Abstract
Signaling mediated by ErbB2 is thought to play a critical role in numerous developmental processes. However, due to the embryonic lethality associated with the germ line inactivation of erbB2, its role in adult tissues remains largely obscure. Given the expression of ErbB2 at the neuromuscular junction, we have created a muscle-specific knockout to assess its role there. This resulted in viable mice with a progressive defect in proprioception due to loss of muscle spindles. Interestingly, a partial reduction of ErbB2 levels also reduced the number of muscle spindles. Although histological analysis of the muscle revealed an otherwise normal architecture, induction of muscle injury revealed a defect in muscle regeneration. Consistent with these observations, primary myoblasts lacking ErbB2 exhibit extensive apoptosis upon differentiation into myofibers. Taken together, these results illustrate a dual role for ErbB2 in both muscle spindle maintenance and survival of myoblasts.
ErbB2 (also known as Neu and HER2) is a member of the epidermal growth factor receptor (EGFR) family of receptor tyrosine kinases (7, 13, 23). This family is comprised of EGFR (48), ErbB2 (4, 12, 36, 39, 48, 51), ErbB3 (27), and ErbB4 (35). Gene targeting experiments have revealed that each of these EGFR family members plays a critical role in regulating embryonic development. For example, germ line elimination of erbB2 or erbB4 results in embryonic lethality at day 10.5 of embryogenesis due to defects in cardiac and neural development (19, 28). Although inactivation of erbB3 function has a less severe impact on cardiac development, embryonic lethality is also observed due to defects in neural and Schwann cell differentiation (5, 14, 38). Interestingly, elimination of EGFR receptor function results in a strain-dependent perinatal lethality (21, 41, 46).
Given the importance of EGFR family members in embryonic development, it has been difficult to elucidate the relative contribution of this family to the maintenance and development of adult tissues. The embryonic lethality associated with inactivation of erbB2 has previously been rescued by myocardial expression of an erbB2 transgene (29, 33, 49). However, these mice die at birth due to loss of motor neurons and defects in Schwann cell development. To avoid this perinatal lethality, specific deletions may be created to address the role of ErbB2 in various adult tissues through the use of the Cre/LOXP1 recombinase system. Indeed this approach has been used to generate a peripheral-nerve-specific deletion of erbB2 that resulted in the extensive demyelination of the nerves (17, 18).
In addition to its role in Schwann cell development, ErbB2 is expressed in skeletal muscle and is concentrated at the neuromuscular junction (NMJ) along with ErbB3 and ErbB4 EGFR family members (1, 34, 52). Indeed, EGFR family members have been implicated as important functional components of the NMJ (24). Neuregulins serve as ligands for ErbB3 and ErbB4 and are released from the motor neuron end plate, where they are thought to activate these receptor tyrosine kinases (34). Heterodimerization and tyrosine phosphorylation of ErbB2/ErbB3 has been shown to regulate the expression of various subunits of the acetylcholine receptor (40, 45, 50). In addition to stimulating acetylcholine receptor subunit expression, neuregulin-mediated ErbB2/ErbB3 heterodimers can stimulate the phosphatidylinositol 3′-kinase cell survival pathway (45). ErbB2/ErbB3-mediated activation of phosphatidylinositol 3′-kinase (25, 43) has been implicated in stimulation of a number of important cell survival elements, including Akt serine kinase (8, 16). Indeed, ErbB2 is thought to play a central role in the EGFR family signaling pathway, since it is the preferred heterodimeric partner for the other EGFR members (22). However, the precise roles of the individual EGFR family members, including ErbB2, in muscle physiology have not been previously elucidated.
Given the expression pattern and potential role of ErbB2 in NMJ development and its importance in mediating EGFR family signaling, we sought to determine whether a functional ErbB2 was required for muscle development and maintenance. In this report, we describe a conditional knockout of erbB2 in adult muscle using mice expressing Cre recombinase under the control of the muscle creatine kinase (Mck) promoter enhancer. Strikingly, mice devoid of ErbB2 in skeletal muscle had proprioception defects and lacked muscle spindles. Muscle-specific elimination of erbB2 also correlated with the induction of apoptosis in myoblasts upon differentiation to myofibers, which correlated with an inhibition of regeneration after muscle injury. These results illustrate a dual role for ErbB2 in survival during myoblast differentiation and for maintenance of muscle spindles.
MATERIALS AND METHODS
Generation of ErbB2Flox/Flox Mice.
ErbB2 genomic sequences were isolated from a 129/SvJ genomic library. A 2.5-kb 5′ arm of homology (SphI to NarI), 5′ to exon 1, and an 8-kb 3′ arm of homology (KpnI to SalI) including exons 2 and 3 were designed. The complete cDNA for neuN was flanked by loxP sites and was followed by a simian virus 40 polyadenylation sequence. 3′ to the cDNA and poly(A), a PGK-neomycin-herpes simplex virus poly(A) selectable marker was added. This loxP-flanked neuN poly(A) PGK-neo-poly(A) sequence replaced exon 1 of the genomic allele. All subsequent techniques to generate the mice were performed as described previously (2).
Excision analysis.
Upon interbreeding with the Mck/Cre transgenic mice, DNA from ErbB2Flox/Flox Mck/Cre muscle was prepared using standard phenol-chloroform isolation methods and was digested using HindIII. The DNA was electrophoresed on a 0.8% gel, and the Southern blot was probed using a PstI fragment of neomycin. Quantification of the extent of excision was measured and compared by PhosphorImager analysis (Molecular Dynamics) using the illustrated controls to determine the extent of excision by comparison of intensity of the wild-type and loxP-flanked alleles.
Track analysis.
To obtain a record of the footpad placement during a normal gait, the hind limb was dipped in India ink and the mouse was allowed to walk down a 15-cm-wide corridor on several pieces of Whatman paper. The track was then scanned into Adobe Photoshop and adjusted for light and contrast.
Histology.
Fixation of all hind limb extensor muscle was achieved by cardiac perfusion using phosphate-buffered saline (PBS) followed by 10% formalin. The muscle was then fixed in 10% formalin for at least 24 h. Horizontal sections (6 μm thick) of paraffin-embedded sample were cut and stained with hematoxylin and eosin according to standard methods. Urea-silver nitrate staining followed the method described for sensory neurons (9). Briefly, the extensor digitorum longus (EDL) was fixed in Bouins solution for 12 to 18 h and embedded in paraffin wax, and serial longitudinal sections were cut at a thickness of 12 or 14 μm and stained as previously described.
Induced regeneration of skeletal muscle.
Skeletal muscle regeneration was induced by an applied crush injury as previously described (31). Briefly, after mice were anesthetized, the tibialis anterior (TA) muscle was exposed and a crush injury was applied. Two weeks post-crush injury, the crushed TA muscle and the contralateral control were examined through serial hematoxylin-and-eosin-stained sections.
Cell culture.
