Abstract
Adaptor proteins assemble multiprotein signaling complexes, enabling the transduction of intracellular signals. While many adaptor proteins positively regulate signaling in this manner, a subgroup of adaptors function as negative regulators. Here we report the identification of a hematopoiesis-specific adaptor protein that we have designated Src-like adaptor protein 2 (SLAP-2). SLAP-2 is most closely related to SLAP and contains a Src homology 3 (SH3) domain and an SH2 domain, as well as an amino-terminal myristoylation site that mediates SLAP-2 association with membranes. Following stimulation of primary thymocytes with anti-CD3 and anti-CD28, SLAP-2 coimmunoprecipitates with tyrosine-phosphorylated c-Cbl and an unidentified protein of approximately 72 kDa. In activated Jurkat T cells, SLAP-2 also binds an additional 70-kDa phosphoprotein, identified as ZAP-70. Binding of SLAP-2 to both p72 and ZAP-70 is dependent on its SH2 domain, while c-Cbl interacts with the carboxy-terminal region. Overexpression of wild-type SLAP-2 alone or in combination with c-Cbl in Jurkat T cells leads to inhibition of T-cell antigen receptor-induced activation of nuclear factor of activated T cells. The inhibitory effect of SLAP-2 requires the carboxy-terminal c-Cbl binding region. Expression of SLAP-2 with SYK or ZAP-70 in COS cells or Jurkat T cells causes the degradation of these kinases, and SLAP-2 overexpression in Jurkat T cells reduces the surface expression of CD3. These results suggest that the mechanism of action of SLAP-2 and the related protein SLAP is to promote c-Cbl-dependent degradation of the tyrosine kinases SYK and ZAP-70 and down-regulation of CD3 at the cell surface.
Engagement of the T-cell antigen receptor (TCR) is directly coupled to the activation of nonreceptor protein tyrosine kinases of both the Src and the SYK/ZAP-70 families, leading to the phosphorylation of intracellular signaling proteins (5, 13). Among the downstream substrates of these activated kinases are signal-transducing enzymes, such as phospholipase Cγ1, and adaptor proteins, such as SLP-76 and linker of activated T cells (LAT) (7, 45). Adaptor proteins play a critical role in mediating the formation of multiprotein signaling complexes and allowing the propagation of the TCR signal (36). Phosphorylated LAT recruits Src homology 2 (SH2) domain-containing proteins phospholipase Cγ, Grb2, and Gads (Grb2-related adaptor dowstream of Shc), while phosphorylated SLP-76 forms complexes with Vav, Nck, and p130SLAP (ADAP) (7, 20, 50). Formation of these multiprotein complexes initiates a cascade of signaling events downstream of the TCR, resulting in the up-regulation of interleukin 2 (IL-2) expression via activation of nuclear transcription factors, such as nuclear factor of activated T cells (NFAT), reorganization of the actin cytoskeleton, and adhesion (48).
Proteins that negatively regulate TCR signaling are essential for the maintenance of T-cell homeostasis, the prevention of aberrant lymphocyte activation, and regulation of the duration of immune responses (13, 37). Adaptor proteins also function in assembling inhibitory complexes that play a role in mediating this down-regulation (16). Transmembrane proteins, such as SIT and PAG, for example, recruit the tyrosine kinase Csk to the membrane (4, 33). Csk acts as a negative regulator of the Src family kinases Lck and Fyn by phosphorylating the negative regulatory site found in the tail of these enzymes (6, 29). Cytosolic adaptors of the Dok family down-regulate activated antigen receptor complexes through recruitment of the inhibitory molecules RasGAP, Csk, and SHIP (15).
c-Cbl is a ubiquitously expressed protein, initially characterized as an adaptor that functions as a negative regulator of both receptor and nonreceptor tyrosine kinases (22, 43). In addition to its adaptor function, c-Cbl also possesses a RING finger domain and has E3 ubiquitin ligase activity, which promotes the ubiquitination of activated tyrosine kinases (12, 43, 51). Following TCR activation, c-Cbl is recruited to the activated TCR complex and tyrosine phosphorylated (8). The activation of TCR signaling also leads to c-Cbl association with the SYK family kinases SYK and ZAP-70 (9, 24, 26). The association between c-Cbl and the SYK family kinases results in a decrease in the activities and protein levels of these kinases (23, 28, 34) and thus in an overall down-regulation of signaling from the TCR. The mechanism by which c-Cbl negatively regulates SYK and ZAP-70 is not fully understood; however, it has been proposed that c-Cbl ubiquitin ligase activity is involved in this process, since the RING finger domain is essential for its inhibitory activity (30, 44).
Through its association with ZAP-70, c-Cbl has been demonstrated to ubiquitinate the zeta (ζ) chain of the TCR (TCR-ζ) (46). Therefore, c-Cbl-mediated ubiquitination of components of the TCR either could result in degradation via the proteasome or could serve as a signal for trafficking of the activated TCR complex to the lysosome. In agreement with this hypothesis, thymocytes from mice deficient in c-Cbl exhibit constitutively elevated tyrosine phosphorylation levels and have increased levels of cell surface TCR (28, 42). Additional support for this model derives from the observation that c-Cbl ubiquitinates and promotes the internalization and subsequent degradation of receptor protein tyrosine kinases, such as the epidermal growth factor receptor (18), the colony-stimulating factor 1 receptor (CSF-1R) (14), and the platelet-derived growth factor receptor (PDGFR) (27).
Recently, it was revealed that Src-like adaptor protein (SLAP) is a negative regulator of the TCR (40). Originally identified as a protein that interacted with the cytoplasmic domain of EphA2, SLAP was shown to inhibit mitogenic signals downstream of the PDGFR (31, 35). Subsequently, SLAP was found to inhibit both NFAT and AP-1 activation when transiently overexpressed in Jurkat T cells (40). While the mechanism by which SLAP mediates inhibitory effects remains to be elucidated, Sosinowski et al. have found that targeted disruption of the SLAP gene in mice results in increased surface TCR expression on doubly positive thymocytes (39).
Here we describe the cloning and characterization of SLAP 2 (SLAP-2), an adaptor protein that exhibits structural and sequence similarities to SLAP. SLAP-2 is expressed predominantly in hematopoietic cells and interacts with both c-Cbl and ZAP-70 in activated T cells. We have found that SLAP-2 inhibits NFAT activation, can induce the degradation of ZAP-70 and SYK, and reduces CD3 surface levels in a manner dependent upon its interaction with c-Cbl. We propose that both SLAP-2 and the related protein SLAP function in concert with c-Cbl to down-regulate signaling by promoting the internalization and degradation of activated receptor complexes.
MATERIALS AND METHODS
Cloning of murine SLAP-2 cDNA.
A search of high-throughput genomic sequences in the GenBank sequence database was conducted in an attempt to identify novel SH2 domain-containing proteins. Using the amino acid sequence of the GADS SH2 domain and the National Center for Biotechnology Information tBLASTn search algorithm, a genomic clone containing the partial sequence of a novel SH2 domain-containing protein bearing weak homology to GADS was identified (GenBank accession number AL050318). Subsequently, the human genomic clone was used with the BLASTn search algorithm to search the mouse expressed sequence tag (EST) database, leading to the identification of two overlapping ESTs encoding the 5′ end of a novel murine cDNA. Nested primers were designed on the basis of the 5′ untranslated region of the cDNA sequence and were used for 3′ rapid amplification of cDNA ends (RACE) of Marathon Ready mouse day 15 embryo cDNA (Clontech). A single RACE product of 1,348 bp was amplified by using the following PCR primers: 5′-CACGCTCTTTGTCCCTGCTGTGCTG-3′ (sense) and 5′-GGCCCAATCTGGTTTCTCTGAGAAGC-3′ (nested sense).
Cell lines.
COS-1, COS-7, Raw264.7, RBL-2H3, MEF, and NIH 3T3 cells were cultured in Dulbecco modified Eagle medium supplemented with 10% (vol/vol) fetal bovine serum (Gibco-BRL), 200 mM l-glutamine, 5 U of penicillin C/ml, and 5 mg of streptomycin sulfate/ml. Jurkat E6.1, Jurkat p116, WEHI, DO11.10, and P815 cells were cultured in RPMI 1640 medium supplemented as described above. BaF3 cells were maintained in RPMI 1640 medium supplemented as described above but with the addition of 10 ml of WEHI cell supernatant (IL-3), while CTLL-2 cells were maintained in RPMI 1640 medium supplemented as described above but with the addition of recombinant IL-2 (2 U/ml; Roche). Primary mouse thymocytes from a 6-week-old female mouse were cultured in RPMI 1640 medium supplemented as described above.
Mammalian expression constructs and GST fusion proteins.
Wild-type and mutant SLAP-2 cDNAs were cloned in frame into pcDNA3.1-MYC/His (EcoRI/BamHI), pEF-MYC/His (ClaI/XbaI), and pEGFP-IRES2 (EcoRI/BamHI) mammalian expression vectors. Both the pcDNA3.1 and the pEF constructs possess carboxy-terminal MYC/His epitope tags so as to avoid interference with the amino-terminal myristoylation of SLAP-2. Similarly, wild-type and mutant SLAP-2 cDNAs were cloned in frame into pGEX-4T1 (EcoRI/XhoI) in order to produce glutathione S-transferase (GST) fusion proteins in bacteria. All SLAP-2 mutants were generated by PCR-based mutagenesis; the myristoylation mutant (G2A) had glycine at amino acid position 2 mutated to alanine, methionine mutant 1 (M1V) had methionine at amino acid position 1 mutated to valine, methionine mutant 2 (M27V) had methionine at amino acid position 27 mutated to valine, SH2-inactivating mutant (SH2*) had the arginine residue at amino acid position 120 mutated to lysine, and ΔC contained a deletion of the carboxy-terminal 70 amino acids. All SLAP-2 expression constructs were confirmed by DNA sequencing. c-Cbl-GST fusion constructs were a gift from Hamid Band and were previously described (24). SYK and ZAP cDNAs in expression vector pXM were provided by Andre Veillette (McGill University, Montreal, Quebec, Canada) and were previously described.
Transient transfections and NFAT-luciferase assays.
COS-1, COS-7, and HeLa cells were grown to ∼60% confluence in 10-cm tissue culture dishes and transfected with 2 to 4 μg of each expression vector by use of Lipofectin reagent (Gibco-BRL) according to the manufacturer's instructions. Jurkat p116 cells were electroporated (250 V, 960 μF) with 20 to 40 μg of empty vector or SLAP-2 or ZAP-70 expression constructs. For NFAT-luciferase assays, Jurkat E6.1 cells (20 × 106) were electroporated (250 V, 960 μF) with 20 to 40 μg of empty vector or c-Cbl or SLAP-2 expression constructs and 10 μg of NFAT-luciferase reporter construct. NFAT-luciferase assays were conducted as previously described (3, 20).
In vitro binding assays.
