Abstract
The EGF-CFC gene Cripto encodes an extracellular protein that has been implicated in the signaling pathway for the transforming growth factor beta (TGFβ) ligand Nodal. Although recent findings in frog and fish embryos have suggested that EGF-CFC proteins function as coreceptors for Nodal, studies in cell culture have implicated Cripto as a growth factor-like signaling molecule. Here we reconcile these apparently disparate models of Cripto function by using a mammalian cell culture assay to investigate the signaling activities of Nodal and EGF-CFC proteins. Using a luciferase reporter assay, we found that Cripto has activities consistent with its being a coreceptor for Nodal. However, Cripto can also function as a secreted signaling factor in cell coculture assays, suggesting that it may also act as a coligand for Nodal. Furthermore, we found that the ability of Cripto to bind to Nodal and mediate Nodal signaling requires the addition of an O-linked fucose monosaccharide to a conserved site within EGF-CFC proteins. We propose a model in which Cripto has dual roles as a coreceptor as well as a coligand for Nodal and that this signaling interaction with Nodal is regulated by an unusual form of glycosylation. Our findings highlight the significance of extracellular modulation of ligand activity as an important means of regulating TGFβ signaling pathways during vertebrate development.
During vertebrate gastrulation, intercellular signaling events mediate the establishment of the basic body plan and formation of the three primary germ layers. Many aspects of these embryonic patterning events, including embryonic mesoderm induction, anterior-posterior axis patterning, and left-right axis specification, require the function of members of the EGF-CFC family in conjunction with Nodal, a ligand of the transforming growth factor beta (TGFβ) family (reviewed in references 52 and 63). EGF-CFC genes encode small extracellular proteins that contain a divergent epidermal growth factor (EGF) motif and a novel conserved cysteine-rich domain termed the CFC motif, with most of the sequence similarity occurring in the central EGF and CFC motifs (54). To date, two members of this family, Cripto and Cryptic, have been isolated in mammals, while only a single EGF-CFC gene has been found in zebra fish, namely one-eyed pinhead (oep). Although the overall level of sequence conservation is relatively low (approximately 30% identity), all EGF-CFC family members appear to have functionally similar activities in assays for phenotypic rescue of oep mutant fish embryos by mRNA microinjection (21).
The Nodal gene was originally identified in genetic studies that demonstrated its requirement for formation of the primitive streak and embryonic mesoderm in the mouse embryo (15, 68). Similar to other ligands of the TGFβ family, including activin and bone morphogenetic proteins (BMPs), Nodal is believed to signal through heteromeric complexes of type I and type II transmembrane receptors with serine-threonine kinase activity (reviewed in references 33 and 34). Molecular genetic studies of mice, fish, and frogs have suggested that Nodal signaling utilizes the type I activin receptor ActRIB (ALK4) and the type II receptors ActRIIA (also known as ActRII) and ActRIIB, leading to phosphorylation and nuclear accumulation of the cytoplasmic signal transducers Smad2 and/or Smad3 together with Smad4 (reviewed in references 52 and 63). The activated Smad complex interacts with nuclear transcription factors that include winged-helix transcription factors of the FAST (FoxH) subfamily (10, 11), which are essential for many aspects of Nodal signaling (24, 44, 57, 62, 65). Downstream targets of the Nodal pathway include members of the Lefty family that inhibit Nodal signaling as well as Nodal itself as part of a positive feedback loop (1, 6, 41, 49).
The intimate relationship of EGF-CFC activity to Nodal signaling was originally deduced from the phenotypic similarity of zebra fish mutants lacking oep activity with those lacking nodal activity as well as the finding that oep activity is essential for Nodal signaling (21). In particular, the combined activities of Nodal and EGF-CFC proteins resemble that of the TGFβ factor activin while they appear to be inactive by themselves (21, 67). These observations have raised the issue of whether EGF-CFC proteins represent ligand cofactors for Nodal or instead act as components of a Nodal receptor.
Consistent with their potential role as a receptor component, EGF-CFC proteins contain a C-terminal hydrophobic region that confers association with the cell surface (3, 55, 67). At least in the case of Cripto, association with the cell membrane has been shown to take place through glycosylphosphatidylinositol (GPI) linkage (36). Cell surface association appears to be important for EGF-CFC activity at physiological concentrations, based on observations that loss-of-function alleles for fish oep and human Cryptic correspond to frameshift mutations that remove the C-terminal hydrophobic region (3, 67). In addition, cell transplantation assays with zebra fish have shown that oep acts cell autonomously in vivo (21, 51, 58). Furthermore, cell culture and biochemical studies have indicated that Nodal signaling is mediated by Smad2 and Smad3 in an EGF-CFC- and FAST-dependent manner (28, 49, 66). Recent experiments with frog embryos have shown that Cripto can associate with the type I receptor ActRIB (ALK4) and can form a complex together with Nodal and the type II receptor ActRIIB (47, 66). Thus, these findings support the idea that EGF-CFC proteins mediate Nodal signaling by forming membrane-associated components of a receptor complex.
Despite this evidence for EGF-CFC proteins functioning as receptor components, earlier studies of Cripto in mammalian cell culture have suggested that it can act as a secreted growth factor-like molecule (reviewed in references 50 and 54). For example, purified human Cripto protein as well as a refolded peptide that contains the EGF-like motif display high-affinity binding and mitogenic activity for mammary cell lines (5), resulting in activation of the ras/raf/MAPK signaling pathway (4, 26). Furthermore, the human Cripto gene has been implicated in the autocrine or paracrine stimulation of tumor cell growth, based on its ability to transform NOG-8 mouse mammary epithelial cells (13) and on its overexpression in a high percentage of human breast, colorectal, gastric, and pancreatic carcinomas (14, 20, 29, 45, 48). However, it has remained unclear how the signaling properties attributed to Cripto protein in cell culture can correlate with its known in vivo roles in mediating Nodal signaling.
To investigate the molecular mechanisms of EGF-CFC and Nodal function in mammalian cells, we have expressed active Cripto and Nodal proteins and have utilized a luciferase reporter assay for analyzing their activity in cell culture. Using coculture experiments, we show that Cripto can act as both a coreceptor and a secreted ligand to stimulate the Nodal signal transduction pathway. Moreover, as was previously noted (37), Cripto contains a consensus sequence for a rare form of glycosylation found in a small subset of EGF motif-containing proteins. We show that Cripto is modified by O fucosylation and that this glycosylation is required for physical interaction with Nodal as well as signaling activity. Taken together, these findings underscore the complexity of the extracellular modulation of TGFβ signaling pathways.
MATERIALS AND METHODS
Transfection of mammalian cells and assay for EGF-CFC and Nodal activities.
Plasmids for expression of EGF-CFC, Nodal, and type I receptors are described in Table 1. The hemagglutinin (HA) and FLAG epitope-tagged mouse Cripto and Cryptic as well as Cripto point mutants were generated by PCR. Human embryonic kidney 293T cells were cultured under 5% CO2 at 37°C in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 50 U of penicillin/ml, and 50 μg of streptomycin/ml. Transient transfection assays were performed with Lipofectamine reagent in Opti-MEM I reduced-serum medium (Invitrogen). Cell lysates and conditioned media were collected after incubation for 2 to 3 days. Protein expression was monitored by Western blotting with the indicated antibodies; Western blot detection was performed with SuperSignal West Pico chemiluminescent substrate (Pierce).
TABLE 1.
