Abstract
CNAP1 (hCAP-D2/Eg7) is an essential component of the human condensin complex required for mitotic chromosome condensation. This conserved complex contains a structural maintenance of chromosomes (SMC) family protein heterodimer and three non-SMC subunits. The mechanism underlying condensin targeting to mitotic chromosomes and the role played by the individual condensin components, particularly the non-SMC subunits, are not well understood. We report here characterization of the non-SMC condensin component CNAP1. CNAP1 contains two separate domains required for its stable incorporation into the complex. We found that the carboxyl terminus of CNAP1 possesses a mitotic chromosome-targeting domain that does not require the other condensin components. The same region also contains a functional bipartite nuclear localization signal. A mutant CNAP1 missing this domain, although still incorporated into condensin, was unable to associate with mitotic chromosomes. Successful chromosome targeting of deletion mutants correlated with their ability to directly bind to histones H1 and H3 in vitro. The H3 interaction appears to be mediated through the H3 histone tail, and a subfragment containing the targeting domain was found to interact with histone H3 in vivo. Thus, the CNAP1 C-terminal region defines a novel histone-binding domain that is responsible for targeting CNAP1, and possibly condensin, to mitotic chromosomes.
Condensation of chromatin into compact mitotic chromosomes is a fundamental function of eukaryotic cells. This complex sequence of events allows the cell to proceed through mitosis and distribute complete genetic information to each daughter cell. Failure to perform this process correctly can result in deleterious chromosome segregation defects. Characterization of the cellular factors involved in chromosome condensation is an active area of research. One key factor is a five-protein complex called condensin, which has been described in eukaryotes ranging from yeast to humans (9, 11, 12, 17, 28, 31). The condensin complex was first identified in Xenopus to be required for mitotic chromosome condensation (12). In Xenopus, condensin contains two structural maintenance of chromosomes (SMC) family proteins, Xenopus chromosome-associated protein C (XCAP-C) and XCAP-E, as well as three non-SMC components—XCAP-D2, XCAP-G, and XCAP-H (a homolog of Drosophila Barren) (11, 12, 15, 16). Immunodepletion or antibody blocking of condensin interfered with mitotic chromosome condensation in an in vitro Xenopus oocyte extract system (12). Condensin appears to coat condensed chromosomes, indicating its direct participation in condensation (12). In both budding and fission yeasts, the condensin subunits are conserved and are essential for cell viability (9, 31). Previously, we identified a similar condensin complex in human cells and identified the non-SMC component CNAP1, a human homolog of XCAP-D2 (thus, also designated hCAP-D2), as part of human condensin (28). CNAP1 was also termed Eg7, and blocking the function of its Xenopus homolog was shown to have deleterious effects on mitotic chromosome condensation and metaphase progression (6, 30). Thus, human condensin contains a heterodimer of hCAP-C and hCAP-E, as well as the three non-SMC components: CNAP1 (hCAP-D2/Eg7), hCAP-G, and hCAP-H (17, 28).
The function of condensin has been addressed extensively in vitro by using the immunopurified endogenous complex and naked plasmid DNA (18). In the presence of ATP, condensins from both Xenopus and humans facilitate positive DNA supercoiling with topoisomerase I and induce chiral knotting with topoisomerase II (17, 18). Among the condensin subunits, the SMC proteins are thought to play a central role in structural changes of chromosomes due to their unique motor-like protein structure. Each SMC protein is composed of conserved head and tail domains with ATPase motifs and two coiled-coil regions separated by a flexible hinge that allows a wide range of motion (2, 23, 34). Taken together with the previous observation that SMC proteins bind to DNA in vitro (1), it was postulated that SMC proteins bind DNA and potentially generate the force to move it, as would be required for chromosome condensation (10). However, the SMC heterodimer alone is not sufficient to perform the in vitro activity of condensin (11). Furthermore, condensin activity was detected only with the mitotic (as opposed to the interphase) condensin, which results from mitosis-specific phosphorylation of the non-SMC proteins XCAP-H, XCAP-D2, and XCAP-G by cdc2-cyclin B (15). These results suggest that the observed in vitro function of condensin on naked DNA reflects the basic mechanism of chromosome condensation in the cell and that the non-SMC components of condensin play important roles in the mitotic activation of condensin.
We demonstrated previously that human condensin localizes predominantly to the cytoplasm during interphase and relocalizes to chromosomes at the G2/M transition (28). Thus, the mitotic function of condensin appears to be in part regulated by cell cycle-specific subcellular targeting in human cells. A similar interphase-specific cytoplasmic localization of condensin was observed in Schizosaccharomyces pombe (31). Interestingly, a small population of human condensin is found in the nucleus as chromosome-associated foci throughout interphase, which become larger and fewer during G2 phase (28). These foci appear to be part of a condensation intermediate structure that forms around the centromeres, together with clusters of phosphorylated histone H3 at late G2 and early prophase (28). Phosphorylation of histone H3 was shown to be required for proper condensation and segregation of mitotic chromosomes in Tetrahymena (33). Thus, the nuclear condensin foci in interphase cells appear to be involved in reinitiation of mitotic chromosome condensation.
The mechanism of condensin targeting to chromosomes remains an enigma. In S. pombe, Cdc2 phosphorylation of an N-terminal residue (T19) of Cut3 (an hCAP-C homolog) is required for relocalization of condensin from the cytoplasm to the mitotic chromosomes (31). However, this site is not conserved in the human homolog. More recently, the nuclear matrix-associated protein kinase A anchoring protein (AKAP95) was shown to be important for Eg7 (CNAP1) targeting to mitotic chromosomes, although it has not been demonstrated whether this interaction is direct (30). An in vitro study of Xenopus condensin revealed that the non-SMC components function in stable chromatin binding of the condensin complex, suggesting the importance of the non-SMC components for chromosome association of condensin (16). However, the exact roles of the individual non-SMC components, particularly in vivo, are not well understood.
We focused here on the in vivo characterization of human CNAP1 (hCAP-D2/Eg7), using green fluorescent protein (GFP)- and FLAG-tagged CNAP1 deletion mutants expressed in human cells to identify key features of this protein. We found that CNAP1 possesses a distinct domain in its carboxyl (C) terminus that is necessary and sufficient for direct binding to chromosomes. The same region also contains a functional bipartite nuclear localization signal (NLS), whose activity appears to be suppressed by the middle portion of the protein. A mutant CNAP1 missing this C-terminal chromosome-targeting domain was able to form a complete condensin complex but failed to associate with mitotic chromosomes. The chromosome-targeting domain specifically interacts with histones H1 and H3 in vitro and coprecipitates with phosphorylated histone H3 in vivo, thus defining a novel histone-binding domain. Our results indicate that CNAP1 is capable of targeting itself to mitotic chromosomes and raises the possibility that this activity may be important for chromosome targeting of condensin in human cells.
MATERIALS AND METHODS
Cell lines.
HeLa and 293T cells were grown in Dulbecco’s modified Eagle medium (Sigma Chemical Co.) supplemented with 10% fetal bovine serum, l-glutamate, and penicillin-streptomycin.
Antibodies.
The human chromosome-associated protein H (hCAPH-N) polyclonal rabbit antibody was generated against a 211-amino-acid (aa) amino terminus fragment. The antibody was affinity purified with the same peptide. The anti-hCAP-E and hCAP-C antibodies were described previously (27). The affinity-purified polyclonal hCAP-G antibody was raised against a peptide representing the carboxyl-terminal 18 aa of hCAP-G. For Western blotting, we used either anti-FLAG M2 monoclonal antibody (Sigma, St. Louis, Mo.), a monoclonal anti-GFP antibody (Clontech, Palo Alto, Calif.), polyclonal anti-phosphorylated H3 antibody, or polyclonal anti-phosphorylated H1 antibody (both from Upstate Biotechnology, Lake Placid, N.Y.). The secondary antibodies used for Western analyses were anti-rabbit or anti-mouse conjugated with alkaline phosphatase (Promega, Madison, Wis.). Antibodies for immunofluorescence included the anti-FLAG M2 monoclonal antibody with an anti-mouse fluorescein isothiocyanate-conjugated secondary antibody (Vector Labs, Inc., Burlingame, Calif.). Additional antibodies utilized for immunoprecipitation included an anti-CNAP1 antibody described previously (28) and the Living Colors full-length A.v. polyclonal antibody (Clontech) against GFP.
