Abstract
The molecular mechanism for the regulated exocytosis of dense-core granules in endocrine cells remains relatively uncharacterized compared to that of synaptic vesicles in neurons. A novel set of Rab and its effector, Rab27a/granuphilin, which is localized on insulin granules in pancreatic beta cells, was recently identified. Here we demonstrate that granuphilin directly binds to syntaxin 1a on the plasma membrane, and this interaction is regulated by Rab27a. Granuphilin shows affinity to syntaxin 1a with a closed conformation but not to mutant syntaxin 1a, which adopts an open conformation constitutively. Overexpression of granuphilin significantly enhances basal insulin secretion but profoundly inhibits high K+-induced insulin secretion. The effect of granuphilin on insulin secretion was impaired by its mutation that disrupts the binding to either Rab27a or syntaxin 1a. Thus, granuphilin is the first regulator in the exocytotic pathway that functions by directly connecting two critical vesicle transport proteins, Rab and SNARE.
The fusion of intracellular vesicles with their target membranes is an essential feature of the compartmental structure of eukaryotic cells, and the protein families involved in this process, such as Rab GTPase and soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE), are conserved from yeast to humans. Different Rabs and SNAREs are localized to distinct membrane compartments, and both have been proposed to ensure the fidelity of fusion. Rab GTPases and their effectors, which interact with Rabs in a manner dependent on the state of the bound nucleotide, mediate tethering of vesicles to their cognate target membranes (20). They are often incorporated into distinct multiprotein complexes, called tethering factors, that function on different classes of vesicles (8). Unlike SNAREs and Rabs, Rab effectors appear to share no detectable sequence similarity, and this uniqueness may in part ensure the specificity of the targeting process. During subsequent docking and priming processes, SNAREs anchored in the vesicle membrane form stable, membrane bridging complexes with SNAREs anchored in the target membrane. Critical to the formation of these complexes is a dynamic structural change of syntaxin, one of the SNAREs on the target membrane, from a closed conformation to an open conformation (15). Because SNARE assembly is inhibited by the closed configuration adopted by syntaxin in vitro, additional factors are thought to be necessary to catalyze its conformational change. Although the compartmentalization of Rabs, Rab effectors, and SNAREs within specific domains should ensure the fidelity and the efficiency of membrane pairing and fusion (32), the mechanistic links between the tethering machinery and the activation of SNAREs have not been well characterized.
In contrast to vesicle trafficking routes, such as endoplasmic reticulum (ER)-to-Golgi transport, and endocytotic pathways that are conserved in all eukaryotic cells, a regulated exocytosis is developed only in a subset of tissues in multicellular organisms. These tissues are equipped with special organelles to store and secrete bioactive molecules, such as synaptic vesicles in neurons, secretory granules in endocrine and exocrine cells, and melanosomes in melanocytes. These organelles themselves are not essential for the life of host cells but are fundamental to intercellular communications at organ and body levels. Thus, specific proteins that are involved in the organization of these membrane compartments might be differentiated in multicellular organisms. Consistent with this idea, whole-genome sequence projects for several eukaryotic organisms reveal that the Rab family uniquely expands from the unicellular yeast to the multicellular organisms among the protein families that are involved in vesicle trafficking (3). A novel protein, granuphilin, was recently discovered that is expressed primarily in pancreatic beta cells and pituitary tissue (28). Granuphilin has a domain structure similar to that of the Rab3 effector rabphilin-3. Furthermore, we identified Rab27a as a binding partner of granuphilin by yeast two-hybrid, in vitro binding, and coimmunoprecipitation experiments (31). Granuphilin and Rab27a are colocalized on the membrane of insulin granules in pancreatic beta cells. Overexpression of Rab27a causes a significant increment of high K+-induced insulin secretion in the pancreatic beta cell line MIN6. Collectively, granuphilin likely functions as a Rab27a effector protein and may be involved in the tethering and/or fusion process of secretory granules in pancreatic beta cells.
In the present study we demonstrate that granuphilin directly associates with the plasma membrane SNARE, syntaxin 1a, and that this interaction is regulatable by Rab27a. Overexpression of granuphilin in pancreatic beta cell lines enhances insulin secretion at the basal state and inhibits it at the depolarization-stimulated state. Analyses using mutant proteins indicate that the effect on insulin secretion by transfected granuphilin is dependent on its ability to bind syntaxin 1a and Rab27a. Furthermore, we show that granuphilin has affinity toward syntaxin 1a exclusively with the closed conformation, which may correlate with its bidirectional effects on insulin secretion. Granuphilin is the first Rab effector that functions through a direct interaction with SNARE protein in the regulated exocytotic pathway.
MATERIALS AND METHODS
DNA construction.
Full-length mouse granuphilin-a and -b cDNAs (28) were subcloned into the EcoRI and XhoI sites of pcDNA3-HA (27). Site-directed mutagenesis of granuphilin was performed by using the following primers: 5′;-AGCAACTGGAGATTCGTTTTATGAC-3′; and 5′;-TACTTTCTGGTCATAAAACGAATCTC-3′; for W118S and 5′;-GCGACTGAAGAATGAGGCCCTGGAG-3′; and 5′;-CTTTTGATCTCCAGGGCCTCATTC-3′; for L43A. The resulting PCR products were cloned into restriction enzyme sites of mouse granuphilin-a cDNA. All the subcloned PCR products were directly sequenced. Expression plasmids encoding enhanced green fluorescent protein (EGFP)-fused wild-type Rab27a and EGFP-Rab27a T23N were constructed by cloning Rab27a cDNAs in pcDNA3.1/HisC vector (31) into the pEGFP-C2 vector (Clontech Laboratories, Palo Alto, Calif.). Recombinant adenovirus bearing granuphilin-a cDNA was prepared as described previously (31).
Cell culture and transfection.
MIN6 mouse insulinoma cell lines were grown in high-glucose (25 mM) Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. INS-1 rat insulinoma cells were cultured in RPMI 1640 medium supplemented with 50 μM 2-mercaptoethanol and 10% fetal calf serum. Transfections were performed with Lipofectamine 2000 reagent (Invitrogen, Groningen, The Netherlands) according to the manufacturer's instructions. MIN6 cells that stably express syntaxin 1a were established by using pcDNA3 vector (Invitrogen) containing syntaxin 1a cDNA with the myc epitope tag at the N terminus (12). Tetracycline-inducible INS-1/tet-on Rab27a Q78L cells were established by methods described previously (7). Briefly, Rab27a Q78L cDNA (31) was subcloned into the downstream of the tetO/cytomegalovirus (CMV) promoter in pUHD10-3(Tet-On) vector and then was introduced into INS-1 cells simultaneously with the pUHD172-1neo plasmid. After stable transfectants were selected in the presence of 0.2 mg of G418 (Sigma Chemical Co., St. Louis, Mo.)/ml, Rab27a-inducible cell clones were evaluated by immunoblotting with anti-Rab27a antibodies. Selected INS-1/Rab27a Q78L cell clones were treated with doxycycline (Sigma Chemical Co.) to induce exogenous Rab27a expression. The optimal concentration and time of doxycycline treatment were 5 μg/ml and 48 h, respectively (data not shown).
