Abstract
GeneCalling, a genome-wide method of mRNA profiling, reveals that endothelial cells adhering to fibronectin through the α5β1 integrin, but not to laminin through the α2β1 integrin, undergo a complex program of gene expression. Several of the genes identified are regulated by the NF-κB transcription factor, and many are implicated in the regulation of inflammation and angiogenesis. Adhesion of endothelial cells to fibronectin activates NF-κB through a signaling pathway requiring Ras, phosphatidylinositol 3-kinase, and Rho family proteins, whereas adhesion to laminin has a limited effect. Retroviral transfer of the superrepressor of NF-κB, IκB-2A, blocks basic fibroblast growth factor-induced angiogenesis in vivo. These results suggest that engagement of the α5β1 integrin promotes an NF-κB-dependent program of gene expression that coordinately regulates angiogenesis and inflammation.
Angiogenesis, the formation of new blood vessels from preexisting ones, requires endothelial cells to migrate, proliferate, and ultimately assemble into tubes that regulate selective transport of white blood cells and solutes from their lumen to the interstitium and vice versa (20, 40). Several observations suggest that angiogenesis and inflammation proceed in a coordinate fashion and sustain one another during wound healing and tissue repair as well as in a variety of chronic inflammatory diseases and in cancer (23). Although it is increasingly clear that endothelial cells mediate angiogenesis and also have broad immune functions (37), the signaling pathways and gene expression mechanisms that allow a coordinate regulation of angiogenesis and inflammation by endothelial cells are incompletely understood.
Angiogenesis requires the interaction of endothelial cells with both angiogenic growth factors and extracellular matrix components (13, 22, 56). The process can be subdivided into two phases. During the invasive and proliferative phase, endothelial cells undergo multiple interactions with a fibronectin-rich interstitial matrix, whereas during the maturation phase they assemble a laminin-rich basement membrane and form a capillary (41). Gene knockout studies have indicated that the α5β1 integrin and its ligand fibronectin are required for vasculogenesis in the mouse (15, 57), and peptide and antibody blocking experiments have also implicated this receptor-ligand pair in postnatal angiogenesis (27). The relatively promiscuous αv integrins are largely dispensable for vascular development in the embryo (2) but are thought to participate in postnatal angiogenesis in response to growth factors, such as basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF), or tumors (6). In particular, αvβ3 promotes the survival and maturation of newly formed blood vessels through inhibition of p53 (7, 50). Finally, antibodies to the collagen- and laminin-binding integrins α1β1 and α2β1 inhibit VEGF-induced angiogenesis, suggesting that these integrins may also play a role in vascular development (44).
Integrins have multiple adhesive and signaling functions that may play a crucial role in angiogenesis. In addition to stable adhesion, migration, and matrix assembly, integrins promote cell survival and regulate cell cycle progression (17). Because each integrin has its own binding specificity and signaling properties, the outcome of the interaction with the matrix depends on the repertoire of integrins on the cell and the composition of the matrix to which it adheres (18). The complexity and specificity of integrin signaling has complicated attempts to define the pathways crucial for angiogenesis. We have used a genome-wide method of mRNA profiling to examine the gene expression program induced by adhesion to the matrix in endothelial cells. Our results indicate that engagement of the α5β1 integrin promotes an NF-κB-dependent program of gene expression that is important for both angiogenesis and inflammation.
MATERIALS AND METHODS
Cells, constructs, and transfections.
Primary human umbilical vein endothelial cells (HUVECs) (Clonetics) were cultured on gelatin-coated dishes in serum-free medium (SFM) (Gibco-BRL) supplemented with 20% fetal calf serum, 20 ng of bFGF/ml, 10 ng of EGF/ml, and 1 μg of heparin/ml and used between passages 2 and 5. After growth factor deprivation, HUVECs were detached with 0.02% EDTA and kept in suspension in SFM containing 0.1% bovine serum albumin (BSA) and 1 μg of cycloheximide (Sigma)/ml for 1 h. [35S]methionine-cysteine incorporation experiments indicated that this treatment results in >90% inhibition of protein synthesis. They were then plated in the continued presence of cycloheximide on dishes coated with 15 μg of human fibronectin (Collaborative Research)/ml, 15 μg of mouse laminin-1 (Collaborative Research)/ml, or 3 μg of poly-l-lysine (Sigma)/ml. The three substrates promoted attachment of HUVECs to the same extent. While fibronectin promoted extensive cell spreading, laminin was less effective and poly-l-lysine did not induce spreading. After incubation at 37°C in SFM with 0.1% BSA and cycloheximide for 1 or 4 h, HUVECs were scraped in Trizol (Gibco-BRL).
For transfection, HUVECs (5 × 106) were suspended in 300 μl of SFM containing 25 μg of pZip Ras N17 (encoding dominant-negative Ras) (33), pcDNA3 Rac N17 (encoding dominant-negative Rac) (33), or pEBB-IκBα32/36A (encoding the NF-κB superrepressor IκB-2A) (from I. Stancowsky and D. Baltimore, California Institute of Technology, Pasadena) in combination with 2.5 μg of pEGFP-F and electroporated at 300 V and 450 μF as previously described (33). The transfection efficiency was approximated at 35% by epifluorescence microscopy. Vectors encoding activated Rac and dominant-negative Cdc42 or Rho (33) were also transfected as above. The NF-κB-luciferase reporter vectors containing two tandem repeats of the intact or mutant NF-κB binding site from the major histocompatibility class promoter were obtained from I. Stancowsky and D. Baltimore (California Institute of Technology). Transfection efficiencies were verified by cotransfection of a vector encoding β-galactosidase. The cells were lysed, and luciferase activity was measured by using a Promega kit.
For the retrovirus studies in the subcutaneous murine Matrigel angiogenesis assay, the cDNA encoding the NF-κB superrepressor IκB-2A was subcloned into the retroviral vector pFB-Neo (Stratagene). 293T cells were transfected with this construct or control empty vector in combination with plasmids encoding Gag/Pol (pCMV-GAGPOL) and the envelope protein G of vesicular stomatitis virus (pMD.G) to allow production of vesicular stomatitis virus G protein-pseudotyped vectors, as previously described (34). Twenty hours after transfection, the cells were detached with trypsin, washed with phosphate-buffered saline (PBS), and included in the Matrigel assay.
GeneCalling.
GeneCalling reactions were performed as described previously (45). Briefly, double-stranded cDNAs were synthesized from poly(A)+ RNA and subdivided into 96 identical pools. Each pool was digested with a distinct pair of restriction enzymes. The resulting fragments were ligated to complementary adapters, one labeled with biotin and the other labeled with fluorescamine (FAM), and amplified by PCR. After affinity purification on streptavidin columns, the fragments were loaded onto 5% polyacrylamide ultrathin gels and electrophoresed on a Niagara instrument mounted in a platform employing stationary laser excitation and a multicolor charge-coupled device imaging system. PCR fragments were visualized by virtue of the fluorescent label at the 5′ end of one of the PCR primers, which ensured that all detected fragments had been digested by both enzymes. Each triplicate sample was subjected to three separate identical restriction enzyme digestions in order to generate a composite profile based on average peak height and variance. The 96 composite digestion profiles from two different cDNA preparations (i.e., fibronectin versus poly-l-lysine, laminin versus poly-l-lysine, and fibronectin versus laminin) were compared to identify differentially expressed fragments. The electrophoresis profiles were processed by using the Java-based, internet-ready, Open Genome Initiative software suite. Linkage of a differentially expressed cDNA fragment to a gene was made through knowledge of the restriction enzymes used to generate the two asymmetric ends and the length of the fragment itself. Before the human genome sequence became available, data were submitted as point-by-point length versus amplitude addresses to an Oracle 8 database.
Gene confirmation by oligonucleotide poisoning.
