Abstract
In mammals, the catabolic pathway of phenylalanine and tyrosine is found in liver (hepatocytes) and kidney (proximal tubular cells). There are well-described human diseases associated with deficiencies of all enzymes in this pathway except for maleylacetoacetate isomerase (MAAI), which converts maleylacetoacetate (MAA) to fumarylacetoacetate (FAA). MAAI is also known as glutathione transferase zeta (GSTZ1). Here, we describe the phenotype of mice with a targeted deletion of the MAAI (GSTZ1) gene. MAAI-deficient mice accumulated FAA and succinylacetone in urine but appeared otherwise healthy. This observation suggested that either accumulating MAA is not toxic or an alternate pathway for MAA metabolism exists. A complete redundancy of MAAI could be ruled out because substrate overload of the tyrosine catabolic pathway (administration of homogentisic acid, phenylalanine, or tyrosine) resulted in renal and hepatic damage. However, evidence for a partial bypass of MAAI activity was also found. Mice doubly mutant for MAAI and fumarylacetoacetate hydrolase (FAH) died rapidly on a normal diet, indicating that MAA could be isomerized to FAA in the absence of MAAI. Double mutants showed predominant renal injury, indicating that this organ is the primary target for the accumulated compound(s) resulting from MAAI deficiency. A glutathione-mediated isomerization of MAA to FAA independent of MAAI enzyme was demonstrated in vitro. This nonenzymatic bypass is likely responsible for the lack of a phenotype in nonstressed MAAI mutant mice.
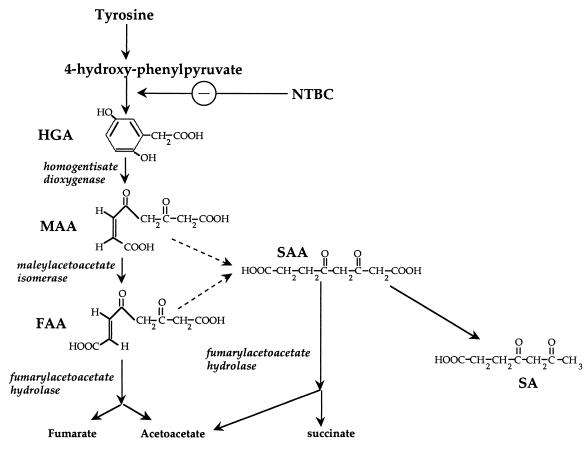
In mammals, the amino acids phenylalanine and tyrosine are both ketogenic and glucogenic. The complete phenylalanine/tyrosine degradation pathway (Fig. 1) is expressed in hepatocytes and renal proximal tubular cells (26). It consists of six sequential enzymatic reactions resulting in the final products acetoacetate (ketogenic) and fumaric acid (glucogenic). Human genetic diseases corresponding to deficiencies of all enzymes in this pathway have been reported, with the exception of the fifth enzyme, maleylacetoacetate isomerase (MAAI). Of these disorders, phenylketonuria is the most common, affecting 1 in 15,000 newborns in the United States (32). Alkaptonuria (deficiency of homogentisic acid [HGA] dioxygenase [HGD]) was the first autosomal recessive disease reported in humans (10, 15).
FIG. 1.
Catabolic pathway of tyrosine.
Hereditary tyrosinemia type I (HT1) is the most severe of the tyrosine catabolic defects and results from deficiency of fumarylacetoacetate hydrolase (FAH) (16, 22). Patients suffer from liver failure and renal proximal tubular dysfunction. In addition, they have a very high rate of hepatocellular carcinoma (3, 31). FAH deficiency results in the accumulation of fumarylacetoacetate (FAA) and possibly maleylacetoacetate (MAA) (17, 26). FAA is very electrophilic and induces oxidative damage by reacting with sulfhydryl groups (27, 42). Therefore, it is highly cytotoxic and is responsible for the liver and renal damage in HT1. In addition, FAA is also mutagenic and hence responsible for the hepatocarcinoma seen in HT1 (19, 25). Both MAA and FAA can be reduced to succinylacetoacetate (SAA) and further decarboxylated to succinylacetone (SA), the diagnostic compound for HT1 (Fig. 1).
MAAI is a 29-kDa cytoplasmic protein which isomerizes MAA to FAA in a GSH-dependent fashion (12). MAAI was also isolated independently as GSH transferase zeta (GSTZ1) (6). In addition to its function in tyrosine degradation, MAAI/GSTZ1 therefore is involved in detoxification of xenobiotic compounds such as dichloroacetic acid (DCA) (7, 39).
Deficiency of MAAI is predicted to result in the accumulation of MAA (cis isomer), and this compound is expected to have toxicity similar to that of its trans isomer, FAA (26, 36). However, to date only one case of putative MAAI deficiency in humans has been reported. This patient had liver failure and kidney dysfunction similar to that in tyrosinemia type I but had normal FAH enzyme activity, and there was no SA accumulation (4). This biochemical phenotype was consistent with MAAI deficiency, because the FAH enzyme not only can hydrolyze FAA but also can convert SAA (formed from FAA and MAA) to succinic acid and acetoacetate and thereby prevent SA formation (Fig. 1) (20, 24). Previously the MAAI gene and MAAI activity were tested in patients manifesting “pseudotyrosinemia.” However, no individuals with MAAI mutations were found, although several cDNA polymorphisms were discovered (11). Therefore, it remains unclear whether MAAI deficiency actually exists in human patients and what the phenotype of human MAAI deficiency may be.
It was also shown previously that a knockout of MAAI in the fungus Aspergillus nidulans blocks the growth of this microorganism on media supplemented with phenylalanine. This indicates that accumulation of MAA is toxic for the fungus (12). However, the phenotype of MAAI deficiency in Aspergillus was less severe than that of FAH deficiency, in agreement with a report indicating that MAA may be less toxic than FAA (19).
In order to better define the in vivo function of mammalian MAAI, we generated mice with a targeted deletion of MAAI. Here we report the phenotype of MAAI knockout mice and describe the existence of a alternate pathway for the isomerization of MAA.
MATERIALS AND METHODS
Targeting construct and generation of mutant mice.