Plasmids.
Constructs were generated by either PCR with specific primers or restriction digests with a full-length clone of CNAP1 (KIAA0159; kindly provided by Kazusa DNA Research Institute, Chiba, Japan). Fusion proteins used for far-Western and peptide-binding experiments were cloned into pGEXGFP, a plasmid combining a derivative of pGEX (Amersham, Piscataway, N.J.) with GFP cloned in-frame with the glutathione S-transferase (GST) reading frame. The GFP-CNAP1 fusion mutants were also cloned into the Tet-Off vector. The specific amino acids of CNAP1 included in each construct are indicated in the figures. For the FLAG constructs, restriction fragments of the full-length molecule or specific PCR fragments were first cloned into pBluescript (Stratagene, La Jolla, Calif.) with an added FLAG tag. Like the GFP-CNAP1 fusions mentioned above, the resulting FLAG-CNAP1 constructs were then cloned into the Tet-Off plasmid. Point mutants were generated with PCR primers incorporating the desired mutations, and introduction of these mutations into each construct was confirmed by DNA sequencing.
Transfection and synchronization.
Cells were transfected by using either a calcium phosphate protocol or Effectene (Qiagen, Valencia, Calif.). For the calcium phosphate procedure, cells were seeded 12 to 20 h prior to transfection. The transfected constructs were all in the Tet-Off vector (Clontech). The DNA-calcium phosphate precipitate was left on the cells for 7 to 11 h, and the media were then changed. The transfected cells were examined or processed for extracts at 24 to 48 h posttransfection. Transfections with Effectene were done according to manufacturer's instructions. DNA in live cells was visualized with Hoechst 33342 (Molecular Probes, Eugene, Oreg.). Photographs of live cells were obtained with an Olympus IX70 microscope and a MagnaFire digital charge-coupled device camera system.
In order to enrich for mitotic cells, HeLa cells were transfected with the expression plasmids concomitant with cell synchronization to M phase by double thymidine block and a brief nocodazole treatment. Synchronization of transfected HeLa cells to M phase was modified from the procedure described previously (19, 28). Briefly, we did a double thymidine block with transfection performed immediately after washing off the first thymidine treatment. The transfected cells underwent a second thymidine treatment 9 h later. The cells were subsequently released from the thymidine and were treated with nocodazole. The nocodazole was removed several hours later, and the cells were fixed or extracts were made when the cells had progressed well into metaphase. Experiments were repeated multiple times. There was no difference between the localization patterns of the mutants in mitotic cells obtained by the synchronization and those found in the asynchronous population.
Immunofluorescence.
Cells were grown on glass coverslips in 24-well plates until 50 to 70% confluent. Mitotic chromosome spreads were prepared essentially as described previously (27). The coverslips were washed in phosphate-buffered saline twice and then fixed with 4% paraformaldehyde at 4°C. Cells and spreads were subjected to immunofluorescent staining as described previously (27). For DNA detection, DAPI (4′,6′-diamidino-2-phenylindole) was used. The coverslips were then rinsed in distilled H2O and mounted onto slides with “antifade” (0.055 mM p-phenylenediamine dihydrochloride in phosphate-buffered saline with glycerol added to 90%) (13).
Image analysis was performed by using a Zeiss Axioplan 2 microscope with a Photometrics Sensys CCD Camera.
Co-IP, Western blotting, and GST pull-downs.
Extracts from transfected cells were prepared as previously described (27). Coimmunoprecipitations (Co-IPs) were performed essentially as described previously (28) with a 1 M KCl salt wash for the anti-hCAP-E and anti-hCAP-H Co-IPs. The anti-hCAP-G Co-IPs were washed five times with 0.2 M KCl buffer prior to the guanidine-HCl elution. For the anti-GFP Co-IPs and the anti-phosphorylated H3 Co-IP, five 0.4 M KCl buffer washes were used. Precipitated proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blotting as described previously (28). The GST pull-down experiments were done as described, but sequential washes of 0.2, 0.4, and 0.5 M NaCl were performed (14). Purified H1 was the kind gift of C. P. Verrijzer (University of Leiden, Leiden, The Netherlands).
Far-Western blot analysis.
Nuclear extracts and nuclear pellet preps from HeLa cells were prepared as described previously (27). Nuclear pellet samples were applied to 15% gels. After SDS-PAGE the denatured proteins were transferred to nitrocellulose (in the absence of methanol). The filters were placed in a blocking buffer consisting of HBB (25 mM HEPES, pH 7.7; 25 mM NaCl; 5 mM MgCl2; 1 mM dithiothreitol [DTT]), 5% milk, and 0.05% NP-40. The proteins on the filters were then treated with 6 M guanidine-HCl-HBB and then slowly renatured by serial dilution of the guanidine-HCl with HBB. The membranes were then reblocked and probed. The probes were GST-GFP (GG) fusions with the desired region of CNAP1. The chimeric proteins were expressed in bacteria and purified by using glutathione-Sepharose beads (Amersham), and samples of each were checked for expression by SDS-PAGE. Comparable amounts of each protein were then labeled in vitro with protein kinase A and [γ-32P]ATP. Labeled proteins were eluted from the glutathione beads, and an equal amount (in counts per minute [cpm]) of each probe was applied to the filters described above in hybridization buffer (20 mM HEPES, pH 7.7; 75 mM KCl; 0.1 mM EDTA; 2.5 mM MgCl2; 1% milk; 1 mM DTT; 0.05% NP-40). After overnight hybridization at 4°C, the filters were washed three times with fresh hybridization buffer and exposed to film. For the purified bovine H1 and purified bovine total histone (Roche, Indianapolis, Ind.) far-Western analyses, 5 μg of total histone and 1 μg of H1 were loaded. For the core histone far-Western analysis, 1 μg of purified Xenopus histone H3 (Upstate) and 10 μg of total histone were loaded.
Peptide-binding assay.
Three peptides encoding different variants of the histone H3 tail were used (4). The unmodified H3 peptide corresponds to the first 20 aa of the H3 N terminus (i.e., ARTKQTARKSTGGKAPRKQLC). Both the phosphorylated and the acetylated peptides correspond to aa 7 to 20 and thus are shorter than the unmodified peptide. All three peptides were the generous gift of C. David Allis (University of Virginia). The peptides were each cross-linked to Sulfolink Coupling Gel (Pierce, Rockford, Ill.) according to the manufacturer's instructions. The same probes used in far-Western analysis were allowed to bind to the coupled peptides in HEMG buffer (25 mM HEPES, pH 7.6; 12.5 mM MgCl2; 0.1 mM EDTA; 10% glycerol) with 100 mM KCl, 1 mM DTT, and 0.1% NP-40. Binding was done on a nutator at 4°C for 2.5 h. The samples were then washed with fresh binding buffer four times, combined with loading buffer, boiled, and applied to an SDS-10% PAGE gel. The gel was dried and exposed to film. The signals were quantitated by using ImageQuant (Molecular Dynamics, Sunnyvale, Calif.).
RESULTS
CNAP1 contains the nuclear localization and chromosome-targeting domains in the carboxyl terminus.
We established previously that CNAP1, a 1,401-aa component of the human condensin complex, is predominantly in the cytoplasm during interphase and associates with chromosomes during mitosis (28). To dissect the domains of CNAP1 that may be involved in such dynamic subcellular distribution, CNAP1 full-length and deletion mutants were fused to either GFP or FLAG, and their localization in transfected human 293T cells was examined. GFP-CNAP1 (full-length) localizes to the cytoplasm in interphase cells and associates with condensed chromosomes in mitotic cells similar to the endogenous CNAP1 (Fig. 1, panels 1 to 3; compare with GFP and untransfected controls, panels 10 to 14). A construct representing the first 603 aa of CNAP1 (GFP-NM) is strictly cytoplasmic in interphase and does not bind mitotic chromosomes (Fig. 1, panels 4 to 6). Interestingly, we found that the C-terminal 400 aa of the protein (GFP-C) associates with chromosomes as the full-length form does in mitotic cells but localizes exclusively to the nucleus in interphase cells (Fig. 1, panels 7 to 9). Similar results were obtained when the “C” and “NM” constructs were fused to FLAG instead of GFP, or when they were transfected into HeLa cells (data not shown). Thus, protein domains sufficient for both nuclear localization and chromosome binding reside in the C terminus of CNAP1.
