Abstract
M-cadherin is a classical calcium-dependent cell adhesion molecule that is highly expressed in developing skeletal muscle, satellite cells, and cerebellum. Based on its expression pattern and observations in cell culture, it has been postulated that M-cadherin may be important for the fusion of myoblasts to form myotubes, the correct localization and function of satellite cells during muscle regeneration, and the specialized architecture of adhering junctions in granule cells of cerebellar glomeruli. In order to investigate the potential roles of M-cadherin in vivo, we generated a null mutation in mice. Mutant mice were viable and fertile and showed no gross developmental defects. In particular, the skeletal musculature appeared essentially normal. Moreover, muscle lesions induced by necrosis were efficiently repaired in mutant mice, suggesting that satellite cells are present, can be activated, and are able to form new myofibers. This was also confirmed by normal growth and fusion potential of mutant satellite cells cultured in vitro. In the cerebellum of M-cadherin-lacking mutants, typical contactus adherens junctions were present and similar in size and numbers to the equivalent junctions in wild-type animals. However, the adhesion plaques in the cerebellum of these mutants appeared to contain elevated levels of N-cadherin compared to wild-type animals. Taken together, these observations suggest that M-cadherin in the mouse serves no absolutely required function during muscle development and regeneration and is not essential for the formation of specialized cell contacts in the cerebellum. It seems that N-cadherin or other cadherins can largely compensate for the lack of M-cadherin.
Classical cadherins constitute a family of transmembrane proteins that mediate homophilic cell-cell interactions in a calcium-dependent fashion. Individual members of the cadherin family usually exhibit distinct and regulated tissue distribution during development and are thought to control morphogenetic processes, such as the separation of cell layers, epithelial-mesenchymal transitions, and condensation or dispersion of cells (17, 18, 32, 34).
M-cadherin has been identified in skeletal muscle cell lines and in developing and regenerating muscle, hence its name (5, 13, 19, 24, 28). In postnatal mature myofibers and after completion of myotube formation in vitro, M-cadherin expression is downregulated, but the protein remains present in quiescent satellite cells (4, 23, 25), although M-cadherin transcripts can only be detected in a small subset of resting satellite cells (11). M-cadherin protein in myoblasts appears evenly spread throughout the plasma membrane and not junction bound; however, it becomes clustered at distinct membrane locations of fetal myofibers when the basal lamina is forming (28). This observation has been interpreted as an indication for the possible involvement of M-cadherin in determining the correct location of satellite cells along the muscle fiber (19, 21, 37). In activated satellite cells of regenerating muscle, M-cadherin expression is markedly induced, suggesting a potential function in the repair process (19).
Based on the preferential expression of M-cadherin in developing skeletal muscle and in satellite cells, it has been postulated that M-cadherin may be essential for the fusion of myoblast to multinucleated myofibers (20, 37). This hypothesis has been supported by the observation that overexpression of M-cadherin in cadherin-deficient mouse fibroblasts that fail to adhere to each other results in enhanced calcium-dependent cell adhesion, an obvious prerequisite for cell fusion (23). Additionally, synthetic peptides that bind to the extracellular interaction domain of M-cadherin and block homophilic cell-cell interactions seem to inhibit myoblast fusion in a dose-dependent manner but do not affect biochemical differentiation of myoblasts, such as the expression of muscle-specific genes (37).
The cytoplasmic domain of M-cadherin, like that of other classical cadherins, is associated with the actin cytoskeleton via complexation with α- and β-catenins and plakoglobin (9, 23). Specifically in muscle cells, the M-cadherin/catenin complex also seems be able to interact with the microtubular system, suggesting that it is involved in the correct longitudinal alignment of myoblasts during myotube formation (20). These observations in cell culture and the expression pattern in vivo led to the current hypothesis that M-cadherin may play a role in muscle development and regeneration.
An additional function of M-cadherin in the cerebellum of mice and rats may be inferred from the finding that M-cadherin protein is also present in specialized adherence junctions, referred to as contactus adherens, in the granular cell layer of the cerebellar glomerulus (3, 27). These small plaque-bearing junctions are part of complex synapse terminals connecting granular cells with mossy fibers that originate from neurons in different areas of the pons, the medulla oblongata, the spinal cord, and γ-aminobutyratergic Golgi cells.
In order to examine the specific role of M-cadherin in skeletal muscle development, muscle regeneration, and cerebellar adhering junctions, we generated an M-cadherin-deficient mouse by targeted gene disruption. Unexpectedly, we found that M-cadherin is apparently not required for normal skeletal muscle development. Moreover, mice lacking M-cadherin are perfectly able to regenerate damaged muscle. Furthermore, satellite cells isolated from homozygous M-cadherin-lacking mutant mice proliferate and form myotubes in culture indistinguishably from satellite cells isolated from heterozygous or wild-type mice. Contactus adherens junctions are also present in the cerebellum of M-cadherin-negative mutants and are similar in number and size to the M-cadherin-bearing junctions in wild-type mice. Interestingly, adherens plaques in the mutant seem to contain increased amounts of N-cadherin, suggesting that this molecule may substitute for M-cadherin.
MATERIALS AND METHODS
Construction of the targeting vector, electroporation of ES cells, and generation of mutant mice.
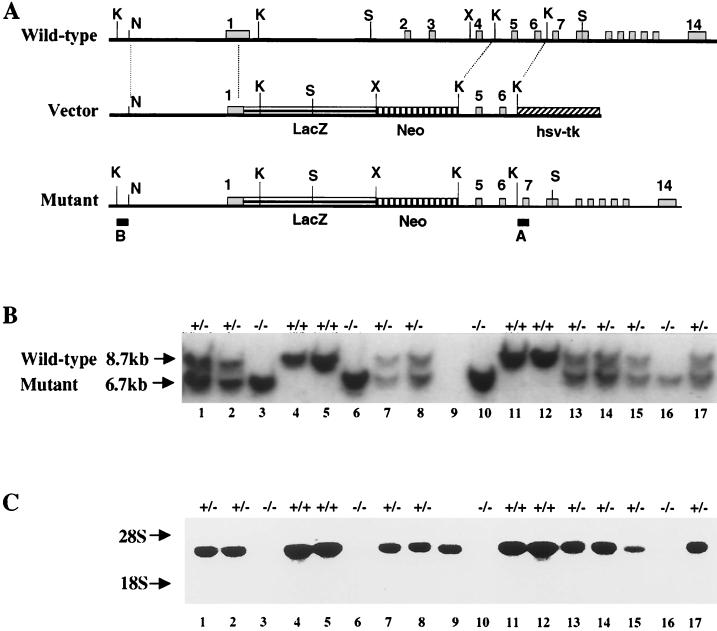
The M-cadherin gene was isolated from a 129/Ola genomic cosmid library (Resource Center/Primary Database Berlin, library no. 121) using the complete cDNA as the probe. Several overlapping cosmid clones were obtained, spanning about 26 kb of the locus. A 4.6-kb NruI-KpnI fragment containing 5′ upstream sequences and part of exon 1 (up to the AUG start codon) was cloned in frame with the nls-lacZ gene isolated from vector pPD46.21 (14). The combined fragment was cut out with NruI and NotI restriction enzymes, blunt ended, and inserted into the blunt-ended XhoI site of the transfer vector pPNT (33). A 1.8-kb KpnI fragment encompassing most of intron 4 and exons 5 and 6 was inserted downstream of the phosphoglycerate kinase (PGK)-neomycin resistance cassette into the corresponding restriction site of the pPNT vector to generate the right arm for homologous recombination.
