Abstract
The yeast Saccharomyces cerevisiae undergoes a dimorphic filamentous transition in response to nutrient cues that is affected by both mitogen-activated protein kinase and cyclic AMP-protein kinase A signaling cascades. Here two transcriptional regulators, Flo8 and Sfl1, are shown to be the direct molecular targets of protein kinase A. Flo8 and Sfl1 antagonistically control expression of the cell adhesin Flo11 via a common promoter element. Phosphorylation by the protein kinase A catalytic subunit Tpk2 promotes Flo8 binding and activation of the Flo11 promoter and relieves repression by prohibiting dimerization and DNA binding by Sfl1. Our studies illustrate in molecular detail how protein kinase A combinatorially effects a key developmental switch. Similar mechanisms may operate in pathogenic fungi and more complex multicellular eukaryotic organisms.
Cells possess a network of signal transduction pathways that enable them to sense the environment and respond to different stimuli. Signals from distinct pathways need to be coordinated so that cells can survive, proliferate, and differentiate properly under particular conditions. Signal integration occurs at several levels of signal transduction, including transcriptional control of gene expression, translational regulation, and posttranslational modifications. One example of such integration is the regulation of interleukin-2 gene expression during T-cell activation, in which both the calcineurin and protein kinase C pathways function. The transcription factors NF-AT and AP-1 are controlled by the calcineurin and protein kinase C pathways and bind to a composite site on the interleukin-2 promoter to activate gene expression (9). The coordinately controlled assembly of transcriptional regulators on the interleukin-2 gene promoter ensures expression when both pathways are engaged. In this regard, studies of FLO11 expression in Saccharomyces cerevisiae similarly serve as a paradigm to understand how combinatorial control of gene expression by multiple signals effects a complex physiological process like pseudohyphal differentiation.
Pseudohyphal differentiation in diploid cells of the yeast S. cerevisiae occurs in response to nitrogen limitation and the presence of fermentable carbon sources (14, 28). Both a mitogen-activated protein (MAP) kinase pathway and the cyclic AMP (cAMP)-dependent protein kinase A cascade are required for pseudohyphal differentiation (for reviews, see references 13 and 37), and both pathways converge to control expression of the cell wall flocculin Flo11 (35, 39). Flo11 is a glycerol phosphoinositol-anchored cell surface protein that promotes mother-daughter cell adhesion and allows cells to bind to and penetrate growth substrates (21, 23). Flo11 is required for both diploid pseudohyphal differentiation and haploid invasive growth (23, 28, 29, 35, 38, 39), and protein kinase A plays critical roles in both processes.
In S. cerevisiae, protein kinase A comprises a single regulatory subunit, Bcy1, and three catalytic subunits, Tpk1, Tpk2, and Tpk3. The Tpk2 catalytic subunit plays a unique positive role and activates pseudohyphal differentiation, whereas the more distantly related Tpk1 and Tpk3 subunits play negative roles, inhibiting filamentous growth (35, 38). Previous genetic studies suggested that Tpk2 activates FLO11 expression by activating Flo8, inhibiting Sfl1, or both (35, 38, 39).
Flo8 is a transcriptional activator, and flo8 mutations abolish FLO11 expression and pseudohyphal growth and account for the inability of the common laboratory strain S288C to undergo filamentous growth (22, 35, 39). flo8 mutations block the effects of activated protein kinase A signaling but not activated MAP kinase on FLO11 expression and pseudohyphal differentiation (35, 39). This result suggests that Flo8 acts downstream of the protein kinase A pathway to promote FLO11 expression and filamentous growth.
Sfl1 was originally identified as a negative regulator of flocculation in yeast cells (12). The N-terminal region of the Sfl1 protein shows extensive similarity to the DNA-binding domains of the yeast heat shock transcription factor Hsf1 (12) and several other yeast transcription factors (Mga1, Hms2, and Skn7) that enhance pseudohyphal differentiation when overexpressed (25). Sfl1 functions with the Srb/mediator complex of RNA polymerase II holoenzyme to repress gene expression (43). sfl1 mutations enhance FLO11 expression and pseudohyphal growth and restore filamentous growth in tpk2 mutants (38). These results suggest that Sfl1 acts downstream of the protein kinase A pathway and that Tpk2 inactivates Sfl1 to stimulate FLO11 expression and filamentous growth.
In this study, we have elucidated the mechanisms by which Flo8 and Sfl1 control FLO11 expression and how Tpk2 controls both transcription factors. We report that Flo8 and Sfl1 are the direct targets of Tpk2 that control FLO11 expression and pseudohyphal growth. Both Flo8 and Sfl1 interact with and are phosphorylated by Tpk2. Flo8 and Sfl1 are both localized to the nucleus, and mutation or activation of Tpk2 had no effect on this localization. The ability of Flo8 and Sfl1 to alter gene expression when targeted to heterologous promoters via fusion to the Gal4 or LexA DNA-binding domains was similarly unaffected by increased or decreased protein kinase A signaling. Flo8 and Sfl1 were both found to act on a 250-bp region of the FLO11 promoter, and both proteins bind to this DNA region in vivo and in vitro. Tpk2 phosphorylates Flo8 and activates its binding to the FLO11 promoter. In contrast, phosphorylation of Sfl1 by Tpk2 inhibits binding to the FLO11 promoter, in accord with a recent report (6).
In summary, our studies illustrate how protein kinase A effects a key developmental switch by stimulating an activator and inhibiting a repressor. Similar mechanisms are likely to operate during signal integration and combinatorial transcriptional control of gene expression in other eukaryotic organisms.
MATERIALS AND METHODS
Yeast strains and plasmids.
The yeast strains used in this study are listed in Table 1. All mutant strains were created by the PCR-mediated gene disruption technique (27, 46), using either the G418 resistance cassette from plasmid pFA6-KanMX2 (46) or the hygromycin B resistance cassette from plasmid pGA32 (15). Homozygous diploid strains were produced by crossing independent isogenic haploid mutant strains (see Table 1). Haploid strains with single or double gene deletions were crossed, sporulated, and dissected to produce double or triple mutant strains. To construct the glutathione S-transferase (GST)-Tpk1- and GST-Tpk2-expressing strains, a G418-pGAL1-GST cassette was generated by PCR (24) and integrated in frame upstream of one copy of the chromosomal TPK1 and TPK2 genes in the protease-deficient diploid strain BJ5627.
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| Σ1278b strains | ||
| MLY40α | MATα ura3-52 | Lorenz and Heitman (26) |
| MLY41a | MATaura3-52 | Lorenz and Heitman (26) |
| MLY42α | MATα ura3-52 leu2::hisG | Lorenz and Heitman (26) |
| MLY61a/α | MATa/α ura3-52/ura3-52 | Lorenz and Heitman (26) |
| MLY97a/α | MATa/α ura3-52/ura3-52 leu2::hisG/leu2::hisG | Lorenz and Heitman (26) |
| MLY162a/α | MATa/α pde2Δ::KanMX/pde2Δ::KanMX ura3-52/ura3-52 | Lorenz and Heitman (26) |
| MLY183α | MATα tec1Δ::KanMX ura3-52 | Lorenz and Heitman (25) |
| MLY183a/α | MATa/α tec1Δ::KanMX/tec1Δ::KanMX ura3-52/ura3-52 | Lorenz and Heitman (25) |
| XPY5α | MATα tpk2Δ::KanMX ura3-52 | Pan and Heitman (35) |
| XPY5a/α | MATa/α tpk2Δ::KanMX/tpk2Δ::KanMX ura3-52/ura3-52 | Pan and Heitman (35) |
| XPY95α | MATα flo8Δ::HygB ura3-52 | Pan and Heitman (35) |
| XPY95a/α | MATa/α flo8Δ::HygB/flo8Δ::HygB ura3-52/ura3-52 | Pan and Heitman (35) |
| XPY108α | MATα sfl1Δ::HygB ura3-52 | This study |
| XPY108a/α | MATa/α sfl1Δ::HygB/sfl1Δ::HygB ura3-52/ura3-52 | This study |
| XPY116α | MATα flo8Δ::HygB sfl1Δ::HygB ura3-52 | This study |
| XPY116a/α | MATa/α flo8Δ::HygB/flo8Δ::HygB sfl1Δ::HygB/sfl1Δ::HygB ura3-52/ura3-52 | This study |
| XPY132α | MATα tpk2Δ::KanMX sfl1Δ::HygB ura3-52 | This study |
| XPY132a/α | MATa/α tpk2Δ::KanMX/tpk2Δ::KanMX sfl1Δ::HygB/sfl1Δ::HygB ura3-52/ura3-52 | This study |
| XPY133α | MATα tec1Δ::KanMX sfl1Δ::HygB ura3-52 | This study |
| XPY133a/α | MATa/α tec1Δ::KanMX/tec1Δ::KanMX sfl1Δ::HygB/sfl1Δ::HygB ura3-52/ura3-52 | This study |
| XPY142a/α | MATa/α tpk2Δ::KanMX/tpk2Δ::KanMX flo8Δ::HygB/flo8Δ::HygB ura3-52/ura3-52 | This study |
| XPY219a/α | MATa/α tpk2Δ::KanMX/tpk2Δ::KanMX ura3-52/ura3-52 leu2::hisG/leu2::hisG | This study |
| XPY305a/α | MATa/α flo8Δ::HygB/flo8Δ::HygB ura3-52/ura3-52 leu2::hisG/leu2::hisG | This study |
| XPY307a/α | MATa/α sfl1Δ::HygB/sfl1Δ::HygB ura3-52/ura3-52leu2::hisG/leu2::hisG | This study |
| XPY308a/α | MATa/α flo8Δ::HygB/flo8Δ::HygB sfl1Δ::HygB/sfl1Δ::HygB ura3-52/ura3-52 leu2::hisG/leu2::hisG | This study |
| XPY309a/α | MATa/α tpk2Δ::KanMX/tpk2Δ::KanMX sfl1Δ::HygB/sfl1Δ::HygB ura3-52/ura3-52 leu2::hisG/leu2::hisG | This study |
| Protease-deficient strains | ||
| BJ2168a | MATaleu2 trp1 ura3-52 prb1-1122 pep4-3 gal2 | Jones (20) |
| XPY247a | MATaleu2 trp1 ura3-52 prb1-1122 pep4-3 gal2 tpk2Δ::KanMX | This study |
| BJ5627a/α | MATa/α pep4::HIS3/pep4::HIS3 prb1Δ1.6R/prb1Δ1.6R can1/can1 ura3-52/ura3-52 his3Δ200/his3Δ200 | Jones (20) |
| XPY310a/α | MATa/α pep4::HIS3/pep4::HIS3 prb1Δ1.6R/prb1Δ1.6R can1/can1 ura3-52/ura3-52 his3Δ200/his3Δ200 GAL1-GST-TPK1/TPK1 | This study |
| XPY311a/α | MATa/α pep4::HIS3/pep4::HIS3 prb1Δ1.6R/prb1Δ1.6R can1/can1 ura3-52/ura3-52 his3Δ200/his3Δ200 GAL1-GST-TPK2/TPK2 | This study |
| Two-hybrid strains | ||
| PJ69-4A | MATatrp1-901 leu2-3,112 ura3-52 his3-200 gal4Δ gal80Δ GAL2-ADE2 LYS2::GAL1-HIS3 met2::GAL7-lacZ | James et al. (20) |
| XPY100a | MATatrp1-901 leu2-3,112 ura3-52 his3-200 gal4Δ gal80Δ GAL2-ADE2 LYS2::GAL1-HIS3 met2::GAL7-lacZ pde2Δ::KanMX | This study |
| XPY220a | MATatrp1-901 leu2-3,112 ura3-52 his3-200 gal4Δ gal80Δ GAL2-ADE2 LYS2::GAL1-HIS3 met2::GAL7-lacZ tpk2Δ::KanMX | This study |
Plasmids used in this study are listed in Table 2. Except for Flo8, all two-hybrid plasmids were constructed by PCR amplification of DNA sequences encoding the corresponding open reading frame (ORFs) and cloning in frame in either pGAD424 or pGBT9 with the restriction enzymes described in Table 2. Flo8 two-hybrid plasmids pXP28 and pXP29 contain FLO8 gene sequences encoding amino acids 206 to 799. The kinase-inactive allele of the TPK2 two-hybrid plasmid pXP96 was created by PCR overlap mutagenesis.