FIG. 1.
Subcellular localization of GFP-CNAP1 and GFP-CNAP1 deletion mutants. GFP constructs were transfected into 293T or HeLa cells, and their localization in both interphase and mitosis was observed. DNA was stained with either DAPI or Hoechst 33342. The GFP signal is shown for the interphase cells (panels 1, 4, 7, and 10), whereas images for both GFP (panels 2, 5, 8, 11, and 13) and DNA (panels 3, 6, 9, 12, and 14) staining are presented for mitotic cells. A schematic of each construct is shown with the amino acids indicated.
The bipartite NLS in CNAP1-C is functional.
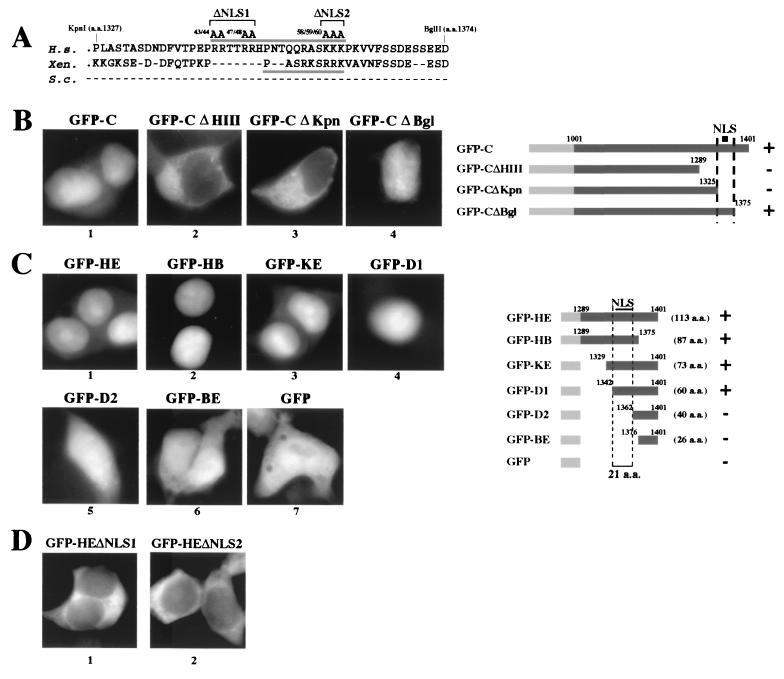
Amino acid sequence analysis revealed that, within the 21 aa between residues 1342 and 1362 in the C terminus of CNAP1, there is a potential bipartite NLS sequence (Fig. 2A). One-half of the site is conserved in Xenopus, but the entire region is missing in both budding and fission yeasts (9, 11, 31). Further deletion analysis revealed that the nuclear localization of GFP-C is entirely dependent on the putative NLS (Fig. 2B), and the NLS is sufficient to direct the nuclear localization of GFP (Fig. 2C). Thus, this bipartite NLS is functional. Point mutations (Fig. 2A) of the basic residues in either the first half or the second half of the bipartite sequence (ΔNLS1 and ΔNLS2, respectively) abolished nuclear localization, demonstrating that both portions are required for NLS function in human cells (Fig. 2D).
FIG. 2.
CNAP1 contains a functional bipartite NLS near its C terminus. (A) Comparison of the primary sequence between residues 1327 and 1374 of human (H.s.) CNAP1 with the same region in the Xenopus (Xen.) XCAP-D2 protein. Note that there is no comparable sequence found in S. cerevisiae (S.c.) or S. pombe (not shown). The bipartite NLS in the human protein is indicated by the bar over the human sequence, whereas the likely NLS in the frog protein is underlined. The positions of the ΔNLS1 and ΔNLS2 alanine substitution mutations are indicated. (B) The putative NLS region is required for the nuclear localization of GFP-C. Transfected 293T cells were fixed on slides and stained with DAPI. A schematic of the constructs used is on the right. A “+” indicates specific nuclear localization, whereas a “−” indicates no specific nuclear localization. (C) Deletion analysis of the NLS. Constructs containing subfragments of the C terminus fused to GFP were expressed in 293T cells, and their behavior was observed. A 21-residue sequence that contains the putative bipartite NLS is required for nuclear localization. A summary schematic is shown. (D) Alanine substitution of basic residues in either half of the putative bipartite NLS demonstrates that mutation of either basic region severely compromises nuclear accumulation. The mutations were made in the context of the GFP-HE construct, which is constitutively nuclear when expressed (see panel C, frame 1). The locations of the alanine substitutions are indicated in panel A.
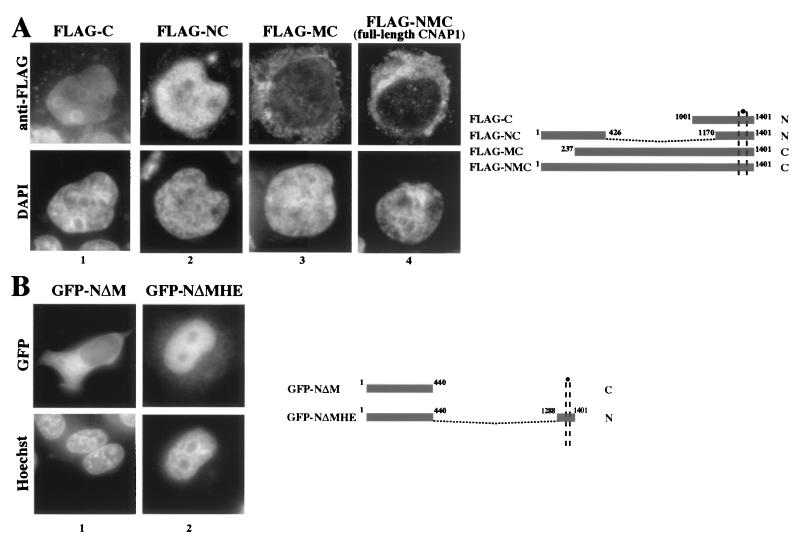
Although the NLS in the C terminus is functional, the endogenous CNAP1 is largely in the cytoplasm during interphase (Fig. 1, panel 1) (28). It is possible that other regions of CNAP1 regulate NLS function. To test this, FLAG-tagged full-length CNAP1 and various deletion mutants were transfected into 293T cells, and their localization was examined (Fig. 3A). We observed nuclear staining only with the two mutant proteins that were missing the middle portion of the molecule, namely, FLAG-C and FLAG-NC, whereas FLAG-NMC (full-length CNAP1) and FLAG-MC were cytoplasmic. To confirm that this was not an artifact of the fixation process, we also examined the localization of GFP-tagged mutants in live cells. We constructed a GFP fusion protein (GFP-NΔMHE) that was similar (but not identical) to FLAG-NC and found that it, too, was primarily nuclear, in contrast to the GFP-NΔM fusion (Fig. 3B). Both GFP-C and GFP-CNAP1 (full-length) demonstrate the same interphase localization as their FLAG-tagged counterparts (compare Fig. 3A, panels 1 and 4, with Fig. 1, panels 1 and 7). These results indicate that CNAP1 contains only one functional NLS at the C terminus and the middle portion of the protein appears to suppress the function of this NLS.
FIG. 3.