Antibodies.
Rabbit antigranuphilin antibodies, αGrp-aC, which recognizes granuphilin-a (28), and αGrp-N, which recognizes both granuphilin-a and -b (31), were previously described and characterized. The rabbit antigranuphilin antibody αGrp-bC was raised against a synthetic peptide covering the C-terminal 15 amino acids of mouse granuphilin-b, (C)VMAKWWTGWIRLVKK, and specifically recognize granuphilin-b. Anti-GFP polyclonal antibodies were purchased from MBL (Nagoya, Japan). Anti-syntaxin 1a (HPC-1) mouse monoclonal antibodies were purchased from Sigma Chemical Co. Anti-Rab27a, anti-SNAP-25, and anti-Munc-18 mouse monoclonal antibodies were purchased from BD Transduction Laboratories (Lexington, Ky.). Antihemagglutinin (anti-HA) rat (clone 3F10) and anti-c-myc mouse (clone 9E10) monoclonal antibodies were purchased from Roche Diagnostics (Mannheim, Germany).
Indirect immunofluorescence microscopy.
Indirect immunofluorescence analysis was performed as described previously (26). Briefly, cells on 8-well Lab-Tek chamber slides were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. The cells were then incubated with primary antibodies followed by fluorescein isothiocyanate- or indocarbocyanine-conjugated, species-specific anti-immunoglobulin G secondary antibodies (Jackson ImmunoResearch, West Grove, Pa.). The cells were observed with an epifluorescence microscope (BX-50; Olympus Optical Co., Tokyo, Japan) equipped with a SenSys charge-coupled device camera (Photometrics, Tucson, Ariz.) or a laser-scanning confocal system (MRC-1024; Bio-Rad, Hercules, Calif.).
Immunoprecipitation and immunoblot analysis.
Cell extracts were prepared in lysis buffer A (10 mM Tris [pH 7.5], 150 mM NaCl, 2 mM MgCl2, 1 mM EGTA, 0.2% NP-40, 0.5 mM phenylmethylsulfonyl fluoride, 5 μg of aprotinin/ml). The antibodies were collected by agitation with protein G-Sepharose 4FF (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). Immunoprecipitates and original cell extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then were transferred onto an Immobilon-P membrane (Millipore, Bedford, Mass.). Antibody detection was accomplished by using enhanced chemiluminescent Western blotting detection reagents (Amersham Pharmacia Biotech).
In vitro binding assay with GST fusion proteins.
cDNAs encoding cytoplasmic portions of mouse syntaxin 1a (amino acids 1 to 264) and syntaxin 3 (amino acids 1 to 263) were amplified from the MIN6 cell cDNA library by PCR and were subcloned into pGEX4T-1 vector (Amersham Pharmacia Biotech). A fragment containing point mutations of rat syntaxin 1a (L165A/E166A) cDNA in the pCMV vector (6) was cut by BamHI and DraIII and ligated into wild-type rat syntaxin 1a cDNA in the pGEX-KG vector. Bacterially expressed glutathione S-transferase (GST)-fused proteins were affinity-purified with glutathione-Sepharose beads. MIN6 cell extracts were prepared in lysis buffer B (10 mM Tris [pH 7.5], 150 mM KCl, 2.5 mM MgCl2, 1 mM EGTA, 0.5% Triton X-100). HA-granuphilin was translated in vitro with the TNT Coupled Reticulocyte Lysate System (Promega, Madison, Wis.). HA-tagged caspase 10CA, as previously described (27), was similarly translated and used as a control protein. Purified GST-fused proteins immobilized on glutathione beads were incubated at 4°C with either MIN6 cell extracts (2 mg of total proteins) in buffer B for 12 h or 10 μl of in vitro-translated proteins in 0.1 ml of buffer B without Triton X-100 for 30 min. The bound proteins were analyzed by immunoblotting.
Measurement of insulin secretion.
Insulin secretion measurement was performed exactly as previously described (31). Briefly, MIN6 cells were infected with recombinant adenoviruses. Forty hours later the cells were incubated for 2 h in modified Krebs-Ringer buffer (120 mM NaCl, 5 mM KCl, 24 mM NaHCO3, 1 mM MgCl2, 2 mM CaCl2, 15 mM HEPES [pH 7.4], 0.1% bovine serum albumin, 2 mM glucose) and then were incubated for 30 min in the same buffer or the buffer modified to include high K+ (60 mM KCl-65 mM NaCl). Insulin in the medium was measured in duplicate.
RESULTS
Granuphilin is colocalized with syntaxin 1a at the peripheral space.