Primary myoblast cells were generated from FVB, ErbB2Flox/Flox, and Mck/Cre ErbB2Flox/Flox mice using previously established techniques (37). Following isolation, cells were cultured in proliferation medium composed of Ham's medium, 20% fetal calf serum, bovine fibroblast growth factor (2.5 ng/ml), penicillin-streptomycin (200 U/ml), and 0.002% amphotericin B (Fungizone; Gibco). To induce differentiation, the medium was switched to 5% horse serum in Ham's F-10 medium with the same antibiotic supplements. The proliferation medium was changed daily, while the differentiation medium was changed every second day. To achieve complete excision of the loxP-flanked allele, an adenovirus containing Cre recombinase was added at a multiplicity of infection of 20 in PBS supplemented with CaCl2 (to 0.7 M) and MgCl2 (to 0.5 M) (3).
Immunohistochemistry.
Complete differentiation of myofibers was detected with MF20, an antibody against myosin heavy chain MF20 (Developmental Studies Hybridoma Bank, Iowa City, Iowa). At day 5 of differentiation, cells were washed with PBS, fixed in ice-cold 90% methanol for 2 min, rinsed three times in PBS with 5% skim milk, and incubated with MF20 for 1 h at a 1:10 dilution. Following incubation with the primary antibody, cells were washed with PBS and were incubated with the goat anti-mouse immunoglobulin G-horseradish peroxidate conjugate secondary antibody (Bio-Rad) for 1 h. After being rinsed three times in PBS, the reaction was developed with DAB (0.6 mg/ml; Sigma) and images were captured using the Zeiss Axiovision system. Expression of ErbB2 in intrafusal muscle fibers was detected using an antibody against ErbB2 (C18; Santa Cruz Biotechnology, Inc.), and a biotinylated goat anti-rabbit secondary antibody was used (Vector Laboratories) in conjunction with the Vectastain Elite ABC kit and DAB substrate (Vector Laboratories). These sections were counterstained with hematoxylin.
Immunoprecipitation and immunoblotting.
Cell lysates were prepared according to previously described methods (42). ErbB2 was detected by a mouse monoclonal antibody (AB-3; Oncogene Research Products, Inc.). Immunoblotting for ErbB3 (C-17) and Grb-2 (C-23) was performed using rabbit polyclonal antibodies (Santa Cruz Biotechnology, Inc.). Immunoprecipitations were completed in accordance with standard protocols using a rabbit polyclonal antibody for ErbB3 (C-17) and were probed using an antiphosphotyrosine antibody (PY20; Transduction Labs).
RESULTS
Muscle-specific elimination of erbB2 is associated with a background-dependent defect in proprioception.
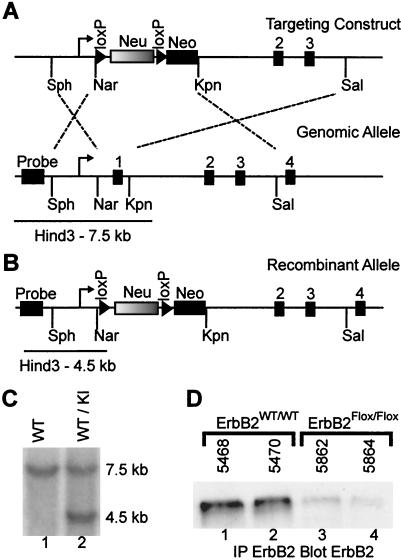
To assess the effect of a lack of erbB2 in muscle development and maintenance, we employed a strategy in which the first coding exon of the endogenous erbB2 gene was replaced by a loxP-flanked wild-type cDNA. Replacement of the first coding exon of mouse erbB2 with the wild-type rat erbB2 cDNA completely rescued the embryonic lethality associated with the germ line inactivation of erbB2. However, it should be noted that these mice express only 10% of the expected amount of ErbB2 protein and are thus hypomorphic for ErbB2 expression (11). Using a similar strategy, we replaced the first coding exon of erbB2 with the loxP-flanked rat c-erbB2 cDNA (neu) followed immediately downstream by a PGK-neomycin (Neo) selection cassette (Fig. 1A). The targeting vector was electroporated into R1 embryonic stem (ES) cells, and independent clonal cell lines were isolated. To confirm that a successful targeting event had occurred (Fig. 1B), genomic DNA from individual cell lines was subjected to Southern blot analyses with a probe external to the targeting event. The results of these analyses revealed that six independent ES cell lines contained evidence of a successful recombination event. After blastocyst injection of ES cell lines and derivation of resultant chimeric mice, germ line transmission of the LOXP1-flanked erbB2 allele yielded mice that were heterozygous for the recombinant allele (Fig. 1C). Subsequent interbreeding of mice heterozygous for this mutation (ErbB2WT/Flox) yielded the expected Mendelian ratio of wild-type, heterozygous, and homozygous (ErbB2Flox/Flox) mutant mice. Although the level of ErbB2 protein was reduced in pooled hind limb muscle (Fig. 1D, lanes 1 and 2 versus lanes 3 and 4), the ErbB2Flox/Flox mice exhibited no obvious phenotypic or histological abnormalities. Further, immunoblot analysis for ErbB3 did not show a difference in protein levels when muscle (data not shown) or primary myoblasts (see Fig. 7A) were examined.
FIG. 1.
Targeting of the loxP-flanked erbB2 allele. (A) A schematic representation of the targeting construct and the genomic allele is shown. The 2.5-kb 5′ arm of homology (Sph to Nar) and the 8-kb 3′ arm of homology (Kpn to Sal) were employed to direct the homologous recombination to the wild-type allele, illustrated by the dashed lines. Exon 1 of the endogenous allele was replaced by a loxP (triangle)-flanked neuN cDNA followed by a simian virus 40 poly(A) (Neu) and a PGK-neomycin-herpes simplex virus poly(A) (Neo). The targeted allele has replaced exon 1 contained within the Nar/Kpn fragment. The size of the genomic HindII-restricted fragment when detected by a probe 5′ to the site of homologous recombination is also depicted. (B) The recombinant allele containing the loxP-flanked neuN allele in place of exon 1 and the corresponding size of the HindIII restriction fragment detected by the external probe are shown. (C) A representative Southern blot of tail DNA from mice that are wild type (WT) and heterozygous for the knockin (KI) allele illustrates the various alleles. (D) Immunoprecipitation of ErbB2 followed by immunoblotting for ErbB2 revealed that the ErbB2Flox/Flox muscle expresses a reduced level of protein compared to the wild-type controls (lanes 1 and 2 versus lanes 3 and 4).
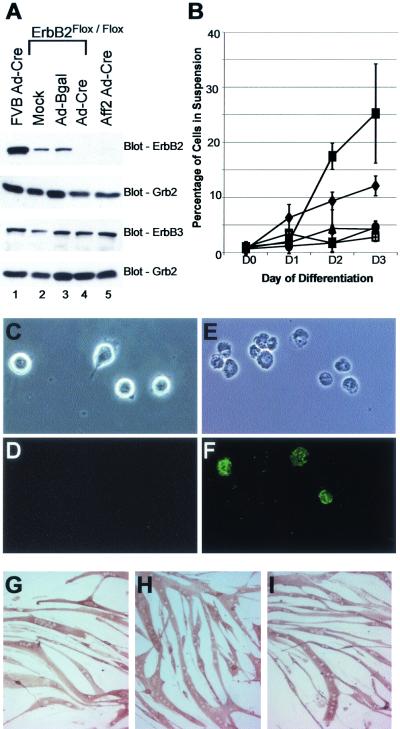
FIG. 7.
