Abstract
Early in vitro cell culture studies suggested that testicular orphan nuclear receptor 2 (TR2), a member of the nuclear receptor superfamily, may play important roles in the control of several pathways including retinoic acids, vitamin D, thyroid hormones, and ciliary neurotrophic factor. Here we report the surprising results showing that mice lacking TR2 are viable and have no serious developmental defects. Male mice lacking TR2 have functional testes, including normal sperm number and motility, and both male and female mice lacking TR2 are fertile. In heterozygous TR2+/− male mice we found that β-galactosidase, the indicator of TR2 protein expression, was first detected at the age of 3 weeks and its expression pattern was restricted mainly in the spermatocytes and round spermatids. These protein expression patterns were further confirmed with Northern blot analysis of TR2 mRNA expression. Together, results from TR2-knockout mice suggest that TR2 may not play essential roles in spermatogenesis and normal testis development, function, and maintenance. Alternatively, the roles of TR2 may be redundant and could be played by other close members of the nuclear receptor superfamily such as testicular orphan receptor 4 (TR4) or unidentified orphan receptors that share many similar functions with TR2. Further studies with double knockouts of both orphan nuclear receptors, TR2 and TR4, may reveal their real physiological roles.
The nuclear receptors are ligand-inducible transcription factors that regulate the expression of target genes by binding to their specific hormone response elements. They are characterized by a central DNA binding domain, which binds to the hormone response element. The C-terminal half of the receptor encompasses the ligand-binding domain, which mediates ligand binding, dimerization, and transactivation function. Nuclear receptors play roles in various aspects of physiology including metabolism, development, homeostasis, and reproduction (8, 18). Orphan nuclear receptors embody structures of nuclear receptors but are without identified ligands and make up the vast majority of the nuclear receptor superfamily (7). With genetic knockout approaches, several orphan nuclear receptors have been demonstrated elsewhere to have important physiological functions (5, 10, 21-23).
Orphan nuclear receptors testicular receptor 2 (TR2) (2) and testicular receptor 4 (TR4) (1) constitute a subfamily of nuclear receptors. TR2 was isolated from testis and prostate cDNA libraries, and its cDNA encodes a protein of 603 amino acids with a calculated molecular mass of 67 kDa (2, 3). An abundance of TR2 mRNA detected in developing mouse embryos and by in situ hybridization revealed that TR2 is highly expressed in the active proliferating zone of the developing nervous system and other developing organs (11, 25). TR2 has been shown previously to be specifically expressed in adult mice testes and is confined to advanced germ cells (11). The role of TR2 has been demonstrated previously in the regulation of several signaling pathways including retinoic acid (17), thyroid hormone (4), and ciliary neurotrophic factor (CNTF) (25). TR2-mediated repression has been shown elsewhere to be, in part, a direct interaction with both class I and class II histone deacetylase proteins (9). The expression of TR2 was completely repressed in surgery-induced cryptorchidism of the rhesus monkey through a pathway which involves p53 and the retinoblastoma gene product (Rb). Such dramatic repression of TR2 in cryptorchidism suggests that TR2 may play important roles in male infertility associated with cryptorchidism (20). In vitamin A-depleted mice, spermatogenesis was blocked at an early stage. In testes losing advanced germ cells, the expression of TR2 could not be detected, suggesting a biological relationship between TR2 and male germ cell differentiation (11). These studies strongly indicate that TR2 may play a role in embryogenesis and male germ cell differentiation. To evaluate the in vivo physiological significance of TR2, we used gene targeting to generate mice with a null mutation of TR2. The results indicate that TR2 is dispensable for mouse development, since homozygous mutant mice (TR2−/−) are viable with no detectable abnormalities. The TR2−/− male mice display normal testis development and spermatogenesis.
MATERIALS AND METHODS
Generation of TR2-knockout mice.
To generate TR2-deficient mice, the lambda KOS system (24) was used to derive a TR2 targeting vector. Two independent genomic lambda KOS clones were isolated that spanned exons 3 to 7. The targeting vector was derived from one clone and contained an 1,865-bp deletion that included most of exon 4 and all of exon 5. This region was replaced with an internal ribosome entry site (IRES) LacZ/MC1-Neo selection cassette. The NotI-linearized vector was electroporated into 129 Sv/Evbrd(LEX1) embryonic stem (ES) cells and G418-fialuridine (FIAU)-resistant ES cell clones were isolated and analyzed for homologous recombination by Southern blot analysis. Targeted ES cell clones were injected into C57BL/6(albino) blastocysts, and the resulting chimeras were mated to C57BL/6(albino) females to generate animals heterozygous for the mutation.