Plasmids used in this work
| Plasmid | Use | Reference |
|---|---|---|
| Cripto | ||
| pcDNA3-FLAG-Cripto | Mammalian expression of mouse Cripto (full-length coding region only) with FLAG epitope tag inserted at N terminus following signal sequence | This work |
| pcDNA3-HA-Cripto | Mammalian expression of mouse Cripto with HA epitope tag inserted at N terminus following signal sequence | This work |
| pFLAG-Cripto/IRES-GFP | Generation of stable clones expressing mouse Cripto (full-length coding region with FLAG epitope tag) | This work |
| pcDNA3-FLAG-Cripto(L75A/S77A/F78A) | Expression of triple point mutant in EGF motif of mouse Cripto | This work |
| pcDNA3-FLAG-Cripto(P82A/F85A/R88A) | Expression of triple point mutant in EGF motif of mouse Cripto | This work |
| pcDNA3-FLAG-Cripto(H104A/W107A/R116A) | Expression of triple point mutant in CFC motif of mouse Cripto | This work |
| pcDNA3-FLAG-Cripto(L122A/P126A/D134A) | Expression of triple point mutant in CFC motif of mouse Cripto | This work |
| pcDNA3-FLAG-Cripto(T72A) | Expression of O-fucosylation site mutant of mouse Cripto | This work |
| pcDNA3-HA-Cripto(T72A) | Expression of O-fucosylation site mutant of mouse Cripto | This work |
| Cryptic | ||
| pcDNA3-FLAG-Cryptic | Mammalian expression of mouse Cryptic (full-length coding region only) with FLAG epitope tag inserted at N terminus following signal sequence | This work |
| pEFneo-HA-hCryptic | Expression of human Cryptic with HA epitope tag inserted at N terminus following signal sequence | 3 |
| pEFneo-HA-hCryptic(R112C) | Expression of human Cryptic mutant associated with left-right laterality defects | 3 |
| pEFneo-HA-hCryptic(G174del1) | Expression of human Cryptic mutant associated with left-right laterality defects | 3 |
| pEFneo-HA-hCryptic(R78W) | Expression of human Cryptic mutant associated with left-right laterality defects | 3 |
| pEFneo-HA-hCryptic(R189C) | Expression of human Cryptic mutant associated with left-right laterality defects | 3 |
| oep | ||
| pcDNA3-FLAG-oep | Mammalian expression of zebra fish Oep with FLAG epitope tag inserted at N terminus following signal sequence | 67 |
| Nodal | ||
| pcDNA3-Nodal | Mammalian expression of mouse Nodal (coding region only; without epitope tag) | This work |
| pNodal/IRES-GFP | Generation of stable clones expressing mouse Nodal | This work |
| Type 1 receptors | ||
| pCMV5-ActRIB-HA | Mammalian expression of human ActRIB with HA epitope tag at C terminus | 2 |
| pCMV5-ActRI-HA | Expression of human ActRI with HA epitope tag at C terminus | 8 |
| pCMV5-BMPRIB-HA | Expression of human BMPRIB with HA epitope tag at C terminus | 32 |
| pCMV5-TβRI-HA | Expression of human TβRI with HA epitope tag at C terminus | 9 |
| Luciferase reporters | ||
| A3-lux | Luciferase reporter containing three tandem copies of Nodal and activin responsive element from the Xenopus Mix2 promoter | 31 |
| 3TP-lux | Luciferase reporter containing three tandem copies of TPA response element and part of PAI-1 promoter | 9 |
| (n2)-lux | Luciferase reporter containing seven tandem copies of Nodal-responsive element from the mouse Nodal promoter | 49 |
To establish stable clones expressing Cripto or Nodal, 293T cells were cotransfected with pFLAG-Cripto/IRES-GFP or pNodal/IRES-GFP together with PGK-Hyg (40); control stable clones were generated by using the parental pIRES2-EGFP vector (Clontech). Clones were selected in the presence of 300 μg of hygromycin/ml for 2 weeks followed by sorting of green fluorescent protein-positive cells by flow cytometry with an EPICS Elite instrument (Beckman Coulter). Stable clones were maintained in the presence of 150 μg of hygromycin/ml; conditioned media were harvested after 3 to 4 days of culture in Opti-MEM I medium in the absence of hygromycin.
For assaying EGF-CFC and Nodal activities, transfection mixtures contained 0.2 μg of each expression construct, 0.2 μg of the luciferase reporter plasmid, 50 to 100 ng of CMV-β-gal plasmid, and various amounts of pcDNA3 vector to maintain a constant amount of total DNA. Luciferase activity was measured 24 h posttransfection with a Berthold Lumat LB9507 luminometer; activities were normalized to that of the β-galactosidase control. When used, recombinant human activin A (400 pM; R & D Systems) and TGFβ (500 pM; R & D Systems) were added to the culture medium 7 to 8 h posttransfection. Relative luciferase activities represent averages of the results of at least three independent experiments performed in triplicate. For the coculture assay, signaling cells and responsive cells were transfected individually with the indicated DNA. After 6 h, the transfection media were removed and the signaling and responsive cells were split, plated together for 12 h in complete media, and then changed to Opti-MEM I media for 24 h prior to the assay.
Production and glycosylation analysis of Cripto protein.
Since Cripto is insoluble under typical extraction conditions (Y.-T. Yan and M. M. Shen, unpublished data), analysis of its expression and glycosylation was performed by extraction of membrane-associated proteins from transfected cells at 4°C for 12 h in RIPA114 buffer (50 mM Tris-Cl [pH 8.0], 5 mM EDTA, 100 mM NaCl, 1% Triton X-114, 0.2% sodium dodecyl sulfate [SDS]) containing a protease inhibitor cocktail (Complete Mini; Roche). Solubilized proteins were collected following centrifugation (15,000 × g) for 15 min at 4°C. To release cell surface-associated Cripto protein from intact cells, transfected cells were harvested by scraping (rather than trypsinization), resuspended in phosphate-buffered saline, and incubated with phosphatidylinositol-specific phospholipase C (PI-PLC) (Sigma) at a final concentration of 0.5 U/ml at 37°C for 30 min.
To analyze Cripto glycosylation, transfected 293T cells expressing HA-tagged mouse Cripto or the T72A mutant were metabolically radiolabeled with [3H]fucose as described previously (39). After 24 h, proteins were immunopurified from cell lysates by using an anti-HA antibody (Covance). Samples were treated with or without PNGase F as described previously (39) to remove N-glycans, subjected to SDS-polyacrylamide gel electrophoresis, and analyzed by Western blotting and fluorography. β-Elimination and gel filtration chromatography were performed as described previously (39).
Cross-linking and coimmunoprecipitation analysis.
For reversible chemical cross-linking, intact transfected cells were incubated in culture medium containing 0.5 mM DTSSP [3,3′-dithiobis(sulfosuccinimidylpropionate)] (Pierce) at room temperature for 30 min. The reaction was stopped by the addition of 50 mM Tris-Cl (pH 8.0, final concentration), and the solubilized membrane proteins were prepared as described above. Following cross-linking, coimmunoprecipitation was performed by incubation of the solubilized membrane proteins with anti-FLAG M2-agarose affinity gel (Sigma) or anti-HA affinity matrix HA.11 (Covance) for 4 h at 4°C. Protein complexes were washed with RIPA114 buffer, and cross-linking was reversed by boiling in SDS-polyacrylamide gel electrophoresis loading buffer with 25 mM β-mercaptoethanol. The polyclonal anti-Nodal antiserum was generated by using a GST-Nodal (mouse) fusion protein as an immunogen (Cocalico Biologicals).
RESULTS
Expression of Cripto and Nodal proteins in transfected mammalian cells.
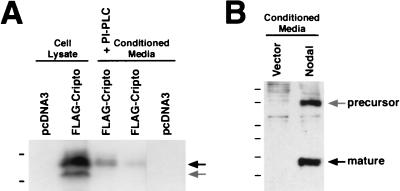
To investigate their activities in mammalian cells, we expressed Cripto and Nodal proteins in human embryonic kidney 293T cells, which do not express the endogenous Cripto or Nodal genes as determined by reverse transcription-PCR analysis (Y.-T. Yan and M. M. Shen, unpublished data). We found that Cripto protein was expressed at high levels in lysates of transfected cells but only at low levels in the conditioned media (Fig. 1A); this finding is consistent with the results of a previous study (36) as well as with our analysis of Cryptic protein expression (55). However, treatment of transfected cells with PI-PLC resulted in increased levels of Cripto protein in the culture supernatants (Fig. 1A), consistent with the previous report that Cripto is GPI linked (36).
FIG. 1.
Expression of EGF-CFC and Nodal proteins in mammalian cells. (A) Expression of Cripto in whole-cell lysates or conditioned media following transient transfection of 293T cells; proteins were detected by Western blotting with anti-FLAG antibodies. Low levels of Cripto were detected in conditioned media in the absence of PI-PLC, while treatment with PI-PLC resulted in the release of increased amounts of Cripto protein into the conditioned media. The upper arrow indicates processed mature Cripto, while the lower arrow indicates an immature, unmodified form (Yan and Shen, unpublished). The dashes from top to bottom indicate molecular size standards at 26 and 19.6 kDa. (B) Expression of mouse Nodal following transient transfection of 293T cells. Note that the mature processed Nodal is produced in conditioned media, as detected by using a polyclonal antiserum to mouse Nodal. The positions of the precursor and mature forms are indicated. The dashes from top to bottom indicate molecular size standards at 50, 38, 25, 20, 14, and 9 kDa.
