Abstract
The cyclin-dependent kinase 2 (Cdk2) inhibitors p21CIP1 and p27KIP1 are negatively regulated by anchorage during cell proliferation, but it is unclear how integrin signaling may affect these Cdk2 inhibitors. Here, we demonstrate that integrin ligation led to rapid reduction of p21CIP1 and p27KIP1 protein levels in three distinct cell types upon attachment to various extracellular matrix (ECM) proteins, including fibronectin (FN), or to immobilized agonistic anti-integrin monoclonal antibodies. Cell attachment to FN did not rapidly influence p21CIP1 mRNA levels, while the protein stability of p21CIP1 was decreased. Importantly, the down-regulation of p21CIP1 and p27KIP1 was completely blocked by three distinct proteasome inhibitors, demonstrating that integrin ligation induced proteasomal degradation of these Cdk2 inhibitors. Interestingly, ECM-induced proteasomal proteolysis of a ubiquitination-deficient p21CIP1 mutant (p21K6R) also occurred, showing that the proteasomal degradation of p21CIP1 was ubiquitin independent. Concomitant with our finding that the small GTPases Cdc42 and Rac1 were activated by attachment to FN, constitutively active (ca) Cdc42 and ca Rac1 promoted down-regulation of p21CIP1. However, dominant negative (dn) Cdc42 and dn Rac1 mutants blocked the anchorage-induced degradation of p21CIP1, suggesting that an integrin-induced Cdc42/Rac1 signaling pathway activates proteasomal degradation of p21CIP1. Our results indicate that integrin-regulated proteasomal proteolysis might contribute to anchorage-dependent cell cycle control.
Anchorage to the extracellular matrix (ECM) is required for proliferation of all untransformed tissue cells (38). To control cell proliferation, anchorage regulates key cell cycle components mainly occurring in the G1 phase (31). Progression through the G1 phase is mediated by the cyclin-dependent kinases Cdk4/6 and Cdk2, whose activities are controlled by their associated cyclins and Cdk inhibitors (43). To this end, attachment to ECM results in up-regulation of cyclin E-Cdk2 activity and down-regulation of the Cdk2 inhibitors p21CIP1 and p27KIP1 (11, 45, 52). Importantly, cells in suspension display higher levels of the Cdk2 inhibitors p21CIP1 and p27KIP1 than do attached cells, leading to impaired cyclin E-Cdk2 activity in suspended cells concomitant with a cell cycle block in late G1 phase (11, 45, 52). In fact, cyclin E-Cdk2 activity is considered the last control in the G1 phase, since activated cyclin E-Cdk2 triggers cyclin A synthesis and thereby promotes S-phase entry. This suggests that the regulation of cyclin E-Cdk2 activity in late G1 phase by anchorage may represent a control stage through which attached cells must maintain low levels of Cdk2 inhibitors until this point is passed. Therefore, the regulation of the Cdk2 inhibitors p21CIP1 and p27KIP1 appears to be an important step for anchorage-dependent G1-phase progression of the cell cycle.
Proteasome-dependent proteolysis represents a cellular pathway for rapid down-regulation of specific proteins for which distinct temporal expression is required. Importantly, many cell cycle components are targets for proteasomes, including cyclins (A, B, D, and E), Rb, E2F, and the Cdk2 inhibitors p21CIP1 and p27KIP1, as well as p53, and proteasomal degradation of these components determines the periodicity of the cell cycle (1, 3, 16, 20, 21, 32, 41). In most of the cases studied, proteasomal degradation is preceded by ubiquitination. However, although p21CIP1 can be ubiquitinated, proteasomal degradation of p21CIP1 does not require ubiquitination (42), which may be related to the capacity of the C8-α subunit of the 20S proteasome to directly interact with the C terminus of p21CIP1, leading to rapid degradation of p21CIP1 (47). However, it is not known if anchorage to ECM might regulate proteasome-dependent proteolysis.
Integrins are the major cell surface receptors mediating cell anchorage to ECM proteins. Integrins activate a variety of signaling cascades, including focal adhesion kinase (FAK), mitogen-activated protein kinases of the ERK and JNK types, phosphoinositide 3-kinase (PI-3K), and integrin-linked kinase, all of which affect cell proliferation (13). These integrin-induced signals regulate key G1-phase components, including induction of cyclin D1 mRNA and protein, thereby promoting G1-phase progression of the cell cycle (8, 35, 51).
The small GTPases Cdc42, Rac, and Rho are also involved in integrin-activated signaling events (7, 9, 33). These small GTPases functionally switch intracellular signaling pathways by cycling between an inactive GDP-bound conformation and an active GTP-bound conformation. The GTP/GDP cycle is controlled by guanine nucleotide exchange factors, GTPase-activating proteins, and guanine nucleotide dissociation inhibitors. One basic function of these small GTPases is to regulate cytoskeleton polymerization and cell morphology and motility (17, 34). Furthermore, Rho, Cdc42, and Rac play critical roles in cell cycle progression through the G1 phase (23, 25, 29, 49, 50). To this end, ectopic overexpression of constitutively active (ca) Rac or ca Cdc42 can induce cyclin D1 expression, pRB hyperphosphorylation, and E2F transcriptional activity and promote G1-phase progression and subsequent DNA synthesis in the absence of growth factors (14). In addition, a recent study indicates that integrin-mediated activation of Rac1 controls G1-phase progression of the cell cycle by promoting cyclin D1 synthesis (25) while Rho appears to maintain the correct timing of cyclin D1 expression in G1 phase for control of cell cycle progression (50). In fact, within the G1 phase of the cell cycle, the small GTPases not only regulate the expression of cyclins to control Cdk activity but also influence levels of Cdk2 inhibitors. To this end, ca Ras induces the cyclin-dependent kinase inhibitor p21CIP1 while Rho signaling suppresses the induction of p21CIP1 by Ras and thereby promotes DNA synthesis (30). In addition, the RhoA protein has been implicated in the mid- to late-G1 phase and the down-regulation of the Cdk inhibitor p27KIP1 (15). However, it is not clear if endogenous Cdc42 and/or Rac signaling induced by integrins may also affect Cdk2 inhibitors.
Our study aimed to elucidate if and how integrin-induced signaling, per se, could regulate the Cdk2 inhibitors p21CIP1 and p27KIP1. To this end, we found that cell attachment to ECM through integrins induced a rapid reduction of the Cdk2 inhibitors p21CIP1 and p27KIP1 in three distinct cell types, caused by proteasomal proteolysis. In addition, we identified an integrin-to-Cdc42/Rac1 signaling pathway that mediated anchorage-induced proteasomal p21CIP1 degradation. Our results indicate that integrin-regulated proteasomal proteolysis might contribute to the control of cell proliferation by anchorage.
MATERIALS AND METHODS
Antibodies.
The following antibodies were utilized in this study. Anti-p21CIP1 (Ab-5) polyclonal antibody (PAb) and anti-p53 (Ab-7) PAb were from Oncogene (Cambridge, Mass.), while anti-p21CIP1 PAb (C19), anti-p27KIP1 (F-8) monoclonal antibody (MAb), anti-cyclin E (M-20) PAb, anti-Myc (9E10) MAb, and anti-Cdc42 (P1) PAb were from Santa Cruz Biotechnology (Santa Cruz, Calif.). We purchased anti-p21CIP1 (SX118) MAb from Pharmingen (San Diego, Calif.), anti-HA (12CA5) MAb from Boehringer Mannheim (Mannheim, Germany), anti-FLAG (M2) MAb from Sigma (St. Louis, Mo.), anti-Rac1 (clone 102) MAb from Transduction Laboratories (Lexington, Ky.), anti-FAK (pY397) phosphospecific PAb from Biosource (Camarillo, Calif.), and anti-p44/42MAP kinase (Thr202/Thr204) phosphospecific PAb from New England BioLabs (Beverly, Mass.). Anti-actin (JLA 20) MAb was developed by J. J.-C. Lin and obtained from the Developmental Studies Hybridoma Bank at the University of Iowa. Secondary antibodies for horseradish peroxidase-conjugated anti-mouse and anti-rabbit immunoglobulin were from Jackson Labs (West Grove, Pa.).
Cell culture.
Human primary umbilical vein endothelial cells (HUVECs) were obtained from Clonetics Corp. (San Diego, Calif.) and cultured in M199 medium containing 20% fetal bovine serum, 100 μg of gentamicin per ml, 4 mM l-glutamine, and 0.9 mg of endothelial cell growth supplements (Upstate Biotechnology, Lake Placid, N.Y.) per ml. Before experiments, HUVEC cultures were grown to confluence and used between passages three and five. ECV 304/T24 human bladder carcinoma cells (4) (American Type Culture Collection, Rockville, Md.) and murine NIH 3T3 fibroblasts were cultured in Dulbecco's modified Eagle's medium (DMEM; Life Technologies, Gaithersburg, Md.) containing 10% fetal calf serum and 5 μg of gentamicin per ml at 37°C in 5% CO2.
