Abstract
Increases in type 1 phosphatase (PP1) activity have been observed in end stage human heart failure, but the role of this enzyme in cardiac function is unknown. To elucidate the functional significance of increased PP1 activity, we generated models with (i) overexpression of the catalytic subunit of PP1 in murine hearts and (ii) ablation of the PP1-specific inhibitor. Overexpression of PP1 (threefold) was associated with depressed cardiac function, dilated cardiomyopathy, and premature mortality, consistent with heart failure. Ablation of the inhibitor was associated with moderate increases in PP1 activity (23%) and impaired β-adrenergic contractile responses. Extension of these findings to human heart failure indicated that the increased PP1 activity may be partially due to dephosphorylation or inactivation of its inhibitor. Indeed, expression of a constitutively active inhibitor was associated with rescue of β-adrenergic responsiveness in failing human myocytes. Thus, PP1 is an important regulator of cardiac function, and inhibition of its activity may represent a novel therapeutic target in heart failure.
Cardiac muscle function is regulated on a beat-to-beat basis through the sympathetic nervous system. In seconds, the heart may respond to increases in workload by enhancing cardiac output to support the demands of peripheral, metabolizing tissues. The inotropic state of the heart is controlled in large part by the catecholamine-dependent activation of myocardial β-adrenoreceptors, which results in cyclic AMP (cAMP) increases, activation of the cAMP-dependent protein kinase (PKA), and phosphorylation of enzymes involved in energy metabolism, as well as key regulatory proteins recruited to modulate contractility. The main regulatory phosphoproteins include phospholamban, the ryanodine receptor, the L-type Ca2+ channel, troponin I, and C protein (44). Phospholamban has been shown to be the major regulator of basal contractility and a key mediator of the inotropic and lusitropic effects of β-agonists in the mammalian heart (10, 29, 46). Phosphorylation of phospholamban relieves its inhibition of the sarcoplasmic reticulum (SR) Ca2+-ATPase (SERCA), which greatly stimulates the rate and amount of cytosolic Ca2+ resequestered into the SR, enhancing myocardial relaxation (29, 49). This profile of increased Ca2+ cycling is associated with enhanced SR Ca2+ content, allowing for increased quantal Ca2+ release during subsequent contractions. Collectively, these events result in enhanced systolic and diastolic function.
The role of protein kinases in cardiac contractility has been well characterized, while the protein phosphatases involved are poorly understood. Increases in protein phosphorylation and enhanced cardiac function are reversed by protein phosphatases in a highly regulated manner. Two main classes of serine/threonine phosphatases, referred to as types 1 and 2, have been shown to regulate cardiac contractile performance (39). The type 1 phosphatase (PP1) has been implicated in the regulation of the β-agonist responses (13, 26). This enzyme is localized to SR membranes and glycogen by the noncatalytic targeting subunit, RGL, also called GM, which enhances substrate availability and specificity (27, 47, 48). Furthermore, PP1 is regulated by two heat- and acid-stable proteins, inhibitor 1 (I-1) and I-2 (25). I-1 becomes active upon phosphorylation on threonine-35 by PKA (15). This results in inhibition of PP1 and therefore enhanced PKA-mediated protein phosphorylation, leading to amplification of the β-agonist responses in the heart (1, 17, 41).
The fine-tuning regulation of cardiac regulatory protein phosphorylation by protein kinases and phosphatases becomes even more critical in pathological states. Heart failure is associated with enhanced noradrenergic activity; this may be compensatory early in the disease state, but long-term neurohormonal activation induces significant damage to cardiomyocytes. Decreases in cAMP levels by desensitization of β-adrenoreceptors (7) lead to inactivation of PKA, while the levels and activity of the SR-associated PP1 are increased (24, 38, 40). Since kinases and phosphatases are in a tight balance in the myocardium, the increase in phosphatases may be compensatory or detrimental. To directly determine the physiological and pathophysiological significance of increased cardiac PP1 activity, we used a two-pronged approach and generated animal models with alterations in PP1 activity and/or levels. Our findings demonstrate the physiological relevance of this enzyme in modulation of cardiac contractility and remodeling and provide novel insights into the functional significance of regulating PP1 activity in heart failure.
MATERIALS AND METHODS
Donor and failing human heart samples.
Tissues were obtained from 9 nonfailing donor and 10 failing left ventricles (LV) (51). LV ejection fractions were 59% ± 4% for nonfailing human hearts and 19% ± 3% for failing human hearts. Samples were handled in a manner approved by the Cleveland Clinic and University of Cincinnati Institutional Review Boards.
Generation of mice.
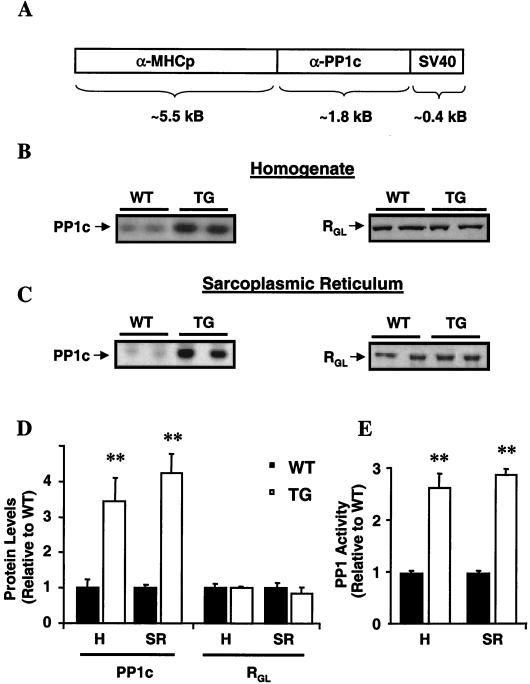
I-1-deficient mice (C57BL/6) were generated as previously described (2). For cardiac tissue-specific overexpression of PP1, the cDNA fragment containing the coding sequence for the α-isoform of the PP1 catalytic subunit (PP1c) (50) was inserted downstream of the mouse α myosin heavy chain (αMHC) promoter (see Fig. 1A). The ∼6.5-kb NruI fragment, comprising the promoter, the cDNA, and the simian virus 40 polyadenylation site, was gel purified and microinjected into the pronuclei of one-cell inbred C3Heb/FeJ embryos (22). The TG mice were generated at the Indiana University School of Medicine Transgenic Facility, directed by L. J. Field. Mice were handled according to protocols approved by the Institutional Animal Care and Use Committees at the University of Cincinnati and at Indiana University School of Medicine.
FIG. 1.
Generation of PP1 overexpression mice and their characterization at 3 months of age. (A) Cardiac tissue-specific overexpression of the α-isoform of the catalytic subunit of PP1 was driven by the α-MHC promoter. SV40, simian virus 40. (B and C) Representative Western blots of cardiac homogenates (B) and SR preparations (C) revealed similar increases in PP1c protein levels (left) in TG hearts without alterations in RGL, the SR targeting subunit (right). (D) Quantitation of PP1c and RGL levels (left) in whole-heart homogenates (H; n = 5) and SR preparations (SR; n = 6) in TG relative to WT hearts. (E) Quantitation of PP1 activity in homogenates and SR preparations from TG hearts relative to activity in those from WT hearts. Phosphatase activity was 1.3 ± 0.1 nmol/min/mg (n = 5) for the WT homogenates and 3.6 ± 0.3 nmol/min/mg (n = 5) for the TG homogenates and 0.21 ± 0.01 nmol/min/mg (n = 6) for WT SR preparations and 0.61 ± 0.02 nmol/min/mg (n = 6) for TG SR preparations. ∗∗, P < 0.001 versus WT.
PP1 activity.
Protein phosphatase activity was assayed with 32P-labeled rabbit glycogen phosphorylase a as the substrate (47). To maintain the phosphorylation status of I-1, PP2A (okadaic acid, 4 nM) and calcineurin phosphatase (EDTA, 0.5 mM) inhibitors were included in preparations of tissue extracts and in the enzyme reactions.
Heart perfusions.
Working-heart perfusions were performed as described previously (29). For Starling curves, each data point represents the mean ± standard error of the mean of three to five values from each wild-type (WT) or knockout heart. Linear regression lines were plotted with Prizm software.
