Abstract
We report the characterization of two signal transduction proteins related to Bam32, known as TAPP1 and TAPP2. Bam32, TAPP1, and TAPP2 share several characteristics, including small size (32 to 47 kDa), lack of enzymatic domains, high conservation between humans and mice, and the presence of pleckstrin homology (PH) domains near their C termini which contain the 3-phosphoinositide-binding motif. Unlike Bam32, the N-terminal regions of TAPP1 and TAPP2 contain a second PH domain. TAPP1 and TAPP2 transcripts are expressed in a variety of tissues including lymphoid tissues. Using live-cell imaging, we demonstrate that TAPP1 and TAPP2 are recruited to the plasma membrane of BJAB human B-lymphoma cells upon activation through the B-cell antigen receptor (BCR). The C-terminal PH domain is necessary and sufficient for BCR-induced membrane recruitment of both TAPP1 and TAPP2. Blockade of phosphatidylinositol 3-kinase (PI3K) activity completely abolished BCR-induced recruitment of TAPP1 and TAPP2, while expression of active PI3K is sufficient to drive constitutive membrane localization of TAPP1 and TAPP2. TAPP1 and TAPP2 preferentially accumulate within ruffled, F-actin-rich areas of plasma membrane, suggesting a potential role in PI3K-driven cytoskeletal reorganization. Like Bam32, BCR-driven TAPP1 and TAPP2 recruitment is a relatively slow and sustained response, in contrast to Btk recruitment and Ca2+ mobilization responses, which are rapid and transient. Consistent with recent studies indicating that Bam32, TAPP1, and TAPP2 can bind to PI(3,4)P2, we find that membrane recruitment correlates well with production of PI(3,4)P2 but not with that of PI(3,4,5)P3. Our results indicate that TAPP1 and TAPP2 are direct targets of PI3K signaling that are recruited into plasma membranes with distinctive delayed kinetics and accumulate within F-actin-rich membrane ruffles. We postulate that the TAPPs function to orchestrate cellular responses during the sustained phase of signaling.
Enzymes such as phospholipase C (PLC) and phosphatidylinositol 3-kinase (PI3K) that act on inositol phospholipids in the plasma membrane play key roles in the intracellular propagation of signals generated by a large variety of cell surface receptors (52). Activation of these enzymes is dependent on protein tyrosine kinase activation and is regulated by complex mechanisms involving tyrosine kinases, phosphatases, and adaptor molecules. PLC and PI3K both act upon the phospholipid phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2]: PLC cleaves PI(4,5)P2 to produce soluble inositol 1,4,5-trisphosphate (IP3) and membrane-anchored diacylglycerol (DAG), while PI3K phosphorylates PI(4,5)P2 on the D3 position of the inositol ring to generate the lipid PI(3,4,5)P3 (PIP3). The functions of IP3 and DAG as second messengers have been relatively well characterized: IP3 stimulates an increase in cytosolic free Ca2+ levels by activating release from the endoplasmic reticulum through IP3-gated calcium channels, while DAG binds to and activates protein kinase C (PKC) isoforms. While PI3K has long been known to be an important component of signal transduction through many receptors, the function of the 3-phosphoinositide second messengers remained elusive until the recent discovery that some pleckstrin homology (PH) domains can specifically bind PIP3 (27, 38, 44). PH domains are protein modules of about 100 amino acids which are present in more than 200 named proteins involved in signal transduction, cytoskeletal organization, and membrane dynamics (26, 27), and dozens of these have been shown to directly bind various phosphoinositides, including PIP3 (24). Binding of PH domain-containing proteins to PIP3 can serve to transiently recruit these signaling proteins to the plasma membrane at sites of PI3K activation (4, 34, 56, 58). Thus, activated PI3K can regulate the function of a significant number of signaling proteins, consistent with the diverse array of biological responses downstream of PI3K (39, 52).
The importance of the PI3K pathway in B-lymphocyte activation and differentiation responses is well established. Studies examining the effects of PI3K inhibitors on B-cell responses in vitro indicate that PI3K is involved in CD40-induced immunoglobulin secretion in human B cells (1) and lipopolysaccharide (LPS)-induced proliferation in murine B cells (57). Human B cells pretreated with the PI3K inhibitor wortmannin show reduced anti-immunoglobulin M (anti-IgM)-induced thymidine incorporation and increased apoptosis, suggesting that PI3K-dependent signaling events deliver a critical component of survival and mitogenic signaling through the BCR. Studies examining mice deficient in the PI3K p85α subunit (15, 48) have demonstrated that PI3K activity is involved in both early B-cell differentiation and B-cell activation. p85-deficient B cells show impaired proliferation in response to anti-IgM, anti-CD40, interleukin 4, or LPS, suggesting that PI3K is required for mitogenic responses through a variety of activating receptors on B cells. Underlining the functional importance of the PI3K pathway in B cells is the fact that it is subject to negative regulation by the inhibitory Fc receptor FcγRII (37, 49). FcγRII is a physiologically important regulator of B-cell responses to antigens, since coligation of FcγRII inhibits B-cell antigen receptor (BCR)-induced Ca2+ mobilization (9) and proliferation (37), and FcγRII-deficient mice show exaggerated antibody responses (49). At least some aspects of FcγRII-mediated inhibition of BCR signal transduction appear to be mediated by SHIP (6, 36); SHIP specifically antagonizes PIP3 generation by removing the 5-phosphate from PIP3 to generate the lipid product PI(3,4)P2 (11, 29). The importance of SHIP in regulating B-cell activation is supported by studies of SHIP-deficient cell lines and mice, which have defects in B-cell maturation and exaggerated BCR signaling responses (21, 36).
Given the importance of PI3K and SHIP in B-cell activation, a current, critical question is, what are the targets of 3-phosphoinositide second messengers in B cells and how do they function to regulate B-cell activation and differentiation? Two PH domain-containing targets of PI3K are known to be activated by the BCR: the Tec family protein tyrosine kinase Bruton's tyrosine kinase (Btk) (41, 42) and the serine/threonine kinase Akt (2, 5, 10, 20). We have recently identified and characterized Bam32, a novel PH domain-containing protein that is recruited to the plasma membrane of activated B lymphocytes in a PI3K-dependent manner (31). Here we report the characterization of TAPP1 and TAPP2, two PH domain-containing adaptor proteins related to Bam32 that have recently been shown to bind specifically to PI(3,4)P2 (12). We demonstrate that these proteins represent targets of activated PI3K and are recruited to the plasma membranes of B lymphocytes in a sustained fashion after activation through the BCR.
MATERIALS AND METHODS
Isolation of human TAPP2 and TAPP1 cDNAs.
In the course of isolating the murine homologue of Bam32 (31), we fortuitously cloned a cDNA fragment encoding the C-terminal PH domain of TAPP2, which we initially referred to as Phad47, for PH domain-containing adaptor of 47 kDa (Marshall et al., FASEB J. 14:A967, 2000). To avoid confusion, we here refer to Phad47 as TAPP2, in accordance with the nomenclature of Dowler and colleagues, who recently reported the identification of this same gene by use of a database-searching approach (12). The 5′ sequence was subsequently isolated by using rapid amplification of cDNA ends (RACE) with an adaptor-ligated cDNA template derived from a BALB/c mouse spleen (Clontech). This RACE product contains an in frame stop codon at the 5′ end, indicating that it represents the full-length sequence. Our assembled TAPP2 sequence, including approximately 700 bp of 3′ untranslated sequence, can be accessed in GenBank under the name Phad47 (accession number AF418551). We found that the expressed sequence tag (EST) database contained sequences corresponding to the human homologue of our murine TAPP2 sequence and to a second gene closely related to TAPP2, which we have designated TAPP1. We obtained IMAGE clones encoding human TAPP2 and human and murine TAPP1 (Research Genetics) and fully sequenced the inserts. IMAGE clone 1884429 (GenBank accession number AI216176) contains the full-length human TAPP1 sequence shown in Fig. 1. We currently have only partial sequence information for human TAPP2 and murine TAPP1.
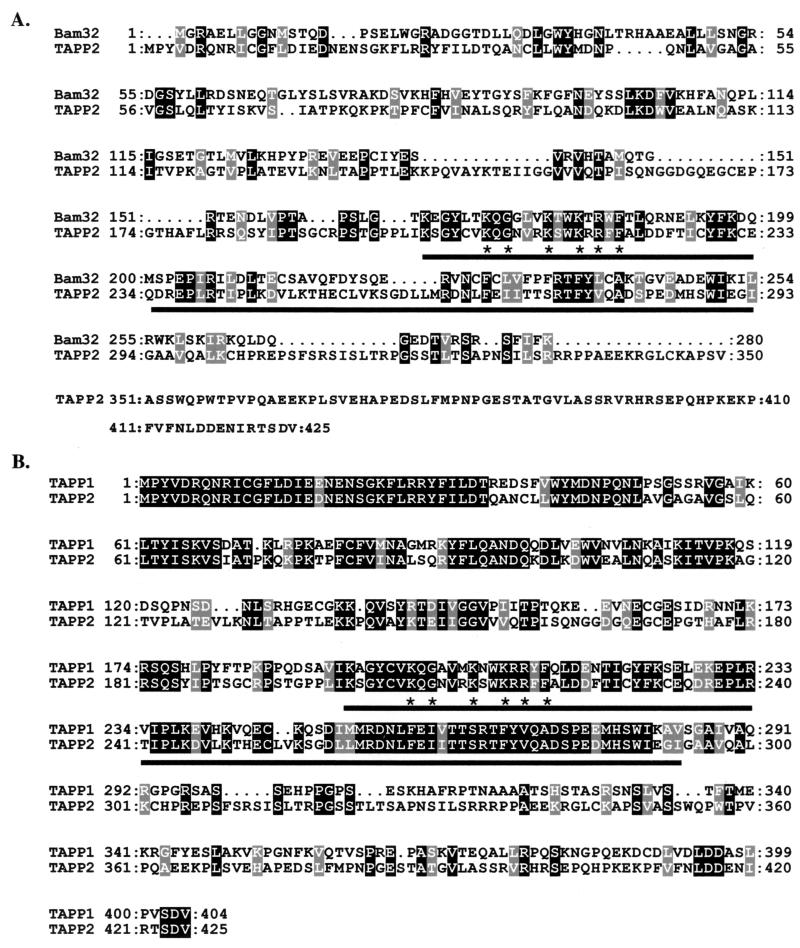
FIG. 1.