Increased apoptosis in ErbB2-null myoblasts during differentiation. (A) Myoblast cells were prepared from adult FVB wild-type controls, ErbB2Flox/Flox, and Mck/Cre ErbB2Flox/Flox mice. Given the residual ErbB2 expression in Mck/Cre ErbB2Flox/Flox cell lines (not shown), we infected ErbB2Flox/Flox and Mck/Cre ErbB2Flox/Flox (Aff2) cell lines with Ad-Cre. A mock infection and Ad-Bgal were included as controls. At day 5 of differentiation, ErbB2 was detected in the wild-type and ErbB2Flox/Flox infection controls (lanes 1 to 3) but was not seen in the ErbB2Flox/Flox Ad-Cre or Aff2 Ad-Cre cells (lanes 4 and 5). ErbB3 was found to be present at equal levels in all cells, and Grb2 was included as an internal loading control. (B) During differentiation, it was noted that a large number of cells were lifting off the plates in cell lines lacking ErbB2. Quantification of the percentage of cells in suspension at each differentiation time point revealed that in cells containing ErbB2, usually less than 5% of the cells were in suspension (•, FVB; □, mock; ▴, Ad-Bgal). In contrast, by day 3 of differentiation, on average 12 and 25% of cells were in suspension in the ErbB2Flox/Flox Ad-Cre (♦) and Aff2 Ad-Cre (▪) cell lines, respectively. Apoptosis was confirmed through annexin-propidium iodide staining for FVB (C and D) and Aff2 Ad-Cre (E and F) (by light microscopy [C and E], annexin fluorescence [D and F], and propidium staining [not shown]). Although many cells lacking ErbB2 underwent apoptosis, the remaining myoblasts were still capable of terminal differentiation. Mock-infected (G), Ad-Bgal-infected (H), and Ad-Cre-infected (I) ErbB2Flox/Flox cells at day 5 of differentiation are shown after immunostaining for myosin heavy chain (MF20). No differences were noted in the ability of the cells to reach terminal differentiation.
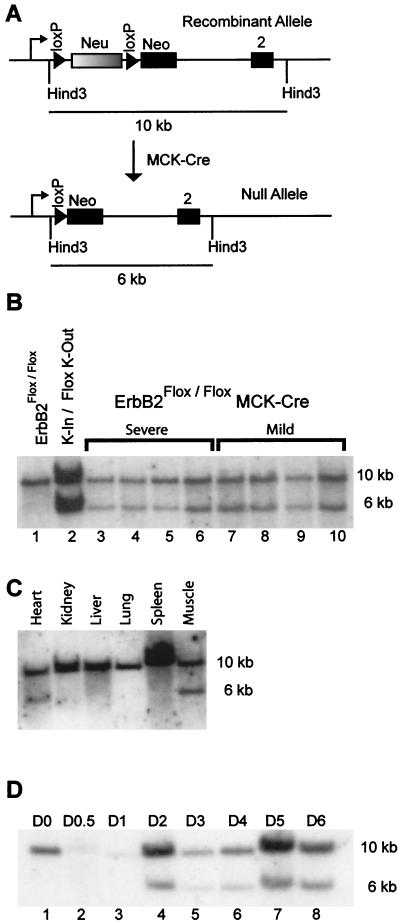
To elucidate the role of ErbB2 in muscle development, we interbred the ErbB2Flox/Flox mice with transgenic mice expressing Cre recombinase under the transcriptional control of the Mck promoter enhancer (Mck/Cre) (6). To determine the extent of excision of the LOXP1-flanked allele in the muscle (Fig 2A), genomic DNA was prepared from pooled hind limb muscle of Mck/Cre ErbB2Flox/Flox mice and subjected to Southern analysis (Fig. 2B). The results revealed that approximately 40% of the muscle-derived DNA exhibited evidence of Cre-mediated excision as measured by PhosphorImager analysis. To ensure that this was a muscle-specific deletion of ErbB2, DNA was prepared from various tissues and was subjected to a Southern analysis. This revealed that excision was limited to cardiac and skeletal muscle (Fig. 2C). To ensure that the moderate level of excision seen in Fig. 2B and C was not due to contamination by nonmuscle components, three independent myoblast cell lines were derived from pooled hind limb muscle of adult Mck/Cre ErbB2Flox/Flox mice. These cell lines were then differentiated to induce Mck-regulated Cre expression, which should peak at 24 h after the initiation of differentiation of the myoblasts to myofibers (10). During the induction of differentiated myofibers, excision was first noted at day 2 of differentiation. However, upon terminal differentiation only 40% of the myofibers exhibited evidence of Cre-mediated excision of the LOXP1-flanked erbB2 allele (Fig. 2D). The extent of excision shown in Fig. 2D is consistent across the three primary cell lines that were surveyed (data not shown).
FIG. 2.
Mck/Cre-mediated excision of loxP-flanked ErbB2. (A) The schematic of the recombinant allele under the control of the endogenous promoter is shown, as is the effect of Cre-mediated recombination. Using a HindIII digest and the PstI fragment of the neomycin cassette as a probe, the size of the recombinant and null alleles in a Southern analysis are shown. (B) A Southern analysis on pooled hind limb muscle from eight Mck/Cre ErbB2Flox/Flox mice is shown. Also included are a control for no excision in the ErbB2Flox/Flox (lane 1) and a control to illustrate when excision is complete for one allele (lane 2). This complete excision control is a knockin/Flox knockout (K-In/Flox K-out), has one recombinant allele and one null allele, and was gen-erated from an embryonic control (Chan and Muller, unpublished observations). Quantification of the extent of excision by PhosphorImager analysis revealed that excision varied from 30 to 40% complete for the muscle samples (lanes 3 to 10). The extent of the observed phenotype is stated above the lanes and does not correlate with an increased level of excision (severe, lanes3 to 6; mild, lanes 7 to 10). (C) To examine the specificity of the excision, DNA was harvested from various organs and was examined through a Southern analysis for excision of the loxP-flanked cDNA. This reveals that excision is limited to skeletal and cardiac muscle. (D) To determine whether excision was complete in the myofibers, several myoblast cell lines were prepared from the Mck/Cre ErbB2Flox/Flox mice and were allowed to differentiate. The number of days in differentiation medium is shown from day 0 (D0) to day 6 (D6). Upon initiation of differentiation, the Mck promoter is activated, and excision is noted by day 2 of differentiation (lane 4). However, when differentiation is complete at day 6, excision remains incomplete (lane 8).
