Abstract
Estrogen receptors are phosphoproteins which can be activated by ligands, kinase activators, or phosphatase inhibitors. Our previous study showed that p38 mitogen-activated protein kinase was involved in estrogen receptor activation by estrogens and MEKK1. Here, we report estrogen receptor-dependent p38 activation by estrogens in endometrial adenocarcinoma cells and in vitro and in vivo phosphorylation of the estrogen receptor α mediated through p38. The phosphorylation site was identified as threonine-311 (Thr311), located in helix 1 of the hormone-binding domain. The mutation of threonine-311 to alanine did not affect estrogen binding of the receptor but compromised its interaction with coactivators. Suppression of p38 activity or mutation of the site inhibited the estrogen-induced receptor nuclear localization as well as its transcriptional activation by estrogens and MEKK1. The inhibition of the p38 signal pathway by a specific chemical inhibitor blocked the biological activities of estrogens in regulating endogenous gene expression as well as endometrial cancer cell growth. Our studies demonstrate the role of estrogen receptor phosphorylation induced by the natural ligand in estrogen receptor's cellular distribution and its significant contribution to the growth-stimulating activity of estrogens in endometrial cancer cells.
Estrogens are female sex steroid hormones that control development, maintenance, and regulation of the female reproductive phenotype and behavior. They also stimulate the growth of normal and transformed epithelial cells of the female reproductive systems. The effect of estrogens is mediated through both estrogen receptors α and β (ERα and ERβ), which belong to the nuclear hormone receptor superfamily, a group of ligand-regulated, zinc finger-containing transcription factors (11, 40). The superfamily includes not only receptors for classical steroids such as estrogens, androgens, progesterones, and glucocorticoids, but also receptors for steroid analogues and nonsteroid ligands such as vitamin D, thyroid, and retinoic acids, as well as orphan receptors for which the ligand is unknown.
Unlike the thyroid and vitamin D receptors, which reside in the nucleus in the absence of ligands, receptors for classical steroids such as ERα are targeted to the nucleus after binding with estrogens or selective estrogen receptor modulators such as tamoxifen. In contrast, the pure ERα antagonist ICI 182,780 directs the ERα to the cytoplasm (6). Of importance in this respect are three clusters of basic amino acids, similar to the nuclear localization signals found in simian virus 40 large T antigen, which were identified in the DNA binding domain and the hinge region of ERα (44). The nuclear localization signals are constitutively active and do not seem to explain the estrogen effect on the ERα nuclear localization (44). It is therefore possible that estrogen-induced targeting of ERα to the nucleus is mediated through other mechanisms.
Studies in recent years have provided increasing evidence that nuclear localization is also controlled through nuclear export signals (17, 38, 42). A number of studies on exported proteins have shown that typical nuclear export signals are hydrophobic, leucine-rich sequences that signal the nuclear export complex containing exportin/Crm1 and RanGTP to transfer nuclear export signal-carrying proteins to the cytoplasm (8, 12, 29, 37).
Besides ligands, nonsteroid stimuli such as kinase activators, phosphatase inhibitors, neurotransmitters, and growth factors were also shown to activate the ERα (35). They either activate the receptor ligand independently or enhance the ligand-induced activity. Consistent with the cross talk with kinase/phosphatase pathways, ERα has been found to be phosphorylated at different sites by various kinases, including the external signal-regulated kinase (4, 19), cyclin A-CDK2 (32), c-SRC (25), protein kinase A (5), and pp90RSK1 (16). Except for tyrosine-537, all known ERα phosphorylation sites are on serine residues (1). Single-site mutation or simultaneous mutation at multiple sites reduced the transcriptional activity of the receptor (13). With the exception of the serine-236 phosphorylation by protein kinase A (5), most studies indicated the general role of ERα phosphorylation to be the regulation of the transcriptional activity of the receptor by modulating the interaction between the ERα activation domains and transcriptional coactivators (9, 28, 39).
In the present studies, we report that, in ERα-expressing endometrial cancer cells, 17β-estradiol activates the p38 mitogen-activated protein kinase (MAPK) pathway, which in turn mediates the phosphorylation of the ERα on threonine-311 (Thr311), promoting the receptor's nuclear localization and interaction with steroid receptor coactivators. Additional studies show that Thr311 phosphorylation in ERα is a critical determinant for its transcriptional and biological activities in endometrial cancer cells.
MATERIALS AND METHODS
Plasmids.
pLEN-hERα (2, 36), pLENβgal (36), Flag-p38 (43), SRαMEKK1 (CT) (26), and EREe1bLuc have been described (20, 36). pBind expression vectors for SRC1 and TIF2 have been described (24). T311A was generated by site-directed mutagenesis with the QuickChange mutagenesis kit (Stratagene, La Jolla, Calif.) and confirmed by direct sequencing. pLEN-hERα was used as the template for the mutagenesis. The primers for the PCR were synthetic oligonucleotides with the sequences 5′-CCTTGTCCCTGGCAGCCGACCAGATG-3′ and 5′-CATCTGGTCGGCTGCCAGGGACAAGG-3′.
Transfection and reporter assays.
Cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Transfection was performed with Lipofectamine (Gibco-BRL Life Technologies, Rockville, Md.) as described previously (20). After transfection, cells were placed in Dulbecco's modified Eagle's medium containing 5% charcoal-stripped fetal bovine serum, treated with different reagents, and assayed for luciferase and β-galactosidase activity as described previously (21). ERα transcriptional activity was measured by normalizing the luciferase activity to the corresponding β-galactosidase activity.
Immunoprecipitation and immunoblotting analysis.
Detection of Flag-p38 and ERα expression by immunoblotting has been described (20). To determine the level of Thr phosphorylation of ERα, cells were lysed in modified radioimmunoprecipitation assay (RIPA) buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 2 mM EDTA, 1% NP-40, 5 mM sodium fluoride, and protease inhibitor cocktail. The lysates were immunoprecipitated with F-10 anti-ERα monoclonal antibody (Santa Cruz Biotechnology Inc., Santa Cruz, Calif.). The ERα precipitates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE, 8% polyacrylamide), transferred to nitrocellulose, probed with the antiphosphothreonine antibody (Sigma, St. Louis, Mo.) overnight, and visualized with the ECL kit (Amersham Pharmacia Biotech Inc., Piscataway, N.J.).
To detect the binding of coactivators to ERα, Ishikawa cells were transfected with pBind-SRC1, pBind-TIF2, or pBind as a control vector together with either wild-type or mutant ERα. Following immunoprecipitation with anti-ERα F-10 antibody, coactivator in the precipitates was detected with anti-Gal4 DNA-binding domain antibody (Santa Cruz).
In vitro kinase assays.
p38 in vitro immunocomplex kinase assays were described before (20). For in vitro kinase assays with purified p38 kinase, recombinant ERα protein was incubated with 25 ng of active recombinant human p38α/SAPK2a (2.4 U/μg; Upstate Biotechnology, Lake Placid, N.Y.).
Phosphoamino acid analysis, phosphopeptide mapping, and Edman degradation.
Phosphoamino acid analysis, phosphopeptide mapping, and manual Edman degradation were performed as described previously (5, 13, 33).
Hormone binding assays.
Cells were incubated for 2 h with 3H-labeled 17β-estradiol (New England Nuclear, Boston, Mass.) at the indicated concentrations. To determine nonspecific binding, a 200-fold excess of unlabeled diethylstilbestrol (DES; Sigma, St. Louis, Mo.) was added in addition to 3H-labeled 17β-estradiol in a parallel set of samples. Cells were washed five times with ice-cold PBS and extracted in ethanol, and radioactivity was counted in a scintillation counter. Specific binding was calculated by subtracting nonspecific binding in cells incubated with excess DES from the total binding in cells incubated with [3H]17β-estradiol alone. Each data point was analyzed in duplicate.
Preparation of nuclear and cytoplasmic extracts.
