Abstract
Differentiating male germ cells express a testis-specific form of cytochrome c (Cyt cT) that is distinct from the cytochrome c expressed in somatic cells (Cyt cS). To examine the role of Cyt cT in germ cells, we generated mice null for Cyt cT. Homozygous Cyt cT−/− pups were statistically underrepresented (21%) but developed normally and were fertile. However, spermatozoa isolated from the cauda epididymis of Cyt cT-null animals were less effective in fertilizing oocytes in vitro and contain reduced levels of ATP compared to wild-type sperm. Sperm from Cyt cT-null mice contained a greater number of immotile spermatozoa than did samples from control mice, i.e., 53.1% ± 13.7% versus 33.2% ± 10.3% (P < 0.0001) for vas deferens sperm and 40.1% ± 9.6% versus 33.2% ± 7.5% (P = 0.0104) for epididymal sperm. Cyt cT-null mice often exhibit early atrophy of the testes after 4 months of age, losing germ cells as a result of increased apoptosis. However, no difference in the activation of caspase-3, -8, or -9 was detected between the Cyt cT−/− testes and controls. Our data indicate that the Cyt cT-null testes undergo early atrophy equivalent to that which occurs during aging as a consequence of a reduction in oxidative phosphorylation.
Cytochrome c, a heme-containing protein, resides in the space between the outer and inner membranes of mitochondria and transports electrons from complex III to complex IV in the respiratory chain. Mammalian testes express two types of cytochrome c, somatic cytochrome c (Cyt cS) and testis-specific cytochrome c (Cyt cT) (6, 12). Within the epithelium of seminiferous tubules, differentiation of the germ cells progresses towards the luminal surface. Spermatogonia, diploid germ cells which reside along the basement membrane, express only Cyt cS. From the preleptotene to the pachytene stages of meiotic prophase, expression of Cyt cS decreases while levels of Cyt cT protein increase (13). Expression of Cyt cT becomes dominant by the pachytene stage, and Cyt cT is the sole cytochrome c expressed in all postmeiotic male germ cells, including mature spermatozoa. Cyt cT localizes in the mitochondria in a manner similar to that of Cyt cS.
Cytochrome c also plays an important role during apoptosis (27). Activated mitochondria release cytochrome c from the intermembrane space into the cytoplasm, and the released cytochrome c binds to apoptosis protease-activating factor 1 (Apaf-1) (33). The resulting cytochrome c-Apaf-1 complex can then activate caspase-9 (20), followed by activation of downstream death effectors such as caspase-3, caspase-6, and caspase-7 (31). In the testis, apoptosis is considered an important physiological mechanism that regulates the number of sperm produced (26), and it has also been suggested that apoptosis removes germ cells with damaged DNA (2). Fas is expressed in germ cells and the Fas ligand (FasL) is expressed in Sertoli cells. The Fas system is known to regulate apoptosis in the testis of young rats (16). Ectopic expression as well as inactivation of apoptosis-related genes have been shown to cause abnormalities in murine spermatogenesis. For example, accumulation of spermatogonia and degeneration of germ cells were observed in transgenic mice misexpressing Bcl-2 (5). Likewise, the testis of the Bax knockout (KO) mouse showed accumulation of atypical premeiotic germ cells and an absence of elongated spermatids (15), suggesting that the mitochondria-mediated cascade of apoptosis is important in spermatogenesis.
Apoproteins of both Cyt cT and Cyt cS are encoded in the nuclear genome; in the mouse, the Cyt cT gene localizes to chromosome 2 and the Cyt cS gene localizes to chromosome 6 (10). While the cDNAs of the two genes are 74% homologous, their genomic organization is quite different. The Cyt cT gene contains four exons and three introns and spans a length of about 6 kb, while the Cyt cS gene contains only one intron and is only about 1.4 kb long. Multiple pseudogenes of Cyt cS exist in the genome of humans and mice (21); however, no pseudogene of Cyt cT has been reported. In the testis, both Cyt cT and Cyt cS show alternative splicing mRNA variants, although the biological significance of this variability is unclear (8, 9). Cyt cT and Cyt cS both encode apoproteins consisting of 104 amino acids, which are about 86% identical. The carboxyl-terminal regions show the least similarity. Both Cyt cT and Cyt cS sequences contain a mitochondria anchoring site; however, it is not known whether the retention of Cyt cT to the mitochondrial inner membrane is different from that of Cyt cS. It is not clear whether Cyt cT has functions distinct from those of Cyt cS.
We have generated and characterized KO mice deficient in Cyt cT. Our analysis reveals that mature spermatozoa expressing no cytochrome c retain motility and are capable of fertilization. However, the testes of mice lacking Cyt cT undergo apoptosis and display early onset atrophy, likely due to a dysfunction in oxidative phosphorylation.
MATERIALS AND METHODS
Targeting vector.
A genomic fragment containing intron 2 and intron 3 was amplified by PCR from genomic DNA from the 129sv+/+ mouse strain by using a 5′ primer from exon 1 (5′-GGC CCA GGG CAC GGC TGC TGT GAT TGT G-3′) and a 3′ primer from exon 3 (5′-CAA AGA TCT TCT TGC CTG CTT CAG CAT CTC CC-3′). The PCR was carried out with a combination of AmpliTaq (PE Applied Biosystems, Foster City, Calif.) and TaqExtender (Stratagene, La Jolla, Calif.) following the manufacturers' instructions. After 40 rounds of annealing and extension at 72°C for 12 min and denaturing at 94°C for 1.5 min, a 4.2-kb fragment was amplified. The 4.2-kb DNA was subcloned into pCR2.1 (Invitrogen, Carlsbad, Calif.) and sequenced. An intermediate KS plasmid vector (Stratagene) containing the pMCNeo poly(A) and the diphtheria toxin A cassette was cut with SpeI and NotI, and the 4.2-kb fragment was ligated to the 5′ end of the Neo cassette. To delete the second half of exon 3, which contains the heme binding site of Cyt cT, a 2.7-kb KpnI-HindIII fragment was isolated from the 3.5-kb genomic DNA fragment and used as the right arm for homologous recombination, as shown in Fig. 1c.
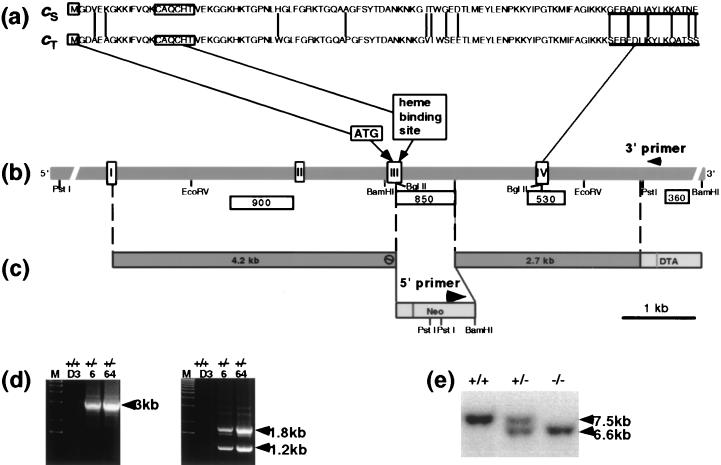
FIG. 1.
