Abstract
CTP:phosphocholine cytidylyltransferase alpha (CCTα) is a nuclear enzyme that catalyzes the rate-limiting step in the CDP-choline pathway, the primary route for synthesis of phosphatidylcholine (PtdCho) in eukaryotic cells. Induction of apoptosis by farnesol (FOH) and other cytotoxic drugs has been shown to alter PtdCho synthesis via the CDP-choline pathway. Here we report that FOH-induced apoptosis in CHO cells caused a dose-dependent activation of CCTα and inhibition of the final step in the pathway, resulting in a biphasic effect on PtdCho synthesis. Activation of CCTα was accompanied by enzyme translocation to the nuclear envelope within 30 min of FOH addition to cells. Following translocation to membranes, CCTα was exported from the nucleus and underwent caspase-mediated proteolysis that coincided with poly(ADP-ribose) polymerase cleavage. Site-directed mutagenesis and in vivo and in vitro expression studies mapped a caspase 6 and/or 8 cleavage site to TEED28↓G, the final residue in the CCTα nuclear localization signal. Nuclear export of CCTα appeared to be an active process in FOH-treated CHO cells that was independent of caspase removal of the nuclear localization signal. Caspase cleavage of CCTα occurred during UV or chelerythrine-induced apoptosis; however, nuclear membrane translocation and nuclear export were not evident under these conditions. Thus, caspase cleavage of CCTα was a late feature of several apoptotic programs that occurred in the nucleus or at the nuclear envelope. Activation and nuclear export of CCTα were early events in FOH-induced apoptosis that contributed to altered PtdCho synthesis and, in conjunction with caspase cleavage, excluded CCTα from the nucleus.
Phosphatidylcholine (PtdCho), the major phospholipid in eukaryotic cells, serves vital roles in membrane function and bile and lipoprotein secretion and as a precursor for lipid second messengers (9, 21). With the exception of liver, the CDP-choline pathway (Fig. 1) is the sole source of de novo PtdCho synthesis in mammalian tissues (33). CTP:phosphocholine cytidylyltransferase (CCT) is the rate-limiting enzyme in the CDP-choline pathway and is primarily regulated by translocation of a soluble inactive form to an active membrane-associated form in response to lipid activators and membrane structure. An amphipathic helix immediately adjacent to the catalytic domain mediates CCT translocation and activation in response to membrane lipid activators such as fatty acids and diacylglycerol (DAG) (9). Lipid activation of CCT provides a homeostatic response to PtdCho degradation by phospholipases, thus balancing synthesis and degradation and maintaining PtdCho levels (4, 38, 42). The extreme C terminus of CCTα contains 16 serine residues that are phosphorylated in a cell cycle-dependent manner (19) and interfere with activation by lipid modulators (53). The C-terminal phosphorylation domain was also shown to regulate CCTα activation by anionic lipids, suggesting a complex interplay between phosphorylation and lipid activation (25).
FIG. 1.
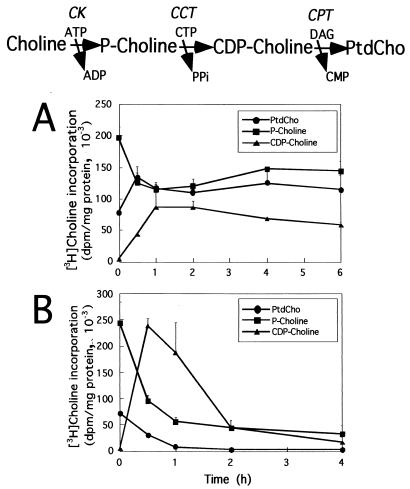
Influence of FOH on the CDP-choline pathway in CHO-K1 cells. The enzymes and intermediates in the CDP-choline pathway are shown at the top (CK, choline kinase). CHO cells were cultured in medium B for 24 h prior to the addition of 20 μM (A) or 60 μM (B) FOH to the culture medium for up to 6 h. Prior to harvest, cells were pulse-labeled with [3H]choline (2 μCi/ml) for 30 min. The samples at time zero were labeled for 30 min with [3H]choline in the absence of FOH. [3H]choline incorporation into PtdCho and the water-soluble choline metabolites (choline, phosphocholine, and CDP-choline) was quantitated as described in Materials and Methods. Results are the means and standard deviation of three separate experiments.
There are three CCT isoforms in mammalian cells encoded by two genes: CCTα, the major isoform ubiquitously expressed in all tissues, and the CCTβ1 and CCTβ2 splice variants, which show restricted tissue expression (23, 24). Unlike the CCTβ isoforms, which are localized to the endoplasmic reticulum-cytoplasm (24), CCTα is localized to the nucleus in many cell types by virtue of an N-terminal basic nuclear localization signal (46-48). While the precise function of nuclearly localized CCTα remains unknown, it may facilitate the coordination of PtdCho synthesis with other cell cycle events in the nucleus (19) or serve as a storage area for inactive enzyme in quiescent cells (30). Since the prior and subsequent enzymes in the CDP-choline pathway are primarily in the cytoplasm and endoplasmic reticulum, there must be effective transport of intermediates between enzymes or enzyme localization could be altered under certain conditions. A study showed that deletion of the nuclear localization signal on CCTα does not affect the ability to restore PtdCho synthesis and rescue growth in CCTα-deficient CHO cells (47). However, interpretation of this result was confounded by the incomplete exclusion from the nucleus of nuclear localization signal mutants of CCTα.
Activity of the CDP-choline pathway is coordinated with the cell cycle via modulation of CCT activity (19, 30), but its integration with the cell cycle-related process of apoptosis is unknown. A variety of cytotoxic and apoptotic agents inhibit PtdCho synthesis (2). One such agent is farnesol (FOH), an isoprenoid that preferentially induces apoptosis in transformed cells (1, 35). FOH is a by-product of the mevalonate/cholesterol biosynthetic pathway and is a potent inhibitor of the CDP-choline pathway, imposing a metabolic block at the terminal reaction catalyzed by CDP-choline:1,2-diacylglycerol cholinephosphotransferase (CPT) (28, 44). Thus, FOH could be a signal to coordinately regulate cholesterol and PtdCho synthesis under in vivo conditions or suppress PtdCho synthesis and induce apoptosis when added exogenously to cells.
There is evidence that FOH acts as a direct inhibitor of CPT in vitro (2, 28), although this has recently been challenged (50). In vivo effects of FOH on CPT could be due to limiting levels of DAG (3, 20) or cellular acidification that occurs during apoptosis (2). Inhibition of PtdCho synthesis was proposed to be a primary mechanism for FOH-induced apoptosis (2, 28, 44). This is supported by studies where apoptosis was induced by disruption of the CDP-choline pathway following genetic inactivation of CCTα (13) or inhibition of CCTα with drugs (5, 6). The addition of PtdCho or exogenous DAG to FOH-treated cells was sufficient to prevent the initiation of apoptosis, suggesting that depletion of PtdCho or inhibition of de novo synthesis was responsible (27, 45). However, a recent study showed that restoration of PtdCho synthesis by overexpression of CPT during FOH treatment was insufficient to rescue CHO cells from apoptosis (50). This implies that cessation of PtdCho synthesis is not the critical determinant in the apoptotic pathway initiated by FOH. Phorbol esters also delayed the induction of apoptosis in response to geranylgeraniol in a protein kinase C-dependent manner (27), indicating that isoprenoids disrupt DAG-signaling events. This could result from effects of FOH on DAG generation by phospholipase D and/or a putative PtdCho-specific phospholipase C (45).
An intriguing feature of the apoptotic program induced by FOH, as well as other drugs, is the massive increase in the biosynthetic intermediate CDP-choline (2, 28, 44). As mentioned above, this could result solely from inhibition of CPT; however, FOH and other apoptotic agents also reduced phosphocholine labeling, suggesting activation of CCT (2). In another study, CCT activity was translocated from cytosol to membranes in FOH- and geranylgeraniol-treated A549 cells, an indication of enzyme activation (28).
Since CCTα is ubiquitously expressed in mammalian tissues and is the rate-limiting step in PtdCho synthesis, we were interested in studying its regulation during FOH-induced apoptosis and comparing this to other apoptotic programs. Our results show that CCTα is translocated to the nuclear envelope and activated in FOH-treated CHO cells. The subsequent export of CCTα from the nucleus and removal of the N-terminal nuclear localization signal by caspase(s), two mutually exclusive events, suggest a novel mechanism to disrupt compartmentalization of the CDP-choline pathway and PtdCho synthesis during apoptosis.
MATERIALS AND METHODS
Materials.