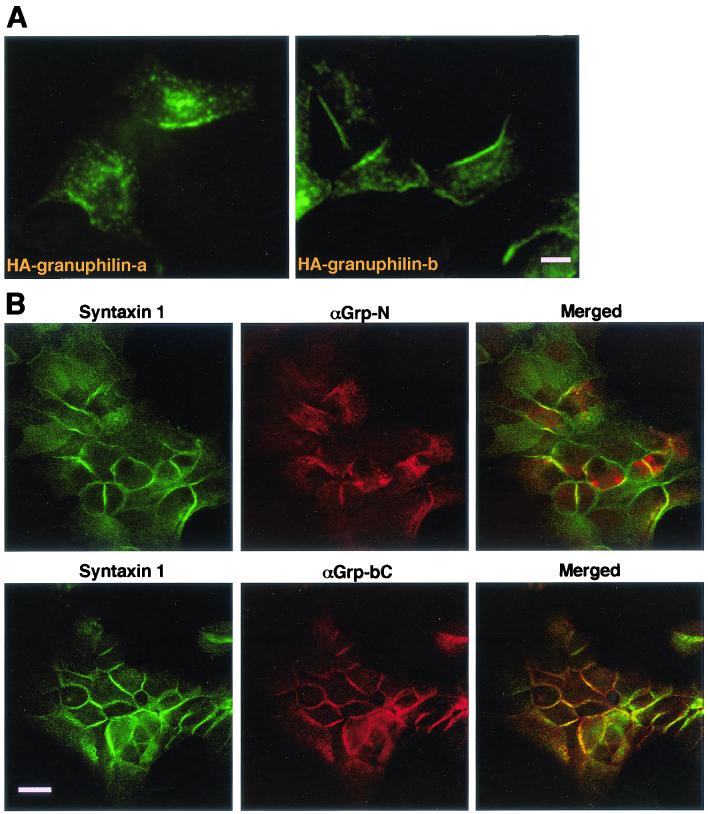
We recently demonstrated that Rab27a and its possible effector, granuphilin, are peripherally localized on the membrane of insulin-containing dense-core granules by using both subcellular fractionation and immunogold electron microscopy (28, 31). We have also recognized, by using immunofluorescence microscopy, that both proteins are often concentrated just beneath the plasma membrane as well as on secretory granules. As shown in Fig. 1A, exogenously expressed HA-tagged granuphilin-a and -b revealed punctate signals concentrated in a region along the plasma membrane in MIN6 cells. The peripheral staining was more prominent for granuphilin-b, which lacks the second C-terminal C2 domain of granuphilin-a, possibly because of alternative splicing (28). The intracellular distribution of endogenous granuphilins is identical to that of exogenous ones: a peripheral localization was more evident by immunostaining with antibodies specific to granuphilin-b (αGrp-bC) than with those recognizing both granuphilin-a and -b (αGrp-N) (Fig. 1B, center panels). These observations combined with previous findings suggest that granuphilins are associated with mature secretory granules that are ready to dock at the plasma membrane in pancreatic beta cells.
FIG. 1.
Granuphilin-a and -b associate with secretory granules in a peripheral region and are colocalized with syntaxin 1a. (A) MIN6 cells were transfected with a plasmid encoding HA-tagged granuphilin-a (left panel) or granuphilin-b (right panel). After 24 h, the cells were fixed and stained with anti-HA antibodies. Bar, 10 μm. (B) MIN6 cells were fixed with methanol and double-immunostained with anti-syntaxin 1a and an antigranuphilin antibody, αGrp-N, which recognizes both granuphilin-a and -b (upper panels), or αGrp-bC, which recognizes only granuphilin-b (lower panels). Bar, 20 μm.
In general, Rab and its effector protein are considered to direct transport vesicles to their appropriate target membranes and then to associate directly or indirectly with SNARE machinery (20, 32). In the case of endosomal homotypic fusion, a Rab5 effector EEA1 tethers two membranes upon assembly into transient oligomeric complexes with Rabaptin-5, Rabex-5, NSF, and the local endosome SNARE, syntaxin 13 (14). Because syntaxin 1a, but not 1b, is expressed and functions at the plasma membrane of pancreatic beta cells (18), we examined whether the localization of granuphilin-a and -b at the cell periphery is overlapped with that of syntaxin 1a. Analysis by confocal microscopy revealed that granuphilin and syntaxin 1a are colocalized within the peripheral plasma membrane region in MIN6 cells (Fig. 1B), suggesting that granuphilin associates with secretory granules that are tethered to the plasma membrane. Similar colocalization was observed in another pancreatic beta cell line, INS-1 (data not shown).
Granuphilin interacts with syntaxin 1a.
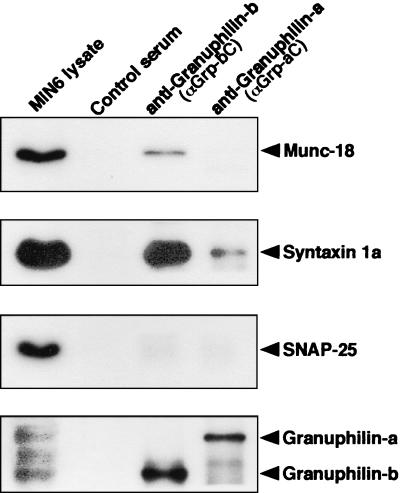
To explore the possibility that granuphilin and syntaxin 1a physiologically interact in cells, coimmunoprecipitation experiments were performed with MIN6 cell lysates. Both granuphilin-a and granuphilin-b immunoprecipitates contained a significant amount of syntaxin 1a, although the latter had a larger amount (Fig. 2). Absence of syntaxin 1a in the immunoprecipitates of control serum suggests that the interaction between granuphilin and syntaxin 1a is specific. Because syntaxins bind various proteins to form a variety of structural states, e.g., ternary trans-SNARE complex consisting of synaptobrevin2 (VAMP 2) and SNAP-25, we examined the existence of other proteins, such as Munc-18, SNAP-25, synaptobrevin2, synaptotagmin I, and syntaxin 4, in granuphilin immunoprecipitates. However, none of them was detected in this assay condition, except that a small mount of Munc-18 was often visible (Fig. 2; data not shown).
FIG. 2.
Granuphilin interacts with syntaxin 1a in intact cells. MIN6 cell extracts (1 mg of each) were incubated with either anti-granuphilin-a antibody (αGrp-aC), anti-granuphilin-b antibody (αGrp-bC), or control rabbit serum. Each immunoprecipitate and an aliquot of the original lysates (10 μg) were analyzed by immunoblotting with antibodies against Munc-18, syntaxin 1a, or SNAP-25. The bottom panel shows the level of granuphilins immunoprecipitated. Ten percent of each immunoprecipitate and the original lysates (10 μg) were analyzed by immunoblotting with anti-granuphilin antibody (αGrp-N).
