Abstract
In mammals, UVB radiation is of biological relevance primarily for the cells of the epidermis. We report here the existence of a UVB response that is specific for proliferating human epidermal keratinocytes. Unlike other cell types that also display a UVB response, keratinocytes respond to UVB irradiation with a transient but potent downregulation of the Ras-extracellular signal-regulated kinase (ERK) signaling cascade. The downregulation of ERK precedes a profound decrease in the steady-state levels of cyclin D1, a mediator of the proliferative action of ERK. Keratinocytes exhibit high constitutive activity of the Ras-ERK signaling cascade even in culture medium lacking supplemental growth factors. The increased activity of Ras and phosphorylation of ERK in these cells are maintained by the autocrine production of secreted molecules that activate the epidermal growth factor receptor (EGFR). Irradiation of keratinocytes increases the phosphorylation of EGFR on tyrosine residues Y845, Y992, Y1045, Y1068, Y1086, Y1148, and Y1173 above the basal levels and leads to the increased recruitment of the adaptor proteins Grb2 and ShcA and of a p55 form of the regulatory subunit of the phosphatidylinositide 3-kinase to the UVB-activated EGFR. Paradoxically, however, UVB causes, at the same time, the inactivation of Ras and a subsequent dephosphorylation of ERK. By contrast, the signaling pathway leading from the activated EGFR to the phosphorylation of PKB/Akt1 is potentiated by UVB. The UVB response of keratinocytes appeared to be a manifestation of the more general ribotoxic stress response inasmuch as the transduction of the UVB-generated inhibitory signal to Ras and ERK required the presence of active ribosomes at the time of irradiation.
Exposure to UVB radiation is the major cause of cutaneous malignancies in the United States (45). UVB is a complete carcinogen that is known to act both as a tumor initiator and a tumor promoter (43, 59). Exposure of the skin to UVB induces the stereotypic early responses of genes such as c-fos and c-jun (6, 26, 27, 63) and delayed responses, including hyperplasia and skin cancer (21, 26, 48, 72). The induction of the early response genes results from a cascade of signaling events that eventually modulates the activity of specific transcription factors. The signaling pathways that transduce UVB-initiated signals to modulate gene expression involve the superfamily of the proline-directed mitogen-activated protein (MAP) kinases (6, 55). The stress-activated protein kinase (SAPK) family of MAP kinases includes the c-Jun-NH2-terminal kinases (JNK; SAPK1) and the p38 MAP kinases (SAPK2) (15, 46, 73). The activation of SAPK in mammalian cells leads to decision making via the activation of competing prosurvival and proapoptotic pathways (15). Activation of SAPK has also been shown to be a major pathway leading to the synthesis and release of proinflammatory cytokines, which function to communicate the presence of cellular damage to other cells of the organism (16, 57). The extracellular signal-regulated kinases, p44 ERK1 and p42 ERK2 (ERKs), represent the other family of MAP kinases. Although ERKs may play specific roles in specific cell types, it is generally accepted that mitogen-induced sustained activation of ERKs mediates cell cycle progression through G1- and S-phase entry by regulating the expression of cyclin D1 (2, 3, 12, 60, 66, 76).
The epidermal growth factor receptor (EGFR) plays a crucial role in mediating both the proliferative and prosurvival programs of keratinocytes, the main cell type of the epidermis (reference 68 and references therein). At least five mitogenic growth factors bind to and activate EGFR in the skin. In addition to epidermal growth factor (EGF), these factors include transforming growth factor α (TGF-α) (11), amphiregulin (13), heparin-binding EGF (HB-EGF) (25), and epiregulin (67). The classical signal-transduction cascade that translates EGFR activation into the increased activity of ERK has been investigated at length (10, 40, 64). The binding of a cognate ligand stabilizes receptor dimerization and stimulates the tyrosine kinase activity of the intracellular portion of the receptor, resulting in the increased auto(trans-)phosphorylation of several tyrosine residues within the receptor dimer (7, 65). The phosphorylated tyrosines serve as recruitment (docking) sites for several adaptor proteins, among which the adaptor proteins Grb2 (19, 42) and Shc (44, 53, 62) are of particular importance for the activation of ERK (60). Grb2 contains one Src-homology 2 (SH2) domain flanked by two Src-homology 3 (SH3) domains (42). The SH2 domain of Grb2 binds to tyrosine-phosphorylated residues in growth factor receptors, such as Y1068 and Y1086 of EGFR (5, 50, 74), whereas the SH3 domains bind to proline-rich motifs found in many signaling molecules, such as the Son of Sevenless (Sos) protein (9, 19, 52). Sos is a guanine nucleotide exchange factor that facilitates, in response to activation of growth factor receptors, the conversion of the inactive (GDP-bound) form of the cell membrane-localized small GTPase Ras into the activated (GTP-bound) form of Ras (9). Activated Ras recruits to the cell membrane the serine/threonine protein kinase c-Raf, an event that leads to the activation of c-Raf itself (37). c-Raf, in turn, phosphorylates and activates the MAP/ERK kinases MEK1 and MEK2, eventually leading to the activation of ERK (10, 37, 40, 64). Grb2 also binds EGFR indirectly via EGFR-bound ShcA (4). ShcA, however, binds to different phosphotyrosine residues of EGFR, namely Y1148, Y1173, and Y992 (4, 5, 49, 74).
The study of the molecular basis of the UV response (encompassing the cellular responses to both UVC and UVB) in a tissue culture system has traditionally been performed in rodent and human fibroblasts and in HeLa cells (6, 30, 31, 34, 36, 55, 63) under the assumption that the UV response is conserved in all cell types. By use of NIH 3T3 fibroblasts and HeLa cells, ERK was identified as the first signal transduction kinase activated early after exposure to UVC radiation (55). The identification of the ability of UVC and UVB to trigger the activation of growth factor receptors (36, 63) favored the conclusion that an operational growth factor receptor-ERK cascade is an important mediator of UV-induced gene expression (6, 26).
In the search for novel and relevant UV-induced signal-transduction pathways, we recently discovered that the activation of SAPK by UVC and UVB is initiated in, or in close proximity to, the functional center of actively translating eukaryotic ribosomes (31, 34). This center contains the 3′ end of 28S rRNA and its proteinaceous environment and is responsible for aminoacyl-tRNA binding, peptidyl transfer, and ribosome translocation. This region of the 28S rRNA is the target of the antibiotics anisomycin and blasticidin S and of the enzymatic ribotoxins ricin A chain and α-sarcin, all of which strongly activate SAPK (reference 33 and references therein). The activation of SAPK by the foregoing agents was termed the ribotoxic stress response and is characterized by the absolute requirement for the presence of actively translating ribosomes at the moment of cellular encounter with the antibiotic or ribotoxin acting on 28S rRNA (33). Cells whose ribosomes are not engaged in translational elongation fail to activate SAPK in response to these agents. In contrast, the activation of SAPK by nonribotoxic stressors, such as inflammatory cytokines, osmotic stress, and some DNA-damaging drugs, is intact in cells containing nontranslating ribosomes (31-33). Interestingly, UVB requires the presence of active ribosomes to activate SAPK. Furthermore, nucleotide- and position-specific damage to the 3′ end of 28S rRNA was detected in UVB-irradiated cells, and the time of appearance and degree of this damage correlated with the activation of SAPK (34). It was concluded therefore that UVB triggers the ribotoxic stress response that leads to the activation of SAPK. In cells that respond to UVB with the activation of ERK (e.g., fibroblasts or HeLa cells), however, the activation of ERK was not dependent on ribotoxic stress inasmuch as it did not require active ribosomes (34).
In the present study, we employed normal human keratinocytes to investigate whether these cells (that are frequently exposed to solar radiation) possess a specific UVB response. We demonstrate for the first time that the UVB response of human keratinocytes is characterized by both stimulatory (activation of SAPK) and inhibitory (inactivation of ERK) signal transduction components. The elevated basal levels of ERK activity in these cells resulted from the autocrine and paracrine actions of extracellular molecules that bind to and activate the EGFR. Despite the activation of EGFR resulting from UVB irradiation, UVB interfered with signals that lead to ERK activation at the level of a signal-transduction step(s) located downstream of the EGFR-bound Grb2 and ShcA but upstream of Ras. At the same time, the activation of EGFR by UVB triggered the phosphorylation of the protein kinase B (PKB/Akt1) that is involved in mediating cell survival (14). Finally, we show that both the stimulatory and the inhibitory UVB responses of MAP kinases are downstream of a ribosome-initiated signaling event.
MATERIALS AND METHODS
Cell culture.
Human epidermal keratinocytes, neonatal (HEKn) were maintained in EpiLife basal keratinocyte medium (BKM) supplemented with a semidefined human keratinocyte growth supplement (HKGS) (the final concentrations of the components in the supplemented medium were as follows: 0.2% [vol/vol] bovine pituitary extract, 5 mg of bovine insulin per ml, 0.18 mg of hydrocortisone per ml, 5 mg of bovine transferrin per ml, and 0.2 ng of human epidermal growth factor per ml). The BKM supplemented with HKGS is referred to as BKM+exoGF (BKM plus exogenous growth factors), and BKM lacking HKGS is referred to as BKM-exoGF (BKM minus exogenous growth factors). The cells and all cell culture reagents were obtained from Cascade Biologics, Inc. Immortalized HEKn (HEKn-E6/E7) were established through infection with an amphitropic recombinant retroviral vector encoding the E6 and E7 transforming proteins of human papillomavirus serotype 16 as previously described (35). HEKn-E6/E7 were maintained in culture in a way identical to what was done with HEKn. The HaCaT cell line (a gift from Tim Bowden) and HeLa cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum and antibiotics. For experiments, HaCaT and HeLa cells were plated in BKM+exoGF.
Irradiation of cells and other treatments.
The spectral characterization of the UVB source and the mode of the irradiation of cells with UVB have been described previously (31, 34). Human recombinant EGF (Sigma) was kept as a 100-μg/ml stock solution in 5 × 10−3 N HCl at −70°C. AG1478 and UO126 (both from Calbiochem), anisomycin, emetine, actinomycin D (all from Sigma), and pactamycin (a gift from Pharmacia and Upjohn) were dissolved in dimethyl sulfoxide (DMSO) and kept at −70°C. Sodium arsenite (Sigma) was freshly prepared before use as a 40 mM stock solution in double-distilled deionized water. To prepare conditioned media, the cells (originally plated in BKM+exoGF) were washed extensively with BKM-exoGF and then placed for 20 to24 h in BKM-exoGF (2 ml for each 60-mm-diameter cell culture plate). Conditioned media were never frozen and were used only once. The EGFR-neutralizing monoclonal antibody LA1 was obtained from Upstate Biotechnology.
Immunocomplex kinase assay.
HEKn from 6-cm-diameter tissue culture dishes were harvested by lysis on ice in an ice-cold solution containing 20 mM HEPES-KOH (pH 7.4), 2 mM EGTA, 50 mM β-glycerophosphate, 1 mM dithiothreitol (DTT), 10% glycerol, 1% Triton X-100, 1 mM sodium vanadate, and 1× Complete protease inhibitors (Roche Molecular Biochemicals). ERK1/ERK2 was immunoprecipitated for 3 h at 4°C with an anti-ERK1/ERK2 antibody (C-16; Santa Cruz Biotechnology) precoupled to protein A-agarose (Santa Cruz Biotechnology). The immunoprecipitates were washed once with lysis buffer; once with a solution consisting of 100 mM Tris-HCl (pH 7.6), 500 mM LiCl, 1 mM DTT, and 0.1% Triton X-100; and once with a buffer containing 20 mM morpholinepropanesulfonic acid (MOPS; pH 7.2), 10 mM MgCl2, 2 mM EGTA, 1 mM DTT, and 0.1% Triton X-100. For the kinase reaction, the immunoprecipitates were incubated with 1 mg of glutathione S-transferase (GST)-Elk1 fusion proteins in the presence of 10 mM MOPS (pH 7.2), 20 mM MgCl2, 1 mM EGTA, 0.5 mM DTT, 0.05% Triton X-100 and 1 mCi of [γ-32P]ATP for 20 min at 30°C. After the reactions were stopped by adding 10 μl of 4× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer, the samples were resolved by SDS-13% PAGE. The phosphorylated GST-Elk1 was quantified from dried gels with a Molecular Dynamics PhosphorImager and IP Lab Gel software.
