stoichiometry of phosphorylation of the p85 binding sites on Gab1 will be required to demonstrate this unambiguously.
In contrast, Grb2 association with Gab1 appears to be decreased slightly but consistently in Shp2−/− cells. The physiological significance of this observation remains to be determined, but notably, Shp2−/− (35, 38, 39) (Fig. 2B and 6) and Gab1−/− (14, 34) fibroblasts have defective Ras→Erk pathway activation in response to EGF and other growth factors. Since Grb2 binds the Ras exchange factor Sos, it will be important to determine whether decreased Gab1-Grb2 association contributes to decreased Ras→Erk activation. The observed decrease in Gab1-Grb2 interaction has two other potentially interesting implications. First, since Gab1-Shc interaction and EGFR tyrosyl phosphorylation (and presumably kinase activity) are unaffected by Shp2 deficiency (Fig. 1A and C), these results suggest that different tyrosine kinases catalyze the phosphorylation of distinct sites on Gab1. Indeed, Gab2 is reportedly phosphorylated by both colony-stimulating factor 1 receptor and Src family kinases in macrophages (17). More intriguingly, however, since Gab1-Grb2 binding is decreased in the absence of Shp2, it is possible that Shp2 regulates (directly or indirectly) the activity of the kinase that phosphorylates the Grb2 binding site(s) on Gab1. Further studies will be required to address these issues.
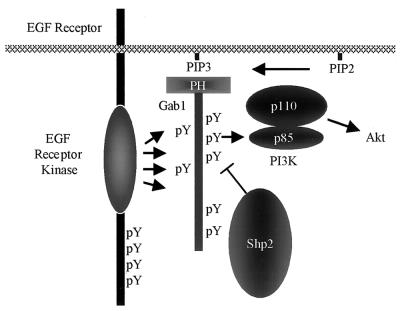
Recently, it was reported that the recruitment of Gab1 to the EGFR initiates a positive feedback loop in which initial tyrosyl phosphorylation of Gab1 leads to p85 recruitment, PI3K activation, and PIP3 production, which then leads to further recruitment (and phosphorylation) of Gab1 via PIP3 binding to the Gab1 PH domain (31). Our data suggest that, by dephosphorylating p85 binding sites on Gab1, Shp2 helps to regulate the Gab1-PI3K positive feedback loop, thereby controlling the extent, kinetics, and location of PI3K activation in response to EGF (Fig. 8). Indeed, in the absence of Shp2, EGF induces sustained translocation of Gab1 to the plasma membrane (Fig. 7). Since Gab1 activation of the Erk pathway also requires membrane recruitment (and Gab1 signaling to Erk requires Shp2), Shp2 may contribute to both Erk activation and inactivation as well. However, we do not exclude the possibility that other mechanisms, such as the recruitment and activation of the 5′-inositol phosphatase Ship-2 and/or serine/threonine phosphorylation of Gab1 and/or PI3K, also contribute to negative regulation of the Gab1-PI3K positive feedback loop. A recent report also indicated that phosphorylation of Gab1 by Erk enhances p85 association with Gab1 (45). Importantly, the increased interaction of p85 with Gab1 in EGF-stimulated Shp2−/− cells cannot be explained by differential Erk activity, since EGF-evoked Erk activation is diminished in the absence of functional Shp2 (e.g., Fig. 2B). However, it is possible that decreased Erk activation may explain or help explain the decreased association of p85 with Gab1 in PDGF- and/or IGF-1-stimulated Shp2−/− cells (compared to WT cells).
FIG. 8.
Model for regulation of kinetics of EGF-induced PI3K activation by Gab1-Shp2 interaction. Following EGF stimulation, the docking protein Gab1 is recruited to the plasma membrane through binding of its Met binding domain to the EGFR directly and through indirect recruitment via the Grb2 adapter protein. Phosphorylation of Gab1 by the EGFR and possibly other tyrosine kinases leads to recruitment and activation of multiple signal relay molecules, including PI3K. Activated PI3K catalyzes the production of PIP3. This initiates a positive feedback loop by recruiting more Gab1 molecules to the plasma membrane through binding of the PH domain of Gab1 to PIP3. By dephosphorylating the p85 binding sites on Gab1, Shp2 downregulates the Gab1-PI3K positive feedback loop, thereby controlling the extent, kinetics, and location of PI3K activation in response to EGF.