Prior to the experiments, ECV 304 and NIH 3T3 cells were grown to confluence and starved for 12 h in serum-free medium in order to silence any sustained effects from growth factor signaling. After washing and trypsinization for 2 min, trypsin was neutralized with soybean trypsin inhibitor (1 mg/ml). Cells were then washed and suspended in DMEM containing 2% bovine serum albumin (BSA) at 37°C for 45 min as previously described to silence active anchorage-dependent signaling (6). For preparation of ECM-coated dishes, cell culture suspension dishes were coated with 10 μg of fibronectin (FN; cell binding fragment; Upstate Biotechnology) per ml, 10 μg of collagen type I (ColI; Upstate Biotechnology) per ml, 5 μg of vitronectin (VN) per ml, 5 μg of laminin (LM; Sigma) per ml, or 100 μg of poly-l-lysine (P-L-L; Sigma) per ml in phosphate-buffered saline (PBS) overnight at 4°C and then blocked with 1% heat-denatured BSA (Sigma) in PBS (pH 7.4) for 1 h at 37°C. For preparation of specific-integrin-coated dishes, cell culture suspension dishes were first coated with 25 μg of goat anti-mouse PAb (Jackson Labs) per ml at 37°C for 2 h and then blocked with 1% heat-denatured BSA for 1 h at 37°C. Anti-α5β1 integrin MAb JBS5 (Chemicon Int., Temecula, Calif.), anti-β1 integrin MAb P4C10 (Life Technologies), or anti-αvβ3 integrin MAb LM609 (Chemicon) was then allowed to bind to immobilized anti-mouse antibody overnight at 4°C. In certain experiments, we maintained cells in suspension by precoating the dishes with 1% heat-denatured BSA. When proteasome inhibitors were used, ECV 304 cells were pretreated with 10 μM clasto-lactacystin β-lactone (β-lactone; Calbiochem-Novabiochem Corp., La Jolla, Calif.), 10 μM lactacystin, or 50 μM N-acetyl-Leu-Leu-norleucinal (LLnL; Calbiochem-Novabiochem Corp.) in serum-free medium for 2, 4, or 10 h, respectively. The cells were then plated and incubated at 37°C for various times. Finally, the cells were harvested and rinsed in cold PBS and then frozen at −20°C.
DNA construct and transient transfections.
An N-terminally hemagglutinin (HA) epitope-tagged human p21CIP1 cDNA was generated by PCR based on a p21CIP1 cDNA template provided by Steven I. Reed, and then this HA-p21CIP1 cDNA was subcloned into the HindIII and EcoRI sites of pCDNA3. p21K6R cDNA was a generous gift from Markus Welcker that was subcloned into the HindIII and XbaI sites of the p3XFLAG-CMV-10 vector (Sigma). ECV 304 cells were transiently transfected with 4 μg each of various expression plasmids by using Lipofectamine Plus (Life Technologies) in accordance with the manufacturer's protocols and used at 36 h after transfection. Typically, 70% of ECV 304 cells were transfected this way, as detected by flow cytometry (data not shown). In some experiments, ECV 304 cells were transfected with 20 μg of a plasmid by using Lipofectamine 2000 (Life Technologies), resulting in approximately 80% transfection efficiency. Vector constructs for dominant negative (dn) N17 Cdc42, dn N17 Rac1, ca L61Rac1, ca L61Cdc42, and wild-type Cdc42 were kindly provided by Pontus Aspenström. His-tagged ubiquitin cDNA was a gift from Markus Welcker.
Analysis of active GTPases.
Cdc42 and Rac1 activities were analyzed by a glutathione S-transferase (GST)-PAK pull-down assay as previously described (39). The bacterial strain that expresses the GST-PAK-CRIB domain (GST-PAK CD) fusion protein was kindly provided by John G. Collard. ECV 304 cells were harvested and kept in serum-free medium for 3 h to silence endogenous active GTPases. Cells were then plated on FN-coated dishes for 20 min. After cells were lysed, lysates were pulled down by glutathione-Sepharose 4B beads (Pharmacia Biotech, Uppsala, Sweden) saturated with GST-PAK-CD. Finally, eluates were analyzed by Western blot analysis to detect GTP-Rac1 or GTP-Cdc42. Total Cdc42, Rac1, and actin levels in the primary lysates were detected in parallel.
Western blotting.
Cells were lysed in a PBS-TDS buffer (PBS with 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride [PMSF], 1× complete inhibitor cocktail [Boehringer Mannheim]). Protein concentrations in lysates were measured with a bicinchoninic acid protein quantification kit (Pierce, Rockford, Ill.) with BSA as the standard. In most of cases, 15 to 30 μg of total proteins was separated by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to an Immobilon PVDF membrane (Millipore, Bedford, Mass.). After blocking of membranes with 10% dry milk in TBS-T (20 mM Tris-HCl, 0.5 M NaCl, 0.2% Tween 100), blots were incubated with primary antibodies for 1 h at room temperature. Following washes and 30 min of incubation with horseradish peroxidase-conjugated secondary antibodies, blots were visualized by an enhanced chemiluminescence detection system (NEN Life Science Products, Boston, Mass.). Actin protein levels were analyzed as a control for constant loading and transfer. Densitometry was determined by Kodak Digital Science 1D Image Analysis Software.
Pulse-chase analysis.
After 24 h of transfection with HA-tagged p21CIP1, ECV 304 cells were starved for 12 h in serum-free medium. Cells were washed with PBS and incubated for 40 min in DMEM depleted of methionine (DMEM-meth; ICN Biomedicals). Following trypsinization and neutralization, cells were suspended in DMEM-meth containing 2% BSA and 300 μCi of [35S]methionine (Tran35S-label; ICN Biomedicals) per ml for 40 min, allowing the [35S]methionine to be incorporated. Unincorporated [35S]methionine was removed by three washes with 20 ml of DMEM, and cells were subsequently resuspended in DMEM containing 2 mM unlabeled methionine (time point zero). The cells were immediately put onto dishes precoated with FN or BSA as described above. At the indicated times, cells were washed in cold PBS and frozen at −70°C. For immunoprecipitation, cells were lysed in PBS-TDS buffer for 15 min on ice and then lysates were clarified by centrifugation at 13,000 rpm (Biofuge Fresco; Heraeus) for 20 min at 4°C. Furthermore, lysates were precleared for 1 h with 25 μl of protein G-Sepharose beads (Santa Cruz Biotech) at 4°C. A 200-μg sample of precleared lysates was added to protein G-Sepharose beads precoated with anti-HA MAb (12CA5) and precipitated for 2 h at 4°C. Following six washes with PBS-TDS, eluted proteins were separated by SDS-13% PAGE, fixed, and dried and the signal from radiolabeled HA-p21CIP1 was quantified with a PhosphorImager (Cyclone Phosphor System; Packard Instrument Co., Meriden, Conn.) with OptiQuant Image Analysis Software.
Ubiquitination assay.
The in vivo p21CIP1 ubiquitination assay was carried out as described previously (48). ECV 304 cells were transfected with p21CIP1, His-ubiquitin, or mock cDNA alone or cotransfected with p21CIP1 and His-ubiquitin cDNA. After 24 h, cells were starved in serum-free medium for 12 h. These transfected cells were pretreated with or without β-lactone for 2 h at the end of the starvation period. Cells cotransfected with p21CIP1 and His-ubiquitin were then allowed to attach to FN- or P-L-L-coated dishes for 30 min. Cells transfected with p21CIP1, His-ubiquitin, or mock cDNA were used as controls.
For the ubiquitination assay, cells were lysed in 6 M guanidinium hydrochloride (GuaCl), 5 mM imidazole, and 100 mM sodium phosphate (NaP, pH 8.0) per 100-mm dish. Following sonication of lysates by three bursts, 50 μl of lysates was removed and used as a whole-cell lysate input control. His-ubiquitin-conjugated proteins were pulled down by the Ni2+-agarose beads (Qiagen) for 2 h at 4°C in accordance with the manufacturer's instructions. The beads were loaded onto columns and sequentially washed with lysis buffer, 6 M GuaCl in NaP, 6 M GuaCl in protein buffer (50 mM NaP [pH 8.0], 20% glycerol, 0.2% Igepal, 100 mM KCl) containing 50% NaP, 6 M GuaCl in protein buffer containing 25% NaP, 10 mM imidazole in protein buffer, and 100 mM imidazole in protein buffer. Finally, bound proteins were eluted with 200 mM imidazole in protein buffer and precipitated with trichloroacetic acid as described by the manufacturer (Qiagen). Eluted proteins were separated by SDS-PAGE (upper part of gel,7%; lower part, 13%) and then transferred onto a PVDF membrane that was immunoblotted with anti-p21CIP1 MAb SX118.
Isolation of total RNA and Northern blotting.