A competitive PCR was used to confirm the identity of each differentially expressed fragment. One end of each restriction fragment mapping in end sequence and length to a known human gene was used as a template for the design of an unlabeled oligonucleotide primer extending into the specific complementary gene sequence 9 to 19 nucleotides (based on estimated melting temperature) further than the original labeled oligonucleotide. The original PCR was reamplified in the presence or absence of an excess amount of the unlabeled oligonucleotide. The two samples were electrophoresed, and the resulting profiles were compared to evaluate the selective suppression of the peak of interest (45).
Immunocytochemistry.
Cells were plated on fibronectin-, laminin-1-, or poly-l-lysine-coated coverslips in SFM. Two hours after plating, cells were rinsed with PBS, fixed with 4% paraformaldehyde for 20 min, and permeabilized with 0.2% Triton X-100 for 20 min. Nonspecific binding was minimized by blocking with 2.5% BSA in Tris-buffered saline and 5% normal goat and donkey serum in PBS. Anti-NF-κB p65 polyclonal antibodies (sc-372; Santa Cruz) were used at concentrations of 1 μg/ml followed by Texas Red- or fluorescein isothiocyanate (FITC)-conjugated anti-rabbit immunoglobulin Gs (Vector Labs). Nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI). Nuclear translocation of NF-κB was quantified by counting the percentage of nuclei that were stained by anti-p65 antibodies more intensely than the surrounding cytoplasm.
Semiquantitative RT-PCR.
For reverse transcription-PCR (RT-PCR) experiments, the HUVECs were not incubated with cycloheximide to inhibit protein synthesis. The cells were either left untreated or treated with 20 μM LY-294002 (Biomol), 50 μM PD98059 (BioLabs), 180 μM pyrrolidine dithiocarbamate (PDTC) (Sigma), 20 mM sodium salicylate (Fisher Scientific), or 5 μM AG-490 (Biomol) for 1 h. They were then detached and kept in suspension or replated on fibronectin for 4 h in the presence of the inhibitors. Total RNA was extracted from HUVECs by using Trizol, and first-strand cDNA was prepared with Superscript II reverse transcriptase (Gibco-BRL). Primer sets specific to ELAM-1, VCAM-1, CYR-61, endothelin 1, c-193, A20, interferon regulatory factor 1 (IRF-1), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used to direct the PCR (94°C for 5 min and 30 cycles of 94°C for 30 s, 54°C for 30 s, and 72°C for 30 s, and then 72°C for 7 min). The linear expression range of each gene was obtained after serial dilution of each cDNA pool and normalization to the expression of GAPDH.
Matrigel assay.
Angiogenesis was induced by subcutaneous injection of growth factor-depleted Matrigel (Becton Dickinson) (400 μl) supplemented with bFGF (200 ng) and heparin sulfate (500 ng) in 6-week-old athymic nude mice, and it was analyzed after 5 days, essentially as described previously (36). To test the role of NF-κB in this model, 2 × 106 293T cells producing either a retroviral vector encoding the superrepressor of IK-Bα or the empty retrovirus were injected along with the Matrigel, bFGF, and heparin. The Matrigel plugs were harvested and analyzed either by immunoprecipitation followed by immunoblotting with a VEGF receptor antibody (flk-1) (sc-315; Santa Cruz) that specifically reacts with mouse endothelial cells or by histological analysis of paraffin-embedded sections stained with hematoxylin and eosin. To control for variability among individual mice, we injected 3 plugs per mouse: Matrigel supplemented with bFGF, Matrigel with bFGF and cells producing the empty retrovirus, and Matrigel with bFGF and cells producing the retrovirus expressing the superrepressor IkB-2A. In additional control animals, we injected 2 plugs of Matrigel alone either in the presence or in the absence of bFGF. As an independent means of quantifying angiogenesis, we injected the mice intravenously with 20 μg of griffonia (bandeiraea) simplicifolia lectin I-isolectin B4 (Vector Laboratories) 30 min prior to harvesting the plugs. Identical aliquots of plug homogenates were subjected to spectrophotometric analysis. Only plugs from the same animal were compared by immunoblotting, histological analysis, or FITC-lectin incorporation.
RESULTS AND DISCUSSION
Adhesion to fibronectin through the α5β1 integrin is sufficient to activate a broad gene expression program in HUVECs.
We have used GeneCalling to determine if adhesion to the extracellular matrix is sufficient to induce gene expression in HUVECs and, if so, to evaluate the extent and integrin-specificity of this event. GeneCalling couples expression profiling with a database query, which utilizes fragment length and end sequence information to provide feedback on expressed genes. This method requires no a priori gene sequence and provides >95% coverage of the expressed genome with a sensitivity of detection greater than 1:100,000 (45). GeneCalling has recently been used to identify genes linked to cardiac hypertrophy, obesity, and endothelial cell differentiation in vitro and it has been extensively confirmed by Northern blot analysis, semiquantitative RT-PCR, and real-time PCR (25, 39, 45).
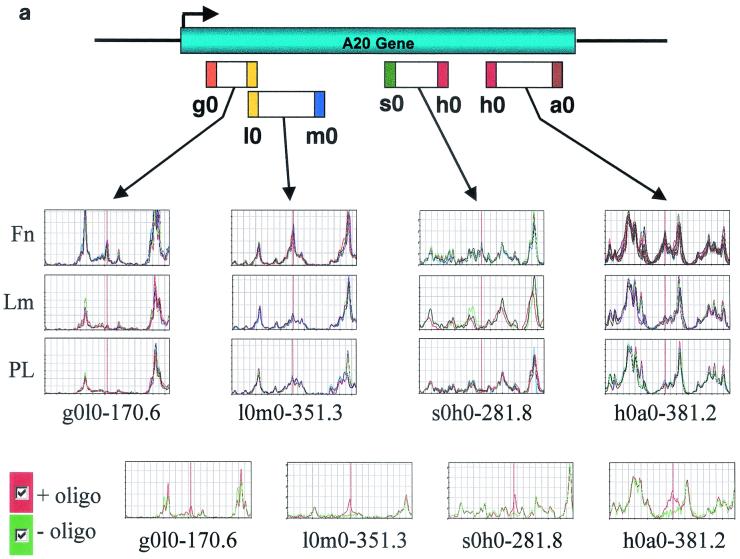
It is known that HUVECs adhere to fibronectin through the α5β1 integrin and to laminin-1 through α2β1 (30). To limit the scope of the search to genes that are directly regulated by integrin signals, HUVECs were treated with the protein synthesis inhibitor cycloheximide and then plated on fibronectin or laminin-1 in the absence of mitogens. As a control, the cells were plated on poly-l-lysine under conditions that do not cause activation of either focal adhesion kinase or Shc signaling (54). Under our experimental conditions, HUVECs attached equally well to all three substrates but spread only on fibronectin and laminin-1. Adhesion to fibronectin caused more extensive cell spreading than adhesion to laminin-1, in accordance with the observation that α2β1 does not activate efficiently Rac, at least in endothelial cells (33). Following double-stranded cDNA synthesis of poly(A)+ RNA, cDNA pools were digested with 96 pairs of restriction enzymes. The resulting fragments were ligated to complementary adapters, one labeled with biotin and the other labeled with FAM, and amplified by PCR. After affinity purification on streptavidin and separation by Niagara polyacrylamide gel electrophoresis, the FAM-labeled fragments were detected by laser excitation. A composite trace, based on average peak height and variance, was calculated for each triplicate sample, and the different traces were compared to one another with software designed to identify differentially expressed peaks. Since each mRNA yields fragments of specific predicted lengths, a database query allowed identification of genes whose sequences are deposited in the database, whereas fragments derived from novel genes were flagged by their absence from the database. Figure 1a shows an example of multiple band differences corresponding to the transcript encoding A20, which is induced 4.2-fold in HUVECs plated for 4 h on fibronectin but not on laminin-1.