Despite the moderate levels of excision observed in the Mck/Cre ErbB2Flox/Flox muscle, approximately 50% of the mice exhibited loss of motor coordination and displayed alterations of gait in the initial crosses. Further, these mice often maintained their limbs in an abnormal posture, progressing from a flexor to an extensor posture. The phenotypic difference between severely affected and mildly affected mice was not due to the extent of excision of erbB2, since hind limb muscle DNA exhibited comparable levels of excision for mice with and without the severe manifestation of the phenotype (Fig. 2B, lanes 3 to 6 versus lanes 7 to 10). Because the initial interbreedings were conducted on a mixed SV129/FVB/BALB/c background, we have selectively bred affected animals and achieved an approximately 80% penetrance of the phenotype. However, attempts to achieve full penetrance of the phenotype were hampered due to an inability of severely affected animals to breed. Backcrossing the mice into a pure FVB background also revealed a deficit in proprioception, and these animals exhibited the same level of penetrance. The variable penetrance of the phenotype is likely due to genetic modifiers inherent in the genetic background of the mice.
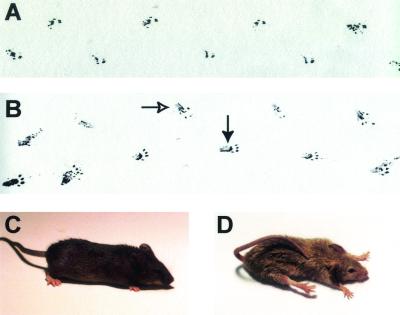
In order to examine alterations in the gait, the hind limbs of control and conditional null mice were dipped into India ink and the mice were allowed to move across a sheet of blotting paper to create a record of their footprints. In contrast to the wild-type control (Fig. 3A), the Mck/Cre ErbB2Flox/Flox track (Fig. 3B) clearly revealed that a large portion of the leg is in contact with the surface during movement. Additionally, the footpad is no longer aligned with the direction of travel but is rotated medially at an unusual angle. This altered gait is observed early in the progression of the phenotype when the mice tend to maintain the hind limb in a flexed position during movement. As the affected mice aged, the phenotype increased in severity and the posture of the mice was dramatically altered. In comparison to the wild-type control (Fig. 3C), the Mck/Cre ErbB2Flox/Flox mice (Fig. 3D) frequently maintained their hind limbs in extension, and the forelimbs were also occasionally extended. Additionally, these mice lacked coordinated motor control and frequently suffered from malocclusions, poor body condition, and wasting, sporadically resulting in early mortality in severely affected mice. In many respects the observed phenotype resembles proprioception defects characteristic of strains deficient for EGR-3, NT-3, and TrkC (15, 26, 47).
FIG. 3.
Altered gait and posture in ErbB2Flox/Flox Mck/Cre mice. (A) The hind limbs of a wild-type mouse were immersed in India ink, and the mouse was permitted to move across a sheet of blotting paper. The mouse was moving from left to right across the sample area in a normal gait where only the footpad was in contact with the surface that it moved across. (B) The track from a mildly affected Mck/Cre ErbB2Flox/Flox mouse as it moves across the blotting paper reveals several distinct differences. In addition to the footpad coming in contact with the surface the mouse was traversing, a large region of the leg also was in contact due to incomplete extension of the leg during movement (closed arrow). Additionally, due to abnormal weight bearing on the hocks, the toes are turned in during movement, resulting in the pigeon-toed track (open arrow). These alterations in the gait appear to be due to a lack of complete extension of the hind limbs during movement. In addition to the altered gait, the Mck/Cre ErbB2Flox/Flox mice have an abnormal posture at rest. In comparison to wild-type (C) and ErbB2Flox/Flox controls, the severely affected ErbB2Flox/Flox Mck/Cre mice (D) suffer from an extensor posture, extending their limbs and resting upon the abdomen.
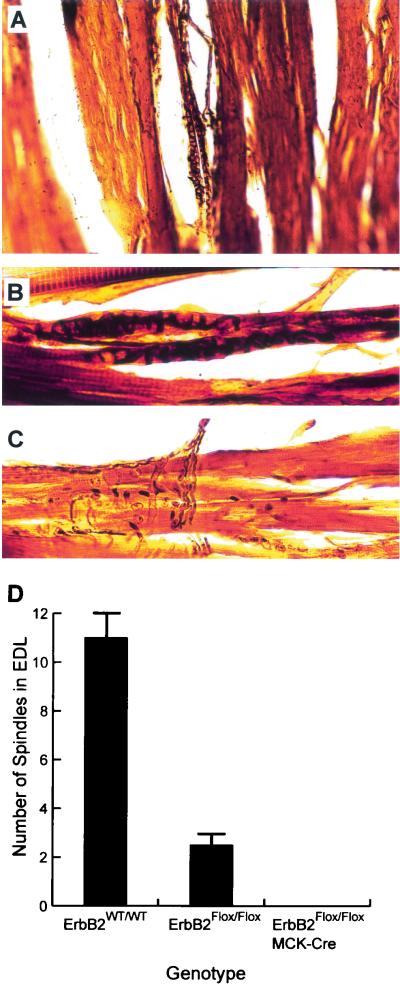
To explore the deficit in proprioception, we examined the mice for a lack of muscle spindles through serial longitudinal sections of the EDL muscle after urea-silver nitrate staining (9). The nonselective silver stain is preferentially taken up by the annulospiral primary sensory endings in the equatorial region of the intrafusal muscle fibers and is a reliable method to easily assess the presence of muscle spindles (Fig. 4A ). While spindles were readily observed in wild-type controls (Fig. 4A and B), spindles were difficult to locate (though occasionally present) in ErbB2Flox/Flox mice and were not observed at all in Mck/Cre ErbB2Flox/Flox mice (n = 6 for each). The only neural structures observed in these mice were associated with motor innervation (Fig. 4C). Additionally, muscle spindle counts from serial sections showed a significant reduction in spindles in ErbB2Flox/Flox mice compared to the wild-type counts (Fig. 4D). A further reduction in the levels of ErbB2 due to introduction of the Mck/Cre transgene resulted in a complete loss of spindles (Fig. 4D). Interestingly, although the ErbB2Flox/Flox mice had on average 2.3 spindles in the EDL, versus 11 in the wild type, they were phenotypically indistinguishable from the wild-type controls.
FIG. 4.
Muscle spindle loss due to ErbB2 reduction. (A) Longitudinal sections of the EDL muscle were examined for the presence of muscle spindles through urea-silver nitrate staining. This method results in readily visible muscle spindles in the thick sections. (B) Muscle spindles are easily detected in wild-type muscle when the nonselective silver stain is preferentially taken up by the neurons. The primary sensory annulospiral endings surrounding the equatorial region ofintrafusal muscle fibers are shown (A and B). (C) While neurons were readily observed in EDL from the ErbB2Flox/Flox Mck/Cre mice, no muscle spindles were found in serial sections through the entire muscle. Only motor innervation was observed in the serial sections. Interestingly, it was also difficult to detect muscle spindles in the EDL muscle from ErbB2Flox/Flox mice. Accordingly, we counted the number of spindles in EDL muscles from ErbB2WT/WT, ErbB2Flox/Flox and ErbB2Flox/Flox Mck/Cre mice. (D) The result of the spindle count is shown. Strikingly, the number of spindles observed in ErbB2Flox/Flox mice was significantly reduced compared to those observed in wild-type mice, although no phenotypic effects were visible in these mice. The further reduction in ErbB2 levels in ErbB2Flox/Flox Mck/Cre mice correlated with a complete loss of spindles and the associated proprioception defects. Error bars, standard deviations.