Total cellular RNA was isolated with a Qiagen RNeasy kit in accordance with the manufacturer's protocol. For each sample, 10 μg of total RNA was denatured and separated in a formaldehyde-containing 1% agarose gel and then transferred to a BrightStar-Plus positively charged nylon membrane (Ambion, Austin, Tex.). After fixation and prehybridization with Prehyb/Hyb buffer (Ambion) at 65°C for 1 h, the membrane was hybridized with 32P-labeled probes including human p21CIP1 cDNA (provided by Steven I. Reed) and chicken actin cDNA in Prehyb/Hyb buffer at 42°C overnight. The membrane was washed twice for 15 min each time at room temperature in 5× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA)-0.5% SDS, twice for 15 min each time at 37°C in 1× SSPE-0.5% SDS, and three times for 15 min each at 65°C in 0.1× SSPE-1% SDS. The extent of hybridization was analyzed with a PhosphorImager (Cyclone Phosphor System; Packard Instrument Co.) with OptiQuant Image Analysis Software.
RESULTS
Down-regulation of Cdk2 inhibitors by attachment to ECM.
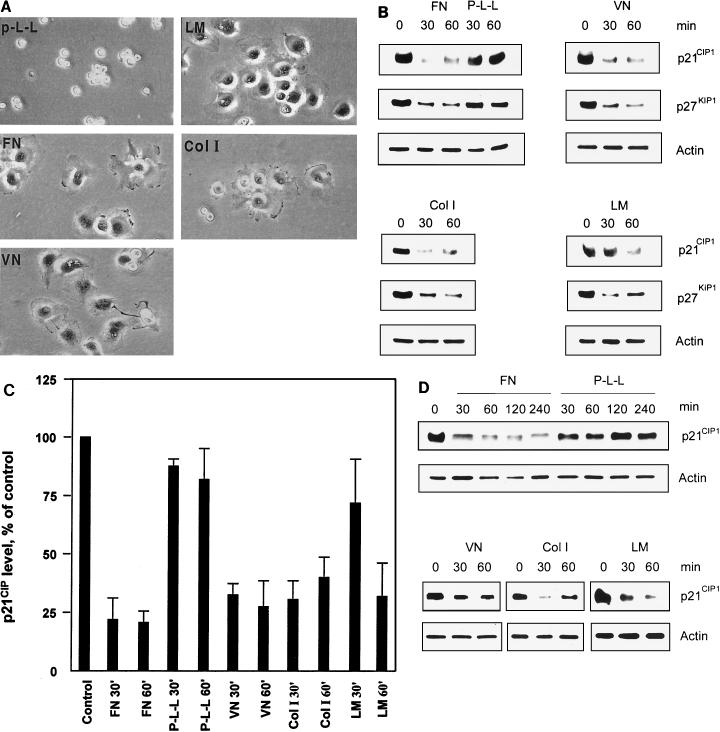
Previous studies have suggested that regulation of the Cdk2 inhibitors p21CIP1 and p27KIP1 by anchorage may function during cell cycle progression by controlling cyclin E-Cdk2 activity (11). To elucidate whether integrin-mediated cell adhesion, by itself, regulates the Cdk2 inhibitors p21CIP1 and p27KIP1, ECV 304 bladder carcinoma cells were allowed to attach to various ECM proteins, including FN, VN, ColI, and LM, with P-L-L as a control. The cells were plated in the absence of serum to avoid any contribution from growth factor signaling in order to analyze exclusively the influence of anchorage-induced signaling. To this end, cells were also starved for 12 h before plating to eliminate any sustained growth factor signaling. Firstly, the morphology of ECV 304 cells attached to distinct ECM proteins and control P-L-L was observed (Fig. 1A). ECV 304 cells spread well on FN, VN, ColI, and LM within 30 min. However, cells plated on P-L-L attached but did not spread. This can be explained by the fact that attachment to P-L-L is mediated by charge in an unspecific manner and does not involve integrins. The effect by cell attachment to various ECM components on p21CIP1 and p27KIP1 protein levels was then determined by Western blotting (Fig. 1B and C). We found the p21CIP1 and p27KIP1 protein levels to be markedly reduced within 30 min when ECV 304 cells were attached to FN, VN, or ColI, while attachment to P-L-L did not influence p21CIP1 or p27KIP1 levels. In addition, we observed a similar down-regulation of p21CIP1 and p27KIP1 by attachment to ECM upon serum-free replating of regularly cultured, nonstarved cells (data not shown). The levels of p21CIP1 were also reduced by attachment to LM but with somewhat slower kinetics compared to those seen after adhesion to the other ECM proteins (Fig. 1B and C). To test whether integrin-mediated regulation of p21CIP1 is similar in different cell types, p21CIP1 levels were also analyzed in primary HUVECs and in murine NIH 3T3 fibroblasts. The levels of p21CIP1 protein were rapidly decreased in HUVECs by attachment to FN, VN, or ColI within 30 min and were kept low for at least 4 h on FN (Fig. 1D). Attachment to LM also decreased p21CIP1 levels in HUVECs but within 60 min, while attachment to P-L-L did not influence the levels of p21CIP1 (Fig. 1D). Furthermore, a similar down-regulation of p21CIP1 was observed in NIH 3T3 fibroblasts plated on FN (data not shown). Taken together, our results demonstrate that cell attachment to ECM initiates down-regulation of p21CIP1 in various cell types, both transformed cells and primary cells of different origin, suggesting that this down-regulation constitutes a general phenomenon regardless of the cell type.
FIG. 1.
Down-regulation of the Cdk2 inhibitors p21CIP1 and p27 KIP1 upon attachment to ECM components. (A) Human bladder carcinoma ECV 304 cells were allowed to attach to dishes precoated with FN, VN, LM, ColI, or P-L-L under serum-free conditions. Photographs (20× objective) show the representative morphology of ECV 304 cells after attachment to the distinct ECM proteins for 30 min. (B) p21CIP1, p27KIP1, and actin protein levels in ECV 304 cell lysates were analyzed by Western blotting after attachment for the indicated times to different ECM components or P-L-L under serum-free conditions in order to analyze the exclusive influence of integrin ligation without intervening growth factor signaling. Actin levels were detected as a loading control. (C) Estimation of p21CIP1 levels in ECV 304 cells by densitometry of Western blots. Bars represent mean values of three independent experiments ± the standard error of the mean. The level at time zero is defined as 100% (control). (D) HUVECs were allowed to attach to FN, P-L-L, VN, ColI, or LM for various times. Cell lysates were analyzed for p21CIP1, p27KIP1, and actin protein levels by Western blotting.
Integrin ligation induces down-regulation of Cdk2 inhibitors.
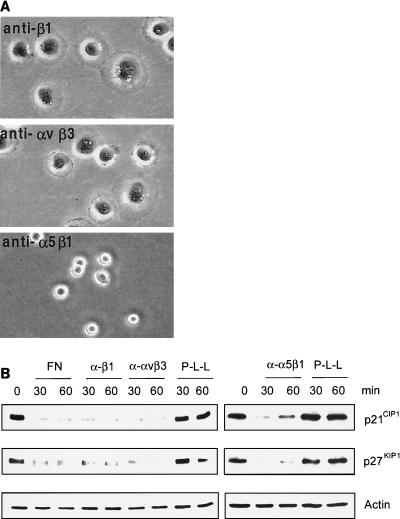
To investigate whether cell attachment-induced down-regulation of p21CIP1 and p27KIP1 is mediated specifically by integrin ligation, ECV 304 cells were plated on immobilized anti-α5β1, anti-β1, or anti-αvβ3 MAbs. Under these conditions, these anti-integrin MAbs act as agonistic integrin-ligating proteins, as shown previously (43). Cells plated on anti-β1 or anti-αvβ3 MAb both attached and spread within 30 min, whereas cells plated on anti-α5β1 MAb attached but did not spread within 30 min (Fig. 2A). Interestingly, the p21CIP1 and p27KIP1 proteins were almost eliminated by specific ligation to all three of these anti-integrin MAbs within 30 min (Fig. 2B), suggesting that specific-integrin ligation results in rapid down-regulation of the Cdk2 inhibitors p21CIP1 and p27KIP1. Because attachment to P-L-L did not induce any change in the levels of the Cdk2 inhibitors (Fig. 2B), we can exclude the action from any additional factors coinciding with integrin ligation. Also, given that attachment to the anti-α5β1 MAb did not cause cell spreading but still down-regulated p21CIP1 and p27KIP1, this down-regulation appears to be specific for integrin ligation regardless of cell spreading.
FIG. 2.
Integrin ligation specifically down-regulates p21CIP1 and p27KIP1. (A) ECV 304 cells were plated on immobilized anti-β1 (P4C10), anti-αvβ3 (LM609), or anti-α5β1 (JBS5) for 30 min and photographed with a 20× objective. (B) ECV 304 cells attached to immobilized anti-β1, anti-αvβ3, or anti-α5β1 MAb were analyzed for p21CIP1, p27KIP1, and actin protein levels by Western blotting.