For preparation of nuclear and cytosolic extracts, cells were scraped into hypotonic buffer containing 20 mM HEPES (pH 7.9), 1 mM EDTA, 1 mM EGTA, 20 mM NaF, 1 mM Na3VO4, 1 mM Na2P2O7, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 1% NP-40, and protease cocktail. Nuclei were separated from the cytosol by centrifugation at 11,750 × g for 20 min at 4°C. After centrifugation, pellets containing nuclei were resuspended in the same hypotonic buffer but containing 420 mM KCl and 20% glucose. To be representative of the ERα distribution in a cell, the same volume of buffer was used to prepare the nuclear and cytoplasmic fractions for each preparation, and equal volumes of cytosolic and nuclear extracts were subjected to immunoblotting analyses.
Immunofluorescence staining.
Ishikawa cells were seeded onto chamber slides and transfected with 0.1 μg of either wild-type or T311A mutant ERα expression constructs. Forty-eight hours later, the cells were fixed in 2% paraformaldehyde, permeabilized with phosphate-buffered saline (PBS) containing 1% Triton X-100 and 1% bovine serum albumin, and blocked in blocking buffer (PBS containing 1% bovine serum albumin and 0.1% NP-40). Cells were then incubated with anti-ERα antibody F-10. After extensive washing with PBS, anti-mouse immunoglobulin-fluorescein isothiocyanate (FITC) conjugate (Sigma, St. Louis, Mo.) was applied in blocking buffer as the secondary antibody. Nuclei were stained with 2′,6′-diamidino-2-phenylindole (DAPI) in antifade mounting medium (Vector laboratories, Burlingame, Calif.) before analysis under a fluorescence microscope.
Alkaline phosphatase and MTT assays.
Alkaline phosphatase activity was determined in ERα-positive Ishikawa cells as described previously (22). Cell growth was measured in methylthiazole tetrazolium (MTT) assays as described previously (21).
RESULTS
Activation of p38 by estrogens in endometrial adenocarcinoma cells.
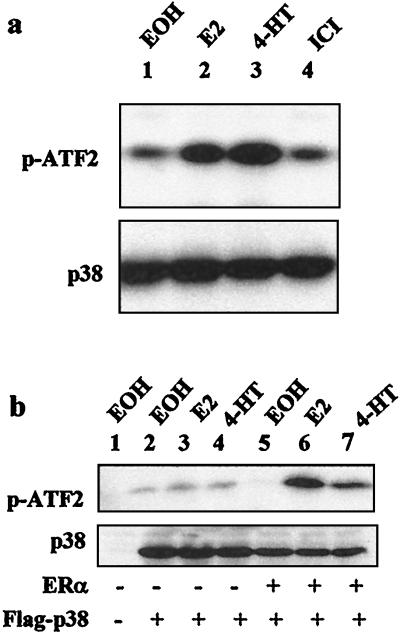
Our previous studies (20) showed that estrogens require p38 to activate ERα in endometrial cancer cells. To determine whether estrogens activate p38 in these cells, Ishikawa cells lacking ERα expression (20) were transfected with ERα and Flag-p38 expression vectors. Following treatment with 17β-estradiol, 4-hydroxytamoxifen, or ICI 182,780, the p38 protein was immunoprecipitated with the M2 anti-Flag antibody, and its activity was determined by immunocomplex kinase assays with glutathione S-transferase (GST)-ATF2 as a substrate. As shown in Fig. 1a, treatment with 17β-estradiol induced p38 activity (Fig. 1a, lane 2 of top panel) compared to the vehicle control (Fig. 1a, lane 1 of top panel). Interestingly, 4-hydroxytamoxifen also increased the p38 activity (Fig. 1a, lane 3 of top panel), supporting our previous data showing the involvement of the MEKK1-p38 MAPK pathway in the uterine-specific agonistic activity of tamoxifen (20). On the other hand, the pure ERα antagonist ICI 182,780 did not induce p38 activity (Fig. 1a, lane 4 of top panel). The level of p38 expression was not affected by the hormonal treatment (Fig. 1a, bottom panel), demonstrating that 17β-estradiol and 4-hydroxytamoxifen increased its specific activity.
FIG. 1.
17β-Estradiol and 4-hydroxytamoxifen activate p38 MAPK in endometrial cancer cells. (a) Activation of p38 by ERα agonists but not pure antagonist. ERα-negative Ishikawa cells were transfected with 0.5 μg of Flag-p38 and 0.5 μg of pLEN-hERα and treated with 10−8 M 17β-estradiol (E2), 10−6 M 4-hydroxytamoxifen (4-HT), 10−7 M ICI 182,780 (ICI), or ethanol (EOH) as a vehicle control for 30 min. The kinase activity was determined by immunocomplex kinase assays with GST-ATF2 as a substrate (upper panel). The level of Flag-p38 expression was determined by Western blotting with the M2 antibody (lower panel). p-ATF2, phosphorylated GST-ATF2. (b) ERα dependency of p38 activation by ERα agonists. ERα-negative Ishikawa cells were transfected with Flag-p38 and pLEN-hERα or control vectors. Cells were treated and the p38 activity was assayed as explained for panel a.
To show that p38 activation by estrogens requires ERα, the induction of p38 activity by 17β-estradiol or 4-hydroxytamoxifen was assayed in the ERα-negative Ishikawa cells transfected or not with ERα. As shown in Fig. 1b, 17β-estradiol and 4-hydroxytamoxifen did not affect p38 activity in the absence of ERα (Fig. 1b, lanes 3 and 4), whereas in ERα-transfected cells, treatment with either 17β-estradiol (Fig. 1b, lane 6) or 4-hydroxytamoxifen (Fig. 1b, lane 7) again increased p38 activity compared to the vehicle control (Fig. 1b, lane 5). No phosphorylation of ATF2 was detected in the absence of Flag-p38 (Fig. 1b, lane 1), showing the specificity of ATF2 phosphorylation to p38. These data demonstrate that ERα agonists activate p38 via ERα in endometrial cancer cells.
Phosphorylation of ERα by p38 immunocomplex but not recombinant p38 purified from bacteria.
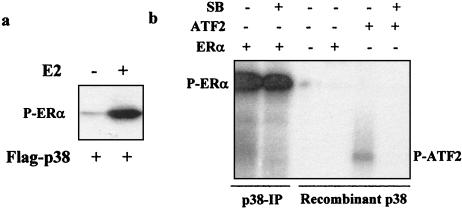
Since MEKK1-activated p38 immunoprecipitated from cells phosphorylated the recombinant ERα protein in in vitro immunocomplex kinase assays (20), we next tested whether p38 immunoprecipitates from estrogen-treated cells would also phosphorylate the ERα protein. ERα-negative Ishikawa cells were transfected with p38 and ERα expression vectors, and p38 immunocomplex kinase assays were performed as described for Fig. 1 with the exception that recombinant human ERα protein was used as a substrate. As shown in Fig. 2a, the ERα protein was weakly phosphorylated by the basal p38 immunoprecipitated from cells treated with vehicle (Fig. 2a, lane l). Treatment with 17β-estradiol induced ERα phosphorylation considerably (Fig. 2a, lane 2), suggesting that the ERα protein is a substrate for the p38 immunocomplex isolated from estrogen-treated cells.
FIG. 2.
Phosphorylation of purified ERα protein by p38 immunocomplex isolated from estrogen-treated cells. (a) ERα-negative Ishikawa cells were transfected with 0.5 μg of Flag-p38 and 0.5 μg of pLEN-hERα and treated with 17β-estradiol (E2) or not for 30 min. The phosphorylation of recombinant ERα protein by p38 immunoprecipitates was determined in in vitro immunocomplex kinase assays. P-ERα, phosphorylated ERα. (b) Lack of ERα phosphorylation by purified recombinant p38. Phosphorylation of recombinant ERα by p38 immunocomplex (p38-IP) or recombinant human p38α purified from bacteria in the presence or absence of 50 μM SB 203580 (SB) was determined. As controls, recombinant ATF2 was phosphorylated by purified p38, and the phosphorylation was inhibited by SB 203580.