Southern blot, Northern blot, and PCR analyses of TR2-knockout mice.
Genomic DNA isolated from mouse tail biopsy samples was digested with EcoRV, separated by electrophoresis through an 0.8% agarose gel, and transferred to a positively charged nylon membrane. A wild-type 5′ probe external to the target vector sequence was labeled with a random primer labeling kit (Amersham) and used in hybridizations. Wild-type and mutant alleles were identified by predicted restriction fragment size differences.
PCR was also used to screen genotypes by using DNA isolated from mouse tail biopsy samples. The primers used for the wild-type allele were 5′-CCCTGACTAGTTTCTGCGATC-3′ (A) and 5′-GCCTACTCATGGAAATATAACC-3′ (B). Primers C, 5′-GCTGATGCTACCAAGTCCACG-3′, and D, 5′-GCAGCGCATCGCCTTCTATC-3′, were used to detect the mutant allele. RNA was extracted from testes of mice at different ages with Trizol reagent (GIBCO BRL). Thirty micrograms of RNA was loaded into 1.2% agarose gels. Northern blot analysis of TR2 and β-actin transcripts was performed as previously described (25).
Necropsy studies.
Gross and histopathological analyses were performed at the Diagnostic Laboratories, Division of Laboratory Animal Medicine, University of Rochester.
Epididymal sperm numbers.
Epididymides were removed from 6-week-old males under sterile conditions and placed in a dish containing 5 ml of Dulbecco modified Eagle medium with 10% fetal calf serum. Sperm was allowed to disperse into the medium for 1 h at 32°C. Sperm motility was examined by phase-contrast microscopy. Sperm numbers were determined by counting with a hemocytometer.
Histological analysis.
Tissues were fixed in fresh 10% neutral buffered formalin and embedded in paraffin. Tissue sections (5 μm) were stained with hematoxylin and eosin (H&E) and examined by light microscopy. For β-galactosidase (β-Gal) activity staining, testes at different ages of mice were dissected and fixed in 0.2% glutaraldehyde for 2 h at room temperature. The tissues were then washed with phosphate buffer and incubated overnight at room temperature in phosphate buffer containing 2 mM MgCl2, 5 mM K4Fe(CN)6, 5 mM K3Fe(CN)6, and 1 mg of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside)/ml. After washing with phosphate-buffered saline, testes were dehydrated, embedded in paraffin, and sectioned at 5 μm. The sections were then counterstained with H&E and examined by light microscopy.
RESULTS
Generation of TR2-deficient mice.
Unlike the human TR2 gene, which is located on human chromosome 12 at band q22 (16), the mouse TR2 gene is located in the distal region of chromosome 10 and is organized into 13 exons spanning more than 50 kb (12). A replacement vector was designed to delete most of exon 4, which encodes the second zinc finger domain of the TR2 DNA binding domain, and all of exon 5. The deleted sequence was replaced with an IRES LacZ/MC1-Neo selection cassette (Fig. 1A). The targeting vector was electroporated into 129 Sv/Evbrd(LEX1) cells, and colonies were selected in the presence of G418 and FIAU. G418-FIAU-resistant ES cell clones were isolated and analyzed for homologous recombination by Southern blot analyses. Targeted ES cell clones were injected into C57BL/6(albino) blastocysts, and the resulting chimeras were mated to C57BL/6(albino) females to generate heterozygous mice. The heterozygous (TR2+/−) mice were then interbred to produce null-mutation (TR2−/−) mice.
