Abstract
MEKK2 is a member of the mitogen-activated protein kinase (MAPK) kinase kinase gene family involved in regulating multiple MAPK signaling pathways. To elucidate the in vivo function of MEKK2, we generated mice carrying a targeted mutation in the Mekk2 locus. Mekk2−/− mice are viable and fertile. Major subsets of thymic and spleen T cells in Mekk2-deficient mice were indistinguishable from those in wild-type mice. B-cell development appeared to proceed similarly in the bone marrow of Mekk2-deficient and wild-type mice. However, Mekk2−/− T-cell proliferation was augmented in response to anti-CD3 monoclonal antibody (MAb) stimulation, and these T cells produced more interleukin 2 and gamma interferon than did the wild-type T cells, suggesting that MEKK2 may be involved in controlling the strength of T-cell receptor (TCR) signaling. Consistently, Mekk2−/− thymocytes were more susceptible than wild-type thymocytes to anti-CD3 MAb-induced cell death. Furthermore, TCR-mediated c-Jun N-terminal kinase activation was not blocked but moderately enhanced in Mekk2−/− T cells. Neither extracellular signal-regulated kinase nor p38 MAPK activation was affected in Mekk2−/− T cells. In conclusion, we found that MEKK2 may be required for controlling the strength of TCR/CD3 signaling.
MEKK2, a member of the MEKK/Ste11 subgroup of the mitogen-activated protein kinase (MAPK) kinase kinase gene family, is a major upstream activator of the c-Jun N-terminal kinase (JNK) MAPK cascade (2, 4, 6, 10). In addition to activating JNKs, MEKK2 has also been shown to activate the extracellular signal-regulated kinase (ERK) and p38 MAPKs (2, 6, 20). MEKK2 is expressed in multiple tissues (23), but its functions in different tissues are largely unknown. In murine T-cell line D10, MEKK2 was redistributed to the plasma membrane region where T cells and antigen-presenting cells were conjugated following stimulation, indicating that MEKK2 is important in transducing T-cell receptor (TCR)/CD3-mediated T-cell-activating signals (20). Interestingly, ERK but not JNK was suggested to be the target of this activation process (20). Using Jurkat T cells, we found that MEKK2 was involved in TCR-mediated JNK activation and interleukin 2 (IL-2) gene induction (23). However, ERK activation in Jurkat T cells was not mediated by MEKK2 (23). Gene-targeting studies with embryonic stem (ES) cells showed that MEKK2 played a role in immunoglobulin E (IgE) and c-Kit-induced cytokine gene expression (11). Although these in vitro studies suggest that MEKK2 may play an important role in T cells and other hematopoietic cells, it is not known which MAPK cascade is involved in transducing MEKK2 signals in vivo.
The ERK, JNK, and p38 MAPK cascades are crucial signal transducers downstream of the TCR/CD3 complex and play important roles in regulating T-cell function (12, 13, 19, 24, 25, 28, 33). Activation of the c-Jun/AP-1 transcription complex, a major target of the JNK pathway, also depends on costimulation of TCR and CD28 in resting T cells or by treatment with the pharmacological reagents tetradecanoyl phorbol acetate and Ca2+ ionophore (16, 24). In anergic T cells, whose activation is impaired, activation of JNK and its target AP-1 is also blocked (7, 13). By using mutant mice carrying targeted mutations of the genes encoding JNKs, JNKK1 (SEK1 or MKK4) and JNKK2 (MKK7), it has been demonstrated that the JNK MAPK cascade plays an essential role in T-cell activation and differentiation but not thymic T-cell development (8, 9, 14, 15, 17, 18, 26, 30). In contrast, the related ERK cascade has been shown elsewhere to play a crucial role in thymic T-cell development (1, 5, 15). However, how these MAPK cascades are activated and through which upstream activators they are activated during lymphocyte development and activation remain largely unknown.
In this study, we generated mice with a targeted Mekk2 mutation by homologous recombination. T-cell development and activation in these mice were investigated. We found that, although MEKK2 was dispensable for normal T- and B-cell development, Mekk2−/− T cells exhibited a stronger proliferative response than did the wild-type T cells to anti-CD3 MAb stimulation and produced more of the cytokines IL-2 and gamma interferon (IFN-γ). Mekk2−/− thymocytes were more susceptible than wild-type thymocytes to cell death induced by anti-CD3 MAb but not to that induced by Fas, UV light, or dexamethasone. Interestingly, disruption of Mekk2 in T cells did not inhibit TCR/CD3-induced JNK activation, nor did it affect the activation of ERK and p38. Instead, Mekk2−/− T cells exhibit a higher JNK activation than that in wild-type T cells to anti-CD3 MAb stimulation. Taken together, our findings suggest that MEKK2 may play a complex role in controlling the TCR signaling strength in part through the JNK but not the ERK and p38 MAPK cascade during T-cell stimulation.
MATERIALS AND METHODS
Generation of Mekk2-deficient mice by gene targeting.