Immunoblot analysis of proteins.
For immunoblot analyses, the cells were lysed directly in 2× SDS-PAGE loading buffer and lysates corresponding to 1 × 105 to 1.5 × 105 cells were resolved in SDS-PAGE with the appropriate percentage of acrylamide (7.5 to 15%). The antibodies against ERK1 (C-16), EGFR (EGFR-1005), the phosphorylated form of EGFR (pY1173), Sos1 (C-23 and D-21), ShcA (PG-797), c-Myc (N-262), p63 (4A4-HRP), p53 (DO-1-HRP), cyclin D1 (M-20), MEK2 (N-20), JNK1 (C-17), p38 MAP kinase (C-20), p85 PI-3K (Z-8), and PKB/Akt1/2 (H-136) were obtained from Santa Cruz Biotechnology. The antibodies against the phosphorylated forms of EGFR (pY1148 and pY1086) were obtained from BioSource International. The antibodies against the phosphorylated forms of EGFR (pY845, pY992, pY1045, and pY1068) were obtained from Cell Signaling Technology. The pan-Ras antibody (31-43) was obtained from Calbiochem. The antibodies against the phosphorylated forms of ERK, p38 MAP kinase, JNK, MEK, PKB/Akt1 (S473), and FKHR were obtained from Cell Signaling Technology. The antibody against Grb2 was obtained from BD-Transduction Laboratories. The separation of proteins in SDS-PAGE and the electrotransfer onto polyvinylidene difluoride membranes (Millipore) were performed by standard procedures. Immunodetections with phosphoepitope-specific antibodies were performed according to the instructions of the respective manufacturers.
Coimmunoprecipitations.
The coimmunoprecipitation of EGFR with associated proteins was performed as described by Dulin et al. (17), and the coimmunoprecipitation of Sos1 with associated proteins was performed as described by Gross et al. (23).
Ras activity assay.
Recombinant protein composed of the Ras-binding domain of c-Raf fused to GST (GST/Raf-RBD; a gift from Rudolf Juliano) was bacterially expressed in advance, affinity bound to glutathione-coupled Sepharose 4B beads (Amersham Pharmacia Biotech AB) shortly before use, and kept on ice. All subsequent procedures were performed with the protein on ice or at 4°C. Cells were lysed by scraping and vigorous mixing in 1 ml (on a 100-mm-diameter cell culture plate) of lysis buffer (50 mM Tris [pH 8.0], 150 mM NaCl, 1% IGEPAL [Sigma; equivalent to Nonidet P-40], 10% glycerol, 5 mM MgCl2, 10 mM NaF, 1 mM NaVO4, 1× Complete protease inhibitors [Roche Molecular Biochemicals], and 1 μM DTT). The lysates were precleared for 10 min at 14,000 rpm in an Eppendorf table-top centrifuge. One hundred microliters of GST/Raf-RBD-bound slurry was mixed with cell lysate (typically corresponding to 3 × 106 to 4 × 106 cells per experimental point) and incubated, with continuous mixing, for 30 min. After the beads were washed three times with lysis buffer, GTP-bound Ras proteins were eluted by boiling in 100 μl of 2× SDS-PAGE loading buffer. Ras proteins were resolved with SDS-15% PAGE and detected by immunoblot analysis.
RESULTS
Inactivation of ERK by UV in human epidermal keratinocytes.
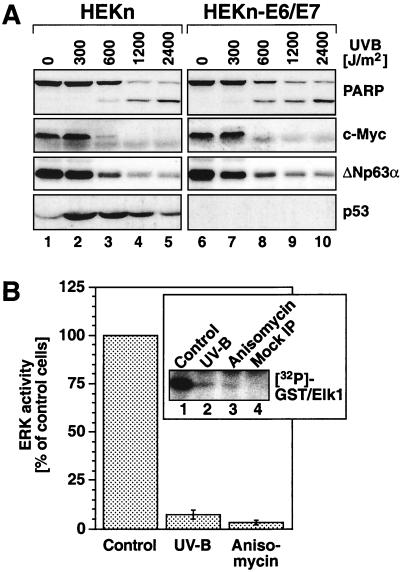
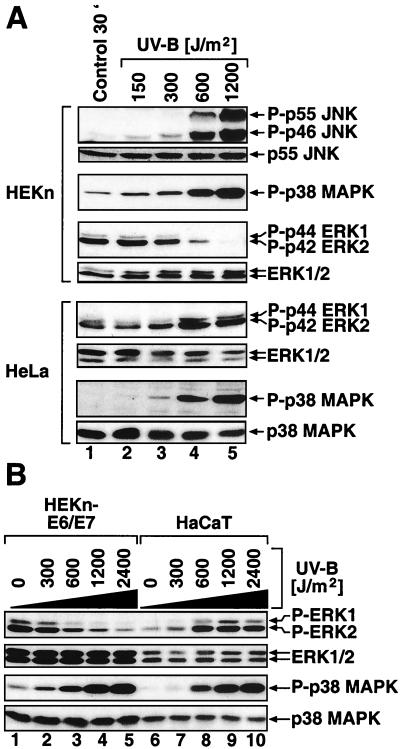
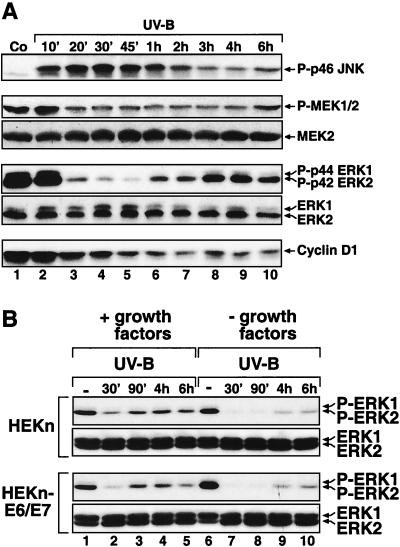
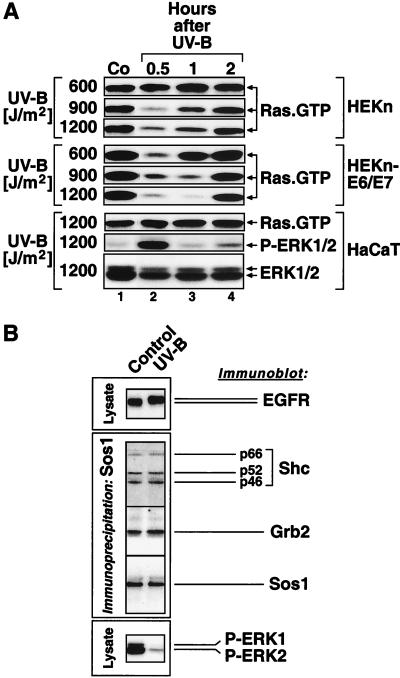
In order to investigate the regulation of MAP kinases by UVB in a cell type that naturally encounters UV radiation, we employed freshly explanted HEKn. We also employed uncloned HEKn-E6/E7 (see Materials and Methods). Both HEKn and HEKn-E6/E7 display the expression of basal keratinocyte markers (ΔNp63α) (54, 77) (Fig. 2A, lanes 1 and 6) and integrin β 1 (75) (data not shown). When cultured in serum-free BKM+exoGF (see Materials and Methods), both HEKn and HEKn-E6/E7 displayed detectable levels of double-phosphorylated p42 ERK2 and, to a much lesser extent, p44 ERK1 (Fig. 1A, lane 1, and B, lane 1), as detected in immunoblot assays with phosphorylation-specific antibodies. To investigate the regulation of ERK activity by UV in keratinocytes, we subjected HEKn and HEKn-E6/E7 to UVB. Irradiation of HEKn and HEKn-E6/E7 induced a dose-dependent increase in the phosphorylation of SAPK (JNK and p38α MAP kinase) at 30 min postirradiation (Fig. 1A and B, lanes 1 to 5 of each). Surprisingly, we found that, instead of activation, UVB-irradiated HEKn and HEKn-E6/E7 displayed a dose-dependent downregulation of the phosphorylation state of ERK at 30 min postirradiation (Fig. 1A and B, lanes 1 to 5 of each). The dephosphorylation of ERK in HEKn (Fig. 1A) occurred after doses of UVB (600 to 1,200 J/m2) that caused both cell cycle withdrawal (evident by an inhibition of DNA synthesis [data not shown] and a decrease in the steady-state levels of c-Myc [Fig. 2B ]) and apoptosis (“sunburn cells” [38, 47]) [evident by the cleavage of the caspase substrate poly-(ADP)ribose polymerase (Fig. 2A) and the decrease in the steady-state levels of ΔNp63α (41) (Fig. 2A)]. The UVB-induced dephosphorylation of ERK resulted in a substantial inhibition of ERK activity (93% ± 2% inhibition) as determined by immunocomplex kinase activity assays 30 min postirradiation (Fig. 2B). The dephosphorylation and inactivation of ERK following exposure to UVB became detectable 20 min after the irradiation (Fig. 3A, lane 3) and persisted, with some variation from experiment to experiment, for typically 2 to 6 h after irradiation (Fig. 3A, lanes 3 to 10). The UV-induced inactivation of ERK has been observed invariably in all six independent preparations of HEKn (each derived from a different individual) that we have examined. For instance, Fig. 1A, 3A, and 9 depict the UVB responses of three independent HEKn preparations (i.e., three different individuals). ERK1 and ERK2 proteins were expressed at similar steady-state levels in HEKn and HEKn-E6/E7, and these levels were not affected by exposure to UVB (Fig. 1, 3, 5, 6A, 7, 8, 10, 11, and 12A). Therefore, the decrease in ERK phosphorylation and activity after exposure to UV was due to a bona fide dephosphorylation event and not to reduced ERK levels. In summary, these results demonstrated that, in contrast to what occurs in other cell types that have been investigated, the activity of ERK in keratinocytes was transiently, but potently, suppressed in response to UVB.
FIG. 2.
(A) Effects of UVB irradiation on cell cycle and apoptosis markers in HEKn and HEKn-E6/E7. Cells grown in BKM+exoGF were irradiated with the indicated doses of UVB, and the expression of the indicated proteins was determined in immunoblot analyses 21 h postirradiation. PARP, poly(ADP)ribose polymerase. (B) Inhibition of ERK activity by UVB and anisomycin. HEKn (grown in BKM+exoGF) were treated with UVB (1,200 J/m2) or anisomycin (10 μg/ml). ERK activity was assessed 30 min later in immunocomplex kinase assays (see Materials and Methods). Lane 4 of the boxed insert shows the control immunoprecipitation (IP) with an irrelevant antibody. Error bars represent the standard deviations from experimental points in triplicates.
FIG. 1.
Dose-dependent regulation of the phosphorylation of MAP kinases by UVB. HEKn and HeLa cells (A) and HEKn-E6/E7 and HaCaT cells (B) (grown in BKM+exoGF) were treated with the indicated doses of UVB and were harvested 30 min later. Shown are the results of immunoblot analyses using phosphorylation-specific antibodies against the indicated proteins.
FIG. 3.
Time-dependent inhibition of ERK phosphorylation by UVB. Shown are the results of immunoblot analyses using phosphorylation-specific antibodies against the indicated proteins. (A) HEKn were treated with UVB (1,200 J/m2) UVB and later harvested at the indicated times. Co, control. (B) HEKn and HEKn-E6/E7, placed 20 h earlier in either BKM+exoGF or BKM-exoGF, were irradiated with UVB (1,200 J/m2) and harvested at the indicated times after the irradiation. -, control cells. Note that a prior hybridization of a membrane with antibodies against the phosphorylated forms of ERK or EGFR interferes with a subsequent hybridization of the same membrane with the antibodies against nonphosphorylated ERK or EGFR at any location in the membrane where the phospho-specific signal was strong. This explains the apparent weaker signal of the nonphosphorylated p42 ERK2 seen in the bottom panel of lane 6. The same phenomenon can also be seen in Fig. 4A, lanes 3 and 10 (ERK2); Fig. 5A, lane 2 (EGFR and ERK2); Fig. 6A, lanes 1 to 3 and 7 (ERK2); Fig. 7A, lanes 5 to 7 (ERK2); Fig 9, panel l, lanes 7 and 12 (ERK2); and Fig. 12A, lanes 2 to 4 (ERK2).