TABLE 2.
Plasmids used in this study
| Plasmid | Descriptiona | Reference |
|---|---|---|
| YCplac33 | CEN URA3 | Gietz and Sugino (13) |
| YEplac195 | 2μm URA3 | Gietz and Sugino (13) |
| YEplac181 | 2μm LEU2 | Gietz and Sugino (13) |
| pGAD424 | 2μm LEU2 GAL4 (aa 768-881) | Fields and Song (10) |
| pGBT9 | 2μm TRP1 GAL4 (aa 1-147) | Fields and Song (10) |
| JK1621 | 2μm URA3 4lexA-CYC1-lacZ | Keleher et al. (20) |
| pLG132ΔS | 2μm URA3 CYC1-lacZ | Guarente and Hoar (18) |
| pMAL2c | MBP expression vector | New England Biolabs |
| pRD56 | GAL1-GST CEN URA3 | Mitchell et al. (32) |
| pSLFΔ-178K | 2μm URA3 CYC1-lacZ | Forsburg and Guarente (11) |
| pWS41 | 2μm HIS3 lexA-SFL1 | Song and Carlson (43) |
| pXP3 | TPK2 in YEplac195 | Pan and Heitman (35) |
| pXP23 | TPK1 in pGBT9 (EcoRI/BamHI) | This study |
| pXP25 | TPK2 in pGBT9 (BamHI/SalI) | This study |
| pXP26 | BCY1 in pGAD424 (EcoRI/BamHI) | This study |
| pXP28 | FLO8 (aa 206-799) in pGAD424 (BamHI/PstI) | This study |
| pXP29 | FLO8 (aa 206-799) in pGBT9 (BamHI/PstI) | This study |
| pXP38 | SOK2 in pGAD424 (BamHI/SalI) | This study |
| pXP40 | PHD1 in pGAD424 (BamHI/PstI) | This study |
| pXP42 | SFL1 in pGAD424 (BamHI/SalI) | This study |
| pXP70 | FLO8-GFP in YEplac195 | This study |
| pXP94 | FLO8 in YEplac195 | This study |
| pXP96 | TPK2 (K99R) kinase-inactive allele in pGBT9 | This study |
| pXP100 | Myc12-SWE1 3′UTR in YEplac195 | This study |
| pXP101 | GFP-FLO8 3′UTR in YEplac195 | This study |
| pXP110 | FLO8-MYC12 in YEplac195 | This study |
| pXP112 | SFL1-MYC12 in YEplac195 | This study |
| pXP113 | SFL1-GFP in YEplac195 | This study |
| pXP116 | ADH1-GFP in YCplac33 | This study |
| pXP121 | pADH1-GFP-TPK2 in YCplac33 | This study |
| pXP142 | MBP-FLO8-HIS6 in pMAL2c | This study |
| pXP143 | MBP-SFL1-HIS6 in pMAL2c | This study |
| pXP160 | pADH1-HA3-SFL1 in YEplac195 | This study |
| pXP179 | TPK2 in YEplac181 | This study |
| pXP181 | SFL1-MYC12 in YEplac181 | This study |
| pXP184 | pFLO8-HA3-FLO8 in YCplac33 | This study |
| pXP189 | FLO8 in YEplac181 | This study |
| pXP217 | FLO8-2 (R155G) in YEplac195 | This study |
| pXP223 | bp −1400 to −1150 region of FLO11 promoter in pCR2.1 | This study |
| pXP233 | bp −2000 to −1750 region of FLO11 promoter in pSLFΔ-178K | This study |
| pXP234 | bp −1800 to −1550 region of FLO11 promoter in pSLFΔ-178K | This study |
| pXP235 | bp −1600 to −1350 region of FLO11 promoter in pSLFΔ-178K | This study |
| pXP236 | bp −1400 to −1150 region of FLO11 promoter in pSLFΔ-178K | This study |
| pXP237 | bp −1200 to −950 region of FLO11 promoter in pSLFΔ-178K | This study |
| pXP238 | bp −1000 to −750 region of FLO11 promoter in pSLFΔ-178K | This study |
| pXP239 | bp −800 to −550 region of FLO11 promoter in pSLFΔ-178K | This study |
| pXP240 | bp −600 to −350 region of FLO11 promoter in pSLFΔ-178K | This study |
| pXP241 | bp −400 to −150 region of FLO11 promoter in pSLFΔ-178K | This study |
| pXP242 | bp −200 to 0 region of FLO11 promoter in pSLFΔ-178K | This study |
| pXP287 | pGAL1-GST-TPK2 (K99R) in YEplac195 | This study |
| pXP291 | FLO8-MYC12 in YEplac181 | This study |
Overlap PCR was performed to insert the yeast green fluorescent protein gene (yGFP) in frame immediately in front of the stop codon of the FLO8 gene, and the fusion gene including the 5′ and 3′ untranslated regions (UTRs) of the FLO8 gene was cloned into YEplac195 with BamHI and XbaI to create plasmid pXP70. The GFP and 3′ UTR portion of this fusion was amplified by PCR and subcloned into YEplac195 with SalI and HindIII digestion to create plasmid pXP101. A 12× Myc and 3′ UTR of the SWE1 gene (42) were PCR amplified and cloned into YEplac195 with SalI and HindIII to create plasmid pXP100. The SFL1 gene, including the 5′ UTR and coding sequence, was PCR amplified and cloned immediately in front of the 12× Myc coding sequence of pXP100 and GFP coding sequence of pXP101 with BamHI and SalI to construct plasmids pXP112 and pXP113.
Plasmid pXP110, which contains the 12X Myc-tagged FLO8 gene, was constructed in a similar way with BamHI and XbaI. The GFP- and Myc-tagged FLO8 and SFL1 alleles complemented the corresponding flo8 and sfl1 mutations, respectively. A DNA fragment corresponding to 400 bp of the ADH1 promoter and the yGFP coding region was PCR amplified and digested with HindIII and SalI and with SalI and XbaI, respectively. The digested DNA fragments were combined and cloned into the YCplac33 vector digested with HindIII and XbaI to create plasmid pXP116. The coding sequence and 3′ UTR of the TPK2 gene was PCR amplified and cloned with XbaI and BamHI into plasmid pXP116 to create the GFP-tagged Tpk2.
For Flo8 and Sfl1 expression in Escherichia coli, the protein coding sequences were PCR amplified and cloned downstream of the maltose-binding protein (MBP) coding sequence of plasmid pMAL2c (New England Biologicals). To introduce a His6 epitope tag on the carboxyl termini of both the MBP-tagged Sfl1 and Flo8 proteins, a His6 coding sequence was included in the reversed PCR primers. Genomic DNA of strain XPY311a/α was used as the template in an overlap PCR to create the GST-Tpk2(K99R) expression plasmid pXP287.
To clone the FLO8 gene, genomic DNA of wild-type strain MLY61a/α was digested with SphI and BamHI and resolved in a 1% agarose gel. DNA fragments with sizes ranging from 4 to 6 kb were recovered and cloned into plasmid YEplac195. The FLO8 gene was identified by hybridization and named plasmid pXP94. This wild-type allele of FLO8 was subcloned into plasmid YEplac181 to form plasmid pXP189. The TPK2 and SFL1-Myc12 alleles were subcloned into plasmid YEplac181 to form plasmids pXP179 and pXP181, respectively. An error-prone PCR protocol was used to mutagenize the FLO8 gene. The FLO8-2 mutant allele (pXP217) was identified via its ability to confer more prominent filamentation than the wild-type allele and found to contain an arginine 155-to-glycine substitution.
Plasmids pXP233 to pXP242 are a series of lacZ reporter plasmids that contain different segments of the FLO11 promoter. Different 250-bp (pXP233 to pXP241) or 200-bp (pXP242) fragments of the FLO11 promoter that overlap by 50 bp were PCR amplified and inserted into the SmaI site of the pSLFΔ-178K lacZ reporter plasmid (11) to create plasmids pXP233 to pXP242. This series of DNA fragments covers the immediate 2,000-bp region of the FLO11 promoter. The bp −1400 to −1150 region of the FLO11 promoter was PCR amplified and purified, an A overhang was added with Extaq (Takara), and the resulting product was cloned into the pCR2.1 TA-cloning vector (Stratagen) to create plasmid pXP223. Except for the error-prone PCR, in which Extaq (Takara) was used, all PCRs were performed with Pfu Turbo high-fidelity DNA polymerase (Stratagene).
Media and growth conditions.
Standard yeast media and genetic manipulations were used (41). Limiting nitrogen medium was used as described (26). Selective synthetic complete medium with either dextrose (SD) or raffinose (SR) as the carbon source was used to maintain plasmids.
Photomicroscopy and fluorescence microscopy.
All single-colony photographs were taken at a magnification of ×25. Yeast cells expressing GFP-tagged Flo8, Sfl1, or Tpk2 fusion proteins were grown in synthetic liquid medium until mid-log phase or in SLAD liquid medium. Cells were harvested, washed once with phosphate-buffered saline (PBS), permeabilized with 0.1% Triton X-100 in PBS for 5 min, and washed twice with PBS. Cell pellets were resuspended in the residual PBS solution and mixed with an equal volume (2 μl) of 4′,6′-diamidino-2-phenylindole (DAPI) solution (1 mg/ml). In the case of pde2 mutant strains, cAMP was added at a concentration of 10 mM to cell suspensions and incubated at room temperature for 10 to 60 min. GFP or DAPI staining was studied by examining the sample-bearing glass slides with a GFP or DAPI filter under a fluorescence microscope (Nikon). Representative cells were photographed at a magnification of ×100.
Northern (RNA) analysis.
Northern blot analysis of expression of the FLO11 and ACT1 genes was performed as previously described (35).
β-Galactosidase assays.
β-Galactosidase activity was assayed in chloroform-permeabilized cells and expressed in Miller units (18). In the assays for Sfl1 repression of CYC1-lacZ expression, cultures were grown to mid-log phase in SD-Ura-His selective medium (43). In all other assays, cultures were grown overnight in selective synthetic dextrose medium to saturation.
Protein purification from E. coli.