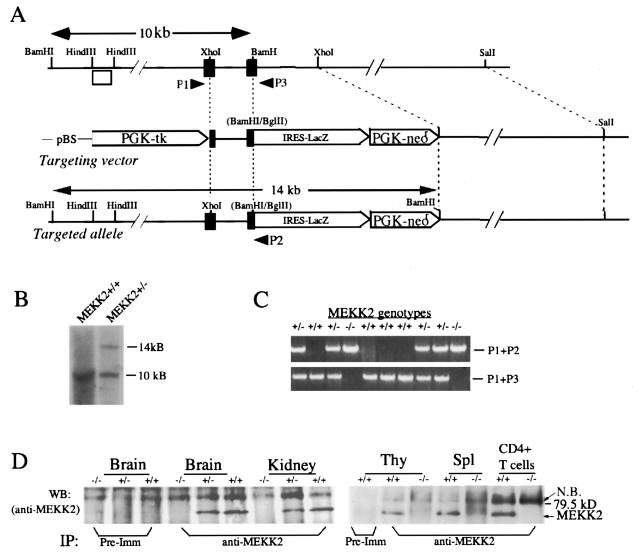
A 14.5-kb murine Mekk2 genomic DNA clone containing sequence coding for part of the catalytic domain of MEKK2 was isolated from a 129sv library and used to construct a Mekk2 targeting vector by standard techniques. The targeting vector (Fig. 1A) was constructed in pBluescript containing a phosphoglycerate kinase-thymidine kinase cassette and an internal ribosome entry site (IRES)-LacZ-Neor cassette flanked at the 5′ end by a 2-kb XhoI-BamHI genomic DNA fragment with exons encoding MEKK2 amino acids 351 to 426 and at the 3′ end by a 7-kb XhoI-SalI DNA fragment. Thus, in the targeted allele, the fragment containing partial Mekk2 exons was replaced by the IRES-LacZ-Neor cassette (Fig. 1A). Insertion of this cassette also destroyed a BamHI site (as a result of BamHI-BglII ligation) and so created a new 14-kb BamHI fragment at this locus in place of the endogenous 10-kb fragment. P1 (5′-CAGGGATCTGAGTGAATCA), P2 (5′-AATTCTCTAGAGTCCAGATCCCTCA), and P3 (5′-GGCAGGCAATGTCAGTACAAAA) were the primers used for genotyping. Targeted ES cells were generated by standard procedures and genotyped by Southern blotting with the HindIII DNA fragment shown in Fig. 1A as a probe. The targeted ES cells were injected into C57BL/6 blastocysts to generate chimeric mice, and the targeted mice were confirmed by PCR genotyping and by immunoprecipitation (IP)-Western blot analysis (see below).
FIG. 1.
Targeted mutation of the murine Mekk2 gene. (A) Targeting strategy for the Mekk2 locus. Murine Mekk2 genomic DNA containing exons (black boxes) encoding amino acids 349 to 438 in the catalytic domain of MEKK2 is shown (top panel). The targeting vector is shown in the middle panel, and the targeted allele is shown in the bottom panel. The new 14-kb BamHI fragment created by the targeting event is shown. The open box is the HindIII fragment used as a probe for Southern blot genotyping. P1, P2, and P3 are three primers used for genotyping. pBS, pBluescript. (B) Southern blot analysis of genomic DNA from wild-type and targeted ES cells with an outside HindIII fragment as a probe (see panel A). (C) PCR genotyping of a litter from Mekk2+/− parents. (D) IP-Western blot (WB) analysis of MEKK2 protein expression. Whole-cell extracts were prepared from 100 mg of brain, kidney, thymus (Thy), and spleen (Spl) or 107 purified spleen T cells from either wild-type, Mekk2+/−, or Mekk2−/− mice and subjected to IP with either control rabbit serum or anti-MEKK2 antiserum 1128. The immunocomplexes were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and further analyzed by Western blotting with anti-MEKK2 antiserum 1129. Pre-Imm, preimmune serum; N.B., nonspecific band.
IP-Western blot analysis.
Tissues dissected from 6- to 8-week-old mice were genotyped by PCR, and 100 mg of tissue was homogenized in 1 ml of whole-cell lysis buffer. Cell debris was removed by centrifugation at 14,000 rpm in an Eppendorf microcentrifuge for 10 min at 4°C. The supernatant was subjected to IP with either preimmune serum (control) or anti-MEKK2-specific antiserum 1128 (raised against the catalytic domain of MEKK2). The immunocomplexes were thoroughly washed and then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis before being further analyzed by Western blotting with the MEKK2-specific antibody 1129 (raised against the N-terminal noncatalytic domain of MEKK2).
Cell preparation and flow cytometric analysis.
Single-cell suspensions of thymus, spleen, and lymph node cells were prepared, stained by standard procedures, and analyzed on a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, N.J.). Total T cells from spleens and lymph nodes were purified with a nylon wool column. CD4+ T cells were purified by negative selection with antibody cocktails and magnetic microbeads (Stem Cell Technologies, Vancouver, British Columbia, Canada) according to the manufacturer's recommendations.
Proliferation assays.
T cells prepared from wild-type and Mekk2−/− mice (6 to 8 weeks old) were plated in U-bottomed 96-well microtiter plates (Costar Co., Cambridge, Mass.) at 105 cells/well in RPMI supplemented with 10% fetal calf serum, 1× antibiotics (Sigma, St. Louis, Mo.), and 50 mM β-mercaptoethanol. The plates were incubated at 37°C in a CO2 incubator and pulsed with 1 μCi of tritiated thymidine (Amersham, Arlington Heights, Ill.) per well for the last 8 h of a 48-h culture period. The cells were harvested with a Tomtec cell harvester and counted with a 1205 Betaplate liquid scintillation counter. All results are expressed as means ± standard deviations of triplicate cultures.