Growth of the embryonic stem (ES) cell line J1, electroporation of the vector, and selection of recombinant clones were performed as described previously (7). For genotyping, DNA was digested with SacI and probed with the 5′-flanking 430-bp KpnI-XhoI fragment on Southern blots. The 6.7- and 8.7-kb SacI fragments are indicative of mutant and wild-type alleles, respectively. Five independent homologous recombination events were detected among 2,900 isolated ES cell clones. Upon injection into C57BL/6 mouse blastocysts, two ES cell clones carrying the mutation yielded chimeras that transmitted the mutation to their offspring. Mouse mutants were established in 129 and C57BL/6 strains without apparent phenotypic differences.
Induction of skeletal muscle regeneration.
Mice at 3 months of age were anaesthetized with 17 μl of a 25-mg/ml Avertin solution (Sigma-Aldrich) per g of body weight. Muscle regeneration was induced by unilateral injection of 0.1 ml of 10 μM cardiotoxin (Calbiochem) dissolved in phosphate-buffered saline (PBS) into the hind limb muscle (16). The contralateral muscle was injected with PBS alone as a control. The soleus and gastrocnemius muscles were dissected at various times after the injection (6 h to 20 days) and prepared for cryostat sections, RNA analysis (reverse transcription [RT]-PCR or Northern blot), and Western blot analysis.
Culture of isolated satellite cells.
Satellite cells were isolated from 3-month-old male wild-type, heterozygous, and homozygous M-cadherin mutant mice by methods described previously (1, 36). Briefly, hind limb muscles were excised, trimmed of fat and connective tissue, worked to a pulp with scissors, and digested with 1.25 mg of protease type XIV (Sigma) per ml at 37°C for 45 min. The suspension was triturated every 15 min, filtered through 50- and 200-mesh sieves, and centrifuged at 1,500 × g for 15 min. The cell pellet was resuspended in satellite cell medium (Dulbecco's modified Eagle's medium [DMEM], 20% fetal calf serum [FCS] supplemented with 5 ng of heparin Σ, 10 ng of human growth factor [R&D Systems], 200 U of penicillin per ml, 200 μg of streptomycin per ml, 0.2 mM glutamine, 0.002% amphotericin B [Fungizone]) and preplated on tissue culture plates for 3 h to remove fibroblasts. Satellite cells remaining in the supernatant were plated on collagen-coated cell culture dishes. After 48 h, bovine fetal growth factor (5 ng/ml) was added to the medium. For induction of differentiation, 1.2 × 105 satellite cells were plated per 35-mm dish for 24 h when differentiation medium (DMEM, 5% horse serum, 200 U of penicillin per ml, 200 μg of streptomycin per ml, 0.2 mM glutamine, 0.002% amphotericin B) was applied. Cells were analyzed after 1, 2, 3, 4, and 5 days in differentiation medium.
Immunohistochemistry and staining for LacZ activity.
For β-galactosidase staining, whole embryos, cryosections of tissues, or cultivated satellite cells were fixed with 0.2% glutaraldehyde, permeabilized with 0.01% sodium desoxycholate and 0.02% Nonidet P-40, and stained with 0.5 mg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal)-10 mM K3[Fe(CN)6]-10 mM K4[Fe(CN)6]. Occasionally sections were counterstained with hematoxylin and eosin. For immunohistochemical stainings, cells were refixed with acetone for 10 min at −20°C and blocked with 0.1% bovine serum albumin-20% fetal calf serum-1.5% goat serum in PBS containing 0.1% Triton X-100. Polyclonal anti-M-cadherin serum (37), monoclonal antidesmin antibody (MAbD3; Developmental Studies Hybridoma Bank) (12), and the monoclonal antibody MF 20 against sarcomeric major histocompatibility complex (2) were used. Antibody binding was detected by using the avidin-biotin peroxidase system (Vectastain ABC kit; Vector Laboratories).
RNA isolation, Northern blot analysis, and RT-PCR.
Total RNA was prepared from cell lines or tissues using the RNA-Clean isolation system (AGS GmbH). For Northern blot analysis, samples of 10 μg of RNA were separated electrophoretically on 0.8% agarose gels containing 2.2 M formaldehyde and 1× RNA-borate buffer. Gels were stained with ethidium bromide to ensure equal loading and blotted onto Genescreen-plus nylon membranes (NEN Life Science Products). Prehybridization and hybridization were carried out in Church buffer (250 mM Na2HPO4 [pH 7], 7% sodium dodecyl sulfate [SDS]) at 65°C. As the M-cadherin-specific hybridization probe, the EcoRV/SpeI restriction fragment of the cDNA (13), representing the 3′ sequence from nucleotides 1271 to 2375, was used. Probes were labeled with a random prime labeling kit (Prime-itII; Stratagene) with [α-32P]dCTP. Washed membranes were exposed to Kodak XAR films at −80°C with an intensifying screen.
Each cDNA for RT-PCR analysis was synthesized from 2 μg of total RNA (avian myeloblastosis virus reverse transcriptase; Promega) in a 25-μl reaction volume; 2.5 μl of the 1:4 diluted cDNA was then used as the template for PCR in a 25-μl reaction volume, including 25 pmol of each primer, 1× Taq buffer, 200 nM deoxynucleoside triphosphates, and 1.25 U of Taq polymerase (Takara Biomedicals). PCR products were analyzed on 2% agarose gels. Primer sequences were as described previously (10, 11). The primer for Myf-5 was 5′-TGCCATCCGCTACATTGAGAG, 3′-CCGGGGTAGCAGGCTGTGATTG; that for myogenin was 5′-GGGCCCCTGGAAGAAAAG, 3′-AGGAGGCGCTGTGGGAGTT; that for MyoD was 5′-GCCCGCGCTCCAACTGCTCTGAT, 3′-CCTACTGTGGTGCGCCCTCTGC; that for MRF4 was 5′-CTGCGCGAAAGGAGGAGACTAAAG, 3′-ATGGAAGAAAGGCGCTGAAGACTG; and that for M-cadherin was 5′-CCACAAACGCCTCCCCTACCCACTT, 3′-TCGTCGATGCTGAAGAACTCAGGGC.
Western blot analysis.
Protein extracts were prepared from whole cells and tissues in radioimmunoprecipitation assay (RIPA) buffer (1× PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) containing protease inhibitors (Boehringer Mannheim). Cell lysates were separated on SDS-7.5% polyacrylamide gels and electrophoretically transferred onto polyvinylidene difluoride microporous membranes (Immobilon-P; Millipore Corp., Bedford, Mass.) at 0.8 mA/cm2 for 2 h under semidry conditions in transfer buffer that contained 50 mM Tris, 40 mM glycine, 375 mg of SDS per ml, and 20% (vol/vol) methanol. Equal gel loading was monitored by protein staining with Ponceau S and quantification by densitometry using the Aida 2.1 software package.