Plasmids expressing the MBP (pMAL2c), MBP-Flo8-His6 (pXP142), and MBP-Sfl1-His6 (pXP143) fusion proteins were transformed into E. coli TB1 cells (New England Biologicals). Cells were grown in 500 ml of YT liquid medium containing 100 μg of ampicillin per ml at 37°C to an optical density at 600 nm of 0.5. The cultures were supplemented with 50 μg of ampicillin per ml and 0.3 μM isopropyl-β-d-thiogalactopyranoside (IPTG) and incubated for 2 h. Cells were collected and homogenized in lysis buffer (40 mM HEPES [pH 7.4], 100 mM KCl, 10% glycerol, 0.5 mM phenylmethylsulfonyl fluoride) with 10 30-s sonications (Branson Sonifier 250; VWR). The fusion proteins were purified by Ni2+-agarose resin chromatography (Qiagen) and further purified on an amylose column (New England Biologicals). MBP was purified on an amylose column alone.
Purification of GST and GST fusion proteins from yeast cells.
Protease-deficient yeast strain BJ5627 containing plasmids pRD56 (URA3 GAL1-GST) and pXP287 (URA3 GAL1-GST-TPK2 [R99K]) and strains XPY310a/α (GAL1-GST-TPK1/TPK1) and XPY311a/α (GAL1-GST-TPK2/TPK2) containing plasmid pRS316 (URA3) were grown in 50 ml of S-raffinose-Ura liquid medium overnight at 30°C to an optical density at 600 nm of 0.6 to 0.8. The cultures were then supplemented with 3% galactose and incubated for 2 h. Cells were collected, washed with 1× chilled PBS solution, and homogenized in 500 μl of phosphatase-inhibiting lysis buffer (20 mM K2HPO4 [pH 7.4], 0.5% Triton X-100, 25 mM β-glycerophosphate, 25 mM NaF, 100 μM Na3VO4, 2 mM EGTA, 2 mM EDTA, 1 mM dithiothreitol, freshly added 1 mM phenylmethylsulfonyl fluoride, and 1× complete cocktail protease inhibitor [10]) with bead beating for six strokes for 1 min (Mini-beadbeater; Biospec).
Cell extracts were collected and cleared by centrifuging at top speed for 20 min and incubated with 100 μl of blank Sepharose beads for 30 min, followed by a 1-min spin at top speed. Then 100 μl of glutathione-Sepharose beads was incubated with the supernatant for 1 h and washed three times with 1 ml of the lysis buffer and twice with the lysis buffer containing 10 mM cAMP to remove Bcy1 copurified with GST fusion proteins, and once with 1× protein kinase A phosphorylation buffer (see below).
Protein mobility shift analysis and coimmunoprecipitation.
The isogenic wild-type (BJ2168a) and tpk2 mutant (XPY247a) protease-deficient strains expressing Flo8-Myc12 (pXP110) or Sfl1-Myc12 (pXP112) and the tpk2 mutant strain complemented with the wild TPK2 gene (pXP179) expressing Sfl1-Myc12 were each grown in 50 ml of SD-Ura-Leu liquid medium to an optical density at 600 nm of 0.8 to 1.0. Cells were harvested and homogenized in phosphatase-inhibiting lysis buffer as described above. Samples containing 1,000 μg of total protein were cleared with 50 μl of blank Sepharose beads and then incubated with 25 μl of anti-c-Myc conjugated to Sepharose beads (2 μg/μl; Santa Cruz) for 3 h for immunoprecipitation. The immunoprecipitation samples were washed five times with 1 ml of lysis buffer and resuspended in 50 μl of lysis buffer.
For phosphatase treatment, 10 μl of the Sfl1-Myc12 fusion protein purified from the wild-type strain BJ2168 was washed with phosphatase buffer and incubated with 1.0 U of calf intestinal phosphatase at 37°C for 1 h. Then 5 μl of each immunoprecipitation sample was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (6% gels; 7-in. by 8-in. [ca. 18-cm by 20-cm] plates, 8 V/cm for 12 h at 4°C) and analyzed by Western blotting with the anti-c-Myc monoclonal antibody (Santa Cruz).
Coimmunoprecipitation analysis of the protein-protein interaction between the hemagglutinin 3× (HA3)-Flo8 and Flo8-Myc12, HA3-Sfl1, and Sfl1-Myc12 or HA3-Sfl1 and Flo8-Myc12 in wild-type or tpk2 mutant cells was carried out essentially as described above. Immunoprecipitation with 15 μl of anti-c-Myc conjugated to Sepharose beads was performed in cell extracts containing the epitope-tagged proteins (1,000 μg of total protein). Immunoprecipitation samples were washed five times and resuspended in 25 μl of lysis buffer. Then 5 μl of each sample was resolved by SDS-8% PAGE and analyzed by Western blotting with anti-HA or anti-c-Myc antibodies (Santa Cruz).
Protein kinase A phosphorylation assay with GST-kinase fusion proteins.
Phosphorylation of the bacterially purified proteins was performed essentially as described by Heitman et al. (20) with minor modifications. For each 20-μl 32P incorporation protein kinase A phosphorylation reaction, ∼50 ng of MBP, MBP-Flo8-His6, or MBP-Sfl1-His6 purified from E. coli was used with 5 μl of GST, GST-Tpk1, GST-Tpk2(K99R), or GST-Tpk2 bound to glutathione-Sepharose beads as the kinase in the presence of 1 μCi of 32P. The phosphorylation reaction mixtures were incubated at 30°C for 30 min, resolved by SDS-PAGE (8%), transferred to a polyvinylidene difluoride membrane, and exposed to film for autoradiography. After the signal decayed, the same membrane was probed with anti-MBP antibody to identify the substrates. In the absence of 32P, 100 ng of MBP or MBP fusion protein sample was used in a phosphorylation assay with or without ATP, GST, and GST-Tpk2. One-tenth of the reaction mixture was employed in the DNA-binding assay described below.
Chromatin immunoprecipitation assays.
Chromatin immunoprecipitation for detecting protein-DNA interaction was done essentially as described by Hecht and Grunstein (19) with minor modifications. In the study of Sfl1-FLO11 promoter interactions, HA3-Sfl1 (pXP160) was expressed in wild-type BJ2168, an isogenic tpk2 strain, or the wild-type strain overexpressing TPK2 (pXP179). In the study of Flo8-FLO11 promoter interactions, HA3-Flo8 (pXP184) expressed in the isogenic wild-type, tpk2, or tpk2 sfl1 mutant strain was treated with 1% formaldehyde and immunoprecipitated with monoclonal mouse anti-HA antibody (12CA5). DNA fragments coimmunoprecipitated with the HA3-tagged proteins were used as the template in PCRs to amplify the bp −1400 to −1150 region of the FLO11 promoter and, as a negative control, the bp +20 to +280 coding region of the TPK1 gene. A PCR program with 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s was applied. The PCR products were fractionated by 2% agarose gel electrophoresis, stained with ethidium bromide, and photographed.
In vitro DNA-binding assays.
The 250-bp DNA fragment encompassing the bp −1400 to −1150 region of the FLO11 promoter was PCR amplified and cloned into plasmid pCR2.1. The cloned sequence (pXP223) was excised with EcoRI, dephosphorylated with calf intestinal phosphatase, purified from an agarose gel, and labeled with T4 polynucleotide kinase (New England Biologicals) to a specificity of 2 × 104 cpm/ng. A protein kinase A phosphorylation mixture containing 10 ng of purified MBP, MBP-Flo8-His6, or MBP-Sfl1-His6 protein was used for each DNA-binding reaction [50 mM HEPES (pH 8.0), 100 mM NaCl, 1 mM EDTA, 10 μg of poly(dI-dC)·poly(dI-dC) nonspecific carrier DNA per ml, 1 mg of bovine serum albumin per ml, 10% glycerol, and 5 × 104 cpm of probe). The binding reaction mixture was incubated at 4°C for 2 h and subjected to electrophoresis on a 4% native polyacrylamide gel in 1 × Tris-glycine running buffer for 3.5 h at 4°C in a cold room with a voltage of 13 V/cm. The gels were dried and autoradiographed.
RESULTS
Flo8 and Sfl1 both specifically interact with the Tpk2 catalytic subunit of protein kinase A.
Previous reports suggested that the Tpk2 catalytic subunit of protein kinase A activates yeast pseudohyphal differentiation and FLO11 expression via multiple transcription factors, including Flo8, Sfl1, Sok2, and Phd1 (35, 38, 39, 47). We therefore examined the relationship between Tpk2 and these factors.
We first tested which of these proteins interact with Tpk2 in a modified yeast two-hybrid system. To sensitize cells to exogenous cAMP, the PDE2 gene, encoding the high-affinity cAMP phosphodiesterase, was disrupted in the two-hybrid strain PJ69-4A. The resulting pde2 mutant two-hybrid reporter strain was used to identify specific interactions between the transcription factors and Tpk2 and Tpk1. Tpk2 and Tpk1 were fused to the Gal4 DNA-binding domain and cotransformed with the individual transcription factors fused to the Gal4 activation domain. Transformants were tested for the ability to grow on synthetic medium with or without exogenous cAMP as a measure of expression of the Gal4-dependent GAL2-ADE2 reporter gene.
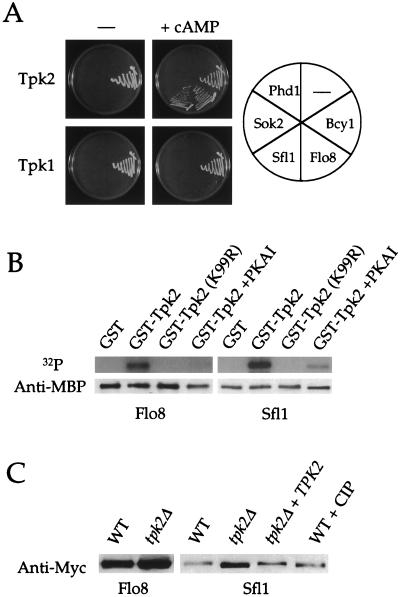
As shown in Fig. 1A, none of the transcription factors tested interacted with either Tpk2 or Tpk1 in the absence of exogenous cAMP, presumably because the kinase is bound by the endogenous protein kinase A regulatory subunit Bcy1 and is inactive. As a positive control, Bcy1 interacted robustly with both Tpk2 and Tpk1 under the same conditions. The addition of 5 mM exogenous cAMP reduced the interaction between Bcy1 and either Tpk2 or Tpk1. cAMP also now allowed Flo8 and Sfl1 to interact specifically with Tpk2 but not with either Tpk1 (Fig. 1A) or a kinase-inactive Tpk2 mutant (K99R) (data not shown). A specific interaction between Sfl1 and Tpk2 has also been reported previously (38). In contrast, Phd1 and Sok2 both failed to interact with either Tpk2 or Tpk1 under these conditions (Fig. 1A), consistent with recent findings that Phd1 and Sok2 may act in a distinct pathway from protein kinase A (36).
FIG. 1.