ELISA for cytokines.
T cells purified from the spleens of wild-type and Mekk2−/− mice were stimulated with anti-CD3 antibody (2C11) for 24 h in 96-well plates as described above, and the supernatants were harvested for measurement of IL-2, IL-4, IL-5, IL-10, and IFN-γ by enzyme-linked immunosorbent assay (ELISA) (Pharmingen, San Diego, Calif.) according to the manufacturer's instructions. All experiments were performed in triplicate, and the data are expressed as means ± standard deviations.
In vivo thymocyte death assay.
Wild-type and Mekk2−/− littermates (8 weeks old) were injected intraperitoneally with control normal mouse IgG or anti-CD3 antibody 2C11 and killed 18 h later. Thymic T cells were isolated by standard procedures and stained with propidium iodide (PI) for fluorescence-activated cell sorting analysis. The results shown are the averages of three mice in each group.
In vitro apoptosis assays.
T cells isolated from the thymus of wild-type and Mekk2−/− mice were seeded at 106 cells/well in 2 ml of RPMI medium with 10% fetal bovine serum into six-well plates precoated with 10 μg of control IgG or anti-CD3 antibody/ml and incubated for 12 and 24 h or with 0.5 μg of anti-Fas antibody (clone jo-2; Pharmingen)/ml or 50 nM dexamethasone (Fluka Chemical Corp., Ronkonkoma, N.J.) for various time points or with 60 J of UVC/m2 and then incubated for 24 h in complete medium. Cell death was determined by staining cells with fluorescein isothiocyanate (FITC)-annexin V (Pharmingen) and analyzing them with a FACSan cytometer. Thymocytes cultured in medium alone served as controls for the spontaneous cell death. Cell death is expressed as the percentage of stimulus-induced annexin V-positive cells. The experiments were performed in triplicate, and the data are representative of four independent experiments.
Western blotting with anti-phosphor-JNK, -ERK, and -p38 antibodies.
T cells were isolated from wild-type and Mekk2−/− littermates (6 to 8 weeks old), and 100 μg of whole-cell extracts prepared from T cells was analyzed with anti-phosphor-JNK, -ERK, and -p38 antibodies (New England Biolabs, Boston, Mass.) according to the manufacturer's instructions.
RESULTS
Disruption of Mekk2 in mice by homologous recombination.
To investigate the physiological role of MEKK2 in vivo, we disrupted the Mekk2 gene by homologous recombination. The targeting vector was constructed by replacing an XhoI-BamHI fragment from the Mekk2 locus with an IRES-lacZ-Neor gene cassette (Fig. 1A). Replacement of the XhoI-BamHI fragment resulted in the deletion of 192 amino acids from the MEKK2 catalytic domain. Southern blot analysis (Fig. 1B) of 956 neomycin-resistant ES cell clones identified three that carried the targeted Mekk2 mutation. One was injected into C57BL/6 blastocysts to produce chimeric mice. The targeted Mekk2 allele was successfully transmitted from the chimeric mice to their offspring, as demonstrated by PCR genotyping (Fig. 1C). β-Galactosidase activity was detected in both Mekk2+/− and Mekk2−/− mice but not wild-type mice (data not shown). Western blot analysis with two MEKK2-specific antibodies raised against the NH2-terminal (1129) and partial catalytic (1128) domains of recombinant MEKK2 protein did not detect any truncated MEKK2 protein in either Mekk2+/− or Mekk2−/− mice, indicating that the N-terminal noncatalytic portion of MEKK2 protein was not expressed (Fig. 1D).
The Mekk2−/− mice appeared normal and were fertile. Thus, these results demonstrated either that MEKK2 was not involved in normal embryonic development or that its function was compensated for by another likely homologue gene.
Disruption of MEKK2 does not alter normal T-cell and B-cell development.
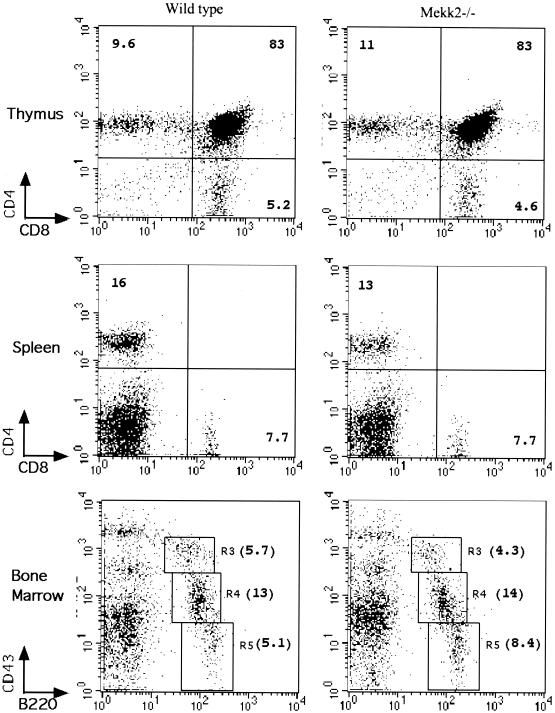
To investigate the role of MEKK2 in lymphocyte development and function, we examined T cells and B cells from the thymuses, spleens, and bone marrow of wild-type and Mekk2−/− mice by flow cytometry analysis. As shown in Fig. 2, the profiles of subsets of T cells and B cells isolated from Mekk2−/− mouse thymuses, spleens, and bone marrow were indistinguishable from those of wild-type mice. In addition, there were no significant differences in the sizes of thymuses and spleens from 0- to 12-week-old Mekk2−/− and wild-type mice (data not shown). The numbers of thymic and peripheral T cells in Mekk2−/− and wild-type mice at these ages were also not significantly different (data not shown). We also analyzed the profiles of subsets of T cells and B cells from Mekk2+/− mice and found no difference from those of wild-type mice (data not shown). Thus, these results demonstrated that the major lymphocyte development could proceed in the absence of the Mekk2 gene product.