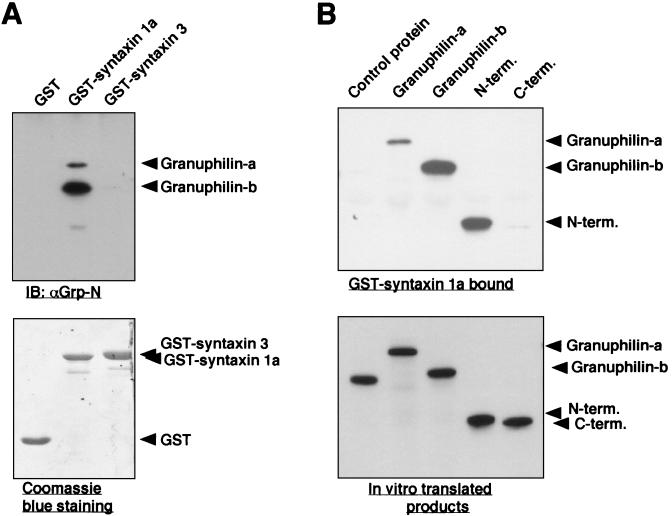
To further investigate the interaction between granuphilin and syntaxin 1a, an in vitro binding assay was performed. First, MIN6 cell extracts were incubated with bacterially expressed GST fusion proteins, and bound granuphilin was detected by immunoblotting with anti-granuphilin antibodies. Granuphilin-a and -b were specifically bound to GST-syntaxin 1a but not to GST alone or to GST-syntaxin 3 (Fig. 3A). Although expression levels of granuphilin-a and -b were similar in MIN6 cells (31), more granuphilin-b was cosedimented with syntaxin 1a. We next analyzed whether the interaction between granuphilin and syntaxin 1a is direct or indirect. GST-syntaxin 1a bound to HA-tagged granuphilin translated in vitro but did not bind to HA-tagged control protein similarly produced (Fig. 3B), suggesting that the interaction is direct and specific. It should be noted that granuphilin-b again shows a stronger interaction than granuphilin-a. The region within granuphilin required for binding to syntaxin 1a was mapped to the N-terminal portion containing a zinc finger motif (residues 1 to 300). Because Rab27a also interacts with the N-terminal domain of granuphilin (31), we pursued the possibility that syntaxin 1a and Rab27a share the binding region of granuphilin. However, no competitive inhibition was found on the binding to granuphilin between syntaxin 1a and Rab27a (data not shown).
FIG. 3.
Granuphilin directly binds to syntaxin 1a in vitro. (A) GST fusion proteins (1 μg of each) containing syntaxin 1a or syntaxin 3 as well as GST alone immobilized to glutathione beads were incubated with MIN6 cell extracts and then were washed four times. Proteins that bound to the GST fusion proteins were analyzed by immunoblotting (IB) with αGrp-N antibody (upper panel). Total GST protein levels were visualized by Coomassie blue staining (lower panel). (B) GST-fused samples of syntaxin 1a (0.5 μg) were incubated with 10 μl of in vitro-translated HA-tagged caspase 10CA (control protein), granuphilin-a, granuphilin-b, and N-terminal (N-term.; amino acids 1 to 300) or C-terminal (C-term.; amino acids 331 to 673) granuphilin-a. Bound proteins (upper panel) as well as 1 μl of each of the in vitro-translated products (lower panel) were analyzed by immunoblotting with anti-HA antibodies.
Rab27a stimulates interaction of granuphilin with syntaxin 1a.
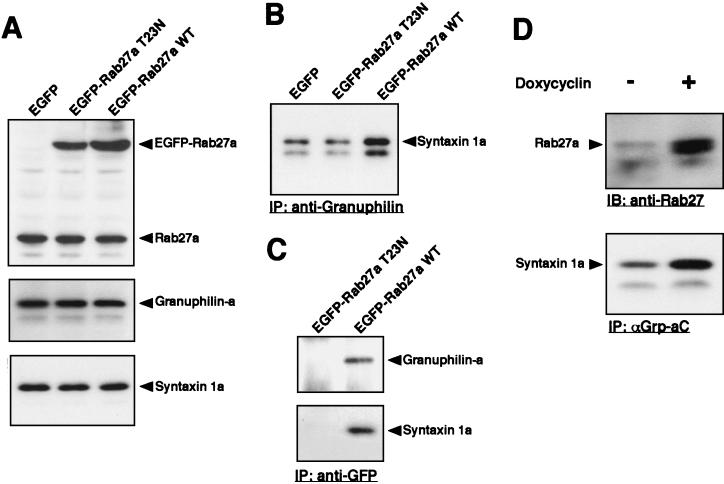
Regarding competition, we observed that Rab27a potentiates the interaction of granuphilin with syntaxin 1a. EGFP-tagged wild-type Rab27a and dominant-negative Rab27a T23N, as well as control EGFP, were transiently overexpressed in MIN6 cells. Although untagged or Xpress-tagged Rab27a T23N was poorly expressed in MIN6 cells (31), EGFP-Rab27a T23N was produced as efficiently as wild-type EGFP-Rab27a Fig. 4A). When wild-type Rab27a was overexpressed, the amount of syntaxin 1a coprecipitated with anti-granuphilin antibodies was significantly increased (Fig. 4B). In contrast, overexpression of Rab27a T23N slightly suppressed the level of syntaxin 1a bound to granuphilin compared to that of the control. Immunoprecipitation with anti-GFP antibodies further demonstrated that wild-type Rab27a, but not the T23N mutant, forms a complex with granuphilin and syntaxin 1a (Fig. 4C). Because direct binding of Rab27a to syntaxin 1a was not detected by the in vitro binding assay (data not shown), it is likely that the interaction of Rab27a with syntaxin 1a found in MIN6 cells is mediated through granuphilin.
FIG. 4.
Rab27a potentiates the interaction of granuphilin with syntaxin 1a. (A to C) MIN6 cells were transiently transfected for 24 h with expression plasmids encoding EGFP-tagged wild-type (WT) Rab27a, EGFP-Rab27a T23N, or control EGFP. (A) The expression levels of EGFP-Rab27a and endogenous Rab27a, granuphilin-a, and syntaxin 1a in each cell lysate were determined by immunoblotting with anti-Rab27a, αGrp-aC, and anti-syntaxin 1a antibodies. (B) Coimmunoprecipitates of antigranuphilin antibodies (a mixture of αGrp-aC and αGrp-bC) were analyzed by immunoblotting with anti-syntaxin 1a antibodies. (C) EGFP-Rab27a-associated proteins were examined by immunoblotting with αGrp-aC and anti-syntaxin 1a antibodies. (D) INS-1/tet-on Rab27a Q78L cells were incubated in the presence or absence of 2 μg of doxycycline/ml for 48 h. Cell lysates were immunoblotted with anti-Rab27a antibodies to examine the inducible expression of Rab27a protein (upper panel). Coimmunoprecipitates of αGrp-aC were analyzed by immunoblotting with anti-syntaxin 1a antibodies (lower panel). IP, immunoprecipitation.
To exclude cell line specificity or any influence of the EGFP epitope tag, we used the rat insulinoma cell line INS-1 and untagged Rab27a for the next analysis. In addition, the tetracycline-induced expression system (Tet-On) was employed to exclude variability in individual cells and to overcome low transfection efficiency. cDNA encoding a constitutive active form, Rab27a Q78L, under the tetO-CMV promoter (7) was introduced into INS-1 cells. One of the stable clones displayed a significant induction of Rab27a Q78L expression in the presence of doxycycline (Fig. 4D, upper panel). Upon the induction, the amount of syntaxin 1a coprecipitated with anti-granuphilin antibodies was significantly increased (Fig. 4D, lower panel). These findings indicate that Rab27a stimulates the interaction of granule-associated granuphilin with plasma membrane syntaxin 1a when activated to a GTP-bound form.