Flo8 and Sfl1 interact with and are phosphorylated by Tpk2. (A) Flo8 and Sfl1 interact with Tpk2 but not with Tpk1. The Gal4 DNA-binding domain (Gal4DB) fused to Tpk2 or Tpk1 was coexpressed with the Gal4 activation domain (Gal4AD) fused to Flo8, Sfl1, Sok2, or Phd1 in the pde2 mutant two-hybrid strain XPY100a. Transformants were tested for growth on SD-Leu-Trp-Ade medium with and without 5 mM cAMP. As controls, the interaction between GalDB-Tpk2 and Gal4DB-Tpk1 with the Gal4 activation domain alone or Gal4-Bcy1 was tested. Transformants were incubated at 30°C for 7 days and photographed. We note that for the Tpk-Bcy1 interaction, colony formation was delayed by the presence of cAMP, indicating a weaker interaction. (B) Bacterially purified MBP-Flo8-His6 and MBP-Sfl1-His6 fusion proteins were tested for in vitro 32P incorporation in the presence of GST, GST-Tpk2 (K99R), or GST-Tpk2 purified from yeast cells with or without 10 μM protein kinase A inhibitor (PKAI). After the signal decayed, the membrane was probed with an anti-MBP polyclonal antibody. (C) Sfl1 is a Tpk2-dependent phosphoprotein in vivo. Flo8-Myc12 or Sfl1-Myc12 expressed from their native promoters in an isogenic wild-type (WT, BJ2168), tpk2 mutant (XPY247a), or tpk2 mutant complemented with the wild-type TPK2 gene (XPY247a + TPK2) strain were immunoprecipitated with anti-c-Myc conjugated to agarose beads. Sfl1-Myc12 from wild-type cells was also treated with calf intestinal phosphatase (WT + CIP). Samples were probed with an anti-c-Myc monoclonal antibody.
Flo8 and Sfl1 are both phosphorylated by Tpk2.
Because Tpk2 is a protein kinase and a kinase-inactive Tpk2 allele failed to restore pseudohyphal growth in tpk2 mutant strains (not shown), we hypothesized that Tpk2 controls Flo8 and Sfl1 via phosphorylation. To address this, we tested whether Flo8 and Sfl1 can be phosphorylated by Tpk2 in vitro. MBP-Sfl1-His6 and MBP-Flo8-His6 fusion proteins were purified from bacteria and used as the substrate for 32P incorporation assays with GST-Tpk2 purified from yeast cells as the kinase. Both Flo8 and Sfl1 were phosphorylated by GST-Tpk2 but not by GST alone or a kinase-inactive Tpk2 mutant (K99R) (Fig. 1B). Phosphorylation of Sfl1 and Flo8 by GST-Tpk2 was markedly reduced in the presence of protein kinase A inhibitor, providing additional evidence that protein phosphorylation is mediated by GST-Tpk2 itself rather than by other yeast proteins that may copurify with GST-Tpk2. In control experiments, the MBP protein alone was not phosphorylated by Tpk2 (not shown). These results indicate that both Flo8 and Sfl1 can be directly phosphorylated by Tpk2 in vitro.
Next, we examined whether Flo8 and Sfl1 are phosphorylated in vivo by Tpk2. Functional Flo8-Myc12 and Sfl1-Myc12 fusion proteins were expressed from the endogenous FLO8 or SFL1 promoter from a 2μm plasmid in the protease-deficient yeast strain BJ2168, an isogenic tpk2 mutant, and the tpk2 mutant complemented with the wild-type TPK2 gene. Western analysis was performed to detect any mobility shift of the Myc12-tagged proteins in these isogenic strains. As shown in Fig. 1C, there was no significant SDS-PAGE mobility shift of the Flo8-Myc12 protein from wild-type compared to tpk2 mutant cells. In contrast, the Sfl1-Myc12 fusion protein reproducibly migrated slightly more rapidly when expressed in tpk2 mutant cells. The slower wild-type electrophoretic mobility of Sfl1 was restored when the wild-type TPK2 gene was reintroduced into the tpk2 mutant, indicating that the mobility shift of Sfl1-Myc12 depends on the Tpk2 kinase. When the Sfl1-Myc12 fusion protein was isolated from the wild-type strain and treated with calf intestinal phosphatase, the mobility of Sfl1-Myc12 was increased to that observed in the tpk2 mutant, demonstrating that the mobility shift of the Sfl1 protein results from phosphorylation.
Because no mobility shift was observed with Flo8 in vivo, it is possible either that Flo8 is not a physiological target of Tpk2 or that phosphorylation of Flo8 occurs but cannot be detected by a shift in mobility. In support of the latter possibility, when the Flo8-Myc12 fusion protein was purified from the tpk2 mutant strain and phosphorylated in vitro with GST-Tpk2, no SDS-PAGE mobility shift of the protein was observed (not shown). We conclude that both Sfl1 and Flo8 are directly phosphorylated by Tpk2 in vitro and that Sfl1 is a physiologically relevant substrate of Tpk2 in vivo.
Genetic evidence that Flo8 and Sfl1 act downstream of Tpk2.
To further understand the consequence of Sfl1 phosphorylation by Tpk2 and investigate Flo8 regulation, we employed genetic approaches to test the relationships between Tpk2 and Sfl1 and Flo8. In particular, we sought to test a model in which Tpk2 phosphorylates and thereby inactivates the Sfl1 repressor, resulting in FLO11 expression and pseudohyphal growth. In this model, Tpk2 might also phosphorylate and activate Flo8 to promote FLO11 expression and filamentous growth. We addressed this model by conducting genetic epistasis tests in which Tpk2, Sfl1, and Flo8 were mutated or overexpressed.
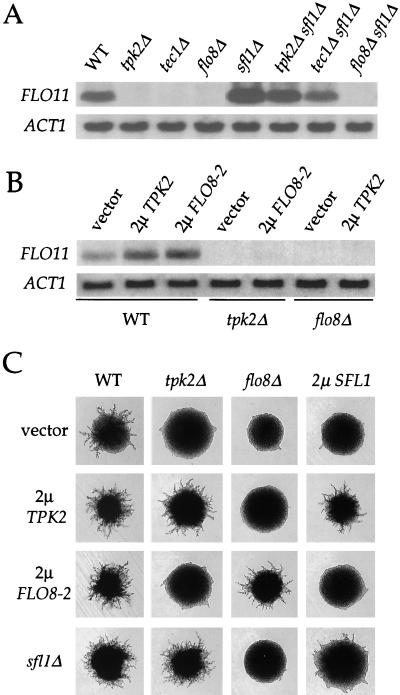
In accord with previous results (38), an sfl1 mutation suppressed the defect in FLO11 expression and restored pseudohyphal growth of tpk2 mutant strains (Fig. 2A and C). Interestingly, overexpression of the SFL1 gene blocked pseudohyphal growth in wild-type cells, and this effect was reversed by concomitant overexpression of TPK2 (Fig. 2C). These results support the model that Tpk2 inactivates Sfl1 during pseudohyphal differentiation.
FIG. 2.
TPK2, FLO8, and SFL1 genes exhibit reciprocal epistasis in controlling FLO11 expression and filamentous growth. (A) sfl1 mutations enhance expression of the FLO11 gene only in the presence of Flo8. Total RNA was isolated from isogenic wild-type (WT) and tpk2Δ, tec1Δ, flo8Δ, sfl1Δ, sfl1Δ tpk2Δ, sfl1Δ tec1Δ, and sfl1Δ flo8Δ mutant strains (see Table 1), fractionated, and probed with portions of the FLO11 and ACT1 genes. (B) Tpk2 and Flo8 activate FLO11 expression in the presence of each other. A wild-type (WT) strain containing a control plasmid (vector), a 2μm TPK2 plasmid, or a 2μm FLO8-2 plasmid, a tpk2 mutant (tpk2Δ) containing a control plasmid (vector) or a 2μm FLO8-2 plasmids and a flo8 mutant (flo8Δ) containing a control plasmid (vector) or a 2μm TPK2 plasmid were grown in selective medium. Total RNA was isolated and analyzed by Northern blotting with portions of the FLO11 and ACT1 genes. (C) Tpk2, Sfl1, and Flo8 exhibit reciprocal epistasis in pseudohyphal differentiation. Isogenic diploid wild-type (WT), tpk2Δ and flo8Δ mutant, and 2μm SFL1 overexpression strains containing a control plasmid (vector, row 1), a 2μm TPK2 (row 2) or 2μm FLO8-2 (row 3) plasmid, or an sfl1 mutation (row 4) were grown on SLAD medium for 3 days at 30°C. Representative colonies were photographed at ×25 magnification.
We also found that the flo8 mutation blocked the stimulating effects of TPK2 overexpression on both FLO11 expression and pseudohyphal growth (Fig. 2B and C), consistent with previous observations that flo8 mutations abolish the effect of activated protein kinase A (35, 39). More importantly, overexpression of either the wild-type FLO8 gene (not shown) or the more active FLO8-2 allele (R155G) enhanced filamentation and FLO11 expression in wild-type cells but failed to restore FLO11 expression (Fig. 2B) or pseudohyphal growth (Fig. 2C) in tpk2 mutant strains. These observations indicate that Flo8 requires the presence of Tpk2 to activate FLO11 expression and filamentous growth in wild-type cells.
Interestingly, Flo8 and Sfl1 have antagonistic actions. Overexpression of the SFL1 gene blocked pseudohyphal growth in strains overexpressing either the wild-type FLO8 gene or the more active FLO8-2 allele (Fig. 2C and not shown). On the other hand, flo8 mutations also blocked the effect of sfl1 mutations on both FLO11 expression and filamentous growth (Fig. 2A and C). In contrast, sfl1 mutations restored FLO11 expression (Fig. 2A) and pseudohyphal growth (data not shown) in cells lacking the MAP kinase pathway component Tec1. These results suggest that Sfl1 acts together with Flo8 in the protein kinase A pathway and that the functions of Sfl1 are distinct from the MAP kinase cascade. Sfl1 might function by antagonizing the effects of Flo8 on FLO11 expression and pseudohyphal growth.
Tpk2 does not control the nuclear localization of Sfl1 or Flo8.
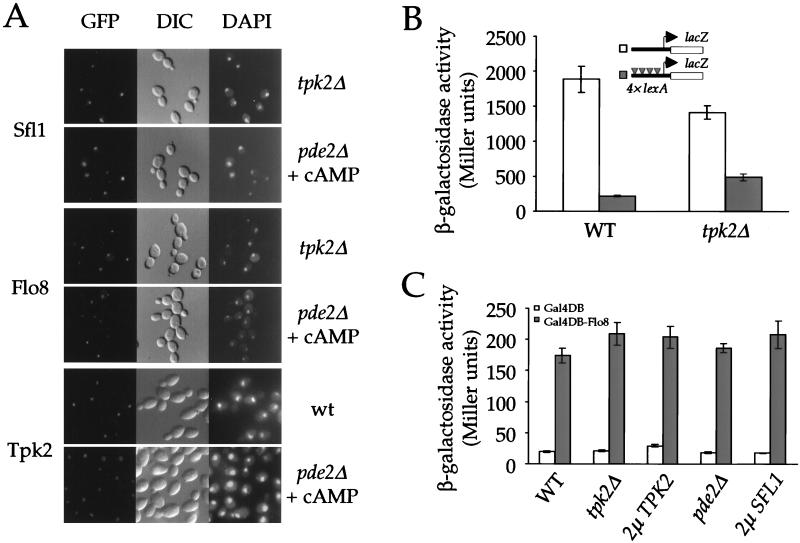
Next, we investigated the mechanism by which Tpk2 controls Sfl1 and Flo8. Protein kinase A has been shown to prohibit the nuclear localization of Msn2 and Msn4, two transcription factors required for stress responses (16). We therefore tested whether Tpk2 controls Sfl1 or Flo8 in a similar fashion. Sfl1 and Flo8 were tagged with the enhanced green fluorescent protein GFPS65T at their carboxy termini and expressed from their native promoters from 2μm plasmids in the wild-type and tpk2/tpk2 and pde2/pde2 mutant strains, and localization of the GFP signals was examined by direct fluorescence. As reported previously, Flo8 localized to the nucleus in wild-type cells (22) (not shown). In accord with its presumptive function as a transcriptional repressor, Sfl1 was also nucleus localized (not shown). Mutation of the TPK2 gene, activation of protein kinase A by treating pde2 mutant strains with exogenous cAMP for 10 and 60 min, or growth in liquid SLAD low ammonium medium did not alter the nuclear localization of either Flo8 or Sfl1 (Fig. 3A and data not shown).
