Abstract
While hyperosmolality of the kidney medulla is essential for urinary concentration, it imposes a great deal of stress. Cells in the renal medulla adapt to the stress of hypertonicity (hyperosmotic salt) by accumulating organic osmolytes. Tonicity-responsive enhancer (TonE) binding protein (TonEBP) (or NFAT5) stimulates transcription of transporters and a synthetic enzyme for the cellular accumulation of organic osmolytes. We found that dominant-negative TonEBP reduced expression of HSP70 as well as the transporters and enzyme. Near the major histocompatibility complex class III locus, there are three HSP70 genes named HSP70-1, HSP70-2, and HSC70t. While HSP70-1 and HSP70-2 were heat inducible, only HSP70-2 was induced by hypertonicity. In the 5′ flanking region of the HSP70-2 gene, there are three sites for TonEBP binding. In cells transfected with a reporter plasmid containing this region, expression of luciferase was markedly stimulated in response to hypertonicity. Coexpression of the dominant-negative TonEBP reduced the luciferase expression. Mutating all three sites in the reporter plasmid led to a complete loss of induction by hypertonicity. Thus, TonEBP rather than heat shock factor stimulates transcription of the HSP70-2 gene in response to hypertonicity. We conclude that TonEBP is a master regulator of the renal medulla for cellular protection against high osmolality via organic osmolytes and molecular chaperones.
Genetic studies of hypertension have shown that fluid reabsorption in the kidney is essential for maintaining proper blood pressure (21). The driving force for water reabsorption in the renal medullary collecting duct is provided by the high osmolarity of the interstitium, the degree of which changes widely depending on the hydration status of the animal (12). In the inner medulla of rat kidney, the tissue osmolality routinely rises over 2,500 mosmol/kg and as high as over 4,000 mosmol/kg when the animal is severely dehydrated.
Salt and urea are primary solutes in the interstitial fluid of mammalian kidney medulla (1). In rat kidney medulla under most physiological conditions, urea concentration varies widely from less than 100 mM to over 2,000 mM while salt concentration varies relatively less—from ∼250 to ∼500 mM. Salt and urea differ in their effects on cell volume due to differences in membrane permeability. Hyperosmolar salt, i.e., NaCl concentration greater than 150 mM, is hypertonic in that cells shrink in it due to osmosis. On the other hand, cells do not shrink appreciably when ambient osmolarity is raised by addition of urea because membrane permeability of urea is comparable to that of water, and therefore, osmosis does not occur. Hypertonicity and a high concentration of urea each imposes a distinct form of stress to cells. When a cell is exposed to high concentrations (>300 mM) of urea, cells die via apoptosis in a dose-dependent manner (see below for more). On the other hand, hypertonicity causes an immediate increase in cellular ionic strength as a result of osmosis (19). The rise in intracellular ionic strength causes double-stranded DNA breaks (17), which in turn cause apoptosis and activation of p53 (5, 25). When challenged with severe hypertonicity (osmolality greater than 650 mosmol/kg), a cell goes through apoptosis outright. On the other hand, in response to mild hypertonicity, activated p53 prevents apoptosis. In addition, cell cycle arrest and DNA repair pathways are activated (6, 18), presumably representing checkpoint regulation for repair of the damaged DNA. A cell can adapt to the high concentrations of salt and urea observed in the renal medulla when the tonicity and urea are increased progressively over time (39).
Long-term adaptation to hypertonicity is achieved by cellular accumulation of organic osmolytes (also called compatible osmolytes) that results in lowering of the intracellular ionic strength via osmotic replacement (for a recent review, see reference 8). Cellular accumulation of compatible osmolytes blunts activation of caspases and apoptosis in hypertonic conditions (10). On the other hand, when the accumulation of organic osmolytes is prevented, cell death occurs in hypertonic culture conditions (13) and in the hypertonic kidney medulla, leading to deadly acute renal failure (14). The accumulation of organic osmoloytes is regulated in large part by the transcription factor tonicity-responsive enhancer (TonE) binding protein (TonEBP) (30). TonEBP stimulates genes coding for transporters and an enzyme that catalyzes cellular accumulation of organic osmolytes via concentrative uptake across plasma membrane and synthesis, respectively: the sodium/myo-inositol cotransporter (SMIT) (40), the sodium/chloride/betaine cotransporter (BGT1) (28), and aldose reductase (AR) (15). The abundance of TonEBP (3) and mRNA of SMIT, BGT1, and AR (27) is much higher in the hypertonic renal medulla compared to the renal cortex or other tissues.
TonEBP is also called nuclear factor of activated T cells (NFAT5) or NFAT-related protein (NFATL1) because its expression is markedly induced in T cells following activation of the T-cell receptors (44). TonEBP directly stimulates transcription of several cytokines, including lymphotoxin-β, tumor necrosis factor alpha, and possibly interleukin-8 (23). As such, TonEBP might play a major role in T-cell activation following hypertonic saline infusion that is often used to treat trauma patients (22). Thus, tonicity-responsive regulation of TonEBP is not limited to the kidney medulla.
Recent studies revealed that TonEBP also plays a major role in generating the high concentration of urea in the renal medulla. TonEBP stimulates transcription of the vasopressin-regulated urea transporter (UT-A) that is exclusively expressed in the renal medulla (35). UT-A facilitates the countercurrent accumulation of urea between the ascending limb of Henle's loop and the collecting ducts. Thus, hypertonicity contributes to the generation of the high urea concentration in the renal medulla via stimulation of TonEBP.