In vivo echocardiography.
Noninvasive assessment of LV function and dimensions were obtained by M-mode and Doppler echocardiography (23).
Generation of a constitutively active I-1 peptide (1-65AA).
A construct encoding a constitutively active and truncated form of I-1 (15), containing the first 65 amino acids, was generated by using a 5′ primer homologous to the I-1 coding sequence with a flanking SalI site (italics) before the initiation start site (boldface) (CGCCGCTGGTCGACCTGACCGGGAGCCATGGAG) and a 3′ primer (GTGGAGACATGCGGCCGCTCATGACAAGGTGGA) containing a NotI site (italics) and translational stop site (boldface) following the codon for amino acid 65. This was subcloned (SalI-NotI) into pBluescript SK II(−) and amplified. The construct was then used to convert threonine-35 to aspartic acid-35 with T7, T3, and a mutant primer engineered with appropriate nucleotide changes (parentheses) GGCAGGG(T→G)(C→T)GGGGCGGC (5).
Adenovirus gene transfer in failing human myocytes.
Adenoviruses containing either the constitutively active I-1 (Ad.I-1T35D) and green fluorescent protein (GFP) or β-galactosidase and GFP (Ad.GFP), controlled by separate cytomegalovirus promoters, were constructed (12). The titers of stocks as measured by plaque assays were 1 × 1012 PFU/ml for Ad.GFP and 1.5 × 1012 PFU/ml for Ad.I-1T35D, with particle/PFU ratios of 10:1 and 20:1, respectively (viral particles per milliliter determined by using the relationship that 1 absorbance unit at 260 nm is equal to 1012 viral particles/ml). Contraction measurement and adenovirus delivery in failing human myocytes were performed as described previously (19).
Other methods.
SR-enriched preparations were isolated (9), and Western analysis of PP1c and RGL was performed (47) with a monoclonal antibody that recognized all isoforms of PP1c and a polyclonal antibody raised against the mouse RGL protein, respectively. Whole-cell Ca2+ channel currents were measured as described previously (33). Kaplan-Meier analysis was performed using Statview software, and significance was indicated by the log rank test. Total cAMP levels in heart homogenates were measured with a commercially available kit (NEN Life Sciences). Antibodies used were I-1 phosphorylated on Thr35 (P. Greengard), calsequestrin (a gift from L. R. Jones), SERCA (in-house), phospholamban (Affinity Bioreagents), and phospho-phospholamban (Phosphoprotein Research) antibodies.
Statistical analysis.
Values represent means ± standard errors of the means. Statistical comparisons were made with Student's t test or two-way repeated-measures analysis of variance (Student-Newman Keuls), with significance imparted at P values <0.05.
RESULTS
Increased PP1 levels result in impaired cardiac contractility.
Alterations in the activity of the SR-associated PP1 have been implicated in the diminished stimulatory effects of β-adrenoreceptor agonists (39) and deteriorated function in failing hearts (40). To determine the physiological and pathophysiological significance of increased PP1 activity, mice with cardiomyocyte-restricted overexpression of PP1c were generated (Fig. 1A). Nine founders were identified, but five died with heart failure symptoms. Two lines of transgenic (TG) mice (TG1 and TG2), expressing similar levels of PP1c protein and activity, were propagated for further characterization. The two lines exhibited identical cardiac phenotypes (see below), and results for the TG1 line are presented, unless otherwise indicated. The PP1c protein levels in TG cardiac homogenates (Fig. 1B) were increased threefold compared to WT levels, and similar increases were observed in SR preparations (Fig. 1C and D). Consistent with the protein level, PP1 activity was threefold higher in SR membranes from TG hearts (Fig. 1E). The increases in PP1 expression levels were not associated with alterations in RGL, its major SR targeting subunit (Fig. 1B and C).
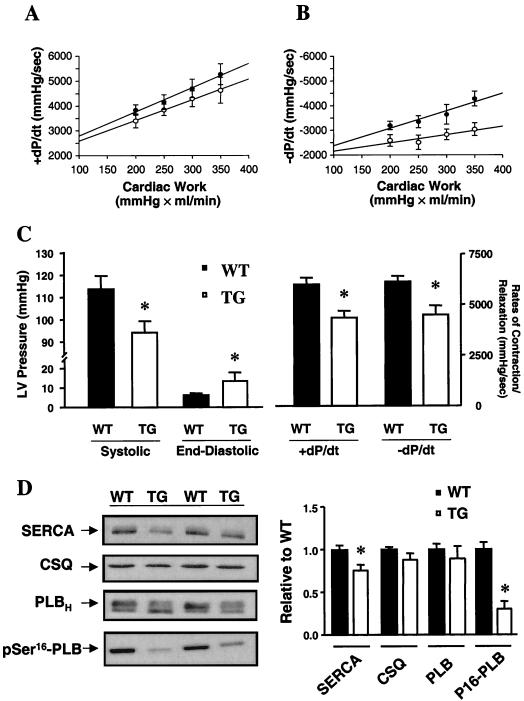
To determine the effect of increased PP1 activity on intrinsic myocardial contractility, in the absence of autonomic or hemodynamic feedback, work-performing cardiac preparations were utilized. Following equilibration of the hearts at 250 ml × mm Hg/min (preload: 5 ml of venous return/min; afterload: 50 mm Hg mean aortic pressure) the rates of contraction (+dP/dt) and relaxation (−dP/dt) in response to acute alterations in mean aortic pressure were examined. Bivariate regression plots revealed that increases and decreases in cardiac work were paralleled by alterations in the rates of contraction in both WT and TG hearts (Fig. 2A). However, the relaxation rates were severely blunted in TG hearts (Fig. 2B), demonstrating an impaired diastolic contractile reserve, particularly under increased cardiac workload. To further examine the ability of TG hearts to respond to stress, a maximal stimulatory concentration of isoproterenol (8 × 10−8 M) was administered in these preparations. The heart rate increases for TG and WT hearts were similar (data not shown), but TG hearts exhibited a significant reduction in peak systolic pressure and elevated end-diastolic pressure (Fig. 2C). The +dP/dt and −dP/dt values were also significantly attenuated (30%; P < 0.05; Fig. 2C), suggesting diminished contractile reserve in PP1 hearts, compared to WT hearts. Furthermore, assessment of the times to peak pressure (WT: 0.28 ± 0.02 s/mm Hg [n = 5]; TG: 0.39 ± 0.03 s/mm Hg [n = 7]; P < 0.05) and to 50% relaxation (WT: 0.28 ± 0.02 s/mm Hg [n = 5]; TG: 0.41 ± 0.04 s/mm Hg [n = 7]; P < 0.05) revealed significant prolongation in TG hearts. To determine whether the decreased response to β-adrenergic receptor stimulation reflected impaired adrenergic signaling, the levels of cAMP were examined. Under isoproterenol stimulation, the cAMP contents for WT (15.5 ± 2.1 pmol/mg, n = 4) and TG (14.4 ± 2.0 pmol/mg, n = 5) hearts were similar, suggesting no alterations in the β-signaling pathway. Furthermore, troponin I phosphorylation, as assessed by back-phosphorylation (31), in TG hearts (1.25 ± 0.14, n = 5) was not altered relative to that in WT hearts (1.00 ± 0.05, n = 5). However, phospholamban exhibited significantly depressed (69%) phosphorylation of the Ser16 site in TG hearts (0.35 ± 0.10, n = 5) compared to that in WT hearts (1.14 ± 0.09, n = 5; P < 0.05) (Fig. 2D), while there were no alterations in Thr17 phosphorylation (TG: 1.03 ± 0.17, n = 5; WT: 0.89 ± 0.26, n = 5; P > 0.05). The lack of effect on the Thr17 site may reflect increased Ca2+/calmodulin-dependent kinase II (CaMKII) activity as a consequence of elevated diastolic calcium levels, due to impaired SR calcium sequestration (see below). Previous studies have shown that phospholamban is phosphorylated by PKA on Ser16 and by CaMKII on Thr17; these events relieve its inhibitory effects on SERCA, leading to enhanced Ca2+ cycling and cardiac contractility during β-agonist stimulation (49). However, it has been suggested that phosphorylation of Ser16 is the major mediator of β-adrenergic effects (11). Thus, the decreased contractile responses to β-agonists in PP1 hearts were, at least partially, due to an inability to relieve phospholamban's inhibition of SERCA. Furthermore, the SERCA protein levels were significantly reduced (25%) in PP1 hearts without any alterations in calsequestrin. The decreases in SERCA and phospholamban phosphorylation are consistent with the impaired basal relaxation rates and attenuated contractile parameters for β-agonists (9, 43).