To determine whether it is the p38 or an associated kinase that phosphorylates ERα, the phosphorylation of ERα protein was tested with active p38α purified from bacteria. As shown in Fig. 2b, while recombinant p38 phosphorylated ATF2, which was blocked by SB 203580, it did not phosphorylate ERα in parallel reactions. In parallel analysis, p38 immunocomplexes phosphorylated ERα protein, as expected, but p38 inhibitor added to the kinase reactions did not block this phosphorylation. These analyses suggest that it is a p38-associated kinase that phosphorylated ERα in the immunocomplex.
p38-mediated phosphorylation of ERα on Thr residues in vitro and in vivo.
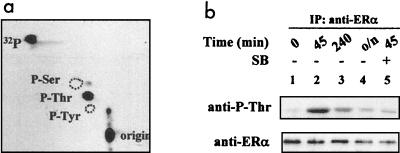
In previous studies (20), none of the presently known ERα phosphorylation sites were found to be required for ERα activation by MEKK1, suggesting that p38 MAPK may induce the phosphorylation of the ERα at novel sites. To determine which type of amino acid is phosphorylated, ERα protein phosphorylated in vitro by p38 immunocomplex (Fig. 2a, lane 2) was excised from the SDS-PAGE gel and subjected to phosphoamino acid analysis. The result revealed that ERα phosphorylation by p38 occurs exclusively on Thr residues (Fig. 3a).
FIG. 3.
ERα is phosphorylated by the p38 immunocomplex on Thr residues. (a) Phosphoamino acid analysis. ERα phosphorylated by the p38 immunocomplex was treated with HCl for hydrolysis, mixed with phosphoamino acid markers, and separated by two-dimensional electrophoresis on a TLC plate. The positions of phosphoamino acids from the markers (indicated by dashed circles) were revealed by ninhydrin spray, and 32P-radiolabeled phosphoamino acids from the sample were located by autoradiography. 32P, free 32P from the hydrolysis; P-Ser, phosphoserine; P-Thr, phosphothreonine; P-Tyr, phosphotyrosine. (b) p38-mediated ERα phosphorylation on Thr residues in vivo induced by 17β-estradiol. ERα-positive Ishikawa cells were treated with 10−8 M 17β-estradiol for the indicated times with 50 μM SB 203580 (SB) or vehicle. o/n, overnight. The Thr phosphorylation of endogenous ERα protein was detected by Western blotting with antiphosphothreonine antibody (anti-P-Thr) (upper panel) following immunoprecipitation with the F-10 anti-ERα antibody. The level of ERα in the immunoprecipitates was determined by immunoblotting with the same F-10 antibody (lower panel).
Since the p38 immunocomplex phosphorylated ERα in vitro exclusively on Thr residues, in contrast to all known ERα phosphorylation sites, we examined whether estrogens induce Thr phosphorylation of ERα in vivo with an antibody that specifically recognizes proteins containing phosphorylated Thr residues. ERα-positive Ishikawa cells (30) were treated with 17β-estradiol for various time periods. The endogenous ERα protein was then immunoprecipitated with an anti-ERα antibody and subjected to immunoblotting with either the anti-ERα or the antiphosphothreonine antibody. As shown in Fig. 3b, treatment with 17β-estradiol for 45 min resulted in a considerable increase in Thr phosphorylation of the endogenous ERα protein (Fig. 3b, lane 2) compared to the control (Fig. 3b, lane 1). The Thr phosphorylation returned to the basal level after the cells were treated for 4 h (Fig. 3b, lane 3) or overnight (Fig. 3b, lane 4), indicating that it is a transient event. Treatment of cells with SB 203580 blocked in vivo ERα phosphorylation on Thr residues (Fig. 3b, lane 5), demonstrating that the endogenous p38 MAPK pathway in endometrial cancer cells is required for the phosphorylation. Compared with the decreased level of the ERα protein (Fig. 3b, lower panel), the increase in the phosphothreonine signal at 45 min cannot be attributed to a change of ERα level and thus must represent an increase in specific Thr phosphorylation.
Identification of Thr311 as the p38-mediated phosphorylation site on ERα.
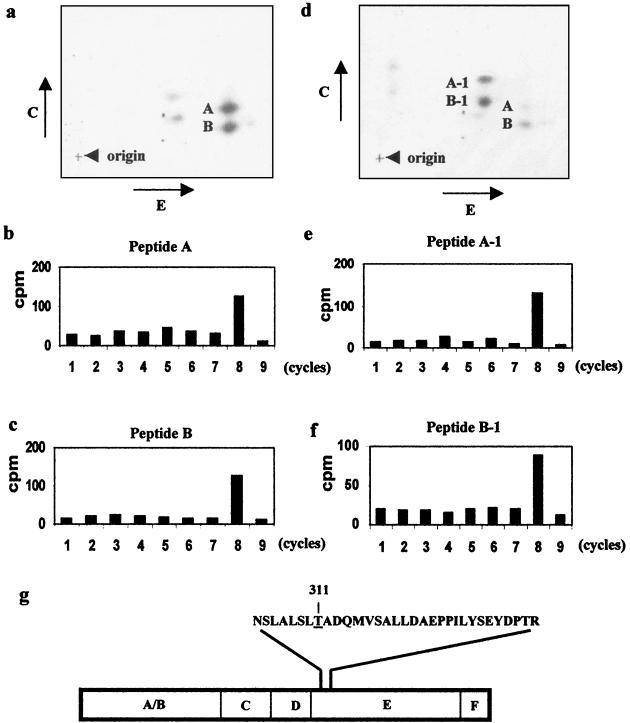
To determine which of the ERα Thr residues is phosphorylated, in vitro-phosphorylated ERα protein was eluted from the gel, digested with trypsin, and subjected to two-dimensional separation on thin-layer chromatography (TLC) plates. The phosphopeptide mapping analysis showed the presence of two major phosphopeptides (Fig. 4a), suggesting that p38 may phosphorylate the ERα at two separate sites. Alternatively, the two spots may represent isoforms or products of partial tryptic digestion of the same phosphopeptide. The minor spots in the chromatogram are likely due to phosphorylation of the ERα protein by background kinases contaminating the p38 immunoprecipitates.
FIG. 4.
Thr311 is the site phosphorylated by the p38 immunocomplex in vitro. (a) Phosphopeptide map of ERα after tryptic digestion. ERα phosphorylated in vitro by p38 was subjected to tryptic digestion and two-dimensional separation. The two major phosphopeptides were labeled A and B. E, electrophoresis; C, chromatography. (b and c) Edman degradation of tryptic peptides. Following autoradiography, peptides A and B were recovered from the TLC plate and subjected to manual Edman degradation. Radioactivity released from each cycle was plotted. (d) Phosphopeptide map of ERα after trypsin/Glu-C double digestion. Tryptic digests of phosphorylated ERα were cut with Glu-C and subjected to two-dimensional separation. The two major peptides were identified as A-1 and B-1. Note the presence of tryptic peptides A and B due to incomplete Glu-C digestion. (e and f) Edman degradation of phosphopeptides after double digestion. (g) Amino acid sequence of ERα tryptic peptide containing Thr311.
Following the tryptic mapping, each of the two major spots was recovered from the TLC plates and subjected to manual Edman degradation. As shown in Fig. 4b and c, both peptides released their 32P at cycle 8, indicating that the phosphorylated amino acids are at the eighth position counting from the amino terminus of the tryptic peptides.
Next, phosphorylated ERα protein was digested with both trypsin and a secondary endoproteinase, Glu-C or Asp-N, which cuts peptides at the carboxyl terminus of glutamate and the amino terminus of aspartate, respectively. As indicated by the altered migration of the peptides after double enzyme digestion, both phosphotryptic peptides were cleaved by Glu-C (Fig. 4d) and Asp-N (data not shown). This suggests that both peptides contain Glu and Asp residues in their sequences. Manual Edman degradation of the two peptides obtained by secondary digestion with either Glu-C (Fig. 4e and 4f) or Asp-N (data not shown) released the 32P at cycle 8, suggesting that the tryptic peptides contain no Asp or Glu residues at positions amino terminal to the phosphothreonine.