As discussed earlier, high concentrations of urea causes apoptosis (25, 47). Heat shock protein 70 (HSP70) appears to play a major role in adaptation to a high level of urea. Hypertonicity induces expression of HSP70 (38). The abundance of HSP70 is over 20-fold higher in the hypertonic renal medulla than in the isotonic cortex (33). A number of experiments have demonstrated that cell survival under a high level of urea increases dramatically as a function of HSP70 expression. Increased expression of HSP70 by treatment with hypertonicity (41) or by stable transfection of HSP70 cDNA (37) promotes cell survival in the presence of a high level of urea, while forced down regulation of HSP70 by using antisense nucleotides (36) renders cells more susceptible to death by urea. In this study, we demonstrate that a major form of heat-inducible HSP70, named HSP70-2, is stimulated by hypertonicity by virtue of TonEBP binding to the 5′ flanking region. Thus, TonEBP is involved in protecting cells from the high urea of the renal medulla in addition to its buildup.
MATERIALS AND METHODS
Cell culture.
mIMCD cells (39) were cultured in 45% Dulbecco's modified Eagle's medium, 45% F12 medium, 10% fetal bovine serum, 2 mM glutamine, and 1% streptomycin/penicillin. MDCK cells were maintained in defined medium made by equal mixing of Dulbecco's modified Eagle's medium and Coon's modified Ham's F-12 medium with additives described previously (46). Hypertonic medium was made by addition of 100 mM NaCl. For heat shock experiments, confluent cells were shifted to 42°C for 2 to 4 h.
Northern blot analysis.
RNA was isolated using Trizol reagent (Life Technologies, Rockville, Md.). Then, 5 μg of RNA from each sample was separated on an 1% agarose gel containing 2.2 M formaldehyde and transferred onto a nitrocellulose membrane. Membranes were hybridized overnight with radiolabeled cDNA probes: human HSP70 (GenBank accession number M11717), canine SMIT (M85068), canine BGT1 (M80403), human AR (J05474), human cyclooxygenase 2 (COX-2) (M90100), mouse αB-crystallin (M63170), and human glyceraldehyde 3-phosphate dehydrogenase (X01677). After washing under stringent conditions (60°C in 75 mM NaCl, 7.5 mM Na3 citrate, and 0.1% sodium dodecyl sulfate), radioactivity was detected using a Phosphorimager (Molecular Dynamics, Sunnyvale, Calif.).
Immunoblot analysis.
Cells were lysed for 30 min at 4°C in a lysis buffer (50 mM Tris-Cl, pH 7.6, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100) with freshly added protease inhibitors: 0.2 μg of aprotinin/ml, 5 μM leupeptin, 1 mM phenylmethylsulfonyl fluoride, and 10 μM E64. After clearing by centrifugation, an aliquot containing 40 μg of protein from each sample was separated on an sodium dodecyl sulfate-7% polyacrylamide gel and blotted onto a nitrocellulose membrane. To detect a specific protein, the blots were incubated with an antiserum or antibody at a 4,000-fold dilution for 1 h in 20 mM Tris-HCl, pH 7.6, 150 mM NaCl, 0.1% Tween 20, and 5% nonfat milk. Monoclonal antibodies for HSP70 and HSC70 were obtained from Stressgene (Victoria, British Columbia, Canada), and polyclonal antiserum against TonEBP was described previously (30). The blots were then incubated with a secondary antibody conjugated with alkaline phosphatase and visualized using a commercial substrate for alkaline phosphatase (Sigma Chemical, St. Louis, Mo.).
Cell lines expressing DN-TonEBP.
Mammalian expression vector pcDNA3.1 (Invitrogen, Carlsbad, Calif.) driving expression of dominant-negative TonEBP (DN-TonEBP) (30), a truncated TonEBP containing the N-terminal amino acids 1 to 472, was transfected into MDCK cells using Lipofectamine (Life Technologies). Colonies were selected in a medium containing 400 μg of G418/ml. More than five colonies that expressed the DN-TonEBP were obtained. Three colonies transfected with empty pcDNA3.1 were mixed and used as vector control.
RPA.
In order to generate RNase protection assay (RPA) probes specific for the three isoforms of HSP70 (see Fig. 3), segments of the genes were cloned by PCR amplification of mouse genomic DNA based on an available genomic sequence (GenBank accession number AF109906). Primers for the PCR cloning were ATGGACGGGATCTCAACAAG and GACGTTCAGGATACCGTTGG for HSC70t, CATCTCCTGGCTGGACTCCA and CCACGTGCAATACACAAAGTAACTG for HSP70-1, and CATCTCCTGGCTGGACTCCA and TATATGCATACAAAATTTAACAGTC for HSP70-2. RPA was performed using a commercial kit according to the instructions from the manufacturer (Ambion, Austin, Tex.). Radioactivity of protected bands was quantified using the PhosphorImager.
FIG. 3.
RPA to detect expression of specific HSP70 isoforms. (A) Genomic structure near the mouse major histocompatibility complex class III region is shown. The rectangles and vertical bar represent exons. HSC70t consists of two exons while HSP70-1 and HSP70-2 each consists of one exon. Bent arrows show the direction of transcription. The region that contains TonE sequences (TonEs) is indicated with a bar. (B) RNA samples of Fig. 1 were analyzed to detect isoform-specific transcripts as indicated.