Amino acid alignment of Bam32, TAPP2, and TAPP1. Sequences were aligned with ClustalX, version 1.81. The PH domains are indicated by underlining. Stars indicate residues within the PH domain that are conserved among PH domains which bind to PI(3,4)P2 or PIP3 (24). Black shading indicates amino acid identities, and grey shading indicates amino acid similarities (as defined by ClustalX). (A) Mouse Bam32 and mouse TAPP2; (B) murine TAPP2 and human TAPP1.
Northern blot and RT-PCR expression analysis.
32P-labeled cDNA probes were hybridized to human multiple tissue Northern blots (Clontech), according to the manufacturer's protocol. Reverse transcription-PCR (RT-PCR) analysis was carried out essentially as described previously (31). For TAPP2 and TAPP1, the following primers were used: TAPP2 sense, GATGTGAGCAGAGCCCAGGAATGCC; TAPP2 antisense, AGGGCTGGAGGAGCTGCTAAGCTC; TAPP1 sense, CATGTTCCTCTGTGTTCTGCATCTC; TAPP1 antisense, CTGAGTGGGAGTAATGATGGGTACG. Serial dilutions of the cDNAs were amplified, and appropriate dilutions were chosen to be within the semiquantitative range of amplification.
Plasmid constructs and antibodies.
Vectors encoding mouse TAPP2 and human TAPP1 fused to enhanced green fluorescent protein (EGFP) at their N termini were constructed by cloning the entire coding sequences into pEGFP-C1 (Clontech). C-terminal PH domain fusions contain amino acids 179 to 311 (TAPP2) or 169 to 329 (TAPP1). N-terminal PH domain fusions contain amino acids 3 to 156 (TAPP2) or 4 to 158 (TAPP1). Point mutations in the TAPP1 or TAPP2 C-terminal PH domains were created by primer-directed mutagenesis. All vector inserts were created by using high-fidelity PCR with restriction-tagged primers, and the final constructs were verified by restriction digestion and DNA sequencing. Constructs encoding the Btk or PLCδ PH domain fused to EGFP were gifts from T. Balla (National Institutes of Health [NIH], Bethesda, Md.). Vectors encoding the PI3K p110-CD2 or PI3K p110-CD2 R916P fusion protein (43) were a gift from D. Cantrell (London, United Kingdom). Expression of this construct was confirmed by surface staining with phycoerythrin-conjugated anti-rat CD2 (BD Pharmingen) and analysis on a FACScalibur instrument (Becton Dickinson).
Transient transfection of BJAB cells with EGFP fusion constructs and live-cell imaging.
BJAB cells (an atypical IgM-positive, Epstein-Barr virus [EBV]-negative Burkitt's lymphoma) were cultured in RPMI 1640 medium containing 10% fetal calf serum (FCS) and antibiotics. For transient transfection experiments, BJAB cells were washed and resuspended in a high-resistance buffer (27 mM sodium phosphate [pH 7.5]-150 mM sucrose) at 2.5 × 107/ml. A 400-μl volume of cells was mixed with 10 μg of the indicated plasmid constructs in a 0.4-cm gap electroporation cuvette (Bio-Rad, Hercules, Calif.) and incubated on ice for 10 min. Cells were electroporated by using a GenePulser II apparatus equipped with an RF module (Bio-Rad) set at 340 V, a 4-ms burst, 100% modulation, 40 kHz, five bursts, and a 1-ms burst interval. Cuvettes were incubated on ice for a further 10 min; then the contents were transferred to a tube containing 10 ml of complete RPMI medium containing 1% FCS and were plated in 8-well LabTek chambered coverglass slides (Nunc). Chamber slides were incubated overnight in a 37°C, 5% CO2 incubator and then directly examined with an inverted laser-scanning confocal microscope (Olympus Fluoview). The EGFP fluorescence signal was detected with 488-nm excitation and a 510-nm emission filter.
For each well, an EGFP-expressing cell was centered within the field and the focal plane was set near the center of the cell. Stimuli were then added to the well, and images were acquired at 30-s intervals. Goat anti-human IgM F(ab′)2 fragments (Jackson ImmunoResearch) were used at a final concentration of 10 μg/ml. In some experiments, the PI3K inhibitor wortmannin (20 ng/ml; Sigma, St. Louis, Mo.) or LY294002 (25 μM; Biomol, Plymouth Meeting, Pa.) was added to the wells and the slides were incubated for 30 min at 37°C prior to stimulation.
Colocalization of EGFP fusions with F-actin.
For F-actin localization experiments, the indicated transfectants were fixed with 3.7% paraformaldehyde and stained with rhodamine-phalloidin (Molecular Probes, Eugene, Oreg.), according to the manufacturer's protocol. Green (EGFP) and red (rhodamine) fluorescence images were acquired simultaneously by using dual 488- and 568-nm excitation and parallel photomultiplier tube detectors equipped with appropriate dichroic and emission filters. Bleedthrough between the red and green channels was minimal, as determined by single-color control samples and single excitation images of dual-color samples (data not shown). Digital overlay images were created by using ImageProPlus software (Media Cybernetics).
Quantitative kinetic analysis of plasma membrane recruitment and [Ca2+] mobilization.
The ratio of membrane to cytoplasmic EGFP fluorescence intensity was determined from digital images by using ImageQuant software (Molecular Dynamics), as previously described (31). For [Ca2+] measurements, ratiometric imaging using Fluo-4 and Fura-Red dyes was employed (14, 30). Briefly, BJAB cells were resuspended in serum-free medium and loaded with Fluo-4-AM and Fura-Red-AM (Molecular Probes). Cells were then plated onto LabTek Chambered Coverglass slides (Nalge Nunc, Naperville, Ill.), stimulated with anti-IgM F(ab′)2, and imaged at 10-s intervals using an excitation wavelength of 488 nm and simultaneous acquisition of green (510-nm longpass filter) and far-red (700- to 775-nm bandpass) channels. The relative [Ca2+] was then determined by dividing the green (Fluo-4) fluorescence by the far-red (Fura-Red) fluorescence for a microscopic field containing 20 to 30 cells. For each run, the data were normalized to those for frame 1 (no stimulus), and normalized data from multiple runs were averaged.
Measurement of PI(3,4)P2, PI(4,5)P2, and PI(3,4,5)P3 levels.
BJAB cells were washed three times with phosphate-free medium before being resuspended at 107/ml in 99% phosphate-free medium, in which the cells were starved for 1 h. Cells were then labeled with 0.5 mCi of orthophosphate (ICN, Costa Mesa, Calif.) per ml for 1.5 h at 37oC and stimulated with 10 μg of anti-human IgM F(ab′)2 fragment/ml for the indicated times. Extraction of inositol phospholipids and high-pressure liquid chromatography (HPLC) analysis of deacylated lipids were performed as described previously (46). The elution times of standard ATP, PI(1,4,5)P3, and PI(1,3,4,5)P3 (Perkin-Elmer, Boston, Mass.) are 85, 95, and 112 min, while the elution times of PI(3,4)P2, PI(4,5)P2, and PI(3,4,5)P3 are 76, 79, and 97 min, respectively. The amounts of radioactivity contained in the elution peak for each lipid (three to six fractions) were summed to give the total counts for each lipid. For each lipid, the fold increase was calculated by dividing the total counts from each stimulated sample by the total counts for the unstimulated control.
RESULTS
Isolation of TAPP2 and TAPP1 cDNAs.
A gene encoding a protein related to the Bam32/DAPP1/PHISH adaptor (3, 13, 31, 40) was isolated through primer cross-hybridization in a 3′ RACE reaction using a primer specific for mouse Bam32 (see Materials and Methods). This gene encodes a 47-kDa protein which, like Bam32, contains a C-terminal PH domain. Searches of the EST database revealed another, closely related gene encoding a 45-kDa PH domain protein. In line with a recent report describing the same pair of related genes (12), we refer to these proteins as TAPP2 and TAPP1, respectively, for tandem PH domain-containing proteins. Database searching revealed that TAPP2 is more closely related to Bam32 than to any other mammalian protein in the GenBank database, with the homology largely confined to the C-terminal PH domain. Figure 1A shows an alignment of the amino acid sequences of murine TAPP2 and Bam32. Like the Bam32 PH domain, the PH domain of TAPP2 contains the residues conserved among PH domains which bind 3-phosphoinositides (24). Unlike Bam32, the N-terminal region of TAPP2 does not contain an SH2 domain but instead contains a second PH domain. The N-terminal PH domain contains the hallmark tryptophan in the C-terminal α-helix but lacks the 3-phosphoinositide-binding motif (24).