The middle domain functionally suppresses the activity of the bipartite NLS. (A) FLAG-tagged CNAP1 constructs were transfected into 293T cells that were subsequently spun onto coverslips. Cells were fixed, and indirect immunofluorescence with anti-FLAG antibody was performed. A schematic of the constructs detailing the amino acids included in each is shown. “N” refers to nuclear localization, whereas “C” refers to cytoplasmic localization. (B) In order to rule out fixation artifact, we tried to duplicate the above results with GFP constructs expressed in live 293T cells. Both GFP-C and GFP-CNAP1 are shown in Fig. 1. The GFP-NΔMHE construct is similar to the FLAG-NC construct shown in panel A and was created by religating an in-frame HindIII deletion of GFP-CNAP1. A schematic is shown at right.
Minimum region of CNAP1-C required for mitotic chromosome targeting.
The C-terminal region of CNAP1 not only contains the functional bipartite NLS but is also sufficient for mitotic chromosome association (Fig. 1, panels 8 and 9). To dissect the minimum region within CNAP1-C required for chromosome targeting, deletion mutants fused to GFP were expressed in human cells, and their mitotic chromosome association was observed (Fig. 4). Observation was made in live cells to avoid potential fixation artifacts. However, we noted similar results by using fixed metaphase chromosome spreads as well (data not shown). We found that GFP fused to the last 60 aa of CNAP1 (GFP-D1) efficiently localized to the mitotic chromosomes, while GFP alone did not (Fig. 4A). Further deletions revealed that the 21-aa region between residues 1342 and 1362, which precisely overlaps with the NLS, is essential for mitotic chromosome association. Mutant proteins that included these residues both moved to the nucleus and were found on chromosomes in mitotic cells (Fig. 2C and 4A, GFP-HE, -HB, and -D1). In contrast, the mutants missing these 21 residues not only failed to go to the nucleus but also did not bind to mitotic chromosomes (GFP-D2 and -BE). Therefore, the 21-aa NLS region is required for both nuclear localization and chromosome association of CNAP1. Does the NLS play a role in chromosome targeting during mitosis? Alanine substitutions of the basic residues within either half of the bipartite NLS, which abolished NLS activity in interphase cells (Fig. 2D), slightly weakened but did not ablate chromosome association during mitosis (Fig. 4B). These results indicate that the critical amino acid residues within the NLS region required for nuclear localization and chromosome targeting are distinct.
FIG. 4.
The mitotic chromosome-targeting domain overlaps the NLS but requires distinct amino acids. Mitotic chromosome association of GFP fusions of C-terminal deletion mutants were examined in live cells. DNA was detected by viable Hoechst 33342 staining. (A) The images demonstrate that a 21-aa segment (between residues 1342 and 1362) that overlaps the bipartite NLS is required for in vivo association with mitotic chromosomes. A construct schematic is shown on the right. A “+” indicates mitotic chromosome localization, whereas a “−” indicates a lack of distinct chromosome staining. (B) Alanine mutations in the NLS (ΔNLS1 and ΔNLS2) that disrupt nuclear localization (Fig. 2D) do not ablate chromosome association. (C) Full-length constructs were transfected into HeLa cells that were subsequently synchronized to mitotic phase. GFP-CNAP1 and GFP-CNAP1ΔNLS2 both bind to mitotic chromosomes, whereas a full-length construct with a deleted HE region (GFP-CNAP1ΔHE) does not bind. Identical results were obtained in asynchronous transfected cells.
In order to determine whether the identified chromosome-targeting domain of CNAP1 is functional in the full-length context, we made two mutants in the GFP-CNAP1 construct. One construct incorporated the NLS point mutation (ΔNLS2). The second construct has a deletion of the HE region (ΔHE, removal of residues 1289 to 1401), which contains the NLS and overlapping mitotic chromosome-targeting domain. We then examined their localization in synchronized mitotic cells and compared this to the wild-type GFP-CNAP1. The GFP-CNAP1, like the endogenous CNAP1, was clearly bound to mitotic chromosomes (Fig. 4C, panels 2). The GFP signal was particularly strong along the core of each chromosome, a finding consistent with an earlier report on the endogenous protein (30). Mitotic chromosome association was also seen for GFP-CNAP1ΔNLS2 (Fig. 4C, panels 3), a finding consistent with the observation with the shorter fragment (Fig. 4B). Importantly, however, GFP-CNAP1ΔHE was unable to bind to mitotic chromosomes (Fig. 4C, panels 4). These results demonstrate that the identified chromosome-targeting domain in the HE region is required for CNAP1 to bind to mitotic chromosomes.
The CNAP1 chromosome-targeting domain does not require the other condensin subunits.
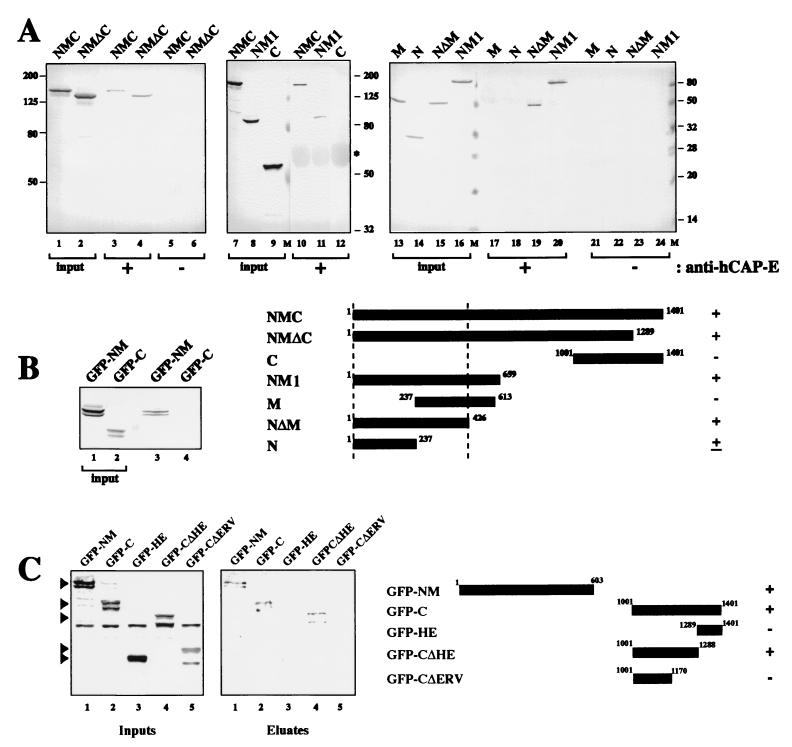
To determine whether the chromosome-targeting activity contained in the HE domain is indirectly mediated by interaction with other condensin components, we attempted to discern which regions of CNAP1 were required for interaction with the hCAP-E-hCAP-C heterodimer, hCAP-G, and hCAP-H. We performed Co-IP of cell extracts expressing either FLAG-tagged or GFP fusion CNAP1 deletion mutants with antibodies specific for hCAP-E, hCAP-H, or hCAP-G, followed by a Western blot with anti-FLAG or anti-GFP antibody to detect the potential association of the CNAP1 deletion mutants (Fig. 5). We have previously demonstrated that almost 100% of the hCAP-C and hCAP-E are engaged in an extremely stable heterodimer independent of the non-SMC condensin components (27). Our anti-hCAP-E antibody was shown to precipitate the hCAP-C-hCAP-E heterodimer in a stoichiometric manner, as well as the three non-SMC-associated proteins (28). Using stringent wash conditions (1 M KCl), which enable the specific purification of condensin (28), the anti-hCAP-E antibody specifically precipitated FLAG-NM1 (aa 1 to 659) but not FLAG-C (Fig. 5A). Analysis of smaller deletion mutants showed that the first 426 aa (FLAG-NΔM) are sufficient for interaction with hCAP-C-hCAP-E. Similarly, we found that our anti-hCAP-H antibody coimmunoprecipitated GFP-NM (aa 1 to 603) but not GFP-C (Fig. 5B). Thus, the amino-terminal half of CNAP1, but not the C-terminal half, is required for the interaction with the hCAP-C-hCAP-E heterodimer and hCAP-H.
FIG. 5.