Human epidermal keratinocytes maintain high steady-state levels of ERK activity via the autocrine production of EGFR ligand(s).
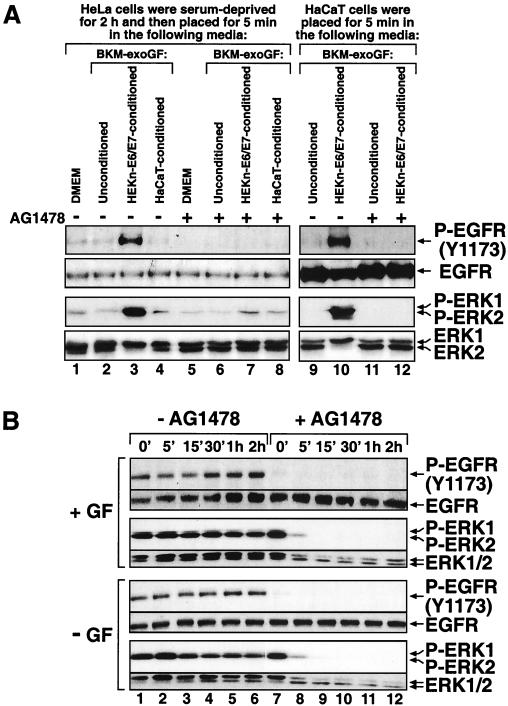
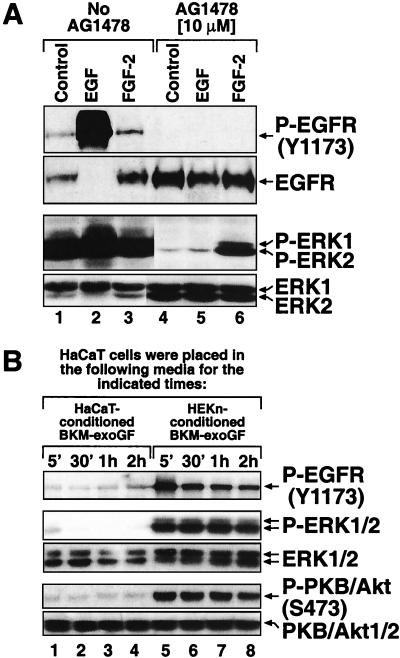
To determine whether the phosphorylated state of ERK in keratinocytes was due to the presence of supplemental growth factors in BKM+exoGF, we assessed the phosphorylation status of ERK after placing HEKn or HEKn-E6/E7 in BKM-exoGF for 20 h. The absence of exogenously supplied growth factors failed to decrease the degree of phosphorylation of ERK (Fig. 3B, compare lanes 1 and 6), suggesting that the activity of ERK is regulated by autocrine mechanisms in these cells. To test this possibility, we transferred HEKn-E6/E7-conditioned BKM-exoGF onto serum-deprived HeLa cells, whose levels of ERK phosphorylation are extremely low. Keratinocyte-conditioned, but not HeLa-cell-conditioned (data not shown), or unconditioned BKM-exoGF stimulated the phosphorylation of ERK in the HeLa cells (Fig. 4A, lane 3). Furthermore, only the keratinocyte-conditioned BKM-exoGF was capable of causing the phosphorylation of EGFR at tyrosine-1173 (Y1173) (Fig. 4A, lane 3). The keratinocyte-conditioned BKM-exoGF was unable to induce the phosphorylation of ERK (Fig. 4A, lane 7) when the conditioned medium was added to HeLa cells that had been pretreated with AG1478, a specific inhibitor of the EGFR tyrosine kinase activity (20, 51). Following the exposure of HeLa cells to keratinocyte-conditioned BKM-exoGF (Fig. 4A, lane 7), the phosphorylation of EGFR at Y1173 was also inhibited by AG1478, suggesting that the receptor phosphorylation was caused by the tyrosine kinase activity of the receptor itself and not by a different tyrosine kinase. These results suggest that keratinocytes produce a soluble ligand(s) that is capable of activating EGFR and, subsequently, ERK. These results were not exclusively specific for the immortalized HEKn-E6/E7, since the mortal HEKn also displayed a potent production of a soluble ligand(s) of EGFR (Fig. 5B). To determine whether this ligand(s) provides the main extracellular signal for the high ERK activity in keratinocytes, we incubated HEKn-E6/E7 with AG1478 and assessed the phosphorylation status of ERK. Immediately (less than 10 s) after the addition of AG1478, the keratinocytes displayed an immediate decrease in the basal phosphorylation of EGFR (Fig. 4B, lane 7) without a concomitant decrease in the phosphorylation of ERK. Five minutes after the addition of AG1478, the basal phosphorylation of ERK dramatically decreased, and the low levels of phosphorylated ERK persisted for the duration of the experiment (Fig. 4B, lanes 7 to 12). The effect of AG1478 was independent of the presence or the absence in BKM of supplemental growth factors (Fig. 4B). To verify the specificity of AG1478, we compared its abilities to inhibit the activation of ERK by EGF and by fibroblast growth factor 2 (FGF-2). FGF-2 also signals through a cognate receptor with intrinsic tyrosine kinase activity but not through EGFR (22). Addition of EGF to cells pretreated with AG1478 failed to induce the phosphorylation of either EGFR or ERK (Fig. 5A, lane 5). However, AG1478 had no effect on the ability of FGF-2 to induce the phosphorylation of ERK (Fig. 5A, lane 6) (FGF-2 did display an ability to trigger the phosphorylation of ERK because the basal phosphorylation of ERK had been reduced to very low levels by AG1478). These results (Fig. 4 and 5) suggested that the elevated activity of ERK in keratinocytes is sustained predominantly through the autocrine production of a soluble ligand(s) that activates EGFR. To verify by a different approach that the ERK activity in keratinocytes is exclusively dependent on the autocrine-maintained activity of EGFR, we employed the EGFR-neutralizing monoclonal antibody LA1. Thirty minutes after the addition of LA1 to the culture medium (BKM-exoGF) of HEKn-E6/E7, the phosphorylation of Y1173 of EGFR was substantially (albeit not completely) decreased and the decrease remained unchanged for as long as 6 h (Fig. 6A, lanes 4 to 6). Importantly, the EGFR-neutralizing antibody completely blocked the phosphorylation of ERK (Fig. 6A, lanes 4 to 6) in a manner similar to the effect of AG1478 (Fig. 6A, lane 8).
FIG. 4.
Production of a soluble ligand(s) of EGFR by HEKn-E6/E7 but not by HaCaT cells. Shown are the results of immunoblot analyses using antibodies specific for the phosphorylated states of EGFR (Y1173) and ERK. (A) The phosphorylation state of EGFR (Y1173) and ERK in HeLa (lanes 1 to 8) or HaCaT (lanes 9 to 12) cells that have been subjected to unconditioned, HEKn-E6/E7-conditioned, or HaCaT-conditioned BKM-exoGF according to the method described in the figure body. The cells were pretreated for 30 min with 10 μM AG1478 (+) or the vehicle solvent DMSO(−). (B) HEKn-E6/E7, placed 20 h earlier in either BKM+exoGF (+ GF) or BKM-exoGF (- GF), were treated with 10 μM AG1478 or the vehicle solvent DMSO (lanes 1 to 6) for the indicated times.
FIG. 5.
Human epidermal keratinocytes maintain high steady-state levels of ERK activity via the autocrine production of EGFR ligand(s). (A) Results of immunoblot analyses showing that AG1478 inhibits specifically the EGF-induced signaling but not the FGF-2-induced signaling to ERK. HEKn-E6/E7 were pretreated for 30 min with 10 μM AG1478 (lanes 4 to 6) or the vehicle solvent DMSO (lanes 1 to 3) and were then treated for 15 min with either EGF (100 ng/ml) or FGF-2 (20 ng/ml). (B) Immunoblot analyses of the phosphorylation state of EGFR (Y1173), ERK, and PKB/Akt1 (Ser473) in HaCaT cells that have been subjected to either HaCaT-conditioned or HEKn-E6/E7-conditioned BKM-exoGF according to the method described in the figure body.
FIG. 6.
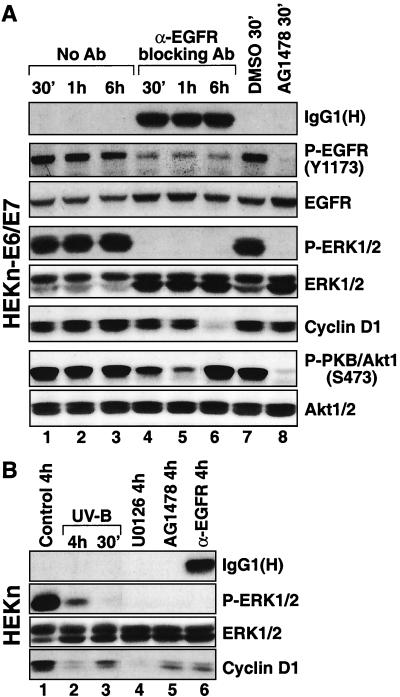
Expression of cyclin D1 in keratinocytes is dependent on the activities of EGFR and MEK. (A) HEKn-E6/E7, placed 20 h earlier in BKM-exoGF, were left untreated or were treated with the EGFR-neutralizing antibody (Ab) LA1 (5 μg/ml), with AG1478 (10 μM), or with DMSO (vehicle control for AG1478). The cells were harvested at the indicated times, and the expression and/or phosphorylation status of the indicated proteins was assayed in immunoblot analyses. IgG1(H), the heavy immunoglobulin chain of the neutralizing anti-EGFR antibody used. (B) HEKn, placed 20 h earlier in BKM-exoGF, were left untreated or were treated with UVB (1,200 J/m2), AG1478 (10 μM), UO126 (10 μM), or the EGFR-neutralizing antibody LA1 (5 μg/ml). The cells were harvested at the indicated times, and the expression and/or phosphorylation status of the indicated proteins was assayed in immunoblot analyses.
To investigate whether the supplemental growth factors present in BKM+exoGF influence the dephosphorylation of ERK by UVB, we irradiated HEKn or HEKn-E6/E7 20 h after placing the cells in BKM-exoGF. The dephosphorylation of ERK by UVB was more pronounced in the cells maintained in BKM-exoGF in terms of both amplitude and duration (Fig. 3B, compare lanes 2 to 5 with lanes 7 to 10). We concluded that the supplemental growth factors present in BKM+exoGF facilitate the recovery of ERK phosphorylation levels following exposure to UVB.
The expression of cyclin D1 in keratinocytes is regulated by the EGFR-MEK-ERK cascade.
To investigate the potential physiological consequences of the UVB-induced inactivation of ERK in keratinocytes, we studied the steady-state levels of cyclin D1. Proliferating HEKn (Fig. 3A, lane 1, and 6B, lane 1) or HEKn-E6/E7 (Fig. 6A, lane 1) displayed detectable levels of cyclin D1. Following UVB irradiation, the steady-state levels of cyclin D1 declined gradually within the first 2 h after irradiation and remained low for as long as 6 h (Fig. 3A, lanes 2 to 10, and 6B, lanes 2 and 3). This was consistent with the withdrawal of the cells from the cell cycle described above (see the legend to Fig. 2A). To investigate whether cyclin D1 expression is determined in keratinocytes by the activity of the EGFR-MEK-ERK cascade, we studied the effects of AG1478, the LA1 EGFR-neutralizing antibody, and UO126, a specific inhibitor of MEK, on cyclin D1 levels. The levels of cyclin D1 were not affected by short (30 to 60 min) treatments with either AG1478 or LA1 (Fig. 6A, lanes 4, 5, and 8). However, 6 h after the application of LA1 (Fig. 6A, lane 6) and 4 h after the application of AG1478 (Fig. 6B, lane 5) a substantial decrease in the cyclin D1 levels was evident. Similarly, 4 h after the application of UO126, we detected a marked decrease in the expression of cyclin D1 (Fig. 6B, lane 4). Taken together, these data are strongly consistent with the conclusion that the linear EGFR-ERK signal transduction cascade is the major determinant of cyclin D1 expression in proliferating keratinocytes.