Blots were then blocked with 5% (wt/vol) nonfat dry milk in TBST (20 mM Tris-HCl [pH 7.5], 0.9% NaCl, 0.05% [vol/vol] Tween 20) for 1 h prior to incubation with primary monoclonal mouse antibodies (Transduction Laboratories Zonula Adherens sampler kit) or with primary polyclonal M-cadherin rabbit antiserum (gift of Anna Starzinski-Powitz). This serum recognizes the following epitopes within the M-cadherin according to peptide scanning analysis (data not shown): amino acids (aa) 88 to 96 in EC1; aa 154 to 162, aa 160 to 168, aa 244 to 255, and aa 256 to 264 in EC2; and aa 265 to 273 and aa 283 to 294 in EC3. Weak interaction was also found with aa 757 to 771 within the cytoplasmic domain. Antibodies were diluted according to the manufacturer's instructions in TBST containing 5% (wt/vol) nonfat dry milk. Incubations were performed for 1 h. Blots were then washed six times in TBST and subsequently incubated for 1 h with secondary anti-mouse or anti-rabbit immunoglobulin antibody conjugated with horseradish peroxidase at 1:2,500 dilution (using 5% [wt/vol] nonfat dry milk in TBST). Blots were washed four times in TBST and two times in TBS prior to visualizing signals with the ECL system (Amersham Pharmacia Biotech). Molecular weights of proteins were determined according to stained size standards (Bio-Rad).
Immunofluorescence microscopy.
Cerebella of wild-type mice as well as of heterozygous (+/−) and homozygous M-cadherin-deficient (−/−) mutants were snap-frozen in isopentane precooled in liquid nitrogen, and 5-μm-thick cryostat sections were fixed with acetone at −20°C for 10 min and incubated with rabbit and goat sera specific for M-cadherin (rabbit sera were kindly provided by Anna Starzinski-Powitz, University of Frankfurt, Frankfurt am Main, Germany). Polyclonal rabbit antibodies against N-, P-, and E-cadherins and p120 were from Transduction Laboratories via Dianova (Hamburg, Germany). Monoclonal murine antibodies against α- and β-catenin were from the same source. Rabbit antibodies to α- and β-catenin were from Sigma (Munich, Germany), the plakoglobin MAb5.1 was from Progen Biotechnics (Heidelberg, Germany), and antibodies against VE-cadherin were from MoBiTec (Göttingen, Germany). Sections were incubated with primary antibodies for 30 min, followed by three washes with PBS. Secondary antibodies coupled to Alexa 594 (MoBiTec, Göttingen, Germany) were then applied for 30 min, again followed by three washes with PBS. The samples were then quickly rinsed with distilled water, dipped in ethanol, air dried, and mounted with Fluoromount (Biozol, Eching, Germany). Fluorescence images were taken with a Zeiss Axiophot.
Electron microscopy.
For morphological studies, cerebella of wild-type mice and homozygous M-cadherin-deficient mutants were fixed with 2.5% glutaraldehyde in 50 mM sodium cacodylate buffer (pH 7.2) containing 50 mM KCl and 2.5 mM MgCl for 30 min, followed by repeated rinsing with cacodylate buffer. After additional postfixation with 2% osmium tetroxide in cacodylate buffer for 2 h, samples were block stained overnight in 0.5% uranyl acetate in water, dehydrated, and embedded in Epon (for details, see reference 15).
For immunoelectron microscopy, ca. 5-μm cryosections of snap-frozen cerebella were mounted on cover slips, fixed with 2% formaldehyde (freshly prepared from paraformaldehyde) in PBS for 15 min, and quenched with 50 mM ammonium chloride in PBS for 10 min. After permeabilization of the sections with 0.1% saponin for 5 min, they were incubated with rabbit antibodies specific for M-cadherin for 2 h and, after thorough washes with PBS, a second time with anti-rabbit immunoglobulin G coupled to Nanogold (BioTrend, Cologne, Germany) for 2 h. After another thorough wash with PBS, the primary gold particles were enhanced using the HQ silver enhancement kit (BioTrend) (6, 27). Sections were then fixed with 2.5% glutaraldehyde for 15 min and postfixed with 0.2% osmium tetroxide for 30 min.
Thereafter, sections were dehydrated and flat embedded in Epon as described before (15). Ultrathin sections were prepared with a Reichert-Jung ultramicrotome, Ultracut (Leica, Bensheim, Germany). Electron micrographs were taken with Zeiss electron microscope EM 910.
RESULTS
M-cadherin-null mutant mice exhibit no apparent skeletal muscle phenotype.
The targeting vector for homologous recombination in ES cells was designed to delete the coding region of exons 1 to 4 of the M-cadherin gene (Fig. 1A). These exons carry information for the signal peptide and most of extracellular domain 1 (EC1), which contains the typical interaction motif and is believed to mediate homophilic binding. The bacterial lacZ reporter gene, including a nuclear localization signal (nls), was fused in frame directly to the AUG start codon, followed by the PGK-neomycin resistance cassette in the transcriptional orientation of the M-cadherin gene. The 4.6 and 1.8 kb of DNA flanking the deleted region of the M-cadherin gene were used as left and right recombination arms, respectively. The herpes simplex virus thymidine kinase TK gene was added downstream of the right arm for counterselection. Electroporation of this vector construct in ES cells resulted in five independent homologous recombination events. Three cell clones were injected into mouse blastocysts and resulted in chimeras that transmitted the mutant allele to their offspring. Crossing of heterozygous animals yielded all three genotypes, born with normal Mendelian distribution and no apparent effect of the mutation on viability (Fig. 1B). In fact, heterozygous and homozygous mutants exhibited no visible phenotypic alterations, in particular no signs of impaired mobility or weight differences, suggesting that skeletal muscle development occurred largely normally in mutants. Histological sections of skeletal muscle from mutants were also essentially indistinguishable from wild-type sections (data not shown).
FIG. 1.
Schematic representation of the murine M-cadherin locus and design of the recombination vector. (A) The gene contains 14 exons. The recombination vector was constructed using a 4.6-kb NruI/KpnI fragment from the 5′ end of the gene and a 1.8-kb KpnI fragment encompassing exons 5 and 6 as the left and right recombination arms, respectively. The nls-lacZ gene and the PGK-neomycin resistance cassette were fused directly to the start codon in exon 1. The herpes simplex virus thymidine kinase (hsv-tk) gene was added downstream of the right recombination arm. Homologous recombination results in deletion of the coding region of exons 1 through 4 encoding the signal peptide and most of EC1. Hybridization probes A and B were used to identify wild-type and mutant alleles, respectively. (B) Typical Southern blot from two litters obtained by crossing heterozygous parents. DNA was digested with SacI restriction endonuclease and hybridized with probe A. Wild-type and mutant alleles are indicated by 8.7- and 6.7-kb fragments, respectively. The DNA sample in lane 9 was lost, and therefore no genotype is indicated for this mouse. (C) Northern blot using muscle RNA from the same animals shown in panel B hybridized with the EcoRV-SpeI 3′ fragment of M-cadherin cDNA (nucleotides 1271 to 2375). Mcad mRNA level is reduced in heterozygous and absent in homozygous mutant animals. Restriction sites: K, KpnI; N, NruI; X, XhoI; S, SacI.
