Abstract
Members of the transforming growth factor β (TGF-β) family of proteins signal through cell surface transmembrane serine/threonine protein kinases known as type I and type II receptors. The TGF-β signal is extended through phosphorylation of receptor-associated Smad proteins by the type I receptor. Although numerous investigations have established the sequence of events in TGF-β receptor (TGF-βR) activation, none have examined the role of the endocytic pathway in initiation and/or maintenance of the signaling response. In this study we investigated whether TGF-βR internalization modulates type I receptor activation, the formation of a functional receptor/Smad/SARA complex, Smad2/3 phosphorylation or nuclear translocation, and TGF-β-dependent reporter gene activity. Our data provide evidence that, whereas type I receptor phosphorylation and association of SARA and Smad2 with the TGF-βR complex take place independently of clathrin lattice formation, Smad2 or Smad3 activation and downstream signaling only occur after endocytic vesicle formation. Thus, TGF-βR endocytosis is not simply a way to dampen the signaling response but instead is required to propagate signaling via the Smad pathway.
The transforming growth factor β (TGF-β) superfamily is composed of many multifunctional cytokines including TGF-βs, activins, inhibins, and bone morphogenic proteins. These proteins regulate a variety of biological responses such as proliferation, differentiation, apoptosis, and development (20). Members of this family signal through cell surface transmembrane serine/threonine protein kinases known as type I and type II receptors. Progress over the past few years has led to the elucidation of a novel signal transduction pathway from membrane receptors to nuclear target genes (19, 30). According to this model, TGF-β binds to a heteromeric complex of type I and type II receptors. The constitutively active type II receptor phosphorylates and activates the type I receptor which phosphorylates Smad2 and Smad3 (receptor-associated Smads) (6, 37, 38). Receptor-associated Smads bind the common mediator Smad4 and are translocated to the nucleus, where they associate with DNA-binding cofactors to modulate gene transcription (19, 30, 39). In addition to direct effects of Smads on gene expression, there are also reports of cross talk between the Smad and mitogen-activated protein kinase or Stat pathways and evidence for Smad-independent signaling (13, 32, 40).
Although the series of events leading to activation and inactivation of various components in TGF-β receptor (TGF-βR) signaling have been documented in detail, the endocytic aspect of this receptor system has been explored to a lesser degree. This is in contrast to other receptor systems, such as the epidermal growth factor receptor or G protein-coupled receptors, where specific mechanisms controlling receptor endocytosis and trafficking have been well defined (4, 5, 17, 22). The lack of endocytic studies on TGF-βRs is in part due to the relatively low number of TGF-βRs on the cell surface and a high degree of nonspecific binding. More importantly, both heteromeric (type I/type II) and homomeric (type I/type I or type II/type II) combinations of type I and type II receptors can occur on the cell surface capable of binding ligand (11), but only the heteromers are signaling competent (3, 9, 18, 23). As such, we designed chimeric receptors capable of addressing the specific contributions of various receptor combinations to the overall endocytic and signaling response. These receptors consist of the extracellular ligand binding domain of the granulocyte-macrophage colony-stimulating factor (GM-CSF) α or β receptor fused to the transmembrane and cytoplasmic domain of the type I or type II TGF-βR. The addition of GM-CSF results in dimerization of the α and β subunits and the formation of defined type I and/or type II TGF-βR cytoplasmic domain interactions. The chimeric receptors have been shown to have the identical signaling activity as native TGF-βRs in mesenchymal (AKR-2B) and epithelial (Mv1Lu) cells (3, 9).
Although ligand-mediated signaling can be regulated at many levels, endocytic and trafficking studies have led to the development of at least two general models describing the role of receptor endocytosis in signaling. The first suggests that endocytic activity is primarily a mechanism for dampening the signal; the signaling pathways initiated by these receptor systems are enhanced and/or unaffected by inhibiting endocytosis or retaining the receptors at the cell surface (5, 8, 15, 17, 34). An alternative model indicates that receptor endocytosis is needed to promote the association of activated receptors with various signaling intermediaries (5, 7, 8, 17, 41). In these receptor systems, inhibition of endocytosis prevents ligand-dependent signaling. Although aspects of both models have been reported for tyrosine kinase and G protein-coupled receptors (17), it is currently unknown whether TGF-βR signaling occurs at the plasma membrane and/or in an endosomal compartment. In that regard, a potential endocytic component to TGF-β signaling was suggested in a recent study linking the FYVE-domain protein SARA (for Smad anchor for receptor activation) to activation of the TGF-β/Smad pathway (31). SARA functions to recruit Smad2 or Smad3 to the activated type I receptor via membrane binding of PtdIns(3)P. Although FYVE-domain proteins have been implicated in various aspects of membrane trafficking (28), an endocytic role for SARA or the subcellular location of Smad2 phosphorylation has not been defined.
The objectives of the present study were to examine (i) the relationship between TGF-βR endocytosis and the formation of the activated receptor complex and (ii) the endocytic locale(s) where TGF-β signaling is initiated and/or extended. Our observations support the hypothesis that TGF-β signaling, monitored by Smad2 and Smad3 activation and subsequently by 3TP-Lux activity, occurs in an intracellular compartment distal to the locale(s) for type I receptor phosphorylation and SARA association. As such, the formation of a receptor/Smad/SARA complex, in itself, is not sufficient for Smad signaling but requires the engagement of the endocytic machinery subsequent to coated vesicle formation.
MATERIALS AND METHODS
Internalization assays.
125I-labeled GM-CSF internalization was performed as previously described (1). Briefly, internalization was measured in the absence or presence of potassium depletion and at various temperatures in an Mv1Lu clone (MB102-9) which stably expresses chimeric TGF-β type I and type II receptors (9) as well as functional native TGF-βRs.
Smad2-P immunoblots.
To detect phosphorylated Smad2 (Smad2-P) in vivo, cultures in 0.2% fetal bovine serum (FBS)-Dulbecco’s modified Eagle medium (DME) were stimulated with either 10 ng of GM-CSF/ml to activate the chimeric receptors or 10 ng of TGF-β/ml to activate the endogenous receptors. After ligand treatment, cellular lysates (50 mM Tris [pH 7.4], 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EGTA, 1 mM Na3VO4, 1× Complete protease inhibitor [Boehringer]) were probed with a phospho-specific Smad2 antibody, stripped, and tested for total Smad2 (both from Upstate Biotechnology). Each experiment was done at least three times, and representative blots were chosen. To examine the levels of Smad2-P in the absence of endocytic activity, cells were treated with ligand at 4°C for 30 min and then transferred to the indicated treatment prior to lysis.
In vitro Smad phosphorylation.
The protocol of Wells et al. (35) was followed. Cos-7 cells were transiently cotransfected with hemagglutinin (HA)-tagged type I (wild type or K232R mutant) and type II TGF-βRs. At 24 h posttransfection, the cells were left untreated or incubated with 10 ng of TGF-β/ml in 0.2% FBS at 16°C for 1 h. Cells were lysed, normalized for transfection, incubated with 3 μg of glutathione S-transferase (GST)-Smad2, and precipitated with 25 μl of glutathione-Sepharose 4B (Pharmacia) at 4°C. The resin was washed three times with lysis buffer, once with kinase buffer (20 mM Tris-HCl [pH 7.5], 50 mM NaCl, 12 mM MgCl2, 5 mM dithiothreitol), and then suspended in 40 μl of kinase buffer. The kinase reaction was initiated by addition of 50 μCi of [γ-32P]ATP at the indicated temperatures for 30 min, followed by washing with ice-cold cell lysis buffer. Samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography. A portion of the lysate was analyzed for receptor expression (data not shown).