[Methyl-3H]choline was purchased from NEN Life Sciences Products. FOH was from Sigma-Aldrich. z-VAD-fmk and z-DEVD-fmk were supplied by Calbiochem. Silica gel G thin-layer chromatography (TLC) plates were purchased from Fisher Scientific. Recombinant caspases 3, 6, and 8; acetyl-DEVD-p-nitroanilide; and acetyl-VEID-p-nitroanilide were purchased from BioMol. The activity of recombinant caspases was tested prior to use with appropriate colorimetric substrates. Recombinant rat CCTα expressed in the baculovirus system was provided by Rosemary Cornell, Simon Fraser University, Vancouver, Canada. Lipoprotein-deficient serum (LPDS) was prepared from fetal calf serum by centrifugation, followed by extensive dialysis against PBS (10 mM phosphate [pH 7.4], 150 mM NaCl) (40). Cell culture medium was from Life Technologies, Inc. A rabbit polyclonal antibody raised against the C-terminal 45 amino acids of rat CCTα was kindly provided by Martin Post, Hospital for Sick Children, Toronto, Canada (55). Rabbit polyclonal antibody against poly(ADP-ribose) polymerase (PARP) was from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). Monoclonal antibody 414 directed against a common epitope found in Nup62 and related components of the nuclear pore complex was from Berkeley Antibody Co., Richmond, Calif. Secondary horseradish peroxidase-coupled antibodies were from Bio-Rad. Oregon Green 488 conjugated to dextran and secondary antibodies for immunofluorescence were from Molecular Probes. Complete protease cocktail (used at 50-fold dilution) was from Boehringer Mannheim.
Cell culture and transfections.
CHO cells (ATCC CCL61) were cultured in Dulbecco's modified Eagle's medium (DMEM) with 5% fetal calf serum and 34 μg of proline per ml (medium A) in an atmosphere of 5% CO2 at 37°C. Cells were seeded at a density of 200,000 cells per 60-mm dish in 3 ml of medium A. On day 2, cells received 2 ml of DMEM containing 5% LPDS and 34 μg of proline per ml (medium B) or 2 ml of medium A. Experiments were started 18 to 24 h after this medium change when cells were ≈70% confluent. NIH 3T3 and HEK 293 cells were cultured in DMEM with 10% fetal calf serum. Cells received DMEM with 10% LPDS 24 h before the start of experiments.
CHO MT58 cells with a temperature-sensitive CCTα allele (15) were seeded at 500,000 cells per 60-mm dish in medium A in an atmosphere of 5% CO2 at 33°C. On day 2, cells were transiently transfected by the Lipofectamine method according to the manufacturer's instructions (Life Technologies, Inc.), using 0.25 μg of DNA and 3 μl of Lipofectamine reagent in 1.5 ml of DMEM per dish. After 6 h, an equal volume of medium A was added, and cells were cultured for 24 h before the start of experiments. CHO MT58 cells were stably transfected with pCMV5-CCT (26) or pCMV-CCT D28E and pSV3Neo by selection in medium A containing G418 (700 μg/ml) at 33°C. Clones expressing CCTα were selected by immunoblotting and maintained in medium A with G418 (350 μg/ml) at 37°C.
Site-directed mutagenesis.
Site-directed mutagenesis of rat CCTα cDNA in pCMV5 (26) was performed with the Gene Editor System (Promega) and confirmed by sequencing. The following oligonucleotides were used to mutagenize CCTα aspartate residues to glutamate (mutation in italics): D28E, ACAGAGGAAGAAGGAATTCCTTCC, and D54E, ATTGAAGTTGAATTTAGTAAGCCC.
Analysis of labeled PtdCho and choline metabolites.
After labeling with [3H]choline, cells were immediately placed on ice and rinsed once with 2 ml of cold PBS. Cells were scraped in 1 ml of methanol-water (5:4, vol/vol). The culture dish was rinsed with 1 ml of methanol-water, and the extracts were combined in a glass screw-cap tube. [3H]PtdCho and aqueous [3H]choline metabolites were separated by extraction with chloroform as previously described (40). [3H]PtdCho was measured by scintillation counting of an aliquot of the chloroform phase (>98% of radioactivity was in PtdCho). Aqueous metabolites were separated on silica gel G TLC plates in a solvent system of ethanol-water-ammonia (48:95:6, vol/vol/vol). Choline, phosphocholine, and CDP-choline were identified by comigration with authentic standards and scraped into vials, and radioactivity was measured by scintillation counting.
Digitonin permeabilization and CCT assays.
Digitonin permeabilization was carried out at room temperature as previously described (51) with minor modifications. Briefly, 60-mm dishes were rinsed once with cold PBS and incubated for 1 min without agitation in 0.5 ml of permeabilization buffer (10 mM Tris-HCl [pH 7.4], 0.05 mg of digitonin per ml, 0.25 M sucrose, 2.5 mM EDTA, 1 mM dithiothreitol [DTT], 0.1 mM phenylmethylsulfonyl fluoride, protease inhibitor cocktail). The permeabilization buffer was removed from cells and subjected to centrifugation at 20,000 × g for 15 min at 4°C to remove debris (designated the soluble fraction). Cell ghosts were scraped in 0.5 ml of permeabilization buffer without digitonin and homogenized by 10 passages through a 23-gauge needle (designated the membrane fraction). This protocol recovered >95% of lactate dehydrogenase activity in the soluble fraction.
CCT activity in soluble and membrane fractions was assayed in the presence of PtdCho-oleate vesicles by monitoring the conversion of phospho[3H]choline to CDP-[3H]choline as previously described (12). Recombinant CCTα activity with FOH- and oleate-supplemented PtdCho vesicles was also assayed by this method. PtdCho-FOH and PtdCho-oleate vesicles were prepared by sonication on ice of lipids suspended in 10 mM Tris-HCl (pH 7.4)-1 mM EDTA.
Preparation of cell extracts.
Preparation of cell extracts extracted CCTα and both the native and caspase-cleaved forms of PARP. Cells were rinsed once with cold PBS, scraped in 1 ml of cold PBS, and collected by centrifugation for 1 min at 1,000 × g. Cells were resuspended in 150 μl of high-salt Triton X-100 solubilization buffer (10 mM Tris-HCl [pH 7.4], 1% [wt/vol] Triton X-100, 500 mM NaCl, 2 mM EDTA, 1 mM DTT, 0.1 mM phenylmethylsulfonyl fluoride, protease inhibitor cocktail), incubated on ice for 15 min, and subjected to centrifugation at 4°C at 21,000 × g for 15 min to remove Triton X-100-insoluble material.
Immunoblotting.
Digitonin and Triton X-100 extracts of CHO cells were prepared as described above. Equal amounts of proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 8 or 10% gels and transferred to nitrocellulose membranes. The fidelity of transfer to membranes and assessment of protein loading were verified by staining with Ponceau S. The nitrocellulose membranes were incubated with polyclonal antibodies against CCTα or PARP, followed by a goat anti-rabbit immunoglobulin antibody conjugated to horseradish peroxidase, and developed by the chemiluminescence method according to the manufacturer's instructions (Amersham Pharmacia Biotech). All antibody incubations were in 20 mM Tris-HCl (pH 7.4)-150 mM NaCl-5% (wt/vol) skim milk powder-0.1% (vol/vol) Tween 20.
Immunofluorescence microscopy.
CHO cells were cultured on glass coverslips to 50% confluence, fixed in 3% formaldehyde-PBS for 15 min, and permeabilized in PBS containing 0.05% (wt/vol) Triton X-100 for 10 min at −20°C. Coverslips were incubated for 12 to 18 h at 4°C with an antibody against CCTα in PBS-1% bovine serum albumin. Cells were washed three times with PBS-1% bovine serum albumin and incubated with goat anti-rabbit immunoglobulin fluorescein isothiocyanate (FITC)- or Alexa Fluor 555-labeled antibody for 45 min at 20°C. Following three washes with PBS-1% bovine serum albumin, cells were incubated in PBS containing 10 μg of Hoechst 33258 (Riedel-De Haen, Seelze, Germany) per ml for 10 min at 4°C, rinsed in PBS, and mounted in 50 mM Tris-HCl (pH 9.0)-2.5% (wt/vol) 1,4-diazabicyclo-[2.2.2]-octane (Sigma-Aldrich)-90% (vol/vol) glycerol.
Oregon green 488-labeled dextran (70 kDa) was introduced into CHO cells by scrape-loading. Briefly, cells were scraped from 60-mm dishes with a rubber policeman in 0.5 ml of ice-cold PBS containing fluorescently tagged dextran (1 mg/ml). Cells were allowed to take up dextran for 5 min on ice, diluted with 8 ml of medium A, and collected by centrifugation for 5 min at 300 × g. Cells were reseeded on glass coverslips in medium A for 8 h, switched to delipidated serum for 18 h, and immunostained for CCTα or the nuclear pore complex.
CCTα cleavage assay.
An XbaI/HindIII fragment containing the entire coding region of rat CCTα or the D28E mutant was subcloned into pGEM4Z. These constructs were used as templates to transcribe and translate [35S]methionine-labeled CCTα in a T7-coupled reticulocyte lysate system according to the manufacturer's instructions (TnT system; Promega). Control and apoptotic cell extracts for in vitro assays were prepared by rinsing cells once in cold PBS, scraping from dishes into PBS, and collection of cell pellets by brief centrifugation at 3,000 × g. Cell pellets were resuspended in extraction buffer (25 mM HEPES [pH 7.2], 1 mM EDTA, 5 mM MgCl2, 0.1% Triton X-100, 250 mM phenylmethylsulfonyl fluoride, 2 mM DTT, protease cocktail), and a soluble fraction was collected after centrifugation for 10 min at 10,000 × g. Assays for caspase cleavage of 35S-labeled CCTα routinely contained 3 μl of the CCT translation mixture and 10 to 15 μg of cell extract in a final volume of 20 μl for 60 min at 30°C. Assays with recombinant caspases were performed with 50 mM HEPES (pH 7.4)-100 mM NaCl-10% glycerol-10 mM EDTA-2 mM DTT-0.1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS). Assays were terminated by the addition of SDS-PAGE loading buffer. Samples were heated to 90°C, separated on SDS-10% PAGE gels, treated with fluorography agent, dried, and exposed to film. Caspase 3 and 6 activity was measured using 200 μM acetyl-DEVD-p-nitroanilide or acetyl-VEID-p-nitroanilide, respectively, in 100 μg of cell extract (prepared as described above) for 1 h at 30°C.