The UVB-induced inactivation of ERK is specific for keratinocytes.
Next, we investigated whether UVB induces the inactivation of ERK in cells other than keratinocytes. Irradiation of HeLa cells with UVB in growth medium optimal for HeLa cells (DMEM, with or without 10% fetal bovine serum) resulted in an increased rather than decreased ERK activity (data not shown), as was previously reported for UVC (30, 63) and UVB (34). To investigate the possibility that the type of growth medium (BKM or DMEM) changes the responsiveness of ERK to UV, we plated HeLa cells in BKM+exoGF and investigated their response to UVB 24 h later. Even in BKM+exoGF, HeLa cells responded to UVB irradiation with a dose-dependent increase in ERK phosphorylation (Fig. 1A, lanes 2 to 5).
The human keratinocyte-derived cell line HaCaT (8) has been used to study the stress responses of keratinocytes (e.g., a literature search revealed 84 articles from 1993 to 2001 dealing with the UV response of HaCaT cells) despite the fact that these cells have acquired the ability to grow in the presence of serum and calcium concentrations that are inhibitory for primary keratinocytes. In contrast to HEKn and HEKn-E6/E7, HaCaT cells maintained low basal levels of phosphorylated ERK and responded to UVB irradiation with an increased (rather than decreased) phosphorylation of ERK (Fig. 1B, lanes 6 to 10). Identical results were observed when HaCaT cells were maintained and propagated in DMEM supplemented with fetal calf serum (data not shown) or in BKM+exoGF (Fig. 1B). In contrast to the various responsiveness of ERK phosphorylation, the phosphorylation of SAPK (JNK and p38α MAP kinase) was stimulated by UVB in all cell types tested (HEKn, HEKn-E6/E7, HeLa, and HaCaT) (Fig. 1).
HaCaT cells have lost the ability to produce a ligand(s) that binds to EGFR.
To begin to understand the basis for the difference in the responsiveness of HEKn and HaCaT cells to UVB radiation, we determined whether HaCaT cells have lost the autocrine regulation of the EGFR-ERK signal transduction pathway that is characteristic of HEKn and HEKn-E6/E7. A deficiency in producing an autocrine EGFR ligand(s) or in responding to this ligand(s) may explain the loss of autocrine regulation characteristic of HaCaT cells. We found that, unlike the BKM-exoGF conditioned by HEKn-E6/E7 (which induced an AG1478-inhibitable phosphorylation of EGFR and ERK), the BKM-exoGF conditioned by HaCaT cells was unable to induce the phosphorylation of EGFR and ERK in recipient serum-deprived HeLa cells (Fig. 4A, lanes 3, 4, 7, and 8). However, growth factor-deprived HaCaT cells responded to HEKn- or HEKn-E6/E7-conditioned BKM-exoGF with an AG1478-inhibitable induced phosphorylation of both EGFR and ERK (Fig. 4A, lanes 9 to 12, and 5B). We concluded therefore that HaCaT cells have lost the ability to produce soluble EGFR ligands but not the ability to respond to these ligands.
UVB interferes with ERK phosphorylation upstream of Ras but downstream of EGFR.
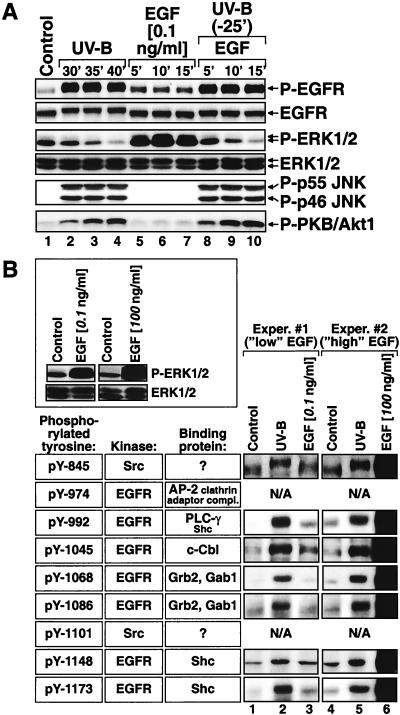
The inhibition of the phosphorylation of ERK following UVB irradiation of keratinocytes was paralleled by a similar decrease in the phosphorylation status of MEK (HEKn [Fig. 3A and see Fig. 9, panel k]; HEKn-E6/E7 [data not shown]). Keratinocytes display the presence of a highly active ERK phosphatase(s), as evidenced by the rapid dephosphorylation of ERK following the application of AG1478 (Fig. 4B). For this reason, the high steady-state activity of ERK appears to be critically dependent on the constant flow of upstream activation signal transmitted by activated MEK. Therefore, the decrease in MEK phosphorylation following UVB irradiation is likely to be responsible for the inactivation of ERK. We speculated that UVB might interfere with the basal activity of MEK and ERK by inhibiting the kinase activity of EGFR. To test this possibility, we investigated the effect of UVB on the basal and EGF-induced tyrosine phosphorylation of EGFR in HEKn-E6/E7. Previously, we had determined that a 0.1-ng/ml dose of EGF is sufficient to trigger reproducibly a detectable, but submaximal, tyrosine phosphorylation of EGFR in HEKn-E6/E7 (Fig. 7A, lanes 5 to 7); this dose was therefore selected to test the ability of UVB to modulate the receptor's kinase activity. Treatment of HEKn-E6/E7 with EGF resulted in the expected coordinated upregulation of the phosphorylation of EGFR and ERK but not of JNK (Fig. 7A, lanes 5 to 7). By contrast, irradiation of the cells with UVB resulted in the upregulation of JNK phosphorylation and the inhibition of ERK phosphorylation (Fig. 7A, lanes 2 to 4). However, the inactivation of ERK by UVB was paralleled by a substantial increase in the phosphorylation of EGFR on Y1173 (Fig. 7A, lanes 2 to 4). The functional potentiation of EGFR's kinase activity by UVB was manifested by the increase in Y1173 phosphorylation and was accompanied by a detectable shift in the electrophoretic mobility of the receptor in SDS-PAGE (Fig. 7A, lanes 2 to 4), suggesting that other tyrosine residues may be phosphorylated in UVB-irradiated keratinocytes as well (Fig. 7B). The UVB-induced inhibitory signal to ERK was apparently dominant over the EGF-induced stimulating signal to ERK (as evidenced by the inability of EGF to trigger the phosphorylation of ERK in the UVB-pretreated HEKn-E6/E7 [Fig. 7A, lanes 8 to 10]). We concluded therefore that UVB interfered with the signaling pathway that leads to the activation of ERK at a point that is located upstream of MEK but downstream of EGFR.
FIG. 9.
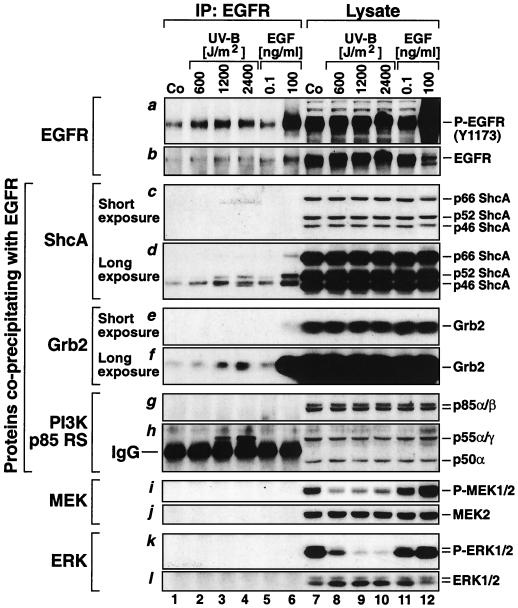
UVB-induced association of the activated EGFR with ShcA, Grb2, and a p55 PI3K. HEKn were maintained in BKM+exoGF and were treated for 30 min with the indicated doses of UVB or for 10 min with the indicated doses of EGF. Lanes 1 to 6, coimmunoprecipitation of the indicated proteins with EGFR; lanes 7 to 12, cell lysates before the immunoprecipitation. Shown are the results of immunoblot analyses using antibodies as indicated in each panel. For the detection of ShcA (panels c and d) and Grb2 (panels e and f), the presentation of short and long film exposures was necessary. IgG, the heavy immunoglobulin chain of the anti-EGFR antibody used for precipitation.
FIG. 7.
UVB activates EGFR but triggers the functional uncoupling of ERK (but not of PKB/Akt1) from EGFR. (A) Immunoblot analyses with HEKn-E6/E7 placed in BKM-exoGF and treated 2 h later with UVB (1,200 J/m2), with a dose of EGF that is submaximal with respect to EGFR phosphorylation (0.1 ng/ml), or with EGF after a pretreatment with UVB. The phosphorylation statuses of EGFR (Y1173), ERK, JNK, and PKB/Akt1 (Ser473) were assessed at the indicated times after the treatments. (B) Immunoblot analyses with HEKn-E6/E7 handled as described for panel A, except that two doses of EGF (0.1 ng/ml for experiment 1 and 100 ng/ml for experiment 2) were applied. The cells were harvested 10 min after the EGF treatments and 30 min after the UVB (1,200 J/m2) treatments (identical UVB treatments were done in experiments 1 and 2). Lanes 1 to 6, each panel represents the analysis of the EGFR phosphorylation with phosphorylation-specific antibodies against the tyrosine residues indicated at left. N/A, antibody not available. The boxed insert shows the EGF-induced phosphorylation of ERK in experiment 1 or 2.
To investigate whether UVB causes a functional activation of EGFR, we employed phospho-specific antibodies and performed analyses of the phosphorylation states of seven of the nine known tyrosine phosphorylation sites within EGFR. It has been shown that these phosphotyrosines serve as specific docking sites for a variety of signal-transducing adaptors and enzymes, such as Grb2 and Gab1 (pY1068 and pY1086 [5, 50, 58, 74]), ShcA (pY992, pY1148, and pY1173 [4, 5, 49, 74]), c-Cbl (pY1045 [39]), PLC-γ (pY992 [61]), and the AP-2 clathrin adaptor complex (pY974 [69]). Figure 7B shows that UVB (1,200 J/m2) caused the substantial phosphorylation of all tyrosine residues, with the exception of Y845, tested at 30 min postirradiation. The levels of tyrosine phosphorylations achieved by UVB were above those achieved by a “low” dose of EGF (0.1 ng/ml) that is fully capable of triggering increased ERK phosphorylation in HEKn-E6/E7 (Fig. 7B, boxed insert). The less pronounced effect of UVB on the phosphorylation state of Y845 (Fig. 7B) is likely to result from the fact that this tyrosine displays a substantial degree of basal phosphorylation in untreated cells. We concluded that UVB triggers a functional activation of EGFR in keratinocytes and that therefore the interference of UVB with the EGFR-dependent ERK activation must occur downstream of EGFR.
Since Ras proteins are involved in coupling the signal generated by the ligated EGFR to the kinase cascade composed of c-Raf, MEK, and ERK, we investigated whether UVB is capable of modulating Ras activity in keratinocytes. To this end, we employed Raf-RBD fused to GST. Ras proteins bind Raf-RBD only in their activated state (i.e., GTP loaded) and are incapable of binding Raf-RBD in their inactive state (i.e., GDP loaded) (71). Using agarose beads coupled to GST/Raf-RBD, we pulled down Ras.GTP from lysates prepared from control or UVB-irradiated keratinocytes (HEKn and HEKn-E6/E7). Previous analysis using discriminating antibodies demonstrated the presence in these cells of p21 H-Ras, p21 K-Ras, and p23 R-Ras (data not shown). In agreement with the results of a previous report (28), p23 R-Ras did not display a detectable Raf-RBD binding activity (data not shown). Therefore, the combined Raf-RBD-binding activity in keratinocytes (Fig. 8A) was composed predominantly of p21 H-Ras and p21 K-Ras (Ras activity, for short). Both HEKn and HEKn-E6/E7 displayed a detectable basal activity of Ras (Fig. 8A, lane 1). Importantly, UVB caused a dose- and time-dependent decrease in Ras activity (GTP loading) in both HEKn and HEKn-E6/E7 (Fig. 8A, lanes 2 to 4). These results demonstrated that UVB irradiation induced a transient but potent inhibition of Ras activity in keratinocytes.