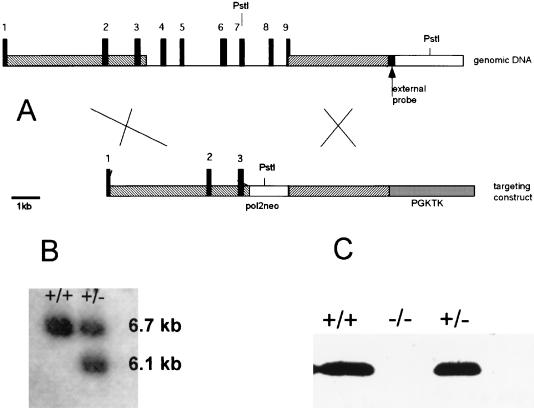
The murine MAAI gene was isolated from a λ-DASH II library (Stratagene) by using a full-length cDNA probe encoding the mouse MAAI (EST AA139820) (12). A 16-kb genomic DNA fragment was cloned into pBluescript as a NotI insert. It contained DNA from the first intron to 5 kb after the stop codon of the MAAI gene. The neo cassette was inserted between the HindIII site in intron 3 and the SalI site 29 nucleotides before the stop codon. The herpes simplex virus thymidine kinase gene (with the PGK promoter) was inserted into a HindIII site about 5 kb downstream from the stop codon. (Fig. 2A).
FIG. 2.
(A) Schematic representation of MAAI knockout. (B) Southern blot of wild-type (+/+) and heterozygote (+/−) DNA. The membrane was hybridized with the external probe shown in panel A. (C) Western blot with liver protein extracts from wild-type (+/+), heterozygote (+/−), and mutant (−/−) mice performed with MAAI antibody.
The plasmid was then linearized by NotI digestion, and 50 μg was electroporated into 129Sv4 ES cells. Twenty-four hours later, selective medium containing G418 and ganciclovir was added to the cells. After 250 colonies of 129Sv4 embryonic stem cells had been screened, seven targeted clones were identified by Southern blotting (Fig. 2B). Two of these clones were injected into blastocysts of the C57/BL6 strain. Chimeric offspring were crossed with 129Sv4 mice, and heterozygotes were used to establish the mouse colony.
PCR genotyping.
To genotype mice, we used three oligonucleotides in the reaction mixture to get two amplified products. With the oligonucleotide MAIM27 (5′-CCAGAGCATCCCAGCCAATGCG-3′), annealing in the last intron of MAAI gene, and oligonucleotide MAIM3 (5′-GGAGCTAAGTCCTCAGCTCGG-3′), which overlaps the stop codon, we amplified a 289-bp fragment from the wild-type allele. Oligonucleotides MAIM3 and BGHPA (5′-GGAAGGTGCCACTCCCACTGTCC-3′), which anneals in the polyadenylation signal of the bovine growth hormone gene located in the neo cassette, amplified a 220-bp fragment from the mutant allele. The conditions were 95°C for 3 min, 35 cycles of 20 s at 95°C, 30 s at 62°C, and 20 s at 72°C, and a last extension of 5 min at 72°C in a Perkin-Elmer thermal cycler 480. The oligonucleotide concentrations were 1 μM for MAIM 3 and BGHPA and 0.1 μM for MAIM27. DNA was isolated from the last 5 mm of the mouse tail according to standard protocols.
Northern, Southern, and Western blotting.
RNA, DNA, and protein blotting was performed by standard methods. Radioactive probes were labeled with a random primed DNA-labeling kit (Roche). Chicken antibodies against MAAI were obtained from Aveslab, Tigard, Oreg. Chickens were immunized with His-tag-purified proteins. Secondary antibodies were horseradish peroxidase-conjugated goat anti-chicken immunoglobulin (Aveslab). The signal was developed by using the Western blot chemiluminescence reagent Renaissance (NEN Life Science Products).
Assay for MAAI activity.
MAAI activity was measured spectrophotometrically in the presence of excess amounts of HGD and FAH. The enzymatic assay was performed as previously reported with slight modifications (11, 12, 14).
There is no commercial substrate (MAA) to assay the MAAI enzymatic activity, and so it is necessary to synthesize MAA enzymatically. We synthesized MAA from HGA, in situ, in the reaction cuvette by using HGD activity. This enzyme converts HGA into MAA, and it was possible to monitor the reaction by measuring the increase in absorbance (330 nm). In the presence of MAAI, MAA was then converted to FAA. These compounds have similar absorbances (at neutral pH). However, if FAH is in the reaction mixture, FAA is converted into fumarate and acetoacetate with a decrease in the absorbance. The reaction mixture contained 50 mM potassium phosphate buffer (pH 7), 1 mM ascorbic acid, 50 μM ferrous sulfate, 50 μM reduced GSH, 100 μM HGA, about 50 μg of crude protein extract of Escherichia coli expressing human HGD, and 25 μg of purified FAH (recombinant His tag protein in E. coli; a gift of Kara Manning [2]). The reaction was initialized by adding HGA. GSH concentration was changed in some experiments. To determine the effect of GSH in MAA isomerization, we used liver protein extract from MAAI-deficient mice as the source of HGD and FAH.
When necessary, 2 μg of recombinant human MAAI (11) was added to the reaction mixture. Recombinant MAAI and FAH were purified by using the pQE-Histag expression system from Qiagen.
Amino acid and organic acid analysis.
Amino acid analysis was performed on urine and serum from mice on a Beckman 6300 automated amino acid analyzer using postcolumn ninhydrin derivatization.
Organic acids analysis was performed on a Hewlett Packard 5890 gas chromatograph (GC)/5970 mass spectrometer (MS) on mouse urine treated as follows: to 500 μl of frozen sample was added about 50 mg of hydroxylamine hydrochloride, and 100 μl of 6 N HCl was added during thawing. The reaction mixture was transferred to a 13- by 100-mm screw-cap tube, which was capped, heated to 85°C for 90 min, and then cooled to room temperature and poured onto 1-ml Chem Elut columns (12198002; Varian). Acids and neutrals were extracted with 5 ml of ethyl acetate, dried with nitrogen at 50 to 60°C, and treated with BSTFA [N,O,-bis(trimethylsilyl)trifluoroacetamide]-1% TMCS (trimethylchlorosilane) (Pierce) at 85°C for 20 min prior to GC-MS analysis. The full-scan GC-MS data were reduced by extracting ion chromatograms for the cyclized oximation products of SA, maleylacetone, fumarylacetone, MAA, and FAA as their trimethylsilyl derivatives (40, 41).