RESULTS
Activation, translocation, and caspase cleavage of CCTα by FOH.
To determine the effect of FOH on PtdCho synthesis, CHO cells were labeled with [3H]choline, and incorporation into the final product PtdCho as well as the pathway intermediates choline, phosphocholine, and CDP-choline was measured. Changes in distribution of these intermediates provide an indication of the relative activity of enzymes in the CDP-choline pathway (top of Fig. 1). Cells were incubated with 20 or 60 μM FOH for up to 6 h and labeled with [3H]choline during the final 30 min of incubation with FOH (Fig. 1). For this and subsequent experiments, we used CHO cells cultured for 18 to 24 h in lipoprotein-deficient medium to reduce the potential influence of exogenous phospholipids and DAG on FOH-induced apoptosis (28, 44, 50).
Incubation of CHO cells with 20 μM FOH (Fig. 1A) caused a modest increase in PtdCho synthesis. This appeared be the result of increased CCT activity, since [3H]phosphocholine levels were reduced. A large increase in PtdCho synthesis was prevented due to apparent inhibition of CPT, as indicated by accumulation of CDP-choline. Consistent with reports using other cultured cells (2, 28, 44), we observed a rapid inhibition of PtdCho synthesis by 30 min in CHO cells treated with 60 μM FOH (Fig. 1B). This was accompanied by a dramatic elevation in [3H]CDP-choline (indicating CPT inhibition) and reduction in [3H]phosphocholine (indicative of CCT activation). The reduction of phosphocholine labeling was not due to inhibition of choline kinase activity by FOH, since this would have inhibited [3H]choline incorporation into all subsequent metabolites and PtdCho. This was clearly not the case except in cells treated with 60 μM FOH for 2 h, after which there appeared to be global suppression of the CDP-choline pathway. [3H]choline in cells was not significantly affected at either concentration of FOH (results not shown).
To determine if the effects of FOH on CDP-choline pathway metabolites were related to increased CCTα activity, the distribution of CCTα activity and mass between the active membrane and inactive soluble pools was measured by digitonin permeabilization (51). In that study (51), digitonin was shown to be effective in releasing soluble CCTα from the nucleus. CCTα in CHO cells is localized primarily in the nucleus and translocates to the inner nuclear membrane in response to lipid activators such as fatty acids and diacylglyceride (9, 10, 22, 38). CHO cells were treated with FOH for up to 8 h, permeabilized with digitonin, and separated into membrane and soluble fractions, and in vitro activity and CCTα protein were measured (Fig. 2).
FIG. 2.
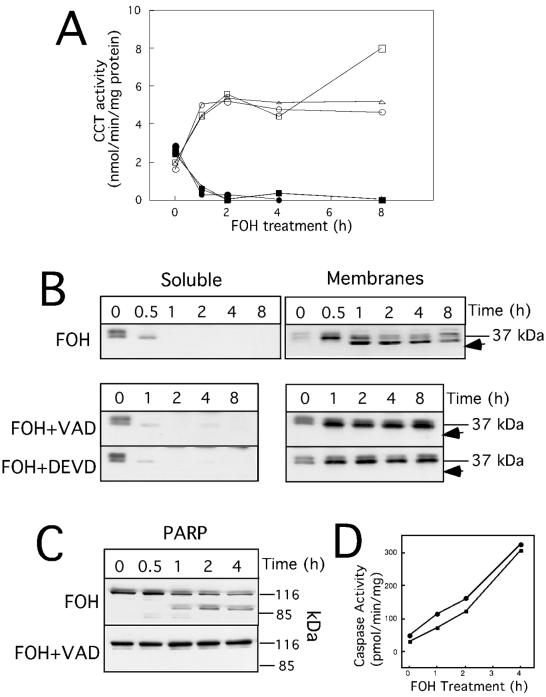
FOH promotes membrane translocation and caspase-mediated cleavage of CCTα. CHO cells were cultured in medium B for 24 h prior to treatment with z-VAD-fmk (75 μM), z-DEVD-fmk (75 μM), or dimethyl sulfoxide control (0.3%, vol/vol) for 15 min, followed by addition of 60 μM FOH for the indicted times. At each time point, soluble and membrane fractions were isolated by digitonin permeabilization as described in Materials and Methods. (A) Soluble (solid symbols) and membrane (open symbols) fractions (10 to 15 μg of protein) from cells treated with FOH (○ and •), FOH plus z-VAD-fmk (□ and ▪), or FOH plus z-DEVD-fmk (Δ and ▴) were assayed for CCT activity in the presence of PtdCho-oleate vesicles. (B) Soluble and membrane fractions (7.5 μg of protein) from digitonin-treated cells were resolved on SDS-10% PAGE gels and immunoblotted with an anti-CCTα antibody. The position of proteolyzed CCTα is indicated by arrows. (C) PARP was detected by immunoblotting of total cell protein (15 μg) from cells treated with FOH (60 μM) or FOH plus z-VAD-fmk (75 μM) for the indicted times. (D) Caspase 3 (•) and 6 (▪) activity was assayed in 100 μg of the 100,000 × g soluble fraction of FOH-treated CHO cells with acetyl-DEVD-p-nitroanilide or acetyl-VEID-p-nitroanilide, respectively. Results for CCT activity and CCT and PARP expression are from one experiment that was repeated three times with similar results.
Treatment of CHO cells with 60 μM FOH resulted in the translocation of >90% of soluble CCTα to membranes within 60 min, as measured by in vitro assays of isolated fractions (Fig. 2A; also see Fig. 7 and 8). We have previously reported the absence of CCTβ isoforms in CHO cells using a CCTβ-specific antibody (22), and therefore we conclude that the stimulation of CCT activity following FOH addition is due to the CCTα isoform. When soluble and membrane fractions were immunoblotted with a CCTα antibody, it was evident that soluble CCTα translocated to membranes within 30 min of FOH addition to cells (Fig. 2B). In cytosol from untreated cells (Fig. 2B), several closely spaced CCTα isoforms were evident on immunoblots. This heterogeneity is the result of differential phosphorylation of multiple serine residues in the C-terminal domain (17). A shift to a lower-molecular-mass CCTα isoform was apparent in membranes following treatment with FOH for 30 min, indicating dephosphorylation of the protein.
FIG. 7.
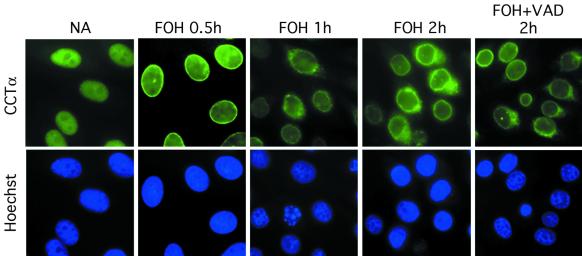
FOH promotes nuclear envelope localization and subsequent nuclear export of CCTα. CHO cells were cultured on glass coverslips in medium B for 24 h prior to the addition of 60 μM FOH. NA, no addition. One set of cells received 75 μM z-VAD-fmk in addition to FOH. At the indicated times, cells were fixed and processed for immunofluorescence localization of CCTα and stained with Hoechst 33258 as described in Materials and Methods. Images were obtained with an Axioplan 2 fluorescence microscope with a Planapo ×100 oil immersion objective and equipped with a Spot charge-coupled device camera.
FIG. 8.
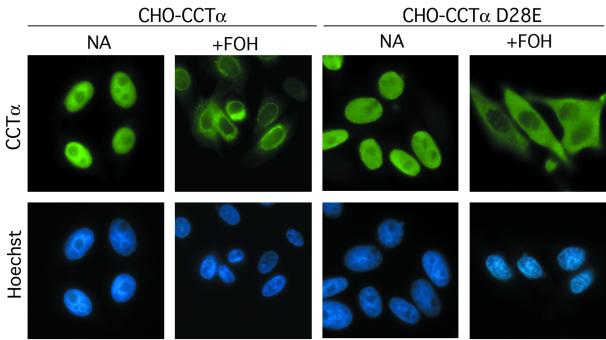
Nuclear export of caspase-resistant CCTα D28E. CHO MT58 cells stably expressing CCTα or CCTα D28E were cultured on glass coverslips in medium B and treated with FOH (60 μM) or solvent (ethanol) for 1 h. Cells were stained for CCTα with a FITC-labeled secondary antibody or Hoechst as described in the legend to Fig. 7.