In vivo type I receptor phosphorylation.
Cos-7 cells were transiently cotransfected with HA-tagged type I and His-tagged type II TGF-βRs and a plasmid expressing β-galactosidase by using Fugene 6 (Boehringer). At 36 h posttransfection, cells were treated with phosphate-free medium for 2 h and then with fresh phosphate-free medium containing 0.5 mCi of 32P-labeled inorganic phosphate/ml for an additional 2 h. Ligand at 10 ng/ml was added to chilled cells for 30 min. The cells were either left at 4°C or transferred to the indicated temperatures for an additional 20 min. Normalized lysates were incubated at 4°C with His-Bind resin (Novagen) overnight, and the receptor complexes were eluted in buffer containing 350 mM imidazole (38). The HA-tagged type I receptor was isolated from the eluted complex by immunoprecipitation with the 12CA5 monoclonal antibody (Boehringer), and a nonradioactive aliquot was subjected to HA-Western analysis to establish equal receptor expression.
Experiments with dominant-negative dynamin 2ab were conducted in a similar manner with R1B cells (an Mv1Lu clone lacking the type I TGF-βR) initially seeded at 1.8 × 106 cells/100-mm dish in 10% FBS-DME (see Fig. 5). After 24 h of incubation, 15 μg of HA-tagged type I TGF-βR was transfected by using Fugene 6 with 45 μg of green fluorescent protein (GFP)-tagged wild-type or K44A mutant dynamin 2ab. At 48 h posttransfection, cells were ligand treated and pulsed with radioactivity as described above. Cultures were lysed in radioimmunoprecipitation assay buffer (50 mM Tris, pH 8.0; 1% Triton X-100; 1% deoxycholate; 0.1% SDS; 1× phosphate-buffered saline [PBS]; 50 mM NaF; 0.1 mM sodium vanadate; 1.48 mg of iodoacetamide/ml; 1× Complete protease inhibitor), and the phosphorylated receptor was detected by SDS-PAGE after overnight incubation with anti-HA affinity matrix (Roche) and an extensive wash with radioimmunoprecipitation assay buffer. To document equal receptor and dynamin expression, parallel nonradioactive plates were subjected to type I receptor (Santa Cruz Biotechnology) and GFP-dynamin (Roche) Western analysis.
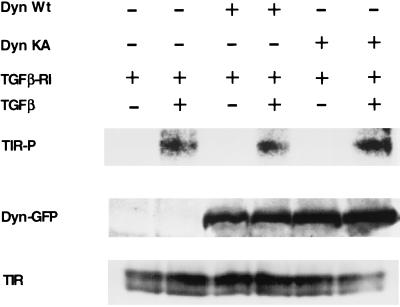
FIG. 5.
Dominant-negative dynamin 2ab functions downstream of type I TGF-βR phosphorylation. R1B cells were transiently transfected with the native HA-tagged type I TGF-βR (TGFβ-RI) and either GFP-tagged wild-type (Dyn Wt) or dominant-negative (Dyn KA) dynamin 2ab isoforms. (Top) After in vivo labeling with 32P, cells were treated as indicated with 15 ng of TGF-β/ml for 25 min. Normalized protein lysates were immunoprecipitated by using anti-HA affinity matrix and processed as described in Material and Methods. (Middle and bottom) Parallel plates were treated identically to those in the top panel but without orthophosphate labeling to verify transfected dynamin (middle panel) and type I receptor (bottom panel) expression. Equivalent protein lysates were processed for Western blotting with antisera to GFP (Dyn-GFP) or the type I TGF-βR (TIR).
Smad phosphorylation in the presence of dominant-negative dynamin.
R1B cells were transiently transfected with plasmids encoding β-galactosidase, the native type I TGF-βR, and GFP-tagged wild-type or K44A mutant dynamin 2ab isoforms by using Lipofectamine 2000 (Gibco-BRL). After 24 h, cultures (in 0.2% serum) were left untreated or were stimulated with 10 ng of TGF-β/ml for 1 h at 37°C. After normalization for transfection efficiency, equivalent amounts of protein (500 μg) were probed with a phospho-specific Smad2 or GFP antibody (Boehringer Mannheim).
Smad3 nuclear translocation.
Nuclear protein fractions were extracted from control and internalization inhibited cells by using the NE-PER reagents (Pierce). Normalized samples were blotted on a polyvinylidene difluoride membrane and probed with a Smad3 (Zymed Laboratories) or Smad2-specific antibody (Upstate Biotechnology). To detect Smad3 by immunofluorescence, MB102-9 cells expressing native and chimeric TGF-β receptors were seeded in six-well plates containing circular glass coverslips at 105 cells/well and incubated overnight. After treatment with or without potassium depletion and after a 20-min treatment at 37°C with or without ligand (10 ng of GM-CSF or TGF-β/ml), cells were rinsed once in PBS and then fixed in 2.5% paraformaldehyde (PBS, pH 7.4) for 20 min at room temperature. Cells were permeabilized with 0.1% Triton X-100 for 2 min at room temperature, washed four times in PBS for 3 min each time, and then blocked for 45 min at 37° in blocking buffer (5% FBS, 5% glycerol, PBS [pH 7.2]). Rabbit anti-Smad3 (Zymed) diluted to 2 μg/ml in blocking buffer was added for 45 min at 37°C, followed by three 10-min washes in PBS. Texas red-conjugated goat anti-rabbit (Molecular Probes) was diluted 1:400 in blocking buffer and incubated for 45 min at room temperature. After three 10-min PBS washes, coverslips were washed in water and mounted with Prolong (Molecular Probes). Images were acquired by using an Olympus AX-70 fluorescence microscope and analyzed by using Metamorph (Universal Imaging Corp., West Chester, Pa.).
Luciferase assays.
R1B cells were plated in six-well dishes at 3 × 105/well prior to transfection. Then, 2 μg of 3TP-Lux, 0.5 μg of cytomegalovirus-β-galactosidase, 2 μg of HA-tagged type I native receptor, and either 6 μg of pCMV5 vector DNA or GFP-tagged wild-type or dominant-negative K44A mutant dynamin 2ab isoforms were transfected/well with Fugene 6 (Boehringer). After transfection (6 h), cells were allowed to recover overnight in 10% FBS-DME and made quiescent by incubation in serum-free DME for 24 h. Cultures were left untreated or stimulated with 10 ng of TGF-β/ml and then assayed for luciferase activity as described previously (3). Parallel lysates (100 μg) were immunoblotted with an anti-GFP antibody (Boehringer Mannheim) or HA-antibody (Boehringer Mannheim) to confirm transfected dynamin and type I receptor expression, respectively.
Receptor cross-linking and SARA association.
Cos-7 cells were serum starved and transfected with HA-tagged type I and/or type II receptors, myc-tagged Smad2, and Flag-tagged SARA. At 12 h posttransfection, 200 pM 125I-labeled TGF-β (NEN) was bound to control or treated cultures at 4°C for 30 min and then kept at the indicated temperatures for 30 min. To inhibit internalization via potassium depletion, cells were K+ depleted and then treated with 200 pM 125I-labeled TGF-β. The ligand-receptor complexes were affinity cross-linked at 4°C with 1 mM DSS (Pierce), lysed (31), and coimmunoprecipitated either with SARA by using the anti-Flag M2 affinity gel (Sigma) or with Smad2 by using anti-myc antibody (Santa Cruz Biotechnology).