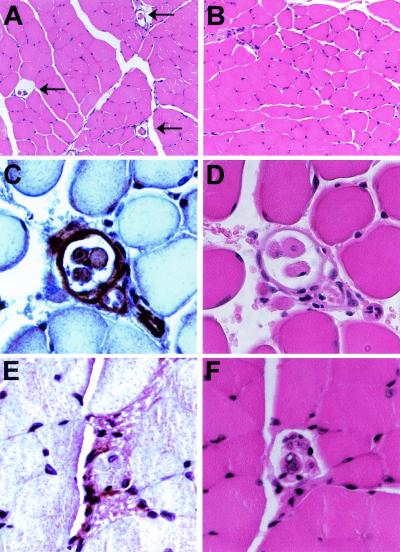
To further explore the pathological basis for the defect in proprioception, the muscle tissue from these mice was subjected to extensive histological analyses. Although no obvious pathological abnormalities were noted in the muscle fibers (compare Fig. 5A and B), these analyses again revealed that muscle spindles were absent in muscle tissue derived from Mck/Cre ErbB2Flox/Flox mice (Fig. 5B). In contrast, the encapsulated muscle spindles were readily detected in the wild-type control animals (Fig. 5A). Consistent with the initial findings, these analyses demonstrated that the observed phenotype correlated with the absence of spindles. Because muscle spindles are required for normal proprioception, the observed progressive defect in gait and posture is likely due to the loss of proprioception associated with the absence of muscle spindles. These observations suggest that a functional ErbB2 product is required for the maintenance of muscle spindles. Therefore, to determine whether ErbB2 was expressed in the muscle spindle, immunohistochemistry for ErbB2 was completed in sections containing multiple muscle spindles. Interestingly, ErbB2 expression was noted in the intrafusal muscle fibers and the capsule in addition to the nervous tissue in the equatorial region of the spindle (Fig. 5C). However, when the distal portion of the muscle spindle was examined, expression of ErbB2 was not detected in the intrafusal muscle fibers (Fig. 5E). Clearly, ErbB2 is concentrated in the intrafusal muscle fibers near the annulospiral primary sensory endings.
FIG. 5.
Histological comparison of ErbB2WT/WT and Mck/Cre ErbB2Flox/Flox muscle. (A) A cross section of TA muscle reveals the presence of numerous muscle spindles in the wild-type controls after hematoxylin and eosin staining (arrows). However, these muscle spindles were not observed in serial sections of Mck/Cre ErbB2Flox/Flox mice (B). In all other aspects, there are no readily discernible differences between histology of wild-type and conditional null muscle (compare panels A and B). (C) Since the targeted deletion of ErbB2 resulted in a loss of muscle spindles, we examined ErbB2 expression in the muscle spindle through immunohistochemistry. ErbB2 expression was readily observed in wild-type intrafusal muscle fibers, in the capsule surrounding the equatorial region, and in the neuron adjacent to the muscle spindle. (D) A serial section is shown after hematoxylin and eosin staining. (E) In contrast to the expression in the intrafusal muscle fibers in the equatorial region of the spindle, ErbB2 is not detected in the intrafusal muscle fibers towards the distal terminus of the muscle spindle. (F) The corresponding hematoxylin-and-eosin-stained serial section is also shown.
ErbB2 provides an important cell survival signal during muscle differentiation.
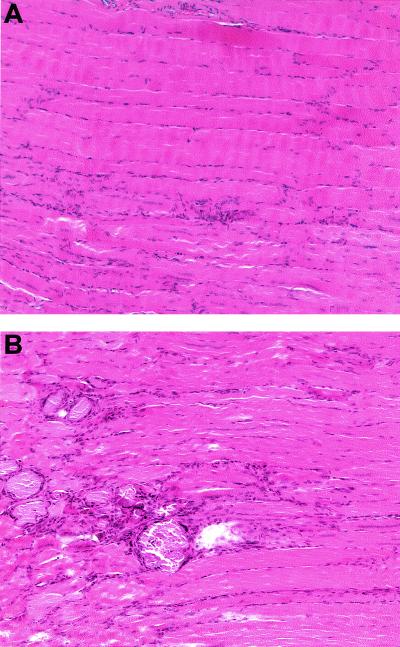
Although the skeletal muscle appeared structurally normal in routine histology, we assessed whether lack of functional ErbB2 was involved in the myogenic differentiation program. Since wild-type muscle should undergo a complete regeneration within 2 weeks after injury (20), we compared the regeneration after an induced muscle injury in ErbB2WT/WT, ErbB2Flox/Flox, and Mck/Cre ErbB2Flox/Flox mice. Two weeks after a muscle crush injury was applied, the ErbB2WT/WT and ErbB2Flox/Flox muscle had regenerated completely with continuous fibers and fiber caliber similar to undamaged muscle when hematoxylin-and-eosin-stained longitudinal sections of the TA muscle were examined (Fig. 6A). However, when the Mck/Cre ErbB2Flox/Flox mice were examined, the injured area was visible during autopsy and by histological examination (Fig. 6B). Clearly, the fibers that have regenerated in the Mck/Cre ErbB2Flox/Flox mice are not continuous and are interspersed with encapsulated cellular debris, indicating a defect in the regeneration of the muscle.
FIG. 6.
Impaired regeneration after an induced muscle injury. Two weeks after a muscle crush injury in the TA muscle, serial sections were examined. Serial longitudinal sections of ErbB2WT/WT and ErbB2Flox/Flox muscle revealed that regeneration was essentially complete, with continuous fibers of normal caliber. (A) The only evidence of the crush injury are the areas with numerous centrally located nuclei shown in the ErbB2Flox/Flox muscle. In contrast, regeneration at the same time point is not complete in the ErbB2Flox/Flox Mck/Cre mice. (B) Numerous regions with encapsulated cellular debris are observed at the site of the injury and there are fewer continuous fibers.
To examine how ErbB2 is involved in the differentiation of myoblasts to myofibers, primary myoblasts were prepared from wild-type controls, ErbB2Flox/Flox, and Mck/Cre ErbB2Flox/Flox pooled adult hind limb muscle. Consistent with our in vivo observations, complete excision of the LOXP1-flanked erbB2 allele was not observed (Fig. 2D), resulting in expression of residual levels of ErbB2 (data not shown). To derive myoblast cell lines that were completely devoid of ErbB2 we introduced an adenovirus containing the Cre recombinase gene (Ad-Cre) (3) into both ErbB2Flox/Flox and Mck/Cre ErbB2Flox/Flox cell lines. Immunoblot analyses revealed that these Ad-Cre-infected cells failed to express significant levels of ErbB2 protein (Fig. 7A, lanes 1 to 3 versus lanes 4 to 5). Interestingly, both the mock-infected cells and myoblasts infected with β-galactosidase (32) control virus (Ad-Bgal) derived from the ErbB2Flox/Flox cells expressed levels of ErbB2 protein about 10% of those observed in the wild-type control line, consistent with the hypomorphic nature of the ErbB2 knockin allele (Fig. 1D). The observed differences in ErbB2 protein levels were clearly not due to inadequate loading, since all cell lines tested expressed comparable levels of ErbB3 and Grb2 (Fig. 7A).