Unlike Cripto, Nodal is expressed as a preprotein that undergoes posttranslational processing to form the mature signaling molecule; previous studies have reported difficulties in expressing active mature Nodal protein in mammalian cells (16). We observed a product of the expected size for the mature processed Nodal protein in the conditioned media of transfected 293T cells by using an unmodified expression vector and Western blot detection with polyclonal Nodal antisera (Fig. 1C). Interestingly, we did not detect mature processed Nodal protein in the conditioned media of other transfected cell lines, such as COS-7 (C. E and M. M. Shen, unpublished data), indicating that its production was cell line dependent and presumably reflecting the expression of a specific proprotein convertase(s).
An assay for Cripto and Nodal activity in mammalian cells.
Our assay for EGF-CFC and Nodal activities is based on known downstream activities of Nodal signaling that are mediated by Smad proteins together with FAST2 at target promoters such as Nodal itself (28, 42, 49, 66). Since 293T cells contain endogenous Smad2, Smad4, and activin receptors (Y.-T. Yan, C. Abate-Shen, and M. M. Shen, unpublished data), the exogenous components of this assay are Nodal, EGF-CFC proteins, FAST2, and an appropriate luciferase reporter gene. For most of our studies, we have utilized the activin-responsive A3-lux reporter gene, which contains a Nodal-sensitive response element from the Xenopus Mix2 homeobox gene (31). Similar results were obtained with both the broad-spectrum TGFβ reporter 3TP-lux and the Nodal-responsive (n2)-lux reporter, which is based on the Nodal autoregulatory enhancer (reference 49 and data not shown).
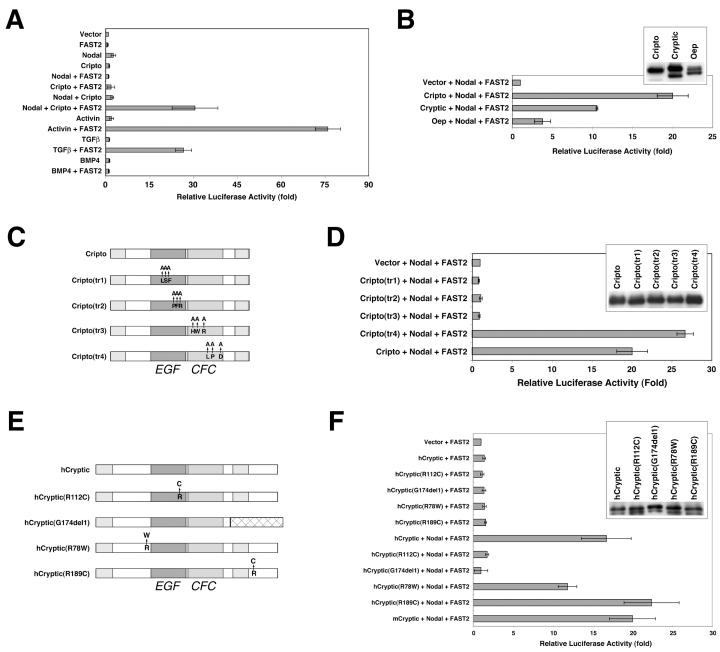
We found that the cotransfection of expression constructs for Nodal, Cripto, and FAST2 produced a strong signaling response (approximately 20- to 30-fold activation) by using the A3-lux reporter in 293T cells (Fig. 2A). Importantly, Nodal activity was strictly dependent upon both EGF-CFC and FAST2 expression (Fig. 2A). (Interestingly, we noted that both N- and C-terminally epitope-tagged mature Nodal proteins displayed greatly reduced activity in this assay [Yan and Shen, unpublished].) In contrast, activin activity was only dependent upon FAST2 expression; this result was expected, since its signaling is EGF-CFC independent (Fig. 2A and data not shown). These findings extend earlier studies showing EGF-CFC- and FAST-dependent Nodal signaling in frog embryos and recombinant Nodal protein function in cell culture (28, 49).
FIG. 2.
Signaling assay for EGF-CFC and Nodal proteins. Transient transfection assays were performed on 293T cells with an A3-lux luciferase reporter plasmid containing three tandem copies of a Nodal-responsive element (31). Data are expressed as the fold difference in luciferase activity relative to that obtained with the control vector (pcDNA3). Experiments were performed in triplicate; error bars represent 1 standard deviation. (A) Cripto and Nodal are mutually required for signaling in a FAST2-dependent manner. Cells were cotransfected with the indicated expression plasmids (Nodal, Cripto, or FAST2) and/or were incubated with the indicated proteins (activin, TGFβ, or BMP4). (B) Activities of other EGF-CFC family members. Cryptic and Oep were also active in this assay, although they displayed lower levels of activity; the inset shows the expression of input EGF-CFC proteins as detected by Western blotting. (C and D) Contribution of EGF and CFC motifs of mouse Cripto for signaling activity. (C) Schematic representation of alanine substitution mutants (tr1 to tr4) (Table 1); (D) activity of Cripto alanine substitution mutants, with the inset showing a Western blot of the input proteins. (E and F) Activities of human Cryptic mutants associated with left-right laterality defects (3). (E) Schematic representation of the mutants (Table 1); (F) activity of human Cryptic mutants, with the inset showing a Western blot of the input proteins.
Specificity for EGF-CFC activity.
To investigate whether our cell culture assay faithfully reflected the in vivo activities of EGF-CFC and Nodal proteins, we examined the activities of other EGF-CFC family members as well as those of various EGF-CFC mutants. We found that mouse Cryptic and zebra fish oep could also mediate Nodal signaling but were less active than mouse Cripto, despite their similar expression levels (Fig. 2B). To investigate the contribution of the conserved EGF and CFC motifs for Cripto function in Nodal signaling, we examined the activities of mutants containing clustered point mutations in these regions (Fig. 2C). We generated triple alanine mutants for selected residues that are conserved in the EGF-CFC family but not in other EGF motifs; moreover, we avoided the conserved cysteines, which are likely to be essential for disulfide bond formation (Table 1 and Fig. 2C). While each of these proteins was expressed at a level similar to that of the wild type, we found that three of these mutants were completely inactive in the Nodal signaling assay, which demonstrates the requirement for both an intact EGF motif and an intact CFC motif in the signaling response (Fig. 2D). Notably, however, the Cripto(tr4) mutant displayed activity comparable to or better than that of the wild type (Fig. 2D).
We also examined the activities of four naturally occurring mutations in human Cryptic (hCryptic) which are associated with congenital left-right laterality defects in children (Fig. 2E) (3). Although wild-type hCryptic displayed activity similar to that of mouse Cryptic in the signaling assay, we found that the hCryptic(R112C) and hCryptic(G174del1) mutants were inactive, while the activities of the hCryptic(R78W) and hCryptic(R189C) mutants were comparable to that of the wild type (Fig. 2F). Notably, these results are completely consistent with their biological activities in rescuing the zebra fish oep mutant phenotype (3). Thus, the biological activities of these EGF-CFC mutants correlate well with their effects on Nodal signaling.
Nodal and Cripto can both act as intercellular signaling factors.
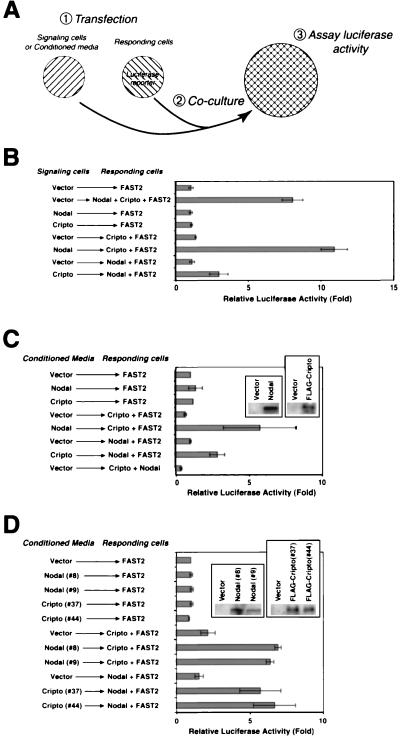
To examine the potential for Nodal and Cripto to act as secreted signaling factors, we performed coculture experiments in which these proteins were expressed either by cells containing the luciferase reporter (responding cells) or by cells lacking the reporter gene (signaling cells) (Fig. 3A). In such an assay, a secreted ligand should be active when expressed in either signaling cells or responding cells, while a receptor (or other cell-associated) component should be active only in responding cells.
FIG. 3.