Disruption of the Cyt cT gene. (a) Amino acid sequences of Cyt cS and Cyt cT. Vertical lines indicate unmatched amino acids. Underlined amino acid sequences shown in this panel were used for the production of antibodies (anti-cS antibody, carboxyl-terminal 16 amino acids; anti-cT antibody, carboxyl-terminal 16 amino acids). (b) Genomic organization of the Cyt cT gene. White boxes are exons and shadowed regions are introns. Translation starts at ATG in exon III. Probes used for Southern blot analysis are shown beneath the genomic DNA. (c) Targeting vector. (d) PCR screening performed for ES cell clones obtained from the vector. PCR using the 5′ primer shown in panel c and the 3′ primer shown in panel b amplified a predicted 3-kb fragment (left panel). EcoRI digestion of this fragment showed the predicted 1.8- and 1.2-kb fragments (right panel). D3, parental ES cell. 6 and 64 are ES clones used for generation of mouse colonies. (e) Southern blot analysis of Cyt cT-targeted mice. The targeted allele in Cyt KO mice showed a 6.6-kb BamHI fragment with probe 360.
Production of KO mice.
Thirty micrograms of linearized targeting vectors was electroporated, and the transfected R1 embryonic stem (ES) cells (23) were processed using standard protocols (25). Fourteen out of 266 G418-resistant clones were confirmed as homologous recombinants by Southern blotting using five restriction enzymes (BamHI, EcoRV, EcoRI, HindIII, and PstI) and four probes (900, 530, 360, and a Neo probe [Fig. 1b]). Homologous recombination was also confirmed by PCR with a 3′ primer (5′-CTC CCA GCC TGA CGC TCC TTC CTC CTC-3′) from the 3′ region of the genomic gene (Fig. 1b) and a 5′ primer (5′-CGT GAC CCA TGG CGA TGC CTG CTT GCC G-3′) from the Neo cassette (Fig. 1c). The PCR conditions were 40 rounds of a cycle consisting of annealing and extension at 72°C for 15 min and denaturing at 94°C for 1.5 min. A combination of AmpliTaq (PE Applied Biosystems) and TaqExtender (Stratagene) was used to amplify the 3-kb fragment (Fig. 1d).
Homologous recombinant clones carrying normal karyotypes were injected into blastocysts of C57BL/6 mice (Charles River, Wilmington, Mass.) to produce chimeric animals. Chimeric males with high contributions of agouti coat color were bred to C57BL/6 females (Charles River). Germ line heterozygote mice, G1, were bred to homozygosity. Most of the mating pairs were siblings, and homozygous animals were analyzed together with littermate controls. Results of the PCR of the ES clones used for production of germ line mice are shown in Fig. 1d. The restriction pattern of DNA derived from the tail of the Cyt cT KO is shown in Fig. 1d (right panel). Two independent mouse colonies, derived from two independent ES clones, were maintained and their genotypes were determined by Southern blot hybridization (Fig. 1e). All male mice used in this study had been individually separated in single cages for at least 10 days to avoid influence from cage-mate males, such as suppression of testosterone production. All mouse experiments complied with all relevant federal guidelines and institutional policies.
Southern and Northern blot hybridizations.
Southern blot hybridization for genotyping mice and Northern blot hybridization for analyses of gene expression were performed using standard protocols (25). The following probes used for Northern blot hybridization were obtained by reverse transcription-PCR by using mouse testis RNA: Pgk2 (a 300-bp fragment amplified with primers provided by John McCarrey [Southwest Foundation for Biomedical Research, San Antonio, Tex.]), cyt b (a 945-bp reverse transcription-PCR fragment amplified with the 5′ primer 5′-CCC ATC CAA CAT TTC ATC ATG ATG A-3′ and 3′ primer 5′-CCA ATT CAG GTT AAG ATA AGT AGG T-3′), cytochrome c oxidase subunit 1 (COX1; a 374-bp fragment amplified with the 5′ primer 5′-GAG GGC ACC CAT GAA GTC ATT C-3′ and 3′ primer 5′-CCA TTT ACA CTA TGT TCC ATC A-3′), Apaf-1 (a 555-bp cDNA fragment amplified with the 5′ primer 5′-CTG CGC GTT CTC CTC AGA CGA CAG-3′ and 3′ primer 5′-GTC ACA GTA CTG GAT GGT GCT GTG ATG-3′), testicular androgen binding protein (a 552-bp fragment amplified with the 5′ primer 5′-GGC GAC TGC TTC TGC TGT TGC TAC TA-3′ and 3′ primer 5′-ATA CAG CCG TCC AGG GCA GGC ACG-3′). The COX3 cDNA probe (250 bp) was prepared from an IMAGE Consortium clone (no. 520010) purchased from the American Type Culture Collection (Manassas, Va.). The protamine 2 probe is a 150-bp fragment from the 3′ untranslated region of the mouse protamine 2 gene. The ldhc probe is a 267-bp partial fragment of the mouse lactate dehydrogenase (LDH)-C4 cDNA.
Antibodies against Cyt cT and Cyt cS.
To generate Cyt cT-specific and Cyt cS-specific antibodies, rabbits were immunized with keyhole limpet hemocyanin-conjugated synthetic peptides of 16 amino acids derived from the carboxy terminal of Cyt cT and Cyt cS (Cyt cT, SEREDLIKYLKQATSS; Cyt cS, GERADLIAYLKKATNE). All antisera were affinity purified with each peptide attached to a solid phase. Peptide synthesis, immunization, and affinity purification were performed by Quality Controlled Biochemicals, Inc. (Hopkinton, Mass.).
Immunostaining, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining, and electron microscopy.
Mouse tissues were fixed in 10% formalin in phosphate-buffered saline for 72 h and processed as previously described (24). The affinity-purified rabbit anti-cS antibody (0.5 μg/ml) and anti-cT antibody (4 μg/ml) were incubated for 60 min. For detection of caspase-3, 5 μg of goat anti-caspase-3 antibody per ml (recognizing the p20 subunit of caspase-3) (Santa Cruz Biotechnology, Santa Cruz, Calif.) was used. To confirm specificity of staining, 10 μg of blocking peptide for the anti-caspase-3 antibody (Santa Cruz Biotechnology) per ml was preincubated for 60 min on sections and added to the primary antibody. To detect caspase-9, 5 μg of rabbit antibody recognizing the p10 subunit of caspase-9 (Santa Cruz Biotechnology) per ml was used. Localization of these primary antibodies was detected by using a Vectastain ABC kit (Vector Laboratories, Burlingame, Calif.). Mayer's hematoxylin was used for the counterstain. Apoptotic fragmentation of DNA was detected using an ApopTag Peroxidase In Situ Apoptosis Detection kit (Intergen Company, Purchase, N.Y.). Testis samples were fixed for electron microscopy using 3.2% paraformaldehyde-2% glutaraldehyde in 0.2 M cacodylate buffer (pH 7.4) for 2 h and processed using standard methods.