In order to ascertain the intended M-cadherin null mutation, Northern blots from muscle tissues were hybridized with an M-cadherin-specific probe containing coding sequence downstream of the deletion. As shown in Fig. 1C, homozygous mutants contained no M-cadherin transcripts, while the correct mRNA was readily detectable in wild-type and heterozygous animals at a reduced level. The lack of M-cadherin in mutants was also confirmed on Western blots by using an M-cadherin-specific antibody. Protein extracts from mutant skeletal muscle and cerebellum revealed no protein, in contrast to extracts from wild-type and heterozygous control mice (see Fig. 3, 4, and 7). Taken together, these data indicate that we have generated an M-cadherin null mutation in mice. Lack of M-cadherin, however, causes no major developmental defects of skeletal musculature.
FIG. 3.
Regeneration of skeletal muscle is not impaired in M-cadherin null mutant mice. Hematoxylin- and eosin-stained sections of hind limb muscle from heterozygous and homozygous mutants were prepared at indicated times after injection with cardiotoxin (A). Blue staining indicates LacZ expression from the mutant M-cadherin locus that is particularly visible in homozygous mutants. Note the infiltration of the necrotic areas by mononucleated cells at day 2 and newly formed fibers with centrally located nuclei at day 10. Regeneration of damaged muscle appears similar in heterozygous and homozygous mutants. A Western blot of protein extracts from regenerating hind limb muscle indicates that components of adhesion junctions are equally upregulated in heterozygous and homozygous animals. Samples were taken at the indicated time points after cardiotoxin injection. Panel C illustrates the induction of muscle-specific transcription factors in regenerating hind limb muscle of homozygous and heterozygous M-cadherin mutants over 20 days following cardiotoxin injection. mRNA levels were determined by semiquantitative RT-PCR with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a control.
FIG. 4.
Differentiation of satellite cells in vitro from wild-type (+/+) and M-cadherin mutant (−/−) mice. Panel A shows satellite cells that were isolated according to a standard procedure, placed in culture, and shifted to differentiation medium. Immunostaining was performed at the indicated times after medium shift. Note that cultured satellite cells are initially positive for desmin (Desm.) and cMet receptor, indicated by brown staining. Under differentiation conditions, cells from wild-type and homozygous mutant mice rapidly form myotubes without apparent distinction and express myosin heavy chain, identified with the MF 20 monoclonal antibody. Panel B demonstrates a Western blot illustrating similar transient upregulation of protein components of adhesive contacts in M-cadherin-containing and -deficient satellite cells.
FIG. 7.
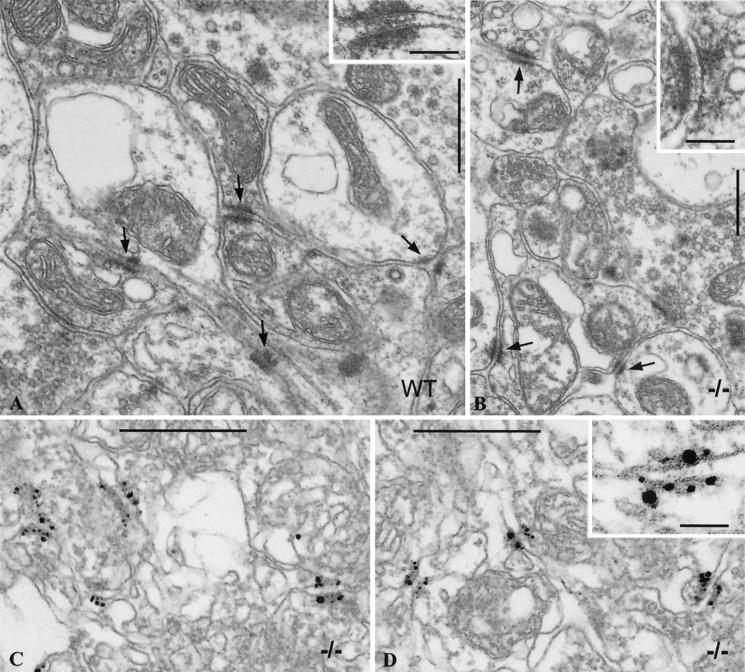
Electron micrographs of ultrathin sections through glomeruli of cerebellum of wild-type (WT, A) and mutant (−/−, B to D) mice without (A and B) and with (C and D) N-cadherin immunolabeling. Note the small, plaque-bearing junctions (arrows in A and B) connecting neuronal extensions. These adhering contacts appear similar in size and ultrastructure in wild-type and mutant animals, revealing the typical midline structure of these junctions (compare insets in panels A and B). Panels C and D present micrographs of sections through different cerebellar regions of M-cadherin-lacking mice, immunolabeled with N-cadherin-specific antibodies. Note that N-cadherin specifically localizes to contactus adherens plaques in the mutant (for higher magnification, see the inset in panel D), whereas it is hardly detected by this method in wild-type animals (data not shown). Bars, 0.5 mm (all panels).
Mcad/LacZ expression recapitulates the developmental pattern in skeletal muscles but shows additional expression in eye and brain.
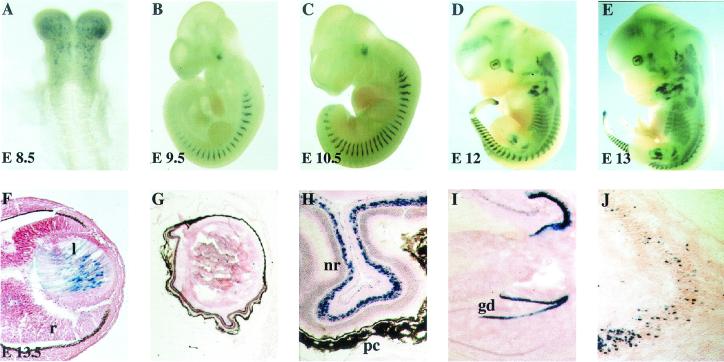
Introduction of the nls-lacZ reporter gene into the M-cadherin locus afforded the reevaluation of its expression during embryonic mouse development and in adult animals. At day 8 of embryogenesis (E8), LacZ-positive cells appeared in head mesenchyme, while the first formed cranial somites did not express the gene (Fig. 2A). With further development (E9 to E13), expression of the mutant allele appeared in myotomes and branchial arches and continued in most if not all epaxial and hypaxial muscles of trunk and limbs (Fig. 2B to E). This result agrees with previously published data obtained by in situ hybridization (24). However, some facial muscles, particularly in the snout, seemed not to express LacZ, which may reflect heterogeneity of individual muscles with respect to M-cadherin expression or dysregulation of the mutant allele. Rather unexpectedly, we also observed strong β-galactosidase activity in embryonic lens fibers (Fig. 2F), in a defined cell layer of the neuroretina (Fig. 2G and H), in regions of the hippocampus (Fig. 2I), and in scattered cells of the brain stem (Fig. 2J). These LacZ-positive cells were never observed in wild-type mice, arguing against a staining artifact.
