Abstract
FOG family zinc finger proteins play essential roles in development through physical interaction with GATA factors. FOG-1, like its interacting partner GATA-1, is required for normal differentiation of erythroid and megakaryocytic cells. Here, we have developed a functional assay for FOG-1 based on its ability to rescue erythroid and megakaryocytic maturation of a genetically engineered FOG-1−/− cell line. We demonstrate that interaction through only one of FOG-1's four GATA-binding zinc fingers is sufficient for rescue, providing evidence against a model in which FOG-1 acts to bridge multiple GATA-binding DNA elements. Importantly, we find that distinct regions of FOG-1 differentially influence erythroid versus megakaryocyte maturation. As such, we propose that FOG-1 may modulate the fate of a bipotential erythroid/megakaryocytic precursor cell.
GATA proteins comprise a family of zinc finger transcription factors that recognize the DNA consensus sequence (T/A)GATA(A/G) and play essential roles in diverse developmental processes (for a review, see reference 27). GATA-1, -2, and -3 are involved in hematopoietic development, whereas GATA-4, -5, and -6 play roles in nonhematopoietic tissues. GATA-1, the founding member of this family, is expressed abundantly in erythroid, megakaryocytic, eosinophilic, mast, and multipotential cells within the hematopoietic system (6, 36). DNA-binding elements for GATA-1 have been identified in the promoters and enhancers of virtually all erythroid- and megakaryocyte-specific genes studied (29, 42). Gene targeting in mice has established that GATA-1 plays an essential role in both erythropoiesis and megakaryopoiesis. GATA-1 knock-out mice die at day 10.5 of gestation due to severe anemia with arrest in erythroid maturation at a proerythroblast-like stage (10). GATA-1− megakaryocytes hyperproliferate and fail to complete their maturation (28, 41).
The FOG (for Friend of GATA) family of proteins comprises a novel class of multitype zinc finger nuclear polypeptides that interact physically with GATA factors and likewise serve essential functions in development. FOG-1, the founding member of this family, was identified through a yeast two-hybrid screen in which the amino zinc finger of GATA-1 was employed as bait (38). The gene for FOG-1 encodes a 998-amino-acid polypeptide with nine predicted zinc fingers. Four of these zinc fingers (fingers 1, 5, 6, and 9) individually are able to mediate an interaction with GATA-1 (9). FOG-1 is expressed abundantly in erythroid and megakaryocytic cells and coexpressed with GATA-1 during embryonic development (38). FOG-1−/− mice die in mid-embryonic gestation (10.5 to 11.5 days postcoitum) from severe anemia with an arrest in erythroid maturation at a stage similar to that observed with the GATA-1− mice (37). This has provided genetic evidence that FOG-1 and GATA-1 function through a common pathway in erythroid development. However, unlike the GATA-1− mice, these mice failed to produce any megakaryocytes, suggesting that FOG-1 also has a GATA-1-independent role in early megakaryopoiesis.
Crispino et al. have shown that a point mutant of GATA-1 with reduced binding to FOG-1 fails to rescue erythropoiesis from a GATA-1-deficient cell line (3). A similar point mutation in humans leads to severe congenital dyserythropoietic anemia and thrombocytopenia (22). Such patients have an overabundance of abnormal megakaryocytes resembling GATA-1− megakaryocytes. Taken together, these findings demonstrate that a direct physical interaction between GATA-1 and FOG-1 is critical for normal erythropoiesis as well as late stages of megakaryopoiesis.
A second mammalian member of the FOG family (FOG-2) is expressed predominantly in heart, brain, lung, and gonadal tissues (33, 19, 31). FOG-2−/− mice exhibit severe defects in cardiac development characterized by a thin ventricular myocardium, common atrioventricular valve, the tetralogy of Fallot malformation, and defective coronary vasculature leading to embryonic lethality (34, 32). Knock-in mice harboring a FOG noninteracting substitution in GATA-4, one of the heart-expressed GATA factors, display a remarkably similar constellation of defects (4, 18, 20, 33). Thus, interactions between members of the FOG and GATA families are essential for multiple developmental processes. These interactions have also been conserved during evolution, as demonstrated by the ability of the Drosophila FOG orthologue u-shaped to heterodimerize with and negatively regulate the Drosophila GATA factors pannier and serpent (5, 8, 11).
While a FOG-GATA interaction is critical for many GATA factor activities, the mechanism behind this requirement remains unknown. Here, we have developed a cellular assay for FOG-1 function based on the ability of retrovirally expressed FOG-1 to rescue erythropoiesis and megakaryopoiesis from a bipotential FOG-1−/− hematopoietic cell line. We have exploited this assay for structure-function analysis of FOG-1. We demonstrate that interaction with GATA-1 through a single GATA-binding zinc finger is sufficient for rescue activity, disfavoring a simple model in which FOG-1 acts to bridge multiple GATA-binding DNA elements. Importantly, we show that distinct domains of FOG-1 differentially influence erythroid versus megakaryocytic maturation. Based on these findings, we propose that FOG-1 may modulate the fate of a bipotential precursor cell to differentiate along an erythroid or megakaryocytic pathway.
MATERIALS AND METHODS
Fluorescein isothiocyanate- or phycoerythrin-conjugated anti-murine CD34, c-Kit, TER-119, CD41, Mac-1, Gr-1, and CD19 were purchased from BD PharMingen, Inc. Antihemagglutinin (anti-HA), anti-GATA-1 (N6), and anti-FOG-1 (A-20) antibodies were purchased from Santa Cruz Biotechnology, Inc. Preparation of anti-FOG-1 antisera has previously been described (38). All chemical reagents were purchased from Sigma Co. (St. Louis, Mo.) unless specified otherwise. The NIH 3T3 hph-HOX11 retroviral producer cell line was a generous gift from Robert Hawley.
Generation of FOG-1−/− cell line.
The generation of FOG-1−/− embryonic stem (ES) cells has already been described (37). Hematopoietic in vitro differentiation of the FOG-1−/− ES cells was performed based on previously described procedures (15). Briefly, gel-adapted FOG-1−/− ES cells were grown to about 25 to 50% confluency in IMDM containing 15% preselected fetal calf serum, 100-U/ml penicillin-streptomycin, 12-μl/liter monothioglycerol, and 1,000-U/ml leukemia inhibitory factor. The cells were trypsinized, and 5 × 104 cells were plated into each of three 100-mm petri dishes containing 10 ml of differentiation medium (IMDM, 7.5% fetal calf serum [HyClone], 7.5% plasma-derived serum, 2 mM glutamine, 0.004% [vol/vol] monothioglycerol, 50-μg/ml ascorbic acid, 5% protein-free hybridoma medium, 100-U/ml penicillin-streptomycin, 5-ng/ml interleukin 11 [IL-11], 50-ng/ml kit-ligand). The cells were incubated for 7 days at 37°C in 5% CO2 without a change of media. The resulting embryoid bodies were pooled and disaggregated by treatment with trypsin-EDTA and passage through a 20-gauge needle. The disaggregated cells were cocultivated with irradiated (3,000 rads) 90 to 100% confluent NIH 3T3 hph-HOX11 retroviral producer cells in 100-mm tissue culture dishes containing 10 ml of infection medium (IMDM, 15% fetal calf serum [FCS], 100-U/ml penicillin-streptomycin, 2 mM glutamine, 10-ng/ml IL-3, 2-ng/ml IL-6, 5-ng/ml IL-11, 50-ng/ml kit-ligand for 3 days at 37°C in 5% CO2 (12). The cells growing in suspension were plated into methylcellulose containing 15% FCS and 10-ng/ml IL-3 at low density. Well-separated clones were picked, expanded, and maintained in FOG-1−/− growth medium (IMDM, 15% FCS, 100-U/ml penicillin-streptomycin, 2 mM glutamine, 10-ng/ml IL-3) at 37°C in 5% CO2.
Immunophenotyping of FOG-1−/− cells.
FOG-1−/− clone 4 cells were immunostained following standard procedures using fluorescein isothiocyanate- or phycoerythrin-conjugated anti-murine CD34, c-Kit, Ter119, CD41, Mac1, Gr-1, CD19, or isotype-matched control antibody. The cells were washed in phosphate-buffered saline and analyzed on a FACScalibur flow cytometer. The percentage of fluorescent cells was calculated using FACscan software.