Attachment to FN decreases p21CIP1 protein stability.
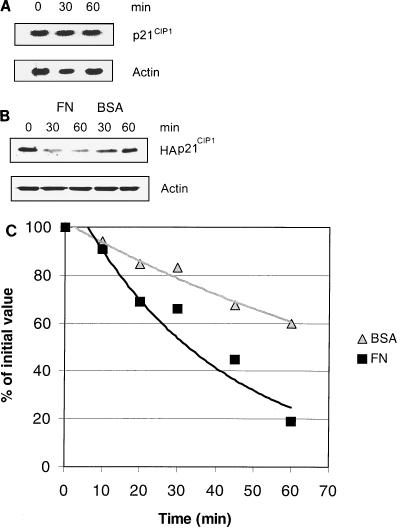
Because down-regulation of p21CIP1 by anchorage occurred as a general phenomenon in cells of various origins, we aimed to elucidate the underlying molecular mechanisms. To this end, we utilized one of the cell lines, ECV304, that is readily transfectable with high efficiency. To examine whether the rapid down-regulation of p21CIP1 by cell attachment occurs at the mRNA level, Northern blotting was performed (Fig. 3A). We found that the p21CIP1 mRNA levels were not influenced by attachment to FN within 60 min, indicating that the rapid anchorage-induced down-regulation of p21CIP1 could not be explained by modulation of p21CIP1 transcription or mRNA stability. However, another possibility is that p21CIP1 might be controlled at the level of protein stability. To test this, the stability of the p21CIP1 protein was analyzed by 35S incorporation-based pulse-chase analysis. To successfully immunoprecipitate p21CIP1, we transiently overexpressed HA-tagged p21CIP1 in ECV 304 cells. We first examined the levels of HA-p21CIP1 protein after attachment to FN and compared it to that in cells kept in suspension. By Western blotting, we found that the levels of HA-p21CIP1 were down-regulated on FN within 30 min, similar to the regulation of endogenous p21CIP1 (Fig. 3B). We then examined the stability of HA-p21CIP1 by examining levels of 35S-HA-p21CIP1. We found that the stability of the HA-p21CIP1 protein was markedly decreased in cells attached to FN compared to that of HA-p21CIP1 protein in cells kept in suspension (Fig. 3C). These results suggest that integrin-mediated cell adhesion stimulates p21CIP1 protein degradation.
FIG. 3.
Cell attachment to FN affects p21CIP1 protein stability. (A) Total mRNA isolated from ECV 304 cells previously plated on FN for the indicated times was analyzed for p21CIP1 mRNA levels by Northern blotting. β-Actin mRNA levels were measured as a loading control. (B) ECV 304 cells transiently transfected with HA-tagged p21CIP1 were allowed to attach to FN or suspended upon BSA, and then HA-p21CIP1 levels were analyzed by Western blotting. Actin levels were used as a loading control. (C) ECV 304 cells transfected with HA-p21CIP1 were plated on FN or kept in suspension (BSA), and then the stability of HA-p21CIP1 was detected by pulse-chase analysis with incorporated [35S]methionine. The values shown are the means of two or three independent experiments.
Integrin-mediated cell attachment induces proteasomal degradation of p21CIP1.
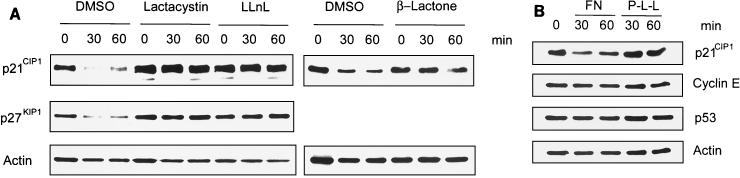
Considering that the integrin-induced rapid down-regulation of p21CIP1 coincided with changed protein stability, we hypothesized that Cdk2 inhibitors might be proteolyzed as a response to integrin ligation. In fact, both p21CIP1 and p27KIP1 have previously been found to be targeted by proteasomal degradation (1, 31, 40, 45). Therefore, we analyzed whether p21CIP1 and p27KIP1 are subjected to proteasomal degradation upon attachment to FN. For this, we utilized three specific proteasome inhibitors, lactacystin, β-lactone, and LLnL. Interestingly, the reduction of p21CIP1 and p27KIP1 by adhesion to FN was completely blocked by each of these inhibitors (Fig. 4A) while cells did not display any obvious defects and spread normally (data not shown). This indicates that integrin-mediated attachment to FN activates proteasomal proteolysis of p21CIP1 and p27KIP1.
FIG. 4.
Proteasome inhibitors block FN-induced down-regulation of p21CIP1 and p27KIP1. (A) ECV 304 cells were pretreated with lactacystin, β-lactone, or LLnL or with the dimethyl sulfoxide (DMSO) vehicle as a control and then plated on FN for the indicated times. p21CIP1, p27 KIP1, and actin protein levels were analyzed by Western blotting. (B) ECV 304 cells were allowed to attach to FN or P-L-L, and then the levels of cyclin E and p53 were examined by Western blotting. The levels of p21CIP1 were used as a comparison and determined with anti-p21CIP1 MAb SX118.
To assess whether other cell cycle regulators known to be targeted by proteasomes are also regulated by integrin-mediated cell attachment, the levels of cyclin E and p53 were examined in the same experiments as the Cdk2 inhibitors (Fig. 4B). Interestingly, cyclin E and p53 protein levels were not changed by attachment to FN within 60 min, suggesting that integrin ligation selectively induces degradation of p21CIP1 and p27KIP1.
Integrin-mediated proteasomal degradation of p21CIP1 is independent of ubiquitination.
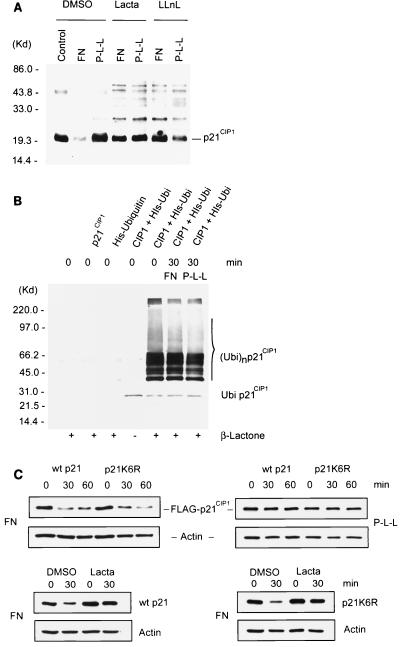
Targeted proteolysis by proteasomes preceded by ubiquitination regulates diverse biological systems (36). To analyze whether integrin-mediated cell attachment regulates ubiquitination of p21CIP1, potential ubiquinated-p21CIP1 conjugates were detected by Western blotting (Fig. 5A). We found that native p21CIP1 was degraded by attachment to FN and that this degradation was blocked by either lactacystin or LLnL. In addition, inhibition of proteolysis by these inhibitors led to an increase in high-molecular-weight bands of p21CIP1. The rapid appearance in the presence of proteasome inhibitors and the pattern of these bands, which appear similar to previously described polyubiquitinated p21CIP1 (42), indicate that these bands may represent ubiquitinated p21CIP1. However, we did not detect any differences in the degree of p21CIP1-associated high-molecular-weight bands after specific attachment to FN compared to nonspecific adhesion to P-L-L. To further clarify if the degree of p21CIP1 ubiquitination was affected by cell attachment, a more specific ubiquitination assay was performed to detect ubiquitinated p21CIP1 conjugates. p21CIP1 and His-ubiquitin were cotransfected into ECV 304 cells, and His-ubiquitin was then trapped by an Ni2+ column, followed by Western blotting for p21CIP1, specifically detecting ubiquitinated p21CIP1 (Fig. 5B). No ubiquitinated bands appeared in cells transfected with p21CIP1 or mock transfected, even in the presence of β-lactone. However, intensive bands representing ubiquitinated p21CIP1 were found when ECV 304 cells were cotransfected with p21CIP1 and His-ubiquitin in the presence of β-lactone compared to non-inhibitor treatment. These bands appeared similar in size and pattern to the p21CIP1-associated bands in Fig. 5A, suggesting that the high-molecular-weight bands in both of these experiments represent ubiquitinated p21CIP1. Interestingly, we found no difference in the levels of accumulated ubiquitin-p21CIP1 bands between cells attached to FN and those attached to P-L-L, suggesting that proteasomal proteolysis of p21CIP1 induced by specific attachment to FN is not preceded by increased ubiquitination. Importantly, the levels of a ubiquitination-deficient p21CIP1 mutant (p21K6R) (42, 47) were also reduced upon attachment to FN in a manner similar to that of wild-type p21 (Fig. 5C). Furthermore, this reduction of p21K6R was completely blocked by lactacystin (Fig. 5C), demonstrating that integrin-activated proteasomal proteolysis of p21CIP1 is independent of ubiquitination.