Based on the above analysis, the p38 phosphorylation site(s) should satisfy the following criteria. The site(s) is a Thr residue. It should be located at the eighth position from an arginine or lysine. The tryptic peptides should contain both Glu and Asp residues. The Glu and Asp residues must be located at positions carboxyl terminal but not amino terminal to the phosphothreonine. Examination of the ERα protein sequence revealed that only Thr311, located in the amino terminus of the ERα ligand-binding domain, satisfies all these criteria and is therefore the p38 phosphorylation site (Fig. 4g). It becomes obvious that the two major spots generated by protease digestions both contain Thr311, suggesting that they are most likely two isoforms of the same peptide.
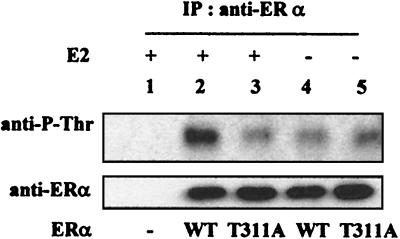
To determine whether Thr311 is the major threonine phosphorylated in vivo, we mutated the site to alanine and analyzed the threonine phosphorylation of the mutant ERα (Τ311A). As shown in Fig. 5, the mutation eliminated the 17β-estradiol-induced ERα phosphorylation on Thr residues (Fig. 5, upper panel) but did not affect the level of ERα expression (Fig. 5, lower panel), suggesting that Thr311 is the major site phosphorylated in vivo. Together with the data in Fig. 3b showing that the p38 inhibitor blocks 17β-estradiol-induced ERα phosphorylation on Thr residues, the analysis shows that the p38 pathway induced by estrogens mediates Thr311 phosphorylation in vivo.
FIG. 5.
Thr311 is phosphorylated in vivo. ERα-negative Ishikawa cells were transfected with 0.5 μg of pLEN-ERα expressing either wild-type (WT) or T311A mutant ERα, as indicated, and treated with 17β-estradiol (E2) or vehicle. The level of Thr phosphorylation was determined as for Fig. 2c. WT, wild-type ERα; T311A, ERα in which Thr311 was replaced with an alanine. IP, immunoprecipitation.
Regulation of ERα transcriptional activity by Thr311 phosphorylation.
Since our previous study showed that inhibition of p38 prevented estrogen- and MEKK1-induced ERα activation (20), it was expected that mutation of Thr311 would decrease the ERα activity and abolish the receptor's response to MEKK1 and p38. Therefore, the transcriptional activities of wild-type ERα and the T311A mutant were compared.
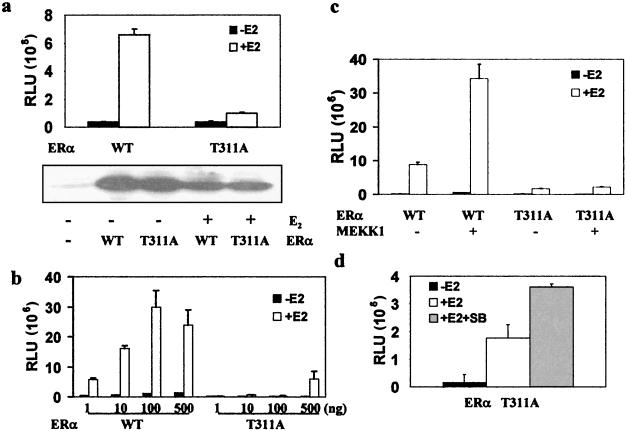
As shown in the upper panel of Fig. 6a, 17β-estradiol activated the wild-type ERα about 15-fold but induced negligible activity in the mutant ERα. Since the level of ERα expression was not affected by the mutation (Fig. 6a, lower panel), this demonstrates that the mutation decreased the specific activity of the receptor. The activity of the mutant receptor was essentially undetectable unless a very high level of the expression plasmids was transfected into the cells, at which point the activity of the wild-type receptor started decreasing (Fig. 6b), presumably due to the squelching effect of excessively expressed receptors.
FIG. 6.
Mutation of Thr311 to alanine decreases the transcriptional activity of ERα. (a) Lack of T311A activation by 17β-estradiol. ERα-negative Ishikawa cells were transfected with 0.1 μg of either wild-type (WT) or mutant (T311A) pLEN-hERα together with 0.5 μg of pLENβGal and 0.5 μg of EREe1bLuc and then treated overnight with 17β-estradiol (+E2) or vehicle (−E2). ERα transcriptional activity was normalized to β-galactosidase activity and is expressed as relative luciferase units (RLU) (upper panel), and the level of ERα protein was analyzed in parallel (lower panel). (b) Transcriptional activity of the T311A mutant at different dosages. Cells were transfected with 0.5 μg of EREe1bLuc, 0.1 μg of pLENβGal, and different amounts of ERα plasmid as indicated. ERα activity was determined as for panel a. (c) Lack of T311A activation by MEKK1. Cells were transfected with 0.5 μg of EREe1bLuc, 0.1 μg of pLEN-hERα, 0.5 μg of pLENβGal, and 0.2 μg of SRαMEKK1(CT). ERα activity was determined as for panel a. (d) Effect of SB 203580 on T311A. Cells were transfected with 0.5 μg of EREe1bLuc, 0.5 μg of pLENβGal, and 0.1 μg of pLEN-hERα expressing T311A and treated with 10−8 M 17β-estradiol and 50 μM SB 203580 or vehicle. ERα activity was determined as for panel a.
Further studies showed that the activity of the T311A mutant was not increased by MEKK1, whereas wild-type ERα was activated threefold by MEKK1 (Fig. 6c). In addition, SB 203580 did not decrease the activity of the T311A mutant (Fig. 6d), while the transcriptional activity of wild-type ERα induced by 17β-estradiol was decreased by 60% by the p38 inhibitor (data not shown). These studies demonstrate that Thr311 phosphorylation is required for ERα activation by both ligands and kinases.
Lack of effect of Thr311 mutation on receptor's ability to bind ligand.
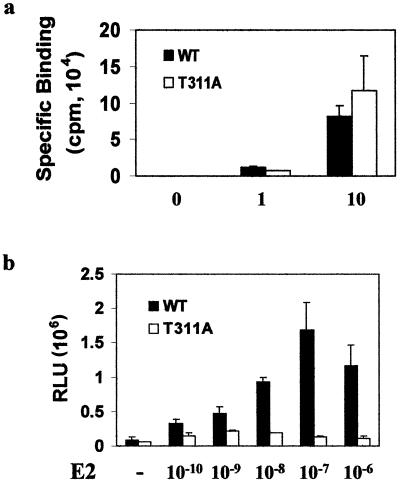
Since Thr311 is located in helix 1 of the receptor's ligand-binding domain, we first determined whether the mutation of Thr311 to alanine affected hormone binding. Wild-type or mutant ERα was transfected into ERα-negative Ishikawa cells, and hormone binding was determined after the cells were incubated with 3H-labeled 17β-estradiol at different concentrations. As shown in Fig. 7a, wild-type and mutant receptors exhibited no difference in specific estrogen binding at both saturating and nonsaturating doses. Consistent with the lack of an effect of the mutation on hormone binding activity, the decreased receptor activity caused by the mutation was not recovered by treatment with excess amounts of 17β-estradiol (Fig. 7b).
FIG. 7.
Decreased transcriptional activity of T311A mutant ERα is not due to a change in hormone binding ability. (a) Similar hormone binding abilities of wild-type (WT) and T311A ERαs. ERα-negative Ishikawa cells at a density of 106 cells/100-mm dish were transfected with 5 μg of wild-type or T311A mutant ERα. Forty-eight hours posttransfection, in vivo hormone binding was performed as described in the text. Specific binding was calculated by subtracting nonspecific binding ([3H]17β-estradiol plus a 200-fold excess of unlabeled DES) from total binding ([3H]17β-estradiol alone). The data are representative of three independent experiments, and each data point was analyzed in duplicate. (b) Transcriptional activity of the T311A mutant was not restored by treatment with excessive estrogen concentrations. ERα-negative Ishikawa cells were transfected as described for Fig. 6a and treated with 17β-estradiol (E2) at the indicated concentrations. ERα activity was determined as for Fig. 6a.
Regulation of ERα nuclear export by Thr311 phosphorylation.