FIG. 2.
Cardiac function in PP1 mice at 3 months of age. (A and B) Bivariate regression plots of the positive (A; +dP/dt) and negative (B; −dP/dt) first derivatives of intraventricular pressure versus cardiac work in five WT (•) and five TG (○) isolated work-performing hearts. (C) Systolic pressure, end-diastolic pressure, and +dP/dt and −dP/dt in isoproterenol-stimulated perfused hearts. (D) Western blots of SERCA (6 μg), calsequestrin (CSQ; 6 μg), phospholamban (PLB; 6 μg), and pSer16-PLB (10 μg) in individual WT and TG hearts. Immunoreactivity for each protein was compared to a linear standard (2, 4, 8, and 12 μg) consisting of five pooled WT hearts on each blot. Quantitation revealed decreases in SERCA and pSer16-PLB in TG hearts (n = 5) compared to WT hearts (n = 5). ∗, P < 0.05 versus WT.
Decreased in vivo function, dilated cardiomyopathy, and early mortality in PP1 mice.
To gain further insight into the role of increased PP1 activity in intact animals, cardiac function was assessed using two-dimensional guided M-mode and Doppler echocardiography. For mice at 3 months of age, the heart rates were similar and there were no significant changes in LV mass or geometry between TG and WT mice (Table 1). In agreement with these data, gravimetric heart-to-body mass ratios for WT (5.7 ± 0.3 mg/g of body weight, n = 5) and TG (6.2 ± 0.5 mg/g, n = 5) mice were similar. Functionally, PP1 hearts demonstrated a substantial reduction in fractional shortening (FS) and heart rate-corrected mean velocity of circumferential fiber shortening (Vcfc; Table 1), indicating impaired basal cardiac function. Administration of isoproterenol (2.0 μg/g, intraperitoneally) stimulated both FS (WT: 69.5% ± 0.6% [n = 7]; TG: 52.9% ± 3.2% [n = 10]; P < 0.05) and Vcfc (WT: 14.4 ± 0.6 circumference (circ)/s [n = 7]; TG: 10.9 ± 0.3 circ/s [n = 10]; P < 0.05), but the maximally stimulated parameters remained attenuated in PP1 hearts, consistent with observations for perfused hearts.
TABLE 1.
Cardiac contractility in PP1-overexpressing mice characterized by in vivo echocardiographya
| Mouse type, age (mo) | n | Heart rate (beats/min) | LV EDD (mm) | LV ESD (mm) | FS (%) | Vcfc (circ/s) | LVM/body wt (mg/g) | h/r ratio | Body wt (g) |
|---|---|---|---|---|---|---|---|---|---|
| WT, 3 | 7 | 408 ± 34 | 3.44 ± 0.12 | 2.11 ± 0.19 | 39.1 ± 4.2 | 7.2 ± 0.9 | 2.00 ± 0.13 | 0.29 ± 0.02 | 19.2 ± 0.8 |
| PP1, 3 | 10 | 402 ± 15 | 3.80 ± 0.13 | 2.80 ± 0.14∗ | 26.5 ± 2.1∗ | 4.9 ± 0.5∗ | 2.40 ± 0.24 | 0.29 ± 0.02 | 18.9 ± 0.9 |
| WT, 6 | 12 | 410 ± 20 | 3.56 ± 0.12 | 2.11 ± 0.10 | 40.8 ± 2.2 | 7.0 ± 0.5 | 2.21 ± 0.12 | 0.33 ± 0.01 | 23.6 ± 1.0 |
| PP1, 6 | 10 | 345 ± 26 | 4.03 ± 0.19† | 3.10 ± 0.21† | 23.5 ± 2.0† | 4.3 ± 0.4† | 3.19 ± 0.27† | 0.32 ± 0.01 | 22.2 ± 0.9 |
Abbreviations: EDD, end-diastolic dimension; ESD, end-systolic dimension; LVM, calculated LV mass; h/r ratio, relative wall thickness, where h is wall thickness and r is LV radius. ∗, P < 0.05 versus the value for the WT at 3 months; †, P < 0.05 versus the value for the WT at 6 months.
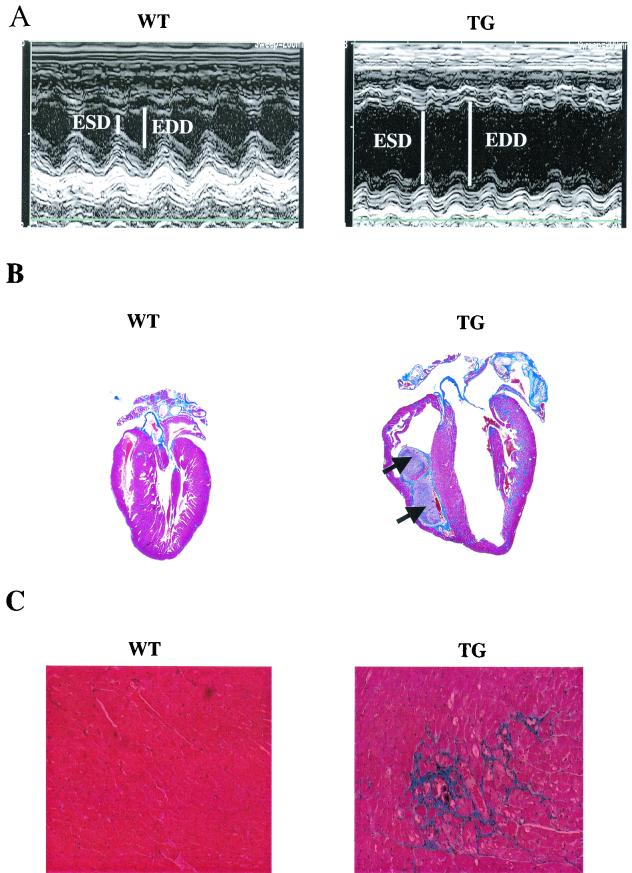
It was of special interest to determine whether the marked reduction in cardiac function of 3-month-old PP1 mice would lead to cardiac remodeling and pathology in older animals. Thus, echocardiography was repeated at 6 months of age. TG mice displayed cardiac enlargement, as evidenced by significant increases in the dimensions of their LV chambers at end systole and end diastole (Table 1 and Fig. 3A ). Histological examination revealed evidence of biventricular chamber dilation (Fig. 3B), extensive interstitial fibrosis (Fig. 3C), and increased LV mass/body mass ratios (Table 1). Increased heart-to-body mass ratios were also confirmed gravimetrically (WT: 6.07 ± 0.48 mg/g [n = 11]; TG: 10.59 ± 1.05 mg/g [n = 8]; P < 0.05). Examination of cardiac function indicated a progressive decrease in contractile parameters (FS and Vcfc) in hearts of 6-month-old PP1 mice, compared to values for age-matched WT mice (Table 1). Isoproterenol responsiveness measured in vivo was also blunted (FS values: WT, 67.1% ± 1.7% [n = 12]; TG, 46.9% ± 4.6% [n = 10]; P < 0.05; Vcfc values: WT, 14.48 ± 0.61 circ/s [n = 12]; TG, 8.70 ± 0.90 circ/s [n = 10]; P < 0.05).
FIG. 3.