FIG. 3.
Tpk2 does not control nuclear localization of Sfl1 or Flo8 or transcriptional activation or repression activity of Sfl1 or Flo8 on heterologous promoters. (A) Protein kinase A does not regulate localization of Sfl1 or Flo8. Plasmids expressing Sfl1-GFP and Flo8-GFP fusion proteins were transformed into isogenic diploid tpk2Δ or pde2Δ mutant strains. Localization of the fusion proteins was visualized by direct immunofluorescence microscopy (GFP, left panels), and cells and nuclei were visualized by differential interference contrast microscopy (DIC, middle panels) and DAPI staining (DAPI, right panel). The pde2Δ mutant cells were treated with 10 mM cAMP for 10 min before GFP was visualized. Localization of the GFP-Tpk2 fusion protein in the wild-type and the isogenic pde2Δ mutant strains was analyzed in a similar fashion. (B) Tpk2 does not prevent LexA-Sfl1 from repressing gene expression. A plasmid expressing the LexA-Sfl1 fusion protein was cotransformed with either a CYC1-lacZ or a lexA-CYC1-lacZ reporter plasmid into the isogenic wild-type (PJ69-4A) and tpk2Δ mutant (XPY220a) strains. Three independent colonies from each transformation were tested for β-galactosidase activity. Error bars in this and the following figures indicate the variation in reporter gene expression (standard error of the mean) among colonies from the same transformations. (C) A Gal4DB-Flo8 (amino acids 206 to 799) fusion protein activates Gal4-dependent lacZ reporter expression independently of Tpk2. Plasmids expressing the Gal4 DNA-binding domain or Gal4DB-Flo8 fusion protein were individually transformed into isogenic wild-type (WT, PJ69-4A), a tpk2Δ mutant (XPY220a), a 2μm TPK2 overexpression, a pde2Δ mutant (XPY100a), or a 2μm SFL1 overexpression strain. Three independent colonies from each transformation were tested for β-galactosidase expression.
In a similar experiment, we found that a functional GFP-Tpk2 fusion protein is also predominantly localized to the nucleus, and this localization was not affected by exogenous cAMP (Fig. 3A). The localization of Tpk2 is distinct from that of Tpk1, which has been shown to translocate from the nucleus to the cytoplasm in response to cAMP (17). The localization of Tpk2 with Flo8 and Sfl1 in the nucleus further supports a model in which Tpk2 directly controls Flo8 and Sfl1 to effect pseudohyphal differentiation. In vitro, we found that either GST-Tpk2 or GST-Tpk1 that was purified from yeast cells was capable of phosphorylating both Sfl1 and Flo8 (not shown). Thus, the differences in localization between Tpk2 and Tpk1 may contribute to their distinct activating and inhibitory roles in pseudohyphal differentiation by allowing Tpk2 but not Tpk1 access to the nuclear substrates Sfl1 and Flo8 (35).
Tpk2 does not affect the activity of Sfl1 or Flo8 on heterologous promoters.
To further investigate how Tpk2 regulates gene expression via Sfl1 and Flo8, we tested whether Tpk2 controls the transcriptional regulatory activity of either Sfl1 or Flo8 targeted to heterologous promoters. Sfl1, when fused to LexA, is known to repress gene expression in a lexA operator-dependent manner (43). If Tpk2 antagonized the interaction between Sfl1 and its corepressors, a tpk2 mutation should enhance repression by the LexA-Sfl1 fusion protein. We tested the ability of LexA-Sfl1 to repress expression of a CYC1-lacZ reporter with lexA operators upstream of its upstream activation sequence in isogenic wild-type and tpk2 mutant strains. In the wild-type strain, the LexA-Sfl1 fusion protein repressed reporter gene expression by 8.2-fold. Under the same conditions, LexA-Sfl1 repressed expression by only 2.9-fold in the tpk2 mutant strain (Fig. 3B). In conclusion, Tpk2 does not antagonize the inhibitory effect of Sfl1 on gene expression when Sfl1 is localized to a heterologous gene promoter by LexA.
A similar approach was taken to investigate whether Tpk2 regulates the interaction between Flo8 and its coactivators. A region of Flo8 encompassing amino acids 206 to 799 was fused to the Gal4 DNA-binding domain. The resulting Gal4DB-Flo8 fusion protein activated expression of a Gal4-dependent lacZ reporter gene (Fig. 3C). A plasmid expressing the Gal4DB-Flo8 protein was introduced into isogenic wild-type, tpk2, and pde2 mutant strains or cells overexpressing Tpk2. If Tpk2 were required for Flo8 to interact with its coactivators, alterations in Tpk2 activity should cause a change in transcriptional activation by the fusion protein. As shown in Fig. 3C, neither overexpression nor deletion of TPK2 had any significant effect on the activity of the Gal4-Flo8 fusion protein. Activation of protein kinase A by exogenous cAMP in a pde2 mutant also did not alter activity of the fusion protein.
Although this Gal4DB-Flo8 fusion lacks the amino-terminal 205 amino acids of the Flo8 protein, it is not likely that Tpk2 acts through this portion to activate Flo8 because full-length Flo8 has also been shown to activate gene expression in a cAMP-independent fashion when fused to the LexA DNA-binding domain (39). These results indicate that Tpk2 is not required for interactions between Flo8 and its coactivators. In a similar experiment, overexpression of the SFL1 gene also did not affect Gal4-Flo8-dependent expression of the lacZ reporter gene (Fig. 3C), suggesting that antagonism between Flo8 and Sfl1 is specific to the FLO11 promoter.
Tpk2, Sfl1, and Flo8 act on a small region of the FLO11 promoter.
The FLO11 gene has one of the largest (>3,000 bp) and most complex promoters in the yeast genome, and myriad trans regulators control its expression (29, 35, 36, 38, 39). Previous studies revealed that Ste12, Tec1, cAMP, and Flo8 act on distinct and overlapping regions of the FLO11 promoter (39). To further determine the DNA region where Tpk2, Flo8, and Sfl1 act, the bp −2000 to 0 region of the FLO11 promoter was divided into a series of 250-bp sequence elements that overlap by 50 bp. These 250-bp DNA fragments were individually inserted upstream of a CYC1-lacZ reporter gene. The resulting constructs were tested for expression of the lacZ reporter gene in the wild-type and tpk2, flo8, sfl1, tpk2 sfl1, and flo8 sfl1 homozygous diploid mutant strains, as well as in strains overexpressing TPK2, FLO8, or SFL1.
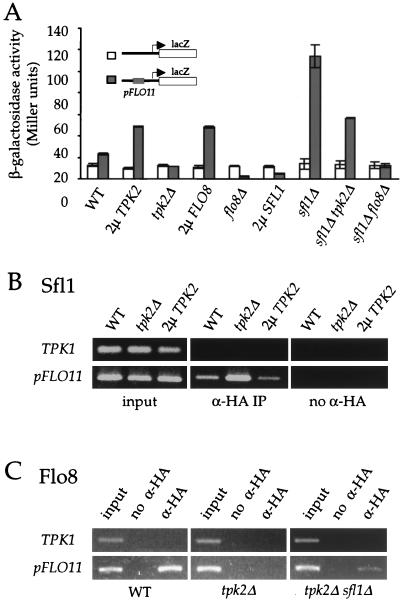
Tpk2, Sfl1, and Flo8 all acted on only a common 250-bp element corresponding to the bp −1400 to −1150 region of the FLO11 promoter. As shown in Fig. 4A, this 250-bp DNA fragment modestly increased expression of the CYC1-lacZ reporter by 1.8-fold in a wild-type strain, and this effect was dependent upon the presence of TPK2 because no increase in lacZ gene expression was observed in the tpk2/tpk2 mutant strain. Correspondingly, overexpression of TPK2 further enhanced lacZ gene expression above the wild-type level by >2-fold. Overexpression of the FLO8 gene also increased expression of this reporter gene by >2-fold (Fig. 4A). In contrast, the flo8 mutant not only prevented the enhancing effect of this 250-bp DNA fragment, but also revealed a repressive activity that abolished lacZ reporter expression. Overexpression of Sfl1 repressed expression of the lacZ reporter, and this effect required the 250-bp FLO11 promoter element. Consistently, deletion of the SFL1 gene enhanced lacZ gene expression from the same reporter construct by >5-fold (Fig. 4A).
FIG. 4.
Tpk2, Flo8, and Sfl1 converge on a 250-bp region of the FLO11 promoter. (A) Tpk2, Flo8, and Sfl1 act on a common region of the FLO11 promoter to regulate gene expression. The CYC1-lacZ and pFLO11-CYC1-lacZ reporter genes were individually transformed into the isogenic diploid wild-type (WT), 2μm TPK2 overexpression, tpk2Δ mutant, 2μm FLO8 overexpression, flo8Δ mutant, 2μm SFL1 overexpression, sfl1Δ mutant, sfl1Δ tpk2Δ mutant, and sfl1Δ flo8Δ mutant strains. In each case, three independent colonies were tested for β-galactosidase activity. (B) Tpk2 inhibits Sfl1 binding to the FLO11 promoter in vivo. HA3-Sfl1 fusion protein was expressed in the isogenic wild-type (WT), tpk2Δ mutant, and 2μm TPK2 overexpression strains. Cells were treated with formaldehyde to cross-link proteins and DNA. Chromatin immunoprecipitation analysis of Sfl1 binding to the FLO11 promoter was performed by PCR amplification with DNA immunoprecipitated by anti-HA3-Sfl1 (α-HA immunoprecipitation) or a no-antibody control (no α-HA). (C) Tpk2 is required for Flo8 binding to the FLO11 promoter in vivo. The HA3-Flo8 fusion protein was expressed in the isogenic wild-type (WT), tpk2Δ mutant, and tpk2Δ sfl1Δ mutant strains. Flo8 binding to the FLO11 promoter was analyzed by chromatin immunoprecipitation assays as above.