GSH determination.
GSH content in liver was determined by using a GSH assay kit (Calbiochem). Mice were starved for 18 h and lethally anaesthetized with 2,2,2-tribromoethanol (Avertin), and the liver was treated as described by the assay kit manufacturer.
RESULTS
Phenotype of MAAI-deficient mice on a regular diet.
Mutant mice were born at the expected Mendelian ratios and had no obvious phenotype under standard nutritional conditions, i.e., mouse chow ad libitum. The livers and kidneys of homozygous mutants were analyzed by Western blotting using MAAI antibodies. This test showed a complete absence of MAAI (Fig. 2C), consistent with deletion of the majority of the gene. MAAI enzyme activity was also completely obliterated, indicating that liver cell extracts did not contain an alternate enzyme capable of metabolizing MAA to FAA in the in vitro assay. The MAAI-deficient mice were monitored for as long as 22 months and had the same weights and fertility as littermate controls. No hepatic or other tumors developed. Homozygous mutant MAAI mice could breed and produced litter sizes (4.3 ± 0.41, n = 20) similar to those of wild-type controls (3.93 ± 0.45, n = 13). Histology was performed on the liver, kidney, lung, heart, spleen, pancreas, prostate, seminal glands, muscle, small intestine, thymus, and testis of 2- and 6-month-old mutant mice, and no abnormalities were seen (data not shown). In addition, liver function tests (Table 1) and blood and urine amino acid analysis did not reveal any pathology associated with MAAI deficiency. Hepatic GSH and blood fatty acid levels were similar in mutant mice and controls.
TABLE 1.
Biochemical parameters of wild-type and mutant micea
| Diet and mouse type | Concn of:
|
|||
|---|---|---|---|---|
| ALT (U/liter) | Bilirubin U (mg/dl) | Bilirubin C (mg/dl) | Creatinine (mg/liter) | |
| RD | ||||
| Wild type | 21 ± 5 | <0.1 | <0.1 | 0.22 ± 0.08 |
| Mutant | 19 ± 4 | <0.1 | <0.1 | 0.18 ± 0.06 |
| RD + Phe | ||||
| Wild type | 25 ± 4 | <0.1 | <0.1 | 0.13 ± 0.01 |
| Mutant | 1,897 ± 17 | <0.1 | 0.56 ± 0.4 | 0.71 ± 0.7 |
Six mice per group. RD, regular diet; Phe, phenylalanine (20 mg/ml) added to drinking water for 10 days; ALT, alanine transaminase; bilirubin U and C, free and conjugated bilirubin, respectively.
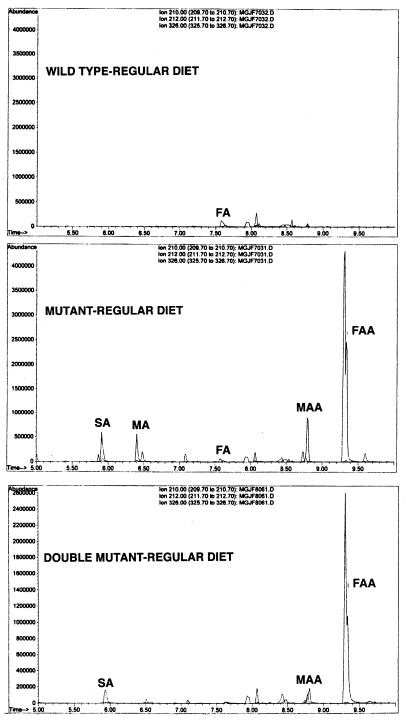
Despite the absence of a major phenotype, several abnormalities were discovered at the molecular and biochemical level. First, GC-MS analysis of the urine from mutant mice showed the presence of both FAA and SA (Fig. 3), compounds which are not present in normal controls. This indicated that intermediates of tyrosine metabolism did accumulate in mutant mice and that MAA could be converted to FAA. However, this conversion likely occurs outside the liver, because any FAH present in hepatocytes would hydrolyze the FAA to fumarate and acetoacetate.
FIG. 3.
Gas chromatography of urine organic acids of wild-type, MAAI mutant, and MAAI/FAH double-mutant mice. Urine from three mice was pooled to avoid individual and time differences. FA, fumarylacetone; MA, maleylacetone.
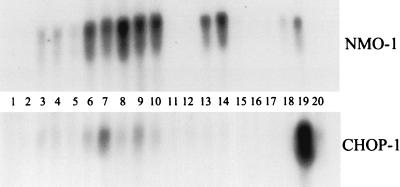
Mutant mice also had a small induction of NMO-1 mRNA (18) levels in liver, a marker for oxidative stress (Fig. 4, lanes 1 to 5). This indicates that oxidative damage occurs in MAAI knockout mice but at a level which causes no clinical effects, such as hepatocyte death or hepatocarcinoma.
FIG. 4.
Liver RNA Northern blot from mice on regular or high-phenylalanine (20 mg/ml in drinking water) diet. Lanes: 1 and 2, wild type on regular diet; 3 to 5, MAAI mutant on regular diet; 6, FAH mutant on regular diet without NTBC for 6 days; 7, FAH mutant on regular diet without NTBC for 2 weeks; 8 to 10, MAAI/FAH double mutant on regular diet without NTBC for 6 days; 11 and 12, wild type on high-phenylalanine diet; 13 and 14, mutant mice on high-phenylalanine diet; 15, MAAI heterozygote on high-phenylalanine diet; 16, wild type on high-phenylalanine diet plus NTBC; 17; MAAI mutant on high-phenylalanine diet plus NTBC; 18, mice 18 months old on regular diet; 19, MAAI mutant treated with DEM plus HGA; 20, wild type treated with DEM plus HGA.