FIG. 5.
Ubiquitination of p21CIP1 upon cell attachment. (A) ECV 304 cells were treated with the proteasome inhibitor lactacystin (Lacta) or LLnL and then plated on FN or P-L-L for 30 min. Cell lysates were analyzed by Western blotting with anti-p21CIP1 PAb (Ab5; Oncogene). DMSO, dimethyl sulfoxide; MW, molecular mass; Kd, kilodaltons. (B) ECV 304 cells transiently mock transfected or transfected with p21CIP1 or His-ubiquitin or cotransfected with p21CIP1 and His-ubiquitin were treated with or without the proteasome inhibitor β-lactone, as indicated. Cells cotransfected with p21CIP1 and His-ubiquitin were allowed to attach to FN or P-L-L, and then His-ubiquitin was trapped by an Ni2+ column and ubiquitinated p21CIP1 was detected with anti-p21CIP1 MAb SX118 by Western blotting. (C, upper portion) ECV 304 cells transiently transfected with FLAG-tagged wild-type (wt) p21 or with the ubiquitination-deficient FLAG-tagged p21K6R mutant were plated on FN or P-L-L for the times indicated. The levels of overexpressed p21CIP1 were detected by Western blotting with anti-FLAG MAb (M2; Sigma). Actin levels were analyzed as a loading control. (C, lower portion) ECV 304 cells transiently transfected with FLAG-tagged wild-type p21 or the FLAG-tagged p21K6R mutant were treated with the specific proteasome inhibitor lactacystin or with the dimethyl sulfoxide vehicle and plated on FN for 30 min. The levels of wild-type p21 and p21K6R were determined by Western blotting with anti-FLAG MAb (M2).
Cell attachment to FN activates Cdc42 and Rac1.
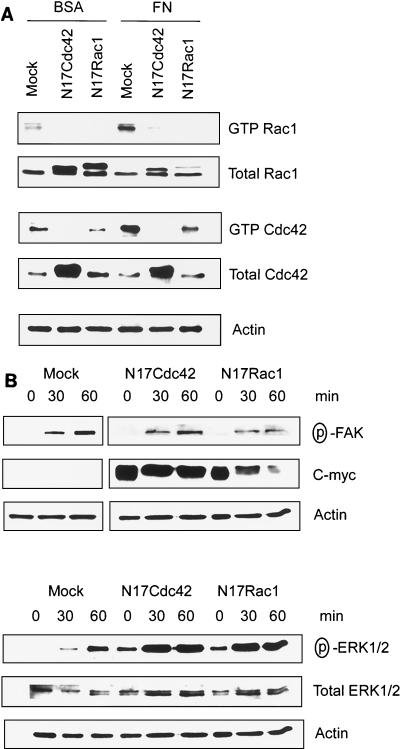
The small GTPases Cdc42 and Rac1 can be activated in response to integrin ligation to FN and collagen (7, 19, 33). To investigate potential activation of Cdc42 and Rac1 in ECV 304 cells by adhesion to FN, we used a GST-PAK1-CRIB domain fusion protein pull-down assay to quantify activated Cdc42 and Rac1. As shown in Fig. 6A, both Cdc42 and Rac1 were activated by adhesion to FN within 20 min, indicating that both of these signaling components are activated by an integrin-dependent mechanism in ECV 304 cells. However, both Rac1 and Cdc42 activities (GTP-Rac1 and GTP-Cdc42) induced by cell attachment were completely inhibited by transient expression of a dn N17Cdc42 mutant, which indicates that blocking of Cdc42 might lead to inhibition of Rac1 activation by cell attachment. Meanwhile, expression of a dn N17 Rac1 mutant blocked Rac1 activity and led to somewhat decreased endogenous levels of active Cdc42. However, Cdc42 was still activated by cell attachment to FN in the presence of dn N17Rac1 (Fig. 6A). Taken together, this could be interpreted as induction of a pathway by cell attachment in which Cdc42 is upstream of Rac1. Alternatively, dn Cdc42 and dn Rac1 might bind to and sequester the same GTP exchange factor(s) needed for activation of both Cdc42 and Rac1 but bind to these exchange factors with different affinities. In any case, our results demonstrate that attachment to FN leads to rapid activation of both Cdc42 and Rac1.
FIG. 6.
Cell attachment to FN activates a Cdc42/Rac1 signaling pathway. (A) ECV 304 cells transiently transfected with dn N17Cdc42 or dn N17Rac1 cDNA or mock transfected were plated on FN for 20 min. Cell lysates were analyzed for active GTPases by binding to a GST-PAK-CRIB domain fusion protein, followed by Western blotting with anti-Rac1 or anti-Cdc42 antibodies. Total levels of Rac1, Cdc42, and actin were analyzed by Western blotting of original cell lysates. (B) ECV 304 cells transfected with Cdc42 or dn Rac1 cDNA or mock transfected were allowed to attach to FN for the indicated times, and then activated FAK and ERK1/2 were analyzed by Western blotting with anti-phospho-FAKY397 PAb or anti-phospho-ERK1/2 PAb. The levels of c-myc were detected with anti-c-Myc MAb 9E10. Actin levels were analyzed as a loading control.
To examine whether dn Cdc42 or dn Rac1 may also block the capacity of cells to activate other integrin-dependent signaling components, we analyzed the degree of activated FAK and ERK1/2 in ECV 304 cells transfected with dn Cdc42 or dn Rac1 mutants upon attachment to FN (Fig. 6B). Both phospho-FAKY397 and phospho-ERK1/2 appeared upon attachment to FN within 60 min. Importantly, induction of phospho-FAKY397 and phospho-ERK1/2 was also detected in the presence of dn Cdc42 or dn Rac1 after attachment to FN, indicating that these integrin-induced signaling components are still activated by attachment when Cdc42 and Rac1 are blocked within our system.
An anchorage-activated Cdc42/Rac1 signaling pathway mediates down-regulation of p21CIP1.
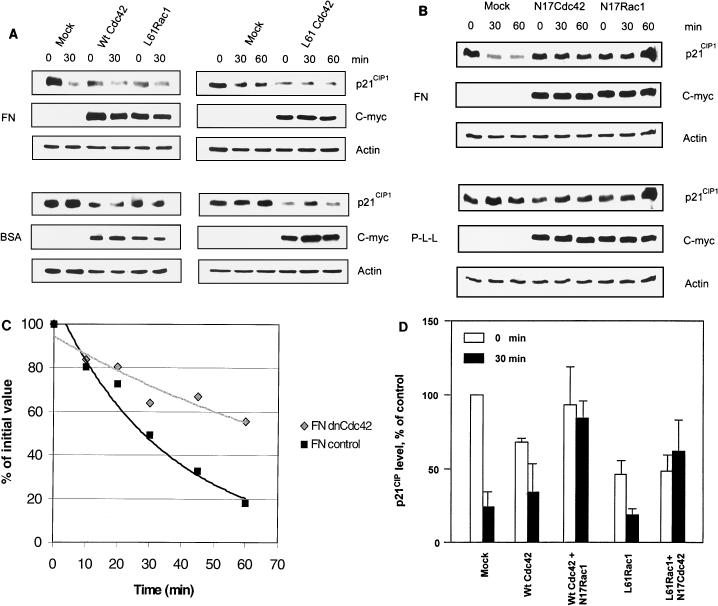
It has been suggested that both Cdc42 and Rac1 play critical roles in progression through the G1 phase of the cell cycle (23, 25, 29). However, it is not entirely clear how the effects on the cell cycle of endogenous Cdc42 and Rac signaling could be executed in terms of regulation of specific cell cycle components. To explore this, we investigated if Cdc42 and/or Rac1 might regulate p21CIP1. Transient overexpression of ca Cdc42 or ca L61Rac1 promoted reduction of p21CIP1 levels compared to those in a mock-transfected control (Fig. 7A), indicating that both of these GTPases are able to down-regulate p21CIP1. To elucidate the role of endogenous Cdc42 and Rac1 in the integrin-dependent regulation of p21CIP1, the dn N17Cdc42 and dn N17Rac1 mutants were utilized. Transient overexpression of N17Cdc42 or N17Rac1 blocked the reduction of p21CIP1 caused by adhesion to FN (Fig. 7B). Moreover, overexpression of N17Cdc42 increased the stability of p21CIP1 in cells attached to FN (Fig. 7C), indicating that integrin-induced signaling through Cdc42 increases the proteolysis rate of p21CIP1. Taken together, these results indicate that Cdc42/Rac1 signaling is required for integrin-mediated p21CIP1 degradation through proteasomal proteolysis.
FIG. 7.