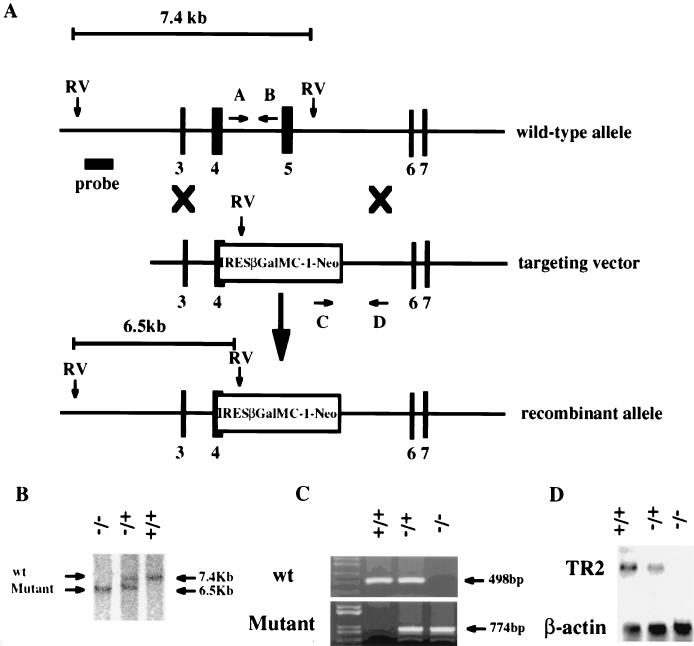
FIG. 1.
Targeted disruption of the murine TR2 gene. (A) Structure of wild-type allele, targeting construct, and recombinant locus. Black boxes represent exons of TR2. The expected fragments after EcoRV digestion are 7.4 kb for the wild-type allele and 6.5 kb for the mutant allele. Arrows indicate the primers used for PCR genotyping, and the bar represents the region used for probe. RV, EcoRV. (B) Southern blot analysis of mouse tail DNA isolated from the progeny of a mating between heterozygous parents. DNA was digested with EcoRV and hybridized with the probe indicated. (C) PCR analysis of mouse genomic DNA. The wild-type and targeted alleles give 498- and 774-bp PCR products, respectively. (D) Northern blot analysis of total RNA isolated from testis tissue from 6-week-old mice. The same membrane was sequentially hybridized with 32P-labeled TR2 and β-actin cDNA probes. +/+, wild-type mouse; +/−, heterozygous mutant mouse; −/−, homozygous mutant mouse; wt, wild type.
Southern blot, Northern blot, and PCR analyses of TR2 mutant mice.
Genomic DNA isolated from mouse tail biopsy samples was used for Southern blot analyses to confirm genotypes, with the expectation that a TR2+/+ animal would have a 7.4-kb DNA fragment after digestion with restriction enzyme EcoRV. The disrupted TR2 gene in TR2−/− animals displayed a 6.5-kb DNA fragment (Fig. 1B). Genotypes were confirmed by PCR with two sets of primers: both primers in the first set (A and B) are located on the TR2 gene between exons 4 and 5, which was replaced by the IRES LacZ/MC1-Neo selection cassette (Fig. 1A). For the second set of primers (C and D), primer C is located within the Neo cassette and primer D is outside the Neo cassette and is located on the 3′ end of the TR2 gene (Fig. 1A). With primers A and B, PCR amplification of the TR2 gene without interruption by the Neo cassette will produce a 498-bp PCR DNA fragment and indicate a wild-type TR2 allele. With the use of primers C and D, PCR amplification of the TR2 gene with the Neo cassette interruption should produce a 774-bp PCR DNA fragment and indicate the presence of the Neo cassette. As expected, TR2+/+ mice produced a single 498-bp DNA fragment, TR2−/− mice produced a single 774-bp fragment, and heterozygous TR2+/− mice produced both 498-bp and 774-bp DNA fragments (Fig. 1C). To confirm the loss of expression of TR2 in the knockout mice, total RNAs from testes of 6-week-old TR2+/+, TR2+/−, and TR2−/− mice were analyzed by Northern blotting with a mouse TR2 cDNA fragment as a probe. Figure 1D clearly demonstrates that TR2−/− mice lack TR2 mRNAs, which were knocked out by replacement of the Neo cassette. Together, results in Fig. 1B to D clearly demonstrate that the TR2 gene was successfully disrupted in the TR2−/− mice.
TR2−/− mice are viable and fertile.