Together these findings show that the phenotype of MAAI deficiency is markedly more mild than that of FAH deficiency. This could be explained in two different ways. First, it is possible that accumulating MAA (or any derivative) is not cytotoxic despite the chemical similarities between MAA and FAA. Second, MAA may be metabolized by an alternate pathway and hence not accumulate to cytotoxic levels.
Effects of overloading the phenylalanine/tyrosine catabolic pathway.
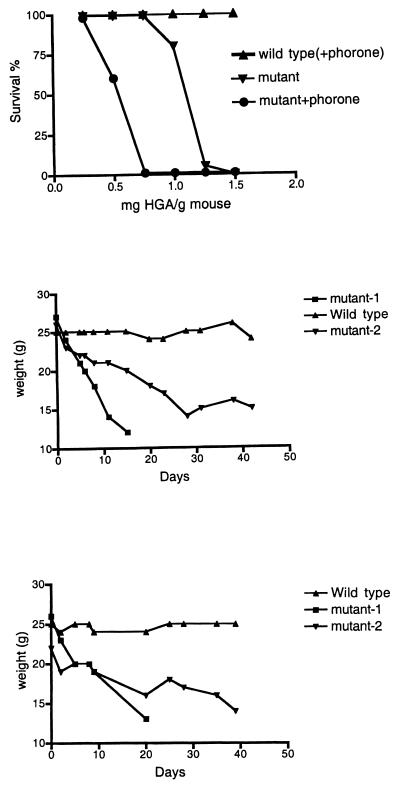
We wished to determine whether MAAI is completely redundant for tyrosine catabolism in mice and hypothesized that the lack of phenotype may be due to the relatively low phenylalanine and tyrosine content in laboratory mouse chow. Mutant mice and controls were therefore given HGA, the metabolite immediately upstream of MAA in the tyrosine degradation pathway. HGA was much more toxic for MAAI mutant mice than controls (Fig. 5A). Also, supplementation of the mouse food with phenylalanine (or tyrosine) or drinking water with phenylalanine resulted in rapid loss of weight and death in mutants, while wild-type mice remained healthy (Fig. 5). The survival time of mutants depended on the phenylalanine (Phe) dose. Typically, mutants survived for about 20 to 30 days when the drinking water was supplemented with 20 mg of Phe per ml. Survival periods varied from 5 to 50 days, with some mutants dying rapidly and others dying much later (Fig. 5B). Most of this variability could be explained by gender and strain differences: females were more resistant to Phe overloading than males (data not shown), and C57/BL6 mutants were more resistant than 129SvJ mutants (data not shown). With the Phe diet, blood amino acid analysis showed no differences between wild-type and mutant mice, but liver function tests indicated hepatocellular damage (elevated transaminases) and renal injury (increased creatinine) (Table 1). Histopathology revealed necrosis, micro- and macrosteatosis, and some calcification in the liver and epithelial hyperplasia, vacuolization, apoptosis (determined by tunnel assay), and necrosis in the kidney. Addition of NTBC [2-(2-nitro-4-fluoromethylbenzoyl)-1,3-cyclohexanedione] to the drinking water prevented this phenotype, demonstrating the relation of this phenotype and overloading of phenylalanine catabolic pathway.
FIG. 5.
(A) Sensitivity to HGA (intraperitoneal injections). Ten mice were injected for each point. (B) Mouse weight variation on phenylalanine (20 mg/ml) in drinking water. (C) Mouse weight variation on bean diet. Diet was 25% (each) soy beans, peas, lentils, and white beans.
On a high-phenylalanine diet, the expression of a liver RNA induced by oxidative stress (NMO-1 RNA) was increased, but unlike in FAH-deficient mice, the expression of the CHOP-1 gene, which is induced by DNA damage (18), was not elevated (Fig. 4).
To simulate nutritional conditions experienced by mice in the wild, experimental animals were fed a diet consisting of beans and lentils, which are high in protein. Under these conditions, MAAI mutant mice also developed renal and hepatic injury and had reduced survival (Fig. 5C). This indicates that MAAI may be redundant for tyrosine catabolism in the laboratory setting but not in nature.
The increased sensitivity to overload of the Phe/Tyr catabolic pathway described above demonstrated that some toxic compound(s) results from MAAI deficiency in spite of the possible existence of a bypass for the enzyme activity. We speculate that this compound is MAA (or some derivative), but to date we have not succeeded in its direct identification. GC-MS analysis of urine of mutant and wild-type mice on a high-Phe diet did not reveal any metabolites which were not also present in mice on a regular diet.
Figure 6B and C illustrate the models for tyrosine catabolism in MAAI mutant mice on a regular and Phe loading diet.
FIG. 6.
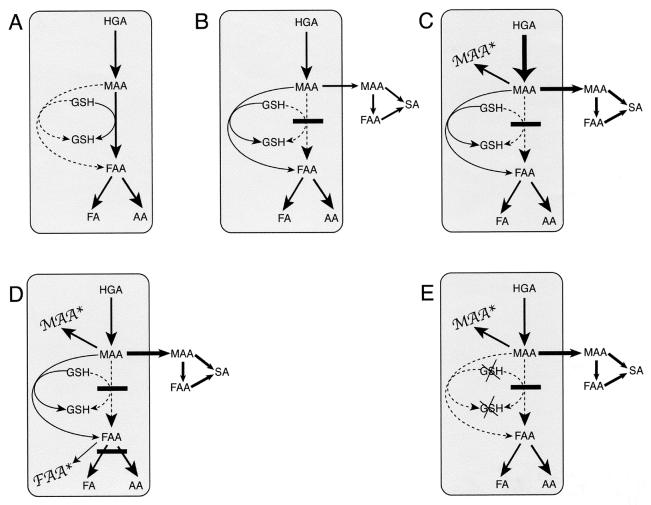
Schematic representation of metabolites in the distal tyrosine catabolic pathway. FA, fumaric acid; AA, acetoacetate. Arrows with solid lines indicate active reactions, and the thickness of the arrow correlates to the relative quantities. Arrows with dashed lines indicate blocked reactions. (A) Normal tyrosine metabolism distal to HGA. MAA is converted to FAA by MAAI in a GSH-dependent reaction. FAA is hydrolyzed to FA and AA by FAH. (B) MAAI deficiency with normal diet. Modest amounts of MAA are formed and then isomerized to FAA by a nonenzymatic bypass reaction. Small amounts of MAA accumulate, leave the hepatocyte, and are converted to FAA and SA, which appear in the urine. (C) MAAI deficiency on a high-phenylalanine diet. Large amounts of MAA are produced from the dietary load, thus overwhelming the nonenzymatic bypass of MAAI. Free MAA accumulates (MAA∗) and results in cytotoxicity. (D) FAH/MAI double mutants on a regular diet. MAA is converted to FAA by the nonenzymatic bypass. MAA and FAA accumulate (MAA∗ and FAA∗) and are cytotoxic. (E) MAI deficiency with GSH depletion. MAA cannot be converted to FAA, because both MAI and the nonenzymatic pathway are defective. MAA accumulates (MAA∗) and is cytotoxic.