Identification of CNAP1 subdomains required for interactions with other condensin components. (A) Extracts from 293T cells transfected with the indicated FLAG fusion proteins were used for immunoprecipitation (IP) with anti-hCAP-E antibody, followed by anti-FLAG antibody-Western analysis. Blots are shown with indicated “input” (lanes 1, 2, 7 to 9, and 13 to 16), immunoprecipitation with anti-hCAP-E (lanes 3, 4, 10 to 12, and 17 to 20), and immunoprecipitation without anti-hCAP-E (lanes 5, 6, and 21 to 24). A summary schematic is shown. +, coprecipitation; −, no coprecipitation; ±, weak coprecipitation. (B) Co-IP analysis was done with anti-hCAP-H antibody for immunoprecipitation, and an anti-GFP antibody was used for the Western blot. The schematic for the two GFP fusions (i.e., GFP-NM and GFP-C) is shown below in panel C. (C) Co-IP of CNAP1 deletion mutants with anti-hCAP-G antibody. Cytoplasmic extracts of 293T cells transfected with the constructs indicated in the schematic were immunoprecipitated with anti-hCAP-G antibody, followed by anti-GFP Western analysis.
Interestingly, a similar set of experiments with anti-hCAP-G antibody revealed that hCAP-G interacts with both GFP-NM and GFP-C (Fig. 5C, “Eluates” lanes 1 and 2). Since antibodies against neither hCAP-C-hCAP-E nor hCAP-H coprecipitated CNAP1-C, the interaction between CNAP1-C and hCAP-G in the condensin complex is most likely direct. Further deletions of GFP-C revealed that removing a region between residues 1170 and 1288 severely compromised interaction with hCAP-G (Fig. 5C, eluate lanes 3, 4, and 5). Importantly, this region is upstream of the HE region, and hCAP-G failed to bind to GFP-HE (Fig. 5C, eluate lane 3). Therefore, we conclude that the HE region is not involved in any interaction with the hCAP-C-hCAP-E heterodimer, hCAP-H, or hCAP-G and that its chromosome-targeting function is not elicited through interaction with other condensin components.
To substantiate the results, we next tested whether the identified interaction domains, excluding the HE region, are sufficient for stable condensin complex formation in vivo. We performed reciprocal Co-IP with anti-GFP antibody to precipitate the GFP-CNAP1 mutants and probed for the presence of other condensin components by a Western blot analysis with a mixture of antibodies against hCAP-C, hCAP-E, hCAP-G, and hCAP-H. By this method, only those condensin components that were interacting with the GFP mutant proteins would be coimmunoprecipitated. In this method, minor interactions may not be detectable, since the unincorporated GFP fusion protein monomers will titrate the anti-GFP antibody. As a positive control we performed a Co-IP with an anti-CNAP1 antibody that was shown previously to precipitate the endogenous condensin complex (Fig. 6A, lanes 1 and 5) (28). The results show that GFP-CNAP1 clearly coprecipitated the rest of the condensin in a manner similar to the endogenous CNAP1, indicating that it is efficiently incorporated into the complex (Fig. 6A, lanes 2 and 6). GFP-CNAP1ΔNLS2, which is able to bind to mitotic chromosomes (Fig. 4C), was also assembled into a complete condensin complex (Fig. 6B, lanes 2 and 5). Significantly, we found that GFP-CNAP1ΔHE, which does not associate with mitotic chromosomes (Fig. 4C), forms a stable complex with the rest of the condensin components similar to the wild type (Fig. 6A, lanes 3 and 7), demonstrating that the HE region is not required for stable condensin complex formation.
FIG. 6.
Co-IP of condensin components with GFP fusion proteins. (A) Cells were transfected with either GFP-CNAP1, GFP-CNAP1ΔHE, or GFP-NM (see schematic on the right). Cytoplasmic extracts from these cells were immunoprecipitated with anti-GFP antibody, and the coprecipitated condensin subunits were detected with antibodies to hCAP-C, hCAP-E, hCAP-G, and hCAP-H. The beads were boiled and loaded onto a separate gel, and the GFP fusions were detected with a monoclonal anti-GFP antibody. (B) Same procedure as in panel A but with cytoplasmic extracts from cells transfected with GFP-CNAP1ΔNLS2 or GFP-CNAP1ΔERV.
Interestingly, GFP-NM, which was shown above to interact with the other four condensin components (Fig. 5), is poorly incorporated into a stable condensin complex (Fig. 6A, lanes 4 and 8). To test the possibility that the C-terminal second hCAP-G binding site (Fig. 5C) is important for stable condensin formation, we further truncated the C terminus of the full-length CNAP1 so that the second hCAP-G binding site was removed (GFP-CNAP1ΔERV). Indeed, this mutant protein was not incorporated into the complex, indicating that the C-terminal hCAP-G binding site is essential for condensin assembly (Fig. 6B, lanes 3 and 6).
Taken together, these results demonstrate that the portion of CNAP1 required for condensin complex formation is distinct from the chromosome-targeting domain contained in the HE (final 113 aa) region of the protein. This region does not interact with other components of condensin, and therefore its targeting function is independent of the rest of the condensin components.
The chromosome-targeting domain of CNAP1 interacts with histones H1 and H3.
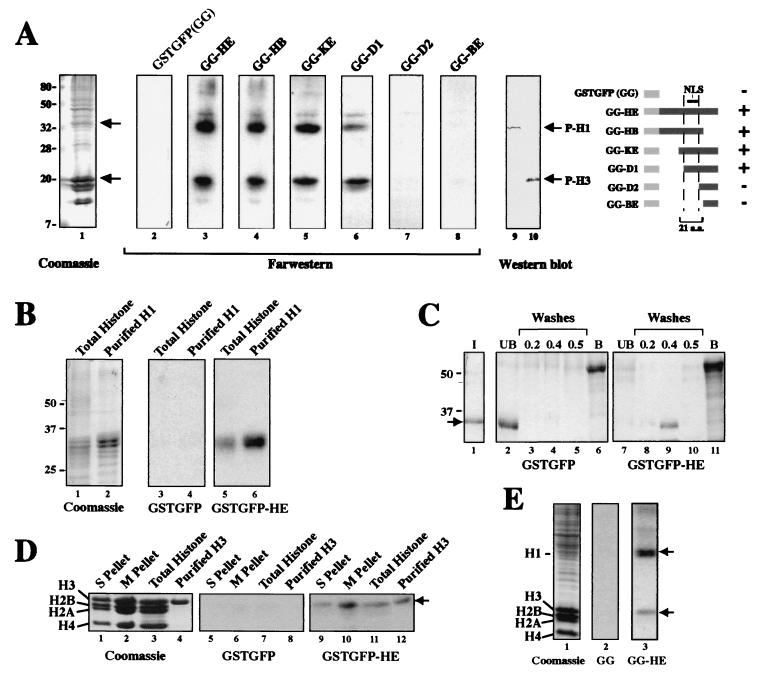
What mediates the chromosome targeting of CNAP1? To address this question, far-Western analysis was carried out against HeLa insoluble fractions enriched in chromatin. Using GG-HE expressed as a bacterial fusion protein probe, two major protein species in the chromatin fraction were specifically labeled (Fig. 7A, lane 3). The smaller deletion mutants demonstrated that the signals were dependent on the presence of the 21-aa NLS region in the probes (Fig. 7A, lanes 4 to 8). By comparing the Coomassie staining (Fig. 7A, lane 1) and Western blot analysis of the same HeLa fraction with antibodies specific for phosphorylated histones H1 and H3 (Fig. 7A, lanes 9 and 10), these two species were identified as histones H1 and H3. Therefore, CNAP1-HE interacts with histones H1 and H3 in vitro, which is dependent upon the presence of the 21-aa segment within the HE region required for mitotic chromosome-targeting in vivo.
FIG. 7.