Granuphilin mutants defective in binding to syntaxin 1a or Rab27a.
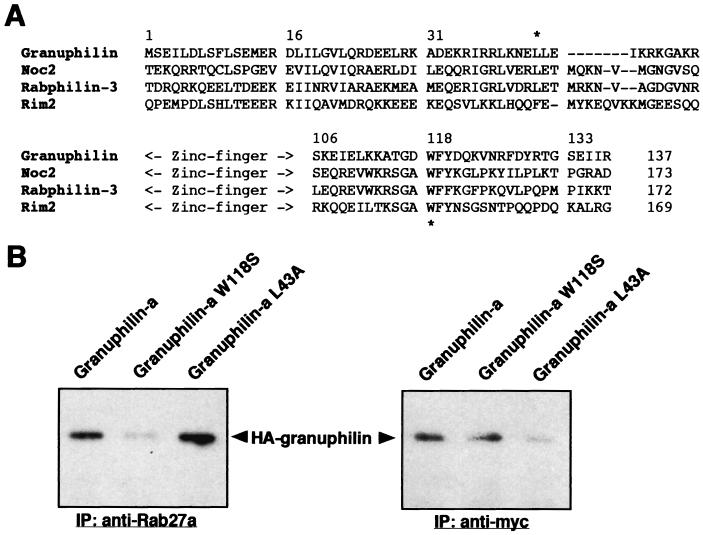
We have demonstrated that both Rab27a and syntaxin 1a bind to the N-terminal half of granuphilin (31) (Fig. 3B) and that their ternary (or more) complexes exist in intact cells (Fig. 4C). To explore the amino acids critical for binding to Rab27a or syntaxin 1a, point mutations were introduced into granuphilin. In one mutant granuphilin, L43A, the leucine residue upstream of a zinc finger region was replaced with alanine, whereas in another mutant granuphilin, W118S, the tryptophan residue conserved among Rab3-binding proteins was altered to serine (Fig. 5A). W118 of granuphilin corresponds to the tryptophan residue in an SGAWFF structural element of rabphilin-3, which was suggested, by stereology, to be an important region for binding with Rab3a (19).
FIG. 5.
The mutations of tryptophan 118 and leucine 43 prevent granuphilin from binding to Rab27a and syntaxin 1a, respectively. (A) Amino acid sequences around the N-terminal zinc finger motif of mouse granuphilin (amino acids 1 to 137), rat Noc2 (amino acids 38 to 173), mouse rabphilin-3 (amino acids 37 to 172), and mouse Rim2 (amino acids 23 to 169) are aligned. Asterisks indicate the positions of the amino acids mutated in this study. (B) MIN6 cells stably expressing myc-tagged syntaxin 1a were transiently transfected with plasmids encoding HA-tagged wild-type granuphilin-a or its mutants, W118S and L43A. Immunoprecipitates (IP) of anti-Rab27a or anti-myc antibodies were analyzed by immunoblotting with anti-HA rat monoclonal antibodies.
Because the syntaxin antibodies we used were not suitable for immunoprecipitation, MIN6 cells stably expressing myc-tagged syntaxin 1a were generated and employed for the coimmunoprecipitation studies (Fig. 5B). Transfection of wild-type and mutant HA-granuphilin followed by immunoblot analysis revealed that each form of HA-granuphilin was similarly expressed (data not shown). As expected, Rab27a or syntaxin 1a immunoprecipitates contained a significant amount of wild-type HA-granuphilin. In contrast, mutant W118S was barely detectable in Rab27a immune complexes but was recovered to a degree comparable to that of the wild type in syntaxin 1a immune complexes. On the other hand, mutant L43A was readily detected in Rab27a immunoprecipitates but was not detected in syntaxin 1a immunoprecipitates. These findings indicate that the W118S and L43A mutations specifically disrupt the interaction of granuphilin with Rab27a and that with syntaxin 1a, respectively.
Granuphilin modulates the regulated exocytosis of secretory granules.
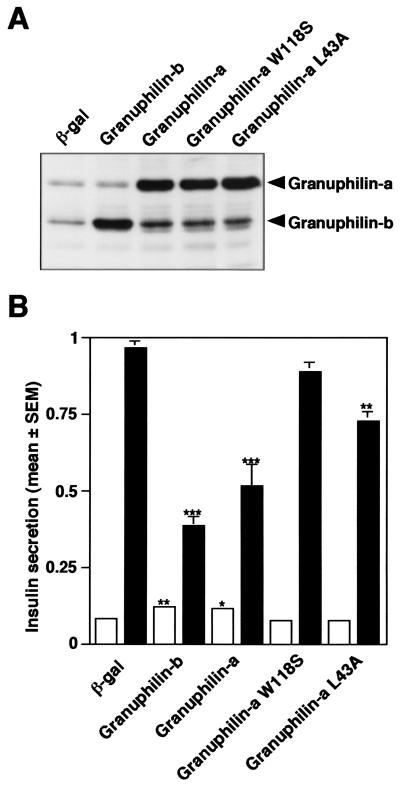
The data presented above indicate that granuphilin seems to tether two key transport factors, Rab and SNARE, that function on insulin granules. We then investigated the effect of overexpressed granuphilin on the final fusion process by measuring the amount of insulin secreted in media. Because the transfection efficiency in MIN6 cells was not high by conventional transfection methods, we employed recombinant adenovirus to overexpress granuphilin as previously done for Rab27a (31). In our experiment almost all cells were infected, and exogenous proteins were expressed much more abundantly than the endogenous ones (Fig. 6A). While overexpression of granuphilin-a caused a 1.4-fold increase in basal insulin secretion, it significantly inhibited high K+-induced insulin secretion (Fig. 6B; 46% decrease). Immunofluorescence analysis revealed that insulin was clearly accumulated inside the cells (data not shown). Compared with granuphilin-a, granuphilin-b had stronger effects on both basal and stimulated secretion states (1.5-fold enhancement and 59% inhibition, respectively), which may correlate with the intracellular localization and syntaxin 1a-binding activity of this isoform. Thus, overexpression of granuphilin elicits distinct effects on basal and depolarization-stimulated exocytosis of insulin granules.