PP1 is associated with pathology at 6 months of age. (A) In vivo, M-mode echocardiography performed in hearts from 6-month-old TG mice (right) revealed increased end-systolic (ESD) and end-diastolic dimensions (EDD) indicating LV dilation, compared to results for age-matched WT mice (left). (B) Masson's trichrome-stained longitudinal sections from WT (left) and TG (right) hearts showed dilated cardiomyopathy and the presence of intracardiac thrombi (arrows) in TG hearts. (C) Higher magnification (×170) indicated widespread interstitial fibrosis (blue) in the TG, but not WT, hearts. (D) Representative dot blot of ventricular gene expression showed increases in atrial natriuretic factor (ANF), β-MHC, and α-skeletal actin (α-sk. actin) in TG hearts. Blots are from three animals in each group. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (E) Survival curves indicated premature mortality (P < 0.00001) in TG (n = 46) compared to WT (n > 100) mice. Survival statistics were obtained by using the log rank test. Cum., cumulative.
At the molecular level, cardiac hypertrophy and heart failure are characterized by a reexpression of fetal gene isoforms, including β-MHC, α-skeletal actin, and atrial natriuretic factor. Consistent with this, ventricular mRNA expression patterns revealed a significant increase in all three transcripts in hearts of 6-month-old TG mice (Fig. 3D). Cardiac hypertrophy coupled with a significant increase in LV end-diastolic dimension (Table 1), without a change in the relative wall thickness, suggested the presence of dilated cardiomyopathy. The depressed function and dilated cardiomyopathy culminated in early lethality, approaching 50% by 194 days (Fig. 3E).
A separate line of PP1-overexpressing mice (TG2), which exhibited increases in cardiac PP1 activity (threefold) similar to those of TG1, also revealed decreases in function (FS: 26.6% ± 2.9%; Vcfc: 5.15 ± 0.5 circ/s; n = 4) and increases in end-diastolic dimension (4.33 ± 0.24 mm; n = 4), as well as LV mass/body mass (3.28 ± 0.23 mg/g; n = 4) similar to those for TG1. These findings indicate that the observed phenotype is not due to insertional effects of the transgene. Attempts to further characterize TG hearts from 6-month-old mice by ex vivo work-performing preparations were precluded by the severely depressed contractile parameters (+dP/dt, <1,900 mm Hg/s) and increased end-diastolic pressure (>30 mm Hg).
Ablation of I-1 results in increased phosphatase activity and impaired cardiac function.
Since PP1 is regulated by I-1, which functions as an inhibitor upon PKA phosphorylation, it was of special interest to generate mice deficient in I-1 (2) and examine the physiological significance of inactive I-1. The hypothesis was that β-adrenergic receptor stimulation and PKA activation would not be able to inhibit PP1 through I-1, leading to attenuated protein phosphorylation and contractility in the knockout hearts. Indeed, examination of in vivo cardiac function revealed significantly reduced basal parameters in knockout mice (FS: 30.2% ± 1.1%; Vcfc: 4.77 ± 0.15 circ/s; n = 10; P < 0.05) compared to WT mice (FS: 40.8% ± 2.0%; Vcfc: 6.70 ± 0.38 circ/s; n = 12) at 3 months of age, without alterations in heart rate. The degree of depressed cardiac function in knockouts did not progress further upon aging to 15 months, and there was no evidence of remodeling, as indicated by normal LV wall thickness and calculated LV mass, compared to values for WT mice (data not shown). Following isoproterenol (2.0 μg/g, intraperitoneally) stimulation, contractility was enhanced to a lower extent in knockout mice (FS: 56.9% ± 0.9%; Vcfc: 9.93 ± 0.34 circ/s; n = 10; P < 0.05) than in WT mice (FS: 68.0% ± 1.4%; Vcfc: 12.43 ± 0.48 circ/s; n = 10; P < 0.05). Interestingly, the degree of basal contractile depression in I-1-deficient hearts was similar to that observed in the PP1 hearts, while the magnitudes of the isoproterenol responses were greater in the knockout mice than in PP1 mice. These differences may reflect the phosphorylation state of phospholamban, which is a major phosphoprotein substrate in the heart. Under basal conditions, phospholamban may be maximally dephosphorylated by the increased phosphatase activity in hearts of either knockout or PP1 mice. However, upon isoproterenol stimulation, the effects are more attenuated in the overexpressing hearts exhibiting higher PP1 activity (see below).
To gain further insight into the mechanisms responsible for the depressed in vivo cardiac function of I-1 knockout mice, cardiac contractility in isolated perfused hearts was assessed and Ca2+-cycling regulatory phosphoproteins were examined. Interestingly, while knockout hearts displayed depressed baseline contractility in vivo, there was only a mild decrease (∼10%; P > 0.05) in cardiac parameters ex vivo, compared to those for WT hearts, which may reflect dephosphorylation of the various phosphoproteins during the isolation procedure (16). Indeed, examination of PP1 activity revealed no differences between knockout and WT hearts, suggesting dephosphorylation and inactivation of I-1 in WT hearts. Thus, perfused hearts were stimulated with a maximal inotropic dose of isoproterenol (8 × 10−8 M) to allow phosphorylation and activation of I-1 in WT hearts. Increases in heart rate for knockout mice (505 ± 15 beats/min; n = 7) were similar to those for WT mice (506 ± 17 beats/min; n = 5), but the maximal rates of contraction and relaxation were significantly attenuated in the knockout hearts compared to WT hearts (Fig. 4A and B). To determine whether the decreased cardiac contractile response of knockout hearts was associated with alterations in PP1, we assessed this enzymatic activity in the stimulated perfused hearts. The phosphorylation status of I-1, and thus its inhibitory capacity, were maintained by including inhibitors of PP2A and calcineurin in all buffers, since these phosphatases dephosphorylate I-1 (42). PP1 activity was not altered under basal conditions, while it was significantly higher (23%) in the knockout hearts than in WT isoproterenol-stimulated hearts (Fig. 4C). These increases in PP1 activity occurred independently of any alterations in the total protein levels of PP1c or RGL, assessed in cardiac homogenates and SR preparations (data not shown). Furthermore, the attenuated isoproterenol response of the knockout hearts did not appear to reflect any alterations in the β-adrenergic signaling cascade, since myocardial cAMP contents in WT and knockout hearts were similar (Fig. 4D).
FIG. 4.
Protein phosphatase I-1 in the heart. (A and B) Rates of cardiac contraction (A) and relaxation (B) obtained in isoproterenol-stimulated work-performing hearts from WT (n = 5) and knockout (KO; n = 7) mice. (C and D) PP1 activity and cAMP levels in perfused hearts. (E) Representative immunoblots (left) and quantitation (right) of SERCA (6 μg), calsequestrin (CSQ; 6 μg), and phospholamban (PLB; 6 μg) protein levels and PKA phosphorylation of PLB (pSer16-PLB; 10 μg) in perfused hearts. Immunoreactivity for each protein was compared to a linear standard (2, 4, 8, and 12 μg) consisting of five pooled WT hearts on each blot. ∗, P < 0.05 versus WT.
The increased PP1 activity was associated with a significant reduction in PKA phosphorylation of phospholamban (Fig. 4E). Phospholamban phosphorylation at the CaMKII site, Thr17 (WT: 1.0 ± 0.15 [n = 6]; knockout: 0.46 ± 0.05 [n = 6]; P < 0.05), was also reduced in knockout hearts. There were no alterations in the expression levels of phospholamban, SERCA, or calsequestrin (Fig. 4E). Furthermore, we examined L-type Ca2+ channel activity and troponin I phosphorylation, since they also contribute to the heart's contractile responses to β-agonists and may be regulated by PP1. Ablation of I-1 did not affect L-type Ca2+ channel current density, time to 50% channel activation, or current-voltage relationships in the absence or presence of isoproterenol (data not shown). In addition, the levels of troponin I phosphorylation, assessed in 32P-labeled cardiomyocytes and expressed as percentages of dibutyryl cAMP phosphorylation (100%) in WT and knockout mice were similar under conditions of basal (WT: 39% ± 10%; knockout: 42% ± 12%; n = 3; P > 0.05) or maximal isoproterenol stimulation (WT: 76% ± 7%; knockout: 92% ± 18%; n = 3; P > 0.05). These data suggest that I-1 is an important regulator of β-adrenergic stimulation in heart.
Regulation of PP1 by its inhibitor, I-1, in the failing human heart.