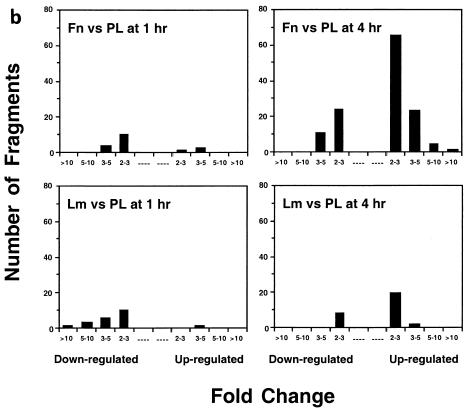
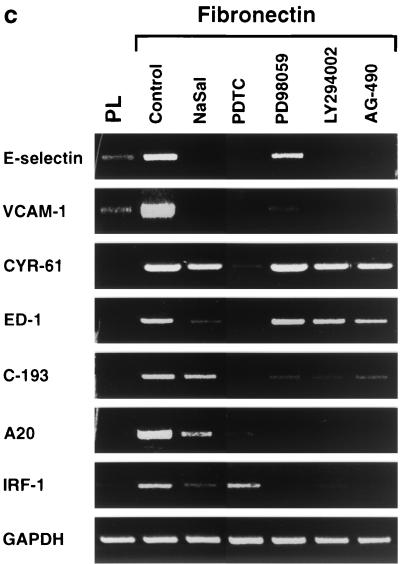
FIG. 1.
Generation and confirmation of GeneCalling profiles. (a) Multiple band differences corresponding to the induction of the NF-κB target A20 in HUVECs adhering to fibronectin. The labels g0, l0, m0, s0, h0, and a0 are arbitrary symbols corresponding to the restriction enzymes used. The expression profile of each sample was generated as a composite of at least three reactions. Four of the differences due to induction of A20 on fibronectin (Fn) compared to laminin-1 (Lm) and poly-l-lysine (PL) are depicted. Only the relevant portions of the electrophoresis output for a set of triplicate amplification reactions from a single sample are shown. The bottom panels include the control oligonucleotide poisoning reaction. The red trace was generated by reamplification without the addition of the gene-specific oligonucleotide while the green trace was generated by reamplification in the presence of the gene-specific primer. (b) Distribution by change (n-fold) of differentially expressed fragments upon HUVEC adhesion to matrix components. Cells plated on fibronectin or laminin-1 for 1 or 4 h were compared to cells plated on poly-l-lysine for the same amount of time. Differentially expressed fragments are grouped by number per range and direction of change (n-fold). (c) Semiquantitative RT-PCR analysis of seven fibronectin-induced genes that had been identified by GeneCalling. HUVECs were plated for 4 h on poly-l-lysine or on fibronectin in the absence (control) or in the presence of 20 mM sodium salicylate (NaSal), 180 μM PDTC, 50 μM PD98059, 20 μM LY294002, or 5 μM AG-490. The level of expression of the indicated transcripts was assessed by semiquantitative RT-PCR analysis. Colors were inverted during reproduction. Fibronectin-dependent gene expression requires NF-κB and distinct combinations of other integrin-dependent signals.
Table 1 indicates the total number of fragments detected and the number of differentially expressed fragments (± twofold) for each comparison made in this study. Attachment of HUVECs to fibronectin or laminin-1 for 1 h resulted in 16 and 19 differentially expressed fragments, respectively. These fragments were mostly downregulated and corresponded to very modest changes in gene expression. The transcript of ornithine aminotransferase increased on laminin, whereas the growth factor-inducible immediate-early gene CYR-61 was induced on fibronectin. CYR-61 is an Arg-Glu-Asp (RGD) containing secreted protein and is thought to promote angiogenesis by binding to the αvβ3 integrin (31). The mRNAs encoding various ribosomal proteins decreased on both substrates. Adhesion of HUVECs to fibronectin for 4 h resulted in 126 fragments being differentially expressed relative to cells cultured on poly-l-lysine. Figure 1b shows that the expression of 28 of these fragments was increased by >3-fold and that of 10 fragments was decreased by >3-fold. In marked contrast, only 28 fragments were differentially regulated on laminin. Among these, only 1 (corresponding to Pyst-1/MPK-3, a mitogen-activated protein kinase phosphatase) was upregulated >3-fold. We positively identified the genes corresponding to 111 of the most-highly regulated fragments on fibronectin by using competitive PCR (oligonucleotide poisoning) (45). The confirmation for the A20 transcript is shown in Fig. 1a. As shown in Table 2, the 111 fragments corresponded to 55 known genes, seven previously sequenced cDNAs, and one novel open reading frame. Using the same method, 24 of the 28 fragments on laminin were positively identified and found to correspond to 16 known genes and three previously sequenced cDNAs.
TABLE 1.
Direct effect of extracellular matrix-mediated attachment on endothelial cell gene expressiona
| Comparison | Time (h) | No. of fragments detectedb | No. of fragments differentially expressedc |
|---|---|---|---|
| Fibronectin vs poly-l-lysine | 1 | 27,025 | 16 |
| Laminin vs poly-l-lysine | 1 | 26,813 | 19 |
| Fibronectin vs poly-l-lysine | 4 | 23,514 | 126 |
| Laminin vs poly-l-lysine | 4 | 24,805 | 28 |
The indicated comparisons were made, and the magnitude of gene expression change is reflected by the number of gene fragments that are differentially expressed compared to the total detected.
Values represent all fragments detected.
Values represent the number of fragments differentially expressed by twofold or more.
TABLE 2.
Endothelial cell attachment to fibronectin activates the NF-κB pathway of gene inductiona
| Comparison | Time (h) | No. of fragments detectedb | No. of fragments differentially expressedc |
|---|---|---|---|
| Fibronectin vs poly-L-lysine | 1 | 27,025 | 16 |
| Laminin vs poly-L-lysine | 1 | 26,813 | 19 |
| Fibronectin vs poly-L-lysine | 4 | 23,514 | 126 |
| Laminin vs poly-L-lysine | 4 | 24,805 | 28 |
The indicated comparisons were made, and the magnitude of gene expression change is reflected by the number of gene fragments that are differentially expressed compared to the total detected.
Values represent all fragments detected.
Values represent the number of fragments differentially expressed by twofold or more.
As shown in Table 2, adhesion to fibronectin for 4 h increased the abundance of 49 mRNAs and decreased that of 17 mRNAs by twofold or more, whereas laminin induced increased accumulation of only 16 mRNAs and downregulated 5 mRNAs. All the mRNAs that accumulated in response to laminin also did so in response to fibronectin and generally to a much larger extent. Employing real-time PCR on independent experimental samples, we confirmed the differential expression of RICK, A20, and interleukin 8 (IL-8) (data not shown). In addition, we used semiquantitative PCR to verify the upregulation of seven additional genes (E-selectin, VCAM-1, CYR-61, ED-1, C-193, A20, and IRF-1) (Fig. 1c). These results argue that adhesion to fibronectin is sufficient to activate a relatively broad gene expression program in HUVECs, whereas laminin-1 is not, suggesting that the α5β1 integrin is a more-potent regulator of gene expression than the α2β1 integrin, at least in endothelial cells.
Many of the genes identified are regulated by NF-κB and encode proteins involved in angiogenesis and inflammation.