RESULTS
Smad2 phosphorylation requires TGF-βR internalization.
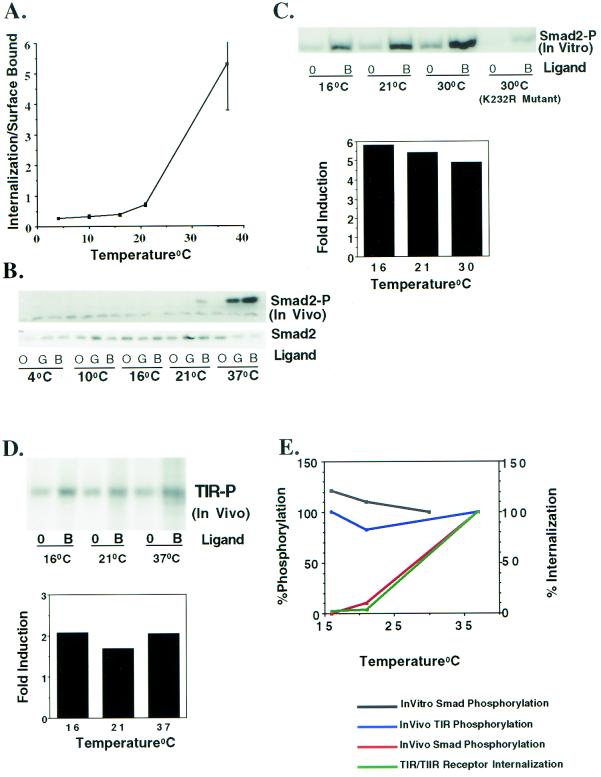
Defining the role(s) of the endocytic system in modulating ligand-dependent signaling has been difficult (5, 8, 17). One way of determining whether endocytic activity is coupled to receptor signaling is to characterize the effect(s) of a block in receptor endocytosis on the subsequent signaling response. Low temperatures have been shown to prevent association of cell surface receptors with components of the endocytic machinery and block internalization of many receptor systems (7, 12, 26). Since we had similarly documented impaired TGF-βR endocytic activity at temperatures below 37°C (1), we examined the effect of low temperature and impaired TGF-βR internalization on Smad2 phosphorylation (Smad2-P). Cells were maintained at various temperatures and parallel internalization (Fig. 1A) and Smad2 phosphorylation (Fig. 1B) assays were performed. There was a marked reduction in GM-CSF-stimulated Smad2 phosphorylation at temperatures (4, 10, 16, and 21°C) at which internalization of the chimeric receptors was impaired. Since the Mv1Lu cell line (MB102-9) used for Fig. 1 stably expresses both the chimeric and native TGF-βRs, the biological response to TGF-β activation of endogenous TGF-βRs was also examined. Similar to findings observed with the chimeric receptors, TGF-β stimulation of Smad2 phosphorylation was inhibited below 37°C (Fig. 1B). When intermediate temperatures such as 25 or 30°C were tested, there was a corresponding increase in both internalization and Smad2 phosphorylation (data not shown). Moreover, ligand treatment at 16°C for an extended period of time (4 h) did not show the presence of Smad2-P (data not shown). Thus, as shown for the chimeric receptor system, Smad2 phosphorylation by native TGF-βRs shows a temperature dependence identical to that of the receptor internalization.
FIG. 1.
Effect of temperature on TGF-βR internalization and Smad2 phosphorylation. (A) Internalization of 125I-labeled GM-CSF through chimeric TGF-βRs was performed in MB102-9 cells (an Mv1Lu clone stably expressing chimeric and endogenous TGF-βRs) at the indicated temperatures. The ratio of internalized to surface bound ligand was determined at 30 min as described previously (36). The results represent the mean ± the standard error of the mean (SEM) of three different experiments performed in duplicate. (B) Serum-starved MB102-9 cells were either left untreated (lanes O) or were treated with 10 ng of GM-CSF (lanes G; activates the chimeric receptors) or TGF-β (lanes B; activates the native receptors)/ml for 30 min at the indicated temperatures. Lysates were prepared from parallel plates, and equivalent protein (100 μg) was immunoblotted with a phospho-specific Smad2 antibody. The blot was stripped and probed with an antibody to Smad2 (lower panel) to confirm equal loading. (C) Type I receptor kinase activity is functional at temperatures where in vivo Smad2 phosphorylation does not occur. In the upper panel, Cos-7 cells were transiently cotransfected with native HA-tagged type I (wild-type or K232R mutant) and type II TGF-βRs. At 24 h posttransfection the cells were left untreated or incubated with 10 ng of TGF-β/ml in 0.2% FBS at 16°C for 1 h. Cells were lysed, normalized for transfection, and incubated with 3 μg of GST-Smad2, and then Smad2 phosphorylation was determined in vitro as described in Materials and Methods. In the lower panel, the bands in the upper panel were quantified by NIH Image software, and the fold ligand induction for the indicated temperature was determined. (D) In vivo TGF-βR phosphorylation occurs at lower temperatures. In the top panel, Cos-7 cells were transiently cotransfected with native HA-tagged type I and type II TGF-βRs. Cells were either left untreated (lanes 0) or were stimulated with 10 ng of TGF-β/ml (lanes B) for 20 min at the indicated temperatures to assay the in vivo phosphorylation state of the endogenous type I receptor (TIR-P). Lysates were subjected to immunoprecipitation (after normalization for transfection) and analyzed by SDS-PAGE, followed by autoradiography. In the lower panel, the bands in panel A were quantified, and the fold ligand induction was determined. (E) Quantitation of the data shown in panels A to D. The responses observed at 37°C (in panels A, B, and D) or 30°C (in panel C) were considered as 100% and compared to that seen at lower temperatures.
One potential concern related to conducting experiments at temperatures lower than 37°C is that the inhibition of Smad2-P might reflect impaired kinase activity of the type I receptor and not a direct inhibition of receptor internalization. Although previous studies have indicated that receptor kinases are functionally active at temperatures varying from 4 to 30°C (12), we tested the kinase activity of the type I receptor in vitro at temperatures where internalization does not occur (Fig. 1A and C). In this way, the kinase activity of the type I receptor can be directly tested in the absence of any endocytic requirement(s). Lysates from Cos-7 cells transiently transfected with HA-tagged native type I and type II receptors were used to perform in vitro kinase assays with GST-Smad2 as a substrate. As shown in Fig. 1C, ligand-dependent Smad2 phosphorylation was observed at 16, 21, and 30°C. Quantitation of Smad2-P indicated that a five- to sixfold ligand-dependent induction of Smad2 phosphorylation was observed at all tested temperatures (Fig. 1C, lower panel). Smad2-P was absent when a type I kinase-impaired receptor (K232R mutation in the ATP binding site) was used (Fig. 1C). Thus, the lack of in vivo Smad2 phosphorylation (Fig. 1B) does not reflect an impaired type I receptor kinase but rather the absence of a temperature-dependent endocytic response.
Type I receptor phosphorylation occurs independent of internalization.