FIG. 8.
(A) Inhibition of Ras activity by UVB in HEKn and HEKn-E6/E7 but not in HaCaT cells. Cells (plated and maintained in BKM+exoGF) were irradiated with the indicated doses of UVB, and the levels of GTP loading of Ras (and ERK phosphorylation in the case of HaCaT cells) were determined by immunoblot analysis at the indicated times after the irradiation as described in the text. (B) Existence of a multiprotein complex containing Sos1, Grb2, and ShcA in untreated or UVB-treated HEKn. HEKn (grown in BKM+exoGF) were treated with UVB (1,200 J/m2). Sos1 was immunoprecipitated 30 min later, and the presence of Sos1, Grb2, and the three forms of ShcA (p46, p52, and p66) in the immunoprecipitate was detected in immunoblot analyses.
Curiously, HaCaT cells also displayed easily detectable levels of basal Ras activity, despite the almost undetectable levels of ERK phosphorylation in these cells (Fig. 8A, lane 1). Furthermore, following UVB irradiation, HaCaT cells failed to display an inhibition of their Ras activity (Fig. 8A, lanes 2 to 4). Consistent with the results reported above (Fig. 1B, lanes 6 to 10), the lack of Ras inactivation was paralleled by a transient activation of ERK (Fig. 8A, lane 2). These results further demonstrate that the regulations of the Ras-ERK cascade by UVB in keratinocytes and HaCaT cells are markedly different.
Next, we investigated the possibility that in HEKn UVB effected a functional uncoupling of the pathway that joins the activated EGFR to Ras. The intermediary proteins that constitute this pathway are Grb2, Shc, and Sos. Using an antibody against Sos1, we were able to coimmunoprecipitate from untreated HEKn a multiprotein complex containing Sos1, Grb2, and all three forms of ShcA (p46, p52, and p66) (Fig. 8B). Furthermore, under conditions of strong UVB-induced inhibition of ERK, we failed to detect any disruption of this preexisting complex (Fig. 8B), suggesting that UVB did not cause the inhibition of Ras by disrupting the interactions of Sos with the adaptor protein Grb2.
We next investigated whether UVB would interfere with the recruitment of the adaptor proteins Grb2 and ShcA to the activated EGFR. As expected, the treatment of HEKn with EGF (100 ng/ml) for 10 min led to the increased association of ShcA and Grb2 with the phosphorylated EGFR, as demonstrated by the immunoprecipitation of lysates with an antibody directed against EGFR and immunodetected, subsequently, with antibodies directed against ShcA and Grb2 (Fig. 9, lanes 1 and 6 of panels c, d, e, and f). Interestingly, exposure of cells to UVB (600 to 2,400 J/M2) also led to a dose-dependent increase in the association of ShcA and Grb2 with EGFR (Fig. 9, lanes 1 to 4 of panels c, d, e, and f), despite the fact that in the same cells the phosphorylation of MEK and ERK was strongly inhibited by UVB (Fig. 9, lanes 8 to 10 of panels i and k).
Inactivation of ERK by ribotoxic stressors in keratinocytes.
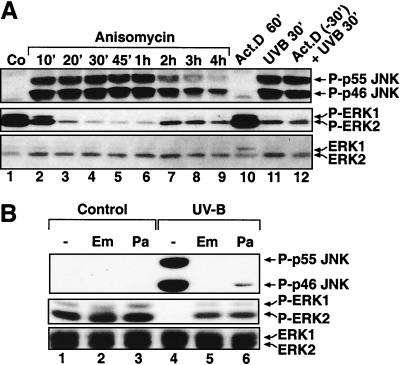
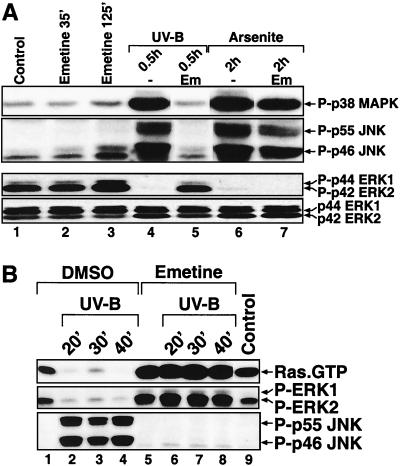
We next investigated whether the inactivation of ERK by UV in keratinocytes is a manifestation of the more general UV-ribotoxic stress response (31, 33, 34). The ribotoxic stressor anisomycin (33) triggered a profound dephosphorylation of ERK in HEKn that was contemporaneous with the induced phosphorylation of JNK (Fig. 10A, lanes 1 to 9). This anisomycin-triggered dephosphorylation of ERK reflected a strong (>95%) inhibition in the basal catalytic activity of ERK (Fig. 2B). The decrease in the levels of phosphorylated ERK was detectable between 10 and 20 min after the addition of anisomycin, and it persisted for at least 4 h after treatment (Fig. 10A, lanes 1 to 9). Emetine and pactamycin, two inhibitors of the UV-ribotoxic stress response (31, 33, 34), completely prevented both the activation of JNK and the inactivation of ERK by UVB in HEKn (Fig. 10B, lanes 4 to 6). In contrast, actinomycin D, an inhibitor of mRNA transcription that does not affect the ribotoxic stress response (31, 33, 34), failed to prevent UVB from causing both the activation of JNK and the inactivation of ERK in HEKn (Fig. 10A, lanes 10 to 12). We have reported previously that emetine specifically inhibits the ribotoxic, but not the oxidative, stress response in Rat-1 cells (31, 33, 34). To test whether emetine is a specific inhibitor of the ribotoxic, but not of the oxidative, stress response in keratinocytes as well, we investigated the regulation of ERK in HEKn by sodium arsenite, an oxidative stressor. Two hours after addition, arsenite triggered the activation of the SAPKs JNK1, JNK2, and p38 MAPK and, simultaneously, the inactivation of ERK (Fig. 11A, lane 6). However, emetine was unable to inhibit any of the effects of arsenite in HEKn (Fig. 11A, compare lanes 6 and 7).
FIG. 10.
The ribotoxic stress response of human keratinocytes. (A) Immunoblot analyses with HEKn (plated and maintained in BKM+exoGF) treated with anisomycin (10 μg/ml) (lanes 1 to 9) or with UVB (1,200 J/m2) (lanes 11 and 12) in the absence or in the presence of a pretreatment (30 min) with actinomycin D (20 μM). The phosphorylation statuses of JNK and ERK were determined at the indicated times after the treatments. (B) Immunoblot analyses with HEKn treated with UVB (1,200 J/m2) in the absence or in the presence of a pretreatment (5 min) with either emetine (Em; 100 μg/ml) or pactamycin (Pa; 0.2 μg/ml). The phosphorylation statuses of JNK and ERK were determined 30 min after the irradiation.
FIG. 11.
The ribotoxic stress response of human keratinocytes. (A) Immunoblot analyses with HEKn (plated and maintained in BKM+exoGF) treated with UVB (1,200 J/m2) or sodium arsenite (200 μg/ml) in the absence or in the presence of a pretreatment (5 min) with emetine (Em, 100 μg/ml). The phosphorylation statuses of p38α MAP kinase, JNK, and ERK were determined at the indicated times after the treatments. -, cells pretreated with the vehicle solvent DMSO. (B) Immunoblot analyses with HEKn-E6/E7 (plated and maintained in BKM+exoGF) treated with UVB (1,200 J/m2) in the absence or in the presence of a pretreatment (5 min) with emetine (100 μg/ml). RasGTP levels were determined at the indicated times after the treatments as shown in Fig. 8A. The phosphorylation statuses of JNK and ERK were assessed from the same cell lysates before the precipitation of Ras with GST/Raf-RBD.
Finally, we determined whether emetine would be able to block the UVB-induced inactivation of Ras. As demonstrated in Fig. 11B, emetine completely prevented the inactivation of Ras triggered by UVB (Fig. 11B, compare lanes 1 to 4 with lanes 5 to 9). Emetine also prevented the UVB-induced inactivation of ERK and the activation of JNK (Fig. 11B, compare lanes 1 to 4 with lanes 5 to 9). The results presented in Fig. 10 and 11 prompted us to conclude that in keratinocytes the UVB response of MAP kinases is indeed a manifestation of the more general ribotoxic stress response.
The phosphorylation of PKB/Akt1 in keratinocytes is maintained by autocrine mechanisms via EGFR and is potentiated, not downregulated, by UVB.
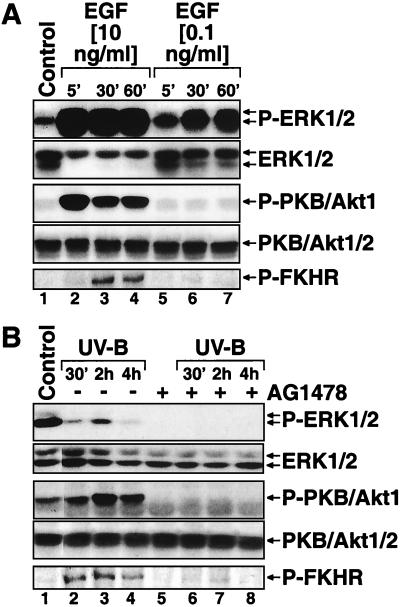
Keratinocyte-conditioned medium (BKM-exoGF) contains a secreted factor(s) that triggered a sustained (>2 h) phosphorylation of PKB/Akt1 in the recipient HaCaT cell line (Fig. 5B, lanes 5 to 8), which displays very low levels of basal phosphorylation of PKB/Akt1 (Fig. 5B, lanes 1 to 4). Addition of AG1478 to keratinocytes in BKM-exoGF triggered a complete dephosphorylation of PKB/Akt1 (Fig. 12B, compare lanes 1 and 5), suggesting that the activity of PKB/Akt1 in keratinocytes is maintained via the autocrine stimulation of EGFR. In support of this conclusion, treatment of HEKn-E6/E7 with EGF potently increased the levels of phosphorylated PKB/Akt1. Interestingly, the lowest dose (0.1 ng/ml) of EGF employed (that was able to cause a detectable phosphorylation of ERK [Fig. 12A, lanes 5 to 7, and 7A, lanes 5 to 7]) was unable to cause a similar phosphorylation of PKB/Akt1 (Fig. 12B, lanes 5 to 7, and 7A, lanes 5 to 7). In contrast to the inhibitory effect of UVB on the Ras-ERK cascade, UVB irradiation induced the phosphorylation of PKB/Akt1 (Fig. 7A, lanes 1 to 4, and 12B, lanes 1 to 4). Both the EGF- and the UVB-induced phosphorylations of PKB/Akt1 led to the functional activation of the kinase, since both treatments caused the phosphorylation (on serine 256) of the transcription factor forkhead in rhabdomyosarcoma (FKHR), a known downstream effector of PKB/Akt1 (56) (Fig. 12A, lanes 1 to 4, and 12B, lane 1 to 4). Pretreatment of cells with AG1478 abolished the ability of UVB to cause the phosphorylation of PKB/Akt1 (Fig. 12B, lanes 5 to 8), suggesting that UVB requires the kinase activity of EGFR to trigger PKB/Akt1 phosphorylation. The EGFR-neutralizing antibody LA1 inhibited the phosphorylation of PKB/Akt1 within 1 h after application (Fig. 6A, lane 5). Intriguingly, however, the phosphorylation of PKB/Akt1 was restored to the original basal levels 6 h after the application of LA1 (Fig. 6A, compare lanes 5 and 6), suggesting that a compensatory, EGFR-independent, mechanism of maintaining the phosphorylated status of PKB/Akt1 is activated in keratinocytes in response to a prolonged inhibition of the activity of EGFR.