As was the case in regulation of the native FLO11 gene, the sfl1 mutation suppressed the effect of tpk2 mutations on reporter expression driven by the 250-bp FLO11 element, and β-galactosidase activity in the sfl1 tpk2 double mutant was increased by ∼2.5-fold (Fig. 4A). However, the increase in reporter expression in the sfl1 tpk2 double mutant strain (2.5-fold) was lower than that observed in the sfl1 single mutant stain (>5-fold), suggesting that Tpk2 has at least one target in addition to Sfl1. This could represent a basal activity of Flo8 in the absence of Tpk2 that is only revealed in the sfl1 mutant background. The 250-bp FLO11 promoter region repressed expression of the CYC1-lacZ reporter gene in a flo8 mutant strain (>5-fold). However, this repressive effect was abolished when both the SFL1 and FLO8 genes were deleted (Fig. 4A). The experiments presented here show that Flo8 and Sfl1 have antagonizing effects on FLO11 gene expression.
Tpk2 modulates Sfl1 and Flo8 binding to the FLO11 promoter in vivo.
Because both Sfl1 and Flo8 act on the bp −1400 to −1150 region of the FLO11 promoter to govern gene expression, we used chromatin immunoprecipitation assays to establish whether both proteins bind to this promoter in vivo. Sfl1 and Flo8 were tagged with triple HA epitope tags on their N termini, and these tagged alleles complemented the corresponding mutations (data not shown). The HA3-Sfl1 protein was expressed from the ADH1 gene promoter, and the HA3-Flo8 protein was expressed from the native FLO8 promoter. To test the effect of Tpk2 on DNA binding, the HA3-Sfl1 and HA3-Flo8 expression plasmids were separately transformed into a wild-type protease-deficient strain and an isogenic tpk2 mutant. Transformants expressing the fusion proteins were identified by Western blotting with anti-HA antibody; mutation of the TPK2 gene had no effect on the amount of either protein (data not shown).
As shown in Fig. 4B, the FLO11 promoter immunoprecipitated with HA3-Sfl1, indicating that Sfl1 interacts with this DNA sequence. Tpk2 inhibits the binding of Sfl1, and the amount of FLO11 promoter DNA that immunoprecipitated with Sfl1 was increased in the tpk2 mutant compared to wild-type cells. Overexpression of Tpk2 reduced the ability of Sfl1 to bind to the FLO11 promoter (Fig. 4B). In contrast, Tpk2 was required for Flo8 to bind to the promoter of the FLO11 gene, and the HA3-Flo8 protein failed to coimmunoprecipitate with this DNA sequence when expressed in a tpk2 mutant strain (Fig. 4C). Interestingly, the sfl1 mutation partially restored binding of Flo8 to the FLO11 promoter in a tpk2 mutant (Fig. 4C), consistent with the observation that sfl1 mutations suppress the pseudohyphal growth and FLO11 expression defects of the tpk2 mutant but not of the tpk2 flo8 double mutant strains (Fig. 2 and data not shown). This suggests that Sfl1 and Flo8 may compete to occupy the FLO11 promoter. In control assays, a nonspecific DNA fragment from the TPK1 gene did not interact with either Sfl1 or Flo8.
Tpk2 represses Sfl1 and activates Flo8 binding to the FLO11 promoter in vitro.
Our chromatin immunoprecipitation analysis indicated that Sfl1 and Flo8 are associated with the FLO11 promoter in vivo. We next tested whether these proteins bind directly to DNA and how DNA binding is controlled by Tpk2. MBP-Flo8-His6 and MBP-Sfl1-His6 fusion proteins were purified from bacteria and then incubated in a protein kinase A phosphorylation assay with GST or a GST-Tpk2 fusion protein immobilized on glutathione beads in the presence or absence of ATP. The beads were pelleted to separate the GST or GST-Tpk2 protein from the reaction mixture, and the supernatant containing either Flo8 or Sfl1 was then employed in DNA-binding assays.
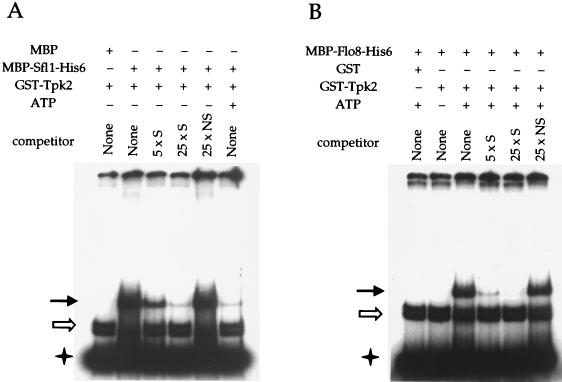
Sfl1 bound to the bp −1400 to −1150 region of the FLO11 promoter and yielded a DNA mobility shift (Fig. 5A). This complex was specific and was inhibited by unlabeled specific DNA (Fig. 5A, lanes 2, 3, and 4), but not by a nonspecific DNA fragment (the bp −2000 to −1750 region of the FLO11 promoter; Fig. 5A, lane 5). Phosphorylation of the MBP-Sfl1-His6 protein by GST-Tpk2 dramatically reduced specific binding (Fig. 5A, lane 6). In these experiments, the MBP-Sfl1-His6 fusion protein but not MBP alone formed a specific protein-DNA complex with the FLO11 promoter. This specific DNA mobility shift was not caused by the GST-Tpk2 fusion protein or copurified yeast proteins that might be present in the protein-DNA binding reaction mixture (compare lanes 1 and 2 of Fig. 5A). In independent experiments, we found that the MBP-Sfl1-His6 fusion protein formed the same protein-DNA complex with the FLO11 promoter in vitro in the absence of GST-Tpk2 (data not shown).
FIG. 5.
Tpk2 regulates Sfl1 and Flo8 binding to the FLO11 promoter in vitro. (A) Phosphorylation of Sfl1 by Tpk2 inhibits its binding to the FLO11 promoter. MBP and MBP-Sfl1-His6 purified from E. coli were used as substrates in a phosphorylation reaction with GST-Tpk2 kinase (purified from yeast cells on glutathione beads) in the presence (+) or absence (−) of ATP. Following incubation at 30°C for 30 min, beads were pelleted and separated from the reaction mixture. The supernatant was used in DNA-binding assays with the labeled FLO11 promoter fragment in the presence or absence of specific (S) or nonspecific (NS) competitor DNA, as indicated. The solid arrow indicates a mobility shift caused by Sfl1 or Flo8 (panel B) binding to the probe. The open arrow indicates nonspecific background binding, and the star indicates free probe. (B) Flo8 binds to the FLO11 promoter in vitro only when phosphorylated by Tpk2. MBP-Flo8-His6 protein purified from E. coli was used as the substrate in a phosphorylation reaction with GST or GST-Tpk2 kinase (purified from yeast cells on glutathione beads) in the presence (+) or absence (−) of ATP. The reaction mixture was separated, and the supernatant used in DNA-binding assays with the labeled FLO11 promoter in the presence or absence of specific (S) or nonspecific (NS) competitor DNA, as indicated.
On the other hand, native unphosphorylated Flo8 did not bind to this DNA sequence, whereas phosphorylation by Tpk2 dramatically enhanced DNA binding by Flo8 (Fig. 5B, lanes 2 and 3). Again, Flo8 binding to the FLO11 promoter was specific and readily inhibited by unlabeled specific DNA but not by even a 25-fold excess of a nonspecific DNA fragment (Fig. 5B, lanes 4, 5, and 6). In control reactions, the MBP-Flo8-His6 fusion protein that was treated with GST did not bind to this DNA fragment (Fig. 5B, lane 1). We also note that the specific DNA mobility shift in lanes 3 through 6 of Fig. 5B was not caused by potential phosphorylation of copurified proteins by GST-Tpk2, because this specific complex was absent in lane 6 of Fig. 5A, in which GST-Tpk2 and ATP were present in the absence of Flo8. No supershift to a higher-molecular-weight DNA-protein complex was observed when the MBP-Flo8-His6 protein was added to the Sfl1-DNA complex (data not shown). Taken together, these results demonstrate that phosphorylation by Tpk2 inactivates Sfl1 binding and activates Flo8 binding to the same region of the FLO11 promoter.
Tpk2 inhibits Sfl1-Sfl1 interactions.
In mammals, cAMP signaling controls gene expression via the cAMP response element binding protein (CREB) and related transcription factors (32). Dimerization of CREB and its associated protein CBP is increased by protein kinase A phosphorylation, which promotes DNA binding by the heterodimer (34). We therefore tested whether Tpk2 inhibits multimerization of Sfl1 or promotes that of Flo8.
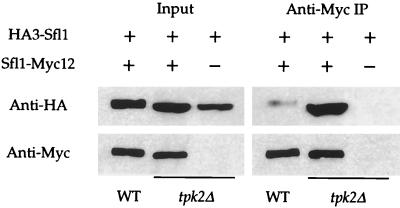
Differentially epitope-tagged forms of Sfl1 (Sfl1-Myc12 and HA3-Sfl1) and Flo8 (Flo8-Myc12 and HA3-Flo8) were expressed in isogenic wild-type and tpk2 mutant strains. The epitope-tagged proteins were immunoprecipitated with anti-c-Myc antibody, and dimerization (or oligomerization) of either Sfl1 or Flo8 was determined by probing for the presence of HA3-tagged proteins in the immunoprecipitates. Any effect of Tpk2 on these interactions would be apparent by a difference between the amount of HA3 fusion protein present in the immunoprecipitates from wild-type compared to tpk2 mutant cells.
As shown in Fig. 6, an interaction between HA3-Sfl1 and Sfl1-Myc12 was clearly detected. More importantly, the interaction between the two differentially epitope-tagged forms of Sfl1 was inhibited in the presence of Tpk2 (Fig. 6). In control experiments, HA3-Sfl1 was not detected in the immunoprecipitates from cells coexpressing HA3-Sfl1 and an empty vector (Fig. 6) or HA3-Sfl1 and Flo8-Myc12 (data not shown). In accord with the finding that Sfl1 forms multimers, the Sfl1 protein contains a coiled-coil domain (amino acids 336 to 371), which is known to mediate subunit oligomerization of other proteins (5). In contrast, we were not able to detect any interaction between HA3-Flo8 and Flo8-Myc12 coexpressed in either wild-type or tpk2 mutant strains (not shown).
FIG. 6.
Tpk2 inhibits Sfl1-Sfl1 interactions. The HA3-Sfl1 fusion protein was coexpressed with Sfl1-Myc12 or an empty plasmid in the isogenic wild-type and tpk2Δ mutant strains. Immunoprecipitation with anti-c-Myc conjugated to Sepharose beads (anti-Myc immunoprecipitation) was performed in each sample (1,000 μg of total protein). Whole-cell extracts (input) and immunoprecipitated samples were probed with anti-HA and anti-c-Myc monoclonal antibodies.