Western blotting.
Tissue samples were homogenized in lysis buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 0.1% sodium dodecyl sulfate (SDS), 1 mM phenylmethylsulfonyl fluoride, and protein inhibitor cocktail (50 μl/0.1 g of tissue; Sigma, St. Louis, Mo.). The protein concentration was determined with bicinchoninic reagent (Pierce, Rockford, Ill.). Sperm samples were dissolved in buffer containing 125 mM Tris-HCl (pH 6.8), 4% SDS, and 0.05% β-mercaptoethanol. SDS-polyacrylamide gel electrophoresis was performed under denaturing and reducing conditions. Peroxidase-labeled secondary antibodies were purchased from Santa Cruz Biotechnology. Bound peroxidase was detected using the ECL Plus system (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, England).
Sperm collection, in vitro fertilization, and motility assay.
Cauda epididymides and vasa deferentia were minced in 1.5 ml of TYH medium, a modified Krebs-Ringer bicarbonate solution (11) and incubated at 33°C in a 7% CO2 incubator for 15 min. Tissue debris was removed with a 40-μm-pore-size nylon filter (BD Falcon, Franklin Lakes, N.J.), and samples were incubated at 33°C. The number of spermatozoa was determined using a Neubauer hemacytometer. One set of samples was diluted with TYH medium to count immotile spermatozoa, and another was diluted with 10% formalin-phosphate-buffered saline to determine total sperm number. The number of sperm acrosomes per 10−4 ml was counted six times to calculate the median value.
For in vitro fertilization, spermatozoa were isolated from 6.5-month-old male mice. Donor eggs were collected from female mice of C57/B6 × 129sv+/+ F1 hybrids purchased from Taconic (Germantown, N.Y.) and superovulated with progesterone and human chorionic gonadotropin. The numbers of fertilized eggs reaching the two-cell stage were determined by using a microscope.
For motility assays, plastic cell culture inserts with 3-μm pores (BD Falcon) were placed in wells of 12-well plates (Costar, Corning, N.J.). One milliliter of TYH medium with or without 0.05 mM iodoacetamide (Sigma) and/or 0.33 mM oxamic acid (Sigma) was added to each outer well. The 3-μm-pore-size inserts were filled with equal numbers of motile spermatozoa (4.5 × 106 sperm/insert) isolated from epididymides and suspended in 250 μl of TYH medium containing the same reagents as the outer well. After 20 h of incubation at 33°C in a 7% CO2 incubator, the number of spermatozoa that moved to the outer well was determined with a Neubauer hemacytometer. The number of sperm acrosomes per 10−3 ml was determined three times to calculate the mean.
Caspase activity assay.
Protein extracts from testes were measured for caspase activity using as substrates carbobenzoxy-Asp-Glu-Val-Asp-7-amino-4-trifluoromethyl coumarin (Z-DEVD-AFC) and carbobenzoxy-Ile-Glu-Thr-Asp-amino-4-trifluoromethyl coumarin (Z-IETD-AFC) (30). Testes extracts, containing 10 μg of total protein/μl, from eight pairs of homozygotes and their littermate controls were measured by release of AFC as emission at 505 nm upon excitation with 400 nm. Average Vmax values from duplicated wells for each animal were compared.
ATP measurements.
Each testicle was homogenized in 1 ml of 3% trichloroacetic acid (TCA)-2 mM EDTA, and spermatozoa collected from epididymides and vas deferentia from each animal were resuspended in 70 μl of 3% TCA-2 mM EDTA (22). After incubation for 15 min at room temperature and centrifugation at 16,000 × g, the ATP content in the supernatants was measured by using a Bioluminescent Somatic Cell Assay kit (Sigma) (28).
RESULTS AND DISCUSSION
Inactivation of the cytochrome cT gene.
The construction of the targeting vector, production of targeted mice, and results of the Southern blot analysis of the targeted allele are presented in Fig. 1 and were described in Materials and Methods. Northern blot analysis of testis RNA revealed that no mRNA of Cyt cT was detected in the testis of Cyt cT−/− mice, and Cyt cT+/− mice expressed Cyt cT mRNA at half the level of wild-type controls (Fig. 2A ). The expression of Cyt cS was unchanged in the testes of Cyt cT KO mice (Fig. 2A).
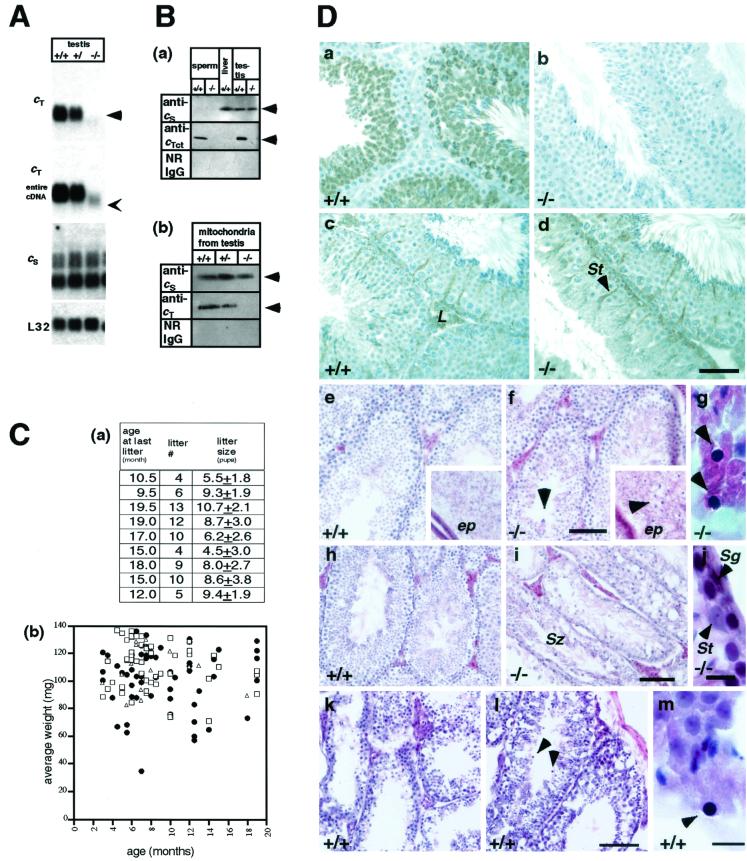
FIG. 2.