To assess the effects of elimination of erbB2 on myoblast differentiation, these cell lines were incubated under conditions that induced differentiation. During differentiation, an abnormally large number of dead cells were found in the supernatant in the cell lines lacking ErbB2 (Fig. 7B). Importantly, wild-type myoblast cell lines infected with Ad-Cre and ErbB2Flox/Flox cell lines infected with Ad-Bgal did not exhibit an abnormal number of cells in the supernatant. To determine whether the cells in the supernatant were undergoing apoptosis, the cell lines were subjected to annexin-propidium iodide staining (Fig. 7C to F). This clearly illustrated that the cells lacking ErbB2 were undergoing apoptosis during the course of differentiation (Fig. 7F). Quantification of annexin-propidium iodide staining revealed that apoptosis correlated with the number of cells in the supernatant the following days for all cell lines. While up to 35% of differentiating myoblasts underwent apoptosis during the course of differentiation in the ErbB2-null cell lines, it appeared that the remaining myoblasts could fuse into myofibers. To verify that terminal differentiation was complete, cells were examined for myosin heavy chain by immunohistochemistry using MF20. The results revealed that all cell lines tested were capable of terminal differentiation (Fig. 7G to I). Taken together, these observations suggest that although ErbB2 function is dispensable for terminal myotube differentiation, it provides an important cell survival signal during the differentiation process.
DISCUSSION
To explore the importance of erbB2 in muscle development, we employed Mck/Cre transgenic mice (6) to delete a loxP-flanked erbB2 cDNA in skeletal muscle. The initial phenotypic analyses of these strains revealed that these mice exhibited a background-dependent deficit in proprioception due to the loss of muscle spindle cells. It should be noted that the 80% penetrance of the proprioception defect was not related to the extent of excision, since affected and unaffected mice exhibited comparable levels of excision. Despite the dramatic phenotype and prior reports of the utility of the Mck/Cre transgenic mice (6, 53), only 40% of pooled skeletal muscle tissue exhibited excision of the LOXP1-flanked allele. Consistent with these observations, examination of myoblast lines from these mice revealed comparable levels of excision that correlated with residual levels of ErbB2 protein. Since the ErbB2Flox/Flox mice already express reduced levels of ErbB2, the observed muscle-specific excision results in a further 40% reduction in ErbB2 levels and results in the dramatic phenotype. The progressive nature of the proprioception phenotype in the ErbB2Flox/Flox Mck/Cre mice and the complete lack of spindles in the EDL suggest an essential role for ErbB2 in spindle maintenance. Interestingly, the number of muscle spindles observed in the ErbB2Flox/Flox mice was significantly reduced compared to that in wild-type mice. Taken together, these observations suggest a crucial threshold of ErbB-2 is required to maintain the muscle spindles. This argument is further supported by the detection of ErbB2 expression in the intrafusal muscle fibers at the equatorial region of the muscle spindle. Additionally, a global reduction from 10% of wild-type ErbB2 levels to 5% was achieved by interbreeding the ErbB2Flox/Flox mice with the ErbB2 knockout mice. Interestingly, this resulted in embryonic lethality in the heterozygotes, which again supports the argument for a critical threshold of ErbB2 for proper development (R. Chan and W. J. Muller, unpublished data).
The reduction of spindle numbers, without a discernible alteration in gait or posture, in the ErbB2Flox/Flox mice has raised the important question of how many spindles are required for normal proprioception. However, the loss of proprioception observed in Mck/Cre ErbB2Flox/Flox mice bears a striking similarity to that in other knockout mutants. For example, germ line elimination of EGR3 results in a proprioception defect associated with a loss of spindles (47). Given the similarity of two phenotypes, it is conceivable that EGR3 and ErbB2 are on an identical signaling pathway that is required for normal spindle maintenance. Interestingly, previous work has shown that EGR3 is a downstream target of ErbB2-mediated signaling (44). In addition, it has recently been reported that ErbB2 and another EGR family member known as EGR2 are required for Schwann cell development (17, 18). These observations suggest that a conserved ErbB2/EGR signaling axis may be required for development or maintenance of multiple cell lineages.
Previous studies have implicated a role for ErbB2 in the induction of acetylcholine receptor subunits at the NMJ. Although clear defects in cell survival were noted in the ErbB2-deficient myoblast cell lines, we failed to note any effect on induction of acetylcholine receptor subunits in ErbB2-null myoblasts or myofibers (data not shown). These observations argue that the presence of functional ErbB2 is dispensable for induction of acetylcholine receptor subunits. Further, given the presence of other members of the EGFR family at the NMJ, including ErbB3 and ErbB4, these data argue that the other EGFR family members may compensate for loss of ErbB2 in the induction of acetylcholine receptor subunits. Future studies with muscle cell-specific eliminations of other EGFR family members should allow this hypothesis to be tested. However, the previous studies that described ErbB2 expression at the NMJ did not document expression of ErbB2 in the muscle spindle. Given the lack of spindles in the mice with a targeted deletion, we examined ErbB2 expression in the muscle spindle itself. In addition to expression in the primary afferent adjacent to the spindle, we noted expression of ErbB2 in the capsule and in the intrafusal muscle fibers in the equatorial region of the spindle.
Apart from the dramatic loss of muscle spindle cells, histological sections of the muscle of Mck/Cre ErbB2Flox/Flox mice did not reveal any apparent abnormalities. However, upon induction of muscle injury the mice exhibited incomplete regeneration, with numerous areas containing encapsulated cellular debris. Consistent with these in vivo observations, induction of differentiation in ErbB2-null myoblast cell lines stimulated a large increase in the number of apoptotic cells. These results suggest that although differentiation can occur in the absence of a functional ErbB2, it provides an important cell survival signal that is critical during myoblast differentiation. However, terminal differentiation is still possible for the myoblasts that survive the early stages of differentiation.
In addition to EGFR family, other receptor tyrosine kinases have been implicated in muscle spindle development. For example, signaling through specific neurotrophins and their receptors plays an integral role in muscle spindle formation. Consistent with this view, germ line elimination of NT-3 and its receptor, TrkC, both resulted in a loss of muscle spindle cells (15, 26). Our results indicate that the reciprocal signaling loop proposed to occur between intrafusal fibers and their afferents to maintain the spindle (30) may well funnel through an ErbB2/ErbB3 heterodimer. Indeed, our observations suggest that other distinct growth factor signaling pathways may cooperate with ErbB2 in promoting development and maintenance of this cell lineage. Further exploration of the role of cross talk between these distinct growth factor signaling pathways should provide important insight into molecular basis for muscle spindle development and maintenance.
Acknowledgments
We thank Dinsdale Gooden for oligonucleotide synthesis and Brian Allore for automated DNA sequence analysis (MOBIX Central Facility, McMaster University). We thank John Hassell for critically reviewing the manuscript and Monica Graham for technical support.