DISCUSSION
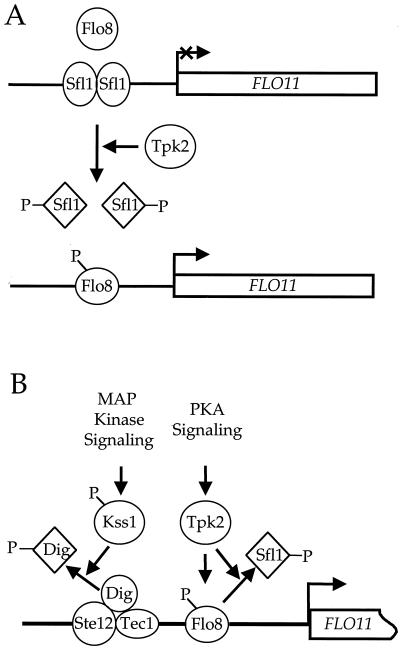
Previous genetic studies have suggested that the cAMP pathway controls FLO11 expression and pseudohyphal growth in S. cerevisiae through the transcription factors Sfl1 and Flo8 (35, 38, 39). However, at the outset of these studies, the molecular mechanism of action was unclear. Here we present genetic and biochemical evidence elucidating the role of the Tpk2 catalytic subunit of protein kinase A during this complex differentiation process. The Sfl1 repressor and Flo8 activator play antagonistic roles in controlling FLO11 expression. The protein kinase A catalytic subunit Tpk2 phosphorylates Sfl1 and inhibits its binding to the FLO11 promoter. Additionally, Tpk2 binds to and phosphorylates Flo8 (Fig. 1) and stimulates binding to the same region of the FLO11 promoter (Fig. 4C and 5B). Recently, similar findings on protein kinase A-dependent inhibition of Sfl1 binding to target promoters were reported, and the Ssn6/Tup1 complex was implicated in repression by Sfl1 (6). Therefore, the Tpk2 catalytic subunit of protein kinase A controls the balance between transcriptional repression by Sfl1 and activation by Flo8 (Fig. 7A).
FIG. 7.
Tpk2 modulates the assembly of transcription factors on the FLO11 promoter. (A) Tpk2 controls binding of Sfl1 and Flo8 to the FLO11 promoter. In the model presented, the transcriptional repressor Sfl1 and transcriptional activator Flo8 bind to the same or adjacent regions of the FLO11 promoter to effect gene expression. Phosphorylation (P) by Tpk2 removes Sfl1 and promotes Flo8 binding to the target DNA. (B) The MAP kinase and protein kinase A (PKA) pathways employ analogous mechanisms to control FLO11 expression, and both pathways drive gene expression by inactivating repressors and stimulating activators.
Our findings that the Tpk2 catalytic subunit of protein kinase A controls gene expression via both a transcriptional repressor and a transcriptional activator is unusual. Typically, either activating an activator or inactivating a repressor is sufficient to govern gene expression. The double-barreled mechanism described here may provide a finer network of checks and balances to modulate gene expression, possibly by controlling the ratios of phosphorylated and unphosphorylated forms of both Sfl1 and Flo8. Several signaling pathways control FLO11 expression, likely in response to different environmental stimuli; this may again serve to provide a finer level of regulation of gene expression via cross talk between pathways. The ability of cells to finely tune Flo11 expression may be important for survival in nature by enabling cells to appropriately effect a key developmental switch: choosing to adhere to each other and invade growth substrates when nutrients are limiting or to rapidly adopt a normal budding growth when nutrients are encountered.
Protein kinase A controls binding of transcription factors to target promoters.
In this study, we found that Tpk2 does not regulate either the intracellular localization or the transcriptional activity of either Flo8 or Sfl1 on heterologous promoters. In fact, Tpk2 phosphorylates Flo8 in vitro and Sfl1 both in vitro and in vivo, and phosphorylation by Tpk2 prevents DNA binding by Sfl1 and facilitates Flo8 binding to the same region of the FLO11 promoter. Additionally, we found that the Sfl1 protein forms multimers and this multimerization is inhibited by Tpk2. In mammalian systems, protein kinase A phosphorylates the transcription factor CREB at serine 133 and increases dimerization between CREB and the associated protein CBP, which promotes binding of the heterodimer to DNA (34). Using the dimeric b/ZIP vitellogenin promoter-binding protein, Szilak and colleagues designed a leucine zipper that is stabilized when a serine residue is phosphorylated by protein kinase A (45). The phosphorylated protein binds to DNA with a 15-fold-higher affinity, and in a transient transfection assay, protein kinase A-dependent activation of a reporter gene was observed (45). The ability of Tpk2 to inhibit multimerization of Sfl1 and impair DNA binding may be accomplished by similar mechanisms.
Functions of the Tpk2 and Tpk1 catalytic subunits are distinct.
Previous studies reveal that the three catalytic subunits of protein kinase A play distinct roles in filamentous growth, with Tpk2 serving as an activator and Tpk1 and Tpk3 functioning as inhibitors under most conditions (35, 38). Our findings suggest that two molecular mechanisms distinguish the functions of Tpk2 from those of Tpk1. First, we found that Tpk2 binds to both Flo8 and Sfl1 in the two-hybrid assay, whereas Tpk1 does not (Fig. 1A). In control experiments, Tpk1 and Tpk2 bound equally well to the Bcy1 regulatory subunit, suggesting that the two differ in ability to associate with substrates. This difference is not absolute, since we also found that GST-Tpk1 and GST-Tpk2 fusion proteins were both capable of phosphorylating Flo8 and Sfl1 in vitro. A second molecular mechanism that distinguishes the functions of Tpk2 and Tpk1 is their different intracellular localizations. Tpk1 is rapidly exported from the nucleus in response to cAMP (17), whereas we found that Tpk2 is exclusively nuclear (Fig. 3A). Thus, differences in both substrate binding and intracellular localization likely contribute to the unique activating function of Tpk2 compared to the inhibitory role of Tpk1.
Under certain conditions, Flo8 can promote filamentous growth in the absence of Tpk2. For example, Flo8 promotes FLO11 expression and pseudohyphal differentiation in sfl1 tpk2 mutant strains, in which Tpk2 is not present (Fig. 2). Moreover, in chromatin immunoprecipitation assays, Flo8 binding to the FLO11 promoter was significantly reduced but not abolished in sfl1 tpk2 mutant strains (Fig. 4C). Under these conditions, the two other protein kinase A catalytic subunits (Tpk1 and Tpk3) may play a role. In previous studies, we found that in cells in which protein kinase A was constitutively activated by loss of the Bcy1 regulatory subunit, Tpk1 and Tpk3 could partially promote filamentous growth in the absence of Tpk2 (35). Based on this finding, Tpk1 and Tpk3 may play a role in promoting Flo8 action in sfl1 tpk2 mutants. In support of this hypothesis, we found that a GST-Tpk1 fusion protein purified from yeast cells could phosphorylate both Sfl1 and Flo8 in vitro (not shown). In a recent report, bovine protein kinase A was also shown to phosphorylate Sfl1 and inhibit binding to target DNA in vitro (6).
Taken together, our observations support a model in which Tpk2 reciprocally controls the activity of Sfl1 and Flo8 in wild-type cells and the functions of Tpk2 are distinguished from those of Tpk1 by their different intracellular localizations and affinity for substrates.
Similarities between protein kinase A and MAP kinase pathways.
Although the protein kinase A and MAP kinase signaling pathways are distinct and respond to different extracellular stimuli, there are several common features in how they control pseudohyphal differentiation in S. cerevisiae. First, the protein kinases in both pathways have dual functions. In the protein kinase A pathway, Tpk2 activates filamentous growth, whereas Tpk1 and Tpk3 play negative roles (35, 38). Similarly, in the MAP kinase pathway, Kss1 has a dual role in which unactivated Kss1 inhibits pseudohyphal growth, whereas activated Kss1 promotes filamentation (8, 30). Second, both the protein kinase A and MAP kinase pathways converge to control expression of the FLO11 gene required for filamentous growth (35, 38, 39). Third, both pathways employ similar mechanisms — removing repressors and stimulating activators — to control FLO11 expression (Fig. 7B). When the MAP kinase pathway is inactive, the MAP kinase Kss1 and the repressors Dig1 and Dig2 bind to and inhibit Ste12/Tec1 heterodimers. Firing of MAP kinase signaling activates Kss1 and prevents it from binding to and inhibiting the Ste12/Tec1 complex. In addition, activated Kss1 phosphorylates Dig1 and Dig2 and might further reduce interactions between the Dig repressors and Ste12/Tec1 heterodimers (1, 2, 7, 8, 30).
Whether Kss1 directly phosphorylates Ste12 to activate transcription and whether the Dig repressors inhibit DNA binding or mask transactivation activity of the Ste12/Tec1 heterodimers remains to be determined. In the protein kinase A pathway, we show here that Tpk2 inhibits the Sfl1 transcriptional repressor and activates the Flo8 transcriptional activator to promote FLO11 expression and that Tpk2 directly controls their assembly on the FLO11 promoter (Fig. 7). These results set the stage for further analysis of how different signaling pathways coordinately and combinatorially control gene expression critical for the complex developmental switch to pseudohyphal differentiation.
Coordinated repression and activation of gene expression.
Although the double-barreled mechanism in which a single kinase inactivates a transcriptional repressor and activates an activator is so far unique to Tpk2 control of FLO11 expression, the combination of dual control by activation and relief of repression employed here is fairly ubiquitous. In bacteria, the lactose operon is under control of both the lactose repressor and the CAP activator, ensuring that genes required for lactose metabolism are induced only when lactose but not glucose is present (31, 33). Similarly, in Candida albicans, filamentous growth and hypha-specific gene expression are inhibited by homologs of the Ssn6/Tup1 general repressor and activated by the Efg1 transcription factor (4, 40).
Both positive and negative control of filamentous growth is important for virulence because both hyperfilamentous tup1 and nonfilamentous efg1 mutant strains of C. albicans are avirulent (3, 23a). Interestingly, the Ssn6/Tup1 repressor and the Efg1 activator converge to control expression of cell wall glycerol phosphinositol-anchored proteins that are functional homologs of Flo11 in S. cerevisiae (4, 40). It will be of significant interest to determine whether the known role of the protein kinase A catalytic subunit Tpk2 in C. albicans virulence (44) also involves a double-barreled mechanism of transcriptional control.
Acknowledgments
We thank Maria Cardenas, Daniel Lew, John McCusker, Robin Wharton, Chris Counter, John Rohde, Miguel Arevalo-Rodriguez, and Christina Hull for advice and discussions; Keri Forrester for technical assistance; Christina Hull, John Rohde, and Robin Wharton for comments on the manuscript; Mike Lorenz and Marjorie Brandriss for strains; Chris Armstrong and Namjin Chung in the Guarente laboratory and Marian Carlson for plasmids; and Tina Wilkins for assistance with preparation of the manuscript.