Anchorage-dependent Cdc42/Rac1 signaling regulates proteolysis of p21CIP1. (A) ECV 304 cells transiently transfected with c-myc-tagged wild-type (Wt) Cdc42 or c-myc-tagged mutant ca Cdc42 or ca L61Rac1 were plated on FN- or BSA-coated plates for the indicated times. Levels of p21CIP1, c-myc, and actin were analyzed by Western blotting. Levels of c-myc were analyzed for transfection efficiency with anti-c Myc MAb 9E10, and actin was used as a loading control. (B) ECV 304 cells transiently overexpressing c-myc-tagged mutant dn N17Cdc42 or dn N17Rac1 were plated on FN or P-L-L. p21CIP1, c-myc, and actin levels were analyzed by Western blotting. (C) ECV 304 cells transiently transfected with HA-p21CIP1 or cotransfected with HA-p21CIP1 and dn Cdc42 were allowed to attach to FN for the times indicated, and then the stability of HA-p21CIP1 was examined by pulse-chase analysis. (D) Quantitative estimations of p21CIP1 levels in ECV 304 cells by densitometry of Western blots from single mock transfections or transfections with L61Rac1 or wild-type Cdc42 or cotransfections with ca L61Rac1 and dn N17Cdc42 or wild-type Cdc42 and dn N17Rac1, respectively, before and 30 min after plating onto FN. Bars represent mean values of three independent experiments ± the standard error of the mean.
On the basis of analysis of integrin-induced Cdc42 and Rac1 signaling (Fig. 6A), we hypothesized that Cdc42 might act upstream of Rac1 in the integrin-induced signaling pathway, leading to degradation of p21CIP1. To examine whether Cdc42 and Rac1 are linked into the same pathway for the regulation of p21CIP1 and to elucidate their internal order, we transiently transfected cells with combinations of wild-type Cdc42 and dn Rac1 or ca Rac1 and dn Cdc42, respectively (Fig. 7D). Overexpression of wild-type Cdc42 was used to enhance the response of Cdc42 signaling after cell attachment. This was performed in order to test if blocking of Rac1 would block Cdc42-induced down-regulation of p21CIP1, i.e., if Cdc42 and Rac1 are ordered into the same integrin-induced signaling pathway and if Rac1 acts downstream of Cdc42 in this pathway. The combination of ca L61Rac1 and dn N17Cdc42 was used to test if blocking of Cdc42 could block the effects of L61Rac1, which would reveal if Rac1 acts upstream of Cdc42 in the pathway. Coexpression of ca L61Rac1 and dn N17Cdc42 resulted in a low initial level of p21CIP1 that was comparable to levels in cells transfected with L61Rac1 alone. However, in the cotransfected cells, p21CIP1 levels were not markedly influenced by cell attachment to FN. This is consistent with the notion that L61Rac1 induces down-regulation of p21CIP1 independently of Cdc42 but with N17Cdc42 blocking any additional decrease in p21CIP1 caused by attachment to FN. Because N17Cdc42 did not block the initial down-regulation of p21CIP1 induced by ca L61Rac1, Rac1 must act independently or downstream of Cdc42. Furthermore, transient transfection of dn N17Rac1 blocked the down-regulation of p21CIP1 by attachment to FN even when wild-type Cdc42 was used to enhance the Cdc42 signaling response (Fig. 7D). Because N17Rac1 was not able to block the attachment-induced activation of endogenous Cdc42 (Fig. 6A), its capacity to block down-regulation of p21CIP1 by attachment to FN in the presence of overexpressed wild-type Cdc42 indicates that Rac1 acts downstream of Cdc42 in regulating p21CIP1 degradation. Taken together, our results point to a novel mechanism linking integrins, Cdc42, and Rac1 into the same signaling pathway, leading to p21CIP1 proteolysis, with Cdc42 ordered upstream of Rac1 within the pathway.
DISCUSSION
Integrin ligation to ECM components leads to the activation of a variety of signaling pathways. These signals are essential for cell proliferation in all tissue cells, suggesting that integrin-activated signaling events regulate key cell cycle components that are important for the control of cell cycle progression (13). To this end, we found that cell attachment to FN induced proteasomal proteolysis of the Cdk2 inhibitors p21CIP1 and p27KIP1.
Cell adhesion to FN, in some cases, engages both integrins and additional receptors, including syndecans (40). However, we used a purified 120-kDa RGD-containing cell-binding fragment of FN for which integrins are the only identified cellular receptors and that does not contain the heparin-binding domain involved in syndecan engagement (37). Furthermore, attachment to three specific immobilized agonistic anti-integrin MAbs gave the same response on the regulation of Cdk2 inhibitor levels as adhesion to FN. This occurred while attachment to P-L-L under the same conditions in the presence of all other potential regulators did not influence p21CIP1 or p27KIP1 levels, showing that integrin ligation is specifically responsible for the down-regulation of p21CIP1 and p27KIP1. Besides cell adhesion, cell spreading is also an important factor in regulation of cellular functions, including cell proliferation and survival (5). Although cell spreading could induce down-regulation of p27KIP1 (18), our results showed that both p21CIP1 and p21KIP1 levels were strongly reduced by attachment to an immobilized anti-α5β1 MAb to which the cells adhered but did not spread. This suggests that the degradation of p21CIP1 and p27KIP1 is influenced primarily by specific integrin-induced signaling and not by the degree of cell spreading.
Integrin ligation to the ECM also plays an important role in the control of anchorage-dependent cell proliferation by facilitating progression through the G1 phase of the cell cycle (31). To this end, cell anchorage regulates the levels and/or activity states of various cell cycle components in the G1 phase of the cell cycle, including cyclins D and E, Rb, p53, and the Cdk2 inhibitors p21CIP1 and p27KIP1 (11, 25, 45, 49, 52). Importantly, cell anchorage promotes the activity of cyclin E-Cdk2 kinase by controlling Cdk2 inhibitors p21CIP1 and p27KIP1 (11), suggesting that suppression of Cdk2 inhibitor levels by cell attachment is required during the cyclin E-Cdk2 point in late G1 phase and is needed until this point is passed. In fact, down-regulation of p27KIP1 in late G1 phase has been identified as central for S-phase entry (43). Taken together, this suggests that regulation of the Cdk2 inhibitors p21CIP1 and p27KIP1 by cell attachment is important for progression through late G1 phase. In addition to binding and blocking of cyclin E-Cdk2 activity in G1 phase, p21CIP1 also associates with proliferating-cell nuclear antigen, thereby inhibiting DNA replication (12). Furthermore, p21CIP1 can assert an inhibitory function in the G2/M transition (27). However, it is unclear if the regulation of p21CIP1 by cell anchorage may influence these functions of p21CIP1 in the S and G2/M phases of the cell cycle.
Our finding that cell attachment regulates proteasomal proteolysis of p27KIP might be predicted, since proteasomal degradation is considered the major p27KIP1 level regulation pathway (32, 44). However, regulation of p21CIP1 has been associated mostly with transcriptional control, including the induction of p21CIP1 by p53 that causes cell cycle arrest after UV irradiation, and the regulation of p21CIP1 in fibroblasts by cell anchorage in the presence of serum factors, where no changes in protein stability were detected (2, 10, 11, 52). However, our experiments that were performed without serum in order to examine the exclusive effect of integrin-mediated signaling showed that integrin ligation to ECM per se did not rapidly influence mRNA levels. Instead, we found that p21CIP1 is regulated by ECM at the level of proteolysis since the stability of p21CIP1 was decreased by attachment to ECM and the down-regulation of p21CIP1 could be blocked by three distinct specific proteasome inhibitors. Although we cannot exclude the possibility of an additional contribution by regulation of the p21CIP1 translational rate, our results that demonstrate that cell attachment to the ECM induces rapid proteasomal degradation of p21CIP1 is the first example of regulation of proteasomal proteolysis by integrin signaling. The difference in the regulation of p21CIP1 stability observed by us compared to the study by Bottazzi et al. (2) in a more complex setup may depend on the different methods and/or cell types used, where we examined the isolated effects of integrin signaling. Our setup, analyzing the exclusive effects of cell anchorage, may limit our conclusions to situations with very active integrin signaling, such as after replating of cells onto the ECM. However, the effects of such signals are likely to represent a contribution by integrins also in more complex situations.
In most studied cases, proteasomal proteolysis is preceded by ubiquitination, where targeted proteins covalently link to multiple ubiquitin molecules and form ubiquitinated protein conjugates, leading to rapid degradation by 26S proteasomes (34). However, certain proteins, including ornithine decarboxylase and p21CIP1, do not require ubiquitination for proteasomal processing (26, 42). In the case of p21CIP1, the lack of a need for ubiquitination may be explained by the binding of the C terminus of p21CIP1 directly to the C8-α subunit of the 20S proteasome complex, leading to degradation of p21CIP1 (47). To this end, our findings indicate that the activation of proteasomal degradation of p21CIP1 by specific integrin-mediated cell anchorage is not preceded by increased ubiquitination. Importantly, the p21CIP1 mutant p21K6R, lacking all of the potential ubiquitination lysine residues, was still degraded upon attachment to FN, a degradation that was blocked by the specific proteasome inhibitor lactacystin. This suggests that integrin-induced proteolysis of p21CIP1 may represent a physiological proteasomal pathway that is independent of ubiquitination.