Histocytochemistry.
Cells (0.5 × 106 to 1 × 106) were washed three times in phosphate-buffered saline, cytocentrifuged, and stained for either hemoglobin (24) or acetylcholinesterase (AChE) (14) as previously described. For each sample, >1,000 cells were evaluated for benzidine or AChE activity, respectively.
Western blot analysis.
Nuclear extracts were prepared from the cells as previously described (1). Between 7.5 and 30 μg of total protein (determined by Bio-Rad microassay; catalog no. 500-0006) was electrophoresed on 7.5% polyacrylamide gels and transferred to nitrocellulose following standard procedures. The blots were probed with anti-murine FOG-1 polyclonal antisera (1:1,000 dilution) (38), anti-murine FOG-1 (A-20) (1:1,000 dilution), anti-HA (1:1,000 dilution), or anti-murine GATA-1 (N6) (1:500 dilution) antibodies, washed extensively in Tris-buffered saline-0.05% Tween 20, incubated with horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (IgG), anti-rat IgG, or α-goat IgG antibodies, and developed using the ECL detection system (Amersham).
Northern blot analysis.
Total RNA was isolated from FOG-1−/− cells 8 days after infection with retrovirus (6 days after sorting for green fluorescent protein [GFP]) using Trizol reagent following the manufacturer’s instructions (GibcoBRL). RNA (20 μg of total RNA/lane) was fractionated on 1% agarose-formaldehyde gels, transferred to Hybond-C extra (Amersham Life Science) membranes, and hybridized to the appropriate radiolabeled cDNA probes. Quantitation was performed using a phosphorimager (Molecular Dynamics).
FOG-1 mutant construct cloning.
Each FOG-1 construct was cloned into the murine myeloproliferative (MMP) retroviral vector (17) between the viral ATG and an internal ribosome start site (IRES)-GFP element. The 5′ ends of all constructs are identical, with an HA tag immediately following the viral ATG. All recombinant DNA work was done using standard techniques. Each cloning junction and region cloned by PCR (with Pfu [Stratagene]) was sequenced. Details of plasmid constructions and oligonucleotide sequences are available upon request.
Production of high-titer retroviruses.
Vesicular stomatitis virus G protein-pseudotyped retroviral particles were generated via transient transfection of the stable packaging cell line 293 GPG and concentrated by centrifugation as previously described (25). Standard precautions were taken in handling the retroviral supernatants, and all media and instruments that were in contact with the supernatants were treated appropriately to inactivate viruses.
Retroviral infection, sorting, and culture of the FOG-1−/− cells.
FOG-1−/− cells (107) were incubated with the appropriate retroviral supernatants at a multiplicity of infection of approximately 2 to 3 in FOG-1−/− growth medium containing 8 μg of Polybrene/ml for 2 h at 0°C followed by 4 h at 37°C in 5% CO2. The cells were washed and incubated in FOG-1−/− growth medium for 2 days. GFP+ cells were isolated by two sequential rounds of fluorescence-activated cell sorting (FACS) using a Beckman-Coulter high-speed sorter. Analysis of the final sorted cells demonstrated that they were typically >98% GFP+. The sorted cells were centrifuged and resuspended in 5 ml of FOG-1−/− growth medium containing 2-U/ml human erythropoietin and 1% (vol/vol) recombinant human thrombopoietin tissue culture supernatant (39). The cells were incubated for 6 days at 37°C in 5% CO2 and expanded as necessary when the media changed to an orange color (typically on the third day after FACS).
Semiquantitative RT-PCR.
Total RNA was isolated from 2 × 106 cells 6 days after sorting for GFP expression using Trizol reagent and following the manufacturer's instructions. cDNA was prepared by incubating the RNA with mouse mammary tumor virus reverse transcriptase (MMTV-RT) in 1× reaction buffer (Gibco-BRL), nucleotides, cloned RNase inhibitor (Promega), and oligo dT15 at 42°C for 1 h in a total volume of 40 μl. PCRs were performed with 2 μl of the cDNA preparation using Taq polymerase, nucleotides, [32P]dCTP, and hypoxanthine phosphoribosyl transferase (HPRT) (5′-CACAGGACTAGAACACCTGC-3′ and 5′-GCTGGTGAAAAGGACCTCT-3′), and von Willebrand factor (vWF) (5′-CCTTCAATGGATCCCAGTCCAAGGAGGAGG-3′ and 5′-GTTCTAGACTCAAGCTTCTGGATCTGTGTG-3′) oligonucleotides (23) and 0.5 μl of Perfectmatch (Stratagene) in a total volume of 50 μl in 0.2-ml thin-walled PCR tubes. The tubes were heated at 95°C for 3 min and then cycled for 1 min at 94°C, 1 min at 55°C, and 1.5 min at 72°C. After 18, 20, 22, 24, and 26 cycles, 5-μl aliquots were removed and stored at 4°C. The samples were electrophoresed on 6% polyacrylamide gels, dried, and exposed to Kodak Bio-max film. Quantitation was performed using a PhosphorImager (Molecular Dynamics).
RESULTS
Generation of FOG-1−/− cell line.
To develop a cell-based assay of FOG-1 function, we first generated a hematopoietic cell line lacking expression of FOG-1 (outlined in Fig. 1A). Murine FOG-1−/− ES cells were differentiated in vitro to produce hematopoietic progenitor cells (15, 37). After 7 days of primary differentiation, embryoid body cells were immortalized by retroviral expression of HOX-11. Cells were cloned in methylcellulose. A single, representative clonal line was chosen for further studies (hereafter referred to as FOG-1−/− cells).
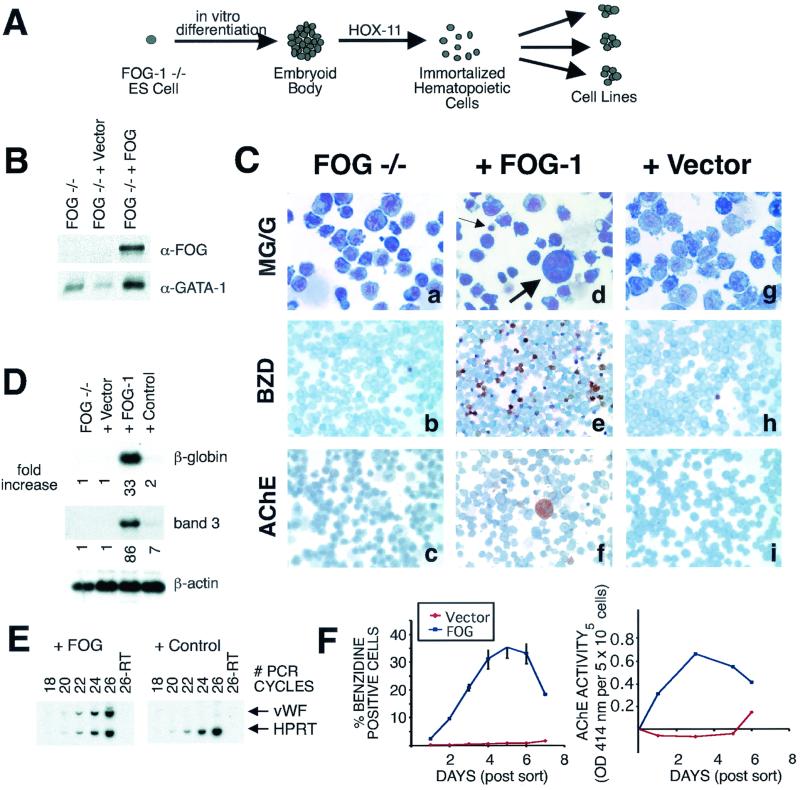
FIG. 1.