FIG. 2.
MEKK2 is not required for normal T-cell and B-cell development. Shown are results from a flow cytometry analysis of cells from wild-type and Mekk2−/− thymus, spleen, and bone marrow. The antibodies used in the assays are shown. The numbers in each profile are the percentages of the total cell population. Cells shown in boxes R3, R4, and R5 are pro-B cells, pro- and pre-B cells, and mature B cells, respectively.
Hyperproliferation of Mekk2−/− T cells in response to anti-CD3 antibody stimulation.
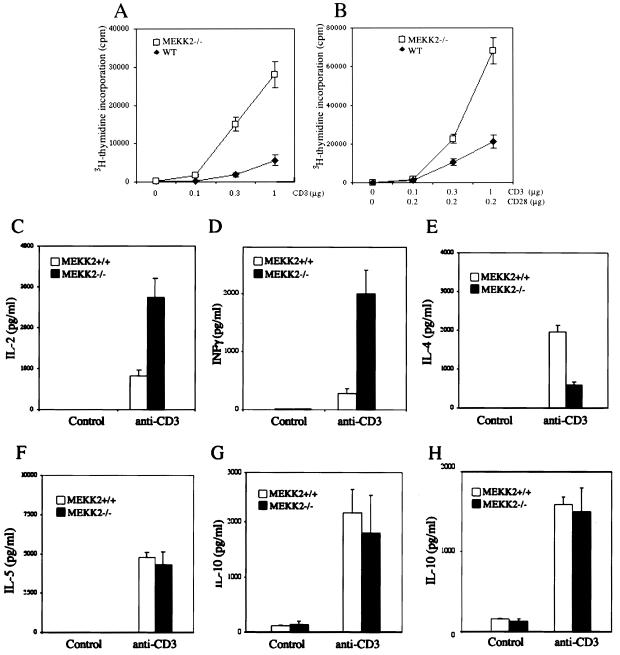
It was reported previously that targeted mutation of the MAPKs JNK1 and JNK2 did not block T-cell development but did impair T-cell activation and differentiation (9, 17, 30). Since in vitro studies showed that MEKK2 is a major upstream activator of JNKs in T cells, it is possible that Mekk2−/− T cells may be affected similarly to the JNK-deficient T cells. To investigate this possibility, we purified total spleen T cells from 6- to 8-week-old Mekk2−/− mice and control wild-type littermates and then stimulated these T cells with anti-CD3 MAb 2C11 or 2C11 plus anti-CD28 MAb. To our surprise, we found that Mekk2−/− T cells proliferated more vigorously in response to anti-CD3 MAb stimulation than did the wild-type T cells (Fig. 3A and B). We also analyzed CD4+ subset T cells purified from draining lymph nodes of Mekk2−/− and wild-type mice and found that the CD4+ subset of Mekk2−/− T cells also had an augmented proliferative response to anti-CD3 MAb (data not shown). Thus, these results suggested that MEKK2 might negatively regulate T-cell activation.
FIG. 3.
Proliferation and cytokine production by wild-type (WT) and Mekk2−/− T cells. (A and B) Purified spleen T cells from 4- to 8-week-old wild-type and Mekk2−/− mice were stimulated with anti-CD3 antibody alone (A) and with anti-CD3 plus anti-CD28 antibodies (B) for 48 h with [3H]thymidine added for the final 8 h before the cells were harvested for measurement of [3H]thymidine incorporation. (C to G) Purified T cells from the spleens of 4- to 8-week-old wild-type and Mekk2−/− mice were stimulated with anti-CD3 antibody for 24 h, and the production of the cytokines IL-2 (C), IFN-γ (D), IL-4 (E), IL-5 (F), and IL-10 (G) was determined by ELISA. Production of IL-10 (H) in thymic T cells was measured as described above.
Alteration in cytokine expression in Mekk2−/− T cells.
To further examine the phenotypes of Mekk2−/− T cells following stimulation of the TCR/CD3 complex, we measured the production of major T-cell cytokines involved in T-cell proliferation and/or T-helper-cell differentiation, including IL-2, IFN-γ, IL-4, IL-5, and IL-10. As shown in Fig. 3C to E, we found consistently that Mekk2−/− T cells produced significantly more IL-2 and IFN-γ but less IL-4 than wild-type T cells did. Mekk2−/− T cells and wild-type T cells produced comparable amounts of IL-5 and IL-10 (Fig. 3F to H). These data suggested that the increased T-cell proliferation may be due to the augmented cytokine production.
Protection of thymocytes by MEKK2 from AICD.