Cloning of HSP70-2 promoter and site-directed mutagenesis.
Mouse genomic DNA covering nucleotide positions −4,148 to −43 relative to the start codon of the HSP70-2 gene was cloned by PCR as described above using primers CATTGGGTAGCCAAGGAAAA and GATGCTCCGGGGAAAGTT. The PCR product was cloned into pCRII-TOPO (Invitrogen) and sequenced for verification. The active TonE's in the promoter were individually inactivated by site-directed mutagenesis using the Quick Change XL site-directed mutagenesis kit (Stratagene, La Jolla, Calif.): TGGAAANNYNY to TttAAANNYNY (mutated nucleotides are indicated by lowercase letters). All the mutations were verified by sequencing. Where indicated, individual mutants were pasted to generate promoters with various combinations of multiple mutants (see Fig. 6). The wild-type and mutant versions of the promoter were moved pGL2 basic vector (Promega, Madison, Wis.) to generate Photinus luciferase reporter constructs.
FIG. 6.
Role of TonE in activity of HSP70-2 promoter. TonEA, TonEB, and TonED in the reporter plasmid described in Fig. 5 was inactivated by site-directed mutagenesis in various combinations as shown. Mutated TonE's are indicated by filled ovals. Relative luciferase activity in mIMCD cells cultured in isotonic and hypertonic medium is shown. Data are mean ± standard deviation (n = 3).
EMSA.
Nuclear extracts were prepared from confluent MDCK cells cultured in isotonic or hypertonic medium as described previously (43). To prepare probes for electrophoretic mobility shift assays (EMSA), single-stranded oligonucleotides were synthesized and purified (Genetic Core Facility, Johns Hopkins University). To obtain a double-stranded probe, 200 pmol of each complementary oligonucleotide was annealed in 100 μl containing 150 mM NaCl, 10 mM MgCl2, and 50 mM Tris-Cl, pH 7.9. Then, 10 μg of protein of nuclear extract was incubated for 10 min at 25°C in 20 μl containing 20 mM HEPES, pH 7.9, 50 mM KCl, 1 mM EDTA, 5% glycerol, 1 mM dithiothreitol, 5 mM MgCl2, and 1.5 μg of poly(dA-dT). After that, 10 fmol of 32P-labeled probe was added and the mixture was incubated for an additional 20 min. The reaction mixtures were electrophoresed on a 4.5% polyacrylamide gel in a buffer containing 45 mM Tris, 45 mM borate, and 1 mM EDTA. Radioactivity of the gels was visualized and quantified using the PhosphorImager.
Transfection and analysis of luciferase expression.
The HSP70-2 promoter constructs were transfected into mIMCD cells using Lipofectamine 2000 (Life Technologies). Each construct (0.1 μg) was transfected with 0.01 μg of pRL-SV40, in which the simian virus 40 (SV40) promoter drove expression of the Renilla luciferase. After transfection, the cells were maintained in isotonic medium for 24 h and then switched to hypertonic medium or were maintained in isotonic medium for another 24 h. For heat shock treatment, transfected cells were maintained at 37°C for 24 h, switched to 42°C for 2 h, and returned to 37°C for 6 h before analysis. Activity of the Photinus and Renilla luciferase in extracts of the transfected cells was determined using a commercial kit, Dual-Luciferase Reporter Assay System (Promega). For each sample, the activity of the Photinus luciferase is divided by the activity of the Renilla luciferase to correct for transfection efficiency. The corrected Photinus luciferase activity of experimental constructs was expressed relative to that of the β-actin promoter-driven Photinus luciferase, as previously described (43).
RESULTS
Induction of HSP70 by hypertonicity.
We examined induction of HSP70 in response to hypertonicity in confluent monolayers of mIMCD cells, an epithelial cell line established from the renal medulla of transgenic mice expressing the SV40 T antigen (39). Raising osmolality of the culture medium by addition of 100 mM NaCl led to a 5.8-fold increase in HSP70 mRNA abundance and a 2.5-fold increase in HSP70 protein over the course of 4 h (n = 4; Fig. 1). This induction was due to hypertonicity rather than hyperosmolality because addition of 200 mM urea to the control medium did not affect HSP70 expression (not shown) as reported earlier (42). The induction by hypertonicity was slower and smaller in magnitude than the induction by heat (42°C), which led to a 24-fold increase in mRNA and a 5-fold increase in protein in 4 h (n = 4). As expected, expression of the noninducible isoform HSC70 was not affected by heat or hypertonicity.
FIG. 1.
Expression of HSP70 and TonEBP in mIMCD cells. Confluent mIMCD cells were treated with hypertonic medium (lane HT; made by addition of 100 mM NaCl), were heat shocked (lane HS; 42°C), or were untreated (C) for 4 h. Then, 5 μg of total RNA was used for Northern blot detection of HSP70 mRNA (top) and 40 μg of protein of cell lysate was used for immunoblot detection of HSP70, HSC70, and TonEBP.
DN-TonEBP reduces expression of HSP70 mRNA.