Confirmation of CytcT inactivation. (A) Northern blot analysis of the following fragments: cT, partial cDNA probe corresponding to a DNA fragment between BglII in exon III and BglII in exon IV of the Cyt cT shown in Fig. 1b; cT entire cDNA, cT probe using entire Cyt cT cDNA; cS, cS probe using entire Cyt cS cDNA; Neo, Neo gene probe; L32, ribosomal protein L32-A4, used as a standard. In the Cyt cT−/− mice, the Cyt cT probe gave no signal (arrowhead); however, the entire Cyt cT cDNA probe detected a faint shorter signal (thin arrowhead), suggesting that there was transcription until the stop codon in exon III. The expression pattern of Cyt cS in the Cyt cT−/− testis was normal and not distinguishable from Cyt cS expression in heterozygotes and wild-type mice. Samples are 10 μg of total RNA from testes. (B) Western blotting using anti-Cyt cS and Cyt cT antibodies. Sperm cells were isolated from wild-type and Cyt cT−/− mice (2 × 105 sperm/lane); liver extract was from wild-type mice (100 μg/lane); testis extracts were from mice (30 μg of protein/lane). Sperm cells were purified on a Percoll gradient (Amersham Pharmacia, Uppsala, Sweden) and washed in 0.45% NaCl hypotonic solution to remove contaminating somatic cells. (a) Black arrowheads indicate the predicted 12-kDa Cyt cT or Cyt cS protein. (b) Enriched population of mitochondria extracted from testes of wild-type, heterozygous, and Cyt cT KO mice (12.5 μg of protein/lane). (C) Fertility and weight of testes. (a) Breeding record of Cyt cT KO homozygous males. Litter size is the mean number of pups in each litter. (b) Average weight of testes from each individual mouse. Wild-type (+/+), n = 50, mean = 110.8 ± 17.1 mg; heterozygote (+/−), n = 21, mean = 113.0 ± 15.7 mg; homozygote (−/−), n = 60, mean = 102.9 ± 22.2 mg. Homozygous animals and their littermate controls, either wild type or heterozygotes, were analyzed at the same time. The ages of the mice subjected to analyses were 3 to 19 months. (D) Immunohistochemistry and morphology of the Cyt cT−/− testis. Panels on the right (b, d, f, g, i, and j) show Cyt cT−/− sections, and panels on the left (a, c, e, and h) show wild-type control sections. (a to d) Immunohistochemistry of testes fixed with Bouin's and embedded in paraffin. Panels a and b were stained with anti-cT antibody; no Cyt cT protein was seen in spermatocytes and spermatids of the Cyt cT−/− testes. Panels c and d were stained with anti-cS antibody showing expression of Cyt cS in spermatogonia, Sertoli cells, and Leydig cells in both +/+ and −/− mice. The mice are 3 months old. Bar, 100 μm. (e to j) Hematoxylin-eosin staining of testis and epididymis (ep; square insets in panels e and f) fixed with formalin and embedded in OCT compound. (e to g) Three-month-old mice. Testis weight of Cyt cT−/− mice (f) was the same as control (e) weights. At this age, homozygote testes appeared to be similar to controls except for the unusual basophilic structures in the luminal region of the seminiferous tubules and epididymis (arrowheads). Panel g is an enlarged view of the basophilic structure in homozygote testis (arrowheads). (h to j) Seven-month-old mice. At this age, testis weight of Cyt cT−/− mice (i) was approximately 27% of controls (h). Seminiferous tubules showed a dramatic loss of spermatocytes, spermatids, and spermatozoa, although some seminiferous tubules in the same testis still contain mature spermatozoa (i). Spermatogonia, Sertoli cells, and Leydig cells appeared unchanged in Cyt cT−/− and wild-type mice (i and j). Bars: e, f, h, and i, 100 μm; j, 10 μm. L, Leydig cell; St, Sertoli cell; Sg, spermatogonia. (k to m) Hematoxylin-eosin staining of testes of 17- to 19-month-old wild-type mice. (k) Testis of a 17-month-old mouse, showing marked loss of germ cells. (l) Testis of a 19-month-old mouse with reduced germ cells and basophilic structures (arrowheads). (m) High magnification of the basophilic structure from the same testis as shown in panel l. Bars: k and l, 100 μm; m, 10 μm.
To confirm the inactivation of the Cyt cT gene at the protein level, we produced, characterized, and used peptide-specific antibodies in Western blotting and immunohistochemistry. No Cyt cT protein (12 kDa) was detected in protein extracts from either whole testes or enriched testicular mitochondrial fractions of Cyt cT−/− mice (Fig. 2B). Expression of endogenous Cyt cT in Cyt cT-heterozygous mice was lower than that seen in wild-type controls (Fig. 2B, panel b). The expression levels of Cyt cS in the Cyt cT KO testes were not different from those in wild-type controls. Figure 2B, panel b, indicates that no Cyt cT protein was expressed in either the testis or the sperm of Cyt cT−/− mice and that Cyt cS levels were unchanged in Cyt cT KO samples (Fig. 2B).
Ratio, fertility, and testicular weight of the homozygous mice.
While both male and female Cyt cT KO mice were fertile, the ratio of homozygous animals was less than 25%, suggesting that the targeted allele does not transmit in a Mendelian fashion. Thus, of 434 offspring from Cyt cT heterozygote parents, the genotype frequencies were 133 Cyt cT+/+ (30.6%), 209 Cyt cT+/− (48.2%), and 92 Cyt cT−/− (21.1%). Nevertheless, all homozygous males tested were able to produce offspring (Fig. 2C, panel a).
We examined whether the testes from Cyt cT−/− mice were of normal size by plotting the average wet tissue weight of each testicle for each individual mouse (Fig. 2C, panel b). The mean values were as follows: Cyt cT+/+, 110.8 ± 17.1 mg (n = 50); Cyt cT+/−, 113.0 ± 15.7 mg (n = 21); Cyt cT−/−, 102.9 ± 22.2 mg (n = 60). Several Cyt cT KO testes were small in size and low in weight, as reflected in the larger standard deviation for the Cyt cT−/− group. We considered a Cyt cT KO mouse testis weighing as much as a wild-type mouse testis to be a normal-sized testis, and a testis weighing less than 70 mg or less than 80% of the littermate control weight was considered a small-sized testis. Nine of 60 testes from Cyt cT KO mice were small (15%) (Fig. 2C, panel b).
Lack of Cyt cT and abnormal morphology in the testes.
No Cyt cT protein was detected in the seminiferous tubules of Cyt cT KO mice by immunohistochemistry using the anti-cT antibody, while germ cells after the pachytene spermatocyte stage in control testes stained positive for Cyt cT (Fig. 2D, panel b). In contrast, the anti-Cyt cS antibody detected Cyt cS in spermatogonia, Sertoli cells, and Leydig cells of KO and control testes, and the expression pattern of Cyt cS remained unchanged in the Cyt cT KO testis (Fig. 2D, panels c and d).