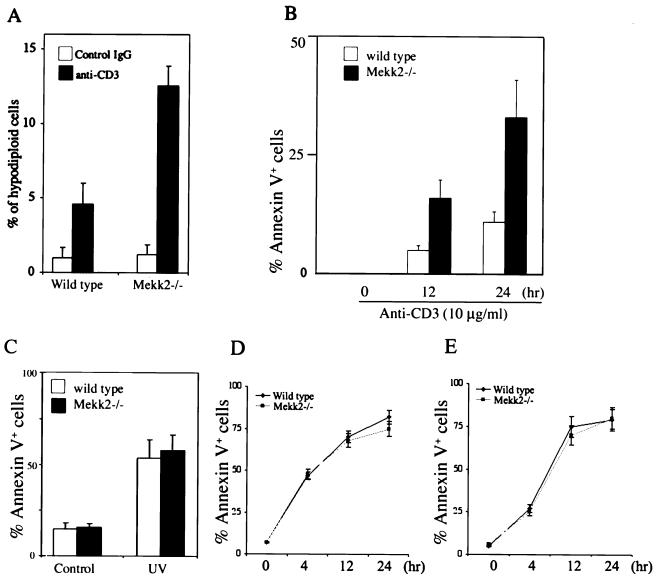
The above results showed that Mekk2-deficient T cells were hyperreactive to TCR/CD3 stimulation. This suggested a possibility that MEKK2 may be involved in negatively modulating the strength of TCR/CD3 signaling. Since stimulation of the TCR/CD3 complex has been shown previously to cause activation-induced cell death (AICD) of thymocytes (21, 22), if MEKK2 negatively modulates the TCR signaling, we would expect that Mekk2−/− thymocytes might be more susceptible to anti-CD3-induced AICD than the wild-type thymocytes. To investigate this possibility, Mekk2−/− and control wild-type mice were injected with anti-CD3 MAb and thymocyte apoptosis was examined 18 h later. As shown in Fig. 4A, we found that there was a significant increase of apoptotic cells in Mekk2−/− thymus over those in the wild-type thymus. We also found more DNA fragmentation in thymus from anti-CD3 antibody-induced Mekk2−/− mice than in that from wild-type mice (data not shown), suggesting that there was more anti-CD3 MAb-induced apoptosis in Mekk2−/− thymus. To examine whether this increased AICD in thymus was intrinsic to the thymic T cells, we incubated total thymocytes from Mekk2−/− mice and wild-type mice with a plate coated with anti-CD3 MAb in vitro, followed by three-color flow cytometry analysis with phycoerythrin-anti-CD4, Cy-chrome-anti-CD8, and FITC-annexin V. As shown in Fig. 4B, more anti-CD3 antibody-induced cell death was observed in Mekk2−/− CD4/CD8 double-positive thymocytes than in wild-type double-positive thymocytes. We also determined the anti-CD3-induced AICD in peripheral T cells in Mekk2−/− and wild-type mice and found no difference (data not shown). The augmented AICD in Mekk2−/− thymic T cells seemed specific to the TCR signaling cascade, as we found that UV irradiation-induced cell death and dexamethasone-induced cell death were not affected (Fig. 4C and D). Furthermore, Fas-induced thymocyte apoptosis was also not affected in Mekk2−/− T cells (Fig. 4E).
FIG. 4.
Susceptibility of Mekk2-deficient thymocytes to cell death caused by cell activation or stress. (A) Wild-type mice (8 weeks old) and Mekk2−/− mice were injected intraperitoneally with 100 μg of control IgG or anti-CD3 antibody. The mice were killed 18 h later, and the thymocytes were analyzed by PI staining. The percentages of hypodiploid cells are shown. (B) Wild-type or Mekk2−/− thymocytes were prepared from 8-week-old mice; stimulated with anti-CD3 antibody for 0, 12, and 24 h; and then monitored by three-color flow cytometry staining with phycoerythrin-anti-CD4, cychrome-anti-CD8, and FITC-annexin V. The percentages shown are after subtraction of background (annexin V+ thymocytes in unstimulated culture). (C to E) Wild-type and Mekk2−/− thymocytes isolated from 8-week-old littermates were stimulated with UVC (60 J/m2) (C), 50 nM dexamethasone (D), or 0.5 μg of anti-Fas/ml (E) for various time points before being assayed for apoptosis as described for panel B.
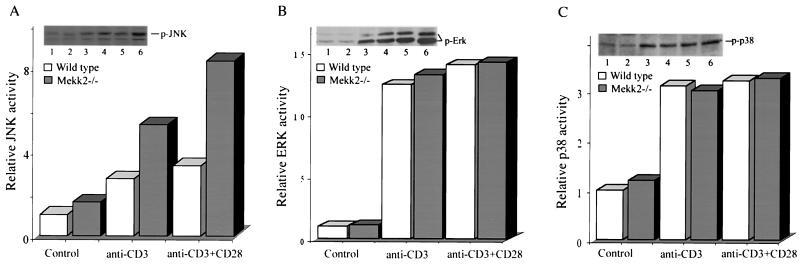
Disruption of Mekk2 did not block TCR-mediated JNK, ERK, or p38 activation.