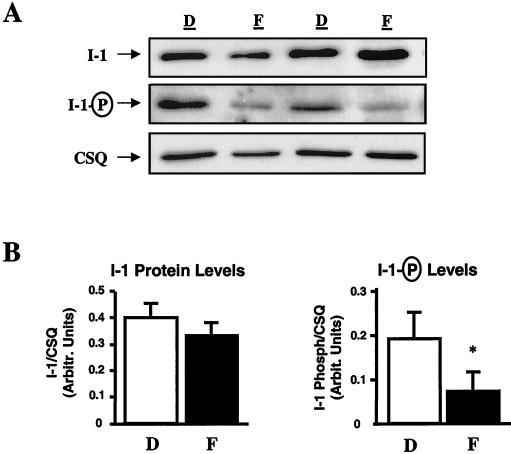
The decreased contractile parameters in I-1-deficient mouse hearts suggested that the reported increases in PP1 activity in human heart failure may be, at least partly, associated with inactivation or dephosphorylation of I-1. Thus, we examined the levels and the phosphorylation state of I-1 in biopsy samples from human nonfailing (n = 9) and failing (n = 10) hearts with dilated cardiomyopathy. To ensure that any observed differences were not due to protein loading, the data were normalized to calsequestrin, since the levels of this SR protein in failing and nonfailing samples were similar (Fig. 5A). There were no alterations in total I-1 protein levels, but I-1's degree of phosphorylation was significantly reduced (∼60%) in failing hearts (Fig. 5B), indicating that I-1 was predominantly inactive and thus incapable of inhibiting PP1 activity. Examination of SR proteins in the same failing and nonfailing hearts indicated a decrease in SERCA (failing hearts: 0.181 ± 0.019; nonfailing hearts: 0.385 ± 0.051 [arbitrary units]) and no alterations in phospholamban (failing hearts: 0.748 ± 0.135; nonfailing hearts: 0.618 ± 0.097), while the phosphorylation status of phospholamban on both Ser16 (failing hearts: 0.253 ± 0.038; nonfailing hearts: 0.427 ± 0.068) and Thr17 (failing hearts: 0.922 ± 0.288; nonfailing hearts: 2.856 ± 0.621) was decreased. The decreased I-1 and phospholamban phosphorylation may reflect impaired β-adrenergic signaling and decreased PKA activation due to reduced cAMP levels in failing hearts (5.8 ± 0.7 pmol/mg; n = 9) compared to those in normal hearts (10.9 ± 1.3 pmol/mg; n = 10; P < 0.05) and/or to increased calcineurin activity, which dephosphorylates I-1 (42).
FIG. 5.
I-1 in human heart failure. (A) Representative immunoblots of the protein levels (top) and phosphorylation (middle) of I-1 in 9 donor (D) and 10 failing (F) heart homogenates. The calsequestrin levels (CSQ; bottom) in the same blots were assessed as an internal control. (B) Quantitation of I-1 protein levels revealed no alterations. However, I-1 phosphorylation in failing hearts was significantly decreased. ∗, P < 0.05 versus donor hearts.
Inhibition of PP1 by a constitutively active I-1 enhances contractile responses to β-agonists in failing human cardiomyocytes.
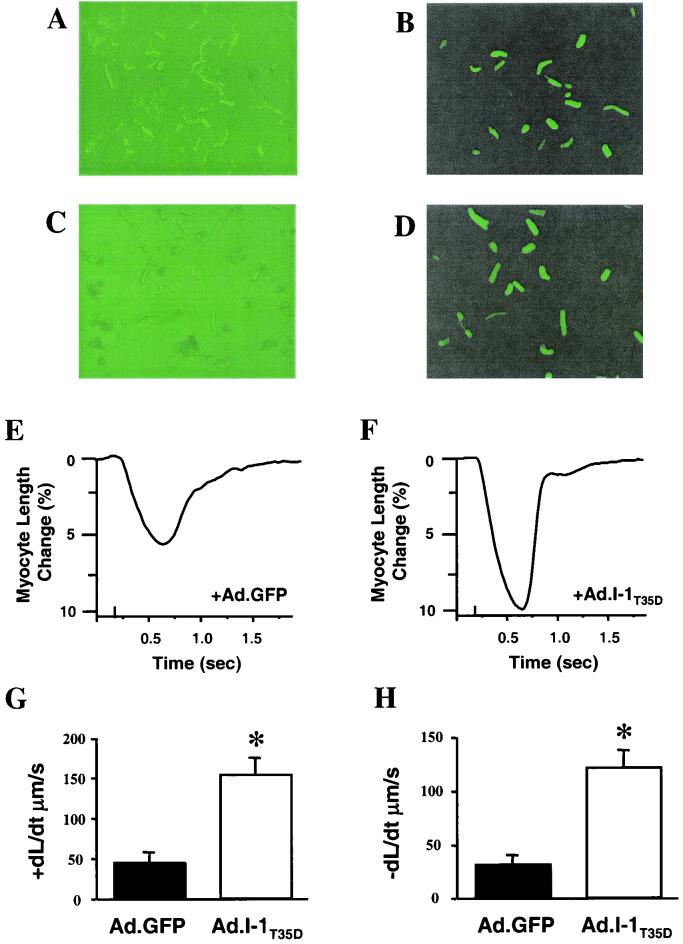
The observed decreases in I-1 phosphorylation in human failing hearts lead to the hypothesis that increases in I-1's activity may be beneficial in restoring the attenuated β-adrenergic responsiveness in failing human cardiomyocytes. To test this hypothesis, we used adenovirus-mediated expression of a constitutively active I-1 protein (I-1T35D) in myocytes isolated from failing human hearts (12). The design of the I-1T35D construct entailed truncation of the I-1 cDNA to encode the first 65 amino acids and introduction of nucleotide changes to replace the PKA phosphorylation site (GGT: Thr35) with aspartic acid (GTC: D), resulting in a constitutively active inhibitor (15). To serve as a control, cardiomyocytes were infected in parallel with an adenovirus encoding β-galactosidase. Both constructs also contained sequences encoding GFP, which served as a marker of transfection (Fig. 6B and D). Failing human cardiomyocytes infected with either β-galactosidase or I-1T35D constructs exhibited similar contractile functions under basal conditions. This finding is consistent with the dephosphorylated state of I-1 in isolated myocytes due to the absence of adrenergic drive. However, in response to isoproterenol (100 nM), myocytes infected with I-1T35D displayed significantly increased shortening (Fig. 6E and F), rates of cell shortening (Fig. 6G), and relengthening (Fig. 6H) and a lower time constant for relaxation (τ) (I-1T35D: 0.16 ± 0.05 s [n = 8]; GFP: 0.37 ± 0.09 s [n = 10]; P < 0.05) compared to controls. Additionally, the time to 50% decay of the Ca2+ signal (I-1T35D: 0.33 ± 0.06 s [n = 8]; GFP: 0.52 ± 0.06 s [n = 10]; P < 0.05) and τ for the Ca2+ signal decay (I-1T35D: 0.36 ± 0.10 s [n = 8]; GFP: 0.70 ± 0.09 s [n = 10]; P < 0.05) were accelerated in the I-10-transfected cells compared to controls. These beneficial effects of inhibiting PP1 activity were observed only upon β-adrenergic stimulation, since isolated myocytes are deprived of endogenous sympathetic stimulation.
FIG. 6.
Adenovirus expression of a constitutively active I-1 protein in cardiomyocytes from failing human hearts. (A to D) Isolated failing human myocytes expressing either β-galactosidase-GFP (Ad.GFP; top) or I-1T35D-GFP (Ad.I-1T35D) were visualized with direct light (left) or fluorescent light (right). Successfully infected cells appear green. (E and F) Representative traces of cardiomyocyte cell shortening in Ad.GFP- (E) and Ad.I-1T35D-infected (F) cells in response to a maximal concentration of isoproterenol (100 nM). (G and H) Rates of cell shortening (G) and relengthening (H) in Ad.GFP- and Ad.I-1T35D-infected cells in response to isoproterenol. Values are averages of at least 8 to 12 cells from three to five human hearts. ∗, P < 0.05.
DISCUSSION
Cross talk of cAMP and Ca2+ through PP1 in cardiac contractility.