GST fusion proteins were produced in bacteria and purified on glutathione-Sepharose beads (Pharmacia). In vitro binding assays were done with Jurkat T-cell lysates (20 × 106 cells) either unstimulated or stimulated with anti-CD3 antibody (anti-CD3ɛ monoclonal antibody [MAb] clone UCHT1; Pharmingen). Lysates were incubated with ∼4 μg of GST fusion proteins for 90 min at 4°C. Following several washes, bound proteins were eluted in 2× sodium dodecyl sulfate (SDS) sample buffer and resolved by SDS-polyacrylamide gel electrophoresis (PAGE). Membranes were stained with Coomassie blue dye to ensure equal loading of fusion proteins.
Cell stimulation.
Jurkat E6.1 cells were stimulated with anti-CD3 antibody (MAb clone UCHT1; 2 μg) for 2 min at 37°C. Mouse thymocytes (5 × 107) were stimulated with anti-CD3ɛ alone (MAb clone 145-2C11; Pharmingen; 10 μg) or anti-CD28 alone (MAb clone 37.51; Pharmingen; 10 μg) or costimulated with anti-CD3 and anti-CD28 for either 2, 5, or 15 min in the presence of a rabbit anti-mouse secondary reagent. Following stimulation, Jurkat T cells and mouse primary thymocytes were lysed in Nonidet P-40 (NP-40) lysis buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 10% [vol/vol] glycerol, 1% [vol/vol] NP-40, 0.1 mM ZnCl2, 2 mM EDTA) containing complete protease inhibitors (Roche) and 1 mM sodium orthovanadate.
Antibodies.
Polyclonal SLAP-2 antisera were produced by immunizing rabbits with a GST fusion protein containing the carboxy-terminal domain of murine SLAP-2. Five microliters of crude SLAP-2 antisera or preimmune serum was used for immunoprecipitation, and crude serum was used at a 1:500 dilution for blotting; 3 μl of affinity-purified SLAP-2 antibody was used in immunoprecipitation experiments, and a 1:1,000 dilution was used for immunoblotting. Affinity-purified GADS antibody was used at a 1:1,000 dilution for immunoblotting as previously described (21). Anti-MYC MAb 9E10 was purchased from the Developmental Studies Hybridoma Bank (University of Iowa); 2 μg was used for immunoprecipitation, and a dilution of 1:1,000 was used for immunoblotting. Anti-ZAP-70 MAb was purchased from Transduction Laboratories and used at a dilution of 1:1,000 for immunoblotting. Rabbit polyclonal anti-SYK/ZAP-70 pan antibody was a kind gift from Andre Veillette; 5 μl was used in immunoprecipitation experiments, and a dilution of 1:500 was used for immunoblotting. Antiphosphotyrosine MAb 4G10 (Upstate Biotechnology Inc.) and anti-hemagglutinin (HA) MAb 12CA5 (Roche) were used at a dilution of 1:1,000 for immunoblotting. Rabbit anti-c-Cbl polyclonal antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.); 10 μl of anti-c-Cbl antibody was used in immunoprecipitation experiments, and a 1:1,000 dilution was used for immunoblotting. Sheep anti-SLP-76 polyclonal antibody was a kind gift from Gary Koretzky (University of Pennsylvania, Philadelphia) and was used at a dilution of 1:1,000 for immunoblotting. Anti-TCR-ζ antibody was purchased from Zymed and was used at a 1:500 dilution for immunoblotting. Mouse anti-LAT MAb was purchased from Upstate Biotechnology Inc. and used at a 1:500 dilution for immunoblotting. Sheep anti-mouse antibody (1:6,000 dilution) and protein A (1:3,000 dilution) conjugated to horseradish peroxidase were used to detect bound primary mouse MAbs and polyclonal antibodies, respectively.
Immunoprecipitation and Western blotting.
Cells and murine tissue samples were lysed in 1 ml of PLC lysis buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 10% [vol/vol] glycerol, 1% [vol/vol] Triton X-100, 1.5 mM MgCl2, 1 mM EDTA, 10 mM NaPPi, 100 mM NaF) or 1 ml of NP-40 lysis buffer containing complete protease inhibitors and 1 mM sodium orthovanadate. Cell lysates were cleared by centrifugation at 14,000 rpm (20,800 × g and 4°C for 10 min and precleared by incubation with 50 μl of 20% (vol/vol) protein G-Sepharose beads (Sigma, for immunoprecipitation with MAbs) or with protein A-Sepharose beads (Sigma) and 2 μg of rabbit anti-mouse immunoglobulin G (for immunoprecipitation with polyclonal antibodies) at 4°C for 30 min. Precleared lysates were incubated with antibodies and either protein G- or protein A-Sepharose beads as described above and then incubated at 4°C with gentle rotation for 90 min or overnight. Immune complexes were washed four times in 1 ml of cold 0.1% NP-40 lysis buffer, and bound proteins were eluted by boiling for 5 min in 2× SDS sample buffer. Eluted proteins were resolved by SDS-PAGE on 10% gels. Proteins were electrophoretically transferred to polyvinylidene difluoride membranes (NEN Life Science) and incubated in a blocking solution of either 5% (wt/vol) skim milk powder in 1× Tris-buffered saline with 0.05%. Tween 20 (TBST) or 1% bovine serum albumin (wt/vol) in 1× TBST (antiphosphotyrosine blotting) for a minimum of 30 min prior to the addition of antibodies. Blocked membranes were incubated with primary antibodies at room temperature for 1 h or at 4°C overnight. Membranes were incubated for 45 min with an appropriate secondary antibody conjugated to horseradish peroxidase. Bound antibodies were detected by using enhanced chemiluminescence reagent (NEN Life Science).
Subcellular fractionation.
DO11.10 cells (2.5 × 107) were washed with phosphate-buffered saline (PBS) and lysed in 1 ml of hypotonic lysis buffer (10 mM Tris-HCl [pH 8.0], 1 mM MgCl2) containing complete protease inhibitors and 1 mM sodium orthovanadate. Cells were sufficiently lysed upon vortexing, as checked by trypan blue staining. Lysed cells were adjusted back to isotonic conditions by the addition of 5 M NaCl to a final concentration of 150 mM. Lysates were centrifuged at 3,000 rpm (960 × g for 10 min at 4°C. The pellet representing the nuclear fraction was resuspended in extraction buffer (1% SDS, 1% Triton X-100, 1% sodium deoxycholate) in 10 mM Tris-HCl (pH 8.0)-150 mM NaCl-1 mM MgCl2. The supernatant from the first spin was centrifuged in a Beckman tabletop ultracentrifuge by using the TLA-45 rotor at 43,000 rpm (100,000 × g) for 30 min at 4°C. The pellet representing the membrane fraction was resuspended in extraction buffer as described above. The supernatant representing the soluble or cytoplasmic fraction was adjusted to 0.1% SDS-0.1% Triton X-100-0.1% sodium deoxycholate. A 250-μg quantity of protein lysate was immunoprecipitated and immunoblotted with affinity-purified anti-SLAP-2 antibody. To confirm the integrity of the individual fractions, 40 μg of lysate was resolved by SDS-PAGE and immunoblotted with both anti-GADS (soluble protein control) and anti-TCR-ζ (membrane protein control) antibodies.
Immunofluorescence.
HeLa cells were transiently transfected with MYC-tagged wild-type SLAP-2 or G2A mutant SLAP-2 expression constructs as described above and seeded onto untreated glass coverslips. Jurkat E6.1 cells were transfected with MYC-tagged wild-type SLAP-2 expression construct and seeded onto glass coverslips that had been pretreated with 0.01% polyornithine (Sigma). Coverslips were washed two times with PBS plus Ca or Mg. Cells were fixed with 4% paraformaldehyde for 30 min at room temperature and permeabilized with 0.2% Triton X-100 in PBS for 10 min. Permeabilized HeLa cells were incubated with anti-MYC primary MAb (9E10) (1:1,000) and subsequently with Alexa488-labeled (green fluorescence) anti-mouse secondary antibody (1:500) at 37°C for 30 min. Cell nuclei were stained with propidium iodide (red fluorescence). Permeabilized Jurkat T cells were incubated with anti-MYC primary polyclonal antibody (Santa Cruz; 1:300) and anti-mannose-6-phosphate receptor (M6PR) MAb (Research Diagnostics; 1:250) and subsequently with Alexa488-labeled anti-rabbit (1:500) and Cy3-labeled (red fluorescence) anti-mouse secondary antibodies at 37°C for 30 min. Coverslips were mounted on glass slides and visualized by confocal microscopy.
Flow cytometry.
Jurkat E6.1 cells were electroporated as previously described with either empty vector pEGFP-IRES2 or vector pEGFP-IRES2 containing the wild-type SLAP-2 coding region. At 24 h after electroporation, cells were harvested and either left unstimulated or stimulated with anti-human CD3ɛ antibody for 1 h at 37°C. A total of 106 cells were subsequently stained with 2 μg of anti-human CD3ɛ primary antibody and 1 μg of phycoerythrin-labeled anti-mouse immunoglobulin G (Pharmingen). Stained cells were analyzed by flow cytometry with CellQuest software. CD3 expression was determined with cells that received the plasmids and expressed green fluorescent protein. Dead cells were excluded from the analysis by staining with propidium iodide. Data from a representative experiment are shown (see Fig. 10b and c>). The expression of transfected SLAP-2 was confirmed by immunoprecipitation and Western blotting with affinity-purified antibodies against epitopes in the SLAP-2 carboxy terminus (anti-SLAP-2-C) (see Fig. 10d). The average fold reduction in mean fluorescence observed for four independent experiments is shown (see Fig. 10e and f).
Nucleotide sequence accession number.
The sequence data determined here have been submitted to GenBank and are available under accession number AF287467.
RESULTS
Cloning of murine SLAP-2 cDNA.