FIG. 6.
Overexpression of granuphilin modulates the exocytosis of insulin granules. MIN6 cells were infected with adenoviruses bearing either β-galactosidase (β-gal), granuphilin-b, or wild-type or mutant granuphilin-a cDNA. (A) The expression levels of endogenous and exogenous granuphilin proteins were determined by immunoblotting with antigranuphilin antibody (αGrp-N). (B) The cells were incubated for 30 min in either modified Krebs-Ringer buffer (open bars) or the buffer modified to include high K+ (solid bars). Values were normalized to the release of insulin from uninfected MIN6 cells stimulated by high K+. The results are given as means ± standard errors of the means (SEM) of five independent experiments. ∗, P < 0.05; ∗∗, P < 0.005; ∗∗∗, P < 0.0005, versus high K+-stimulated MIN6 cells infected with the same titer of the virus bearing β-gal cDNA. Note that the effect of granuphilin on insulin secretion was either completely (W118S) or partially (L43A) lost in the mutants defective in binding with Rab27a or syntaxin 1a.
We then compared the effects of wild-type and mutant forms of granuphilin on insulin secretion. In contrast to wild-type granuphilin-a, overexpression of either W118S or L43A granuphilin-a did not significantly change insulin secretion at the basal state (Fig. 6B). Furthermore, the inhibitory effect of granuphilin on exocytosis at the stimulated state was either completely (W118S) or partially (L43A) lost. Immunostaining of mutant W118S revealed a diffuse cytosolic distribution, which may reflect the inert binding activity of this mutant to Rab27a (data not shown). These observations suggest that interactions with both Rab27a and syntaxin 1a are critical for granuphilin to affect insulin secretion.
Granuphilin binds to syntaxin 1a with a closed conformation.
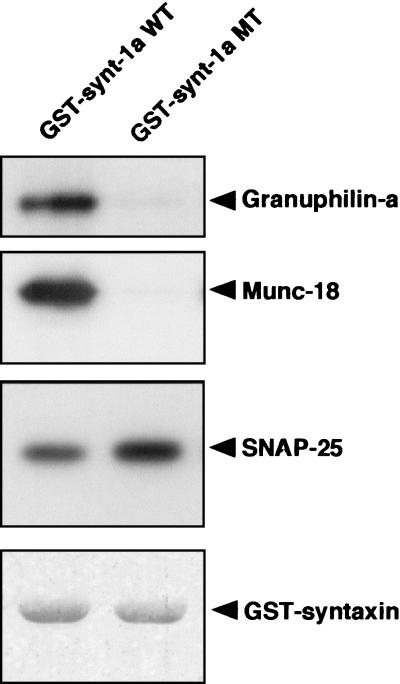
While the enhancement of basal secretion by granuphilin may reflect an increase in the spontaneously releasable pool by its ability to target insulin granules close to the plasma membrane where syntaxin 1a is localized, its inhibitory effect on high K+-induced insulin secretion was unexpected. Structural analyses indicate that syntaxin in solution exists in equilibrium between a closed form and an open form that is capable of forming a trans-SNARE complex (15). We next examined whether granuphilin interacts with the closed form or the open form of syntaxin 1a. For this purpose, we used a syntaxin 1a mutant, L165A/E166A, that is known to adopt a constantly open conformation in vitro (6). MIN6 cell extracts were incubated with GST-fused wild-type or mutant (L165A/E166A) syntaxin 1a protein, and bound granuphilin was detected by immunoblotting. Like Munc-18 as previously reported (6), granuphilin showed essentially no binding activity to the open form of syntaxin 1a, whereas SNAP-25 bound both wild-type and mutant syntaxin 1a (Fig. 7). This feature of granuphilin in binding with syntaxin 1a may cause, in part, the inhibitory effect on insulin secretion, because the closed conformation of syntaxin inhibits trans-SNARE assembly (15, 16).
FIG. 7.
Granuphilin exclusively binds to the closed form of syntaxin 1a. GST fusion proteins (1 μg of each) containing wild-type (WT) or mutant (MT; L165A/E166A) rat syntaxin 1a immobilized to glutathione beads were incubated with MIN6 cell extracts and then washed four times. Proteins that bound to the GST fusion proteins were analyzed by immunoblotting with antibodies against granuphilin (αGrp-aC), Munc-18, or SNAP-25. Total GST protein levels were visualized by Coomassie blue staining (bottom panel).
DISCUSSION
In the present study we demonstrate that granuphilin is involved in the exocytotic transport of insulin granules through direct interactions with both granule-associated Rab27a and plasma membrane syntaxin 1a. Recent studies of other transport pathways indicate that Rabs and their effectors regulate the initial membrane recognition and tethering process (20). For example, TRAPP I acts as a specific receptor for ER-derived COP II vesicles, and once bound, Ypt1p/Rab1 is then activated and binds to its effector protein Uso1p/p115, which in turn interacts directly with a select set of SNARE proteins, including syntaxin 5, and tethers vesicles with target membranes (1, 4, 21). In the case of endosome fusion, the Rabaptin-5/Rabex-5 complex activates Rab5, and then its effector protein, EEA1, is recruited to the endosome membrane and assembles into a macromolecular complex with Rabaptin-5, Rabex-5, NSF, and syntaxin 13 (14). These results suggest that the tethering and docking process may be mediated in part by the interaction of Rab effector proteins with syntaxins. The interaction of the Rab27a/granuphilin complex with syntaxin 1a may also mediate the tethering of secretory granules to the plasma membrane. Consistent with this hypothesis, our preliminary morphological data suggest that transfected granuphilin induces the translocation of granules to the plasma membrane, and this activity is highly correlated with its ability to bind syntaxin 1a (S. Torii and T. Izumi, unpublished observations). The emerging discovery of molecular interactions between Rab effectors and components of the SNARE machinery will provide a new understanding of how Rab proteins directly regulate SNARE function and ensure the fidelity of membrane fusion.
We showed that active Rab27a enhances the interaction of granuphilin with syntaxin 1a. A recent characterization of Rab27a-mutated cells derived from Griscelli patients or ashen mice has shown that Rab27a is required for the peripheral localization of melanosomes in melanocytes (2) and for the tight clustering of lytic granules at the immunological synapse in cytotoxic T lymphocytes (25). Furthermore, both genetic and biochemical evidence suggests that Rab27a functions in conjunction with the actin-based motor myosin-Va (10, 17, 30). Genetic alterations that cause pigmentary dilution in leaden mice, whose phenotypes are indistinguishable from those of ashen mice, have recently been discovered (13). The responsible gene encodes a novel protein, melanophilin, which has homology to the N-terminal Rab27a-binding region of granuphilin and is thus considered a possible Rab27a effector in melanocytes. These previous studies, combined with our own findings, suggest that Rab27a and its effectors, possibly with myosin-Va, regulate the translocation and tethering and docking processes of granule-like organelles.