Heat shock did not increase the abundance of TonEBP (Fig. 1, bottom) nor induce other TonEBP target genes, such as SMIT and AR (not shown). On the other hand, hypertonicity induced the abundance of TonEBP (Fig. 1, bottom) and its activity (not shown) as reported earlier (30, 45), raising the possibility that TonEBP might play a role in the induction of HSP70 by hypertonicity. To explore this, we generated several lines of MDCK cells, an epithelial cell line from canine kidney, that express DN-TonEBP, an N-terminal truncated TonEBP (30). Cells of one of the lines, named R6, and cells transfected with vector alone (V) are shown in Fig. 2. In R6 and V cells, TonEBP expression was comparable in magnitude and inducible by hypertonicity (Fig. 2A). Increased abundance of DN-TonEBP in hypertonic medium was due to stimulation of the cytomegalovirus promoter by hypertonicity (unpublished observation). Northern blot analyses of mRNA for SMIT, BGT1, and AR revealed that the mRNA abundance decreased by 70 to 80% (P < 0.05 for all three) in R6 cells compared to V cells in hypertonic conditions, confirming the role of TonEBP in hypertonicity induction of these genes. Under isotonic conditions, the mRNA abundance of SMIT, BGT1, and AR also decreased slightly (10 to 20%) but consistently in R6 cells compared to V cells, in keeping with our previous observation that TonEBP is active in isotonicity (45). DN-TonEBP contains the DNA binding domain (30) but lacks the transactivation domains in the C terminus (23). Furthermore, the abundance of TonEBP was not affected by expression of DN-TonEBP (Fig. 2A). Thus, the inhibitory effects of DN-TonEBP must be due to competition with endogenous TonEBP for binding to the TonE sites.
FIG. 2.
Effects of DN-TonEBP on mRNA expression. MDCK cells stably transfected with pcDNA3.1 (lanes V) or pcDNA3.1 driving expression of DN-TonEBP (lanes R6, a clonal line) were cultured overnight in control isotonic (lanes C) or hypertonic medium (lanes HT). (A) Cell lysate containing 40 μg of protein was used in each lane for immunoblot analysis of TonEBP. Bands representing TonEBP and DN-TonEBP are indicated on the right. (B) Five micrograms of RNA was used for Northern blot detection of mRNA for SMIT, BGT1, AR, HSP70, COX-2, αB-crystallin, and glyceraldehyde 3-phosphate dehydrogenase.
Next, we examined other genes whose expression was known to be higher in the hypertonic renal medulla compared to the isotonic renal cortex. The mRNA abundance of αB-crystallin was not affected by hypertonicity or expression of DN-TonEBP. Although the COX-2 mRNA was induced by hypertonicity, it was not reduced by expression of DN-TonEBP. On the other hand, the abundance of HSP70 mRNA was reduced by 50% in isotonicity (P < 0.05, n = 4) and 42% in hypertonicity (P < 0.05, n = 4) by expression of DN-TonEBP. Similar observations were made in other MDCK cell lines expressing DN-TonEBP (not shown), removing doubts that these changes were due to clonal variation. We conclude that TonEBP is involved in the transcription of heat-inducible HSP70 in addition to SMIT, BGT1, and AR.
HSP70-2 is regulated by tonicity.
A human HSP70 cDNA (GenBank accession number M11717; 11) was used for the Northern probe shown in Fig. 2 and for the antigen to raise the commercial antibody used in the immunsoblot analysis in Fig. 1. Within the major histocompatibility complex class III locus in chromosome 6, there is a cluster of three genes in the HSP70 family—HSP70-1, HSP70-2, and HSC70t (26). A genomic clone containing this region was sequenced (GenBank accession number AF134726). HSP70-1 and HSP70-2 are almost identical in that only two amino acids out of 641 differ. Nucleotide sequence comparison suggests that the M11717 cDNA represent HSP70-1. However, the M11717 probe should detect mRNA of both HSP70-1 and HSP70-2 due to greater than 99% identity in nucleotide sequence in the open reading frames. We used the mouse cell line mIMCD to determine which isoform—HSP70-1 or HSP70-2—was induced by hypertonicity because the gene cluster is conserved in the mouse genome (GenBank accession number AF109906; Fig. 3A). In mouse, HSP70-1 and HSP70-2 differ by one amino acid. In order to discriminate mRNA for HSP70-1, HSP70-2, and HSC70t, specific RPA probes were made by PCR from the divergent 3′ untranslated regions as described in Materials and Methods. As shown in Fig. 3B, HSC70t mRNA was not detected in all conditions. The mRNA for HSP70-1 was in very low abundance and was often undetectable under control conditions. In contrast, the HSP70-2 mRNA was detected consistently, albeit at low levels. The mRNA for both HSP70-1 and HSP70-2 were vigorously induced by heat shock as reported previously (26). In response to hypertonicity, the HSP70-2 mRNA but not HSP70-1 was consistently induced. We conclude that the increase in HSP70 mRNA in response to hypertonicity (Fig. 1 and 2) is due to HSP70-2.
TonE sites in 5′ flanking region of HSP70-2 gene.
Reduced expression of the HSP70 mRNA by DN-TonEBP (Fig. 2B) indicated that there might be sites for TonEBP binding in the promoter of the HSP70-2 gene. We examined 4 kb upstream of the gene and found four sites named TonEA to TonED that fit the consensus of TonE (40), as shown in Fig. 4A. In EMSA, all the TonE's formed a complex that was competed by an active TonE but not by an inactive TonE (Fig. 4B). This complex contained TonEBP because it was specifically supershifted by a TonEBP antibody (Fig. 4B, lanes PI and IM). The affinity of the TonE's to TonEBP was determined by a competition assay shown in Fig. 4C. TonEC displayed the lowest affinity in correlation the weakest binding to TonEBP (Fig. 4B). Since the apparent affinity (Kd) of TonEC was significantly lower than 50 nM, TonEC was not expected to be active (29).
