Abstract
The caspase 8 inhibitor c-FLIPL can act in vitro as a molecular switch between cell death and growth signals transmitted by the death receptor Fas (CD95). To elucidate its function in vivo, transgenic mice were generated that overexpress c-FLIPL in the T-cell compartment (c-FLIPL Tg mice). As anticipated, FasL-induced apoptosis was inhibited in T cells from the c-FLIPL Tg mice. In contrast, activation-induced cell death of T cells in c-FLIPL Tg mice was unaffected, suggesting that this deletion process can proceed in the absence of active caspase 8. Accordingly, c-FLIPL Tg mice differed from Fas-deficient mice by showing no accumulation of B220+ CD4− CD8− T cells. However, stimulation of T lymphocytes with suboptimal doses of anti-CD3 or antigen revealed increased proliferative responses in T cells from c-FLIPL Tg mice. Thus, a major role of c-FLIPL in vivo is the modulation of T-cell proliferation by decreasing the T-cell receptor signaling threshold.
Death receptors belonging to the tumor necrosis factor receptor family (e.g., Fas/CD95, TNFR1, TRAIL-R1, TRAIL-R2, TRAMP/DR3, and DR6) play an important role in the regulation of lymphocyte homeostasis (35, 42, 44). Upon encounter of specific antigenic peptides presented by antigen-presenting cells, T cells become activated and enter the cell cycle. Concomitantly with proliferation, T cells differentiate into effector cells that are either cytolytic or able to provide help to B lymphocytes. Once the effector cells have successfully performed their prescribed function, the expanded pool of antigen-specific T-cell clones needs to be reduced to its original size. Both for the effector function of cytolytic T cells and in the elimination of expanded T cells, cell death signals transmitted by Fas/CD95 are of key importance (30). This is underscored by the observation that mice and humans lacking a functional Fas receptor or Fas ligand develop a lymphoproliferative disease and systemic autoimmunity accompanied by the production of autoantibodies (12, 40, 52, 59).
As with all death receptors, the prototypic death receptor Fas/CD95 contains within its cytoplasmic tail a 60-amino-acid death domain (DD) motif (35, 44). Upon activation of Fas by its ligand, the DD undergoes homotypic interaction with a DD in the adaptor protein FADD, which then recruits the initiator caspase 8 via their mutual N-terminal death effector domains (DED) (3). A high local concentration of caspase 8 zymogens is thought to facilitate self-processing and cleavage to the active enzyme (34). Activated caspase 8 then initiates apoptosis by cleavage of the downstream effector caspases 3, 6, and 7 (10).
A number of gammaherpesviruses and molluscipoxviruses encode a molecule termed FLIP (FLICE inhibitory protein) that can inhibit FasL-induced cell death (5, 53). v-FLIP resembles caspase 8 in containing two DED but lacks the enzymatic C-terminal portion. As such, v-FLIP can be recruited into the death-inducing signaling complex (DISC) of Fas, thereby competing with recruitment of caspase 8 to FADD. In this manner, v-FLIP may function to promote viral persistence and dissemination by inhibiting death receptor-mediated elimination of infected cells (56). A mammalian cellular homologue (c-FLIP) has been described that exists in at least two splice variants, c-FLIPS and c-FLIPL (15, 16, 20-22, 38, 47, 49). Like v-FLIP, the 26-kDa c-FLIPS has two DED and functions in a similar manner to inhibit death receptor-induced apoptosis (22). The full-length 55-kDa form of c-FLIP (c-FLIPL) shows overall structural homology to caspase 8. It contains two DED that interact with FADD but bears a mutation in the caspase-like domain that renders it enzymatically inactive. Following Fas ligation, both c-FLIPL and caspase 8 are recruited into the DISC and are subsequently partially cleaved. The affinity for FADD of the c-FLIPL/caspase 8 heterodimer appears to be considerably greater than that of the caspase 8 homodimer; therefore, the ratio of c-FLIPL to caspase 8 is critical in determining cell fate after Fas ligation (24, 43).
c-FLIPL is expressed in resting T cells, but after activation its expression is gradually decreased in an interleukin-2 (IL-2)-dependent manner (2, 39). Paralleling this, resting T cells are resistant to Fas-induced death, whereas cycling T cells, especially in the presence of exogenous IL-2, become highly sensitive to Fas-induced death, as occurs during activation-induced cell death (AICD) following restimulation of cycling T cells (39). This agrees with the view that AICD is mediated in part by functional interactions between Fas and FasL (1, 6, 9, 23, 37, 63). In view of its potent capacity to inhibit FasL-induced apoptosis in vitro (22, 61), c-FLIPL is suggested to play a central role in the regulation of T-lymphocyte homeostasis in vivo.
In addition to inhibition of cell death, we recently observed that c-FLIPL is also capable of binding Raf-1, which leads to the activation of the mitogen-activated protein kinase, ERK, and of binding TRAF-1 and -2, which activate NF-κB. Overexpression of c-FLIPL in Jurkat T cells increased ERK and NF-κB activities upon T-cell receptor (TCR) ligation. Hence, c-FLIPL not only has the capacity to block Fas-mediated cell death but can divert Fas signals toward pathways leading to cell growth and differentiation. To assess how these two aspects of c-FLIPL function in primary T cells, murine c-FLIPL was overexpressed transgenically. As expected, overexpression of c-FLIPL in T cells decreased Fas-mediated cell death but surprisingly had no influence on AICD. Rather, c-FLIPL overexpression appears to render primary T cells more sensitive to TCR-triggered proliferation.
MATERIALS AND METHODS
Abs and reagents.
The following antibodies (Abs) were used for fluorescence-activated cell sorting (FACS) staining: fluorescein isothiocyanate (FITC)-anti-CD3, phycoerythrin (PE)- and Cychrome (CyChr)-anti-CD8, CyChr and antigen-presenting cell anti-CD4, PE-anti-Vα2, FITC-anti-Vβ8, FITC-anti-Vβ14, PE-anti-αβTCR, PE-anti-heat-stable antigen, and biotinylated anti-Fas Jo2 (all purchased from Pharmingen, San Diego, Calif.) and PE-anti-Thy1 (Caltag Laboratories, South San Francisco, Calif.). Anti-FLAG polyclonal Ab (Zymed, South San Francisco, Calif.), anti-FLIP monoclonal Ab (MAb) Dave-2 (Alexis, Lausanne, Switzerland), and anti-FLIP polyclonal Ab (R & D Systems, Abingdon, England) were used for Western blotting. Anti-CD3 (145-2C11), anti-CD28 (37.51), and anti-IL-2R MAb (PC66) were purified from hybridoma supernatants and used in T-cell proliferation assays. Recombinant human FasL, staurosporine, and z-VAD-fmk were obtained from Alexis. Bromodeoxyuridine (BrdU) was purchased from Sigma (St. Louis, Mo.), and Staphylococcus enterotoxin B (SEB) was from Toxin Technology (Sarasota, Fla.).
Generation of c-FLIPL transgenic mice.
FLAG-tagged mouse FLIPL cDNA was inserted into a target vector containing the β-globin promoter and a downstream human CD2 locus enhancer element (11). The resulting construct was injected into BALB/c × C57BL/6 single-cell-stage embryos, and transgenic founders were screened by PCR of tail DNA with the following primers: JT766 (5′-GGAGCCAGGGCTGGGCATAAAA-3′) and JT767 (5′-GACTCACCCTGAAGTTCTCAGGATCC-3′). Western blotting using anti-FLIP MAb (Dave-2) and anti-FLAG Ab (Zymed) further confirmed expression of the transgene. The c-FLIPL Tg mouse strain was maintained by serially mating heterozygous transgenic animals with wild-type C57BL/6 mice, and the Tg mice used for the indicated experiments were backcrossed at least six times. For certain experiments c-FLIPL Tg mice were crossed with DBA/2 mice, expressing the endogenous superantigens Mtv 1, 6, 7, 8, 11, 13, 14, and 17 together with I-E major histocompatibility complex (MHC) class II, or with OT-1 Tg mice (C57BL/6 background) expressing a transgenic TCR specific for ovalbumin (Ova) peptide 257-264 (SIINFEKL) in the context of H-2Kb (18). Expression of the transgenic TCR was determined by FACS analysis of peripheral blood with CyChr anti-CD8 and PE anti-Vα2 Abs. For most experiments, mice were used between 6 and 12 weeks of age.
Purification and activation of T cells.
Single-cell suspensions of pooled mesenteric, inguinal, and axillary lymph nodes were depleted of B cells and monocytes by incubation with anti-B220 and anti-MHC class II immunoglobulin M Abs and rabbit complement (Saxon Europe, Cambridge, United Kingdom) for 45 min at 37°C. The remaining viable cells were collected by Ficoll-Hypaque density centrifugation. T-cell purity was 95% as confirmed by FACS analysis with FITC-labeled anti-CD3. Lymph node T cells (1.5 × 105/well) were stimulated with graded amounts of plate-bound anti-CD3 (145-2C11) in the absence or presence of recombinant mouse IL-2 (50 U/ml; Roche Diagnostics, Rotkreutz, Switzerland). Cells were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 50 μM β-mercaptoethanol, and antibiotics (Life Technologies, Paisley, Scotland) for 4 or 6 days. To measure proliferation, 0.5 μCi of [3H]thymidine (Amersham, Little Chalfont, Buckinghamshire, England) was added during the last 16 h of culture. IL-2 production was measured by enzyme-linked immunosorbent assay in culture supernatants 24 h after activation with anti-CD3. To assess AICD, purified T cells were stimulated with 0.1 μg of anti-CD3/ml and IL-2 (20 U/ml). After 3 days cells were collected, extensively washed, and put back into culture in the presence of IL-2 (50 U/ml). After 24 h viable cells were recovered by Ficoll-Hypaque density centrifugation and subsequently restimulated with 5 μg of plate-bound anti-CD3/ml for 24 h. The percentage of viable cells was determined by annexin V staining and FACS analysis.