MEKK2 has been shown elsewhere to be an activating MAPK kinase kinase for the JNK, ERK, and p38 MAPK cascades (2, 4, 6, 20). Schaefer and coworkers reported that MEKK2 used the ERK pathway to transduce TCR signals in murine T-cell lines (20). However, we found that MEKK2 was involved in JNK activation in response to TCR stimulation in Jurkat T cells (23). Therefore, it was possible that activation of these kinase pathways might be defective in Mekk2−/− T cells. To investigate this possibility, we stimulated thymic T cells isolated from Mekk2−/− mice and wild-type mice with anti-CD3 MAb alone or together with anti-CD28 antibody and then measured the enzymatic activities of JNK, ERK, and p38. Surprisingly, activation of the JNK MAPK was not blocked in Mekk2−/− T cells, nor was the ERK and p38 activation. Rather, we consistently observed a three- to fourfold increase of JNK activation in Mekk2-deficient thymic T cells over that in wild-type T cells (Fig. 5A). However, the ERK and p38 activation was not changed (Fig. 5B and C). JNK activation was also slightly augmented in the peripheral T cells from Mekk2−/− mice (data not shown). Thus, MEKK2 may negatively regulate the JNK but not ERK and p38 MAPKs during TCR stimulation.
FIG. 5.
Hyperactivation of JNK MAPK by anti-CD3 antibody stimulation in Mekk2−/− T cells. Thymocytes prepared from 8-week-old wild-type (lanes 1, 3, and 5) and Mekk2−/− (lanes 2, 4, and 6) littermates were stimulated with control antibody (lanes 1 and 2), anti-CD3 (lanes 3 and 4), or anti-CD3 plus anti-CD28 antibodies (lanes 5 and 6) for 30 min before being assayed for JNK, ERK, and p38 activation. Relative JNK, ERK, and p38 activities are presented as bar graphs after normalization to the protein levels.
DISCUSSION
We generated Mekk2-deficient mice by homologous recombination. These mice developed normally and were fertile, in contrast to the mice lacking Mekk3, a closely related homologue of Mekk2, which die around embryonic day 11 (31). Normal T- and B-cell development can proceed in Mekk2-deficient mice. However, we found that T cells from Mekk2-deficient mice exhibit enhanced proliferation in response to anti-CD3 MAb and produced more of the cytokines IL-2 and IFN-γ. In addition, we found that anti-TCR-induced thymic T-cell apoptosis is augmented in the absence of MEKK2. Furthermore, the TCR-mediated JNK activation appeared to be elevated rather than blocked in the absence of MEKK2. Thus, these studies suggest that MEKK2 may be involved in modulating TCR signaling strength partially through the MEKK2-JNK pathway.
We showed previously in Jurkat T cells that MEKK2 was required for TCR signaling (23). A similar conclusion was reported by Schaefer et al., using murine T-cell lines (20). In the present study, we found an increased TCR signaling in Mekk2-deficient T cells. One possible explanation for these apparently contradictory results is that MEKK2 may be involved in both positive and negative regulation of the TCR signaling. Since TCR signaling is tightly controlled, it is conceivable that, following TCR stimulation and MEKK2 signal transduction, the TCR signaling cascade needs to be down regulated. The reason that we do not observe a complete blockage of TCR signal in Mekk2-deficient T cells may be due to other Mekk2-related kinases such as MEKK3, which could partially compensate for the loss of MEKK2. However, this compensation may compensate for only the positive role of MEKK2 and not for its negative role in TCR signaling, resulting in augmented T-cell responses. However, since previous work was carried out in cell lines with transient transfection and overexpression of dominant-negative MEKK2 constructs, which could interfere with pathways utilizing other MAPK kinase kinases, whereas the present work was carried out with normal T cells in mice, it is possible that the main function of MEKK2 is to balance the TCR signal during T-cell activation.
It was shown recently that compound mutations of both Jnk1 and Jnk2 lead to augmented T-cell proliferation and IL-2 and IFN-γ production (8). Whereas this phenotype appeared to resemble that of our Mekk2-deficient T cells, it is clear that the Mekk2−/− T-cell phenotype was not caused by a defective JNK pathway, as we found that JNK activation was not blocked following TCR stimulation. In fact, we observed a consistent augmentation of JNK activation in Mekk2−/− T cells. It is not known at this time how both JNK deficiency and increased JNK activity could cause these seemingly similar phenotypes in T cells. One possibility is that, in Mekk2-deficient T cells, Mekk2 may target not only the JNK cascade and that the phenotype observed is the result of alterations in both the JNK pathway and another, yet-unidentified pathway. In this regard, we found recently that MEKK3, a closely related homologue of MEKK2, plays a crucial role in NF-κB activation (32).
Increased and prolonged JNK activation has been suggested to activate the cell death pathway, whereas blocking the JNK activation protects cells from both activation-induced and stress-induced cell death (3, 9, 17, 27, 29). Thus, the increased JNK activation in Mekk2-deficient thymocytes may partially explain why Mekk2-deficient thymocytes were more susceptible to anti-CD3-induced cell death. The involvement of the MEKK2 signaling pathway in apoptosis seems to be dependent on the specific death-inducing stimulus, as disruption of Mekk2 did not significantly alter the cell death induced by anti-Fas antibody, UVC irradiation, and dexamethasone treatment. These results also suggest a potential role of MEKK2 signaling in the negative selection of TCR repertoire since TCR-mediated apoptosis has been demonstrated to be essential in such a process. Future experiments crossing the MEKK2-knockout mice to different TCR transgenic mice will allow us to further examine the role of MEKK2 signaling in T-cell thymic selection.
How MEKK2 is involved in modulating TCR signaling is not known. It is possible that this pattern of negative regulation may not be limited to lymphocytes, since MEKK2 is expressed in various tissues. In this regard, we recently found that mast cells isolated from MEKK2-deficient mice also exhibited an augmented proliferation response (unpublished data). Future analysis of these cells and other MEKK2-deficient cells established from the MEKK2-knockout mice may provide crucial clues for the molecular mechanisms of MEKK2 signaling.