In addition to changes in localization and phosphorylation, immunoblots of membrane fractions from FOH-treated cells revealed a 3- to 5-kDa reduction in CCTα molecular mass (Fig. 2B), indicating proteolysis near the N or C terminus of the enzyme. This putative CCTα proteolysis product appeared after translocation to membranes. Since FOH is a potent apoptotic agent that activates caspase-mediated proteolytic cascades (29), we tested whether proteolytic cleavage of CCTα could be prevented by the addition of the broad-range caspase inhibitor z-VAD-fmk or the caspase 3-specific inhibitor z-DEVD-fmk. Treatment of cells with these caspase inhibitors for 15 min prior to the addition of 60 μM FOH completely prevented CCTα cleavage for up to 8 h. CCTα membrane translocation and dephosphorylation (Fig. 2B), as well as enzyme activity (Fig. 2A), were not affected by caspase inhibitors. FOH-stimulated cleavage of PARP and CCTα occurred within the same time frame and was completely blocked by z-VAD-fmk (Fig. 2C). Caspase 3 and 6 activity (measured using colorimetric substrates) in cell extracts from FOH-treated cells also increased linearly over 4 h (Fig. 2D).
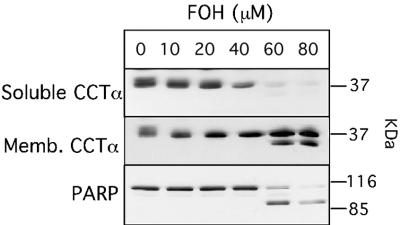
Dose-dependent CCTα translocation and caspase cleavage by FOH is shown in Fig. 3. In digitonin-treated control cells, CCTα was equally distributed between soluble and membrane fractions. Exposure to 20 to 80 μM FOH for 60 min resulted in almost complete CCTα translocation to membranes. CCTα and PARP cleavage (indicated by arrow) was evident at higher FOH concentrations (60 to 80 μM) that caused dramatic inhibition of PtdCho synthesis (Fig. 1). Interestingly, CCTα and PARP processing by caspases did not appear to go to completion in FOH-treated cells (Fig. 2 and 3).
FIG. 3.
Dose-dependent activation and caspase processing of CCTα induced by FOH. CHO cells were cultured in medium B for 24 h prior to addition of indicated concentrations of FOH for 1 h. Cells were subsequently permeabilized with digitonin, and the soluble and membrane (Memb.) fractions were collected. Samples (7.5 μg of protein) were resolved on SDS-10% PAGE gels and immunoblotted for CCTα. The position of proteolyzed CCTα is indicated by an arrow. PARP was immunoblotted in total cell extracts (15 μg).
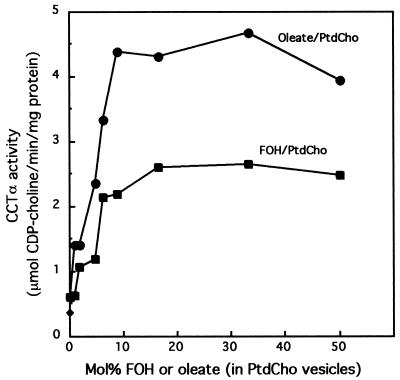
Results shown in Fig. 2 and 3 suggest that FOH activates CCTα, but it is uncertain if this is a direct activation or the result of indirect effects. To determine whether FOH activated CCTα in vitro, the recombinant rat enzyme was assayed in the presence of PtdCho vesicles containing increasing amounts of FOH and, for comparison, a known activator, oleate (Fig. 4). Relative to activity in the presence of PtdCho vesicles, CCTα activity was stimulated fourfold in PtdCho vesicles supplemented with 10 to 20 mol% FOH (20 mol% FOH corresponds to 20 μM). Activation of CCTα was also maximal with 10 mol% oleate but was sevenfold greater than activity observed with PtdCho vesicles alone.
FIG. 4.
In vitro activation of CCTα by FOH. Recombinant rat CCTα (70 ng) was assayed in the presence of PtdCho vesicles containing an increasing concentration of FOH or oleate as described in Materials and Methods. The total concentration of lipid in the assay was 100 μM. Results are the means of duplicate assays from a representative experiment.
Identification of the caspase site in CCTα.
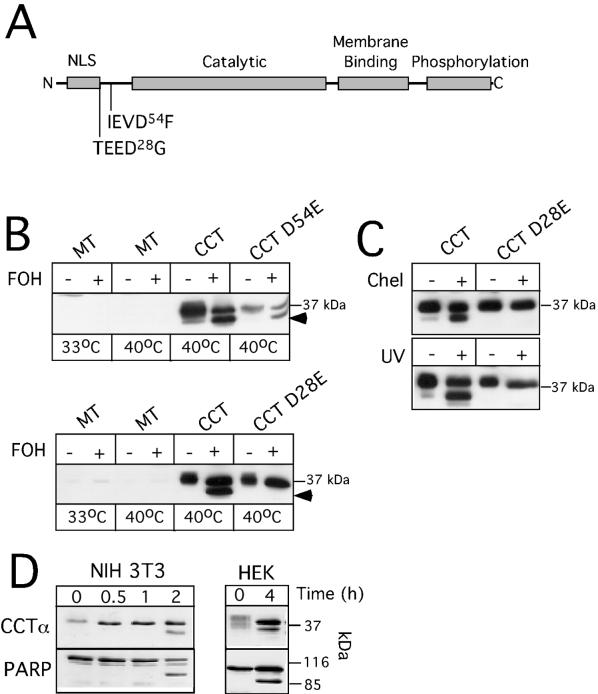
Caspases recognize four amino acid consensus sequences, with cleavage occurring on the carboxyl side of an absolutely conserved aspartate residue at the fourth position (49). Removal of 3 to 4 kDa from the C terminus of CCTα would remove the antibody epitope, indicating proteolysis was close to the N terminus. Inspection of the CCTα protein sequence from several species revealed two candidate caspase sites within the N-terminal region, TEED28G and IEVD54F (Fig. 5A). Interestingly, both of these sites are absent in the β-isoforms of CCT and yeast CCT but are conserved in CCTα from other mammalian species.
FIG. 5.
Caspase-dependent cleavage of CCTα at D28 in FOH-treated cells. (A) Schematic of CCTα, showing the positions of domains and putative caspase cleavage sites adjacent to the N terminus (NLS, nuclear localization signal). (B) cDNAs encoding wild-type (wt) CCTα, CCTα D54E, CCTα D28E, or empty vector (MT, mock transfected) were transiently transfected into CHO MT58 cells cultured at 33°C. After 24 h, cells received 3 ml of medium B at 33°C. Forty-eight hours after transfection, MT58 cells were either kept at 33°C or switched to 40°C and treated with 60 μM FOH or solvent (ethanol) alone for 2 h. Cells were harvested, and total homogenates (10 μg of protein) were resolved on SDS-10% PAGE gels and immunoblotted for CCTα. The position of proteolyzed CCTα is indicated by arrows. (C) cDNAs were transfected into MT58 cells as described above and treated with 20 μM chelerythrine for 2 h or exposed to UV light (254 nm) for 10 min and cultured for an additional 12 h. Cells were shifted to 40°C 60 min prior to harvest to reduce expression of endogenous CCTα. (D) NIH 3T3 and HEK 293 cells were cultured in DMEM with 10% LPDS for 24 h prior to addition of 60 μM FOH for the indicated times. CCTα and PARP were immunoblotted in 15 μg of cell extract as described in the legend to Fig. 2.
To assess if these were caspase sites in vivo, aspartate residues were changed to glutamate by site-directed mutagenesis. CHO MT58 cells were transiently transfected with the cDNAs and treated with FOH (Fig. 5B). CHO MT58 cells were chosen for these experiments because of low expression of the temperature-sensitive allele of CCTα (15), thus negating interference by endogenous CCTα. Indeed, in mock-transfected CHO MT58 cells, endogenous CCTα was virtually undetectable under permissive (33°C) and nonpermissive (40°C) temperatures (a faint band was detected upon prolonged overexposure of the blots). Similar to results with endogenous CCTα (Fig. 2B), wild-type transfected CCTα was partially cleaved in response to FOH. CCT D54E was also cleaved in response to FOH, indicating that this is not a caspase site.
TEED28↓G was identified as the caspase cleavage site, as indicated by resistance of CCTα D28E to proteolysis in response to FOH. Interestingly, D28 is the final residue in the nuclear localization sequence for CCTα (47), and caspase cleavage would generate a protein unable to enter the nucleus. We also tested whether CCTα was cleaved at this site in response to other apoptotic stimuli (Fig. 5C) and in other cell lines (Fig. 5D). Exposure of CHO MT58 cells to chelerythrine (a nonspecific protein kinase C inhibitor) or UV light resulted in cleavage of transfected wild-type CCTα, but not the D28E mutant. Thus, in CHO cells, proteolysis of CCTα is a common feature in different apoptotic programs. The in vitro activity of CCT D54E and CCT D28E transiently expressed in MT58 cells was similar to that of wild-type controls (data not shown), and both proteins were localized to the nucleus under normal conditions (see Fig. 8). Proteolytic cleavage of endogenous CCTα was also observed in CHO cells exposed to UV or chelerythrine (data not shown).
To confirm that caspase cleavage of CCTα is a general feature of FOH-induced apoptosis, proteolysis of CCTα and PARP was determined by SDS-PAGE and immunoblotting of NIH 3T3 and HEK 293 cells treated with 60 μM FOH for up to 4 h (Fig. 5D). In both cell lines, FOH caused the partial cleavage of CCTα to a low-molecular-mass fragment similar to that observed in CHO cells. Particularly evident in HEK 293 cells was FOH-induced CCTα dephosphorylation, as indicated by the conversion of several diffuse CCTα isoforms to the 38- to 39-kDa species.