FOG-1-mediated rescue of erythroid and megakaryocytic terminal maturation of a FOG-1−/− hematopoietic cell line. (A) Schematic depiction of derivation of the FOG-1−/− cell line. (B) Western blot analysis of nuclear extracts from uninfected FOG-1−/− cells or FOG-1−/− cells infected with vector alone or FOG-1 cDNA packaged retroviruses. The blots were probed with a polyclonal antibody against FOG-1 and then stripped and reprobed with an antibody against GATA-1. (C) Comparison of the morphology of uninfected FOG-1−/− cells (a, b, and c) with those infected with retroviruses encoding FOG-1 cDNA (d, e, and f) or vector alone (g, h, and i) by May-Grunwald-Giemsa (MG/G) staining (a, d, and g), benzidine (BZD) (o-dianisodine) staining (b, e, and h), or AChE staining (c, f, and i). Hemoglobinized cells stain a dark brown color with BZD, and megakaryocytes develop an orange color with AChE staining. Small and large arrows indicate maturing erythrocytes and megakaryocytes, respectively, in panel d. Original magnification, ×1,000 (panels a, d, and g) or ×400 (remaining panels). (D) Northern blot analysis for erythroid markers β-globin and band 3 from retrovirally infected cells. The cDNA encoding a GFP/Cre fusion was used as a negative control. The fold increase in the signal compared to the uninfected FOG-1−/− cells and normalized to the β-actin loading controls is shown below each panel. (E) Semiquantitative RT-PCR analysis for the megakaryocyte marker vWF or ubiquitously expressed HPRT in cells rescued with FOG-1 cDNA or a control plasmid (GFP/Cre fusion). (F). Time course of erythroid and megakaryocytic maturation of rescued cells transduced with vector (shown in red) or FOG-1 (shown in blue). The percentages of BZD+ cells and total AChE enzymatic activity (normalized to uninfected cells) are indicated. Day zero of the time course represents the day the cells were sorted for GFP expression (2 days following retroviral infection). The results represent those of two independent experiments. OD 414 nm, optical density at 414 nm.
FOG-1−/− cells are IL-3 dependent and grow in continuous suspension culture as either single cells or clusters with a doubling time of ∼36 to 48 h. As expected, FOG-1 is not detected in nuclear extracts of these cells by immunoblotting, whereas GATA-1 is expressed and stable (Fig. 1B). FOG-1−/− cells exhibit primitive morphology with irregularly lobulated nuclei, prominent nucleoli, and basophilic cytoplasm (Fig. 1C, panel a). Many cells display cytoplasmic blebbing at their cell borders and contain cytoplasmic vacuoles and violaceous granules. On immunophenotyping, FOG-1−/− cells express the progenitor surface marker c-kit, the early erythroid marker Ter-119, and the megakaryocytic marker CD41 (GP IIb) but only weakly express the granulocyte/macrophage markers Gr-1 and Mac-1; the progenitor marker CD34 and the B lymphoid marker CD19 are not detectable (data not shown). RT-PCR analysis demonstrated the presence of mRNA transcripts for the hematopoietic factors GATA-1, GATA-2, EKLF, NF-E2 p45, and PU.1 (data not shown).
In contrast to early markers of erythroid/megakaryocytic lineage cells, practically no expression of mature erythroid or megakaryocytic markers was detected. Only 0.6% ± 0.1% of the cells stained positively with benzidine (o-dianosidine), a reagent that identifies hemoglobinized cells (Fig. 1C, panel b). Northern blot analysis failed to detect significant amounts of mRNA transcripts for either β-globin or the erythroid membrane anion transporter, band 3 (Fig. 1D). Likewise, histochemical and RT-PCR analysis failed to detect significant expression of the megakaryocyte marker AChE (Fig. 1C, panel c) or vWF (Fig. 1E, control panel), respectively. Based on these data, we conclude that the FOG-1−/− cells represent hematopoietic cells of the erythroid/megakaryocytic lineage arrested in their terminal maturation at a precursor stage.
Retroviral expression of FOG-1 rescues erythroid and megakaryocytic terminal differentiation.
To validate use of the immortalized FOG-1−/− cells for functional studies, we infected cells with FOG-1-expressing retrovirus and assessed terminal maturation. The FOG-1 cDNA followed by an IRES and the cDNA encoding GFP was inserted into an MMP-based retroviral vector (17). High-titer retroviral supernatants were generated using a vesicular stomatitis virus glycoprotein packaging cell line. Two days following infection, GFP-expressing cells were isolated by two sequential rounds of FACS to recover cells that were >98% GFP+ (data not shown). GFP+ cells were then incubated in growth medium containing IMDM, 15% FCS, IL-3, erythropoietin, and thromobopoietin. A progressive increase in the percentage of benzidine-positive cells was observed, reaching a maximum of 35 ± 3.9% on day 5 following FACS (Fig. 1C, panel e, and Fig. 1F). This correlated with the appearance of maturing erythroid cells (Fig. 1C, panel d, small arrow) and 33- and 86-fold increases in the mRNA levels for β-globin and band 3, respectively (Fig. 1D). FOG-1 protein was readily detected in the rescued cells by Western blot analysis using an anti-FOG-1 antibody (Fig. 1B). In contrast, only 0.8% ± 0.6% of cells infected with vector alone containing retrovirus stained with benzidine after 5 days of culture, and these cells lacked morphological evidence of maturation (Fig. 1C, panels g and h, and Fig. 1F) or significant accumulation of β-globin or band 3 mRNA transcripts (Fig. 1D). Cells rescued with a control nuclear protein (a fusion protein between GFP and Cre recombinase) also failed to undergo significant terminal erythroid maturation (Fig. 1D). After 5 days of culture, the percentage of benzidine-positive cells from the rescued FOG-1−/− cells declined, likely representing overgrowth of the culture from nondifferentiating cells (Fig. 1F).
Total AChE enzymatic activity from the pool of cells rescued with FOG-1, but not the vector alone, also increased progressively over the course of the incubation, reaching a maximum level 3 days following FACS (Fig. 1F). This correlated with the appearance of large cells with multilobed nuclei and cytoplasmic AChE staining consistent with mature megakaryocytes (maximum, 13% ± 1.6%) (Fig. 1C, panels d [large arrow] and f). The rescued cells also accumulated mRNA transcripts for the megakaryocyte-specific marker vWF to levels 30-fold greater than those for controls (Fig. 1E). Taken together, these data indicate that the FOG-1−/− cell line represents an erythroid/megakaryocytic bipotential precursor cell blocked in its terminal maturation in a FOG-1 dependent manner.
A FOG-1-GATA interaction is required for rescue of the FOG-1−/− cell line.
GATA-1 requires a physical interaction with FOG-1 in order to serve its essential function in erythropoiesis and late megakaryopoiesis (3, 22). We therefore examined whether FOG-1's ability to rescue erythroid and megakaryocyte terminal maturation of the FOG-1−/− cell line likewise requires interaction with GATA-1. Four of FOG-1's nine zinc fingers (fingers 1, 5, 6, and 9) can individually mediate a physical interaction with GATA-1 (9). These interactions depend on a critical tyrosine residue immediately preceding the final cysteine in each of their zinc fingers. Substitution of alanine for this tyrosine markedly impairs each finger's ability to bind GATA-1 (9).
To test dependence of erythroid and megakaryocytic maturation on FOG-1-GATA-1 interaction, we expressed a mutant form of FOG-1 containing tyrosine-to-alanine substitution in all four GATA-binding zinc fingers (m1,5,6,9). This mutant failed to rescue terminal erythroid maturation of the FOG-1−/− cells (Fig. 2B, panels a to c). Only 2.0% ± 0.7% of the cells became benzidine positive, which was similar to the level obtained with viruses generated from the vector alone or GFP/Cre control constructs (0.6% ± 0.1% and 2.2% ± 0.3%, respectively), compared to 26% ± 1.2% of the wild-type FOG-1 rescued cells. Similarly, levels of mRNA transcripts for β-globin or band 3 were minimally increased compared to wild-type rescue (Fig. 2C). Western blot analysis confirmed that the m1,5,6,9 construct was expressed at least as well as the wild-type molecule (Fig. 2F), and indirect immunofluorescence demonstrated appropriate nuclear localization (data not shown). These data provide evidence that a direct physical interaction between FOG-1 and a GATA factor is necessary for FOG-1-mediated erythroid rescue of the FOG-1−/− cells.