FIG. 2.
Expression pattern of the Mcad/lacZ mutant allele in embryonic and adult mice. Whole-mount preparations of heterozygous animals from E8.5 to E13 (A to E), sagittal section of the eye of an E13.5 embryo (F), and sagittal sections of adult eyes (G and H) and adult brains (I and J) were stained for β-galactosidase activity. Blue cells indicate expression of the mutant allele in head mesenchyme (A), in myotomes and individual muscles (B to E), and in nuclei of lens fibers during embryonic development (F). Cryotome sections of isolated eyes (G and H) and brains (I and J) of adult animals show expression in a distinct cell layer of the neuroretina, gyrus dentatus of the hippocampus (I), and undefined single dispersed cells of the brain stem (J). l, lens; r, retina; nr, neuroretina; pc, pigment cells; gd, gyrus dentatus.
At present, it is unclear whether M-cadherin is normally expressed in these structures, since it has never been described previously and so far we have been unable to demonstrate M-cadherin gene transcripts or protein in eye and brain. Therefore, it cannot be excluded that ectopic expression of Mcad/lacZ is the result of the deletion within the mutant allele. This allele lacks several introns which might contain important regulatory elements. Moreover, the PGK-neomycin resistance cassette remaining in the mutated locus may also affect its correct expression. In any case, the Mcad/lacZ gene in these mice can serve as a convenient marker for embryonic lens fibers and a distinct subpopulation of retina cells. It also marks specific cells in the hippocampus and brain stem.
Regeneration of skeletal muscle appears unaffected in M-cadherin-deficient mice.
Mature skeletal muscle fibers in adult mice express very little M-cadherin, but the protein is generally present in quiescent satellite cells. When satellite cells are activated during muscle regeneration, M-cadherin expression is markedly upregulated (5, 11, 19). These observations have led to the hypothesis that M-cadherin plays a role in processes of muscle repair.
We tested this idea in the M-cadherin-deficient mouse mutant by unilateral injection of cardiotoxin into the hind limb muscle, which leads to rapid necrosis of muscle fibers (16). Regeneration of the damaged muscle was then compared on hematoxylin-eosin-stained histological sections of wild-type, heterozygous, and homozygous mutants over 3 weeks. At least three animals of each genotype were analyzed for each time point.
As illustrated in Fig. 3A, regeneration of new fibers was readily observed within 10 days following cardiotoxin injection in both heterozygous and M-cadherin-deficient mice, with no difference from wild-type animals (data not shown). The newly formed myofibers were identified by centrally located nuclei and nuclear LacZ staining that indicated activation of the M-cadherin gene locus, most visible in homozygous mutants. The existence of such fibers suggests that, in the absence of M-cadherin, mutant muscle harbors satellite cells that can be activated to proliferate and form new myotubes, very similar to their wild-type counterparts. As we have never observed blue nuclei in quiescent satellite cells or in peripherally located nuclei of existing fibers, we are confident that the new fibers actually have been formed by activated satellite cells.
To search for alternative molecules that may mediate cell adhesiveness in the absence of M-cadherin, we analyzed known components of adhering plaques on Western blots of proteins from regenerating muscle (Fig. 3B). Interestingly, heterozygous and homozygous animals contained significant levels of N- and E-cadherins that were both markedly upregulated during regeneration. Similarly, the canonical catenins usually associated with the cytoplasmic tail of cadherins were present and increased in concentration during regeneration. These observations are consistent with the view that in the absence of M-cadherin, sufficient adhesion contacts can be formed between muscle cells, and that this protein therefore does not serve an obligatory function during myofiber formation.
We also investigated the expression of the myogenic transcription factors MyoD, Myf-5, and myogenin during regeneration, as they are known to be induced in activated satellite cells (11, 31, 35). RT-PCR analysis indicated that expression of all three markers was upregulated with similar kinetics in mutants and heterozygous controls, confirming the histological data (Fig. 3C). Downregulation of Myf-5 and myogenin after approximately 10 days of ongoing repair suggested that the process had been essentially concluded by that time. Minor differences in concentrations of myogenic factor mRNAs among different animals were not found reproducibly and may be due to slight variations in dissecting regenerating tissues from the individual animals and/or slightly varying input of RNA. By and large, our results indicated that in the necrosis model chosen, muscle regeneration occurred quite normally in the absence of M-cadherin, most likely because N-cadherin or another related glycoprotein(s) performs the required functions in satellite cells. The marginally delayed onset of Myf-5 and myogenin upregulation in regenerating muscle of homozygous mutants compared to heterozygous controls may suggest that even though other cadherins can substitute for M-cadherin, they possibly do so less efficiently.
In vitro experiments with satellite cells isolated from wild-type and mutant mice indeed support this idea. When desmin and cMet-receptor-positive satellite cells in culture were subjected to differentiation medium, both wild-type and homozygous mutant cells readily formed large multinucleated myotubes expressing myosin heavy chain (Fig. 4A). Similar to the in vivo situation, these cells upregulated the components of adhesion plaques with no significant differences between wild-type and mutant cells (Fig. 4B). We also did not observe any appreciable differences in numbers of satellite cells obtainable from wild-type and mutant muscle, nor did we see any significant differences in proliferation. Thus, we have concluded that lack of M-cadherin does not alter the growth and fusion potential of satellite cells.
M-cadherin-deficient mice form cerebellar contactus adherens junctions.