Cripto and Nodal act as secreted signaling factors. (A) Design of a coculture assay to assess the signaling activities of Cripto and Nodal. Two populations of 293T cells were transiently transfected and replated together to assay luciferase activity (panel B). Responding cells are distinguished from signaling cells by transfection with the A3-lux luciferase reporter plasmid. Alternatively (panel C), conditioned media from signaling cells were added without direct coculture. (B) Nodal is active when expressed by either signaling or responding cells. Cripto is active when expressed by responding cells, but also displays detectable activity when expressed by signaling cells. (C) Both Nodal and Cripto proteins expressed in conditioned media of transiently transfected 293T cells are active in this signaling assay; the left and right insets show the expression of Nodal and Cripto protein in conditioned media, respectively. (D) Conditioned media from two independent stable clones (#8 and #9) expressing mouse Nodal display activity; similarly, conditioned media from two independent stable clones (#37 and #44) expressing mouse Cripto are also active. The left and right insets show the expression of Nodal and Cripto protein, respectively, in conditioned media from these stable cell lines or from control stable lines containing the parental vector alone.
Using this coculture assay, we found that Nodal was active when expressed by either the signaling or the responding cells (Fig. 3B), a finding consistent with its known role as a ligand that acts as a morphogenetic signal in vivo (12). Furthermore, Nodal protein secreted to the conditioned media of transfected 293T cells was active in signaling to cells transfected with Cripto and FAST2 expression constructs (Fig. 3C). To our knowledge, this represents the first demonstration of active secreted mouse Nodal protein in mammalian cell culture.
In the coculture assay, Cripto was highly active when expressed in the responding cells (Fig. 3B), as expected for a putative receptor component. However, Cripto also displayed reduced but significant activity when expressed by signaling cells, suggesting that it can act as a secreted signaling molecule. Consistent with this observation, we found that conditioned media from Cripto-transfected cells were similarly active in signaling to 293T cells expressing Nodal and FAST2 (Fig. 3C). Furthermore, conditioned media from two independent Cripto-expressing stable 293T clones were active in signaling to cells expressing Nodal and FAST2 (Fig. 3D); similarly, conditioned media from two independent Nodal-expressing stable clones were active on cells expressing Cripto and FAST2 (Fig. 3D). These findings indicate that secreted Cripto protein can successfully mediate Nodal signaling and can thereby act as a diffusible ligand.
Physical interactions between Nodal, Cripto, and type I receptors.
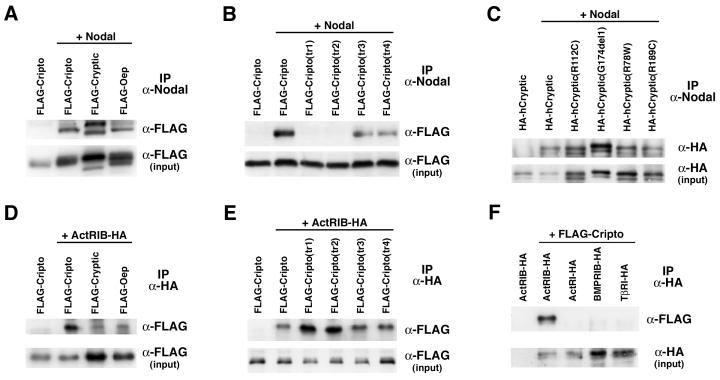
Given their mutually dependent signaling activities, we next investigated whether Nodal and EGF-CFC proteins could physically interact. Our strategy was to cross-link the proteins in situ in their extracellular milieu by using the membrane-impermeable reversible cross-linking agent DTSSP. Following cross-linking, we found that Cripto could be coimmunoprecipitated with an antiserum directed against mouse Nodal (Fig. 4A); a weaker but reproducible interaction could be observed in the absence of the cross-linking agent (data not shown). Similar results were obtained for mouse Cryptic and zebra fish Oep (Fig. 4A). Interestingly, the ability of the four Cripto triple point mutants to interact with Nodal correlated with their activity in the cell culture assay (Fig. 2D and 4B). In contrast, all four human Cryptic mutants interacted with Nodal (Fig. 4C). Thus, our results show that extracellular Nodal and EGF-CFC proteins can interact in situ in transfected mammalian cells.
FIG. 4.
EGF-CFC proteins interact with Nodal and ActRIB. Transfected 293T cells were treated with the membrane-impermeable cross-linking agent DTSSP followed by immunoprecipitation (IP). Cross-linking was reversed, and proteins were analyzed by Western blotting. The inputs represent 10% of the total protein used in each case. (A) EGF-CFC family members interact with Nodal in cotransfected 293T cells. (B) Contribution of the EGF and CFC motifs to Nodal interaction. Cripto mutants in the EGF motif (tr1 and tr2) do not interact with Nodal, whereas mutants in the CFC motif (tr3 and tr4) do interact. (C) All four human Cryptic mutants interact with Nodal; the decreased electrophoretic mobility of HA-hCryptic(G174del1) is due to the increased size of this protein. In panels A to C, immunoprecipitations were performed with anti-Nodal and Western blot detection with anti-FLAG or anti-HA antibodies. (D) EGF-CFC proteins interact with ActRIB, although Cripto interaction is more robust than that of Cryptic or Oep. (E) All four Cripto mutants interact with ActRIB. (F) Interaction of Cripto with type I receptors is specific for ActRIB. In panels C to E, immunoprecipitations were performed with anti-HA and Western blot detection with anti-FLAG or anti-HA antibodies as indicated.
We also used chemical cross-linking followed by coimmunoprecipitation to examine the interaction of EGF-CFC proteins with epitope-tagged type I receptors by cotransfection of 293T cells. We found that Cripto could cross-link and coimmunoprecipitate with the type I receptor ActRIB (ALK4) (Fig. 4D), a result consistent with previous findings involving microinjected frog embryos (66) or soluble forms of Cripto and ActRIB (47). Cryptic and Oep proteins showed a similar but lower-level interaction, correlating with their relative activities in the cell culture assay (Fig. 2B and 4D). Notably, all four Cripto mutants interacted with ActRIB in a manner similar to that of the wild type, suggesting that this interaction was unaffected by these alanine substitution mutations (Fig. 4E) and contrasting with their signaling activities and abilities to interact with Nodal (Fig. 2D and 4B). (However, the weaker interaction of Cryptic with ActRIB precluded our analysis of human Cryptic mutants.) Finally, Cripto did not interact with the type I receptors ActRI (ALK2), BMPRIB (ALK6), and TβRI (ALK5), which are not thought to be involved in Nodal signaling, indicating that the interaction of EGF-CFC proteins with type I receptors is relatively specific (Fig. 4F). Taken together, these findings suggest that EGF-CFC proteins interact specifically with Nodal and with ActRIB and that the regions involved in these interactions are distinct.
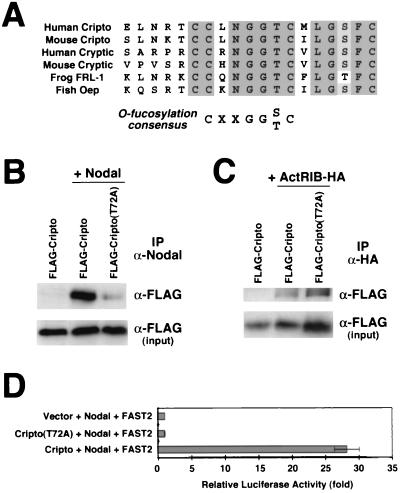
Requirement of O fucosylation for Cripto activity.
During our analysis of point mutants for Cripto, we noted the presence at amino acids 67 to 73 of a conserved sequence (CXXGG[S/T]C) for O-linked fucose modification (Fig. 5A), which is a rare glycosylation event found only in a small subset of EGF motif-containing proteins (22, 37). This sequence is conserved in all EGF-CFC family members identified to date (54), but its functional significance has been unclear. Therefore, we generated an alanine substitution mutation (T72A) in this site in Cripto and found that the mutant protein displayed a greatly decreased ability to interact with Nodal (Fig. 5B) but interacted well with ActRIB (Fig. 5C). Consistent with this observation, the Cripto(T72A) mutant was completely inactive in facilitating Nodal signaling in a cotransfection assay (Fig. 5D).
FIG. 5.