Acknowledgments
We thank Melynda Borem and Guizhi Sun for their excellent technical support and Mureen Goode for editing the manuscript.
This work is supported by an NIH grant (AI44016) and by a grant from the Kleberg Fund to B.S. Y.Z. is supported by the Leukemia and Lymphoma Society and NIH grant GM59638. Z.G. was partially supported by a fellowship from PUMC hospital, CAMS & PUMC. The animal facility at the University of Texas M. D. Anderson Cancer Center is supported in part by an NCI Core grant (CA16672).
REFERENCES
- 1.Alberola-Ila, J., K. A. Forbush, R. Seger, E. G. Krebs, and R. M. Perlmutter. 1995. Selective requirement for MAP kinase activation in thymocyte differentiation. Nature 373:620-623. [DOI] [PubMed] [Google Scholar]
- 2.Blank, J. L., P. Gerwins, E. M. Elliott, S. Sather, and G. L. Johnson. 1996. Molecular cloning of mitogen-activated protein/ERK kinase kinases (MEKK) 2 and 3. Regulation of sequential phosphorylation pathways involving mitogen-activated protein kinase and c-Jun kinase. J. Biol. Chem. 271:5361-5368. [DOI] [PubMed] [Google Scholar]
- 3.Chen, Y. R., C. F. Meyer, and T. H. Tan. 1996. Persistent activation of c-Jun N-terminal kinase 1 (JNK1) in gamma radiation-induced apoptosis. J. Biol. Chem. 271:631-634. [DOI] [PubMed] [Google Scholar]
- 4.Cheng, J., J. Yang, Y. Xia, M. Karin, and B. Su. 2000. Synergistic interaction of MEK kinase 2, c-Jun N-terminal kinase (JNK) kinase 2, and JNK1 results in efficient and specific JNK1 activation. Mol. Cell. Biol. 20:2334-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crompton, T., K. C. Gilmour, and M. J. Owen. 1996. The MAP kinase pathway controls differentiation from double-negative to double-positive thymocyte. Cell 86:243-251. [DOI] [PubMed] [Google Scholar]
- 6.Deacon, K., and J. L. Blank. 1997. Characterization of the mitogen-activated protein kinase kinase 4 (MKK4)/c-Jun NH2-terminal kinase 1 and MKK3/p38 pathways regulated by MEK kinases 2 and 3. MEK kinase 3 activates MKK3 but does not cause activation of p38 kinase in vivo. J. Biol. Chem. 272:14489-14496. [DOI] [PubMed] [Google Scholar]
- 7.DeSilva, D. R., W. S. Feeser, E. J. Tancula, and P. A. Scherle. 1996. Anergic T cells are defective in both jun NH2-terminal kinase and mitogen-activated protein kinase signaling pathways. J. Exp. Med. 183:2017-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong, C., D. D. Yang, C. Tournier, A. J. Whitmarsh, J. Xu, R. J. Davis, and R. A. Flavell. 2000. JNK is required for effector T-cell function but not for T-cell activation. Nature 405:91-94. [DOI] [PubMed] [Google Scholar]
- 9.Dong, C., D. D. Yang, M. Wysk, A. J. Whitmarsh, R. J. Davis, and R. A. Flavell. 1998. Defective T cell differentiation in the absence of Jnk1. Science 282:2092-2095. [DOI] [PubMed] [Google Scholar]
- 10.Fanger, G. R., P. Gerwins, C. Widmann, M. B. Jarpe, and G. L. Johnson. 1997. MEKKs, GCKs, MLKs, PAKs, TAKs, and tpls: upstream regulators of the c-Jun amino-terminal kinases? Curr. Opin. Genet. Dev. 7:67-74. [DOI] [PubMed] [Google Scholar]
- 11.Garrington, T. P., T. Ishizuka, P. J. Papst, K. Chayama, S. Webb, T. Yujiri, W. Sun, S. Sather, D. M. Russell, S. B. Gibson, G. Keller, E. W. Gelfand, and G. L. Johnson. 2000. MEKK2 gene disruption causes loss of cytokine production in response to IgE and c-Kit ligand stimulation of ES cell-derived mast cells. EMBO J. 19:5387-5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacinto, E., G. Werlen, and M. Karin. 1998. Cooperation between Syk and Rac1 leads to synergistic JNK activation in T lymphocytes. Immunity 8:31-41. [DOI] [PubMed] [Google Scholar]
- 13.Li, W., C. D. Whaley, A. Mondino, and D. L. Mueller. 1996. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science 271:1272-1276. [DOI] [PubMed] [Google Scholar]
- 14.Nishina, H., K. D. Fischer, L. Radvanyi, A. Shahinian, R. Hakem, E. A. Rubie, A. Bernstein, T. W. Mak, J. R. Woodgett, and J. M. Penninger. 1997. Stress-signalling kinase Sek1 protects thymocytes from apoptosis mediated by CD95 and CD3. Nature 385:350-353. [DOI] [PubMed] [Google Scholar]
- 15.Pages, G., S. Guerin, D. Grall, F. Bonino, A. Smith, F. Anjuere, P. Auberger, and J. Pouyssegur. 1999. Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science 286:1374-1377. [DOI] [PubMed] [Google Scholar]