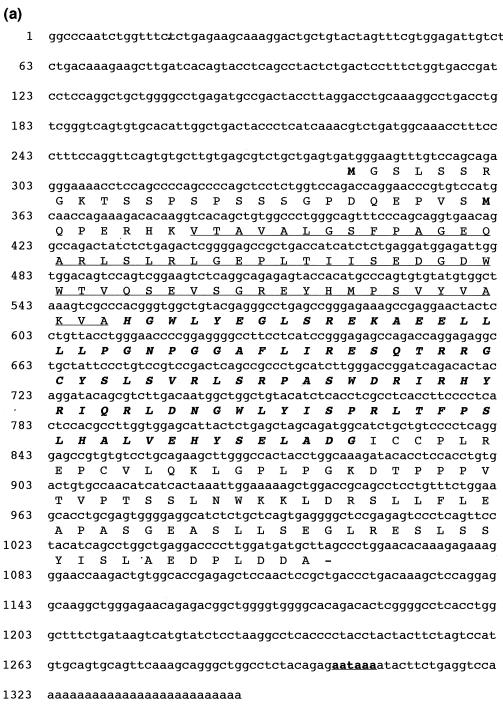
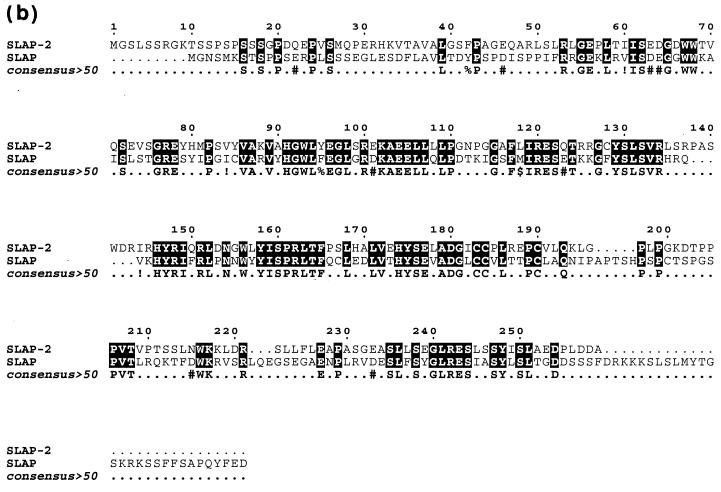
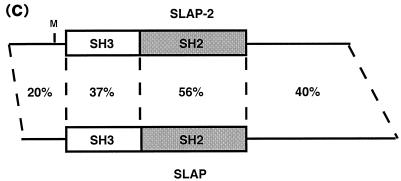
The SH2 domain sequence of the hematopoietic adaptor molecule Gads (21) was used to carry out an in silico screen of high-throughput genomic sequences in the National Center for Biotechnology Information GenBank sequence database by using the BLAST algorithm. A human genomic clone harboring the partial sequence of a novel protein with an SH2 domain that had weak similarity to the Gads SH2 domain was identified (GenBank accession number AL050318). The genomic clone was subsequently used to search the GenBank mouse EST database for related cDNAs. Two mouse ESTs encoding the 5′ end of a novel cDNA were identified (GenBank accession numbers AI510095 and AA959151), and the full-length 1,348-bp cDNA was cloned by 3′ RACE. The full-length mouse cDNA contains relatively short 5′ and 3′ untranslated regions and an open reading frame of 777 bp which encodes a putative protein of 259 amino acids with a predicted molecular mass of 28.5 kDa (Fig. 1a ). Based on sequence and structural similarities to SLAP (30), we named this new protein SLAP-2 and propose that it represents a second member of a SLAP family of adaptors (Fig. 1b and c). Like SLAP, SLAP-2 contains a serine-rich amino terminus with a myristoylation sequence (MGX1-4S) at the extreme amino terminus, SH3 and SH2 protein interaction domains, and a unique carboxy-terminal domain.
FIG. 1.
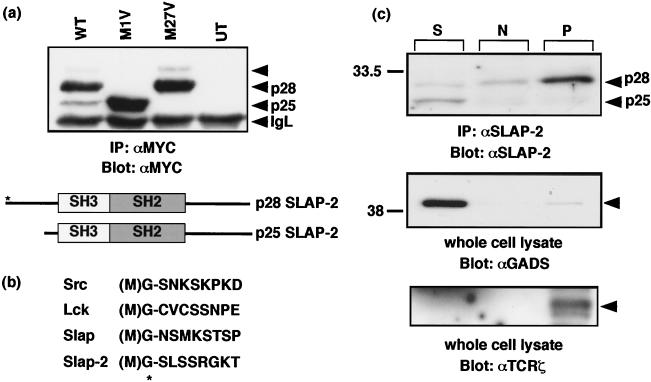
Molecular cloning of murine SLAP-2. (a) Full-length murine SLAP-2 cDNA and translated amino acid sequence. Initiation methionines are indicated in bold type; a consensus polyadenylation signal sequence is indicated in bold type and underlined. The residues comprising the SH3 domain are underlined, and the residues comprising the SH2 domain are indicated in bold italic type. (b) Alignment of the amino acid sequences of murine SLAP-2 and SLAP. Conserved residues are blocked in black, while similar residues are denoted by exclamation points and number symbols in the consensus row. (c) Schematic representations of murine SLAP-2 and SLAP. The percent amino acid identity between the individual domains is indicated. M, site of alternative translation initiation, which leads to the expression of a SLAP-2 translation isoform (see Fig. 3a).
Expression of SLAP-2 in murine tissues and cell lines.
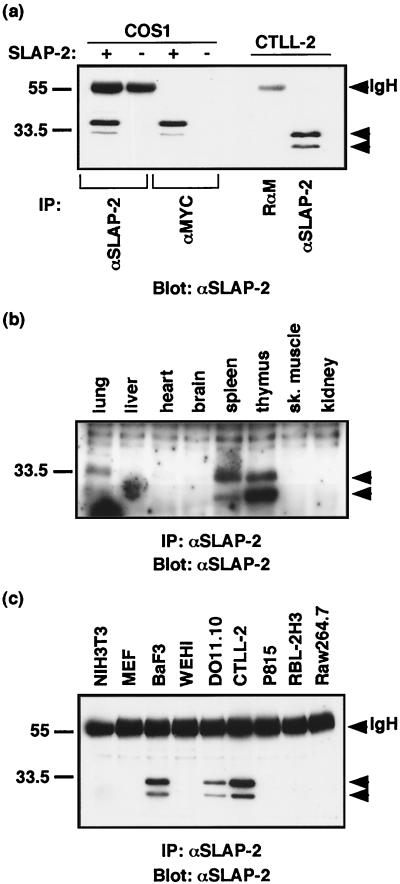
In order to profile SLAP-2 expression, we generated anti-SLAP-2-C. The antibodies were tested for their ability to recognize both transfected SLAP-2-MYC and endogenous SLAP-2 in CTLL-2 cells (Fig. 2a). In both SLAP-2-MYC-transfected COS-1 cells and untransfected CTLL-2 cells, the anti-SLAP-2-C antibodies specifically detected a protein doublet which migrated with a slower mobility in the transfected COS-1 cells, presumably as a result of the added epitope tag. The upper band observed in CTLL-2 cells migrated with an apparent molecular mass of 28 kDa, in keeping with the predicted molecular mass of 28.5 kDa. The smaller form of SLAP-2, with an apparent molecular mass of 25 kDa, arose from an internal translation initiation site (Fig. 3).
FIG. 2.
SLAP-2 expression in murine tissues and cell lines. (a) Generation of SLAP-2-specific antisera. Rabbit antisera generated against a GST fusion protein containing the murine SLAP-2 carboxy terminus (anti-SLAP-2-C) were tested against transfected MYC-tagged SLAP-2 (crude antibody) and endogenous SLAP-2 (affinity-purified antibody). IgH, immunoglobulin heavy chain; IP, immunoprecipitation; RαM, rabbit anti-mouse IgG. (b) Expression of SLAP-2 in murine tissues. One milligram of total protein lysate isolated from the tissues of an 8-week-old mouse was immunoprecipitated and immunoblotted with crude anti-SLAP-2-C antibody. sk., skeletal. (c) Expression of SLAP-2 in murine cell lines. One milligram of total protein lysate from various hematopoietic and nonhematopoietic cell lines was immunoprecipitated and immunoblotted with affinity-purified anti-SLAP-2-C antibody.
FIG. 3.
Subcellular localization of SLAP-2 isoforms. (a) Identification of SLAP-2 alternative translation isoforms via mutagenesis. Methionine residues at positions 1 and 27 in the primary amino acid sequence were mutated to valine residues by PCR-based mutagenesis (denoted by M1V and M27V, respectively). Mutant proteins expressed in COS cells were immunoprecipitated and immunoblotted with anti-MYC antibody (9E10). IgL, immunoglobulin light chain. Alternative translation initiation from the SLAP-2 transcript results in protein isoforms of ∼28 (p28) and ∼25 (p25) kDa. The p28 isoform contains a myristoylation sequence at its amino terminus (asterisk). WT, wild type; UT, untransfected; IP, immunoprecipitation. (b) Alignment of Src family (Src and Lck), SLAP, and SLAP-2 amino-terminal amino acid sequences indicating a consensus myristoylation sequence (MGX1-4S). (c) Subcellular localization of endogenous SLAP-2 in DO11.10 murine T cells, as determined by subcellular fractionation. Soluble (S), nuclear (N), and pellet (P) fractions were immunoprecipitated and immunoblotted with anti-SLAP-2-C antibody (top panel). Additionally, lysates were resolved by SDS-PAGE and immunoblotted with anti-GADS (middle panel) and anti-TCR-ζ (bottom panel) antibodies as soluble and pellet fraction controls.
The expression of SLAP-2 in murine tissues and murine hematopoietic and nonhematopoietic cell lines was assessed by immunoprecipitation and Western blot analysis. In murine tissues, SLAP-2 was detected in extracts of both the thymus and the spleen and at lower levels in the lungs (Fig. 2b). SLAP-2 was not detected in the other tissues surveyed, including the liver, heart, and brain, indicating that SLAP-2 expression is hematopoiesis pecific. Among the cell lines, SLAP-2 was abundantly expressed in murine BaF3 cells (pro-B cells) and DO11.10 and CTLL-2 cells (T cells) but not in mast cell lines (RBL-2H3 and p815), macrophages (RAW264.7), or either of the two nonhematopoietic cell lines tested (NIH 3T3 and MEF cell lines) (Fig. 2c).
Identification and differential subcellular localization of SLAP-2 isoforms.
Inspection of the SLAP-2 cDNA sequence revealed a putative alternative translation initiation site at methionine 27 (M27) of the SLAP-2 sequence, suggesting that the two prominent anti-SLAP-2-reactive protein species that we observed may represent alternative translation products of SLAP-2. SLAP-2 mutants in which each of the methionine residues (M1 and M27) was mutated to valine were generated and transiently transfected into COS cells. The M1V mutant produced a protein that comigrated with the 25-kDa form of SLAP-2 (p25), while the M27V product comigrated with the 28-kDa species (p28), confirming that the use of alternative translation initiation gives rise to two forms of SLAP-2 (Fig. 3a). An alignment of the amino acid sequences of Src, Lck, and SLAP with the SLAP-2 sequence revealed the presence of a consensus myristoylation sequence at the SLAP-2 amino terminus (Fig. 3b). The smaller, 25-kDa SLAP-2 isoform lacks both the amino-terminal myristoylation sequence and the serine-rich region present at the amino terminus of the long isoform. p25 is expressed at low levels relative to p28 both in transfected cells and endogenously in mouse cell lines. Interestingly, in murine tissues, the relative expression levels are reversed in the thymus, suggesting that the utilization of the internal translation initiation site may be regulated (Fig. 2b). In transiently transfected COS cells, we also observed an additional higher-molecular-weight form of SLAP-2 that was associated with the expression of p28 (Fig. 3a). p28 retains both the myristoylation sequence and the serine-rich region; therefore, the slower-migrating form of SLAP-2 may arise from posttranslational modification. The slower-migrating band is no longer detected following phosphatase treatment of SLAP-2 immunoprecipitates and is not detected by antiphosphotyrosine antibodies, suggesting that phosphorylation on serine and/or threonine residues in the amino terminus contributes to the formation of this species (data not shown). The functional consequences of SLAP-2 phosphorylation remain to be investigated.