FIG. 12.
Activation of PKB/Akt1 in response to EGF and UVB in keratinocytes. (A) Immunoblot analyses with HEKn-E6/E7 placed in BKM-exoGF overnight and treated with low (0.1 ng/ml) or high (10 ng/ml) doses of EGF. The phosphorylation statuses of ERK, PKB/Akt1 (Ser473), and FKHR (Ser256) were assessed at the indicated times after the treatments. (B) Immunoblot analyses with HEKn-E6/E7 placed in BKM-exoGF and treated 2 h later with UVB (1,200 J/M2) in the absence (-) or presence (+) of a 30-min pretreatment with AG1478 (10 μM). The phosphorylation statuses of ERK, PKB/Akt1 (Ser473), and FKHR (Ser256) were assessed at the indicated times after the treatments.
UVB-specific recruitment of a 55-kDa form of the p85 phosphatidylinositide 3-kinase regulatory subunit (PI3K p85 RS) to the activated EGFR.
To investigate the molecular basis for the UVB-induced activation of PKB/Akt1 in keratinocytes and the role of EGFR in this process, we studied the recruitment of PI3K p85 RS to EGFR after UVB and EGF treatments of HEKn. Mammalian PI3K p85 RS is a family of five different polypeptides encoded by three different genes: (i) the p85 α protein; (ii) the p85 β protein, a product of a different gene; (iii) the p50 α protein, a splice variant of p85 α; (iv) the p55 α protein, another splice variant of p85 α; and (v) the p55 γ protein, a product of the p55pik gene (18). All PI3K p85 RS proteins share a highly homologous region consisting of two SH2 domains and an inter-SH2 domain (18). Using a pan-p85 RS antibody that recognizes all five polypeptides, we observed that a single form of PI3K p85 RS with an apparent molecular mass of 55 kDa associated with EGFR specifically after the UVB irradiation but, surprisingly, not after the treatment with EGF (Fig. 9, lanes 1 to 6 of panels g and h [the binding of the 55 kDa form of PI3K p85 RS is obvious in lanes 3 and 4 above the interfering immunoglobulin heavy-chain band]). Whether the p55 protein represents p55 α or p55 γ remains to be elucidated. Neither the p85 α/β nor the p50 α forms associated with EGFR under the experimental conditions employed for immunoprecipitation (Fig. 9, lanes 1 to 6 of panels g and h).
DISCUSSION
The most important findings of the research presented here can be summarized as follows: (i) normal human keratinocytes display high levels of ERK activity that is sustained via the autocrine production of an EGFR ligand(s); (ii) keratinocytes respond to UVB irradiation with a stimulation of EGFR activity above the levels normally maintained by autocrine activation; (iii) the UVB-induced activation of EGFR is followed, unexpectedly, by a rapid and potent inactivation of ERK; (iv) the inactivation of ERK correlates with a UVB-induced (and yet-to-be-identified) signal transduction event that uncouples the EGFR-recruited ShcA and Grb2 from Ras; and (v) the UVB-induced inactivation of the Ras-ERK cascade in keratinocytes belongs to the more general ribotoxic stress response. To our knowledge, this is the first report showing that the functional activation of EGFR need not invariably result in an elevated ERK activity and that it may coincide even with an inhibition of ERK activity. We discuss here both the mechanisms of this inhibition and its potential physiological implications.
We have found that both HEKn and HEKn-E6/E7 appeared to maintain high basal activity of ERK even in the absence of exogenous growth factors (Fig. 3B and 4B). The most likely reason for the high basal activity of ERK in proliferating keratinocytes is the autocrine production of soluble growth factor(s) (Fig. 4A and 5B). Furthermore, based on the use of AG1478 and of the EGFR-neutralizing antibody, we concluded that these factors signal through EGFR. On the other hand, HaCaT cells, a human keratinocyte-derived cell line, (i) have lost the ability to produce an autocrine EGFR ligand(s) (Fig. 4A and 5B), (ii) proliferate poorly even in complete keratinocyte medium (BKM+exoGF) (data not shown), and (iii) proliferate significantly better in the presence of growth factors supplied by fetal calf serum (data not shown). Taken together, these observations make a convincing argument that the autocrine production of an EGFR ligand(s) is critically important for the proliferation and homeostasis of human keratinocytes. Evidence from mice with manipulated egfr alleles (hypomorphic, dominant-negative, or null) suggests that EGFR provides both proliferative and survival signals for the basal keratinocytes in mouse skin (reference 68 and references therein). It has been reported that keratinocytes produce four EGFR ligands, namely TGF-α (11), amphiregulin (13), HB-EGF (25), and epiregulin (67). We are currently investigating the identity of the factors that maintain the high basal levels of ERK activity in HEKn and HEKn-E6/E7.
Using NIH 3T3 mouse fibroblasts and HeLa cells, ERK was identified as the first MAP kinase whose activation was able to be detected early after irradiation with UVC radiation (55). The identification of the ability of UVC to trigger the activation of EGFR (36, 63) gave further credence to the concept of an operational EGFR-ERK cascade as an important mediator of the UV-induced gene expression (6, 26). Evidence has been presented that the ability of UVC and UVB to activate EGFR resulted from the UV-induced incapacitation of protein phosphatases (36). We demonstrate here for the first time that UVB causes the inactivation, rather than activation, of ERK in normal human keratinocytes. The wavelength of the radiation used (UVC versus UVB) cannot account for the differences in ERK behavior, since we have observed the same potent inactivation of ERK in keratinocytes with UVC and with UVB (data not shown) and since both UVC and UVB activate EGFR (36). Therefore, we conclude that it is the cell type and not the type of UV radiation that determines the direction of the response of ERK.
A striking finding in our experiments was that the activation of EGFR in keratinocytes by UVB (Fig. 7B) did not result in the subsequent activation of Ras and ERK but rather in their inactivation. We propose that an important role in this process is played by a UVB-triggered event that uncouples the functionally EGFR-recruited adaptor proteins ShcA and Grb2 (Fig. 9) from the activation of Ras (Fig. 8A). Grb2 contains one SH2 domain flanked by two SH3 domains (42). The SH2 domain of Grb2 binds to tyrosine-phosphorylated residues in growth factor receptors, such as Y1068 and Y1086 of EGFR (5, 50, 74), whereas the SH3 domains bind to proline-rich motifs found in many signaling molecules, such as Sos (9, 52). Sos is a guanine nucleotide exchange factor that greatly increases the activation of Ras in response to activation of growth factor receptors (9). Since we failed to detect a UVB-induced disruption of the interaction of Sos with Grb2 and ShcA (Fig. 8B), it is likely that the Sos-Grb2 association is not the relevant target of the UVB-triggered inhibitory signal. It is possible that UVB interferes with the enzymatic activities of Sos and/or Ras GAP proteins (for a review, see reference 71).
Since the UVB response of keratinocytes has both stimulatory (activation of SAPK) and inhibitory (inactivation of ERK) signal transduction components, it is reasonable to ask whether the two components originate from the same primary event (e.g., the interaction of UVB with the same sensory chromophore). Recent studies using nonepidermal cells have suggested that ribosomes actively engaged in translational elongation may be the cellular structures where the UV-induced signal leading to the activation of SAPK is initiated (31, 34). The results presented in Fig. 10 and 11 strongly suggest that both the stimulatory (activation of SAPK) and the inhibitory (inactivation of ERK) signal transduction components of the UVB response in keratinocytes require active ribosomes to occur. Interestingly, while the activation of SAPK by UVB is invariant in nonepidermal cells as well as in keratinocytes (31, 34), the UVB response of ERK is markedly different. In nonepidermal cells, UVC and UVB stimulated the activity of ERK in a way that required growth factor receptors but did not require active ribosomes (31, 34; unpublished data). In contrast, keratinocytes require EGFR to maintain the basal activity of ERK but require active ribosomes for the UVB-induced inhibition of ERK activity.
Although the UVB-induced inhibition of ERK is invariant in its occurrence in all experiments that we have performed in HEKn or HEKn-E6/E7, the duration of this inhibition was subject to some variation from experiment to experiment. Furthermore, while we have observed a precise temporal correlation between the onsets of inhibitions of Ras and of ERK whenever these analyses have been performed in parallel, we have observed that the basal activity of Ras tends to be restored faster than that of ERK. For instance, 2 h after irradiation of keratinocytes with 1,200 J/m2 of UVB, the activity of Ras was typically similar to the basal Ras activity (Fig. 8A). At the same time, the phosphorylation of ERK in most experiments did not reach basal levels until at least 4 to 6 h after the irradiation (Fig. 3A). These results suggest that the activity of ERK in keratinocytes may be regulated by UVB at a more complex level than the activity of Ras alone.
Observations with mice with a genetic inactivation of the egfr gene have demonstrated the involvement of this receptor in the development of normal mouse skin (reference 68 and references therein). However, similar genetic evidence for the possible roles of ERK in the development of the mouse skin is still lacking. Particularly insufficient is our knowledge of the involvement of the EGFR-Ras-ERK cascade in the maintenance of skin homeostasis in long-lived mammals such as humans. However, indirect evidence strongly suggests that the proliferative state of basal keratinocytes in human skin crucially depends on the activity of the EGFR-Ras-ERK cascade. For instance, phosphorylated ERK has been detected, via immunohistochemical methods, almost exclusively in the stratum basale, the proliferative compartment of the skin harboring the keratinocyte stem cells (see below) (1, 70). Furthermore, abnormally increased phosphorylation of ERK in the stratum spinosum has been observed in cases of psoriasis, a disorder that is characterized by a keratinocyte hyperproliferation in the suprabasal layers (24). Finally, patients enrolled in clinical trials of blocking anti-EGFR antibodies for the treatment of head-and-neck cancer displayed a marked decrease of ERK phosphorylation in the stratum basale. This decrease in ERK phosphorylation correlated with a dramatic decrease in the proliferation of basal keratinocytes (1). In view of the abovementioned observations, we propose that the inactivation of the Ras-ERK pathway in proliferating basal keratinocytes (particularly in epidermal stem cells; see below) may provide an important initial signal arresting the cell cycle progression of cells that have not yet entered S phase (Fig. 13). The model shown in Fig. 13 is supported by the rapid (>50% within 2 h) decrease in the proportion of keratinocytes engaged in DNA synthesis after irradiation with UVB (unpublished data). It is thought that the major mediator of cell cycle arrest in UV-irradiated cells is the p53 tumor suppressor (29). Surprisingly, we found that UVB irradiation of HEKn (p53-proficient cells [Fig. 2A, lanes 1 to 5]) and HEKn-E6/E7 (p53-deficient cells [Fig. 2A, lanes 6 to 10]) resulted in a similar rapid decrease in the [3H]thymidine incorporation in both cell types (unpublished data). These results suggest that a p53-independent mechanism of cell cycle arrest is operational in UVB-irradiated keratinocytes. In support of the hypothesis that this p53-independent signal triggering a cell cycle arrest in irradiated keratinocytes may indeed be provided by the inhibition of ERK activity, we have found that the steady-state levels of cyclin D1 decline substantially in UVB-irradiated keratinocytes. It has been found that the expression of cyclin D1 requires the sustained activity of ERK (3, 76). Therefore, it is intriguing to speculate that even a transient inhibition of ERK activity in response to UVB may trigger a prolonged decline in the cyclin D1 levels (Fig. 3A).
FIG. 13.