Histological examination of the testes of 2- to 3-month-old Cyt cT KO mice showed the presence of abnormal basophilic structures near the luminal region of the seminiferous tubules (Fig. 2D, panels f and g). Older (4- to 10-month-old) Cyt cT KO mice tended to have small testes. Examination of these small testes revealed that they were highly atrophic and had a reduced number of spermatocytes, spermatids, and spermatozoa (Fig. 2D, panel i). The spermatogonia, Sertoli cells, and Leydig cells appeared normal, and some seminiferous tubules still contained mature spermatozoa (Fig. 2D, panels i and j).
Loss of germ cells from the seminiferous tubules was also observed in normal testes of aged wild-type animals (Fig. 2D, panels k and l). In these older control mice, we observed basophilic round structures that were indistinguishable from those structures observed in the Cyt cT−/− testis (Fig. 2D, panels l and m). However, in the case of wild-type mice, such basophilic structures were recognized only in animals older than 17 months, while these structures were clearly evident in the testes of 6-week-old Cyt cT−/− mice.
Evidence of accelerated apoptosis in homozygous Cyt cT KO testes.
To examine if the loss of germ cells in Cyt cT−/− testes occurs as a result of apoptosis, we used TUNEL staining to analyze DNA fragmentation. The seminiferous tubules of small testes from Cyt cT KO mice contained a substantial number of pachytene stage spermatocytes undergoing apoptosis (Fig. 3b and c). Electron microscopy confirmed the presence of apoptotic chromatin condensation in the smaller testes of Cyt cT KO animals (Fig. 3d). In contrast, no differences were seen in 2- to 3-month-old Cyt cT KO mouse testes compared to littermate controls (data not shown).
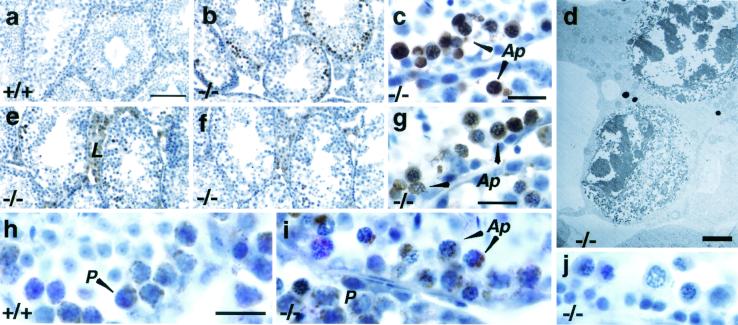
FIG. 3.
Apoptosis in the Cyt cT−/− testis. (a to c) TUNEL staining of testes from 4.5-month-old mice; (b) Cyt cT−/− mouse; (a) Cyt cT+/+ littermate control of animal shown in panel b (weight of the −/− testis was approximately 71% of control); (c) high magnification of image shown in panel b. Arrowheads in panel c indicate positive cells. (d) Electron microscopic view of apoptotic cells in testis of 5.5-month-old Cyt cT−/− mouse (weight of −/− testis was approximately 53% of control). (e to g) Immunostaining with anti-caspase-3 antibody on the same testis as shown in panel b. (f) Control inhibition of immunoreaction by a specific blocking peptide for the epitope; (g) high magnification of panel e. (h and i) Immunostaining with anti-caspase-9 antibody on testes from the same animals as shown in panels a and b. (j) Staining with normal rabbit immunoglobulin G (4 μg/ml) using same testis samples as shown in panels b, e, and i. Ap, apoptotic cell; P, pachytene spermatocyte; L, Leydig cell. Bars: a, b, e, and f, 100 μm; c, g, h, i, and j, 20 μm; d, 2.5 μm.
Since release of cytochrome c from mitochondria is required for the activation of downstream caspases in the mitochondria-mediated apoptotic cascade, we analyzed the expression of two key proteases, caspase-3 and caspase-9. Using an antibody against the p20 subunit of caspase-3, we found positive staining in Leydig cells and in apoptotic cells in the Cyt cT KO testes (Fig. 3e, f, and g). This reaction was inhibited in the presence of the caspase-3 peptide used to raise the antibody, indicating the specificity of the signal (Fig. 3e and f). TUNEL staining and caspase-3 were detected in the same seminiferous tubule in the same testis (Fig. 3c and g). Immunostaining with an antibody against the p10 subunit of caspase-9 showed that pachytene spermatocytes in wild-type testes stained positive, as did apoptotic cells in the Cyt cT KO testes (Fig. 3h and i). Western blotting experiments showed that there is activation of caspase-3 and caspase-9 in wild-type testis, but that their activation is not accelerated in the Cyt cT-null testes (data not shown).
The main extrinsic apoptotic pathway operating in the testis is the Fas-FasL system (16). Fas is expressed in spermatogenic cells, and FasL is expressed in Sertoli cells of 4-week-old rats. Interactions between the germ cells and Sertoli cells through the Fas-FasL system serve as an important regulator of apoptosis in the testis (17) via caspase-8 activation. However, we observed no induction of Fas protein or caspase-8 in our apoptotic Cyt cT-null testis. Caspase activity assays using Z-DEVD-AFC, a substrate preferred by caspase-3 and -7, and Z-IETD-AFC, a substrate preferred by caspase-8, yielded no significant differences in activity between wild-type and cyt cT−/− testes. We have also examined mRNA levels of apoptosis-related molecules using the GEArray system (SuperArray, Bethesda, Md.). Neither the membrane containing the cDNAs associated with mouse apoptosis nor the signal transduction pathways showed significant differences when hybridized with probes from homozygote testes and littermate controls. These cDNA arrays include the tumor necrosis factor (TNF) ligand family, TNF receptor family, and caspase family. These additional data are consistent with the Western blotting and caspase activity assays that showed no evidence of enhanced activation of caspase-8 in the Cyt cT−/− testes. We found no significant difference in the expression levels of other apoptosis-related molecules, including apoptosis-inducing factor, Apaf-1, Nip3 (a cell death-inducing protein) (3), and Fas, as shown in Fig. 4B, panel a.
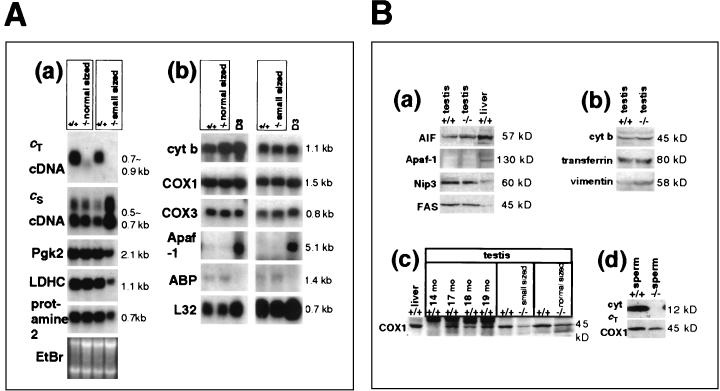
FIG. 4.