Several integrin-activated signaling pathways promote cell proliferation, including FAK, mitogen-activated protein kinase (ERK1/2), PI-3K, and integrin-linked kinase (13). However, a FAK, MEK1/ERK1/2, or PI-3K block in our system did not reverse the integrin-induced down-regulation of p21CIP1 (W. Bao and S. Strömblad, unpublished data). Instead, we linked this down-regulation of p21CIP1 to signaling by the small GTPases Cdc42 and Rac. One feature of these GTPases is that Cdc42 activation can induce subsequent activation of Rac (22, 28). This may be brought about by the exchange factor PIX, which is enriched in Cdc42- and Rac1-driven focal complexes and has been suggested to link Cdc42 to Rac activation by coupling of the Cdc42 effector PAK (22, 24, 34). Furthermore, it has been indicated that an attachment-activated Cdc42 block inhibits cell spreading, a function that could be restored by transfection with ca Rac, indicating that Rac may act downstream of Cdc42 in integrin signaling (33). However, activation of the Cdc42 and Rac1 signaling components in this putative integrin signaling pathway and their potential interdependence have not been thoroughly examined. To this end, by analysis of the interdependence of Cdc42 and Rac1 activation upon attachment, and by cotransfecting various combinations of Cdc42 and Rac1 mutants for analysis of p21CIP1 regulation, we found that integrin ligation coordinates Cdc42 and Rac1 signaling in the same pathway that regulates p21CIP1, with Cdc42 ordered upstream of Rac1.
It is known that small GTPases influence the cell cycle in terms of regulation of specific cell cycle components since overexpression of ca Cdc42 and Rac mutants promotes cyclin D transcription and pRB hyperphosphorylation, induces E2F transcriptional activity, and contributes to S-phase entry (14). Integrin-activated Rac controls progression through the G1 phase of the cell cycle by regulating cyclin D1 synthesis, while Rho affects the timing of cyclin D (25, 49). In addition, Rho and Ras signaling stimulates p27KIP1 degradation and regulates p21CIP1 expression in response to growth factors (15, 30, 46). However, although the small GTPases Rho, Rac, and Cdc42 may be functionally involved in G1-phase progression (15, 25, 30, 49), it was not previously clear if small GTPases could affect Cdk2 inhibitors as a response to cell anchorage. Thus, our finding that integrin activation of endogenous Cdc42/Rac1 signaling induces proteasomal degradation of the Cdk2 inhibitor p21CIP1 might contribute to clarification of the complex function of small GTPases in cell cycle progression.
Rho, Rac, and Cdc42 also regulate the assembly of multimolecular focal adhesion complexes that are associated with the formation of actin stress fibers, lamellipodia, and filopodia, respectively (28, 34). In addition, integrin-activated Cdc42 and Rac1 are involved in the regulation of cell spreading (7, 33). However, our results indicate that inhibition of Cdc42 or Rac1 did not block activation of FAK or ERK1/2 induced by cell attachment to FN. Considering also that down-regulation of p21CIP1 and p27KIP1 also occurred in the absence of cell spreading, we conclude that an integrin-induced signaling pathway through Cdc42/Rac1 specifically causes proteasomal proteolysis of p21CIP1.
In conclusion, our results demonstrate that integrin-mediated cell attachment to the ECM induces proteasomal proteolysis of the Cdk2 inhibitors p21CIP1 and p27KIP1 and that degradation of p21CIP1 is independent of ubiquitination. The integrin-induced Cdc42/Rac1 signaling pathway activates proteasomal degradation of p21CIP1. Integrin regulation of proteasomal proteolysis might contribute to the control of anchorage-dependent cell proliferation.
Acknowledgments
We are most grateful for the contribution of various cDNAs by colleagues, including Pontus Aspenström, John G. Collard, Markus Welcker, and Steven I. Reed. Many thanks go to Annica Ekström and Zhilun Li for excellent technical assistance and Pernilla Lång for computer guidance.
This study was supported by grants to S.S. from the Swedish Cancer Society, the Swedish Medical Research Council, and the Magnus Bergvall Foundation. M.T. was supported by the Swedish Cancer Society, and H.Z. was supported by the Wenner-Gren Foundation and the Swedish Medical Society.
REFERENCES
- 1.Blagosklonny, M. V., G. S. Wu, S. Omurand, and W. S. El-Deiry. 1996. Proteasome-dependent regulation of p21WAF1/CIP1 expression. Biochem. Biophys. Res. Commun. 227:564-569. [DOI] [PubMed] [Google Scholar]
- 2.Bottazzi, M. E., X. Y. Zhu, R. M. Böhmer, and R. K. Assoian. 1999. Regulation of p21cip1 expression by growth factors and the extracellular matrix reveals a role for transient ERK activity in G1 phase. J. Biol. 146:1255-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyer, S. N., D. E. Wazer, and V. Band. 1996. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 56:4620-4624. [PubMed] [Google Scholar]
- 4.Brown, J., S. J. Reading, S. Jones, C. J. Fitchett, J. Howl, A. Martin, C. Longland, F. Michelangeli, Y. E. Dubrova, and C. A. Brown. 2000. Critical evaluation of ECV 304 as a human endothelial cell model defined by genetic analysis and functional responses: a comparison with the human bladder cancer derived epithelial cell line T24/83. Lab. Investig. 80:37-45. [DOI] [PubMed] [Google Scholar]
- 5.Chen, C. S., M. Marksich, S. Huang, G. M. Whitesides, and D. E. Ingber. 1997. Geometric control of cell life and death. Science 276:1425-1428. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Q., T. H. Lin, C. J. Der, and R. L. Juliano. 1996. Integrin-mediated activation of mitogen-activated protein (MAP) or extracellular signal-related kinase kinase (MEK) and kinase is independent of Ras. J. Biol. Chem. 271:18122-18127. [DOI] [PubMed] [Google Scholar]
- 7.Clark, E. A., W. G. King, J. S. Brugge, M. Symons, and R. O. Hynes. 1998. Integrin-mediated signals regulated by members of the Rho family of GTPases. J. Cell Biol. 142:573-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Amico, M., J. Hulit, D. F. Amanatullah, B. T. Zafonte, C. Albanese, B. Bouzahzah, M. Fu, L. H. Augenlicht, L. A. Donehower, K. I. Takemaru, R. T. Moon, R. Davis, M. P. Lisanti, M. Shtutman, J. Zhurinsky, A. Ben-Ze'ev, A. A. Troussard, S. Dedhar, and R. G. Pestell. 2000. The integrin-linked kinase regulates the cyclin D1 gene through glycogen synthase kinase 3β and cAMP-responsive element-binding protein-dependent pathways. J. Biol. Chem. 275:32649-32657. [DOI] [PubMed] [Google Scholar]
- 9.del Pozo, M. A., L. S. Price, N. B. Alderson, X. D. Ren, and M. A. Schwartz. 2000. Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J. 19:2008-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Deiry, W. S., T. Tokino, V. E. Velculescu, D. B. Levy, R. Parsons, J. M. Trent, D. Lin, W. E. Mercer, W. K. Kinzler, and B. Vogelstein. 1993. WAF1, a potential mediator of p53 tumor suppression. Cell 75:817-825. [DOI] [PubMed] [Google Scholar]
- 11.Fang, F., G. Orend, N. Watanabe, T. Hunter, and E. Ruoslahti. 1996. Dependence of cyclin E-cdk2 kinase activity on cell anchorage. Science 271:499-502. [DOI] [PubMed] [Google Scholar]
- 12.Flores-Rozas, H., Z. Kelman, F. B. Dean, Z.-O. Pan, J. W. Harper, S. J. Elldge, M. O'Donnell, and J. Hurwitz. 1994. Cdk-interacting protein 1 directly binds with proliferating cell nuclear antigen and inhibits DNA replication catalyzed by the DNA polymerase δ holoenzyme. Proc. Natl. Acad. Sci. USA 91:8655-8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giancotti, F. G., and E. Ruoslahti. 1999. Integrin signaling. Science 285:1028-1032. [DOI] [PubMed] [Google Scholar]
- 14.Gjoerup, O., J. Lukas, J. Bartek, and B. M. Willumsen. 1998. Rac and Cdc42 are potent stimulators of E2F-dependent transcription capable of promoting retinoblastoma susceptibility gene product hyperphosphorylation. J. Biol. Chem. 273:18812-18818. [DOI] [PubMed] [Google Scholar]
- 15.Hirai, A., S. Nakamura, Y. Noguchi, T. Yasuda, M. Kitagawa, I. Tatsuno, T. Oeda, K. Tahara, T. Terano, S. Narumiya, L. D. Kohn, and Y. Saito. 1997. Geranylgeranylated Rho small GTPase(s) are essential for the degradation of p27Kip1 and facilitate the progression from G1 to S phase in growth-stimulated rat FRTL-5 cells. J. Biol. Chem. 272:13-16. [PubMed] [Google Scholar]