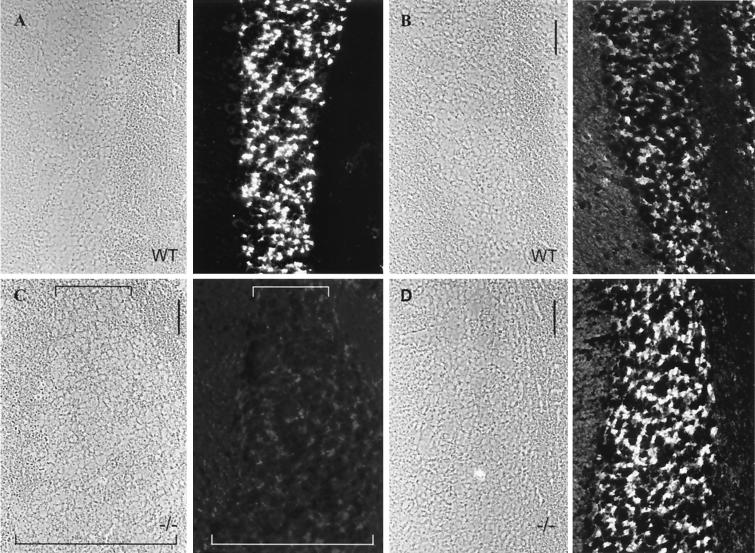
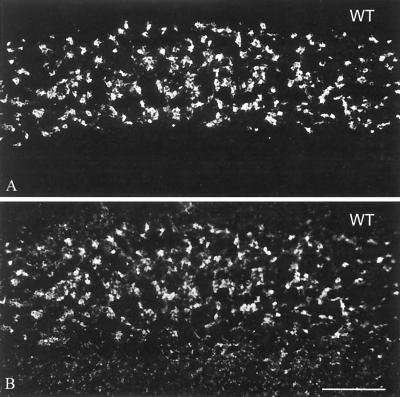
M-cadherin has been identified as the predominant transmembrane component of contactus adherens junctions representing a specialized type of adherens plaques in the cerebellum of mice and rats (3, 27). Therefore, we examined these structures in M-cadherin-deficient mice. M-cadherin-specific antibodies readily immunostained the granular cell layer of cerebellar glomeruli in wild-type mice but failed to detect the antigen in mutant mice, as expected for the null mutation (Fig. 5A and C). To test for other adhesion molecules that might compensate for the lack of M-cadherin, adjacent sections were analyzed with N-cadherin-specific antibodies. Immunofluorescence revealed low levels of N-cadherin in granular cells of wild-type mice (Fig. 5B) but remarkably intense immunoreaction in M-cadherin null mutants (Fig. 5D). Reactions for various other adhering junction proteins, such as α- and β-catenin and plakoglobin, were in the normal range (27), whereas reactions for P-, VE-, and E-cadherin and p120 were negative in both wild-type and mutant mice.
FIG. 5.
Immunofluorescence micrographs of frozen sections through the cerebellum of wild-type (WT, A and B) and mutant (−/−, C and D) mice, showing immunoreactions of granule cells with antibodies specific for M-cadherin (A and C) or N-cadherin (B and D). Fields in phase-contrast optics are shown on the left side of each pair of pictures. The layer of granule cells is demarcated by brackets in panel C. Note that wild-type granule cells are intensely labeled for M-cadherin (A) but very weakly for N-cadherin (B), while the −/− mutant cells are rich in N-cadherin (D). Bars, 25 μm.
To ensure that only one type of granular cells exist in the glomeruli coexpressing the genes of both M- and N-cadherin, double-label immunofluorescence microscopy was performed (Fig. 6).
FIG. 6.
Double-label immunofluorescence microscopy of frozen sections through cerebellum of wild-type (WT) mice, showing colocalization of M-cadherin (A) and N-cadherin (B) in the same granular cells. Bar, 50 μm.
Electron micrographs of cerebellum sections confirmed the presence of contactus adherens in mutant (−/−) mice similar in number and size and with a structure indistinguishable from that of wild-type animals (Fig. 7A and B). Immunogold staining of such sections showed very intense N-cadherin immunostaining, with the majority of immunoreaction products located on the junctional plaques (Fig. 7C and D).
Here it should be mentioned that by immunolabeling of wild-type cerebellum with the same N-cadherin-specific antibody, hardly any N-cadherin was detectable (data not shown) (27).
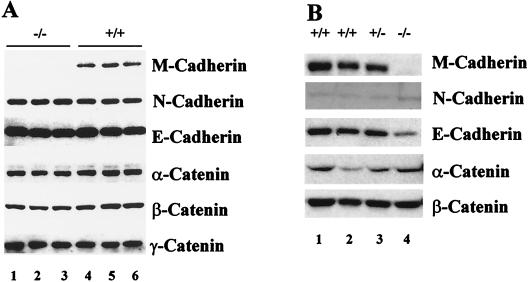
Western blot analysis of cerebellar protein extracted from wild-type and mutant animals, however, revealed only a 30 to 50% increase in N-cadherin levels in the M-cadherin null mutants compared to wild-type mice, while α- and β-catenin appeared moderately (25%) reduced (Fig. 8). Interestingly, we found considerable levels of E-cadherin in cerebellum but did not determine its precise location. A previous study, however, had excluded E-cadherin from contactus adherens junctions in the cerebellum of mice and rats (27, 28). With the possible exception of N-cadherin in cerebellum we found no evidence that the absence of M-cadherin would lead to profound alterations in the global composition of adherence plaque-associated proteins either in muscle or in cerebellum (Fig. 8). In contrast to adult skeletal muscle with very low concentrations of N-cadherin present, N-cadherin appears to be a prime candidate to substitute for M-cadherin in the contactus adherens junctions of mutant mouse.
FIG. 8.
Immunoblot of various cadherins and catenins in protein extracts of cerebellum (A) and skeletal muscle (B) from wild-type and homozygous mouse mutants. The M-cadherin null mutation is confirmed by the complete absence of M-cadherin in homozygous animals. Concentrations of canonical catenins present in microfilament-anchoring adherens junctions appear largely unaffected by the mutation. N-cadherin is moderately upregulated (35 to 50%) in the cerebellum of M-cadherin null mutants. Note the very low level of N-cadherin present in nonregenerating adult skeletal muscle.
DISCUSSION
M-cadherin has been proposed to function as a crucial and selective mediator of cell-cell interactions in skeletal muscle and cerebellum, the two organs in mice in which M-cadherin is mainly expressed. Sequence homology and conservation of the domain structure of M-cadherin, compared with other “classical” cadherins, is in agreement with the concept of a possible function in cell adhesion. Moreover, it has been shown that the cytoplasmic domain of M-cadherin interacts with canonical components of microfilament-anchoring adherens junctions, such as α- and β-catenin and plakoglobin (23). Synthetic peptides that block the homotypic interaction motif in extracellular domain 1 of M-cadherin inhibit fusion of myoblasts to myotubes in culture (37), and forced expression of M-cadherin in mouse L cells that normally do not express any cadherins confers adhesiveness on these cells, supporting the idea that M-cadherin is a major transmembrane constituent of adhering junctions in muscle cells (23).
In this report we present evidence that targeted disruption of the M-cadherin gene in mice has no apparent effect on tissues that normally express this cell adhesion molecule. In particular, development and maintenance of skeletal muscle and regeneration of damaged muscle from satellite cells appear essentially unaffected by the mutation. The lack of M-cadherin protein also does not prevent the formation of contactus adherens, the specialized small plaque-bearing junctions connecting neuronal extensions within the granule cell layer of glomeruli in the cerebellum (27).
Myoblasts can form myofibers in the absence of M-cadherin.
Establishment and maintenance of tissue organization are believed to involve semistable intercellular junction structures that mediate cell-cell adhesion on one side and affect intracellular architecture of the cytoskeleton on the other side. The preferential expression of the transmembrane protein M-cadherin in developing muscle has made it a prime candidate to fulfill at least some of these functions in skeletal muscle, although its presence precisely in adhering junctions has not yet been demonstrated rigorously. During myogenesis and muscle regeneration, myoblasts fuse to form multinucleated myotubes that eventually develop into mature contractile myofibers. As myoblasts normally fuse exclusively with each other, proper cell recognition and adhesion appear to be prerequisites for the fusion process itself. Based on this consideration, it was postulated that M-cadherin represents the molecule conferring specific surface properties to myoblasts and thereby participates in the formation of myotubes. Similarly, the presence of M-cadherin on satellite cells and in clusters on myofibrillar membranes adjacent to quiescent satellite cells has been interpreted as a possible mechanism to specifically localize satellite cells to discrete spots along muscle fibers (19, 21, 37). Interestingly, satellite cells from MyoD-deficient mice display a major deficit in M-cadherin expression and fail to fuse efficiently, which may be taken as support for the notion that M-cadherin is instrumental in the fusion process (10, 29, 30, 36).