Comparison of murine TAPP2 to the EST database revealed the human counterpart of TAPP2 and clones encoding human and murine TAPP1. Alignment of the TAPP2 and TAPP1 sequences (Fig. 1B) shows that these proteins are approximately 60% identical over the region containing the N-terminal and C-terminal PH domains but share little identity in the C-terminal 100 amino acids. Strikingly, the N-terminal 34 amino acids are nearly identical, suggesting a strong conservation of function in this region.
Expression of TAPP1 and TAPP2 mRNAs.
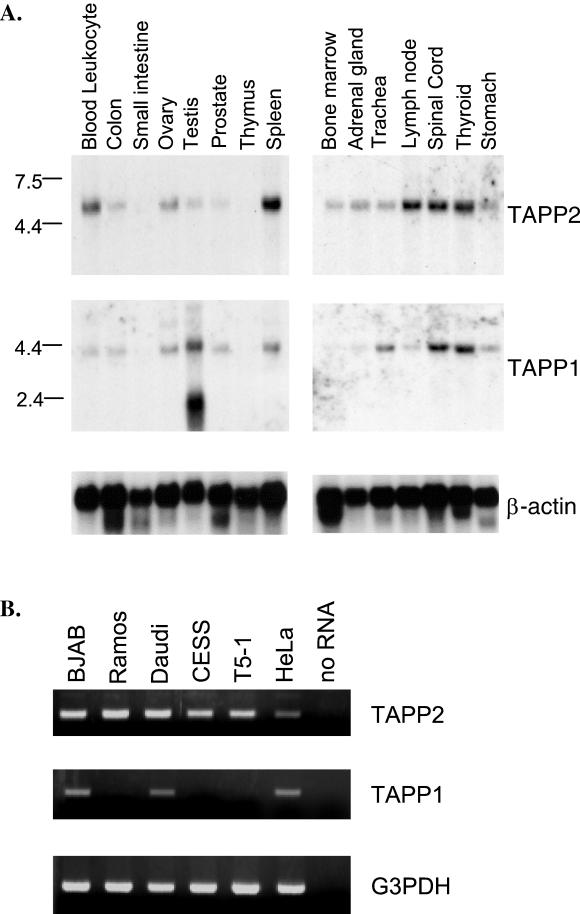
Expression of TAPP2 and TAPP1 transcripts in human tissues was determined by Northern blot analysis. The TAPP2 probe detected a single 5.5-kb transcript, which is predominantly expressed in lymphoid tissues such as peripheral blood leukocytes, spleen, and lymph node, but at much lower levels in the thymus (Fig. 2A). TAPP2 is also expressed at high levels in the spinal cord and thyroid gland, and at lower levels in several other tissues. The TAPP1 probe detects a single 4.5-kb transcript, except in the testis, where an abundant 2.5-kb transcript is detected. TAPP1 is expressed at the highest levels in the testis, spinal cord, and thyroid gland but is also expressed in all lymphoid tissues, though only very weakly in the thymus.
FIG. 2.
Expression of TAPP1 and TAPP2 in human tissues and cell lines. (A) Northern blots containing 2 μg of poly(A)+ RNA from the indicated human tissue (Clontech) were sequentially hybridized with radiolabeled TAPP2, TAPP1, or actin cDNA probes. (B) Total RNA from the indicated cell lines (0.1 μg) was reverse transcribed, and PCR was amplified for 28 cycles with primers specific for TAPP2, TAPP1, or the housekeeping gene G3PDH. No PCR product was observed by using a genomic DNA template, indicating that the TAPP2 and TAPP1 primer sets span an intron-exon boundary. BJAB, Ramos, and Daudi are Burkitt's lymphoma lines, CESS and T5-1 are lymphoblastoid cells, and HeLa is an epithelial-cell line.
RT-PCR analysis of TAPP1 and TAPP2 expression in immortalized cell lines shows that both TAPP2 and TAPP1 are expressed in B-cell and epithelial-cell lines (Fig. 2B). Among B-cell lines, both genes appear to be more highly expressed in Burkitt's lymphoma lines than in EBV-transformed lymphoblastoid cells; in fact, TAPP1 was not detected in either of the lymphoblastoid cells tested. These results suggest that, like Bam32 (31), the TAPPs may be differentially expressed during B-cell activation or differentiation.
TAPP2 and TAPP1 are recruited to the plasma membrane after BCR ligation.
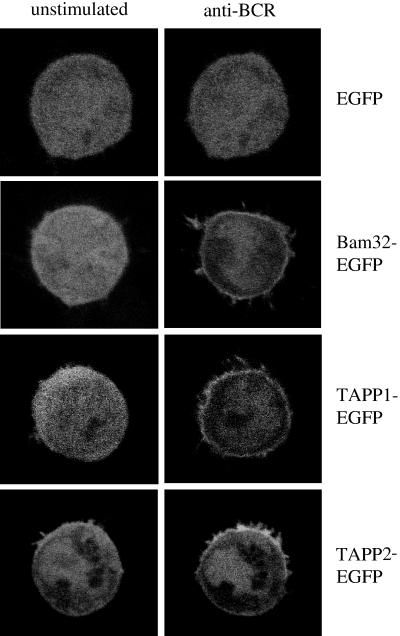
Given that the PH domains of TAPP1 and TAPP2 are closely related to that of Bam32 and contain the 3-phosphoinositide-binding motif, we hypothesized that these domains function to target these molecules to the plasma membrane upon activation of PI3K. To test this possibility, we generated plasmid constructs encoding TAPP1 or TAPP2 fused to the fluorescent marker protein EGFP. These fusion proteins were expressed in human B-lymphoma cells, and the cells were activated by antibody cross-linking of the endogenous BCR, which is known to activate PI3K (17, 18). The subcellular localizations of the fluorescent proteins were assessed by live-cell imaging with a confocal microscope (Fig. 3). Before stimulation, these proteins generally showed a diffuse cytoplasmic and nuclear distribution, similar to EGFP alone. After stimulation, the Bam32, TAPP1, and TAPP2 fusion proteins showed a clear redistribution of the plasma membrane within 5 min, while EGFP alone showed no change in distribution. This result demonstrates that the TAPPs can be recruited to the plasma membranes of B lymphocytes by stimulation through a receptor known to activate PI3K.
FIG. 3.
TAPP1 and TAPP2 are recruited to the plasma membrane during B-lymphocyte activation. Constructs encoding fusion proteins of EGFP with Bam32, TAPP2, or TAPP1 were transfected into BJAB B-lymphoma cells. Localizations of the fusion proteins in live cells were imaged with a confocal microscope both prior to stimulation and 5 min after BCR cross-linking [10 μg of anti-human IgM F(ab′)2 fragments/ml]. Results are representative of 10 or more experiments.
Active PI3K is necessary and sufficient for membrane recruitment of TAPP1 and TAPP2.
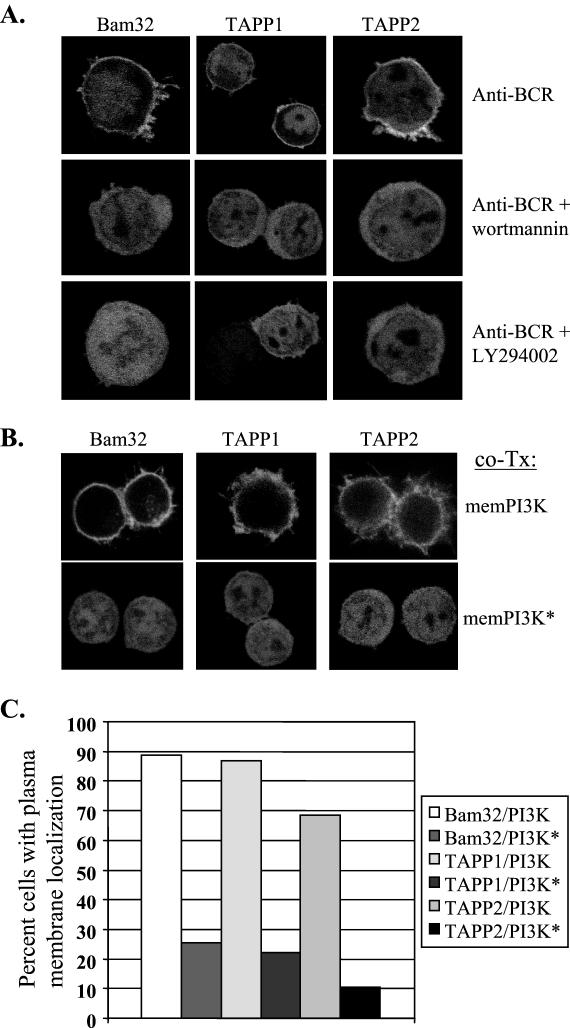
In order to test whether BCR-induced redistribution is dependent on activation of PI3K, cells were preincubated with one of two distinct PI3K inhibitors, wortmannin or LY294002, prior to BCR cross-linking. We found that either of these inhibitors can completely block BCR-induced redistribution to the plasma membrane (Fig. 4A). In addition, stimulation of the cells with agents not known to activate PI3K, such as LPS, did not lead to recruitment of either protein to the membrane (data not shown).