Mating of TR2+/− mice generates pups of three possible genotypes (TR2+/+, TR2+/−, and TR2−/−) that fit well with normal Mendelian frequencies, suggesting that TR2 is not required for normal embryonic development. The external and internal phenotypes of TR2−/− mice appear normal. Furthermore, breeding studies showed that both male and female TR2−/− mice are fertile and can produce normal offspring. Additionally, the histopathological analyses from necropsy studies of more than 30 tissues, including brain and prostate in four adult TR2−/− animals, revealed no gross anatomical defects or significant lesions (data not shown).
Normal testis development and spermatogenesis in TR2−/− mice.
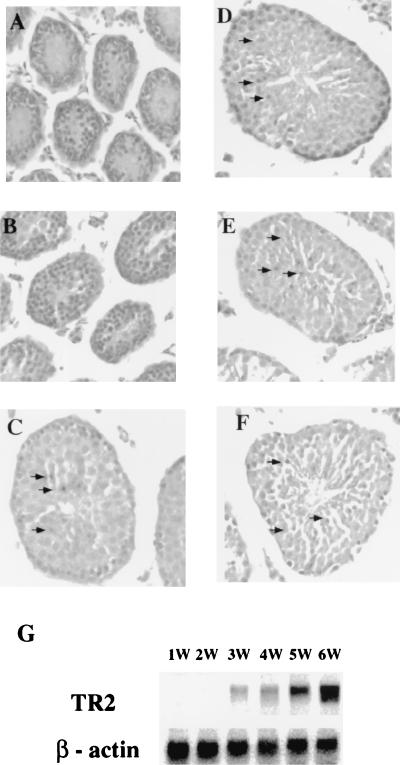
TR2 expression has been demonstrated previously for mature testes and is mainly present in the advanced germ cell population (12). The β-Gal activity from the lacZ reporter gene present in the knockout construct and in TR2+/− or TR2−/− mice allows for the analysis of TR2 expression. Testes collected from TR2+/− mice at different ages were examined for the distribution of β-Gal activity, which represents TR2 protein expression. The results show that TR2 expression was observed in the testis only after the first wave of spermatogenetic cells had completed both meiotic division I and meitoic division II, which occurs around 3 weeks after birth. β-Gal activity localized predominantly in more advanced germ cells (pachytene spermatocytes and round spermatids) and was not found in spermatogonia and elongated spermatids (Fig. 2C to F). In contrast, β-Gal activity is undetectable in the testes from 1- and 2-week-old TR2+/− mice (Fig. 2A and B). To further prove this distinct expression pattern of TR2 in testes, total RNAs were isolated from the TR2+/+ mouse testes at different ages for Northern blotting analysis to compare the TR2 mRNA expression with β-Gal expression in TR2+/− mice. As shown in Fig. 2G, the expression of TR2 mRNA in TR2+/+ mouse testes is similar to β-Gal activity in TR2+/− mouse testes. In both cases, the expression is detected starting at 3 weeks of age and increases as testes develop into maturity.
FIG. 2.
(A to F) Expression of TR2 represented by β-Gal activity in TR2+/− heterozygous mice. Testes from postnatal mice of different ages were processed through whole-mount X-Gal staining, embedded in paraffin, sectioned, and counterstained with H&E. Magnification, ×100. Panels A to F show samples from mice aged 1, 2, 3, 4, 5, and 6 weeks, respectively. Positive staining is indicated by arrows. (G) Northern blot analysis of total RNA from testis tissue of mice at 1, 2, 3, 4, 5, and 6 weeks of age. The same membrane was sequentially hybridized with 32P-labeled TR2 and β-actin probes.
As shown in Fig. 3A and B, examination of H&E-stained paraffin-embedded sections from 6-week-old TR2−/− mice did not reveal any abnormal histological features. The diameter of the seminiferous tubules, the thickness of the seminiferous epithelium, and the size of the lumen in TR2−/− mice appear normal. Spermatogenesis also appears to progress normally in TR2−/− mice.