Although Gab1-Shp2 interaction is critical for regulation of EGF-evoked PI3K activation, Shp2 is dispensable for, and may potentiate, PDGF and IGF-1 stimulation of PI3K-dependent pathways. Notably, unlike the EGFR, the PDGF receptor (PDGFR) has direct binding sites for p85, and p85 binding to these sites is believed to provide the major route to PI3K activation from the PDGFR (15, 43). Previous studies suggest that there is no preferential dephosphorylation of these sites by Shp2 (16), which could explain why there is no increase in Akt activation in response to PDGF in Shp2−/− cells. Less clear is why PDGF-evoked Akt activation appears to be decreased in these cells. Conceivably, as discussed above, a tyrosine kinase regulated by Shp2 could contribute to phosphorylation of the p85 binding sites on the PDGFR. Alternatively, and perhaps more likely, decreased Ras activation may lead to lower PI3K activation in response to PDGF, since Ras binds to and activates the catalytic subunit (p110) of PI3K (32). Like the EGFR, the IGF-1 receptor lacks direct binding sites for PI3K. In the IGF pathway, however, insulin receptor substrate (IRS) proteins, particularly IRS-1, are more important for PI3K activation than is Gab1. Previous studies of the role of Shp2 in regulating insulin or IGF-1 activation of PI3K yielded conflicting results. One group, studying the effects of IRS-1 mutants that cannot bind Shp2, reported that Shp2 negatively regulates p85 binding to IRS-1, presumably by dephosphorylating its p85 binding sites (23). Another group demonstrated that expression of dominant-negative Shp2 attenuates IRS-1-associated PI3K activity (42). Our results are consistent with the latter report and suggest that Shp2 is a positive regulator of PI3K activation by IGF-1 or insulin, at least in fibroblast cell lines. How and why Shp2 can act as a negative regulator of EGF-induced PI3K activation and a positive regulator of PI3K activation by other RTKs remain topics for future work.
It is increasingly clear that signal thresholds and the temporal aspects of signaling are important for determining the cellular response. One way that cells can utilize the same (or similar) signaling pathways to control a wide range of cellular processes is to vary the amplitude and duration of pathway activation and to convert this into qualitatively different biological responses. In perhaps the best studied example, the EGFR induces transient Erk activation and stimulates proliferation of PC12 cells, whereas the NGF receptor and fibroblast growth factor receptor evoke a sustained Erk response, culminating in neuronal differentiation (21). By regulating the extent and magnitude of p85 association with Gab1 and thereby helping to regulate the extent and kinetics of PI3K activation in response to some, but not all, RTKs, Shp2 may play an important role in directing the varied effects of different growth factors.
Acknowledgments
We thank Haiyan Liu for generating the inducible NIH 3T3 Shp2 C/S cell lines and Haihua Gu, Kenneth D. Swanson, and Heike Keilhack of the Neel laboratory for their helpful suggestions. We also thank Lewis C. Cantley (Beth Israel-Deaconess Medical Center) for generously providing the anti-p85 antibodies and the activated p110, Christiane R. Maroun and Morag Park (McGill University, Montreal, Quebec, Canada) for the pEGFP-Gab1 and HA-Gab1ΔShp2 constructs, Brian S. Schaffhausen (Tufts University Medical School) for the expression vector for GST-p85-N-SH2, and Garry P. Nolan (Stanford University School of Medicine) for the Phoenix-Ecotropic packaging cells.
This work was supported by NIH grant R01CA49152 to B.G.N. T.A. was supported by a postdoctoral fellowship from the Japan Society for the Promotion of Science for Young Scientists.