The dynamics of contraction and relaxation in cardiac muscle are highly regulated by finely tuned cross talk between second messengers Ca2+ and cAMP. Synergism of Ca2+ and cAMP is mediated through the intricate balance of protein kinase and phosphatase activities, resulting in modulation of protein phosphorylation. Importantly, increased PKA phosphorylates I-1, which inhibits PP1 activity, allowing for amplification of the cAMP-signaling cascade. The resultant enhancement of Ca2+ cycling leads to activation of Ca2+-dependent phosphatase calcineurin and dephosphorylation of I-1, reinstating PP1 activity. However, this elegant balance of regulating cardiac function is shifted in favor of the phosphatase(s) in heart failure due to decreased PKA activation and increased PP1 activity (8, 40).
A unique property of PP1 is its localization to specific subcellular loci by noncatalytic subunits, such as the glycogen and SR targeting subunit, RGL (27, 47, 48). The isoforms of PP1c (α, δ, γ1, and γ2) are associated with a large variety of targeting/regulatory components to localize the enzyme to distinct subcellular locales. Cardiac overexpression of the α isoform in this study resulted in quantitatively similar increases (threefold) in the levels of PP1 in either cardiac homogenates or SR preparations, without alterations in RGL levels. These findings indicate that some of the overexpressed protein was targeted to the SR compartment by “spare” RGL subunits in this membrane. Alternatively, PP1 may be also targeted to SR by an unknown anchoring protein(s). Overexpression of PP1 was associated with significant decreases in phospholamban phosphorylation (pSer16) and depressed function, as assessed in isolated work-performing hearts or in vivo. The depressed function was not due to impaired glycogen metabolism, since glycogen content was increased threefold in PP1-overexpressing hearts.
We anticipated that ablation of the cytosolic I-1 protein would modulate PP1 activity toward its substrates localized in the SR, myofilaments, and the sarcolemma. Interestingly, I-1-deficient hearts exhibited alterations only in phospholamban phosphorylation, not in myofilament phosphorylation or L-type Ca2+ channel activity. A plausible mechanism for such fine-tuning regulation of PP1 by I-1 may involve the anchoring subunit, RGL, or other yet-unknown targeting subunits. Administration of isoproterenol was associated with both inotropic and lusitropic responses in the PP1-overexpressing and I-1 knockout hearts, although these effects were attenuated relative to those in WT hearts, indicating that inhibition of PP1 activity is important and necessary in the heart's contractile responses to β-adrenergic stimulation. It has been suggested that, besides PP1, other phosphatases, such as PP2A, which dephosphorylates troponin I (37), and the ATP-sensitive K+ channels (52), regulate cardiac function. However, the activity of PP2A does not appear to change in vivo, even upon isoproterenol stimulation (1). Furthermore, PP2A activity is unaffected in the PP1 TG mice (data not shown).
PP1 activity and cardiac remodeling.
The role of serine/threonine protein kinases, such as PKA, protein kinase C, and Ca2+/calmodulin kinase, in the regulation of cardiac contractility and remodeling has been well characterized (35). However, the function of their counterparts, the serine/threonine phosphatases, is not well understood. Recently, overexpression of a dominant-negative mutant of the A subunit of PP2A or a constitutively active form of PP2B, calcineurin, was shown to induce hypertrophy (6, 36). In the present study, moderate increases in PP1 activity through ablation of I-1 resulted in significantly depressed function, but there was no remodeling observed up to 15 months of age. However, threefold increases in PP1 activity, similar to those observed in human heart failure, were associated with dilated cardiomyopathy in 6-month-old mice. The cardiac phenotype of the PP1-overexpressing mice exhibited several characteristics similar to those found in human heart failure, including biventricular dilatation, intracardiac thrombi, interstitial fibrosis, myocyte hypertrophy, and premature mortality. Interestingly, decreases in the phosphorylation status of phospholamban and lower SERCA protein levels coincided with depressed function at 3 months and preceded the development of dilated cardiomyopathy, suggesting that dysfunction at the level of SR Ca2+ transport may be one of the factors contributing to the onset and progression of cardiac remodeling. Consistent with these observations, several reports have indicated that impaired Ca2+ homeostasis is a primary stimulus for cardiac hypertrophy and failure (35). Indeed, recent studies on restoring SR Ca2+ handling in the failing heart have shown that cardiac contractility can be enhanced and remodeling can be reversed through SERCA overexpression or phospholamban downregulation (12, 14, 21, 34). Thus, by altering Ca2+ homeostasis, PP1 may induce a hypertrophic response as an adaptive mechanism to enhance the depressed cardiac function. Alternatively, PP1 may directly dephosphorylate signaling molecules or transcriptional factors, similar to dephosphorylation of NFAT-3 by calcineurin, leading to the observed hypertrophic response. Examination of the phosphorylation state of the cAMP response element binding protein, CREB, a proposed downstream substrate for PP1 (18), revealed no alterations in our TG model (data not shown). Regardless of the mechanism by which PP1 leads to remodeling, it will be important to elucidate the hypertrophic cascade involved and identify the signaling molecules responsible for regulation of cardiac growth by this pathway.
PP1 activity and its regulator I-1 in human heart.
The role of I-1 phosphorylation in the regulation of synaptic mechanisms involved in learning and memory (2), as well as regulation of muscarinic cholinergic effects in the heart (1), has been previously defined. The present study has also demonstrated an important role for I-1 in the regulation of basal contractility in vivo. Importantly, examination of failing human hearts revealed that I-1 was predominantly dephosphorylated or inactive. This decrease in I-1 phosphorylation levels may reflect attenuated PKA activity, due to reduction of cAMP levels, or enhanced calcineurin activity (35). The consequent increase in PP1 activity could be self-perpetuating, since it is expected to dephosphorylate phospholamban, leading to impaired Ca2+ resequestration by the SR and, ultimately, elevation of diastolic Ca2+. High Ca2+ levels in the cytosol would promote calcineurin activation and I-1 inactivation, further augmenting PP1 activity. Thus, whether increased PP1 activity represents an initial insult or is secondary to decreased β-receptor signaling in the failing heart, this enzyme could represent a nodal point in the progression of cardiac dysfunction.
In chronic heart failure, increased adrenergic signaling is initially beneficial because it helps maintain myocardial function at a level that can support the circulatory needs of the body. However, continuous activation of β-adrenergic signaling leads to desensitization of these receptors and the response becomes maladaptive. Furthermore, the PP1 activity in the failing human heart is elevated (40), resulting in depressed phospholamban phosphorylation (3, 20, 45). Thus, besides the decreased ratio of SERCA to phospholamban, which is critical for cardiac function (28, 29, 34), depressed phospholamban phosphorylation serves as an additional insult to SR Ca2+ transport function. Interestingly, the ryanodine receptor appears to be hyperphosphorylated in failing human hearts, resulting in increased sensitivity to Ca2+-dependent activation (32), which would further depress the Ca2+ load of the SR. The apparently paradoxical findings on decreased phospholamban phosphorylation and increased ryanodine receptor phosphorylation in the cardiac SR may reflect the complex interplay between kinases and phosphatases in the microenvironment of each phosphoprotein and/or compartmentalized increases of PP1 with phospholamban. Indeed, the anchoring proteins for PP1 and PP2A, associated with the ryanodine receptor, are spinophilin and RR130, respectively (31), while the anchoring subunit for the phospholamban phosphatase is reported to be RGL (4, 30). Furthermore, inhibition of PP1 activity by a constitutively active I-1 restored the contractile responses to β-agonists in failing human cardiomyocytes. The enhanced contraction appeared to be mainly due to SR function, since alterations in L-type Ca2+ channel activity would have resulted in arrhythmogenicity, which was not observed in these failing cardiomyocytes. Thus, the elevated PP1 activity may be at least one of the modifiers in the impaired function of dilated cardiomyopathy, and modulation of this activity may represent a potential target for therapeutic intervention.
Acknowledgments
We gratefully acknowledge L. Cooper and G. Newman for excellent technical assistance, L. J. Field for assistance in generation of the PP1 mice, and J. Robbins for the α-MHC promoter.