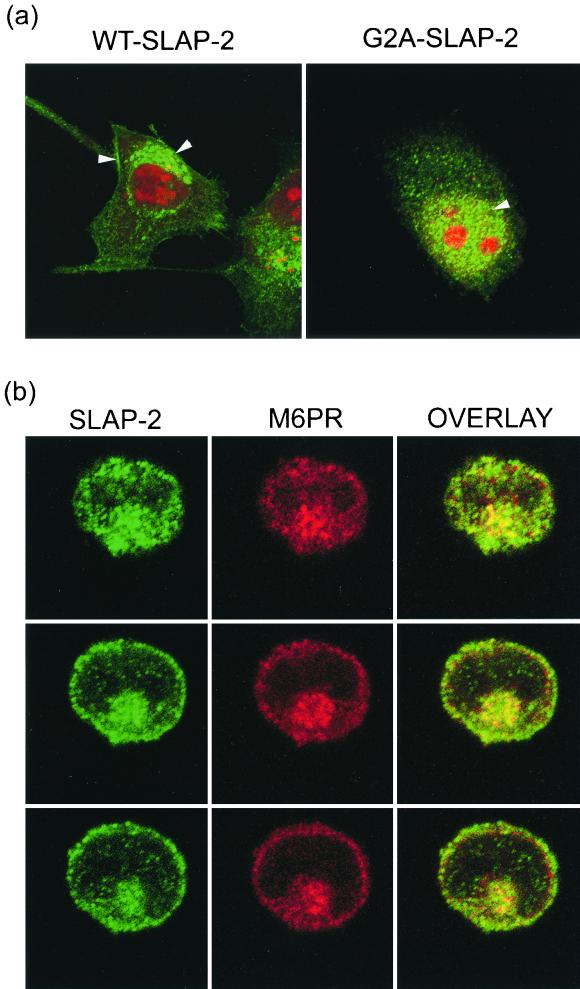
The localization of endogenous SLAP-2 isoforms was assessed by subcellular fractionation of DO11.10 T cells (Fig. 3c). The p28 isoform of SLAP-2 was found predominantly in the pellet fraction, indicative of association with cell membranes, presumably due to its amino-terminal myristoylation. In contrast, the p25 isoform, which lacks the myristoylation sequence, was found predominantly in the soluble cytosolic fraction. The subcellular localization of SLAP-2 was also assessed by immunofluorescence staining of SLAP-2-transfected HeLa cells and Jurkat T cells (Fig. 4). In HeLa cells, wild-type SLAP-2 localized to both the plasma membrane and intracellular vesicles (Fig. 4a, left panel). In contrast, mutant SLAP-2 lacking the amino-terminal myrisotylation site (SLAP-2-G2A) no longer associated with cell membranes and appeared mislocalized to the nucleus, confirming the notion that the myristoylation of p28 is important for its subcellular localization (Fig. 4a, right panel). In order to identify the vesicular structures with which SLAP-2 colocalizes, Jurkat T cells expressing wild-type SLAP-2 were costained for transfected MYC-tagged SLAP-2 and endogenous M6PR, a marker for late endosomes (Fig. 4b). Similar to observations made with HeLa cells, SLAP-2 was observed to localize to both the plasma membrane and intracellular vesicles, and a subpopulation of intracellular SLAP-2 colocalized with M6PR.
FIG. 4.
SLAP-2 localizes to both the plasma membrane and intracellular vesicles. (a) Differential localization of wild-type (WT) SLAP-2 (left panel) and a SLAP-2 myristoylation mutant (G2A-SLAP) (right panel) in HeLa cells, as assessed by immunostaining. MYC-tagged SLAP-2 was expressed in HeLa cells. Transfected cells were immunostained with an anti-MYC primary antibody (9E10) in conjunction with an Alexa488-labeled anti-mouse secondary antibody (green fluorescence), while nuclei were stained with propidium iodide (red fluorescence). Stained cells were visualized by confocal microscopy as described in Materials and Methods. Arrowheads indicate the areas of SLAP-2 localization. (b) SLAP-2 colocalizes with endosomes. MYC-tagged wild-type SLAP-2 was expressed in Jurkat T cells. Transfected cells were coimmunostained with an anti-MYC primary polyclonal antibody in conjunction with an Alexa488-labeled anti-rabbit secondary antibody (green fluorescence) and an anti-M6PR primary monoclonal antibody in conjunction with a Cy3-labeled anti-mouse secondary antibody (red fluorescence). Stained cells were visualized by confocal microscopy, and images taken of three different 1-μm sections of a representative cell are shown. The green and red channels were merged in order to show the colocalization of SLAP-2 and M6PR (overlay).
SLAP-2 associates with c-Cbl and ZAP-70 following TCR cross-linking.
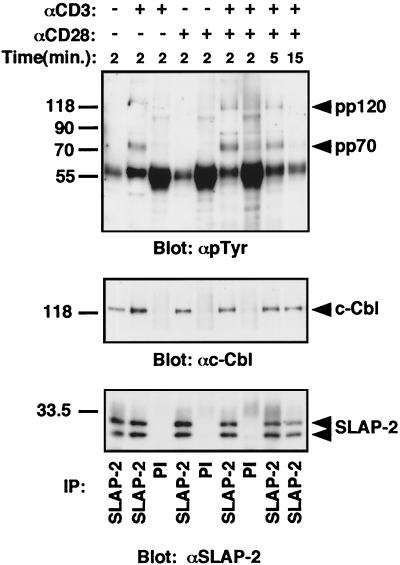
Given the domain composition of SLAP-2, including the presence of an SH2 domain, and its endogenous expression in T cells, we tested its ability to interact with tyrosine-phosphorylated signaling proteins downstream of the activated TCR. Following the stimulation of mouse primary thymocytes with anti-CD3 alone or costimulation with anti-CD3 and anti-CD28, anti-SLAP-2-C antibodies specifically immunoprecipitated tyrosine-phosphorylated proteins of approximately 70 and 120 kDa (Fig. 5). Subsequent immunoblotting identified the 120-kDa protein as c-Cbl. The interaction between SLAP-2 and c-Cbl was also observed in unstimulated cells. ZAP-70 has been reported to associate with c-Cbl; therefore, we tested whether the p72 phosphoprotein represented ZAP-70 or the related family member SYK. While we were unable to identify the coprecipitating 72-kDa phosphoprotein in primary thymocytes, experiments performed with Jurkat T cells (Fig. 6) suggested that a similar set of bands migrating in the 70- to 72-kDa region contained ZAP-70. Therefore, the faint phosphotyrosine-reactive bands migrating in the 70- to 72-kDa region in SLAP-2 immunoprecipitates might have represented a very low level of activated ZAP-70 or SYK or might in fact have represented distinct proteins.
FIG. 5.
SLAP-2 associates in vivo with tyrosine-phosphorylated proteins following TCR cross-linking. Thymocytes were isolated from 6-week-old mice and stimulated with either anti-CD3 alone or anti-CD28 alone or costimulated with anti-CD3 and anti-CD28 antibodies for the indicated periods of time. Lysates from stimulated thymocytes (5 × 107) were immunoprecipitated with affinity-purified anti-SLAP-2-C antibody and immunoblotted. (Top panel) Antiphosphotyrosine immunoblot. (Middle panel) Anti-cCbl immunoblot. (Bottom panel) Results obtained after the blot was stripped and reprobed with affinity-purified anti-SLAP-2-C antibody. IP, immunoprecipitation; PI, preimmune sera.
FIG. 6.
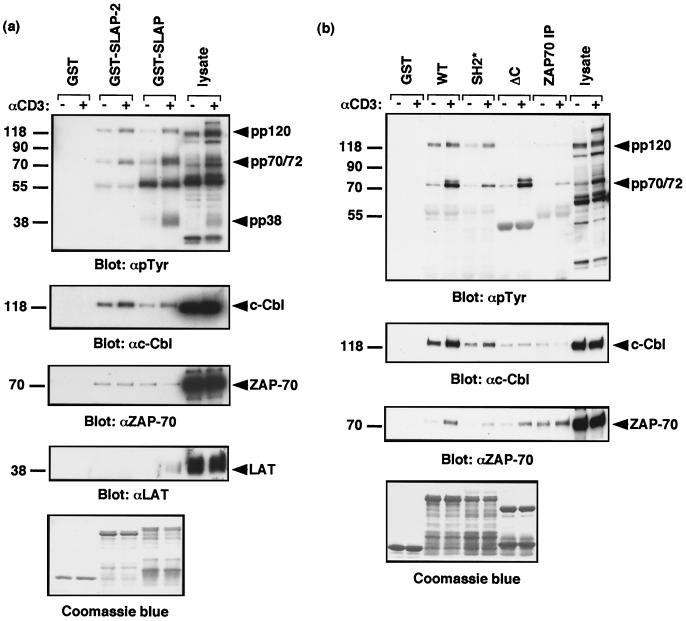
SLAP-2 associates in vitro with tyrosine-phosphorylated ZAP-70 and c-Cbl following TCR activation. (a) SLAP-2 and SLAP associate with similar subsets of T-cell phosphoproteins following TCR activation. Purified immobilized GST fusion proteins with SLAP-2 and SLAP were incubated with lysates from Jurkat T cells that were either left unstimulated (−) or stimulated with anti-CD3 antibody (+). Immunoblotting was performed with the indicated antibodies. (b) Mapping of the SLAP-2 interaction with ZAP-70 and c-Cbl. Purified immobilized GST fusion proteins with wild-type (WT) SLAP-2 or mutant SLAP-2 with an inactivating mutation in the SH2 domain (SH2*) or a deletion of the carboxy terminus (ΔC) were incubated with lysates from Jurkat T cells that were either left unstimulated (−) or stimulated with anti-CD3 antibody (+). Immunoblotting was performed with the indicated antibodies. Equal loading of GST fusion proteins was assessed by Coomassie blue staining. IP, immunoprecipitation.
Recombinant GST-SLAP-2 interacted with a set of tyrosine-phosphorylated proteins from anti-CD3-stimulated Jurkat T cells that were similar to those observed binding to endogenous SLAP-2 in primary thymocytes (Fig. 6a). Furthermore, when we compared the profiles of T-cell phosphoproteins bound by GST-SLAP-2 and GST-SLAP, we found that both proteins interacted predominantly with phosphoproteins of approximately 70 to 72 kDa and 120 kDa which were subsequently identified as ZAP-70 and c-Cbl, respectively (Fig. 6a). The interaction between SLAP-2 and c-Cbl was constitutive but was increased following anti-CD3 stimulation. The observed interaction between SLAP and c-Cbl is in agreement with a previous report that identified SLAP as a c-Cbl-interacting protein in a yeast two-hybrid screen (41). Surprisingly, this interaction was not observed in two different studies of SLAP function in T cells (40, 41). Interestingly, SLAP, but not SLAP-2, appears to inducibly associate with the transmembrane adaptor LAT following anti-CD3 stimulation. The interaction between SLAP and LAT in T cells was described in previous studies (40, 41).