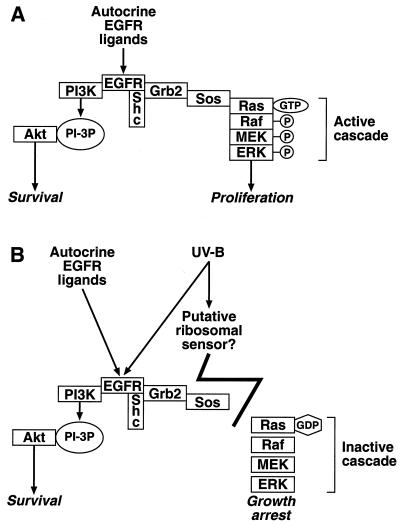
A model for the selective interference of UVB radiation with the EGFR-dependent signal transduction in keratinocytes. (A) In the absence of UVB, the autocrine stimulation of EGFR maintains an active Ras-ERK cascade that is involved in cell proliferation (see Discussion). (B) EGFR also generates an antiapoptotic signal that is mediated via the PI3K-PKB/Akt1 cascade (reference 68 and references therein). UVB irradiation causes the selective inactivation of the Ras-ERK cascade, whereas the signal transduction pathway leading to the activation of PKB/Akt1 remains intact (or is even stimulated [Fig. 12]).
Epidermal stem cells (i.e., cells that remain in stratum basale after each cell division and retain their full proliferative potential) in the skin of long-lived mammalian organisms are likely to have developed unique ways to deal with repeated episodes of acute encounter with solar radiation. Suprabasal cells, such as differentiating or fully differentiated keratinocytes, can be eliminated by apoptosis (sunburn cells) without lasting consequences for the organism. However, shunting stratum basale stem cells into apoptosis after repeated episodes of acute irradiation may lead to a gradual depletion of the epidermis of stem cells, thereby severely hampering the continuous renewal of the skin. At the other extreme, tipping the balance toward increased survival after an encounter with a potent mutagen such as UVB carries the risk of a malignant transformation of the epidermal stem cell (thus leading to epidermal carcinomas). It seems likely that one sound strategy for fostering the survival of UVB-irradiated epidermal stem cells without the accumulation of mutations would be to arrest their cell cycle progression before they enter S phase (while actively suppressing apoptosis) until the DNA damage has been repaired. Upon completion of DNA repair, the epidermal stem cells (having been spared apoptotic cell death) can resume their important role of providing a continuous supply of differentiation-committed suprabasal keratinocytes. The newly described phenomenon of the UVB-induced uncoupling of the signaling pathway leading from EGFR to the activation of ERK presented here may suggest one possible mechanism executing exactly such a complex response to UVB. This hypothesis is outlined in Fig. 13. In proliferating basal keratinocytes, the low-level but persistent activation of EGFR (due to autocrine EGFR ligands) provides survival (antiapoptotic) and proliferative signals. The antiapoptotic program may be mediated through the sustained activity of the EGFR-PI3K-PKB/Akt1 cascade, whereas the mitogenic program may be mediated through the EGFR-Ras-ERK cascade (reference 68 and references therein) (Fig. 13A). By uncoupling EGFR from the Ras-ERK cascade, UVB may trigger the growth arrest program of the cell (Fig. 13B). At the same time, by stimulating the intrinsic tyrosine kinase activity of EGFR, UVB may potentiate the necessary survival signals, mediated by an EGFR-driven activation of PKB/Akt1, to prevent apoptosis (Fig. 13B). The experimental testing of this hypothesis is under way.
Finally, it should be noted that with regard to the responsiveness of ERK signaling to UVB, the HaCaT cell line behaves like fibroblasts and HeLa cells but unlike HEKn and HEKn-E6/E7. Cell lines selected by atypical culture conditions (e.g., serum-dependent growth in the case of HaCaT cells) thus may not provide useful (under some circumstances) models for the study of tissue-specific responses to toxic metabolites, carcinogens, and environmental stresses. The results presented here underscore the need for the development of suitable human tissue explants for studying these responses.
Acknowledgments
We thank Paul Spitz for the excellent technical assistance, Gail Clinton for the FGF-2, and Tim Bowden for the HaCaT cell line.
This work was supported by U.S. Public Health Service grants CA-39360 and ES-08456 to B.E.M. and CA-93718 to M.S.I.
REFERENCES
- 1.Albanell, J., J. Codony-Servat, F. Rojo, J. M. Del Campo, S. Sauleda, J. Anido, G. Raspall, J. Giralt, J. Rosello, R. I. Nicholson, J. Mendelsohn, and J. Baselga. 2001. Activated extracellular signal-regulated kinases: association with epidermal growth factor receptor/transforming growth factor alpha expression in head and neck squamous carcinoma and inhibition by anti-epidermal growth factor receptor treatments. Cancer Res. 61:6500-6510. [PubMed] [Google Scholar]
- 2.Assoian, R. K., and M. A. Schwartz. 2001. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr. Opin. Genet. Dev. 11:48-53. [DOI] [PubMed] [Google Scholar]
- 3.Balmanno, K., and S. J. Cook. 1999. Sustained MAP kinase activation is required for the expression of cyclin D1, p21Cip1 and a subset of AP-1 proteins in CCL39 cells. Oncogene 18:3085-3097. [DOI] [PubMed] [Google Scholar]
- 4.Batzer, A. G., P. Blaikie, K. Nelson, J. Schlessinger, and B. Margolis. 1995. The phosphotyrosine interaction domain of Shc binds an LXNPXY motif on the epidermal growth factor receptor. Mol. Cell. Biol. 15:4403-4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batzer, A. G., D. Rotin, J. M. Urena, E. Y. Skolnik, and J. Schlessinger. 1994. Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol. Cell. Biol. 14:5192-5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bender, K., C. Blattner, A. Knebel, M. Iordanov, P. Herrlich, and H. J. Rahmsdorf. 1997. UV-induced signal transduction. J. Photochem. Photobiol. B 37:1-17. [DOI] [PubMed] [Google Scholar]
- 7.Bogdan, S., and C. Klambt. 2001. Epidermal growth factor receptor signaling. Curr. Biol. 11:R292-R295. [DOI] [PubMed] [Google Scholar]
- 8.Boukamp, P., R. T. Petrussevska, D. Breitkreutz, J. Hornung, A. Markham, and N. E. Fusenig. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106:761-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chardin, P., J. H. Camonis, N. W. Gale, L. van Aelst, J. Schlessinger, M. H. Wigler, and D. Bar-Sagi. 1993. Human Sos1: a guanine nucleotide exchange factor for Ras that binds to GRB2. Science 260:1338-1343. [DOI] [PubMed] [Google Scholar]
- 10.Cobb, M. H. 1999. MAP kinase pathways. Prog. Biophys. Mol. Biol. 71:479-500. [DOI] [PubMed] [Google Scholar]
- 11.Coffey, R. J., Jr., R. Derynck, J. N. Wilcox, T. S. Bringman, A. S. Goustin, H. L. Moses, and M. R. Pittelkow. 1987. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. Nature 328:817-820. [DOI] [PubMed] [Google Scholar]
- 12.Coleman, M. L., and C. J. Marshall. 2001. A family outing: small GTPases cyclin' through G1. Nat. Cell Biol. 3:E250-E251. [DOI] [PubMed] [Google Scholar]
- 13.Cook, P. W., P. A. Mattox, W. W. Keeble, M. R. Pittelkow, G. D. Plowman, M. Shoyab, J. P. Adelman, and G. D. Shipley. 1991. A heparin sulfate-regulated human keratinocyte autocrine factor is similar or identical to amphiregulin. Mol. Cell. Biol. 11:2547-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 15.Davis, R. J. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103:239-252. [DOI] [PubMed] [Google Scholar]
- 16.Dong, C., R. J. Davis, and R. A. Flavell. 2001. Signaling by the JNK group of MAP kinases. c-jun N-terminal kinase. J. Clin. Immunol. 21:253-257. [DOI] [PubMed] [Google Scholar]
- 17.Dulin, N. O., A. Sorokin, and J. G. Douglas. 1998. Arachidonate-induced tyrosine phosphorylation of epidermal growth factor receptor and Shc-Grb2-Sos association. Hypertension 32:1089-1093. [DOI] [PubMed] [Google Scholar]
- 18.Funaki, M., H. Katagiri, K. Inukai, M. Kikuchi, and T. Asano. 2000. Structure and function of phosphatidylinositol-3,4 kinase. Cell. Signal. 12:135-142. [DOI] [PubMed] [Google Scholar]
- 19.Gale, N. W., S. Kaplan, E. J. Lowenstein, J. Schlessinger, and D. Bar-Sagi. 1993. Grb2 mediates the EGF-dependent activation of guanine nucleotide exchange on Ras. Nature 363:88-92. [DOI] [PubMed] [Google Scholar]
- 20.Gazit, A., P. Yaish, C. Gilon, and A. Levitzki. 1989. Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J. Med. Chem. 32:2344-2352. [DOI] [PubMed] [Google Scholar]
- 21.Gilchrest, B. A., H. Y. Park, M. S. Eller, and M. Yaar. 1996. Mechanisms of ultraviolet light-induced pigmentation. Photochem. Photobiol. 63:1-10. [DOI] [PubMed] [Google Scholar]
- 22.Goldfarb, M. 2001. Signaling by fibroblast growth factors: the inside story. Science's STKE 2001:PE37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gross, I., B. Bassit, M. Benezra, and J. D. Licht. 2001. Mammalian sprouty proteins inhibit cell growth and differentiation by preventing ras activation. J. Biol. Chem. 276:46460-46468. [DOI] [PubMed] [Google Scholar]
- 24.Haase, I., R. M. Hobbs, M. R. Romero, S. Broad, and F. M. Watt. 2001. A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis. J. Clin. Investig. 108:527-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hashimoto, K., S. Higashiyama, H. Asada, E. Hashimura, T. Kobayashi, K. Sudo, T. Nakagawa, D. Damm, K. Yoshikawa, and N. Taniguchi. 1994. Heparin-binding epidermal growth factor-like growth factor is an autocrine growth factor for human keratinocytes. J. Biol. Chem. 269:20060-20066. [PubMed] [Google Scholar]
- 26.Herrlich, P., K. Bender, A. Knebel, F. D. Bohmer, S. Gross, C. Blattner, H. J. Rahmsdorf, and M. Gottlicher. 1999. Radiation-induced signal transduction. Mechanisms and consequences. C. R. Acad. Sci. Ser. III 322:121-125. [DOI] [PubMed] [Google Scholar]
- 27.Herrlich, P., C. Sachsenmaier, A. Radler-Pohl, S. Gebel, C. Blattner, and H. J. Rahmsdorf. 1994. The mammalian UV response: mechanism of DNA damage induced gene expression. Adv. Enzyme Regul. 34:381-395. [DOI] [PubMed] [Google Scholar]
- 28.Herrmann, C., G. Horn, M. Spaargaren, and A. Wittinghofer. 1996. Differential interaction of the ras family GTP-binding proteins H-Ras, Rap1A, and R-Ras with the putative effector molecules Raf kinase and Ral-guanine nucleotide exchange factor. J. Biol. Chem. 271:6794-6800. [DOI] [PubMed] [Google Scholar]
- 29.Inohara, S., K. Kitagawa, and Y. Kitano. 1996. Coexpression of p21Waf1/Cip1 and p53 in sun-exposed normal epidermis, but not in neoplastic epidermis. Br. J. Dermatol. 135:717-720. [PubMed] [Google Scholar]
- 30.Iordanov, M., K. Bender, T. Ade, W. Schmid, C. Sachsenmaier, K. Engel, M. Gaestel, H. J. Rahmsdorf, and P. Herrlich. 1997. CREB is activated by UVC through a p38/HOG-1-dependent protein kinase. EMBO J. 16:1009-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iordanov, M. S., and B. E. Magun. 1999. Different mechanisms of c-Jun NH(2)-terminal kinase-1 (JNK1) activation by ultraviolet-B radiation and by oxidative stressors. J. Biol. Chem. 274:25801-25806. [DOI] [PubMed] [Google Scholar]