This work was supported by National Institutes of Health grants HL64018, HL26057, HL52318, and P40RR12358 (E.G.K.), HL07382 (A.N.C.), MH40899 and DA10044 (P.G. and P.B.A.), and HL06308, Subp.4, and DK36569 (A.A.D.P.-R.).
REFERENCES
- 1.Ahmad, Z., F. J. Green, H. S. Subuhi, and A. M. Watanabe. 1989. Autonomic regulation of type 1 protein phosphatase in cardiac muscle. J. Biol. Chem. 264:3859-3863. [PubMed] [Google Scholar]
- 2.Allen, P. B., O. Hvalby, V. Jensen, M. L. Errington, M. Ramsay, F. A. Chaudhry, T. V. Bliss, J. Storm-Mathisen, R. G. Morris, P. Andersen, and P. Greengard. 2000. Protein phosphatase-1 regulation in the induction of long-term potentiation: heterogeneous molecular mechanisms. J. Neurosci. 20:3537-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartel, S., B. Stein, T. Eschenhagen, U. Mende, J. Neumann, W. Schmitz, E. G. Krause, P. Karczewski, and H. Scholz. 1996. Protein phosphorylation in isolated trabeculae from nonfailing and failing human hearts. Mol. Cell. Biochem. 157:171-179. [DOI] [PubMed] [Google Scholar]
- 4.Berrebi-Bertrand, I., M. Souchet, J. C. Camelin, M. P. Laville, T. Calmels, and A. Bril. 1998. Biophysical interaction between phospholamban and protein phosphatase 1 regulatory subunit GM. FEBS Lett. 439:224-230. [DOI] [PubMed] [Google Scholar]
- 5.Bowman, S., J. A. Tischfield, and P. J. Stambrook. 1990. An efficient and simplified method for introducing site-directed mutatations by PCR. Technique 2:254-260. [Google Scholar]
- 6.Brewis, N., K. Ohst, K. Fields, A. Rapacciuolo, D. Chou, C. Bloor, W. Dillmann, H. Rockman, and G. Walter. 2000. Dilated cardiomyopathy in transgenic mice expressing a mutant A subunit of protein phosphatase 2A Am. J Physiol Heart Circ Physiol. 279:H1307-H1318. [DOI] [PubMed] [Google Scholar]
- 7.Bristow, M. R. 2000. Beta-adrenergic receptor blockade in chronic heart failure. Circulation 101:558-569. [DOI] [PubMed] [Google Scholar]
- 8.Bristow, M. R., R. Ginsburg, W. Minobe, R. S. Cubicciotti, W. S. Sageman, K. Lurie, M. E. Billingham, D. C. Harrison, and E. B. Stinson. 1982. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N. Engl. J. Med. 307:205-211. [DOI] [PubMed] [Google Scholar]
- 9.Brittsan, A. G., A. N. Carr, A. G. Schmidt, and E. G. Kranias. 2000. Maximal inhibition of SERCA2 Ca(2+) affinity by phospholamban in transgenic hearts overexpressing a non-phosphorylatable form of phospholamban. J. Biol. Chem. 275:12129-12135. [DOI] [PubMed] [Google Scholar]
- 10.Brittsan, A. G., and E. G. Kranias. 2000. Phospholamban and cardiac contractile function. J. Mol. Cell. Cardiol. 32:2131-2139. [DOI] [PubMed] [Google Scholar]
- 11.Chu, G., J. W. Lester, K. B. Young, W. Luo, J. Zhai, and E. G. Kranias. 2000. A single site (Ser16) phosphorylation in phospholamban is sufficient in mediating its maximal cardiac responses to β-agonists. J. Biol. Chem. 275:38938-38943. [DOI] [PubMed] [Google Scholar]
- 12.del Monte, F., S. E. Harding, U. Schmidt, T. Matsui, Z. B. Kang, G. W. Dec, J. K. Gwathmey, A. Rosenzweig, and R. J. Hajjar. 1999. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation 100:2308-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Depaoli-Roach, A. A., I. K. Park, V. Cerovsky, C. Csortos, S. D. Durbin, M. J. Kuntz, A. Sitikov, P. M. Tang, A. Verin, and S. Zolnierowicz. 1994. Serine/threonine protein phosphatases in the control of cell function. Adv Enzyme Regul. 34:199-224. [DOI] [PubMed] [Google Scholar]
- 14.Eizema, K., H. Fechner, K. Bezstarosti, S. Schneider-Rasp, A. van der Laarse, H. Wang, H. P. Schultheiss, W. C. Poller, and J. M. Lamers. 2000. Adenovirus-based phospholamban antisense expression as a novel approach to improve cardiac contractile dysfunction: comparison of a constitutive viral versus an endothelin-1-responsive cardiac promoter. Circulation 101:2193-2199. [DOI] [PubMed] [Google Scholar]
- 15.Endo, S., X. Zhou, J. Connor, B. Wang, and S. Shenolikar. 1996. Multiple structural elements define the specificity of recombinant human inhibitor-1 as a protein phosphatase-1 inhibitor. Biochemistry 35:5220-5228. [DOI] [PubMed] [Google Scholar]
- 16.Garvey, J. L., E. G. Kranias, and R. J. Solaro. 1988. Phosphorylation of C-protein, troponin I and phospholamban in isolated rabbit hearts. Biochem. J. 249:709-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta, R. C., J. Neumann, A. M. Watanabe, M. Lesch, and H. N. Sabbah. 1996. Evidence for presence and hormonal regulation of protein phosphatase inhibitor-1 in ventricular cardiomyocyte. Am. J. Physiol. 270:H1159-H1164. [DOI] [PubMed] [Google Scholar]
- 18.Hagiwara, M., A. Alberts, P. Brindle, J. Meinkoth, J. Feramisco, T. Deng, M. Karin, S. Shenolikar, and M. Montminy. 1992. Transcriptional attenuation following cAMP induction requires PP-1-mediated dephosphorylation of CREB. Cell 70:105-113. [DOI] [PubMed] [Google Scholar]
- 19.Hajjar, R. J., U. Schmidt, J. X. Kang, T. Matsui, and A. Rosenzweig. 1997. Adenoviral gene transfer of phospholamban in isolated rat cardiomyocytes. Rescue effects by concomitant gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase. Circ. Res. 81:145-153. [DOI] [PubMed] [Google Scholar]
- 20.Hasenfuss, G., M. Meyer, W. Schillinger, M. Preuss, B. Pieske, and H. Just. 1997. Calcium handling proteins in the failing human heart. Basic Res. Cardiol. 92:87-93. [DOI] [PubMed] [Google Scholar]
- 21.He, H., M. Meyer, J. L. Martin, P. M. McDonough, P. Ho, X. Lou, W. Y. Lew, R. Hilal-Dandan, and W. H. Dillmann. 1999. Effects of mutant and antisense RNA of phospholamban on SR Ca(2+)-ATPase activity and cardiac myocyte contractility. Circulation 100:974-980. [DOI] [PubMed] [Google Scholar]
- 22.Hogan, B., R. Beddington, F. Constantini, and E. Lacy. 1994. Manipulating the mouse embryo: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 23.Hoit, B. D., S. F. Khoury, E. G. Kranias, N. Ball, and R. A. Walsh. 1995. In vivo echocardiographic detection of enhanced left ventricular function in gene-targeted mice with phospholamban deficiency. Circ. Res. 77:632-637. [DOI] [PubMed] [Google Scholar]
- 24.Huang, B., S. Wang, D. Qin, M. Boutjdir, and N. El-Sherif. 1999. Diminished basal phosphorylation level of phospholamban in the postinfarction remodeled rat ventricle: role of beta-adrenergic pathway, G(i) protein, phosphodiesterase, and phosphatases. Circ. Res. 85:848-855. [DOI] [PubMed] [Google Scholar]
- 25.Huang, F. L., and W. H. Glinsmann. 1976. Separation and characterization of two phosphorylase phosphatase inhibitors from rabbit skeletal muscle. Eur. J. Biochem. 70:419-426. [DOI] [PubMed] [Google Scholar]
- 26.Hubbard, M. J., and P. Cohen. 1993. On target with a new mechanism for the regulation of protein phosphorylation. Trends Biochem. Sci. 18:172-177. [DOI] [PubMed] [Google Scholar]