To determine the regions of SLAP-2 required for interactions with c-Cbl and ZAP-70, GST-SLAP-2 fusion proteins containing mutations that disrupt the SH2 domain (SH2*) or that delete the carboxy terminus of SLAP-2 (ΔC) were generated. The SLAP-2 mutants were tested for their ability to form complexes with tyrosine-phosphorylated proteins from anti-CD3-stimulated Jurkat T cells (Fig. 6b, top panel). Inactivation of the SLAP-2 SH2 domain specifically disrupted binding to the 70- to 72-kDa proteins, indicating that the interaction of SLAP-2 with these proteins is mediated by the SH2 domain. Notably, deletion of the SLAP-2 carboxy terminus enhanced p72 binding or possibly its phosphorylation. Immunoblotting of the GST-SLAP-2-bound proteins with anti-ZAP-70 revealed that ZAP-70 is present in these complexes and corresponds to the lower (∼70-kDa) band of the doublet (bottom panel). Although the extent of binding of the SLAP-2 SH2 mutant to ZAP-70 was significantly decreased, binding was not completely abolished. This result suggests either that SLAP-2 and ZAP-70 make additional contacts or perhaps that ZAP-70 is recruited indirectly through binding to c-Cbl. p72 appears to represent a protein species distinct from ZAP-70; however, we have been unable to confirm its identity. ITK, a Tec family tyrosine kinase expressed in T cells, has an approximate molecular mass of 72 kDa; however, immunoblotting failed to detect any ITK protein in these experiments (data not shown). Reprobing of the membrane with anti-c-Cbl antibodies revealed that the carboxy terminus of SLAP-2 contains the primary c-Cbl binding region (Fig. 6b, second panel from top). The SH2* mutant appeared to bind slightly less c-Cbl, while c-Cbl did not bind the ΔC mutant of SLAP-2 (Fig. 6b). Since ZAP-70 still bound the SLAP-2 ΔC mutant and c-Cbl binding was only weakly affected by the SH2 mutation, these proteins likely bind SLAP-2 independently.
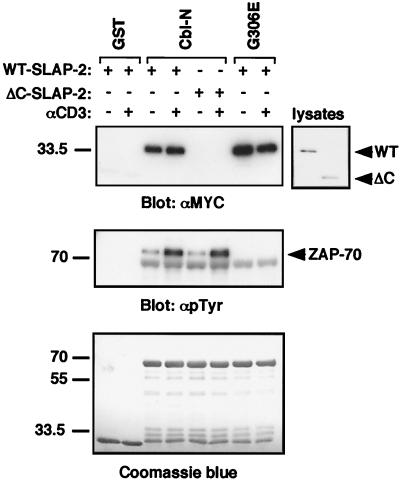
To determine the region of c-Cbl that interacts with SLAP-2, GST-Cbl fusion proteins were incubated with lysates from Jurkat T cells that had been transfected with wild-type or ΔC mutant SLAP-2. The GST-Cbl-N fusion protein, which includes the amino-terminal four-helix bundle, the EF hand, and a variant SH2 domain, associated with wild-type SLAP-2 independently of anti-CD3 stimulation but was unable to associate with the ΔC mutant form of SLAP-2 (Fig. 7, top panel). Additionally, a fusion protein containing a mutant form of the Cbl amino terminus and possessing an inactivating point mutation in the variant SH2 domain (G306E) exhibited no decrease in its ability to bind to wild-type SLAP-2. In contrast, the interaction between ZAP-70 and c-Cbl was observed to be inducible upon anti-CD3 stimulation and to require a functional Cbl SH2 domain, as has been reported elsewhere (24) (Fig. 7, middle panel).
FIG. 7.
The c-Cbl amino terminus mediates binding to SLAP-2 in a phosphorylation-independent manner. Jurkat T cells transfected with either wild-type (WT) or ΔC mutant SLAP-2 expression constructs were either left unstimulated (−) or stimulated with anti-CD3 antibody (+). Transfected cell lysates were subsequently incubated with GST fusion proteins containing either the wild-type c-Cbl amino terminus (Cbl-N) or a mutant form of the amino terminus with an inactivating mutation in the SH2 domain (G306E). Immunoblotting was performed with the indicated antibodies. Equal loading of GST fusion proteins was assessed by Coomassie blue staining.
SLAP-2 inhibits TCR-mediated NFAT activation.
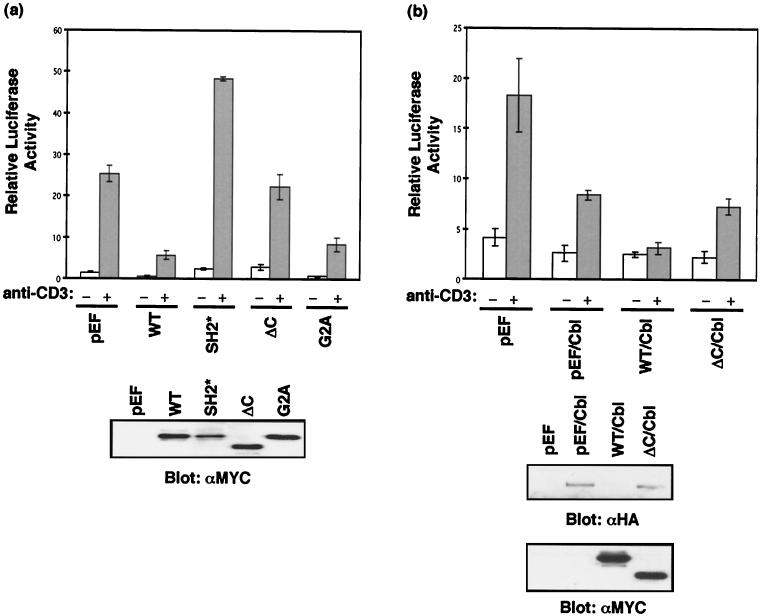
Activation of the TCR triggers an intracellular signaling cascade that leads to the activation of specific nuclear transcription factors and subsequent transcriptional up-regulation and expression of IL-2. NFAT is one of the transcription factors that is activated in response to TCR activation. To determine the effect of SLAP-2 on TCR signaling, we measured NFAT activation in the presence of overexpressed SLAP-2 or SLAP-2 mutants (G2A, SH2*, and ΔC) with a reporter gene consisting of the NFAT-responsive IL-2 promoter fused to the luciferase cDNA. The transient overexpression of wild-type SLAP-2 in Jurkat T cells blocked the anti-CD3-triggered activation of NFAT (Fig. 8a). Both the myristoylation mutant (G2A) and the SLAP-2 ΔC mutant had intermediate inhibitory effects compared to the wild-type protein, indicating that both c-Cbl binding and membrane localization are important for SLAP-2 function. In contrast, the SH2 mutant dramatically enhanced NFAT activation, suggesting that this mutant acts in a dominant-negative manner, blocking the inhibitory activity of endogenous SLAP-2.
FIG. 8.
SLAP-2 is a negative regulator of signaling from the TCR. (a) Inhibition of NFAT activation by SLAP-2 requires a functional SH2 domain and the c-Cbl binding region. Jurkat E6.1 cells were cotransfected with empty vector or SLAP-2 expression constructs as well as an NFAT-luciferase reporter construct. Relative NFAT activation was assessed in unstimulated (−) and anti-CD3 antibody-stimulated (+) cells. The data shown are representative of results obtained in at least three independent experiments. Error bars indicate standard deviations. Cell lysates were immunoblotted with anti-MYC antibody (9E10) to check relative levels of expression of the SLAP-2 constructs. WT, wild type. (b) Wild-type SLAP-2 augments Cbl-mediated inhibition of NFAT activation following TCR stimulation. Jurkat E6.1 cells were cotransfected with empty vector or c-Cbl or SLAP-2 expression constructs as well as an NFAT-luciferase reporter construct. The data shown are representative of results obtained in at least three independent experiments. Error bars indicate standard deviations. NFAT activation and levels of expression of MYC-tagged SLAP-2 were assessed as described for panel a. The expression of ectopically expressed HA-tagged c-Cbl was detected by immunoblotting with anti-HA antibody.
The overexpression of c-Cbl has also been shown to inhibit NFAT activation downstream of the activated TCR (34). Therefore, we tested whether SLAP-2 could influence c-Cbl-mediated down-regulation of TCR signaling. As previously reported, the expression of c-Cbl resulted in a significant decrease in NFAT activation in response to anti-CD3 stimulation (Fig. 8b). Cotransfection of c-Cbl with wild-type SLAP-2 further enhanced this effect, completely blocking TCR-mediated NFAT activation. Conversely, the SLAP-2 ΔC mutant, which does not interact with c-Cbl, did not enhance c-Cbl inhibitory activity. Interestingly, we have consistently observed a decrease in c-Cbl protein levels in cells overexpressing wild-type SLAP-2 (Fig. 8b, bottom panel). This decrease in c-Cbl protein expression is not observed in cells expressing the SLAP-2 ΔC mutant, suggesting that SLAP-2 association with c-Cbl may trigger its degradation.
SLAP-2 down-regulates ZAP-70 and CD3ɛ expression levels.
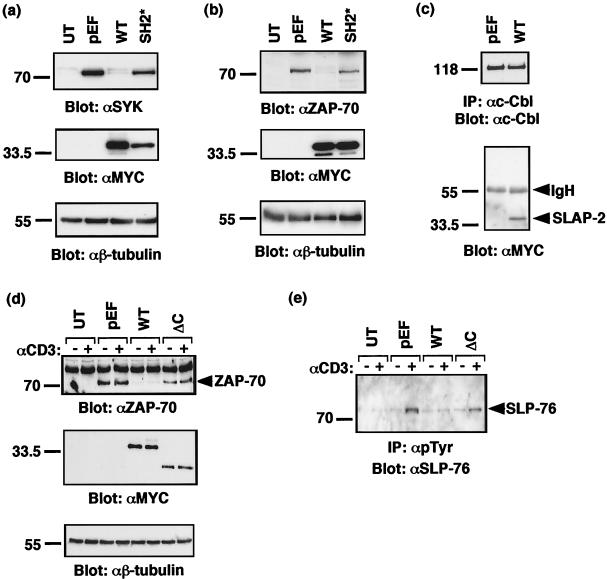
The mechanism by which c-Cbl carries out its inhibitory function in T cells relies on its ability to associate with the Y292 negative regulatory site in ZAP-70 (34). c-Cbl expression inhibits the activation and tyrosine phosphorylation of ZAP-70 and the related family member SYK, and in some cell types, c-Cbl reduces SYK and ZAP-70 protein levels (23, 34, 44). Similar to the effect of c-Cbl overexpression, cotransfection of SLAP-2 with either SYK or ZAP-70 caused a dramatic reduction in the levels of both proteins (Fig. 9a and b). This effect was specific to SLAP-2, since the equivalent expression of a SLAP-2 mutant (SH2*) which does not bind efficiently to ZAP-70 and which does not inhibit NFAT activation did not reduce the levels of the SYK and ZAP-70 proteins. We confirmed in this experiment that transfected SLAP-2 was bound to endogenous c-Cbl, as well as the related Cbl-b protein, suggesting that the effect of SLAP-2 on SYK and ZAP-70 likely is mediated through c-Cbl or Cbl-b (Fig. 9c and data not shown).