REFERENCES
- 1.Barford, D., and B. G. Neel. 1998. Revealing mechanisms for SH2 domain-mediated regulation of the protein tyrosine phosphatase SHP-2. Structure 6:249-254. [DOI] [PubMed] [Google Scholar]
- 2.Cunnick, J. M., J. F. Dorsey, T. Munoz-Antonia, L. Mei, and J. Wu. 2000. Requirement of SHP2 binding to Grb2-associated binder-1 for mitogen-activated protein kinase activation in response to lysophosphatidic acid and epidermal growth factor. J. Biol. Chem. 275:13842-13848. [DOI] [PubMed] [Google Scholar]
- 3.Cunnick, J. M., L. Mei, C. A. Doupnik, and J. Wu. 2001. Phosphotyrosines 627 and 659 of Gab1 constitute a bisphosphoryl tyrosine-based activation motif (BTAM) conferring binding and activation of SHP2. J. Biol. Chem. 276:24380-24387. [DOI] [PubMed] [Google Scholar]
- 4.Feng, G. S. 1999. Shp-2 tyrosine phosphatase: signaling one cell or many. Exp. Cell Res. 253:47-54. [DOI] [PubMed] [Google Scholar]
- 5.Frangioni, J. V., and B. G. Neel. 1993. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion proteins. Anal. Biochem. 210:179-187. [DOI] [PubMed] [Google Scholar]
- 6.Freeman, R. M., Jr., J. Plutzky, and B. G. Neel. 1992. Identification of a human src-homology 2 (SH2)-containing tyrosine phosphatase: a putative homolog of Drosophila corkscrew. Proc. Natl. Acad. Sci. USA 89:11239-11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu, H., J. D. Griffin, and B. G. Neel. 1997. Characterization of two SHP-2-associated binding proteins and potential substrates in hematopoietic cells. J. Biol. Chem. 272:16421-16430. [DOI] [PubMed] [Google Scholar]
- 8.Gu, H., J. C. Pratt, S. J. Burakoff, and B. G. Neel. 1998. Cloning and characterization of the major SHP-2 binding protein in hematopoietic cells (p97) reveals a novel pathway for cytokine-induced gene activation. Mol. Cell 2:729-740. [DOI] [PubMed] [Google Scholar]
- 9.Herbst, R., P. M. Carroll, J. D. Allard, J. Schilling, T. Raabe, and M. A. Simon. 1996. Daughter of sevenless is a substrate of the phosphotyrosine phosphatase Corkscrew and functions during sevenless signaling. Cell 85:899-909. [DOI] [PubMed] [Google Scholar]
- 10.Holgado-Madruga, M., D. R. Emlet, D. K. Moscatello, A. K. Godwin, and A. J. Wong. 1996. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature 379:560-564. [DOI] [PubMed] [Google Scholar]
- 11.Holgado-Madruga, M., D. K. Moscatello, D. R. Emlet, R. Dieterich, and A. J. Wong. 1997. Grb2-associated binder-1 mediates phosphatidylinositol 3-kinase activation and the promotion of cell survival by nerve growth factor. Proc. Natl. Acad. Sci. USA 94:12419-12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter, T. 2000. Signaling—2000 and beyond. Cell 100:113-127. [DOI] [PubMed] [Google Scholar]
- 13.Ingham, R. J., M. Holgado-Madruga, C. Siu, A. J. Wong, and M. R. Gold. 1998. The Gab1 protein is a docking site for multiple proteins involved in signaling by the B cell antigen receptor. J. Biol. Chem. 273:30630-30637. [DOI] [PubMed] [Google Scholar]
- 14.Itoh, M., Y. Yoshida, K. Nishida, M. Narimatsu, M. Hibi, and T. Hirano. 2000. Role of Gab1 in heart, placenta and skin development and growth factor- and cytokine-induced extracellular signal-related kinase mitogen-activated protein kinase activation. Mol. Cell. Biol. 20:2695-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazlauskas, A., A. Kashishian, J. A. Cooper, and M. Valius. 1992. GTPase-activating protein and phosphatidylinositol 3-kinase bind to distinct regions of the platelet-derived growth factor receptor β subunit. Mol. Cell. Biol. 12:2534-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klinghoffer, R., and A. Kazlauskas. 1995. Identification of a putative Syp substrate, the PDGF beta receptor. J. Biol. Chem. 270:22208-22217. [DOI] [PubMed] [Google Scholar]