Adhesion to fibronectin induced accumulation of a significant number of transcripts encoded by genes known to be controlled by the NF-κB transcription factor or to be induced by inflammatory cytokines, presumably through NF-κB (Table 2). In addition, a significant subset of the transcripts that accumulated on fibronectin consisted of components of cytokine receptor signaling pathways capable of activating NF-κB (heparin-binding epidermal growth factor [HB-EGF], IL-1α, TRAF-1, RICK, cIAP-2). Although prior evidence suggests that the α5β1 integrin activates the NF-κB pathway in fibroblasts and possibly also endothelial cells (38, 43), these findings illustrate the centrality of NF-κB signaling in α5β1-mediated control of gene expression in endothelial cells. In addition, they reveal that adhesion to fibronectin enhances the expression of signaling molecules involved in the activation and propagation of the NF-κB signal, thus providing a mechanism for the amplification of the response. Among the NF-κB-dependent genes induced by fibronectin, A20 encodes a zinc finger protein, which interferes with cytokine receptor signaling (47). Its transcription may thus provide negative feedback to NF-κB activation. In addition, HUVECs adhesion to fibronectin caused accumulation of transcripts known to be regulated by JAK-STAT signaling, such as those encoding IRF-1, STAT5A, and guanilate binding protein-2 (GBP-2). Accordingly, prior studies have indicated that adhesion to fibronectin activates JAK2-STAT5A signaling in HUVECs (5).
A significant number of NF-κB-dependent transcripts induced by fibronectin encoded proteins involved in angiogenesis (Table 2). These transcripts could be assigned to three groups. The first included granulocyte colony-stimulating factor, IL-8, and the receptor protein tyrosine kinase Eck (ephrin A1 receptor), which can exert a direct angiogenic effect by promoting the migration, proliferation, and survival of endothelial cells (8, 28, 35). In addition to participating in the assembly of focal adhesions, the membrane proteoglycan syndecan-4 functions as a coreceptor for the heparin-binding angiogenic growth factor bFGF (53) and may thus be included in this group. Connective tissue growth factor, which, like the homologous protein CYR-61, may promote angiogenesis by binding to the αvβ3 integrin on endothelial cells (31), and CXCR4, the seven-transmembrane-spanning receptor for the chemokine PBSF/SDF-1 (52), potentially also belong to this group. The second group of transcripts consisted of tissue factor, HB-EGF, and endothelin 1, which have been implicated in the recruitment of pericytes and smooth muscle cells during angiogenesis (1, 10, 55). These mural cells not only contribute to the assembly of the endothelial basement membrane but also provide the endothelial cells with factors, such as transforming growth factor β, which promote the completion of angiogenesis (40). A third group of transcripts included adhesion receptors (VCAM-1, E-selectin, ICAM-1) as well as cytokines and chemokines (IL-1α, MCP-3, Groα, and Groβ) involved in the recruitment of leukocytes (3, 12, 48). Macrophages, and possibly other leukocytes, are thought to participate in angiogenesis during inflammation, wound healing, and cancer by secreting cytokines and growth factors acting on endothelial cells (tumor necrosis factor alpha [TNF-α], IL-8, bFGF) and mural cells (transforming growth factor β, platelet-derived growth factor, acidic fibroblast growth factor) (51). The ability of soluble forms of VCAM-1 and E-selectin to promote angiogenesis in vivo (29) is consistent with this notion. Thus, adhesion to fibronectin induces the expression of genes that encode proteins able to regulate angiogenesis by both a direct mechanism and an indirect mechanism. The upregulation of B94 and A20, two mRNAs strongly upregulated during angiogenesis (42), lends further support to the scope of the gene expression program induced by fibronectin.
The α5β1 integrin activates NF-κB through Rho family proteins and reactive oxygen species.
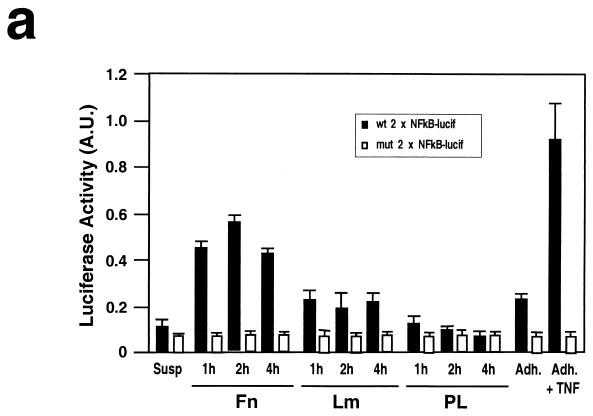
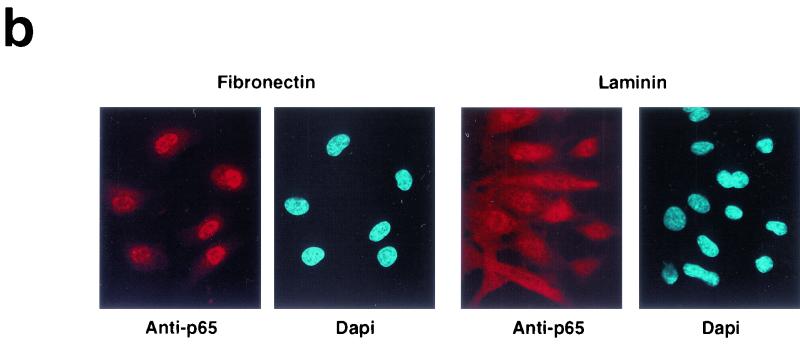
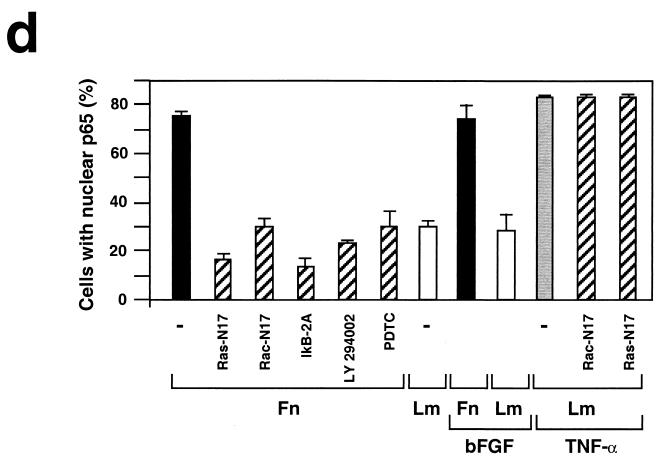
We directly tested fibronectin-mediated activation of the NF-κB pathway by measuring transcriptional activation of NF-κB in a luciferase reporter assay. Figure 2a illustrates that adhesion to fibronectin induces NF-κB transcriptional activity, whereas adhesion to laminin-1 exerts a much more modest effect. In addition, we monitored nuclear translocation of the p65 subunit of NF-κB upon matrix adhesion. As shown in Fig. 2b and d, p65 translocated to the nucleus in the majority of HUVECs adhering to fibronectin but only in a minor fraction of those adhering to laminin. Treatment with angiogenic concentrations of bFGF neither activated NF-κB in stably adherent HUVECs nor enhanced the response to fibronectin (Fig. 2d), indicating that the receptor for bFGF does not activate NF-κB, whereas α5β1 can efficiently activate NF-κB in the absence of concurrent signals.
FIG. 2.