In vitro interaction of the CNAP1-C mutants and histones H1 and H3. (A) Far-Western analysis. The same GFP chimeric proteins used in the nuclear localization study (see Fig. 2 and schematic) were fused in-frame to the C terminus of GST, labeled with [γ-32P]ATP, and used to probe filters containing a HeLa protein fraction enriched for chromatin. A schematic of the constructs used for probes is also shown. A “+” indicates binding with histones H1 and H3, whereas a “−” indicates no binding. (B) Far-Western analysis with the GG and GG-HE probes described above against purified bovine total histone and purified bovine H1. (C) GST pull-down assay with glutathione bead-bound GG or GG-HE and purified H1. The “I” (lane 1) represents 30% of the input of H1 (see arrow). “UB” (lanes 2 and 7) refers to the unbound fraction, whereas the “washes” (lanes 3 to 5 and 8 to 10) refer to the stepwise 0.2, 0.4, and 0.5 M NaCl washes. “B” (lanes 6 and 11) indicates the GG or GG-HE bound to the beads. (D) Far-Western analysis with the same probes as in panel B against core histones derived from an S-phase-insoluble fraction (S pellet, lanes 1, 5, and 9), an M-phase insoluble pellet (M pellet, lanes 2, 6, and 10), purified bovine total histone (lanes 3, 7, and 11), and purified Xenopus histone H3 (lanes 4, 8, and 12). The arrow indicates the position of histone H3. (E) Far-Western analysis with the same probes as in panel B against the S-phase insoluble pellet. The upper and lower arrows indicate the positions of histone H1 and histone H3, respectively.
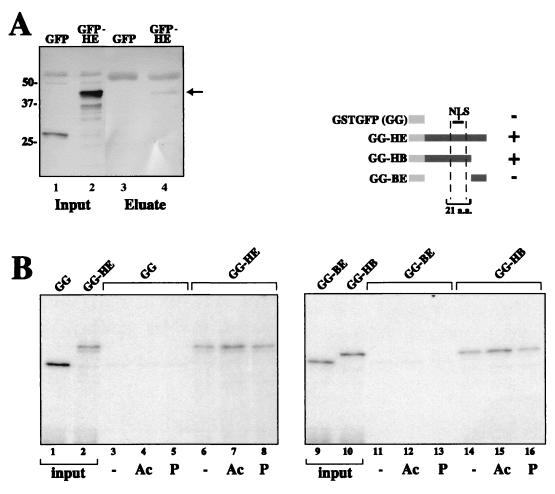
Specificity of H1 binding was confirmed by the far-Western analysis of purified total histones and histone H1 probed with GG and GG-HE (Fig. 7B). The interaction between CNAP1-HE and histone H1 was further demonstrated by a GST pull-down assay (Fig. 7C). The purified H1 was added to either GG or GG-HE on beads, which were washed with buffers of increasing salt (NaCl) concentrations. We consistently observed that histone H1 binds to the GG-HE in a 0.4 M NaCl wash-sensitive manner, while it was found in the unbound fraction of GG (Fig. 7C, compare lanes 2 and 9). Thus, the HE region specifically binds to histone H1, a finding consistent with the results of the far-Western analyses.
To confirm the binding to histone H3, we repeated the far-Western analysis of an S-phase insoluble fraction, an M-phase insoluble fraction (same as in Fig. 7A), purified total histones, and purified histone H3 with GG and GG-HE (Fig. 7D). H3 binding was found in both interphase and mitotic insoluble fractions, and GG-HE binding was specific for H3 and not other core histones. Similarly, the interaction of GG-HE with histone H1 was seen with an interphase insoluble fraction, demonstrating that the interactions of CNAP1-HE with histones H1 and H3 are not dependent on mitosis-specific phosphorylation of H1 and H3 (Fig. 7E).
Previously, by using reciprocal Co-IPs of mitotic extracts with antibodies against CNAP1 and phosphorylated histone H3, we showed that the endogenous condensin complex associates with histone H3 in vivo (28). Using a similar strategy, antibody against phosphorylated histone H3 specifically coprecipitated GFP-HE but not GFP alone from mitotically synchronized and transfected HeLa extracts, indicating that CNAP1-HE is capable of interacting with histone H3 in vivo (Fig. 8A).
FIG. 8.
Interaction of histone H3 and the CNAP1 chromosome-targeting domain. (A) In vivo Co-IP of GFP-HE with antibody against phosphorylated histone H3. Nuclear extracts derived from cells expressing either GFP-HE or GFP alone were immunoprecipitated with anti-phosphor-H3 antibody, and the immunoprecipitated proteins were subjected to SDS-PAGE. The proteins were transferred to nitrocellulose, and a Western blot with anti-GFP was performed. The arrow indicates the position of the immunoprecipitated GFP-HE (lane 4). (B) An in vitro peptide binding assay suggests that the H3 interaction of GG-HE is mediated through the H3 tail. Beads cross-linked to H3 tail peptides that are either phosphorylated (P), acetylated (Ac), or unmodified (−) were each incubated with each of the four probes shown in the schematic (specifically GG, GG-HE, GG-HB, and GG-BE), washed, and run on SDS-PAGE. Input lanes represent 5% of the input probes. +, binding; −, no specific binding.
To better characterize the binding between CNAP1 and histone H3 and to assess the role of the H3 tail, three different N-terminal histone H3 tail peptides (a generous gift from C. David Allis) were conjugated to resin and used for in vitro peptide-binding assays. Only the deletion mutants containing the essential 21-aa region found in the HE segment were able to specifically bind to the H3 tails (Fig. 8B). This interaction is not affected by covalent modification of the H3 tail by either acetylation on lysine 14 or phosphorylation on serine 10 (4). Taken together, these results indicate that, in addition to binding to histone H1, CNAP1 also associates with the histone H3 tail.
DISCUSSION
Although the SMC proteins are known to play central roles in the activity of the condensin complex, it is now clear that the non-SMC components play equally critical roles in regulating SMC functions and stabilizing condensin binding to chromatin (11, 12, 15-17, 30). Consistent with this, two recent studies provided visual evidence of the association of the non-SMC components with the N- and C-terminal globular domains of SMC proteins, which contain ATPase motifs and in vitro DNA-binding domains (2, 34). Previous studies demonstrated that CNAP1 is a target for mitosis-specific phosphorylation by cdc2 and that blocking its function with antibody resulted in the disruption of condensation in Xenopus (6, 15). Nevertheless, the exact role of this protein was unclear. In the present study, we dissected the functional domains of human CNAP1 and identified the domain required for its mitotic chromosome targeting. Importantly, this domain is distinct from those involved in interactions with other condensin components (Fig. 9). A full-length CNAP1 missing the chromosome-targeting domain was efficiently incorporated into the condensin complex but failed to associate with mitotic chromosomes, indicating the indispensable role of this domain in CNAP1 targeting to mitotic chromosomes. We found that this domain interacts directly with histones H1 and H3 in vitro. It also interacts with histone H3 in vivo, which is consistent with our previous observation that the condensin complex interacts with histone H3 in vivo (28). Thus, our results define a novel histone-binding domain in one of the condensin components, which may be critical for mediating the chromosome association of condensin.
FIG. 9.
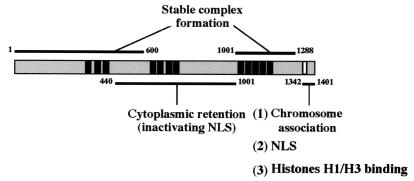
Schematic diagram of CNAP1 functional subdomains based on the data presented in the present study. The N terminus (residues 1 to 600) interacts with the hCAP-C-hCAP-E heterodimer, hCAP-H, and hCAP-G. The C-terminal region (residues 1001 to 1288) upstream of the chromosome-targeting domain interacts with hCAP-G. The mitotic chromosome-targeting domain (residues 1342 to 1401) includes the 21-aa essential domain (white box) superimposed upon the bipartite NLS. The NLS activity is suppressed by residues contained in the middle portion (residues 440 to 1001) of the CNAP1 molecule. The black boxes represent the locations of sequence homologous to the HEAT repeats originally described in Xenopus XCAP-D2 (24).
Condensin complex formation.