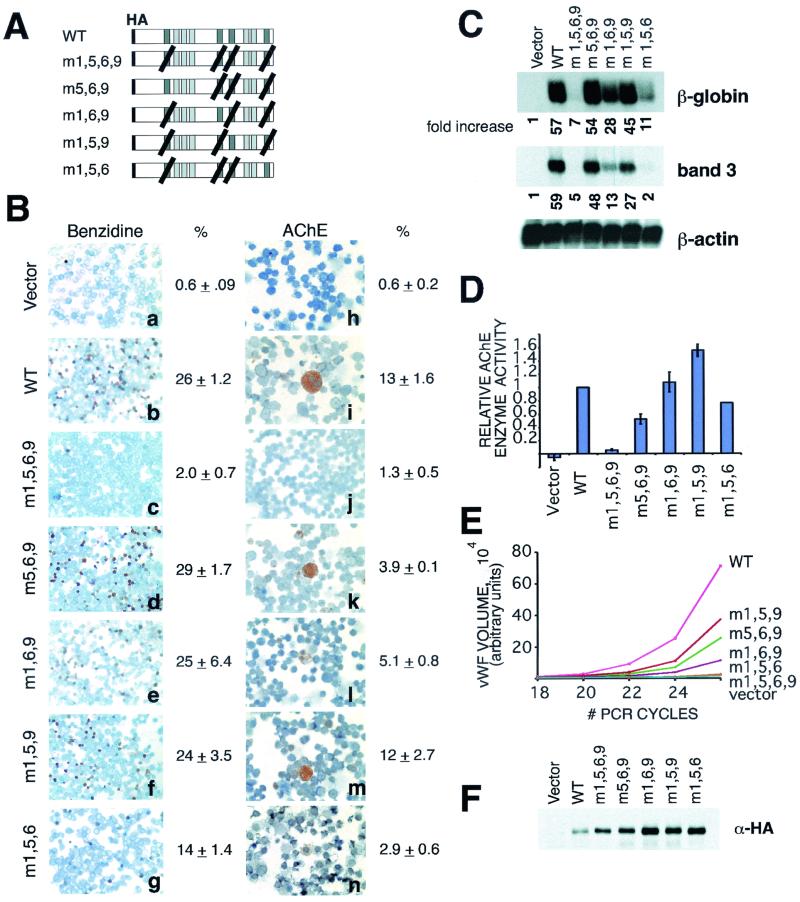
FIG. 2.
GATA-binding requirements for FOG-1-mediated rescue of erythropoiesis and megakaryopoiesis. (A) Schematic representation of mutant FOG-1 constructs. Darker shaded boxes represent GATA-binding zinc fingers. Black diagonal lines indicate substitution of alanine for the tyrosine residue that immediately precedes the final cysteine of each zinc finger structure. (B) Benzidine and AChE stains of FOG-1−/− cells rescued with each of the mutant constructs. Original magnification, ×400. (C) Northern blot analysis for β-globin and band 3 from the FOG-1−/− cells rescued with each of the mutant constructs. The fold increase in signal for each construct relative to the vector-alone control and normalized to the β-actin samples is shown below each panel. (D) Total AChE enzymatic activity from cells rescued with each of the mutant constructs. Total AChE activity was measured in triplicate from 5 × 105 of the rescued cells. Basal activity measured from uninfected FOG-1−/− cells was subtracted, and the data are displayed relative to the wild-type construct. (E) Semiquantitative RT-PCR analysis of cells rescued with each of the mutant constructs. RT-PCR using vWF-specific and HPRT-specific primers was performed utilizing [32P]dCTP for radioisotope incorporation. The products were separated by polyacrylamide gel electrophoresis, and the labeled bands were quantified using a PhosphorImager. The data are displayed as vWF signal normalized to the HPRT control signal. (F) Western blot analysis of rescued cells for expression of mutant constructs. Nuclear extracts were prepared from a portion of the rescued cells 1 day after sorting for GFP expression. Equivalent amounts of total protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with an anti-HA antibody.
Comparison of the phenotypes of FOG-1 and GATA-1 knock-out mice indicates that FOG-1 plays a GATA-1-independent role in early megakaryopoiesis (37). To test this model, we examined the ability of the m1,5,6,9 molecule to rescue megakaryocyte differentiation. Surprisingly, this molecule failed to rescue megakaryocyte maturation any better than erythroid development. (Fig. 2B, panels h to j, and Fig. 2D and E). These data imply that FOG-1 requires an interaction with a GATA factor for its role in early megakaryopoiesis, but this presumably occurs through a GATA factor other than GATA-1 (see Discussion, below).
Mutant FOG-1 molecules capable of binding to GATA-1 through only a single zinc finger can rescue both erythropoiesis and megakaryopoiesis.
The cis-acting regulatory regions of many erythroid and megakaryocytic-specific genes contain multiple GATA-binding sites, often situated in both promoter and enhancer elements and at considerable distance from each other (27, 42). The presence of multiple GATA-binding fingers in FOG-1 suggests that it might act as a molecular bridge between these elements, bringing regulatory elements into proximity of the promoter region through a DNA looping mechanism. To examine this model, we expressed various FOG-1 molecules containing the tyrosine-to-alanine substitutions (as described above) in three of four GATA-binding zinc fingers and examined their ability to rescue the FOG-1−/− cells. As shown in Fig. 2, several combinations of triple mutants were able to rescue both erythroid and megakaryocyte terminal differentiation to levels considerably above those attained by the quadruple mutant (m1,5,6,9). The m5,6,9 mutant, which leaves only zf1 available for binding to GATA-1, rescued erythroid differentiation as well as the wild type (Fig. 2B, panels b and d, and C). The m1,5,9 molecule, which leaves only zf6 available for binding to GATA-1, was also highly active in rescue of megakaryocyte differentiation (Fig. 2D and E). With only a single, intact GATA-binding zinc finger available, bridging of two GATA-binding DNA elements by these molecules would not be possible. Therefore, these findings provide evidence against a simple DNA looping model of FOG-1's mechanism of action.
Although each of the triple mutants rescues FOG-1−/− cells, significant differences were observed among the mutants. Interaction through either zf1 or zf6 led to more substantial rescue, in general, than interaction through either zf5 or zf9; zf5 (m1,6,9) exhibited intermediate activity, whereas zf9 (m1,5,6) was less active (Fig. 2). This indicates that interactions through different GATA-binding zinc fingers of FOG-1 are not functionally equivalent.
Lack of essential domain of FOG-1 outside of its GATA-binding zinc fingers required for rescue of erythroid maturation.
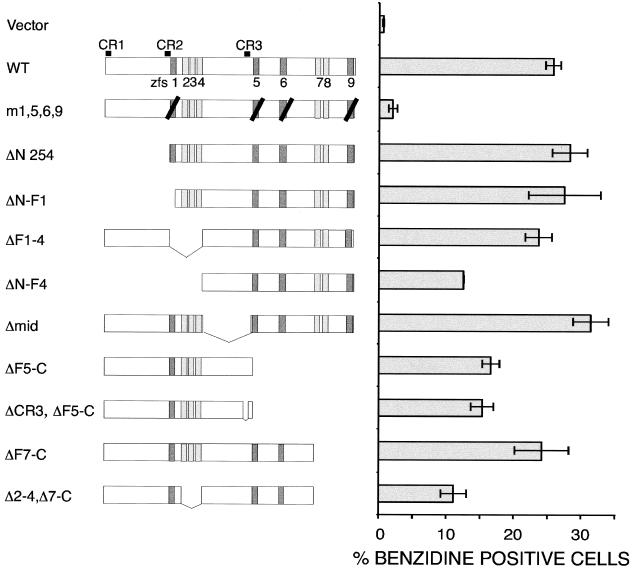
As a cofactor for GATA-1, FOG-1 might recruit transcriptional activators to the GATA-1-DNA complex through interaction with a specific protein domain. To examine this possibility, we tested deletion mutants of FOG-1 spanning the entire molecule for rescue activity. As shown in Fig. 3, no region (other than a GATA-binding zinc finger) is strictly required for rescue activity of erythroid differentiation (as assessed by the production of benzidine-positive cells). Even the construct with the lowest activity (ΔF2-4, ΔF7-C) rescued erythropoiesis ∼15-fold above vector alone and 5-fold above the m1,5,6,9 construct. The instability of expressed single FOG-1 fingers (or single fingers fused to a control nuclear protein) has precluded the assessment of whether a simple interaction between FOG-1 and GATA-1 is sufficient for promoting erythroid maturation.