The M-cadherin null mutation, however, unequivocally demonstrates that M-cadherin plays no essential role in cell fusion. Skeletal muscles developing in the absence of M-cadherin are fully functional and show no signs of increased susceptibility to fiber decay, at least under the conditions of the cage habitat. However, challenging muscle performance may reveal subtle differences in the physiology of wild-type and mutant muscle that have previously escaped detection. In any case, myoblasts lacking M-cadherin are able to selectively fuse with each other to form skeletal muscle that is anatomically and histologically indistinguishable from wild-type muscle. This also applies to mutant satellite cells that readily form myotubes both in vivo and in vitro, without apparent differences from their wild-type counterparts. We also found no evidence for misplacement or loss of satellite cells in M-cadherin mutant mice. These observations, however, do not rule out the possibility that under conditions of permanent degeneration and regeneration, such as in dystrophic mdx mice, impaired regeneration capacity in the absence of M-cadherin may become apparent.
A possible explanation for the lack of a muscle phenotype in mutant animals may come from the observation that other cadherins, such as N-cadherin and R-cadherin, are present in skeletal muscle and may substitute for M-cadherin function (21). Although the N-cadherin concentration appears to be low in normal adult muscle, its expression is drastically upregulated in activated satellite cells during muscle regeneration. Under these conditions, N-cadherin may compensate for the lower level of M-cadherin, as it is also induced during muscle regeneration in wild-type mice. Interestingly, mouse myoblasts lacking N-cadherin can also fuse to myotubes, indicating that it has no specific function in this process and may possibly be functionally replaced by M-cadherin (8). However, a monoclonal anti-N-cadherin antibody or synthetic peptides containing the specific interaction motif inhibit myoblast fusion in chickens, suggestive of its requirement for myogenesis in this species.
It is not possible to test the potentially overlapping functions of M- and N-cadherin because N-cadherin mutations are embryonically lethal prior to skeletal myogenesis and conditional mutations are currently not available (26). Although the M-cadherin null mutation described here exhibits no apparent muscle phenotype, it should be interesting to investigate the developmental potential of M-cadherin-lacking cells under competitive conditions, such as in mouse chimeras that contain both M-cadherin-positive and -negative cells. For N-cadherin-lacking cells, it has been shown that under these conditions, mutant cells are sorted out and fail to participate properly in organ development (22). Thus, it is conceivable that hypomorphic mutations of M-cadherin may display a more severe phenotype than the null mutation.
M-cadherin is not essential for contactus adherens junctions in the cerebellum.
Granule cells in the cerebellar glomerulus contain a special type of plaque-bearing adhering junctions that have been characterized as small, nearly isodiametric structures that contain M-cadherin as the principal transmembrane glycoprotein. Previously classified as puncta adherentia related to microfilament-anchoring junctions of epithelial cells, these contacts fail to contain E- and VE-cadherin or desmosomal cadherins and desmoplakin, and they contain little if any vinculin and α-actinin (27). Analysis of contactus adherens in M-cadherin null mutant mice has now revealed that they contain considerable levels of N-cadherin, which appears somewhat upregulated in comparison with that in wild-type mice. Other regular components of these adhesion plaques are not appreciably altered, suggesting that M- and N-cadherin exert similar adhesion properties and can organize cytoskeletal architecture in a similar way. This may explain how these cerebellar contact structures can be established and maintained in the absence of M-cadherin.
Since the locomotion and behavior of mutant mice appears grossly normal, it can be assumed that these complex synapse terminals also function fairly normally in connecting neurons with granular cells in the cerebellum. If then N-cadherin can substitute for M-cadherin without any major functional consequences, it seems that both cadherins are equally effective in mediating adhesive functions between such cells. Whether or not M-cadherin distinctly affects cell-cell interactions or cell migration at some point during organ development remains to be seen. The mere existence and evolutionary conservation of the M-cadherin gene argue strongly for an important, although possibly subtle function that will still have to be identified.
Acknowledgments
We thank A. Starzinski-Powitz and her colleagues (University of Frankfurt, Frankfurt am Main, Germany) for the generous gift of M-cadherin antiserum. We also acknowledge R. Frank for help with the peptide scanning analysis.
This work was supported by the Deutsche Forschungsgemeinschaft, grant Jo 55/7-3, and the Fond der Chemischen Industrie.
REFERENCES
- 1.Allen, R. E., S. M. Sheehan, R. G. Taylor, T. L. Kendall, and G. M. Rice. 1995. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J. Cell. Physiol. 165:307-312. [DOI] [PubMed] [Google Scholar]
- 2.Bader, D., T. Masaki, and D. A. Fischman. 1982. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J. Cell Biol. 95:763-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahjaoui-Bouhaddi, M., F. Padilla, M. Nicolet, C. Cifuentes-Diaz, D. Fellmann, and R. M. Mege. 1997. Localized deposition of M-cadherin in the glomeruli of the granular layer during the postnatal development of mouse cerebellum. J. Comp. Neurol. 378:180-195. [PubMed] [Google Scholar]
- 4.Beauchamp, J. R., L. Heslop, D. S. Yu, S. Tajbakhsh, R. G. Kelly, A. Wernig, M. E. Buckingham, T. A. Partridge, and P. S. Zammit. 2000. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J. Cell Biol. 151:1221-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bornemann, A., and H. Schmalbruch. 1994. Immunocytochemistry of M-cadherin in mature and regenerating rat muscle. Anat. Rec. 239:119-125. [DOI] [PubMed] [Google Scholar]
- 6.Borrmann, B. 2000. Ph.D. thesis. University of Heidelberg, Heidelberg, Germany.