In case of peptide stimulation, total splenocytes (1.5 × 106/well) of single OT-1 Tg mice or double (c-FLIPL Tg × OT-1) Tg mice were stimulated with various concentrations of Ova peptide 257-264 (SIINFEKL) for 4 days. During the final 4 h of culture 0.5 μCi of [3H]thymidine was added.
Western blot analysis.
Cells were washed once in ice-cold phosphate-buffered saline (PBS) and solubilized in lysis buffer (1% NP-40, 50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 2 mM dithiothreitol, protease inhibitor cocktail [Complete; Boehringer Mannheim, Indianapolis, Ind.]). Postnuclear lysates were collected after centrifugation (15,000 × g), and proteins (60 μg) were separated in sodium dodecyl sulfate-12% polyacrylamide gels. Proteins were transferred to nitrocellulose (Hybond-ECL; Amersham), and blots were blocked and probed with the indicated Abs in 5% nonfat milk in PBS-Tween 20 (0.1%). Immunoreactive proteins were visualized with horseradish peroxidase-labeled conjugates (Jackson Laboratories, West Grove, Pa.) and ECL blotting substrate (Amersham)
Flow cytometry.
Cells (5 × 105) were washed with ice-cold FACS buffer (PBS, 3% fetal calf serum, 0.02% sodium azide) and incubated with saturating amounts of conjugated Abs or isotype-matched controls for 30 min at 4°C. After two washes with FACS buffer, PE-labeled streptavidin was added to samples that had been incubated with biotinylated Abs. After washing, 104 viable cells were analyzed on a FACScan apparatus (Becton Dickinson, San Jose, Calif.) with the CellQuest program. Phosphatidylserine exposure on apoptotic cells was measured by washing cells once in ice-cold HEPES buffer (10 mM HEPES, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, and 1.8 mM CaCl2, pH 7.4) supplemented with 1 mg of glucose/ml and 0.5% bovine serum albumin (BSA) and subsequent incubation with FITC-labeled annexin V (2.5 μg/ml). Cells were incubated for 20 min at 4°C and washed twice with HEPES buffer. Before analysis on a FACScan apparatus, propidium iodide (PI) was added (final concentration, 5 μg/ml) to the samples to discriminate necrotic cells (annexin V−, PI+) from apoptotic cells (annexin V+, PI−, annexin V+, PI+).
Detection of apoptosis by TUNEL and cell cycling by BrdU.
To analyze apoptotic cells by flow cytometry, the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay was used as described previously (8, 41, 46). Thymocytes were cultured at 37°C for 4 h to reveal apoptotic cells. Cells (0.75 × 106) were stained for TCRβ, CD4, and CD8 expression by using a MAb in PBS containing 1% BSA for 30 min at 4°C and then washed with cold PBS. The cells were fixed with 1% methanol-free formaldehyde for 15 min on ice, washed with cold PBS, and pelleted by spinning at 10,000 × g. The thymocytes were resuspended in cold 70% ethanol for 15 min and then washed twice with cold PBS. For the TUNEL reaction, the cells were incubated in TUNEL reaction mix containing 10 U of terminal deoxyribosyltransferase, 10 mM dUTP-biotin, and 2.5 mM cobalt chloride in 1× terminal transferase reaction buffer (Boehringer Mannheim) for 1 h at 37°C. The samples were washed twice with PBS-1% BSA and incubated with streptavidin tricolor (Caltag Laboratories) for 20 min on ice. Cells were washed twice, fixed in 1% methanol-free formaldehyde in PBS-1% BSA, and stored at 4°C until analysis. Cells stained with the same protocol in the absence of dUTP-biotin were used as negative controls.
To determine proliferation of thymocyte subsets, mice received 4 intraperitoneal (i.p.) injections of 1 mg of BrdU (Sigma) over a 24-h period with the last injection given 1 h prior to tissue harvest. Single-cell suspensions were stained for TCRβ, CD4, and CD8 expression with a MAb in PBS containing 1% BSA for 30 min at 4°C, washed with cold PBS, and fixed with 70% ethanol for 30 min on ice. By a modification of published methods (54), the thymocytes were washed, fixed with 1% methanol-free formaldehyde for 15 min on ice, washed, and fixed overnight in PBS containing 1% methanol-free formaldehyde and 0.01% Tween 20. The following day, the samples were washed and incubated with 50 U of DNase I (Sigma)/ml in 0.15 M NaCl-4.2 mM MgCl2 (pH 5) for 15 min at 37°C. After two washes with PBS-1% BSA, the cells were incubated with anti-BrdU-FITC (Becton Dickinson) for 30 min on ice. The samples were washed twice with PBS-1% BSA, fixed in 1% methanol-free formaldehyde in PBS-1% BSA, and stored at 4°C until analysis. Samples were analyzed on a FACScalibur apparatus with the CellQuest program.
In vivo experiments.
SEB (100 μg) was injected into each of eight c-FLIPL Tg mice and eight C57BL/6 control littermates. Blood was collected from tail vein on the indicated days, and peripheral T-cell subsets were analyzed. Cells were stained for Vβ8, Vβ14, CD4, and CD8, and the percentages of the responsive Vβ8+ T cells and nonresponsive Vβ14+ T cells were determined within the CD4+ and CD8+ subsets. The mean percentage ± standard deviation of eight different animals per group was calculated.
To determine the in vivo proliferative responses of T cells in c-FLIPL Tg mice, c-FLIPL Tg × OT-1 double Tg mice (four mice per group) and OT-1 Tg control littermates (four mice per group) received i.p. injections of 2 mg of BrdU and 250 μl of 100 μM Ova peptide or the same volume of PBS and BrdU. BrdU (1 mg/ml) and 5% glucose were added to the drinking water, and 36 h after injection mice were sacrificed and spleens were removed. Single-cell suspensions were surface stained with PE-labeled anti-Vα2 and CyChr-labeled anti-CD8 followed by nuclear staining of BrdU-labeled DNA with FITC-labeled anti-BrdU (Pharmingen). Samples were analyzed on a FACScan apparatus, and the percentage of cells incorporating BrdU was determined within the CD8+ Vα2+ T-cell subset.
RESULTS
Generation of transgenic mice that constitutively express murine c-FLIPL in T cells.
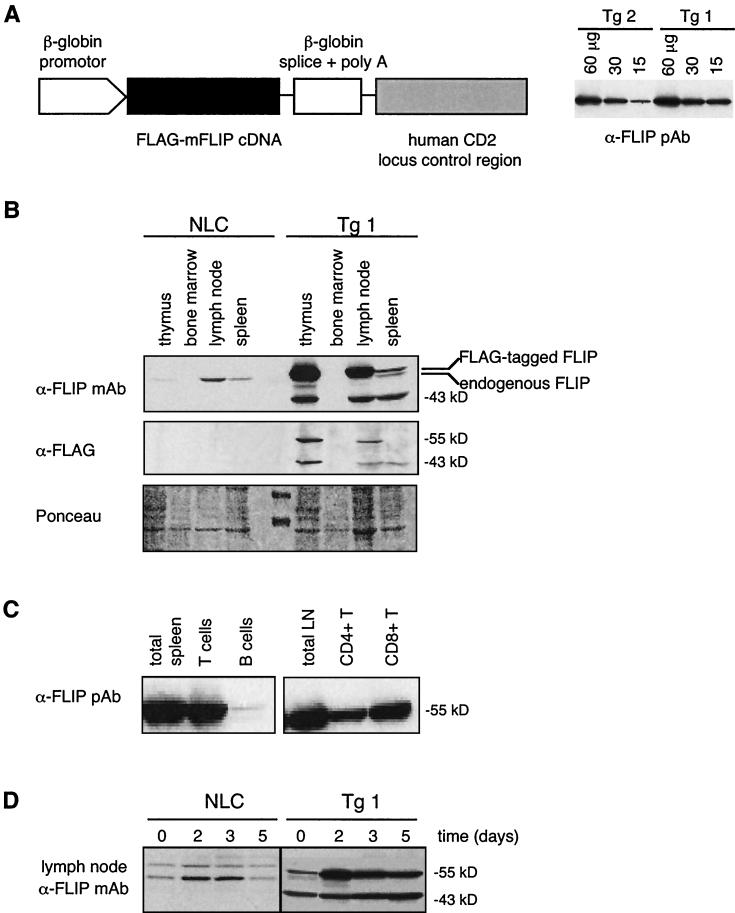
To investigate the role of c-FLIPL in T-cell development and activation in vivo, we generated transgenic mice expressing mouse c-FLIPL under the control of a β-globin promoter and the downstream human CD2 enhancer element (11) (Fig. 1A). Two independent transgenic lines were obtained, both expressing high levels of transgenic protein (Fig. 1A). Transgenic mice expressed the transgene at levels approximately 5 (Tg 2)- to 10 (Tg 1)-fold higher than endogenous c-FLIPL (Fig. 1A and B). One line (Tg 1), expressing slightly higher concentrations of transgenic FLIP, was chosen for detailed analysis, but similar results were also obtained with the second line. Whereas endogenous lymphoid expression of c-FLIPL is mainly found in lymph node, with lowest levels in spleen and thymus (Fig. 1B), transgenic FLAG-tagged c-FLIPL expression was highest in the thymus and lymph node, low in the spleen, and absent in bone marrow, consistent with the expected T-cell-directed expression of the human CD2 enhancer element (11) (Fig. 1B). Sorting of T and B cells from spleen confirmed the T-cell-specific expression of the transgene. Within T-cell compartments, the transgene was equally expressed in CD4+ and CD8+ T cells (Fig. 1C). Interestingly, in addition to its full-length 55-kDa form, an additional 43-kDa cleavage product of transgenic c-FLIPL was observed. This fragment is usually generated by caspase 8 when the caspase 8/FLIPL heterodimeric complex is recruited to death receptors (24, 43). Finally, whereas the expression of endogenous c-FLIPL was only transiently induced after T-cell activation (Fig. 1D) (2, 39), transgenic c-FLIPL was increased after T-cell stimulation and remained highly expressed in activated T cells (Fig. 1D).
FIG. 1.