FIG. 3.
Lack of an essential subdomain of FOG-1 required for terminal erythroid maturation of the FOG-1−/− cell line (excluding a GATA interacting domain). A schematic representation of each construct is shown next to the percentage of benzidine-positive cells obtained from at least two independent rescue experiments and displayed as the mean value ± the standard error of the mean. Three regions of high sequence conservation (outside of the zinc fingers) between FOG-1 and other members of the FOG family are indicated above the top of the wild-type construct as CR1, CR2, and CR3. Darker shaded boxes represent GATA-binding zinc fingers, and black diagonal lines represent substitution of alanine for the tyrosine residue immediately preceding the final cysteine residue of the zinc finger.
Deletion of an amino-terminal region impairs FOG-1's ability to rescue terminal megakaryocyte maturation.
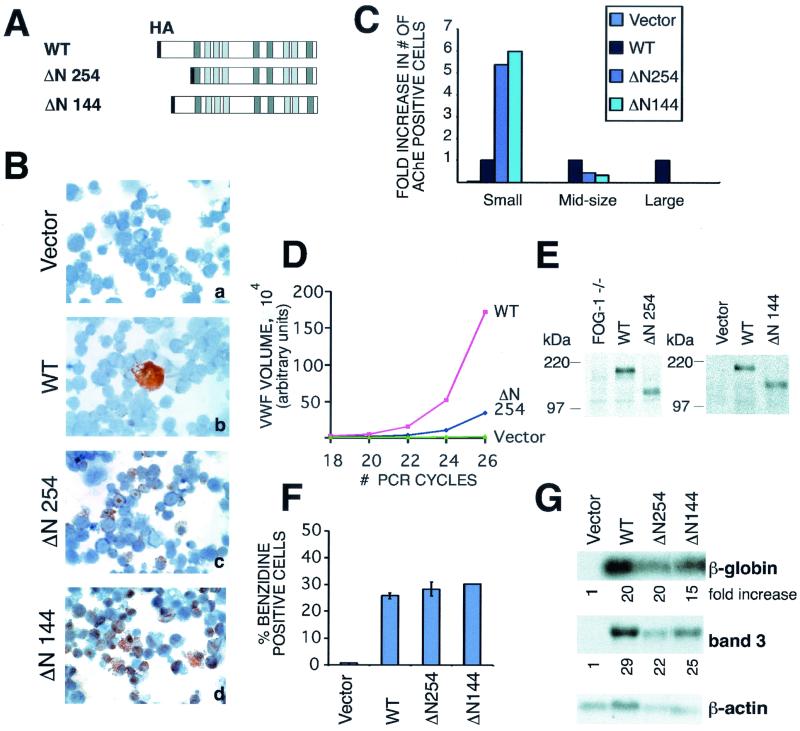
In contrast to the lack of an essential domain required for erythroid rescue, deletion of the amino terminus of FOG-1 markedly impaired ability to rescue terminal megakaryocyte maturation. Expression of a construct lacking amino acids 1 to 254 (ΔN 254) led to a fivefold increase in the number of AChE-positive cells compared to the wild-type construct (Fig. 4A to C). Yet, the resulting cells were small, lacked multilobulated nuclei, exhibited a nuclear AChE staining pattern, and contained about fivefold fewer mRNA transcripts for the terminal maturation marker vWF than megakaryocytes rescued with the wild-type construct (Fig. 4B, panels b and c, and D). Similar findings were observed with a construct lacking amino acids 1 to 144 (ΔN 144) (Fig. 4A to C), further localizing this activity to the extreme amino terminus of FOG-1. The differences in rescue activity were not due to differences in expression levels as determined by Western blot analysis (Fig. 4E). Remarkably, these deletions had no appreciable effect on rescue of erythroid maturation (Fig. 4F and G).
FIG. 4.
Amino terminus of FOG-1 is required for rescue of terminally differentiated megakaryocytes. (A) Schematic representation of constructs. (B) AChE stains of FOG-1−/− cells rescued with the mutant FOG-1 constructs. Original magnification, ×600. (C) Fold increase in the number of AChE+ cells compared to the wild type for each of the constructs. The data have been categorized for small, medium, and large cells. (D) Semiquantitative RT-PCR for vWF mRNA from FOG-1−/− cells rescued with each of the mutant constructs. The data have been normalized to the HPRT controls included in each sample. (E) Western blot analysis from nuclear extracts prepared from FOG-1−/− cells rescued with the different N-terminal deletion constructs using an antibody directed against the C terminus of FOG-1 (Santa Cruz Biotechnology; A-20) (right panel) or the HA epitope (left panel). Equivalent amounts of total protein were loaded into each lane for sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The migration of molecular mass standards is indicated. (F) Percentage of benzidine-positive cells from FOG-1−/− cells rescued with each of the mutant constructs. Error bars represent ± standard error of the mean. (G) Northern blot analysis for β-globin and band 3 mRNAs from FOG-1−/− cells rescued with each construct. The fold increase in signal (after normalization to the β-actin control) relative to vector alone is shown beneath each panel.
Deletion of zf1 to zf4 enhances FOG-1's ability to rescue terminal megakaryocyte but not erythroid maturation.
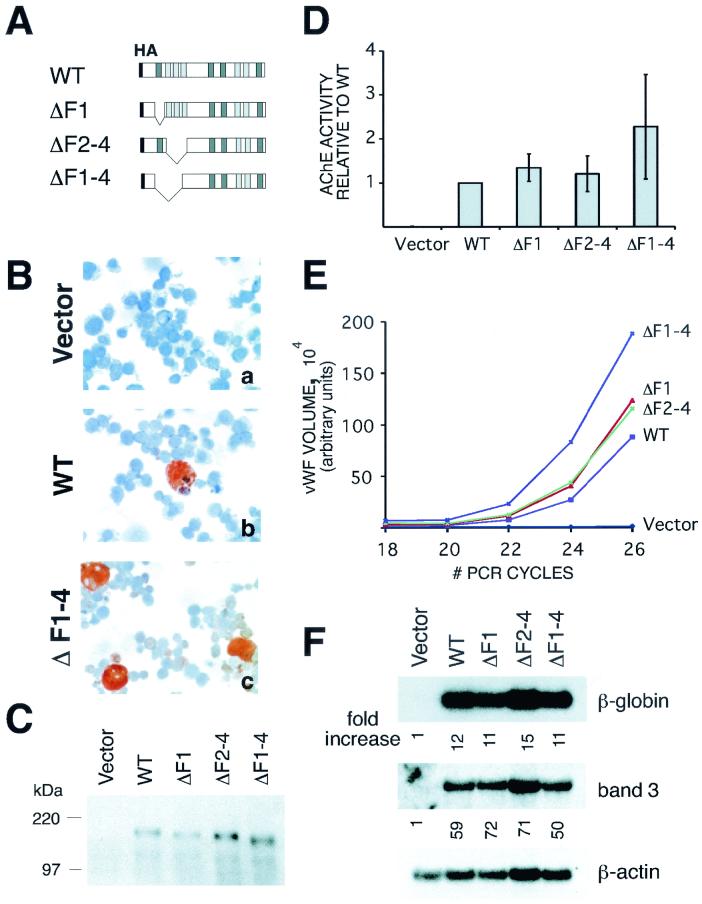
In the course of our deletional analysis of FOG-1, we observed that the deletion of zinc fingers 1 to 4 (amino acids 257 to 384) of FOG-1 led to enhanced rescue of megakaryocyte maturation. As shown in Fig. 5B (panels b and c), the number of AchE-positive cells rescued with the ΔF1-4 versus the wild-type construct was enhanced (42% ± 2.0% versus 13% ± 1.2%); in addition, many of these cells were large and, hence, mature in appearance. This correlated with a 2.3-fold ± 1.2-fold increase in total AchE enzymatic activity and ∼2-fold increase in the number of vWF transcripts (Fig. 5D and E). Again, these differences were not related to expression levels of the variant FOG-1 molecules (Fig. 5C). In the same transduction experiments the extent of erythroid rescue by these contructs was indistinguishable from that with wild-type controls, either in terms of benzidine-positive cells (24% ± 2.0% versus 26% ± 1.2% for ΔF1-4 and wild type, respectively) or mRNA transcripts for β-globin and band 3 (Fig. 5F). Thus, the effects of N-terminal deletions of FOG-1 appear to be lineage restricted.