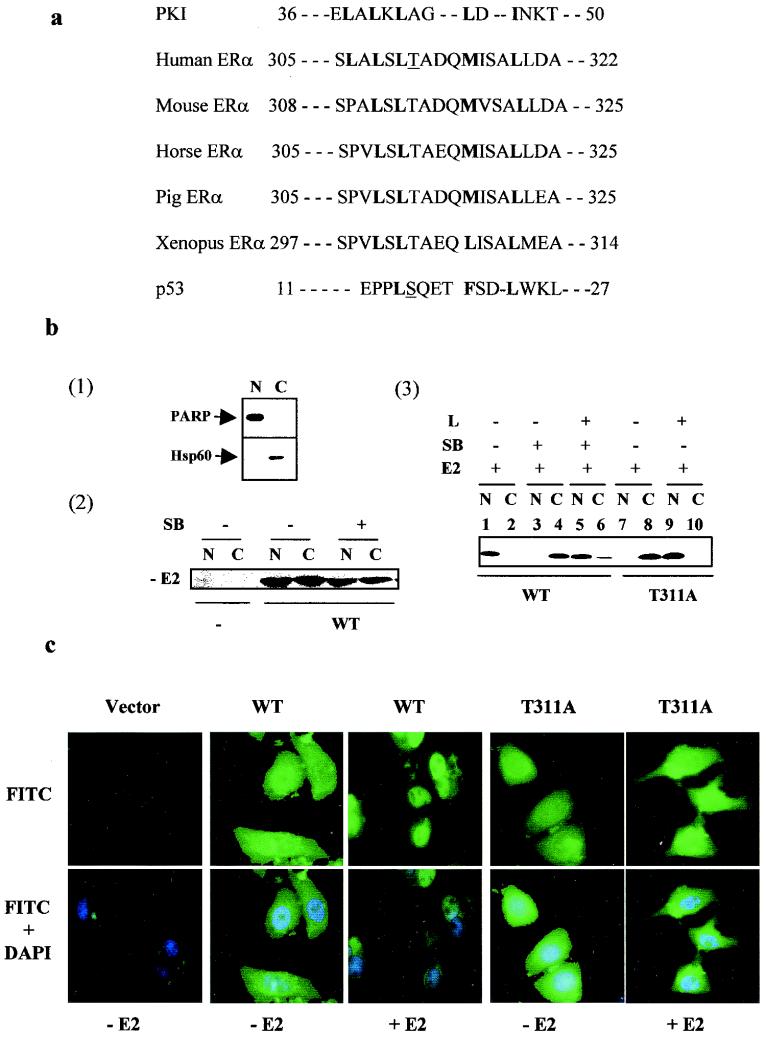
Nucleus-cytoplasm shuttling of proteins, including nuclear hormone receptors and their cofactors, has been shown to be regulated by phosphorylation (15, 18, 27, 47). In ERα, Thr311 appears to be located adjacent to the third nuclear localization signal (amino acids 299 to 303) (44) and within a putative nuclear export signal similar to that described for the inhibitor of protein kinase A (42) and p53 (46). The putative nuclear export signal is conserved in ERα from different species (Fig. 8a). Accordingly, it is possible that Thr311 phosphorylation controls the nuclear localization of ERα.
FIG. 8.
Nuclear export of ERα is regulated by Thr311 phosphorylation. (a) The putative leucine-rich nuclear export signal sequence that is highly conserved among ERαs from different species. The amino acids critical for the nuclear export signal function of the protein kinase I (PKI) and conserved in ERα and p53 are bold. Note the close similarity between the conserved Thr311 and serine-15 of p53, which are both underlined. (b) Panel 1, separation of nuclear and cytosolic proteins. Nuclear (N) and cytosolic (C) extracts were separated as described in the text and confirmed by immunoblotting with antibodies against known nuclear (PARP) and cytoplasmic (Hsp60) markers. Panel 2, lack of effect of p38 inhibition on ERα nuclear localization in the absence of estrogens. ERα-negative Ishikawa cells were transfected with parental vector or pLEN-hERα and treated with vehicle or SB 203580 for 45 min. Cellular localization of ERα was determined with anti-ERα antibody. Panel 3, regulation of Crm1-dependent nuclear export of ERα by p38 MAPK-mediated Thr311 phosphorylation. Cells were transfected with pLEN-hERα and treated with 17β-estradiol (E2), SB 203580 (SB), leptomycin B (L, 5 ng/ml), or a combination. Leptomycin B was added to the cells 30 min before treatment with 17β-estradiol or SB 203580. (c) Visualization of subcellular distribution of wild-type (WT) and T311A mutant ERαs by direct immunofluorescence staining. ERα-negative Ishikawa cells were plated on coverslips and transfected with vector or wild-type or T311A mutant ERα DNA. Forty-eight hours posttransfection, cells were treated with 17β-estradiol for 30 min and fixed. Following immunostaining as described in the text, ER subcellular distribution (FITC signal) and cell nucleus (DAPI signal) were visualized, and images were captured under a fluorescence microscope.
To address this possibility, ERα-negative Ishikawa cells were transfected with ERα vector and treated with SB 203580, and the nuclear-cytoplasmic distribution of the ERα was analyzed. As shown in Fig. 8b(1), poly(ADP-ribose) polymerase (PARP) and Hsp60 were clearly separated into the nuclear and cytosolic fractions under our conditions, demonstrating the efficiency of our fractionation procedure. As can be seen in Fig. 8b(2), in the absence of estrogens, ERα was evenly distributed between the nucleus and the cytoplasm, which is consistent with our data that p38 activity is induced by 17β-estradiol. No ERα signal was detected in cells transfected with the control vector, showing the ERα-negative status of the cells as well as the specificity of the antibody for the ERα. After 17β-estradiol treatments, the receptor was located in the nucleus, as shown in Fig. 8b(3), lanes 1 and 2, whereas it remained in the cytoplasm after cotreatment with the p38 inhibitor, as shown in Fig. 8b(3), lanes 3 and 4. This demonstrates that p38 is involved in ERα nuclear localization.
The p38 MAPK may regulate the nuclear localization of ERα by either increasing nuclear import or decreasing export. In order to distinguish between these possibilities, ERα-positive Ishikawa cells were treated with leptomycin B, a known exportin inhibitor, prior to analysis of the cellular distribution of endogenous ERα. As shown in Fig. 8b(3), lanes 5 and 6, leptomycin B restored the 17β-estradiol-induced ERα nuclear localization in the presence of the p38 inhibitor, demonstrating that phosphorylation mediated through p38 inhibits Crm1-dependent ERα nuclear export. Accordingly, mutation of Thr311 to alanine blocked estrogen-induced nuclear localization, as shown in Fig. 8b(3), lanes 7 and 8, which was restored by leptomycin B treatment, as shown in Fig. 8b(3), lanes 9 and 10. It appears, therefore, that it is nuclear export that is controlled by Thr311 phosphorylation of ERα mediated through the p38 MAPK pathway.
Since the separation of nucleus and cytoplasm by fractionation is considered to determine only nuclear retention, the cellular distribution of the wild-type and T311A mutant ERα forms was analyzed by direct immunofluorescence staining. As shown in Fig. 8c, treatment with 17β-estradiol induced the nuclear distribution of the wild-type receptor but not the T311A mutant. By comparing the FITC and DAPI signals, T311A in the presence of estrogens appeared to accumulate in the areas surrounding the nuclei. The exact meaning of this observation is unclear, but it may reflect signals from the newly exported mutant ERα which have not been fully dissociated from the exporting complex. The lack of FITC signal in cells transfected with the control vector demonstrated that the FITC signal is specific to the ERα.
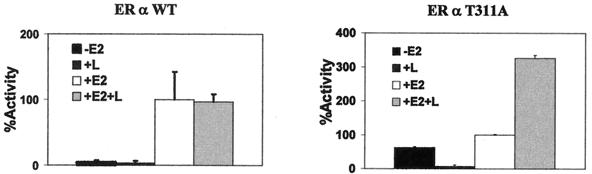
If the lack of transcriptional activity of T311A were due to its cytoplasmic dislocation, its restored nuclear localization would be expected to restore its transcriptional activity. The transcriptional activities of the wild-type ERα and the T311A mutant were measured in the presence and absence of leptomycin B (Fig. 9). While leptomycin B did not affect the estrogen-induced activity of wild-type ERα, the activity of the T311A mutant was increased threefold. The decreased basal activity might be due to the cytotoxicity of the drug. The lack of an effect of leptomycin B on the activity of the wild-type receptor was expected because, with respect to the effect on ERα localization, leptomycin B and ERα Thr311 phosphorylation should be redundant.