Expression of the c-FLIPL transgene. (A) FLAG-tagged mouse c-FLIPL was cloned into a targeting vector, which is driven by the β-globin promoter and controlled by the human CD2 enhancer element. Protein levels of transgenic c-FLIPL in thymocytes derived from two independent transgenic mice were compared. Numbers indicate the amount of total protein loaded per lane. (B) Western blot analysis of transgene protein expression in different immunological compartments. Cell lysates were prepared from single-cell suspensions of thymus, bone marrow, lymph nodes, and spleen of a transgenic mouse (Tg 1) and an NLC. The blots were probed with an anti-FLIP MAb that recognizes both endogenous and transgenic c-FLIPL (Dave-2, top panel) and with an anti-FLAG Ab detecting only the transgenic form of c-FLIPL (middle panel). Ponceau staining was included to show the presence of similar amounts of protein in each lane (bottom panel). (C) T and B cells were FACS sorted from single-cell suspensions of spleens, and purified CD4+ and CD8+ T cells were sorted from lymph nodes of c-FLIPL Tg mice (line 1). Lysates were subjected to Western blotting with a polyclonal anti-FLIP Ab. (D) Lymph node cells of transgenic (line 1) and control littermate mice were stimulated with plastic-coated anti-CD3 MAb (1 μg/ml). At the indicated time points cell lysates were prepared and subjected to Western blotting. The blot was probed with an anti-FLIP MAb. In order to view differences in expression, less protein was loaded than in panel B.
Overexpression of c-FLIPL protects T cells against FasL-induced apoptosis.
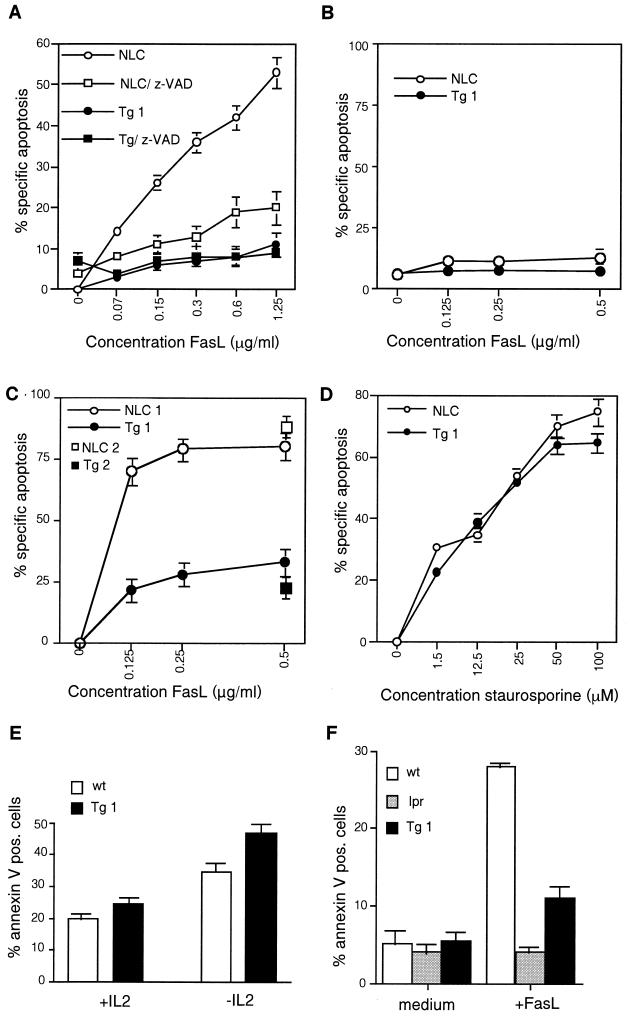
In vitro, Fas-sensitive cell lines are protected against FasL-induced cell death when c-FLIPL is overexpressed (22, 25). Primary T cells become sensitive to FasL-induced apoptosis 3 to 5 days after activation, which coincides with downregulation of c-FLIPL protein levels (22, 28, 39) (Fig. 1D). Consistent with this, we found that activated T cells from control mice were rapidly killed after incubation with recombinant FasL, whereas the majority of day 3 T-cell blasts derived from c-FLIPL Tg mice still expressed high levels of transgenic c-FLIPL (Fig. 1D) and were protected against FasL-induced apoptosis (Fig. 2A). Similar to peripheral T cells, the high expression levels of c-FLIPL in the thymi of Tg mice derived from both lines (Fig. 1A and B) were sufficient to inhibit FasL-induced apoptosis of thymocytes (Fig. 2C). As opposed to T-cell blasts, resting T cells from either c-FLIPL Tg or control mice were insensitive to killing by recombinant FasL (Fig. 2B). Resting and activated T cells from both strains of mice expressed similar levels of Fas on the cell surface (data not shown). In contrast to FasL-induced cell death, death receptor-independent apoptosis, as induced by staurosporine, was unaffected by c-FLIPL transgene expression (Fig. 2D). Likewise, apoptosis induced by an established Bcl-2 family-regulated pathway (e.g., IL-2 withdrawal) (51) was also not inhibited by c-FLIPL but appeared to be somewhat enhanced (Fig. 2E). These data show that the transgene-encoded c-FLIPL is functional. However, as shown in Fig. 2A and F, protection against FasL-induced cell death in the c-FLIPL Tg mice was not complete, whereas it was completely inhibited in Fas-deficient lpr mice (Fig. 2F). To investigate whether this difference was due to insufficient expression and caspase-inhibitory function of the c-FLIPL transgene, z-VAD was added to cultures of activated T cells of c-FLIPL Tg mice and normal littermate controls (NLC). z-VAD was found to protect T cells of NLC from recombinant FasL-induced death to a level similar to, but no better than, c-FLIPL Tg T cells (Fig. 2A). Moreover, z-VAD did not significantly affect the remaining cell death in T cells derived from the c-FLIPL Tg mice. These experiments indicate that the transgene-encoded c-FLIPL levels are sufficient to block completely the Fas-triggered, caspase-mediated cell death pathway and that the remaining FasL-induced cell death in T cells is most likely caspase independent, in agreement with previous observations (17, 19). However, it is also possible that the lack of full inhibition of FasL-mediated death with z-VAD reflects incomplete permeability of the blocker or caspase-dependent cell death mediated by a z-VAD-insensitive caspase.
FIG. 2.
T cells from c-FLIPL Tg mice are resistant to FasL-induced cell death. T cells purified from lymph nodes (A, B, and D to F) or thymocytes isolated from thymi (C) of c-FLIPL Tg mice (Tg 1 and Tg 2), NLC, or lpr mice (F) were either directly incubated with recombinant FasL for 3 h (B and C) or stimulated with plastic-coated anti-CD3 (1 μg/ml) and recombinant mouse IL-2 (50 U/ml) (A and D to F). After 2 to 3 days T-cell blasts were harvested and depleted of dead cells by Ficoll gradient centrifugation. Viable cells were incubated with titrated amounts of recombinant FasL, either in the absence or in the presence of 50 μM z-VAD (A), with staurosporine (D), or with or without IL-2 (50 U/ml) (E). After 6 h of incubation the cells were stained with FITC-labeled annexin V and PI, and the percentage of apoptotic cells was determined by FACS analysis. In panel F activated T cells were exposed to 0.5 μg of FasL/ml for 3 h. The percentage of spontaneous cell death 6 h after incubation with medium only was 11% for controls and 23% for c-FLIPL Tg T cells (in panels A and D). wt, wild type.
c-FLIPL Tg mice have a decreased proportion of CD8+ T cells.
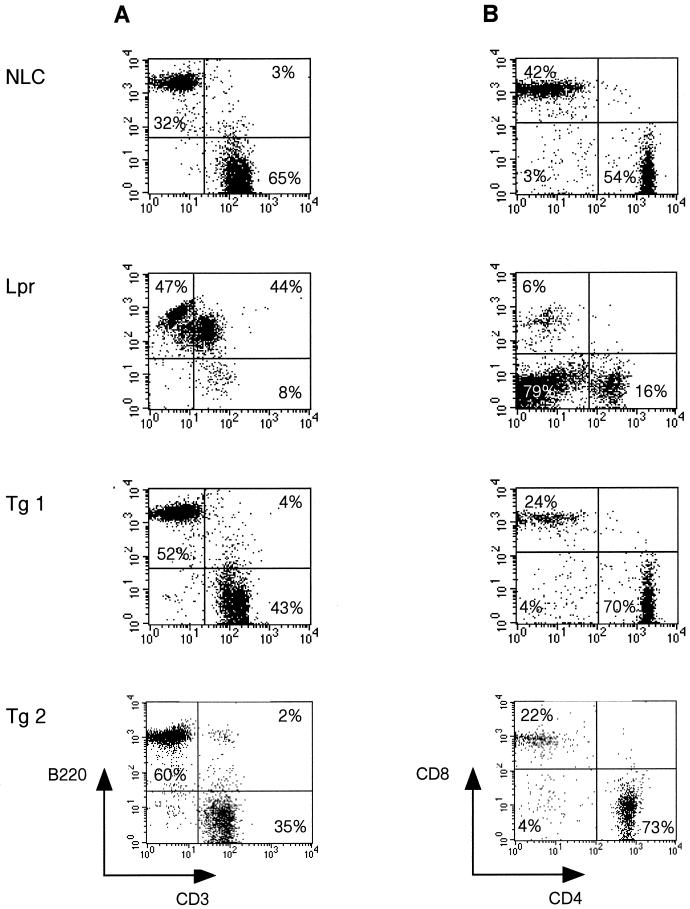
Although T cells from c-FLIPL Tg mice were significantly protected against Fas-induced cell death, c-FLIPL Tg mice did not develop profound lymphadenopathy and age-dependent autoimmune disease characteristic of Fas-deficient lpr and FasL-deficient gld mice (7). The c-FLIPL Tg mice appear healthy with normal cell counts for spleen and lymph nodes even at 12 to 18 months of age (Table 1 and data not shown). Consistent with these data, FACS analysis of mature lymphocyte populations from lymph node and spleen did not reveal an accumulation of B220+ CD4− CD8− T cells, characteristic of lpr and gld mice (Fig. 3). However, the c-FLIPL Tg mice demonstrated a small reduction in the number of T cells in the spleen and lymph node compared to control mice, which was primarily confined to the CD8+ T-cell subset (Fig. 3B and Table 1).