Expression of testis-specific proteins and apoptosis-related proteins. (A) Northern blot hybridization of testis RNA. (a) Analysis of testis-specific genes. The first pair of samples are from normal-sized testes of Cyt cT−/− and control (+/+) mice. The second pair of samples are from small testes of Cyt cT−/− and control (+/+) mice. Weight of the small testis was approximately 27% of its control littermate. (b) Analysis of genes related to oxidative phosphorylation or apoptosis. The first pair of samples are testes from a Cyt cT−/− mouse and his littermate control (both testes were approximately the same weight). For the second pair of samples the weight of the Cyt cT−/− testis was approximately 60% of the littermate control value. D3 is RNA from D3 ES cells and was included as a positive control for Apaf-1. (B) Western blotting of protein extracts from testes and sperm. (a and b) Testis extracts from a Cyt cT−/− mouse (weight of −/− testis was approximately 75% of the +/+ control), with antibodies against apoptosis-related proteins (AIF, Apaf-1, Nip3, and Fas) (a) or antibodies to cytochrome b, transferrin, and vimentin (b); 100 μg of protein/lane. (c) Western blotting with anti-COX1 antibody on testis extracts from aged wild-type mice (14, 17, 18, or 19 months old) and Cyt cT−/− mice (weight of −/− testis was approximately 75% of the +/+ control), and control and Cyt cT−/− mice with testes of comparable weight; 100 μg of protein/lane. (d) Sperm cells isolated from epididymides of Cyt cT−/− and control mice with anti-cT and anti-COX1 antibody. Extracts are of 3.3 × 105 sperm/lane.
Are there changes in the glycolytic and/or oxidative phosphorylation pathways?
Normal expression of two meiotic and one postmeiotic marker, pgk2 (phosphoglycerate kinase 2), ldhc (lactate dehydrogenase c), and prot2 (protamine 2), was seen in the RNA from a normal-sized testis of a 6-month-old Cyt cT mouse. PGK-2 (EC 2.7.2.3) and LDH-C4 (EC 1.1.1.27) are glycolytic enzymes, and the fact that their mRNA levels remained unchanged indicates that the disruption of oxidative phosphorylation resulting from inactivation of the Cyt cT gene does not accelerate the synthesis of glycolytic enzymes in normal-sized Cyt cT KO testes. A small testis of a 7-month-old Cyt cT KO mouse, weighing only 24% of the control, showed reduced expression of pgk2, ldhc, and prot2, while levels of Cyt cS mRNA were increased. However, these changes are due to the reduction in the number of germ cells and the altered ratio of germ cells to Sertoli cells in these atrophic testes (Fig. 2D, panel i), since markers of Sertoli cells, i.e., testicular androgen binding protein and vimentin, also appeared to increase (Fig. 4A and B, panels b). The level of transferrin, another marker of Sertoli cells, was not increased (Fig. 4B, panel b), but this most likely reflects a down-regulation of transferrin expression caused by the loss of germ cells (29).
To determine whether the deletion of Cyt cT alters the expression levels of molecules that participate in oxidative phosphorylation, we analyzed the expression of three mitochondrial genes. Cytochrome b (cyt b) is a component of complex III in the electron transport chain, while COX1 and COX3 are components of complex IV of the electron transport chain. Northern blot analysis revealed no changes in the mRNA levels of either cyt b, COX1, or COX3 in small testes (Fig. 4A, panel b). However, Western blotting with protein extracts from small (4-month-old) or normal-sized (3-month-old) testes of Cyt cT KO mice and from spermatozoa isolated from Cyt cT KO mice revealed a significant reduction of COX1 protein (Fig. 4B, panels c and d). This suggests that the synthesis or stability of the COX1 protein is reduced in the Cyt cT−/− germ cells and spermatozoa. Similar changes in COX1 expression were also observed in the testes of aged wild-type mice (14 to 19 months old), as shown in Fig. 4B, panel c.
It has been reported that aging damages complex III in oxidative phosphorylation in the human heart and decreases the heart's tolerance for ischemia and reperfusion (18). Aging mammals often show testicular atrophy with loss of germ cells (7). We found corresponding basophilic structures in the luminal surface of the seminiferous tubules and detected a reduction in COX1 mRNA levels in both aging normal testis and the atrophic Cyt cT-null testis from young mice. Thus, the Cyt cT−/− testis appears to mimic a dysfunction in oxidative phosphorylation which normally occurs with aging but which in the Cyt cT-null testis results in early testicular atrophy.
Cyt cT−/− sperm show reduced motility, ATP concentrations, and fertility.
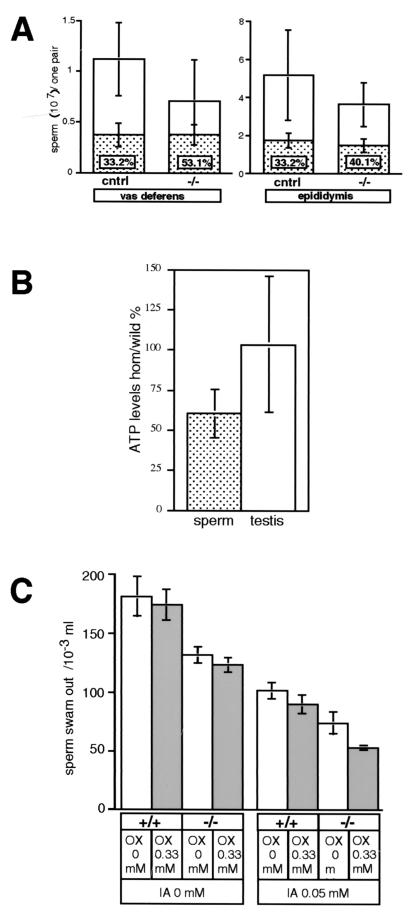
To examine if the production of spermatozoa by the Cyt cT−/− testis is normal, we studied the number and motility of Cyt cT−/− spermatozoa. Isolating sperm from the vas deferens, we found an average of (0.71 ± 0.4) × 107 Cyt cT−/− sperm, compared to (1.1 ± 0.36) × 107 for control samples (P = 0.0014) (Fig. 5A, left). Furthermore, the Cyt cT−/− samples contained 53.1% ± 13.7% immotile sperm, while the control samples contained 33.2% ± 10.3% immotile sperm (P < 0.001). Similar sperm counts were obtained from the epididymis (Fig. 5A, right), in agreement with the results obtained for the vas deferens sperm; i.e., we found (3.6 ± 1.2) × 107 sperm for the Cyt cT−/− samples, compared to (5.2 ±2.4) × 107 sperm for the controls (P = 0.0104). The epididymal Cyt cT−/− samples had 40.1% ± 9.6% immotile sperm, while only 33.2% ± 7.5% immotile sperm were seen in the control samples (P = 0.0146). A total of 23 Cyt cT−/− mice and a total of 20 control mice were examined for these experiments (age range, 6 to 15 months; average, 10.5 months).