FIG. 9.
Exportin inhibitor increases the activity of T311A but not the wild-type (WT) ERα. ERα-negative Ishikawa cells were transfected with 0.5 μg of EREe1bLuc, 0.5 μg of pLENβGal, and 0.1 μg of pLEN-hERα expressing wild-type or T311A ERα and treated overnight with vehicle, 17 β-estradiol (E2), leptomycin B (L), or a combination. ERα activity was expressed as a percentage of the activity in cells treated with 10−8 M 17β-estradiol but without leptomycin B.
Regulation of ERα interaction with steroid receptor coactivators by Thr311 phosphorylation.
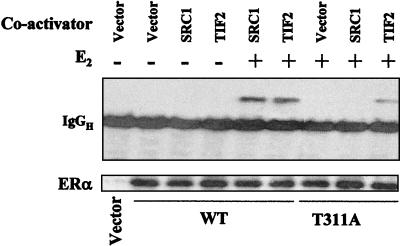
Although leptomycin B selectively increased the activity of the T311A mutant, the activity of the mutant in the presence of leptomycin B was still less than that of the wild type, suggesting that the mutation affected other steps of the receptor activation process in addition to its effect on estrogen-induced nuclear targeting. Therefore, the ability of the wild-type and mutant ERαs to bind members of the p160 SRC family was compared in coimmunoprecipitation assays. As shown in the upper panel of Fig. 10, the wild-type ERα coprecipitated with both SRC1 and TIF2 in an estrogen-dependent manner, while the T311A mutant showed no binding to SRC1 and significantly reduced binding to TIF2. In parallel studies, the expression levels of the wild-type ERα and the T311A mutant were comparable (Fig. 10, lower panel), demonstrating that the decreased interaction with steroid receptor coactivators and the selective effect of Thr311 mutation on the interaction with SRC1 are not due to variation in the level of receptor protein expression or variation in the efficiency of the immunoprecipitations.
FIG. 10.
Effect of T311A mutation on coactivator binding. Ishikawa cells were plated in 35-mm dishes at 3 × 105/dish and transfected with 0.5 μg of pBind-SRC-1 or pBind-TIF2 and 0.5 μg of wild-type (WT) or T311A mutant DNA. Cells were treated for 30 min with 17β-estradiol (E2) or not, and cellular extracts from duplicate dishes were pooled and subjected to immunoprecipitation with anti-ERα antibody, followed by immunoblotting with the anti-Gal4 DNA-binding domain (upper panel) or anti-ERα (lower panel) antibody.
Regulation of biological activity of ERα in endometrial cancer cells by p38 MAPK.
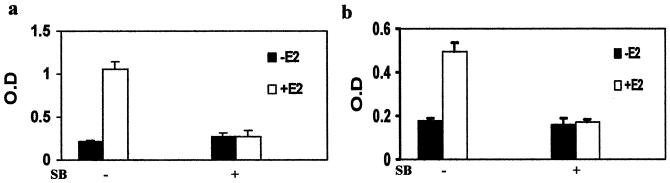
In endometrial cancer cells, estrogens have been shown to induce alkaline phosphatase (14). To demonstrate the significance of p38 phosphorylation on estrogen regulation of endogenous gene expression, alkaline phosphatase activity was measured in ERα-positive Ishikawa cells after estrogen treatment in the presence or absence of SB 203580. In the analysis, 17β-estradiol caused a threefold increase in alkaline phosphatase activity (Fig. 11a), which was blocked by SB 203580. The p38 inhibitor did not decrease the basal activity of alkaline phosphatase, indicating the absence of general cytotoxicity.
FIG. 11.
p38 MAPK signaling pathway regulates the biological activity of ERα in endometrial adenocarcinoma cells. (a) Inhibition of estrogen induction of alkaline phosphatase by p38 inhibitor. ERα-positive Ishikawa cells were treated for 72 h with vehicle, 17β-estradiol (E2), SB 203580 (SB), or a combination, and alkaline phosphatase activity was measured by colorimetric enzyme-linked immunosorbent assay. (b) Inhibition of estrogen stimulation of cell growth by the p38 inhibitor. ERα-positive Ishikawa cells were treated as for panel a for 4 days, and cell growth was measured by MTT assays. O.D., optical density.
To further demonstrate the significance of p38 phosphorylation in the biological function of the ERα, the growth response of the ERα-positive Ishikawa cells to estrogens was assayed in the presence and absence of the p38 inhibitor. As shown in Fig. 11b, 17β-estradiol stimulated the growth of the endometrial cancer cells, which was blocked by SB 203580. Taken together with the data from Fig. 11a, these findings support the important role of the p38 MAPK as a determinant for the activation of transcriptional activity of endogenous genes through the ERα as well as its biological importance in mediating the regulation of endometrial cancer cell growth.
DISCUSSION
In summary, our studies demonstrate that the signaling pathway mediated through p38 MAKP plays an important role in ER action in endometrial cancer cells. The pathway exerts its effect by inducing ERα phosphorylation at Thr311, the first threonine residue reported to be phosphorylated in ERα in response to estrogens. Although located in helix 1 of the ligand-binding domain, phosphorylation does not affect the ability of the receptor to bind the natural ligand. Instead, it blocks ERα nuclear export and promote the interaction of ERα with p160 steroid receptor coactivators, which may explain both the observed effect of the p38 MAPK pathway on the transcriptional activity of ERα in regulating endogenous gene expression as well as the biological activity of estrogens in stimulating endometrial cancer cell growth.
Although our studies clearly suggest that the p38 MAPK induced by the natural ERα agonist mediates Thr311 phosphorylation, it appears unlikely that p38 is the kinase that directly phosphorylates ERα at Thr311. First, the Ser-Pro motif is a feature shared by substrates for known MAPKs, and Thr311 is not followed by a proline. Second, SB 230580, when added directly to the kinase reactions, failed to block the in vitro phosphorylation of the recombinant ERα by the p38 immunocomplex (Fig. 2b), although the same inhibitor blocked estrogen stimulation of Thr311 phosphorylation in vivo and ATF-2 phosphorylation by purified human recombinant p38 kinase in vitro. Third, purified recombinant p38 did not phosphorylate ERα protein in in vitro kinase assays, whereas it phosphorylated ATF2 in parallel reactions (Fig. 2b). However, under the same conditions in which ERα phosphorylation by p38 immunocomplexes was demonstrated (Fig. 2a), large-scale affinity chromatography failed to detect any specific p38-bound protein after silver staining, even though the amount of purified p38 was readily detectable by Coomassie blue staining (data not shown). These studies suggest that the kinase phosphorylating Thr311 may be a member of the p38 family or a separate kinase that has a high specificity toward ERα but binds to p38 at a level too low to be detected by traditional protein-staining methods after affinity purification.
One of the main findings in these studies is that the transient ERα phosphorylation at Thr311 induced by the natural ligand is required for the stable localization of the ERα protein in the nucleus. The transient phosphorylation may initiate a more stable secondary event that keeps the receptor in the nucleus. For example, Thr311 phosphorylation may only prolong the retention of the liganded ERα in the nucleus, permitting more persistent binding of the receptor to nuclear protein or DNA components, which then obviates the requirement for phosphorylation.
From the data in Fig. 8, it appears that, in the presence of the p38 inhibitor, 17β-estradiol promotes the accumulation of ERα in the cytoplasm. It is conceivable that, in the absence of ligands, equilibrium between basal nuclear import and export determined the approximately equal distribution of ERα in the nucleus and cytoplasm in endometrial cancer cells. The binding of ligands accelerates both import and export activities, but nuclear export is blocked by the ligand-induced Thr311 phosphorylation. It is the suppression of nuclear export induced by ligand-activated receptor phosphorylation, instead of acceleration of import, that shifts the steady state in favor of the nuclear localization of the ERα. According to our data, the pure antagonist ICI 182,780 may target ERα to the cytoplasm because of the inability of the pure estrogen antagonist to activate the p38 MAPK.