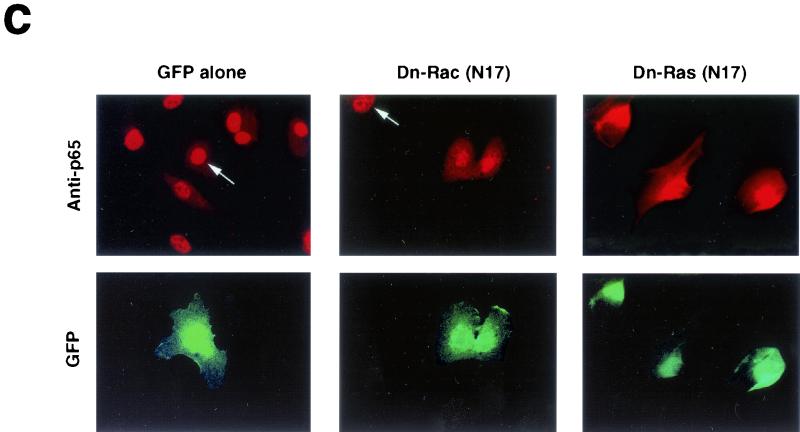
Adhesion to fibronectin activates NF-κB through a signaling pathway that requires Ras, PI-3K, and Rac. (a) Adhesion to fibronectin induces NF-κB-dependent transcription. HUVECs were transiently transfected with luciferase reporter constructs containing two tandem wild-type (wt 2 x NFkB-lucif) or inactivated (mut 2 x NFkB-lucif) κB sites and then either kept in suspension (Susp) or plated on fibronectin (Fn), laminin-1 (Lm), or poly-l-lysine (PL) for the indicated times. For comparison, stably adherent cells were either left untreated (Adh.) or treated with 50 ng of TNF-α/ml (Adh. + TNF). The data (± standard deviations) shown are from one representative experiment performed in triplicate. (b) HUVECs were plated on fibronectin or laminin-1. They were then subjected to immunofluorescent staining with anti-p65 antibodies. Nuclei were counterstained with Dapi. (c) HUVECs were transiently transfected with a vector encoding enhanced GFP (EGFP) alone or in combination with plasmids encoding the indicated dominant-negative (Dn) constructs and then plated on fibronectin for 2 h. The cells were subjected to immunofluorescent staining with anti-p65 antibodies followed by Texas Red-conjugated anti-rabbit immunoglobulin Gs and observed by epifluorescence microscopy. Arrows point to cells that had been transfected with and were expressing EGFP alone or to control untransfected cells within fields also containing cells that were expressing EGFP in combination with the indicated dominant-negative construct. (d) HUVECs were transiently transfected with a vector encoding EGFP alone (−) or in combination with plasmids encoding the indicated dominant-negative constructs and then plated on fibronectin (Fn), laminin-1 (Lm), or laminin-1 in the presence of 50 ng of TNF-α/ml for 2 h. Untransfected HUVECs were plated on fibronectin or laminin-1 for 2 h in the presence of 10 ng of bFGF/ml and 1 μg of heparin/ml. When indicated, untransfected cells were treated with 20 μM LY294002 or 180 μM PDTC and then plated on fibronectin for 2 h in the presence of the inhibitors. The graph illustrates the mean percentages (± standard deviations) of cells displaying nuclear translocation of p65. The means ± standard deviations of values from multiple experiments are shown.
Previous studies have provided evidence that Ras and Rho family proteins are coordinately regulated by integrins and growth factor receptors and can activate NF-κB (4, 17). We therefore examined the role of these small G proteins in fibronectin-induced activation of NF-κB in HUVECs. As shown in Fig. 2c and d, expression of green fluorescent protein (GFP) alone did not prevent nuclear accumulation of p65 in HUVECs adhering to fibronectin. By contrast, expression of dominant-negative Ras (N17) and Rac (N17) inhibited fibronectin-induced nuclear translocation of p65 to a significant extent (79 and 60% inhibition, respectively). Dominant-negative forms of Cdc42 and Rho also exerted an inhibitory effect, but it was smaller than that exerted by Rac (data not shown). There is evidence that Ras can regulate Rac through activation of phosphatidylinositol 3-kinase (PI-3K) (4). Pretreatment of HUVECs with the specific inhibitor of PI-3K LY294002 prevented nuclear translocation of p65 upon adhesion to fibronectin (68% inhibition), suggesting that this process requires the coupling of Ras to Rac through PI-3K (Fig. 2d). Expression of either dominant-negative Ras (N17) or Rac (N17) did not inhibit TNF-α-induced p65 translocation, indicating that these small G proteins participate specifically in matrix-induced activation of NF-κB (Fig. 2d). This result is consistent with the observation that cytokine receptors activate NF-κB through TRAF proteins, independent of Ras signaling (9). Expression of an activated form of Rac (L61) increased nuclear accumulation of p65 by approximately threefold in cells adhering to laminin (data not shown). As anticipated, expression of the superrepressor of I-κBα, IκB-2A (a phosphorylation-defective mutant which inhibits NF-κB activation by retaining NF-κB subunits in the cytoplasm), suppressed nuclear translocation of p65 induced by fibronectin (81% inhibition). Treatment with the antioxidant PDTC or the IKK-β inhibitor, sodium salicylate, exerted a similar inhibition (60%) (Fig. 2d). In contrast, treatment with the MEK kinase inhibitor PD98059 did not affect nuclear translocation of p65 on fibronectin (not shown). Our findings in HUVECs suggest that the activation of NF-κB in response to adhesion to fibronectin requires Ras, PI-3K, Rac, and oxygen radicals. By contrast, previous studies had implicated Rac and reactive oxygen species in detachment-induced signaling to NF-κB in primary fibroblasts (26).
Inhibitors of NF-κB signaling suppress fibronectin-induced gene expression.
To further delineate the molecular pathways involved in the regulation of gene expression by adhesion to fibronectin, we performed a semiquantitative RT-PCR analysis of fibronectin-induced gene expression in HUVECs treated with a panel of pharmacological inhibitors (Fig. 1c). In these experiments, the HUVECs were not pretreated with cycloheximide. Exposure to the antioxidant and NF-κB signaling inhibitor PDTC prevented the fibronectin-induced accumulation of mRNAs encoding E-selectin, VCAM-1, CYR-61, endothelin 1, C-193, A20, and IRF-1. With the exception of CYR-61, the genes encoding these transcripts are controlled by NF-κB or induced by inflammatory cytokines, presumably through NF-κB. The IKK-β inhibitor sodium salicylate prevented the induction by fibronectin of approximately half of the above mRNAs, suggesting that some of the integrin-induced signals regulating NF-κB may not be transmitted through IKK-β. The MEK kinase inhibitor PD98059 blocked the upregulation by fibronectin of VCAM-1, C-193, A20, and IRF-1, but not of ELAM-1, CYR-61, and endothelin 1. Finally, the PI-3K inhibitor LY294002 and the JAK2 inhibitor AG-490 inhibited expression of the genes encoding ELAM-1, VCAM-1, C-193, A20, and IRF-1. These results indicate that NF-κB is necessary for transcription of a large fraction of genes induced upon HUVEC adhesion to fibronectin. However, they also suggest that other transcription factors, which are targets of integrin signals, participate in the regulation of distinct subsets of fibronectin-induced transcripts.
Inhibition of NF-κB suppresses bFGF-mediated angiogenesis.