Funding for this work was provided by the MRC grant MT10594. M.A.R. is supported by grants from the NIH, CIHR, and Muscular Dystrophy Association.
REFERENCES
- 1.Altiok, N., J. L. Bessereau, and J. P. Changeux. 1995. ErbB3 and ErbB2/neu mediate the effect of heregulin on acetylcholine receptor gene expression in muscle: differential expression at the endplate. EMBO J. 14:4258-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrechek, E. R., W. R. Hardy, P. M. Siegel, M. A. Rudnicki, R. D. Cardiff, and W. J. Muller. 2000. Amplification of the neu/erbB-2 oncogene in a mouse model of mammary tumorigenesis. Proc. Natl. Acad. Sci. USA 97:3444-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anton, M., and F. L. Graham. 1995. Site-specific recombination mediated by an adenovirus vector expressing the Cre recombinase protein: a molecular switch for control of gene expression. J. Virol. 69:4600-4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bargmann, C. I., M. C. Hung, and R. A. Weinberg. 1986. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature 319:226-230. [DOI] [PubMed] [Google Scholar]
- 5.Britsch, S., L. Li, S. Kirchhoff, F. Theuring, V. Brinkmann, C. Birchmeier, and D. Riethmacher. 1998. The ErbB2 and ErbB3 receptors and their ligand, neuregulin-1, are essential for development of the sympathetic nervous system. Genes Dev. 12:1825-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruning, J. C., M. D. Michael, J. N. Winnay, T. Hayashi, D. Horsch, D. Accili, L. J. Goodyear, and C. R. Kahn. 1998. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol. Cell 2:559-569. [DOI] [PubMed] [Google Scholar]
- 7.Burden, S., and Y. Yarden. 1997. Neuregulins and their receptors: a versatile signaling module in organogenesis and oncogenesis. Neuron 18:847-855. [DOI] [PubMed] [Google Scholar]
- 8.Burgering, B. M., and P. J. Coffer. 1995. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376:599-602. [DOI] [PubMed] [Google Scholar]
- 9.Butler, R. 1979. A new grouping of intrafusal muscle fibers based on developmental studies of muscle spindles in the cat. Am. J. Anat. 156:115-120. [DOI] [PubMed] [Google Scholar]
- 10.Chamberlain, J. S., J. B. Jaynes, and S. D. Hauschka. 1985. Regulation of creatine kinase induction in differentiating mouse myoblasts. Mol. Cell. Biol. 5:484-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan, R., W. R. Hardy, M. A. Laing, S. E. Hardy, and W. J. Muller. 2002. The catalytic activity of the ErbB-2 receptor tyrosine kinase is essential for embryonic development. Mol. Cell. Biol. 22:1073-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coussens, L., T. L. Yang-Feng, Y. C. Liao, E. Chen, A. Gray, J. McGrath, P. H. Seeburg, T. A. Libermann, J. Schlessinger, U. Francke, et al. 1985. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science 230:1132-1139. [DOI] [PubMed] [Google Scholar]
- 13.Dougall, W. C., X. Qian, N. C. Peterson, M. J. Miller, A. Samanta, and M. I. Greene. 1994. The neu-oncogene: signal transduction pathways, transformation mechanisms and evolving therapies. Oncogene 9:2109-2123. [PubMed] [Google Scholar]
- 14.Erickson, S. L., K. S. O'Shea, N. Ghaboosi, L. Loverro, G. Frantz, M. Bauer, L. H. Lu, and M. W. Moore. 1997. ErbB3 is required for normal cerebellar and cardiac development: a comparison with ErbB2-and heregulin-deficient mice. Development 124:4999-5011. [DOI] [PubMed] [Google Scholar]
- 15.Ernfors, P., K. F. Lee, J. Kucera, and R. Jaenisch. 1994. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell 77:503-512. [DOI] [PubMed] [Google Scholar]
- 16.Franke, T. F., S. I. Yang, T. O. Chan, K. Datta, A. Kazlauskas, D. K. Morrison, D. R. Kaplan, and P. N. Tsichlis. 1995. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81:727-736. [DOI] [PubMed] [Google Scholar]
- 17.Garratt, A. N., S. Britsch, and C. Birchmeier. 2000. Neuregulin, a factor with many functions in the life of a Schwann cell. Bioessays 22:987-996. [DOI] [PubMed] [Google Scholar]
- 18.Garratt, A. N., O. Voiculescu, P. Topilko, P. Charnay, and C. Birchmeier. 2000. A dual role of erbB2 in myelination and in expansion of the Schwann cell precursor pool. J. Cell Biol. 148:1035-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gassmann, M., F. Casagranda, D. Orioli, H. Simon, C. Lai, R. Klein, and G. Lemke. 1995. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature 378:390-394. [DOI] [PubMed] [Google Scholar]
- 20.Grounds, M. D., and Z. Yablonka-Reuveni. 1993. Molecular and cell biology of skeletal muscle regeneration. Mol. Cell. Biol. Hum. Dis. Ser. 3:210-256. [DOI] [PubMed] [Google Scholar]
- 21.Hansen, L. A., N. Alexander, M. E. Hogan, J. P. Sundberg, A. Dlugosz, D. W. Threadgill, T. Magnuson, and S. H. Yuspa. 1997. Genetically null mice reveal a central role for epidermal growth factor receptor in the differentiation of the hair follicle and normal hair development. Am. J. Pathol. 150:1959-1975. [PMC free article] [PubMed] [Google Scholar]
- 22.Harari, D., and Y. Yarden. 2000. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene 19:6102-6114. [DOI] [PubMed] [Google Scholar]
- 23.Hynes, N. E., and D. F. Stern. 1994. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim. Biophys. Acta 1198:165-184. [DOI] [PubMed] [Google Scholar]
- 24.Jo, S. A., X. Zhu, M. A. Marchionni, and S. J. Burden. 1995. Neuregulins are concentrated at nerve-muscle synapses and activate ACh-receptor gene expression. Nature 373:158-161. [DOI] [PubMed] [Google Scholar]
- 25.Kita, Y. A., J. Barff, Y. Luo, D. Wen, D. Brankow, S. Hu, N. Liu, S. A. Prigent, W. J. Gullick, and M. Nicolson. 1994. NDF/heregulin stimulates the phosphorylation of Her3/erbB3. FEBS Lett. 349:139-143. [DOI] [PubMed] [Google Scholar]
- 26.Klein, R., I. Silos-Santiago, R. J. Smeyne, S. A. Lira, R. Brambilla, S. Bryant, L. Zhang, W. D. Snider, and M. Barbacid. 1994. Disruption of the neurotrophin-3 receptor gene trkC eliminates la muscle afferents and results in abnormal movements. Nature 368:249-251. [DOI] [PubMed] [Google Scholar]
- 27.Kraus, M. H., W. Issing, T. Miki, N. C. Popescu, and S. A. Aaronson. 1989. Isolation and characterization of ERBB3, a third member of the ERBB/epidermal growth factor receptor family: evidence for overexpression in a subset of human mammary tumors. Proc. Natl. Acad. Sci. USA 86:9193-9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee, K. F., H. Simon, H. Chen, B. Bates, M. C. Hung, and C. Hauser. 1995. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature 378:394-398. [DOI] [PubMed] [Google Scholar]