- 32.Iordanov, M. S., and B. E. Magun. 1998. Loss of cellular K+ mimics ribotoxic stress. Inhibition of protein synthesis and activation of the stress kinases SEK1/MKK4, stress-activated protein kinase/c-Jun NH2-terminal kinase 1, and p38/HOG1 by palytoxin. J. Biol. Chem. 273:3528-3534. [DOI] [PubMed] [Google Scholar]
- 33.Iordanov, M. S., D. Pribnow, J. L. Magun, T. H. Dinh, J. A. Pearson, S. L. Chen, and B. E. Magun. 1997. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the alpha-sarcin/ricin loop in the 28S rRNA. Mol. Cell. Biol. 17:3373-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iordanov, M. S., D. Pribnow, J. L. Magun, T. H. Dinh, J. A. Pearson, and B. E. Magun. 1998. Ultraviolet radiation triggers the ribotoxic stress response in mammalian cells. J. Biol. Chem. 273:15794-15803. [DOI] [PubMed] [Google Scholar]
- 35.Iordanov, M. S., J. Wong, D. L. Newton, S. M. Rybak, R. K. Bright, R. A. Flavell, R. J. Davis, and B. E. Magun. 2000. Differential requirement for the stress-activated protein kinase/c-Jun NH(2)-terminal kinase in RNA damage-induced apoptosis in primary and in immortalized fibroblasts. Mol. Cell Biol. Res. Commun. 4:122-128. [DOI] [PubMed] [Google Scholar]
- 36.Knebel, A., H. J. Rahmsdorf, A. Ullrich, and P. Herrlich. 1996. Dephosphorylation of receptor tyrosine kinases as target of regulation by radiation, oxidants or alkylating agents. EMBO J. 15:5314-5325. [PMC free article] [PubMed] [Google Scholar]
- 37.Kolch, W. 2000. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J. 351:289-305. [PMC free article] [PubMed] [Google Scholar]
- 38.Kulms, D., and T. Schwarz. 2000. Molecular mechanisms of UV-induced apoptosis. Photodermatol. Photoimmunol. Photomed. 16:195-201. [DOI] [PubMed] [Google Scholar]
- 39.Levkowitz, G., H. Waterman, S. A. Ettenberg, M. Katz, A. Y. Tsygankov, I. Alroy, S. Lavi, K. Iwai, Y. Reiss, A. Ciechanover, S. Lipkowitz, and Y. Yarden. 1999. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol. Cell 4:1029-1040. [DOI] [PubMed] [Google Scholar]
- 40.Lewis, T. S., P. S. Shapiro, and N. G. Ahn. 1998. Signal transduction through MAP kinase cascades. Adv. Cancer Res. 74:49-139. [DOI] [PubMed] [Google Scholar]
- 41.Liefer, K. M., M. I. Koster, X. J. Wang, A. Yang, F. McKeon, and D. R. Roop. 2000. Down-regulation of p63 is required for epidermal UVB-induced apoptosis. Cancer Res. 60:4016-4020. [PubMed] [Google Scholar]
- 42.Lowenstein, E. J., R. J. Daly, A. G. Batzer, W. Li, B. Margolis, R. Lammers, A. Ullrich, E. Y. Skolnik, D. Bar-Sagi, and J. Schlessinger. 1992. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell 70:431-442. [DOI] [PubMed] [Google Scholar]
- 43.Matsui, M. S., and V. A. DeLeo. 1991. Longwave ultraviolet radiation and promotion of skin cancer. Cancer Cells 3:8-12. [PubMed] [Google Scholar]
- 44.Migliaccio, E., S. Mele, A. E. Salcini, G. Pelicci, K. M. Lai, G. Superti-Furga, T. Pawson, P. P. Di Fiore, L. Lanfrancone, and P. G. Pelicci. 1997. Opposite effects of the p52shc/p46shc and p66shc splicing isoforms on the EGF receptor-MAP kinase-fos signalling pathway. EMBO J. 16:706-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller, D. L., and M. A. Weinstock. 1994. Nonmelanoma skin cancer in the United States: incidence. J. Am. Acad. Dermatol. 30:774-778. [DOI] [PubMed] [Google Scholar]
- 46.Minden, A., and M. Karin. 1997. Regulation and function of the JNK subgroup of MAP kinases. Biochim. Biophys. Acta 1333:F85-F104. [DOI] [PubMed] [Google Scholar]
- 47.Murphy, G., A. R. Young, H. C. Wulf, D. Kulms, and T. Schwarz. 2001. The molecular determinants of sunburn cell formation. Exp. Dermatol. 10:155-160. [DOI] [PubMed] [Google Scholar]
- 48.Norris, D. A., K. Whang, K. David-Bajar, and S. D. Bennion. 1997. The influence of ultraviolet light on immunological cytotoxicity in the skin. Photochem. Photobiol. 65:636-646. [DOI] [PubMed] [Google Scholar]
- 49.Okabayashi, Y., Y. Kido, T. Okutani, Y. Sugimoto, K. Sakaguchi, and M. Kasuga. 1994. Tyrosines 1148 and 1173 of activated human epidermal growth factor receptors are binding sites of Shc in intact cells. J. Biol. Chem. 269:18674-18678. [PubMed] [Google Scholar]
- 50.Okutani, T., Y. Okabayashi, Y. Kido, Y. Sugimoto, K. Sakaguchi, K. Matuoka, T. Takenawa, and M. Kasuga. 1994. Grb2/Ash binds directly to tyrosines 1068 and 1086 and indirectly to tyrosine 1148 of activated human epidermal growth factor receptors in intact cells. J. Biol. Chem. 269:31310-31314. [PubMed] [Google Scholar]
- 51.Osherov, N., and A. Levitzki. 1994. Epidermal-growth-factor-dependent activation of the src-family kinases. Eur. J. Biochem. 225:1047-1053. [DOI] [PubMed] [Google Scholar]
- 52.Pawson, T., and J. D. Scott. 1997. Signaling through scaffold, anchoring, and adaptor proteins. Science 278:2075-2080. [DOI] [PubMed] [Google Scholar]
- 53.Pelicci, G., L. Lanfrancone, F. Grignani, J. McGlade, F. Cavallo, G. Forni, I. Nicoletti, T. Pawson, and P. G. Pelicci. 1992. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell 70:93-104. [DOI] [PubMed] [Google Scholar]
- 54.Pellegrini, G., E. Dellambra, O. Golisano, E. Martinelli, I. Fantozzi, S. Bondanza, D. Ponzin, F. McKeon, and M. De Luca. 2001. p63 identifies keratinocyte stem cells. Proc. Natl. Acad. Sci. USA 98:3156-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Radler-Pohl, A., C. Sachsenmaier, S. Gebel, H. P. Auer, J. T. Bruder, U. Rapp, P. Angel, H. J. Rahmsdorf, and P. Herrlich. 1993. UV-induced activation of AP-1 involves obligatory extranuclear steps including Raf-1 kinase. EMBO J. 12:1005-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rena, G., S. Guo, S. C. Cichy, T. G. Unterman, and P. Cohen. 1999. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J. Biol. Chem. 274:17179-17183. [DOI] [PubMed] [Google Scholar]
- 57.Rincon, M., R. A. Flavell, and R. J. Davis. 2001. Signal transduction by MAP kinases in T lymphocytes. Oncogene 20:2490-2497. [DOI] [PubMed] [Google Scholar]
- 58.Rodrigues, G. A., M. Falasca, Z. Zhang, S. H. Ong, and J. Schlessinger. 2000. A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol. Cell. Biol. 20:1448-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romerdahl, C. A., L. C. Stephens, C. Bucana, and M. L. Kripke. 1989. The role of ultraviolet radiation in the induction of melanocytic skin tumors in inbred mice. Cancer Commun. 1:209-216. [PubMed] [Google Scholar]
- 60.Roovers, K., and R. K. Assoian. 2000. Integrating the MAP kinase signal into the G1 phase cell cycle machinery. Bioessays 22:818-826. [DOI] [PubMed] [Google Scholar]
- 61.Rotin, D., B. Margolis, M. Mohammadi, R. J. Daly, G. Daum, N. Li, E. H. Fischer, W. H. Burgess, A. Ullrich, and J. Schlessinger. 1992. SH2 domains prevent tyrosine dephosphorylation of the EGF receptor: identification of Tyr992 as the high-affinity binding site for SH2 domains of phospholipase C gamma. EMBO J. 11:559-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rozakis-Adcock, M., J. McGlade, G. Mbamalu, G. Pelicci, R. Daly, W. Li, A. Batzer, S. Thomas, J. Brugge, P. G. Pelicci, et al. 1992. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature 360:689-692. [DOI] [PubMed] [Google Scholar]
- 63.Sachsenmaier, C., A. Radler-Pohl, R. Zinck, A. Nordheim, P. Herrlich, and H. J. Rahmsdorf. 1994. Involvement of growth factor receptors in the mammalian UVC response. Cell 78:963-972. [DOI] [PubMed] [Google Scholar]
- 64.Schaeffer, H. J., and M. J. Weber. 1999. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol. Cell. Biol. 19:2435-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schlessinger, J. 2000. Cell signaling by receptor tyrosine kinases. Cell 103:211-225. [DOI] [PubMed] [Google Scholar]
- 66.Schwartz, M. A., and R. K. Assoian. 2001. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J. Cell Sci. 114:2553-2560. [DOI] [PubMed] [Google Scholar]
- 67.Shirakata, Y., T. Komurasaki, H. Toyoda, Y. Hanakawa, K. Yamasaki, S. Tokumaru, K. Sayama, and K. Hashimoto. 2000. Epiregulin, a novel member of the epidermal growth factor family, is an autocrine growth factor in normal human keratinocytes. J. Biol. Chem. 275:5748-5753. [DOI] [PubMed] [Google Scholar]
- 68.Sibilia, M., A. Fleischmann, A. Behrens, L. Stingl, J. Carroll, F. M. Watt, J. Schlessinger, and E. F. Wagner. 2000. The EGF receptor provides an essential survival signal for SOS-dependent skin tumor development. Cell 102:211-220. [DOI] [PubMed] [Google Scholar]
- 69.Sorkin, A., M. Mazzotti, T. Sorkina, L. Scotto, and L. Beguinot. 1996. Epidermal growth factor receptor interaction with clathrin adaptors is mediated by the Tyr974-containing internalization motif. J. Biol. Chem. 271:13377-13384. [DOI] [PubMed] [Google Scholar]
- 70.Takahashi, H., M. Honma, A. Ishida-Yamamoto, K. Namikawa, H. Kiyama, and H. Iizuka. 2001. Expression of human cystatin A by keratinocytes is positively regulated via the Ras/MEKK1/MKK7/JNK signal transduction pathway but negatively regulated via the Ras/Raf-1/MEK1/ERK pathway. J. Biol. Chem. 276:36632-36638. [DOI] [PubMed] [Google Scholar]
- 71.Takai, Y., T. Sasaki, and T. Matozaki. 2001. Small GTP-binding proteins. Physiol. Rev. 81:153-208. [DOI] [PubMed] [Google Scholar]
- 72.Taylor, C. R., R. S. Stern, J. J. Leyden, and B. A. Gilchrest. 1990. Photoaging/photodamage and photoprotection. J. Am. Acad. Dermatol. 22:1-15. [DOI] [PubMed] [Google Scholar]
- 73.Tibbles, L. A., and J. R. Woodgett. 1999. The stress-activated protein kinase pathways. Cell. Mol. Life Sci. 55:1230-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ward, C. W., K. H. Gough, M. Rashke, S. S. Wan, G. Tribbick, and J. Wang. 1996. Systematic mapping of potential binding sites for Shc and Grb2 SH2 domains on insulin receptor substrate-1 and the receptors for insulin, epidermal growth factor, platelet-derived growth factor, and fibroblast growth factor. J. Biol. Chem. 271:5603-5609. [DOI] [PubMed] [Google Scholar]
- 75.Watt, F. M. 2001. Stem cell fate and patterning in mammalian epidermis. Curr. Opin. Genet. Dev. 11:410-417. [DOI] [PubMed] [Google Scholar]
- 76.Weber, J. D., D. M. Raben, P. J. Phillips, and J. J. Baldassare. 1997. Sustained activation of extracellular-signal-regulated kinase 1 (ERK1) is required for the continued expression of cyclin D1 in G1 phase. Biochem. J. 326:61-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang, A., M. Kaghad, Y. Wang, E. Gillett, M. D. Fleming, V. Dotsch, N. C. Andrews, D. Caput, and F. McKeon. 1998. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol. Cell 2:305-316. [DOI] [PubMed] [Google Scholar]