It should be noted, however, that granuphilin expression per se does not lead to final fusion. In fact, overexpression of granuphilin significantly inhibits high K+-induced insulin secretion, although it enhances basal insulin secretion. These findings suggest that other unknown factors are involved in further downstream prefusion and fusion events. Granuphilin immune complexes contain syntaxin 1a but not other SNARE proteins, such as SNAP-25 and synaptobrevin2, suggesting that granuphilin binds to unpaired syntaxin 1a. This is similar to the interaction of the Class C Vps protein complex with the syntaxin homolog Vam3 in Golgi-to-vacuole transport (22). Our GST-pull-down assay revealed that granuphilin interacts with the closed form of syntaxin 1a and is not incorporated into the SNARE complex. Structural analyses indicate that the N-terminal Habc domain interacts with the C-terminal H3 SNARE motif region in the closed conformation of syntaxin, which is thought to inhibit SNARE complex formation (15, 16). Our data thus suggest that granuphilin promotes the plasma membrane targeting of secretory granules through the direct binding to syntaxin 1a, but additional factors are required to promote the subsequent structural rearrangements within syntaxin 1a to prime the fusion reaction. This sequential model may explain our findings regarding the enhancement of insulin secretion at the basal state and the inhibition of high K+-stimulated secretion when granuphilin is overexpressed.
It has recently been shown that overexpression of active Rab27a enhances high K+-induced insulin secretion in MIN6 cells (31), in contrast to the inhibitory effect of granuphilin presented here. The inhibitory effect of granuphilin can be partially reversed upon the cooverexpression of wild-type Rab27a (Z. Yi and T. Izumi, unpublished observations). Similar opposite effects of Rab and its effector on regulated secretion have been reported previously. While overexpression of Rab3a inhibits agonist- or high-K+-stimulated exocytosis in chromaffin cells and PC12 cells, overexpression of its effector rabphilin-3 or Rim enhances it (5, 9, 29). Rabphilin-3 and Rim are localized to distinct subcellular compartments in synapses (29). Rab27a could also have multiple downstream effectors. Alternatively, granuphilin may constitute Rab27a-independent protein complexes. It has been recently demonstrated that both rabphilin-3 and Rim have Rab3a-independent functions in vivo (23, 24). Thus, it is possible that overexpression of a single component produces complex effects on the sum of exocytotic events. Nonetheless, it is noteworthy that the effects of wild-type and mutant granuphilins on insulin secretion correlate precisely with their ability to bind syntaxin 1a and Rab27a.
The mutational analysis of granuphilin and the competitive binding assay in vitro suggest that Rab27a and syntaxin 1a have different binding sites. The crystal structure of activated Rab3a bound to the effector domain of rabphilin-3 reveals an interface that consists of a hydrophobic surface pocket in Rab3a and an SGAWFF structural element of rabphilin-3 (19). Since the corresponding sequence of granuphilin is TGDWFY, we generated a mutant, W118S, and used it for the interaction analysis. Defective binding of W118S to Rab27a provides biochemical evidence that this tryptophan-based motif is functional for the granuphilin/Rab27a interaction. Another mutant granuphilin, L43A, holds the binding activity to Rab27a but loses the activity to syntaxin 1a. This leucine corresponds to leucine 83 of rabphilin-3, which was considered to be within an amphipathic α-helix of the N-terminal region and demonstrated a partial determinant of its membrane localization (11).
In summary, the present study identifies granuphilin as the first Rab effector in exocytosis that directly links Rab GTPase and SNARE machinery. We provide evidence that this interaction is regulatable by Rab27a and modulates the exocytosis of insulin granules. Granuphilin likely regulates the targeting and subsequent priming and fusion processes through the interaction with syntaxin 1a. The identification and characterization of proteins involved in possible granuphilin complexes will further elucidate the molecular mechanism of the exocytosis of secretory granules.
Acknowledgments
We thank M. Hosaka (Gunma University) for generous support and useful advice. We also thank K. Kasai and K. Akagawa (Kyorin University School of Medicine) for the expression vector of myc-tagged syntaxin 1a, T. C. Südhof (The University of Texas Southwestern Medical Center) for rat syntaxin 1a cDNAs, and H. Bujard (University of California Berkeley) for tetracycline-inducible expression vectors.
This work was supported by a grant in aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and in part by grants from the Yamanouchi Foundation for Research on Metabolic Disorders and a Japan Insulin Study Group Award to T.I.