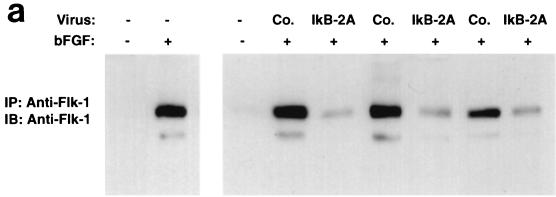
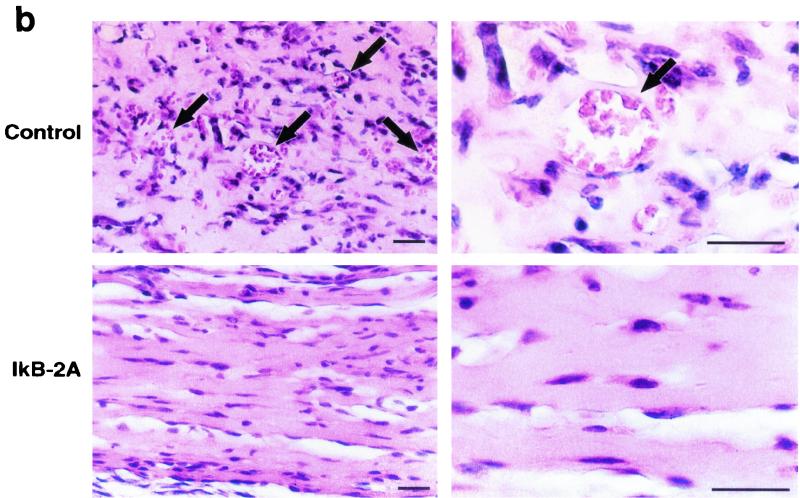
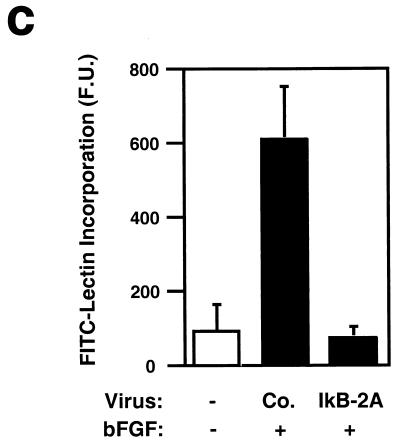
It is known that NF-κB is activated in rat aortic endothelial cells regenerating after injury (32). In addition, inhibition of NF-κB impairs capillary tube formation in vitro in response to hydrogen peroxide and 12-HETrE (46, 49). However, the role of NF-κB during angiogenesis in vivo has not been tested with specific inhibitors. To examine the role of NF-κB signaling in endothelial cell function in vivo, we induced angiogenesis in mice by subcutaneous injection of growth factor-depleted Matrigel supplemented with bFGF (36). We included in the Matrigel assay 293 HEK cells producing a control retrovirus or a retrovirus encoding the superrepressor of NF-κB, IκB-2A. Angiogenesis was quantitated by removing and extracting the Matrigel plugs and then subjecting equal amounts of total proteins to immunoprecipitation followed by immunoblotting with a VEGF receptor antibody (Flk-1). As shown in Fig. 3a, the IκB-2A retrovirus consistently suppressed bFGF-induced angiogenesis, as measured by Flk-1 expression. Histological analysis of paraffin-embedded sections revealed that the control virus did not interfere with the formation of mature blood vessels containing erythrocytes in response to bFGF (Fig. 3b). By contrast, such structures were almost completely absent in the samples containing 293 cells producing the IκB-2A retrovirus. As an independent means of quantifying the effect of IκB 2A on bFGF-induced angiogenesis, we measured the incorporation of an FITC-conjugated endothelial cell-binding lectin in the Matrigel plugs. As shown in Fig. 3c, the IκB-2A retrovirus suppressed FITC-lectin incorporation in bFGF-treated Matrigel plugs. These results strongly suggest that NF-κB signaling is required for bFGF-mediated angiogenesis.
FIG. 3.
Retroviral delivery of I-κBα superrepressor inhibits bFGF-induced angiogenesis in the Matrigel plug assay. (a) Mice were injected with growth factor-depleted Matrigel supplemented with bFGF and 293T cells infected with virus encoding the superrepressor I-κBα (IkB-2A) or vector alone (Co.). Angiogenesis was quantitated 5 days later by immunoprecipitating (IP) and immunoblotting (IB) equal amounts of total proteins from plug homogenates with a VEGF receptor antibody (Flk-1) that is specific for endothelial cells. The blot includes the results from three different experiments chosen as representatives of the seven performed. +, present; −, absent. (b) Paraffin sections of Matrigel plugs were stained with hematoxylin and eosin. Arrows indicate the formation of vessel-like structures containing erythrocytes in response to bFGF in plugs containing 293T cells expressing the control retrovirus. These structures are absent in the samples containing bFGF and 293T cells producing retrovirus encoding the I-κBα superrepressor (IkB-2A). Most of the interspersed nonendothelial cells present in the sections are 293T cells because they are lacking on sections derived from Matrigel plugs supplemented with bFGF but not 293T cells. These photographs are representative of those obtained from three different experiments. Bar, 8 μm. (c) Matrigel plugs from mice that had been injected intravenously with FITC-labeled lectin were homogenized, and identical aliquots were subjected to spectrophotometric analysis. The data are shown in arbitrary fluorescence units (F.U.). +, present; −, absent.
In conclusion, the results in this paper provide evidence that integrin signaling through NF-κB is sufficient to induce an angiogenic and proinflammatory phenotype in endothelial cells. Prior studies had indicated that integrin signals contribute to the regulation of individual genes or small groups of genes in monocytes and fibroblasts (14, 21, 24). Our present findings indicate that adhesion to fibronectin is sufficient to elicit a broad and complex NF-κB-dependent program of gene expression in endothelial cells. These results are in good agreement with those of a recent study conducted in monocytes (11) and provide a particularly vivid illustration of the ability of integrins to activate an entire gene expression program in the absence of concurrent signals. We have limited the scope of our search to genes directly targeted by integrin signals. However, many of the genes upregulated by fibronectin encode components of the NF-κB signaling pathway, thus providing a mechanism for amplification of the response. In addition, several other genes up- or downregulated on fibronectin encode signaling molecules with the potential of generating a second wave of transcription. Thus, it is likely that the full gene expression program elicited by the α5β1 integrin in endothelial cells in vivo is much larger and more complex than it can be anticipated from the present results.
The results of gene knockout and antibody inhibition experiments have established a role for the α5β1 integrin in vasculogenesis and angiogenesis (19, 27, 57). We find that most of the genes induced by α5β1 are activated through NF-κB and encode proteins involved in angiogenesis and inflammation, suggesting that signaling by α5β1 through NF-κB may help to coordinate these two related and often concurring processes (23). Ligation of the αvβ3 integrin, which is thought to be involved in angiogenesis, similarly activates NF-κB signaling (43). These findings suggest that integrin-mediated activation of NF-κB in endothelial cells plays a major role during angiogenesis and inflammation and potentially explain the results of gene knockout and antibody inhibition experiments.
We have used a dominant-negative approach to explore the mechanism by which the α5β1 integrin activates NF-κB in endothelial cells. Our results suggest central roles for Ras, PI-3K, Rac, and oxygen radicals in this process. By contrast, cytokine-mediated stimulation of NF-κB does not require Ras or Rac, as anticipated. Thus, the α5β1 integrin and cytokine receptors are each sufficient to activate NF-κB, and they do so through largely distinct signaling pathways. It is possible that ligation of α5β1 on endothelial cells by fibronectin in the interstitial matrix provides a mechanism to activate NF-κB when angiogenesis is not initiated by an inflammatory stimulus. Expression of the NF-κB-dependent gene expression program described here would then provide endothelial cells with the machinery required to recruit leukocytes and to acquire an additional source of proangiogenic and proinflammatory signals.
We have observed that retroviral expression of an inhibitor of NF-κB suppresses bFGF-induced angiogenesis in the Matrigel plug assay. By contrast, targeted deletion of various components of the NF-κB signaling system does not impair angiogenesis during development (16). Although it is possible that NF-κB plays a role only during postnatal angiogenesis, it is more likely that the genetic experiments underestimate the role of NF-κB during angiogenesis because of the overlapping functions of various NF-κB signaling components or of compensatory mechanisms.
Angiogenesis requires the coordinate interaction of endothelial cells with both extracellular matrix components and angiogenic growth factors (13, 22, 56). Since activation of the bFGF receptor does not activate NF-κB signaling, it is likely that the endothelial cells undergoing angiogenesis in the Matrigel plug assay activate NF-κB primarily in response to interaction with the matrix. Thus, our results imply that α5β1-mediated NF-κB signaling is important for angiogenesis.
In summary, the results of this study implicate the α5β1 integrin in the control of endothelial cell gene expression during angiogenesis and inflammation and potentially explain the crucial role of this integrin in these interrelated processes.