FIG. 4.
PI3K activation is necessary and sufficient for membrane recruitment of TAPP1 and TAPP2. (A) BCR-induced membrane recruitment of TAPP1 and TAPP2 is blocked by inhibitors of PI3K. BJAB cells were transfected with the indicated EGFP fusion constructs. Where indicated, cells were preincubated for 30 min with the PI3K inhibitor wortmannin or LY294002 prior to stimulation. (B) Expression of activated PI3K leads to constitutive membrane localization of TAPP1 and TAPP2. BJAB cells were cotransfected (co-Tx) with the indicated EGFP fusion constructs and a 10-fold excess of vectors encoding either membrane-bound PI3K (memPI3K) or a control kinase-dead protein (memPI3K∗). (C) The frequency of cells showing membrane localization in the absence of stimulation was assessed by visual scoring on a high-resolution epifluorescence microscope. Only cells that could be definitively categorized as showing membrane association or not were included in the scoring count. A coded labeling system was employed to negate observer bias. Results are representative of three or more experiments.
To determine whether the presence of active PI3K at the plasma membrane is sufficient for recruitment of the TAPPs, BJAB cells were cotransfected with the TAPP-EGFP fusion constructs and vectors encoding either membrane-bound PI3K (memPI3K) or a control kinase-inactive protein (memPI3K∗) (Fig. 4B). Under the conditions used, 70 to 90% of the EGFP-expressing cells coexpressed detectable amounts of the membrane-bound PI3K proteins, as assessed by fluorescence-activated cell sorter (FACS) staining of the extracellular portion of rat CD2 used in the constructs. We found that, in the absence of stimulation, 77 to 89% of cells cotransfected with active PI3K had an observable accumulation of the EGFP fusion proteins at the plasma membrane, compared to 15 to 27% of cells expressing kinase-inactive PI3K (Fig. 4C). These results provide direct evidence that active PI3K is sufficient for membrane recruitment of TAPP1 and TAPP2.
TAPP1 and TAPP2 colocalize with F-actin within membrane ruffles.
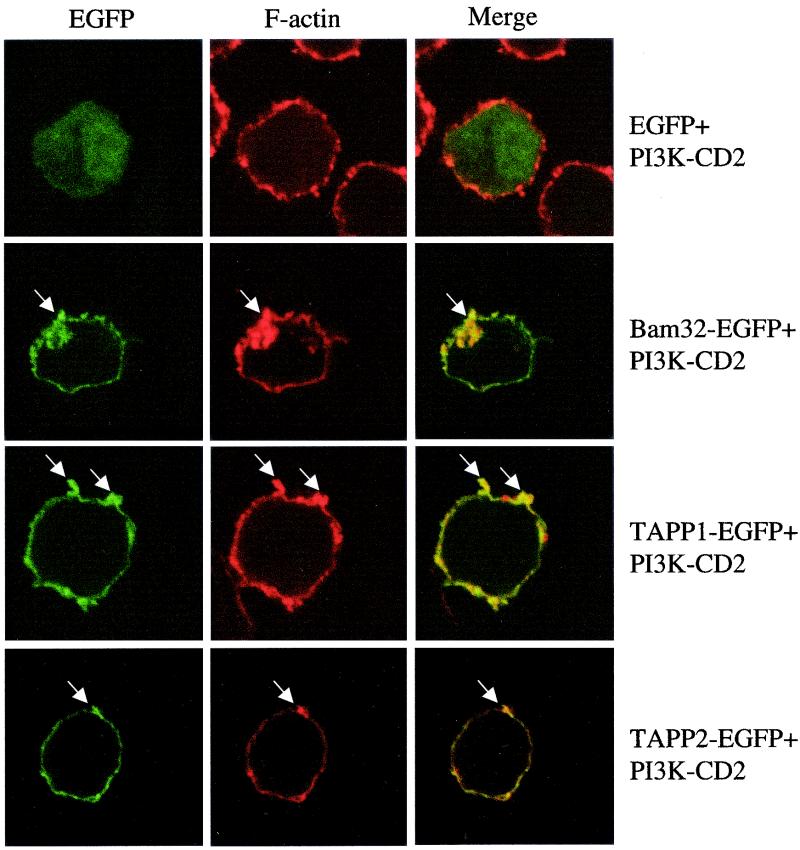
A striking feature of TAPP1 and TAPP2 membrane recruitment induced by either anti-IgM treatment or transmembrane PI3K expression was the strong association of these molecules with rapidly moving plasma membrane structures that appear to represent membrane ruffles or filopodia. To examine whether these structures represent areas of cytoskeletal reorganization and actin polymerization, we examined the relative distributions of filamentous actin and TAPP1 or TAPP2 in cells overexpressing active PI3K (Fig. 5). Clear colocalization of EGFP-TAPP1 or EGFP-TAPP2 and filamentous actin could be observed within these structures. EGFP-Bam32 showed a similar pattern, while EGFP alone showed no preferential colocalization with F-actin. The N-terminal PH domain of TAPP1 or TAPP2 also failed to show colocalization with F-actin (data not shown). This result indicates that this group of PH domain adaptors preferentially associates with membrane ruffles in activated B lymphocytes, as has been reported for other PH domains in other cell types (33, 51, 59).
FIG. 5.
Bam32, TAPP1, and TAPP2 colocalize with F-actin within PI3K-induced membrane ruffles. BJAB cells were cotransfected with the indicated EGFP fusion proteins and PI3K-CD2 constructs and then fixed, permeabilized, stained with rhodamine-phalloidin to detect filamentous actin, and observed under the confocal microscope. Yellow color on the merged images indicates areas of colocalization of red and green signals. Arrows indicate areas of ruffled plasma membrane which stain intensely for F-actin and show accumulation of the EGFP fusion proteins. Note that membrane ruffles have a more condensed appearance in these images due to the fixation-permeabilization processing prior to imaging. Data are representative of three experiments.
Membrane recruitment is mediated solely by the C-terminal PH domains of TAPP1 and TAPP2.
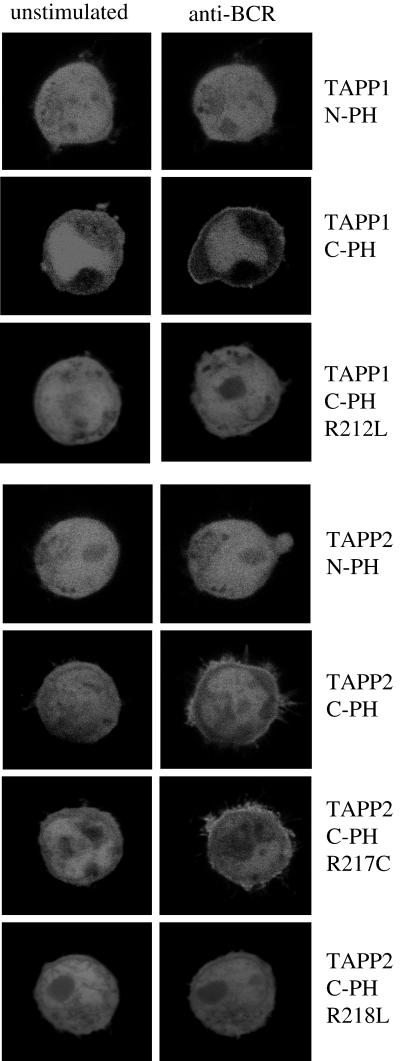
To determine which domain(s) of TAPP1 or TAPP2 is required for membrane localization, we constructed EGFP fusion vectors encoding either the N-terminal PH domain or the C-terminal PH domain of either TAPP2 or TAPP1. When these proteins were expressed in B-lymphoma cells, we found that the C-terminal PH domain fusions, but not the N-terminal PH domain fusions, were recruited to the plasma membrane after stimulation (Fig. 6). The N-terminal PH domains also failed to target to the plasma membrane when coexpressed with transmembrane PI3K (data not shown). Thus, the C-terminal PH domains, which contain the 3-phosphoinositide-binding motif, appear to be solely responsible for the PI3K-dependent membrane targeting of the TAPPs. To determine whether the 3-phosphoinositide-binding motif (24) is indeed required for membrane recruitment, we generated mutations of conserved arginine residues within this motif to leucines. We found that these mutations (R212L for TAPP1 or R218L for TAPP2) completely abolish BCR-induced membrane recruitment (Fig. 6), indicating that direct interaction of the C-terminal PH domains with 3-phosphoinositides is required. Interestingly, mutation of an adjacent nonconserved arginine in the TAPP2 C-terminal PH domain (R217C) did not completely block membrane recruitment, further supporting the specific function of this conserved motif in membrane recruitment.
FIG. 6.