The type II receptor, a constitutively active kinase, phosphorylates the type I receptor in the juxtamembrane GS domain (6, 37, 38). Since type I receptor phosphorylation is one of the most proximal events known to regulate receptor signaling, we sought to determine the endocytic requirement(s) for type I receptor activation. To accomplish this, Cos-7 cells were transiently transfected with native type I and type II TGF-β receptors, and the phosphorylation state of the type I receptor in vivo was determined at 37°C and at temperatures previously established to inhibit receptor internalization (16 and 21°C) (Fig. 1A). A twofold ligand-dependent increase in type I receptor phosphorylation was observed at all temperatures irrespective of whether internalization occurred (Fig. 1A and D). A comparison of the temperature requirement for in vitro Smad2 phosphorylation with that required for in vivo Smad2 and type I receptor phosphorylation is shown in Fig. 1E. Although in vitro Smad2 phosphorylation and in vivo type I receptor phosphorylation are relatively constant at the different temperatures, indicating functional type I and type II TGF-βR kinase activity independent of receptor internalization, Smad2 phosphorylation in vivo is essentially absent at temperatures where receptor internalization is impaired.
Clathrin-coated pit formation is required for Smad2 phosphorylation.
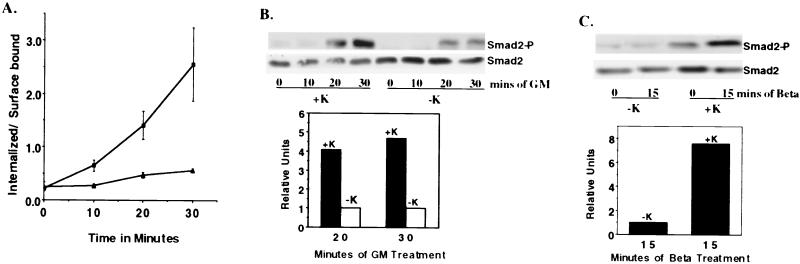
Previous work from our laboratory has established that TGF-βRs are endocytosed primarily via a clathrin-dependent pathway(s) (1). Since potassium depletion combined with hypotonic shock has been shown to specifically inhibit clathrin-coated pit uptake of various receptor systems (14, 16, 24, 26), we examined the effect of inhibiting clathrin-mediated endocytosis via potassium depletion on TGF-βR signaling. As shown in Fig. 2A, potassium depletion, which prevents clathrin lattice formation (16, 27), inhibits the internalization of chimeric TGF-βRs expressed on Mv1Lu cells (clone MB102-9). Parallel plates were treated in an identical manner (−K and +K) and analyzed for Smad2 phosphorylation. As shown in Fig. 2B and C, Smad2 phosphorylation by chimeric and native TGF-βRs, respectively, mimicked the observed internalization pattern and was significantly reduced in −K cells versus +K cells. Quantification after normalization for total Smad2 (Fig. 2B and C, lower panel) showed an approximate 75 to 80% inhibition of Smad2-P in the absence of potassium. This observation demonstrates that when clathrin-dependent internalization is prevented, there is a coincident loss in the ability of native or chimeric TGF-βRs to activate Smad2.
FIG. 2.
Effect of potassium depletion on TGF-βR internalization and/or Smad2 phosphorylation. (A) Internalization of 125I-labeled GM-CSF through the chimeric TGF-βRs was performed in MB102-9 cells in the absence (▴) or presence (▪) of potassium. At the indicated times, the ratio of internalized to surface bound ligand was determined. The results represent the mean ± the SEM of three experiments done in duplicate. (B and C) At the top, serum-starved MB102-9 cells were left untreated (lanes 0) or were treated with 10 ng of GM-CSF/ml (B) (to activate chimeric TGF-βRs) or 10 ng of TGF-β/ml (C) (to activate endogenous TGF-βRs) for the indicated times. Immunoblotting with a phospho-specific Smad2 antibody was performed with equivalent protein lysates (100 μg) prepared from parallel plates treated with (+K) or without (−K) potassium as in panel A. The blot was stripped and probed with Smad2 antibody to confirm equal loading (lower half). In the bottom portions of panels B and C, the amount of Smad2-P relative to total Smad2 observed at the indicated time of ligand stimulation was quantified. The ratio obtained for the −K treatment was given the relative value of 1.0.
Role of internalization in Smad3 activation.
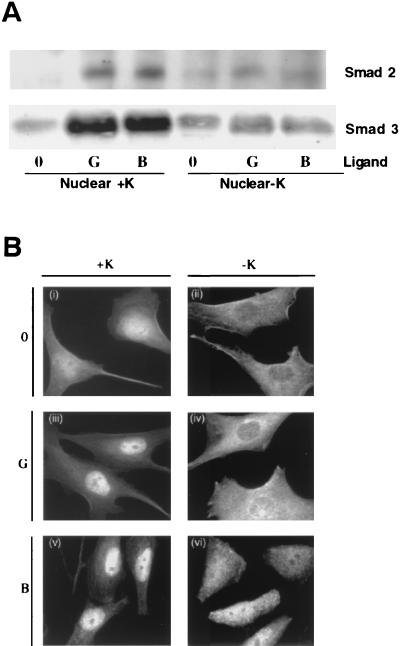
The role of receptor internalization in signaling has been found to vary for defined substrates. For instance, whereas activation of mitogen-activated protein kinase and Shc proteins by insulin-like growth factor I (IIGF-I) has been reported to require receptor endocytosis, insulin receptor substrate 1 (IRS-1) activation can occur at the plasma membrane independent of IGF-I receptor internalization (7). An analogous situation may exist for activation of Smad3, an additional early mediator of TGF-β signaling. Like Smad2, Smad3 is phosphorylated by the activated type I receptor and translocates to the nucleus complexed with Smad4 (21). To define the role of TGF-βR internalization in Smad3 activation, nuclear accumulation of Smad3 in response to ligand was determined in the presence or absence of endocytic inhibitors. Endocytic assays were conducted in parallel to confirm that receptor internalization was inhibited (data not shown). As shown in Fig. 3, there was a significant decrease in ligand-dependent (TGF-β and GM-CSF) nuclear translocation of Smad2 and Smad3 when receptor internalization was impaired by potassium depletion (Fig. 3A). An identical result was observed when Smad3 translocation was visualized by fluorescence microscopy (Fig. 3B) or by incubating cells at temperatures (16 or 21°C) that inhibit internalization (data not shown). Thus, activation of Smad3 is similar to that of Smad2 and is dependent upon receptor endocytic activity.
FIG. 3.
Smad2 and Smad3 nuclear translocation requires receptor internalization. (A) MB102-9 cells were left untreated (lanes 0) or were treated with 10 ng of GM-CSF/ml (lanes G) or 10 ng of TGF-β/ml (lanes B) with (+K) or without (−K) potassium at 37°C to activate chimeric or endogenous TGF-βRs, respectively. Nuclear fractions were analyzed by immunoblotting with a Smad2 (top) and Smad3 (bottom) antibody. (B) Cells with (i, iii, and v) or without (ii, iv, and vi) potassium were left untreated (i and ii), treated with 10 ng of GM-CSF/ml (iii and iv) to examine the response to chimeric TGF-βR activation, or treated with 10 ng of TGF-β/ml (v and vi) to examine the response to endogenous TGF-βR activation for 20 min at 37°C. Smad3 was visualized by immunofluorescence as described in Materials and Methods.
Dynamin GTPase is required for Smad2 phosphorylation and TGF-β-dependent transcriptional activity.