- 16.Hofmann, F., F. Martelli, D. M. Livingston, and Z. Wang. 1996. The retinoblastoma gene product protects E2F-1 from degradation by the ubiquitin-proteasome pathway. Genes Dev. 10:2949-2959. [DOI] [PubMed] [Google Scholar]
- 17.Hotchin, N. A., and A. Hall. 1995. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J. Cell Biol. 131:1857-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang, S., C. S. Chen, and D. E. Ingber. 1998. Control of cyclin D1, p27KIP1, and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension. Mol. Biol. Cell 9:3179-3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivaska, J., H. Reunanen, J. Westermarck, L. Koivisto, V. M. Kähäri, and J. Heino. 1999. Integrin α2β1 mediates isoform-specific activation of p38 and upregulation of collagen gene transcription by a mechanism involving the α2 cytoplasmic tail. J. Cell Biol. 147:401-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King, R. W., R. J. Deshaies, J. M. Peters, and M. W. Kirschner. 1996. How proteolysis drives the cell cycle. Science 274:1652-1658. [DOI] [PubMed] [Google Scholar]
- 21.Koepp, D. M., J. W. Harper, and S. J. Elledge. 1999. How the cyclin became a cyclin: regulated proteolysis in the cell cycle. Cell 97:431-434. [DOI] [PubMed] [Google Scholar]
- 22.Kozma, R., S. Ahmed, A. Best, and L. Lim. 1995. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol. Cell. Biol. 15:1942-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamarche, N., N. Tapon, L. Stowers, P. D. Burelo, P. Aspenström, T. Bridges, J. Chant, and A. Hall. 1996. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell 87:519-529. [DOI] [PubMed] [Google Scholar]
- 24.Manser, E., T. H. Loo, C. G. Koh, Z. S. Zhao, X. Q. Chen, L. Tan, I. Tan, T. Leung, and L. Lim. 1998. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol. Cell 1:83-192. [DOI] [PubMed] [Google Scholar]
- 25.Mettouchi, A., S. Klein, W. J. Guo, M. Lopez-Lago, E. Lemichez, J. K. Westwick, and F. G. Giancotti. 2001. Integrin-specific activation of Rac controls progression through the G1 phase of the cell cycle. Mol. Cell 8:115-127. [DOI] [PubMed] [Google Scholar]
- 26.Murakami, Y., S. Matsufuji, T. Kameji, S. Hayashi, K. Igarashi, T. Tamura, K.Tanaka, and A. Ichihara. 1992. Ornithine decarboxylase is degraded by the 26S proteasome without ubiquitination. Nature 360:597-599. [DOI] [PubMed] [Google Scholar]
- 27.Niculescu, A. B., III, A. Chen, M. Smeets, L. Hengst, C. Prives, and S. I. Reed. 1998. Effects of p21cip1/waf1 at both the G1/S and G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol. Cell. Biol. 18:629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nobes, C. D., and A. Hall. 1995. Rho, Rac and Cdc42 GTPases regulate the assembly of multi-molecular focal complexes associated with actin stress fibers, lamellipodia and filopodia. Cell 81:53-62. [DOI] [PubMed] [Google Scholar]
- 29.Olson, M. F., A. Ashworth, and A. Hall. 1995. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science 269:1270-1272. [DOI] [PubMed] [Google Scholar]
- 30.Olson, M. F., H. F. Paterson, and C. J. Marshall. 1998. Signals from Ras and Rho GTPases interact to regulate expression of p21Waf1/Cip1. Nature 394:295-299. [DOI] [PubMed] [Google Scholar]
- 31.O'Neill., C. P. Jordan, and G. Ireland. 1986. Evidence for two distinct mechanisms of anchorage stimulation in freshly explanted and 3T3 Swiss mouse fibroblasts. Cell 44:489-496. [DOI] [PubMed] [Google Scholar]
- 32.Pagano, M., S. Tam, A. Theodoras, P. Beer, G. Delsal, V. Chau, R. Yew, G. Draetta, and M. Rolfe. 1995. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 269:682-685. [DOI] [PubMed] [Google Scholar]
- 33.Price, L. S., J. Leng, M. A. Schwartz, and G. M. Bokoch. 1998. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol. Biol. Cell 9:1863-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ridley, A. J., and A. Hall. 1992. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70:389-399. [DOI] [PubMed] [Google Scholar]
- 35.Roovers, K., G. Davey, X. Zhu, M. E. Bottazzi, and R. K. Assoian. 1999. α5β1 Integrin controls cyclin D1 expression by sustaining mitogen-activated protein kinase activity in growth factor-treated cells. Mol. Biol. Cell 10:3197-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubin, D. M., and D. Finley. 1995. The proteasome: a protein-degrading organelle? Curr. Biol. 5:854-858. [DOI] [PubMed] [Google Scholar]
- 37.Ruoslahti, E. 1988. Fibronectin and its receptors. Annu. Rev. Biochem. 57:375-413. [DOI] [PubMed] [Google Scholar]
- 38.Ruoslahti, E., and J. C. Reed. 1994. Anchorage dependence, integrins, and apoptosis. Cell 77:477-478. [DOI] [PubMed] [Google Scholar]
- 39.Sander, E. E., S. van Delft, J. P. ten Klooster, T. Reid, R. A. van der Kammen, F. Michiels, and J. G. Collard. 1998. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J. Cell Biol. 143:1385-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saoncella, S., F. Echtermeyer, F. Denhez, J. K. Nowlen, D. F. Mosher, S. D. Robinson, R. O. Hynes, and P. F. Goetinck. 1999. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc. Natl. Acad. Sci. USA 96:2805-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheffner, M., J. M. Huibregtse, R. D. Vierstra, and P. M. Howley. 1993. The HPV 16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 75:495-505. [DOI] [PubMed] [Google Scholar]
- 42.Sheaff, R. J., J. D. Singer, J. Swanger, M. Smitherman, J. M. Roberts, and B. E. Clurman. 2000. Proteasomal turnover of p21Cip1 does not require p21Cip1 ubiquitination. Mol. Cell 5:403-410. [DOI] [PubMed] [Google Scholar]
- 43.Sherr, C. J., and J. M. Roberts. 1999. Cdk inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13:1501-1512. [DOI] [PubMed] [Google Scholar]
- 44.Shirane, M., Y. Harumiya, N. Ishida, A. Hirai, C. Miyamoto, S. Hatakeyama, K. Nakayama, and M. Kitagawa. 1999. Down-regulation of p27Kip1 by two mechanisms, ubiquitin-mediated degradation and proteolytic progressing. J. Biol. Chem. 274:13886-13893. [DOI] [PubMed] [Google Scholar]
- 45.Strömblad, S., J. C. Becker, M. Yebra, P. C. Brooks, and D. A. Cheresh. 1996. Suppression of p53 activity and p21WAF1/CIP1 expression by vascular cell integrin αVβ3 during angiogenesis. J. Clin. Investig. 98:426-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takuwa. N., and Y. Takuwa. 1997. Ras activity late in G1 phase required for p27kip1 downregulation, passage through the restriction point, and entry into S phase in growth factor-stimulated NIH 3T3 fibroblasts. Mol. Cell. Biol. 17:5348-5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Touitou, R., J. Richardson, S. Bose, M. Nakanishi, J. Rivett, and M. J. Allday. 2001. A degradation signal located in the C-terminus of p21WAF1/CIP1 is a binding site for the C8-α-subunit of the 20S proteasome. EMBO J. 20:2367-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Treier, M., L. M, Sstaszewski, and D. Bohmann. 1994. Ubiquitin-dependent c-Jun degradation in vivo is mediated by the d domain. Cell 78:787-798. [DOI] [PubMed] [Google Scholar]
- 49.Welsh, G. F., K. Roovers, J. Villanueva, Y. Q. Liu, M. A. Schwartz, and R. K. Assoian. 2001. Timing of cyclin D1 expression within G1 phase is controlled by Rho. Nat. Cell Biol. 3:950-957. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto, M., N. Marui, T. Sakai, N. Morii, S. Kozaki, K. Ikai, S. Imamura, and S. Narumiya. 1993. ADP-ribosylation of the rhoA gene product by botulinum C3 exoenzyme causes Swiss 3T3 cells to accumulate in the G1 phase of the cell cycle. Oncogene 8:1449-1455. [PubMed] [Google Scholar]
- 51.Zhao, J. H., R. Pestell, and J. L. Guan. 2001. Transcriptional activation of cyclin D1 promoter by FAK contributes to cell cycle progression. Mol. Biol. Cell 12:4066-4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu, X., M. Ohtsubo, R. M. Böhmer, J. M. Roberts, and R. K. Assoian. 1996. Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2, and phosphorylation of the retinoblastoma protein. J. Cell Biol. 133:391-403. [DOI] [PMC free article] [PubMed] [Google Scholar]