TABLE 1.
Absolute cell numbers of CD3+ (T) and B220+ (B) cells and CD4+ and CD8+ T cells in lymph nodes and spleens of c-FLIPL Tg mice (line 1) and NLCa
| Cell type | Value for:
|
|||
|---|---|---|---|---|
| Lymph node (106)
|
Spleen (106)
|
|||
| NLC (n = 7) | c-FLIPL Tg (n = 7) | NLC (n = 7) | c-FLIPL Tg (n = 7) | |
| CD3+ cells | 1.53 ± 0.43 | 1.45 ± 0.33 | 28.5 ± 1.15 | 18.6 ± 0.61 |
| B220+ cells | 0.67 ± 0.42 | 1.37 ± 0.43 | 58.1 ± 7.76 | 50.2 ± 0.52 |
| Ratio T/B | 2.30 ± 0.6 | 1.08 ± 0.2 | 0.50 ± 0.1 | 0.37 ± 0.1 |
| CD3+ gate | ||||
| CD4+ T cells | 0.86 ± 0.05 | 0.94 ± 0.06 | 19.3 ± 0.54 | 14.3 ± 0.9 |
| CD8+ T cells | 0.63 ± 0.04 | 0.44 ± 0.05 | 7.81 ± 0.63 | 3.16 ± 0.8 |
| Ratio CD4/CD8 | 1.37 ± 0.3 | 2.14 ± 0.6 | 2.47 ± 0.35 | 4.52 ± 0.2 |
Spleens and inguinal lymph nodes of 8- to 12-week-old mice were removed, and lymphocytes were counted. The number of cells in each subset was calculated from flow cytometric analysis of two- and three-color immunofluorescence on 10,000 cells per sample. Values are the means ± standard deviations.
FIG. 3.
Flow cytometric analysis of peripheral lymphocytes in c-FLIPL Tg mice. Lymph node cells from 8- to 12-week-old c-FLIPL Tg mice and control littermates were stained with PE-B220 and FITC-CD3 MAb (A) or with FITC-CD3, PE-CD8, and CyChr-CD4 MAb (B). The percentages of B (B220+) and T (CD3+) cells and CD4+ and CD8+ T cells within the CD3+ subset are shown and are representative of seven independent experiments.
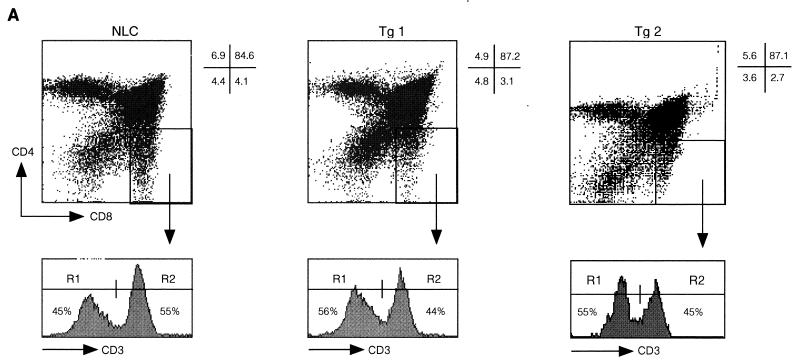
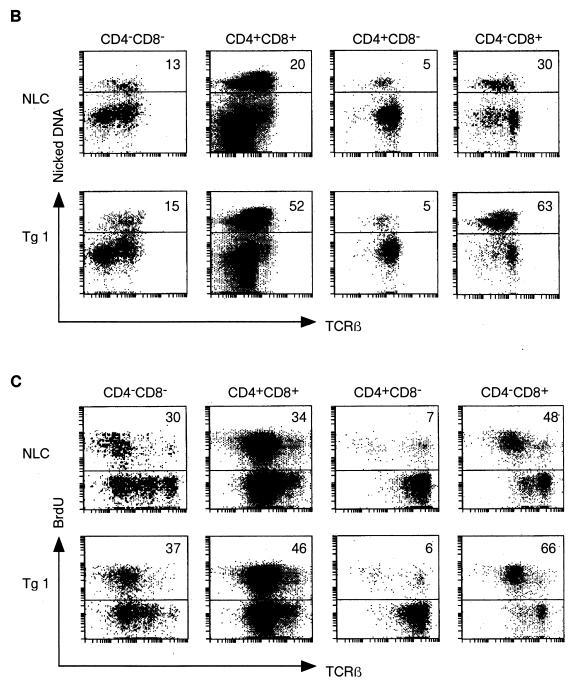
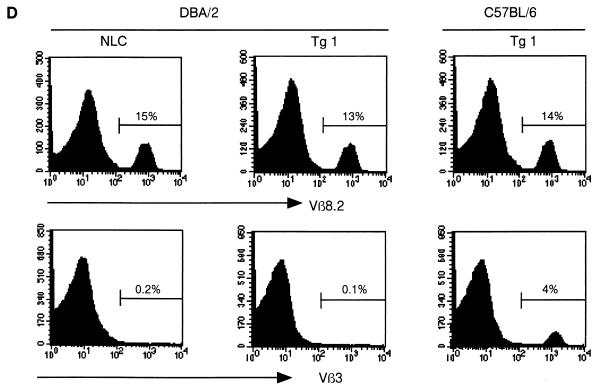
Analysis of thymi from c-FLIPL Tg mice did not reveal overt deviations in thymus size or thymocyte differentiation (Fig. 4A and data not shown). However, similar to peripheral T cells, we found a small reduction in the percentage of mature single-positive CD8 thymocytes. These are discriminated from immature single-positive CD8+ thymocytes by the expression of high surface levels of CD3 and TCR and decreased expression of Thy1 and heat-stable-antigen (Fig. 4A and data not shown). Since the upregulation of CD5, CD69, and Bcl-2 expression coincident with increasing surface TCR expression on c-FLIPL Tg CD4+ CD8+ thymocytes was comparable to that for control mice, defects in positive selection do not appear to explain the reduced percentage of single-positive CD8 thymocytes in c-FLIPL Tg mice (data not shown). However, TUNEL analysis revealed an increased percentage of apoptotic cells within the CD4+ CD8+ double-positive and single-positive CD8 thymocyte subsets of c-FLIPL Tg mice compared to the same subsets in control mice (Fig. 4B). This suggests that the reduced percentages of mature CD8+ thymocytes and peripheral CD8+ T cells in c-FLIPL Tg mice are likely caused by an increased cell death turnover of single-positive CD8 thymocytes. The increased spontaneous thymocyte death was balanced by augmented proliferation based on in vivo BrdU labeling (Fig. 4C). This balance most likely accounts for the overall similar thymus size of c-FLIPL Tg and control mice. Finally, when c-FLIPL Tg mice were backcrossed onto the DBA/2 background, which expresses endogenous mammary tumor virus (mtv) superantigen as well as I-E MHC class II (31), deletion of the mtv-reactive Vβ3 and Vβ17 TCR-expressing thymocytes and T cells was unaffected (Fig. 4D and data not shown), demonstrating that c-FLIPL does not affect negative selection.
FIG. 4.
Thymic development and negative selection in c-FLIPL Tg mice. (A) Single-cell suspensions of thymi of 4-week-old c-FLIPL Tg mice and control littermates were prepared and stained with FITC-CD3, CyChr-CD4, or PE-CD8 MAb. The percentages of double-negative, double-positive, and single-positive thymocytes of the total thymocyte population are shown and are representative of four independent experiments. (B) Thymi of 8- to 12-week-old Tg mice or NLC were isolated, and single-cell suspensions were surface stained for TCRβ, CD4, and CD8. Spontaneous apoptosis in the different thymocyte subsets was determined by TUNEL assay. The experiment shown is one representative of three. (C) c-FLIPL Tg mice and NLC received four i.p. injections of BrdU (1 mg/ml) over a 24-h period. Thymi were harvested, and single-cell suspensions were surface stained prior to staining with anti-BrdU. One representative experiment of three is shown. (D) c-FLIPL Tg mice were backcrossed into a DBA/2 background that expresses the endogenous superantigens mtv 1, 6, 7, 8, 11, 13, 14, and 17 as well as I-E MHC class II. Deletion of Vβ3 TCR-expressing T cells was monitored by staining total splenocytes of DBA/2 mice with Vβ3-specific Abs followed by FACS analysis. Since C57BL/6 mice do not express I-E MHC class II molecules, Vβ3-expressing T cells are present in the spleen. Vβ8.2 T cells are not deleted by the above-mentioned mtv subtypes and are thus present both in C57BL/6 and in DBA/2 mice.
Increased proliferation of T cells from c-FLIPL Tg mice upon suboptimal triggering of the TCR/CD3 complex.