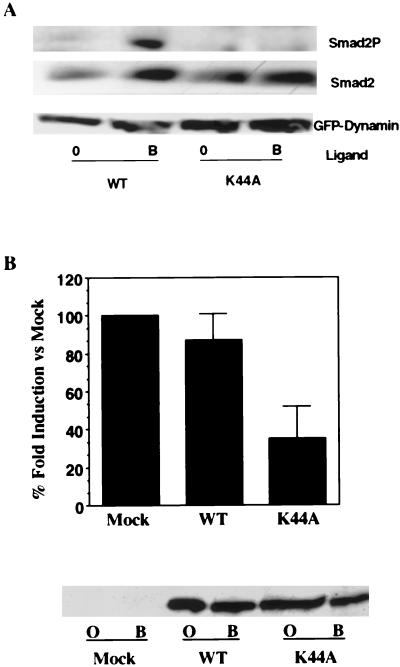
The endocytic process proceeds through a defined series of events with vesicle scission facilitated by the GTPase dynamin (25). Although the results presented thus far (Fig. 1B, 2, and 3) indicate that Smad2 phosphorylation occurs subsequent to clathrin lattice formation, they do not resolve whether Smad2 phosphorylation occurs within pits, per se, or in vesicles formed after pit budding. Since a recent study demonstrated that the Drosophila TGF-β homologue decapentaplegic is internalized by dynamin-dependent endocytosis (10), we examined the effect of the dominant-negative K44A dynamin 2ab isoform on TGF-β-dependent signaling. R1B cells were transiently transfected with the native type I TGF-βR and either GFP-tagged wild type or dominant-negative dynamin 2ab and then assayed for Smad2 phosphorylation. As shown in Fig. 4A, expression of the K44A dynamin 2ab isoform diminished Smad2 phosphorylation in response to TGF-β.
FIG. 4.
Dominant-negative dynamin 2ab inhibits Smad2 phosphorylation and TGF-β transcriptional activity. (A) Effect of dominant-negative dynamin 2ab on TGF-β-stimulated Smad2 phosphorylation. R1B cells (an Mv1Lu clone lacking the type I TGF-βR) were transiently transfected with the type I TGF-βR and GFP-tagged wild type or mutant (K44A) dynamin 2ab. Cultures were left untreated (lanes 0) or stimulated with 10 ng of TGF-β/ml for 1 h at 37°C. After normalization for transfection efficiency, equivalent protein (500 μg) was probed with a phospho-specific Smad2 antibody (Smad2P), Smad2 antibody (Smad2), or GFP antibody (GFP-Dynamin). (B) Dynamin regulates TGF-β-stimulated 3TP-Lux transcription. R1B cells were cotransfected with HA-tagged native type I receptor, 3TP-Lux, cytomegalovirus-β-galactosidase, and either pCMV5 vector (mock) DNA or GFP-tagged wild-type (WT) or dominant-negative K44A mutant dynamin 2ab isoforms. Cells were left untreated or were stimulated with 10 ng of TGF-β/ml for 24 h. The fold ligand induction in vector (mock)-transfected cells was assigned a value of 100%, and the effect of wild-type or K44A dynamin 2ab transfection is shown as the percentage of mock induction and represents the mean ± the SEM of two separate experiments performed in duplicate. In the lower panel, parallel lysates were immunoblotted with a GFP antibody to confirm the expression of transfected GFP-tagged wild type (WT) or dominant-negative dynamin 2ab (K44A) in untreated (lanes O) or TGF-β-stimulated (lanes B) cultures.
In order to determine whether decreased Smad phosphorylation due to the absence of receptor endocytosis modulated downstream signaling, the effect of dominant-negative dynamin 2ab on TGF-β-mediated transcriptional activity was examined (Fig. 4B). Although a 20- to 30-fold induction in luciferase activity was observed in R1B cells transfected with HA-tagged type I receptors and either vector (mock) DNA or wild-type dynamin 2ab, coexpression of the dominant-negative K44A dynamin 2ab mutant decreased TGF-β-stimulated luciferase activity ca. 60 to 70% relative to mock-transfected cells. Thus, expression of dominant-negative dynamin 2ab prevents the activation of Smad2 and similarly inhibits TGF-β-dependent transcriptional responses. These observations (Fig. 1 to 4) suggest that (i) the formation of a clathrin-coated pit is not sufficient for Smad2 or Smad3 activation, (ii) ligand-stimulated Smad2 phosphorylation occurs at a step(s) downstream of dynamin 2ab function, and (iii) TGF-β-dependent reporter gene expression requires the action of dynamin 2ab.
Dynamin 2ab functions downstream of type I receptor activation.
We previously showed that, while internalization and in vivo Smad2 phosphorylation are inhibited by incubation at 16 or 21°C, type I receptor phosphorylation occurs similarly as at 37°C (Fig. 1A, B, and D). Although these results indicate that receptor phosphorylation does not depend upon endocytic activity, we wanted to determine whether the inhibitory effect on Smad signaling by dominant-negative dynamin 2ab (Fig. 4) would function through an analogous mechanism. R1B cells were transfected with the type I TGF-βR and either wild-type or dominant-negative dynamin 2ab, and the effect of TGF-β on type I receptor phosphorylation was determined. Ligand-dependent type I receptor phosphorylation was similar in cells transfected with either dynamin isoform (Fig. 5). Thus, the formation and activation of the TGF-βR complex is not sufficient for Smad signaling; an activity(s) downstream of dynamin 2ab function is also required.
Association of SARA, Smad2, and the TGF-βR complex is not sufficient for receptor signaling.
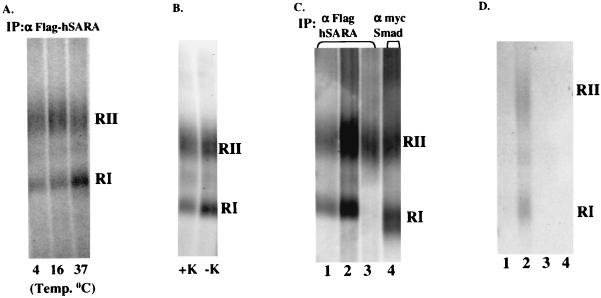
SARA is a FYVE-finger protein that interacts with and presents Smad2 to the TGF-βR complex (31). Immunofluorescence profiles have demonstrated SARA and the receptors colocalize at the plasma membrane and to punctate subcellular domains (presumably endosomes) (28, 31). A possible explanation for our data (i.e., requirement for receptor endocytosis in Smad activation) might be that internalization facilitates the interaction between TGF-βRs and the SARA/Smad complex. Since kinase-impaired type I receptors have been reported to show enhanced SARA binding (31), Cos-7 cells were cotransfected with HA-tagged type II and the kinase-deficient type I receptors (K232R), Flag-SARA, and myc-Smad2. The receptors expressed on the cell surface were bound to 125I-labeled TGF-β at 4°C, internalized at 37°C (or retained at the surface by inhibiting endocytosis), and cross-linked to the radioactive ligand by using the cell-permeable cross-linker disuccinimidyl suberate (DSS). If internalization was an obligate prerequisite for receptors to associate with the Smad/SARA complex, then it should not be possible to immunoprecipitate TGF-βRs with SARA or Smad2 in the absence of endocytosis. However, as shown in Fig. 6, TGF-βRs coimmunoprecipitate with SARA under conditions in which internalization is inhibited by either decreased temperature (4 and 16°C; Fig. 6A), potassium depletion (Fig. 6B), or use of a kinase-inactive type II receptor at 16°C (Fig. 6C, lane 3). In order to determine whether Smad2 is also associated with the receptor complex in the absence of internalization, TGF-βRs were coimmunoprecipitated with Smad2 after 16°C incubation. As shown in Fig. 6C (lane 4), Smad2 is similarly associated with the receptor complex.
FIG. 6.