FIG. 9.
SLAP-2 expression promotes degradation of c-Cbl substrates SYK and ZAP-70. Mammalian expression constructs for SYK (a) or ZAP-70 (b) were cotransfected into COS-7 cells with either empty vector or expression constructs encoding MYC-tagged wild-type (WT) SLAP-2 and SH2 mutant (SH2*) SLAP-2. Lysates from transfected cells were subjected to SDS-PAGE and immunoblotted with either anti-SYK or anti-ZAP-70 (top panels), anti-MYC (middle panels), and anti-β-tubulin (bottom panels) antibodies. UT, untransfected. (c) Transfected SLAP-2 binds to endogenous c-Cbl in COS cells. A lysate from COS-7 cells transfected with MYC-tagged wild-type SLAP-2 was immunoprecipitated with anti-c-Cbl antibody. Membranes were immunoblotted with anti-c-Cbl (top panel) and anti-MYC (bottom panel) antibodies. IP, immunoprecipitation; IgH, immunoglobulin heavy chain. (d) Jurkat p116 T cells were either left untransfected (UT) or transfected with empty vector pEF or wild-type (WT) SLAP-2, ΔC mutant SLAP-2, or ZAP-70 expression constructs. Cells were either left unstimulated (−) or stimulated with anti-CD3 antibody (+). A 40-μg portion of whole-cell lysate was subjected to SDS-PAGE and immunoblotted with anti-ZAP-70 (top panel), anti-MYC (middle panel), and anti-β-tubulin (bottom panel) antibodies. (e) A 500-μg portion of lysate from the experiment described in panel d was immunoprecipitated with antiphosphotyrosine antibody and immunoblotted with anti-SLP-76 antibody.
Evidence that SLAP-2 facilitates ZAP-70 degradation was further demonstrated with Jurkat T cells. Jurkat p116 cells, deficient for the expression of ZAP-70, were cotransfected with either empty vector or SLAP-2 and ZAP-70. Similar to the observations made with COS cells, the expression of wild-type SLAP-2 resulted in the degradation of ZAP-70 protein. In contrast, the level of ZAP-70 remained unaffected in cells overexpressing the SLAP-2 ΔC mutant, suggesting that the ability of SLAP-2 to recruit c-Cbl is essential for the observed effect on ZAP-70. Correspondingly, the level of tyrosine phosphorylation of SLP-76, a major ZAP-70 substrate, was selectively decreased in cells overexpressing wild-type versus ΔC mutant SLAP-2 (Fig. 9d and e). We were unable to block the effect of SLAP-2 with proteasome inhibitors (data not shown). This finding is consistent with SLAP-2 activity being mediated through c-Cbl, since the down-regulation of activated receptor tyrosine kinases by c-Cbl seems to occur predominantly through lysosomal rather than proteasomal degradation.
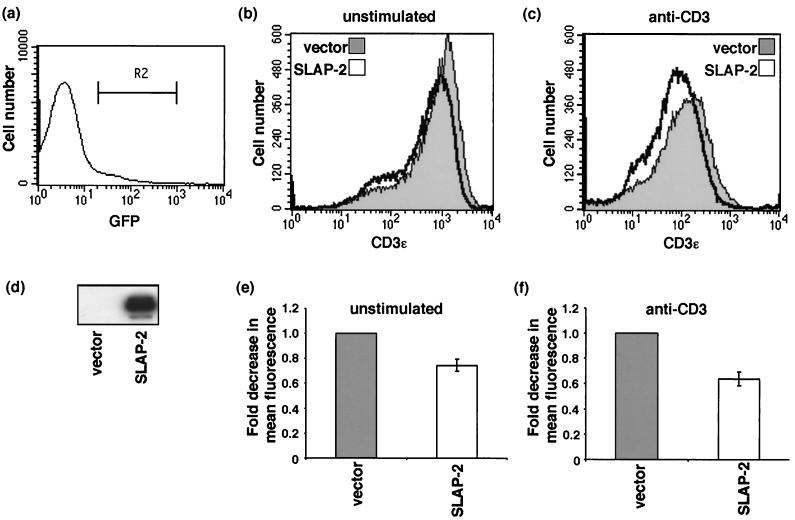
c-Cbl promotes the down-regulation of activated transmembrane receptor tyrosine kinases by promoting their ubiquitination (14, 17, 18). It is currently unknown whether c-Cbl regulates the trafficking of the activated TCR in addition to its role as a negative regulator of ZAP-70. However, c-Cbl-deficient thymocytes have increased levels of surface CD3, CD4, and CD69, suggesting that c-Cbl may also have an effect on receptor levels in T cells (28, 42). Since SLAP-2 inhibitory activity appears to be closely linked to its association with c-Cbl, we tested whether SLAP-2 expression affected surface CD3 levels on Jurkat T cells. Transient expression of wild-type SLAP-2 consistently resulted in a reduction in CD3ɛ levels, as assessed by mean fluorescence, compared with the results obtained for empty vector-transfected control cells (Fig. 10). SLAP-2 expression caused a reduction in surface CD3ɛ levels in both unstimulated and stimulated Jurkat T cells, although the effect was more pronounced following TCR activation. A decrease in the surface expression of TCR-αβ (∼30%) in cells overexpressing SLAP-2 was also observed (data not shown). These results suggest that SLAP-2 and c-Cbl could be part of a complex that regulates the internalization and trafficking of the TCR-CD3 complex.
FIG. 10.
SLAP-2 expression causes reduced surface expression of CD3ɛ in Jurkat T cells. (a) GFP-positive populations of transiently transfected Jurkat cells were gated as indicated (R2). GFP, green fluorescent protein. (b and c) Jurkat T cells were transiently transfected with either empty vector IRES-GFP (shading) or SLAP-2-IRES-GFP (no shading). After 16 h, cells were either left unstimulated (b) or stimulated with anti-CD3 antibody (c) for 1 h. Cells were stained with a phycoerythrin-labeled anti-CD3ɛ antibody and analyzed by flow cytometry. Histograms for green fluorescent protein-positive cells (R2 gated) were created by using CellQuest software. Representative data from one of four independent experiments are shown. (d) Expression of murine SLAP-2 in transiently transfected Jurkat T cells was confirmed by immunoprecipitation and Western blot analysis with affinity-purified anti-SLAP-2-C antibody. (e and f) Histograms displaying the fold decrease in mean fluorescence observed in four independent experiments for both unstimulated (e) and stimulated (f) conditions. Error bars indicate standard deviations.
DISCUSSION
We have cloned and characterized SLAP-2, an SH3 and SH2 domain-containing adaptor protein, which exhibits sequence and structural similarities to SLAP and to members of the Src family of tyrosine kinases. Two protein isoforms of SLAP-2, p28 and p25, are products of alternative translation initiation and are expressed in hematopoietic tissues and cell lines. SLAP-2 functions as an inhibitor of TCR-mediated signaling, and SLAP-2 activity is directly correlated with its binding to c-Cbl, a protein with E3 ubiquitin ligase activity. In keeping with this inhibitory function of SLAP-2, we found that it promotes both the degradation of SYK and ZAP-70 tyrosine kinases and the down-regulation of cell surface CD3ɛ.
Our results show that a functional SLAP-2 SH2 domain, the SLAP-2 carboxy terminus, and the membrane association of SLAP-2 are all required for the full inhibitory effects of SLAP-2. These results are both similar and contradictory to those reported for the related protein SLAP. SLAP overexpression in Jurkat T cells was similarly shown to inhibit TCR-mediated NFAT, AP-1, and IL-2 promoter activity, although only the SLAP SH2 and SH3 domains were found to be necessary and the carboxy terminus of SLAP was not required for inhibitory activity (40). In fibroblasts, SLAP has also been shown to associate with the activated PDGFR via its SH2 domain and to negatively regulate PDGFR-mediated DNA synthesis. In this context, both the SLAP SH2 domain and the carboxy-terminal region were important for the inhibitory effects of SLAP (25).
SLAP-2 and SLAP share both sequence and structural similarities, except in the carboxy-terminal region, where the amino acid sequences of these two proteins diverge. Despite the low level of sequence similarity, the carboxy-terminal regions of both proteins mediate interactions with c-Cbl. We have found that the interaction between SLAP-2 or SLAP and c-Cbl is constitutive in T cells and therefore is likely to be independent of the tyrosine phosphorylation status of c-Cbl. While the precise nature of the SLAP-2-Cbl interaction remains to be elucidated, we have shown that like that of SLAP, the SLAP-2 carboxy terminus associates in a phosphotyrosine-independent manner with the c-Cbl amino-terminal region, containing the four-helix bundle, the EF hand, and a variant SH2 domain. Further studies are required to identify the specific region within the Cbl amino terminus with which these proteins interact.
We have found that tyrosine phosphorylated ZAP-70 binds to SLAP-2 through the SLAP-2 SH2 domain. The similarity that exists between the SH3 and SH2 protein interaction modules in SLAP-2 and SLAP suggests that these proteins might associate with a similar set of proteins. Indeed, SLAP also associates with both SYK and ZAP-70 tyrosine kinases through its SH2 domain (40, 41). While inactivation of the SLAP-2 SH2 domain does not completely ablate the interaction between SLAP-2 and ZAP-70, it greatly diminishes the association between these two proteins. As c-Cbl also binds to Y292 on ZAP-70 via its TKB domain (24), the small amounts of ZAP-70 observed to associate with the SLAP-2 SH2 mutant might be indirectly recruited via c-Cbl. Alternatively, the partial binding of ZAP-70 might also involve the SH3 domain of SLAP-2. However, we have been unable to analyze SH3 mutant forms of SLAP-2 expressed in either bacteria or mammalian cells due to their instability. In addition to the interaction with ZAP-70, an inducible association between SLAP-2 and an unidentified protein, p72, appears to be mediated exclusively by the SLAP-2 SH2 domain. Interestingly, SLAP, but not SLAP-2, appears to specifically associate with the transmembrane adaptor protein LAT following anti-CD3 stimulation, highlighting a potentially important difference between these two closely related adaptors.
Overexpression of wild-type SLAP-2 in Jurkat T cells results in a marked reduction in the activation of NFAT following TCR activation. The ΔC mutant and, to a lesser degree, the myristoylation site mutant (G2A) of SLAP-2 are impaired in their ability to inhibit NFAT activation, indicating that both the membrane localization of SLAP-2 and its association with c-Cbl via its carboxy terminus are required for the full inhibitory effect. Interestingly, a mutant form of SLAP-2 possessing an inactivating point mutation in the SH2 domain (SH2*) appears to act in a dominant-negative manner, inhibiting endogenous SLAP-2 function and enhancing NFAT activation. The SLAP-2 SH2 mutant fails to interact with the unidentified 72-kDa phosphoprotein, is severely compromised in its ability to bind ZAP-70, but still binds to c-Cbl, suggesting that this mutant may engage c-Cbl in an inactive complex uncoupled from ZAP-70 and p72. The expression of SLAP-2 with c-Cbl can further decrease NFAT activation. This additive effect is dependent upon the presence of an intact c-Cbl binding region, suggesting that SLAP-2 and c-Cbl act in the same complex.