Our in vivo Co-IP experiments with CNAP1 deletion mutants revealed the structural basis for stable condensin complex formation. The N-terminal half of CNAP1 is involved in interactions with the hCAP-C-hCAP-E heterodimer, hCAP-H, and hCAP-G. However, stable complex formation appears to require the interaction of hCAP-G at an additional C-terminal region (Fig. 9). Interestingly, both the N- and C-terminal interaction domains of CNAP1 contain clustered HEAT repeats conserved in humans and Xenopus (24). HEAT repeats are flexible, bihelical, tandemly organized structures that function as scaffolds to which other factors can bind (24). Significant effects on protein-protein interactions after even partial truncation of a single HEAT repeat were demonstrated in studies characterizing the binding between importin-β, importin-α, and Ran (5, 20). Therefore, the interactions with other condensin components may be mediated by the HEAT repeats in these two regions.
Role of CNAP1 NLS.
The structure of the CNAP1 bipartite NLS closely resembles the motif originally described in nucleoplasmin, in which both basic domains are interdependent (26). Currently, it is not clear why there is a functional NLS in CNAP1, since the majority of CNAP1 (and condensin) is cytoplasmic during interphase, and the nuclear membrane is being disassembled when the majority of condensin is associating with mitotic chromosomes. However, it is important to recall that mitotic chromosome condensation is initiated during G2 phase prior to nuclear membrane breakdown. It is possible that a subpopulation of condensin must be imported into the nucleus to maintain the chromatin-associated focus structures during interphase (28). Based on the colocalization of condensin foci with the phosphorylated H3 clusters on locally condensed chromosomes in late G2/early prophase, it was hypothesized that the nuclear condensin may be important for reinitiation of condensation (28). Alternatively, it may play a role in organizing interphase higher order chromatin structure important for regulating gene expression (21) and/or nucleolar organization (3, 28).
How CNAP1's NLS is inactivated in the cytoplasmic population of CNAP1/condensin is another open question. This appears to require the middle region of CNAP1. Further study will be needed to determine whether this region is involved in masking the NLS intramolecularly or in sequestration of CNAP1 (or condensin) by interacting with another cytoplasmic protein(s).
Interestingly, a PROSITE analysis revealed that hCAP-G has no bipartite NLS and is predicted to be a cytoplasmic protein. Although hCAP-H contains a putative bipartite NLS, deletion mutant analysis failed to show its function (J. E. Huh, A. R. Ball, and K. Yokomori, data not shown). Hence, it appears that among the non-SMC components of condensin, CNAP1 uniquely possesses a functional bipartite NLS.
The chromosome-targeting domain of CNAP1.
We found that the last 60 residues of the C terminus of CNAP1 are sufficient for mitotic chromosome binding. Within this region, a stretch of 21 aa is absolutely required for mitotic chromosome binding, which correlates with in vitro histone H1 and H3 binding and in vivo H3 binding. Previously, we demonstrated an in vivo interaction between endogenous condensin and histone H3 (28). The present study shows how CNAP1 may be responsible for this interaction.
Several recent studies have provided data that question the role of phosphorylated H3 in the condensation process (7, 8, 22). Although our data showed that H3 binding by the CNAP1 chromosome-targeting domain appears to be mediated by the interaction through the H3 tail, it is not affected by different tail modifications, including phosphorylation. This is consistent with our previous observation that condensin interacts with H3 in both interphase and mitosis (28) and with our current finding that the chromosome-targeting domain interacts with interphase histone H3 as well. Taken together, these findings show clearly that the histone H3 tail permits condensin binding but that its phosphorylation does not serve as a direct targeting signal.
It was somewhat surprising that the linker histone H1 appears to be one of the two histone targets for CNAP1. Although the dispensability of histone H1 in mitotic chromosome condensation has been demonstrated in Xenopus (25), H1 was shown to affect in vivo chromatin condensation in Tetrahymena (29). The functions of H1 in human cells are not fully understood. Since histone H1 is suggested to play a role in higher order chromatin folding (32), it will be interesting to determine whether this interaction is important for chromosome condensation in human cells.
Although closely overlapping, the amino acid residues important for nuclear localization in the CNAP1 chromosome-targeting domain are distinct from those required for chromosome association and histone binding, indicating that they are functionally separate. There is no other known structural motif and no obvious consensus sequence for histone- or DNA-binding identified in this region. Therefore, this domain defines a novel histone-binding motif that is critical for CNAP1 targeting to mitotic chromosomes.
Currently, it is not clear whether the chromosome-targeting function of the CNAP1 C-terminal domain can be explained solely by its ability to interact with histones. Preliminary results suggest the same domain is engaged in yet another interaction in the cell (J. T. Heale, A. R. Ball, Jr., and K. Yokomori, unpublished). It is likely that, although the interactions of the chromosome-targeting domain of CNAP1 with histones H1 and H3 may be important for facilitating the bulk targeting and/or stable chromosome association of CNAP1, additional factors may be necessary for the specific targeting of CNAP1 (and condensin) to mitotic chromosomes.
AKAP95 was shown to play a role in the recruitment of Eg7 (CNAP1) to chromosomes during early mitosis (30). However, it is not clear whether this interaction is direct. In the present study we did not observe a clear interaction of AKAP95 with condensin in HeLa cells by Co-IP with either anti-CNAP1 or anti-hCAP-E antibody, indicating that the interaction of AKAP95 and CNAP1 (or condensin) may be indirect (data not shown). Furthermore, we did not detect binding between AKAP95 and the C-terminal chromosome-targeting domain of CNAP1 (A. R. Ball, Jr., and K. Yokomori, unpublished). Thus, the relationship with AKAP95 is currently unclear. It is possible that although the C-terminal chromosome-targeting domain is relevant for stable association of condensin with mitotic chromosomes, the condensin-AKAP95 interaction may be important for loading of the complex onto chromatin.
Evolution of the C terminus of CNAP1.
A recent study in Xenopus described the isolation of a “regulatory subcomplex” termed 11SR that consists of all three non-SMC condensin subunits, although 11SR was not found in human cells and its in vivo relevance in human cells is not clear (16). It was shown that 11SR is incapable of binding to in vitro-assembled chromatin in the absence of the SMC heterodimer. This is in contrast to our in vivo observation that the CNAP1 mitotic chromosome-targeting domain is self-sufficient. It is possible that the other two non-SMC components of 11SR may repress the targeting function of CNAP1 while the three of them are in the subcomplex. This repression may be released with formation of the condensin holocomplex. Alternatively, stable association of CNAP1 with chromatin may require an additional factor(s) which may be missing in the in vitro system. It is also possible that there may be species-specific functional differences between human CNAP1 and its Xenopus homolog (XCAP-D2/Eg7), since Xenopus XCAP-D2 is missing the upstream basic sequence of the NLS, whereas the downstream motif is intact (Fig. 2A). Interestingly, in both Saccharomyces cerevisiae and S. pombe, the CNAP1 homologs are smaller and completely lack the 113-aa HE region (9, 31). The mouse CNAP1 homolog, on the other hand, has a bipartite organization similar to that found in the human protein (accession no. AK019259). Since the cdc2 phosphorylation sites also cluster to this region in both the human and Xenopus homologs, we speculate that this additional sequence in the vertebrate protein may reflect the requirement for more sophisticated regulation of nuclear localization and chromatin association of condensin, which may have further evolved in a distinct way in mammals.
It is conceivable that the other components of condensin, both SMC and non-SMC, also exert influence on the localization of condensin in the cell through the cell cycle. However, we found here that CNAP1 is capable of targeting itself to mitotic chromosomes, strongly suggesting that it plays a significant role in condensin association with mitotic chromosomes. Determining how the identified function of CNAP1 is controlled in the context of the condensin holocomplex remains an area for future work.
Acknowledgments
We thank C. D. Allis and C. P. Verrijzer for the histone H3 peptides and purified histone H1, respectively. We thank H. Liu, M. Nomura, and S. Sandmeyer for access to their Zeiss microscope.
This work was supported by GM59150 from NIH to K.Y. K.Y. is a Scholar of the Leukemia and Lymphoma Society.