Interaction of SARA with receptor complexes in the absence of receptor internalization. (A) Cos-7 cells were transiently cotransfected with native kinase-impaired (K232R) type I and wild-type type II receptors, Flag-tagged SARA, and myc-tagged Smad2. Chilled cells were treated with 125I-labeled TGF-β for 30 min at 4°C, transferred to the indicated temperatures for 30 min, and cross-linked (at 4°C) as described in Materials and Methods. Lysates were immunoprecipitated with anti-Flag affinity matrix, and coprecipitating receptor-ligand complexes were analyzed by SDS-PAGE and autoradiography. (B) Cos-7 cells were transiently transfected as described for panel A. Cells with (+K) and without (−K) potassium were treated with 125I-labeled TGF-β at 37°C for 30 min. After cross-linking and anti-Flag immunoprecipitation, the complexes were visualized as described for panel A. (C) Cos-7 cells were transiently transfected with Flag-tagged SARA, myc-tagged Smad2, and various native TGF-βR combinations as follows: lane 1, wild-type type I and type II receptors; lanes 2 and 4, kinase-impaired (K232R) type I and wild-type type II receptors; lane 3, kinase-impaired (K277R) type II receptor alone. Serum-starved cells were treated with 125I-labeled TGF-β at 16°C, and the receptors were affinity labeled as described for panel A. Lysates were immunoprecipitated with either anti-Flag affinity resin (lanes 1 to 3) or anti-myc (lane 4) to visualize the receptor complex coprecipitating with SARA or Smad2, respectively. (D) For lane 1, equivalent lysates from Cos-7 cells transfected with native type I and type II receptors (cross-linked to 125I-labeled TGF-β as described for panel A) or Flag-SARA and myc-Smad2 were mixed in vitro and subjected to immunoprecipitation with anti-Flag affinity matrix. For lane 2, cells were treated and receptors were coimmunoprecipitated exactly as described for panel A. For lane 3, Cos-7 cells were transfected with type I and II receptors, myc-Smad2, and cross-linked with 125I-labeled TGF-β as described for panel A. Lysates were prepared and immunoprecipitated with anti-Flag resin. For lane 4, Cos-7 cells were transfected as for panel A and subjected to immunoprecipitation with control beads alone.
Although unlikely, it is possible that the association of TGF-βRs with SARA and Smad2 is an in vitro effect occurring after cell lysis. This possibility was excluded by mixing equal amounts of lysates expressing only Flag-SARA and myc-Smad2 with lysates expressing only tagged receptors affinity labeled with 125I-labeled TGF-β. Under these conditions the receptors do not coimmunoprecipitate with SARA (Fig. 6D, lane 1). In addition, no complexes were precipitated without the expression of Flag-SARA (Fig. 6D, lane 3) or without the Flag resin (Fig. 6D, lane 4). Since the kinase activity of the type II TGF-βR is required for optimal endocytosis (2), these findings provide substantive evidence that, while Smad activation requires endocytic activity (Fig. 1 to 4), formation of the SARA/Smad/TGF-βR complex can occur independent of receptor internalization (Fig. 6).
Endocytic requirement for TGF-β activation of the Smad pathway.
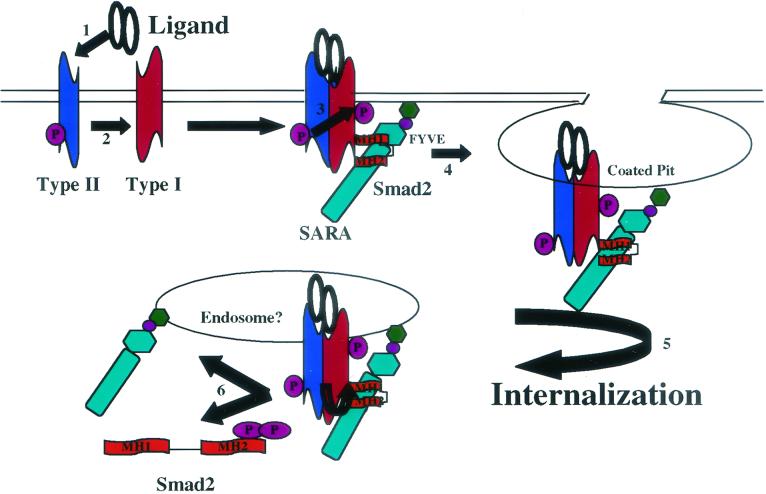
Based on our observations, we propose a model depicting defined events in TGF-βR activation of the Smad pathway (Fig. 7). Ligand binding induces the association of phosphorylated type I and type II TGF-βRs with SARA and Smad2 (or presumably Smad3) at the plasma membrane in the absence of internalization (steps 1 to 3). A coated pit is then formed with the associated receptor complex (step 4). Phosphorylation (step 5) and separation-nuclear translocation (step 6) of Smad2 or Smad3 do not occur until dynamin has excised the budded vesicle from the plasma membrane. Although ligand is shown bound to the TGF-βR complex throughout, our current observations do not address the status of ligand association or dissociation after internalization.
FIG. 7.
Endocytic requirement for TGF-β activation of the Smad pathway. A model depicting defined events in TGF-βR activation and Smad2 phosphorylation is shown. Steps 1 to 3 reflect ligand binding inducing the association of phosphorylated type I and type II TGF-βRs with SARA and Smad2 at the plasma membrane (i.e., occurs in the absence of internalization). Step 4 depicts the formation of a coated pit with the associated receptor complex. Phosphorylation (step 5) and separation and/or nuclear translocation (step 6) of Smad2 (or Smad3) does not occur until dynamin has excised the budded vesicle from the plasma membrane. Although ligand is shown bound to the TGF-βR complex throughout, our data do not address the relationship of ligand dissociation and/or degradation to Smad activation.
DISCUSSION
The initial events in TGF-βR activation are mediated by ligand binding to the type II receptor; this results in type I receptor recruitment and subsequent Smad phosphorylation. Although this study addresses many aspects of TGF-β action, the receptor locale(s) for either the initiation or the extension of the various activities required for TGF-βR signaling has not been clearly defined. A potential endocytic component to TGF-β signaling was suggested in a recent study showing that SARA, Smad, and TGF-βRs colocalized at the plasma membrane, as well as to punctate subcellular domains which were speculated to be endosomes (28, 31). Although the role of endocytic activity in signaling was not examined in this study, it is suggestive of a scenario whereby Smad, SARA, and receptors assemble at the plasma membrane and subsequently engage the signaling machinery in a post-plasma membrane compartment. As such, in the present study we sought to directly test this model by determining whether receptor endocytic activity was required for defined events in receptor activation, complex formation with other proteins, and/or Smad phosphorylation. To accomplish this, we used various treatments (low temperatures, potassium depletion, and dominant-negative dynamin 2ab) that block receptor endocytosis (7, 14, 16, 25, 26). We demonstrated that these endocytic blocks do not affect early events in TGF-β signaling such as ligand binding, type I receptor phosphorylation, type II kinase activity, and SARA/Smad receptor complex formation. However, ligand-dependent activation of Smad2 and/or Smad3 requires interaction with the endocytic machinery. These findings document that (i) endocytic inhibitors can define specific internalization-dependent and -independent requirements of the TGF-βR complex, (ii) Smad2 or Smad3 activation is dependent upon receptor internalization (Fig. 1 to 4), (iii) TGF-βR complex formation and type I receptor phosphorylation occur independently of receptor internalization (Fig. 1D and 5), (iv) Smad2 phosphorylation and TGF-β-dependent transcriptional activity occur distal to dynamin 2ab action (Fig. 4), and (v) the formation of a SARA/Smad2/receptor complex in the absence of internalization is not sufficient for Smad phosphorylation (Fig. 6).