Joseph Heitman is a Burroughs Wellcome Scholar in Molecular Pathogenic Mycology and an associate investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Bardwell, L., J. G. Cook, J. X. Zhu-Shimoni, D. Voora, and J. Thorner. 1998. Differential regulation of transcription: repression by unactivated mitogen-activated protein kinase Kss1 requires the Dig1 and Dig2 proteins. Proc. Natl. Acad. Sci. USA 95:15400-15405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bardwell, L., J. G. Cook, D. Voora, D. M. Baggott, A. R. Martinez, and J. Thorner. 1998. Repression of yeast Ste12 transcription factor by direct binding of unphosphorylated Kss1 MAPK and its regulation by the Ste7 MEK. Genes Dev. 12:2887-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun, B. R., and A. D. Johnson. 1997. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science 277:105-109. [DOI] [PubMed] [Google Scholar]
- 4.Braun, B. R., and A. D. Johnson. 2000. TUP1, CPH1 and EFG1 make independent contributions to filamentation in Candida albicans. Genetics 155:57-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burkhard, P., S. V. Strelkov, and J. Stetefeld. 2001. Coiled coils: a highly versatile protein folding motif. Trends Cell Biol. 11:82-88. [DOI] [PubMed] [Google Scholar]
- 6.Conlan, R. S., and D. Tzamarias. 2001. Sfl1 functions via the corepressor Ssn6-Tup1 and the cAMP-dependent protein kinase Tpk2. J. Mol. Biol. 309:1007-1015. [DOI] [PubMed] [Google Scholar]
- 7.Cook, J. G., L. Bardwell, S. J. Kron, and J. Thorner. 1996. Two novel targets of the MAP kinase Kss1 are negative regulators of invasive growth in the yeast Saccharomyces cerevisiae. Genes Dev. 10:2831-2848. [DOI] [PubMed] [Google Scholar]
- 8.Cook, J. G., L. Bardwell, and J. Thorner. 1997. Inhibitory and activating functions for MAPK Kss1 in the S. cerevisiae filamentous-growth signalling pathway. Nature 390:85-88. [DOI] [PubMed] [Google Scholar]
- 9.Crabtree, G. R. 2001. Calcium, calcineurin and the control of transcription. J. Biol. Chem. 276:2313-2316. [DOI] [PubMed] [Google Scholar]
- 10.Dhand, R., I. Hiles, G. Panayotou, S. Roche, M. J. Fry, I. Gout, N. F. Totty, O. Truong, P. Vicendo, and K. Yonezawa. 1994. PI 3-kinase is a dual specificity enzyme: autoregulation by an intrinsic protein-serine kinase activity. EMBO J. 13:522-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Fields, S., and O.-K. Song. 1989. A novel genetic system to detect protein-protein interactions. Nature 340:245-246. [DOI] [PubMed] [Google Scholar]
- 11.Forsburg, S. L., and L. Guarente. 1988. Mutational analysis of upstream activation sequence 2 of the CYC1 gene of Saccharomyces cerevisiae: a HAP2-HAP3-responsive site. Mol. Cell. Biol. 8:647-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujita, A., Y. Kikuchi, S. Kuhara, Y. Misumi, S. Matsumoto, and H. Kobayashi. 1989. Domains of the SFL1 protein of yeasts are homologous to Myc oncoproteins or yeast heat-shock transcription factor. Gene 85:321-328. [DOI] [PubMed] [Google Scholar]
- 13.Gancedo, J. M. 2001. Control of pseudohyphae formation in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 25:107-123. [DOI] [PubMed] [Google Scholar]
- 13a.Gietz, R. D., and A. Sugino. 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74:527-534. [DOI] [PubMed] [Google Scholar]
- 14.Gimeno, C. J., P. O. Ljungdahl, C. A. Styles, and G. R. Fink. 1992. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell 68:1077-1090. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein, A. L., X. Pan, and J. H. McCusker. 1999. Heterologous URA3MX cassettes for gene replacement in Saccharomyces cerevisiae. Yeast 15:507-511. [DOI] [PubMed] [Google Scholar]
- 16.Görner, W., E. Durchschlag, M. T. Martinez-Pastor, F. Estruch, G. Ammerer, B. Hamilton, H. Ruis, and C. Schüller. 1998. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 12:586-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffioen, G., P. Anghileri, E. Imre, M. D. Baroni, and H. Ruis. 2000. Nutritional control of nucleocytoplasmic localization of cAMP-dependent protein kinase catalytic and regulatory subunits in Saccharomyces cerevisiae. J. Biol. Chem. 275:1449-1456. [DOI] [PubMed] [Google Scholar]
- 18.Guarente, L. 1983. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 101:181-191. [DOI] [PubMed] [Google Scholar]
- 18a.Guarente, L., and E. Hoar. 1984. Upstream activation sites of the CYC1 gene of Saccharomyces cerevisiae are active when inverted but not when placed downstream of the “TATA box.” Proc. Natl. Acad. Sci. USA 81:7860-7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hecht, A., and M. Grunstein. 1999. Mapping DNA interaction sites of chromosomal proteins using immunoprecipitation and polymerase chain reaction. Methods Enzymol. 304:399-414. [DOI] [PubMed] [Google Scholar]
- 20.Heitman, J., A. Koller, M. E. Cardenas, and M. N. Hall. 1993. Identification of immunosuppressive drug targets in yeast. Methods Companion Methods Enzymol. 5:176-187. [Google Scholar]
- 20a.James, P., J. Halladay, and E. A. Craig. 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144:1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20b.Jones, E. W. 1991. Tackling the protease problem in Saccharomyces cerevisiae. Methods Enzymol. 194:428-453. [DOI] [PubMed] [Google Scholar]
- 20c.Keleher, C., M. J. Redd, J. Schultz, M. Carlson, and A. D. Johnson. 1992. Ssn6-Tup1 is a general repressor of transcription in yeast. Cell 68:709-719. [DOI] [PubMed] [Google Scholar]
- 21.Lambrechts, M. G., F. F. Bauer, J. Marmur, and I. S. Pretorius. 1996. Muc1, a mucin-like protein that is regulated by Mss10, is critical for pseudohyphal differentiation in yeast. Proc. Natl. Acad. Sci. USA 93:8419-8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, H., C. A. Styles, and G. R. Fink. 1996. Saccharomyces cerevisiae S288C has a mutation in FLO8, a gene required for filamentous growth. Genetics 144:967-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo, W.-S., and A. M. Dranginis. 1998. The cell surface flocculin Flo11 is required for pseudohyphae formation and invasion by Saccharomyces cerevisiae. Mol. Biol. Cell 9:161-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.Lo, H.-J., J. R. Köhler, B. DiDomenico, D. Loebenberg, A. Cacciapuoti, and G. R. Fink. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90:939-949. [DOI] [PubMed] [Google Scholar]
- 24.Longtine, M. S., I. A. McKenzie, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953-961. [DOI] [PubMed] [Google Scholar]
- 25.Lorenz, M. C., and J. Heitman. 1998. Regulators of pseudohyphal differentiation in Saccharomyces cerevisiae identified through multicopy suppressor analysis in ammonium permease mutant strains. Genetics 150:1443-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorenz, M. C., and J. Heitman. 1997. Yeast pseudohyphal growth is regulated by GPA2, a G protein α homolog. EMBO J. 16:7008-7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorenz, M. C., R. S. Muir, E. Lim, J. McElver, S. C. Weber, and J. Heitman. 1995. Gene disruption with PCR products in Saccharomyces cerevisiae. Gene 158:113-117. [DOI] [PubMed] [Google Scholar]
- 28.Lorenz, M. C., X. Pan, T. Harashima, M. E. Cardenas, Y. Xue, J. P. Hirsch, and J. Heitman. 2000. The G protein-coupled receptor GPR1 is a nutrient sensor that regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Genetics 154:609-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madhani, H. D., and G. R. Fink. 1997. Combinatorial control required for the specificity of yeast MAPK signaling. Science 275:1314-1317. [DOI] [PubMed] [Google Scholar]
- 30.Madhani, H. D., C. A. Styles, and G. R. Fink. 1997. MAP kinases with distinct inhibitory functions impart signaling specificity during yeast differentiation. Cell 91:673-684. [DOI] [PubMed] [Google Scholar]
- 31.Malan, T. P., and W. R. McClure. 1984. Dual promoter control of the Escherichia coli lactose operon. Cell 39:173-180. [DOI] [PubMed] [Google Scholar]
- 32.Meyer, T. E., and J. F. Habener. 1993. Cyclic adenosine 3′,5′-monophosphate response element binding protein (CREB) and related transcription-activating deoxyribonucleic acid-binding proteins. Endocr. Rev. 14:269-290. [DOI] [PubMed] [Google Scholar]
- 32a.Mitchell, A. D., T. K. Marshall, and R. J. Deschenes. 1993. Vectors for the inducible overexpression of glutathione S-transferase fusion proteins in yeast. Yeast 9:715-722. [DOI] [PubMed] [Google Scholar]
- 33.Monod, J., J.-P. X. Changeux, and F. Jacob. 1963. Allosteric proteins and cellular control systems. J. Mol. Biol. 6:306-329. [DOI] [PubMed] [Google Scholar]
- 34.Nichols, M., F. Weih, W. Schmid, C. DeVack, E. Kowenz-Leutz, B. Luckow, M. Boshart, and G. Schutz. 1992. Phosphorylation of CREB affects its binding to high and low affinity sites: implications for cAMP induced gene transcription. EMBO J. 11:3337-3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan, X., and J. Heitman. 1999. Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:4874-4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan, X., and J. Heitman. 2000. Sok2 regulates yeast pseudohyphal differentiation via a transcription factor cascade that regulates cell-cell adhesion. Mol. Cell. Biol. 20:8364-8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan, X., T. Harashima, and J. Heitman. 2000. Signal transduction cascades regulating pseudohyphal differentiation of Saccharomyces cerevisiae. Curr. Opin. Microbiol. 3:567-572. [DOI] [PubMed] [Google Scholar]
- 38.Robertson, L. S., and G. R. Fink. 1998. The three yeast A kinases have specific signaling functions in pseudohyphal growth. Proc. Natl. Acad. Sci. USA 95:13783-13787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rupp, S., E. Summers, H. Lo, H. Madhani, and G. Fink. 1999. MAP kinase and cAMP filamentation signaling pathways converge on the unusually large promoter of the yeast FLO11 gene. EMBO J. 18:1257-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharkey, L. L., M. D. McNemar, S. M. Saporito-Irwin, P. S. Sypherd, and W. A. Fonzi. 1999. HWP1 functions in the morphological development of Candida albicans downstream of EFG1, TUP1, and RBF1. J. Bacteriol. 181:5273-5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherman, F. 1991. Getting started with yeast. Methods Enzymol. 194:3-21. [DOI] [PubMed] [Google Scholar]
- 42.Sia, R. A. L., E. S. Bardes, and D. J. Lew. 1998. Control of Swe1p degradation by the morphogenesis checkpoint. EMBO J. 17:6678-6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song, W., and M. Carlson. 1998. Srb/mediator proteins interact functionally and physically with transcriptional repressor Sfl1. EMBO J. 17:5757-5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sonneborn, A., D. P. Bockmuhl, M. Gerads, K. Kurpanek, D. Sanglard, and J. F. Ernst. 2000. Protein kinase A encoded by TPK2 regulates dimorphism of Candida albicans. Mol. Microbiol. 35:386-396. [DOI] [PubMed] [Google Scholar]
- 45.Szilak, L., J. Moitra, and C. Vinson. 1997. Design of a leucine zipper coiled coil stabilized 1.4 kcal mol-1 by phosphorylation of a serine in the e position. Protein Sci. 6:1273-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wach, A., A. Brachat, R. Pohlmann, and P. Philippsen. 1994. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10:1793-1808. [DOI] [PubMed] [Google Scholar]
- 47.Ward, M. P., C. J. Gimeno, G. R. Fink, and S. Garrett. 1995. SOK2 may regulate cyclic AMP-dependent protein kinase-stimulated growth and pseudohyphal development by repressing transcription. Mol. Cell. Biol. 15:6854-6863. [DOI] [PMC free article] [PubMed] [Google Scholar]