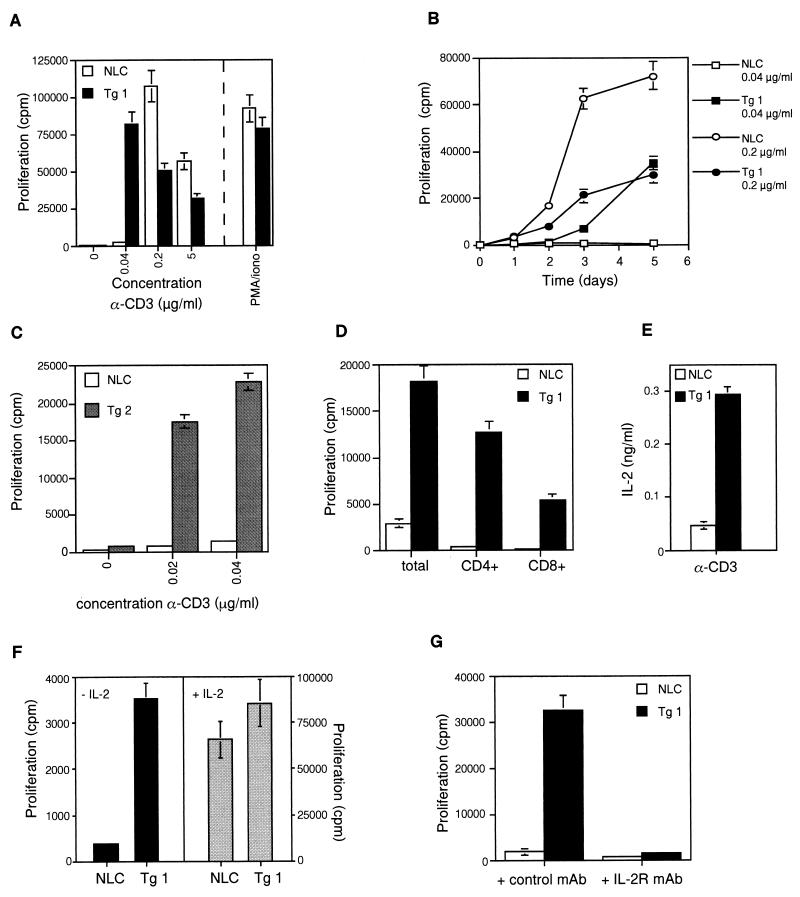
In addition to its antiapoptotic function, we recently showed that c-FLIPL can also promote IL-2 production in T-cell lines by connecting with the ERK and NF-κB pathways (24). To investigate this alternative function of c-FLIPL in nontransformed primary T cells, proliferation and IL-2 production were measured in purified lymph node T cells stimulated with plate-bound anti-CD3. Interestingly, the dose-response curve for CD3 stimulation of c-FLIPL Tg T cells was shifted toward a lower dose of anti-CD3. At high doses of anti-CD3, T cells from c-FLIPL Tg mice actually proliferated less well than T cells derived from control mice (Fig. 5A). Since increased percentages of annexin V-positive cells (47% in NLC versus 65% in Tg 1 with 0.2 μg of anti-CD3/ml and 50% in NLC versus 74% in Tg 1 with 5 μg of anti-CD3/ml) were observed in the cultures of c-FLIPL Tg T cells, enhanced cell death is the likely reason for the reduced proliferation rates at higher doses of anti-CD3.
FIG. 5.
Enhanced in vitro proliferative responses in c-FLIPL Tg mice. (A) Purified lymph node T cells from c-FLIPL Tg mice and NLC were activated with the indicated concentrations of plate-bound anti-CD3 or phorbol myristate acetate (1 ng/ml) and ionomycin (0.5 μM) for 6 days. [3H]thymidine (0.5 μCi/well) was added during the final 16 h of culture. (B) Purified lymph node T cells from c-FLIPL Tg mice and NLC were activated with a high (0.2-μg/ml) or suboptimal (0.04-μg/ml) dose of plate-bound anti-CD3 for the indicated number of days, and [3H]thymidine (0.5 μCi/well) was added during the final 16 h of culture. (C) Purified lymph node T cells of NLC and Tg mice derived from the second c-FLIPL Tg line (Tg 2) were stimulated with suboptimal doses of anti-CD3 for 4 days, and [3H]thymidine was added during the final 16 h of culture. (D) Total lymph node cells and FACS-sorted CD4+ and CD8+ T cells were stimulated with a suboptimal dose of plate-bound anti-CD3 (0.04 μg/ml) for 4 days, and [3H]thymidine was added during the final 16 h of culture. (E) Purified lymph node T cells were stimulated with 0.04 μg of plate-bound anti-CD3/ml, and IL-2 production was measured in 24-h culture supernatants. (F) Purified lymph node T cells were stimulated with 0.04 μg of plate-bound anti-CD3/ml in the presence or absence of IL-2 for 4 days, and [3H]thymidine was added during the final 16 h of culture. (G) Purified lymph node T cells were stimulated for 4 days with a low dose of coated anti-CD3 (0.04 μg/ml) in the presence or absence of a blocking anti-IL-2R MAb (10 μg/ml). [3H]thymidine was added during the final 16 h of culture.
Kinetics of the proliferative response did not appear to be faster in the c-FLIPL Tg mice at high doses of anti-CD3. Addition of [3H]thymidine 1, 2, and 3 days after stimulation revealed no early increase in proliferation in c-FLIPL Tg mice (Fig. 5B). However, with the use of doses of anti-CD3 (0.02 and 0.04 μg/ml) at which T cells from control mice no longer proliferated, the c-FLIPL Tg T cells still proliferated vigorously and produced significant levels of IL-2 (Fig. 5A, B, C, and E). The increased proliferative potential at low doses of anti-CD3 was observed in both CD4+ and CD8+ T cells of c-FLIPL Tg mice, but the amplitude of the response was higher in CD4+ Tg T cells than in CD8+ Tg T cells (Fig. 5D). When TCR signaling was circumvented by direct activation of protein kinase C with phorbol myristate acetate and addition of the calcium ionophore ionomycin, T cells from the two types of mice showed similar proliferative responses (Fig. 5A). To examine whether the enhanced IL-2 production was responsible for the increased proliferation of c-FLIPL Tg T cells, IL-2 was added to the cultures. In the presence of exogenous IL-2, T cells of control mice were able to proliferate at low doses of anti-CD3, and, more importantly, their proliferative response was similar to the response of FLIPL Tg T cells (Fig. 5F). In line with this, addition of a blocking anti-IL-2R MAb to suboptimally triggered c-FLIPL Tg T cells completely eliminated proliferation, showing that the increased proliferation rate of c-FLIPL Tg T cells at low doses of anti-CD3 is IL-2 mediated (Fig. 5G).
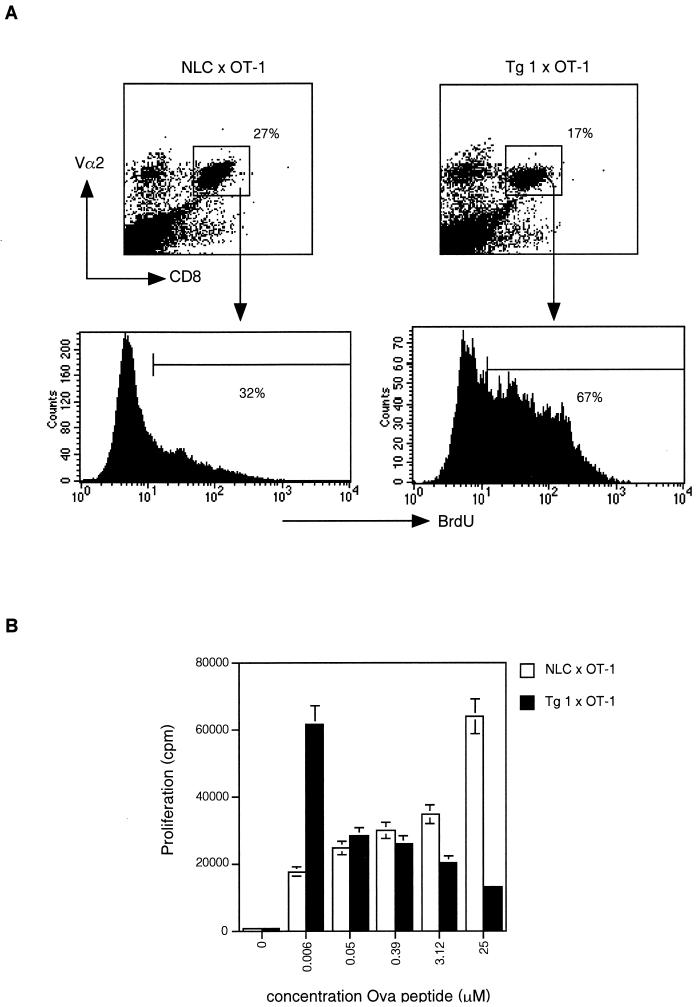
Finally, to assess whether these in vitro observations could also be made in vivo, c-FLIPL Tg mice were crossed with OT-1 mice expressing a transgenic TCR specific for the Ova peptide 257-264 restricted to MHC class I Kb. Cell cycling following injection of Ova peptide was monitored by BrdU incorporation. As shown in Fig. 6A, c-FLIPL × OT-1 mice manifested enhanced BrdU incorporation in the responsive CD8+ Vα2+ T cells compared to the same subset in OT-1 control littermates. Furthermore, when splenocytes of OT-1 Tg mice and double (c-FLIPL Tg × OT-1) Tg mice were stimulated in vitro with Ova peptide, we observed a similar effect as that with anti-CD3 stimulation: higher proliferative responses in the c-FLIPL Tg mice at low doses of peptide and lower proliferative responses at high doses of peptide (Fig. 6B). Thus, T cells from c-FLIPL Tg mice have increased proliferative potential both in vitro and in vivo.
FIG. 6.
c-FLIPL Tg mice show enhanced proliferative responses in vivo. (A) c-FLIP Tg mice were crossed with OT-1 mice that express an MHC class I-restricted transgenic TCR specific for Ova peptide 257-264. Four OT-1 c-FLIP Tg mice or four single OT-1 Tg littermates were injected with 2 mg of BrdU together with 100 μM Ova peptide or the carrier PBS. After 36 h, single-cell suspensions of spleens were prepared and stained with anti-CD8, anti-Vα2, and anti-BrdU. BrdU expression within the responsive CD8+ Vα2+ T cells is shown and is representative of four experiments. (B) Splenocytes derived from single OT-1 transgenic mice or double OT-1 × c-FLIPL Tg mice were stimulated in vitro with the indicated amount of Ova peptide for 4 days. [3H]thymidine was added during the final 4 h of culture.
AICD of T cells is not inhibited by overexpression of c-FLIPL in vivo.