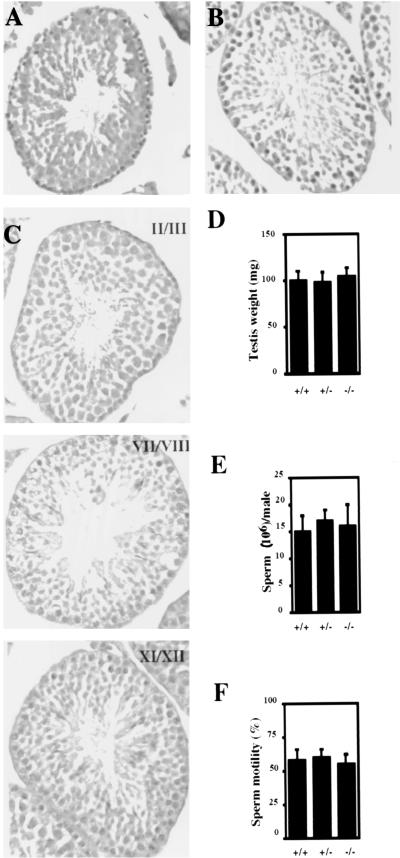
FIG. 3.
(A and B) Histological analysis of testes from 6-week-old wild-type (A) and mutant (B) mice. Testes were fixed in neutral buffered formalin, sectioned, and stained with H&E. Magnification, ×100. (C) Selected stages of the seminiferous cycle of 6-week-old TR2 homozygous mutant mice. Testis sections were stained with periodic acid-Schiff stain and counterstained with hematoxylin. Magnification, ×100. (D to F) Testis weight, sperm count, and sperm motility assays, respectively, of 6-week-old wild-type mice (+/+), heterozygous mutant mice (+/−), and homozygous mutant mice (−/−) (n = 6 from each genotype). Error bars represent the standard errors of the means.
In mouse spermatogenesis, there are 12 designated cell associations or stages, which are defined groupings of germ cell types at particular phases of development in cross-sectioned tubules. The staging of spermatogenesis allows us to understand normal spermatogenesis and what might go wrong with spermatogenesis. Figure 3C presents selected stages of TR2-homozygous mutant mice. We did not observe any abnormal cell type present in any stage. Additionally, there were no significant differences in testis weight (Fig. 3D), sperm count (Fig. 3E), or sperm motility (Fig. 3F) among TR2+/+, TR2+/−, and TR2−/− mice.
TR4 expression is normal in the TR2−/− mice.
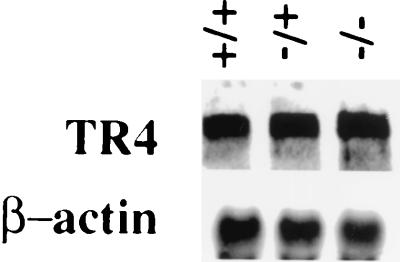
One explanation for the absence of abnormality in testis development and spermatogenesis in TR2−/− mice involves functional compensation by another orphan receptor, TR4, which exhibits high homology (65% overall) with TR2 (1). Early studies also indicate that these two orphan receptors share many functions in regulating several signaling pathways (4, 13-15). To determine whether TR4 expression is increased in TR2+/− and/or TR2−/− mice, total RNAs were isolated from testes of 6-week-old TR2+/+, TR2+/−, and TR2−/− mice, and Northern blot analysis was carried out with mouse TR4 cDNA as a probe. The results show that TR4 mRNA is expressed at similar levels among TR2+/+, TR2+/−, and TR2−/− mice (Fig. 4). Whether these results indicate that TR4 does not compensate for the loss of TR2 in the TR2−/− mice or that the original normal amount of TR4 available in the TR2+/+, TR2+/−, and TR2−/− mice is already at a sufficient level for the function of TR2 and TR4 needed in testis development or spermatogenesis, however, remains unclear.
FIG. 4.
Analysis of TR4 expression. Total RNA from 6-week-old wild-type mice (+/+), heterozygous mutant mice (−/+), and homozygous mutant mice (−/−) was sequentially hybridized with 32P-labeled TR4 and β-actin probes.
The central nervous system is normal in TR2−/− mice.