Phenotype of MAAI/FAH double-mutant mice.
The lack of phenotype in MAAI mutant mice on a regular laboratory diet could be explained if a low-efficiency pathway for the isomerization of MAA existed. To address this question, mice doubly mutant for MAAI and FAH were generated by cross-breeding the two mutant strains. FAH-deficient mice have a severe phenotype and can survive only with treatment by NTBC, an inhibitor of the Phe/Tyr catabolic pathway upstream of FAH (Fig. 1) (18, 23). In absence of this inhibitor, the accumulating metabolites FAA and SA produce a chronic injury in the liver and kidney resulting in death in 20 to 40 days. It has been shown that loss-of-function mutations in the HGD or 4-hydroxy-phenylpyruvate dioxygenase genes coding for enzymes upstream of FAH in the pathway also rescue FAH deficiency (9, 25).
Two possibilities for the phenotype of double mutants were considered. If the mild phenotype was due to low toxicity of accumulating MAA rather than an MAAI-independent bypass, no difference between MAAI mutants and MAAI/FAH double mutants was expected. MAAI deficiency would protect the animals from FAH deficiency. On the other hand, if MAA could be isomerized to FAA even in the absence of MAAI, the double-mutant mice were predicted to have a severe, HT1-like phenotype. An intermediate phenotype might result if the bypass was only partial.
Interestingly, double-mutant animals developed a phenotype even more severe than purely FAH-deficient mice when taken off NTBC. Double mutants died after about 1 week (range, 5 to 11 days), and blood tests showed severe liver and kidney injury (Table 2). The marked elevation of plasma creatinine indicated severe renal glomerular dysfunction, a phenotype not seen in FAH-deficient animals. In addition, histology revealed acute necrosis in kidney, not found in FAH mutant mice. Very high concentrations of amino acids in the urine of double-mutant mice confirmed the severe kidney malfunction (Table 3). In the liver, a strong induction of NMO-1 mRNA and a small induction of CHOP-1 were found (Fig. 4, lanes 8 to 10).
TABLE 2.
Biochemical parameters of mutant micea
| Mutation | Concn of:
|
|||
|---|---|---|---|---|
| ALT (U/liter) | Bilirubin U (mg/dl) | Bilirubin C (mg/dl) | Creatinine (mg/liter) | |
| MAAI | 20 ± 14 | <0.1 | <0.1 | 0.16 ± 0.05 |
| FAH | 78 ± 54 | 0.12 ± 0.4 | <0.1 | 0.25 ± 0.06 |
| MAAI/FAH | 75 ± 49 | <0.1 | 0.36 ± 0.7 | 1.42 ± 0.7 |
Six mice per group. Mice receiving NTBC were taken off NTBC for 6 days before harvesting. ALT, alanine transaminase; bilirubin U and C, free and conjugated bilirubin, respectively.
TABLE 3.
Urine amino acids (n = 3)
| Amino acid | Concn (μM) in mice with genotype
|
||
|---|---|---|---|
| +/+ | MAAI | MAAI/FAH | |
| Phe | 69 | 45 | 123 |
| Tyr | 103 | 232 | 3,377 |
| Met | 59 | 75 | 402 |
| Ala | 268 | 228 | 1,387 |
| Gly | 457 | 446 | 1,531 |
| Leu | 282 | 285 | 1,263 |
| Arg | 88 | 166 | 312 |
| Gln | 0 | 320 | 4,963 |
| Orn | 53 | 33 | 260 |
| Glu | 284 | 72 | 0 |
| Tau | 511 | 715 | 1,723 |
| Pho | 1,345 | 1,134 | 1,970 |
| Arg | 88 | 170 | 312 |
| His | 56 | 59 | 824 |
| Lys | 148 | 136 | 797 |
| Asp | 207 | 172 | 1,075 |
| Thr | 0 | 237 | 2,037 |
| GlcNH2a | 199 | 345 | 747 |
GlcNH2, glucosamine.
These facts clearly showed two things. First, there is a bypass for MAAI activity in hepatocytes and renal tubular cells. Second, MAAI deficiency must result in the accumulation of toxic compounds not present in pure FAH deficiency, because double mutants had a more severe and also different phenotype than FAH-deficient mice. The severity of renal damage indicates that the kidney rather than the liver is the main target organ for these compounds.
Tyrosine metabolism in double-mutant mice is schematically shown in Fig. 6D.
GSH is responsible for a nonenzymatic bypass of MAAI activity.
MAAI has been described as a new class of GSH S-transferase enzymes, class Z (GSTZ1) (6). However, a clear GSH S-transferase activity of the MAAI enzyme has not been shown to date (6, 8). The crystal structure of MAAI is in general similar to other GSH S-transferase structures, but there are some special features in MAAI which make this enzyme different from the GST superfamily enzymes (29, 37).
GST enzymes are known to transfer some substrates to the thiol group of the GSH molecule (5, 30). However, GSH binds to MAA spontaneously, and the bound MAA-GSH is the substrate of the MAAI enzyme. Therefore, a transferase reaction is not needed. After isomerization, free GSH and FAA are the products of the reaction (21, 33-35). MAA can bind GSH with or without enzyme and it has also been shown that GSH alone is able to catalyze the conversion of MAA to FAA in vitro (35). This reaction occurs slowly but it could be responsible for the bypass of MAAI enzymatic activity.