REFERENCES
- 1.Akhmedov, A. T., C. Frei, M. Tsai-Pflugfelder, B. Kemper, S. M. Gasser, and R. Jessberger. 1998. Structural maintenance of chromosomes protein C-terminal domains bind preferentially to DNA with secondary structure. J. Biol. Chem. 273:24088-24094. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, D. E., A. Losada, H. P. Erickson, and T. Hirano. 2002. Condensin and cohesin display different arm conformations with characteristic hinge angles. J. Cell Biol. 156:419-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabello, O. A., E. Eliseeva, W.-G. He, H. Youssoufian, S. E. Plon, B. R. Brinkley, and J. W. Belmont. 2001. Cell cycle-dependent expression and nucleolar localization of hCAP-H. Mol. Biol. Cell 12:3527-3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung, P., K. G. Tanner, W. L. Cheung, P. Sassone-Corsi, J. M. Denu, and C. D. Allis. 2000. Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol. Cell 5:905-915. [DOI] [PubMed] [Google Scholar]
- 5.Cingolani, G., C. Petosa, K. Weis, and C. W. Müller. 1999. Structure of importin-β bound to the IBB domain of importin-α. Nature 399:221-229. [DOI] [PubMed] [Google Scholar]
- 6.Cubizolles, F., V. Legagneux, R. Le Guellec, I. Chartrain, R. Uzbekov, C. Ford, and K. Le Guellec. 1998. pEg7, a new Xenopus protein required for mitotic chromosome condensation in egg extracts. J. Cell Biol. 143:1437-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de la Barre, A.-E., D. Angelov, A. Molla, and S. Dimitrov. 2001. The N terminus of histone H2B, but not that of histone H3 or its phosphorylation, is essential for chromosome condensation. EMBO J. 20:6383-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Barre, A.-E., V. Gerson, S. Gout, M. Creaven, C. D. Allis, and S. Dimitrov. 2000. Core histone N termini play an essential role in mitotic chromosome condensation. EMBO J. 19:379-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman, L., L. Aragon-Alcaide, and A. Strunnikov. 2000. The condensin complex governs chromosome condensation and mitotic transmission of rDNA. J. Cell Biol. 149:811-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirano, T. 1999. SMC-mediated chromosome mechanics: a conserved scheme from bacteria to vertebrates? Genes Dev. 13:11-19. [DOI] [PubMed] [Google Scholar]
- 11.Hirano, T., R. Kobayashi, and M. Hirano. 1997. Condensins, chromosome condensation protein complexes containing XCAP-C, XCAP-E, and a Xenopus homolog of the Drosophila Barren protein. Cell 89:511-521. [DOI] [PubMed] [Google Scholar]
- 12.Hirano, T., and T. J. Mitchison. 1994. A heterodimeric coiled-coil protein required for mitotic chromosome condensation in vitro. Cell 79:449-458. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, G. D., and G. M. Nogueira Araujo. 1981. A simple method of reducing the fading of immunofluorescence during microscopy. J. Immunol. Methods 43:349-350. [DOI] [PubMed] [Google Scholar]
- 14.Katsani, K. R., J. J. Arredondo, A. J. Kal, and C. P. Verrijzer. 2001. A homeotic mutation in the trithorax SET domain impedes histone binding. Genes Dev. 15:2197-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura, K., M. Hirano, R. Kobayashi, and T. Hirano. 1998. Phosphorylation and activation of 13S condensin by Cdc2 in vitro. Science 282:487-490. [DOI] [PubMed] [Google Scholar]
- 16.Kimura, K., and T. Hirano. 1977. 2000. Dual roles of the 11S regulatory subcomplex in condensin functions. Proc. Natl. Acad. Sci. USA 97:11972-11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura, K., O. Cuvier, and T. Hirano. 2001. Chromosome condensation by a human condensin complex in Xenopus egg extracts. J. Biol. Chem. 276:5417-5420. [DOI] [PubMed] [Google Scholar]
- 18.Kimura, K., V. V. Rybenkov, N. J. Crisona, T. Hirano, and N. R. Cozzarelli. 1999. 13S condensin actively reconfigures DNA by introducing global positive writhe: implication for chromosome condensation. Cell 98:239-248. [DOI] [PubMed] [Google Scholar]
- 19.Knehr, M., M. Poppe, M. Enulescu, W. Eickelbaum, M. Stoehr, D. Schroeter, and N. Paweletz. 1995. A critical appraisal of synchronization methods applied to achieve maximal enrichment of HeLa cells in specific cell cycle phases. Exp. Cell Res. 217:546-553. [DOI] [PubMed] [Google Scholar]
- 20.Kutay, U., E. Izaurralde, F. R. Bischoff, I. W. Mattaj, and D. Görlich. 1997. Dominant-negative mutants of importin-β block multiple pathways of import and export through the nuclear pore complex. EMBO J. 16:1153-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lupo, R., A. Breiling, M. E. Bianchi, and V. Orlando. 2001. Drosophila chromosome condensation proteins topoisomerase II and Barren colocalize with Polycomb and maintain Fab-7 PRE silencing. Mol. Cell 7:127-136. [DOI] [PubMed] [Google Scholar]
- 22.MacCallum, D. E., A. Losada, R. Kobayashi, and T. Hirano. 2002. ISWI remodeling complexes in Xenopus egg extracts: identification as major chromosomal components that are regulated by INCENP-aurora B. Mol. Biol. Cell 13:25-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melby, T. E., C. N. Ciampaglio, G. Briscoe, and H. P. Erickson. 1998. The symmetrical structure of structural maintenance of chromosomes (SMC) and MukB proteins: long, antiparallel coiled coils, folded at a flexible hinge. J. Cell Biol. 142:1595-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neuwald, A. F., and T. Hirano. 2000. HEAT repeats associated with condensins, cohesins, and other complexes involved in chromosome-related functions. Genome Res. 10:1445-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohsumi, K., C. Katagiri, and T. Kishimoto. 1993. Chromosome condensation in Xenopus mitotic extracts without histone H1. Science 262:2033-2035. [DOI] [PubMed] [Google Scholar]
- 26.Robbins, J., S. M. Dilworth, R. A. Laskey, and C. Dingwall. 1991. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell 64:615-623. [DOI] [PubMed] [Google Scholar]
- 27.Schmiesing, J. A., A. R. Ball, H. C. Gregson, J. Alderton, S. Zhou, and K. Yokomori. 1998. Identification of two distinct human SMC protein complexes involved in mitotic chromosome dynamics. Proc. Natl. Acad. Sci. USA 95:12906-12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmiesing, J. A., H. C. Gregson, S. Zhou, and K. Yokomori. 2000. A human condensin complex containing hCAP-C/hCAP-E and CNAP1, a homolog of Xenopus XCAP-D2, colocalizes with phosphorylated histone H3 during the early stage of mitotic chromosome condensation. Mol. Cell. Biol. 20:6996-7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen, X., L. Yu, J. W. Weir, and M. A. Gorovsky. 1995. Linker histones are not essential and affect chromatin condensation in vivo. Cell 82:47-56. [DOI] [PubMed] [Google Scholar]
- 30.Steen, R. L., F. Cubizolles, K. Le Guellec, and P. Collas. 2000. A kinase-anchoring protein (AKAP)95 recruits human chromosome-associated protein (hCAP)-D2/Eg7 for chromosome condensation in mitotic extract. J. Cell Biol. 149:531-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutani, T., T. Yuasa, T. Tomonaga, N. Dohmae, K. Takio, and M. Yanagida. 1999. Fission yeast condensin complex: essential roles of non-SMC subunits for condensation and Cdc2 phosphorylation of Cut3/SMC4. Genes Dev. 13:2271-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thoma, F., T. Koller, and A. Klug. 1979. Involvement of histone H1 in the organizaiton of the nucleosome and of the salt-dependent superstructures of chromatin. J. Cell Biol. 83:403-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei, Y., L. Yu, J. Bowen, M. A. Gorovsky, and C. D. Allis. 1999. Phosphorylation of histone H3 is required for proper chromosome condensation and segregation. Cell 97:99-109. [DOI] [PubMed] [Google Scholar]
- 34.Yoshimura, S. H., K. Hizume, A. Murakami, T. Sutani, K. Takeyasu, and M. Yanagida. 2002. Condensin architecture and interaction with DNA: regulatory non-SMC subunits bind to the head of SMC heterodimer. Curr. Biol. 12:508-513. [DOI] [PubMed] [Google Scholar]