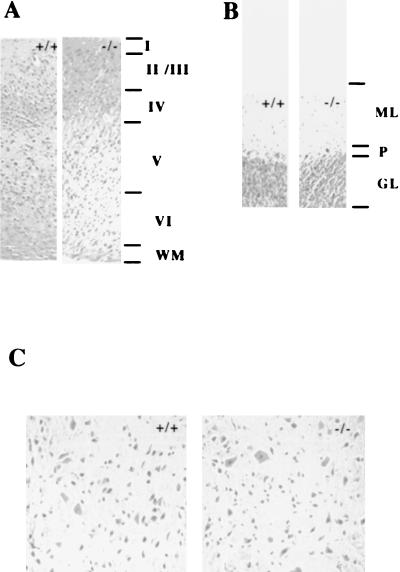
Although TR2 is expressed in neural epithelia, spinal cord, cerebellar primordium, and the periventricular area of the developing brain (25), no gross abnormalities were found with the examination of the serial sections of cerebra, cerebella, and spinal cords in 3-month-old wild-type mice (+/+) and homozygous mutant mice (−/−). The six-cortical-layer laminar structure is well formed, and the cerebellar cortex consists of three layers in both mice (Fig. 5A and B). TR2 not only is highly expressed in the developing nervous system but also induces CNTF receptor α (CNTFRα) gene transcriptional activity, and the expression of TR2 is increased in P19 cells treated with CNTF (25). Mice lacking the CNTF gene exhibit a progressive atrophy and loss of motor neurons in adult mice (19). Motor neuron number is dramatically reduced in mice homozygous for null mutations in the CNTFRα gene (6). These studies indicate the importance of the CNTF signaling pathway in the maintenance of motor neurons. However, in contrast to mice lacking CNTF and CNTFRα, lumbar motor neurons in TR2−/− mice showed no obvious reduction in number (Fig. 5C). Also, since the CNTFRα gene was suggested as a putative target gene for TR2, we isolated RNAs from brains of newborn pups of TR2+/+, TR2+/−, and TR2−/− mice and examined CNTFRα mRNA expression by Northern blot assay. The results showed that, in correlation with their normal phenotypes in the nervous system, the expression of CNTFRα mRNA in mutant mice was not significantly changed compared to that in heterozygous or wild-type control mice at the same age (data not shown).
FIG. 5.
Comparison of the cerebral cortex, cerebellar cortex, and lumbar spinal cord motor neurons in 3-month-old wild-type mice (+/+) and homozygous mutant mice (−/−). (A) Coronal sections of the cerebral cortex in wild-type and mutant mice. Six-layer laminar structures are seen in both mice. Magnification, ×100. (B) Coronal sections of cerebellar cortex. Three layers are seen in both mice. Magnification, ×100. (C) Transverse sections of lumbar spinal cord in wild-type and mutant mice. Magnification, ×400. Brains and spinal cords were fixed in neutral buffered formalin, sectioned, and stained with H&E. Abbreviations: I to VI, cortical layers I through VI; WM, white matter; ML, molecular layer; P, Purkinje cells; GL, granular cell layer.
DISCUSSION
Previous studies indicated that TR2 might be able to modulate many signaling pathways (4, 17, 25). TR2 may also be involved in male infertility associated with cryptorchidism (20) and the testis degeneration that occurred in vitamin A-deficient mice (11). Results from TR2−/− mice, however, did not display any detectable abnormality in testis development or spermatogenesis. Homozygous TR2−/− male mice were fertile and had normal testis weight and histology. The epididymal sperm number and motility of homozygous TR2−/− mice were similar to those for wild-type TR2+/+ mice.
These results, contrasting with what might be expected based on earlier studies showing no obvious abnormal phenotypes in TR2−/− mice, may suggest that the TR2 gene could be dispensable with respect to embryonic development, testis development, and spermatogenesis. Another explanation could involve the compensation by TR4, which has functions similar to TR2, as demonstrated in many in vitro cell culture studies (4, 13, 14, 17, 25, 26). Although results from Fig. 4 indicate that TR4 mRNA expression did not increase in TR2−/− mice compared to that in TR2+/+ mice, it is still possible that the original normal amount of TR4 available in either TR2+/+ or TR2−/− could be enough to fulfill most functional roles of TR2 and TR4. Alternatively, there may still be other possible unidentified close members of the nuclear receptor superfamily with functions that are redundant with both TR2 and TR4.