REFERENCES
- 1.Allan, B. B., B. D. Moyer, and W. E. Balch. 2000. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science 289:444-448. [DOI] [PubMed] [Google Scholar]
- 2.Bahadoran, P., E. Aberdam, F. Mantoux, R. Buscà, K. Bille, N. Yalman, G. de Saint-Basile, R. Casaroli-Marano, J.-P. Ortonne, and R. Ballotti. 2001. Rab27a: a key to melanosome transport in human melanocytes. J. Cell Biol. 152:843-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bock, J. B., H. T. Matern, A. A. Peden, and R. H. Scheller. 2001. A genomic perspective on membrane compartment organization. Nature 409:839-841. [DOI] [PubMed] [Google Scholar]
- 4.Cao, X., N. Ballew, and C. Barlowe. 1998. Initial docking of ER-derived vesicle requires Uso1p and Ypt1 but is independent of SNARE proteins. EMBO J. 17:2156-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung, S.-H., Y. Takai, and R. W. Holz. 1995. Evidence that the Rab3a-binding protein, Rabphilin3a, enhances regulated secretion: studies in adrenal chromaffin cells. J. Biol. Chem. 270:16714-16718. [DOI] [PubMed] [Google Scholar]
- 6.Dulubova, I., S. Sugita, S. Hill, M. Hosaka, I. Fernandez, T. C. Südhof, and J. Rizo. 1999. A conformational switch in syntaxin during exocytosis: role for munc18. EMBO J. 16:4372-4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gossen, M., S. Freundlieb, G. Bender, G. Müller, W. Hillen, and H. Bujard. 1995. Transcriptional activation by tetracyclines in mammalian cells. Science 268:1766-1769. [DOI] [PubMed] [Google Scholar]
- 8.Guo, W., M. Sacher, J. Barrowman, S. Ferro-Novick, and P. Novick. 2000. Protein complexes in transport vesicle targeting. Trends Cell Biol. 10:251-255. [DOI] [PubMed] [Google Scholar]
- 9.Holz, R. W., W. H. Brondyk, R. A. Senter, L. Kuizon, and I. G. Macara. 1994. Evidence for the involvement of Rab3A in Ca2+-dependent exocytosis from adrenal chromaffin cells. J. Biol. Chem. 269:10229-10234. [PubMed] [Google Scholar]
- 10.Hume, A. N., L. M. Collinson, A. Repak, A. Q. Gomes, C. R. Hopkins, and M. C. Seabra. 2001. Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J. Cell Biol. 152:795-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joberty, G., P. F. Stabila, T. Coppola, I. G. Macara, and R. Regazzi. 1999. High affinity Rab3 binding is dispensable for Rabphilin-dependent potentiation of stimulated secretion. J. Cell Sci. 112:3579-3587. [DOI] [PubMed] [Google Scholar]
- 12.Kasai, K., and K. Akagawa. 2001. Roles of the cytoplasmic and transmembrane domains of syntaxins in intracellular localization and trafficking. J. Cell Sci. 114:3115-3124. [DOI] [PubMed] [Google Scholar]
- 13.Matesic, L. E., R. Yip, A. E. Reuss, D. A. Swing, T. N. O'Sullivan, C. F. Fletcher, N. G. Copeland, and N. A. Jenkins. 2001. Mutations in Mlph, encoding a member of the Rab effector family, cause the melanosome transport defects observed in leaden mice. Proc. Natl. Acad. Sci. USA 98:10238-10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McBride, H. M., V. Rybin, C. Murphy, A. Giner, R. Teasdale, and M. Zerial. 1999. Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell 98:377-386. [DOI] [PubMed] [Google Scholar]
- 15.Misura, K. M. S., A. P. May, and W. I. Weis. 2000. Protein-protein interactions in intracellular membrane fusion. Curr. Opin. Struct. Biol. 10:662-671. [DOI] [PubMed] [Google Scholar]
- 16.Munson, M., X. Chen, A. E. Cocina, S. M. Schultz, and F. M. Hughson. 2000. Interactions within the yeast t-SNARE Sso1p that control SNARE complex assembly. Nat. Struct. Biol. 7:894-902. [DOI] [PubMed] [Google Scholar]
- 17.Ménasché, G., E. Pastural, J. Feldmann, S. Certain, F. Ersoy, S. Dupuis, N. Wulffraat, D. Bianchi, A. Fischer, F. Le Deist, and G. de Saint Basile. 2000. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat. Genet. 25:173-176. [DOI] [PubMed] [Google Scholar]
- 18.Nagamatsu, S., T. Fujiwara, Y. Nakamichi, T. Watanabe, H. Kitahira, H. Sawa, and K. Akagawa. 1996. Expression and functional role of syntaxin 1/HPC-1 in pancreatic β cells: syntaxin 1A, but not 1B, plays a negative role in regulatory insulin release pathway. J. Biol. Chem. 271:1160-1165. [DOI] [PubMed] [Google Scholar]
- 19.Ostermeier, C., and A. T. Brunger. 1999. Structural basis of Rab effector specificity: crystal structure of the small G protein Rab3A complexed with the effector domain of rabphilin-3A. Cell 96:363-374. [DOI] [PubMed] [Google Scholar]
- 20.Pfeffer, S. R. 1999. Transport-vesicle targeting: tethers before SNAREs. Nat. Cell Biol. 1:E17-E22. [DOI] [PubMed] [Google Scholar]
- 21.Sacher, M., J. Barrowman, W. Wang, J. Horecka, Y. Zhang, M. Pypaert, and S. Ferro-Novick. 2001. TRAPP I implicated in the specificity of tethering in ER-to-Golgi transport. Mol. Cell 7:433-442. [DOI] [PubMed] [Google Scholar]
- 22.Sato, T. K., P. Rehling, M. R. Peterson, and S. D. Emr. 2000. Class C Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol. Cell 6:661-671. [DOI] [PubMed] [Google Scholar]
- 23.Schoch, S., P. E. Castillo, T. Jo, K. Mukherjee, M. Geppert, Y. Wang, F. Schmitz, R. C. Malenka, and T. C. Südhof. 2002. RIM1α forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature 415:321-326. [DOI] [PubMed] [Google Scholar]
- 24.Staunton, J., B. Ganetzky, and M. L. Nonet. 2001. Rabphilin potentiates soluble N-ethylmaleimide sensitive factor attachment protein receptor function independently of rab3. J. Neurosci. 21:9255-9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stinchcombe, J. C., D. C. Barral, E. H. Mules, S. Booth, A. N. Hume, L. M. Machesky, M. C. Seabra, and G. M. Griffiths. 2001. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J. Cell Biol. 152:825-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torii, S., T. Banno, T. Watanabe, Y. Ikehara, K. Murakami, and K. Nakayama. 1995. Cytotoxicity of breferdin A correlates with its inhibitory effect on membrane binding of COP coat proteins. J. Biol. Chem. 270:11574-11580. [DOI] [PubMed] [Google Scholar]
- 27.Torii, S., D. A. Egan, R. A. Evans, and J. C. Reed. 1999. Human Daxx regulates Fas-induced apoptosis from nuclear PML oncogenic domains (PODs). EMBO J. 18:6037-6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, J., T. Takeuchi, H. Yokota, and T. Izumi. 1999. Novel rabphilin3-like protein associates with insulin-containing granules in pancreatic beta cells. J. Biol. Chem. 274:28542-28548. [DOI] [PubMed] [Google Scholar]
- 29.Wang, Y., M. Okamoto, F. Schmitz, K. Hofmann, and T. C. Südhof. 1997. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature 388:593-598. [DOI] [PubMed] [Google Scholar]
- 30.Wu, X., K. Rao, M. B. Bowers, N. G. Copeland, N. A. Jenkins, and J. A. Hammer III. 2001. Rab27a enables myosin Va-dependent melanosome capture by recruiting the myosin to the organelle. J. Cell Sci. 114:1091-1100. [DOI] [PubMed] [Google Scholar]
- 31.Yi, Z., H. Yokota, S. Torii, T. Aoki, M. Hosaka, S. Zhao, K. Takata, T. Takeuchi, and T. Izumi. 2002. The Rab27a/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules. Mol. Cell. Biol. 22:1858-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2:107-118. [DOI] [PubMed] [Google Scholar]