A role of the p38 MAPK signaling pathway in promoting nuclear export of MAPK-AP kinase 2 (10) and the nuclear factor of activated T cells (NFAT) (7) has been described. On the other hand, p38 binds p53 and induces phosphorylation of serine-15 within the N-terminal nuclear export signal of p53 that blocks nuclear export (3, 46). As shown in Fig. 8, there is a close sequence homology between the leucine-rich nuclear export signal in the amino terminus of p53 and the putative nuclear export signal in the ERα containing Thr311. Therefore, the effect of Thr311 phosphorylation on the nuclear export of the ERα is likely due to an alteration in the function of the putative nuclear export signal. However, our data do not rule out the possibility that the phosphorylation may affect nuclear export through nuclear export signal-independent mechanisms. This is particularly true because the role of the putative nuclear export signal in the intracellular traffic of ERα has not been characterized, and the existence of a functional nuclear export signal in steroid receptors is controversial (23, 41). In the study of progesterone receptor nuclear export, sequences with homology to leucine-rich nuclear export signals do not appear to be functional (41). In addition, studies by Liu et al. (23) showed that glucocorticoid receptors did not utilize the exportin/Crm1 pathway for nuclear export.
Besides the effect on ER nuclear localization, our studies demonstrated that Thr311 phosphorylation of ERα regulates the receptor's interaction with steroid receptor coactivators. More interestingly, Thr311 phosphorylation preferentially affected the interaction with SRC-1, which has recently been shown to be expressed at a higher level in uterine than in breast cells and may contribute to the uterine-selective agonistic activity of tamoxifen (34). Our earlier studies demonstrated that MEKK1 increases the agonistic activity and decreases the antagonistic activity of 4-hydroxytamoxifen (20). Together with the stimulation of p38 activity by 4-hydroxytamoxifen shown in Fig. 1, our data support a role of the p38 MAPK signaling pathway in determining the tissue-selective agonistic activity of tamoxifen in endometrial cancer cells.
Our study is the first to link hormone, kinase pathway, specific receptor phosphorylation site, and receptor transcriptional activity with biological function in one comprehensive investigation. The activation of p38 by estrogens is fast and transient, obviously due to the nongenomic effect of the hormone. Taking all the data together, the study provides a good example of how the genomic and nongenomic effects of estrogens work together to fulfill biological functions. Consistent with our finding that the p38 pathway is positively involved in estrogen stimulation of endometrial cancer cell growth, studies by Razandi et al. (31) showed that estrogen activation of p38 is associated with protection of endothelial cells from hypoxia-induced apoptosis. However, Zhang et al. (45) reported that activation of p38 is coupled with ER-mediated apoptosis. The different data may be explained by the possibility that estrogen-induced p38 activity regulates estrogen action in different cells through distinct mechanisms. Consistent with this idea, studies by Razandi et al. (31) suggest that the protective effect of estrogen-activated p38 on vascular cells may be mediated through Hsp27 phosphorylation by MAPK-AP2, which is obviously different from the ERα phosphorylation by the p38 MAPK pathway proposed in our studies.
Acknowledgments
We thank Carolyn Smith and Zafar Nawaz for the pBind-SRC-1 and pBind-TIF2 expression vectors. We thank Paul Byvoet for critical reading of the manuscript and Edward Haller for processing the fluorescence pictures.
This study was supported by R29 grant CA79530 (W.B.) from the National Cancer Institute and a Conceptual Award DAMD17-01-1-0630 (W.B.) from the United States Department of Defense Breast Cancer Program.
REFERENCES
- 1.Arnold, S. F., J. D. Obourn, H. Jaffe, and A. C. Notides. 1994. Serine 167 is the major estradiol-induced phosphorylation site on the human estrogen receptor. Mol. Endocrinol. 8:1208-1214. [DOI] [PubMed] [Google Scholar]
- 2.Bai, W., B. Oliveros-Saunders, Q. Wang, M. E. Acevedo-Duncan, and S. V. Nicosia. 2000. Estrogen stimulation of ovarian surface epithelial cell proliferation. In Vitro Cell. Dev. Biol. Anim. 36:657-666. [DOI] [PubMed] [Google Scholar]
- 3.Bulavin, D. V., S. Saito, M. C. Hollander, K. Sakaguchi, C. W. Anderson, E. Apella, and A. J. Fornace, Jr. 1999. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 18:6845-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunone, G., P. A. Briand, R. J. Milsicek, and D. Picard. 1996. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 15:2174-2183. [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, D., P. E. Pace, C. Coombes, and S. Ali. 1999. Phosphorylation of human estrogen receptor α by protein kinase A regulates dimerization. Mol. Cell. Biol. 19:1002-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dauvois, S., R. White, and G. Parker. 1993. The antiestrogen ICI 182,780 disrupts estrogen receptor nucleocytoplasmic shuttling. J. Cell Sci. 106:1377-1388. [DOI] [PubMed] [Google Scholar]
- 7.del Arco, P. G., S. Martinez-Martinez, J. L. Maldonado, I. Ortega-Perez, and J. M. Redondo. 2000. A role for the p38 MAP kinase pathway in the nuclear shuttling of NFATp. J. Biol. Chem. 275:13872-13878. [DOI] [PubMed] [Google Scholar]
- 8.Elfgang, C., O. Rosorius, L. Hofer, H. Jaksche, J. Hauber, and D. Bevec. 1999. Evidence for specific nucleocytoplasmic transport pathways used by leucine-rich nuclear export signals. Proc. Natl. Acad. Sci. USA 96:6229-6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endoh, H., K. Maruyama, Y. Masuhiro, Y. Kobayashi, M. Goto, H. Tai, J. Yanagisawa, D. Metzger, S. Hashimoto, and S. Kato. 1999. Purification of p68 RNA helicase acting as a transcriptional coactivator specific for the activation function of human estrogen receptor α. Mol. Cell. Biol. 19:5363-5372. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Engel, K., A. Kotlyarov, and M. Gaestel. 1998. Leptomycin B-sensitive nuclear export of MAPKAP kinase 2 is regulated by phosphorylation. EMBO J. 17:3363-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans, R. M. 1988. The steroid and thyroid receptor superfamily. Science 240:889-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Formerod, M., M. Ohno, M. Yoshida, and L. Mattaj. 1997. Crm1 is an export receptor for leucine-rich nuclear export signals. Cell 90:1051-1060. [DOI] [PubMed] [Google Scholar]
- 13.Goff, P., M. M. Montano, D. J. Schodin, and B. S. Katzenellenbogen. 1994. Phosphorylation of the human estrogen receptor: Identification of hormone regulated sites and examination of their influence on transcriptional activity. J. Biol. Chem. 269:4458-4466. [PubMed] [Google Scholar]
- 14.Holinka, C. F., H. Hata, H. Kuramoto, and E. Gurpide. 1986. Effects of steroid hormones and antisteroids on alkaline phosphatase activity in human endometrial cancer cells (Ishikawa line). Cancer Res. 46:2771-2774. [PubMed] [Google Scholar]