Interaction between Cripto and Nodal requires O fucosylation of Cripto. (A) The EGF motif of all known EGF-CFC proteins contains a consensus site for O fucosylation (22, 37). Shown is the N-terminal portion of the EGF-like motif; the shaded residues are strictly conserved. (B) A Cripto mutant for the O-fucosylation site (T72A) has greatly decreased interaction with Nodal, as assessed by cross-linking followed by immunoprecipitation (IP). (C) The Cripto(T72A) mutant interacts with ActRIB in a manner similar to that of the wild type. (D) The Cripto(T72A) mutant lacks signaling activity, as assessed by cotransfection with Nodal and FAST2 in the luciferase assay.
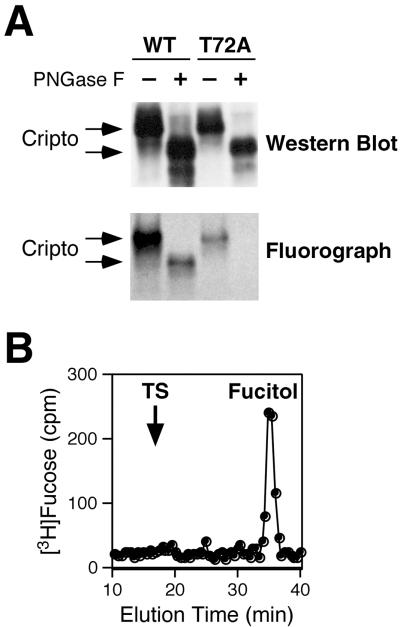
To establish the nature of the possible glycan modification on this site, we expressed HA-tagged wild-type and Cripto(T72A) mutant proteins in 293T cells in the presence of [3H]fucose. We treated the purified HA-tagged proteins with PNGase F to remove N-glycans, followed by Western blotting and fluorography to detect 3H-labeled proteins. PNGase F treatment of both wild-type and T72A mutant Cripto proteins resulted in a significant shift in electrophoretic mobility, consistent with extensive N-linked glycosylation (Fig. 6A). However, only the wild-type Cripto protein contained labeled fucose following PNGase F treatment, indicating that wild-type Cripto expressed in 293T cells is modified by O-linked fucose while the T72A mutant is not (Fig. 6A). Since O-linked fucose can exist in either a monosaccharide form (e.g., Factor VII) (22) or a tetrasaccharide form (e.g., Notch or Factor IX) (22, 38, 39), we next examined the form present on Cripto. O-linked sugars were released from wild-type Cripto by alkali-induced β-elimination, a treatment that cleaves the bond between carbohydrates and the hydroxyl groups of serine or threonine residues. Analysis of the released sugars by gel filtration chromatography showed only [3H]fucitol (Fig. 6B), the expected product from the β-elimination of an O-linked fucose monosaccharide (39).
FIG. 6.
Mouse Cripto is modified with the monosaccharide form of O-linked fucose on Thr-72. 293T cells were transiently transfected with HA-tagged Cripto or the T72A mutant. Following radiolabeling with [3H]fucose, proteins were isolated by immunoprecipitation with anti-HA antibodies. (A) Proteins were treated with or without PNGase F to remove N-glycans and were analyzed by Western blotting and fluorography. The large shift in molecular weight (apparent in the Western blot) after PNGase F treatment is due to the removal of N-glycans; the remaining radiolabel (apparent in the fluorograph) corresponds to O-linked fucose, which is absent in the T72A mutant. WT, wild type. (B) Following PNGase F treatment, the radiolabeled Cripto protein was analyzed by β-elimination and gel filtration chromatography to demonstrate that the O-linked sugar corresponds to the monosaccharide form of O-linked fucose (fucitol). The migration position of the tetrasaccharide form of O-linked fucose is indicated by TS.
DISCUSSION
Our study has investigated the mechanisms by which members of the EGF-CFC family modulate Nodal signaling. We have demonstrated that Cripto may have complex activities in the Nodal signaling pathway, having potential roles either as a coreceptor or as a coligand. Moreover, the activity of Cripto is itself modulated at the posttranslational level by O fucosylation, which could provide yet another mechanism for regulating Nodal activity in vivo. Thus, our findings underscore the multifaceted regulation of Nodal signaling at the extracellular level, including the regulation of ligand processing, ligand heterodimerization, and competition for receptor binding (reviewed in references 34 and 63).
Signaling activity of Cripto.
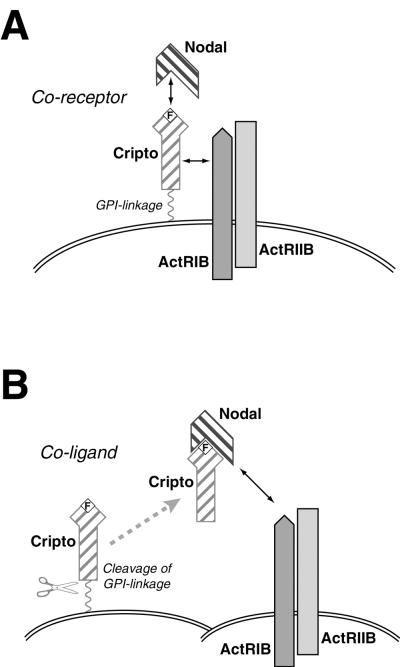
Our findings are consistent with a model supported by previous genetic and biochemical studies in which EGF-CFC proteins act as membrane-associated coreceptors for type I and type II activin receptors (Fig. 7A) (21, 28, 47, 49, 66). In this view, Cripto can bind Nodal directly to recruit this ligand to type I receptors, leading to the formation of an active EGF-CFC-Nodal-type I receptor-type II receptor signaling complex. Furthermore, we propose an alternative mechanism for Cripto function, as a coligand together with Nodal, presumably following release from the cell membrane (Fig. 7B).
FIG. 7.
Dual roles of Cripto. A schematic model for the interaction of Cripto with Nodal, ActRIB, and ActRIIB is shown. The wavy line indicates GPI linkage, and the boxed F represents O-linked fucose modification of Cripto. (A) Cripto acts as a coreceptor for Nodal. (B) Cripto can act as a coligand together with Nodal. Following cleavage of the GPI linkage of Cripto, Nodal and Cripto can act together as a paracrine signal.
Consistent with the role of EGF-CFC proteins as coreceptors for Nodal, the cell autonomy of EGF-CFC function has been indicated by cell transplantation experiments on zebra fish, in which cells expressing wild-type oep are unable to rescue the phenotype of adjacent oep mutant cells (21, 51, 58). On the other hand, the situation for the mouse is less clear, since chimeric mice generated with homozygous Cripto−/− embryonic stem (ES) cells display no phenotypic consequences, which led to the suggestion that Cripto can act non-cell autonomously (64). However, it is difficult to determine the extent to which Cripto can act non-cell-autonomously, since the contribution of mutant ES cells in this chimera experiment was not evaluated at cellular resolution. Thus, the potential for Cripto (and Cryptic) to act non-cell autonomously in vivo as a coligand with Nodal is still unresolved.
Since Cripto is GPI linked, its potential non-cell autonomy could be explained by active or passive shedding from the cell membrane (17, 43). In support of this idea, microinjection of C-terminally truncated oep mRNA or protein can rescue the phenotype of oep null mutants, indicating that diffusible EGF-CFC proteins are potentially active (35, 67). An alternative possibility is that Cripto could undergo intermembrane transfer, in which GPI-linked proteins can move from the membrane of one cell to those of adjacent cells (19, 27). Thus, the in vivo shedding and/or transfer of EGF-CFC proteins could result in the formation of Nodal receptor complexes in trans on neighboring cells that might not themselves express the EGF-CFC gene (Fig. 7B). A precedent for such a mechanism has been provided by the GFRα protein, which is a GPI-linked protein that heterodimerizes with the c-RET tyrosine kinase to form a receptor for GDNF, a distant member of the TGFβ superfamily (25, 43, 59).
Indeed, numerous studies of Cripto activity have suggested that Cripto can act as a growth factor-like molecule in cell culture, although the basis for this activity has not been previously elucidated (reviewed in reference 50). Salomon and colleagues have demonstrated that a refolded synthetic human Cripto peptide as well as full-length recombinant protein can bind to mammary epithelial cells, resulting in tyrosine phosphorylation of the ErbB4 receptor and activation of the Ras/Raf/MAPK signaling pathway (4, 26); it is plausible that many or all of these effects represent downstream effects of Nodal signaling. However, the synthetic Cripto peptide contains the EGF-like motif but lacks the CFC motif (5) and presumably would not be O fucosylated. These findings contrast with our mutational analysis and with in vivo data on the fish and biochemical data on the frog (66, 67) which indicate that the CFC motif and O fucosylation are essential for EGF-CFC activity.