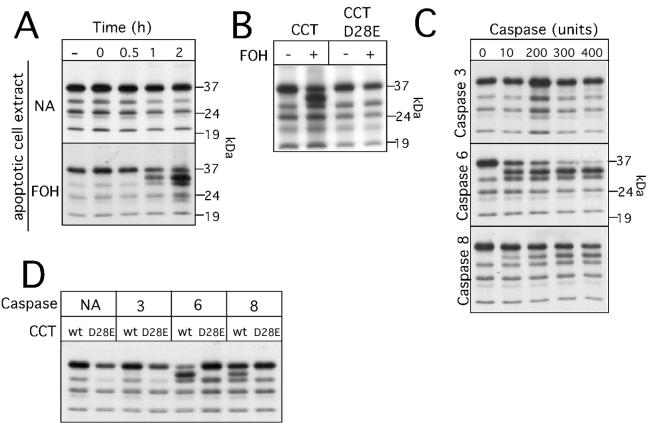
To further verify that CCTα is a caspase substrate and to identify the caspase(s) involved, we examined the processing of in vitro-translated CCTα by apoptotic extracts and recombinant caspases (Fig. 6). CCTα was translated in vitro in the presence of [35S]methionine, and the labeled protein was incubated for various times with extracts from control or FOH-treated CHO cells (Fig. 6A). The major in vitro-translated CCTα had a molecular mass similar to that of the endogenous 38-kDa protein (several minor labeled proteins likely correspond to initiation from internal methionines). Incubation with apoptotic cell extracts generated a CCTα that migrated on SDS-PAGE just below the full-length protein. Proteolyzed CCTα was not evident in incubations with extracts from untreated CHO cells. Cleavage of CCTα by apoptotic cell extracts was prevented by the D28E mutation (Fig. 6B), showing that the in vitro system faithfully reproduced results from transfected cells.
FIG. 6.
In vitro proteolysis of CCTα by apoptotic cell extracts and recombinant caspases. (A) In vitro-translated 35S-labeled CCTα was incubated with cell extract (10 μg) prepared from control (NA, no addition) or FOH-treated cells (60 μM for 3 h) for the indicated times and resolved by SDS-PAGE and fluorography as described in Materials and Methods. (B) FOH-treated and control cell extracts (15 μg) were incubated with in vitro-translated CCTα or CCTα D28E for 2 h and analyzed by SDS-PAGE and fluorography. (C) The indicated units of recombinant caspases 3, 6, and 8 were incubated with in vitro-translated 35S-labeled CCTα for 30 min and analyzed by SDS-PAGE and fluorography. One unit of activity refers to 1 pmol/min with tetrapeptide colorimetric substrates specific for the caspases. (D) Recombinant caspases (300 U) were incubated with in vitro-translated 35S-labeled CCTα or CCTα D28E for 30 min and resolved by SDS-PAGE. Fluorograms were the result of exposure of dried gels to Kodak XAR film for 8 to 12 h at −70°C.
With this information, we determined which caspase was responsible for cleavage at the TEED28 site. Based on specificity with fluorogenic peptide substrates (41), the best candidates are the class III caspases 6, 8, and 9. Due to limited specificity of caspase inhibitors, initial experiments with cells or in vitro assays did not provide conclusive data on the identity of the CCTα-specific caspase. To directly assess whether CCTα was a substrate for class III caspases, in vitro-translated wild-type and D28E enzymes were incubated with increasing amounts of recombinant caspases 6 and 8 as well as caspase 3 as a control (Fig. 6C). As expected, caspase 3 did not cleave CCTα at the aspartate 28 site, nor were other proteolyzed forms of CCT generated. In contrast, both caspases 6 and 8 generated a CCTα product consistent with proteolysis at aspartate 28. However, caspase 6 displayed the highest activity toward CCTα, with complete digestion in 30 min with 200 to 300 U of enzyme. Caspases 6 and 8 did not cleave the D28E mutant, indicating absolute preference for that site (Fig. 6D).
Release of CCTα from the nucleus by FOH.
Digitonin permeabilization and immunoblotting experiments indicated that CCTα rapidly translocated to membranes in response to FOH. We used indirect immunofluorescence to more precisely determine the membrane binding site for CCTα. Figure 7 shows fluorescence micrographs of cells treated with FOH for different times and immunostained for CCTα and the DNA binding dye Hoechst 33258 to visualize the nucleus and changes in chromatin structure. As reported previously, CCTα in CHO cells is dispersed throughout the nucleus under basal conditions (46, 47). Treatment with FOH for 30 min promoted CCTα translocation to the nuclear envelope. A portion of CCTα was evident outside the nucleus at 1 and 2 h of FOH treatment, as indicted by an absence of overlap with the nuclear stain. However, there was still substantial CCTα on the nuclear envelope, although staining was now more fragmented and localized to discrete regions. Cells that showed significant extranuclear staining of CCTα also displayed abnormal chromatin staining and appearance of nuclear bodies.
To determine if caspase-mediated removal of the nuclear localization signal of CCTα played a role in its release from the nucleus, we pretreated cells with z-VAD-fmk and analyzed CCTα intracellular localization after FOH treatment for 2 h. Although the broad-spectrum caspase inhibitor z-VAD-fmk completely prevented CCTα and PARP cleavage (see Fig. 2), cells still displayed CCTα staining of the perinuclear region. This showed that inhibition of caspases and removal of the CCTα nuclear localization signal were not necessary for movement to the nuclear membrane or export from the nucleus.
To more precisely address the role of caspase cleavage in release of CCTα from the nucleus, CHO MT58 cells were stably transfected with wild-type CCTα or the D28E mutant and treated with FOH for 1 h, and the localization of CCTα was assessed by immunofluorescence (Fig. 8). In untreated cells, the wild-type CCTα and D28E mutant were exclusively in the nucleus, indicating that the D28E mutation does not affect the import signal. In cells treated with FOH for 1 h, both the wild-type and D28E CCTα were released from the nucleus and were diffusely localized throughout the cell. The localization of wild-type CCTα overexpressed in FOH-treated cells was similar to that of the endogenous enzyme (see Fig. 7). In contrast, D28E CCTα staining was more diffuse and excluded from the nucleus and nuclear envelope.
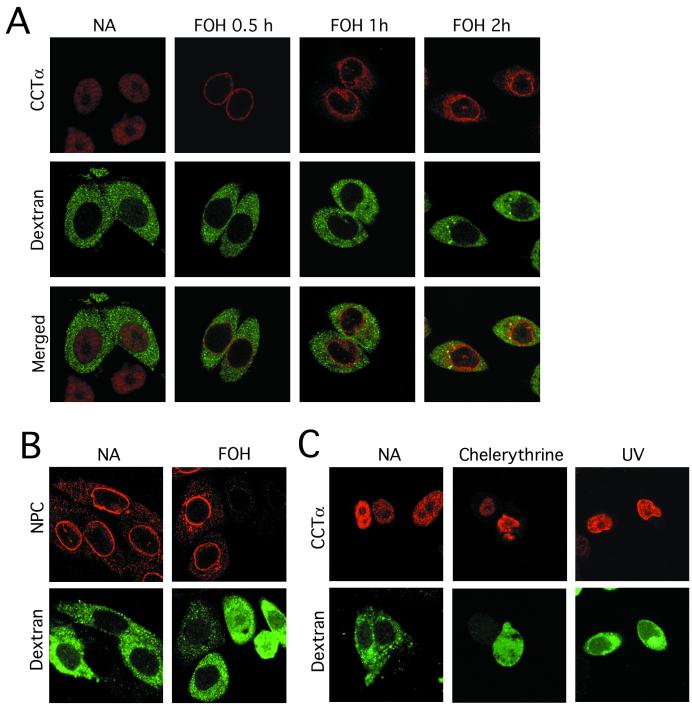
A possible explanation for release of CCTα from the nucleus during apoptosis is loss of integrity of the nuclear envelope. To address this question, CHO cells were scrape-loaded with soluble Oregon green 488-labeled 70-kDa dextran, and its uptake into the nucleus was monitored during FOH-mediated CCTα export as an index of nuclear integrity (36). This dextran is similar in molecular mass to that of dimeric CCTα (12) and provided a good index of passive diffusion across the nuclear envelope. In untreated CHO cells, Oregon green 488-labeled dextran was localized in the cytoplasm and excluded from the nucleus, while CCTα was entirely in the nucleus (Fig. 9A). After FOH treatment for 30 min, CCTα was translocated to the nuclear envelope, while dextran was maintained in the cytoplasm. Following 1 h of FOH treatment, CCTα was evident outside the nucleus, but fluorescent dextran was still excluded from the nucleus. The same pattern was observed at 2 h; however, more CCTα was outside the nucleus and there was weak dextran staining of the nucleus in 20 to 40% of cells.
FIG. 9.