FIG. 4.
TonE's found upstream of the HSP70-2 gene and their binding to TonEBP. (A) Sequences and locations (relative to the start codon of the HSP70-2 gene) of four sequences (TonEA to TonED) that fit the TonE consensus. (B) EMSA was performed using 0.5 nM (each) 32P-labeled hTonE, TonEA, TonEB, TonEC, and TonED. Nuclear extracts were isolated from confluent MDCK cells treated with isotonic (lane C) or hypertonic medium (lanes HT) overnight. In some lanes, 50 nM hTonE (lane WT [TGGAAAATTAC]) or inactive mutant hTonE (lane m [TGGAtAATTAC]) was added as a competitor. TonEBP antiserum (lane IM) or preimmune serum (lane PI) was used to confirm the presence of TonEBP in the bands. (C) In order to compare their affinity to TonEBP, 50 nM hTonE (lane WT), mutant hTonE (lane m), TonEA (lane A), TonEB (lane B), TonEC (lane C), or TonED (lane D) was used to compete with 0.5 nM 32P-hTonE for TonEBP binding.
Stimulation of HSP70-2 promoter by TonE.
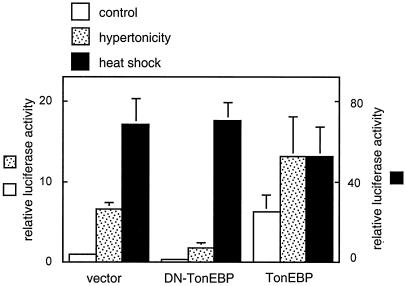
In order to examine the activity of the TonE sites upstream of the HSP70-2 gene, a luciferase reporter construct containing ∼4 kb of the 5′ flanking region was made. In mIMCD cells transfected with the construct, luciferase expression was stimulated over 60-fold by heat shock, as expected (Fig. 5). This heat induction of luciferase was not affected by coexpression of the DN-TonEBP or a full-length TonEBP, indicating that TonEBP was not involved. Of interest, the luciferase expression increased 7.5-fold when the cells were cultured in hypertonic medium. Coexpression of the DN-TonEBP reduced the expression of luciferase both in isotonic and hypertonic conditions while coexpression of a full-length TonEBP increased the luciferase expression only in isotonic conditions. These results are the same as for other TonE-driven luciferase constructs (24, 30), indicating that the TonE/TonEBP system was responsible for the luciferase expression in isotonic and hypertonic conditions. In order to investigate the role of the TonEA, TonEB, and TonED, these sites were mutated to inactivate their activity as shown in Fig. 6. Mutation of all the three TonE sites completely obliterated the induction by hypertonicity indicating that TonE was an essential cis-element. When one or two TonE was mutated, the induction was progressively lower indicating that all the three TonE's contributed to the induction by hypertonicity. In addition, luciferase expression in isotonicity was also lower as the TonE's were mutated. Take together, the data in Fig. 5 and 6 demonstrated that TonEA, TonEB, and TonED stimulated the promoter of the HSP70-2 gene.
FIG. 5.
Activity of HSP70-2 promoter in response to hypertonicity and heat shock. A 4.1-kb genomic DNA fragment covering nucleotides −4,158 to −43 upstream of the start codon was cloned into a luciferase reporter vector. mIMCD cells were transfected with the reporter plasmid along with pcDNA3.1 (vector) or pcDNA3.1 driving expression of DN-TonEBP or TonEBP as indicated. The transfected cells were treated with hypertonic medium or heat shock. The activity of luciferase is shown relative to the vector control. Data are the mean ± standard deviation (n = 4). Note that the scale for the heat shock-treated group is shown on the right.
DISCUSSION
The heat-induced stimulation of HSP70 transcription has been the paradigm for investigation of transcriptional response to environmental stresses. HSP70 and other heat-inducible molecular chaperones respond to accumulation of nonnative (unfolded, misfolded, or aggregates) proteins (31). A family of proteins called heat shock factor (HSF) are activated by the nonnative proteins that accumulate in response to a variety of insults, including heat. Activated HSF binds to heat shock element (HSE) and stimulates transcription of HSP70 and other chaperones. There are three major isoforms of HSP70 that are induced vigorously by heat shock—HSP70-1, HSP70-2, and HSP70B′. HSP70B′ shares 81% of amino acid identity with HSP70-1 or HSP70-2 (20). Each of the three genes is encoded by a single exon, enhancing the efficiency and speed of the induction. HSP70-1 and HSP70-2 are clustered in the major histocompatibility complex class III locus in chromosome 6 (Fig. 3) while HSP70B′ is in chromosome 1 (annotated by the Human Genome project with GenBank accession number XP033999).
In this study, we have shown that HSP70-2 but not HSP70-1 is specifically induced by hypertonicity (Fig. 3). The data presented in Fig. 5 and 6 demonstrate that the promoter of HSP70-2 is specifically stimulated in response to hypertonicity by virtue of the TonEBP binding rather than HSF binding. The heat-responsive HSF proteins are not activated in response to hypertonicity because the promoter of HSP70-1 (Fig. 3) and other heat-inducible chaperones (not shown) are not stimulated. On the other hand, heat does not activate TonEBP (see Results). Thus, the signal for the induction of HSP70-2 by hypertonicity, i.e., signal for stimulation of TonEBP, is not related to the accumulation of nonnative proteins.