The identification of c-Cbl as an E3 ubiquitin ligase has provided further insight into the mechanisms that control the down-regulation of receptor-mediated signaling. c-Cbl has been implicated in the direct ubiquitination of activated growth factor receptors, such as epidermal growth factor receptor, CSF-1R, and PDGFR (14, 17, 27, 38, 47), regulating both internalization and trafficking from the recycling compartment to multivesicular bodies, the gateway to lysosomal destruction (10). c-Cbl is known to negatively regulate signaling from the TCR, and recent evidence has led to speculation that the mechanism underlying this activity may be due in part to ubiquitin-dependent alterations in TCR trafficking. Recently, it was shown that c-Cbl mediates the ubiquitination of TCR-ζ via its interaction with ZAP-70 (46). Furthermore, c-Cbl-deficient thymocytes have been reported to have up-regulated CD3, CD4, and CD69, suggesting that c-Cbl may also have an inhibitory effect on receptor levels in T cells. The TCR-CD3 complex is constitutively internalized and recycled back to the cell surface (2, 19). However, following TCR activation, a decrease in cell surface CD3 expression accompanies the down-regulation of signaling from the TCR-CD3 complex. It has been proposed that following activation, the internalized activated receptor complex is sorted to the lysosomal pathway for degradation rather than recycling back to the cell surface (19). Together, this evidence strongly suggests that c-Cbl regulates TCR down-regulation in a manner analogous to that reported for activated receptor tyrosine kinases. Interestingly, thymocytes from SLAP-deficient mice were reported to have elevated levels of surface TCR (39). We have found that the overexpression of SLAP-2 decreases cell surface CD3ɛ expression in Jurkat T cells. Collectively, these results suggest that the mechanism by which SLAPs act to negatively regulate signaling from the TCR involves their cooperation with c-Cbl to actively down-modulate the levels of the TCR-CD3 complex on the T-cell surface.
A number of adaptor proteins have been shown to bind to c-Cbl. Many of these, including CrkL, Grb2, Nck, and CAP, associate through either SH2- or SH3-mediated interactions with the c-Cbl carboxy terminus (43). We propose that SLAP-2 and SLAP represent a distinct class of c-Cbl-associated adaptors which associate with the Cbl-N domain and function in cooperation with c-Cbl, enhancing its ability to negatively regulate receptor-mediated signaling. This class may also include APS, which has been shown to facilitate c-Cbl-mediated down-regulation of both PDGFR and the insulin receptor (1, 49).
These adaptors could facilitate c-Cbl function via a number of mechanisms. In T cells, SLAP-2 may direct the localization of c-Cbl to the activated TCR complex or to specific membrane compartments, such as lipid rafts or endocytic vesicles. Targeting of c-Cbl to specific subcellular locations could, in turn, regulate substrate selection. The p28 form of SLAP-2 is anchored to cell membranes by myristoylation. In transiently transfected HeLa cells, we observed both plasma membrane and perinuclear staining in vesicular structures resembling recycling endosomes. As with SLAP, we also observed the colocalization of SLAP-2 with an endosomal marker in Jurkat T cells, supporting a role for these proteins in endocytosis. Whether SLAP-2 or SLAP influences the membrane compartmentalization of c-Cbl remains unknown. In contrast to p28 SLAP-2, we have shown that p25 SLAP-2, which lacks the amino-terminal myristoylation sequence, is cytosolic. Interestingly, the relative levels of expression of p25 and p28 SLAP-2 translation isoforms are variable. While the p28 isoform is consistently expressed at a higher level in cell lines, the expression of the p25 isoform is higher than that of the p28 isoform in the thymus. The consequences of fluctuations in the relative levels of expression of these two isoforms remain unknown; however, p25 SLAP-2 could target c-Cbl to a different subcellular location or antagonize the function of p28.
In addition to a potential role in localizing c-Cbl to the activated TCR and in close proximity to its substrates, SLAP-2 could alternatively participate in substrate recognition by the formation of a ternary complex involving c-Cbl and a substrate molecule. A mechanism for c-Cbl substrate recognition requiring two separate interactions was recently described. c-Cbl-induced ubiquitination of Met requires both indirect binding through the adaptor protein Grb2 that promotes c-Cbl tyrosine phosphorylation and direct binding of the c-Cbl SH2 domain to tyrosine 1003 in the juxtamembrane region of Met (32). Through its SH2 domain, c-Cbl associates with and down-regulates the SYK family tyrosine kinases SYK and ZAP-70. We have shown that SLAP-2 is capable of associating independently with both ZAP-70 and c-Cbl following TCR stimulation. Subsequently, we have determined that SLAP-2 functions in a manner analogous to that of c-Cbl, causing the specific degradation of both ZAP-70 and SYK. In T cells, the observed decrease in the level of ZAP-70 protein is dependent upon the ability of SLAP-2 to associate with c-Cbl. The formation of a stabilized complex involving SLAP-2, c-Cbl, and ZAP-70 thus may promote the ubiquitination of ZAP-70 as well as components of the TCR, such as TCR-ζ.
Although SLAP-2 and SLAP share some structural and functional similarities and may function by a common mechanism, there are also a number of indications that these proteins have distinct biologic activities. While both SLAP-2 and SLAP are expressed in thymocytes, SLAP-2 does not appear to fully compensate for SLAP deficiency. This finding might reflect differences in the levels of expression during T-cell development. Interestingly, SLAP-2 is abundantly expressed in Jurkat T cells, while SLAP is undetectable. In in vitro binding experiments, we have observed an inducible association between SLAP and the transmembrane adaptor protein LAT, as was previously reported (39, 40), while no interaction between SLAP-2 and LAT was observed. Furthermore, we have also detected SLAP-2 expression in myeloid cell lines (M. P. Loreto et al., submitted for publication). SLAP expression in myeloid cells has not been reported. Finally, another distinguishing feature is the expression of a cytoplasmic SLAP-2 isoform generated by alternative translation initiation. Further comparative studies will be required to delineate the specific functions of these two protein isoforms.
We have described the cloning and characterization of the adaptor protein SLAP-2 and have demonstrated its function in the c-Cbl-mediated negative regulation of T-cell receptor signaling. During the preparation of this manuscript, Holland et al. reported the identification of human SLAP-2 as a negative regulator of signaling downstream of the activated B-cell antigen receptor (11). Collectively, these data support a negative regulatory role for SLAP-2 in antigen receptor signal transduction. Our findings that SLAP-2 promotes the degradation of the SYK and ZAP-70 tyrosine kinases and decreases the levels of CD3 at the T-cell surface suggest a mechanism by which SLAP-2 functions to negatively regulate antigen receptor signaling. We propose that SLAP-2 and the related protein SLAP act in concert with c-Cbl to down-regulate signaling by promoting the lysosomal targeting and degradation of activated antigen receptor complexes.
Acknowledgments
We thank Hamid Band and Morag Park for c-Cbl reagents, Gary Koretzky for SLP-76 antibody, and Andre Veillette for SYK and ZAP-70 cDNAs and antiserum. We also thank Sheyun Zhao and Sascha Dho for technical assistance with the flow cytomety and confocal microscopy, Alec Cheng and Cindy Guidos for helpful discussions, and Dwayne Barber and Paul D. Simoncic for comments on the manuscript.
Michael P. Loreto is a recipient of a scholarship from the Natural Science and Engineering Research Council of Canada (NSERC). C. Jane McGlade is a research scientist of the National Cancer Institute of Canada. This work was supported by grants from the Canadian Institutes of Health Research and the Leukemia Research Fund of Canada.
REFERENCES
- 1.Ahmed, Z., B. J. Smith, and T. S. Pillay. 2000. The APS adapter protein couples the insulin receptor to the phosphorylation of c-Cbl and facilitates ligand-stimulated ubiquitination of the insulin receptor. FEBS Lett. 475:31-34. [DOI] [PubMed]
- 2.Alcover, A., and B. Alarcon. 2000. Internalization and intracellular fate of TCR-CD3 complexes. Crit. Rev. Immunol. 20:325-346. [PubMed] [Google Scholar]
- 3.Berry, D. M., S. J. Benn, A. M. Cheng, and C. J. McGlade. 2001. Caspase-dependent cleavage of the hematopoietic specific adaptor protein Gads alters signalling from the T cell receptor. Oncogene 20:1203-1211. [DOI] [PubMed] [Google Scholar]
- 4.Brdicka, T., D. Pavlistova, A. Leo, E. Bruyns, V. Korinek, P. Angelisova, J. Scherer, A. Shevchenko, I. Hilgert, J. Cerny, K. Drbal, Y. Kuramitsu, B. Kornacker, V. Horejsi, and B. Schraven. 2000. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J. Exp. Med. 191:1591-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantrell, D. 1996. T cell antigen receptor signal transduction pathways. Annu. Rev. Immunol. 14:259-274. [DOI] [PubMed] [Google Scholar]
- 6.Chow, L. M., M. Fournel, D. Davidson, and A. Veillette. 1993. Negative regulation of T-cell receptor signalling by tyrosine protein kinase p50csk. Nature 365:156-160. [DOI] [PubMed] [Google Scholar]
- 7.Clements, J. L., N. J. Boerth, J. R. Lee, and G. A. Koretzky. 1999. Integration of T cell receptor-dependent signaling pathways by adapter proteins. Annu. Rev. Immunol. 17:89-108. [DOI] [PubMed] [Google Scholar]
- 8.Donovan, J. A., Wange, R. L., Langdon, W. Y., and Samelson, L. E. 1994. The protein product of the c-cbl protooncogene is the 120-kDa tyrosine-phosphorylated protein in Jurkat cells activated via the T cell antigen receptor. J. Biol. Chem. 269:22921-22924. [PubMed] [Google Scholar]