In principle, our findings implicating Cripto as a coligand in cell culture could be nonphysiological, particularly since oep acts cell autonomously in fish (21, 51, 58). One possibility is that there are fundamental differences between fish Oep and mouse Cripto in their abilities to be released from the cell membrane and/or to diffuse; notably, zebra fish Squint (zNr2) can act as a long-range morphogenetic signal in vivo while the closely related Cyclops (zNr1) protein cannot (12). An alternative possibility, which is perhaps consistent with its overexpression in epithelial cancers (14, 20, 29, 45, 48), is that Cripto acts cell autonomously during embryogenesis but may act nonautonomously in carcinogenesis. Conceivably, alterations of intercellular interactions and the extracellular matrix that occur during cellular transformation may affect the ability of Cripto to be released and/or transferred from the cell surface; in this view, the use of mammalian cell lines such as 293T would be more closely analogous to tumor cells.
Modulation of Cripto activity by O fucosylation.
While posttranslational modification by glycosylation is likely to represent an important mechanism for the regulation of signaling proteins, there are relatively few examples in which glycosylation has been shown to be essential for signaling activity. We have found that mouse Cripto is O fucosylated at Thr-72 and that mutation of this residue abolishes Cripto activity in Nodal signaling. While this manuscript was in preparation, Schiffer and colleagues reported that human Cripto expressed in CHO cells contains a single O-fucose residue and that a similar mutant lacking O fucosylation is inactive (53). We have extended these results to demonstrate that O fucosylation is required for the physical interaction of Cripto with Nodal but not with ActRIB.
Cripto is one of only three proteins known to have an O-linked fucose modification essential for function. Interestingly, O-linked fucose modification of urinary-type plasminogen activator (uPA) is essential for activation of the uPA receptor, but in contrast with Cripto, defucosylated uPA binds to the uPA receptor with the same affinity as fucosylated uPA (46). In addition, recent studies have demonstrated that O-linked fucose modifications on Notch play an essential role (7, 38, 39), since the extension of O-linked fucose with GlcNAc by Fringe glycosyltransferases modulates the interactions of Notch receptors with the ligands Jagged and Delta (23, 38, 56). Although there is no evidence for the modification of Cripto by Fringe at present, other glycosyltransferases that modify O-linked fucose have been described (37) and others may well exist; these glycosyltransferases could potentially add additional sugar residues to EGF-CFC proteins in appropriate contexts. The in vivo functional analysis of the recently cloned GDP-fucose protein O-fucosyltransferase enzyme (61) should prove informative with respect to these possibilities.
To some extent, EGF-CFC proteins might be functionally analogous to betaglycan and endoglin, which are considered to be auxiliary receptors for TGFβ signals (reviewed in reference 33). Both betaglycan and endoglin are large extracellular glycoproteins that can regulate the access of TGFβ ligands to type I and II receptors (33); for example, betaglycan is required for inhibin binding to activin receptors (30). Although EGF-CFC proteins share no sequence similarity to either betaglycan or endoglin, the importance of O fucosylation for their activity may imply possible mechanistic similarities with respect to the importance of sugar modifications.
Finally, we speculate that the O fucosylation of Cripto could represent a posttranslational mechanism for regulating the Nodal signaling pathway. In particular, both Nodal and Cripto are coexpressed at pregastrulation stages of mouse development (6, 18, 60), yet Nodal-induced mesoderm formation does not occur. One possibility is that Nodal may act independently of Cripto, perhaps through interactions with the orphan type I receptor ALK7, which can occur in the absence of Cripto (47). These observations raise the possibility that O fucosylation of Cripto regulates Nodal signaling outputs through the differential utilization of ALK4 versus ALK7 type I receptors. Thus, the unusual glycosylation of Cripto may provide an additional mechanism to fine-tune the outcome of Nodal signaling during embryogenesis.
Acknowledgments
We thank Richard Bamford, Hiroshi Hamada, Michael Kuehn, Fang Liu, Joan Massague, Rick Mortensen, Max Muenke, and Malcolm Whitman for generous gifts of clones. We are particularly indebted to Fang Liu for advice and reagents and to Wen-Feng Chen and Umay Saplakoglu for important contributions at earlier phases of this study. We thank Fang Liu and Peter Lobel for insightful comments on the manuscript.
This work was supported by a DOD Breast Cancer Research Program Pre-doctoral Fellowship (C.E.) and NIH grants GM61126 (R.S.H), HD29446 (C.A.-S.), and HL60212 and HD38766 (M.M.S.).
REFERENCES
- 1.Adachi, H., Y. Saijoh, K. Mochida, S. Ohishi, H. Hashiguchi, A. Hirao, and H. Hamada. 1999. Determination of left/right asymmetric expression of nodal by a left side-specific enhancer with sequence similarity to a lefty-2 enhancer. Genes Dev. 13:1589-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attisano, L., J. L. Wrana, E. Montalvo, and J. Massague. 1996. Activation of signalling by the activin receptor complex. Mol. Cell. Biol. 16:1066-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bamford, R. N., E. Roessler, R. D. Burdine, U. Saplakoglu, J. dela Cruz, M. Splitt, J. Towbin, P. Bowers, B. Marino, A. F. Schier, M. M. Shen, M. Muenke, and B. Casey. 2000. Loss-of-function mutations in the EGF-CFC gene CFC1 are associated with human left-right laterality defects. Nat. Genet. 26:365-369. [DOI] [PubMed] [Google Scholar]
- 4.Bianco, C., S. Kannan, M. De Santis, M. Seno, C. K. Tang, I. Martinez-Lacaci, N. Kim, B. Wallace-Jones, M. E. Lippman, A. D. Ebert, C. Wechselberger, and D. S. Salomon. 1999. Cripto-1 indirectly stimulates the tyrosine phosphorylation of erbB-4 through a novel receptor. J. Biol. Chem. 274:8624-8629. [DOI] [PubMed] [Google Scholar]
- 5.Brandt, R., N. Normanno, W. J. Gullick, J. H. Lin, R. Harkins, D. Schneider, B. W. Jones, F. Ciardiello, M. G. Persico, F. Armenante, N. Kim, and D. S. Salomon. 1994. Identification and biological characterization of an epidermal growth factor-related protein: cripto-1. J. Biol. Chem. 269:17320-17328. [PubMed] [Google Scholar]
- 6.Brennan, J., C. C. Lu, D. P. Norris, T. A. Rodriguez, R. S. Beddington, and E. J. Robertson. 2001. Nodal signalling in the epiblast patterns the early mouse embryo. Nature 411:965-969. [DOI] [PubMed] [Google Scholar]
- 7.Bruckner, K., L. Perez, H. Clausen, and S. Cohen. 2000. Glycosyltransferase activity of Fringe modulates Notch-Delta interactions. Nature 406:411-415. [DOI] [PubMed] [Google Scholar]
- 8.Carcamo, J., F. M. Weis, F. Ventura, R. Wieser, J. L. Wrana, L. Attisano, and J. Massague. 1994. Type I receptors specify growth-inhibitory and transcriptional responses to transforming growth factor beta and activin. Mol. Cell. Biol. 14:3810-3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carcamo, J., A. Zentella, and J. Massague. 1995. Disruption of transforming growth factor beta signaling by a mutation that prevents transphosphorylation within the receptor complex. Mol. Cell. Biol. 15:1573-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, X., M. J. Rubock, and M. Whitman. 1996. A transcriptional partner for MAD proteins in TGF-beta signalling. Nature 383:691-696. [DOI] [PubMed] [Google Scholar]