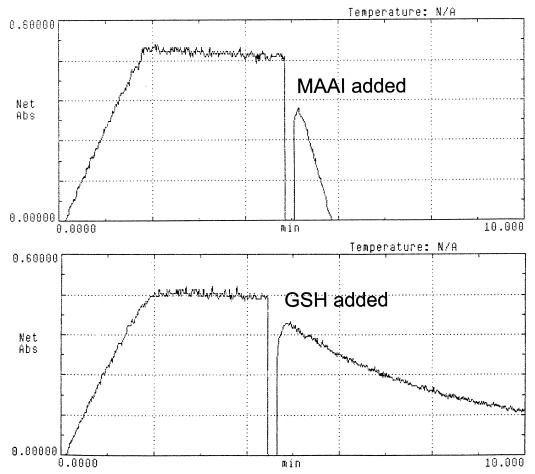
We were able to demonstrate the enzyme-independent bypass of MAAI activity by increasing the GSH concentration in the in vitro reaction using protein extract from MAAI mutant mouse liver (Fig. 7). GSH concentrations lower than 1 mM did not result in the chemical isomerization of MAA to FAA. However, higher concentrations of GSH increased the speed of the reaction until a maximal effect was achieved with 10 mM GSH. GSH at 5 mM produced almost the same effect as 10 mM GSH. Dithiothreitol had a smaller effect in the reaction than GSH, but it was also possible to obtain in vitro conversion of MAA to FAA with 10 mM dithiothreitol. The GSH concentration in the liver of wild-type mice and MAAI mutant mice was not significantly different. It ranged from 3 to 7 mM and hence could be sufficient to prevent accumulation of MAA in the mutant mice. The role for GSH in the nonenzymatic bypass of MAA isomerization was confirmed in vivo by using known GSH-depleting agents (diethylmaleate [DEM] and phorone) (28). One hour after intraperitoneal injections of DEM (0.5 mg/g of mouse body weight) or phorone (0.25 mg/g), the GSH content in the liver decreased to 20 and <5%, respectively, in both wild-type and mutant mice. GSH-depleted mice were then injected with 0.75 mg of HGA per g of body weight 1 h later. This resulted in death of 100% of the mutant mice but had no effect in controls (Fig. 5A). Interestingly, these conditions resulted in a marked induction of CHOP-1 gene expression, a marker of DNA damage, in mutant mice (Fig. 4, lanes 19 to 20). This suggests that MAA (or any derivative) can act as a DNA-damaging agent in the absence of GSH.
FIG. 7.
MAAI activity in MAAI mutant mouse liver. Activity was restored only when 2 μg of purified recombinant MAAI or 10 mM GSH was added to the reaction mixture. Gaps indicate the time of the addition.
Fig. 1E illustrates tyrosine degradation in GSH-depleted MAAI mutant mice.
DISCUSSION
Is tyrosine degradation the main function of the MAAI protein?
The studies of MAAI knockout mice reported here show that the MAAI gene is not an essential gene for the catabolism of phenylalanine and tyrosine in mice under laboratory conditions. It has also has been shown by others that MAAI is able to catalyze the conversion of DCA to glyoxylate (7, 38). Therefore, MAAI has been proposed as a precursor in the evolution of some dehalogenases (1). One could hypothesize that detoxification of xenobiotics and not tyrosine metabolism may be the major function of MAAI. However, in many microorganisms, such as A. nidulans, the MAAI gene is clustered with FAH and HGD (GenBank database). This strongly suggests that tyrosine degradation is actually the major role for the MAAI enzyme (11, 13, 18). Consistent with this, we have shown that MAAI knockout mice become ill when fed a “natural” diet. In the wild, the predominant food source for mice is grass (and crops), but at times it can be legumes (beans, lentils, and peas), which are abundant in many places in Eurasia, where this family of rodents is native. Legumes are very rich in protein (25 to 40%, wt/wt). We therefore speculate that MAAI is indeed essential for survival under some nutritional conditions but dispensable in others.
Human phenotype of MAAI deficiency.
Humans, at least in developed countries, are unlikely to experience nutritional stress severe enough to be affected by MAAI deficiency if this gene defect exists in the human population. MAAI knockout mice stressed with high levels of tyrosine showed primarily renal pathology. Therefore, the most likely manifestation of MAAI deficiency in humans would be renal tubular dysfunction or renal Fanconi syndrome. In addition, the results obtained here with mice predict the presence of FAA and SA in urine of MAAI-deficient individuals. This is in contrast to previous reports which suggested that MAAI-deficient patients would have a phenotype similar to FAH deficiency and would lack SA excretion.
Our data showed clearly that hepatic GSH levels are essential for the MAAI bypass reaction. Therefore, MAAI deficiency may manifest itself as hypersensitivity to GSH-depleting drugs, such as acetaminophen. In humans, MAAI may be important primarily as a pharmacogenetic locus.
Although genetic MAAI deficiency may be very rare or nonexistent, pharmacologically induced MAAI deficiency may be clinically important. It has been shown that DCA, a compound which is in clinical trials for the treatment of lactic acidosis, is detoxified by MAAI (7, 38). It is also known that DCA inactivates MAAI and hence can induce hepatic MAAI deficiency. It can be speculated that some of the side effects of DCA, particularly hepatic injury, might be due to accumulating MAA. If true, the side effects of DCA may be ameliorated by blocking tyrosine degradation upstream of MAAI by using the drug NTBC.
MAAI in detoxification of xenobiotics.
Although MAAI/GSTZ1 clearly can be involved in drug metabolism, we have not yet used the knockout model described here to test its importance in the detoxification of various drugs. It will be particularly interesting to determine whether DCA is differentially toxic to these mice in a long-term exposure. As mentioned above, acetaminophen is another compound which may cause liver injury in MAAI mutant mice.
Acknowledgments
This work was supported by NIH grant RO1-DK48252 to M.G. J.M.F.-C. was in part supported by an EMBO fellowship (ALTF-746).
We thank Ching-Nan Ou for liver function analysis and Angela Major and Billie Smith for their support with histological analysis. We thank Muhsen Al-Dhalimy for technical support.