Membrane recruitment of TAPP1 or TAPP2 is mediated by the C-terminal PH domains and requires an intact 3-phosphoinositide-binding motif. Constructs encoding fusion proteins of EGFP with either the N-terminal PH domain, the C-terminal PH domain, or the indicated mutant of the C-terminal PH domain of TAPP1 or TAPP2 were generated, expressed in BJAB cells, and analyzed as in the experiment for which results are shown in Fig. 3. Images were acquired prior to stimulation and 5 min after BCR cross-linking with antibody. Data are representative of 3 to 10 experiments per construct.
Delayed kinetics of TAPP1 and TAPP2 membrane recruitment relative to other PH domain-mediated redistribution events.
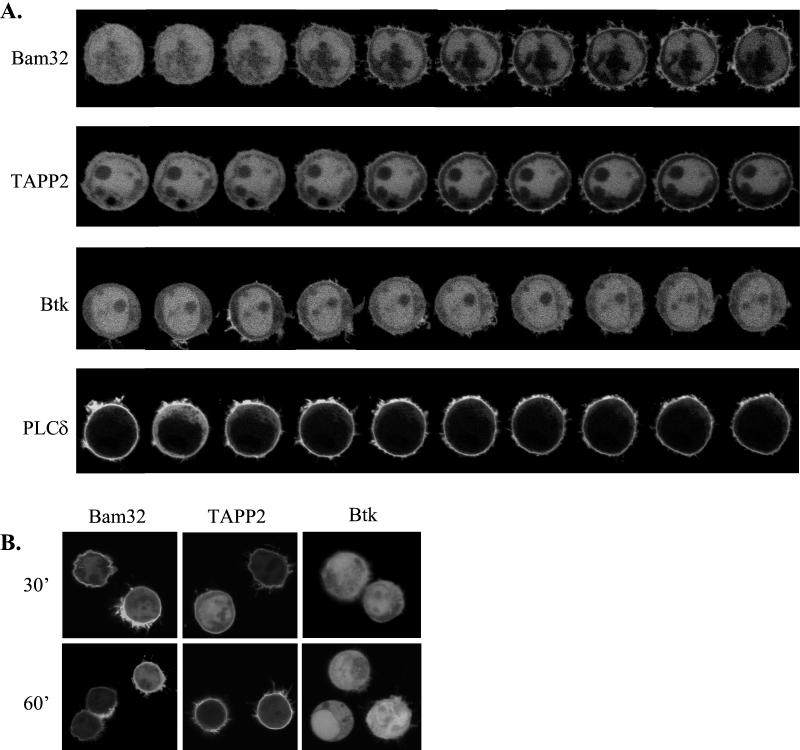
In order to gain insight into the role of TAPP1 and TAPP2 in BCR-mediated signaling events, we took advantage of the live-cell imaging approach to examine the kinetics of TAPP1 and TAPP2 membrane recruitment in single cells (Fig. 7A shows representative cells). It was found that the PH domain-mediated membrane recruitment of these proteins occurred with relatively slow kinetics, with the first visible redistribution becoming visible at 1.5 to 3.5 min after addition of the stimulus. This was surprising, given the reportedly rapid (<1 min) activation of PI3K by the BCR (17, 18). In addition, the recruitment was remarkably sustained; membrane association was clearly observable up to 1 h after stimulation (Fig. 7B). Bam32 recruitment followed kinetics similar to those of the TAPPs, with visible redistribution occurring at 1.5 to 3 min and membrane association still visible after 1 h.
FIG. 7.
TAPP1 and TAPP2 show delayed and sustained kinetics of PH domain-mediated membrane recruitment after BCR cross-linking. BJAB cells were transiently transfected with the indicated PH domain-EGFP fusion and activated by BCR cross-linking. (A) Confocal images were acquired at 30-s intervals after activation. Note the marked differences in the kinetics of PH domain-mediated translocation events. (B) The indicated transfectants were stimulated by BCR cross-linking and imaged after 30 or 60 min. Individual representative cells are shown, while a quantitative analysis over multiple cells is shown in Fig. 8.
The kinetics of TAPP1 and TAPP2 recruitment were also compared with the BCR-induced redistribution of two relatively well-characterized PH domain proteins, Btk and PLCδ (Fig. 7A). Membrane recruitment of the Btk PH domain, which binds to PIP3 (38, 44), was generally weak and occurred rapidly (within 1 min) and transiently, with visible membrane association disappearing by 5 min after stimulation. The PLCδ PH domain, which binds to the constitutively present phosphoinositide PI(4,5)P2 (16, 28), shows constitutive association with the plasma membranes of the B-lymphoma cells (Fig. 7). A transient redistribution of protein to the cytoplasm is clearly visible within 30 s of stimulation, as has been observed for other cell types (47, 54, 55). This redistribution is thought to reflect both the transient depletion of membrane PI(4,5)P2 due to the activation of PLC and PI3K enzymes and the production of the soluble IP3, which can also bind to the PLCδ PH domain (22, 54). Thus, PI3K and PLC activation appears to occur very rapidly in our BJAB live-cell imaging system.
Membrane recruitment of Bam32 and TAPP2 coincides with peak PI(3,4)P2 generation rather than peak PI(3,4,5)P3 or calcium mobilization responses.
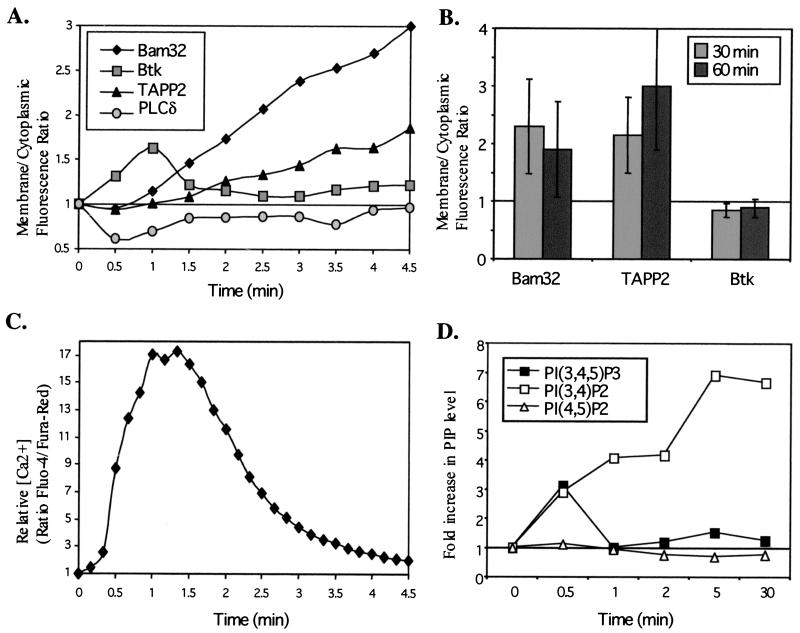
In order to obtain a better measure of the relative kinetics and magnitude of PH domain-mediated redistribution events, we analyzed the fluorescence image data to obtain an averaged quantitative representation of the change in the ratio of membrane to cytoplasmic fluorescence over time (Fig. 8A). We also measured the change in intracellular free [Ca2+] over time in order to compare the kinetics of this response to the PH domain-mediated redistribution events (Fig. 8C). It was found that the Ca2+ mobilization response is initiated with rapid kinetics (within 30 s) that parallel the redistribution of the PLCδ and Btk PH domains. In contrast, membrane recruitment of TAPP2 is relatively delayed, becoming apparent at 2 to 3 min after stimulation. Remarkably, membrane localization of Bam32 and TAPP2 was still strong after 30 and 60 min of stimulation, while no membrane localization of Btk was detectable (Fig. 8B). Similar results were obtained for TAPP1 (data not shown).
FIG. 8.
Membrane recruitment of Bam32 and TAPPs correlates with PI(3,4)P2 production but not with PI(3,4,5)P3 production or calcium mobilization. (A) Digital image data such as those presented in Fig. 7A were quantitatively analyzed to determine changes in the ratio of cytoplasmic to membrane fluorescence intensity over time after addition of stimulus. For each cell, data were normalized to those in frame 1 and the plots shown represent the average for three to five cells from a single transfection experiment. Cells showing no visible redistribution of fluorescence upon stimulation (less than 15% in most experiments) were assumed to be damaged or dead and were excluded from the analysis. Data are representative of at least three experiments per PH domain construct. (B) Quantitative analysis of cells 30 or 60 min poststimulation. Random fields containing several cells were imaged, and membrane/cytoplasmic fluorescence was determined without normalization. Data are averages and standard deviations for 6 to 17 cells per group. (C) Kinetics of [Ca2+] mobilization under live-cell imaging conditions. BJAB cells were loaded with Fluo-4-AM and Fura-Red-AM dyes and were stimulated by BCR cross-linking, and relative intracellular calcium levels were determined by image analysis. Data are representative of five experiments. (D) Kinetics of PI(3,4,5)P3 and PI(3,4)P2 production following BCR ligation. Data are represented as the fold change in phosphoinositide levels at the indicated time after stimulation. Data are representative of three experiments.