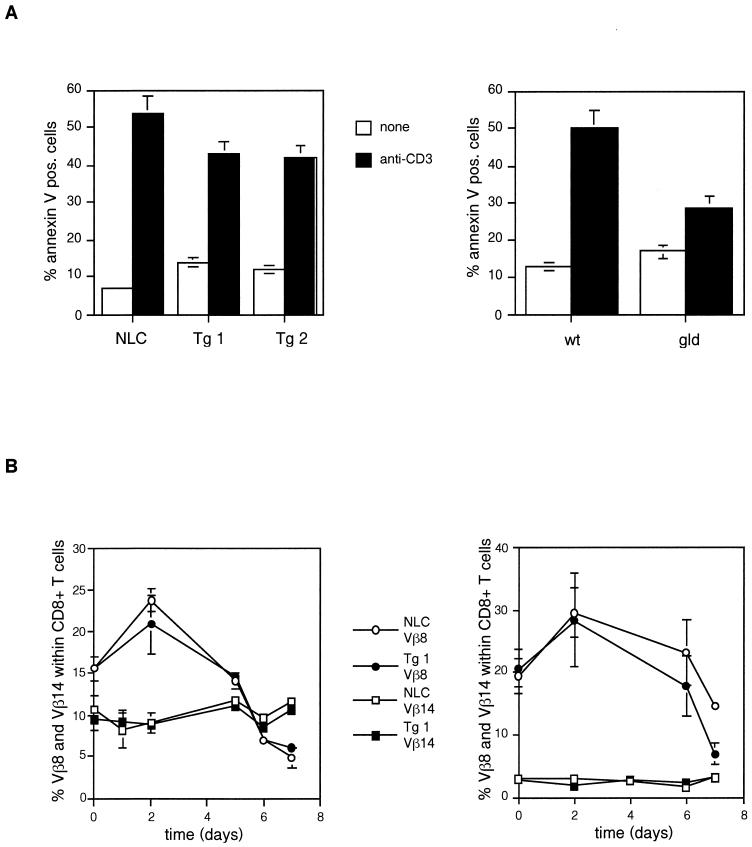
Despite the increased proliferative potential of c-FLIPL Tg T cells and their increased resistance to FasL-induced cell death, c-FLIPL Tg mice did not accumulate vast numbers of T cells in secondary lymphoid organs. T-cell homeostasis after immune activation is complex and maintained both by deprivation of cytokines, which become limiting at the end of an immune response (30), and by AICD that is, at least in part, mediated by Fas-FasL interactions (6, 9, 23). In vitro, AICD is mimicked by restimulation of activated T cells via their antigen receptor (37). Surprisingly, T cells from c-FLIPL Tg mice were not protected against this type of cell death, whereas FasL-deficient gld mice were partially protected (Fig. 7A), in agreement with previous results (14). Injection of the superantigen SEB in mice induces T-cell activation and expansion followed by deletion of the reactive Vβ8+ T cells (27). Injection of SEB resulted in increased percentages of SEB-reactive Vβ8+ T cells at day 2 followed by markedly decreased percentages at day 7 in both strains of mice (Fig. 7B). No effect was found in the nonresponsive Vβ14 TCR-expressing T cells. Thus, both the in vitro and in vivo experiments imply that AICD is not affected by overexpression of c-FLIPL.
FIG. 7.
AICD is not inhibited in c-FLIPL Tg mice. (A) Purified lymph node T cells of c-FLIPL Tg and NLC mice (left panel) and of wild-type C57BL/6 (wt) and gld C57BL/6 mice (right panel) were stimulated with CD3 MAb (0.1 μg/ml) and IL-2 (20 U/ml) for 3 days. Cells were washed and put into culture in the presence of IL-2 (50 U/ml) only. After 24 h dead cells were removed by Ficoll gradient centrifugation and the preactivated T cells were restimulated with plate-bound CD3 MAb (5 μg/ml) for 24 h. Cell viability was determined by annexin V staining and FACS analysis. Means ± standard deviations of triplicate determinations are shown. (B) SEB (100 μg/animal) was injected into eight c-FLIPL Tg mice and eight NLC, and peripheral blood T cells were analyzed on the indicated days. Cells were stained for Vβ8, Vβ14, CD4, and CD8, and the percentages of Vβ8+ and Vβ14+ within CD4+ (left) and CD8+ (right) T cells are shown.
DISCUSSION
The present findings show that constitutive expression of the caspase 8 inhibitor c-FLIPL in primary mouse T cells not only confers protection from FasL-induced cell death but also lowers the threshold for TCR-triggered proliferation and IL-2 production. The former is likely due to the ability of c-FLIPL to bind caspase 8 and block its proapoptotic activity (55). The augmented T-cell activation may reflect the more recently discovered ability of c-FLIP to augment signaling via ERK and NF-κB (24).
Despite the resistance of their T cells to FasL-induced death, the c-FLIPL Tg mice did not accumulate B220+ CD4− CD8− T cells, typical of Fas-deficient lpr mice. The fact that AICD was not impaired in c-FLIPL Tg mice probably explains why progression to T-cell hyperplasia does not occur in c-FLIPL Tg mice. The unaffected AICD stands in contrast to findings of Van Parijs et al. (57) for mice reconstituted with bone marrow cells that had been retrovirally transduced with c-FLIPL. This approach allowed the expression of c-FLIPL in both T and B cells. In this study, both restimulation of activated T cells with anti-CD3 and injection of SEB resulted in impaired cell death responses in the FLIP-expressing mice. Similar to the c-FLIPL Tg mice, however, the retrovirally transduced c-FLIP mice did not accumulate aberrant T cells but rather developed an autoimmune disease due to defects in the B-cell compartment.
Several reasons may explain why AICD was not impaired in c-FLIPL Tg mice. First, T-cell deletion in response to superantigen stimulation is only mildly affected in lpr mice (45, 51). Therefore, this type of cell death most likely has a major death receptor-independent component, which cannot be inhibited by c-FLIPL. Second, we cannot formally exclude the possibility that the levels of c-FLIPL in T cells from c-FLIPL Tg mice are insufficient to block caspase 8 activity required for AICD in vivo, although it efficiently decreases FasL-mediated death in vitro. It is, however, more likely that AICD can occur in the absence of active caspase 8. Recently, Holler et al. (19) and Hildeman et al. (17) have demonstrated that AICD of human and murine T cells occurs in the presence of caspase inhibitors. Since some caspases are not readily blocked by the concentrations that can be achieved in cultured cells, these results do not completely rule out the involvement of caspases. However, FasL, TRAIL, and TNF-α were found to kill both primary and transformed T cells in the absence of caspase 8, inducing morphological changes that are reminiscent of necrosis. This type of cell death is transmitted via the DD-containing kinase RIP (19). Together with the classical caspase-dependent route, this caspase-independent cell death pathway may regulate T-cell homeostasis. The differences in the phenotypes of lpr mice and c-FLIPL Tg mice may therefore be explained as follows. Whereas the loss-of-function mutation lpr affects both the caspase-dependent and -independent cell death pathways due to impaired recruitment of both FADD-caspase 8 and RIP to the DD of Fas, overexpression of c-FLIPL inhibits only the caspase 8-dependent route and leaves the RIP-dependent cell death pathway intact. In line with this, we observed that the pancaspase inhibitor z-VAD inhibited FasL-induced cell death in normal mice nearly to the same extent as in c-FLIPL Tg mice. In addition, the remaining Fas-induced cell death in the c-FLIPL Tg mice was not further blocked by addition of z-VAD. T-cell accumulation leading to hyperplasia is therefore expected to take place only in lpr and gld mice, not in c-FLIP Tg mice. Similarly, T cells from transgenic mice expressing the caspase 8 inhibitor CrmA were protected from FasL-induced cell death in vitro, but these mice also did not develop a lymphoproliferative disease (48, 64). Finally, whereas in the CD2 enhancer-driven c-FLIPL Tg mice Fas-mediated responses are affected only in T cells, in lpr and gld mice Fas and FasL are absent on other cell types, such as B cells, NK cells, and macrophages. Impairment of Fas-induced death in these cell types may also contribute to the development of hyperproliferative disease in these mice
Rather than the anticipated T-cell accumulation, we observed a reduction in the percentage of CD8+ T cells in the periphery of both young and old c-FLIPL Tg mice. This reduction became apparent developmentally as early as the mature CD8 single-positive thymocyte stage. Despite the fact that thymocytes were resistant to FasL-induced death, studies of c-FLIPL Tg thymi showed that neither positive nor negative selection was affected. However, both the double-positive and the CD8 single-positive thymocytes manifested increased cell cycling and, at the same time, were more prone to undergo spontaneous cell death, suggesting that increased (or noncontrolled) proliferation is kept in check by subsequent cell death. Therefore, the decrease in the number of peripheral CD8+ T cells is at least in part due to decreased survival of its thymocyte precursor. Furthermore, the observation that predominantly the CD8+ T cells from c-FLIPL Tg mice are more prone to undergo apoptosis upon IL-2 withdrawal (unpublished observations) suggests also that the mature CD8+ T cells have a decreased survival potential. Why and how overexpression of c-FLIPL decreases the survival of CD8+ T cells are unclear at this point.
A similar duality of proliferation and cell death was observed in activated peripheral T cells. Whereas the proliferative response of c-FLIPL Tg T cells was markedly enhanced at suboptimal doses of anti-CD3, at optimal doses the c-FLIPL Tg T cells had decreased proliferative rates due to increased cell death. This finding may be linked to our recent observation that c-FLIPL can act as an adaptor molecule, associating with Raf-1 and TRAF-1/TRAF-2, which activate ERK and NF-κB, respectively, important mediators of cell proliferation and survival (24). Interestingly, T cells derived from c-FLIPL Tg mice were observed to have increased ERK phosphorylation and NF-κB activation after triggering of the TCR/CD3 complex, resulting in enhanced IL-2 production (24). It can thus be envisaged that at low doses of anti-CD3 the increased ERK and NF-κB activation may help to overcome the signaling threshold necessary for IL-2 production and T-cell proliferation. However, at high doses of anti-CD3, these same TCR signal pathways may provoke excessive proliferation and subsequent cell death. Consistent with this view, enhanced cell death has been observed with both augmented activation of ERK and sustained activation of NF-κB (4, 29, 32, 50).