Acknowledgments
We thank I. Stancowsky and D. Baltimore for the plasmid encoding IκB-2A and the NF-κB-luciferase reporter vectors, J. Westwick for the vectors encoding activated Rac1 and Ras, J. Wood and D. Lyden for their advice, and M. Dans, A. Mariotti, A. Mettouchi, L. Gagnoux, and D. Alpert for many helpful discussions. We are also indebted to the staff of the Sloan-Kettering Institute Molecular Cytology Core Facility. A.D.F. and V.K. would like to thank P. Gotwals, J. Rosa, and R. Lobb for helpful discussions and support.
This study was supported by grants (to F.G.G.) and a fellowship (to S.K.) from the NIH. F.G.G. was an Established Investigator of the American Heart Association.
REFERENCES
- 1.Abramovitch, R., M. Niueman, R. Reich, I. Stein, E. Keshet, J. Abraham, A. Solomon, and M. Marinowsky. 1998. Intercellular communication between vascular smooth muscle and endothelial cells mediated by HB-EGF-like growth factor and VEGF. FEBS Lett. 425:441-447. [DOI] [PubMed] [Google Scholar]
- 2.Bader, B. L., H. Rayburn, D. Crowley, and R. O. Hynes. 1998. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all αv integrins. Cell 95:507-519. [DOI] [PubMed] [Google Scholar]
- 3.Baggiolini, M., B. Dewald, and B. Moser. 1997. Human chemokines: an update. Annu. Rev. Immunol. 15:675-705. [DOI] [PubMed] [Google Scholar]
- 4.Bar-Sagi, D., and A. Hall. 2000. Ras and Rho GTPases: a family reunion. Cell 103:227-238. [DOI] [PubMed] [Google Scholar]
- 5.Brizzi, M. F., P. Defilippi, A. Rosso, M. Venturino, G. Garbarino, A. Miyajima, L. Silengo, G. Tarone, and L. Pegoraro. 1999. Integrin-mediated adhesion of endothelial cells induces JAK2 and STAT5A activation: role in the control of c-fos gene expression. Mol. Biol. Cell 10:3463-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks, P. C., R. A. Clark, and D. A. Cheresh. 1994. Requirement of vascular integrin αvβ3 for angiogenesis. Science 264:569-571. [DOI] [PubMed] [Google Scholar]
- 7.Brooks, P. C., A. M. Montgomery, M. Rosenfeld, R. A. Reisfeld, T. Hu, G. Klier, and D. A. Cheresh. 1994. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 79:1157-1164. [DOI] [PubMed] [Google Scholar]
- 8.Bussolino, F., J. M. Wang, P. Defilippi, F. Turrini, F. Sanavio, C. J. Edgell, M. Aglietta, P. Arese, and A. Mantovani. 1989. Granulocyte- and granulocyte-macrophage-colony stimulating factors induce human endothelial cells to migrate and proliferate. Nature 337:471-473. [DOI] [PubMed] [Google Scholar]
- 9.Cao, Z., M. Tanaka, C. Regier, M. Rothe, A. Yamit-hezi, J. D. Woronicz, M. E. Fuentes, M. H. Durnin, S. A. Dalrymple, and D. V. Goeddel. 1999. NF-κB activation by tumor necrosis factor and interleukin-1. Cold Spring Harbor Symp. Quant. Biol. 64:473-483. [DOI] [PubMed] [Google Scholar]
- 10.Carmeliet, P., N. Mackman, L. Moons, T. Luther, P. Gressens, I. Van Vlaenderen, H. Demunck, M. Kasper, G. Breier, P. Evrard, M. Muller, W. Risau, T. Edgington, and D. Collen. 1996. Role of tissue factor in embryonic blood vessel development. Nature 383:73-75. [DOI] [PubMed] [Google Scholar]
- 11.De Fougerolles, A. R., G. Chi-Rosso, A. Bajardi, P. Gotwals, C. D. Green, and V. E. Koteliansky. 2000. Global expression analysis of extracellular matrix-integrin interactions in monocytes. Immunity 13:749-758. [DOI] [PubMed] [Google Scholar]
- 12.Dinarello, C. A. 1996. Biological basis for interleukin-1 in disease. Blood 87:2095-2147. [PubMed] [Google Scholar]
- 13.Eliceiri, B. P., and D. A. Cheresh. 2001. Adhesion events in angiogenesis. Curr. Opin. Cell Biol. 13:563-568. [DOI] [PubMed] [Google Scholar]
- 14.Fan, S., N. Mackman, M. Cui, and T. S. Edgington. 1995. Integrin regulation of an inflammatory effector gene: direct induction of the tissue factor promoter by engagement of β1 or α4 integrin chains. J. Immunol. 154:3266-3274. [PubMed] [Google Scholar]
- 15.George, E. L., L. E. Georges, K. R. Patel, H. Rayburn, and R. O. Hynes. 1993. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development 119:1079-1091. [DOI] [PubMed] [Google Scholar]
- 16.Gerondakis, S., M. Grossmann, Y. Nakamura, T. Pohl, and R. Grumont. 1999. Genetic approaches in mice to understand Rel/NF-κB and IκB function: transgenics and knock-outs. Oncogene 16:6888-6895. [DOI] [PubMed] [Google Scholar]
- 17.Giancotti, F. G., and E. Ruoslahti. 1999. Integrin signaling. Science 285:1028-1032. [DOI] [PubMed] [Google Scholar]
- 18.Giancotti, F. G. 2000. Complexity and specificity of integrin signaling. Nat. Cell Biol. 2:E13-E14. [DOI] [PubMed] [Google Scholar]
- 19.Goh, K. L., J. T. Yang, and R. O. Hynes. 1997. Mesodermal defects and cranial neural crest apoptosis in α5 integrin-null embryos. Development 124:4309-4319. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan, D. 1997. Signaling vascular morphogenesis and maintenance. Science 277:48-50. [DOI] [PubMed] [Google Scholar]
- 21.Huhtala, P., M. J. Humphries, J. B. McCarthy, P. M. Tremble, Z. Werb, and C. H. Damsky. 1995. Cooperative signaling by α5β1 and α4β1 integrins regulates metalloproteinase gene expression in fibroblasts adhering to fibronectin. J. Cell Biol. 129:867-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hynes, R. O., B. L. Bader, and K. Hodivala-Dilke. 1999. Integrins in vascular development. Braz. J. Med. Biol. Res. 32:501-510. [DOI] [PubMed] [Google Scholar]
- 23.Jackson, J. R., M. P. Seed, C. H. Kircher, D. A. Willoughby, and J. D. Winkler. 1997. The codependence of angiogenesis and chronic inflammation. FASEB J. 11: 457-465. [PubMed] [Google Scholar]
- 24.Juliano, R. L., and S. Haskill. 1993. Signal transduction from the extracellular matrix. J. Cell Biol. 120:577-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahn, J., F. Mehraban, G. Ingle, X. Xin, J. E. Bryant, J. Vehar, J. Schoenfeld, C. J. Grimaldi, F. Peale, A. Draksharapu, D. A. Lewin, and M. E. Gerritsen. 2000. Gene expression profiling in an in vitro model of angiogenesis. Am. J. Pathol. 156:1887-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kheradmand, F., E. Werner, P. Tremble, M. Simons, and Z. Werb. 1998. Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science 280:898-902. [DOI] [PubMed] [Google Scholar]
- 27.Kim, S., K. Bell, S. A. Mousa, and J. A. Varner. 2000. Regulation of angiogenesis in vivo by ligation of integrin α5β1 with the central cell-binding domain of fibronectin. Am. J. Pathol. 156:1345-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koch, A. E., P. J. Polverini, S. L. Kunkel, L. A. Harlow, L. A. DiPietro, V. M. Elner, S. G. Elner, and R. M. Strieter. 1992. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 258:1798-1801. [DOI] [PubMed] [Google Scholar]