In order to compare the membrane recruitment kinetics with 3-phosphoinositide generation, we measured the relative levels of PI(4,5)P2, PI(3,4,5)P3, and PI(3,4)P2 before and after BCR cross-linking (Fig. 8D). It was found that PI(3,4,5)P3 levels are rapidly increased following BCR cross-linking (a threefold increase within 30 s) but rapidly return to near-baseline levels. In contrast, PI(3,4)P2 levels are dramatically increased (up to 10-fold), peak much later (>5 min), and are remarkably sustained, with little diminution of the response apparent at 30 min poststimulation. Thus, PI(3,4)P2 generation correlates well with the delayed and sustained recruitment of the TAPPs. Notably, the total amount of labeled PI(3,4)P2 recovered from BCR-activated BJAB cells was substantially higher than that for PI(3,4,5)P3 (4- to 10-fold [data not shown]), perhaps explaining why membrane recruitment of Bam32, TAPP1, and TAPP2 is generally stronger than that of Btk in this model (Fig. 7 and 8). PI(4,5)P2 levels showed a modest decrease after activation, which may partially account for the redistribution of the PLCδ PH domain.
Together, the quantitative kinetics analyses indicate that membrane recruitment of the TAPPs occurs well after an initial series of signal transduction events involving PI3K, including PI(3,4,5)P3 production, Btk membrane recruitment, PI(4,5)P2 depletion, and Ca2+ mobilization. This suggests that these proteins are unlikely to play a critical role in Ca2+ responses but are recruited later, presumably in response to peak PI(3,4)P2 levels, perhaps functioning to link the BCR to mitogen-activated protein kinase (MAPK) activation or cytoskeletal rearrangement.
DISCUSSION
In this study we have characterized two Bam32-related signal transduction molecules and provided strong evidence that these proteins function as targets of PI3K in activated B cells. TAPP1 and TAPP2 appear to be related to Bam32 both in structure and in placement in the hierarchy of signal transduction pathways. However, their expression patterns are markedly different: TAPP1 and TAPP2 are widely expressed, and Bam32 is expressed primarily in hematopoietic cells. We have also noted apparent differences in overall mRNA levels, with TAPP2 being the most abundant, TAPP1 being the least abundant, and Bam32 having intermediate abundance in those tissues in which it is expressed (data not shown). Searches against the public draft human genome sequence indicate that these are all single-copy genes residing on distinct chromosomes (TAPP1 on chromosome 10, TAPP2 on chromosome 8, and Bam32 on chromosome 4, in agreement with our previous fluorescent in situ hybridization [FISH] analysis [31]).
Phospholipid-binding ability appears to be a function shared by the majority of PH domains; however, only a subset of PH domains possess the ability to bind with high affinity to the D3 phosphoinositides generated by PI3K (24, 27). The C-terminal, but not the N-terminal, PH domains of TAPP1 and TAPP2 contain the sequence motif that is associated with D3 binding. Consistent with the hypothesis that the C-terminal PH domains mediate binding to D3 phosphoinositides, we find that these domains can target EGFP to the plasma membrane in a PI3K-dependent fashion. Furthermore, we show that active PI3K is sufficient for membrane recruitment through the C-terminal PH domain, showing that recruitment does not require activation of additional pathways and may be a direct outcome of PI3K activity. Consistent with the latter idea, mutations in the phosphoinositide-binding pocket of the TAPPs abolish membrane recruitment.
A recent study defining the in vitro lipid-binding properties of recombinant TAPP1 and TAPP2 PH domains concluded that both of these proteins bind specifically to the PI3K product PI(3,4)P2 (12). Consistent with our in vivo membrane recruitment studies, the N-terminal PH domains of TAPP1 and TAPP2 did not show significant phosphoinositide-binding activity in vitro. Thus, our findings on BCR-activated membrane recruitment of the TAPPs are consistent with a model where BCR ligation leads to PI3K activation and production of PI(3,4)P2, which drives the initial membrane recruitment response. PH domains have also been shown to be involved in protein-protein interactions, such as binding to the βγ subunits of heterotrimeric G proteins (53), PKC isoforms (60), or F-actin (59). Since active PI3K is both necessary and sufficient for membrane recruitment of Bam32 and the TAPPs, it appears that protein ligands may not play an essential role in membrane recruitment of these molecules. Alternatively, putative protein ligands may be constitutively present and accessible in the plasma membrane or may themselves be targets of PI3K.
Expression of active PI3K in COS cells has been shown to induce actin reorganization and membrane ruffling through activation of Rac (43). Recent studies indicate that some PH domain proteins are selectively recruited to sites of cytoskeletal activity such as ruffles, filopodia, and lamellipodia (33, 35, 51), suggesting that PI3K effectors recruited through PH domains may play a role in cytoskeletal reorganization. We find that TAPP1, TAPP2, and Bam32 strongly colocalize with F-actin in PI3K-induced membrane ruffles in B cells. One possible explanation for this finding is that cytoskeletal elements may play a role in membrane recruitment of the TAPPs by stabilizing the PH domain-lipid interaction and promoting stable docking in membrane-associated complexes. However, actin reorganization does not appear to be essential for membrane recruitment, since inhibition of F-actin turnover using cytochalasin D fails to inhibit recruitment (data not shown). Alternatively, PI(3,4)P2 may selectively accumulate in cytoskeletally active, ruffled areas of the plasma membrane. We hypothesize that the TAPPs may be functionally linked to cytoskeletal rearrangement downstream of the initial phosphoinositide-driven membrane recruitment.
To our knowledge, this is the first study reporting the visualization and quantitation of PI3K-dependent membrane recruitment of PH domain proteins in live human B cells. The ability to clearly visualize membrane recruitment of Bam32 and the TAPPs in response to BCR stimulation is likely due to the inherent properties of their PH domains, since recruitment of these proteins is considerably stronger than that of other PH domain proteins such as Btk (Fig. 7 and 8) and Grp1 (data not shown). Membrane recruitment of Bam32 and the TAPPs in BJAB cells follows relatively slow kinetics and is sustained for prolonged periods after activation. This, again, appears to be due to the inherent properties of this group of adaptors, since BCR-induced Btk recruitment, PLCγ activation, and Ca2+ mobilization occur with rapid and transient kinetics. One common property of Bam32, TAPP1, and TAPP2 which may explain these observations is their ability to bind to PI(3,4,)P2 (12, 13). In agreement with previous work on other B-cell lines (17), we find that PI(3,4)P2 is produced later and in higher quantities than PIP3 following BCR cross-linking, consistent with the observed recruitment kinetics of PIP3-binding (Btk) versus PI(3,4)P2-binding (TAPP) proteins. One important factor regulating the conversion of PIP3 to PI(3,4)P2 is the inositol phosphatase SHIP (7, 11, 23), which may favor membrane recruitment of the TAPPs over that of PIP3-specific PI3K targets such as Btk (6). Interestingly, we have recently generated evidence that PI(3,4)P2 has a function in regulating Akt activation in mast cells (46), supporting the idea that this lipid is not simply a by-product of PI(3,4,5)P3 breakdown but rather has specific signaling functions, which may be mediated in part through specific interactions with PH domain proteins such as the TAPPs. Thus, while rapid PI3K-driven recruitment of Btk plays a critical role in PLCγ activation, IP3 generation, and Ca2+ mobilization (8, 25, 32, 45, 50), the TAPPs likely have a distinct set of functions during the response, such as activation of Akt or MAPKs, receptor desensitization, receptor internalization, or linkage of the activated membrane to cytoskeletal rearrangement.
The distinct membrane-targeting characteristics of the PH domains examined in our study demonstrate the underlying complexity in PH domain-membrane interactions. The tagged TAPP1 and TAPP2 PH domain proteins described here provide new tools for dissecting the complex temporal and spatial regulation and function of 3-phosphoinositides during cellular activation. Given the large number of PH domain proteins in the human genome (26), the cell biology of PH domain-membrane interactions will no doubt have great functional importance in many areas of biology.
Acknowledgments
We thank Edward Clark for supporting the early stages of this work and for critical reading of the manuscript. We thank Tomas Balla and Doreen Cantrell for providing plasmid constructs and John Rutherford for help with confocal microscopy.
This work was supported by grants from the Canadian Institutes of Health Research (to A.J.M. and V.D.), Manitoba Health Research Council (to A.J.M.), and NIH (AI44257). A.J.M. is a CIHR New Investigator. V.D. is a CIHR/BCLA Scientist and a Michael Smith Foundation for Health Research Senior Scholar.