The study of mice lacking orphan nuclear receptor TR2 has revealed some important scientific implications. First, it is always possible to have different results from in vitro cell culture studies compared with in vivo animal studies. Second, our results suggest that the roles of TR2 could be compensated for by TR4 or other unidentified similar nuclear receptors. More-definitive results regarding the involvement of TR4 in this compensation will be obtained in the near future when double-knockout TR2-TR4 mice are available for study. Until then, we may have to assume that there are distinctly different results between in vitro cell culture and in vivo animal studies of TR2's physiological roles. Nevertheless, even TR2-knockout mice did not provide us the expected correlation of results with previous in vitro cell culture studies. We foresee these animals becoming a valuable tool for future in vivo studies involving double-knockout strategies aimed toward the characterization of the physiological roles of TR2 and related members of the nuclear receptor superfamily.
Acknowledgments
This work was supported by the George Whipple professorship endowment and NIH grant DK 47258.
The TR2-knockout mice were contracted from and generated by Lexicon Genetics.
REFERENCES
- 1.Chang, C., S. L. Da Silva, R. Ideta, Y. Lee, S. Yeh, and J. P. Burbach. 1994. Human and rat TR4 orphan receptors specify a subclass of the steroid receptor superfamily. Proc. Natl. Acad. Sci. USA 91:6040-6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang, C., and J. Kokontis. 1988. Identification of a new member of the steroid receptor super-family by cloning and sequence analysis. Biochem. Biophys. Res. Commun. 155:971-977. [DOI] [PubMed] [Google Scholar]
- 3.Chang, C., J. Kokontis, L. Acakpo-Satchivi, S. Liao, H. Takeda, and Y. Chang. 1989. Molecular cloning of new human TR2 receptors: a class of steroid receptor with multiple ligand-binding domains. Biochem. Biophys. Res. Commun. 165:735-741. [DOI] [PubMed] [Google Scholar]
- 4.Chang, C., and H. J. Pan. 1998. Thyroid hormone direct repeat 4 response element is a positive regulatory element for the human TR2 orphan receptor, a member of steroid receptor superfamily. Mol. Cell. Biochem. 189:195-200. [DOI] [PubMed] [Google Scholar]
- 5.Chen, W. S., K. Manova, D. C. Weinstein, S. A. Duncan, A. S. Plump, V. R. Prezioso, R. F. Bachvarova, and J. E. Darnell, Jr. 1994. Disruption of the HNF-4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gastrulation of mouse embryos. Genes Dev. 8:2466-2477. [DOI] [PubMed] [Google Scholar]
- 6.DeChiara, T. M., R. Vejsada, W. T. Poueymirou, A. Acheson, C. Suri, J. C. Conover, B. Friedman, J. McClain, L. Pan, N. Stahl, et al. 1995. Mice lacking the CNTF receptor, unlike mice lacking CNTF, exhibit profound motor neuron deficits at birth. Cell 83:313-322. [DOI] [PubMed] [Google Scholar]
- 7.Enmark, E., and J. A. Gustafsson. 1996. Orphan nuclear receptors—the first eight years. Mol. Endocrinol. 10:1293-1307. [DOI] [PubMed] [Google Scholar]
- 8.Evans, R. M. 1988. The steroid and thyroid hormone receptor superfamily. Science 240:889-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franco, P. J., M. Farooqui, E. Seto, and L. N. Wei. 2001. The orphan nuclear receptor TR2 interacts directly with both class I and class II histone deacetylases. Mol. Endocrinol. 15:1318-1328. [DOI] [PubMed] [Google Scholar]
- 10.Ingraham, H. A., D. S. Lala, Y. Ikeda, X. Luo, W. H. Shen, M. W. Nachtigal, R. Abbud, J. H. Nilson, and K. L. Parker. 1994. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev. 8:2302-2312. [DOI] [PubMed] [Google Scholar]
- 11.Lee, C. H., L. Chang, and L. N. Wei. 1996. Molecular cloning and characterization of a mouse nuclear orphan receptor expressed in embryos and testes. Mol. Reprod. Dev. 44:305-314. [DOI] [PubMed] [Google Scholar]