- 17.Lee, A. W., and D. J. States. 2000. Both Src-dependent and -independent mechanisms mediate phosphatidylinositol 3-kinase regulation of colony-stimulating factor 1-activated mitogen-activated protein kinases in myeloid progenitors. Mol. Cell. Biol. 20:6779-6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maroun, C. R., M. Holgado-Madruga, I. Royal, M. A. Naujokas, T. M. Fournier, A. J. Wong, and M. Park. 1999. The Gab1 PH domain is required for localization of Gab1 at sites of cell-cell contact and epithelial morphogenesis downstream from the Met receptor tyrosine kinase. Mol. Cell. Biol. 19:1784-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maroun, C. R., D. K. Moscatello, M. A. Naujokas, M. Holgado-Madruga, A. J. Wong, and M. Park. 1999. A conserved inositol phospholipid binding site within the pleckstrin homology domain of the Gab1 docking protein is required for epithelial morphogenesis. J. Biol. Chem. 274:31719-31726. [DOI] [PubMed] [Google Scholar]
- 20.Maroun, C. R., M. A. Naujokas, M. Holgado-Madruga, A. J. Wong, and M. Park. 2000. The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the Met receptor tyrosine kinase. Mol. Cell. Biol. 20:8513-8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall, C. 1995. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80:179-185. [DOI] [PubMed] [Google Scholar]
- 22.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myers, M. G., Jr., R. Mendez, P. Shi, J. H. Pierce, R. Rhoads, and M. F. White. 1998. The COOH-terminal tyrosine phosphorylation sites on IRS-1 bind SHP-2 and negatively regulate insulin signaling. J. Biol. Chem. 273:26908-26914. [DOI] [PubMed] [Google Scholar]
- 24.Neel, B. G., and N. K. Tonks. 1997. Protein tyrosine phosphatases in signal transduction. Curr. Opin. Cell Biol. 9:193-204. [DOI] [PubMed] [Google Scholar]
- 25.Nishida, K., Y. Yoshida, M. Itoh, T. Fukada, T. Ohtani, T. Shirogane, T. Atsumi, M. Takahashi-Tezuka, K. Ishihara, M. Hibi, and T. Hirano. 1999. Gab-family adapter proteins act downstream of cytokine and growth factor receptors and T- and B-cell antigen receptors. Blood 93:1809-1816. [PubMed] [Google Scholar]
- 26.Noguchi, T., T. Matozaki, K. Horita, Y. Fujioka, and M. Kasuga. 1994. Role of SH-PTP2, a protein-tyrosine phosphatase with Src homology 2 domains, in insulin-stimulated Ras activation. Mol. Cell. Biol. 14:6674-6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh, E. S., H. Gu, T. M. Saxton, J. F. Timms, S. Hausdorff, E. U. Frevert, B. B. Kahn, T. Pawson, B. G. Neel, and S. M. Thomas. 1999. Regulation of early events in integrin signaling by protein tyrosine phosphatase SHP-2. Mol. Cell. Biol. 19:3205-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pawson, T., and J. D. Scott. 1997. Signaling through scaffold, anchoring and adapter proteins. Science 278:2075-2080. [DOI] [PubMed] [Google Scholar]
- 29.Qu, C. K., Z. Q. Shi, R. Shen, F. Y. Tsai, S. H. Orkin, and G. S. Feng. 1997. A deletion mutation in the SH2-N domain of Shp-2 severely suppresses hematopoietic cell development. Mol. Cell. Biol. 17:5499-5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raabe, T., J. Riesgo-Escovar, X. Liu, B. S. Bausenwein, P. Deak, P. Maroy, and E. Hafen. 1996. DOS, a novel pleckstrin homology domain-containing protein required for signal transduction between sevenless and Ras1 in Drosophila. Cell 85:911-920. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigues, G. A., M. Falasca, Z. Zhang, S. H. Ong, and J. Schlessinger. 2000. A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol. Cell. Biol. 20:1448-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Viciana, P., P. H. Warne, R. Dhand, B. Vanhaesebroeck, I. Gout, M. J. Fry, M. D. Waterfield, and J. Downward. 1994. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 370:527-532. [DOI] [PubMed] [Google Scholar]