REFERENCES
- 1.Aagaard Tillery, K. M., and D. F. Jelinek. 1996. Phosphatidylinositol 3-kinase activation in normal human B lymphocytes: differential sensitivity of growth and differentiation to wortmannin. J. Immunol. 156:4543-4554. [PubMed] [Google Scholar]
- 2.Aman, M. J., T. D. Lamkin, H. Okada, T. Kurosaki, and K. S. Ravichandran. 1998. The inositol phosphatase SHIP inhibits Akt/PKB activation in B cells. J. Biol. Chem. 273:33922-33928. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, K. E., P. Lipp, M. Bootman, S. H. Ridley, J. Coadwell, L. Ronnstrand, J. Lennartsson, A. B. Holmes, G. F. Painter, J. Thuring, Z. Lim, H. Erdjument-Bromage, A. Grewal, P. Tempst, L. R. Stephens, and P. T. Hawkins. 2000. DAPP1 undergoes a PI 3-kinase-dependent cycle of plasma-membrane recruitment and endocytosis upon cell stimulation. Curr. Biol. 10:1403-1412. [DOI] [PubMed] [Google Scholar]
- 4.Astoul, E., S. Watton, and D. Cantrell. 1999. The dynamics of protein kinase B regulation during B cell antigen receptor engagement. J. Cell Biol. 145:1511-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beitz, L. O., D. A. Fruman, T. Kurosaki, L. C. Cantley, and A. M. Scharenberg. 1999. SYK is upstream of phosphoinositide 3-kinase in B cell receptor signaling. J. Biol. Chem. 274:32662-32666. [DOI] [PubMed] [Google Scholar]
- 6.Bolland, S., R. N. Pearse, T. Kurosaki, and J. V. Ravetch. 1998. SHIP modulates immune receptor responses by regulating membrane association of Btk. Immunity 8:509-516. [DOI] [PubMed] [Google Scholar]
- 7.Brauweiler, A. M., I. Tamir, and J. C. Cambier. 2000. Bilevel control of B-cell activation by the inositol 5-phosphatase SHIP. Immunol. Rev. 176:69-74. [DOI] [PubMed] [Google Scholar]
- 8.Buhl, A. M., C. M. Pleiman, R. C. Rickert, and J. C. Cambier. 1997. Qualitative regulation of B cell antigen receptor signaling by CD19: selective requirement for PI 3-kinase activation, inositol-1,4,5-trisphosphate production and Ca2+ mobilization. J. Exp. Med. 186:1897-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choquet, D., M. Partiseti, S. Amigorena, C. Bonnerot, W. H. Fridman, and H. Korn. 1993. Cross-linking of IgG receptors inhibits membrane immunoglobulin-stimulated calcium influx in B lymphocytes. J. Cell Biol. 121:355-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craxton, A., A. Jiang, T. Kurosaki, and E. A. Clark. 1999. Syk and Bruton's tyrosine kinase are required for B cell antigen receptor-mediated activation of the kinase Akt. J. Biol. Chem. 274:30644-30650. [DOI] [PubMed] [Google Scholar]
- 11.Damen, J. E., L. Liu, P. Rosten, R. K. Humphries, A. B. Jefferson, P. W. Majerus, and G. Krystal. 1996. The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-triphosphate 5-phosphatase. Proc. Natl. Acad. Sci. USA 93:1689-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowler, S., R. A. Currie, D. G. Campbell, M. Deak, G. Kular, C. P. Downes, and D. R. Alessi. 2000. Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochem. J. 351:19-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowler, S., R. A. Currie, C. P. Downs, and D. R. Alessi. 1999. DAPP1: a dual adaptor for phosphotyrosine and 3-phosphoinositides. Biochem. J. 342:7-12. [PMC free article] [PubMed] [Google Scholar]
- 14.Floto, R. A., M. P. Mahaut-Smith, B. Somasundaram, and J. M. Allen. 1995. IgG-induced Ca2+ oscillations in differentiated U937 cells; a study using laser scanning confocal microscopy and co-loaded fluo-3 and fura-red fluorescent probes. Cell Calcium 18:377-389. [DOI] [PubMed] [Google Scholar]
- 15.Fruman, D. A., S. B. Snapper, C. M. Yballe, L. Davidson, J. Y. Yu, F. W. Alt, and L. C. Cantley. 1999. Impaired B cell development and proliferation in the absence of phosphoinositide 3-kinase p85α. Science 283:393-397. [DOI] [PubMed] [Google Scholar]
- 16.Garcia, P., R. Gupta, S. Shah, A. J. Morris, S. A. Rudge, S. Scarlata, V. Petrova, S. McLaughlin, and M. J. Rebecchi. 1995. The pleckstrin homology domain of phospholipase C-δ1 binds with high affinity to phosphatidylinositol 4,5-bisphosphate in bilayer membranes. Biochemistry 34:16228-16234. [DOI] [PubMed] [Google Scholar]
- 17.Gold, M., and R. Aebersold. 1994. Both phosphatidylinositol 3-kinase and phosphatidylinositol 4-kinase products are increased by antigen receptor signaling in B cells. J. Immunol. 152:42-50. [PubMed] [Google Scholar]
- 18.Gold, M., V. Chan, C. W. Turck, and A. DeFranco. 1992. Membrane Ig cross-linking regulates phosphatidylinositol 3-kinase in B lymphocytes. J. Immunol. 148:2012-2022. [PubMed] [Google Scholar]
- 19.Gold, M. R. 2000. Intermediary signaling effectors coupling the B-cell receptor to the nucleus. Curr. Top. Microbiol. Immunol. 245:77-134. [DOI] [PubMed] [Google Scholar]
- 20.Gold, M. R., M. P. Scheid, L. Santos, M. Dang-Lawson, R. A. Roth, L. Matsuuchi, V. Duronio, and D. L. Krebs. 1999. The B cell antigen receptor activates the Akt (protein kinase B)/glycogen synthase kinase-3 signaling pathway via phosphatidylinositol 3-kinase. J. Immunol. 163:1894-1905. [PubMed] [Google Scholar]
- 21.Helgason, C. D., C. P. Kalberer, J. E. Damen, S. M. Chappel, N. Pineault, G. Krystal, and R. K. Humphries. 2000. A dual role for Src homology 2 domain-containing inositol-5-phosphatase (SHIP) in immunity: aberrant development and enhanced function of B lymphocytes in SHIP−/− mice. J. Exp. Med. 191:781-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirose, K., S. Kadowaki, M. Tanabe, H. Takeshima, and M. Iino. 1999. Spatiotemporal dynamics of inositol 1,4,5-trisphosphate that underlies complex Ca2+ mobilization patterns. Science 284:1527-1530. [DOI] [PubMed] [Google Scholar]
- 23.Huber, M., C. D. Helgason, M. P. Scheid, V. Duronio, R. K. Humphries, and G. Krystal. 1998. Targeted disruption of SHIP leads to Steel factor-induced degranulation of mast cells. EMBO J. 17:7311-7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isakoff, S. J., T. Cardozo, J. Andreev, Z. Li, K. M. Ferguson, R. Abagyan, M. A. Lemmon, A. Aronheim, and E. Y. Skolnik. 1998. Identification and analysis of PH domain-containing targets of phosphatidylinositol 3-kinase using a novel in vivo assay in yeast. EMBO J. 17:5374-5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurosaki, T. 1999. Genetic analysis of B cell antigen receptor signaling. Annu. Rev. Immunol. 17:555-592. [DOI] [PubMed] [Google Scholar]
- 26.Lander, E. S., L. M. Linton, B. Birren, C. Nusbaum, M. C. Zody, J. Baldwin, K. Devon, K. Dewar, M. Doyle, W. FitzHugh, R. Funke, D. Gage, K. Harris, A. Heaford, J. Howland, L. Kann, J. Lehoczky, R. LeVine, P. McEwan, K. McKernan, J. Meldrim, J. P. Mesirov, C. Miranda, W. Morris, J. Naylor, C. Raymond, M. Rosetti, R. Santos, A. Sheridan, C. Sougnez, N. Stange-Thomann, N. Stojanovic, A. Subramanian, D. Wyman, J. Rogers, J. Sulston, R. Ainscough, S. Beck, D. Bentley, J. Burton, C. Clee, N. Carter, A. Coulson, R. Deadman, P. Deloukas, A. Dunham, I. Dunham, R. Durbin, L. French, D. Grafham, S. Gregory, T. Hubbard, S. Humphray, A. Hunt, M. Jones, C. Lloyd, A. McMurray, L. Matthews, S. Mercer, S. Milne, J. C. Mullikin, A. Mungall, R. Plumb, M. Ross, R. Shownkeen, S. Sims, R. H. Waterston, R. K. Wilson, L. W. Hillier, J. D. McPherson, M. A. Marra, E. R. Mardis, L. A. Fulton, A. T. Chinwalla, K. H. Pepin, W. R. Gish, S. L. Chissoe, M. C. Wendl, K. D. Delehaunty, T. L. Miner, A. Delehaunty, J. B. Kramer, L. L. Cook, R. S. Fulton, D. L. Johnson, P. J. Minx, S. W. Clifton, T. Hawkins, E. Branscomb, P. Predki, P. Richardson, S. Wenning, T. Slezak, N. Doggett, J. F. Cheng, A. Olsen, S. Lucas, C. Elkin, E. Uberbacher, M. Frazier, et al. 2001. Initial sequencing and analysis of the human genome. Nature 409:860-921. [DOI] [PubMed] [Google Scholar]
- 27.Lemmon, M. A., and K. M. Ferguson. 2000. Signal-dependent membrane targeting by pleckstrin homology (PH) domains. Biochem. J. 350:1-18. [PMC free article] [PubMed] [Google Scholar]