- 29.Lin, W., H. B. Sanchez, T. Deerinck, J. K. Morris, M. Ellisman, and K. F. Lee. 2000. Aberrant development of motor axons and neuromuscular synapses in erbB2-deficient mice. Proc. Natl. Acad. Sci. USA 97:1299-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loeb, J. A., and G. D. Fischbach. 1997. Neurotrophic factors increase neuregulin expression in embryonic ventral spinal cord neurons. J. Neurosci. 17:1416-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Megeney, L. A., B. Kablar, K. Garrett, J. E. Anderson, and M. A. Rudnicki. 1996. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 10:1173-1183. [DOI] [PubMed] [Google Scholar]
- 32.Mittal, S. K., A. J. Bett, L. Prevec, and F. L. Graham. 1995. Foreign gene expression by human adenovirus type 5-based vectors studied using firefly luciferase and bacterial beta-galactosidase genes as reporters. Virology 210:226-230. [DOI] [PubMed] [Google Scholar]
- 33.Morris, J. K., W. Lin, C. Hauser, Y. Marchuk, D. Getman, and K. F. Lee. 1999. Rescue of the cardiac defect in ErbB2 mutant mice reveals essential roles of ErbB2 in peripheral nervous system development. Neuron 23:273-283. [DOI] [PubMed] [Google Scholar]
- 34.Moscoso, L. M., G. C. Chu, M. Gautam, P. G. Noakes, J. P. Merlie, and J. R. Sanes. 1995. Synapse-associated expression of an acetylcholine receptor-inducing protein, ARIA/heregulin, and its putative receptors, ErbB2 and ErbB3, in developing mammalian muscle. Dev. Biol. 172:158-169. [DOI] [PubMed] [Google Scholar]
- 35.Plowman, G. D., J. M. Culouscou, G. S. Whitney, J. M. Green, G. W. Carlton, L. Foy, M. G. Neubauer, and M. Shoyab. 1993. Ligand-specific activation of HER4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc. Natl. Acad. Sci. USA 90:1746-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plowman, G. D., G. S. Whitney, M. G. Neubauer, J. M. Green, V. L. McDonald, G. J. Todaro, and M. Shoyab. 1990. Molecular cloning and expression of an additional epidermal growth factor receptor-related gene. Proc. Natl. Acad. Sci. USA 87:4905-4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rando, T. A., and H. M. Blau. 1994. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol. 125:1275-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riethmacher, D., E. Sonnenberg-Riethmacher, V. Brinkmann, T. Yamaai, G. R. Lewin, and C. Birchmeier. 1997. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature 389:725-730. [DOI] [PubMed] [Google Scholar]
- 39.Schechter, A. L., D. F. Stern, L. Vaidyanathan, S. J. Decker, J. A. Drebin, M. I. Greene, and R. A. Weinberg. 1984. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature 312:513-516. [DOI] [PubMed] [Google Scholar]
- 40.Si, J., Z. Luo, and L. Mei. 1996. Induction of acetylcholine receptor gene expression by ARIA requires activation of mitogen-activated protein kinase. J. Biol. Chem. 271:19752-19759. [DOI] [PubMed] [Google Scholar]
- 41.Sibilia, M., J. P. Steinbach, L. Stingl, A. Aguzzi, and E. F. Wagner. 1998. A strain-independent postnatal neurodegeneration in mice lacking the EGF receptor. EMBO J. 17:719-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siegel, P. M., D. L. Dankort, W. R. Hardy, and W. J. Muller. 1994. Novel activating mutations in the neu proto-oncogene involved in induction of mammary tumors. Mol. Cell. Biol. 14:7068-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soltoff, S. P., K. L. Carraway, 3rd, S. A. Prigent, W. G. Gullick, and L. C. Cantley. 1994. ErbB3 is involved in activation of phosphatidylinositol 3-kinase by epidermal growth factor. Mol. Cell. Biol. 14:3550-3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sweeney, C., D. Fambrough, C. Huard, A. J. Diamonti, E. S. Lander, L. C. Cantley, and K. L. Carraway III. 2001. Growth factor-specific signaling pathway stimulation and gene expression mediated by ErbB receptors. J. Biol. Chem. 276:22685-22698. [DOI] [PubMed] [Google Scholar]
- 45.Tansey, M. G., G. C. Chu, and J. P. Merlie. 1996. ARIA/HRG regulates AChR epsilon subunit gene expression at the neuromuscular synapse via activation of phosphatidylinositol 3-kinase and Ras/MAPK pathway. J. Cell Biol. 134:465-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Threadgill, D. W., A. A. Dlugosz, L. A. Hansen, T. Tennenbaum, U. Lichti, D. Yee, C. LaMantia, T. Mourton, K. Herrup, R. C. Harris, et al. 1995. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science 269:230-234. [DOI] [PubMed] [Google Scholar]
- 47.Tourtellotte, W. G., and J. Milbrandt. 1998. Sensory ataxia and muscle spindle agenesis in mice lacking the transcription factor Egr3. Nat. Genet. 20:87-91. [DOI] [PubMed] [Google Scholar]
- 48.Ullrich, A., L. Coussens, J. S. Hayflick, T. J. Dull, A. Gray, A. W. Tam, J. Lee, Y. Yarden, T. A. Libermann, J. Schlessinger, et al. 1984. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature 309:418-425. [DOI] [PubMed] [Google Scholar]
- 49.Woldeyesus, M. T., S. Britsch, D. Riethmacher, L. Xu, E. Sonnenberg-Riethmacher, F. Abou-Rebyeh, R. Harvey, P. Caroni, and C. Birchmeier. 1999. Peripheral nervous system defects in erbB2 mutants following genetic rescue of heart development. Genes Dev. 13:2538-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Won, S., J. Si, M. Colledge, K. S. Ravichandran, S. C. Froehner, and L. Mei. 1999. Neuregulin-increased expression of acetylcholine receptor epsilon-subunit gene requires ErbB interaction with Shc. J. Neurochem. 73:2358-2368. [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto, T., S. Ikawa, T. Akiyama, K. Semba, N. Nomura, N. Miyajima, T. Saito, and K. Toyoshima. 1986. Similarity of protein encoded by the human c-erb-B-2 gene to epidermal growth factor receptor. Nature 319:230-234. [DOI] [PubMed] [Google Scholar]
- 52.Zhu, X., C. Lai, S. Thomas, and S. J. Burden. 1995. Neuregulin receptors, erbB3 and erbB4, are localized at neuromuscular synapses. EMBO J. 14:5842-5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zisman, A., O. D. Peroni, E. D. Abel, M. D. Michael, F. Mauvais-Jarvis, B. B. Lowell, J. F. Wojtaszewski, M. F. Hirshman, A. Virkamaki, L. J. Goodyear, C. R. Kahn, and B. B. Kahn. 2000. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat. Med. 6:924-928. [DOI] [PubMed] [Google Scholar]