FIG. 5.
Deletion of zinc fingers 1 to 4 enhances FOG-1-mediated rescue of megakaryopoiesis but not erythropoiesis. (A) Schematic representation of FOG-1 deletion constructs. (B) AChE staining of FOG-1−/− cells rescued with each of the constructs. Magnification, ×600. (C) Western blot analysis of a portion of FOG-1−/− cells rescued with each of the constructs using an anti-HA antibody. Nuclear extracts were prepared from GFP+ cells one day following FACS, and equivalent amounts of total protein for each construct were loaded for sodium dodecyl sulfate-polyacrylamide gel electrophoresis. (D) AChE enzymatic activity from infected cells. AChE enzymatic activity was measured in triplicate for 5 × 105 of the rescued cells obtained 6 days following FACS. Background activity from the uninfected FOG-1−/− cells was subtracted, and the activity is reported relative to the wild-type molecule. (E) Semiquantitative RT-PCR analysis for vWF mRNA from FOG-1−/− cells rescued with each of the constructs. The vWF signals were normalized to the HPRT control values for each sample. (F) Northern blot analysis for β-globin and band 3 mRNAs from FOG-1−/− cells rescued with each of the constructs. Fold increase in signal (after normalization to the β-actin control for each sample) relative to the vector alone is shown below each panel.
To define further the domain responsible for enhanced megakaryocyte rescue, we also evaluated constructs lacking zf1 or zf2 to zf4 by themselves (Fig. 5A). Zinc fingers 2 to 4 were treated as a single domain since these are closely-spaced, C2H2-type zinc fingers and likely function as a unit. As shown in Fig. 5D and E, these deletions yielded only very modest effects. We conclude, therefore, that zf1 and zf2 to zf4 may act synergistically to restrict FOG-1's activity in rescuing megakaryopoiesis.
DISCUSSION
Lineage-specific transcription factors play essential roles in hematopoiesis, as in many other developmental systems. In addition to their DNA interactions, protein-protein interactions of these factors modulate positive or negative functions (26). Here, we have developed an in vivo functional assay for the essential GATA-1 cofactor FOG-1 and have used it to probe FOG-1's activity in promoting erythroid and megakaryocytic differentiation pathways. Our results tend to disfavor a model in which FOG-1 acts as a molecular bridge between multiple GATA DNA-binding sites found in the cis-acting regulatory sequences of erythroid and megakaryocytic genes. We observe, however, that distinct subdomains of FOG-1 differentially modulate its ability to rescue erythroid versus megakaryocytic differentiation in this assay. These findings provide new insight into the complexity of lineage control by GATA-1 and FOG-1 and suggest that precise features of their interaction determine the developmental fate of a bipotential erythroid/megakaryocytic precursor cell.
Generation of immortalized cell lines from gene-targeted murine ES cells.
Rather than relying on artificial cellular contexts in which to examine the contribution of FOG-1 to GATA-1's transcriptional activities, we sought to develop a cellular rescue assay for erythroid and megakaryocytic differentiation. To this end, we employed retrovirally expressed Hox-11 to immortalize hematopoietic progenitors (12, 13) generated in vitro from FOG-1−/− ES cells. Hox-11-immortalized cells retain characteristics of distinct lineages, including some with multipotentiality (16). Moreover, Hox-11-transformed cell lines retain growth factor dependency and some capacity to spontaneously differentiate. These properties warrant the use of Hox-11-immortalized cells for functional analysis, such as that described here.
Requirement for a FOG-1:GATA interaction.
Prior evidence establishes a critical role for direct physical interaction of FOG-1 with GATA-1 in both erythroid and megakaryocytic differentiation. GATA-1 mutants with reduced affinity for FOG-1 fail to rescue erythroid terminal maturation of GATA-1− cells (3). Moreover, Nichols et al. have recently reported a family with congenital dyserythropoietic anemia and thrombocytopenia whose affected members harbor a similar missense mutation in their GATA-1 gene (22). In the present study, we have examined the converse question: does FOG-1 require a GATA factor interaction for its activity in erythropoiesis and megakaryopoiesis?
A FOG-1 mutant with reduced affinity for GATA-1 (by substitution of alanine for a key tyrosine residue present in each of the four GATA-binding zinc fingers [m1,5,6,9]) failed to rescue terminal erythroid maturation of the FOG-1−/− cells. Though one might argue that this effect is due to changes in the molecule unrelated to the loss of interaction with GATA-1, we believe this is unlikely since mutant FOG-1 molecules containing three of the substitutions in all combinations (m5,6,9, m1,6,9, m1,5,9, and m1,5,6) retained activity. Our data further support a model in which direct physical interaction between GATA-1 and FOG-1 is critical for erythroid maturation.
In vitro and in vivo studies have shown that GATA-1 plays an essential role relatively late, rather than early, in megakaryocytic development (28, 41). GATA-1 megakaryocytes hyperproliferate and express the early marker AChE but fail to undergo proper endomitosis or express normal levels of terminal megakaryocyte markers (41). In contrast, megakaryopoiesis is absent in FOG-1−/− mice (37). Based on these observations, it has been proposed that FOG-1 serves a GATA-1-independent role in early megakaryopoiesis (37). Surprisingly, however, we find that a mutant FOG-1 molecule with impaired binding to GATA-1 (m1,5,6,9) also fails to rescue even early megakaryocytic cells (no AChE-positive cells). This finding implies that FOG-1 requires interaction with another GATA factor in early megakaryopoiesis. A possible candidate is GATA-2, which is highly expressed in multipotential hematopoietic cells and early megakaryocytes (21, 40). Prior work indicates, though, that GATA-2 is dispensable for megakaryocyte formation (35). Hence, it remains possible that GATA-1 and GATA-2 play overlapping FOG-dependent roles in early megakaryopoiesis. Studies are currently under way to test this hypothesis.
Models of FOG-1's mechanism of action.
Virtually all erythrocyte- and megakaryocyte-expressed genes contain GATA DNA-binding sites in both their promoters and enhancer elements (27). Since a single FOG-1 molecule can potentially bind more than one GATA-1 molecule, FOG-1 might function as a molecular bridge between distant GATA-1-DNA complexes (9). This could bring distal enhancer elements into proximity of the promoter through DNA looping, a mechanism invoked in models of enhancer-promoter interactions (for a review, see reference 2). Our data tend to argue against this model of FOG-1's action in its simplest form in that mutant FOG-1 molecules capable of binding only a single GATA-1 molecule rescue erythropoiesis and megakaryopoiesis nearly as well as the wild-type FOG-1 (Fig. 2). Although it is possible that the tyrosine-to-alanine substitutions do not completely disrupt the FOG-1-GATA-1 interaction, we feel that this is an unlikely explanation, since a FOG-1 mutant containing substitutions in all four GATA-binding zinc fingers is virtually inactive. Looping mediated by FOG-1 might yet occur if FOG-1 proteins were to dimerize. At present, there are no data in support of this possibility.
We then began to explore other possible mechanisms by which FOG-1 might influence GATA-1 function. Previously we hypothesized that FOG-1 either provides a transactivation function itself or recruits a transactivator to the GATA-1-DNA complex (43). According to this model, one would anticipate being able to define specific domains, other than the zinc fingers themselves, required for FOG-1 function. In contrast, however, we observe that no domain (other than a GATA-binding zinc finger) is required for rescue of terminal erythroid maturation. Although it is conceivable that this merely reflects functional redundancy in the molecule, we consider this possibility unlikely given the extensive and overlapping deletions tested. In addition, the degree of amino acid sequence conservation between FOG-1 and FOG-2 outside of the zinc fingers is relatively low, yet FOG-2 rescues erythroid maturation of the FOG-1−/− cell line (33).