- 33.Ruderman, N. B., R. Kapeller, M. F. White, and L. C. Cantley. 1990. Activation of phosphatidylinositol 3-kinase by insulin. Proc. Natl. Acad. Sci. USA 87:1411-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sachs, M., H. Brohmann, D. Zechner, T. Muller, J. Hulsken, I. Walther, U. Schaeper, C. Birchmeier, and W. Birchmeier. 2000. Essential role of Gab1 for signaling by the c-Met receptor in vivo. J. Cell Biol. 150:1375-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saxton, T. M., M. Henkemeyer, S. Gasca, R. Shen, D. J. Rossi, F. Shalaby, G.-S. Feng, and T. Pawson. 1997. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase SHP-2. EMBO J. 16:2352-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaeper, U., N. H. Gehring, K. P. Fuchs, M. Sachs, B. Kempkes, and W. Birchmeier. 2000. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J. Cell Biol. 149:1419-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlessinger, J. 2000. Cell signaling by receptor tyrosine kinases. Cell 103:211-225. [DOI] [PubMed] [Google Scholar]
- 38.Shi, Z., W. Lu, and G. Feng. 1998. The Shp-2 tyrosine phosphatase has opposite effects in mediating the activation of extracellular signal-regulated and c-Jun NH2-terminal mitogen-activated protein kinases. J. Biol. Chem. 273:4904-4908. [DOI] [PubMed] [Google Scholar]
- 39.Shi, Z. Q., D. H. Yu, M. Park, M. Marshall, and G. S. Feng. 2000. Molecular mechanism for the Shp-2 tyrosine phosphatase function in promoting growth factor stimulation of Erk activity. Mol. Cell. Biol. 20:1526-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shockett, P. E., and D. G. Schatz. 1996. Diverse strategies for tetracycline-regulated inducible gene expression. Proc. Natl. Acad. Sci. USA 93:5173-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tajahashi-Tezuka, M., Y. Yoshida, T. Fukada, T. Ohtani, Y. Yamanaka, K. Nishida, K. Nakajima, M. Hibi, and T. Hirano. 1998. Gab1 acts as an adapter molecule linking the cytokine receptor gp130 to ERK mitogen-activated protein kinase. Mol. Cell. Biol. 18:4109-4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ugi, S., H. Maegawa, A. Kashiwagi, M. Adachi, J. M. Olefsky, and R. Kikkawa. 1996. Expression of dominant negative mutant SHPTP2 attenuates phosphatidylinositol 3′-kinase activity via modulation of phosphorylation of insulin receptor substrate-1. J. Biol. Chem. 271:12595-12602. [DOI] [PubMed] [Google Scholar]
- 43.Valius, M., and A. Kazlauskas. 1993. Phospholipase C-γ1 and phosphatidylinositol 3 kinase are the downstream mediators of the PDFG receptor's mitogenic signal. Cell 73:321-334. [DOI] [PubMed] [Google Scholar]
- 44.Weidner, K. M., S. Di Cesare, M. Sachs, V. Brinkmann, J. Behrens, and W. Birchmeier. 1996. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature 384:173-176. [DOI] [PubMed] [Google Scholar]
- 45.Yu, C. F., B. Roshan, Z. X. Liu, and L. G. Cantley. 2001. ERK regulates the hepatocyte growth factor-mediated interaction of Gab1 and the phosphatidylinositol 3-kinase. J. Biol. Chem. 276:32552-32558. [DOI] [PubMed] [Google Scholar]
- 46.Yu, D.-H., C.-K. Qu, O. Henegariu, X. Lu, and G.-S. Feng. 1998. Protein-tyrosine phosphatase SHP-2 regulates cell spreading, migration and focal adhesion. J. Biol. Chem. 273:21125-21131. [DOI] [PubMed] [Google Scholar]
- 47.Zhao, C., D. Yu, R. Shen, and G. Feng. 1999. Gab2, a new pleckstrin homology domain-containing adapter protein, acts to uncouple signaling from ERK kinase to Elk-1. J. Biol. Chem. 274:19649-19654. [DOI] [PubMed] [Google Scholar]