Nuclear envelope integrity and CCTα export in FOH-, UV-, and chelerythrine-induced apoptosis. CHO cells were scrape loaded with Oregon green 488-labeled dextran as described in Materials and Methods. (A) Cells were subsequently treated with FOH (60 μM) or solvent alone (NA, no addition) for the indicated times and processed for immunofluorescence localization of CCTα with an Alexa Fluor 647-labeled secondary antibody. (B) Oregon green 488-dextran-loaded CHO cells were treated with FOH (60 μM) for 1 h and immunostained for the nuclear pore complex (NPC) with an Alexa Fluor 555-labeled secondary antibody. (C) CHO cells were exposed to UV (10-min exposure, followed by 10 h of incubation) or chelerythrine (20 μM for 1 h) and analyzed for localization of CCTα and dextran as described above. Images are single optical sections (0.5 to 0.7 μm) taken with a Zeiss LSM510 confocal microscope with an ×100 oil immersion objective.
To confirm that the nuclear pore complex was intact in cells excluding dextran and permeable to dextran in those cells where integrity was lost, CHO cells loaded with dextran were treated with FOH and immunostained with an antibody directed against the nuclear pore complex. It is evident from the results shown in Fig. 9B that cells which excluded dextran during FOH treatment for 1 h had an intact nuclear pore complex, while those cells with diminished nuclear pore complex staining had increased nuclear staining by fluorescent dextran. Thus, 70-kDa fluorescent dextran is a good indicator of nuclear envelope integrity, and increased permeability of the nuclear envelope during FOH treatment cannot account for nuclear export of CCTα.
We also compared nuclear envelope translocation and nuclear export of CCTα in other apoptotic programs (Fig. 9C). CHO MT58 cells stably overexpressing CCTα were exposed to UV or chelerythrine for a duration that caused CCTα and PARP cleavage (results not shown) but allowed for maintenance of nuclear integrity. Under these conditions, CCTα was exclusively in the nucleus in UV-treated cells, and there was no evidence of translocation to the nuclear envelope. Chelerythrine-treated cells rapidly lost nuclear integrity, as indicated by uptake of Oregon green 488-dextran, but CCTα was maintained in the nucleus. This shows that caspase cleavage of CCTα can occur in the nucleus and that membrane translocation and nuclear export are unique to FOH-induced apoptosis.
DISCUSSION
PtdCho synthesis via the CDP-choline pathway is inhibited by numerous cytotoxic drugs, such as FOH, apparently at the terminal step in the pathway catalyzed by CPT. In this study we demonstrated that FOH rapidly and potently stimulates the step prior to CPT catalyzed by the rate-limiting enzyme CCTα. FOH influenced CCTα on several levels, causing rapid activation and translocation of CCTα to the nuclear envelope, release from the nucleus, and activation of caspases that remove the nuclear localization signal. Nuclear export and caspase-mediated removal of the nuclear localization signal would exclude CCTα from the nucleus and disrupt the compartmentalization of the CDP pathway during FOH-induced apoptosis.
[3H]choline labeling experiments in CHO cells were used to demonstrate the overall effects of FOH on the CDP-choline pathway. Both 20 and 60 μM FOH caused a reduction in [3H]choline incorporation into phosphocholine, indicating activation of CCTα, while at the same time elevating CDP-[3H]choline levels, indicative of CPT inhibition. The net effect on PtdCho synthesis was complex and dependent on the balance between these two effects. Thus, 20 μM FOH caused greater activation of CCT relative to inhibition of the terminal step in the pathway and no induction of apoptosis (Fig. 3), resulting in stimulation of PtdCho synthesis. Induction of apoptosis by 60 μM FOH was correlated with activation of CCT and profound inhibition of CPT and PtdCho synthesis. Since CCT and CPT catalyze concurrent and tightly coupled steps in the CDP-choline pathway, the accumulation of intermediates and reduction in PtdCho synthesis in response to FOH could result from changes in enzyme activity and/or localization. Our results suggest that altered PtdCho synthesis and CDP-choline levels are due in part to persistent activation and mislocalization of CCTα during FOH-induced apoptosis, effectively uncoupling it from the subsequent step in the pathway and disrupting PtdCho synthesis.
CCTα is an amphitrophic protein that binds to membranes via an amphipathic helix in response to changes in membrane composition (9). This increases enzyme activity by lowering the Km for CTP by 30-fold (54). Lipids with a single hydroxyl group and acyl moieties, such as fatty alcohols and DAG, as well as fatty acids, promote membrane localization and activation of CCT (10, 11). Isoprenoids are structurally related to these lipids and could promote CCT membrane association by a similar mechanism. In support of this conclusion, in vitro activity of recombinant CCT was stimulated by PtdCho vesicles containing FOH. The magnitude of activation was about twofold less than that afforded by PtdCho vesicles containing an equivalent amount of oleate but was evident at low concentrations of FOH (2 to 10 mol%).
Similar to the effects of exogenous fatty acids on CHO cells (22), FOH appears to alter the membrane properties of the nuclear envelope to favor the association of CCTα. CCTα was also rapidly dephosphorylated during FOH-mediated translocation in several cell types, as indicated by increased lower-molecular-mass isoforms on SDS-PAGE and immunoblots. CCTα dephosphorylation is correlated with but is not necessary for membrane binding (17). Activation and membrane association of CCTα were unique to FOH-induced apoptosis. In two other apoptotic programs induced by UV exposure or the protein kinase inhibitor chelerytherine, CCT was not translocated to the nuclear envelope and remained in the nucleus. Of several apoptotic drugs tested in a prior study (2), chelerythrine was the most potent inhibitor of PtdCho synthesis in HL-60 cells. This indicates that CCT activation does not contribute to inhibition of PtdCho synthesis in that apoptotic program.
Following FOH-induced translocation of CCTα to the nuclear membrane, the enzyme was detected outside the nucleus by immunofluorescence. It is controversial whether CCTα is strictly nuclear or exits the nucleus under specific conditions. While CCTα is endonuclear in many cultured cells (46-48; this study), CCTα in pulmonary epithelial cells and lung tissue was in the cytoplasm or on the endoplasmic reticulum, reflecting the increased demand for PtdCho in surfactant synthesis (34). The role of CCTα in the nucleus is enigmatic, since the prior and subsequent steps in the CDP-choline pathway are cytosolic or on the endoplasmic reticulum, respectively. However, the identification of an intact CDP-choline pathway in the nucleus of neuroblastoma cells that synthesized a distinct pool of PtdCho suggests a unique role in chromatin structure or nuclear signal transduction (8, 18).
It is also feasible that CCTα shuttles between the nucleus and cytoplasm. Northwood et al. (30) showed by immunofluorescence analysis that CCTα exited the nucleus and localized to the endoplasmic reticulum, concurrent with an increase in CCTα activity and PtdCho synthesis upon release of a G0 cell cycle block in IIC9 fibroblasts. CCTα eventually reentered the nucleus. This suggested that nuclear CCTα was largely a reservoir for inactive enzyme that was activated upon translocation to the endoplasmic reticulum. The signals that mediate the wave of CCTα release from the nucleus have not been identified. In contrast, CCTα-green fluorescent protein was localized in the nucleus at all stages of the cell cycle and in different cell types, including CHO cells (14). However, CCTα-green fluorescent protein did not translocate to membranes in response to activation by oleate, suggesting fundamental differences between cell models or the green fluorescent protein fusion protein and native CCTα.
Our results suggest that nuclear release of CCTα is coupled to an apoptotic program that activated CCTα and disrupted PtdCho synthesis. Membrane translocation, or events following translocation, appears to be required for nuclear export of CCTα. In the same manner that release from a G0 block increased nuclear export of CCTα (30), activation of CCT by FOH could indirectly alter nuclear export pathways or convert the enzyme to an exportable form. In the former case, it is feasible that both membrane localization and dephosphorylation are coupled to CCTα export. The change in compartmentalization of CCTα during FOH-induced apoptosis in CHO cells could serve to disrupt the normal flow of CDP-choline to CPT and block PtdCho synthesis. In addition, activation of CCTα could further the apoptotic program by trapping cytidine nucleotide in the CDP-choline pool, thus causing depletion of cellular CTP levels and disruption of other CTP-dependent functions
Ultrastructure of the nuclear envelope is maintained late in apoptosis (52) despite the caspase 3-mediated proteolysis of a subset of nuclear pore complex proteins (7). Examples of protein import into the nucleus during apoptosis (caspase 9 and mammalian STE20-like kinase 1) indicate that the nuclear pore is functional (39, 43). In FOH-treated CHO cells, fluorescent-tagged dextran was excluded from the nucleus during CCTα export, indicating that a permeability barrier to 70-kDa molecules was maintained. In addition, the integrity of the nuclear pore complex (as determined by immunostaining for Nup62 and related epitopes [Fig. 9B]) was unaffected under conditions in which fluorescent dextran was excluded from the nucleus. This suggests that CCTα was actively exported to membranes sites outside the nucleus during FOH-induced apoptosis. The enzyme does not have typical leucine-rich export motifs recognized by the leptomycin-sensitive CRM-1 pathway (16), and thus, other export mechanisms must be involved. CCTα export was not dependent on caspases (Fig. 7), suggesting that it is triggered by an early event in FOH-induced apoptosis or results from direct effects of FOH on the nuclear envelope. CCT appeared to be exported to endoplasmic reticulum membrane sites, but derangement of endoplasmic reticulum markers by FOH precluded definitive identification by immunofluorescence.