- 11.Chen, X., E. Weisberg, V. Fridmacher, M. Watanabe, G. Naco, and M. Whitman. 1997. Smad4 and FAST-1 in the assembly of activin-responsive factor. Nature 389:85-89. [DOI] [PubMed] [Google Scholar]
- 12.Chen, Y., and A. F. Schier. 2001. The zebrafish Nodal signal Squint functions as a morphogen. Nature 411:607-610. [DOI] [PubMed] [Google Scholar]
- 13.Ciardiello, F., R. Dono, N. Kim, M. G. Persico, and D. S. Salomon. 1991. Expression of cripto, a novel gene of the epidermal growth factor gene family, leads to in vitro transformation of a normal mouse mammary epithelial cell line. Cancer Res. 51:1051-1054. [PubMed] [Google Scholar]
- 14.Ciardiello, F., N. Kim, T. Saeki, R. Dono, M. G. Persico, G. D. Plowman, J. Garrigues, S. Radke, G. J. Todaro, and D. S. Salomon. 1991. Differential expression of epidermal growth factor-related proteins in human colorectal tumors. Proc. Natl. Acad. Sci. USA 88:7792-7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conlon, F. L., K. M. Lyons, N. Takaesu, K. S. Barth, A. Kispert, B. Herrmann, and E. J. Robertson. 1994. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development 120:1919-1928. [DOI] [PubMed] [Google Scholar]
- 16.Constam, D. B., and E. J. Robertson. 1999. Regulation of bone morphogenetic protein activity by pro domains and proprotein convertases. J. Cell Biol. 144:139-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis, S., T. H. Aldrich, N. Y. Ip, N. Stahl, S. Scherer, T. Farruggella, P. S. DiStefano, R. Curtis, N. Panayotatos, H. Gascan, S. Chevalier, and G. D. Yancopolous. 1993. Released form of CNTF receptor alpha component as a soluble mediator of CNTF responses. Science 259:1736-1739. [DOI] [PubMed] [Google Scholar]
- 18.Ding, J., L. Yang, Y. T. Yan, A. Chen, N. Desai, A. Wynshaw-Boris, and M. M. Shen. 1998. Cripto is required for correct orientation of the anterior-posterior axis in the mouse embryo. Nature 395:702-707. [DOI] [PubMed] [Google Scholar]
- 19.Dunn, D. E., J. Yu, S. Nagarajan, M. Devetten, F. F. Weichold, M. E. Medof, N. S. Young, and J. M. Liu. 1996. A knock-out model of paroxysmal nocturnal hemoglobinuria: pig-a(−) hematopoiesis is reconstituted following intercellular transfer of GPI-anchored proteins. Proc. Natl. Acad. Sci. USA 93:7938-7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friess, H., Y. Yamanaka, M. Buchler, M. S. Kobrin, E. Tahara, and M. Korc. 1994. Cripto, a member of the epidermal growth factor family, is over-expressed in human pancreatic cancer and chronic pancreatitis. Int. J. Cancer 56:668-674. [DOI] [PubMed] [Google Scholar]
- 21.Gritsman, K., J. Zhang, S. Cheng, E. Heckscher, W. S. Talbot, and A. F. Schier. 1999. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell 97:121-132. [DOI] [PubMed] [Google Scholar]
- 22.Harris, R. J., and M. W. Spellman. 1993. O-linked fucose and other post-translational modifications unique to EGF modules. Glycobiology 3:219-224. [DOI] [PubMed] [Google Scholar]
- 23.Hicks, C., S. H. Johnston, G. diSibio, A. Collazo, T. F. Vogt, and G. Weinmaster. 2000. Fringe differentially modulates Jagged1 and Delta1 signalling through Notch1 and Notch2. Nat. Cell Biol. 2:515-520. [DOI] [PubMed] [Google Scholar]
- 24.Hoodless, P. A., M. Pye, C. Chazaud, E. Labbe, L. Attisano, J. Rossant, and J. L. Wrana. 2001. FoxH1 (Fast) functions to specify the anterior primitive streak in the mouse. Genes Dev. 15:1257-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jing, S., D. Wen, Y. Yu, P. L. Holst, Y. Luo, M. Fang, R. Tamir, L. Antonio, Z. Hu, R. Cupples, J. C. Louis, S. Hu, B. W. Altrock, and G. M. Fox. 1996. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell 85:1113-1124. [DOI] [PubMed] [Google Scholar]
- 26.Kannan, S., M. De Santis, M. Lohmeyer, D. J. Riese II, G. H. Smith, N. Hynes, M. Seno, R. Brandt, C. Bianco, G. Persico, N. Kenney, N. Normanno, I. Martinez-Lacaci, F. Ciardello, D. F. Stern, W. J. Gullick, and D. S. Salomon. 1997. Cripto enhances the tyrosine phosphorylation of Shc and activates mitogen-activated protein kinase (MAPK) in mammary epithelial cells. J. Biol. Chem. 272:3330-3335. [DOI] [PubMed] [Google Scholar]
- 27.Kooyman, D. L., G. W. Byrne, S. McClellan, D. Nielsen, M. Tone, H. Waldmann, T. M. Coffman, K. R. McCurry, J. L. Platt, and J. S. Logan. 1995. In vivo transfer of GPI-linked complement restriction factors from erythrocytes to the endothelium. Science 269:89-92. [DOI] [PubMed] [Google Scholar]
- 28.Kumar, A., V. Novoselov, A. J. Celeste, N. M. Wolfman, P. ten Dijke, and M. R. Kuehn. 2001. Nodal signaling uses activin and transforming growth factor-beta receptor-regulated Smads. J. Biol. Chem. 276:656-661. [DOI] [PubMed] [Google Scholar]
- 29.Kuniyasu, H., K. Yoshida, H. Yokozaki, W. Yasui, H. Ito, T. Toge, F. Ciardiello, M. G. Persico, T. Saeki, D. S. Salomon, and E. Tahara. 1991. Expression of cripto, a novel gene of the epidermal growth factor family, in human gastrointestinal carcinomas. Jpn. J. Cancer Res. 82:969-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis, K. A., P. C. Gray, A. L. Blount, L. A. MacConell, E. Wiater, L. M. Bilezikjian, and W. Vale. 2000. Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature 404:411-414. [DOI] [PubMed] [Google Scholar]
- 31.Liu, F., C. Pouponnot, and J. Massagué. 1997. Dual role of the Smad4/DPC4 tumor suppressor in TGFβ-inducible transcriptional complexes. Genes Dev. 11:3157-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu, F., F. Ventura, J. Doody, and J. Massague. 1995. Human type II receptor for bone morphogenic proteins (BMPs): extension of the two-kinase receptor model to the BMPs. Mol. Cell. Biol. 15:3479-3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massagué, J. 1998. TGF-β signal transduction. Annu. Rev. Biochem. 67:753-791. [DOI] [PubMed] [Google Scholar]
- 34.Massague, J., and Y. G. Chen. 2000. Controlling TGF-beta signaling. Genes Dev. 14:627-644. [PubMed] [Google Scholar]
- 35.Minchiotti, G., G. Manco, S. Parisi, C. T. Lago, F. Rosa, and M. G. Persico. 2001. Structure-function analysis of the EGF-CFC family member Cripto identifies residues essential for nodal signalling. Development 128:4501-4510. [DOI] [PubMed] [Google Scholar]
- 36.Minchiotti, G., S. Parisi, G. Liguori, M. Signore, G. Lania, E. D. Adamson, C. T. Lago, and M. G. Persico. 2000. Membrane-anchorage of Cripto protein by glycosylphosphatidylinositol and its distribution during early mouse development. Mech. Dev. 90:133-142. [DOI] [PubMed] [Google Scholar]
- 37.Moloney, D. J., and R. S. Haltiwanger. 1999. The O-linked fucose glycosylation pathway: identification and characterization of a uridine diphosphoglucose: fucose-beta1,3-glucosyltransferase activity from Chinese hamster ovary cells. Glycobiology 9:679-687. [DOI] [PubMed] [Google Scholar]
- 38.Moloney, D. J., V. M. Panin, S. H. Johnston, J. Chen, L. Shao, R. Wilson, Y. Wang, P. Stanley, K. D. Irvine, R. S. Haltiwanger, and T. F. Vogt. 2000. Fringe is a glycosyltransferase that modifies Notch. Nature 406:369-375. [DOI] [PubMed] [Google Scholar]
- 39.Moloney, D. J., L. H. Shair, F. M. Lu, J. Xia, R. Locke, K. L. Matta, and R. S. Haltiwanger. 2000. Mammalian Notch1 is modified with two unusual forms of O-linked glycosylation found on epidermal growth factor-like modules. J. Biol. Chem. 275:9604-9611. [DOI] [PubMed] [Google Scholar]