FIG. 5.
Reduced motility of Cyt cT−/− spermatozoa. (A) Numbers of spermatozoa collected from vas deferens (left graph) and cauda epididymis (right graph) from a single mouse. White bars indicate total spermatozoa. Control samples (cntrl) consist of 16 wild-type and four heterozygous mice (n = 20; mean = [1.1 ± 1] × 107 per mouse) while homozygote samples were from 23 mice (mean = [0.71 ± 0.40] × 107 per mouse). Bars with hatch marks show immotile spermatozoa in each sample, and percent numbers within the hatched bar show the ratio of immotile spermatozoa in the total sperm count. (B) Ratio of ATP levels in testes and isolated spermatozoa of Cyt cT−/− mice relative to values of littermate wild-type controls. The average ATP level in sperm from Cyt cT−/− mice was approximately 60% of control, while the average ATP level in testis was approximately the same as in controls (about 100%). (C) Motility assay of spermatozoa isolated from epididymis. Total numbers of spermatozoa passing through 3-μm pores in media with or without 0.33 mM oxamic acid (OX) were determined. The same assay was conducted with or without 0.05 mM iodoacetamide (IA).
We then measured and compared the ATP content in the testes and sperm samples from seven pairs of Cyt cT−/− mice and matched controls (3 to 19 months old). As shown in Fig. 5B, ATP content per single sperm in homozygotes was 60% ± 14.8% of the controls, while ATP content per testis weight (in milligrams) in homozygotes was 103% ± 42.7% of the controls.
Next, sperm motility was quantified in the presence or absence of agents that are known to inhibit glycolysis, such as oxamic acid, an inhibitor of LDHs, and iodoacetamide, an inhibitor of glyceraldehyde-3-phosphate dehydrogenase (EC 1.2.1.12). The Cyt cT KO samples contained reduced numbers of highly motile sperm with and without oxamic acid (approximately 73%) (Fig. 5C). In the presence of 0.05 mM iodoacetamide but without oxamic acid, both homozygote and control sperm were less motile (approximately 56%); however, in the presence of both iodoacetamide and oxamic acid, wild-type sperm still contained 48.8% ± 1.2% motile sperm, while Cyt cT KO sperm contained only 39.9% ± 0.6% motile sperm (P = 0.012).
To determine whether Cyt cT KO spermatozoa are capable of fertilizing normal eggs in vitro as efficiently as wild-type spermatozoa, we used an in vitro fertilization assay. We found that out of 180 oocytes incubated with spermatozoa isolated from a 6.5-month-old Cyt cT KO mouse, 14.5% ± 6.1% developed to the two-cell stage. In contrast, 60.5% ± 15.6% (n = 2; 157 out of 263 eggs) of the oocytes that were incubated with spermatozoa from a littermate wild-type mouse developed to the two-cell stage.
It has been suggested that deficiencies in the respiratory chain could affect spermatogenesis, producing nonfunctional sperm (4). However, a causal relationship between a dysfunction of the mitochondria in germ cells and male infertility is still unclear (1). Our data indicate that oxidative phosphorylation is not essential to support sperm motility and fertility under conditions where fructose, lactic acid, and/or glucose are available to maintain glycolysis.
Concluding remarks.
Cytochrome c is an essential protein in the electron transport chain functioning during oxidative phosphorylation. Since Cyt cT is highly expressed in meiotic prophase during spermatogenesis and is the only cytochrome c expressed in mature spermatozoa, we predicted severe consequences to the function of the spermatozoa upon inactivation of Cyt cT. Contrary to our expectations, Cyt cT-null mice are fertile, even though a high percentage of their spermatozoa are immotile. Other investigators have shown that mice lacking Cyt cS die in utero, and cells derived from these embryos are viable only under conditions that compensate for the resulting dysfunction in oxidative phosphorylation (19). The authors reported that Cyt cS−/− cells showed reduced caspase-3 activation, were resistant to proapoptotic stimuli, and became more susceptible to the extrinsic TNF-α-mediated pathway of apoptosis. Spermatogenesis in our Cyt cT-null mice was ostensibly unaffected in young mice, although their germ cells underwent apoptosis in an age-related fashion. The fact that removal of cytochrome c accelerates apoptosis seems paradoxical. Cyt cS in germ cells declines as the cells enter meiosis, with pachytene spermatocytes containing low levels of Cyt cS at an approximate Cyt cS to Cyt cT ratio of 1:4 (13). One would have expected that a compensatory Cyt cS induction in the Cyt cT−/− pachytene cells might explain the accelerated apoptosis. However, our data show no evidence of such a compensatory induction of Cyt cS. Thus, it seems that Cyt cT may play a role distinct from that of Cyt cS in germ cell mitochondria. It has been reported that cells lacking mitochondrial DNA expression and lacking a functional respiratory chain undergo apoptosis (14, 32). The Cyt cT−/− testis appears to mimic a dysfunction in oxidative phosphorylation which normally occurs with aging.
Acknowledgments
This work was supported in part by grants CA 42595 and HD 05863 from the National Institutes of Health.
We thank the Animal Facility at the Burnham Institute for mouse husbandry and Ling Wang of the Mouse Genetics Laboratory for IVF experiments.