- 29.Koch, A. E., M. M. Halloran, C. J. Haskell, M. R. Shah, and P. J. Polverini. 1995. Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule-1. Nature 376:517-519. [DOI] [PubMed] [Google Scholar]
- 30.Languino, L. R., K. R. Gehlsen, E. Wayner, W. G. Carter, E. Engvall, and E. Ruoslahti. 1989. Endothelial cells use α2β1 integrin as a laminin receptor. J. Cell Biol. 109:2455-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lau, L. F., and S. C.-T. Lam. 1999. The CCN family of angiogenic regulators: the integrin connection. Exp. Cell Res. 248:44-57. [DOI] [PubMed] [Google Scholar]
- 32.Lindner, V., and T. Collins. 1996. Expression of NF-κB and IκB-α by aortic endothelium in an arterial injury model. Am. J. Pathol. 148:427-438. [PMC free article] [PubMed] [Google Scholar]
- 33.Mettouchi, A., S. Klein, W. Guo, M. Lopez-Lago, E. Lemichez, J. K. Westwick, and F. G. Giancotti. 2001. Integrin-specific control of cell cycle progression mediated by Rac. Mol. Cell 8:115-127. [DOI] [PubMed] [Google Scholar]
- 34.Naldini, L., U. Blomer, P. Gallay, D. Ory, R. Mulligan, F. H. Gage, I. M. Verma, and D. Trono. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272:263-267. [DOI] [PubMed] [Google Scholar]
- 35.Pandey, A., H. Shao, R. M. Marks, P. J. Polverini, and V. M. Dixit. 1995. Role of B61, the ligand for the Eck receptor tyrosine kinase, in TNF-α-induced angiogenesis. Science 268:567-569. [DOI] [PubMed] [Google Scholar]
- 36.Passaniti, A., R. M. Taylor, R. Pili, Y. Guo, P. V. Long, J. A. Haney, R. R. Pauly, D. S. Grant, and G. R. Martin. 1992. A simple quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab. Investig. 67:519-528. [PubMed] [Google Scholar]
- 37.Pober, J. S. 1999. Immunobiology of human vascular endothelium. Immunol. Res. 19:225-232. [DOI] [PubMed] [Google Scholar]
- 38.Quarnström, E. E., C. O. Ostberg, G. L. Turck, C. A. Richardson, and K. Bomsztyk. 1994. Fibronectin attachment activates the NF-κB p50/p65 heterodimer in fibroblasts and smooth muscle cells. J. Biol. Chem. 269:30765-30768. [PubMed] [Google Scholar]
- 39.Renz, M., E. Tomlinson, B. Hultgren, N. Levin, Q. Gu, R. A. Shimkets, D. A. Lewein, and T. A. Stewart. 2000. Quantitative expression analysis of genes regulated by both obesity and leptin reveals a regulatory loop between leptin and pituitary-derived ACTH. J. Biol. Chem. 275:10429-10436. [DOI] [PubMed] [Google Scholar]
- 40.Risau, W. 1997. Mechanisms of angiogenesis. Nature 386:671-674. [DOI] [PubMed] [Google Scholar]
- 41.Risau, W., and V. Lemmon. 1988. Changes in the vascular extracellular matrix during embryonic vasculogenesis and angiogenesis. Dev. Biol. 125:441-450. [DOI] [PubMed] [Google Scholar]
- 42.Sarma, V., F. W. Wolf, R. M. Marks, T. B. Shows, and V. M. Dixit. 1992. Cloning of a novel tumor necrosis factor-α-inducible primary response gene that is differentially expressed in development and capillary tube-like formation in vitro. J. Immunol. 148:3302-3312. [PubMed] [Google Scholar]
- 43.Scatena, M., M. Almeida, M. L. Chaisson, N. Fausto, R. F. Nicosia, and C. M. Giachelli. 1998. NF-κB mediates αvβ3 integrin-induced endothelial cell survival. J. Cell Biol. 141:1083-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Senger, D. R., K. P. Claffey, J. E. Benes, C. A. Perruzzi, A. P. Sergiou, and M. Detmar. 1997. Angiogenesis promoted by vascular endothelial growth factor: regulation through α1β1 and α2β1 integrins. Proc. Natl. Acad. Sci. USA 94:13612-13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shimkets, R. A., D. J. Lowe, J. T. Tai, P. Sehl, H. Jin, R. Yang, P. F. Predki, P. E. Rothberg, M. T. Murtha, M. E. Roth, S. G. Shenoy, A. Windemuth, J. W. Simpson, J. F. Simons, M. P. Daley, S. A. Gold, M. P. McKenna, K. Hillan, G. T. Went, and J. M. Rothberg. 1999. Gene expression analysis by transcript profiling coupled to a gene database query. Nat. Biotechnol. 17:798-803. [DOI] [PubMed] [Google Scholar]
- 46.Shono, T., M. Ono, H. Izumi, S. I. Jimi, K. Matsushima, T. Okamoto, K. Kohno, and M. Kuwano. 1996. Involvement of the transcription factor NF-κB in tubular morphogenesis of human microvascular endothelial cells by oxidative stress. Mol. Cell. Biol. 16:4231-4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song, H. Y., M. Rothe, and D. V. Goeddel. 1996. The tumor necrosis factor-inducible zinc finger protein A20 interacts with TRAF1/TRAF2 and inhibits NF-κB activation. Proc. Natl. Acad. Sci. USA 93:6721-6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Springer, T. 1995. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 57:827-872. [DOI] [PubMed] [Google Scholar]
- 49.Stoltz, R. A., N. G. Abraham, and M. Laniado-Schwartzman. 1996. The role of NF-kB in the angiogenic response of coronary microvessel endothelial cells. Proc. Natl. Acad. Sci. USA 93:2832-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stromblad, S., J. C. Becker, M. Yebra, P. C. Brooks, and D. A. Cheresh. 1996. Suppression of p53 activity and p21WAF1/CIP1 expression by vascular cell integrin αvβ3 during angiogenesis. J. Clin. Investig. 98:426-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sunderkötter, C., K. Steinbrink, M. Goebeler, R. Bhardwaj, and C. Sorg. 1994. Macrophages and angiogenesis. J. Leukoc. Biol. 55:410-422. [DOI] [PubMed] [Google Scholar]
- 52.Tachibana, K., S. Hirota, H. Iizasa, H. Yoshida, K. Kawabata, Y. Kataoka, Y. Kitamura, K. Matsushima, N. Yoshida, S. Nishikawa, T. Kishimoto, and T. Nagasawa. 1998. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 393:591-594. [DOI] [PubMed] [Google Scholar]
- 53.Volk, R., J. J. Schwartz, J. Li, R. D. Rosenberg, and M. Simons. 1999. The role of syndecan cytoplasmic domain in basic fibroblast growth factor-dependent signal transduction. J. Biol. Chem. 274:24417-24424. [DOI] [PubMed] [Google Scholar]
- 54.Wary, K. K., F. Mainiero, S. Isakoff, E. E. Marcantonio, and F. G. Giancotti. 1996. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell 87:733-743. [DOI] [PubMed] [Google Scholar]
- 55.Yamanishi, S., C. C. Hsu, K. Kobayashi, and H. Yamamoto. 1993. Endothelin 1 mediates endothelial cell-dependent proliferation of vascular pericytes. Biochem. Biophys. Res. Commun. 191:840-846. [DOI] [PubMed] [Google Scholar]
- 56.Yancopoulos, G. D., S. Davis, N. W. Gale, J. C. Rudge, S. J. Wiegand, and J. Holash. 2000. Vascular-specific growth factors and blood vessel formation. Nature 407:242-248. [DOI] [PubMed] [Google Scholar]
- 57.Yang, J. T., H. Rayburn, and R. O. Hynes. 1993. Embryonic mesodermal defects in α5 integrin-deficient mice. Development 119:1093-1105. [DOI] [PubMed] [Google Scholar]