The relationship between receptor endocytic activity and the subsequent signaling response is a concept requiring greater investigation (5, 8, 17). In the TGF-βR system, it is currently unknown whether TGF-β signaling occurs entirely at the plasma membrane, in an early and/or late endosomal compartment, or all three, depending upon the particular signaling pathway or cell type being examined. Moreover, since the signaling response actually represents a number of discrete events, the possibility that distinct activities occur at defined locales within a particular pathway would also have to be considered. To this end, we directly addressed the question of endosomal signaling and examined whether ligand-induced receptor internalization would influence the most proximal events necessary for generating an active TGF-βR complex. Successive steps required for optimal internalization were blocked by using inhibitors that function through various mechanisms, and the effect on TGF-β signaling was determined. Our results demonstrated internalization-dependent and -independent components to TGF-βR signaling. For instance, while type I receptor phosphorylation by the type II receptor and association of the ligand-activated TGF-βR complex with Smad2 and SARA did not require receptor endocytosis (Fig. 1D, 5, and 6), Smad2 phosphorylation and Smad3 nuclear translocation were inhibited by lower temperatures and potassium depletion (Fig. 1B, 2, and 3). Although these studies illustrate a requirement for clathrin lattice formation, they do not differentiate whether Smad phosphorylation only requires the formation of a coated pit or whether an endocytic vesicle (i.e., excised from the plasma membrane) is needed. For this reason, we examined Smad2 phosphorylation in cells expressing dominant-negative dynamin 2ab since dynamin has been shown to be necessary for vesicle scission (29). As shown in Fig. 4 and 5, dominant-negative dynamin 2ab prevented Smad phosphorylation and TGF-β-stimulated 3TP-Lux activity but had no effect on type I receptor phosphorylation. Thus, while type I receptor recruitment and activation occurs proximal to clathrin lattice formation, our data are consistent with a model wherein Smad phosphorylation occurs predominantly in a compartment formed after scission of clathrin-coated pits.
SARA has been shown to recruit Smad2 or Smad3 to activated TGF-β receptors (31). Since our findings demonstrated an obligate endocytic requirement for optimal TGF-β signaling (Fig. 1 to 4), we sought to determine whether this might be due to the absence of a SARA/Smad/receptor complex forming when internalization was inhibited. Surprisingly, our data show that SARA and Smad2 associate with the receptors independent of receptor internalization (Fig. 6). As such, assembly of the complex at the plasma membrane, in itself, is not sufficient for Smad activation. One scenario to explain this observation may be that the SARA/Smad/receptor complex undergoes some conformational change in the more acidic endosomal environment (after internalization) which facilitates Smad phosphorylation. Alternatively, there may be additional proteins or factors that associate with the complex in an internalization-dependent manner required for Smad activation. A model is presented in Fig. 7 that depicts the interrelationship between the cellular endocytic and signaling machinery for TGF-β activation of the Smad pathway.
It has been suggested that distinct signaling responses can occur at various steps in the internalization process. For example, tumor necrosis factor generates ceramide in the plasma membrane to activate serine/threonine protein kinases and phospholipase A2; however, ceramide generated in the endosome directs the activation of NF-κB (33). In a complex system such as TGF-β it is reasonable to speculate that some signaling pathways are entirely initiated at the plasma membrane and have no endosomal component to them, whereas others require some form of vesicular trafficking. It is also possible that components of both plasma membrane and endosomes (early, late, or recycling) contribute to signal diversity depending on the pathway and/or the cell type. We provide evidence here for both plasma membrane and endosomal components controlling defined events in TGF-β signal transduction. Defining this relationship may be crucial for understanding the biological actions of TGF-β in various cell types since previous studies from our laboratory indicated that endocytic responses in epithelial (growth inhibited by TGF-β) and mesenchymal (growth stimulated by TGF-β) cells were differentially regulated (1, 9).
Acknowledgments
We thank the laboratories of Richard Pagano and Mark McNiven for helpful comments during the course of these studies. We also thank John DiGuglielmo in the laboratory of Jeffrey Wrana for constructs and for help with the SARA association assays.
This work was supported by Public Health Service grants GM54200 and GM55816 from the National Institute of General Medical Science and the Mayo Foundation. S.G.P. was partially supported by National Cancer Institute postdoctoral training grant CA09441.
REFERENCES
- 1.Anders, R. A., S. L. Arline, J. J. E. Doré, Jr., and E. B. Leof. 1997. Distinct endocytic responses of heteromeric and homomeric transforming growth factor β receptors. Mol. Biol. Cell 8:2133-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anders, R. A., J. J. E. Doré, Jr., S. A. Arline, N. Garamszegi, and E. B. Leof. 1998. Differential requirements for type I and type II TGFβ receptor kinase activity in ligand-mediated receptor endocytosis. J. Biol. Chem. 273:23118-23125. [DOI] [PubMed] [Google Scholar]
- 3.Anders, R. A., and E. B. Leof. 1996. Chimeric granulocyte/macrophage colony-stimulating factor/transforming growth factor-β (TGF-β) receptors define a model system for investigating the role of homomeric and heteromeric receptors in TGF-β signaling. J. Biol. Chem. 271:21758-21766. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter, G. 2000. The EGF receptor: a nexus for trafficking and signaling. Bioessays 22:697-707. [DOI] [PubMed] [Google Scholar]
- 5.Ceresa, B. P., and S. L. Schmid. 2000. Regulation of signal transduction by endocytosis. Curr. Opin. Cell Biol. 12:202-210. [DOI] [PubMed] [Google Scholar]
- 6.Chen, F., and R. A. Weinberg. 1995. Biochemical evidence for the autophosphorylation and transphosphorylation of transforming growth factor beta receptor kinases. Proc. Natl. Acad. Sci. USA 92:1565-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow, J. C., G. Condorelli, and R. J. Smith. 1998. Insulin-like growth factor-1 receptor internalization regulates signaling via the Shc/mitogen-activated protein kinase pathway, but not the insulin receptor substrate-1 pathway. J. Biol. Chem. 273:4672-4680. [DOI] [PubMed] [Google Scholar]
- 8.DiFiore, P. P., and G. N. Gill. 1999. Endocytosis and mitogenic signaling. Curr. Opin. Cell Biol. 11:483-488. [DOI] [PubMed] [Google Scholar]