Cross-linked recombinant FasL can costimulate proliferation and IL-2 production of primary human T cells, triggered with suboptimal doses of anti-CD3. Furthermore, addition of Fas-Fc as well as caspase inhibitors to cultures of anti-CD3-activated T cells was shown to inhibit their proliferation, implying that Fas-induced caspase activity can result in augmentation of T-cell proliferation (26). Since c-FLIPL is expressed in resting T cells, activation of these cells might first result in FasL expression, and subsequent Fas-FasL interactions may lead to the recruitment of both caspase 8 and c-FLIPL into the DISC. Caspase 8 is known to process c-FLIPL between its p10 and p18 caspase-like subunits, resulting in a cleaved c-FLIPL protein of 43 kDa (22, 43). Cleaved c-FLIPL may now be able to activate ERK and NF-κB and thus contribute to proliferation. In this context, it is noteworthy that thymocytes or T cells deficient in FADD or overexpressing a dominant negative form of FADD show highly impaired proliferation capacities (36, 58, 62). Since FADD is essential for c-FLIPL recruitment to death receptors, it is tempting to speculate that c-FLIPL-dependent ERK and NF-κB activation is required for T-cell growth. The costimulatory effect observed in the c-FLIPL Tg mice, however, was already apparent without further triggering of the Fas receptor by exogenously added FasL. Interestingly, in both resting and activated T cells of these mice, a large proportion of transgenic c-FLIPL is constitutively cleaved at its C terminus, giving rise to a product of 43 kDa. This cleavage is normally induced by death receptor-activated caspase 8 (43), but the high expression levels of c-FLIPL in the transgenic mice may lead to spontaneous processing of c-FLIPL that is sufficient to support CD3-triggered IL-2 production and proliferation in the absence of exogenous death receptor ligation. Alternatively, death ligands may constitutively activate death receptor-dependent signaling pathways in thymocytes and T cells. In line with this, high FasL expression has been detected in stromal cells of the thymus (13). The function of such signals, however, remains to be determined.
Collectively, our observations demonstrate that c-FLIPL possesses the potential of modulating signals leading to both cell death and cell growth. These crucial functions of c-FLIPL require tight control of c-FLIPL expression levels. The c-FLIPL protein is short lived (60), and its transcription is regulated by IL-2 in T cells (39). Since c-FLIPL is also upregulated in various types of tumors (22), rendering tumor cells resistant to immune surveillance (33), the understanding of the regulation of c-FLIPL will be of utmost importance.
Acknowledgments
We thank Anne Wilson for her generous gifts of Abs and helpful discussions.
S.M.A.L. is a fellow of the Dutch Cancer Society. This work was supported in part by grants AI36333 and AI45666 from the National Institutes of Health (R.C.B.).
REFERENCES
- 1.Alderson, M. R., T. W. Tough, T. Davis-Smith, S. Braddy, B. Falk, K. A. Schooley, R. G. Goodwin, C. A. Smith, F. Ramsdell, and D. H. Lynch. 1995. Fas ligand mediates activation-induced cell death in human T lymphocytes. J. Exp. Med. 181:71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Algeciras-Schimnich, A., T. S. Griffith, D. H. Lynch, and C. V. Paya. 1999. Cell cycle-dependent regulation of FLIP levels and susceptibility to Fas-mediated apoptosis. J. Immunol. 162:5205-5211. [PubMed] [Google Scholar]
- 3.Ashkenazi, A., and V. M. Dixit. 1998. Death receptors: signaling and modulation. Science 281:1305-1308. [DOI] [PubMed] [Google Scholar]
- 4.Beg, A. A., W. C. Sha, R. T. Bronson, and D. Baltimore. 1995. Constitutive NF-κB activation, enhanced granulopoiesis, and neonatal lethality in IκBα-deficient mice. Genes Dev. 9:2736-2746. [DOI] [PubMed] [Google Scholar]
- 5.Bertin, J., R. C. Armstrong, S. Ottilie, D. A. Martin, Y. Wang, S. Banks, G. H. Wang, T. G. Senkevich, E. S. Alnemri, B. Moss, M. J. Lenardo, K. J. Tomaselli, and J. I. Cohen. 1997. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc. Natl. Acad. Sci. USA 94:1172-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunner, T., R. J. Mogil, D. LaFace, N. J. Yoo, A. Mahboubi, F. Echeverri, S. J. Martin, W. R. Force, D. H. Lynch, C. F. Ware, et al. 1995. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature 373:441-444. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, P. L., and R. A. Eisenberg. 1991. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu. Rev. Immunol. 9:243-269. [DOI] [PubMed] [Google Scholar]
- 8.Darzynkiewicz, Z., X. Li, and J. Gong. 1994. Assays of cell viability: discrimination of cells dying by apoptosis. Methods Cell Biol. 41:15-38. [DOI] [PubMed] [Google Scholar]
- 9.Dhein, J., H. Walczak, C. Baumler, K. M. Debatin, and P. H. Krammer. 1995. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95). Nature 373:438-441. [DOI] [PubMed] [Google Scholar]
- 10.Earnshaw, W. C., L. M. Martins, and S. H. Kaufmann. 1999. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 68:383-424. [DOI] [PubMed] [Google Scholar]
- 11.Esslinger, C. W., A. Wilson, B. Sordat, F. Beermann, and C. V. Jongeneel. 1997. Abnormal T lymphocyte development induced by targeted overexpression of IκBα. J. Immunol. 158:5075-5078. [PubMed] [Google Scholar]
- 12.Fisher, G. H., F. J. Rosenberg, S. E. Straus, J. K. Dale, L. A. Middleton, A. Y. Lin, W. Strober, M. J. Lenardo, and J. M. Puck. 1995. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell 81:935-946. [DOI] [PubMed] [Google Scholar]
- 13.French, L. E., A. Wilson, M. Hahne, I. Viard, J. Tschopp, and H. R. MacDonald. 1997. Fas ligand expression is restricted to nonlymphoid thymic components in situ. J. Immunol. 159:2196-2202. [PubMed] [Google Scholar]
- 14.Gillette-Ferguson, I., and C. L. Sidman. 1994. A specific intercellular pathway of apoptotic cell death is defective in the mature peripheral T cells of autoimmune lpr and gld mice. Eur. J. Immunol. 24:1181-1185. [DOI] [PubMed] [Google Scholar]
- 15.Goltsev, Y. V., A. V. Kovalenko, E. Arnold, E. E. Varfolomeev, V. M. Brodianskii, and D. Wallach. 1997. CASH, a novel caspase homologue with death effector domains. J. Biol. Chem. 272:19641-19644. [DOI] [PubMed] [Google Scholar]
- 16.Han, D. K., P. M. Chaudhary, M. E. Wright, C. Friedman, B. J. Trask, R. T. Riedel, D. G. Baskin, S. M. Schwartz, and L. Hood. 1997. MRIT, a novel death-effector domain-containing protein, interacts with caspases and BclXL and initiates cell death. Proc. Natl. Acad. Sci. USA 94:11333-11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hildeman, D. A., T. Mitchell, T. K. Teague, P. Henson, B. J. Day, J. Kappler, and P. C. Marrack. 1999. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity 10:735-744. [DOI] [PubMed] [Google Scholar]
- 18.Hogquist, K. A., S. C. Jameson, W. R. Heath, J. L. Howard, M. J. Bevan, and F. R. Carbone. 1994. T cell receptor antagonist peptides induce positive selection. Cell 76:17-27. [DOI] [PubMed] [Google Scholar]
- 19.Holler, N., R. Zaru, O. Micheau, M. Thome, A. Attinger, S. Valitutti, J.-L. Bodmer, P. Schneider, B. Seed, and J. Tschopp. 2000. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 1:489-495. [DOI] [PubMed] [Google Scholar]
- 20.Hu, S., C. Vincenz, J. Ni, R. Gentz, and V. M. Dixit. 1997. I-FLICE, a novel inhibitor of tumor necrosis factor receptor-1- and CD-95-induced apoptosis. J. Biol. Chem. 272:17255-17257. [DOI] [PubMed] [Google Scholar]
- 21.Inohara, N., T. Koseki, Y. Hu, S. Chen, and G. Nunez. 1997. CLARP, a death effector domain-containing protein interacts with caspase-8 and regulates apoptosis. Proc. Natl. Acad. Sci. USA 94:10717-10722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irmler, M., M. Thome, M. Hahne, P. Schneider, K. Hofmann, V. Steiner, J. L. Bodmer, M. Schroter, K. Burns, C. Mattmann, D. Rimoldi, L. E. French, and J. Tschopp. 1997. Inhibition of death receptor signals by cellular FLIP. Nature 388:190-195. [DOI] [PubMed] [Google Scholar]
- 23.Ju, S. T., D. J. Panka, H. Cui, R. Ettinger, M. el-Khatib, D. H. Sherr, B. Z. Stanger, and A. Marshak-Rothstein. 1995. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature 373:444-448. [DOI] [PubMed] [Google Scholar]
- 24.Kataoka, T., R. C. Budd, N. Holler, M. Thome, F. Martinon, M. Irmler, K. Burns, M. Hahne, N. Kennedy, M. Kovacsovics, and J. Tschopp. 2000. The caspase-8 inhibitor FLIP promotes activation of NF-κB and Erk signaling pathways. Curr. Biol. 10:640-648. [DOI] [PubMed] [Google Scholar]
- 25.Kataoka, T., M. Schroter, M. Hahne, P. Schneider, M. Irmler, M. Thome, C. J. Froelich, and J. Tschopp. 1998. FLIP prevents apoptosis induced by death receptors but not by perforin/granzyme B, chemotherapeutic drugs, and gamma irradiation. J. Immunol. 161:3936-3942. [PubMed] [Google Scholar]