- 16.Rincon, M., and R. A. Flavell. 1994. AP-1 transcriptional activity requires both T-cell receptor-mediated and co-stimulatory signals in primary T lymphocytes. EMBO J. 13:4370-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabapathy, K., Y. Hu, T. Kallunki, M. Schreiber, J. P. David, W. Jochum, E. F. Wagner, and M. Karin. 1999. JNK2 is required for efficient T-cell activation and apoptosis but not for normal lymphocyte development. Curr. Biol. 9:116-125. [DOI] [PubMed] [Google Scholar]
- 18.Sabapathy, K., T. Kallunki, J. P. David, I. Graef, M. Karin, and E. F. Wagner. 2001. c-Jun NH(2)-terminal kinase (JNK)1 and JNK2 have similar and stage-dependent roles in regulating T cell apoptosis and proliferation. J. Exp. Med. 193:317-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salmon, R. A., I. N. Foltz, P. R. Young, and J. W. Schrader. 1997. The p38 mitogen-activated protein kinase is activated by ligation of the T or B lymphocyte antigen receptors, Fas or CD40, but suppression of kinase activity does not inhibit apoptosis induced by antigen receptors. J. Immunol. 159:5309-5317. [PubMed] [Google Scholar]
- 20.Schaefer, B. C., M. F. Ware, P. Marrack, G. R. Fanger, J. W. Kappler, G. L. Johnson, and C. R. Monks. 1999. Live cell fluorescence imaging of T cell MEKK2: redistribution and activation in response to antigen stimulation of the T cell receptor. Immunity 11:411-421. [DOI] [PubMed] [Google Scholar]
- 21.Shi, Y. F., R. P. Bissonnette, N. Parfrey, M. Szalay, R. T. Kubo, and D. R. Green. 1991. In vivo administration of monoclonal antibodies to the CD3 T cell receptor complex induces cell death (apoptosis) in immature thymocytes. J. Immunol. 146:3340-3346. [PubMed] [Google Scholar]
- 22.Smith, C. A., G. T. Williams, R. Kingston, E. J. Jenkinson, and J. J. Owen. 1989. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature 337:181-184. [DOI] [PubMed] [Google Scholar]
- 23.Su, B., J. Cheng, J. Yang, and Z. Guo. 2001. Mekk2 is required for T-cell receptor signals in JNK activation and interleukin-2 gene expression. J. Biol. Chem. 276:14784-14790. [DOI] [PubMed] [Google Scholar]
- 24.Su, B., E. Jacinto, M. Hibi, T. Kallunki, M. Karin, and Y. Ben-Neriah. 1994. JNK is involved in signal integration during costimulation of T lymphocytes. Cell 77:727-736. [DOI] [PubMed] [Google Scholar]
- 25.Su, B., and M. Karin. 1996. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr. Opin. Immunol. 8:402-411. [DOI] [PubMed] [Google Scholar]
- 26.Swat, W., K. Fujikawa, S. Ganiatsas, D. Yang, R. J. Xavier, N. L. Harris, L. Davidson, R. Ferrini, R. J. Davis, M. A. Labow, R. A. Flavell, L. I. Zon, and F. W. Alt. 1998. SEK1/MKK4 is required for maintenance of a normal peripheral lymphoid compartment but not for lymphocyte development. Immunity 8:625-634. [DOI] [PubMed] [Google Scholar]
- 27.Tournier, C., P. Hess, D. D. Yang, J. Xu, T. K. Turner, A. Nimnual, D. Bar-Sagi, S. N. Jones, R. A. Flavell, and R. J. Davis. 2000. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science 288:870-874. [DOI] [PubMed] [Google Scholar]
- 28.Werlen, G., E. Jacinto, Y. Xia, and M. Karin. 1998. Calcineurin preferentially synergizes with PKC-theta to activate JNK and IL-2 promoter in T lymphocytes. EMBO J. 17:3101-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia, Z., M. Dickens, J. Raingeaud, R. J. Davis, and M. E. Greenberg. 1995. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326-1331. [DOI] [PubMed] [Google Scholar]
- 30.Yang, D. D., D. Conze, A. J. Whitmarsh, T. Barrett, R. J. Davis, M. Rincon, and R. A. Flavell. 1998. Differentiation of CD4+ T cells to Th1 cells requires MAP kinase JNK2. Immunity 9:575-585. [DOI] [PubMed] [Google Scholar]
- 31.Yang, J., M. Boerm, M. McCarty, C. Bucana, I. J. Fidler, Y. Zhuang, and B. Su. 2000. Mekk3 is essential for early embryonic cardiovascular development. Nat. Genet. 24:309-313. [DOI] [PubMed] [Google Scholar]
- 32.Yang, J., Y. Lin, Z. Guo, J. Cheng, J. Huang, L. Deng, W. Liao, Z. Chen, Z. Liu, and B. Su. 2001. The essential role of MEKK3 in TNF-induced NF-κB activation. Nat. Immunol. 2:620-624. [DOI] [PubMed] [Google Scholar]
- 33.Zhang, J., K. V. Salojin, J. X. Gao, M. J. Cameron, I. Bergerot, and T. L. Delovitch. 1999. p38 mitogen-activated protein kinase mediates signal integration of TCR/CD28 costimulation in primary murine T cells. J. Immunol. 162:3819-3829. [PubMed] [Google Scholar]