- 9.Fournel, M., D. Davidson, R. Weil, and A. Veillette. 1996. Association of tyrosine protein kinase Zap-70 with the protooncogene product p120c-cbl in T lymphocytes. J. Exp. Med. 183:301-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hicke, L. 2001. A new ticket for entry into budding vesicles-ubiquitin. Cell 106:527-530. [DOI] [PubMed] [Google Scholar]
- 11.Holland, S. J., X. C. Liao, M. K. Mendenhall, X. Zhou, J. Pardo, P. Chu, C. Spencer, A. Fu, N. Sheng, P. Yu, E. Pali, A. Nagin, M. Shen, S. Yu, E. Chan, X. Wu, C. Li, M. Woisetschlager, G. Aversa, F. Kolbinger, M. K. Bennett, S. Molineaux, Y. Luo, D. G. Payan, H. S. Mancebo, and J. Wu. 2001. Functional cloning of Src-like adapter protein-2 (SLAP-2), a novel inhibitor of antigen receptor signaling. J. Exp. Med. 194:1263-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joazeiro, C. A., S. S. Wing, H. Huang, J. D. Leverson, T. Hunter, and Y. C. Liu. 1999. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science 286:309-312. [DOI] [PubMed] [Google Scholar]
- 13.Latour, S., and A. Veillette. 2001. Proximal protein tyrosine kinases in immunoreceptor signaling. Curr. Opin. Immunol. 13:299-306. [DOI] [PubMed] [Google Scholar]
- 14.Lee, P. S., Y. Wang, M. G. Dominguez, Y. G. Yeung, M. A. Murphy, D. D. Bowtell, and E. R. Stanley. 1999. The Cbl protooncoprotein stimulates CSF-1 receptor multiubiquitination and endocytosis, and attenuates macrophage proliferation. EMBO J. 18:3616-3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemay, S., D. Davidson, S. Latour, and A. Veillette. 2000. Dok-3, a novel adapter molecule involved in the negative regulation of immunoreceptor signaling. Mol. Cell. Biol. 20:2743-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leo, A., and B. Schraven. 2001. Adapters in lymphocyte signalling. Curr. Opin. Immunol. 13:307-316. [DOI] [PubMed] [Google Scholar]
- 17.Levkowitz, G., H. Waterman, S. A. Ettenberg, M. Katz, A. Y. Tsygankov, I. Alroy, S. Lavi, K. Iwai, Y. Reiss, A. Ciechanover, S. Lipkowitz, and Y. Yarden. 1999. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol. Cell 4:1029-1040. [DOI] [PubMed] [Google Scholar]
- 18.Levkowitz, G., H. Waterman, E. Zamir, Z. Kam, S. Oved, W. Y. Langdon, L. Beguinot, B. Geiger, and Y. Yarden. 1998. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 12:3663-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, H., M. Rhodes, D. L. Wiest, and D. A. Vignali. 2000. On the dynamics of TCR:CD3 complex cell surface expression and downmodulation. Immunity 13:665-675. [DOI] [PubMed] [Google Scholar]
- 20.Liu, S. K., N. Fang, G. A. Koretzky, and C. J. McGlade. 1999. The hematopoietic-specific adaptor protein Gads functions in T-cell signaling via interactions with the SLP-76 and LAT adaptors. Curr. Biol. 9:67-75. [DOI] [PubMed] [Google Scholar]
- 21.Liu, S. K., and C. J. McGlade. 1998. Gads is a novel SH2 and SH3 domain-containing adaptor protein that binds to tyrosine-phosphorylated Shc. Oncogene 17:3073-3082. [DOI] [PubMed] [Google Scholar]
- 22.Lupher, M. L., Jr., N. Rao, M. J. Eck, and H. Band. 1999. The Cbl protooncoprotein: a negative regulator of immune receptor signal transduction. Immunol. Today 20:375-382. [DOI] [PubMed] [Google Scholar]
- 23.Lupher, M. L., Jr., N. Rao, N. L. Lill, C. E. Andoniou, S. Miyake, E. A. Clark, B. Druker, and H. Band. 1998. Cbl-mediated negative regulation of the Syk tyrosine kinase. A critical role for Cbl phosphotyrosine-binding domain binding to Syk phosphotyrosine 323. J. Biol. Chem. 273:35273-35281. [DOI] [PubMed] [Google Scholar]
- 24.Lupher, M. L., Jr., Z. Songyang, S. E. Shoelson, L. C. Cantley, and H. Band. 1997. The Cbl phosphotyrosine-binding domain selects a D(N/D)XpY motif and binds to the Tyr292 negative regulatory phosphorylation site of ZAP-70. J. Biol. Chem. 272:33140-33144. [DOI] [PubMed] [Google Scholar]
- 25.Manes, G., P. Bello, and S. Roche. 2000. Slap negatively regulates Src mitogenic function but does not revert Src-induced cell morphology changes. Mol. Cell. Biol. 20:3396-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng, W., S. Sawasdikosol, S. J. Burakoff, and M. J. Eck. 1999. Structure of the amino-terminal domain of Cbl complexed to its binding site on ZAP-70 kinase. Nature 398:84-90. [DOI] [PubMed] [Google Scholar]
- 27.Miyake, S., K. P. Mullane-Robinson, N. L. Lill, P. Douillard, and H. Band. 1999. Cbl-mediated negative regulation of platelet-derived growth factor receptor-dependent cell proliferation. A critical role for Cbl tyrosine kinase-binding domain. J. Biol. Chem. 274:16619-16628. [DOI] [PubMed] [Google Scholar]
- 28.Murphy, M. A., R. G. Schnall, D. J. Venter, L. Barnett, I. Bertoncello, C. B. Thien, W. Y. Langdon, and D. D. Bowtell. 1998. Tissue hyperplasia and enhanced T-cell signaling via ZAP-70 in c-Cbl-deficient mice. Mol. Cell. Biol. 18:4872-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okada, M., S. Nada, Y. Yamanashi, T. Yamamoto, and H. Nakagawa. 1991. CSK: a protein-tyrosine kinase involved in regulation of src family kinases. J. Biol. Chem. 266:24249-24252. [PubMed] [Google Scholar]
- 30.Ota, S., K. Hazeki, N. Rao, M. L. Lupher, Jr., C. E. Andoniou, B. Druker, and H. Band. 2000. The RING finger domain of Cbl is essential for negative regulation of the Syk tyrosine kinase. J. Biol. Chem. 275:414-422. [DOI] [PubMed] [Google Scholar]
- 31.Pandey, A., Duan, H., and Dixit Vm. 1995. Characterization of a novel Src-like adapter protein that associates with the Eck receptor tyrosine kinase. J. Biol. Chem. 270:19201-19204. [DOI] [PubMed] [Google Scholar]
- 32.Peschard, P., T. M. Fournier, L. Lamorte, M. A. Naujokas, H. Band, W. Y. Langdon, and M. Park. 2001. Mutation of the c-Cbl TKB domain binding site on the Met receptor tyrosine kinase converts it into a transforming protein. Mol. Cell 8:995-1004. [DOI] [PubMed] [Google Scholar]
- 33.Pfrepper, K. I., A. Marie-Cardine, L. Simeoni, Y. Kuramitsu, A. Leo, J. Spicka, I. Hilgert, J. Scherer, and B. Schraven. 2001. Structural and functional dissection of the cytoplasmic domain of the transmembrane adaptor protein SIT (SHP2-interacting transmembrane adaptor protein). Eur. J. Immunol. 31:1825-1836. [DOI] [PubMed] [Google Scholar]
- 34.Rao, N., M. L. Lupher, Jr., S. Ota, K. A. Reedquist, B. J. Druker, and H. Band. 2000. The linker phosphorylation site Tyr292 mediates the negative regulatory effect of Cbl on ZAP-70 in T cells. J. Immunol. 164:4616-4626. [DOI] [PubMed] [Google Scholar]
- 35.Roche, S., G. Alonso, A. Kazlauskas, V. M. Dixit, S. A. Courtneidge, and A. Pandey. 1998. Src-like adaptor protein (Slap) is a negative regulator of mitogenesis. Curr. Biol. 8:975-978. [DOI] [PubMed] [Google Scholar]
- 36.Rudd, C. E. 1999. Adaptors and molecular scaffolds in immune cell signaling. Cell 96:5-8. [DOI] [PubMed] [Google Scholar]
- 37.Rudd, C. E., and H. Schneider. 2000. Lymphocyte signaling: Cbl sets the threshold for autoimmunity. Curr. Biol. 10:R344-R347. [DOI] [PubMed]
- 38.Sinha, S., J. Jancarik, V. Roginskaya, K. Rothermund, L. M. Boxer, and S. J. Corey. 2001. Suppression of apoptosis and granulocyte colony-stimulating factor-induced differentiation by an oncogenic form of Cbl. Exp. Hematol. 29:746-755. [DOI] [PubMed] [Google Scholar]
- 39.Sosinowski, T., N. Killeen, and A. Weiss. 2001. The Src-like adaptor protein downregulates the T cell receptor on CD4+CD8+ thymocytes and regulates positive selection. Immunity 15:457-466. [DOI] [PubMed] [Google Scholar]
- 40.Sosinowski, T., A. Pandey, V. M. Dixit, and A. Weiss. 2000. Src-like adaptor protein (SLAP) is a negative regulator of T cell receptor signaling. J. Exp. Med. 191:463-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang, J., S. Sawasdikosol, J. H. Chang, and S. J. Burakoff. 1999. SLAP, a dimeric adapter protein, plays a functional role in T cell receptor signaling. Proc. Natl. Acad. Sci. USA 96:9775-9780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thien, C. B., D. D. Bowtell, and W. Y. Langdon. 1999. Perturbed regulation of ZAP-70 and sustained tyrosine phosphorylation of LAT and SLP-76 in c-Cbl-deficient thymocytes. J. Immunol. 162:7133-7139. [PubMed] [Google Scholar]
- 43.Thien, C. B., and W. Y. Langdon. 2001. Cbl: many adaptations to regulate protein tyrosine kinases. Nat. Rev. Mol. Cell. Biol. 2:294-307. [DOI] [PubMed] [Google Scholar]
- 44.van Leeuwen, J. E., P. K. Paik, and L. E. Samelson. 1999. The oncogenic 70Z Cbl mutation blocks the phosphotyrosine binding domain-dependent negative regulation of ZAP-70 by c-Cbl in Jurkat T cells. Mol. Cell. Biol. 19:6652-6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Leeuwen, J. E., and L. E. Samelson. 1999. T cell antigen-receptor signal transduction. Curr. Opin. Immunol. 11:242-248. [DOI] [PubMed] [Google Scholar]
- 46.Wang, H. Y., Y. Altman, D. Fang, C. Elly, Y. Dai, Y. Shao, and Y. C. Liu. 2001. Cbl promotes ubiquitination of the T cell receptor zeta through an adaptor function of Zap-70. J. Biol. Chem. 276:26004-26011. [DOI] [PubMed] [Google Scholar]
- 47.Waterman, H., G. Levkowitz, I. Alroy, and Y. Yarden. 1999. The RING finger of c-Cbl mediates desensitization of the epidermal growth factor receptor. J. Biol. Chem. 274:22151-22154. [DOI] [PubMed] [Google Scholar]
- 48.Weiss, A., and D. R. Littman. 1994. Signal transduction by lymphocyte antigen receptors. Cell 76:263-274. [DOI] [PubMed] [Google Scholar]
- 49.Yokouchi, M., T. Wakioka, H. Sakamoto, H. Yasukawa, S. Ohtsuka, A. Sasaki, M. Ohtsubo, M. Valius, A. Inoue, S. Komiya, and A. Yoshimura. 1999. APS, an adaptor protein containing PH and SH2 domains, is associated with the PDGF receptor and c-Cbl and inhibits PDGF-induced mitogenesis. Oncogene 18:759-767. [DOI] [PubMed] [Google Scholar]
- 50.Zhang, W., J. Sloan-Lancaster, J. Kitchen, R. P. Trible, and L. E. Samelson. 1998. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 92:83-92. [DOI] [PubMed] [Google Scholar]
- 51.Zheng, N., P. Wang, P. D. Jeffrey, and N. P. Pavletich. 2000. Structure of a c-Cbl-UbcH7 complex: RING domain function in ubiquitin-protein ligases. Cell 102:533-539. [DOI] [PubMed] [Google Scholar]