REFERENCES
- 1.Anandarajah, K., P. M. Kiefer, Jr., B. S. Donohoe, and S. D. Copley. 2000. Recruitment of a double bond isomerase to serve as a reductive dehalogenase during biodegradation of pentachlorophenol. Biochemistry 39:5303-5311. [DOI] [PubMed] [Google Scholar]
- 2.Bateman, R. L., P. Bhanumoorthy, J. F. Witte, R. W. McClard, M. Grompe, and D. E. Timm. 2001. Mechanistic inferences from the crystal structure of fumarylacetoacetate hydrolase with a bound phosphorus-based inhibitor. J. Biol. Chem. 276:15284-15291. [DOI] [PubMed] [Google Scholar]
- 3.Belanger, L., M. Belanger, L. Prive, J. Larochelle, M. Tremblay, and G. Aubin. 1973. Hereditary tyrosinemia and alpha-1-fetoprotein. I. Clinical value of alpha-fetoprotein in hereditary tyrosinemia. Pathol. Biol. (Paris) 21:449-455. [PubMed] [Google Scholar]
- 4.Berger, R., K. Michals, J. Galbraeth, and R. Matalon. 1988. Tyrosinemia type Ib caused by maleylacetoacetate isomerase deficiency: a new enzyme defect. Pediatr. Res. 23:328A. [Google Scholar]
- 5.Berhane, K., M. Widersten, A. Engstrom, J. W. Kozarich, and B. Mannervik. 1994. Detoxication of base propenals and other alpha, beta-unsaturated aldehyde products of radical reactions and lipid peroxidation by human glutathione transferases. Proc. Natl. Acad. Sci. USA 91:1480-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Board, P. G., R. T. Baker, G. Chelvanayagam, and L. S. Jermiin. 1997. Zeta, a novel class of glutathione transferases in a range of species from plants to humans. Biochem. J. 328:929-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornett, R., M. O. James, G. N. Henderson, J. Cheung, A. L. Shroads, and P. W. Stacpoole. 1999. Inhibition of glutathione S-transferase zeta and tyrosine metabolism by dichloroacetate: a potential unifying mechanism for its altered biotransformation and toxicity. Biochem. Biophys. Res. Commun. 262:752-756. [DOI] [PubMed] [Google Scholar]
- 8.Dixon, D. P., D. J. Cole, and R. Edwards. 2000. Characterisation of a zeta class glutathione transferase from Arabidopsis thaliana with a putative role in tyrosine catabolism. Arch. Biochem. Biophys. 384:407-412. [DOI] [PubMed] [Google Scholar]
- 9.Endo, F., S. Kubo, H. Awata, K. Kiwaki, H. Katoh, Y. Kanegae, I. Saito, J. Miyazaki, T. Yamamoto, C. Jakobs, S. Hattori, and I. Matsuda. 1997. Complete rescue of lethal albino c14CoS mice by null mutation of 4-hydroxyphenylpyruvate dioxygenase and induction of apoptosis of hepatocytes in these mice by in vivo retrieval of the tyrosine catabolic pathway. J. Biol. Chem. 272:24426-24432. [DOI] [PubMed] [Google Scholar]
- 10.Fernández-Cañón, J. M., B. Granadino, D. Beltran-Valero de Bernabe, M. Renedo, E. Fernandez-Ruiz, M. A. Penalva, and S. Rodriguez de Cordoba. 1996. The molecular basis of alkaptonuria. Nat. Genet. 14:19-24. [DOI] [PubMed] [Google Scholar]
- 11.Fernández-Cañón, J. M., J. Hejna, C. Reifsteck, S. Olson, and M. Grompe. 1999. Gene structure, chromosomal location, and expression pattern of maleylacetoacetate isomerase. Genomics 58:263-269. [DOI] [PubMed] [Google Scholar]
- 12.Fernández-Cañón, J. M., and M. A. Peñalva. 1998. Characterization of a fungal maleylacetoacetate isomerase gene and identification of its human homologue. J. Biol. Chem. 273:329-337. [DOI] [PubMed] [Google Scholar]
- 13.Fernández-Cañón, J. M., and M. A. Peñalva. 1995. Fungal metabolic model for human type I hereditary tyrosinaemia. Proc. Natl. Acad. Sci. USA 92:9132-9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernández-Cañón, J. M., and M. A. Penalva. 1997. Spectrophotometric determination of homogentisate using Aspergillus nidulans homogentisate dioxygenase. Anal. Biochem. 245:218-221. [DOI] [PubMed] [Google Scholar]
- 15.Garrod, A. E. 1902. The incidence of alkaptonuria: A study in chemical individuality. Lancet ii:1616-1620. [Google Scholar]
- 16.Grompe, M. 2001. The pathophysiology and treatment of hereditary tyrosinemia type 1. Semin. Liver Dis. 21:563-571. [DOI] [PubMed] [Google Scholar]
- 17.Grompe, M., M. Al-Dhalimy, M. Finegold, C. N. Ou, T. Burlingame, N. G. Kennaway, and P. Soriano. 1993. Loss of fumarylacetoacetate hydrolase is responsible for the neonatal hepatic dysfunction phenotype of lethal albino mice. Genes Dev. 7:2298-2307. [DOI] [PubMed] [Google Scholar]
- 18.Grompe, M., S. Lindstedt, M. al-Dhalimy, N. G. Kennaway, J. Papaconstantinou, C. A. Torres-Ramos, C. N. Ou, and M. Finegold. 1995. Pharmacological correction of neonatal lethal hepatic dysfunction in a murine model of hereditary tyrosinaemia type I. Nat. Genet. 10:453-460. [DOI] [PubMed] [Google Scholar]
- 19.Jorquera, R., and R. M. Tanguay. 1997. The mutagenicity of the tyrosine metabolite, fumarylacetoacetate, is enhanced by glutathione depletion. Biochem. Biophys. Res. Commun. 232:42-48. [DOI] [PubMed] [Google Scholar]
- 20.Knox, W. E., and S. W. Edwards. 1955. Enzymes involved in conversion of tyrosine to acetoacetate. Methods Enzymol. 2:287-300. [Google Scholar]