There is another example of HSP70 regulation that does not appear to involve the HSF. The promoter of HSP70B′ is stimulated when 50 mM KCl is added to culture medium (7). Hypertonicity plays a minimal role in that addition of 50 mM NaCl results in a much smaller stimulation of the promoter. Instead, Ca2+ influx triggered by the elevated K+ concentration was necessary for the stimulation. HSF is not stimulated since other heat-inducible chaperones are not upregulated. The presumptive transcription factor that responds to the Ca2+ influx is not known.
In addition to HSP70, other heat shock proteins have been examined for their induction by hypertonicity. A small heat shock protein (αB-crystallin [4]), a HSP40 called HLJ1 (34), and a couple of the HSP110 members (Osp94 [16] and HSP105B [42]) are induced by hypertonicity. More importantly, their expression is higher in the hypertonic kidney medulla than the isotonic kidney cortex. In MDCK cells, αB-crystallin is not induced by hypertonicity (Fig. 2) and Osp94 expression is very low (not shown). TonEBP does not appear to play a role in the induction of HLJ1 based on sensitivity to several inhibitors (34). HSP105B is induced by hypertonicity in MDCK cells (not shown). TonEBP may be involved in the HSP105B induction because in the 5′ flanking region (GenBank accession number for human gene, NT_031889) there are two sites that fit the consensus of TonEBP binding sites.
As discussed earlier, the increased HSP70 expression protects cells from apoptosis caused by high concentrations of urea in the renal medulla (36, 37, 41). HSP70 inhibits the mitochondrial pathway of apoptosis at multiple steps. Stress-induced release of cytochome c from mitochondria is inhibited by HSP70, and this action requires the chaperone function (or ATPase activity) of HSP70 (32). Activation of caspase-9 is also inhibited by HSP70 (2). Significance of this action of HSP70 in protection from the urea-induced apoptosis is not clear because it is not known whether high urea induces the mitochondrial pathway of apoptosis. The requirement for ATPase activity for the antiapoptotic activity of HSP70 suggests that a cochaperone or a HSP40 is also involved (9). We reported recently that the mRNA for HLJ1, an HSP40 isoform, is induced by hypertonicity in mIMCD cells at the mRNA level (34). It remains to be determined whether HLJ1 protein is also involved in protection against urea-induced apoptosis.
Acknowledgments
This work was supported by Grant-in-Aid 0150368N from the American Heart Association and NIH grant DK42479 to HMK. S.K.W. was supported by the Juvenile Diabetes Foundation International fellowship 3-1999-727.
We thank M. Takenaka at the Osaka University for providing the αB crystallin cDNA.
REFERENCES
- 1.Beck, F. X., A. Burger-Kentischer, and E. Müller. 1988. Cellular response to osmotic stress in the renal medulla. Pflügers Arch. 436:814-827. [DOI] [PubMed] [Google Scholar]
- 2.Beere, H. M., B. B. Wolf, K. Cain, D. D. Mosser, A. Mahboubi, T. Kuwana, P. Tailor, R. I. Morimoto, G. M. Cohen, and D. R. Green. 2000. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2:469-475. [DOI] [PubMed] [Google Scholar]
- 3.Cha, J. H., S. K. Woo, K. H. Han, Y. H. Kim, J. S. Handler, J. Kim, J., and H. M. Kwon. 2001. Hydration status affects nuclear distribution of transcription factor tonicity responsive enhancer binding protein in rat kidney. J. Am. Soc. Nephrol. 12:2221-2230. [DOI] [PubMed] [Google Scholar]
- 4.Dasgupta, S., T. C. Hohman, and D. Carper. 1992. Hypertonic stress induces αB-crystallin expression. Exp. Eye Res. 54:461-470. [DOI] [PubMed] [Google Scholar]
- 5.Dmitrieva, N., D. Kultz, L. Michea, J. Ferraris, and M. Burg. 2000. Protection of renal inner medullary epithelial cells from apoptosis by hypertonic stress-induced p53 activation. J. Biol. Chem. 275:18243-18247. [DOI] [PubMed] [Google Scholar]
- 6.Dmitrieva, N., L. Michea, and M. B. Burg. 2001. p53 protects renal inner medullary cells from hypertonic stress by restricting DNA replication. Am. J. Physiol. Renal Physiol. 281:F522-F530. [DOI] [PubMed] [Google Scholar]
- 7.Eickleberg, O., J. Geibel, F. Seebach, G. Giebisch, and M. Kashgarian. 2001. K+-induced HSP-72 expression is mediated via rapid Ca2+ influx in renal epithelial cells. Am. J. Physiol. Renal Physiol. 281:F280-F287. [DOI] [PubMed] [Google Scholar]
- 8.Handler, J. S., and H. M. Kwon. 2001. Cell and molecular biology of organic osmolyte accumulation in hypertonic renal cells. Nephron 87:106-110. [DOI] [PubMed] [Google Scholar]
- 9.Hartl, U. F. 1996. Molecular chaperones in cellular protein folding. Nature 381:571-580. [DOI] [PubMed] [Google Scholar]
- 10.Horio, M., A. Ito, Y. Matsuoka, T. Moriyama, Y. Orita, M. Takenaka, and E. Imai. 2001. Apoptosis induced by hypertonicity in Madin Darley canine kidney cells: protective effect of betaine. Nephrol. Dial. Transplant. 16:483-490. [DOI] [PubMed] [Google Scholar]
- 11.Hunt, C., and R. I. Morimoto. 1985. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc. Natl. Acad. Sci. USA 82:6455-6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jamison, R. L., and W. Kritz. 1981. Urinary concentrating mechanism: structure and function. Oxford, New York, N.Y.