REFERENCES
- 1.Bourgeron, T. 2000. Mitochondrial function and male infertility. Probl. Cell Differ. 28:187-210. [DOI] [PubMed] [Google Scholar]
- 2.Cai, L., B. F. Hales, and B. Robaire. 1997. Induction of apoptosis in the germ cells of adult male rats after exposure to cyclophosphamide. Biol. Reprod. 56:1490-1497. [DOI] [PubMed] [Google Scholar]
- 3.Chen, G., J. Cizeau, C. Vande Velde, J. H. Park, G. Bozek, J. Bolton, L. Shi, D. Bubik, and A. Greenberg. 1999. Nix and Nip3 form a subfamily of pro-apoptotic mitochondrial proteins. J. Biol. Chem. 274:7-10. [DOI] [PubMed] [Google Scholar]
- 4.Cummins, J. M., A. M. Jequier, and R. Kan. 1994. Molecular biology of human male infertility: links with aging, mitochondrial genetics, and oxidative stress? Mol. Reprod. Dev. 37:345-362. [DOI] [PubMed] [Google Scholar]
- 5.Furuchi, T., K. Masuko, Y. Nishimune, M. Obinata, and Y. Matsui. 1996. Inhibition of testicular germ cell apoptosis and differentiation in mice misexpressing Bcl-2 in spermatogonia. Development 122:1703-1709. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg, E., D. Sberna, T. E. Wheat, G. J. Urbanski, and E. Margoliash. 1977. Cytochrome c: immunofluorescent localization of the testis-specific form. Science 196:1010-1012. [DOI] [PubMed] [Google Scholar]
- 7.Gosden, R. G., D. W. Richardson, N. Brown, and D. W. Davidson. 1982. Structure and gametogenic potential of seminiferous tubules in ageing mice. J. Reprod. Fertil. 64:127-133. [DOI] [PubMed] [Google Scholar]
- 8.Hake, L. E., A. A. Alcivar, and N. B. Hecht. 1990. Changes in mRNA length accompany translational regulation of the somatic and testis-specific cytochrome c genes during spermatogenesis in the mouse. Development 110:249-257. [DOI] [PubMed] [Google Scholar]
- 9.Hake, L. E., and N. B. Hecht. 1993. Utilization of alternative transcription initiation site of somatic cytochrome c in the mouse produces a testis-specific cytochrome c mRNA. J. Biol. Chem. 268:4788-4797. [PubMed] [Google Scholar]
- 10.Hake, L. E., N. Kuemmerle, N. B. Hecht, and C. A. Kozak. 1994. The genes encoding the somatic and testis-specific isotypes of the mouse cytochrome c genes map to paralogous regions of chromosomes 6 and 2. Genomics 20:503-505. [DOI] [PubMed] [Google Scholar]
- 11.Hamano, S., K. Naito, Y. Fukuda, and Y. Toyoda. 1989. In vitro capacitation of boar ejaculated spermatozoa: effect of conditioned media prepared from preincubated sperm suspension. Gamete Res. 24:483-489. [DOI] [PubMed] [Google Scholar]
- 12.Hecht, N. B. 1998. Molecular mechanisms of male germ cell differentiation. Bioessays 20:555-561. [DOI] [PubMed] [Google Scholar]
- 13.Hess, R. A., L. A. Miller, J. D. Kirby, E. Margoliash, and E. Goldberg. 1993. Immunoelectron microscopic localization of testicular and somatic cytochromes c in the seminiferous epithelium of the rat. Biol. Reprod. 48:1299-1308. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson, M. D., J. F. Burne, M. P. King, T. Miyashita, J. C. Reed, and M. C. Raff. 1993. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature 361:365-369. [DOI] [PubMed] [Google Scholar]
- 15.Knudson, C. M., K. S. Tung, W. G. Tourtellotte, G. A. Brown, and S. J. Korsmeyer. 1995. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 270:96-99. [DOI] [PubMed] [Google Scholar]
- 16.Lee, J., J. H. Richburg, S. C. Younkin, and K. Boekelheide. 1997. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology 138:2081-2088. [DOI] [PubMed] [Google Scholar]
- 17.Lee, J., J. H. Richburg, E. B. Shipp, M. L. Meistrich, and K. Boekelheide. 1999. The Fas system, a regulator of testicular germ cell apoptosis, is differentially up-regulated in Sertoli cell versus germ cell injury of the testis. Endocrinology 140:852-858. [DOI] [PubMed] [Google Scholar]
- 18.Lesnefsky, E. J., T. I. Gudz, C. T. Migita, M. Ikeda-Saito, M. O. Hassan, P. J. Turkaly, and C. L. Hoppel. 2001. Ischemic injury to mitochondrial electron transport in the aging heart: damage to the iron-sulfur protein subunit of electron transport complex III. Arch. Biochem. Biophys. 385:117-128. [DOI] [PubMed] [Google Scholar]
- 19.Li, K., Y. Li, J. M. Shelton, J. A. Richardson, E. Spencer, Z. J. Chen, X. Wang, and R. S. Williams. 2000. Cytochrome c deficiency causes embryonic lethality and attenuates stress-induced apoptosis. Cell 101:389-399. [DOI] [PubMed] [Google Scholar]
- 20.Li, P., D. Nijhawan, I. Budihardjo, S. M. Srinivasula, M. Ahmad, E. S. Alnemri, and X. Wang. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479-489. [DOI] [PubMed] [Google Scholar]
- 21.Limbach, K. J., and R. Wu. 1985. Characterization of a mouse somatic cytochrome c gene and three cytochrome c pseudogenes. Nucleic Acids Res. 13:617-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyons, G., Y. R. Bilgeri, A. Zanzinger, M. Berzin, and D. Mendelsohn. 1986. Extraction and estimation of ATP from human spermatozoa. Andrologia 18:455-460. [DOI] [PubMed] [Google Scholar]
- 23.Nagy, A., J. Rossant, R. Nagy, W. Abramow-Newerly, and J. C. Roder. 1993. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl. Acad. Sci. USA 90:8424-8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narisawa, S., K. A. Smans, J. Avis, M. F. Hoylaerts, and J. L. Millan. 1993. Transgenic mice expressing the tumor marker germ cell alkaline phosphatase: an in vivo tumor model for human cancer antigens. Proc. Natl. Acad. Sci. USA 90:5081-5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narisawa, S., N. Frohlander, and J. L. Millan. 1997. Inactivation of two mouse alkaline phosphatase genes and establishment of a model of infantile hypophosphatasia. Dev. Dyn. 208:432-446. [DOI] [PubMed] [Google Scholar]
- 26.Print, C. G., and K. L. Loveland. 2000. Germ cell suicide: new insights into apoptosis during spermatogenesis. Bioessays 22:423-430. [DOI] [PubMed] [Google Scholar]
- 27.Reed, J. C. 1997. Cytochrome c: can't live with it—can't live without it. Cell 9:559-562. [DOI] [PubMed] [Google Scholar]
- 28.Rieger, D. 1997. Batch analysis of the ATP content of bovine sperm, oocytes, and early embryos using a scintillation counter to measure the chemiluminescence produced by the luciferin-luciferase reaction. Anal. Biochem. 246:67-70. [DOI] [PubMed] [Google Scholar]
- 29.Roberts, K. P., R. Santulli, J. Seiden, and B. R. Zirkin. 1992. The effect of testosterone withdrawal and subsequent germ cell depletion on transferrin and sulfated glycoprotein-2 messenger ribonucleic acid levels in the adult rat testis. Biol. Reprod. 47:92-96. [DOI] [PubMed] [Google Scholar]
- 30.Stennicke, H. R., and G. S. Salvesen. 1997. Biochemical characteristics of caspases-3, -6, -7, and -8. J. Biol. Chem. 272:25719-25723. [DOI] [PubMed] [Google Scholar]
- 31.Thornberry, N. A., and Y. Lazebnik. 1998. Caspases: enemies within. Science 281:1312-1316. [DOI] [PubMed] [Google Scholar]
- 32.Wang, J., J. P. Silva, C. M. Gustafsson, P. Rustin, and N. G. Larsson. 2001. Increased in vivo apoptosis in cells lacking mitochondrial DNA gene expression. Proc. Natl. Acad. Sci. USA 98:4038-4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou, H., W. J. Henzel, X. Liu, A. Lutschg, and X. Wang. 1997. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90:405-413. [DOI] [PubMed] [Google Scholar]