- 28.Lemmon, M. A., K. M. Ferguson, R. O'Brien, P. B. Sigler, and J. Schlessinger. 1995. Specific and high-affinity binding of inositol phosphates to an isolated pleckstrin homology domain. Proc. Natl. Acad. Sci. USA 92:10472-10476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lioubin, M. N., P. A. Algate, S. Tsai, K. Carlberg, A. Aebersold, and L. R. Rohrschneider. 1996. p150Ship, a signal transduction molecule with inositol polyphosphate-5-phosphatase activity. Genes Dev. 10:1084-1095. [DOI] [PubMed] [Google Scholar]
- 30.Lipp, P., and E. Niggli. 1993. Ratiometric confocal Ca2+ measurements with visible wavelength indicators in isolated cardiac myocytes. Cell Calcium 14:359-372. [DOI] [PubMed] [Google Scholar]
- 31.Marshall, A. J., H. Niiro, C. G. Lerner, T. Y. Yun, S. Thomas, C. M. Disteche, and E. A. Clark. 2000. A novel B lymphocyte-associated adaptor protein, Bam32, regulates antigen receptor signaling downstream of phosphatidylinositol 3-kinase. J. Exp. Med. 191:1319-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marshall, A. J., H. Niiro, T. J. Yun, and E. A. Clark. 2000. Regulation of B cell activation and differentiation by the phosphatidylinositol 3-kinase and phospholipase Cγ pathways. Immunol. Rev. 176:30-46. [DOI] [PubMed] [Google Scholar]
- 33.Mukhopadhyay, S., A. S. Ramars, and D. Dash. 2001. Bruton's tyrosine kinase associates with the actin-based cytoskeleton in activated platelets. J. Cell. Biochem. 81:659-665. [DOI] [PubMed] [Google Scholar]
- 34.Nagel, W., P. Schilcher, L. Zeitlmann, and W. Kolanus. 1998. The PH domain and polybasic domain of cytohesin-1 cooperate specifically in plasma membrane association and cellular function. Mol. Biol. Cell 9:1981-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nore, B. F., L. Vargas, A. J. Mohamed, L. J. Branden, C. M. Backesjo, T. C. Islam, P. T. Mattsson, K. Hultenby, B. Christensson, and C. I. Smith. 2000. Redistribution of Bruton's tyrosine kinase by activation of phosphatidylinositol 3-kinase and Rho-family GTPases. Eur. J. Immunol. 30:145-154. [DOI] [PubMed] [Google Scholar]
- 36.Ono, M., H. Okada, S. Bolland, S. Yanagi, T. Kurosaki, and J. V. Ravetch. 1997. Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell 90:293-301. [DOI] [PubMed] [Google Scholar]
- 37.Phillips, N. E., and D. C. Parker. 1983. Fc-dependent inhibition of mouse B cell activation by whole anti-mu antibodies. J. Immunol. 130:602-606. [PubMed] [Google Scholar]
- 38.Rameh, L. E., A. K. Arvidsson, K. L. Carraway III, A. D. Couvillon, G. Rathbun, A. Crompton, B. VanRenterghem, M. P. Czech, K. S. Ravichandran, S. J. Burakoff, D. S. Wang, C. S. Chen, and L. C. Cantley. 1997. A comparative analysis of the phosphoinositide binding specificity of pleckstrin homology domains. J. Biol. Chem. 272:22059-22066. [DOI] [PubMed] [Google Scholar]
- 39.Rameh, L. E., and L. C. Cantley. 1999. The role of phosphoinositide 3-kinase lipid products in cell function. J. Biol. Chem. 274:8347-8350. [DOI] [PubMed] [Google Scholar]
- 40.Rao, V. R., M. N. Corradetti, J. Chen, J. Peng, J. Yuan, G. D. Prestwich, and J. S. Brugge. 1999. Expression cloning of protein targets for 3-phosphorylated phosphoinositides. J. Biol. Chem. 274:37893-37900. [DOI] [PubMed] [Google Scholar]
- 41.Rawlings, D. J. 1999. Bruton's tyrosine kinase controls a sustained calcium signal essential for B lineage development and function. Clin. Immunol. 91:243-253. [DOI] [PubMed] [Google Scholar]
- 42.Rawlings, D. J., A. M. Scharenberg, H. Park, M. I. Wahl, S. Lin, R. M. Kato, A. C. Fluckiger, O. N. Witte, and J. P. Kinet. 1996. Activation of BTK by a phosphorylation mechanism initiated by SRC family kinases. Science 271:822-825. [DOI] [PubMed] [Google Scholar]
- 43.Reif, K., C. D. Nobes, G. Thomas, A. Hall, and D. A. Cantrell. 1996. Phosphatidylinositol 3-kinase signals activate a selective subset of Rac/Rho-dependent effector pathways. Curr. Biol. 6:1445-1455. [DOI] [PubMed] [Google Scholar]
- 44.Salim, K., M. J. Bottomley, E. Querfurth, M. J. Zvelebil, I. Gout, R. Scaif, R. L. Margolis, R. Gigg, C. I. E. Smith, P. C. Driscoll, M. D. Waterfield, and G. Panayotou. 1996. Distinct specificity in the recognition of phosphoinositides by the pleckstrin homology domains of dynamin and the Bruton's tyrosine kinase. EMBO J. 15:6241-6250. [PMC free article] [PubMed] [Google Scholar]
- 45.Scharenberg, A. M., and J. P. Kinet. 1998. PtdIns-3,4,5-P3: a regulatory nexus between tyrosine kinases and sustained calcium signals. Cell 94:5-8. [DOI] [PubMed] [Google Scholar]
- 46.Scheid, M. P., M. Huber, J. E. Damen, M. Hughes, V. Kang, P. Neilsen, G. D. Prestwich, G. Krystal, and V. Duronio. 2002. Phosphatidylinositol (3,4,5)P3 is essential but not sufficient for protein kinase B (PKB) activation; phosphatidylinositol (3,4)P2 is required for PKB phosphorylation at Ser-473: studies using cells from SH2-containing inositol-5-phosphatase knockout mice. J. Biol. Chem. 277:9027-9035. [DOI] [PubMed] [Google Scholar]
- 47.Stauffer, T. P., S. Ahn, and T. Meyer. 1998. Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr. Biol. 8:343-346. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki, H., Y. Terauchi, M. Fujiwara, S. Aizawa, Y. Yazaki, T. Kadowaki, and S. Koyasu. 1999. Xid-like immunodeficiency in mice with disruption of the p85α subunit of phosphoinositide 3-kinase. Science 283:390-392. [DOI] [PubMed] [Google Scholar]
- 49.Takai, T., M. Ono, M. Hikida, H. Ohmori, and J. V. Ravetch. 1996. Augmented humoral and anaphylactic responses in FcγRII-deficient mice. Nature 379:346-349. [DOI] [PubMed] [Google Scholar]
- 50.Takata, M., and T. Kurosaki. 1996. A role for Bruton's tyrosine kinase in B cell antigen receptor-mediated activation of phospholipase C-γ2. J. Exp. Med. 184:31-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tall, E. G., I. Spector, S. N. Pentyala, I. Bitter, and M. J. Rebecchi. 2000. Dynamics of phosphatidylinositol 4,5-bisphosphate in actin-rich structures. Curr. Biol. 10:743-746. [DOI] [PubMed] [Google Scholar]
- 52.Toker, A., and L. C. Cantley. 1997. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature 387:673-676.9192891 [Google Scholar]
- 53.Touhara, K., J. Inglese, J. A. Pitcher, G. Shaw, and R. J. Lefkowitz. 1994. Binding of G protein β γ-subunits to pleckstrin homology domains. J. Biol. Chem. 269:10217-10220. [PubMed] [Google Scholar]
- 54.van der Wal, J., R. Habets, P. Varnai, T. Balla, and K. Jalink. 2001. Monitoring agonist-induced phospholipase C activation in live cells by fluorescence resonance energy transfer. J. Biol. Chem. 276:15337-15344. [DOI] [PubMed] [Google Scholar]
- 55.Varnai, P., and T. Balla. 1998. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J. Cell Biol. 143:501-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varnai, P., K. L. Rother, and T. Balla. 1999. Phosphatidylinositol 3-kinase-dependent membrane association of the Bruton's tyrosine kinase pleckstrin homology domain visualized in single living cells. J. Biol. Chem. 274:10983-10989. [DOI] [PubMed] [Google Scholar]
- 57.Venkataraman, C., G. Shankar, G. Sen, and S. Bondada. 1999. Bacterial lipopolysaccharide induced B cell activation is mediated via a phosphatidylinositol 3-kinase dependent signaling pathway. Immunol. Lett. 69:233-238. [DOI] [PubMed] [Google Scholar]
- 58.Venkateswarlu, K., F. Gunn-Moore, P. B. Oatey, J. M. Tavare, and P. J. Cullen. 1998. Nerve growth factor- and epidermal growth factor-stimulated translocation of the ADP-ribosylation factor-exchange factor GRP1 to the plasma membrane of PC12 cells requires activation of phosphatidylinositol 3-kinase and the GRP1 pleckstrin homology domain. J. Biochem. 335:139-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yao, L., P. Janmey, L. G. Frigeri, W. Han, J. Fujita, Y. Kawakami, J. R. Apgar, and T. Kawakami. 1999. Pleckstrin homology domains interact with filamentous actin. J. Biol. Chem. 274:19752-19761. [DOI] [PubMed] [Google Scholar]
- 60.Yao, L., Y. Kawakami, and T. Kawakami. 1994. The pleckstrin homology domain of Bruton tyrosine kinase interacts with protein kinase C. Proc. Natl. Acad. Sci. USA 91:9175-9179. [DOI] [PMC free article] [PubMed] [Google Scholar]