- 27.Hubbard, M. J., P. Dent, C. Smythe, and P. Cohen. 1990. Targetting of protein phosphatase 1 to the sarcoplasmic reticulum of rabbit skeletal muscle by a protein that is very similar or identical to the G subunit that directs the enzyme to glycogen. Eur. J. Biochem. 189:243-249. [DOI] [PubMed] [Google Scholar]
- 28.Kadambi, V. J., S. Ponniah, J. M. Harrer, B. D. Hoit, G. W. Dorn II, R. A. Walsh, and E. G. Kranias. 1996. Cardiac-specific overexpression of phospholamban alters calcium kinetics and resultant cardiomyocyte mechanics in transgenic mice. J. Clin. Investig. 97:533-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo, W., I. L. Grupp, J. Harrer, S. Ponniah, G. Grupp, J. J. Duffy, T. Doetschman, and E. G. Kranias. 1994. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ. Res. 75:401-409. [DOI] [PubMed] [Google Scholar]
- 30.MacDougall, L. K., L. R. Jones, and P. Cohen. 1991. Identification of the major protein phosphatases in mammalian cardiac muscle which dephosphorylate phospholamban. Eur. J. Biochem. 196:725-734. [DOI] [PubMed] [Google Scholar]
- 31.Marx, S. O., S. Reiken, Y. Hisamatsu, M. Gaburjakova, J. Gaburjakova, Y. M. Yang, N. Rosemblit, and A. R. Marks. 2001. Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine/isoleucine zippers J. Cell Biol. 153:699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marx, S. O., S. Reiken, Y. Hisamatsu, T. Jayaraman, D. Burkhoff, N. Rosemblit, and A. R. Marks. 2000. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101:365-376. [DOI] [PubMed] [Google Scholar]
- 33.Masaki, H., Y. Sato, W. Luo, E. G. Kranias, and A. Yatani. 1997. Phospholamban deficiency alters inactivation kinetics of L-type Ca2+ channels in mouse ventricular myocytes. Am. J. Physiol. 272:H606-H612. [DOI] [PubMed] [Google Scholar]
- 34.Minamisawa, S., M. Hoshijima, G. Chu, C. A. Ward, K. Frank, Y. Gu, M. E. Martone, Y. Wang, J. Ross, Jr., E. G. Kranias, W. R. Giles, and K. R. Chien. 1999. Chronic phospholamban-sarcoplasmic reticulum calcium ATPase interaction is the critical calcium cycling defect in dilated cardiomyopathy Cell 99:313-322. [DOI] [PubMed] [Google Scholar]
- 35.Molkentin, J. D., and I. G. Dorn II. 2001. Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu. Rev. Physiol. 63:391-426. [DOI] [PubMed] [Google Scholar]
- 36.Molkentin, J. D., J. R. Lu, C. L. Antos, B. Markham, J. Richardson, J. Robbins, S. R. Grant, and E. N. Olson. 1998. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93:215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mumby, M. C., and G. Walter. 1993. Protein serine/threonine phosphatases: structure, regulation, and functions in cell growth. Physiol. Rev. 73:673-699. [DOI] [PubMed] [Google Scholar]
- 38.Netticadan, T., R. M. Temsah, K. Kawabata, and N. S. Dhalla. 2000. Sarcoplasmic reticulum Ca(2+)/calmodulin-dependent protein kinase is altered in heart failure. Circ. Res. 86:596-605. [DOI] [PubMed] [Google Scholar]
- 39.Neumann, J., P. Boknik, S. Herzig, W. Schmitz, H. Scholz, R. C. Gupta, and A. M. Watanabe. 1993. Evidence for physiological functions of protein phosphatases in the heart: evaluation with okadaic acid. Am. J. Physiol. 265:H257-H266. [DOI] [PubMed] [Google Scholar]
- 40.Neumann, J., T. Eschenhagen, L. R. Jones, B. Linck, W. Schmitz, H. Scholz, and N. Zimmermann. 1997. Increased expression of cardiac phosphatases in patients with end-stage heart failure. J. Mol. Cell. Cardiol. 29:265-272. [DOI] [PubMed] [Google Scholar]
- 41.Neumann, J., R. C. Gupta, W. Schmitz, H. Scholz, A. C. Nairn, and A. M. Watanabe. 1991. Evidence for isoproterenol-induced phosphorylation of phosphatase inhibitor-1 in the intact heart. Circ. Res. 69:1450-1457. [DOI] [PubMed] [Google Scholar]
- 42.Oliver, C. J., and S. Shenolikar. 1998. Physiologic importance of protein phosphatase inhibitors. Front. Biosci. 3:D961-D972. [DOI] [PubMed] [Google Scholar]
- 43.Periasamy, M., T. D. Reed, L. H. Liu, Y. Ji, E. Loukianov, R. J. Paul, M. L. Nieman, T. Riddle, J. J. Duffy, T. Doetschman, J. N. Lorenz, and G. E. Shull. 1999. Impaired cardiac performance in heterozygous mice with a null mutation in the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 (SERCA2) gene. J. Biol. Chem. 274:2556-2562. [DOI] [PubMed] [Google Scholar]
- 44.Rapundalo, S. T. 1998. Cardiac protein phosphorylation: functional and pathophysiological correlates. Cardiovasc. Res. 38:559-588. [DOI] [PubMed] [Google Scholar]
- 45.Schwinger, R. H., G. Munch, B. Bolck, P. Karczewski, E. G. Krause, and E. Erdmann. 1999. Reduced Ca(2+)-sensitivity of SERCA 2a in failing human myocardium due to reduced serin-16 phospholamban phosphorylation. J. Mol. Cell. Cardiol. 31:479-491. [DOI] [PubMed] [Google Scholar]
- 46.Simmerman, H. K., and L. R. Jones. 1998. Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol. Rev. 78:921-947. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki, Y., C. Lanner, J. H. Kim, P. G. Vilardo, H. Zhang, J. Yang, L. D. Cooper, M. Steele, A. Kennedy, C. B. Bock, A. Scrimgeour, J. C. Lawrence, Jr., and A. A. DePaoli-Roach. 2001. Insulin control of glycogen metabolism in knockout mice lacking the muscle-specific protein phosphatase PP1G/RGL. Mol. Cell. Biol. 21:2683-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang, P. M., J. A. Bondor, K. M. Swiderek, and A. A. DePaoli-Roach. 1991. Molecular cloning and expression of the regulatory (RG1) subunit of the glycogen-associated protein phosphatase. J. Biol. Chem. 266:15782-15789. [PubMed] [Google Scholar]
- 49.Wegener, A. D., H. K. Simmerman, J. P. Lindemann, and L. R. Jones. 1989. Phospholamban phosphorylation in intact ventricles. Phosphorylation of serine 16 and threonine 17 in response to beta-adrenergic stimulation J. Biol. Chem. 264:11468-11474. (Erratum, 264:15738.) [PubMed]
- 50.Yang, J., T. D. Hurley, and A. A. DePaoli-Roach. 2000. Interaction of inhibitor-2 with the catalytic subunit of type 1 protein phosphatase. Identification of a sequence analogous to the consensus type 1 protein phosphatase-binding motif. J. Biol. Chem. 275:22635-22644. [DOI] [PubMed] [Google Scholar]
- 51.Zakhary, D. R., C. S. Moravec, R. W. Stewart, and M. Bond. 1999. Protein kinase A (PKA)-dependent troponin-I phosphorylation and PKA regulatory subunits are decreased in human dilated cardiomyopathy. Circulation 99:505-510. [DOI] [PubMed] [Google Scholar]
- 52.Zhou, X. B., P. Ruth, J. Schlossmann, F. Hofmann, and M. Korth. 1996. Protein phosphatase 2A is essential for the activation of Ca2+-activated K+ currents by cGMP-dependent protein kinase in tracheal smooth muscle and Chinese hamster ovary cells. J. Biol. Chem. 271:19760-19767. [DOI] [PubMed] [Google Scholar]