- 26.Kennedy, N. J., T. Kataoka, J. Tschopp, and R. C. Budd. 1999. Caspase activation is required for T cell proliferation. J. Exp. Med. 190:1891-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kishimoto, H., C. D. Surh, and J. Sprent. 1998. A role for Fas in negative selection of thymocytes in vivo. J. Exp. Med. 187:1427-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klas, C., K. M. Debatin, R. R. Jonker, and P. H. Krammer. 1993. Activation interferes with the APO-1 pathway in mature human T cells. Int. Immunol. 5:625-630. [DOI] [PubMed] [Google Scholar]
- 29.Lee, E. G., D. L. Boone, S. Chai, S. L. Libby, M. Chien, J. P. Lodolce, and A. Ma. 2000. Failure to regulate TNF-induced NF-κB and cell death responses in A20-deficient mice. Science 289:2350-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lenardo, M., K. M. Chan, F. Hornung, H. McFarland, R. Siegel, J. Wang, and L. Zheng. 1999. Mature T lymphocyte apoptosis—immune regulation in a dynamic and unpredictable antigenic environment. Annu. Rev. Immunol. 17:221-253. [DOI] [PubMed] [Google Scholar]
- 31.Luther, S. A., and H. Acha-Orbea. 1997. Mouse mammary tumor virus: immunological interplays between virus and host. Adv. Immunol. 65:139-243. [PubMed] [Google Scholar]
- 32.Mariathasan, S., S. S. Ho, A. Zakarian, and P. S. Ohashi. 2000. Degree of ERK activation influences both positive and negative thymocyte selection. Eur. J. Immunol. 30:1060-1068. [DOI] [PubMed] [Google Scholar]
- 33.Medema, J. P., J. de Jong, T. van Hall, C. J. Melief, and R. Offringa. 1999. Immune escape of tumors in vivo by expression of cellular FLICE-inhibitory protein. J. Exp. Med. 190:1033-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muzio, M., B. R. Stockwell, H. R. Stennicke, G. S. Salvesen, and V. M. Dixit. 1998. An induced proximity model for caspase-8 activation. J. Biol. Chem. 273:2926-2930. [DOI] [PubMed] [Google Scholar]
- 35.Nagata, S. 1997. Apoptosis by death factor. Cell 88:355-365. [DOI] [PubMed] [Google Scholar]
- 36.Newton, K., A. W. Harris, M. L. Bath, K. G. C. Smith, and A. Strasser. 1998. A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J. 17:706-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radvanyi, L. G., G. B. Mills, and R. G. Miller. 1993. Religation of the T cell receptor after primary activation of mature T cells inhibits proliferation and induces apoptotic cell death. J. Immunol. 150:5704-5715. [PubMed] [Google Scholar]
- 38.Rasper, D. M., J. P. Vaillancourt, S. Hadano, V. M. Houtzager, I. Seiden, S. L. Keen, P. Tawa, S. Xanthoudakis, J. Nasir, D. Martindale, B. F. Koop, E. P. Peterson, N. A. Thornberry, J. Huang, D. P. MacPherson, S. C. Black, F. Hornung, M. J. Lenardo, M. R. Hayden, S. Roy, and D. W. Nicholson. 1998. Cell death attenuation by "Usurpin', a mammalian DED-caspase homologue that precludes caspase-8 recruitment and activation by the CD-95 (Fas, APO-1) receptor complex. Cell Death Differ. 5:271-288. [DOI] [PubMed] [Google Scholar]
- 39.Refaeli, Y., L. Van Parijs, C. A. London, J. Tschopp, and A. K. Abbas. 1998. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity 8:615-623. [DOI] [PubMed] [Google Scholar]
- 40.Rieux-Laucat, F., F. Le Deist, C. Hivroz, I. A. Roberts, K. M. Debatin, A. Fischer, and J. P. de Villartay. 1995. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science 268:1347-1349. [DOI] [PubMed] [Google Scholar]
- 41.Russell, J. Q., T. Mooney, P. L. Cohen, B. MacPherson, R. J. Noelle, and R. C. Budd. 1998. Anti-CD40L accelerates renal disease and adenopathy in MRL-lpr mice in parallel with decreased thymocyte apoptosis. J. Immunol. 161:729-739. [PubMed] [Google Scholar]
- 42.Scaffidi, C., S. Kirchhoff, P. H. Krammer, and M. E. Peter. 1999. Apoptosis signaling in lymphocytes. Curr. Opin. Immunol. 11:277-285. [DOI] [PubMed] [Google Scholar]
- 43.Scaffidi, C., I. Schmitz, P. H. Krammer, and M. E. Peter. 1999. The role of c-FLIP in modulation of CD95-induced apoptosis. J. Biol. Chem. 274:1541-1548. [DOI] [PubMed] [Google Scholar]
- 44.Schulze-Osthoff, K., D. Ferrari, M. Los, S. Wesselborg, and M. E. Peter. 1998. Apoptosis signaling by death receptors. Eur. J. Biochem. 254:439-459. [DOI] [PubMed] [Google Scholar]
- 45.Scott, D. E., W. J. Kisch, and A. D. Steinberg. 1993. Studies of T cell deletion and T cell anergy following in vivo administration of SEB to normal and lupus-prone mice. J. Immunol. 150:664-672. [PubMed] [Google Scholar]
- 46.Sgonc, R., G. Boeck, H. Dietrich, J. Gruber, H. Recheis, and G. Wick. 1994. Simultaneous determination of cell surface antigens and apoptosis. Trends Genet. 10:41-42. [DOI] [PubMed] [Google Scholar]
- 47.Shu, H. B., D. R. Halpin, and D. V. Goeddel. 1997. Casper is a FADD- and caspase-related inducer of apoptosis. Immunity 6:751-763. [DOI] [PubMed] [Google Scholar]
- 48.Smith, K. G., A. Strasser, and D. L. Vaux. 1996. CrmA expression in T lymphocytes of transgenic mice inhibits CD95 (Fas/APO-1)-transduced apoptosis, but does not cause lymphadenopathy or autoimmune disease. EMBO J. 15:5167-5176. [PMC free article] [PubMed] [Google Scholar]
- 49.Srinivasula, S. M., M. Ahmad, S. Ottilie, F. Bullrich, S. Banks, Y. Wang, T. Fernandes-Alnemri, C. M. Croce, G. Litwack, K. J. Tomaselli, R. C. Armstrong, and E. S. Alnemri. 1997. FLAME-1, a novel FADD-like anti-apoptotic molecule that regulates Fas/TNFR1-induced apoptosis. J. Biol. Chem. 272:18542-18545. [DOI] [PubMed] [Google Scholar]
- 50.Stanciu, M., Y. Wang, R. Kentor, N. Burke, S. Watkins, G. Kress, I. Reynolds, E. Klann, M. R. Angiolieri, J. W. Johnson, and D. B. DeFranco. 2000. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J. Biol. Chem. 275:12200-12206. [DOI] [PubMed] [Google Scholar]
- 51.Strasser, A., A. W. Harris, D. C. Huang, P. H. Krammer, and S. Cory. 1995. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 14:6136-6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takahashi, T., M. Tanaka, C. I. Brannan, N. A. Jenkins, N. G. Copeland, T. Suda, and S. Nagata. 1994. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell 76:969-976. [DOI] [PubMed] [Google Scholar]
- 53.Thome, M., P. Schneider, K. Hofmann, H. Fickenscher, E. Meinl, F. Neipel, C. Mattmann, K. Burns, J. L. Bodmer, M. Schroter, C. Scaffidi, P. H. Krammer, M. E. Peter, and J. Tschopp. 1997. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 386:517-521. [DOI] [PubMed] [Google Scholar]
- 54.Tough, D. F., and J. Sprent. 1994. Turnover of naive- and memory-phenotype T cells. J. Exp. Med. 179:1127-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tschopp, J., M. Irmler, and M. Thome. 1998. Inhibition of fas death signals by FLIPs. Curr. Opin. Immunol. 10:552-558. [DOI] [PubMed] [Google Scholar]
- 56.Tschopp, J., M. Thome, K. Hofmann, and E. Meinl. 1998. The fight of viruses against apoptosis. Curr. Opin. Genet. Dev. 8:82-87. [DOI] [PubMed] [Google Scholar]
- 57.Van Parijs, L., Y. Refaeli, A. K. Abbas, and D. Baltimore. 1999. Autoimmunity as a consequence of retrovirus-mediated expression of C-FLIP in lymphocytes. Immunity 11:763-770. [DOI] [PubMed] [Google Scholar]
- 58.Walsh, C. M., B. G. Wen, A. M. Chinnaiyan, K. O'Rourke, V. M. Dixit, and S. M. Hedrick. 1998. A role for FADD in T cell activation and development. Immunity 8:439-449. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe-Fukunaga, R., C. I. Brannan, N. G. Copeland, N. A. Jenkins, and S. Nagata. 1992. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356:314-317. [DOI] [PubMed] [Google Scholar]
- 60.Willems, F., Z. Amraoui, N. Vanderheyde, V. Verhasselt, E. Aksoy, C. Scaffidi, M. E. Peter, P. H. Krammer, and M. Goldman. 2000. Expression of c-FLIP(L) and resistance to CD95-mediated apoptosis of monocyte-derived dendritic cells: inhibition by bisindolylmaleimide. Blood 95:3478-3482. [PubMed] [Google Scholar]
- 61.Yeh, W. C., A. Itie, A. J. Elia, M. Ng, H. B. Shu, A. Wakeham, C. Mirtsos, N. Suzuki, M. Bonnard, D. V. Goeddel, and T. W. Mak. 2000. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity 12:633-642. [DOI] [PubMed] [Google Scholar]
- 62.Zhang, J., D. Cado, A. Chen, N. H. Kabra, and A. Winoto. 1998. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature 392:296-300. [DOI] [PubMed] [Google Scholar]
- 63.Zheng, L., G. Fisher, R. E. Miller, J. Peschon, D. H. Lynch, and M. J. Lenardo. 1995. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature 377:348-351. [DOI] [PubMed] [Google Scholar]
- 64.Zhou, Q., S. Snipas, K. Orth, M. Muzio, V. M. Dixit, and G. S. Salvesen. 1997. Target protease specificity of the viral serpin CrmA. Analysis of five caspases. J. Biol. Chem. 272:7797-7800. [DOI] [PubMed] [Google Scholar]