Following translocation to the nuclear envelope and coincident with release to the cytoplasmic compartment, CCTα was cleaved by a caspase activity at TEED28↓G. Aspartate 28 is the final residue in the CCTα nuclear localization signal 8KVNSRKRRKEASSPNGATEED28 (47), and cleavage at this site will precisely remove this domain. Based on the recognition site TEED, it is likely that the class III caspases are involved in CCTα proteolysis (41). Of these, only caspase 6 had preference for threonine in the P4 position and was capable of digesting CCTα to completion in vitro. Caspase 6 activity was detected in cell extracts from FOH-treated CHO cells (Fig. 2D), and these extracts cleaved CCTα at aspartate 28 (Fig. 6A and B). Caspase 8 also cleaved CCTα at the TEED site but is primarily involved in death receptor signaling (50) and seems unlikely to mediate FOH-induced apoptosis. Caspase 3 did not cleave CCTα, but its inhibition in intact cells by z-DEVD-fmk completely blocked both PARP and CCTα digestion. Thus, a likely sequence of events involves activation of caspase 3 by an unknown mechanism and subsequent activation of caspase 6 and CCTα cleavage (37).
The caspase 6 substrates lamin A and C (32) and several transcription factors (31) are found in the nucleus. Interestingly, lamins colocalized with CCTα at the nuclear envelope in FOH-treated cells, suggesting that both were cleaved in a concerted manner (unpublished results). Caspase proteolysis occurred simultaneously or following release of CCTα from the nucleus in CHO cells, respectively, and was confined to the nucleus in other apoptotic programs. Thus, CCTα cleavage can take place in the nucleus along with other caspase 6 substrates, but whether cleavage occurs in the cytoplasm is unknown. Based on the lack of effect of caspase inhibition and the D28E mutation on CCTα translocation from the nucleus, it is clear that caspase cleavage was not required for export. However, the N-terminal CCT nuclear localization signal is required for import into the nucleus (47), suggesting that nuclear export and removal of the CCTα nuclear localization signal are coordinated events designed to permanently exclude CCTα from the nucleus.
By virtue of its direct control over PtdCho synthesis and indirect control over the levels of lipid signaling molecules derived from PtdCho, CCTα is uniquely positioned to sense or regulate levels of these important lipids and thus control apoptotic or proliferative signals. Our results show that activation and caspase cleavage of CCTα contribute to disruption of the CDP-choline pathway that is a component of isoprenoid-mediated apoptotic programs.
Acknowledgments
We thank Robert Zwicker and Gladys Keddy for maintaining and culturing cells.
This work was supported by a Canadian Institutes of Health grant to N.D.R. and Cancer Care Nova Scotia trainee awards to T.A.L. and J.R.M.
REFERENCES
- 1.Adany, I., E. M. Yazlovitskaya, J. S. Haug, P. A. Voziyan, and G. Melnykovych. 1994. Differences in sensitivity to farnesol toxicity between neoplastically and non-neoplastically derived cells in culture. Cancer Lett. 79:175-179. [DOI] [PubMed] [Google Scholar]
- 2.Anthony, M. L., M. Zhao, and K. M. Brindle. 1999. Inhibition of phosphatidylcholine biosynthesis following induction of apoptosis in HL-60 cells. J. Biol. Chem. 274:19686-19692. [DOI] [PubMed] [Google Scholar]
- 3.Araki, W., and R. J. Wurtman. 1997. Control of membrane phosphatidylcholine biosynthesis by diacylglycerol levels in neuronal cells undergoing neurite outgrowth. Proc. Natl. Acad. Sci. USA 94:11946-11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baburina, I., and S. Jackowski. 1999. Cellular responses to excess phospholipid. J. Biol. Chem. 274:9400-9408. [DOI] [PubMed] [Google Scholar]
- 5.Baburina, I., and S. Jackowski. 1998. Apoptosis by 1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine is prevented by increased expression of CTP:phosphocholine cytidylyltransferase. J. Biol. Chem. 273:2169-2173. [DOI] [PubMed] [Google Scholar]
- 6.Boggs, K. P., C. O. Rock, and S. Jackowski. 1995. Lysophosphatidylcholine and 1-O-octadecl-2-O-methyl-rac-glycero-3-phosphocholine inhibit the CDP-choline pathway for phosphatidylcholine synthesis at the CTP:phosphocholine cytidylyltransferase step. J. Biol. Chem. 270:7757-7764. [DOI] [PubMed] [Google Scholar]
- 7.Buendia, B., A. Santa-Maria, and J. C. Courvalin. 1999. Caspase-dependent proteolysis of integral and peripheral proteins of the nuclear membrane and nuclear pore complex proteins during apoptosis. J. Cell Sci. 112:1743-1753. [DOI] [PubMed] [Google Scholar]
- 8.Cocco, L., A. M. Martelli, R. Stewart Gilmour, S. G. Rhee, and F. A. Manzoli. 2001. Nuclear phospholipase C signaling. Biochim. Biophys. Acta 1530:1-14. [DOI] [PubMed] [Google Scholar]
- 9.Cornell, R. B., and I. C. Northwood. 2000. Regulation of CTP:phosphocholine cytidylyltransferase by amphitropism and relocalization. Trends Biochem. Sci. 25:441-447. [DOI] [PubMed] [Google Scholar]
- 10.Cornell, R. B., and D. E. Vance. 1987. Translocation of CTP:phosphocholine cytidylyltransferase from cytosol to membranes in HeLa cells: stimulation by fatty acids, fatty alcohol, mono-and diacylglycerol. Biochim. Biophys. Acta 919:26-37. [DOI] [PubMed] [Google Scholar]
- 11.Cornell, R. B., and D. E. Vance. 1987. Binding of CTP:phosphocholine cytidylyltransferase to large unilamellar vesicles. Biochim. Biophys. Acta 919:37-48. [DOI] [PubMed] [Google Scholar]
- 12.Cornell, R. B. 1989. Chemical cross-linking reveals a dimeric structure for CTP:phosphocholine cytidylyltransferase. J. Biol. Chem. 264:9077-9082. [PubMed] [Google Scholar]
- 13.Cui, Z., M. Houweling, M. H. Chen, M. Record, H. Chap, D. E. Vance, and F. Terce. 1996. A genetic defect in phosphatidylcholine biosynthesis triggers apoptosis in Chinese hamster ovary cells. J. Biol. Chem. 271:14668-14671. [DOI] [PubMed] [Google Scholar]
- 14.DeLong, C. J., L. Qin, and Z. Cui. 2000. Nuclear localization of enzymatically active green fluorescent protein-CTP:phosphocholine cytidylyltransferase α fusion protein is independent of the cell cycle conditions and cell types. J. Biol. Chem. 275:32325-32330. [DOI] [PubMed] [Google Scholar]
- 15.Esko, J. D., M. M. Wermuth, and C. R. Raetz. 1981. Thermostable CDP-choline synthetase in an animal cell mutant defective in lecithin formation. J. Biol. Chem. 256:7388-7393. [PubMed] [Google Scholar]
- 16.Fornerod, M., M. Ohno, M. Yoshida, and I. W. Mattaj. 1997. CRM-1 is an export receptor for leucine-rich nuclear export signals. Cell 90:1051-1060. [DOI] [PubMed] [Google Scholar]
- 17.Houweling, M., H. Jamil, G. M. Hatch, and D. E. Vance. 1994. Dephosphorylation of CTP:phosphocholine cytidylyltransferase is not required for binding to membranes. J. Biol. Chem. 269:7544-7551. [PubMed] [Google Scholar]
- 18.Hunt, A. N., G. T. Clark, G. S. Attard, and A. D. Postle. 2001. Highly saturated endonuclear phosphatidylcholine is synthesized in situ and colocated with CDP-choline pathway enzymes. J. Biol. Chem. 276:8492-8499. [DOI] [PubMed] [Google Scholar]
- 19.Jackowski, S. 1994. Coordination of membrane phospholipid synthesis with the cell cycle. J. Biol. Chem. 269:3858-3867. [PubMed] [Google Scholar]
- 20.Jamil, H., A. K. Utal, and D. E. Vance. 1992. Evidence that cyclic AMP-induced inhibition of phosphatidylcholine biosynthesis is caused by a decrease in cellular diacylglycerol levels in cultured rat hepatocytes. J. Biol. Chem. 267:1752-1760. [PubMed] [Google Scholar]
- 21.Kent, C. 1999. CTP:phosphocholine cytidylyltransferase. Biochim. Biophys. Acta 257:643-650. [DOI] [PubMed] [Google Scholar]
- 22.Lagace, T. A., M. K. Storey, and N. D. Ridgway. 2000. Regulation of phosphatidylcholine metabolism in Chinese hamster ovary cells by the sterol regulatory element-binding protein (SREBP)/SREBP-cleavage activating protein pathway. J. Biol. Chem. 275:14367-14374. [DOI] [PubMed] [Google Scholar]
- 23.Lykidis, A., K. G. Murti, and S. Jackowski. 1998. Cloning and characterization of a second CTP:phosphocholine cytidylyltransferase. J. Biol. Chem. 273:14022-14029. [DOI] [PubMed] [Google Scholar]