- 9.Doré, J. J. E., Jr., M. Edens, N. Garamszegi, and E. B. Leof. 1998. Heteromeric and homomeric transforming growth factor-β receptors show distinct signaling and endocytic responses in epithelial cells. J. Biol. Chem. 273:31770-31777. [DOI] [PubMed] [Google Scholar]
- 10.Entchev, E. V., A. Schwabedissen, and M. Gonzalez-Gaitan. 2000. Gradient formation of the TGF-β homolog Dpp. Cell 103:981-991. [DOI] [PubMed] [Google Scholar]
- 11.Gilboa, L., R. G. Wells, H. F. Lodish, and Y. I. Henis. 1998. Oligomeric structure of type I and type II transforming growth factor beta receptors-homodimers form in the ER and persist at the plasma membrane. J. Cell Biol. 140:767-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heller-Harrison, R. A., M. Morin, and M. P. Czech. 1995. Insulin regulation of membrane-associated insulin receptor substrate 1. J. Biol. Chem. 270:24442-24450. [DOI] [PubMed] [Google Scholar]
- 13.Hocevar, B. A., T. L. Brown, and P. H. Howe. 1999. TGF-β induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 18:1345-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johannsen, L. E., T. Ringerike, J. Molnes, and I. H. Madshus. 2000. Epidermal growth factor receptor efficiently activates mitogen-activated protein kinase in HeLa cells and Hep2 cells conditionally defective in clathrin-dependent endocytosis. Exp. Cell Res. 2601:136-145. [DOI] [PubMed] [Google Scholar]
- 15.Kao, A. W., B. P. Ceresa, S. R. Santeler, and J. E. Pessin. 1998. Expression of a dominant interfering dynamin mutant in 3T3L1 adipocytes inhibits GLUT4 endocytosis without affecting insulin signaling. J. Biol. Chem. 273:25450-25457. [DOI] [PubMed] [Google Scholar]
- 16.Larkin, J. M., M. S. Brown, J. L. Goldstein, and R. G. W. Anderson. 1983. Depletion of intracellular potassium arrests coated pit formation and receptor mediated endocytosis in fibroblasts. Cell 33:273-285. [DOI] [PubMed] [Google Scholar]
- 17.Leof, E. B. 2000. Growth factor receptor signalling: location, location, location. Trends Cell Biol. 10:343-348. [DOI] [PubMed] [Google Scholar]
- 18.Luo, K., and H. F. Lodish. 1996. Signaling by chimeric erythropoietin-TGFβ receptors: homodimerization of the cytoplasmic domain of the type I TGFβ receptor and heterodimerization with the type II receptor are both required for intracellular signal transduction. EMBO J. 15:4485-4496. [PMC free article] [PubMed] [Google Scholar]
- 19.Massagué, J. 1998. TGF-β signal transduction. Annu. Rev. Biochem. 67:753-791. [DOI] [PubMed] [Google Scholar]
- 20.Massagué, J., S. W. Blain, and R. S. Lo. 2000. TGFβ signaling in growth control, cancer, and heritable disorders. Cell 103:295-309. [DOI] [PubMed] [Google Scholar]
- 21.Massagué, J., A. Hata, and F. Liu. 1997. TGF-β signalling through the Smad pathway. Trends Cell Biol. 7:187-192. [DOI] [PubMed] [Google Scholar]
- 22.Miller, W. E., and R. J. Lefkowitz. 2001. Expanding roles for beta-arrestins as scaffolds and adaptors in GPCR signaling and trafficking. Curr. Opin. Cell Biol. 13:139-145. [DOI] [PubMed] [Google Scholar]
- 23.Muramatsu, M., J. Yan, K. Eto, T. Tomoda, R. Yamada, and K. Arai. 1997. A chimeric serine/threonine kinase receptor system reveals the potential of multiple type II receptors to cooperate with transforming growth factor-β. Mol. Biol. Cell 8:469-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roettger, B. F., R. U. Rentsch, D. Pinon, E. Holicky, E. Hadac, J. M. Larkin, and L. J. Miller. 1995. Dual pathways of internalization of the cholecystokinin receptor. J. Cell Biol. 128:1029-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmid, S. L., M. A. McNiven, and P. De Camilli. 1998. Dynamin and its partners: a progress report. Curr. Opin. Cell Biol. 10:504-512. [DOI] [PubMed] [Google Scholar]
- 26.Sorkin, A., and G. Carpenter. 1993. Interaction of activated EGF receptors with coated pits adaptins. Science 261:612-615. [DOI] [PubMed] [Google Scholar]
- 27.Sorkin, A., T. McKinsey, W. Shih, T. Kirchhausen, and G. Carpenter. 1995. Stoichiometric interaction of the epidermal growth factor receptor with the clathrin-associatied protein complex AP-2. J. Biol. Chem. 270:619-625. [DOI] [PubMed] [Google Scholar]
- 28.Stenmark, H., and R. Aasland. 1999. FYVE-finger proteins—effectors of an inositol lipid. J. Cell Sci. 112:4175-4183. [DOI] [PubMed] [Google Scholar]
- 29.Sweitzer, S. M., and J. E. Hinshaw. 1998. Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell 5:1021-1029. [DOI] [PubMed] [Google Scholar]
- 30.ten Dijke, P., K. Miyazono, and C.-H. Heldiin. 2000. Signaling inputs converge on nuclear effectors in TGF-β signaling. Trends Biochem. Sci. 25:64-70. [DOI] [PubMed] [Google Scholar]
- 31.Tsukazaki, T., T. A. Chiang, A. F. Davison, L. Attisano, and J. L. Wrana. 1998. SARA, a FYVE domain protein that recruits Smad2 to the TGFβ receptor. Cell 95:779-791. [DOI] [PubMed] [Google Scholar]
- 32.Ulloa, L., J. Doody, and J. Massagué. 1999. Inhibition of transforming growth factor-β/Smad signalling by the interferon-gamma/STAT pathway. Nature 397:710-713. [DOI] [PubMed] [Google Scholar]
- 33.Weigmann, K., S. Schutze, T. Machleidt, and D. Witte. 1994. Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling. Cell 78:1005-1015. [DOI] [PubMed] [Google Scholar]
- 34.Wells, A., J. B. Welsh, C. S. Lazar, H. S. Wiley, G. N. Gill, and M. G. Rosenfeld. 1990. Ligand-induced transformation by a noninternalizing epidermal growth factor receptor. Science 247:962-964. [DOI] [PubMed] [Google Scholar]
- 35.Wells, R. G., L. Gilboa, Y. Sun, X. Liu, Y. I. Henis, and H. F. Lodish. 1999. Transforming growth factor-β induces formation of a dithiothreitol-resistant type I/type II receptor complex in live cells. J. Biol. Chem. 274:5716-5722. [DOI] [PubMed] [Google Scholar]
- 36.Wiley, H. S., and D. D. Cunningham. 1982. The endocytotic rate constant. A cellular parameter for quantitating receptor-mediated endocytosis. J. Biol. Chem. 257:4222-4229. [PubMed] [Google Scholar]
- 37.Wrana, J. L., L. Attisano, J. Carcamo, A. Zentella, J. Doody, M. Laiho, X. F. Wang, and J. Massagué. 1992. TGFβ signals through a heteromeric protein kinase receptor complex. Cell 71:1003-1014. [DOI] [PubMed] [Google Scholar]
- 38.Wrana, J. L., L. Attisano, R. Wieser, F. Ventura, and J. Massagué. 1994. Mechanism of activation of the TGF-β receptor. Nature 370:341-347. [DOI] [PubMed] [Google Scholar]
- 39.Xiao, Z., X. Liu, Y. I. Henis, and H. F. Lodish. 2000. A distinct nuclear localization signal in the N terminus of Smad 3 determines its ligand-induced nuclear translocation. Proc. Natl. Acad. Sci. USA 97:7853-7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yae, J., R. S. Frey, and K. Mulder. 1999. Cross-talk between the Smad1 and Ras/MEK signaling pathways for TGF-β. Oncogene 58:4752-4757. [DOI] [PubMed] [Google Scholar]
- 41.York, R. D., D. C. Molliver, S. S. Grewal, P. E. Stenberg, E. W. McCleskey, and P. J. Stork. 2000. Role of phosphoinositide 3-kinase and endocytosis in nerve growth factor-induced extracellular signal-regulated kinase activation via Ras and Rap1. Mol. Cell. Biol. 20:8069-8083. [DOI] [PMC free article] [PubMed] [Google Scholar]