- 13.Kitamura, H., A. Yamauchi, T. Nakanishi, Y. Takamitsu, T. Sugiura, A. Akagi, T. Moriyama, M. Horio, and E. Imai. 1997. Effects of inhibition of myo-inositol transport on MDCK cells under hypertonic environment. Am. J. Physiol. 272:F267-F272. [DOI] [PubMed] [Google Scholar]
- 14.Kitamura, H., A. Yamauchi, T. Sugiura, Y. Matsuoka, M. Horio, M. Tohyama, S. Shimada, E. Imai, and M. Hori. 1998. Inhibition of myo-inositol transport causes acute renal failure with selective medullary injury in the rat. Kidney Int. 53:146-153. [DOI] [PubMed] [Google Scholar]
- 15.Ko, B. C. B., B. Ruepp, K. M. Bohren, K. H. Gabbay, and S. S. M. Chung. 1997. Identification and characterization of multiple osmotic response sequences in the human aldose reductase gene. J. Biol. Chem. 272:16431-16437. [DOI] [PubMed] [Google Scholar]
- 16.Kojima, R., R. Randall, B. M. Brenner, and S. R. Gullans. 1996. Osmotic stress protein 94 (Osp94): a new member of the Hsp110/SSE gene subfamily. J. Biol. Chem. 271:12327-12332. [DOI] [PubMed] [Google Scholar]
- 17.Kültz, D., and D. Chakravarty. 2001. Hyperosmolality in the form of elevated NaCl but not urea causes DNA damage in murine kidney cells. Proc. Natl. Acad. Sci. USA 98:1999-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kültz, D., S. Madhany, and M. Burg. 1998. Hyperosmolality causes growth arrest of murine kidney cells: induction of GADD45 and GADD153 by osmosensing via stress-activated protein kinase 2. J. Biol. Chem. 273:13645-13651. [DOI] [PubMed] [Google Scholar]
- 19.Kwon, H. M., and J. S. Handler. 1995. Cell volume regulated transporters of compatible osmolytes. Curr. Opin. Cell Biol. 7:465-471. [DOI] [PubMed] [Google Scholar]
- 20.Leung, T. K. C., M. Y. Jajendran, C. Monfies, C. Hall, and L. Lim. 1990. The human heat-shock protein family: expression of a novel heat-inducible HSP70 (HSP70B') and isolation of its cDNA and genomic DNA. Biochem. J. 267:125-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lifton, R. P., A. G. Gharavi, and D. S. Geller. 2001. Molecular mechanisms of human hypertension. Cell 104:545-556. [DOI] [PubMed] [Google Scholar]
- 22.Loomis, W. H., S. Namiki, D. B. Hoyt, and W. G. Junger. 2001. Hypertonicity rescues T cells from suppression by trauma-induced anti-inflammatory mediators. Am. J. Physiol. Cell Physiol. 281:C840-C848. [DOI] [PubMed] [Google Scholar]
- 23.López-Rodríguez, C., J. Aramburu, L. Jin, A. S. Rakeman, M. Michino, and A. Rao. 2001. Bridging the NFAT and NF-kappaB families: NFAT5 dimerization regulates cytokine gene transcription in response to osmotic stress. Immunity 15:47-58. [DOI] [PubMed] [Google Scholar]
- 24.Maouyo, D., J. Y. Kim, S. D. Lee, Y. Wu, S. K. Woo, and H. M. Kwon. 2002. Mouse TonEBP/NFAT5: expression in early development and alternative splicing. Am. J. Physiol. Renal Physiol. 282:F802-F809. [DOI] [PubMed] [Google Scholar]
- 25.Michea, L., D. R. Ferguson, E. M. Peters, P. M. Andrews, M. R. Kirby, and M. B. Burg. 2000. Cell cycle delay and apoptosis are induced by high salt and urea in renal medullary cells. Am. J. Physiol. 278:F209-F218. [DOI] [PubMed] [Google Scholar]
- 26.Milner, C. M., and R. D. Campbell. 1990. Structure and expression of the three MHC-linked HSP70 genes. Immunogenetics 32:242-251. [DOI] [PubMed] [Google Scholar]
- 27.Miyai, A., A. Yamauchi, T. Moriyama, T. Kaneko, M. Takenaka, T. Sugiura, H. Kitamura, A. Ando, M. Tohyama, S. Shimada, E. Imai, and T. Kamada. 1996. Expression of betaine transporter mRNA: its localization and rapid regulation in rat kidney. Kidney Int. 50:819-827. [DOI] [PubMed] [Google Scholar]
- 28.Miyakawa, H., J. S. Rim, J. S. Handler, and H. M. Kwon. 1999. Identification of the second tonicity-responsive enhancer for the BGT-1 gene. Biochim. Biophys. Acta 1446:359-364. [DOI] [PubMed] [Google Scholar]
- 29.Miyakawa, H., S. K. Woo, C. P. Chen, S. C. Dahl, J. S. Handler, and H. M. Kwon. 1998. Cis- and trans-acting factors regulating transcription of the BGT1 gene in response to hypertonicity. Am. J. Physiol. 274:F753-F761. [DOI] [PubMed] [Google Scholar]