- 21.Lee, H. E., and S. Seltzer. 1989. cis-β-Acetylacrylate is a substrate for maleylacetoacetate cis-trans isomerase. Mechanistic implications. Biochem. Int. 18:91-97. [PubMed] [Google Scholar]
- 22.Lindblad, B., S. Lindstedt, and G. Steen. 1977. On the enzymic defects in hereditary tyrosinemia. Proc. Natl. Acad. Sci. USA 74:4641-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindstedt, S., E. Holme, E. A. Lock, O. Hjalmarson, and B. Strandvik. 1992. Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet 340:813-817. [DOI] [PubMed] [Google Scholar]
- 24.Mahuran, D. J., R. H. Angus, C. V. Braun, S. S. Sim, and D. E. Schmidt, Jr. 1977. Characterization and substrate specificity of fumarylacetoacetate fumarylhydrolase. Can. J. Biochem. 55:1-8. [DOI] [PubMed] [Google Scholar]
- 25.Manning, K., M. Al-Dhalimy, M. Finegold, and M. Grompe. 1999. In vivo suppressor mutations correct a murine model of hereditary tyrosinemia type I. Proc. Natl. Acad. Sci. USA 96:11928-11933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell, G. A., M. Grompe, M. Lambert, and R. M. Tanguay. 2001. Hypertyrosinemia, p. 1777-1805. In D. Valle (ed.), The metabolic and molecular basis of inherited disease, 9th ed., vol. 2. McGraw-Hill, New York, N.Y. [Google Scholar]
- 27.Nebert, D. W., A. L. Roe, M. Z. Dieter, W. A. Solis, Y. Yang, and T. P. Dalton. 2000. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem. Pharmacol. 59:65-85. [DOI] [PubMed] [Google Scholar]
- 28.Plummer, J. L., B. R. Smith, H. Sies, and J. R. Bend. 1981. Chemical depletion of glutathione in vivo. Methods Enzymol 77:50-59. [DOI] [PubMed] [Google Scholar]
- 29.Polekhina, G., P. G. Board, A. C. Blackburn, and M. W. Parker. 2001. Crystal structure of maleylacetoacetate isomerase/glutathione transferase zeta reveals the molecular basis for its remarkable catalytic promiscuity. Biochemistry 40:1567-1576. [DOI] [PubMed] [Google Scholar]
- 30.Rowe, J. D., Y. V. Patskovsky, L. N. Patskovska, E. Novikova, and I. Listowsky. 1998. Rationale for reclassification of a distinctive subdivision of mammalian class Mu glutathione S-transferases that are primarily expressed in testis. J. Biol. Chem. 273:9593-9601. [DOI] [PubMed] [Google Scholar]
- 31.Russo, P., and S. O'Regan. 1990. Visceral pathology of hereditary tyrosinemia type I. Am. J. Hum. Genet. 47:317-324. [PMC free article] [PubMed] [Google Scholar]
- 32.Scriver, C. R., and S. Kaufman. 2001. Hyperphenylalaninemia: phenylalanine hydroxylase deficiency, p. 1667-1724. In D. Valle (ed.), The metabolic and molecular basis of inherited disease, 9th ed., vol. 2. McGraw-Hill, New York, N.Y. [Google Scholar]
- 33.Seltzer, S. 1989. Maleylacetoacetate cis-trans isomerase, p. 733-751. In D. Dolphin, R. Poulson, and O. Avramovic (ed.), Coenzymes and cofactors, vol. 3, part A. Wiley, New York, N.Y.
- 34.Seltzer, S. 1973. Purification and properties of maleylacetone cis-trans isomerase from vibrio 01. J. Biol. Chem. 248:215-222. [PubMed] [Google Scholar]
- 35.Seltzer, S., and M. Lin. 1979. Maleylacetone cis-trans isomerase. Mechanism of interaction of coenzyme glutathione and substrate maleylacetone in the presence and absence of enzyme. J. Am. Chem. Soc. 101:3091-3097. [Google Scholar]
- 36.Tanguay, R. M., R. Jorquera, J. Poudrier, and M. St-Louis. 1996. Tyrosine and its catabolites: from disease to cancer. Acta Biochim. Pol. 43:209-216. [PubMed] [Google Scholar]
- 37.Thom, R., D. P. Dixon, R. Edwards, D. J. Cole, and A. J. Lapthorn. 2001. The structure of a zeta class glutathione S-transferase from Arabidopsis thaliana: characterisation of a GST with novel active-site architecture and a putative role in tyrosine catabolism. J. Mol. Biol. 308:949-962. [DOI] [PubMed] [Google Scholar]
- 38.Tong, Z., P. G. Board, and M. W. Anders. 1998. Glutathione transferase zeta catalyses the oxygenation of the carcinogen dichloroacetic acid to glyoxylic acid. Biochem. J. 331(Pt. 2):371-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tong, Z., P. G. Board, and M. W. Anders. 1998. Glutathione transferase zeta-catalyzed biotransformation of dichloroacetic acid and other alpha-haloacids. Chem. Res. Toxicol. 11:1332-1338. [DOI] [PubMed] [Google Scholar]
- 40.Tuchman, M., L. D. Bowers, K. D. Fregien, P. J. Crippin, and W. Krivit. 1984. Capillary gas chromatographic separation of urinary organic acids. Retention indices of 101 urinary acids on a 5% phenylmethyl silicone capillary column. J. Chromatogr. Sci. 22:198-202. [DOI] [PubMed] [Google Scholar]
- 41.Tuchman, M., C. B. Whitley, M. L. Ramnaraine, L. D. Bowers, K. D. Fregien, and W. Krivit. 1984. Determination of urinary succinylacetone by capillary gas chromatography. J. Chromatogr. Sci. 22:211-215. [DOI] [PubMed] [Google Scholar]
- 42.Vasiliou, V., A. Puga, C. Y. Chang, M. W. Tabor, and D. W. Nebert. 1995. Interaction between the Ah receptor and proteins binding to the AP-1-like electrophile response element (EpRE) during murine phase II [Ah] battery gene expression. Biochem. Pharmacol. 50:2057-2068. [DOI] [PubMed] [Google Scholar]