- 12.Lee, C. H., N. G. Copeland, D. J. Gilbert, N. A. Jenkins, and L. N. Wei. 1995. Genomic structure, promoter identification, and chromosomal mapping of a mouse nuclear orphan receptor expressed in embryos and adult testes. Genomics 30:46-52. [DOI] [PubMed] [Google Scholar]
- 13.Lee, Y. F., H. J. Pan, J. P. Burbach, E. Morkin, and C. Chang. 1997. Identification of direct repeat 4 as a positive regulatory element for the human TR4 orphan receptor. A modulator for the thyroid hormone target genes. J. Biol. Chem. 272:12215-12220. [DOI] [PubMed] [Google Scholar]
- 14.Lee, Y. F., W. J. Young, J. P. Burbach, and C. Chang. 1998. Negative feedback control of the retinoid-retinoic acid/retinoid X receptor pathway by the human TR4 orphan receptor, a member of the steroid receptor superfamily. J. Biol. Chem. 273:13437-13443. [DOI] [PubMed] [Google Scholar]
- 15.Lee, Y. F., W. J. Young, W. J. Lin, C. R. Shyr, and C. Chang. 1999. Differential regulation of direct repeat 3 vitamin D3 and direct repeat 4 thyroid hormone signaling pathways by the human TR4 orphan receptor. J. Biol. Chem. 274:16198-16205. [DOI] [PubMed] [Google Scholar]
- 16.Lin, D. L., S. Q. Wu, and C. Chang. 1998. The genomic structure and chromosomal location of the human TR2 orphan receptor, a member of the steroid receptor superfamily. Endocrine 8:123-134. [DOI] [PubMed] [Google Scholar]
- 17.Lin, T. M., W. J. Young, and C. Chang. 1995. Multiple functions of the TR2-11 orphan receptor in modulating activation of two key cis-acting elements involved in the retinoic acid signal transduction system. J. Biol. Chem. 270:30121-30128. [PubMed] [Google Scholar]
- 18.Mangelsdorf, D. J., C. Thummel, M. Beato, P. Herrlich, G. Schutz, K. Umesono, B. Blumberg, P. Kastner, M. Mark, P. Chambon, et al. 1995. The nuclear receptor superfamily: the second decade. Cell 83:835-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masu, Y., E. Wolf, B. Holtmann, M. Sendtner, G. Brem, and H. Thoenen. 1993. Disruption of the CNTF gene results in motor neuron degeneration. Nature 365:27-32. [DOI] [PubMed] [Google Scholar]
- 20.Mu, X., Y. Liu, L. L. Collins, E. Kim, and C. Chang. 2000. The p53/retinoblastoma-mediated repression of testicular orphan receptor-2 in the rhesus monkey with cryptorchidism. J. Biol. Chem. 275:23877-23883. [DOI] [PubMed] [Google Scholar]
- 21.Pereira, F. A., Y. Qiu, G. Zhou, M. J. Tsai, and S. Y. Tsai. 1999. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 13:1037-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu, Y., F. A. Pereira, F. J. DeMayo, J. P. Lydon, S. Y. Tsai, and M. J. Tsai. 1997. Null mutation of mCOUP-TFI results in defects in morphogenesis of the glossopharyngeal ganglion, axonal projection, and arborization. Genes Dev. 11:1925-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinmayr, M., E. Andre, F. Conquet, L. Rondi-Reig, N. Delhaye-Bouchaud, N. Auclair, H. Daniel, F. Crepel, J. Mariani, C. Sotelo, and M. Becker-Andre. 1998. Staggerer phenotype in retinoid-related orphan receptor alpha-deficient mice. Proc. Natl. Acad. Sci. USA 95:3960-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wattler, S., M. Kelly, and M. Nehls. 1999. Construction of gene targeting vectors from lambda KOS genomic libraries. BioTechniques 26:1150-1156, 1158, 1160. [DOI] [PubMed] [Google Scholar]
- 25.Young, W. J., Y. F. Lee, S. M. Smith, and C. Chang. 1998. A bidirectional regulation between the TR2/TR4 orphan receptors (TR2/TR4) and the ciliary neurotrophic factor (CNTF) signaling pathway. J. Biol. Chem. 273:20877-20885. [DOI] [PubMed] [Google Scholar]
- 26.Young, W. J., S. M. Smith, and C. Chang. 1997. Induction of the intronic enhancer of the human ciliary neurotrophic factor receptor (CNTFRα) gene by the TR4 orphan receptor. A member of steroid receptor superfamily. J. Biol. Chem. 272:3109-3116. [DOI] [PubMed] [Google Scholar]