- 30.Miyakawa, H., S. K. Woo, S. C. Dahl, J. H. Handler, and H. M. Kwon. 1999. Tonicity-responsive enhancer binding protein, a rel-like protein that stimulates transcription in response to hypertonicity. Proc. Natl. Acad. Sci. USA 96:2538-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morimoto, R. I. 1997. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 12:3788-3796. [DOI] [PubMed] [Google Scholar]
- 32.Mosser, D. D., A. W. Caron, L. Bourget, A. B. Meriin, M. Y. Sherman, R. I. Morimoto, and B. Massie. 2000. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol. Cell. Biol. 20:7146-7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Müller, E., W. Neuhofer, A. Ohno, S. Rucker, K. Thurau, and F. X. Beck. 1996. Heat shock proteins HSP25, HSP60, HSP72, HSP73 in isoosmotic cortex and hyperosmotic medulla of rat kidney. Pflugers Arch. 431:608-617. [DOI] [PubMed] [Google Scholar]
- 34.Nahm, O., S. K. Woo, J. S. Handler, and H. M. Kwon. 2002. Involvement of multiple kinase pathways in stimulation of gene transcription by hypertonicity. Am. J. Physiol. Cell Physiol. 282:C49-C58. [DOI] [PubMed] [Google Scholar]
- 35.Nakayama, Y., T. Peng, J. M. Sands, and S. M. Bagnasco. 2000. The TonE/TonEBP pathway mediates tonicity-responsive regulation of UT-A urea transporter expression. J. Biol. Chem. 275:38275-38280. [DOI] [PubMed] [Google Scholar]
- 36.Neuhofer, W., E. Muller, A. Burger-Kentischer, M. L. Fraek, K. Thurau, and F. X. Beck. 1999. Inhibition of NaCl-induced heat shock protein 72 expression renders MDCK cells susceptible to high urea concentrations. Pflugers Arch. 437:611-616. [DOI] [PubMed] [Google Scholar]
- 37.Neuhofer, W., K. Lugmayr, M. L. Fraek, and F. X. Beck. 2001. Regulated overexpression of heat shock protein 72 protects Madin-Darby canine kidney cells from the detrimental effects of high urea concentrations. J. Am. Soc. Nephrol. 12:2565-2571. [DOI] [PubMed] [Google Scholar]
- 38.Rauchman, M. I., J. Pullman, and S. R. Gullans. 1997. Induction of molecular chaperones by hyperosmotic stress in mouse inner medullary collecting duct cells. Am. J. Physiol. 273:F9-F17. [DOI] [PubMed] [Google Scholar]
- 39.Rauchman, M. I., S. K. Nigam, E. Delpire, and S. R. Gullans. 1993. An osmotically tolerant inner medullary collecting duct cell line from an SV40 transgenic mouse. Am. J. Physiol. 265:F416-F424. [DOI] [PubMed] [Google Scholar]
- 40.Rim, J. S., M. G. Atta, S. C. Dahl, G. T. Berry, J. S. Handler, and H. M. Kwon. 1998. Transcription of the sodium/myo-inositol cotransporter gene is regulated by multiple tonicity-responsive enhancers spread over 50 kilobase pairs in the 5′-flanking region. J. Biol. Chem. 273:20615-20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santos, B. C., A. Chevaile, M. J. Hébert, J. Zagajeski, and S. R. Gullans. 1998. A combination of NaCl and urea enhances survival of IMCD cells to hyperosmolality. Am. J. Physiol. 274:F1167-F1173. [DOI] [PubMed] [Google Scholar]
- 42.Santos, B. C., A. Chevaile, R. Kojima, and S. R. Gullans. 1998. Characterization of the Hsp110/SSE gene family response to hyperosmolality and other stresses. Am. J. Physiol. Renal Physiol. 274:F1054-F1061. [DOI] [PubMed] [Google Scholar]
- 43.Takenaka, M., A. S. Preston, H. M. Kwon, and J. S. Handler. 1994. The tonicity-sensitive element that mediates increased transcription of the betaine transporter gene in response to hypertonic stress. J. Biol. Chem. 269:29379-29381. [PubMed] [Google Scholar]
- 44.Trama, J., Q. Lu, R. G. Hawley, and S. N. Ho. 2000. The NFAT-related protein NFATL1 (TonEBP/NFAT5) is induced upon T cell activation in a calcineurin-dependent manner. J. Immunol. 165:4884-4894. [DOI] [PubMed] [Google Scholar]
- 45.Woo, S. K., S. C. Dahl, J. S. Handler, and H. M. Kwon. 2000. Bidirectional regulation of tonicity-responsive enhancer binding protein in response to changes in tonicity. Am. J. Physiol. 278:F1006-F1012. [DOI] [PubMed] [Google Scholar]
- 46.Yamauchi, A., H. M. Kwon, S. Uchida, A. S. Preston, and J. S. Handler. 1991. Myo-inositol and betaine transporters regulated by tonicity are basolateral in MDCK cells. Am. J. Physiol. 261:F197-F202. [DOI] [PubMed] [Google Scholar]
- 47.Zhang, Z., X.-Y. Yang, S. P. Soltoff, and D. M. Cohen. 2000. PI3K signaling in the murine kidney inner medullary cell response to urea. Am. J. Phsiol. Renal Physiol. 278:F155-F164. [DOI] [PubMed] [Google Scholar]