- 40.Mortensen, R. M., M. Zubiaur, E. J. Neer, and J. G. Seidman. 1991. Embryonic stem cells lacking a functional inhibitory G-protein subunit (αi2) produced by gene targeting of both alleles. Proc. Natl. Acad. Sci. USA 88:7036-7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norris, D. P., and E. J. Robertson. 1999. Asymmetric and node-specific nodal expression patterns are controlled by two distinct cis-acting regulatory elements. Genes Dev. 13:1575-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osada, S. I., Y. Saijoh, A. Frisch, C. Y. Yeo, H. Adachi, M. Watanabe, M. Whitman, H. Hamada, and C. V. Wright. 2000. Activin/nodal responsiveness and asymmetric expression of a Xenopus nodal-related gene converge on a FAST-regulated module in intron 1. Development 127:2503-2514. [DOI] [PubMed] [Google Scholar]
- 43.Paratcha, G., F. Ledda, L. Baars, M. Coulpier, V. Besset, J. Anders, R. Scott, and C. F. Ibanez. 2001. Released GFRalpha1 potentiates downstream signaling, neuronal survival, and differentiation via a novel mechanism of recruitment of c-Ret to lipid rafts. Neuron 29:171-184. [DOI] [PubMed] [Google Scholar]
- 44.Pogoda, H. M., L. Solnica-Krezel, W. Driever, and D. Meyer. 2000. The zebrafish forkhead transcription factor FoxH1/Fast1 is a modulator of nodal signaling required for organizer formation. Curr. Biol. 10:1041-1049. [DOI] [PubMed] [Google Scholar]
- 45.Qi, C. F., D. S. Liscia, N. Normanno, G. Merlino, G. R. Johnson, W. J. Gullick, F. Ciardiello, T. Saeki, R. Brandt, N. Kim, N. Kenney, and D. S. Salomon. 1994. Expression of transforming growth factor alpha, amphiregulin and cripto-1 in human breast carcinomas. Br. J. Cancer 69:903-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabbani, S. A., A. P. Mazar, S. M. Bernier, M. Haq, I. Bolivar, J. Henkin, and D. Goltzman. 1992. Structural requirements for the growth factor activity of the amino-terminal domain of urokinase. J. Biol. Chem. 267:14151-14156. [PubMed] [Google Scholar]
- 47.Reissmann, E., H. Jornvall, A. Blokzijl, O. Andersson, C. Chang, G. Minchiotti, M. G. Persico, C. F. Ibanez, and A. H. Brivanlou. 2001. The orphan receptor ALK7 and the activin receptor ALK4 mediate signaling by Nodal proteins during vertebrate development. Genes Dev. 15:2010-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saeki, T., K. Stromberg, C. F. Qi, W. J. Gullick, E. Tahara, N. Normanno, F. Ciardiello, N. Kenney, G. R. Johnson, and D. S. Salomon. 1992. Differential immunohistochemical detection of amphiregulin and cripto in human normal colon and colorectal tumors. Cancer Res. 52:3467-3473. [PubMed] [Google Scholar]
- 49.Saijoh, Y., H. Adachi, R. Sakuma, C. Y. Yeo, K. Yashiro, M. Watanabe, H. Hashiguchi, K. Mochida, S. Ohishi, M. Kawabata, K. Miyazono, M. Whitman, and H. Hamada. 2000. Left-right asymmetric expression of lefty2 and nodal is induced by a signaling pathway that includes the transcription factor FAST2. Mol. Cell 5:35-47. [DOI] [PubMed] [Google Scholar]
- 50.Salomon, D. S., C. Bianco, and M. De Santis. 1999. Cripto: a novel epidermal growth factor (EGF)-related peptide in mammary gland development and neoplasia. Bioessays 21:61-70. [DOI] [PubMed] [Google Scholar]
- 51.Schier, A. F., S. C. F. Neuhauss, K. A. Helde, W. S. Talbot, and W. Driever. 1997. The one-eyed pinhead gene functions in mesoderm and endoderm formation in zebrafish and interacts with no tail. Development 124:327-342. [DOI] [PubMed] [Google Scholar]
- 52.Schier, A. F., and M. M. Shen. 2000. Nodal signalling in vertebrate development. Nature 403:385-389. [DOI] [PubMed] [Google Scholar]
- 53.Schiffer, S. G., S. Foley, A. Kaffashan, X. Hronowski, A. E. Zichittella, C. Y. Yeo, K. Miatkowski, H. B. Adkins, B. Damon, M. Whitman, D. Salomon, M. Sanicola, and K. P. Williams. 2001. Fucosylation of Cripto is required for its ability to facilitate nodal signaling. J. Biol. Chem. 276:37769-37778. [DOI] [PubMed] [Google Scholar]
- 54.Shen, M. M., and A. F. Schier. 2000. The EGF-CFC gene family in vertebrate development. Trends Genet. 16:303-309. [DOI] [PubMed] [Google Scholar]
- 55.Shen, M. M., H. Wang, and P. Leder. 1997. A differential display strategy identifies Cryptic, a novel EGF-related gene expressed in the axial and lateral mesoderm during mouse gastrulation. Development 124:429-442. [DOI] [PubMed] [Google Scholar]
- 56.Shimizu, K., S. Chiba, T. Saito, K. Kumano, T. Takahashi, and H. Hirai. 2001. Manic fringe and lunatic fringe modify different sites of the Notch2 extracellular region, resulting in different signaling modulation. J. Biol. Chem. 276:25753-25758. [DOI] [PubMed] [Google Scholar]
- 57.Sirotkin, H. I., M. A. Gates, P. D. Kelly, A. F. Schier, and W. S. Talbot. 2000. Fast1 is required for the development of dorsal axial structures in zebrafish. Curr. Biol. 10:1051-1054. [DOI] [PubMed] [Google Scholar]
- 58.Strahle, U., S. Jesuthasan, P. Blader, P. Garcia-Villalba, K. Hatta, and P. W. Ingham. 1997. one-eyed pinhead is required for development of the ventral midline of the zebrafish (Danio rerio) neural tube. Genes Funct. 1:131-148. [DOI] [PubMed] [Google Scholar]
- 59.Treanor, J. J., L. Goodman, F. de Sauvage, D. M. Stone, K. T. Poulsen, C. D. Beck, C. Gray, M. P. Armanini, R. A. Pollock, F. Hefti, H. S. Phillips, A. Goddard, M. W. Moore, A. Buj-Bello, A. M. Davies, N. Asai, M. Takahashi, R. Vandlen, C. E. Henderson, and A. Rosenthal. 1996. Characterization of a multicomponent receptor for GDNF. Nature 382:80-83. [DOI] [PubMed] [Google Scholar]
- 60.Varlet, I., J. Collignon, and E. J. Robertson. 1997. nodal expression in the primitive endoderm is required for specification of the anterior axis during mouse gastrulation. Development 124:1033-1044. [DOI] [PubMed] [Google Scholar]
- 61.Wang, Y., L. Shao, S. Shi, R. J. Harris, M. W. Spellman, P. Stanley, and R. S. Haltiwanger. 2001. Modification of epidermal growth factor-like repeats with O-fucose. Molecular cloning and expression of a novel GDP-fucose protein O-fucosyltransferase. J. Biol. Chem. 276:40338-40345. [DOI] [PubMed] [Google Scholar]
- 62.Watanabe, M., and M. Whitman. 1999. FAST-1 is a key maternal effector of mesoderm inducers in the early Xenopus embryo. Development 126:5621-5634. [DOI] [PubMed] [Google Scholar]
- 63.Whitman, M. 2001. Nodal signaling in early vertebrate embryos. Themes and variations. Dev. Cell 1:605-617. [DOI] [PubMed] [Google Scholar]
- 64.Xu, C., G. Liguori, M. G. Persico, and E. D. Adamson. 1999. Abrogation of the Cripto gene in mouse leads to failure of postgastrulation morphogenesis and lack of differentiation of cardiomyocytes. Development 126:483-494. [DOI] [PubMed] [Google Scholar]
- 65.Yamamoto, M., C. Meno, Y. Sakai, H. Shiratori, K. Mochida, Y. Ikawa, Y. Saijoh, and H. Hamada. 2001. The transcription factor FoxH1 (FAST) mediates Nodal signaling during anterior-posterior patterning and node formation in the mouse. Genes Dev. 15:1242-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yeo, C., and M. Whitman. 2001. Nodal signals to Smads through Cripto-dependent and Cripto-independent mechanisms. Mol. Cell 7:949-957. [DOI] [PubMed] [Google Scholar]
- 67.Zhang, J., W. S. Talbot, and A. F. Schier. 1998. Positional cloning identifies zebrafish one-eyed pinhead as a permissive EGF-related ligand required during gastrulation. Cell 92:241-251. [DOI] [PubMed] [Google Scholar]
- 68.Zhou, X., H. Sasaki, L. Lowe, B. L. Hogan, and M. R. Kuehn. 1993. Nodal is a novel TGF-beta-like gene expressed in the mouse node during gastrulation. Nature 361:543-547. [DOI] [PubMed] [Google Scholar]