- 7.Braun, T., M. A. Rudnicki, H. H. Arnold, and R. Jaenisch. 1992. Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell 71:369-382. [DOI] [PubMed] [Google Scholar]
- 8.Charlton, C. A., W. A. Mohler, G. L. Radice, R. O. Hynes, and H. M. Blau. 1997. Fusion competence of myoblasts rendered genetically null for N-cadherin in culture. J. Cell Biol. 138:331-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cifuentes-Diaz, C., D. Goudou, R. M. Mege, E. Velasco, M. Nicolet, K. Herrenknecht, L. Rubin, and F. Rieger. 1998. Distinct location and prevalence of alpha-, beta-catenins and gamma-catenin/plakoglobin in developing and denervated skeletal muscle. Cell Adhes. Commun. 5:161-176. [DOI] [PubMed] [Google Scholar]
- 10.Cornelison, D. D., B. B. Olwin, M. A. Rudnicki, and B. J. Wold. 2000. MyoD−/− satellite cells in single-fiber culture are differentiation defective and MRF4 deficient. Dev. Biol. 224:122-137. [DOI] [PubMed] [Google Scholar]
- 11.Cornelison, D. D., and B. J. Wold. 1997. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev. Biol. 191:270-283. [DOI] [PubMed] [Google Scholar]
- 12.Danto, S. I., and D. A. Fischman. 1984. Immunocytochemical analysis of intermediate filaments in embryonic heart cells with monoclonal antibodies to desmin. J. Cell Biol. 98:2179-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donalies, M., M. Cramer, M. Ringwald, and A. Starzinski-Powitz. 1991. Expression of M-cadherin, a member of the cadherin multigene family, correlates with differentiation of skeletal muscle cells. Proc. Natl. Acad. Sci. USA 88:8024-8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fire, A., S. W. Harrison, and D. Dixon. 1990. A modular set of lacZ fusion vectors for studying gene expression in Caenorhabditis elegans. Gene 93:189-198. [DOI] [PubMed] [Google Scholar]
- 15.Franke, W. W., C. Grund, M. Osborn, and K. Weber. 1978. The intermediate-sized filaments in rat kangaroo PtK2 cells. I. Morphology in situ. Cytobiologie 17:365-391. [PubMed] [Google Scholar]
- 16.Garry, D. J., Q. Yang, R. Bassel-Duby, and R. S. Williams. 1997. Persistent expression of MNF identifies myogenic stem cells in postnatal muscles. Dev. Biol. 188:280-294. [DOI] [PubMed] [Google Scholar]
- 17.Geiger, B., and O. Ayalon. 1992. Cadherins. Annu. Rev. Cell Biol. 8:307-332. [DOI] [PubMed] [Google Scholar]
- 18.Huber, P., J. Dalmon, J. Engiles, F. Breviario, S. Gory, L. D. Siracusa, A. M. Buchberg, and E. Dejana. 1996. Genomic structure and chromosomal mapping of the mouse VE-cadherin gene (Cdh5). Genomics 32:21-28. [DOI] [PubMed] [Google Scholar]
- 19.Irintchev, A., M. Zeschnigk, A. Starzinski-Powitz, and A. Wernig. 1994. Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev. Dyn. 199:326-337. [DOI] [PubMed] [Google Scholar]
- 20.Kaufmann, U., J. Kirsch, A. Irintchev, A. Wernig, and A. Starzinski-Powitz. 1999. The M-cadherin catenin complex interacts with microtubules in skeletal muscle cells: implications for the fusion of myoblasts. J. Cell Sci. 112:55-68. [DOI] [PubMed] [Google Scholar]
- 21.Kaufmann, U., B. Martin, D. Link, K. Witt, R. Zeitler, S. Reinhard, and A. Starzinski-Powitz. 1999. M-cadherin and its sisters in development of striated muscle. Cell Tissue Res. 296:191-198. [DOI] [PubMed] [Google Scholar]
- 22.Kostetskii, I., R. Moore, R. Kemler, and G. L. Radice. 2001. Differential adhesion leads to segregation and exclusion of N-cadherin-deficient cells in chimeric embryos. Dev. Biol. 234:72-79. [DOI] [PubMed] [Google Scholar]
- 23.Kuch, C., D. Winnekendonk, S. Butz, U. Unvericht, R. Kemler, and A. Starzinski-Powitz. 1997. M-cadherin-mediated cell adhesion and complex formation with the catenins in myogenic mouse cells. Exp. Cell Res. 232:331-338. [DOI] [PubMed] [Google Scholar]
- 24.Moore, R., and F. S. Walsh. 1993. The cell adhesion molecule M-cadherin is specifically expressed in developing and regenerating, but not denervated skeletal muscle. Development 117:1409-1420. [DOI] [PubMed] [Google Scholar]
- 25.Pouliot, Y., M. Gravel, and P. C. Holland. 1994. Developmental regulation of M-cadherin in the terminal differentiation of skeletal myoblasts. Dev. Dyn. 200:305-312. [DOI] [PubMed] [Google Scholar]
- 26.Radice, G. L., H. Rayburn, H. Matsunami, K. A. Knudsen, M. Takeichi, and R. O. Hynes. 1997. Developmental defects in mouse embryos lacking N-cadherin. Dev. Biol. 181:64-78. [DOI] [PubMed] [Google Scholar]
- 27.Rose, O., C. Grund, S. Reinhardt, A. Starzinski-Powitz, and W. W. Franke. 1995. Contactus adherens, a special type of plaque-bearing adhering junction containing M-cadherin, in the granule cell layer of the cerebellar glomerulus. Proc. Natl. Acad. Sci. USA 92:6022-6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rose, O., J. Rohwedel, S. Reinhardt, M. Bachmann, M. Cramer, M. Rotter, A. Wobus, and A. Starzinski-Powitz. 1994. Expression of M-cadherin protein in myogenic cells during prenatal mouse development and differentiation of embryonic stem cells in culture. Dev. Dyn. 201:245-259. [DOI] [PubMed] [Google Scholar]
- 29.Sabourin, L. A., A. Girgis-Gabardo, P. Seale, A. Asakura, and M. A. Rudnicki. 1999. Reduced differentiation potential of primary MyoD−/− myogenic cells derived from adult skeletal muscle. J. Cell Biol. 144:631-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seale, P., and M. A. Rudnicki. 2000. A new look at the origin, function, and stem-cell status of muscle satellite cells. Dev. Biol. 218:115-124. [DOI] [PubMed] [Google Scholar]
- 31.Smith, C. K., II, M. J. Janney, and R. E. Allen. 1994. Temporal expression of myogenic regulatory genes during activation, proliferation, and differentiation of rat skeletal muscle satellite cells. J. Cell. Physiol. 159:379-385. [DOI] [PubMed] [Google Scholar]
- 32.Takeichi, M. 1991. Cadherin cell adhesion receptors as a morphogenetic regulator. Science 251:1451-1455. [DOI] [PubMed] [Google Scholar]
- 33.Tybulewicz, V. L., C. E. Crawford, P. K. Jackson, R. T. Bronson, and R. C. Mulligan. 1991. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell 65:1153-1163. [DOI] [PubMed] [Google Scholar]
- 34.Vleminckx, K., and R. Kemler. 1999. Cadherins and tissue formation: integrating adhesion and signaling. Bioessays 21:211-220. [DOI] [PubMed] [Google Scholar]
- 35.Yablonka-Reuveni, Z., and A. J. Rivera. 1994. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev. Biol. 164:588-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yablonka-Reuveni, Z., M. A. Rudnicki, A. J. Rivera, M. Primig, J. E. Anderson, and P. Natanson. 1999. The transition from proliferation to differentiation is delayed in satellite cells from mice lacking MyoD. Dev. Biol. 210:440-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeschnigk, M., D. Kozian, C. Kuch, M. Schmoll, and A. Starzinski-Powitz. 1995. Involvement of M-cadherin in terminal differentiation of skeletal muscle cells. J. Cell Sci. 108:2973-2981. [DOI] [PubMed] [Google Scholar]