These findings suggest an alternative possibility—that is, physical interaction between FOG-1 and GATA-1 is by itself sufficient to activate GATA-1. The instability of single FOG-1 zinc finger polypeptides in the FOG-1−/− cells precludes a present test of this model. If direct interaction were sufficient, how could the binding of FOG-1 to GATA-1 influence GATA-1's function? One possibility is that binding by FOG-1 elicits an allosteric change in GATA-1 such that its transcriptional activity is enhanced. Alternatively, FOG-1 might displace or prevent a transcriptional corepressor molecule from binding to GATA-1. Indirect support for the latter model might be suggested by the earlier work of Evans and Felsenfeld, who reported that GATA-1's transcriptional activity is dampened in erythroid cells compared to activity in nonhematopoietic cells (7).
Lineage selectivity mediated by different domains of FOG-1.
Erythroid and megakaryocytic cells are thought to arise from a common bipotential precursor cell, but the molecular mechanism(s) underlying this cell fate decision remains unknown (29). The ability of exogenously expressed FOG-1 to rescue both erythroid and megakaryocytic terminal maturation of FOG-1−/− cells has enabled us to examine FOG-1's possible role in this process. Unexpectedly, we found that distinct domains differentially influence rescue of erythroid versus megakaryocytic maturation. Namely, the amino-terminal domain of FOG-1 was required for full megakaryocyte maturation but dispensable for erythroid differentiation. A similar region in FOG-2 mediates GATA-4-dependent transcriptional repression of a cardiac reporter gene in transient transfection experiments (30). Thus, it may be that FOG-1-mediated transcriptional repression of selected target genes (such as erythroid-specific genes) is required for megakaryocyte maturation.
Removal of a region encompassing zinc fingers 1 to 4 actually enhances FOG-1's ability to rescue terminal megakaryocyte maturation without affecting erythroid differentiation. This implies that this region may normally serve to selectively restrict megakaryocyte development from multipotent precursors. Zinc fingers 2 to 4 are closely spaced, C2H2-type fingers, features that suggest a DNA-binding function. Such activity could explain lineage selectivity if it were to influence FOG-1-GATA-1 function at different target genes. However, as of yet, no sequence-specific high-affinity DNA binding activity of FOG proteins has been detected (A. P. Tsang, S. G. Tevosian, and S. H. Orkin, unpublished observation). Instead, it may be that this region (in conjunction with zf1) is involved in protein-protein interactions. Further studies are in progress to examine this possibility.
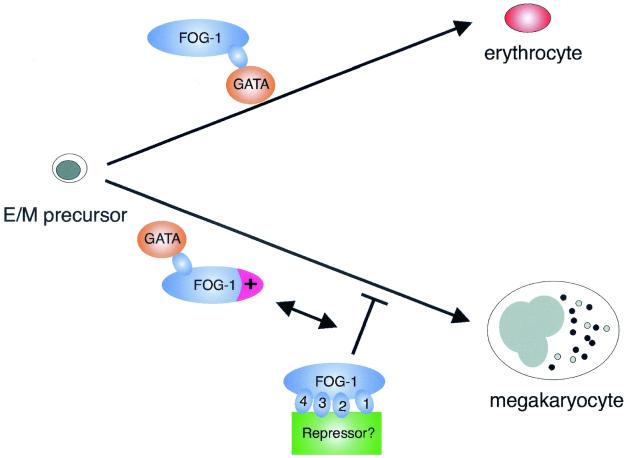
Our results demonstrate that distinct subdomains of FOG-1 differentially influence GATA-1-dependent erythroid and megakaryocyte differentiation. These findings raise the possibility that FOG-1 may participate in fate decisions of the erythroid/megakaryocytic precursor (Fig 6). In this model, interaction with GATA-1 by itself promotes erythroid differentiation, whereas interaction in conjunction with activity provided by a critical domain in the FOG-1 amino terminus promotes megakaryocyte differentiation. Further complex control may be mediated by binding of an unknown factor to zinc fingers 1 to 4 (or a GATA factor to zf1 and some unknown factor to zinc fingers 2 to 4) which normally acts to repress megakaryocyte differentiation. Further studies are aimed at exploring these possibilities. It will also be of interest to determine whether our findings on FOG-1 have functional correlates in other developmental systems which rely on FOG proteins.
FIG. 6.
Models of FOG-1's lineage-selective roles in erythropoiesis and megakaryopoiesis. Model for the lineage-selective effects of distinct regions of FOG-1. For erythroid differentiation, a simple interaction between GATA-1 and FOG-1 is both necessary and sufficient to drive erythroid maturation from a bipotential precursor cell. For megakaryocytic differentiation, activity provided by the amino terminus of FOG-1 (shown in magenta), in addition to an interaction with GATA-1, is required. Further complex control serving to restrict megakaryocyte differentiation may be provided by interaction between an unknown factor (shown in green) and zinc fingers 1 to 4 of FOG-1.
Acknowledgments
The first two authors contributed equally to this work.
We thank Robert Hawley and Richard Mulligan for generously providing us with the HOX-11 retroviral producer cell line and the MMP retroviral vector system, respectively. We thank Merlin Crossley for providing FOG-1 tyrosine-to-alanine point mutant constructs. We also thank Christoph Klein and Hanno Hock for advice on retroviral production and infection and John Daley for his assistance with fluorescence cell sorting.
A.B.C. is supported by National Institutes of Health grant 5K08CA82175. Support was also derived from an NIH grant to S.H.O. S.H.O. is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Andrews, N. C., and D. Faller. 1991. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 19:2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bulger, M., and M. Groudine. 1999. Looping versus linking: toward a model for long-distance gene activation. Genes Dev. 13:2465-2477. [DOI] [PubMed] [Google Scholar]
- 3.Crispino, J. D., M. Lodish, J. P. Mackay, and S. H. Orkin. 1999. Use of altered specificity mutants to probe a specific protein-protein interaction in differentiation: the GATA-1:FOG complex. Mol. Cell 3:219-228. [DOI] [PubMed] [Google Scholar]
- 4.Crispino, J. D., M. Lodish, B. L. Thurberg, S. H. Litovsky, T. Collins, J. D. Molkentin, and S. H. Orkin. 2001. Proper coronary vascular development and heart morphogenesis depend on interaction of GATA-4 with FOG factors. Genes Dev. 15:839-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cubadda, Y., P. Heitzler, R. P. Ray, M. Bourouis, P. Ramain, E. Gelbart, P. Simpson, and M. Haenlin. 1997. u-shaped encodes a zinc finger protein that regulates the proneural genes achaete and scute during the formation of bristles in Drosophila. Genes Dev. 11:3083-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans, T., and G. Felsenfeld. 1989. The erythroid-specific transcription factor Eryf1: a new zinc finger protein. Cell 58:877-885. [DOI] [PubMed] [Google Scholar]
- 7.Evans, T., and G. Felsenfeld. 1991. trans-activation of a globin promoter in nonerythroid cells. Mol. Cell. Biol. 11:843-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fossett, N., S. G. Tevosian, K. Gajewski, Q. Zhang, S. H. Orkin, and R. A. Schulz. 2001. The Friend of GATA proteins U-shaped, FOG-1 and FOG-2 function as negative regulators of blood, heart, and eye development in Drosophila. Proc. Natl. Acad. Sci. USA 98:7342-7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox, A. H., C. Liew, M. Holmes, K. Kowalski, J. Mackay, and M. Crossley. 1999. Transcriptional cofactors of the FOG family interact with GATA proteins by means of multiple zinc fingers. EMBO J. 18:2812-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujiwara, Y., C. Browne, K. Cunniff, S. C. Goff, and S. H. Orkin. 1996. Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc. Natl. Acad. Sci. USA 93:12355-12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haenlin, M., Y. Cubadda, F. Blondeau, P. Heitzler, Y. Lutz, P. Simpson, and P. Ramain. 1997. Transcriptional activity of Pannier is regulated negatively by heterodimerization of the GATA DNA-binding domain with a cofactor encoded by the u-shaped gene of Drosophila. Genes Dev. 11:3096-3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawley, R. G., A. Z. C. Fong, M. Lu, and T. S. Hawley. 1994. The HOX11 homeobox-containing gene of human leukemia immortalizes murine hematopoietic precursors. Oncogene 9:1-12. [PubMed] [Google Scholar]