- 15.Hong, S. H., and M. Privalsky. 2000. The SMRT corepressor is regulated by a MEK-1 kinase pathway: inhibition of corepressor function is associated with SMRT phosphorylation and nuclear export. Mol. Cell. Biol. 20:6612-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joel, P. B., J. Smith, T. Sturgill, T. L. Fisher, J. Blenis, and D. A. Lannigan. 1998. pp90rsk1 regulates estrogen receptor-mediated transcription through phosphorylation of Ser-167. Mol. Cell. Biol. 18:1978-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, C., A. V. Antwerp, and T. J. Hope. 1999. An N-terminal nuclear export signal is required for the nucleocytoplasmic shuttling of IκBα. EMBO J. 18:6682-6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katagiri, Y., K. Takeda, Z.-X. Yu, V. Ferrans, K. Ozato, and G. Guroff. 2000. Modulation of retinoid signaling through NGF-induced nuclear export of NGFI-B. Nat. Cell Biol. 2:435-440. [DOI] [PubMed] [Google Scholar]
- 19.Kato, S., H. Endoh, Y. Masuhiro, T. Kitamoto, S. Uchiyama, H. Sasaki, S. Masushigae, Y. Gotoh, E. Nishida, H. Kawashima, D. Metzger, and P. Chambon. 1995. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 270:1491-1494. [DOI] [PubMed] [Google Scholar]
- 20.Lee, H., F. Jiang, Q. Wang, S. V. Nicosia, J. Yang, B. Su, and W. Bai. 2000. MEKK1 activation of human estrogen receptor α and stimulation of the agonistic activity of 4-hydroxytamoxifen in endometrial and ovarian cancer cells. Mol. Endocrinol. 14:1882-1896. [DOI] [PubMed] [Google Scholar]
- 21.Li, P., S. V. Nicosia, and W. Bai. 2001. Antagonism between PTEN/MMAC1/TEP-1 and androgen receptor in growth and apoptosis of prostatic cancer cells. J. Biol. Chem. 276:20444-20450. [DOI] [PubMed] [Google Scholar]
- 22.Littlefield, B. A., E. Gurpide, L. Markiewicz, B. McKinley, and R. B. Hochberg. 1990. A simple and sensitive microtiter plate estrogen bioassay based on stimulation of alkaline phosphatase in Ishikawa cells. Endocrinology 127:2757-2762. [DOI] [PubMed] [Google Scholar]
- 23.Liu, J., and D. B. DeFranco. 1999. Chromatin recycling of glucocorticoid receptors: implications for multiple roles of heat shock protein 90. Mol. Endocrinol. 13:355-365. [DOI] [PubMed] [Google Scholar]
- 24.Lonard, D. M., Z. Nawaz, C. L. Smith, and B. O'Malley. 2000. The 26S proteasome is required for estrogen receptor-α and coactivator turnover and for efficient estrogen receptor-α transactivation. Mol. Cell 5:939-948. [DOI] [PubMed] [Google Scholar]
- 25.Migliaccio, A., M. Di Domenico, G. Castoria, A. de Falco, P. Bontempo, E. Nola, and F. F. Auricchio. 1996. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 15:1292-1300. [PMC free article] [PubMed] [Google Scholar]
- 26.Minden, A., A. Lin, M. McMahon, C. Lange-Carter, B. Derjard, R. J. Davis, G. L. Johnson, and M. Karin. 1994. Differential activation of ERK and JNK mitogen activated protein kinases by Ras-1 and MEKK. Science 266:1719-1723. [DOI] [PubMed] [Google Scholar]
- 27.Nigg, E. A. 1997. Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature 386:779-787. [DOI] [PubMed] [Google Scholar]
- 28.Onate, S. A., V. Boonyaratanakornkit, T. E. Spencer, S. Y. Tsai, M.-J. Tsai, D. P. Edwards, and B. W. O'Malley. 1998. The steroid receptor coactivator-1 contains multiple receptor interacting and activation domains that cooperatively enhance the activation function 1 (AF1) and AF2 domains of steroid receptors. J. Biol. Chem. 273:12101-12108. [DOI] [PubMed] [Google Scholar]
- 29.Ossareh-Nazzari, B., F. Bachelerie, and C. Dargemont. 1997. Evidence for a role of Crm1 in signal-mediated nuclear protein export. Science 278:141-144. [DOI] [PubMed] [Google Scholar]
- 30.Pinelli, D. M., J. Drake, M. C. Williams, D. Cavanagh, and J. L. Becker. 1998. Hormonal modulation of Ishikawa cells during three-dimensional growth in vitro. J. Soc. Gynecol. Investig. 5:217-223. [DOI] [PubMed] [Google Scholar]
- 31.Razandi, M., A. Pedram, and E. R. Levin. 2000. Estrogen signals to the preservation of endothelial cell form and function. J. Biol. Chem. 275:38540-38546. [DOI] [PubMed] [Google Scholar]
- 32.Rogatsky, I., J. M. Trowbridge, and M. J. Garabedian. 1999. Potentiation of human estrogen receptor transcriptional activation through phosphorylation of serines 104 and 106 by the cyclin A-CDK2 complex. J. Biol. Chem. 274:22296-22302. [DOI] [PubMed] [Google Scholar]
- 33.Rowan, B. G., N. L. Weigel, and B. W. O'Malley. 2000. Phosphorylation of steroid receptor coactivator-1. J. Biol. Chem. 275:4475-4483. [DOI] [PubMed] [Google Scholar]
- 34.Shang, Y., and M. Brown. 2002. Molecular determinants for the tissue specificity of SERMs. Science 295:2465-2468. [DOI] [PubMed] [Google Scholar]
- 35.Smith, C. L. 1998. Cross-talk between peptide growth factor and estrogen signaling pathways. Biol. Rep. 58:627-632. [DOI] [PubMed] [Google Scholar]
- 36.Smith, C. L., O. M. Conneely, and B. M. O'Malley. 1993. Modulation of the ligand-independent activation of the human estrogen receptor α by hormone and antihormone. Proc. Natl. Acad. Sci. USA 90:6120-6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stade, K., C. S. Ford, C. Guthrie, and K. Weis. 1997. Exportin 1 (Crm1) is an essential nuclear export factor. Cell 90:1041-1050. [DOI] [PubMed] [Google Scholar]
- 38.Stommel, J. M., N. D. Marchenko, G. S. Jimenez, U. Moll, T. J. Hope, and G. M. Wahl. 1999. A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by nuclear export signal masking. EMBO J. 18:1660-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suen, C. S., T. J. Berrodin, R. Mastroeni, B. J. Cheskis, C. R. Lyttle, and D. E. Frail. 1998. A transcriptional coactivator, steroid receptor coactivator-3, selectively augments steroid receptor transcriptional activity. J. Biol. Chem. 273:27645-27653. [DOI] [PubMed] [Google Scholar]
- 40.Tsai, M. J., and B. W. O'Malley. 1994. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 63:451-486. [DOI] [PubMed] [Google Scholar]
- 41.Tyagi, R. K., L. Amazit, P. Lescop, E. Milgrom, and A. Guiochon-Mantel. 1998. Mechanisms of progesterone receptor export from nuclei: role of nuclear localization signal, nuclear export signal, and Ran guanosine triphosphate. Mol. Endocrinol. 12:1684-1695. [DOI] [PubMed] [Google Scholar]
- 42.Wen, W., J. L. Meinkoth, R. Y. Tsien, and S. Taylor. 1995. Identification of a signal for rapid export of proteins from the nucleus. Cell 82:463-473. [DOI] [PubMed] [Google Scholar]
- 43.Yang, J., L. New, Y. Jiang, J. Han, and B. Su. 1998. Molecular cloning and characterization of a human protein kinase that specifically activates c-Jun N-terminal kinase. Gene 212:95-102. [DOI] [PubMed] [Google Scholar]
- 44.Yikomi, T., M. T. Bocquel, M. Berry, H. Gronemeyer, and P. Chambon. 1992. Cooperation of proto-signals for nuclear accumulation of estrogen and progesterone receptors. EMBO J. 11:3681-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, C. C., and D. J. Shapiro. 2000. Activation of the p38 mitogen-activated protein kinase pathway by estrogen or by 4-hydroxytamoxifen is coupled to estrogen receptor-induced apoptosis. J. Biol. Chem. 275:479-486. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, Y., and Y. Xiong. 2001. A p53 amino-terminal nuclear export signal inhibited by DNA damage-induced phosphorylation. Science 292:1910-1915. [DOI] [PubMed] [Google Scholar]
- 47.Zhou, X., V. M. Richon, A. H. Wang, X.-J. Yang, R. A. Rifkind, and P. Marks. 2000. Histone deacetylase 4 associates with extracellular signal-regulated kinases 1 and 2, and its cellular localization is regulated by oncogenic Ras. Proc. Natl. Acad. Sci. USA 97:14329-14333. [DOI] [PMC free article] [PubMed] [Google Scholar]