- 24.Lykidis, A., I. Baburina, and S. Jackowski. 1999. Distribution of CTP:phosphocholine cytidylyltransferase (CCT) isoforms. J. Biol. Chem. 274:26992-27001. [DOI] [PubMed] [Google Scholar]
- 25.Lykidis, A., P. Jackson, and S. Jackowski. 2001. Lipid activation of CTP:phosphocholine cytidylyltransferase α: characterization and identification of a second activation domain. Biochemistry 40:494-503. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald, J. I. S., and C. Kent. 1993. Baculovirus-mediated expression of rat liver CTP:phosphocholine cytidylyltransferase. Protein Expr. Purif. 4:1-7. [DOI] [PubMed] [Google Scholar]
- 27.Masuda, Y., M. Yoda, H. Ohizumi, T. Aiuchi, M. Watabe, S. Nakajo, and K. Nakaya. 1997. Activation of protein kinase C prevents induction of apoptosis by geranylgeraniol in human leukemic HL60 cells. Int. J. Cancer 71:691-697. [DOI] [PubMed] [Google Scholar]
- 28.Miquel, K., A. Pradines, F. Terce, S. Selmi, and G. Favre. 1998. Competitive inhibition of choline phosphotransferase by geranylgeraniol and farnesol inhibits phosphatidylcholine synthesis and induces apoptosis in human lung adenocarcinoma A549 cells. J. Biol. Chem. 273:26179-26186. [DOI] [PubMed] [Google Scholar]
- 29.Nakaya, M., M. Yutaka, S. Mihara, T. Aiuchi, T. Shibayama-Imazu, S. Nakajo, and K. Nakaya. 1999. Analysis of caspases that are activated during apoptosis in leukemic U937 cells in response to geranylgeraniol. Anticancer Res. 19:5063-5068. [PubMed] [Google Scholar]
- 30.Northwood, I. C., A. H. Tong, B. Crawford, A. E. Drobnies, and R. B. Cornell. 1999. Shuttling of CTP:phosphocholine cytidylyltransferase between the nucleus and endoplasmic reticulum accompanies the wave of phosphatidylcholine synthesis during the G0/G1 transition. J. Biol. Chem. 274:26240-26248. [DOI] [PubMed] [Google Scholar]
- 31.Nyormoi, O., Z. Wang, D. Doan, M. Ruiz, D. McConkey, and M. Bar-Ele. 2001. Transcription factor AP-2a is preferentially cleaved by caspase 6 and degraded by proteosome during tumor necrosis factor alpha-induced apoptosis in breast cancer cells. Mol. Cell. Biol. 21:4856-4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orth, K., A. M. Chinnaiyan, M. Garg, C. J. Froelich, and V. M. Dixit. 1996. The CED-3/ICE-like protease Mch2 is activated during apoptosis and cleaves the death substrate lamin A. J. Biol. Chem. 271:16443-16446. [PubMed] [Google Scholar]
- 33.Ridgway, N. D. 1989. Phosphatidylethanolamine N-methyltransferase, p. 103-120. In D. E. Vance (ed.), Phosphatidylcholine metabolism. CRC Press, Boca Raton, Fla.
- 34.Ridsdale, R., I. Tseu, J. Wang, and M. Post. 2001. CTP:phosphocholine cytidylyltransferase α is a cytosolic protein in pulmonary epithelial cells and tissues. J. Biol. Chem. 276:49148-49155. [DOI] [PubMed] [Google Scholar]
- 35.Rioja, A., A. R. Pizzey, C. M. Marson, and N. S. Thomas. 2000. Preferential induction of apoptosis of leukemic cells by farnesol. FEBS Lett. 467:291-295. [DOI] [PubMed] [Google Scholar]
- 36.Schirmer, E. C., T. Guan, and L. Gerace. 2001. Involvement of the lamin rod domain in heterotypic interactions important for nuclear organization. J. Cell Biol. 153:479-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slee, E. A., C. Adrain, and S. J. Martin. 2001. Executioner caspase-3, -6 and -7 perform distinct roles during the demolition phase of apoptosis. J. Biol. Chem. 276:7320-7326. [DOI] [PubMed] [Google Scholar]
- 38.Sleight, R., and C. Kent. 1983. Regulation of phosphatidylcholine biosynthesis in mammalian cells. II. Effects of phospholipase C treatment on the activity and subcellular distribution of CTP:phosphocholine cytidylyltransferase in Chinese hamster ovary and cell lines. J. Biol. Chem. 258:831-835. [PubMed] [Google Scholar]
- 39.Stanislaw, K., M. Krajewska, L. M. Ellerby, K. Welsh, Z. Xie, Q. L. Deveraux, G. S. Salvesen, D. E. Bredesen, R. E. Rosenthal, G. Fiskum, and J. C. Reed. 1999. Release of casapse-9 from the mitochondria during neuronal apoptosis and cerebral ischemia. Proc. Natl. Acad. Sci. USA 96:5752-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Storey, M. K., D. M. Byers, H. W. Cook, and N. D. Ridgway. 1997. Decreased phosphatidylcholine biosynthesis and abnormal distribution of CTP:phosphocholine cytidylyltransferase in cholesterol auxotrophic Chinese hamster ovary cells. J. Lipid Res. 38:711-722. [PubMed] [Google Scholar]
- 41.Thronberry, N. A., T. A. Reno, E. P. Peterson, D. M. Rasper, T. Timkey, M. Garcia-Calco, V. M. Houtzager, P. A. Nordstrom, S. Roy, J. P. Vaillancourt, K. T. Chapman, and D. W. Nicholson. 1997. A combinatorial approach defines specificities of members of the caspase family and granzyme B. J. Biol. Chem. 272:17907-17911. [DOI] [PubMed] [Google Scholar]
- 42.Tronchere, H., V. Planat, M. Record, F. Terce, G. Ribbes, and H. Chap. 1995. Phosphatidylcholine turnover in activated human neutrophils. J. Biol. Chem. 270:13138-13146. [DOI] [PubMed] [Google Scholar]
- 43.Ura, S., N. Masuyama, J. D. Graves, and Y. Gotoh. 2001. Caspase cleavage of MST1 promotes nuclear translocation and chromatin condensation. Proc. Natl. Acad. Sci. USA 98:10148-10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voziyan, P. A., C. M. Goldner, and G. Melnykovych. 1993. Farnesol inhibits phosphocholine biosynthesis in cultured cells by decreasing cholinephosphotransferase activity. Biochem. J. 295:757-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voziyan, P. A., J. S. Haug, and G. Melnykovych. 1995. Mechanism of farnesol cytotoxicity: further evidence for the role of PKC-dependent signal transduction in farnesol-induced apoptotic cell death. Biochem. Biophys. Res. Commun. 212:479-486. [DOI] [PubMed] [Google Scholar]
- 46.Wang, Y., T. D. Sweitzer, P. A. Weinhold, and C. Kent. 1993. Nuclear localization of soluble CTP:phosphocholine cytidylyltransferase. J. Biol. Chem. 268:5899-5904. [PubMed] [Google Scholar]
- 47.Wang, Y., J. I. MacDonald, and C. Kent. 1995. Identification of the nuclear localization signal of rat CTP:phosphocholine cytidylyltransferase. J. Biol. Chem. 270:354-360. [DOI] [PubMed] [Google Scholar]
- 48.Watkins, J. D., and C. Kent. 1992. Immunolocalization of membrane-associated CTP:phosphocholine cytidylyltransferase in phosphatidylcholine-deficient Chinese hamster ovary cells. J. Biol. Chem. 267:5686-5692. [PubMed] [Google Scholar]
- 49.Wolf, B. B., and D. R. Green. 1999. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J. Biol. Chem. 274:20049-20052. [DOI] [PubMed] [Google Scholar]
- 50.Wright, M. M., A. L. Henneberry, T. A. Lagace, N. D. Ridgway, and C. R. McMaster. 2001. Uncoupling farnesol-induced apoptosis from its inhibition of phosphatidylcholine synthesis. J. Biol. Chem. 276:25254-25261. [DOI] [PubMed] [Google Scholar]
- 51.Wright, P. S., J. N. Morand, and C. Kent. 1985. Regulation of phosphatidylcholine biosynthesis in Chinese hamster ovary cells by reversible membrane association of CTP:phosphocholine cytidylyltransferase. J. Biol. Chem. 260:7919-7926. [PubMed] [Google Scholar]
- 52.Wyllie, A. H., J. F. R. Kerr, and A. R. Currie. 1980. Cell death: the significance of apoptosis. Int. Rev. Cytol. 68:251-305. [DOI] [PubMed] [Google Scholar]
- 53.Yang, W., and S. Jackowski. 1995. Lipid activation of CTP:phosphocholine cytidylyltransferase is regulated by the phosphorylated carboxyl-terminal domain. J. Biol. Chem. 270:1650-16506. [DOI] [PubMed] [Google Scholar]
- 54.Yang, W., K. P. Boggs, and S. Jackowski. 1995. The association of lipid activators with the amphipathic helical domain of CTP:phosphocholine cytidylyltransferase accelerates catalysis by increasing the affinity of the enzyme for CTP. J. Biol. Chem. 270:23951-23957. [DOI] [PubMed] [Google Scholar]
- 55.Yang, J., J. Wang, I. Tseu, M. Kuliszewski, W. Lee, and M. Post. 1997. Identification of an 11-residue portion of CTP-phosphocholine cytidylyltransferase that is required for enzyme-membrane interactions. Biochem. J. 325:29-38. [DOI] [PMC free article] [PubMed] [Google Scholar]