- 13.Hawley, R. G., A. Z. C. Fong, R. D. Marciano, N. Zhang, M. Lu, and T. S. Hawley. 1997. Transforming function of the HOX11/TCL3 homeobox gene. Cancer Res. 57:337-345. [PubMed] [Google Scholar]
- 14.Karnovsky, M. J., and L. Roots. 1964. A direct colouring thiocholine method for cholinesterases. J. Histochem. Cytochem. 12:219-221. [DOI] [PubMed] [Google Scholar]
- 15.Keller, G., M. Kennedy, T. Papayannopoulou, and M. V. Wiles. 1993. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol. Cell. Biol. 13:473-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller, G., C. Wall, A. Z. C. Fong, T. S. Hawley, and R. G. Hawley. 1998. Overexpression of HOX11 leads to the immortalization of embyronic precursors with both primitive and definitive hematopoietic potential. Blood 92:877-887. [PubMed] [Google Scholar]
- 17.Klein, C., H. Bueler, and R. C. Mulligan. 2000. Comparative analysis of genetically modified dendritic cells and tumor cells as therapeutic cancer vaccines. J. Exp. Med. 191:1699-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuo, C. T., E. E. Morrisey, R. Anandappa, K. Sigrist, M. M. Lu, M. S. Parmacek, C. Soudais, and J. M. Leiden. 1997. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 11:1048-1060. [DOI] [PubMed] [Google Scholar]
- 19.Lu, J.-R., T. McKinsey, H. Xu, D.-Z. Wang, J. A. Richardson, and E. N. Olson. 1999. FOG-2, a heart- and brain-enriched cofactor for GATA transcription factors. Mol. Cell. Biol. 19:4495-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molkentin, J. D., Q. Lin, S. A. Duncan, and E. N. Olson. 1997. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 11:1061-1072. [DOI] [PubMed] [Google Scholar]
- 21.Mouthon, M., O. Bernard, M. T. Mitjavila, P. H. Romeo, W. Vainchenker, and D. Mathieu-Mahul. 1993. Expression of tal-1 and GATA-binding proteins during human hematopoiesis. Blood 81:647-655. [PubMed] [Google Scholar]
- 22.Nichols, K. E., J. D. Crispino, M. Poncz, J. G. White, S. H. Orkin, J. M. Maris, and M. J. Weiss. 2000. Familial dyserythropoietic anaemia and thrombocytopenia due to an inherited mutation in GATA-1. Nat. Genet. 24:266-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nichols, W. C., K. A. Cooney, K. L. Mohike, J. D. Ballew, A. Yang, M. E. Bruck, M. Reddington, E. K. Novak, R. T. Swank, and D. Ginsburg. 1994. von Willebrand disease in the RIIIS/J mouse is caused by a defect outside of the von Willebrand factor gene. Blood 83:3225-3231. [PubMed] [Google Scholar]
- 24.Orkin, S. H., F. L. Harosi, and P. Leder. 1975. Differentiation in erythroleukemic cells and their somatic hybrids. Proc. Natl. Acad. Sci. USA 72:98-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ory, D., B. A. Neugeboren, and R. C. Mulligan. 1996. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc. Natl. Acad. Sci. USA 93:11400-11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orkin, S. H. 2000. Diversification of haematopoietic stem cells to specific lineages. Nat. Rev. 1:57-64. [DOI] [PubMed] [Google Scholar]
- 27.Orkin, S. H. 1992. GATA-binding transcription factors in hematopoietic cells. Blood 80:575-581. [PubMed] [Google Scholar]
- 28.Shivdasani, R. A., Y. Fujiwara, M. A. McDevitt, and S. H. Orkin. 1997. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J. 16:3965-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shivdasani, R. A., and S. H. Orkin. 1996. The transcriptional control of hematopoiesis. Blood 87:4025-4039. [PubMed] [Google Scholar]
- 30.Svensson, E. C., G. S. Huggins, F. B. Dardik, C. E. Polk, and J. M. Leiden. 2000. A functionally conserved N-terminal domain of Friend of GATA-2 (FOG-2) protein represses GATA-4 dependent transcription. J. Biol. Chem. 275:20762-20769. [DOI] [PubMed] [Google Scholar]
- 31.Svensson, E. C., R. L. Tufts, C. E. Polk, and J. M. Leiden. 1999. Molecular cloning of FOG-2: a modulator of transcription factor GATA-4 in cardiomyocytes. Proc. Natl. Acad. Sci. USA 96:956-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svensson, E. C., G. S. Huggins, H. Lin, C. Clendenin, F. Jiang, R. Tufts, F. B. Dardik, and J. M. Leiden. 2000. A syndrome of tricuspid atresia in mice with a targeted mutation of the gene encoding FOG-2. Nat. Genet. 25:353-356. [DOI] [PubMed] [Google Scholar]
- 33.Tevosian, S. G., A. E. Deconinck, A. B. Cantor, H. I. Reiff, Y. Fujiwara, G. Corfas, and S. H. Orkin. 1999. FOG-2: a novel GATA-family cofactor related to multitype zinc-finger proteins Friend of GATA-1 and U-shaped. Proc. Natl. Acad. Sci. USA 96:950-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tevosian, S. G., A. E. Deconinck, M. Tanaka, M. Schinke, L. H. Silvio, S. Izumo, Y. Fujiwara, and S. H. Orkin. 2000. FOG-2, a cofactor of GATA transcription factors, is essential for heart morphogenesis and development of coronary vessels from epicardium. Cell 101:729-739. [DOI] [PubMed] [Google Scholar]
- 35.Tsai, F.-Y., and S. H. Orkin. 1997. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cells formation, but not for erythroid and myeloid terminal differentiation. Blood 89:3636-3643. [PubMed] [Google Scholar]
- 36.Tsai, S. F., D. I. K. Martin, L. I. Zon, A. D. D'andrea, G. G. Wong, and S. H. Orkin. 1989. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature 339:446-451. [DOI] [PubMed] [Google Scholar]
- 37.Tsang, A. P., Y. Fujiwara, D. B. Hom, and S. H. Orkin. 1998. Failure of megakaryocytopoiesis and arrested erythropoiesis in mice lacking the GATA-1 transcription factor cofactor FOG. Genes Dev. 12:1176-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsang, A. P., J. E. Visvader, A. C. Turner, Y. Fujiwara, C. Yu, M. J. Weiss, M. Crossley, and S. H. Orkin. 1997. FOG, a multitype zinc finger protein, acts as a cofactor for transcription factor GATA-1 in erythroid and megakaryocytic differentiation. Cell 90:109-119. [DOI] [PubMed] [Google Scholar]
- 39.Villeval, J.-L., K. Cohen-Solal, M. Tulliez, S. Giraudier, J. Guichard, S. A. Burstein, E. M. Cramer, W. Vainchenker, and F. Wendling. 1997. High thrombopoietin production by hemopoietic cells induces a fatal myeloproliferative syndrome in mice. Blood 90:4369-4383. [PubMed] [Google Scholar]
- 40.Visvader, J., and J. M. Adams. 1993. Megakaryocyte differentiation induced in 416B myeloid cells by GATA-2 and GATA-3 transgenes or 5-azacytidine is tightly coupled to GATA-1 expression. Blood 82:1493-1501. [PubMed] [Google Scholar]
- 41.Vyas, P., K. Ault, C. W. Jackson, S. H. Orkin, and R. A. Shivdasani. 1999. Consequences of GATA-1 deficiency in megakaryocytes and platelets. Blood 93:2867-2875. [PubMed] [Google Scholar]
- 42.Weiss, M. J., and S. H. Orkin. 1995. GATA transcription factors: key regulators of hematopoiesis. Exp. Hematol. 23:99-107. [PubMed] [Google Scholar]
- 43.Weiss, M. J., C. Yu, and S. H. Orkin. 1997. Erythroid-cell-specific properties of transcription factor GATA-1 revealed by phenotypic rescue of a gene-targeted cell line. Mol. Cell. Biol. 17:1642-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]